Abstract
The peregrine falcon (Falco peregrinus) and the gyrfalcon (Falco rusticolus) are top avian predators of Arctic ecosystems. Although existing monitoring efforts are well established for both species, collaboration of activities among Arctic scientists actively involved in research of large falcons in the Nearctic and Palearctic has been poorly coordinated. Here we provide the first overview of Arctic falcon monitoring sites, present trends for long-term occupancy and productivity, and summarize information describing abundance, distribution, phenology, and health of the two species. We summarize data for 24 falcon monitoring sites across the Arctic, and identify gaps in coverage for eastern Russia, the Arctic Archipelago of Canada, and East Greenland. Our results indicate that peregrine falcon and gyrfalcon populations are generally stable, and assuming that these patterns hold beyond the temporal and spatial extents of the monitoring sites, it is reasonable to suggest that breeding populations at broader scales are similarly stable. We have highlighted several challenges that preclude direct comparisons of Focal Ecosystem Components (FEC) attributes among monitoring sites, and we acknowledge that methodological problems cannot be corrected retrospectively, but could be accounted for in future monitoring. Despite these drawbacks, ample opportunity exists to establish a coordinated monitoring program for Arctic-nesting raptor species that supports CBMP goals.
Electronic supplementary material
The online version of this article (10.1007/s13280-019-01300-z) contains supplementary material, which is available to authorized users.
Keywords: Arctic, CBMP, Falco peregrinus, Falco rusticolus, Long-term trends, Occupancy, Productivity
Introduction
The Arctic Council’s Biodiversity Working Group developed a pan-Arctic biodiversity monitoring plan to detect and report on long-term changes in Arctic biodiversity (Christensen et al. 2018). The plan recognizes a suite of Focal Ecosystem Components (FECs) and associated FEC attributes (e.g., abundance, distribution, demography, phenology, health) that are considered to be suitable indicators for ecological monitoring at the scale of the Arctic. The Terrestrial Expert Monitoring Group (TEMG) of the Circumpolar Biodiversity Monitoring Programme (CBMP) identified the peregrine falcon (Falco peregrinus) and the gyrfalcon (Falco rusticolus) as FECs (Christensen et al. 2018) due to their role as top predators within Arctic food webs. Although location-specific surveys vary in spatial and temporal extent, both species have received considerable long-term monitoring effort (Fig. 1). The work presented here is a synopsis of Arctic-wide monitoring of peregrine falcons and gyrfalcons, and addresses the need to integrate the state of knowledge for these species within the context of CBMP monitoring priorities. The objectives of this study were to (1) identify long-term monitoring sites; (2) acquire, share, and collate attribute-specific data for Arctic-based peregrine falcon and gyrfalcon projects; (3) present empirically derived trends associated with FEC attribute demography; (4) summarize information for other essential and recommended CBMP FEC attributes; (5) identify challenges associated with disparate application of survey methods that currently hamper comparisons among research groups, and (6) suggest minimum standards for future coordinated monitoring.
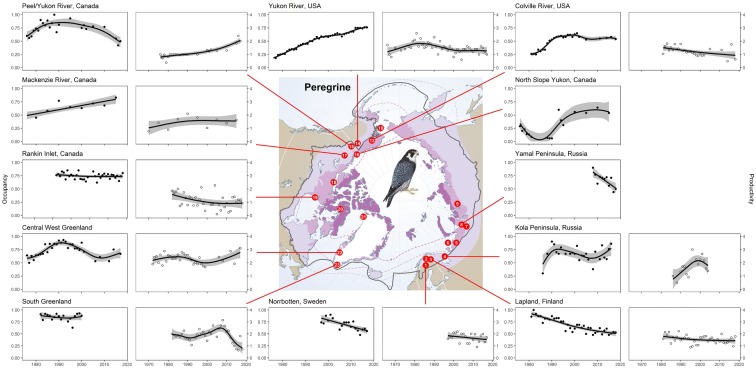
Fig. 1.
