Abstract
Changes in Arctic vegetation can have important implications for trophic interactions and ecosystem functioning leading to climate feedbacks. Plot-based vegetation surveys provide detailed insight into vegetation changes at sites around the Arctic and improve our ability to predict the impacts of environmental change on tundra ecosystems. Here, we review studies of changes in plant community composition and phenology from both long-term monitoring and warming experiments in Arctic environments. We find that Arctic plant communities and species are generally sensitive to warming, but trends over a period of time are heterogeneous and complex and do not always mirror expectations based on responses to experimental manipulations. Our findings highlight the need for more geographically widespread, integrated, and comprehensive monitoring efforts that can better resolve the interacting effects of warming and other local and regional ecological factors.
Electronic supplementary material
The online version of this article (10.1007/s13280-019-01161-6) contains supplementary material, which is available to authorized users.
Keywords: Arctic, Experimental warming, Long-term monitoring, Phenology, Vegetation change
Introduction
A major goal of global change ecology is to document and predict the impacts of environmental change on species, communities, and ecosystems worldwide. In the Arctic, exceptionally rapid warming (IPCC 2013) has the potential to lead to dramatic changes in vegetation through longer growing seasons, increased thaw depth, and altered snow regimes. High latitudes contain up to 50% of the world’s soil carbon stored in permafrost soils; this carbon is vulnerable to loss with warming (Schuur et al. 2015; Crowther et al. 2016; van Gestel et al. 2018). Thus, changes in vegetation carbon and nutrient inputs to tundra soils could have potentially global impacts. For example, shifts in species composition could lead to changes in aboveground carbon storage, nutrient cycling, decomposition rates, and albedo (Callaghan et al. 2004), potentially leading to global climate-based feedbacks (Chapin et al. 2005; Pearson et al. 2013). Changing vegetation could also alter trophic interactions (Post et al. 2009; Gauthier et al. 2013) and thus influence Arctic wildlife populations and the human communities that rely on them for resource provision or cultural purposes (Weller et al. 2004; Henry et al. 2012; Stern and Gaden 2015).
A key source of information about the consequences of climate warming for Arctic vegetation comes from plot-based research at sites across the Arctic (Henry and Molau 1997). This includes both long-term monitoring of species composition, diversity, and phenology over time (up to four decades), as well as experimental manipulation of key abiotic and biotic drivers (e.g., temperature, snow, nutrients, grazing). Community composition, diversity, and phenology have all been identified as “Focal Ecosystem Components” (FECs) by the international Circumpolar Biodiversity Monitoring Programme (Christensen et al. 2013), as monitoring of these attributes facilitates a more rapid detection, communication, and response to significant biodiversity-related trends and pressures affecting the circumpolar world. In addition, comparing the results of observed trends over time with experimental studies can help to elucidate the drivers of observed trends and inform predictions of future change (Elmendorf et al. 2015).
Here, we synthesize what is currently known about plot-based changes in vegetation composition (abundance), phenology, diversity, and functional traits. We compiled information from single-site studies of compositional and phenological changes to document (1) the direction and significance of change over time, and (2) the direction and significance of responses to experimental warming. We compare these results to published syntheses of long-term monitoring and experimental warming. We additionally review studies of plot-based changes in plant functional traits and diversity, for which published observations are relatively scarce. Finally, we discuss the broader implications of observed and predicted Arctic vegetation changes and recommend priorities for future monitoring efforts.
Materials and methods
Literature review of vegetation trends
We conducted a literature review to identify single-site studies of changes in plant community composition (abundance) and phenology both over time and in response to experimental warming. Our search included combinations of the terms “tundra,” “arctic,” “vegetation,” “plot,” “change,” “ITEX,” “cover,” “abundance,” “phenology,” “diversity,” “functional trait,” “warming,” and “experiment”. These terms encompass two FECs included in the Circumpolar Biodiversity Monitoring Program terrestrial monitoring plan: (i) diversity, composition, and abundance and (ii) phenology. We do not include the attributes “diversity and spatial structure,” “productivity,” “Rare species, species of concern,” or “food species” in this review due to a paucity of published plot-based monitoring and/or experimental studies on these topics.
We included only studies at sites above 63°N and identified as “Arctic” or “tundra” by the authors. This latitudinal cutoff includes some sub-Arctic sites but is roughly comparable to areas included in the Arctic Biodiversity Assessment (CAFF 2013). For community composition/abundance, we included measured responses in any variables called abundance, biomass, or percent cover. We included studies that analyzed changes in abundance at both the species and functional group levels. For studies where abundance trends were identified at the species level, we included all species but grouped them by functional group for visualization purposes. All phenological responses were provided at the species level.
For phenological studies, we recorded all phenostages provided by the authors, but here we report only the most commonly observed phenostages: leaf emergence, flowering, and leaf senescence. Leaf emergence is the day at which leaf bud-break first occurs or the first day on which overwintered leaves re-green. Flowering encompasses several phases related to the timing of flowering, including inflorescence elongation, first open flower, onset of pollen release, and peak flowering. Leaf senescence is the date on which leaves change color or die, indicating the end of the growing season for most plants. Studies reporting responses of diversity and/or functional traits were scarce; thus, we review the available information but do not attempt to categorize and quantitate these responses.
For all studies, we recorded the direction (increase/stable/decrease for abundance change, or earlier/stable/later for phenological change) and significance (yes/no) of responses for all species and functional groups identified. A response could be recorded as directional (increase/decrease or earlier/later) and nonsignificant if the authors identified it as such, or if the p value provided was between 0.05 and 0.1. We adopted this approach in order to standardize α levels across all studies (e.g., if some studies used an α level cutoff of 0.05 to assess significance, while others used an α level of 0.1). If a response was identified by the authors as directional but no indication of significance was given (either in the text or in a figure/table), the response was categorized as nonsignificant. The difference between significant and nonsignificant directional changes is shown in the figures and provided in the supplementary data table. We used this “vote-counting” approach, rather than a traditional meta-analysis, in order to include the many studies that do not provide response effect sizes or estimates of error. In addition, this approach allows us to visualize the full distribution of vegetation responses to ambient and experimental warming, as a meta-analysis finding of “no change” could in fact be made up of multiple significant changes in different directions (e.g., context dependency).
Experimental warming was generally conducted through the use of clear-sided, open-top chambers that passively warm air temperatures by ~ 1.5–3 °C, with most of the studies following International Tundra Experiment (ITEX) protocols (Molau and Mølgaard 1996; Marion et al. 1997), though some experiments used greenhouses or other warming methods (Chapin and Shaver 1996; Wang et al. 2017). The seasonal duration of warming also varies by study; some warming chambers were in place only during the summer, while others were present year-round. Both warming chambers and greenhouses can influence environmental factors other than temperature (e.g., soil moisture, wind, snow accumulation), though the magnitude and significance of these effects are variable among sites (Marion et al. 1997).
Comparison to tundra-wide syntheses
In order to evaluate the consistency of patterns revealed by the literature review, we compared the results of our review with tundra-wide syntheses of compositional and phenological changes (Arft et al. 1999; Walker et al. 2006; Elmendorf et al. 2012a, b; Oberbauer et al. 2013), both over time and in response to experimental warming. These syntheses used primary data and were not based on published studies, though some data included in the syntheses may be from the same sites as the single-site studies included in our literature review. However, the synthesis and single-site studies likely include different combinations of sites and years, and use different statistical methods to analyze responses. In addition, many of the synthesis studies included both Arctic and alpine tundra sites, while here we focused exclusively on Arctic and sub-Arctic locations. Thus, evidence that synthesis studies found trends consistent with those documented in this literature review can help evaluate the robustness of observed patterns in Arctic vegetation change.
Results
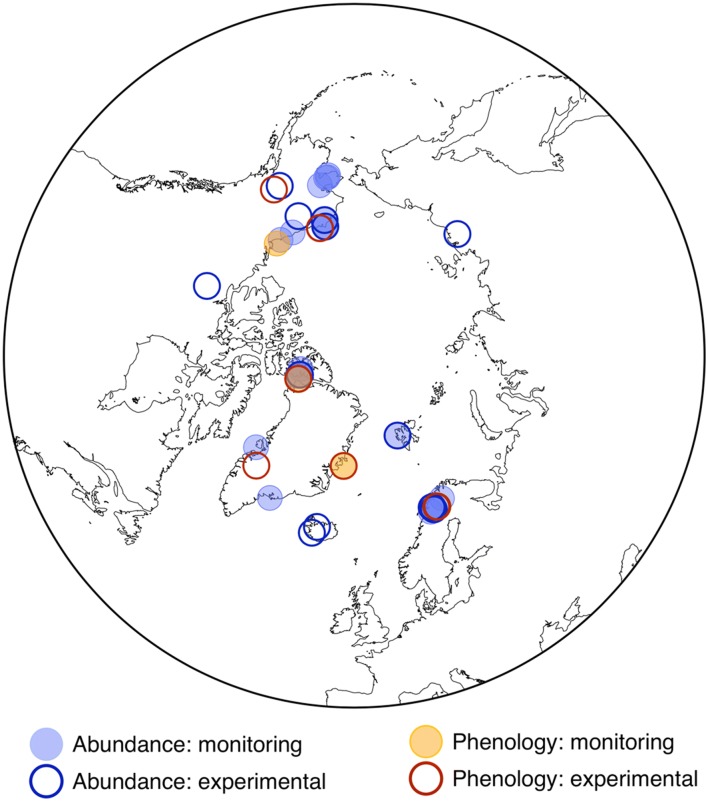
We identified a total of 560 vegetation composition/abundance observations (species or functional group) from 19 studies of long-term monitoring and 209 observations from 14 studies of responses to experimental warming (Fig. 1; Table 1). The duration of monitoring studies ranged from 5 to 43 years, with a median duration of 19 years. We additionally identified long-term monitoring of phenology for 17 species in three studies and responses to experimental warming of 52 species from 9 studies (Fig. 1; Table 1). Phenological monitoring studies ranged from 9 to 21 years in duration, with a median duration of 16 years.
