Abstract
Rock ptarmigan (Lagopus muta) and willow ptarmigan (L. lagopus) are Arctic birds with a circumpolar distribution but there is limited knowledge about their status and trends across their circumpolar distribution. Here, we compiled information from 90 ptarmigan study sites from 7 Arctic countries, where almost half of the sites are still monitored. Rock ptarmigan showed an overall negative trend on Iceland and Greenland, while Svalbard and Newfoundland had positive trends, and no significant trends in Alaska. For willow ptarmigan, there was a negative trend in mid-Sweden and eastern Russia, while northern Fennoscandia, North America and Newfoundland had no significant trends. Both species displayed some periods with population cycles (short 3–6 years and long 9–12 years), but cyclicity changed through time for both species. We propose that simple, cost-efficient systematic surveys that capture the main feature of ptarmigan population dynamics can form the basis for citizen science efforts in order to fill knowledge gaps for the many regions that lack systematic ptarmigan monitoring programs.
Electronic supplementary material
The online version of this article (10.1007/s13280-019-01191-0) contains supplementary material, which is available to authorized users.
Keywords: Arctic, Climate change, Ecosystems, Lagopus spp., Population cycles, Transient dynamics
Introduction
Two ptarmigan species, willow ptarmigan (Lagopus lagopus; “Willow grouse” (Europe)) and rock ptarmigan (Lagopus muta), are among the very few bird species that reside year round in Arctic ecosystems. Both species have circumpolar distributions (Fig. 1), but inhabit different bioclimatic zones and use different habitats (Hannon et al. 1998; Potapov and Sale 2013). Willow ptarmigan prefer shrubby habitats in the low-Arctic tundra and the sub-Arctic tundra-forest ecotone. They are also found further south associated with either mountain ranges, where they inhabit low-alpine tundra and sub-alpine forest, or boreal forest where tree cover is sparse or patchy, for instance in areas with extensive bogs and mires. Rock ptarmigan live in rocky habitats mostly without trees or bushes in high-Arctic (up to 83° N) or high-alpine tundra as far south as southern Europe and Japan. Where the two species are sympatric, rock ptarmigan use higher elevations and more barren habitats than willow ptarmigan, although they may overlap to some extent in winter (Wilson and Martin 2012; Potapov and Sale 2013). On islands not inhabited by willow ptarmigan, the rock ptarmigan can use habitat types more typical of willow ptarmigan, such as areas with shrubs.
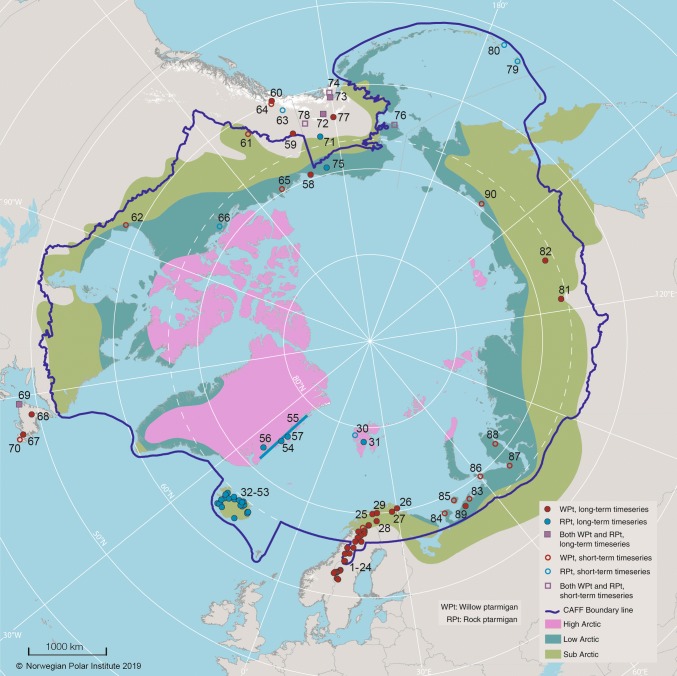
Fig. 1.
Map showing ptarmigan monitoring sites (sites numbered from 1 to 90) considered in the present study. Filled symbols denote sites with continuous long-term data (≥ 7 years) that could be subjected to trend analyses, while open symbols denote sites where the time series were too short (< 6 years) for such analyses or ended before 2010. Red symbols denote willow ptarmigan monitoring sites, blue symbols rock ptarmigan monitoring sites and squares denote sites with monitoring of both species. The blue tick line denotes Conservation of Arctic Flora and Fauna Working Group (CAFF) boundary, the pink colour high-Arctic, dark green low-Arctic and light green sub-Arctic areas
Ptarmigan are browsing herbivores that play important roles in the food web as prey for endemic Arctic predators (Nielsen 1999; Ims and Fuglei 2005; Tape et al. 2010) and as game for local people (Potapov and Sale 2013). Partly for this reason, there has been considerable focus on their population dynamics (Moss and Watson 2001). High-amplitude, multi-annual population cycles are common, but cycle period and amplitude vary considerably among different populations, geographic areas and species. In some areas, ptarmigan population cycles seem to be entrained to the cycles of other herbivores, in particular the 3–5-year cycles of rodents in boreal and Arctic ecosystems in Eurasia (Steen et al. 1988; Ims and Fuglei 2005) or to the 10-year cycle of the snowshoe hare (Lepus americanus) in boreal and sub-Arctic ecosystems of North America (Hannon et al. 1998; Martin et al. 2001; Krebs et al. 2014). In other areas, cyclic dynamics in ptarmigan populations may be caused by interactions with specialized ptarmigan predators such as the gyrfalcon (Falco rusticolus) in Iceland (Nielsen 1999), or intestinal parasites on islands in northern Norway (Holmstad et al. 2005). Some cyclic willow ptarmigan populations may reach high peak densities, with up to 80 breeding pairs/km2 reported for an island in northern Norway (Myrberget 1986) and over 50 pairs/km2 in northern Canada (Hannon et al. 1998). Ptarmigan populations with low-amplitude fluctuations and no evidence for population cycles also exist, such as Svalbard rock ptarmigan (L. m. hyperborea) that occur at 1–5 territorial males/km2 with relatively little temporal variability (Soininen et al. 2016).
Ptarmigan were historically considered to be “well protected” because they often occupy remote habitats, are distributed over vast areas and population estimates have been large (Sandercock et al. 2005; Storch 2007). However, during the last few decades, there are concerns about the status of ptarmigan populations due to disrupted cyclicity (e.g. Kausrud et al. 2008) and regional declines in abundance (Lehikoinen et al. 2014). Ptarmigan have entered national red lists in some countries, particularly south of the Arctic (Storch 2007; International Union for Conservation of Nature, IUCN 2016). In mainland Norway and Finland, which include sub-Arctic areas within the Conservation of Arctic Flora and Fauna Working Group (CAFF) borders, both species are now on the national red lists (https://artsdatabanken.no/Rodliste/Sok, http://www.ymparisto.fi/en-US/Nature/Species/Threatened_species).
For some bird species, the impact of climate change has been explicitly studied and specific climate-related mechanisms have been revealed in the context of recent decreasing trends (Møller et al. 2010; Scridel et al. 2018). Although studies from different regions have pointed out how climate change likely affect future population dynamics (Sandercock et al. 2005; Martin and Wilson 2011) and distribution of ptarmigan (e.g. Revermann et al. 2012; Elmhagen et al. 2015), we lack a good understanding of how ongoing and predicted climate change processes will affect ptarmigan populations, particularly in the Arctic (Henden et al. 2017). Therefore, the Terrestrial Ecosystem Working Group of the Circumpolar Biodiversity Monitoring Program (CBMP) selected ptarmigan as a focal ecosystem component (Christensen et al. 2013).
To provide the first steps towards a better understanding of the current status and future fate of ptarmigan populations in the circumpolar Arctic, we compiled and evaluated existing time series of ptarmigan populations (abundance and/or density estimates) from the entire Arctic (Christensen et al. 2013). Our main goal was to describe the geographic patterns in ptarmigan population dynamics, including evidence for long-term trends in abundance and the prevalence of cyclic behaviour. Second, we discuss likely mechanisms underlying the observed patterns. Last, we highlight the gaps in our current knowledge and provide suggestions as to how they can be filled.
