Abstract
Background & Aims:
Body mass index is a simple anthropometric measure (kg/m2) used as an indirect estimate of body fat in individuals, and in assessments of population health and comparisons between populations. Bioelectrical impedance analysis (BIA) is often used to provide additional information on body fat and fat-free mass, and has been used to generate body composition reference data in national health surveys. However, BIA measurements are known to be device-specific and there are few published studies comparing results from different BIA instruments. Therefore, we compared the performance of two BIA instruments in the Russian Children’s Study (RCS) of male growth, pubertal development and maturation.
Methods:
Paired BIA measurements were obtained using the Tanita BC-418MA (Tanita Corp., Tokyo, Japan) and ABC-01 ‘Medas’ (Medas Ltd, Moscow, Russia) BIA instruments. Cross-sectional data on 236 RCS subjects aged 18–22 years were used for the BIA comparison and the development of a conversion formula between measured resistances; follow-up data (n=96) were used for validation of the conversion formula.
Results:
Whole-body resistances were highly correlated (Spearman rho=0.95), but fat mass (FM) estimates were significantly higher with the Medas than the Tanita device (median difference 3.3 kg, 95% CI: 2.9, 3.6 kg) with large limits of agreement (LoA) for the FM difference (−2.0, 8.6 kg). A conversion formula between the resistances (Res) was obtained: Medas Res = 0.882×Tanita Res+26.2 (r2=0.91, SEE=17.6 Ohm). After applying the conversion formula to Tanita data and application of the Medas assessment algorithm, the ‘converted’ Tanita FM estimates closely matched the Medas original estimates (median difference −0.1 kg, 95% CI: −0.3, 0.2 kg), with relatively small LoA for the FM difference (−2.3 to 2.1 kg), suggesting potential interchangeability of the ABC-01 ‘Medas’ and Tanita BC-418MA data at the group level.
Conclusions:
Our results support the importance of cross-calibration of BIA instruments for population comparisons and proper data interpretation in clinical and epidemiological studies.
Keywords: bioelectrical impedance analysis, instrumentation, consistency of data, cross-calibration, skinfolds, body composition assessment, fat mass, young adult males
Introduction
Body mass index (BMI; kg/m2) is frequently used as an estimate of body fat in both clinical settings and population studies. However, there are acknowledged shortcomings using BMI to evaluate body composition, e.g. fat mass [1]. Bioelectrical impedance analysis (BIA) measures the passive electrical properties of biological tissues [2], and has been used internationally to assess body fat and fat-free mass. BIA can provide a quick, safe, non-invasive, portable and relatively low-cost technique for the assessment of body composition [3]. BIA instruments have been commercially available since the mid-1980s [4], are manufactured in many countries, and used widely throughout the world [5, 6]. At present, BIA is the most common method of body composition assessment in population and clinical studies, providing a potentially useful tool for assessing body fat and lean mass in epidemiological studies [7, 8].
BIA is used to assess cross-sectional variations in body composition as well as changes in body composition over time in prospective cohort studies [9–12]. Body composition parameters, such as total body water, fat-free mass, fat mass and skeletal muscle mass, are derived from population-specific, built-in validated regression formulae which utilize the measured impedance data and some additional inputs, e.g. height, weight, age, and sex. These formulae are constructed by fitting the regression model to a criterion method [e.g., dual-energy X-ray absorptiometry (DXA) or 4-component (4C) model] using body composition data from a reference population of specific ethnicity, age, weight status and physical activity level [7, 13–15]. In general, BIA has high within-instrument reliability [16, 17]. However, measured parameters and body composition estimates using different BIA instruments may not match closely. Differences in electronic design, measurement conditions/standardization and the body composition assessment formulae which depend on the reference population and the criterion method used [18–20] may hinder between-study comparisons and impact clinical relevance and epidemiological inference.
In Russia, the BIA ABC-01 ‘Medas’ (Medas Ltd, Moscow, Russia) is extensively used in population and clinical studies, while the Tanita BC-418MA (Tanita Corp., Tokyo, Japan) is widely used outside Russia. However, there is a lack of published data comparing the ABC-01 ‘Medas’ analyzer with other BIA instruments, including the Tanita system, which limits comparability across populations. The goals of our study were to compare the performance of the ABC-01 ‘Medas’ and the Tanita BC-418MA using a well-characterized, longitudinal Russian male cohort assessed with parallel BIA measurements using these two instruments at ages 18 to 22 years, to develop a conversion formula between these two sets of BIA measurements, and to test comparability of the BIA body composition data after the conversion. We also compared BIA estimates to body composition data from skinfold measures available in a subset of participants.
Materials and Methods
Russian Children’s Study
The Russian Children’s Study (RCS) is a prospective cohort study of male growth, pubertal development and maturation, and semen quality. 516 boys aged 8 and 9 years who were residents in Chapaevsk, Samara region, Russia, were enrolled from 2003 to 2005 and followed to age 18–19 years, with a subset followed to ages 21–22 years [21–23]. Annual visits included anthropometric and pubertal staging assessments, BIA measurements, and biological sample collection. The study was approved by the local Chapaevsk Institutional Review Board, and the Institutional Review Boards of the Harvard T.H. Chan School of Public Health, and the Brigham and Women’s Hospital. Before participation, the parent/guardian provided informed consent and the boys signed assent forms; at age 18 years the young men signed informed consent forms.
Study subjects
From 2014 to 2017, the Medas instrument was used in parallel to measure BIA with the Tanita instrument on the available RCS participants in order to compare the results between the instruments. In total, 332 paired Tanita and Medas BIA measurements of 236 young men aged 18–22 years were made. Of the participants, 151 were measured once, 74 twice and 11 thrice. All measurements were obtained by the same trained physician and research nurse. Skinfold data was available at the same visit for 115 out of 236 study subjects (49%), with 126 skinfold measurements in total. To compare paired bioimpedance and skinfold data, only a single measurement per subject was utilized in our initial analyses. Among those with measurements at multiple study visits with both BIA and skinfold data, one visit was randomly selected [24]. Cross-sectional data was used for the BIA comparison and the derivation of the conversion formula between the resistances (236 paired BIA measurements). Similarly, in individuals with available skinfold data, BIA body composition data was compared cross-sectionally with skinfold-based body composition estimates (n=115). The remaining follow-up paired BIA measurements (n=96) were used for validation of the conversion formula and for comparison with Russian reference health centers’ (HCs’) data [25].
Anthropometry
Anthropometry was performed annually according to a standardized protocol [26, 27]. Standing height was measured to the nearest 0.1 cm without shoes and socks using a wall-mounted Seca stadiometer (Model 226, Hopkins Medical Products, USA). Weight (Wt) was measured to the nearest 0.1 kg in underwear using a Seca digital scale (Model 700, Vogel & Halke GmbH, Germany). Body mass index (BMI) was calculated (kg/m2). Skinfolds at biceps, triceps, subscapula, suprailium, chest, abdomen, and thigh were measured biannually using a Lange skinfold caliper (Beta Technology, USA) according to standardized protocol [26, 27] to the nearest 0.5 mm.