Peregrine falcon (a); gyrfalcon (b, white morph); occupancy survey conducted by snowmobile in Nunavut, Canada (c); gyrfalcon occupancy survey conducted on skis in Finnmark, Norway (d); typical breeding habitat in the peat bogs of Norrbotten, Sweden with helicopter assistance to access nests to estimate productivity (e); gyrfalcon survey in typical habitat in Iceland (f); productivity survey conducted by boat in Low Arctic, Nunavut, Canada (g); continuous monitoring of gyrfalcon productivity using motion sensitive scouting camera (upper left) on the Seward Peninsula, Alaska, United States (h); productivity survey in typical breeding habitat in Low Arctic South Greenland requires climbing equipment to access nests (i); typical sandy cliff breeding habitat for peregrine falcons on the Yamal Peninsula, Russia (j); photos: E. Hedlin, K. Falk, A. Franke, K. Johansen, P. Lindberg, D. Bergman, B. Robinson, S. Møller, and D. Nowak
Natural history of Arctic falcons as ecosystem components
The peregrine falcon is a medium-sized raptor with long, pointed wings, dark hood and face, with distinct dark malar stripe, slate gray back, and barred belly, legs, and tail (see Fig. 1). Compared to the peregrine falcon, the gyrfalcon is larger, with more rounded and broader wings, and longer tail (see Fig. 1). Gyrfalcons have three main color morphs: black, gray, and white. Both species exhibit reverse sex-dimorphism (Ferguson-Lees et al. 2001). Both species are highly territorial, and nesting territories are generally considered to be associated with rugged terrain (particularly coastal, lake-shore and river-side cliffs, and rock outcrops), but peregrine falcons also utilize thermokarst bluffs in northern Alaska (Ritchie 2014), and commonly nest on the ground in the peat bogs of Fennoscandia (Lindberg et al. 1988). Both species occasionally nest in trees where old stick nests (typically built by common ravens Corvus corax or rough-legged buzzards Buteo lagopus) are available. Nesting territories are typically regularly spaced, particularly in areas where breeding habitat and prey availability are uniformly distributed (Newton 1988). Although they share some similarities in ecology and life history attributes (e.g., reproductive life span, number of offspring, survivorship), peregrine falcons and gyrfalcons differ with regard to migratory behavior, foraging and breeding ecology, and degree of specialization. To varying degrees, these factors are influenced by natural disturbance regimes, such as cycles in prey abundance (Barichello and Mossop 2011; Koskimies 2011; Nielsen 2011), or potentially from anthropogenic disturbance (Tucker et al. 2019). Both species, however, are exposed to the effects of climate change, including shifts in weather regimes that can affect breeding phenology and success directly (Franke et al. 2010; Bente 2011; Anctil et al. 2014; Lamarre et al. 2017), or indirectly from habitat loss mediated through shrubification (Johansen and Østlyngen 2011; Wheeler et al. 2018), and changes in food supply (Newton 1979; Poole 1987; Barichello and Mossop 2011; Nielsen 2011).
In the Palearctic, the distribution of the nominate peregrine falcon F. p. peregrinus includes the Sub-Arctic and Low Arctic of northernmost Fennoscandia and westernmost Russia; birds from this population are medium-range migrants wintering in western and southern Europe, or northern Africa (Ganusevich et al. 2004; Lindberg 2008; Saurola et al. 2013). The Siberian peregrine falcon F. p. calidus (hereafter referred to as calidus) is distributed throughout tundra areas of Russia to the Bering Strait—it is considered the Palearctic equivalent to the Arctic peregrine falcon F. p. tundrius (White 1968) in the Nearctic (hereafter referred to as tundrius).