Materials and methods
Selection of study/monitoring sites
Based on a survey of the literature and contact with ptarmigan researchers in 7 CAFF affiliated nations, we identified 90 study sites within, or close to, the CAFF boundary line with current or past population monitoring of rock and willow ptarmigan (see Fig. 1; Table 1; Table S1). Considering only the 73 sites that were located within the CAFF boundary revealed large sections within the circumpolar north where surveys are lacking. To obtain a better geographical coverage, we therefore chose to include 17 sites adjacent to the CAFF border (Fig. 1). The 90 study sites encompassed a latitudinal range from 47 to 83° N and included high-Arctic (n = 6 sites), low-Arctic (n = 22 sites) and sub-Arctic (n = 62 sites) bioclimatic zones (Fig. 1; Table 1; Table S1). Most sites were from Europe (n = 62), with the majority clustered in Iceland and Scandinavia. There were 23 sites from North America and 5 sites from Asia (i.e. Russia east of Ural). Willow ptarmigan were monitored at 53 sites, rock ptarmigan on 35 sites and both species on 6 sites (Table 1, Table S1).
Table 1.
Circumpolar ptarmigan study sites and monitoring series included in the analyses. The sites with ID numbers (#) are located on the map in Fig. 1 and the complete list of study sites is presented in Table S1. Abbreviations: Map# = site number on the map in Fig. 1 and Tables S1 and S2; Species: WPt = willow ptarmigan, RPt = rock ptarmigan, ERPt = Evermann’s rock ptarmigan, – = information is not available; Arctic: sub = sub-Arctic, low = low Arctic, high = high Arctic; Duration = length of time series in years; start–stop = year for start and stop of time series in the analyses; Mean = mean of untransformed time series; CV = coefficient of variation of untransformed time series; Trend.lm = trend estimate from the linear model; Trend.SE = standard error of linear trend estimate
| Map # | Site names | Countries | Species | Arctic | Duration | Start–stop | Mean | CV | Trend.lm | Trend.SE | p values |
|---|---|---|---|---|---|---|---|---|---|---|---|
| 1 | North | Sweden | WPt | Sub | 12 | 1997–2008 | 13.8 | 82.2 | 0.085 | 0.0834 | 0.33 |
| 2 | North | Sweden | WPt | Sub | 12 | 1997–2008 | 19.2 | 66.7 | 0.130 | 0.0769 | 0.11 |
| 3 | North | Sweden | WPt | Sub | 24 | 1994–2017 | 11.1 | 77 | 0.010 | 0.0301 | 0.74 |
| 4 | North | Sweden | WPt | Sub | 24 | 1994–2017 | 5.7 | 71.5 | − 0.017 | 0.0299 | 0.58 |
| 5 | North | Sweden | WPt | Sub | 24 | 1994–2007 | 18.1 | 94.2 | 0.010 | 0.0301 | 0.74 |
| 6 | North | Sweden | WPt | Sub | 24 | 1994–2017 | 17.3 | 66.7 | − 0.005 | 0.0301 | 0.86 |
| 7 | North | Sweden | WPt | Sub | 13 | 1994–2006 | 10.7 | 106.4 | − 0.090 | 0.0724 | 0.24 |
| 8 | North | Sweden | WPt | Sub | 14 | 1994–2007 | 13.4 | 54.3 | − 0.006 | 0.0690 | 0.93 |
| 9 | North | Sweden | WPt | Sub | 17 | 1996–2017 | 9.4 | 114.9 | 0.034 | 0.0355 | 0.35 |
| 10 | North | Sweden | WPt | Sub | 11 | 2007–2017 | 9.4 | 74.1 | − 0.051 | 0.0991 | 0.62 |
| 11 | North | Sweden | WPt | Sub | 7 | 2010–2016 | 6 | 58.3 | − 0.074 | 0.2044 | 0.73 |
| 12 | Middle | Sweden | WPt | Sub | 19 | 1999–2017 | 17.6 | 57 | − 0.045 | 0.0417 | 0.301 |
| 13 | Middle | Sweden | WPt | Sub | 19 | 1999–2017 | 13.7 | 64.9 | − 0.087 | 0.0377 | 0.035 |
| 14 | Middle | Sweden | WPt | Sub | 19 | 1999–2017 | 17.1 | 56.3 | − 0.008 | 0.0431 | 0.854 |
| 15 | Middle | Sweden | WPt | Sub | 10 | 2008–2017 | 7.2 | 35.9 | 0.059 | 0.1149 | 0.621 |
| 16 | South | Sweden | WPt | Sub | 21 | 1996–2017 | 22.3 | 46.1 | 0.026 | 0.0360 | 0.475 |
| 17 | South | Sweden | WPt | Sub | 22 | 1996–2017 | 11.4 | 45.5 | − 0.004 | 0.0344 | 0.912 |
| 18 | South | Sweden | WPt | Sub | 22 | 1996–2017 | 9 | 52 | − 0.040 | 0.0333 | 0.249 |
| 19 | South | Sweden | WPt | Sub | 22 | 1996–2017 | 18 | 41 | − 0.005 | 0.0344 | 0.889 |
| 20 | South | Sweden | WPt | Sub | 22 | 1996–2017 | 13.7 | 51.1 | 0.028 | 0.0339 | 0.423 |
| 21 | South | Sweden | WPt | Sub | 22 | 1996–2017 | 15.2 | 51 | − 0.060 | 0.0317 | 0.071 |
| 22 | South | Sweden | WPt | Sub | 14 | 2004–2017 | 12.1 | 49.7 | 0.023 | 0.0687 | 0.741 |
| 23 | South | Sweden | WPt | Sub | 9 | 2009–2017 | 16.8 | 60.8 | − 0.150 | 0.1262 | 0.28 |
| 24 | South | Sweden | WPt | Sub | 7 | 2009–2015 | 11 | 58.3 | 0.280 | 0.1657 | 0.155 |
| 25 | Troms | Norway | WPt | Sub | 11 | 2007–2017 | 10.1 | 58.3 | 0.197 | 0.0762 | 0.03 |
| 27 | East Finnmark | Norway | WPt | Sub | 17 | 2000–2016 | 10.4 | 47.5 | − 0.072 | 0.0477 | 0.154 |
| 28 | Interior Finnmark | Norway | WPt | Sub | 17 | 2000–2016 | 10.8 | 58.3 | − 0.007 | 0.0511 | 0.887 |
| 29 | West Finnmark | Norway | WPt | Sub | 17 | 2000–2016 | 18.5 | 52.2 | − 0.090 | 0.0455 | 0.067 |
| 31 | Svalbard | Norway | RPt | High | 18 | 2000–2017 | 2.4 | 44.9 | 0.121 | 0.0359 | 0.004 |
| 32 | North-East | Iceland | RPt | Sub | 50 | 1963–2017 | 17.11 | 46.2 | − 0.004 | 0.0087 | 0.647 |
| 33 | North-East | Iceland | RPt | Sub | 35 | 1981–2015 | 13.24 | 44.9 | − 0.049 | 0.0147 | 0.002 |
| 34 | North-East | Iceland | RPt | Sub | 47 | 1963–2015 | 4.07 | 53.8 | − 0.008 | 0.0094 | 0.421 |
| 35 | North-East | Iceland | RPt | Sub | 35 | 1981–2015 | 3.83 | 54.8 | − 0.029 | 0.0162 | 0.083 |
| 36 | North-East | Iceland | RPt | Sub | 35 | 1981–2015 | 2.75 | 51.9 | − 0.012 | 0.0169 | 0.471 |
| 37 | North-East | Iceland | RPt | Sub | 35 | 1981–2015 | 4.58 | 57.2 | − 0.022 | 0.0166 | 0.201 |
| 38 | North-East | Iceland | RPt | Sub | 35 | 1981–2015 | 7.23 | 41.1 | − 0.039 | 0.0156 | 0.017 |
| 39 | North-East | Iceland | RPt | Sub | 15 | 2000–2014 | 4.29 | 30.1 | − 0.070 | 0.0589 | 0.259 |
| 40 | North-West | Iceland | RPt | Sub | 14 | 2004–2017 | 1.37 | 45.9 | − 0.123 | 0.0591 | 0.059 |
| 41 | North-West | Iceland | RPt | Sub | 15 | 2000–2014 | 1.4 | 51.7 | − 0.070 | 0.0589 | 0.257 |
| 42 | North-West | Iceland | RPt | Sub | 14 | 2004–2017 | 0.43 | 70.7 | − 0.170 | 0.0486 | 0.004 |
| 43 | North-West Penn | Iceland | RPt | Sub | 16 | 2000–2015 | 6.14 | 47.66 | 0.173 | 0.0319 | < 0.0001 |
| 44 | North-West Penn | Iceland | RPt | Sub | 27 | 1991–2017 | 8.04 | 44.46 | 0.084 | 0.0188 | 0.0002 |
| 45 | East | Iceland | RPt | Sub | 21 | 1994–2014 | 4.13 | 51.52 | 0.015 | 0.0368 | 0.689 |
| 46 | East | Iceland | RPt | Sub | 15 | 2000–2014 | 3.51 | 57.97 | 0.145 | 0.0472 | 0.009 |
| 47 | East | Iceland | RPt | Sub | 55 | 1963–2017 | 7.1 | 66.9 | − 0.034 | 0.0072 | < 0.0001 |
| 48 | South-East | Iceland | RPt | Sub | 18 | 2000–2017 | 1.5 | 55.03 | 0.005 | 0.0468 | 0.924 |
| 49 | South | Iceland | RPt | Sub | 13 | 2002–2014 | 1.7 | 52.6 | − 0.081 | 0.0735 | 0.295 |
| 50 | West | Iceland | RPt | Sub | 19 | 1999–2017 | 0.98 | 54.9 | − 0.019 | 0.0428 | 0.657 |