BIA measurements
The Tanita BC-418MA instrument has been utilized in the RCS since 2006. This 8-electrode body composition monitor utilizes low-amplitude alternating electric current with a frequency of 50 kHz. At the annual physical exam, after fasting overnight, each subject was invited to participate in morning BIA measurements. Each boy stood on the scale barefoot in contact with the 4 electrodes for the feet, dressed in underwear, hands in contact with the 4 electrodes on the handles, and the arms separated from the trunk to prevent contact, as recommended by the manufacturer.
Beginning in 2014 and continuing through 2017, BIA measurements have been obtained on both instruments at the same visit in each boy. The ABC-01 ‘Medas’ device measurements were collected after the Tanita measurement. Medas measurements were obtained in the supine position according to the conventional wrist-to-ankle 4-electrode scheme at an electric current frequency of 50 kHz [28] with the disposable surface bio-adhesive Ag-AgCl ECG electrodes (F3001ECG, FIAB SpA, Italy) placed on the right side of the body.
Body composition
According to the Tanita instruction manual [29], the fat-free mass estimate was obtained using the manufacturer’s proprietary algorithm utilizing regression formulae based on height, weight, age, sex and whole-body resistance between right hand and foot at the electric current frequency of 50 kHz.
In the Medas open assessment algorithm [25], total body water (TBW) is estimated from measured resistance value R50, height (Ht) and weight (Wt) using the following Kushner and Schoeller equation for males [30]: TBW = 0.3963×Ht2/R50 + 0.143×Wt + 8.399. Fat-free mass (FFM) was calculated from TBW assuming constant hydration of FFM according to the formula FFM=TBW/0.732. For both types of BIA instruments, fat mass (FM) was determined by the subtraction of FFM from Wt. The percentage of body weight as fat (%FM) was then calculated. The fat mass index (FMI) and fat-free mass index (FFMI) were assessed as FM and FFM, respectively, divided by height squared.
We did not have criterion data (e.g. DXA or 4C model) to assess the validity of BIA body composition estimates in our study. For the comparison, we applied respective Durnin-Womersley (D-W) [31] and Jackson-Pollock (J-P) [32] anthropometric equations for males based on skinfolds: %FM = 495/(1.1765 − 0.0744×log S4) − 450, where S4 is the sum of skinfold thicknesses (mm) measured at the biceps, triceps, subscapula, and suprailium [31], and %FM=495/(1.10938 − 0.0008267×S3 + 0.0000016×(S3)2 − 0.0002574×Age) − 450, where S3 is the sum of skinfold thicknesses (mm) measured at the chest, abdomen, and thigh, and Age is the subject’s age (years) [32].
Statistics and data process
Data analysis was performed using Minitab 18.1 and Excel 2010 software programs. For uniformity, standing height was rounded to the nearest integer, as the Tanita BC-418MA instrument utilizes only 3 digits for entering standing height (i.e., without decimals). Descriptive statistics were reported as means and standard deviations (SD), as well as medians and interquartile range (IQR) when data was not normally distributed. The agreement between the Tanita and Medas measured resistance values (Res) and body composition estimates was assessed using Spearman correlation coefficients. A conversion formula between the Medas and Tanita resistances was developed based on fitting a linear regression model. For the conversion formula, a proportion of explained variance (r2) and the standard error of estimate (SEE) were determined. The Tanita ‘converted’ resistance values (i.e., a proxy of paired Medas resistances) were then used in the open Medas body composition assessment algorithm and compared with the Medas original body composition estimates. The RCS original and ‘converted’ bioimpedance data were standardized by calculating z-scores against the Russian reference HCs’ data [25]. For comparisons between the two BIA instruments, a Wilcoxon signed rank test for paired measurements and Bland-Altman analysis [33] were utilized. Mann-Whitney tests were used to conduct comparisons between age groups. Statistical significance was set at p-value ≤ 0.05.
Results
Descriptive statistics of the study participants are shown in Table 1. According to WHO criteria for adults [34], 75% of our subjects were within the normal BMI range, 8.9% were thin, 16.1% overweight, and 4.2% obese.
Table 1.
Anthropometric (n=236) and skinfold (n=115) data for the study population.
| Parameter | Mean ± SD | Median (IQR) |
|---|---|---|
| Age, yrs | 19.7 ± 1.2 | 19.6 (18.6; 20.6) |
| Height, cm | 177.4 ± 6.7 | 178.0 (173.0; 182.0) |
| Weight, kg | 69.9 ± 13.5 | 67.9 (60.8; 75.4) |
| Body mass index, kg/m2 | 22.1 ± 3.8 | 21.6 (19.6; 23.2) |
| Waist circumference, cm | 78.1 ± 8.7 | 76.5 (72.0; 81.5) |
| Hip circumference, cm | 94.8 ± 8.2 | 93.5 (89.5; 98.4) |
| Waist-to-hip ratio | 0.82 ± 0.04 | 0.82 (0.80; 0.85) |
| Skinfold thickness, mm | ||
| Abdominal | 16.9 ± 6.6 | 15.0 (11.5; 21.0) |
| Biceps | 5.7 ± 1.9 | 5.0 (4.0; 6.5) |
| Chest | 12.9 ± 6.3 | 11.5 (7.5; 16.0) |
| Subscapular | 10.5 ± 3.7 | 9.5 (8.0; 12.5) |
| Suprailiac | 10.4 ± 6.0 | 8.5 (6.0; 12.5) |
| Thigh | 14.0 ± 4.8 | 13.5 (10.5; 16.5) |
| Triceps | 9.0 ± 3.1 | 9.0 (6.5; 11.0) |
Abbreviations: n (number of measurement records); SD (standard deviation); IQR (interquartile range).
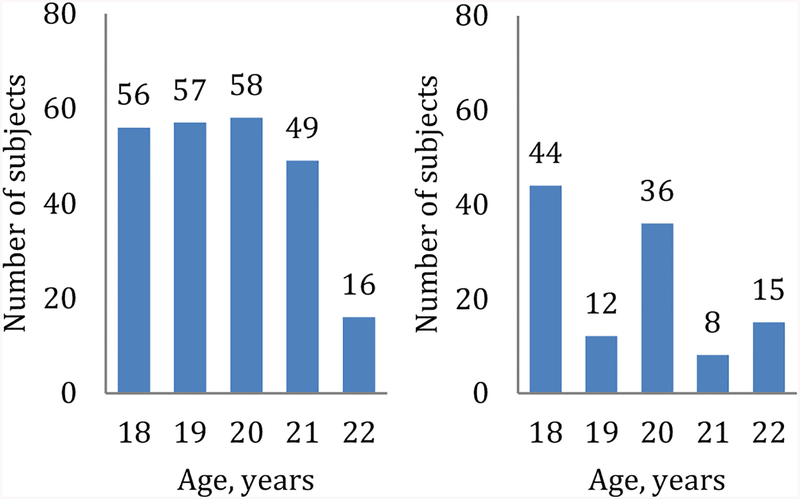
Age distribution of the selected cross-sectional paired BIA measurements (n=236) was nearly uniform, while the associated skinfold data (n=115) were mainly represented by 18 and 20 year olds (see Fig. 1). Most of the remaining paired BIA records (66/96, or 69%) used for validation of the conversion formula were obtained at 19 years of age.