Fig. 1.
Map of plot-based vegetation change studies identified in a review of the literature. Blue points designate studies of community composition (abundance) change, while orange points designate studies of phenological change. Filled circles denote long-term monitoring studies (change over time) while open circles indicate experimental studies (responses to experimental warming)
Table 1.
Studies of abundance and phenology included in this review
| Studies | Site names | Latitude | Longitude | Duration (years) | Abundance | Phenology | ||
|---|---|---|---|---|---|---|---|---|
| Monitoring | Experiment | Monitoring | Experiment | |||||
| Alatalo and Totland (1997) | Latnjajaure, Lapland, Sweden | 68.21 | 18.3 | 1 | x | |||
| Bjorkman et al. (2015) | Alexandra Fiord, Ellesmere Island, Canada | 78.53 | − 75.55 | 21 | x | x | ||
| Boulanger-Lapointe et al. (2014) | Alexandra Fiord, Ellesmere Island, Nunavut | 78.86 | − 75.9 | 13–15 | x | |||
| Boulanger-Lapointe et al. (2014) | Sverdrup Pass, Ellesmere Island, Nunavut | 79.13 | − 79.73 | 5–23 | x | |||
| Callaghan et al. (2011) | Disko Island, Greenland | 69.15 | − 53.34 | 43 | x | |||
| Chapin and Shaver (1996) | Toolik Lake, Alaska | 68.38 | − 149.34 | 4 | x | |||
| Chapin et al. (1995) | Toolik Lake, Alaska | 68.38 | − 149.34 | 9 | x | |||
| Daniëls and de Molenaar (2011) | Tasiilaq, Southeast Greenland | 65.62 | − 37.67 | 41 | x | |||
| Graglia et al. (2001) | Abisko, Sweden | 68.35 | 18.82 | 10 | x | |||
| Hill and Henry (2011) | Alexandra Fiord, Ellesmere Island, Canada | 78.53 | − 75.55 | 25 | x | |||
| Hobbie and Chapin (1998) | Toolik Lake, Alaska | 68.38 | − 149.34 | 3 | x | |||
| Hollister and Webber (2000) | Barrow, Alaska, USA | 71.18 | − 156.4 | 1 | x | |||
| Hollister et al. (2015) | Atqasuk, Alaska | 70.45 | − 157.41 | 16 | x | x | ||
| Hollister et al. (2015) | Barrow, Alaska | 71.29 | − 156.64 | 17 | x | x | ||
| Høye et al. (2007) | Zackenberg, Greenland | 74.28 | − 20.34 | 9 | x | |||
| Hudson and Henry (2009) | Alexandra Fiord, Ellesmere Island, Nunavut | 78.88 | − 75.92 | 28 | x | |||
| Hudson and Henry (2010) | Alexandra Fiord, Ellesmere Island, Nunavut | 78.88 | − 75.92 | 16 | x | |||
| Jägerbrand et al. (2009) | Latnjajaure, Lapland, Sweden | 68.35 | 18.5 | 5 | x | |||
| Jandt et al. (2008) | Northwestern Alaska | 65.1 | − 163.4 | 10–15 | x | |||
| Joly et al. (2007) | Seward Peninsula, Alaska | 64.85 | − 163.7 | 25 | x | |||
| Jonasson et al. (1999) | Abisko, Sweden | 68.35 | 18.82 | 5 | x | |||
| Jones et al. (1997) | Alexandra Fiord, Ellesmere Island, Canada | 78.53 | − 75.55 | 1 | x | |||
| Jones et al. (1997) | Barrow, Alaska, USA | 71.19 | − 156.37 | 1 | x | |||
| Jones et al. (1997) | Latnjajaure, Lapland, Sweden | 68.21 | 18.3 | 1 | x | |||
| Jónsdóttir et al. (2005) | Audkuluheidi, Iceland | 65.27 | − 20.25 | 5 | x | |||
| Jónsdóttir et al. (2005) | Thingvellir, Iceland | 64.28 | − 21.08 | 5 | x | |||
| Jorgenson et al. (2015) | Arctic National Wildlife Refuge, Alaska | 69.8 | − 144.25 | 26 | x | |||
| Marchand et al. (2004) | Zackenberg, Greenland | 74.28 | − 20.34 | 1 | x | |||
| Molau (2010) | Latnjajaure, Lapland, Sweden | 68.35 | 18.5 | 12 | x | |||
| Myers-Smith et al. (2011b) | Qikiqtaruk-Herschel Island, Yukon | 69.57 | − 138.91 | 11 | x | |||
| Myers-Smith et al. (2019) | Qikiqtaruk-Hershel Island, Yukon, Canada | 69.57 | − 138.91 | 16–19 | x | x | ||
| Natali et al. (2012) | Eight Mile Lake, Alaska, USA | 63.52 | − 149.13 | 2 | x | x | ||
| Pattison et al. (2015) | Arctic National Wildlife Refuge, Alaska | 69.8 | − 144.25 | 26 | x | |||
| Post and Pedersen (2008) | Kangerlussuaq, Greenland | 67.6 | − 50.2 | 2 | x | |||
| Richardson et al. (2002) | Abisko Valley, Sweden | 68 | 19 | 9 | x | |||
| Robinson et al. (1998) | Ny Alesund, Svalbard | 78.93 | 11.83 | 5 | x | x | ||
| Rundqvist et al. (2011) | Abisko Valley, Sweden | 68.35 | 18.82 | 35 | x | |||
| Stenström and Jónsdóttir (1997) | Latnjajaure, Lapland, Sweden | 68.22 | 18.13 | 1 | x | |||
| Tømmervik et al. (2004) | Kautokeino, Norway | 69 | 23.1 | 38 | x | |||
| Villarreal et al. (2012) | Barrow, Alaska | 71.3 | − 156.67 | 39 | x | |||
| Vowles et al. (2017) | Ritsem, Sweden | 67.824 | 17.715 | 18 | x | |||
| Wang et al. (2017) | Kytalyk, Siberia | 70.82 | 147.48 | 4 | x | |||
| Wilson and Nilsson (2009) | Cievrratjäkka, Sweden | 68.01 | 18.81 | 21 | x | |||
| Wookey et al. (1993) | Abisko, Sweden | 68.21 | 18.49 | 1 | x | |||
| Zamin et al. (2014) | Daring Lake, NWT | 64.87 | − 111.57 | 8 | x | |||
Our literature review reveals geographic gaps in both long-term monitoring and experimental warming studies. The FEC (Christensen et al. 2013) encompassing composition and abundance is better represented than that encompassing phenology, but both lack published records of change from Siberia and wide swaths of the Canadian Arctic. Intensive, multivariate monitoring is concentrated primarily in Alaska and Scandinavia, with the exception of one site in high-Arctic Canada (Muc et al. 1989; Freedman and Svoboda 1994; Hudson and Henry 2009; Hill and Henry 2011; Bjorkman et al. 2015).
Change in the composition of vegetation
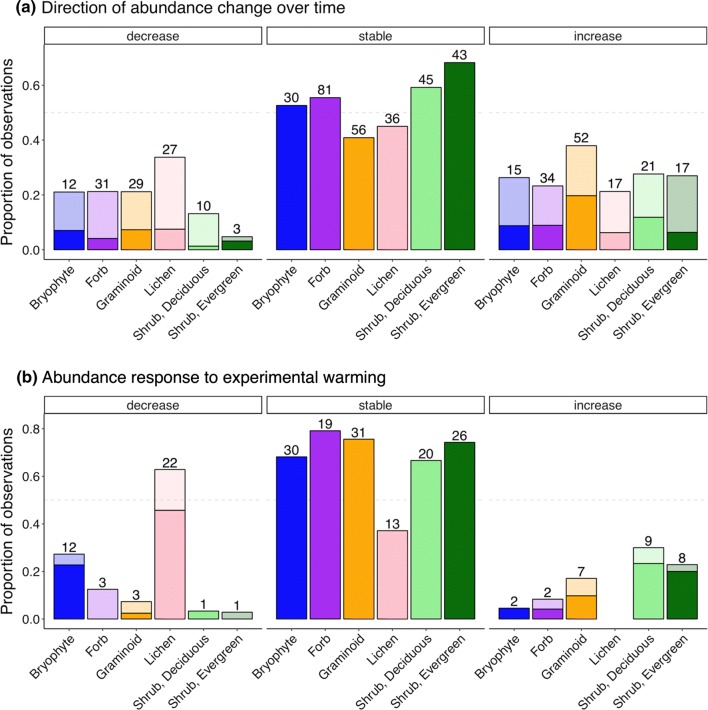
In all cases, the most common response documented by long-term monitoring of compositional change was one of no trend (52–84% of trends did not differ from zero, depending on the significance cutoff used; Fig. 2). This is likely an underestimate of the proportion of no-change responses, as some studies reported results only for species that changed significantly over time (e.g., Tømmervik et al. 2004). Forbs, graminoids, and both evergreen and deciduous shrubs were slightly more likely to increase in abundance over time than decrease, but were most likely to remain stable. Experimental warming led to more dramatic responses, particularly in lichens, which were far more likely to decrease in abundance in response to experimental warming (46–63%) than to increase (0%) or remain stable (37%). Bryophytes also had a tendency to respond negatively to experimental warming, while evergreen and deciduous shrubs were more likely to respond positively.