| 51 | West | Iceland | RPt | Sub | 12 | 2003–2014 | 1.6 | 75.5 | − 0.210 | 0.0573 | 0.004 |
| 52 | South-West | Iceland | RPt | Sub | 15 | 2003–2017 | 1.6 | 82.1 | − 0.085 | 0.0573 | 0.161 |
| 53 | South-West | Iceland | RPt | Sub | 18 | 2000–2017 | 3.4 | 43.1 | − 0.042 | 0.0457 | 0.377 |
| 54 | Zackenberg North-East | Greenland | RPt | High | 22 | 1996–2017 | 1.7 | 140.9 | − 0.093 | 0.0275 | 0.003 |
| 55 | Sirius North-East | Greenland | RPt | High | 36 | 1977–2012 | 25.1 | 114 | 0.007 | 0.0162 | 0.651 |
| 56 | Karupelv North-East | Greenland | RPt | High | 30 | 1988–2017 | 1.2 | 125.5 | − 0.055 | 0.0188 | 0.007 |
| 57 | Hochstetter North-East | Greenland | RPt | High | 9 | 2010–2018 | 0.9 | 164.6 | − 0.243 | 0.1029 | 0.052 |
| 58 | Yukon Northern Slope | Canada | WPt | Low | 35 | 1976–2010 | 2.6 | 110.7 | − 0.013 | 0.0168 | 0.43 |
| 59 | Yukon Ogilvie Mountains | Canada | WPt | Sub | 47 | 1971–2017 | 15.6 | 41.4 | − 0.009 | 0.0108 | 0.41 |
| 60 | Nadahini Chilkat Pass North British Columbia | Canada | WPt | Low | 61 | 1957–2017 | 30.9 | 55.7 | 0.010 | 0.0072 | 0.17 |
| 67 | Fair Haven Newfoundland | Canada | WPt | Sub | 12 | 1999–2012 | 0.95 | 67.8 | − 0.113 | 0.0601 | 0.089 |
| 68 | Gaff Topsails Newfoundland | Canada | WPt | Sub | 12 | 1999–2012 | 0.79 | 65.4 | 0.002 | 0.0747 | 0.982 |
| 69 | Lapoile Newfoundland | Canada | WPt | Sub | 15 | 1995–2012 | 3.3 | 47 | − 0.029 | 0.0463 | 0.545 |
| RPt | 15 | 1995–2012 | 1.1 | 65.6 | 0.004 | 0.0468 | 0.934 | ||||
| 71 | Alaska-Eagle Summit | USA | RPt | Low | 12 | 2007–2017 | 0.27 | 58.9 | − 0.045 | 0.0994 | 0.658 |
| 72 | Alaska–Alaska Range | USA | WPt | Low | 21 | 1997–2017 | 0.9 | 39 | − 0.003 | 0.0370 | 0.942 |
| RPt | 21 | 1997–2017 | 0.23 | 79.7 | 0.052 | 0.0350 | 0.152 | ||||
| 73 | Alaska-South-central Metro | USA | WPt | Sub | 11 | 2008–2017 | 1.27 | 41.9 | 0.209 | 0.0903 | 0.049 |
| 75 | Alaska-Taylor Highway | USA | RPt | Low | 12 | 2007–2017 | 0.23 | 63.3 | 0.272 | 0.0436 | <0.001 |
| 76 | Alaska Denali | USA | WPt | Sub | 29 | 1988–2016 | 10.3 | 48.1 | − 0.020 | 0.0223 | 0.375 |
| 81 | Central Verkhoyansky | Russia | WPt | Low | 29 | 1984–2012 | 4.95 | 73.4 | − 0.016 | 0.0224 | 0.479 |
| 82 | Lower Lena River | Russia | WPt | Sub | 27 | 1986–2012 | 4.7 | 96.5 | − 0.069 | 0.0210 | 0.003 |
| 89 | Nenets Autonomous District Komi Republic | Russia | WPt | Low | 41 | 1973–2014 | 1.7 | 81 | − 0.014 | 0.0129 | 0.28 |
The symbol bold indicate significant values
Monitoring methods
Monitoring of ptarmigan populations at the 90 study sites was conducted in areas ranging from 2 to 48 000 km2, in different seasons of the year, with efforts ranging from 2 to 1400 person days per year, and with greatly varying duration of the monitoring period (Table 1, time series length from 2 to 60 years). Ptarmigan populations are currently being monitored on almost half of the study sites.
The monitoring methods used can be grouped into four main categories (Table S1):
Distance sampling from line or point transects: statistical estimates of population density (birds/km2) corrected for detection probability (Buckland et al. 2001).
Total counts by territory mapping or similar methods: assessment of population densities that are assumed relatively accurate given sufficient field effort.
Transect surveys of minimum number of birds observed: density index not corrected for detection probability or the size of the area surveyed.
Faecal pellet counts on permanent plots: density index.
We did not include harvest statistics because of the difficulty in most cases of separating population trends from variable harvest efforts (Willebrand et al. 2011) and consequently, uncertainty in the covariance between harvest statistics and population size (Cattadori et al. 2003; Ranta et al. 2008). This might be a topic for future studies to make attempt to extract robust information from harvest statistics (see Hjeljord 2015).
Selection of time series for analyses
In the following, we consider only study sites with continuous time series ≥ 7 years as sufficiently long-term to include them in an analyses of temporal trends (n = 72 series; see Tables 1, S2). To assess evidence for cyclicity in the population dynamics, we only included time series ≥ 12 years in the analyses (n = 60 series) (see “Analyses” section for details). The time series analysed for trends and cycles included both estimates of true population densities (methods 1 and 2 mentioned above) and population density/abundance indices (method 3). For simplicity, we will use the term population density to refer to all time series.
Of the 72 population time series, only 17 were from outside Europe. The mean length of the series was 21.7 years with extensive variation (range = [7, 61]). There were 31 time series of rock ptarmigan populations, of which 22 were from Iceland. All rock ptarmigan series were conducted in spring and provide measures of density of breeding males or pairs/km2 or counts per transects or areas surveyed annually (Greenland and Alaska, Table S1). For willow ptarmigan, 28 time series were sampled in autumn, 11 in spring and 2 in winter. Of the 41 willow ptarmigan series, 35 provide some estimates of density (males, pairs or individuals/km2), while 4 provide counts of numbers of males per stop along transects and 2 provide numbers of ptarmigan along winter transects.
Analyses
To assess recent status and trends of circumpolar ptarmigan populations, we restricted the analyses to time series that extended to at least 2010. As a first assessment of the population density dynamics, we calculated the mean and the coefficient of variation (CV) for each time series. Next, we regressed year as a continuous variable against density using linear models (function lm in R, R Core Team 2017) to test for any trends in density across time. To facilitate direct comparison of trend estimates, all time series were scaled (mean = 0, variance = 1 standard deviation). We also obtained region-specific estimates of time trends using the function metagen (i.e. the fixed effects model) in the package meta (Schwarzer 2007) in R. The function metagen can generally be applied to all types of data as long as estimates of the effect size (i.e. slope estimates) and corresponding estimated standard errors are provided. In the metagen function, time series are weighted by the inverse of the variance in order to provide more weight to time series of longer duration and less uncertain trend estimates. Also, we fitted generalized additive models to each time series to aid in visual assessment of non-linearity in time trends, using the gam function in the package mgcv (Wood 2011) in R, with year as a smoothing term (k = 4 to represent the smooth term).