Figure 1.
Age distribution of the study subjects with paired BIA measurements (left panel, n=236) and additional skinfold data (right panel, n=115).
Development of the conversion formula between measured resistances
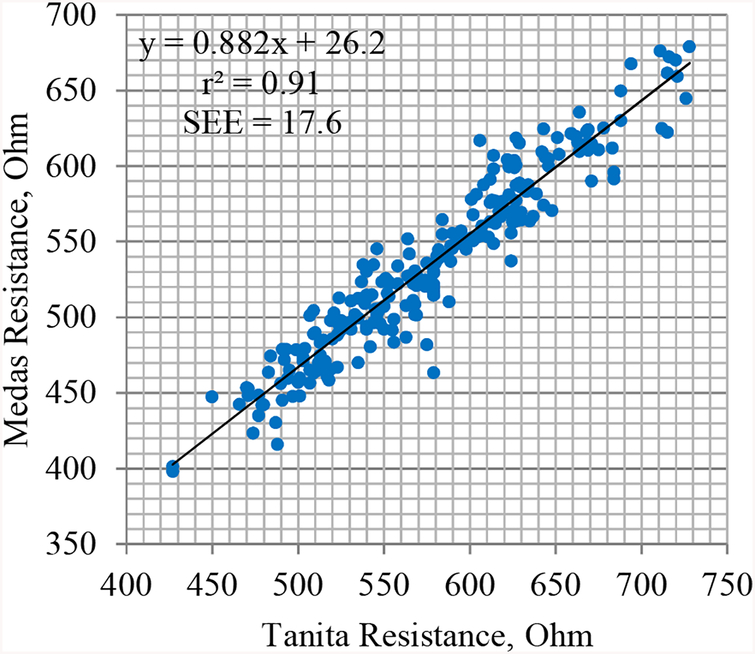
The correlation of ABC-01 ‘Medas’ and Tanita BC-418MA whole-body resistances (Res) was high (Spearman rho=0.95), but the values of Medas Res were significantly lower than the Tanita Res values (see Fig. 2 and Table 2), with the median paired difference of −42.4 Ohm.
Figure 2.
Plot of the Medas vs Tanita whole-body resistance values in our study population, n=236.
Table 2.
Bioimpedance body composition measurements in the Russian Children’s Study participants, n=236.
| Parameter | ABC-01 ‘Medas’ | Tanita BC-418MA | ||
|---|---|---|---|---|
| Mean ± SD | Median (IQR) | Mean ± SD | Median (IQR) | |
| Res, Ohm | 534.9 ± 58.5 | 527.6a (492.0; 577.2) | 577.1 ± 63.3 | 574.5 (526.5; 624.0) |
| FFM, kg | 57.3 ± 6.6 | 56.8a (52.6; 61.1) | 60.6 ± 8.4 | 60.1 (54.8; 65.2) |
| FFMI, kg/m2 | 18.2 ± 1.7 | 18.2a (16.9; 19.4) | 19.2 ± 2.1 | 19.1 (17.8; 20.5) |
| FM, kg | 12.4 ± 8.0 | 10.3a (7.2; 15.0) | 9.1 ± 6.6 | 7.8 (4.5; 11.0) |
| FMI, kg/m2 | 3.9 ± 2.4 | 3.3a (2.3; 4.8) | 2.9 ± 2.0 | 2.5 (1.5; 3.5) |
| %FM | 16.6 ± 6.9 | 15.4a (11.5; 20.5) | 12.2 ± 6.0 | 11.9 (7.2; 15.5) |
Abbreviations: Res (whole-body resistance); FFM (fat-free mass); FFMI (fat-free mass index); FM (fat mass); FMI (fat mass index); %FM (percentage body mass as fat); SD (standard deviation); IQR (interquartile range).
Statistically significant difference according to Wilcoxon signed rank test (p<0.01) as compared to the respective TANITA BC-418MA estimate.
We obtained the following conversion formula between the whole-body resistances from the two instruments:
Similar proportion of explained variance (r2=0.90) and accuracy (SEE=19.3 Ohm) were observed when the Medas original and Tanita converted resistance values were compared for the rest of the paired BIA records, n=96 (data not shown).
Comparison of the original BIA body composition data
Before applying the conversion formula, the Medas body composition estimates in our study population (n=236) differed significantly from those provided by the Tanita instrument, see Table 2. The Medas FFM estimates were significantly lower, while the FM estimates significantly higher than the Tanita estimates (median paired difference=3.2 kg in both cases).
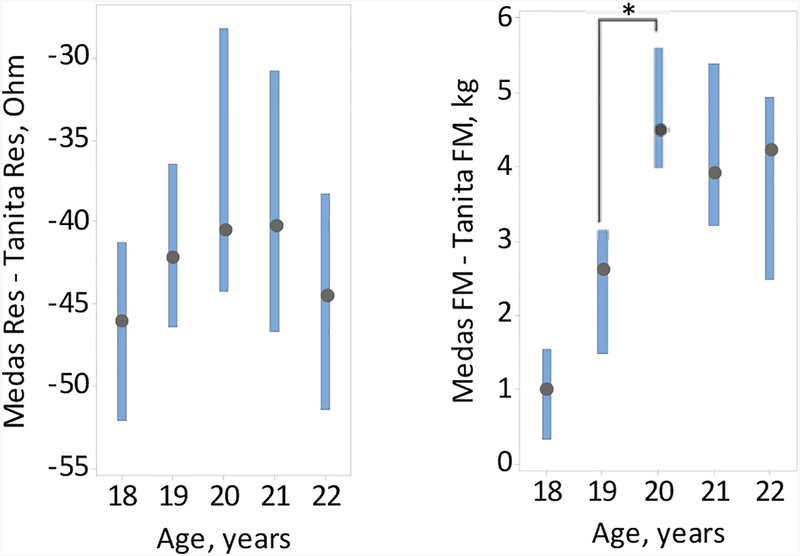
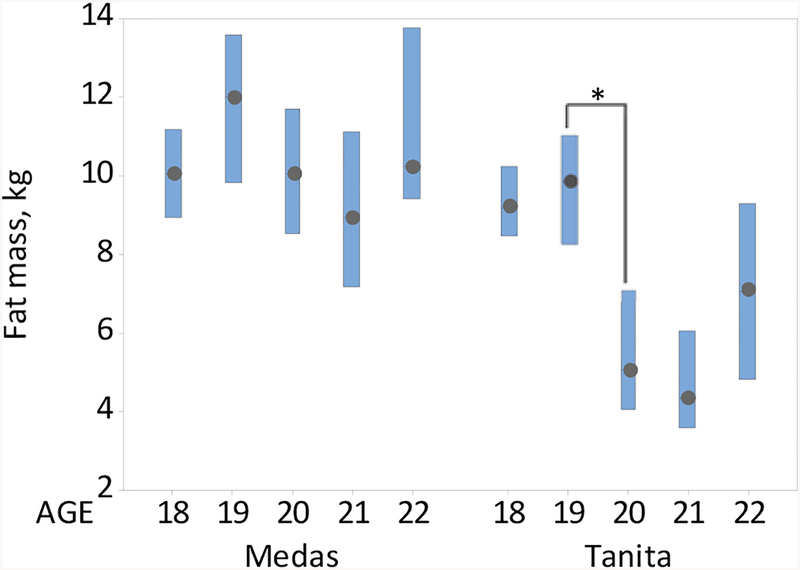
In evaluating age-related trends in BIA as measured by both the Medas and Tanita instruments, the median difference between the Medas and Tanita body composition estimates experienced a jump between age 19 and 20 (see Fig. 3). This jump was associated with the precipitous decline in the Tanita, but not Medas, FM between age 19 and 20 (see Fig. 4), suggesting that the Tanita internal formulas may change starting at age 20. At all ages, overall resistance measures from the Medas instrument were significantly lower and FM estimates were significantly higher than those of the Tanita instrument (see Fig. 4). Accordingly, the Medas FFM estimates were significantly lower than that provided by the Tanita instrument (data not shown).
