Abstract Abstract
Ants play essential roles in most terrestrial ecosystems and may be considered pests for agriculture and agroforestry. Recent morphological and molecular data have challenged conventional ant phylogeny and the interpretation of karyotypic variations. Existing Neotropical ant cytogenetic data focus on Atlantic rainforest species, and provide evolutionary and taxonomic insight. However, there are data for only 18 Amazonian species. In this study, we describe the karyotypes of 16 ant species belonging to 12 genera and three subfamilies, collected in the Brazilian state of Amapá, and in French Guiana. The karyotypes of six species are described for the first time, including that of the South American genus Allomerus Mayr, 1878. The karyotype of Crematogaster Lund, 1831 is also described for the first time for the New World. For other species, extant data for geographically distinct populations was compared with our own data, e.g. for the leafcutter ants Acromyrmex balzani (Emery, 1890) and Atta sexdens (Linnaeus, 1758). The information obtained for the karyotype of Dolichoderus imitator Emery, 1894 differs from extant data from the Atlantic forest, thereby highlighting the importance of population cytogenetic approaches. This study also emphasizes the need for good chromosome preparations for studying karyotype structure.
Keywords: Formicidae , karyotype, Neotropical ants, biodiversity
Introduction
Ants are a diverse group of insects comprising more than 16,000 described species and about 6,000 species yet to be described (Ward 2013), and can represent up to 20% of terrestrial animal biomass in tropical regions (Schultz 2000). Considered good indicators of ecosystem diversity or disturbance (reviewed in Andersen 2018), some ant species play important roles in ecosystems (e.g., seed dispersal, plant protection, predation) whereas other species are considered agricultural pests (Hölldobler and Wilson 1990). However, many ant species belong to cryptic species complexes, making accurate description and the understanding of their biogeographical distribution difficult.
Usually, species identification relies on external morphological traits, but this approach is ineffective in cases where two or more species cannot be morphologically differentiated (Bickford et al. 2007). Complementary biological information can be used in these instances (Schlick-Steiner et al. 2010). Considering recent revisions of higher taxa (Ward et al. 2015, 2016, Sosa-Calvo et al. 2017, 2019), cytogenetics could be used to solve taxonomic issues related to the family Formicidae. Cytogenetics is particularly useful in understanding species evolution and population dynamics because chromosome modifications play a direct role in speciation events and generate heritable variation (King 1993, Aguiar et al. 2017).
More than 800 species of Formicidae have been cytogenetically studied to date (reviewed in Lorite and Palomeque 2010, Mariano et al. 2015, 2019). Cytogenetic research of Neotropical ants has focused on species found in the Atlantic forest biome in Brazil, with few data for other regions and countries. Population studies in ant cytogenetics remain scarce, e.g. Typhlomyrmex rogenhoferi Mayr, 1862 (Mariano et al. 2006), Dinoponera lucida Emery, 1901 (Mariano et al. 2008), Pachycondyla spp. (Mariano et al. 2012), and Camponotus rufipes (Fabricius, 1775) (Aguiar et al. 2017). However, cytogenetic data can be used to identify cryptic species, which are common in Formicidae (Seifert 2009). Cytogenetic data have advanced our understanding of biology, reproduction, phylogeny, taxonomy, and evolution, and facilitated investigation of cryptic and threatened species (Lorite and Palomeque 2010).
Cytogenetic data are only available for 18 ant species from the Amazon region, mostly (13 species) from French Guiana (Mariano et al. 2006, 2011, 2012, Santos et al. 2010), with four species from the state of Pará, Brazil (Sposito et al. 2006, Mariano et al. 2006, Santos et al. 2012, Mariano et al. 2015), and one species from Amapá, Brazil (Aguiar et al. 2017). Until now, only data for T. rogenhoferi, is available for two locations: Pará, Brazil and French Guiana (Mariano et al. 2006). This species shows an interesting cline variation, which highlights the importance of population assays. In the present study, new data for 16 ant species from the Eastern Amazon are presented using cytogenetic analysis (chromosome number and morphology), with phylogenetic insights into three subfamilies.
Material and methods
Ant colonies were collected in French Guiana at three locations: Montagne des Singes, Kourou (5.07225N, 52.69407W), Campus Agronomique, Kourou (5.17312N, 52.65480W), and Sinnamary (5.28482N, 52.91403W). Colonies were collected in Brazil at Oiapoque, state of Amapá (3.84151N, 51.84112W) (Table 1). Sampling permission was given by the Instituto Chico Mendes de Conservação da Biodiversidade (ICMBio) to Luísa Antônia Campos Barros (SISBIO accession number 32459). Specimens were identified by Jacques Hubert Charles Delabie and deposited in the reference collection at the Laboratório de Mirmecologia, Centro de Pesquisas do Cacau (CPDC/Brazil), as items #5802 and #5803.
Table 1.
Ant species cytogenetically studied from North-eastern Amazonia. Diploid (2n) and haploid (n) chromosome numbers, karyotypic formulae, sample sizes (numbers of colonies/individuals) and localities are given.