Fig. 2.
Summary of studies investigating abundance change over time (a) and abundance change in response to experimental warming (b) by species or functional group. Panels represent, from left to right, the proportion of observations decreasing in abundance, stable, or increasing in abundance over time (median 20.5 years) or in response to experimental warming. Species-specific trends were grouped into the relevant functional group category. The darker portions of each bar represent “significant” (p < 0.05) change (decrease or increase) or insignificant (stable, p > 0.1) results, while lighter colors represent borderline or marginally significant change (e.g., p-values between 0.05 and 0.1). The numbers above each bar represent a count of the number of observations included in that group. The proportion of “stable” species is underrepresented in this figure, as some studies only reported results for species that changed in abundance
Few studies included both above- and belowground measurements; of those that did, above- and belowground responses were not always consistent. Aboveground responses to experimental warming in northern Alaska were greater than belowground responses (Chapin and Shaver 1996), but belowground biomass increased more than aboveground biomass over 30 years of monitoring at Alexandra Fiord, Ellesmere Island (Hill and Henry 2011). At Daring Lake, Canada, experimental warming enhanced both above- and belowground biomass in evergreen shrubs, but only aboveground biomass in deciduous shrubs (Zamin et al. 2014).
These responses are largely in line with those revealed in tundra-wide syntheses of vegetation change at the functional group level. In a recent 30-year study of vegetation change across 46 Arctic, alpine, and Antarctic tundra locations (Elmendorf et al. 2012b), only evergreen shrubs (but not deciduous) increased significantly over time. Bryophytes were more likely to decrease than increase, but the response was not significant. Similar to results from our literature review, responses to experimental warming were more dramatic. Deciduous but not evergreen shrubs increased significantly in abundance in response to experimental warming, while both lichens and bryophytes decreased significantly (Elmendorf et al. 2012a). In both monitoring and experimental synthesis studies, the quantity of dead material (litter and attached dead) increased over time or with warming (Elmendorf et al. 2012a, b).
Phenological change
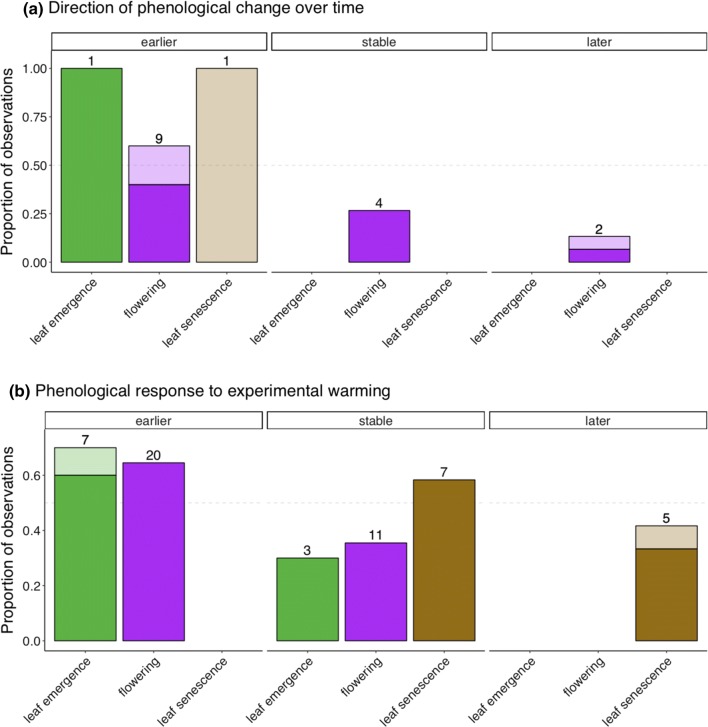
In general, the timing of leaf emergence and flowering advanced both over time and with experimental warming, though a minority of species experienced stable or even delayed flowering over time (Fig. 3). Interestingly, experimental warming led to later leaf senescence in all cases, while the one study that documents long-term trends in leaf senescence (Myers-Smith et al. 2019) found a nonsignificant trend toward earlier leaf senescence over 16 years of monitoring.
Fig. 3.
Summary of studies investigating phenological change over time (a) and in response to experimental warming (b). Panels represent, from left to right, the proportion of observations that advanced (“earlier”) in a given phenological stage, remained stable, or were delayed (“later”) over time or in response to experimental warming. All observations represent species-specific responses. The darker portions of each bar represent “significant” (p < 0.05) change (decrease or increase) or insignificant (stable, p > 0.1) results, while lighter colors represent borderline or marginally significant change (e.g., p-values between 0.05 and 0.1). The numbers above each bar represent a count of the number of observations included in that group
In a 17-year synthesis of phenological trends at 12 tundra sites (including 2 alpine locations), there was no significant change in the timing of flowering or leaf senescence, though both events tended to advance over time (Oberbauer et al. 2013), as we also found in the literature review. Surprisingly, this same synthesis study found that leaf emergence was significantly delayed over time despite increasing temperatures over the same period. In a separate synthesis of responses to experimental warming at 10 Arctic sites, leaf emergence and flowering both occurred significantly earlier when warmed, but senescence was not affected (Arft et al. 1999). This is also in agreement with our literature review, where most sites reported no difference in leaf senescence or a slight delay.
Discussion
Studies of plot-based vegetation change reveal that while some sites and species or functional groups have experienced substantial shifts in vegetation and phenology in response to ambient or experimental warming, the most common response overall is one of no change. Furthermore, vegetation changes over time did not always match responses to experimental warming. There is one pattern that emerges: both long-term monitoring and experimental studies suggest that the graminoid and shrub functional groups respond positively to warming and are slightly more likely to increase in abundance over time. This is in line with studies of shrub infilling and expansion across much of the tundra (Sturm et al. 2001; Myers-Smith et al. 2011a; Martin et al. 2017). Even so, the majority of graminoid and shrub abundance responses in our literature review were that of no significant trend over time and no significant response to experimental warming. Further exploration of these trends reveals that increasing shrub abundance primarily occurs in relatively warm tundra regions with mesic or wet soils, while colder and dry tundra sites have not experienced increasing shrub abundance (Elmendorf et al. 2012b) consistent with patterns in the climate sensitivity of shrub growth (Myers-Smith et al. 2015). Grazing may also influence shrub responses to summer temperature change (Bråthen et al. 2017). The lack of strong trends over time in many sites and for many species suggests that tundra plant communities are remarkably resilient to moderate warming, at least over decadal time spans, and that site-specific factors such as moisture availability and grazing may limit vegetation responses to warming (Elmendorf et al. 2012b; Myers-Smith et al. 2015; Ackerman et al. 2017).
Differing responses to experimental and ambient (natural) warming highlight both the benefits and the challenges of using experimental approaches to understand tundra vegetation responses to climatic change. When experimental and monitoring results agree, experimental studies enable us to pinpoint the likely drivers of change over time (Elmendorf et al. 2015), and improve our confidence in predictions of the impacts of warming on vegetation (e.g., increasing shrub abundance). Diverging responses can challenge our understanding of tundra vegetation change. For example, while experimental warming led to fairly dramatic declines in lichen abundance (Walker et al. 2006; Elmendorf et al. 2012a and this study), lichen abundance did not decline over time in long-term monitoring studies (Elmendorf et al. 2012b and this study). Similarly, while experimental warming generally led to later leaf senescence (Arft et al. 1999 and this study), monitoring studies indicate that senescence is in fact advancing over time, though not significantly (Oberbauer et al. 2013 and this study). Trends in the timing of flowering and leaf emergence are also varied despite a relatively consistent advance in these variables in response to experimental warming.
The reasons underlying these heterogeneous and contrasting trends are not entirely clear, but may have to do with interactions among environmental drivers that are not captured by experimental isolation of a single driver. For example, lichens are sensitive to soil moisture, and may be responding to changes in precipitation, hydrology, or snow regimes over time rather than temperature trends alone (Björk and Molau 2007). Similarly, phenological advance with warming temperatures (Høye et al. 2007) may be limited by concurrent changes in winter snowfall (Bjorkman et al. 2015) and snowmelt date (Cooper et al. 2011). In addition, growing season phenology might be controlled by deterministic leaf age (Starr et al. 2000) or adaptation to photoperiod (Kummerow 1992; Bjorkman et al. 2017) in many Arctic species, thus limiting the impact of temperature change alone. Phenological responses to different drivers may interact or be nonlinear, leading to more complex responses than can be easily detected from simple experiments or ecological monitoring studies (Iler et al. 2013). Finally, experimental warming chambers can alter conditions other than temperature alone (Marion et al. 1997), and vegetation could be responding to these unwanted environmental side-effects.
