Abstract
Lymphotoxin-a (LTA) may be associated with the pathogenesis of inflammatory diseases. To assess the association of the LTA rs909253 A/G polymorphism with plasma level and risk of ankylosing spondylitis (AS) in a Chinese Han population. Genotyping and LTA plasma were tested by mass spectroscopy and enzyme-linked immunosorbent assay (ELISA), respectively. The results showed that the average plasma level of LTA in AS was significantly lower than in the controls (P = 0.000). Our results also indicated that LTA rs909253 A/G was associated with a decreased risk of AS (G vs. A: P = 0.014). Significant differences were also found between the rs909253 A/G genotype and down-regulated plasma level in AS patients, compared with controls. After stratification analysis, a decreased risk of AS was associated with the LTA rs909253 G allele (G vs. A) among female patients, younger patients (Yr. < 30), HLA-B27-positive patients. In addition, In conclusion, LTA rs909253 A/G genotype has a significant relationship with decreased susceptibility to AS.
Subject terms: Genetics research, Ankylosing spondylitis
Introduction
Ankylosing spondylitis (AS) is a common rheumatic chronic inflammatory disease. It often occurs in young men. It begins with the sacroiliac joint and then gradually causes stiffness and pain in the spine. In many patients, the bilateral hip joints are involved. AS often results in limited movement of the spine, sacroiliac joint and hip joint, which can cause serious limitations to the patients’ life and work1. Up to now, the pathogenesis of AS remains unclear. Immune response dysregulation, infectious agents, and genetic factors may cause the development of AS2. A twin study confirmed that AS susceptibility is largely determined by heredity, while HLA-B27 accounts for a small proportion of the whole genetic susceptibility3.
Lymphotoxin-a (LTA), another name is tumor necrosis factor-β (TNF-β), is a close homologue of tumor necrosis factor-α (TNF-a)4. The TNF-α gene is located on chromosome 6, and between HLA-B and HLA-DR5. LTA is a proinflammatory cytokine produced by lymphocytes that causes tissue injury. It also can significantly affect the function of lymphogenesis6,7. Lymphotoxin (LT) signaling plays a key role in lymphogenesis and maintenance8. Studies have shown that the inflammatory factors of IL-22 and IL-23 are associated with the development of AS9,10. Furthermore, IL-22 and IL-23 production for host defense were regulated by the LT pathway in adult innate lymphoid cells11. Therefore, LTA is correlated to the pathogenesis of inflammatory diseases.
The LTA rs909253 A/G polymorphism has been correlated to the risk of developing several autoimmune diseases, such as scleroderma12, multiple sclerosis13 and systemic lupus erythematosus5,14. Single nucleotide polymorphisms (SNPs) of the LTA gene have been suggested to be associated with the susceptibility of AS15,16. For example, a case-control study showed that rs909253 may influence susceptibility to AS16.
Recently, a study by Fabiano Aparecido de Medeiros et al. explored the association between the LTA rs909253 polymorphism with plasma LTA level, the susceptibility for RA, and the presence of autoantibodies. They found that the LTA rs909253 polymorphism was not correlated to RA susceptibility and LTA plasma levels. However, the B1 allele had significantly correlated to the presence of autoantibodies. Furthermore, interaction between the presence of autoantibodies and B1 allele has significantly related to the increase of plasma LTA level in RA patients17.
In this study, we investigated the potential correlation between the plasma level of LTA and AS, and examined associations between the plasma level of LTA and clinical parameters in the Chinese Han population. The correlations between rs909253 and plasma LTA level also have been tested. Finally, we tested the correlation between rs909253 and susceptibility to AS.
Results
Characteristics of the study population
The demographic and clinical characteristics of all subjects are summarized in Table 1. Subjects were adequately matched for age and sex (P = 0.345 and 0.815, respectively). The genotype distributions of LTA rs909253 A/G in all subjects are illustrated in Table 2. The observed genotype frequencies for the polymorphism in controls were in HWE for LTA rs909253 A/G (P = 0.109).