| Species | 2n(n) | Karyotypic formula | Col/Ind | Locality |
|---|---|---|---|---|
| Subfamily Ponerinae | ||||
| Anochetus targionii Emery, 1894* | 30 | 2n = 16m+2sm+2st+10a | 1/5 | Campus Agronomique, Kourou, FG |
| Odontomachus haematodus Linnaeus, 1758 | 44 | 2n = 8sm+18st+18a | 3/8 | Campus Agronomique, Kourou, FG |
| Pseudoponera stigma Fabricius, 1804 | 14 | 2n = 14m | 1/4 | Oiapoque, BR |
| Pseudoponera gilberti (Kempf, 1960) | 12 | 2n = 10m+2sm | 1/6 | Sinnamary, FG |
| Subfamily Myrmicinae | ||||
| Atta sexdens Linnaeus, 1758 | 22 | 2n = 18m+2sm+2st | 2/12 | Campus Agronomique, Kourou, FG; Oiapoque, BR |
| Acromyrmex balzani Emery, 1890 | 38 | 2n = 12m+10sm+14st+2a | 1/10 | Campus Agronomique, Kourou, FG |
| Cyphomyrmex transversus Emery, 1894 | 24(12) | 2n = 14m+6sm+4a (n = 7m+3sm+2a) | 2/8 | Campus Agronomique, Kourou, FG |
| Myrmicocrypta sp. | 30 | 2n = 22m+2sm+6a | 1/6 | Sinnamary, FG |
| Allomerus decemarticulatus Mayr, 1878* ; Hirtella physophora Martius et Zuccarini, 1832 † | 28 | 2n = 18m+6sm+2a | 4/9 | La Montagne des Singes, Kourou, FG |
| Allomerus octoarticulatus var. demerarae Mayr, 1878* ; Cordia nodosa Lamarck, 1792 † | 44 | 2n = 4sm+40a | 5/12 | La Montagne des Singes, Kourou, FG |
| Allomerus octoarticulatus Mayr, 1878*; Hirtella physophora † | 44 | 2n = 4sm+40a | 5/11 | La Montagne des Singes, Kourou FG |
| Crematogaster longispina Emery, 1890* | 24 | 2n = 20m+4sm | 1/4 | Sinnamary, FG |
| Strumigenys diabola Bolton, 2000* | 40 | 2n = 18sm+12st+10a | 1/3 | Sinnamary, FG |
| Wasmannia auropunctata Roger, 1863 | 32 | 2n = 16m+13sm+5st | 1/6 | Campus Agronomique, Kourou, FG |
| Solenopsis geminata Fabricius, 1804 | 32 (16) | 2n = 14m+12sm+6st (n = 7m+6sm+3st) | 1/5 | Sinnamary, FG |
| Subfamily Dolichoderinae | ||||
| Dolichoderus imitator Emery, 1894 | 46 | 2n = 6m+28sm+12a | 1/5 | Sinnamary, FG |
* first cytogenetic report. † host plant. FG: French Guiana, BR: Brazil
Metaphases were obtained from the cerebral ganglia of the larvae after meconium elimination, according to Imai et al. (1988). Chromosome number and morphology of metaphases were analyzed using conventional 4% Giemsa staining. Chromosome morphology was defined according to Levan et al. (1964) using the ratio of chromosome arms (long arm/short arm). Metaphases and chromosomes were karyotyped using Adobe Photoshop CC and measured using Image Pro Plus.
Results and discussion
Sixteen ant species belonging to 12 genera and three subfamilies have been cytogenetically analyzed (Table 1). The karyotypes of six species are described for the first time, including karyotypic information for the genus Allomerus Mayr, 1878. Another genus, Crematogaster Lund, 1831, is cytogenetically analyzed for the first time in the Neotropical region. Karyotypes of ten species, including the leafcutter ants Acromyrmex Mayr, 1865 and Atta Fabricius, 1804, previously described in other localities, were compared with our own data.
Ponerinae: Ponerini: Anochetus and Odontomachus
Anochetus Mayr, 1861 is a monophyletic genus and a sister genus of Odontomachus Latreille, 1804 (Larabee et al. 2016, Fernandes 2017). Morphologically, they belong to the subtribe Odontomachiti of trap-jaw ants (Brown-Jr 1976).
Anochetus targionii has 2n = 30 chromosomes (Fig. 1a), which is considered as a modal number according to Santos et al. (2010). Anochetus chromosome numbers range from 2n = 24–46, which represents higher karyotype diversity than that found in Odontomachus (2n = 32–42) (reviewed in Mariano et al. 2019). However, only 12 morphospecies out of 113 valid species of Anochetus have been cytogenetically analyzed: nine from the Indo-Malayan and three from the Neotropics, A. altisquamis Mayr, 1887 (2n = 30), A. horridus Kempf, 1964 (2n = 46), and A. emarginatus (Fabricius, 1804) (2n = 28) (Santos et al. 2010, Mariano et al. 2015).
Figure 1.

Karyotypes of the tribe Odontomachiti (Ponerinae): aAnochetus targionii (2n = 30) bOdontomachus haematodus (2n = 44). Scale bar: 5µm.
Since Anochetus diversified earlier than Odontomachus (Larabee et al. 2016, Fernandes 2017), higher karyotypic variation in Anochetus would be expected (Santos et al. 2010). Anochetus targionii has the same chromosome number as A. altisquamis, A. modicus Brown, 1978, and A. graeffei Mayr, 1870. It seems that 2n = 30 chromosomes is the plesiomorphic condition since it is found throughout the genus Anochetus and is present in A. altisquamis, which is considered a phylogenetically “basal” clade (Larabee et al. 2016, Fernandes 2017). Anochetus species also share a constant number of acrocentric chromosomes. Within the lineage of A. horridus, chromosome fission seems to have played an important role in recent karyotype evolution, increasing the number of chromosomes in the karyotype. According to Larabee et al. (2016), A. horridus diversified around 25 million years ago (MYA), whereas A. targionii diversified less than 10 MYA.
Odontomachus haematodus has 2n = 44 chromosomes, of which 18 are acrocentric (Fig. 1b), confirming information provided by Santos et al. (2007, unpublished data) and Aguiar et al. (2012, unpublished data) (reviewed in Mariano et al. 2019). Similar to Anochetus, Odontomachus species seem to have characteristic karyotypes that are slightly different between Odontomachus phylogenetic clades.
The Indo-Pacific species, O. rixosus Smith, 1857 and O. latidens Mayr, 1867, have 2n = 30 chromosomes but no further information about their karyotypes is available (reviewed in Lorite and Palomeque 2010). The other known karyotype of Odontomachus species belongs to the haematodus group according to molecular phylogeny (Larabee et al. 2016). All known karyotypes from the haematodus group (reviewed in Santos et al. 2010) have 44 chromosomes, including O. haematodus, whose karyotype is described in this study. Odontomachus chelifer (Latreille, 1802) has plesiomorphic traits and the highest number of acrocentric chromosomes (40) among Indo-Pacific species (reviewed in Santos et al. 2010). The species O. meinerti Forel, 1905 and O. bauri Emery, 1892 have 34 and 14 acrocentric chromosomes out of 44 respectively (Teixeira 2018).
This suggests that heterochromatin growth at telomeric regions of shorter arms of acrocentric chromosomes may be significant in Odontomachus karyotype evolution. This is in accordance with the Minimum Interaction Theory proposed by Imai et al. (1994), which proposes that reduced interactions between different chromosomes inside the nucleus increases the fitness of the individual.