Figure 3.
Boxplots of the median differences, and the respective 95% CI’s, between the Medas and Tanita resistance values (left panel) and fat mass estimates (right panel) against age, n=236. *Statistically significant difference (p<0.05).
Figure 4.
Boxplots of the Medas and Tanita fat mass against age, medians and the respective 95% CI’s, n=236. *Statistically significant difference (p<0.05).
Comparison of the original BIA and skinfold-based body composition data
In the subgroup with available skinfold data (n=115), the overall Medas FFM, FFMI, FM, FMI, and %FM medians were similar to those obtained using the Durnin-Womersley (D-W) equation, whereas the Tanita estimates were similar to those obtained using the Jackson-Pollock (J-P) equation; however, differences in body composition estimates from skinfolds and BIA were statistically significant in all cases, see Table 3.
Table 3.
BIA vs skinfold-based body composition estimates in Russian Children’s Study participants with both BIA and skinfold measures collected at the same time (n=115, Median (IQR)).
| Parameter | ABC-01 ‘Medas’ | D-W equation (4 skinfolds) | J-P equation (3 skinfolds) | Tanita BC-418MA |
|---|---|---|---|---|
| FFM, kg | 56.3a (51.4; 60.2) | 55.3a,b (50.5; 59.9) | 57.7a,b (52.9; 62.7) | 58.8b (53.1; 64.1) |
| FFMI, kg/m2 | 18.0a (16.8; 19.0) | 17.5a,b (16.6; 18.7) | 18.5a,b (17.3; 19.4) | 18.5b (17.2; 20.1) |
| FM, kg | 9.6a (7.1; 12.0) | 9.9a,b (7.2; 13.0) | 7.0a,b (4.8; 10.8) | 7.2b (4.2; 9.9) |
| FMI, kg/m2 | 3.0a (2.2; 3.8) | 3.2a,b (2.3; 4.3) | 2.3a,b (1.5; 3.4) | 2.2b (1.4; 3.1) |
| %FM | 14.7a (11.7; 17.3) | 15.2a,b (11.5; 18.9) | 11.0a,b (7.9; 15.3) | 11.2b (6.8; 13.6) |
Abbreviations: FFM (fat-free mass); FFMI (fat-free mass index); FM (fat mass); FMI (fat mass index); %FM (percentage body mass as fat); IQR (interquartile range); D-W (Durnin-Womersley); J-P (Jackson-Pollock).
Statistically significant difference as compared to the Tanita BC-418MA estimate (p<0.05).
Statistically significant difference as compared to the ABC-01 ‘Medas’ estimate (p<0.05).
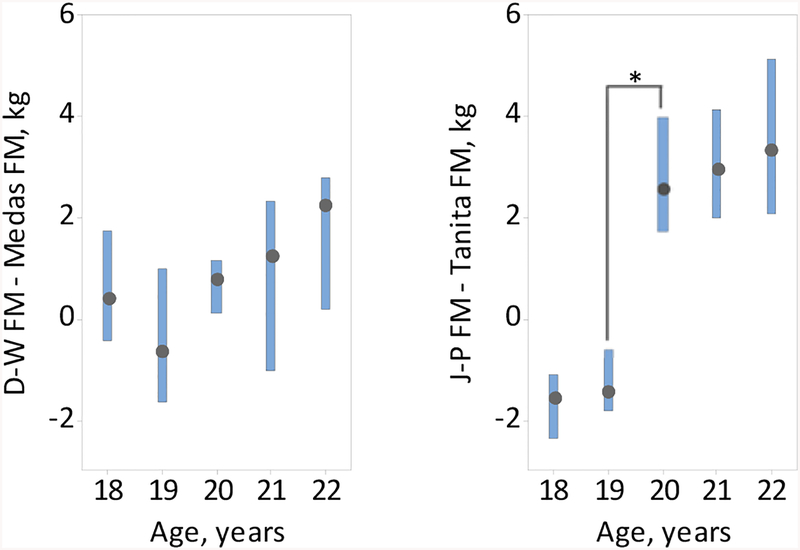
At ages 18–21 years, there was good agreement between the Medas and D-W equation-based FM estimates, but significant differences between the Tanita and J-P equation-based estimates at all ages, see Table 4 and Fig. 5. The observed jump in the Tanita, but not Medas, FM estimate between ages 19 and 20 (see Fig. 4) was not accompanied by a significant change in BMI or skinfold-based FM estimates at the same ages, see Table 4.
Table 4.
BIA vs skinfold-based FM estimates by age at measurement (n=115, Median (IQR)).
| Age, years | BMI, kg/m2 | Medas FM, kg | D-W FM, kg | J-P FM, kg | Tanita FM, kg |
|---|---|---|---|---|---|
| 18 | 20.6 (18.6; 22.3) | 9.2a (8.0; 10.0) | 9.2a (8.0; 10.8) | 6.7a,b (5.0; 7.7) | 8.5b (7.8; 9.2) |
| 19 | 21.4 (18.7; 23.0) | 11.3a (5.7; 13.0) | 10.5 (5.8; 12.1) | 7.9a,b (4.6; 10.0) | 9.0b,c (6.4; 10.6) |
| 20 | 21.3 (19.1; 22.6) | 9.6a (8.0; 10.6) | 9.9a (8.5; 11.6) | 6.8a,b (6.3; 8.6) | 4.1b (3.7; 5.0) |
| 21 | 21.9 (18.8; 24.1) | 7.9a (3.5; 19.4) | 9.4a (5.3; 17.7) | 6.9a (4.3; 15.2) | 4.0b (2.2; 12.2) |
| 22 | 22.1 (20.7; 23.1) | 10.4a (9.3; 14.9) | 12.9a,b (9.6; 17.3) | 11.0a (7.4; 14.4) | 7.1b (4.6; 9.6) |
Abbreviations: FM (fat mass); IQR (interquartile range); BMI (body mass index).