Table 1.
Patient demographics and risk factors in ankylosing spondylitis.
| Variable* | Cases (n = 190) | Controls (n = 190) | P |
|---|---|---|---|
| Age (years) | 32.49 (±10.15) | 33.38 (±8.10) | 0.345 |
| Male/female | 142/48 | 140/50 | 0.815 |
| CRP positive, no. (%) | 118 (62.11%) | NA | NA |
| HLA-B27 positive, no. (%) | 151 (79.47%) | NA | NA |
| Grading of sacroiliac joint, no. (%) | |||
| Grade I | 0 (0.00%) | NA | NA |
| Grade II | 136 (71.58%) | NA | NA |
| Grade III | 31 (16.32%) | NA | NA |
| Grade IV | 23 (12.11%) | NA | NA |
| AA + AG + GG LTA levels∗∗ | 61.20/109.80 | 5202.00/9333.00 | 0.000∗∗∗ |
| AA (25/26) LTA levels | 18.64/33.08 | 466.00/860.00 | 0.001∗∗∗ |
| AG (44/37) LTA levels | 28.80/55.51 | 1267.00/2054.00 | 0.000∗∗∗ |
| GG (16/22) LTA levcls | 15.38/22.50 | 5202.00/9333.00 | 0.052∗∗∗ |
*CRP: C-reactive protein. **LTA levels were available in 85 AS cases (AA: 26; AG: 37; GG: 22 of LTA rs909253 A/G) and 85 controls (AA: 25; AG: 44; GG: 16 of LTA rs909253 A/G), with age, P = 0.214; sex, P = 0.506 (cases vs. controls). ***P value was calculated by non-parametric tests. Bold values are statistically significant (P < 0.05).
Table 2.
Logistic regression analysis of associations between LTA rs909253 A/G polymorphisms and risk of ankylosing spondylitis.
| Genotype | Cases* (n = 190) | Controls (n = 190) | OR (95% CI) | P | OR (95% CI) | P | ||
|---|---|---|---|---|---|---|---|---|
| n | % | n | % | Adjust∗∗ | Adjust | |||
| AA | 65 | 34.39 | 53 | 28.19 | 1.00 | NA | 1.00 | NA |
| AG (AG vs. AA) | 93 | 49.21 | 83 | 44.15 | 0.91 (0.57–1.46) | 0.705 | 0.92 (0.57–1.47) | 0.722 |
| GG (GG vs. AA) | 31 | 16.40 | 52 | 27.66 | 0.49 (0.27–0.86) | 0.014 | 0.46 (0.26–0.83) | 0.010 |
| AG + GG vs. AA | NA | NA | NA | NA | 0.75 (0.48–1.16) | 0.195 | 0.74 (0.48–1.15) | 0.185 |
| GG vs. AG + AA | NA | NA | NA | NA | 0.51 (0.31–0.85) | 0.009 | 0.50 (0.30–0.82) | 0.007 |
| G vs. A | NA | NA | NA | NA | 0.70 (0.53–0.94) | 0.016 | 0.70 (0.52–0.93) | 0.014 |
*The genotyping was successful in: 189 cases and 188 controls for LTA rs909253 A/G. **Adjusted by age and sex. Bold values are statistically significant (P < 0.05).
Association between LTA rs909253 A/G Polymorphisms and the Risk of AS
Logistic regression analyses revealed that LTA rs909253 A/G polymorphism was associated with the risk of AS (Table 2). Using genotypes AA as a reference, genotype GG acted as a protection factor for AS patients (GG vs. AA: OR = 0.46, 95%CI = 0.26–0.83; P = 0.010). Using genotypes AG + AA as a reference, genotype GG acted as a protection factor for AS patients (GG vs. AG + AA: OR = 0.74, 95%CI = 0.48–1.15; P = 0.007). Our analysis also revealed that LTA rs909253 G allele was associated with significantly decreased risk of AS (G vs. A: OR = 0.70, 95%CI = 0.52–0.93; P = 0.014) than the rs909253 A allele in the Chinese Han population.