The peregrine falcon is typically considered to be a generalist predator that predominantly consumes avian prey, but also regularly consumes small mammals where they are available (Lindberg 1983; Court et al. 1988; Bradley and Oliphant 1991; Ganusevich 2006; Dawson et al. 2011). For gyrfalcons, Lagopus spp. (L. lagopus, L. muta and L. leucura) hereafter referred to as ptarmigan (Fuglei et al. 2019) unless specified otherwise are invariably cited as critically important prey, particularly in late winter and early spring (Booms et al. 2008; Barichello and Mossop 2011; Koskimies 2011; Nielsen 2011; Robinson et al. 2019). In some parts of the range, seabirds, Arctic ground squirrels (Urocitellus parryii), Arctic hares (Lepus arcticus), and passerines are also important gyrfalcon prey, especially in the breeding season (Poole 1987).
Methods
Identification of monitoring sites and FEC attributes
An informal network of biologists (Arctic Falcon Specialist Group; AFSG) with a research focus on Arctic-breeding peregrine falcons and gyrfalcons was established on the basis of two CBMP-TEMG workshops (Sweden, 2016, and Iceland, 2017), two gyrfalcon workshops hosted by the Icelandic Institute of Natural History and the Raptor Group Finnmark in cooperation with the Arctic University of Norway (Tromsø 2014 and 2015, respectively), and by inviting known experts to participate in the network. The AFSG identified monitoring sites throughout the circumpolar Arctic, and used web-based applications to acquire, share, and collate data that describe study area characteristics (Tables S1 and S2), as well as details describing survey effort and design, number of surveys completed annually, timing of surveys, type of observation platform, and sampling design (Tables 1 and 2).
Table 1.
Monitoring period (years in bold = ongoing monitoring), sampling regime, and within season survey effort for peregrine falcons
| Site IDa | Monitoring site | Occupancy based on presence ofb | Survey periodc | Sampling regimed | Platform | Pre-laying | Incubation | Brood rearing | Occupancy estimatef | |
|---|---|---|---|---|---|---|---|---|---|---|
| 1 | Norrbotten (Se) | Adult(s), egg/young | 1972–2018 | Stratified | Partial | Ground, air | ✓ | (X + Y + Z + G)/N | ||
| 2 | Finnmark (No) | Adult(s), egg/young, other evidence | 1987–2018 | Random | Full | Ground, air | ✓ | ✓ | ✓ | (X + Y + Z + G)/N |
| 3 | Lapland (Fi) | Adult(s), egg/young | 1981–2018 | Stratified | Partial | Ground, air | ✓ | (X + Y + Z + G)/N | ||
| 4 | Kola Peninsula (Ru) | Adult(s), egg/young, other evidence | 1980–2018 | Census | Partial | Ground | ✓ | (X + Y + Z + G)/N | ||
| 5 | Nenetskiy Ridge (Ru) | Adult(s), egg/young | 2009–2014 | Stratified | Partial | Ground | ✓ | (X + Y)/N | ||
| 6 | Kolguev Island (Ru) | adult(s), egg/young | 2013–2018 | Stratified | Partial | Ground | ✓ | (X + Y)/N | ||
| 7 | Schuchya River (Ru) | Adult(s), egg/young | 1988–2016 | Census | Partial | Ground | ✓ | |||
| 8 | Yamal (Ru) | Adult(s), egg/young | 1999–2018 | Census | Partial | Ground | ✓ | ✓ | (X + Y)/N | |
| 9 | Taimyr (Ru) | Adult(s), egg/young | 2000–2013e | Census | Partial | Ground | ✓ | |||
| 10 | Seward Peninsula (US) | Adult(s), egg/young | 1998–2018 | Census | Fulle | Air, ground | ✓e | ✓e | (X + Y + Z + G)/N | |
| 13 | Colville River (US) | Adult(s), egg/young | 1952–2018e | Census | Full | Ground | ✓ | ✓ | (X + Y + Z + G)/N | |
| 14 | Yukon River (US) | Adult(s), egg/young | 1966–2015 | Census | Full | Ground | ✓ | ✓ | ||