Finally, we used wavelet analysis to assess evidence for multi-annual population cycles, and in that case their length (i.e. cycle period), as well as evidence for any change over time in these characteristics. Wavelet analyses were performed on unscaled time series using the function analyse.wavelet in the package WaveletComp (Roesch and Schmidbauer 2014) in R, which applies the Morlet wavelet to compute the wavelet power spectrum. The analyse.wavelet function internally detrends the time series by specifying the term loess.span = 0.75 (α parameter which controls the degree of time series smoothing). We restricted the range of possible periodicities in the wavelet analyses to 2–16 years to encompass the range of known cycle lengths for Arctic ptarmigan populations (3–5-year and 9–11-year cycles). We include results from wavelet analyses for time series with a length of three to four times the largest dominant periodicity. Hence, for time series with an indication of long periodicity, i.e. 9–11 years, we only included series with a minimum of 27–33 years and for time series with shorter periodicities, i.e. 3–6 years, we included locations with a minimum of 12–18 years. Note, that since the wavelet analyses cannot be conducted on time series with missing values, we did not analyse time series with missing data over two or more consecutive years. We imputed values for a few single missing years for 4 of the longer time series—Yukon Northern Slope (4 times), Yukon Ogilvie Mountains (3 times), north-eastern Russia (3 times) and the Lapoile area on Newfoundland (3 times)—and 1 shorter time series [Jamtland 14 (1 time)], by using the average of the year before and after a missing year (see Table S2).
Results
Mean and temporal variation in density
Rock ptarmigan
For time series providing estimates of population densities, the mean density of males in spring was 4.3/km2; however, mean density varied among the monitoring sites (range = [0.4, 17.1]). With regard to temporal variation in the dynamics, the mean CV was 53.8, but with large variation among sites (range = [30.1, 82.1]).
Willow ptarmigan
For times series providing true population densities, the mean density of males in spring surveys was ~ 8.0/km2 (range = [0.8, 30.9]) with greater temporal variation in spring density than for rock ptarmigan (Willow ptarmigan mean and range of CV was 67.0, and [41.4, 110.7], respectively). Compared to the spring surveys, the mean density in autumn surveys (13.0 individuals/km2) was, as expected, higher, but with less variation among sites (range = [5.7, 22.3]). The temporal variation in density in autumn surveys was similar to spring surveys [mean CV = 62.3], (range = [35.9, 114.9]). Note that the spring and autumn surveys were not conducted in the same areas.
Trends in population density
Rock ptarmigan
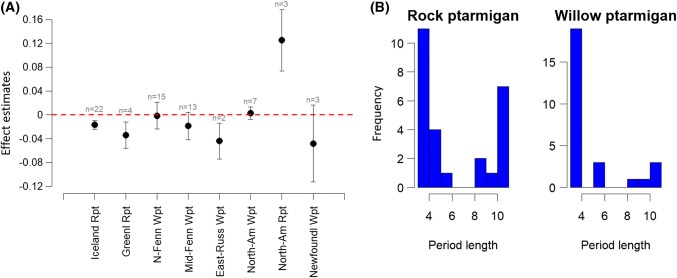
For 10 of 31 time series, there was a significant declining trend in density over years, while 5 series (1 in Svalbard, 2 in east Iceland, 1 in the North-West Peninsula on Iceland and 1 in Alaska) showed a significant increase in density (Table 1; Table S2). While not significant, another 11 series showed negative trend estimates, and in 5 time series there was a positive trend estimate. With regard to regional trends, the meta-analysis indicated an overall negative trend in density on Iceland (Figs. 2a, 3. Estimate = − 0.017, CI = [− 0.024; − 0.001], p = < 0.0001) and Greenland (estimate = − 0.0341, CI = [− 0.056; − 0.012], p = < 0.0022) and an overall positive trend in Alaska (estimate = + 0.125, CI = [0.074; 0.177], p = < 0.0001). The other geographic regions (Svalbard and Newfoundland) contain only 1 series each. Of those, Svalbard showed a significant positive trend (estimate = + 0.121, SE = 0.036, p = 0.004), while Newfoundland showed no significant trend in density over time (Table S2; Fig. 3: Newfoundland Lapoile: estimate = + 0.004, SE = 0.047, p = 0.93).
Fig. 2.
a Linear trend effect estimates (mean, confidence intervals and number of time series in regional meta-analyses) of rock ptarmigan (RPt) and willow ptarmigan (WPt) populations from different regions within or just outside the CAFF area (see Fig. 1; Table S2). b Frequencies (i.e. number of time series) of cycle period length (in years, based on dominant power spectrum) from wavelet analyses of rock ptarmigan and willow ptarmigan time series
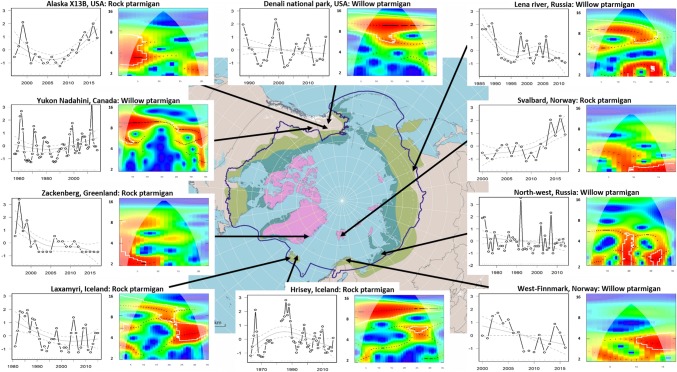
Fig. 3.
Representative examples of 10 long time series and monitoring sites illustrating the variety of populations dynamics and trends displayed by the two ptarmigan species (willow and rock ptarmigan) in the circumpolar Arctic. The left panels for each site show the standardized time series and GAM trend curves (grey lines) with confidence envelopes (grey dotted lines). The right panels show the result from the wavelet analyses where red areas, within white wavelet power contour lines, denote periods with evidence for cyclic dynamics with different cycle lengths. The colour palette in the wavelet plots denotes wavelet power levels, with wavelet power increasing from blue to red
Willow ptarmigan
For 2 time series, there were significant negative trends in density (Table S2), and in 3 series, there were close to significant negative trends. Three series showed a significant positive trend (one in Troms, Norway and two in Alaska). With regard to regional trends, the meta-analyses revealed an overall negative trend in abundance or density in eastern Russia (2 series) (estimate = − 0.044, CI = [− 0.074; − 0.014], p = 0.0039) (Figs. 2a, 3). For the northern and middle part of Fennoscandia, North America (Canada and USA) and Newfoundland there was no significant overall regional trend in density or abundance (Figs. 2a, 3), though there was a tendency for a negative trend in central Sweden (estimate = − 0.017 CI = [− 0.04; 0.0054], p = 0.1).
Population cycles
Rock ptarmigan
Most of the 31 time series deemed adequate for wavelet analyses (Table S2), displayed cyclic dynamics for short or long periods. Only 4 time series [Svalbard, Zackenberg and Alaska (2 series)] did not show any evidence for periods of cyclic dynamics. The wavelet analyses displayed two groups with respect to cycle length, a group of 10 series with long cycles (9–12 years) and another group of 16 series with shorter cycles (3–6 years, Fig. 2b). All the time series displaying longer cycles were from the northern part of Iceland (Fig. 3), except for a ~ 9-year cycle at Karupelv, Greenland.
Of the 10 time series with long cycles, 8 also showed significant shorter cycles (3–6 years) in parts of the time series. One series indicated declining cycle length with time (Laxamyri, north-east Iceland) and one indicated increasing period length with time (Kvisker, south-east Iceland; Fig. 3).
Of the rock ptarmigan series with shorter cycles (i.e. 3–6 years), 12 series displayed periods of non-cyclic dynamics, of which 10 displayed signs of cycle collapse towards the mid- to end of the series. Of those, 1 series showed an indication of declining cycle length with time (Reykholar, North-West Peninsula, Iceland), and conversely 4 showed indications of increasing cycle length with time (Hegranes and Sudvesturland on Iceland, Sirius Subarea C on Greenland and on Newfoundland; Table S2).
Willow ptarmigan
Of the 30 series deemed adequate for wavelet analyses (Table S2), 3 series indicated lack of cyclic dynamics, 5 indicated long 9–12-year cycles and 22 showed shorter cycles with 3–6-year periodicities (Table S2; Fig. 2b). Eleven series were deemed too short with regard to the dominant cycle period or contained too many missing years to be suitable for wavelets analyses (Table S2). For instance, a few series in northern Sweden indicated cycles of 9–10 years, but with a survey duration of only 24 years this indication must be judged with caution. Several series in Newfoundland contained too many missing values to be subjected to wavelets analysis (Table S2).