Ponerinae: Ponerini: Pseudoponera
The genus Pseudoponera Emery, 1900 has six valid species (Bolton 2019). Two species Pseudoponera gilberti and P. stigma are near-identical morphologically. Conflicting cytogenetic analyses (Mariano et al. 2012) due to misidentification have recently been resolved and the two species distinguished in samples from the Atlantic forest (Correia et al. 2016). The chromosome number for P. gilberti is 2n = 12 (10m + 2sm) and for P. stigma was 2n = 14, all of them metacentrics. In spite of minor differences in chromosome morphology of P. stigma between Atlantic forest (Correia et al. 2016) and Amazonia, both karyotypes share the same chromosome number.
Figure 2.
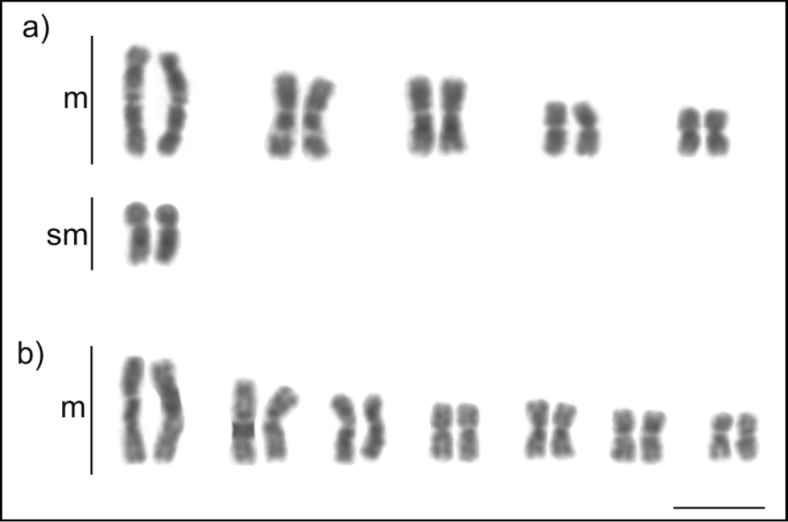
Karyotypes of the genus Pseudoponera (Ponerinae): aP. gilberti (2n = 12) bP. stigma (2n = 14). Scale bar: 5µm.
Myrmicinae: Attini: Attina: Atta and Acromyrmex
The fungus-growing ants from the genus Acromyrmex form a sister group of the genus Atta and together are believed to be monophyletic. There are 33 valid species of Acromyrmex and 18 species of Atta (Bolton 2019), with wide distributions throughout the Neotropics (Delabie et al. 2011). The relationship between Atta and Acromyrmex became clearer under a combined approach using morphological, molecular, and cytogenetic tools (Cristiano et al. 2013). Cytogenetic data are available for five Atta species (Barros et al. 2011, 2014, 2015) from three of the four monophyletic groups according to the molecular phylogeny proposed by Bacci et al. (2009) and 13 species of Acromyrmex (reviewed in Barros et al. 2011, 2016, Teixeira et al. 2017).
The leaf-cutter ant Atta sexdens has 2n = 22 (Fig. 3a), and chromosome morphology is the same (18m + 2sm + 2st) to that of other Atta species from the Brazilian savannah and Atlantic Forest (Barros et al. 2014, 2015). The Amazonian population of Acromyrmex balzani analyzed in this study has 2n = 38 chromosomes and the same karyotype (Fig. 3b) as that of the Brazilian savannah and Atlantic forest populations (Barros et al. 2016). The largest metacentric pair of A. balzani is large, about twice the length of the largest subtelocentric chromosome previously identified in other Brazilian populations of this species (Barros et al. 2016). In all other Acromyrmex species studied so far, the former pair of chromosomes is of similar length. Based on the recent checklist of the ants of French Guiana (Franco et al. 2019) this is the first record for A. balzani in French Guiana and also the first cytogenetic analysis of this species in the region.
Figure 3.

Karyotypes of fungus-growing ants (Myrmicinae, Attini: Attina): aAtta sexdens (2n = 22) bAcromyrmex balzani (2n = 38) cCyphomyrmex transversus (2n = 24) dC. transversus (n = 12, male karyotype) eMyrmicocrypta sp. (2n = 30). Scale bar: 5µm.
So far, all karyotype analyses showed that Atta spp. have 2n = 22 chromosomes and Acromyrmex spp. have 2n = 38 chromosomes (Barros et al. 2016, Teixeira et al. 2017). Acromyrmex striatus (Roger, 1863) is an exception, with 2n = 22 chromosomes, the same as Atta spp. and is considered the sister group of leaf-cutter ants (Cristiano et al. 2013). There are variations between the morphological features of certain chromosomes in Acromyrmex due to heterochromatin growth (Barros et al. 2016). Interpopulation cytogenetic studies for ants are scarce (e.g., Mariano et al., 2006, 2012, Aguiar et al. 2017) and none are available for leaf-cutter ants.
Myrmicinae: Attini: Attina: Cyphomyrmex
The fungus-growing attine Cyphomyrmex transversus has 2n = 24 and n = 12 with mostly metacentric and submetacentric chromosomes (Fig. 3c, d) which differs from 2n = 42, all of them acrocentric, observed by Mariano et al. (2019), highlighting the importance of detailed cytogenetic studies in this species. It has a range from northern Brazil to central Argentina including the northeastern regions of Brazil (Kempf 1965). Cyphomyrmex transversus and the three other Cyphomyrmex species which have been karyotyped (see Sosa-Calvo et al. 2017 for recent taxonomic changes) have chromosome numbers ranging between 2n = 20 and 2n = 42 (reviewed in Mariano et al. 2019). It seems that the high proportion of metacentric chromosomes is characteristic of this genus. In spite of morphological affinity of C. transversus (present study) to C. rimosus (Spinola, 1851), observed by Kempf (1965), the karyotype divergence between them (2n = 24 and 2n = 32, respectively; Murakami et al. 1998) is puzzling because of the numerical difference of their karyotypes coupled with similar morphology of chromosomes. These findings merit further study using advanced chromosome banding and molecular phylogenetic techniques.