Improved monitoring of multiple environmental drivers and experimental studies that manipulate several variables simultaneously (e.g., snow depth, moisture availability) could help to elucidate the importance of these interactions. Multisite syntheses can also help to clarify the context dependency of trends over time. For example, additional syntheses of tundra plant phenology have shown that a plant’s sensitivity to temperature varies by the temperature of the site (greater sensitivity at colder sites, Prevéy et al. 2017) as well as the phenological niche of the species (greater sensitivity in late-flowering species, Prevéy et al. 2019).
Other vegetation trends: functional traits and diversity
While trends in composition and phenology are perhaps the most studied plot-based responses to global change, a handful of studies document changes in other vegetation parameters. Of these, increasing height is likely the most well-documented. Increases in community height have been documented by synthesis studies of responses to experimental warming (Elmendorf et al. 2012a) and over time (Bjorkman et al. 2018), a change driven primarily by the influx of taller species into the monitoring plots (Bjorkman et al. 2018). Some single-site studies have also documented increasing height over time (Hollister et al. 2015) and in response to experimental warming (Hudson et al. 2011; Hollister et al. 2015; Baruah et al. 2017). Changes in other plant traits have also been documented. Experimental warming at Alexandra Fiord in High Arctic Canada resulted in greater leaf size, lower specific leaf area (the ratio of leaf area to leaf dry mass), and decreased leaf carbon content for at least some species-site combinations (Hudson et al. 2011) but did not affect leaf nitrogen (N) content, leaf dry matter content, or nitrogen isotope signatures. Other studies have documented mixed responses of leaf size to experimental warming in the Swedish sub-Arctic tundra (Graglia et al. 1997; Baruah et al. 2017) and one study found trends toward reduced leaf size over time (Barrett et al. 2015). Additional studies of leaf N content responses to experimental warming are also mixed; leaf N content increased in response to winter but not summer warming across six species at Eight Mile Lake, Alaska (Natali et al. 2012) but was either unaffected by temperature or declined in response to warming at Toolik Lake, Alaska (Chapin and Shaver 1996) and Alexandra Fiord, Canada (Tolvanen and Henry 2001). A synthesis of community-weighted mean functional trait change across the tundra biome (including alpine sites) over 27 years found no significant change in leaf area, leaf N content, leaf dry matter content, or specific leaf area (Bjorkman et al. 2018). Overall, species composition has shifted toward more thermophilic (warm-loving) species both over time and in response to experimental warming (Elmendorf et al. 2015).
Over the long term, climatic warming may lead to increased diversity in the Arctic as southern, species-rich floras move northward (Parmesan 2006). However, short-term responses to warming might differ substantially from long-term trends, as immigration is likely to be slow relative to local assembly processes (e.g., competition; Walker et al. 2006). Thus far, evidence of plot-scale diversity change in Arctic ecosystems is mixed. A multisite synthesis found a significant decline in both Shannon diversity and species richness after 3 to 6 years of experimental warming (Walker et al. 2006), but a more recent, longer-term synthesis found no response (Elmendorf et al. 2012a). Lichen diversity was found to decline significantly in response to long-term experimental warming at three sites in northern Sweden and Alaska (Lang et al. 2012). Among monitoring studies, a recent synthesis found no change in vascular plant diversity over three decades of monitoring across dozens of tundra sites (Elmendorf et al. 2012b). This is in stark contrast to ongoing changes in European mountaintop plant communities, which have experienced rapid and accelerating increases in richness over the past century (Steinbauer et al. 2018). The difference between Arctic and alpine responses could indicate that diversity change in nonalpine tundra communities is limited by dispersal rates of southerly, warm-adapted species, or that strong gradients in environmental variables other than temperature (e.g., photoperiod) across latitudes limit the establishment success of warm-adapted species from farther south (Bjorkman et al. 2017).
Consequences of Arctic vegetation change
Changes in tundra vegetation could have far-reaching impacts across trophic levels and to human societies (Weller et al. 2004). Shifts in plant phenology and reproductive success influence individual- and population-level fitness (Berteaux et al. 2004; Cleland et al. 2012) and could lead to trophic mismatches of resources for pollinators (Høye et al. 2013; Wheeler et al. 2015; Prevéy et al. 2019), breeding birds (McKinnon et al. 2012; Gauthier et al. 2013; Boelman et al. 2015) and mammals (Hertel et al. 2017). For example, one long-term study at Zackenberg, Greenland documented a shortening of the flowering season with climatic warming over time and a concurrent decline in the abundance of insect visits to flowers (Høye et al. 2013). Berry-producing (Hertel et al. 2017) and other tundra plants provide forage for hunted or domestic wildlife (Post and Stenseth 1999; Kerby and Post 2013) and represent culturally important resources for Arctic peoples (Henry et al. 2012).
The nearly ubiquitous shifts in phenology in response to experimental warming (Arft et al. 1999 and this study) suggest that many Arctic plant species are inherently sensitive to interannual variations in temperature, though concurrent changes in other environmental variables (e.g., precipitation, cloudiness) might limit the degree of advance over time with warming. A meta-analysis of phenological responses to experimental warming in temperate and alpine regions found that the temperature sensitivity of a species’ phenology correlates with better growth and/or reproductive performance (Cleland et al. 2012), but it is not known if this pattern holds true in the Arctic. A synthesis of responses to 4 years of experimental warming at 10 Arctic sites revealed increased reproductive effort (e.g., number of flowers produced) and success (e.g., number of seeds/fruits produced or seed mass) in experimentally warmed plots, though responses were generally not significant (Arft et al. 1999). Single-site studies have also found evidence of increased reproductive effort in experimentally warmed plots (Welker et al. 1997; Klady et al. 2011). Contrasting responses have been documented for seed germination rates, which increased with experimental warming at Alexandra Fiord, Canada (Klady et al. 2011) but not at Toolik Lake, Alaska (Welker et al. 1997).
Due to the large amount of carbon stored in tundra permafrost soils (Koven et al. 2011; Schuur et al. 2015; Crowther et al. 2016) and well-established links between vegetation and carbon storage, vegetation change in the Arctic can influence regional carbon cycling and feedbacks to the global climate (Callaghan et al. 2004; Sturm and Douglas 2005; Petrenko et al. 2016). For example, increasing shrub abundance and/or plant height can lead to increased winter snow trapping, greater insulation of underlying soils, warmer winter soil temperatures (Myers-Smith and Hik 2013), and potentially increased active layer depth and decomposition (Blok et al. 2016). Taller shrubs may also extend above the snowpack, decreasing winter albedo and increasing absorbed solar radiation (Sturm and Douglas 2005). Bryophytes have also been shown to play an important role in soil insulation and energy fluxes; experimental removal of bryophytes leads to increased evapotranspiration and ground heat flux (Blok et al. 2011). Thus, future declines in bryophytes—observed in warming experiments but not yet in monitoring studies—could also lead to deeper summer permafrost thaw and soil carbon release, representing another positive feedback to climatic warming.
Changing vegetation can also impact carbon cycling through changes in the quantity and decomposability of litter (Callaghan et al. 2004), as litter decomposition contributes nearly 70% of global CO2 fluxes from soils (Raich and Potter 1995). A long-term increase in shrubs, which have relatively recalcitrant litter, could lead to reduced litter decomposability and a negative feedback to climatic warming (Cornelissen et al. 2007). A change in litter composition can also indirectly influence soil carbon storage by driving changes in soil microbial communities (Christiansen et al. 2018) or altering tundra fuel loads. For example, increased woody litter inputs from shrub expansion might also increase flammability, which could lead to positive feedbacks through fire-induced soil carbon loss (Cornelissen et al. 2007; van Altena et al. 2012).
Conclusions
Rapid warming in the Arctic has the potential to cause substantial shifts in vegetation, potentially driving widespread changes across trophic levels and altering tundra ecosystem functions. While our review identifies significant shifts at some sites and in some species, the large variations in the magnitude and even direction of responses illustrate the high degree of context dependency in tundra vegetation change. This context dependency highlights the importance of maintaining multiple monitoring sites in many different habitat types across the entire Arctic, as well as increasing the monitoring of local ecological and environmental conditions that would improve our understanding of how factors other than temperature influence Arctic vegetation change. Thus, we recommend that international bodies such as the Circumpolar Biodiversity Monitoring Program (Christensen et al. 2013) prioritize monitoring efforts that (i) fill current geographic gaps, particularly in Canada and Siberia; and (ii) enable us to better disentangle the relative importance of climatic warming and other environmental factors on the diverging responses reported here.
Electronic supplementary material
Below is the link to the electronic supplementary material.
Acknowledgements
ADB was supported by The Danish Council for Independent Research: Natural Sciences (DFF 4181-00565 to SN); MGC was funded by the University of Edinburgh, IHMS by the UK Natural Environment Research Council (ShrubTundra Project NE/M016323/1); and SN was funded by the Villum Foundation’s Young Investigator Programme (VKR023456).
Biographies
Anne D. Bjorkman
is a Postdoctoral Scholar at the Senckenberg Biodiversity and Climate Research Centre. Her research interests include biogeography, functional ecology, and community ecology, with a particular focus on the ecological consequences of climatic change in tundra ecosystems.