| 15 | Yukon/Peel Rivers (Ca) | Adult(s), egg/young | 1970–2018 | Census | Partial | Ground | ✓ | (X + Y + Z + G)/N | ||
| 16 | North Slope Yukon (Ca) | Adult(s), egg/young | 1973–2015 | Census | Partial | Ground | ✓ | (X + Y + Z + G)/N | ||
| 17 | Mackenzie River (Ca) | Adult(s), egg/young | 1970–2015e | Census | Partial | Air, ground | ✓ | |||
| 18 | Hope Bay (Ca) | Adult(s), egg/young | 1983–1986 | Census | Full | Air, ground | ✓ | ✓ | ||
| 19 | Rankin Inlet (Ca) | Adult(s), egg/young | 1982–2018 | Census | Full | Ground | ✓ | ✓ | ✓ | (X + Y + Z + G)/N |
| 20 | Mary River (Ca) | Adult(s), egg/young | 2011–2018 | Census | Partial | Air, ground | ✓ | ✓ | ✓ | (X + Y + Z + G)/N |
| 21 | Northwest Greenland | Adult(s), egg/young | 1993–2018 | Census | Partial | Air, ground | ✓ | (X + Y + Z + G)/N | ||
| 22 | Central West Greenland | Adult(s), egg/young | 1972–2017e | Census | Partial | Air, ground | ✓ | (X + Y + Z + G)/N | ||
| 23 | South Greenland | Adult(s), egg/young | 1981–2018 | Stratified | Full/partial | Ground | ✓e | ✓ | (X + Y + Z + G)/N | |
aSee Fig. 2
bOther evidence—egg shell fragments, molted feathers or down, recent excrement, fresh prey remains
cBold indicates monitoring is ongoing
dCensus—all known nesting territories checked systematically; stratified—sub-set of all known nesting territories checked systematically; random—random selection of known nesting territories checked; full—known nesting territories receive multiple visits per breeding season; partial—single visits, or only in brood-rearing period
eDiscontinuous
fSensu Nielsen (2011): X = successful nest, nesting attempt, or pair—one in which at least one young reaches the minimum acceptable age for assessing success (Steenhof et al. 2017), Y = unsuccessful nest, nesting attempt, or pair—a laying pair that failed before nestlings reached the minimum acceptable age for assessing success, Z = non-laying Pair—a mated pair that fails to lay at least 1 egg in a given year (Steenhof et al. 2017), G = single non-laying individual—a non-mated individual evidenced by an absence of territorial behavior, or reproductive-related activity, N = count of known nesting territories surveyed
Table 2.
Monitoring period (years in bold = ongoing monitoring), sampling regime, and within season survey effort for gyrfalcons
| Site IDa | Monitoring site | Occupancy based on presence ofb | Survey periodc | Sampling regimed | Platform | Pre-laying | Incubation | Brood rearing | Occupancy estimatef | |
|---|---|---|---|---|---|---|---|---|---|---|
| 1 | Norrbotten (Se) | Adult(s), egg/young | 1997–2018 | Stratified | Full/partial | Ground, air | ✓e | ✓ | (X + Y + Z + G)/N | |
| 2 | Finnmark (No) | Adult(s), egg/young, other evidence | 2000–2018 | Census | Full | Air, ground | ✓ | ✓ | (X + Y + Z + G)/N | |
| 3 | Lapland (Fi) | Adult(s), egg/young, other evidence | 1990–2018 | Census | Full | Ground, air | ✓ | ✓ | (X + Y + Z + G)/N | |
| 4 | Kola Peninsula (Ru) | Adult(s), egg/young, other evidence | 2007–2018 | Census | Partial | Ground | ✓ | (X + Y + Z + G)/N | ||
| 7 | Schuchya River (Ru) | Adult(s), egg/young | 2006–2016 | Census | Partial | Ground | ✓ | (X + Y)/N | ||
| 10 | Seward Peninsula (US) | Adult(s), egg/young | 1998–2018 | Census | Fulle | Air, ground | ✓e | ✓ | (X + Y + Z + G)/N | |
| 11 | Denali Nat. Park (US) | Adult(s), egg/young | 2006–2018 | Census | Full | Air, ground | ✓ | ✓ | ||
| 12 | South Yukon (Ca) | Adult(s), egg/young | 1981–2018 | Random | Partial | Air | ✓ | (X + Y + Z + G)/N | ||