Stratification analyses of LTA rs909253 A/G polymorphisms and the risk of RA
Stratification analyses were performed according to age, sex, HLA-B27 (Table 3). Following stratified analysis, a decreased risk of AS was associated with the LTA rs909253 G allele (G vs. A) among female patients (OR = 0.55, 95%CI = 0.31–0.97, P = 0.040), younger patients (Yr. < 30) (OR = 0.56, 95%CI = 0.35–0.89, P = 0.014), HLA-B27-positive patients (OR = 0.73, 95%CI = 0.54–0.99, P = 0.040).
Table 3.
Stratified Analyses between LTA rs909253 A/G Polymorphisms and the Risk of Ankylosing Spondylitis.
| Variable | LTA rs909253 A/G (case/control) | OR (95% CI); P | ||||||
|---|---|---|---|---|---|---|---|---|
| AA | AG | GG | G versus A | AG versus AA | GG versus AA | AG + GG versus AA | GG versus AG + AA | |
| Sex | ||||||||
| Male | 45/38 | 70/63 | 26/38 | 0.76 (0.55–1.06); 0.110 | 0.94 (0.54–1.63); 0.820 | 0.58 (0.30–1.12); 0.103 | 0.80 (0.48–1.34); 0.402 | 1.66 (0.95–2.93); 0.078 |
| Female | 20/15 | 23/20 | 5/14 | 0.55 (0.31–0.97); 0.040 | 0.86 (0.35–2.12); 0.747 | 0.27 (0.08–0.91); 0.034 | 0.62 (0.27–1.43); 0.258 | 3.44 (1.13–10.48); 0.030 |
| Age (years) | ||||||||
| < 30 | 29/13 | 43/29 | 15/21 | 0.56 (0.35–0.89); 0.014 | 0.67 (0.30–1.49); 0.321 | 0.32 (0.13–0.81); 0.017 | 0.52 (0.24–1.11); 0.090 | 2.40 (1.12–5.15); 0.025 |
| ≥ 30 | 36/40 | 50/54 | 16/31 | 0.78 (0.53–1.13); 0.185 | 1.03 (0.57–1.86); 0.925 | 0.57 (0.27–1.22); 0.148 | 0.86 (0.50–1.50); 0.601 | 1.77 (0.91–3.47); 0.094 |
| HLA-B27 | ||||||||
| Negative | 13/53 | 19/83 | 4/52 | 0.61 (0.36–1.02); 0.059 | 0.93 (0.43–2.05); 0.863 | 0.31 (0.10–1.03); 0.055 | 0.70 (0.33–1.47); 0.341 | 3.06 (1.03–0.08); 0.044 |
| Positive | 52/53 | 74/83 | 27/52 | 0.73 (0.54–0.99); 0.040 | 0.91 (0.55–1.49); 0.704 | 0.53 (0.29–0.97); 0.038 | 0.76 (0.48–1.21); 0.249 | 1.78 (1.06–3.01); 0.030 |
Bold values are statistically significant (P < 0.05).
Production of LTA in AS Patients, Controls and Different Genotypes
The average plasma concentration of LTA was significantly lower in AS patients compared with controls (Table 1). We compared LTA plasma levels on basis of LTA rs909253 A/G genotypes. We found LTA rs909253 A/G genotypes had significant lower levels of LTA in AS patients when compared with control groups except for group genotype GG (Table 1). However, we did not find the significant statistic associations between LTA plasma levels and different LTA rs909253 A/G genotypes in AS patients or control groups (Fig. 1).
Figure 1.