Statistically significant difference as compared to the Tanita BC-418MA estimate for this age group (p<0.05).
Statistically significant difference as compared to the ABC-01 ‘Medas’ estimate for this age group (p<0.05).
Statistically significant difference as compared to the following age group for the same instrument (p<0.05).
Figure 5.
Boxplots of the median differences, and the respective 95% CI’s, between the D-W and Medas (left panel) and J-P and Tanita fat mass estimates (right panel) against age, n=115. *Statistically significant difference (p<0.05).
Comparison of the Tanita ‘converted’ and Medas original body composition estimates
After the conversion of the Tanita resistance values to equivalent Medas resistances and application of the Medas assessment algorithm, the Tanita ‘converted’ body composition averages closely matched the Medas original averages, see Table 5. Bland-Altman analyses of the agreement between the Medas and Tanita original and ‘converted’ resistances (Res) indicated highly significant trends and large limits of agreement (LoA) in both cases, see Fig. 6 (n=96). However, after the conversion, there was no significant trend between the mean and the difference of the Medas and Tanita ‘converted’ FM (r=−0.08, p=0.44) despite the highly significant trend for the Medas and Tanita original FM data (r=0.75, p<0.001). As a result, the limits of agreement for FM significantly narrowed from −2.5 to 7.5 kg to more acceptable levels (−2.3 to 2.1 kg), see Fig. 6. Insignificant trends and relatively small limits of agreement were also observed for the Medas and Tanita ‘converted’ estimates of FMI (r=−0.08, p=0.46; LoA: −0.7 to 0.7 kg/m2) and %FM (r=−0.07, p=0.47; LoA: −3.2 to 3.1%), while the trends for FFMI (r=0.20, p=0.05; LoA: −0.7 to 0.7 kg/m2) and FFM (r=0.19, p=0.06; LoA: −2.1 to 2.3 kg) were marginally significant.
Table 5.
The Medas original vs Tanita ‘converted’ estimates: the resistances and body composition data, n=96.
| Parameter | The Medas original estimates | The Tanita ‘converted’ estimates | ||
|---|---|---|---|---|
| Mean ± SD | Median (IQR) | Mean ± SD | Median (IQR) | |
| Res, Ohm | 539.0 ± 63.3 | 533.3 (487.6; 576.8) | 539.6 ± 58.3 | 533.8 (496.5; 576.8) |
| FFM, kg | 57.7 ± 6.7 | 57.2 (52.4; 61.8) | 57.6 ± 6.5 | 56.8 (52.5; 61.1) |
| FFMI, kg/m2 | 18.1 ± 1.8 | 18.1 (16.7; 19.4) | 18.1 ± 1.7 | 18.0 (17.0; 19.3) |
| FM, kg | 12.7 ± 7.4 | 11.1 (7.5; 16.9) | 12.8 ± 7.4 | 10.9 (7.4; 17.1) |
| FMI, kg/m2 | 4.0 ± 2.3 | 3.2 (2.3; 5.5) | 4.0 ± 2.3 | 3.4 (2.3; 5.4) |
| %FM | 17.0 ± 7.0 | 15.9 (11.8;22.6) | 17.1 ± 7.1 | 15.8 (12.0; 22.4) |
Abbreviations: Res (whole-body resistance); FFM (fat-free mass); FFMI (fat-free mass index); FM (fat mass); FMI (fat mass index); %FM (percentage body mass as fat); SD (standard deviation); IQR (interquartile range).
Figure 6.
Bland-Altman analysis of the agreement between the Medas and Tanita Res (upper panels) and the Medas and Tanita FM (lower panels) before (left panel) and after (right panel) application of the conversion formula (n=96). Solid lines represent the mean difference between methods and regression line; dashed lines represent 95% limits of agreement.
Comparison with the Medas Russian reference HCs’ data
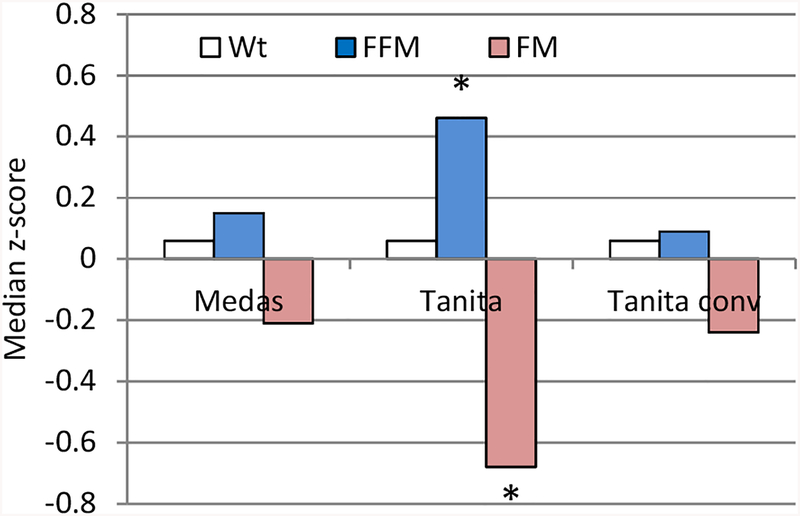
The Tanita ‘converted’ and Medas data on FFM and FM of the RCS study subjects were similar to each other and to the Medas Russian reference HCs’ data [25] while the Tanita original FFM and FM data significantly differed from them (n=96), see Fig. 7.
Figure 7.
Median z-scores of Wt, FFM and FM relative to the Medas body composition Russian HCs’ reference data [29] according to the Medas, Tanita, and Tanita ‘converted’ estimates, n=96. *Statistically significant difference as compared to the Russian reference data (p<0.05).
Discussion
BIA provides a quick, portable, and relatively inexpensive method of estimating body composition [3, 4, 5, 6]. However, potential inconsistency of body composition data because of different instruments [35, 36] is one of the main challenges of using BIA data for clinical nutrition and population studies. BIA equipment currently dominates the market for body composition instruments, with approximately 68% market share in 2017, and is expected to reach even higher rates in the coming years [37] thus making BIA the primary source of body composition data in the present [7, 8] and near future. Therefore, in order to make comparisons across populations, and for possible adjustment of BIA-based diagnostic criteria, it is important to develop methods for the assessment of the data comparability. Our study of potential inter-changeability of BIA data was based on the comparison and adjustment of the physical measurements based on bioimpedance using appropriate formulae. Given our findings, we believe that cross-calibration of BIA instruments is the most convenient way to make existing BIA data from different instruments comparable.