Myrmicinae: Attini: Attina: Myrmicocrypta
The fungus-growing species Myrmicocrypta sp. had 2n = 30 chromosomes, 18 of them metacentric (Fig. 3e). The studied colony was collected from the cavities of a rotten log. Myrmicocrypta Smith, 1860 is the sister genus of Mycocepurus Forel, 1893, and both are members of the clade Palleoattina (Sosa-Calvo et al. 2017). Myrmicocrypta is widely distributed in the Neotropics, from Mexico to Argentina and includes 27 valid species (Bolton 2019). A recent study by Sosa-Calvo et al. (2019) suggests that only two species can nest in rotten logs: M. spinosa Weber, 1937 and the undescribed species, M. JSC001. This is a derived characteristic for Myrmicocrypta and therefore this clade is apparently monophyletic. The only extant cytogenetic data available for this genus are from the Montagne des Singes area, French Guiana (Mariano et al. 2011), about 60 km from where the samples from the present study were collected.
Since the studied sample was identified as an undescribed species, it is possible that the present species is M. JSC001. Myrmicocrypta spinosa has not been recorded in French Guiana: the samples studied by Sosa-Calvo et al. (2019) included only M. JSC001. The species studied by Mariano et al. (2011) had a slightly different karyotype, probably as a result of variation in the chromosome condensation. These results highlight the importance of good chromosome preparations for studying karyotype configuration.
Myrmicinae: Attini: Allomerus
This study represents the first cytogenetic analysis for the genus Allomerus. A. decemarticulatus and the A. octoarticulatus species complex had 2n = 28 and 2n = 44, respectively (Fig. 4). The Allomerus species are specialist ants inhabiting diverse obligate myrmecophytic plants in South America. Allomerus decemarticulatus and A. octoarticulatus, have been intensively studied in French Guiana from an ecological perspective: they build galleries using the fungus Trimmatostroma cordae Sharma & Singh, 1976 (see Dejean et al. 2005, Ruiz-González et al. 2011). The molecular phylogeny of the genus showed that A. octoarticulatus is a complex of two species that cannot be separated morphologically (Orivel et al. 2017). However, these two species are associated with different plants: A. octoarticulatus var. demerarae inhabits only Cordia nodosa, while A. octoarticulatus can be associated with several myrmecophytic plant species throughout its distribution range.
Figure 4.

Karyotypes of the genus Allomerus (Myrmicinae): aA. decemarticulatus (2n = 28) bA. octoarticulatus var. demerarae (2n = 44) associated with Cordia nodosacA. octoarticulatus (2n = 44) associated with Hirtella sp. Scale bar: 5µm.
The number of acrocentric chromosomes is highly different between these two species, even though meta/submetacentric and acrocentric chromosomes predominate in A. decemarticulatus and A. octoarticulatus, respectively. According to the Minimum Interaction Theory, centric fissions may have played an important role in the chromosome evolution of Allomerus; however, the karyotypes of additional species should be investigated to support this conclusion. A comparison between the two species of A. octoarticulatus, which nest in different plant species, was also made (Fig. 4b, c). However, basic cytogenetic techniques (chromosome number and morphology) could not differentiate between the two. Additional banding techniques with molecular probes may further illuminate this question in the future.
Myrmicinae: Attini: Strumigenys
Strumigenys diabola has 2n = 40 (Fig. 5a) with many chromosomes having short arms (submeta/subtelocentrics). The genus Strumigenys Smith, 1860 harbors small cryptic species specialized in preying on collembolans. There are currently more than 800 valid species of Strumigenys (Bolton 2019) of which 190 are from the Neotropics. Strumigenys diabola are reported in northern and northeastern Brazil and in French Guiana (Janicki et al. 2016). For the Neotropics, cytogenetic data was previously only available for Strumigenys louisianae Roger, 1863, which has 2n = 4 (Alves-Silva et al. 2014) and for eight species from Asia and Oceania (Lorite and Palomeque 2010). This is the second cytogenetic record in Neotropics and the absence of data in the Strumigenys mandibularis group sensu Bolton (2000) make comparisons with other species impossible. Further studies of this genus will help understanding chromosome evolution and phylogeny of the group.
Figure 5.
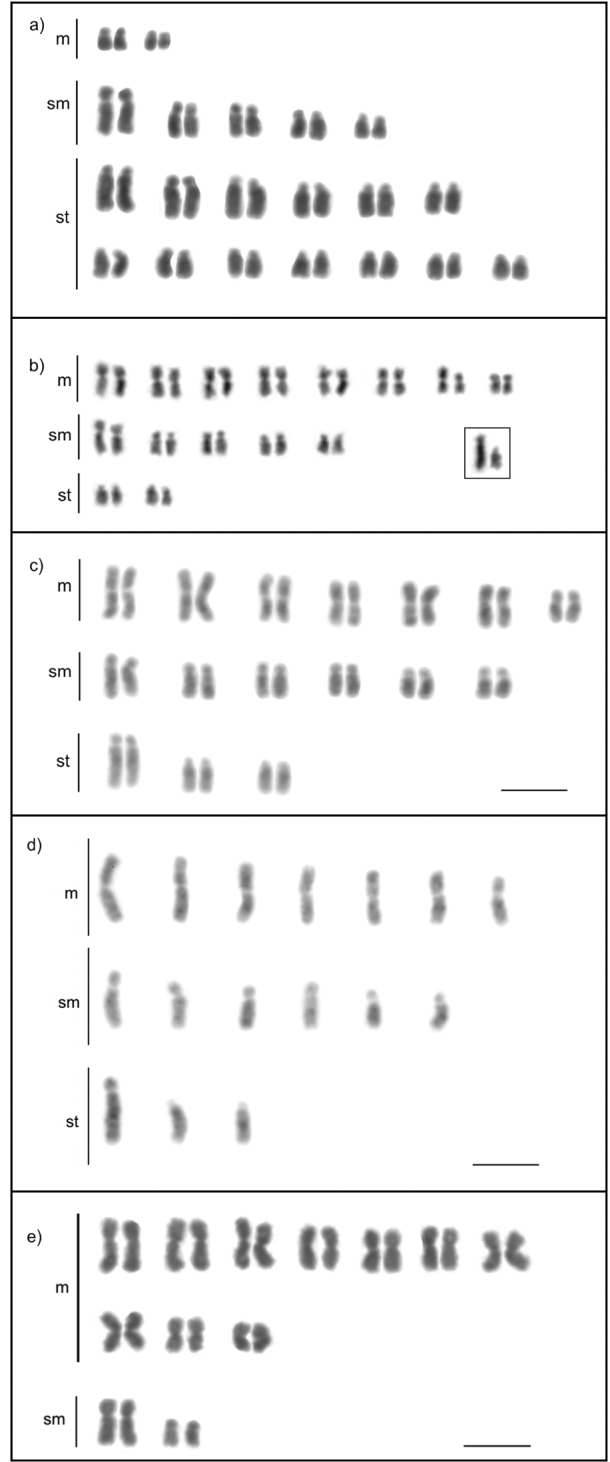
Karyotypes of four genera of Myrmicinae: aStrumigenys diabola (2n = 40) bWasmannia auropunctata (2n = 32) cSolenopsis geminata (2n = 32) dS. geminata (n = 16, male karyotype) eCrematogaster longispina (2n = 24). Box shows the chromosome pair with size heteromorphism. Scale bar: 5µm.