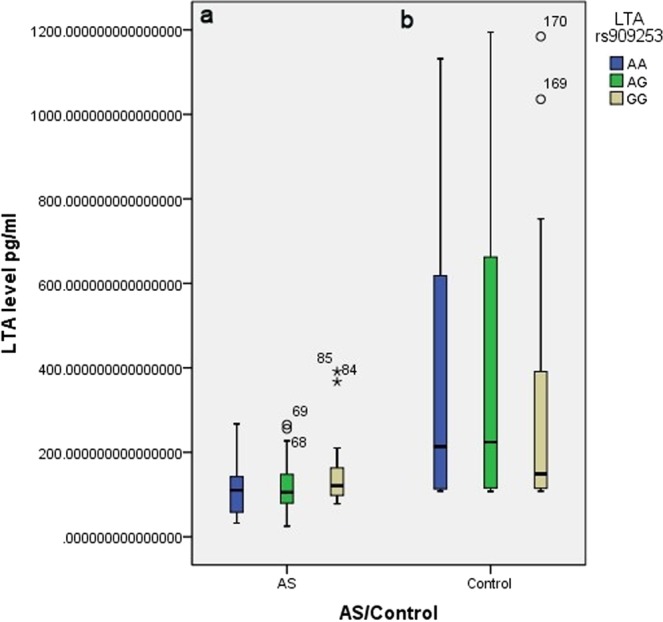
Association between LTA levels and LTA rs909253 A/G genotype frequencies in (a) ankylosing spondylitis patients and (b) controls.
Stratification of association between plasma level of LTA and other biomarkers
Our study indicated the LTA plasma levels of female AS patients were significantly lower than male AS patients. However, no associations were obtained between plasma levels of LTA and sex, age, HLA-B27, C-reactive protein (CRP), or grade of the sacroiliac joint in AS patients (Table 4).
Table 4.
Stratification of association between plasma levels of LTA and other biomarkers in ankylosing spondylitis patients.
| Variable | Case, n | P* | |
|---|---|---|---|
| Age | ≥30Ys | 54 | 0.493 |
| <30Ys | 31 | ||
| Sex | Male | 61 | 0.109 |
| Female | 24 | ||
| HLA-B27 | Negative | 25 | 0.539 |
| Positive | 60 | ||
| CRP status | Negative | 35 | 0.237 |
| Positive | 50 | ||
| Grading of Sacroiliac joint | I + II | 58 | 0.902 |
| III + IV | 27 | ||
*P value was calculated by non-parametric tests. Bold values are statistically significant (P < 0.05).
Combined analysis with recent researches of LTA rs909253 A/G polymorphisms in AS
Combined with recent two other researches found that LTA rs909253 AG increased risk of AS significantly than controls (OR = 1.28; 95% CI = 1.01–1.62; P = 0.038) (Table 5).
Table 5.
Meta-analysis of the association between LTA rs909253 A/G polymorphisms and ankylosing spondylitis risk.
| SNP | Comparison | Category | Category | OR (95% CI) | P-value | P for heterogeneity |
|---|---|---|---|---|---|---|
| LTA rs909253 | G vs. A | Total | 1.20(0.70, 2.07) | 0.512 | 0.000 | |
| 2019 | This study | 0.70(0.53, 0.94) | 0.016 | NA | ||
| 2017 | Jia B | 1.53(1.07, 2.18) | 0.020 | NA | ||
| 2011 | Chen J | 1.62(1.26, 2.08) | 0.000 | NA | ||
| GG vs. AG + AA | Total | 1.38(0.46, 4.12) | 0.564 | 0.000 | ||
| 2019 | This study | 0.51(0.31, 0.85) | 0.009 | NA | ||
| 2017 | Jia B | 2.15(1.02, 4.52 | 0.043 | NA | ||
| 2011 | Chen J | 2.54(1.33, 8.45) | 0.005 | NA | ||
| GG + AG vs. AA | Total | 1.25(0.77, 2.04) | 0.373 | 0.012 | ||
| 2019 | This study | 0.75(0.48, 1.16) | 0.195 | NA | ||
| 2017 | Jia B | 1.53(0.95, 2.45) | 0.080 | NA | ||
| 2011 | Chen J | 1.65(1.21, 2.24) | 0.001 | NA | ||
| GG vs. AA | Total | 1.50(0.45, 5.02) | 0.513 | 0.000 | ||
| 2019 | This study | 0.49(0.27, 0.86) | 0.014 | NA | ||
| 2017 | Jia B | 2.46(1.13, 5.35) | 0.024 | NA | ||
| 2011 | Chen J | 2.95(1.53, 5.70) | 0.001 | NA | ||
| AG vs. AA | Total | 1.28(1.01, 1.62) | 0.120 | 0.241 | ||
| 2019 | This study | 0.91(0.57, 1.46) | 0.705 | NA | ||
| 2017 | Jia B | 1.33(0.80, 2.20) | 0.273 | NA | ||
| 2011 | Chen J | 1.49(1.08, 2.06) | 0.016 | NA |
Bold values are statistically significant of total values (P < 0.05).