Both the lead-type ABC-01 ‘Medas’ and stand-on Tanita BC-418MA models have been used to generate huge datasets of body composition data for various populations [25, 38–42] thus representing a significant portion of the total BIA data pool. We compared the performance of the ABC-01 ‘Medas’ and Tanita BC-418MA instruments using data from the well-characterized Russian Children’s Study male cohort, and derived a conversion formula between the resistances (Res). The observed difference in the Medas and Tanita original whole-body resistances (mean paired difference of −42.2 Ohm) was much higher than that observed in other studies using the lead-type instruments (RJL/Akern, Xitron 4200) [35] (mean paired difference of −9.9 Ohm). This may be due to the shorter electric current path for the conventional wrist-to-ankle measurement scheme compared to the possible counter-effect of the lying vs standing bioimpedance due to hydrostatic fluid shift [43].
The differences between the resistances (SEE=17.6 Ohm) and the proportion of explained variance (r2=0.91) using our conversion formula were lower than previously reported values (r2=0.99, SEE=5.0–5.3 Ohm) in a classical study using the lead-type instruments (RJL, Valhalla) [35], and are most likely because of the difference in measurement techniques. However, despite the differences in the Medas and Tanita original resistances and body composition estimates, after application of the conversion formula, the Tanita ‘converted’ body composition estimates closely matched the Medas original estimates at the group level, thus suggesting that the ABC-01 ‘Medas’ and Tanita BC-418MA data are comparable.
The above findings demonstrate the feasibility of comparing BIA body composition data from different BIA instruments using a relative scale for comparison which is independent of a specific reference method. Importantly, this was made possible due to the open availability of the Medas assessment algorithm.
Our results also suggest that the age-related non-homogeneity of the Tanita BC-418MA body composition data may be due to differences in their proprietary formula between ages 19 and 20. Application of the same formula is, therefore, necessary to assess dynamic changes in body composition. Because different predictive equations are used for the age groups ‘under 18’ and ‘18+’ in the Medas assessment algorithm [25], a similar effect for the Medas BIA body composition data might be expected between ages 17 and 18, but we had no data to verify this assumption.
For this study, knowledge of only the Medas body composition assessment algorithm was needed. The Tanita BC-418MA algorithm can be estimated from the data, as the general structure of the regression formula for FFM is given in the Tanita manual. Similar to the Medas age-based assessment algorithms, our analysis suggests that there exist two Tanita BC-418MA formulae for FFM for the respective age intervals before and after 20 years old.
We used a cross-sectional design for the longitudinal RCS data analysis because there was sparse parallel longitudinal BIA data. Therefore, we were not able to compare individual trajectories of the BIA data from the two instruments. Because of the absence of a criterion method, the accuracy of BIA body composition estimates was not addressed here. Other conventional ‘directly measured’ analyses and indexes used in clinical nutrition, such as BIVA and phase angle, were not available in our study because the reactance is not measured with the Tanita BC-418MA instrument. Given the limited age range, small sample size, and restriction to males in our study population, additional comparisons are needed to confirm applicability of our robust conversion formula across a wider age range of individuals of both sexes.
Although, Silva et al. [36] found a lack of agreement between different BIA instruments in their study of highly physically active individuals and elite athletes and concluded they should not be used interchangeably at the individual level, our results suggest that body composition data for different BIA instruments is comparable at the group level thus providing important knowledge for nutritional epidemiology. Future studies of variation of body composition globally, in addition to reported worldwide trends in BMI [44], would contribute to planning and monitoring effectiveness of various international nutritional intervention initiatives. Contrary to the dominant paradigm about the role of obesity among populations living in cities, the rising BMI in rural populations has recently been shown to be the main driver of the global obesity epidemic in adults [45]. Comparability of global BIA body composition data may be used to establish which constituents of the BMI, namely, FMI or FFMI, are mainly responsible for the observed changes in BMI among different countries and regions, including urban and rural settings.
BMI is not recommended for diagnosis of obesity at the individual level [46, 47]. The true reference for diagnosing obesity is the percentage of body fat, a component of body composition. The BMI is the sum of FFMI and FMI, and the same BMI values may have different ‘composition’ in terms of FFMI and FMI. Lean mass and fat mass have different associations with mortality [48]. Therefore, body composition data can provide important additional information for health assessment.
Until now, there has been a lack of validated national BIA body composition formulae for the Russian population; instead, external age-, sex-, and ethnic-specific formulae have been used [25]. The development of such formulae will make it possible to clarify patterns of age-related changes, sexual dimorphism and spatial variation of body composition in the Russian population using BIA data.
Taken together, our results support the importance of methods for the adjustment of parameters generated by different BIA instruments for population comparisons and proper data interpretation in clinical and epidemiological studies. The analysis also confirms that, without preliminary cross-calibration, longitudinal changes in BIA body composition need to be assessed using the same BIA instrument while checking the consistency of the built-in assessment algorithm.
Highlights.
Two BIA instruments were compared in the longitudinal Russian Children’s Study
Paired ABC-01 Medas and Tanita BC-418MA data were collected in males at 18–22 years
We observed large differences in original resistances and body composition data
After conversion, mean body fat measures were comparable between instruments
Converted BIA data show a potential to assess global variations in body composition
Acknowledgements
The authors would like to thank the study participants, the Chapaevsk government, Chapaevsk Medical Association and Chapaevsk Health Center staff. Our special thanks to Lyubov Sergeyeva for the anthropometric and bioimpedance measurements. OS thanks Dmitry Nikolaev (Medas Ltd, Russia) for the provision of ABC-01 `Medas’ BIA instrument.
Funding
RCS was funded by the U.S. Environmental Protection Agency (EPA grant R82943701), the National Institute of Environmental Health Sciences (NIEHS grants R01 ES014370 and P30 ES000002). SR was supported by the RSF grant no 14-15-01085.
List of Abbreviations
- %FM
the percentage of body weight as fat
- BIA
bioelectrical impedance analysis BMI – body mass index, kg/m2
- CI
confidence interval
- DXA
dual-energy X-ray absorptiometry
- D-W
Durnin-Womersley
- FFM
fat-free mass
- FM
fat mass, kg
- FFMI
fat-free mass index, kg/m2
- FMI
fat mass index, kg/m2
- HCs
health centers
- Ht
height, cm
- IQR
interquartile range
- J-P
Jackson-Pollock
- LoA
limits of agreement
- r2
a proportion of explained variance
- RCS
Russian Children’s Study
- Res
whole-body resistance, Ohm
- SD
standard deviation
- SEE
standard error of estimate
- TBW
total body water, kg
- WHO
World Health Organization
- Wt
weight, kg
Footnotes
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
Conflict of Interest
Formerly, SR has consulted for Medas Ltd and received compensation. Medas Ltd had no input into the conception, design, and conduct of this research or manuscript preparation. Other authors have no conflicts of interest to declare. This work, emphasizing the importance of comparability of BIA instruments at the group level and their cross-calibration for inter-population comparisons, was first presented by the authors at the 11th International Symposium on In Vivo Body Composition Studies (25–27 June 2018, Columbia University Medical Center, New York).