All of the willow ptarmigan series with only long cycles were found in North America (Yukon and Alaska), except for 1 time series from Lena River in eastern Russia. Moreover, the survey from Nadahini in Yukon (Canada) and the Lena River in Russia indicated a decrease in cycle length with time towards 6-year cycle (Fig. 3). Last, a survey from Denali National Park indicated an additional significant shorter 6-year cycle towards the end (Fig. 3).
Of the series with shorter cycles, 14 series showed changes in cyclicity, with 7 indicating a cycle collapse and 6 indicating cycle emergence with time (see examples in Fig. 3). Finally, 4 series indicated a small increase in period length over time, whereas 5 series indicated a small decrease in cycle length with time (see examples in Fig. 3).
Discussion
Several of the insights gained from our analyses of ptarmigan population time series, compiled from many sites across the circumpolar north, are in close agreement with Moss and Watson (2001) in their general review of grouse and ptarmigan population dynamics:
The population dynamics of rock ptarmigan and willow ptarmigan are spatially variable and temporally complex.
Populations that exhibit temporal population cycles appear to be more common than populations with persistently non-cyclic dynamics.
Among populations with cyclic dynamics there is a striking variability in average density, cycle amplitude and cycle length.
Cycle lengths can be both short (3–6 years) and long (9–11 years).
Long cycles (approx. 10 years) appear to be most prevalent in North America where they may be entrained to snowshoe hare cycles or in Iceland where they could be driven by gyrfalcon.
Short cycles (3–6 years) are most prevalent in Scandinavia where they seem to be entrained to rodent cycles.
Despite tendencies for regionalized dynamics, there are divergent patterns of population dynamics among adjacent populations within the same biogeographic region.
Population dynamics are often temporally transient (non-stationary) in the sense that populations might alternate between cyclic and non-cyclic periods, and cycle length might change through time.
Some ptarmigan populations exhibit long-term trends that are likely driven by other forces than those responsible for cycles and normal transient dynamics.
The present study based on a larger sample of long-term time series that could be subjected to a unified analytical approach, reinforces several of Moss and Watson’s conclusions. Moreover, we now have more sophisticated analytical tools than were available two decades ago to aid in our analyses. In particular, the wavelet analyses are sensitive tools for assessing the prevalence and consistency of population cycles in time and space. This allows us to infer that transience (non-stationarity) seems to be common feature of ptarmigan population dynamics. Such transience is evident from frequent changes in cycle length as well as alternating episodes of cyclic and non-cyclic dynamics within the same population. Collapses and emergences of cycles over time within the same population appears to be a novel/emergent feature of ptarmigan population dynamics not highlighted by Moss and Watson (2001), perhaps because fewer and shorter time series were available two decades ago.
In terms of relatively persistent 9–12-year cycles, the most stationary time series were present in willow ptarmigan populations in NW North America and rock ptarmigan in NE Iceland. The NW North American populations reside in sub-Arctic ecosystems where snowshoe hare cycles act as a major driver of the dynamics of many other species including ptarmigan through an alternative prey mechanism (Boonstra et al. 2016; Schmidt et al. 2017), whereas rock ptarmigan in NE-Iceland reside in a simple sub-Arctic ecosystem with one specialist predator, the gyrfalcon (Nielsen 2011). In contrast, many of the populations with a high degree of transience, due to frequently collapsing short cycles, are willow ptarmigan in sub-Arctic Scandinavia. In this region, 3–5-year population cycles of rodents are a key driver of the food web dynamics (Ims and Fuglei 2005; Boonstra et al. 2016). The population cycles in Scandinavian rodents have also been found to exhibit transience, both historically (Henden et al. 2009) and especially in recent decades (Ims et al. 2008; Cornullier et al. 2013). Kausrud et al. (2008) inferred a predation-driven link between a recent collapse of the willow ptarmigan cycle and the concurrent collapse of lemming cycles in an alpine area in southern Norway. Although change in ptarmigan population dynamics in certain cases can be linked to specific drivers, the fact that transient dynamics appear to be a normal feature of ptarmigan populations (Moss and Watson 2001) represents a major challenge to conclude on relatively short time series. Transient population dynamics may have several causes (Bjørnstad and Grenfell 2001; Hastings 2004), including interaction (“resonance”) between non-linear biotic interactions and environmental stochasticity (e.g. weather events). We suspect that ptarmigan may be particularly susceptible to such stochastic resonance (Barraquand et al. 2017), both because of their demographic sensitivity to the highly variable weather regimes in alpine/Arctic environments (Wilson and Martin 2012) and their non-linear interactions with natural enemies in the food web (Henden et al. 2017). In any case, discerning such normal transient dynamics in a stationary environment from abnormal trends forced by environmental change is difficult based on time series analysis alone. This is especially true when the time series are relatively short. Indeed, the presence of long cycles combined with frequent episodes of transient dynamics emphasizes the need for long time series for making reliable assessments of status and trends in ptarmigan populations.
We found temporal trends in average population density during the last 2–6 decades in many of the ptarmigan time series. Both significant negative and positive trends were estimated, although those expressed at a regional scale were most often negative. However, in light of the high degree of natural transience in ptarmigan population dynamics discussed above, we need to be cautious regarding how we should interpret such trend estimates. In the case of ptarmigan, reliable documentation of “true” population trends (e.g. due to environmental change) requires very long time series since an apparent trend in relatively short time series may just be “pseudo-trends” owing to natural transience.
With this caution in mind, we suggest that the negative trends in some of the longest time series analysed in this study to represent true trends. In addition, a negative long-term trend is supported for willow ptarmigan by hunting statistics (> 100 years, catch per day index) from south to south-east of Norway (Hjeljord 2015) and along the Fennoscandian mountain chain (Lehikoinen et al. 2014). The reality of this trend is supported by the presence of equivalent population declines in other alpine bird species from the same region (Lehikoinen et al. 2014; Elmhagen et al. 2015). Several potential causes have been proposed (Lehikoinen et al. 2014), such as dampened small rodent cycles (see Kausrud et al. 2008; Henden et al. 2011, 2017). It is interesting to note that the population decline in rock ptarmigan at Hochstetter, Zackenberg and Karupelv in NE Greenland also appear to coincide with the collapse of the lemming cycle at these two sites (Schmidt et al. 2012). However, an overall long-term negative trend was also evident across the many monitoring sites for rock ptarmigan in Iceland (but heavily influenced by a strong decline in the Kvisker time series in south-east Iceland), where lemmings are missing. Interestingly, there is a great deal of variability in short-term dynamics among the Icelandic time series (e.g. Fig. 3). Accordingly, Moss and Watson (2001) noted that simultaneous long-term declines in ptarmigan could take place across populations with different short-term dynamics and in structurally different ecosystems. They attributed such cases of spatially extensive synchronous declines to climate change.
Spatial replication of monitoring series, both within and among different regions, is required to make robust assessments of regionalized short-term population dynamics and long-term trends. Unfortunately, the limited spatial coverage of ptarmigan monitoring in the circumpolar Arctic does not permit sophisticated spatial analyses. Large regions within Arctic Russia and America lack monitoring series (Fig. 1). In regions with monitoring series present, spatial replication is either missing or insufficient for analyses of spatial population dynamics (Bjørnstad et al. 1999). This is unfortunate because such analyses could facilitate more precise assessments of whether spatial scaling of population trends matches spatial domains of climate change (Stenseth et al. 2004) or food webs with different structures (Henden et al. 2017).