Discussion
In the current case-control association study, our present data suggest that the LTA rs909253 A/G genotype is associated with decreased susceptibility to AS. In addition, we also found LTA rs909253 A/G genotypes had significant lower levels of LTA in AS patients compared to control groups. In stratification analysis, we found a decreased risk of AS was associated with the LTA rs909253 G allele (G vs. A) among female patients, younger patients (Yr. < 30) and HLA-B27-positive patients.
AS can lead to a decrease in the quality of life of patients. However, there is no cure for AS, although treatments and medications can reduce symptoms and pain. Many researchers want to find new ways to prevent and treat AS. Genetic factors may contribute to the development of AS2. Approximately 90% of people with AS are the HLA-B27 genotype, and thus, there is a strong genetic association18. However, only 1–2% of the persons with the HLA-B27 genotype develop AS. Investigating AS-related genetic factors may be helpful in the prevention and diagnosis of AS. Thus, we explored the associations between the LTA rs909253 A/G polymorphism, plasma level and risk of AS in the Chinese Han population.
LTA is located at the HLA-III region of chromosome 6p, is closely linked to TNF-a. LTA gene consists of four exons and three introns. LTA plays a critical role in inflammatory regulation, anti-virus response and immune activation, similar to TNF-α19,20.
There have been studies on rs909253 and gastric cancer, chronic obstructive pulmonary disease (COPD), and coronary heart diseases21–23. A meta-analysis suggested that rs909253 was correlated with the risk of gastric cancer, and especially in Asians21. However, no significantly different genotype frequencies of rs909253 were seen in COPD or coronary heart disease compared with controls22,23. In this study, we found that rs909253 was correlated with a decreased risk of AS (G vs. A: OR = 0.70, 95%CI = 0.52–0.93; P = 0.014). Then, we searched PubMed and meta-analyzed our results with the results of two other studies on this locus15,16. We found that rs909253 had no risk of AS.
LTA is a soluble protein released by lymphocytes and activated by antigens or mitogens. It can inhibit the activity of tumor cells24. Bachmann believed that blocking lymphotoxin might be a promising therapeutic strategy for other autoimmune diseases, such as Hashimoto’s thyroiditis and arthritis25. As far as we know, there have not been any studies on the level of LTA in AS. Thus, we tested the plasma LTA level in AS and healthy controls. We found that the average plasma level of LTA was significantly lower in AS patients, compared with controls (P = 0.000).
We stratified the plasma LTA levels of the AS and control groups according to rs909253 genotype. We found that the LTA levels of the genotypes were significantly lower in the AS group except for group genotype GG (Table 1). Recently, Bolstad AI et al. investigated whether SNPs in the LTA gene clusters were correlated with primary Sjogren ‘s syndrome, and they found that LTA rs909253 and rs1800629 had significantly association with primary Sjogren’s syndrome, and the correlations were mainly due to anti-Ro/SSA and anti-La/SSB antibody-positive primary Sjogren’s syndrome26. Therefore, we hypothesized that LTA rs909253 may affect mechanistic pathways in AS. We will do functional studies of LTA rs909253 in our future research.