Myrmicinae: Attini: Wasmannia
In this study, workers of W. auropunctata had 2n = 32 (Fig. 5b), with one chromosome pair showing considerable size heteromorphism in all individuals analyzed. The genus Wasmannia Forel, 1893 includes 11 species and is endemic to the Neotropics. The “little fire ant” W. auropunctata is notable because of its reproductive mechanism (Fournier et al. 2005). It has three different genetic systems: haplodiploidy, male clonality, and thelytoky (Foucaud et al. 2006). In this study, the same chromosome number and a similar karyotype from colonies from Ilhéus and Una, southeast Bahia, Brazil were observed (Souza et al. 2011), although there are differences in chromosome classification. The heteromorphic pattern was not described for the Atlantic forest population and therefore needs to be investigated further.
Myrmicinae: Solenopsidini: Solenopsis
Our analysis found 2n = 32 in female Solenopsis geminata and n = 16 in males with most chromosomes (26) being metacentric or submetacentric (Fig. 5c, d). The genus Solenopsis Westwood, 1840 is difficult to identify at the species level, although these species form obvious natural groups (Pacheco and Mackay 2013). The chromosome number for this species in our analysis is the same as that observed in five previously described fire ant species including S. geminata, (reviewed in Lorite and Palomeque 2010) which belong to the subgenus Solenopsis (Pacheco and Mackay 2013).
We compared our data with those from colonies of S. geminata from the USA (Crozier 1970) and India (Imai et al. 1984). The karyotype from French Guiana is similar to that from India, despite certain differences in chromosome classification. Differences in karyotypic formula among various localities and colonies were reported by Imai et al. (1984) based on their observation of the presence/absence of the short arm in some chromosomes as a result of C-band polymorphisms. Those patterns demonstrate the importance of understanding heterochromatin dynamics at the population level for analyzing karyotype evolution of ants.
Myrmicinae: Crematogastrini: Crematogaster
The ant genus Crematogaster is a global, widespread, and species-rich clade. It currently comprises 498 valid species and is divided into two subgenera, Crematogaster sensu stricto and Crematogaster (Orthocrema) Santschi, 1918 (Blaimer 2012a, b). The subgenus Orthocrema is more complex, and numerous clades exist within this group. Crematogaster longispina, which belongs to the subgenus Orthocrema, has 2n = 24, and all chromosomes are meta/submetacentrics (Fig. 5e). This is the first New World Crematogaster karyotype ever described, which makes reasonable comparisons difficult. Karyotype data is available for 17 morphospecies of Crematogaster from Malaysia, Indonesia, India, Japan, and Australia (reviewed in Lorite and Palomeque 2010).
Within Crematogaster spp., the chromosome number ranges from 2n = 24–58, with 10 morphospecies having 2n = 24 or 26. Increasing the number of studied species in the Neotropics may help to understand the chromosome evolution of the group.
Dolichoderinae: Dolichoderus
Dolichoderus Lund, 1831 is the most speciose ant genus in the subfamily Dolichoderinae, with 130 valid species (Bolton 2019). Chromosomal data is available for seven species collected from the Atlantic forest (Santos et al. 2016) and four species from the Indo-Malayan region (reviewed in Lorite and Palomeque 2010). The genus demonstrates high chromosome variation, 2n = 10–52, and is the most cytogenetically diverse genus within Dolichoderinae. According to the molecular phylogeny produced by Santos et al. (2016), this species occupies a less derived position, which agrees with previous conclusions that suggest that this species belongs to a separate species group (Mackay 1993). The chromosome number already known for this species is 2n = 38, and it also has many meta/submetacentric chromosomes (Santos et al. 2016). However, in this study, additional acrocentric chromosomes were observed in D. imitator (2n = 46). Chromosomal intraspecific variation in Dolichoderus has not previously been reported. This again emphasizes the importance of karyotypic studies at the level of certain populations, which may represent either geographic clines or a species complex. Enhancing population studies for this species may have important implications for our understanding of both taxonomy and chromosome evolution of Formicidae.
Figure 6.
Karyotype of Dolichoderus imitator (2n = 46) (Dolichoderinae). Scale bar: 5µm.
Conclusion
Our study increased the number of karyotyped Amazonian ant species from 18 to 34. The karyotype of 16 species were analyzed, six of them for the first time, which permitted comparisons with previously studied species, including population studies of leaf-cutting ants (Atta sexdens and Acromyrmex balzani). Although cytogenetic analysis of more than 800 ant species is available, there are no data for many genera, including many Neotropical ones. This paper includes the first description of the karyotype of a Crematogaster species ever reported for the New World.
Conventional cytogenetics constitutes a powerful tool in characterizing cryptic biodiversity (Cioffi et al. 2018). For example, our study of D. imitator showed substantial differences between chromosome numbers of the previously studied Atlantic forest karyotype and that of our study, strongly suggesting the presence of different species. Future studies on molecular cytogenetics will have important implications for understanding the chromosome evolution of ants, focusing especially on the genus Allomerus and fungus-growing ants.
Acknowledgments
Financial support for this study was provided by “Investissement d’Avenir” grants managed by the French Agence Nationale de la Recherche (DRIIHM ref. ANR-11-LABX-0010 and CEBA, ref. ANR-10-LABX-25-01), by the Programa de Auxílio ao Pesquisador – PAPESQ/UNIFAP/2017, by the PO-FEDER 2014-2020, Région Guyane (BiNG, ref. GY0007194), and by the scholarship grant from Conselho Nacional de Desenvolvimento Científico e Tecnológico (CNPq) to LIS.