While there are several reasons for why ecological monitoring in general has poor geographic coverage in the Arctic, there may be additional reasons why ptarmigan monitoring is missing even from sites where many other ecosystem components are monitored. State-of-the-art methods used to estimate ptarmigan population density are either laborious, expensive and/or require particular means or skills such as the use of trained pointing dogs. The fact that such ptarmigan monitoring is difficult to maintain for financial and logistical reasons, may have contributed to the fact that only almost half of the 90 monitoring series compiled in our study are still ongoing. It is worth considering whether more focus should be devoted to developing and validating simpler population index methods that are easier to implement and maintain across the Arctic, than for instance distance sampling and territory mapping. Faecal pellet counts may constitute such an index method with good potential (Krebs et al. 2014). When implemented on seasonal basis (spring and fall) on permanent faecal removal plots with a spatially stratified design that ensures that seasonal habitat use of only one ptarmigan species is included, this method appears to capture the main features of willow ptarmigan population dynamics (see Fig. 4; Henden et al. 2011). Validations of pellet count-derived density indices should be conducted in different regions in order to assess to what extent this method is generally applicable across the Arctic. In our experience, the method appears less suitable for rock ptarmigan populations at low densities. Nevertheless, faecal pellet counts are currently conducted at seven of the sites compiled in Table S2. Pellet counts have been implemented in two circumpolar monitoring network; the Herbivory Network (http://herbivory.biology.ualberta.ca/) and Interactions Working Group (Gilg et al. unpublished). In these networks, the method is also destined to provide information on the relative abundance of other important herbivores in Arctic ecosystems (geese, hares and reindeer/caribou; Ims et al. 2007). There may also be a good potential for implementing faecal counts in citizen science initiatives, as it requires no other skills than distinguishing ptarmigan pellets from those of other species and because the method requires a relatively small effort in terms of observer hours in the field.
Fig. 4.
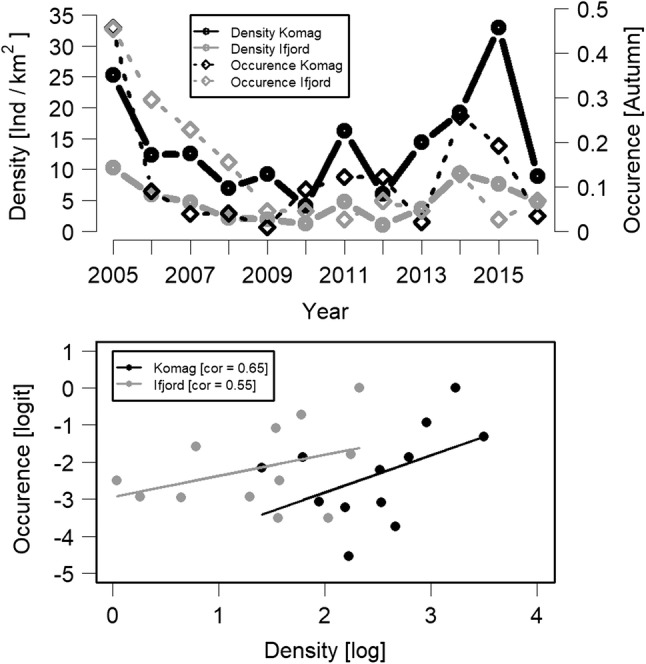
Upper panel: distance sampling-based density estimates of willow ptarmigan from two areas (Komag and Ifjord) in eastern Finnmark, sub-Arctic/low-Arctic Norway (monitoring site 27; Fig. 1; Table 1) based on annual autumn line transect surveys with pointing dogs (solid black and grey lines, respectively) compared to estimates of ptarmigan occurence based on faecal pellet counts from two monitoring sites [indicated with black and grey stippled lines (all from monitoring site 26; Table S2)]. The faecal pellet counts were made on replicated permanent removal plots (pellets removed each year) in early and late summer each year (only the early autumn estimates are shown) (see Henden et al. 2011 for details). Lower panel: distance sampling-based density estimates (log) compared to faecal pellet count-based estimates of ptarmigan occurence (logit) for the two areas. Area-specific correlations are provided in the legend and regression lines are added for visualization
Although the implementation of cost-efficient index methods can contribute substantially to filling current gaps in our knowledge of status and trends in Arctic ptarmigan, a key priority should be to maintain all the ongoing long-term monitoring series that are conducted with methods that provide accurate population density estimates. Obviously, the value of these time series increases tremendously with time, especially considering the difficulty of separating high degrees of natural transience in ptarmigan evident from our analysis and the impact of climate change that certainly impacts the fate of Arctic ptarmigan currently and in the immediate future. Finally, we also stress that ptarmigan monitoring conducted in concert with monitoring of other, likely linked, essential ecosystem components (i.e. ecosystem-based monitoring; Christensen et al. 2013; Ims and Yoccoz 2017; Schmidt et al. 2017) will improve our ability to identify the drivers of Arctic ptarmigan population dynamics.
Electronic supplementary material
Below is the link to the electronic supplementary material.
Acknowledgements
We thank multiple field workers that participated in the ptarmigan data collections, researchers that gave information on study sites not included in the present analysis, specifically K. Christie, C. Braun, S. Ebbert, I. Pokrovsky, D. Ehrich, A. Sokolov, N. Sokolov, the Greenland Ecosystem Monitoring Program for access to ecosystem data from Zackenberg, and Oddveig Ø. Ørvoll, Norwegian Polar Institute for graphical design of maps.
Biographies
Eva Fuglei
is a Researcher at the Norwegian Polar Institute, Norway. Her main interests and special competence are within high-Arctic tundra ecosystems, terrestrial animal ecology, management of Arctic ecosystem services and biodiversity, and adaptive monitoring. Her research is connected to rock ptarmigan and Arctic fox in the high-Arctic tundra ecosystem in Svalbard, Norway.
John-André Henden
is a Researcher in the Northern Populations and Ecosystems Research Groups, at the University of Tromsø, The Arctic University. His research is mainly focused on population, community and food web dynamics in terrestrial tundra ecosystems. He is also responsible for the Ptarmigan Monitoring Module within COAT, a climate–ecological monitoring system of the Arctic tundra in northern Norway and Svalbard.
Chris T. Callahan
is a Biologist with the Newfoundland and Labrador Government. His research interests are primarily wildlife management with special focus on high altitude species. Recent activities include monitoring Labrador willow ptarmigan migration and Arctic hare habitat modeling for the Island of Newfoundland.
Olivier Gilg
is a Associate Researcher at the University of Bourgogne-Franch-Comté (France) and the chairman of the NGO Groupe de Recherche en Ecologie Arctique. He works in NE Greenland since 1990 and is co-leading the “Interactions” Research Program at Hochstetter Forland since 2010.
Jannik Hansen
is a Scientific Officer at Aarhus University, Denmark. He mainly works with ecosystem monitoring, being responsible for bird fauna work, at the Zackenberg Research Station in North-east Greenland.
Rolf A. Ims
is a Professor of Ecology at UiT-Arctic University of Tromsø and a Leader of the Terrestrial Program of the Fram: High North Research Centre for Climate and the Environment as well as the COAT climate-ecological observatory for Arctic tundra. He has in particular conducted research on herbivore species with cyclic population dynamics and the function of such herbivore cycles in boreal and Arctic ecosystems.
Arkady P. Isaev
is the Head of the Laboratory of Ecosystem Researches in Cold Regions of the Institute for Biological Problems of Cryolithozone under Siberian Branch of Russian Academy for Sciences. His main interests are the ecology of grouses and birds of prey in ecosystems of cold regions. His studies are related to the grouse and other birds of Yakutia.
Johannes Lang
is a Researcher at Justus-Liebig-University, Germany and Member of the Arctic Ecology Research Group, France. His main interests are monitoring of mammal and bird species and predator–prey interactions. He is part of the Karupelv Valley Long-Term Monitoring Project in North-east Greenland since 2001.
Carol L. McIntyre
(Ph.D.) is a Wildlife Biologist at Denali National Park and Preserve, Alaska, USA. Among her main interests is the ecology of northern breeding raptors and their prey.
Richard A. Merizon
is the Statewide Small Game Program Coordinator for the Alaska Department of Fish and Game. This program is responsible for research and management of Alaska’s grouse, ptarmigan and hare populations.
Oleg Y. Mineev
is a Researcher at the Institute of Biology of Komi Scientific Centre of Ural Division of Russian Academy of Science, Laboratory of Vertebrate Ecology. His main scientific interests are distribution, number and ecology of birds (waterfowl, waterbirds, grouse, waders, birds of prey), key bird habitats, wetlands, protection of the Arctic as an important area for the reproduction of birds. He has done investigations on the European North-east of Russia. His investigations are mostly connected with typical Arctic birds.
Yuri N. Mineev
is the Chief Researcher at the Institute of Biology of Komi Scientific Centre of Ural Division of Russian Academy of Science, Laboratory of Vertebrate Ecology. His main scientific interests are distribution, number and ecology of birds (waterfowl, waterbirds, grouse, waders, birds of prey), key bird habitats, wetlands, protection of the Arctic as an important area for the reproduction of birds. He has done investigations on the European North-east of Russia. His investigations are mostly connected with typical Arctic birds.