Our study has some limitations. First, because of our hospital-based case–control design, we may have selection bias. Second, we investigated SNPs just based on the functional characteristics, another fine-mapping study is required. Third, we use a medium sample size, so our analytical power is limited, although we also combined the results with other two independent studies. Forth, the control group we recruited was trauma patients, which may have a bias on our study. We will collect healthy patients as control in the future research. Fifth, because of the bias of choice, there is a slight difference in the proportion of HLA-B27 positive patients between the 85 vs. 85 samples and the 190 vs. 190 samples. However, because we use a medium sample size, studies including larger population, more ethnic groups are required.
Materials and Methods
Subjects
We obtained approval of the study protocol from the Ethics Committee of Nanjing Medical University (Nanjing, China). All patients provided written informed consent to be included in the study. We confirmed that all research was performed in accordance with relevant guidelines. One hundred and ninety AS patients were consecutively recruited from the Affiliated Changzhou No.2 People’s Hospital of Nanjing Medical University (Changzhou, China), the Changzhou First Hospital (Changzhou, China), between September 2010 and January 2016. A diagnosis of AS was established by using the classification criteria reported by the American College of Rheumatology (Modified New York Criteria)27. One hundred and ninety controls were traumatic patients without AS, matched AS for age (±5 years) and sex, and recruited from the same institutions during the same period time. Each patient was interviewed by trained personnel using a pre-tested questionnaire to obtain information on demographic data and related risk factors for AS. After the interview, 2 ml of peripheral blood was collected from each subject. Blood samples were collected using vacutainers and transferred to test tubes containing ethylenediamine tetra-acetic acid (EDTA).
Genomic DNA was isolated from whole blood using the QIAamp DNA Blood Mini Kit (Qiagen, Hilden, Germany). Genotyping was done by matrix-assisted laser desorption/ionization time-of-flight mass spectrometry (MALDI-TOF MS) using the MassARRAY system as previously described28.
The blood plasma concentration of LTA in 85 AS patients and 85 randomly selected controls using an enzyme-linked immunosorbent assay Kit (Boster, Wuhan, China). All analytical steps were performed in accordance with the manufacturer’s recommendations. The concentration of LTA was calculated by referring to a standard curve, according to the manufacturer’s instructions.
Statistical analyses
Differences in demographics, variables, and genotypes of LTA rs909253 A/G polymorphism variants were evaluated using a chi-squared test. The associations between LTA rs909253 A/G genotypes and risk of AS were estimated by computing odds ratios (ORs) and 95% confidence intervals (CIs) using logistic regression analyses, and by using crude ORs. The Hardy–Weinberg equilibrium (HWE) was tested by a goodness-of-fit chi-squared test to compare the observed genotype frequencies to the expected frequencies among controls. Differences in LTA gene polymorphism and LTA blood plasma concentrations were evaluated using the Non parametric Tests. All statistical analyses were done with SAS software (version 9.1.3; SAS Institute, Cary, NC, USA).
Acknowledgements
This research was supported in part by Changzhou High-Level Medical Talents Training Project (2016CZLJ011).
Author contributions
A.Z. and Z.Y. wrote the manuscript, H.Z. collected samples, R.L. and A.Z. conceived the experiment. Z.Y. and H.Z. conducted the experiment, Z.Y. and R.L. analyzed the results. All authors reviewed the manuscript.
Competing interests
The authors declare no competing interests.
Footnotes
Publisher’s note Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
These authors contributed equally: Aiping Zhu and Zhicheng Yang.