Citation
Aguiar HJAC, Barros LAC, Silveira LI, Petitclerc F, Etienne S, Orivel J (2019) Cytogenetic data for sixteen ant species from North-eastern Amazonia with phylogenetic insights into three subfamilies. Comparative Cytogenetics 14(1): 43–60. https://doi.org/10.3897/CompCytogen.v14i1.46692
Funding Statement
Agence Nationale de la Recherche, PO-FEDER 2014-2020
References
- Aguiar HJAC, Barros LAC, Alves DR, Mariano CSF, Delabie JHC, Pompolo SG. (2017) Cytogenetic studies on populations of Camponotus rufipes (Fabricius, 1775) and Camponotus renggeri Emery, 1894 (Formicidae: Formicinae). PloS ONE 12(5): e0177702. 10.1371/journal.pone.0177702 [DOI] [PMC free article] [PubMed]
- Alves-Silva AP, Barros LAC, Chaul JCM, Pompolo SG. (2014) The First Cytogenetic Data on Strumigenys louisianae Roger, 1863 (Formicidae: Myrmicinae: Dacetini): The Lowest Chromosome Number in the Hymenoptera of the Neotropical Region. PloS ONE 9(11): e111706. 10.1371/journal.pone.0111706 [DOI] [PMC free article] [PubMed]
- Andersen AN. (2018) Responses of ant communities to disturbance: five principles for understanding the disturbance dynamics of a globally dominant faunal group. Journal of Animal Ecology 2018: 1–3. 10.1111/1365-2656.12907 [DOI] [PubMed] [Google Scholar]
- Bacci Jr M, Solomon SE, Mueller UG, Martins VG, Carvalho AO, Vieira LG, Silva-Pinhati AC. (2009) Phylogeny of leafcutter ants in the genus Atta Fabricius (Formicidae: Attini) based on mitochondrial and nuclear DNA sequences. Molecular Phylogenetics and Evolution 51(3): 427–437. 10.1016/j.ympev.2008.11.005 [DOI] [PubMed] [Google Scholar]
- Barros LAC, Mariano CSF, Pompolo SG, Delabie JHC. (2011) Citogenética de Attini. In: Della Lucia TMC. (Ed.) Formigas-cortadeiras da biotecnologia ao manejo.Editora da Universidade Federal de Viçosa, Viçosa, Brazil, 68–79. [In Portuguese]
- Barros LAC, Teixeira GA, Aguiar HJAC, Mariano CSF, Delabie JHC, Pompolo SG. (2014) Banding patterns of three leafcutter ant species of the genus Atta (Formicidae: Myrmicinae) and chromosomal inferences. Florida Entomologist 97(4): 1694–1701. 10.1653/024.097.0444 [DOI] [Google Scholar]
- Barros LAC, Aguiar HJAC, Teixeira GA, Mariano CSF, Teixeira MC, Delabie JHC, Pompolo SG. (2015) Cytogenetic data on the threatened leafcutter ant Atta robusta Borgmeier, 1939 (Formicidae: Myrmicinae: Attini). Comptes Rendus Biologies 338(10): 660–665. 10.1016/j.crvi.2015.07.006 [DOI] [PubMed] [Google Scholar]
- Barros LAC, Aguiar HJAC, Mariano CSF, Andrade-Souza V, Costa MA, Delabie JHC, Pompolo SG. (2016) Cytogenetic data on six leafcutter ants of the genus Acromyrmex Mayr, 1865 (Hymenoptera, Formicidae, Myrmicinae): insights into chromosome evolution and taxonomic implications. Comparative Cytogenetics 10(2): 229–243. 10.3897/CompCytogen.v10i2.7612 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bickford D, Lohman DJ, Sodhi NS, Ng PK, Meier R, Winker K, Ingram KK, Das I. (2007) Cryptic species as a window on diversity and conservation. Trends in Ecology and Evolution 22(3): 148–155. 10.1016/j.tree.2006.11.004 [DOI] [PubMed] [Google Scholar]
- Blaimer BB. (2012a) A subgeneric revision of Crematogaster and discussion of regional species-groups (Hymenoptera: Formicidae). Zootaxa 3482: 47–67. https://www.mapress.com/j/zt/article/view/zootaxa.3482.1.3 [Google Scholar]
- Blaimer BB. (2012b) Acrobat ants go global – Origin, evolution and systematics of the genus Crematogaster (Hymenoptera: Formicidae). Molecular Phylogenetics and Evolution 65(2): 421–436. 10.1016/j.ympev.2012.06.028 [DOI] [PubMed] [Google Scholar]
- Bolton B. (2019) An Online Catalog of the Ants of the World. http://antcat.org [accessed 31 Jan 2019]
- Brown-Jr WL. (1976) Contributions toward a reclassification of the Formicidae. Part VI. Ponerinae, tribe Ponerini, subtribe Odontomachiti. Section A. Introduction, Subtribal characters. Genus Odontomachus. Studia Entomologica 19(1–4): 66–171. [Google Scholar]
- Cioffi MB, Moreira-Filho O, Ráb P, Sember A, Molina WF, Bertollo LAC. (2018) Conventional Cytogenetic Approaches – Useful and Indispensable Tools in Discovering Fish Biodiversity. Current Genetic Medicine Reports 6(4): 176–186. 10.1007/s40142-018-0148-7 [DOI] [Google Scholar]
- Correia JPSO, Mariano CSF, Delabie JHC, Lacau S, Costa MA. (2016) Cytogenetic analysis of Pseudoponera stigma and Pseudoponera gilberti (Hymenoptera: Formicidae: Ponerinae): a taxonomic approach. Florida Entomologist 99(4)1: 718–721. 10.1653/024.099.0422 [DOI]
- Cristiano MP, Cardoso DC, Fernandes-Salomão TM. (2013) Cytogenetic and molecular analyses reveal a divergence between Acromyrmex striatus (Roger, 1863) and other congeneric species: taxonomic implications. PLoS One 8(3): e59784. 10.1371/journal.pone.0059784 [DOI] [PMC free article] [PubMed]
- Crozier RH. (1970) Karyotypes of twenty-one ant species (Hymenoptera; Formicidae), with reviews of the known ant karyotypes. Canadian Journal of Genetics and Cytology 12(1): 109–128. 10.1139/g70-018 [DOI] [PubMed] [Google Scholar]
- Dejean A, Solano PJ, Ayroles J, Corbara B, Orivel J. (2005) Insect behaviour: arboreal ants build traps to capture prey. Nature 434(7036): 973 10.1038/434973a [DOI] [PubMed] [Google Scholar]
- Delabie JHC, Alves HR, Reuss-Strenzel GM, Carmo AD, Nascimento ID. (2011) Distribuição das formigas cortadeiras dos gêneros Acromyrmex e Atta no Novo Mundo. Formigas Cortadeiras: da Bioecologia ao Manejo (1st edn). Editora da Universidade Federal de Viçosa, Viçosa, 80–101.