References
- 1.Prentice AM, Jebb SA. Beyond body mass index. Obes Rev. 2001; 2:141–147. [DOI] [PubMed] [Google Scholar]
- 2.Grimnes S, Martinsen OG. Bioimpedance and Bioelectricity Basics. 3rd edn (Academic Press, London, 2014). [Google Scholar]
- 3.Thomasset MA. Bioelectric properties of tissue. Impedance measurement in clinical medicine. Significance of curves obtained. Lyon Med. 1962; 94: 107–118 (in French). [PubMed] [Google Scholar]
- 4.Chumlea WC, Guo SS. Bioelectrical impedance: present status and future directions. Nutr Rev. 1994; 52: 123–131. DOI: 10.1111/j.1753-4887.1994.tb01404.x [DOI] [PubMed] [Google Scholar]
- 5.Jaffrin MY. Body composition determination by bioimpedance: an update. Curr Opin Clin Nutr Metab Care. 2009; 12: 482–486. DOI: 10.1097/MCO.0b013e32832da22c [DOI] [PubMed] [Google Scholar]
- 6.Brantlov S, Jødal L, Lange A, Rittig S, Ward LC. Standardisation of bioelectrical impedance analysis for the estimation of body composition in healthy pediatric populations: a systematic review. J Med Eng Technol. 2017; 41: 460–479. DOI: 10.1080/03091902.2017.1333165 [DOI] [PubMed] [Google Scholar]
- 7.Kyle UG, Bosaeus I, DeLorenzo AD, Deurenberg P, Elia M, Gómez JM et al. Bioelectrical impedance analysis – part II: utilization in clinical practice. Clin Nutr. 2004; 23: 1430–1453. DOI: 10.1016/j.clnu.2004.09.012 [DOI] [PubMed] [Google Scholar]
- 8.Böhm A, Heitmann BL. The use of bioelectrical impedance analysis for body composition in epidemiological studies. Eur J Clin Nutr. 2013; 67(Suppl 1): S79–85. DOI: 10.1038/ejcn.2012.168 [DOI] [PubMed] [Google Scholar]
- 9.Ross R, Léger P, Martin P, Roy R. Sensitivity of bioelectrical impedance to detect changes in human body composition. J Appl Physiol. 1989; 67: 1643–1648. DOI: 10.1152/jappl.1989.67.4.1643 [DOI] [PubMed] [Google Scholar]
- 10.Kushner RF, Kunigk A, Alspaugh M, Andronis PT, Leitch CA, Schoeller DA. Validation of bioelectrical-impedance analysis as a measurement of change in body composition in obesity. Am J Clin Nutr. 1990; 52: 219–223. DOI: 10.1093/ajcn/52.2.219 [DOI] [PubMed] [Google Scholar]
- 11.Kyle UG, Zhang FF, Morabia A, Pichard C. Longitudinal study of body composition changes associated with weight change and physical activity. Nutrition. 2006; 22: 1103–1111. DOI: 10.1016/j.nut.2006.08.003 [DOI] [PubMed] [Google Scholar]
- 12.Neovius M, Uddén J, Hemmingsson E. Assessment of change in body fat percentage with DXA and eight-electrode BIA in centrally obese women. Med Sci Sports Exerc. 2007; 39: 2199–2203. DOI: 10.1249/mss.0b013e3181579.38a [DOI] [PubMed] [Google Scholar]
- 13.Silva AM, Fields DA, Sardinha LB. A PRISMA-driven systematic review of predictive equations for assessing fat and fat-free mass in healthy children and adolescents using multicomponent molecular models as the reference method. J Obes. 2013; 2013: 148696 DOI: 10.1155/2013/148696 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Mialich MS, Sicchieri JMF, Junior AAJ. Analysis of body composition: a critical review of the use of bioelectrical impedance analysis. Int J Clin Nutr. 2014; 2: 1–10. DOI: 10.12691/ijcn-2-1-1 [DOI] [Google Scholar]
- 15.Sergi G, De Rui M, Stubbs B, Veronese N, Manzato E. Measurement of lean body mass using bioelectrical impedance analysis: a consideration of the pros and cons. Aging Clin Exp Res. 2017; 29: 591–597. DOI: 10.1007/s40520-016-0622-6 [DOI] [PubMed] [Google Scholar]
- 16.Dittmar M. Reliability and variability of bioimpedance measures in normal adults: effects of age, gender, and body mass. Am J Phys Anthropol. 2003; 122: 361–370. DOI: 10.1002/ajpa.10301 [DOI] [PubMed] [Google Scholar]
- 17.Nagy E, Vicente-Rodriguez G, Manios Y, Béghin L, Iliescu C, Censi L et al. Harmonization process and reliability assessment of anthropometric measurements in a multicenter study in adolescents. Int J Obes. 2008; 32(Suppl 5): S58–65. DOI: 10.1038/ijo.2008.184 [DOI] [PubMed] [Google Scholar]
- 18.Talma H, Chinapaw MJM, Bakker B, HiraSing RA, Terwee CB, Altenburg TM. Bioelectrical impedance analysis to estimate body composition in children and adolescents: a systematic review and evidence appraisal of validity, responsiveness, reliability and measurement error. Obes Rev. 2013; 14: 895–905. DOI: 10.1111/obr.12061 [DOI] [PubMed] [Google Scholar]
- 19.Van Zanten M, Piller N, Ward LC. Inter-changeability of impedance devices for lymphedema assessment. Lymphat Res Biol. 2016; 14: 88–94. DOI: 10.1089/lrb.2015.0026 [DOI] [PubMed] [Google Scholar]
- 20.Brantlov S, Ward LC, Jødal L, Rittig S, Lange A. Critical factors and their impact on bioelectrical impedance analysis in children. J Med Eng Technol. 2017; 41: 22–35. DOI: 10.1080/03091902.2016.1209590 [DOI] [PubMed] [Google Scholar]
- 21.Lee MM, Sergeyev OV, Williams P, Korrick S, Zeilert V, Revich B, Hauser R. Physical growth and sexual maturation of boys in Chapaevsk, Russia. J Pediatr Endocrinol Metab. 2003; 16: 169–178. [DOI] [PubMed] [Google Scholar]
- 22.Burns JS, Williams PL, Sergeyev O, Korrick SA, Lee MM, Revich B et al. Serum concentrations of organochlorine pesticides and growth among Russian boys. Environ Health Perspect. 2012; 120: 303–308. DOI: 10.1289/ehp.1103743 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Sergeyev OV, Burns JS, Williams PL, Korrick SA, Lee MM, Revich B, Hauser R. The association of peripubertal serum concentrations of organochlorine chemicals and blood lead with growth and pubertal development in a longitudinal cohort of boys: a review of published results from the Russian Children’s Study. Rev Environ Health. 2017; 32: 83–92. DOI: 10.1515/reveh-2016-0052 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Randstuff [Internet]. Random number generator. Available from: https://randstuff.ru/number/