References
- 1.Braun J, Sieper J. Ankylosing spondylitis. Lancet. 2007;369:1379–90. doi: 10.1016/S0140-6736(07)60635-7. [DOI] [PubMed] [Google Scholar]
- 2.Li Z, et al. Genome-wide association study in Turkish and Iranian populations identify rare familial Mediterranean fever gene (MEFV) polymorphisms associated with ankylosing spondylitis. PLoS Genet. 2019;15:e1008038. doi: 10.1371/journal.pgen.1008038. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Brown MA, et al. Susceptibility to ankylosing spondylitis in twins: the role of genes, HLA, and the environment. Arthritis and Rheumatism. 1997;40:1823–8. doi: 10.1002/art.1780401015. [DOI] [PubMed] [Google Scholar]
- 4.Posch PE, Cruz I, Bradshaw D, Medhekar BA. Novel polymorphisms and the definition of promoter ‘alleles’ of the tumor necrosis factor and lymphotoxin alpha loci: inclusion in HLA haplotypes. Genes and Immunity. 2003;4:547–58. doi: 10.1038/sj.gene.6364023. [DOI] [PubMed] [Google Scholar]
- 5.Umare VD, et al. Impact of TNF-alpha and LTalpha gene polymorphisms on genetic susceptibility in Indian SLE patients. Human Immunology. 2017;78:201–8. doi: 10.1016/j.humimm.2016.11.002. [DOI] [PubMed] [Google Scholar]
- 6.Kumar P, et al. Association between Lymphotoxin Alpha (-252G/A and -804C/A) Gene Polymorphisms and Risk of Ischemic Stroke: A Meta-Analysis. Acta Neurologica Taiwanica. 2016;25:10–7. [PubMed] [Google Scholar]
- 7.Upadhyay V, Fu YX. Lymphotoxin signalling in immune homeostasis and the control of microorganisms. Nature Reviews: Immunology. 2013;13:270–9. doi: 10.1038/nri3406. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Tang H, Zhu M, Qiao J, Fu YX. Lymphotoxin signalling in tertiary lymphoid structures and immunotherapy. Cellular & Molecular Immunology. 2017;14:809–18. doi: 10.1038/cmi.2017.13. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Rezaiemanesh A, et al. Ankylosing spondylitis M-CSF-derived macrophages are undergoing unfolded protein response (UPR) and express higher levels of interleukin-23. Modern. Rheumatology. 2017;27:862–7. doi: 10.1080/14397595.2016.1259716. [DOI] [PubMed] [Google Scholar]
- 10.Ciccia F, et al. Interleukin-22 and interleukin-22-producing NKp44+ natural killer cells in subclinical gut inflammation in ankylosing spondylitis. Arthritis and Rheumatism. 2012;64:1869–78. doi: 10.1002/art.34355. [DOI] [PubMed] [Google Scholar]
- 11.Upadhyay V, Fu YX. Lymphotoxin organizes contributions to host defense and metabolic illness from innate lymphoid cells. Cytokine and Growth Factor Reviews. 2014;25:227–33. doi: 10.1016/j.cytogfr.2013.12.007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Pandey JP, Takeuchi F. TNF-alpha and TNF-beta gene polymorphisms in systemic sclerosis. Human Immunology. 1999;60:1128–30. doi: 10.1016/S0198-8859(99)00105-6. [DOI] [PubMed] [Google Scholar]
- 13.Kallaur AP, et al. Tumor necrosis factor beta (TNF-beta) NcoI polymorphism is associated with multiple sclerosis in Caucasian patients from Southern Brazil independently from HLA-DRB1. Journal of Molecular Neuroscience. 2014;53:211–21. doi: 10.1007/s12031-014-0287-6. [DOI] [PubMed] [Google Scholar]
- 14.Parks CG, et al. Genetic polymorphisms in tumor necrosis factor (TNF)-alpha and TNF-beta in a population-based study of systemic lupus erythematosus: associations and interaction with the interleukin-1alpha-889 C/T polymorphism. Human Immunology. 2004;65:622–31. doi: 10.1016/j.humimm.2004.03.001. [DOI] [PubMed] [Google Scholar]