Dave Mossop
is a Professor Emeritus and Research Scientist at the Yukon Research Center of Yukon College, in Whitehorse, Canada. His prime research interest is in understanding tundra community, focusing mostly on Willow Ptarmigan as a key-stone species and Gyrfalcon as top predator. His regular annual survey data now span just over 50 years.
Olafur K. Nielsen
is a Wildlife Ecologist at the Icelandic Institute for Natural History. He is responsible for the monitoring of the Icelandic rock ptarmigan population. His main research interests relate to population dynamics of the rock ptarmigan and the role of food–web connections including herbivore–plant, predator–prey and parasite–host interactions.
Erlend B. Nilsen
is a Senior Researcher at the Norwegian Institute for Nature Research. His main research interests are related to understanding the effects of environmental variation and anthropogenic drivers on the demography and population dynamics of wildlife species, including willow ptarmigan. He is Project Leader for the National Tetraonidae Line Transect Survey Program in Norway, which includes the study sites in Finnmark and Troms presented in this paper.
Åshild Ønvik Pedersen
is a Researcher at the Norwegian Polar Institute, Norway. Her main interests and competence are within high-Arctic tundra ecosystems including vertebrate population and community ecology and adaptive management. Her research cover climate effects and trophic interactions of both resident and migratory species in the Svalbard tundra ecosystem. She is currently responsible for the Monitoring Program of Svalbard Reindeer and leads COAT Svalbard (Climate-Ecological Observatory for Arctic Tundra).
Niels Martin Schmidt
is Senior Scientist, Scientific Leader of Zackenberg Research Station, and Manager of the BioBasis Programme in Zackenberg and Nuuk. His research mainly focuses on biotic interactions in a rapidly changing Arctic.
Benoît Sittler
is a Research Scientist at the Chair for Nature Conservation and Landscape Ecology (University of Freiburg, Germany). Co-founder of the Groupe de Recherche en Ecologie Arctique (F), he is also Initiator of the Karupelv Valley Project as an ongoing long-term research on lemming cycles in North-east Greenland.
Maria Hørnell Willebrand
is the Dean of the Faculty of Applied Ecology, Agricultural Sciences and Biotechnology at the Inland Norway University of Applied Science, Norway. Her special competence is within wildlife management, adaptive monitoring, terrestrial animal ecology and population dynamics.
Kathy Martin
is a Professor at the University of British Columbia and a Senior Research Scientist at Environment and Climate Change Canada, Vancouver, Canada. Her main research interests are life history, behaviour and population ecology of ptarmigan and songbirds in alpine and Arctic tundra ecosystems, terrestrial animal ecology, structure and function of tree cavity-nesting vertebrate communities in relation to local and regional disturbances including climate change. She has conducted research on all three ptarmigan species in the low-Arctic and alpine systems in North America.
Footnotes
Publisher's Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Contributor Information
Eva Fuglei, Email: eva.fuglei@npolar.no.
John-André Henden, Email: john-andre.henden@uit.no.
Chris T. Callahan, Email: chriscallahan@gov.nl.ca
Olivier Gilg, Email: ollivier.gilg@gmail.com.
Jannik Hansen, Email: jaha@bios.au.dk.
Rolf A. Ims, Email: rolf.ims@uit.no
Arkady P. Isaev, Email: isaev_ark@rambler.ru
Johannes Lang, Email: Johannes.lang@vetmed.uni-giessen.de.
Carol L. McIntyre, Email: Carol_McIntyre@nps.gov
Richard A. Merizon, Email: richard.merizon@alaska.gov
Oleg Y. Mineev, Email: mineev@ib.komisc.ru
Yuri N. Mineev, Email: mineev@ib.komisc.ru
Dave Mossop, Email: dmossop@yukoncollege.yk.ca.
Olafur K. Nielsen, Email: okn@ni.is
Erlend B. Nilsen, Email: erlend.nilsen@nina.no
Åshild Ønvik Pedersen, Email: aashild.pedersen@npolar.no.
Niels Martin Schmidt, Email: nms@bios.au.dk.
Benoît Sittler, Email: benoit.sittler@nature.uni-freiburg.de.
Maria Hørnell Willebrand, Email: maria.willebrand@inn.no.
Kathy Martin, Email: Kathy.martin@ubc.ca.
References
- Barraquand F, Louca S, Abbott KC, Cobbold CA, Cordoleani F, DeAngelis DL, Eldern BD, Fox JW, et al. Moving forward in circles: Challenges and opportunities in modelling population cycles. Ecology Letters. 2017;20:1074–1092. doi: 10.1111/ele.12789. [DOI] [PubMed] [Google Scholar]
- Bjørnstad ON, Grenfell BT. Noisy clockwork: Time series analysis of population fluctuations in animals. Science. 2001;29:638–643. doi: 10.1126/science.1062226. [DOI] [PubMed] [Google Scholar]
- Bjørnstad ON, Ims RA, Lambin X. Spatial population dynamics: Analysing patterns and processes of population synchrony. Trends in Ecology and Evolution. 1999;14:427–432. doi: 10.1016/S0169-5347(99)01677-8. [DOI] [PubMed] [Google Scholar]
- Boonstra R, Andreassen HP, Boutin S, Husek J, Ims RA, Krebs CJ, Skarpe C, Wabakken P. Why do the boreal forest ecosystems of northwestern Europe differ from those of western North America? BioScience. 2016;66:722–734. doi: 10.1093/biosci/biw080. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Buckland ST, Anderson DR, Burnham KP, Laake JL, Borchers DL, Thomas L. Introduction to distance sampling. Estimating abundance of biological populations. New York: Oxford University Press, Inc.; 2001. [Google Scholar]
- Cattadori IM, Haydon DT, Thirgood SJ, Hudson PJ. Are indirect measures of abundance a useful index of population density? The case of red grouse harvesting. Oikos. 2003;100:439–446. doi: 10.1034/j.1600-0706.2003.12072.x. [DOI] [Google Scholar]
- Christensen, T., J. Payne, M. Doyle, G. Ibarguchi, J. Taylor, N.M. Schmidt, M. Gill, M. Svoboda, et al. 2013. The Arctic Terrestrial Biodiversity Monitoring Plan. CAFF Monitoring Series Report Nr. 7. Akureyri: CAFF International Secretariat.
- Cornullier T, Yoccoz NG, Bretagnolle V, Brommer JE, Butet A, Ecke F, Elston DA, Framstad E, et al. Europe-wide dampening of population cycles in keystone herbivores. Science. 2013;340:63–66. doi: 10.1126/science.1228992. [DOI] [PubMed] [Google Scholar]
- Elmhagen B, Kindberg J, Hellstrom P, Angerbjorn A. A boreal invasion in response to climate change? Range shifts and community effects in the borderland between forest and tundra. Ambio. 2015;44:39–50. doi: 10.1007/s13280-014-0606-8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hannon, S.J., P.K. Eason, and K. Martin. 1998. Willow Ptarmigan. In The birds of North America, No. 369, eds. A. Poole, F. Gill, 1–28. Philadelphia: The Academy of Natural Sciences; Washington, DC: The American Ornithologists’ Union.
- Hastings A. Transients: The key to long-term ecological understanding? Trends in Ecology and Evolution. 2004;19:39–45. doi: 10.1016/j.tree.2003.09.007. [DOI] [PubMed] [Google Scholar]
- Henden J-A, Ims RA, Fuglei E, Pedersen ÅØ. Changed Arctic–alpine food web interactions under rapid climate warming: Implication for ptarmigan research. Wildlife Biology. 2017 doi: 10.2981/wlb.00240. [DOI] [Google Scholar]
- Henden J-A, Ims RA, Yoccoz NG. Nonstationary spatio-temporal small rodent dynamics: Evidence from long-term Norwegian fox bounty data. Journal of Animal Ecology. 2009;78:636–645. doi: 10.1111/j.1365-2656.2008.01510.x. [DOI] [PubMed] [Google Scholar]
- Henden J-A, Ims RA, Yoccoz NG, Killengreen ST. Declining Willow Ptarmigan populations: The role of habitat structure and community dynamics. Basic and Applied Ecology. 2011;12:413–422. doi: 10.1016/j.baae.2011.05.006. [DOI] [Google Scholar]
- Hjeljord, O. 2015. Ryper før og nå. Rypejegernes dagsutbytte fra 1872 til 2013. NINA fagrapport 30 (in Norwegian).