Mariana García Criado
is a PhD Candidate at The University of Edinburgh (Scotland). She is a Conservation Scientist specialized in macroecology and biogeography, and is currently working to quantitate vegetation shifts under climatic change in extreme biomes such as the tundra and the savannah.
Isla H. Myers-Smith
is a Chancellor’s Fellow and Senior Lecturer at the University of Edinburgh. Her research quantifies how global change alters plant communities and ecosystem processes, with a focus on the tundra biome.
Virve Ravolainen
is a researcher at the Norwegian Polar Institute and in the ecosystem monitoring program “Climate-ecological Observatory for Arctic Tundra”. She is a vegetation ecologist focusing on climate-herbivore-vegetation interactions in the Arctic.
Ingibjörg Svala Jónsdóttir
is a Professor of Ecology at the University of Iceland. Her research interests are plant-herbivore interactions, biodiversity, and the effects of climate change and land use on terrestrial ecosystems at northern latitudes.
Kristine Bakke Westergaard
is a Researcher in botany at the Norwegian institute for nature research and a member of the CAFF Flora Expert Group. Her research focus on historical and contemporary plant dispersal and plant conservation in northern and arctic regions.
James P. Lawler
is the Alaska lead for the U.S. National Park Service Inventory and Monitoring program. The intent of the program is to provide an inventory of key natural resources within the Alaska parks, and monitor the health of park units, by focusing on “vital signs” - physical, chemical and biological elements - chosen to provide a broad understanding of park ecosystems.
Mora Aronsson
is a senior advisor and coordinator of the Swedish Species Information Centre (ArtDatabanken) at the Swedish University of Agricultural Sciences in Uppsala, Sweden.
Bruce Bennett
has been a plant taxonomist for over 30 years, residing in Yukon Territory since 1995 when he began research on invasive plants. He is the curator of B.A. Bennett Yukon (BABY) herbarium, the only active herbarium housed in northern Canada with over 11,000 specimens including almost all Yukon species. He is a member of the COSEWIC Vascular Plants Species Specialist Subcommittee, a regional reviewer for the Flora of North America, and works for the Yukon Department of Environment as the coordinator of the Yukon Conservation Data Centre.
Hans Gardfjell
is an Environmental monitoring specialist at the Swedish University of Agricultural sciences in Umeå, Sweden. He is a plant ecologist and data scientist working with large-scale monitoring programs focused on terrestrial vegetation and habitats.
Starri Heiðmarsson
is a lichenologist at the Icelandic Institute of Natural History. His research interest include the lichen funga of Iceland, taxonomy of Verrucariaceae and succession on nunataks in Icelandic glaciers.
Laerke Stewart
is an Arctic ecologist in the Department of Bioscience at Aarhus University.
Signe Normand
is an Associate Professor at the Aarhus University. She is a Macro- and Vegetation Ecologist dedicated to understanding patterns of species’ occurrence and biodiversity. The main goal of her research is to find answers to fundamental questions in ecology, but also to inform nature conservation about the impact of global change on biodiversity. Her current research is focused on understanding global change effects on Arctic ecosystems by dendroecological and drone-based investigations.
Footnotes
Publisher's Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Contributor Information
Anne D. Bjorkman, Email: annebj@gmail.com
Ingibjörg Svala Jónsdóttir, Email: isj@hi.is.
Bruce Bennett, Email: Bruce.Bennett@gov.yk.ca.
References
- Ackerman D, Griffin D, Hobbie SE, Finlay JC. Arctic shrub growth trajectories differ across soil moisture levels. Global Change Biology. 2017;23:4294–4302. doi: 10.1111/gcb.13677. [DOI] [PubMed] [Google Scholar]
- Alatalo JM, Totland Ø. Response to simulated climatic change in an alpine and sub-Arctic pollen-risk strategist, Silene acaulis. Global Change Biology. 1997;3:74–79. doi: 10.1111/j.1365-2486.1997.gcb133.x. [DOI] [Google Scholar]
- Arft AM, Walker MD, Gurevitch JEA, Alatalo JM, Bret-Harte MS, Dale M, Diemer M, Gugerli F, et al. Responses of tundra plants to experimental warming: Meta-analysis of the International Tundra Experiment. Ecological Monographs. 1999;69:491–511. doi: 10.1890/0012-9615(1999)069[0491:ROTPTE]2.0.CO;2. [DOI] [Google Scholar]
- Barrett RTS, Hollister RD, Oberbauer SF, Tweedie CE. Arctic plant responses to changing abiotic factors in northern Alaska. American Journal of Botany. 2015;102:2020–2031. doi: 10.3732/ajb.1400535. [DOI] [PubMed] [Google Scholar]
- Baruah G, Molau U, Bai Y, Alatalo JM. Community and species-specific responses of plant traits to 23 years of experimental warming across sub-Arctic tundra plant communities. Scientific Reports. 2017;7:2571. doi: 10.1038/s41598-017-02595-2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Berteaux D, Reale D, McAdam AG, Boutin S. Keeping pace with fast climate change: Can Arctic life count on evolution? Integrative and Comparative Biology. 2004;44:140–151. doi: 10.1093/icb/44.2.140. [DOI] [PubMed] [Google Scholar]
- Björk RG, Molau U. Ecology of alpine snowbeds and the impact of global change. Arctic, Antarctic, and Alpine Research. 2007;39:34–43. doi: 10.1657/1523-0430(2007)39[34:EOASAT]2.0.CO;2. [DOI] [Google Scholar]
- Bjorkman AD, Elmendorf SC, Beamish AL, Vellend M, Henry GHR. Contrasting effects of warming and increased snowfall on Arctic tundra plant phenology over the past two decades. Global Change Biology. 2015;21:4651–4661. doi: 10.1111/gcb.13051. [DOI] [PubMed] [Google Scholar]
- Bjorkman AD, Myers-Smith IH, Elmendorf SC, Normand S, Rüger N, Beck PSA, Blach-Overgaard A, Blok D, et al. Plant functional trait change across a warming tundra biome. Nature. 2018;562:57–62. doi: 10.1038/s41586-018-0563-7. [DOI] [PubMed] [Google Scholar]
- Bjorkman AD, Vellend M, Frei ER, Henry GHR. Climate adaptation is not enough: Warming does not facilitate success of southern tundra plant populations in the High Arctic. Global Change Biology. 2017;23:1540–1551. doi: 10.1111/gcb.13417. [DOI] [PubMed] [Google Scholar]
- Blok D, Elberling B, Michelsen A. Initial stages of tundra shrub litter decomposition may be accelerated by deeper winter snow but slowed down by spring warming. Ecosystems. 2016;19:155–169. doi: 10.1007/s10021-015-9924-3. [DOI] [Google Scholar]
- Blok D, Heijmans MMPD, Schaepman-Strub G, van Ruijven J, Parmentier FJW, Maximov TC, Berendse F. The cooling capacity of mosses: Controls on water and energy fluxes in a Siberian tundra site. Ecosystems. 2011;14:1055–1065. doi: 10.1007/s10021-011-9463-5. [DOI] [Google Scholar]
- Boelman NT, Gough L, Wingfield J, Goetz S, Asmus A, Chmura HE, Krause JS, Perez JH, et al. Greater shrub dominance alters breeding habitat and food resources for migratory songbirds in Alaskan Arctic tundra. Global Change Biology. 2015;21:1508–1520. doi: 10.1111/gcb.12761. [DOI] [PubMed] [Google Scholar]
- Boulanger-Lapointe N, Lévesque E, Boudreau S, Henry GHR, Schmidt NM. Population structure and dynamics of Arctic willow (Salix arctica) in the High Arctic. Journal of Biogeography. 2014;41:1967–1978. doi: 10.1111/jbi.12350. [DOI] [Google Scholar]
- Bråthen KA, Ravolainen VT, Stien A, Tveraa T, Ims RA. Rangifer management controls a climate-sensitive tundra state transition. Ecological Applications. 2017;27:2416–2427. doi: 10.1002/eap.1618. [DOI] [PubMed] [Google Scholar]
- CAFF . In: Arctic Biodiversity Assessment. Status and Trends in Arctic Biodiversity. Meltofte H, editor. Akureyri: Conservation of Arctic Flora and Fauna; 2013. [Google Scholar]
- Callaghan TV, Björn LO, Chernov Y, Chapin FS, III, Christensen TR, Huntley B, Ims RA, Johansson M, et al. Effects on the function of Arctic ecosystems in the short- and long-term perspectives. Ambio. 2004;33:448–458. doi: 10.1579/0044-7447-33.7.448. [DOI] [PubMed] [Google Scholar]
- Callaghan TV, Tweedie CE, Akerman J, Andrews C, Bergstedt J, Butler MG, Christensen TR, Cooley D, et al. Multi-decadal changes in tundra environments and ecosystems: Synthesis of the International Polar Year-Back to the Future Project (IPY-BTF) Ambio. 2011;40:705–716. doi: 10.1007/s13280-011-0179-8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chapin FS, III, Shaver GR. Physiological and growth responses of Arctic plants to a field experiment simulating climatic change. Ecology. 1996;77:822–840. doi: 10.2307/2265504. [DOI] [Google Scholar]
- Chapin FS, III, Shaver GR, Giblin AE, Nadelhoffer KJ, Laundre JA. Responses of Arctic tundra to experimental and observed changes in climate. Ecology. 1995;76:694–711. doi: 10.2307/1939337. [DOI] [Google Scholar]
- Chapin FS, III, Sturm M, Serreze MC, McFadden JP, Key JR, Lloyd AH, McGuire AD, Rupp TS, et al. Role of land-surface changes in Arctic summer warming. Science. 2005;310:657–660. doi: 10.1126/science.1117368. [DOI] [PubMed] [Google Scholar]
- Christensen, T., J. Payne, M. Doyle, G. Ibarguchi, J. Taylor, N.M. Schmidt, M. Gill, M. Svoboda, et al. 2013. The Arctic Terrestrial Biodiversity Monitoring Plan. CAFF Monitoring Series Report No. 7. Akureyri: CAFF International Secretariat. 10.9752/ts056.10-24-2013.