- 15.Chen J, et al. Identification of a novel lymphotoxin-alpha (LTA) gene associated with ankylosing spondylitis in Ningxia population. Yi Chuan. 2011;33:329–36. doi: 10.3724/SP.J.1005.2011.00329. [DOI] [PubMed] [Google Scholar]
- 16.Jia B, Qi X. The genetic association between polymorphisms in lymphotoxin-alpha gene and ankylosing spondylitis susceptibility in Chinese group: A case-control study. Medicine (Baltimore) 2017;96:e6796. doi: 10.1097/MD.0000000000006796. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.van der Linden S, Valkenburg HA, Cats A. Evaluation of diagnostic criteria for ankylosing spondylitis. A proposal for modification of the New York criteria. Arthritis and Rheumatism. 1984;27:361–8. doi: 10.1002/art.1780270401. [DOI] [PubMed] [Google Scholar]
- 18.Chen B, et al. Role of HLA-B27 in the pathogenesis of ankylosing spondylitis (Review) Mol Med Rep. 2017;15:1943–51. doi: 10.3892/mmr.2017.6248. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Yu X, et al. Positive association between lymphotoxin-alpha variation rs909253 and cancer risk: a meta-analysis based on 36 case-control studies. Tumour Biology. 2014;35:1973–83. doi: 10.1007/s13277-013-1263-4. [DOI] [PubMed] [Google Scholar]
- 20.Daller B, et al. Lymphotoxin-beta receptor activation by lymphotoxin-alpha(1)beta(2) and LIGHT promotes tumor growth in an NFkappaB-dependent manner. International Journal of Cancer. 2011;128:1363–70. doi: 10.1002/ijc.25456. [DOI] [PubMed] [Google Scholar]
- 21.Xu Z, Shi R, Zhang R, Zhang D, Wang L. Association between tumor necrosis factor beta 252 A/G polymorphism and risk of gastric cancer: a meta-analysis. Tumour Biol. 2013;34:4001–5. doi: 10.1007/s13277-013-0989-3. [DOI] [PubMed] [Google Scholar]
- 22.Seifart C, et al. TNF-alpha-, TNF-beta-, IL-6-, and IL-10-promoter polymorphisms in patients with chronic obstructive pulmonary disease. Tissue Antigens. 2005;65:93–100. doi: 10.1111/j.1399-0039.2005.00343.x. [DOI] [PubMed] [Google Scholar]
- 23.Gao H, et al. Association of LT-alpha Ala252Gly gene polymorphism and the genetic predisposition of coronary heart disease in Chinese. Mol Biol Rep. 2010;37:47–50. doi: 10.1007/s11033-009-9509-3. [DOI] [PubMed] [Google Scholar]
- 24.Gray PW, et al. Cloning and expression of cDNA for human lymphotoxin, a lymphokine with tumour necrosis activity. Nature. 1984;312:721–4. doi: 10.1038/312721a0. [DOI] [PubMed] [Google Scholar]
- 25.Bachmann MF. Blocking lymphotoxin: a potential therapy for diabetes? Trends Immunol. 2001;22:420. doi: 10.1016/S1471-4906(01)02009-9. [DOI] [PubMed] [Google Scholar]
- 26.Gong D, et al. Methylenetetrahydrofolate reductase C677T and reduced folate carrier 80 G>A polymorphisms are associated with an increased risk of conotruncal heart defects. Clin Chem Lab Med. 2012;50:1455–61. doi: 10.1515/cclm-2011-0759. [DOI] [PubMed] [Google Scholar]
- 27.van der Linden, S., H. A. Valkenburg & A. Cats. Evaluation of diagnostic criteria for ankylosing spondylitis. A proposal for modification of the New York criteria. Arthritis and Rheumatism27, 361–368 (1984). [DOI] [PubMed]
- 28.Gong, D. et al. Methylenetetrahydrofolate reductase C677T and reduced folate carrier 80 G>A polymorphisms are associated with an increased risk of conotruncal heart defects. Clin Chem Lab Med50, 1455–1461 (2012). [DOI] [PubMed]