- 25.Rudnev SG, Soboleva NP, Sterlikov SA, Nikolaev DV, Starunova OA, Chernykh SP et al. Bioimpedance study of body composition in the Russian population (Federal Research Institute for Health Organization and Informatics, Moscow, 2014, in Russian). [Google Scholar]
- 26.Lohman TG, Roche AF, Martorell R (eds). Anthropometric Standardization Reference Manual (Human Kinetics, Champaign, IL, 1988). [Google Scholar]
- 27.National Health and Nutrition Examination Survey (NHANES). Anthropometry Procedures Manual (CDC, 2007). URL: https://www.cdc.gov/nchs/data/nhanes/nhanes_07_08/manual_an.pdf
- 28.Smirnov AV, Kolesnikov VA, Nikolaev DV, Eryukova TA. ABC-01 ‘Medas’: Analyzer of Body Water Compartments with Software: Instruction Manual (SRC Medas, Moscow, 2009, in Russian). [Google Scholar]
- 29.TANITA Corporation [Internet]. Body Composition Analyzer BC-418MA: Instruction Manual. Available from: https://www.manualslib.com/products/Tanita-Body-Composition-Analyzer-Bc-418ma-2318718.html
- 30.Kushner RF, Schoeller DA. Estimation of total body water by bioelectrical impedance analysis. Am J Clin Nutr. 1986; 44: 417–424. DOI: 10.1093/ajcn/44.3.417 [DOI] [PubMed] [Google Scholar]
- 31.Durnin JVGA, Womersley J. Body fat assessed from total body density and its estimation from skinfold thickness: Measurements on 481 men and women aged from 16 to 72 years. Br J Nutr. 1974; 32: 77–97. [DOI] [PubMed] [Google Scholar]
- 32.Jackson AS, Pollock ML. Generalized equations for predicting body density of men. Br J Nutr. 1978; 40: 497–504. [DOI] [PubMed] [Google Scholar]
- 33.Bland JM, Altman DG. Statistical methods for assessing agreement between two methods of clinical measurement. Lancet. 1986; 327: 307–310. [PubMed] [Google Scholar]
- 34.World Health Organization [Internet]. Body mass index – BMI. Available from: http://www.euro.who.int/en/health-topics/disease-prevention/nutrition/a-healthy-lifestyle/body-mass-index-bmi
- 35.Chumlea WC, Guo SS, Kuczmarski RJ, Flegal KM, Johnson CL, Heymsfield SB et al. Body composition estimates from NHANES III bioelectrical impedance data. Int J Obes. 2002; 26: 1596–1609. DOI: 10.1038/sj.ijo.0802167 [DOI] [PubMed] [Google Scholar]
- 36.Silva AM, Matias CN, Nunes CL, Santos DA, Marini E, Lukaski HC, Sardinha LB. Lack of agreement of in vivo raw bioimpedance measurements obtained from two single and multi-frequency bioelectrical impedance devices. Eur J Clin Nutr. 2019; 73: 1077–1083. DOI: 10.1038/s41430-018-0355-z [DOI] [PubMed] [Google Scholar]
- 37.Data Bridge Market Research Private Ltd [Internet]. Global body composition analyzers market: analysis, companies, profiles, size, share, growth, trends and forecast to 2024. Available from: https://databridgemarketresearch.com/reports/global-body-composition-analyzers-market/
- 38.McCarthy HD, Cole TJ, Fry T, Jebb SA, Prentice AM. Body fat reference curves for children. Int J Obes. 2006; 30: 598–602. DOI: 10.1038/sj.ijo.0803232 [DOI] [PubMed] [Google Scholar]
- 39.McCarthy HD, Samani-Radia D, Jebb SA, Prentice AM. Skeletal muscle mass reference curves for children and adolescents. Pediatr Obes. 2014; 9: 249–259. DOI: 10.1111/j.2047-6310.2013.00168.x [DOI] [PubMed] [Google Scholar]
- 40.Kurtoglu S, Mazicioglu MM, Ozturk A, Hatipoglu M, Cicek B, Ustunbas HB. Body fat reference curves for healthy Turkish children and adolescents. Eur J Pediatr. 2010; 169: 1329–1335. DOI: 10.1007/s00431-010-1225-4 [DOI] [PubMed] [Google Scholar]
- 41.Franssen FM, Rutten EP, Groenen MT, Vanfleteren LE, Wouters EFM, Spruit MA. New reference values for body composition by bioelectrical impedance analysis in the general population: results from the UK Biobank. J Am Med Dir Assoc. 2014; 15: 448.e1–6. DOI: 10.1016/j.jamda.2014.03.012 [DOI] [PubMed] [Google Scholar]
- 42.Pedrera-Zamorano JD, Roncero-Martin R, Lavado-Garcia JM, Calderon-Garcia JF, Rey-Sanchez P, Vera V et al. Segmental fat-free and fat mass measurements by bioelectrical impedance analysis in 2,224 healthy Spanish women aged 18–85 years. Am J Hum Biol. 2015; 27: 468–474. DOI: 10.1002/ajhb.22669 [DOI] [PubMed] [Google Scholar]
- 43.Rush EC, Crowley J, Freitas IF, Luke A. Validity of hand-to-foot measurement of bioimpedance: standing compared with lying position. Obesity. 2006; 14: 252–257. DOI: 10.1038/oby.2006.32 [DOI] [PubMed] [Google Scholar]
- 44.NCD Risk Factor Collaboration. Worldwide trends in body-mass index, underweight, overweight, and obesity from 1975 to 2016: a pooled analysis of 2416 population-based measurement studies in 128·9 million children, adolescents, and adults. Lancet. 2017; 390: 2627–2642. DOI: 10.1016/S0140-6736(17)32129-3 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.NCD Risk Factor Collaboration. Rising rural body-mass index is the main driver of the global obesity epidemic in adults. Nature. 2019; 569: 260–264. DOI: 10.1038/s41586-019-1171-x [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Okorodudu DO, Jumean MF, Montori VM, Romero-Corral A, Somers VK, Erwin PJ, Lopez-Jimenez F. Diagnostic performance of body mass index to identify obesity as defined by body adiposity: a systematic review and meta-analysis. Int J Obes (Lond). 2010; 34: 791–799. DOI: 10.1038/ijo.2010.5 [DOI] [PubMed] [Google Scholar]
- 47.Javed A, Jumean M, Murad MH, Okorodudu D, Kumar S, Somers VK et al. Diagnostic performance of body mass index to identify obesity as defined by body adiposity in children and adolescents: a systematic review and meta-analysis. Pediatr Obes. 2015; 10: 234–244. DOI: 10.1111/ijpo.242 [DOI] [PubMed] [Google Scholar]
- 48.Heitmann BL, Erikson H, Ellsinger BM, Mikkelsen KL, Larsson B. Mortality associated with body fat, fat-free mass and body mass index among 60-year-old Swedish men – a 22-year follow-up. The study of men born in 1913. Int J Obes Relat Metab Disord. 2000; 24: 33–37. [DOI] [PubMed] [Google Scholar]