| 13 | Colville River (US) | Adult(s), egg/young | 1981–2018 | Census | Partial | Ground | ✓ | (X + Y + Z + G)/N | ||
| 16 | North Slope Yukon (Ca) | Adult(s), egg/young | 1974–2015 | Random | Partial | Air | ✓ | (X + Y + Z + G)/N | ||
| 18 | Hope Bay (Ca) | Adult(s), egg/young | 1982–1991 | Census | Partial | Ground | ✓ | (X + Y + Z + G)/N | ||
| 21 | Northwest Greenland | Adult(s), egg/young | 1993–2018 | Census | Partial | Air, ground | ✓ | (X + Y + Z + G)/N | ||
| 22 | Central West Greenland | Adult(s), egg/young | 1972–2005 | Census | Partial | Air, ground | ✓ | (X + Y + Z + G)/N | ||
| 24 | Northeast Iceland | Adult(s), egg/young, other evidence | 1981–2018 | Census | Full | Ground | ✓ | ✓ | (X + Y + Z + G)/N | |
aSee Fig. 3
bOther evidence—egg shell fragments, molted feathers or down, recent excrement, fresh prey remains
cBold indicates monitoring is ongoing
dCensus—all known nesting territories checked systematically; stratified—sub-set of all known nesting territories checked systematically; random—random selection of known nesting territories checked; full—known nesting territories receive multiple visits per breeding season; partial—single visits, or only in brood-rearing period
eDiscontinuous
fX = successful nest, nesting attempt, or pair—one in which at least one young reaches the minimum acceptable age for assessing success (Steenhof et al. 2017), Y = unsuccessful nest, nesting attempt, or pair—a laying pair that failed before nestlings reached the minimum acceptable age for assessing success, Z = non-laying Pair—a mated pair that fails to lay at least 1 egg in a given year (Steenhof et al. 2017), G = single non-laying individual—a non-mated individual evidenced by an absence of territorial behavior, or reproductive-related activity, N = Count of known nesting territories surveyed
AFSG members reviewed the list of ‘essential’ and ‘recommended’ FEC attributes identified by the CBMP-TEMG (Christensen et al. 2013), and selected occupancy and productivity (parameters of FEC attribute ‘demography’; see Millsap 2018 for example) to be of greatest utility for past and ongoing monitoring efforts of Arctic falcon species (see Table S3 for assessment of all FEC attributes). Franke et al. (2017) defined occupancy as the quotient of the count of occupied nesting territories and the count of known nesting territories that were fully surveyed in a given breeding season (i.e., two or more surveys). Productivity is defined as the number of young that reach the minimum acceptable age for assessing success (80 % of normal fledging age), and should be reported as the number of young produced per territorial pair, or per occupied territory in a particular year (Steenhof and Newton 2007). We define a population as a group of organisms of the same species occupying a particular space (i.e., monitoring sites) at a particular time (i.e., monitoring duration), with the potential to breed with each other (Krebs 2001). Terminology used throughout follows recommendations outlined in Franke et al. (2017).
Trend analysis
Data exploration was carried out following the protocol described in Zuur et al. (2010). Specifically, we used histograms and kernel density plots to inspect raw values for normality. In addition, we applied the Shapiro–Wilk test (w) for normality to assess whether p values were > 0.05, and w = 1 (Shapiro and Wilk 1965). We used boxplots to detect outliers; however, none of the extreme values observed were considered to be outside of the range of natural variation, and all data points were retained.