- Holmstad PR, Hudson PJ, Vandvik V, Skorping A. Can parasites synchronise the population fluctuations of sympatric tetraonids? Examining some minimum conditions. Oikos. 2005;109:429–434. doi: 10.1111/j.0030-1299.2005.13702.x. [DOI] [Google Scholar]
- Ims RA, Fuglei E. Trophic interaction cycles in tundra ecosystems and the impact of climate change. BioScience. 2005;55:311–322. doi: 10.1641/0006-3568(2005)055[0311:TICITE]2.0.CO;2. [DOI] [Google Scholar]
- Ims RA, Henden J-A, Killengreen ST. Collapsing population cycles. Trends in Ecology and Evolution. 2008;23:79–86. doi: 10.1016/j.tree.2007.10.010. [DOI] [PubMed] [Google Scholar]
- Ims RA, Yoccoz NG. Ecosystem-based monitoring in the age of rapid climate change and new technologies. Current Opinions in Sustainability Science. 2017;29:170–176. doi: 10.1016/j.cosust.2018.01.003. [DOI] [Google Scholar]
- Ims RA, Yoccoz NG, Bråthen KA, Fauchald P, Tveraa T, Hausner V. Can reindeer overabundance cause a trophic cascade? Ecosystems. 2007;10:607–622. doi: 10.1007/s10021-007-9060-9. [DOI] [Google Scholar]
- International Union for Conservation of Nature, IUCN. 2016. The IUCN Red List of Threatened Species. Version 2016.3.1.
- Kausrud KL, Mysterud A, Steen H, Vik JO, Østbye E, Cazelles B, Framstad E, Eikeset AM, et al. Linking climate change to lemming cycles. Nature. 2008;456:93–98. doi: 10.1038/nature07442. [DOI] [PubMed] [Google Scholar]
- Krebs CJ, Boonstra R, Boutin S, Sinclair ARE, Smith JNM, Gilbert BS, Martin K, O’Donoghue M, et al. Trophic dynamics of the boreal forests of the Kluane Region. Arctic (Supplement) 2014;67:71–81. doi: 10.14430/arctic4350. [DOI] [Google Scholar]
- Lehikoinen A, Green M, Husby M, Kålås JA, Lindström Å. Common montane birds are declining in northern Europe. Journal of Avian Biology. 2014;45:3–14. doi: 10.1111/j.1600-048X.2013.00177.x. [DOI] [Google Scholar]
- Martin K, Doyle C, Hannon S, Mueller F. Forest grouse and ptarmigan. Chapter 11. In: Krebs CJ, Boutin S, Boonstral R, editors. Ecosystem dynamics of the boreal forest: The Kluane Project. Oxford: Oxford University Press; 2001. pp. 240–260. [Google Scholar]
- Martin K, Wilson S. Ptarmigan in North America: Influence of life history and environmental conditions on population persistence. In: Watson RT, Cade TJ, Fuller M, Hunt G, Potapov E, editors. Gyrfalcons and ptarmigan in a changing world. Boise: The Peregrine Fund; 2011. pp. 45–54. [Google Scholar]
- Møller AP, Fiedler W, Berthold P. Effects of climate change on birds. Oxford: Oxford University Press; 2010. [Google Scholar]
- Moss R, Watson A. Population cycles in birds of the grouse family (Tetraonidae) Advances in Ecological Research. 2001;32:53–111. doi: 10.1016/S0065-2504(01)32011-1. [DOI] [Google Scholar]
- Myrberget S. Annual variation in clutch sizes of a population of willow grouse Lagopus lagopus. Fauna norvegica Series C, Cinclus. 1986;9:74–81. [Google Scholar]
- Nielsen ÓK. Gyrfalcon predation on ptarmigan: Numerical and functional responses. Journal of Animal Ecology. 1999;68:1034–1050. doi: 10.1046/j.1365-2656.1999.00351.x. [DOI] [Google Scholar]
- Nielsen ÓK. Harvest and population change of Rock Ptarmigan in Iceland. Abstract. In: Watson RT, Cade TJ, Fuller M, Hunt G, Potapov E, editors. Gyrfalcons and ptarmigan in a changing world. Boise: The Peregrine Fund; 2011. p. 71. [Google Scholar]
- Potapov R, Sale R. Grouse of the world. London: New Holland Publishers; 2013. [Google Scholar]
- Ranta E, Lindstrom J, Linden H, Helle P. How reliable are harvesting data for analyses of spatio-temporal population dynamics? Oikos. 2008;117:1461–1468. doi: 10.1111/j.0030-1299.2008.16879.x. [DOI] [Google Scholar]
- R Core Team. 2017. R: A Language and Environment for Statistical Computing. https://www.R-project.org/.
- Revermann R, Schmid H, Zbinden N, Spaar R, Schröder B. Habitat at the mountain tops: How long can Rock Ptarmigan (Lagopus muta helvetica) survive rapid climate change in the Swiss Alps? A multi-scale approach. Journal of Ornithology. 2012;153:891–905. doi: 10.1007/s10336-012-0819-1. [DOI] [Google Scholar]
- Roesch, A., and R. Schmidbauer. 2014. WaveletComp: Computational Wavelet Analysis. Rpackageversion. https://pdfs.semanticscholar.org/d6f6/6c94fe262be38806ce55ac60215954ff3fbc.pdf.
- Sandercock BK, Martin K, Hannon SJ. Life history strategies in extreme environments: Comparative demography of Arctic and alpine ptarmigan. Ecology. 2005;86:2176–2186. doi: 10.1890/04-0563. [DOI] [Google Scholar]
- Schmidt NM, Christensen TR, Roslin T. A high Arctic experience of uniting research and monitoring. Earth’s Future. 2017;5:650–654. doi: 10.1002/2017ef000553. [DOI] [Google Scholar]
- Schmidt NM, Ims RA, Høye TT, Gilg O, Hansen LH, Hansen J, Lund M, Fuglei E, et al. Response of an Arctic predator guild to collapsing lemming cycles. Proceedings of the Royal Society B. 2012;279:4417–4422. doi: 10.1098/rspb.2012.1490. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Schwarzer G. meta: An R package for meta-analysis. R News. 2007;7:40–45. [Google Scholar]
- Scridel D, Brambilla M, Martin K, Lehikoinen A, Iemma A, Anderle M, Jahnig S, Caprio E, et al. A review and meta-analysis of the effects of climate change on Holarctic mountain and upland bird populations. Ibis. 2018;160:489–515. doi: 10.1111/ibi.12585. [DOI] [Google Scholar]
- Soininen E, Fuglei E, Pedersen ÅØ. Complementary use of density estimates and hunting statistics: Different sides of the same story? European Journal of Wildlife Research. 2016;62:151–160. doi: 10.1007/s10344-016-0987-z. [DOI] [Google Scholar]
- Steen JB, Steen H, Stenseth NC, Myrberget S, Marcstrom V. Microtine density and weather as predictors of chick production in willow ptarmigan, Lagopus l. lagopus. Oikos. 1988;51:367–373. doi: 10.2307/3565320. [DOI] [Google Scholar]
- Stenseth NC, Shabbar A, Chan KS, Boutin S, Rueness EK, Ehrich D, Hurrell JW, Lingjaerde OC, et al. Snow conditions may create an invisible barrier for lynx. Proceedings of the National Academy of Sciences of USA. 2004;101:10632–10634. doi: 10.1073/pnas.0308674101. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Storch, I. 2007. Grouse: Status survey and conservation action plan 2006–2010. Gland: IUCN; Fordingbridge: World Pheasant Association.
- Tape KD, Lord R, Marshall H-P, Ruess RW. Snow-mediated ptarmigan browsing and shrub expansion in Arctic Alaska. Ecoscience. 2010;17:186–193. doi: 10.2980/17-2-3323. [DOI] [Google Scholar]
- Willebrand T, Hornell-Willebrand M, Asmyr L. Willow grouse bag size is more sensitive to variation in hunter effort than variation in willow grouse density. Oikos. 2011;120:1667–1673. doi: 10.1111/j.1600-0706.2011.19204.x. [DOI] [Google Scholar]
- Wilson S, Martin K. Influence of life history strategies on sensitivity, population growth and response to climate for sympatric alpine birds. BMC Ecology. 2012;12:9. doi: 10.1186/1472-6785-12-9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wood SN. Fast stable restricted maximum likelihood and marginal likelihood estimation of semiparametric generalized linear models. Journal of the Royal Statistical Society, Series B (Statistical Methodology) 2011;73:3–36. doi: 10.1111/j.1467-9868.2010.00749.x. [DOI] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.