- Fernandes IO. (2017) Análise filogenética de Anochetus Mayr, 1961 e Odontomachus Latreille, 1804 (Hymenoptera: Formicidae: Ponerinae) e revisão taxonômica de Anochetus para a região Neotropical. PhD Thesis, Instituto Nacional de Pesquisas da Amazônia, Manaus, Brazil. [In Portuguese]
- Foucaud J, Jourdan H, Le Breton J, Loiseau A, Konghouleux D, Estoup A. (2006) Rare sexual reproduction events in the clonal reproduction system of introduced populations of the little fire ant. Evolution 60: 1646–1657. 10.1111/j.0014-3820.2006.tb00509.x [DOI] [PubMed] [Google Scholar]
- Fournier D, Estoup A, Orivel J, Foucaud J, Jourdan H, Le Breton J, Keller L. (2005) Clonal reproduction by males and females in the little fire ant. Nature 435: 1230–1234. 10.1038/nature03705 [DOI] [PubMed] [Google Scholar]
- Franco W, Ladino N, Delabie JHC, Dejean A, Orivel J, Fichaux M, Groc S, Leponce M, Feitosa RM. (2019) First checklist of the ants (Hymenoptera: Formicidae) of French Guiana. Zootaxa 4674(5): 509–543. 10.11646/zootaxa.4674.5.2 [DOI] [PubMed] [Google Scholar]
- Hölldobler B, Wilson EO. (1990) The Ants. Harvard University Press, Cambridge. 10.1007/978-3-662-10306-7 [DOI]
- Imai HT, Urbani CB, Kubota M, Sharma GP, Narasimhanna MN, Das BC, Sharma AK, Sharma A, Deodikar GB, Vaidya VG, Rajasekarasetty MR. (1984) Karyological survey of Indian ants. Japanese Journal of Genetics 59(1): 1–32. 10.1266/jjg.59.1 [DOI] [Google Scholar]
- Imai H, Taylor RW, Crosland MW, Crozier RH. (1988) Modes of spontaneous chromosomal mutation and karyotype evolution in ants with reference to the minimum interaction hypothesis. Japanese Journal of Genetics 63: 159–185. 10.1266/jjg.63.159 [DOI] [PubMed] [Google Scholar]
- Imai HT, Taylor RW, Crozier RH. (1994) Experimental bases for the minimum interaction theory. Chromosome evolution in ants of the Myrmecia pilosula species complex (Hymenoptera: Formicidae: Myrmeciinae). Japanese Journal of Genetics 69: 137–182. 10.1266/jjg.69.137 [DOI] [Google Scholar]
- Janicki J, Narula N, Ziegler M, Guénard B, Economo EP. (2016) Visualizing and interacting with large-volume biodiversity data using client-server web-mapping applications: The design and implementation of antmaps.org. Ecological Informatics 32: 185–193. 10.1016/j.ecoinf.2016.02.006 [DOI] [Google Scholar]
- Kempf WW. (1965) A Revision oí the Neotropical Fungus-Growing Ants of the Genus Cyphomyrmex Mayr. Part II: Group of rimosus (Spinola) (Hymenoptera. Formicidae). Studia Entomologica 8(1–4): 160–200. [Google Scholar]
- King M. (1993) Species Evolution: The role of Chromosome Change. Cambridge University Press, Cambridge.
- Larabee FJ, Fisher BL, Schmidt CA, Matos-Maraví P, Janda M, Suarez AV. (2016) Molecular phylogenetics and diversification of trap-jaw ants in the genera Anochetus and Odontomachus (Hymenoptera: Formicidae). Molecular Phylogenetics and Evolution 103: 143–154. 10.1016/j.ympev.2016.07.024 [DOI] [PubMed] [Google Scholar]
- Levan A, Fredga K, Sandberg AA. (1964) Nomenclature for centromeric position on chromosomes. Hereditas 52: 201–220. 10.1111/j.1601-5223.1964.tb01953.x [DOI] [Google Scholar]
- Lorite P, Palomeque T. (2010) Karyotype evolution in ants (Hymenoptera: Formicidae), with a review of the known ant chromosome numbers. Myrmecological News 13(1): 89–102. [Google Scholar]
- MacKay WP. (1993) A review of the new world ant of the genus Dolichoderus. Sociobiology 22(1): 1–48. [Google Scholar]
- Mariano CSF, Lacau S, Pompolo SG, Sposito EC, Borges DS, Dergam JA, Villemant C, Delabie JHC. (2006) Cytogenetic studies in the rare Neotropical ant genus Typhlomyrmex (Ectatomminae: Typhlomyrmecini). Sociobiology 47(1): 225–234. [Google Scholar]
- Mariano CSF, Pompolo SG, Barros LAC, Mariano-Neto E, Campiolo S, Delabie JHC. (2008) A biogeographical study of the threatened ant Dinoponera lucida Emery (Hymenoptera: Formicidae: Ponerinae) using a cytogenetic approach. Insect Conservation and Diversity 1(3): 161–168. 10.1111/j.1752-4598.2008.00022.x [DOI] [Google Scholar]
- Mariano CSF, Santos ID, Groc S, Leroy C, Malé PJ, Ruiz-González MX, Cerdan P, Dejean A, Delabie JHC. (2011) The karyotypes of Gigantiops destructor (Fabricius) and other ants from French Guiana (Formicidae). Annales de la Société Entomologique de France 47(1–2): 140–146. 10.1080/00379271.2011.10697705 [DOI] [Google Scholar]
- Mariano CSF, Pompolo SG, Silva JG, Delabie JHC. (2012) Contribution of cytogenetics to the debate on the paraphyly of Pachycondyla spp. (Hymenoptera, Formicidae, Ponerinae). Psyche: A Journal of Entomology 2012: 1–9. 10.1155/2012/973897 [DOI] [Google Scholar]
- Mariano CSF, Santos IS, Silva JG, Costa MA, Pompolo SG. (2015) Citogenética e evolução do cariótipo em formigas poneromorfas. In: Delabie JHC, Serrão JE, Feitosa R, Mariano CSF, Majer J. (Eds) As formigas poneromorfas do Brasil.Ilhéus: Editus. Editora da Universidade Estadual de Santa Cruz, 103–125. 10.7476/9788574554419.0010 [DOI]
- Mariano CSF, Barros LAC, Velasco YM, Guimarães IN, Pompolo SG, Delabie JHC. (2019) Citogenética de hormigas de la región neotropical. In: Fernández F, Guerrero R, Delsinne T. (Eds) Hormigas de Colombia.Universidad Nacional de Colombia, Bogotá, 131–157.