Because we anticipated non-linear trends in occupancy and productivity, General Additive Models (GAMs) were used to estimate temporal trends as follows:
| 1 |
where represents the ith observation of the FEC attribute parameter of interest, is the intercept, is the smoothing function, and represents a vector that contains prediction residuals. Each GAM was estimated using the mgcv package (Wood 2016) in R (R Development Core Team 2017). The amount of smoothing was limited to a maximum of 10 degrees of freedom, and estimated using restricted maximum likelihood (Wood 2016). We limited trend analysis to monitoring sites with data spanning 10 or more years, and for each monitoring site we excluded years in which fewer than 10 nesting territory visits were completed.
After fitting each GAM, we validated model fit using histograms and kernel density plots to inspect the residuals for normality. We assessed homogeneity of variance by plotting residual values against fitted values, and inspected each plot to ensure that points were uniformly distributed. Model outputs for each GAM included estimates of the grand mean for each FEC attribute of interest for each monitoring site (i.e., the intercept term). Effective degrees of freedom (edf) dictate the amount of smoothing estimated for each GAM, where higher values indicated less smoothing (more undulating) and lower values indicate more smoothing (less undulating). Numerical smoothing terms must be interpreted in conjunction with the associated GAM graphical output. For example, a trend (where time is the covariate) with edf = 1.0 that is accompanied by significant p value would be interpreted as a straight line that deviates from horizontal. However, inspection of the graphical output would be required to assess the degree of incline or decline, taking into consideration the confidence intervals and deviance explained. Similarly, a trend with edf = 1.0 that is accompanied by non-significant p value would be interpreted as a straight line that does not deviate from horizontal (i.e., neither increasing nor decreasing), but inspection the graphical output (trend line and associated confidence intervals) is recommended to support the conclusion that no incline or decline is present.
Summary of other FEC attributes
To provide an overview of other CBMP FEC attributes, we conducted a literature search using the Web of Science database for journal articles, conference proceedings, and books using the following keywords: abundance, phenology, Arctic, climate change, Falco rusticolus, Falco peregrinus, genetic diversity, gyrfalcon, long-term trends, peregrine, prey cycles, and pollutants.
Results
Identification of monitoring sites
Researchers from the Arctic Council states representing 24 monitoring sites contributed information describing existing monitoring efforts, and assessing trends in occupancy and productivity for peregrine falcons and gyrfalcons. We summarized information from 11 monitoring sites where both species were surveyed, ten involving peregrine falcons only, and three involving gyrfalcons only. Fourteen monitoring sites were in the Nearctic and ten were in the Palearctic. The most significant gaps in coverage exist for eastern Russia, the Arctic Archipelago of Canada, and East Greenland. Monitoring sites consisted of stretches of rivers (or coastline) several hundred kilometers long, or inland areas ranging in size from 100 to 84 000 km2 (Tables S1 and S2) located between 60°N and 77°N. Depending on time of year, landscape type, and spatial extent of study areas, surveys were conducted by snowmobile, on foot (including skis), all-terrain vehicle, boat, and helicopter (Tables 1 and 2; Fig. 1). In almost all areas, monitoring required accessing occupied nests to record productivity data. Duration of monitoring projects ranged from 5 to 66 years; 14 projects covered 30 years or more, and four were up to 10 years in duration. In total, monitoring was conducted over approximately 800 field seasons combined, and as of 2018, 21 projects were active, and these have the greatest potential to form the basis for future coordinated monitoring (Tables 1 and 2).