- Christiansen CT, Mack MC, DeMarco J, Grogan P. Decomposition of senesced leaf litter is faster in tall compared to low birch shrub tundra. Ecosystems. 2018;21:1564–1579. doi: 10.1007/s10021-018-0240-6. [DOI] [Google Scholar]
- Cleland EE, Allen JM, Crimmins TM, Dunne JA, Pau S, Travers SE, Zavaleta ES, Wolkovich EM. Phenological tracking enables positive species responses to climate change. Ecology. 2012;93:1765–1771. doi: 10.1890/11-1912.1. [DOI] [PubMed] [Google Scholar]
- Cooper EJ, Dullinger S, Semenchuk P. Late snowmelt delays plant development and results in lower reproductive success in the High Arctic. Plant Science. 2011;180:157–157. doi: 10.1016/j.plantsci.2010.09.005. [DOI] [PubMed] [Google Scholar]
- Cornelissen JHC, van Bodegom PM, Aerts R, Callaghan TV, van Logtestijn RSP, Alatalo JM, Chapin FS, III, Gerdol R, et al. Global negative vegetation feedback to climate warming responses of leaf litter decomposition rates in cold biomes. Ecology Letters. 2007;10:619–627. doi: 10.1111/j.1461-0248.2007.01051.x. [DOI] [PubMed] [Google Scholar]
- Crowther TW, Todd-Brown KEO, Rowe CW, Wieder WR, Carey JC, Machmuller MB, Snoek BL, Fang S, et al. Quantifying global soil carbon losses in response to warming. Nature. 2016;540:104–108. doi: 10.1038/nature20150. [DOI] [PubMed] [Google Scholar]
- Daniëls FJA, de Molenaar JG. Flora and vegetation of Tasiilaq, formerly Angmagssalik, southeast Greenland: A comparison of data between around 1900 and 2007. Ambio. 2011;40:650–659. doi: 10.1007/s13280-011-0171-3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Elmendorf SC, Henry GHR, Hollister RD, Björk RG, Bjorkman AD, Callaghan TV, Collier LS, Cooper EJ, et al. Global assessment of experimental climate warming on tundra vegetation: Heterogeneity over space and time. Ecology Letters. 2012;15:164–175. doi: 10.1111/j.1461-0248.2011.01716.x. [DOI] [PubMed] [Google Scholar]
- Elmendorf SC, Henry GHR, Hollister RD, Björk RG, Boulanger-Lapointe N, Cooper EJ, Cornelissen JHC, Day TA, et al. Plot-scale evidence of tundra vegetation change and links to recent summer warming. Nature Climate Change. 2012;2:453–457. doi: 10.1038/nclimate1465. [DOI] [Google Scholar]
- Elmendorf SC, Henry GHR, Hollister RD, Fosaa AM, Gould WA, Hermanutz L, Hofgaard A, Jónsdóttir II, et al. Experiment, monitoring, and gradient methods used to infer climate change effects on plant communities yield consistent patterns. Proceedings of the National Academy of Sciences of the United States of America. 2015;112:448–452. doi: 10.1073/pnas.1410088112. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Freedman B, Svoboda J. Alexandra Fiord—An ecological oasis in the polar desert. In: Svoboda J, Freedman B, editors. Ecology of a Polar Oasis. Toronto: Captus University Publications; 1994. [Google Scholar]
- Gauthier G, Bêty J, Cadieux M-C, Legagneux P, Doiron M, Chevallier C, Lai S, Tarroux A, et al. Long-term monitoring at multiple trophic levels suggests heterogeneity in responses to climate change in the Canadian Arctic tundra. Philosophical Transactions of the Royal Society of London. Series B, Biological Sciences. 2013;368:20120482. doi: 10.1098/rstb.2012.0482. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Graglia E, Jonasson S, Michelsen A, Schmidt IK. Effects of shading, nutrient application and warming on leaf growth and shoot densities of dwarf shrubs in two Arctic–alpine plant communities. Écoscience. 1997;4:191–198. doi: 10.1080/11956860.1997.11682395. [DOI] [Google Scholar]
- Graglia E, Jonasson S, Michelsen A, Schmidt IK, Havström M, Gustavsson L. Effects of environmental perturbations on abundance of sub-Arctic plants after three, seven and ten years of treatments. Ecography. 2001;24:5–12. doi: 10.1034/j.1600-0587.2001.240102.x. [DOI] [Google Scholar]
- Henry GHR, Harper KA, Chen W, Deslippe JR, Grant RF, Lafleur PM, Lévesque E, Siciliano SD, et al. Effects of observed and experimental climate change on terrestrial ecosystems in northern Canada: Results from the Canadian IPY Program. Climatic Change. 2012;115:207–234. doi: 10.1007/s10584-012-0587-1. [DOI] [Google Scholar]
- Henry GHR, Molau U. Tundra plants and climate change: The International Tundra Experiment (ITEX) Global Change Biology. 1997;3:1–9. doi: 10.1111/j.1365-2486.1997.gcb132.x. [DOI] [Google Scholar]
- Hertel AG, Bischof R, Langval O, Mysterud A, Kindberg J, Swenson JE, Zedrosser A. Berry production drives bottom-up effects on body mass and reproductive success in an omnivore. Oikos. 2017;127:197–207. doi: 10.1111/oik.04515. [DOI] [Google Scholar]
- Hill GB, Henry GHR. Responses of High Arctic wet sedge tundra to climate warming since 1980. Global Change Biology. 2011;17:276–287. doi: 10.1111/j.1365-2486.2010.02244.x. [DOI] [Google Scholar]
- Hobbie SE, Chapin FS., III The response of tundra plant biomass, aboveground production, nitrogen, and CO2 flux to experimental warming. Ecology. 1998;79:1526–1544. doi: 10.1890/0012-9658(1998)079[1526:trotpb]2.0.co;2. [DOI] [Google Scholar]
- Hollister RD, May JL, Kremers KS, Tweedie CE, Oberbauer SF, Liebig JA, Botting TF, Barrett RT, et al. Warming experiments elucidate the drivers of observed directional changes in tundra vegetation. Ecology and Evolution. 2015;5:1881–1895. doi: 10.1002/ece3.1499. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hollister RD, Webber PJ. Biotic validation of small open-top chambers in a tundra ecosystem. Global Change Biology. 2000;6:835–842. doi: 10.1046/j.1365-2486.2000.00363.x. [DOI] [Google Scholar]
- Høye TT, Post ES, Meltofte H, Schmidt NM, Forchhammer MC. Rapid advancement of spring in the High Arctic. Current Biology. 2007;17:R449–R451. doi: 10.1016/j.cub.2007.04.047. [DOI] [PubMed] [Google Scholar]
- Høye TT, Post E, Schmidt NM, Trøjelsgaard K, Forchhammer MC. Shorter flowering seasons and declining abundance of flower visitors in a warmer Arctic. Nature Climate Change. 2013;3:759–763. doi: 10.1038/nclimate1909. [DOI] [Google Scholar]
- Hudson JMG, Henry GHR. Increased plant biomass in a High Arctic heath community from 1981 to 2008. Ecology. 2009;90:2657–2663. doi: 10.1890/09-0102.1. [DOI] [PubMed] [Google Scholar]
- Hudson JMG, Henry GHR. High Arctic plant community resists 15 years of experimental warming. Journal of Ecology. 2010;98:1035–1041. doi: 10.1111/j.1365-2745.2010.01690.x. [DOI] [Google Scholar]
- Hudson JMG, Henry GHR, Cornwell WK. Taller and larger: Shifts in Arctic tundra leaf traits after 16 years of experimental warming. Global Change Biology. 2011;17:1013–1021. doi: 10.1111/j.1365-2486.2010.02294.x. [DOI] [Google Scholar]
- Iler AM, Høye TT, Inouye DW, Schmidt NM. Nonlinear flowering responses to climate: Are species approaching their limits of phenological change? Philosophical Transactions of the Royal Society B: Biological Sciences. 2013;368:20120489. doi: 10.1098/rstb.2012.0489. [DOI] [PMC free article] [PubMed] [Google Scholar]
- IPCC. 2013. Climate Change 2013: The Physical Science Basis. Contribution of Working Group I to the Fifth Assessment Report of the Intergovernmental Panel on Climate Change, eds. T.F. Stocker, D. Quin, G.-K. Plattner, M. Tignor, S.K. Allen, J. Boschung, A. Nauels, Y. Xia, et al. Cambridge: Cambridge University Press.