- Murakami T, Fujiwara A, Yoshida MC. (1998) Cytogenetics of ten ant species of the tribe Attini (Hymenoptera, Formicidae) in Barro Colorado Island, Panama. Chromosome Science 2: 135–139. [Google Scholar]
- Orivel J, Malé PJ, Lauth J, Roux O, Petitclerc F, Dejean A, Leroy C. (2017) Trade-offs in an ant–plant–fungus mutualism. Proceedings of the Royal Society B 284(1850): 20161679 10.1098/rspb.2016.1679 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pacheco JA, Mackay WP. (2013) The systematics and biology of the New World thief ants of the genus Solenopsis (Hymenoptera: Formicidae). Edwin Mellen Press, Lewiston.
- Ruiz-González MX, Malé PJ, Leroy C, Dejean A, Gryta H, Jargeat P, Quilichini A, Orivel J. (2011) Specific, non-nutritional association between an ascomycete fungus and Allomerus plant-ants. Biology Letters 7(3): 475–479. 10.1098/rsbl.2010.0920 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Santos IS, Costa MA, Mariano CS, Delabie JHC, Andrade-Souza V, Silva JG. (2010) A cytogenetic approach to the study of Neotropical Odontomachus and Anochetus ants (Hymenoptera: Formicidae). Annals of the Entomological Society of America 103(3): 424–429. 10.1603/AN09101 [DOI] [Google Scholar]
- Santos IS, Delabie JHC, Silva JG, Costa MA, Barros LAC, Pompolo SG, Mariano CSF. (2012) Karyotype differentiation among four Dinoponera (Formicidae: Ponerinae) species. Florida Entomologist 95: 737–742. 10.1653/024.095.0324 [DOI] [Google Scholar]
- Santos IS, Mariano CS, Delabie JHC, Costa MA, Carvalho AF, Silva JG. (2016) “Much more than a neck”: karyotype differentiation between Dolichoderus attelaboides (Fabricius, 1775) and Dolichoderus decollatus F. Smith, 1858 (Hymenoptera: Formicidae) and karyotypic diversity of five other Neotropical species of Dolichoderus Lund, 1831. Myrmecological News 23: 61–69. [Google Scholar]
- Schlick-Steiner BC, Steiner FM, Seifert B, Stauffer C, Christian E, Crozier RH. (2010) Integrative taxonomy: a multisource approach to exploring biodiversity. Annual Review of Entomology 55: 421–438. 10.1146/annurev-ento-112408-085432 [DOI] [PubMed] [Google Scholar]
- Schultz TR. (2000) In search of ant ancestors. Proceedings of the National Academy of Sciences 97(26): 14028–14029. 10.1073/pnas.011513798 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Seifert B. (2009) Cryptic species in ants (Hymenoptera: Formicidae) revisited: we need a change in the alpha-taxonomic approach. Myrmecological News 12: 149–166. [Google Scholar]
- Sosa-Calvo J, Ješovnik A, Vasconcelos HL, Bacci Jr M, Schultz TR. (2017) Rediscovery of the enigmatic fungus-farming ant “Mycetosoritis” asper Mayr (Hymenoptera: Formicidae): Implications for taxonomy, phylogeny, and the evolution of agriculture in ants. PloS ONE 12(5): e0176498. 10.1371/journal.pone.0176498 [DOI] [PMC free article] [PubMed]
- Sosa‐Calvo J, Fernández F, Schultz TR. (2019) Phylogeny and evolution of the cryptic fungus‐farming ant genus Myrmicocrypta F. Smith (Hymenoptera: Formicidae) inferred from multilocus data. Systematic Entomology 44(1): 139–162. 10.1111/syen.12313 [DOI] [Google Scholar]
- Souza ALB, Mariano CSFD, Delabie JHC, Pompolo SDG, Serrão JE. (2011) Cytogenetic studies on workers of the Neotropical ant Wasmannia auropunctata (Roger 1863) (Hymenoptera: Formicidae: Myrmicinae). Annales de la Société Entomologique de France 47(3–4): 510–513. 10.1080/00379271.2011.10697742 [DOI] [Google Scholar]
- Sposito EC, Mariano CSF, Pompolo SG, Delabie JHC. (2006) Exploratory studies on the karyotypes of seven species of the ant Neotropical genus Pseudomyrmex (Hymenoptera: Formicidae: Pseudomyrmecinae). Brazilian Journal of Morphological Sciences 23(3–4): 435–440. [Google Scholar]
- Teixeira GA. (2018) (Dissertação) Citogenética Clássica e Molecular de formigas Neotropicais. Master Degree. PhD Thesis, Universidade Federal de Viçosa. [In Portuguese]
- Teixeira GA, Barros LAC, Aguiar HJAC, Pompolo SG. (2017) Comparative physical mapping of 18S rDNA in the karyotypes of six leafcutter ant species of the genera Atta and Acromyrmex (Formicidae: Myrmicinae). Genetica 145: 351–357. 10.1007/s10709-017-9970-1 [DOI] [PubMed] [Google Scholar]
- Ward PS. (2013) AntWeb: Ants of California. http://www.antweb.org/california.jsp [Accessed 4 Sept 2019]
- Ward PS, Brady SG, Fisher BL, Schultz TR. (2015) The evolution of myrmicine ants: phylogeny and biogeography of a hyperdiverse ant clade (Hymenoptera: Formicidae). Systematic Entomology 40(1): 61–81. 10.1111/syen.12090 [DOI] [Google Scholar]
- Ward PS, Blaimer BB, Fisher BL. (2016) A revised phylogenetic classification of the ant subfamily Formicinae (Hymenoptera: Formicidae), with resurrection of the genera Colobopsis and Dinomyrmex. Zootaxa 4072(3): 343–357. 10.11646/zootaxa.4072.3.4 [DOI] [PubMed] [Google Scholar]