- Jägerbrand AK, Alatalo JM, Chrimes D, Molau U. Plant community responses to 5 years of simulated climate change in meadow and heath ecosystems at a sub-Arctic–alpine site. Oecologia. 2009;161:601–610. doi: 10.1007/s00442-009-1392-z. [DOI] [PubMed] [Google Scholar]
- Jandt R, Joly K, Meyers CR, Racine C. Slow recovery of lichen on burned Caribou Winter Range in Alaska Tundra: Potential influences of climate warming and other disturbance factors. Arctic, Antarctic, and Alpine Research. 2008;40:89–95. doi: 10.1657/1523-0430(06-122)[jandt]2.0.co;2. [DOI] [Google Scholar]
- Joly K, Jandt RR, Meyers CR, Cole MJ. Changes in vegetative cover on Western Arctic Herd winter range from 1981 to 2005: Potential effects of grazing and climate change. Rangifer. 2007;17:199–207. doi: 10.7557/2.27.4.345. [DOI] [Google Scholar]
- Jonasson S, Michelsen A, Schmidt IK, Nielsen EV. Responses in microbes and plants to changed temperature, nutrient, and light regimes in the Arctic. Ecology. 1999;80:1828–1843. doi: 10.1890/0012-9658(1999)080[1828:rimapt]2.0.co;2. [DOI] [Google Scholar]
- Jones MH, Bay C, Nordenhäll U. Effects of experimental warming on Arctic willows (Salix spp.): A comparison of responses from the Canadian High Arctic, Alaskan Arctic, and Swedish Sub-Arctic. Global Change Biology. 1997;3:55–60. doi: 10.1111/j.1365-2486.1997.gcb135.x. [DOI] [Google Scholar]
- Jónsdóttir IS, Magnússon B, Gudmundsson J, Elmarsdottir A, Hjartarson H. Variable sensitivity of plant communities in Iceland to experimental warming. Global Change Biology. 2005;11:553–563. doi: 10.1111/j.1365-2486.2005.00928.x. [DOI] [Google Scholar]
- Jorgenson JC, Raynolds MK, Reynolds JH, Benson A-M. Twenty-five year record of changes in plant cover on tundra of northeastern Alaska. Arctic, Antarctic, and Alpine Research. 2015;47:785–806. doi: 10.1657/aaar0014-097. [DOI] [Google Scholar]
- Kerby Jeffrey, Post Eric. Capital and income breeding traits differentiate trophic match–mismatch dynamics in large herbivores. Philosophical Transactions of the Royal Society B: Biological Sciences. 2013;368(1624):20120484. doi: 10.1098/rstb.2012.0484. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Klady RA, Henry GHR, Lemay V. Changes in High Arctic tundra plant reproduction in response to long-term experimental warming. Global Change Biology. 2011;17:1611–1624. doi: 10.1111/j.1365-2486.2010.02319.x. [DOI] [Google Scholar]
- Koven CD, Ringeval B, Friedlingstein P, Ciais P, Cadule P, Khvorostyanov D, Krinner G, Tarnocai C. Permafrost carbon-climate feedbacks accelerate global warming. Proceedings of the National Academy of Sciences of the United States of America. 2011;108:14769–14774. doi: 10.1073/pnas.1103910108. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kummerow J. Phenology, resource allocation, and growth of Arctic vascular plants. In: Chapin FS III, Jefferies R, Reynolds J, Shaver GR, Svoboda J, editors. Arctic Ecosystems in a Changing Climate: An Ecophysiological Perspective. San Diego: Academic; 1992. pp. 193–211. [Google Scholar]
- Lang SI, Cornelissen JHC, Shaver GR, Ahrens M, Callaghan TV, Molau U, Ter Braak CJF, Hölzer A, et al. Arctic warming on two continents has consistent negative effects on lichen diversity and mixed effects on bryophyte diversity. Global Change Biology. 2012;18:1096–1107. doi: 10.1111/j.1365-2486.2011.02570.x. [DOI] [Google Scholar]
- Marchand FL, Nijs I, Heuer M, Mertens S, Kockelbergh F, Pontailler J-Y, Impens I, Beyens L. Climate warming postpones senescence in High Arctic tundra. Arctic, Antarctic, and Alpine Research. 2004;36:390–394. doi: 10.1657/1523-0430(2004)036[0390:CWPSIH]2.0.CO;2. [DOI] [Google Scholar]
- Marion GM, Henry GHR, Freckman DW, Johnstone J, Jones G, Jones MH, Lévesque E, Molau U, et al. Open‐top designs for manipulating field temperature in high‐latitude ecosystems. Global Change Biology. 1997;3:20–32. doi: 10.1111/j.1365-2486.1997.gcb136.x. [DOI] [Google Scholar]
- Martin AC, Jeffers ES, Petrokofsky G, Myers-Smith I, Macias-Fauria M. Shrub growth and expansion in the Arctic tundra: An assessment of controlling factors using an evidence-based approach. Environmental Research Letters. 2017;12:085007–085014. doi: 10.1088/1748-9326/aa7989. [DOI] [Google Scholar]
- McKinnon L, Picotin M, Bolduc E, Juillet C, Bêty J. Timing of breeding, peak food availability, and effects of mismatch on chick growth in birds nesting in the High Arctic. Canadian Journal of Zoology. 2012;90:961–971. doi: 10.1139/z2012-064. [DOI] [Google Scholar]
- Molau U. Long-term impacts of observed and induced climate change on tussock tundra near its southern limit in northern Sweden. Plant Ecology and Diversity. 2010;3:29–34. doi: 10.1080/17550874.2010.487548. [DOI] [Google Scholar]
- Molau U, Mølgaard P. International Tundra Experiment (ITEX) Manual. 2. Copenhagen: Danish Polar Center; 1996. [Google Scholar]
- Muc M, Freedman B, Svoboda J. Vascular plant communities of a polar oasis at Alexandra Fiord (79 N), Ellesmere Island, Canada. Canadian Journal of Botany. 1989;67:1126–1136. doi: 10.1139/b89-147. [DOI] [Google Scholar]
- Myers-Smith IH, Elmendorf SC, Beck PSA, Wilmking M, Hallinger M, Blok D, Tape KD, Rayback SA, et al. Climate sensitivity of shrub growth across the tundra biome. Nature Climate Change. 2015;5:887–891. doi: 10.1038/nclimate2697. [DOI] [Google Scholar]
- Myers-Smith IH, Forbes BC, Wilmking M, Hallinger M, Lantz T, Blok D, Tape KD, Macias-Fauria M, et al. Shrub expansion in tundra ecosystems: Dynamics, impacts and research priorities. Environmental Research Letters. 2011;6:045509. doi: 10.1088/1748-9326/6/4/045509. [DOI] [Google Scholar]
- Myers-Smith IH, Hik DS. Shrub canopies influence soil temperatures but not nutrient dynamics: An experimental test of tundra snow–shrub interactions. Ecology and Evolution. 2013;3:3683–3700. doi: 10.1002/ece3.710. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Myers-Smith IH, Hik DS, Kennedy C, Cooley D, Johnstone JF, Kenney AJ, Krebs CJ. Expansion of canopy-forming willows over the twentieth century on Herschel Island, Yukon Territory, Canada. Ambio. 2011;40:610–623. doi: 10.1007/s13280-011-0168-y. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Myers-Smith IH, Grabowski MM, Thomas H, Angers-Blondin S, Daskalova G, Bjorkman AD, Cunliffe AM, Assmann JJ. Eighteen years of ecological monitoring reveals multiple lines of evidence for tundra vegetation change. Ecological Monographs. 2019 doi: 10.1002/ecm.1351. [DOI] [Google Scholar]
- Natali SM, Schuur EAG, Rubin RL. Increased plant productivity in Alaskan tundra as a result of experimental warming of soil and permafrost. Journal of Ecology. 2012;100:488–498. doi: 10.1111/j.1365-2745.2011.01925.x. [DOI] [Google Scholar]
- Oberbauer SF, Elmendorf SC, Troxler TG, Hollister RD, Rocha AV, Bret-Harte MS, Dawes MA, Fosaa AM, et al. Phenological response of tundra plants to background climate variation tested using the International Tundra Experiment. Philosophical Transactions of the Royal Society, Series B: Biological Sciences. 2013 doi: 10.1098/rstb.2012.0481. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Parmesan C. Ecological and evolutionary responses to recent climate change. Annual Review of Ecology, Evolution and Systematics. 2006;37:637–669. doi: 10.1146/annurev.ecolsys.37.091305.110100. [DOI] [Google Scholar]
- Pattison RR, Jorgenson JC, Reynolds MK, Welker JM. Trends in NDVI and tundra community composition in the Arctic of NE Alaska between 1984 and 2009. Ecosystems. 2015 doi: 10.1007/s10021-015-9858-9. [DOI] [Google Scholar]
- Pearson RG, Phillips SJ, Loranty MM, Beck PSA, Damoulas T, Knight SJ, Goetz SJ. Shifts in Arctic vegetation and associated feedbacks under climate change. Nature Climate Change. 2013;3:673–677. doi: 10.1038/nclimate1858. [DOI] [Google Scholar]
- Petrenko CL, Bradley-Cook J, Lacroix EM, Friedland AJ, Virginia RA. Comparison of carbon and nitrogen storage in mineral soils of graminoid and shrub tundra sites, western Greenland. Arctic Science. 2016;2:165–182. doi: 10.1139/as-2015-0023. [DOI] [Google Scholar]
- Post E, Forchhammer MC, Bret-Harte MS, Callaghan TV, Christensen TR, Elberling B, Fox AD, Gilg O, et al. Ecological dynamics across the Arctic associated with recent climate change. Science. 2009;325:1355–1358. doi: 10.1126/science.1173113. [DOI] [PubMed] [Google Scholar]
- Post E, Pedersen C. Opposing plant community responses to warming with and without herbivores. Proceedings of the National Academy of Sciences of the United States of America. 2008;105:12353–12358. doi: 10.1073/pnas.0802421105. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Post E, Stenseth NC. Climatic variability, plant phenology, and northern ungulates. Ecology. 1999;80:1322–1339. doi: 10.1890/0012-9658(1999)080[1322:cvppan]2.0.co;2. [DOI] [Google Scholar]
- Prevéy J, Vellend M, Rüger N, Hollister RD, Bjorkman AD, Myers-Smith IH, Elmendorf SC, Clark K, et al. Greater temperature sensitivity of plant phenology at colder sites: Implications for convergence across northern latitudes. Global Change Biology. 2017;23:2660–2671. doi: 10.1111/gcb.13619. [DOI] [PubMed] [Google Scholar]
- Prevéy JS, Rixen C, Rüger N, Høye TT, Bjorkman AD, Myers-Smith IH, Elmendorf SC, Ashton IW, et al. Warming shortens flowering seasons of tundra plant communities. Nature Ecology and Evolution. 2019;3:45–52. doi: 10.1038/s41559-018-0745-6. [DOI] [PubMed] [Google Scholar]
- Raich JW, Potter CS. Global patterns of carbon dioxide emissions from soils. Global Biogeochemical Cycles. 1995;9:23–36. doi: 10.1029/94gb02723. [DOI] [Google Scholar]
- Richardson SJ, Press MC, Parsons AN, Hartley SE. How do nutrients and warming impact on plant communities and their insect herbivores? A 9-year study from a sub-Arctic heath. Journal of Ecology. 2002;90:544–556. doi: 10.1046/j.1365-2745.2002.00681.x. [DOI] [Google Scholar]
- Robinson CH, Wookey PA, Lee JA, Callaghan TV, Press MC. Plant community responses to simulated environmental change at a High Arctic polar semi-desert. Ecology. 1998;79:856. doi: 10.2307/176585. [DOI] [Google Scholar]
- Rundqvist S, Hedenås H, Sandström A, Emanuelsson U, Eriksson H, Jonasson C, Callaghan TV. Tree and shrub expansion over the past 34 years at the tree-line near Abisko, Sweden. Ambio. 2011;40:683–692. doi: 10.1007/s13280-011-0174-0. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Schuur EAG, McGuire AD, Schädel C, Grosse G, Harden JW, Hayes DJ, Hugelius G, Koven CD, et al. Climate change and the permafrost carbon feedback. Nature. 2015;520:171–179. doi: 10.1038/nature14338. [DOI] [PubMed] [Google Scholar]
- Starr G, Oberbauer SF, Pop EW. Effects of lengthened growing season and soil warming on the phenology and physiology of Polygonum bistorta. Global Change Biology. 2000;6:357–369. doi: 10.1046/j.1365-2486.2000.00316.x. [DOI] [Google Scholar]
- Steinbauer MJ, Grytnes J-A, Jurasinski G, Kulonen A, Lenoir J, Pauli H, Rixen C, Winkler M, et al. Accelerated increase in plant species richness on mountain summits is linked to warming. Nature. 2018;556:231–234. doi: 10.1038/s41586-018-0005-6. [DOI] [PubMed] [Google Scholar]
- Stenström A, Jónsdóttir IS. Responses of the clonal sedge, Carex bigelowii, to two seasons of simulated climate change. Global Change Biology. 1997;3:89–96. doi: 10.1111/j.1365-2486.1997.gcb134.x. [DOI] [Google Scholar]
- Stern GA, Gaden A. From Science to Policy in the Western and Central Canadian Arctic: An Integrated Regional Impact Study (IRIS) of Climate Change and Modernization. Quebec City: ArcticNet; 2015. [Google Scholar]
- Sturm M, Douglas T. Changing snow and shrub conditions affect albedo with global implications. Journal of Geophysical Research. 2005;110:G01004. doi: 10.1029/2005jg000013. [DOI] [Google Scholar]
- Sturm M, Racine C, Tape K. Increasing shrub abundance in the Arctic. Nature. 2001;411:546–547. doi: 10.1038/35079180. [DOI] [PubMed] [Google Scholar]
- Tolvanen A, Henry GHR. Responses of carbon and nitrogen concentrations in High Arctic plants to experimental warming. Canadian Journal of Botany. 2001;79:711–718. doi: 10.1139/b01-052. [DOI] [Google Scholar]
- Tømmervik H, Johansen B, Tombre I, Thannheiser D, Høgda KA, Gaare E, Wielgolaski FE. Vegetation changes in the Nordic Mountain birch forest: The influence of grazing and climate change. Arctic, Antarctic, and Alpine Research. 2004;36:323–332. doi: 10.1657/1523-0430(2004)036[0323:vcitnm]2.0.co;2. [DOI] [Google Scholar]
- van Altena C, van Logtestijn RSP, Cornwell WK, Cornelissen JHC. Species composition and fire: Non-additive mixture effects on ground fuel flammability. Frontiers in Plant Science. 2012;3:63. doi: 10.3389/fpls.2012.00063. [DOI] [PMC free article] [PubMed] [Google Scholar]
- van Gestel N, Shi Z, van Groenigen KJ, Osenberg CW, Andresen LC, Dukes JS, Hovenden MJ, Luo Y, et al. Predicting soil carbon loss with warming. Nature. 2018;554:E4–E5. doi: 10.1038/nature25745. [DOI] [PubMed] [Google Scholar]
- Villarreal S, Hollister RD, Johnson DR, Lara MJ, Webber PJ, Tweedie CE. Tundra vegetation change near Barrow, Alaska (1972–2010) Environmental Research Letters. 2012;7:015508–015511. doi: 10.1088/1748-9326/7/1/015508. [DOI] [Google Scholar]
- Vowles Tage, Lovehav Cajsa, Molau Ulf, Björk Robert G. Contrasting impacts of reindeer grazing in two tundra grasslands. Environmental Research Letters. 2017;12(3):034018. doi: 10.1088/1748-9326/aa62af. [DOI] [Google Scholar]
- Walker MD, Wahren CH, Hollister RD, Ahlquist LE, Alatalo JM, Bret-Harte MS, Calef MP, Callaghan TV, et al. Plant community responses to experimental warming across the tundra biome. Proceedings of the National Academy of Sciences of the United States of America. 2006;103:1342–1346. doi: 10.1073/pnas.0503198103. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wang P, Limpens J, Mommer L, van Ruijven J, Nauta AL, Berendse F, Schaepman-Strub G, Blok D, et al. Above- and below-ground responses of four tundra plant functional types to deep soil heating and surface soil fertilization. Edited by Etienne Laliberté. Journal of Ecology. 2017;105:947–957. doi: 10.1111/1365-2745.12718. [DOI] [Google Scholar]
- Welker JM, Molau U, Parsons AN, Robinson CH, Wookey PA. Responses of Dryas octopetala to ITEX environmental manipulations: A synthesis with circumpolar comparisons. Global Change Biology. 1997;3:61–73. doi: 10.1111/j.1365-2486.1997.gcb143.x. [DOI] [Google Scholar]
- Weller G, Bush E, Callaghan TV, Corell R, Fox S, Furgal C, Hoel AH, Huntington H, et al. Summary and synthesis of the ACIA. In: Hassol SJ, et al., editors. Impacts of a Warming Arctic: Arctic Climate Impact Assessment. Cambridge: Cambridge University Press; 2004. pp. 990–1020. [Google Scholar]
- Wheeler HC, Høye TT, Schmidt NM, Svenning J-C, Forchhammer MC. Phenological mismatch with abiotic conditions—Implications for flowering in Arctic plants. Ecology. 2015;96:775–787. doi: 10.1890/14-0338.1. [DOI] [PubMed] [Google Scholar]
- Wilson SD, Nilsson C. Arctic alpine vegetation change over 20 years. Global Change Biology. 2009;15:1676–1684. doi: 10.1111/j.1365-2486.2009.01896.x. [DOI] [Google Scholar]
- Wookey PA, Parsons AN, Welker JM, Potter JA, Callaghan TV, Lee JA, Press MC. Comparative responses of phenology and reproductive development to simulated environmental change in sub-Arctic and High Arctic plants. Oikos. 1993;67:490–502. doi: 10.2307/3545361. [DOI] [Google Scholar]
- Zamin TJ, Bret-Harte MS, Grogan P. Evergreen shrubs dominate responses to experimental summer warming and fertilization in Canadian mesic low Arctic tundra. Journal of Ecology. 2014;102:749–766. doi: 10.1111/1365-2745.12237. [DOI] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.