Trends in occupancy
Peregrine falcon data from Rankin Inlet (n = 35 years), Kola Peninsula (n = 25 years), central West Greenland (n = 35 years), South Greenland (n = 18 years), and Peel and Yukon Rivers (n = 24 years), all suggest stable trends in occupancy (Fig. 2, Table 3). GAM results for Rankin Inlet and South Greenland indicate that long-term occupancy has been linear (non-significant p values and low edf scores). GAM results indicate that occupancy in central West Greenland has been non-linear (significant p values associated with high edf scores), showing increases through the 1970s and 1980s, which have since declined from a peak that occurred around 1990 to levels similar to those recorded in the mid-1980s. Results from the Kola Peninsula suggest that occupancy has varied over time (high edf score), but are associated with a non-significant p value and overlapping confidence intervals that suggest overall stability. Although results from the Peel/Yukon River drainages suggest that occupancy has varied through time (high edf score and significant p value), rates of occupancy from 2010 to 2015 were similar to those observed in the 1970s, having declined from the mid-1990s.
Fig. 2.
Trends (black lines) in peregrine falcon occupancy (black circles) and productivity (open circles) estimated using general additive models (see Table 3 numerical outputs) for monitoring sites distributed throughout the circumpolar Arctic. Gray bands represent 95 % confidence intervals. See Methods for definitions of occupancy and productivity. Light purple refers to Sub-Arctic, medium purple to Low Arctic, and dark purple to High Arctic
Table 3.
Model structure, smoothing terms, and overall trends for peregrine falcon occupancy (top panel) and productivity (bottom panel) for monitoring sites distributed throughout the circumpolar Arctic. The intercept estimates the grand mean. Effective degrees of freedom (edf) were estimated using restricted maximum likelihood, where higher values indicate less smoothing and lower value indicate more smoothing. D (%) equals the proportion of the null deviance explained by the model and p is the significance level (p < 0.005 in bold) and n = number of years in which 10 or more nesting territory visits were completed. Trend indicates whether the time series remained stable, or had decreased or increased over the course of the monitoring period
| Site# | Site | Occupancy ~ s(year) | Smoothing terms | Trend | ||||
|---|---|---|---|---|---|---|---|---|
| Intercept | SE | edf | p value | D (%) | n | |||
| 1 | Norrbotten (Se) | 0.69 | 0.02 | 1.00 | 0.001 | 55.1 | 19 | Decrease |
| 3 | Lapland (Fi) | 0.68 | 0.01 | 2.24 | < 0.001 | 81.4 | 38 | Decrease |
| 4 | Kola Peninsula (Ru) | 0.67 | 0.02 | 4.20 | 0.084 | 44.3 | 25 | Stable |
| 8 | Yamal Peninsula (Ru) | 0.67 | 0.03 | 1.00 | 0.006 | 58.2 | 11 | Decrease |
| 13 | Colville River (US) | 0.50 | 0.01 | 6.73 | < 0.001 | 96.8 | 27 | Increase |
| 14 | Yukon River (US) | 0.48 | < 0.01 | 7.48 | < 0.001 | 99.2 | 47 | Increase |
| 15 | Yukon/Peel Rivers (Ca) | 0.73 | 0.02 | 3.68 | < 0.001 | 72.3 | 24 | Stable |
| 16 | North Slope (Ca) | 0.32 | 0.03 | 4.48 | 0.001 | 81.3 | 17 | Increase |
| 17 | Mackenzie River (Ca) | 0.60 | 0.02 | 1.00 | 0.001 | 80.31 | 10 | Increase |
| 19 | Rankin Inlet (Ca) | 0.74 | 0.01 | 1.52 | 0.428 | 6.6 | 35 | Stable |
| 20 | Mary River (Ca) | – | – | – | – | – | – | – |
| 21 | Northwest Greenland | – | – | – | – | – | – | – |
| 22 | Central West Greenland | 0.72 | 0.01 | 5.80 | < 0.001 | 80.3 | 35 | Stable |
| 23 | South Greenland | 0.85 | 0.02 | 1.74 | 0.634 | 12.7 | 18 | Stable |
| Site# | Site | Productivity ~ s(year) | Smoothing terms | Trend | ||||
|---|---|---|---|---|---|---|---|---|
| Intercept | SE | edf | p value | D (%) | n | |||
| 1 | Norrbotten (Se) | 1.69 | 0.08 | 1.00 | 0.230 | 7.5 | 21 | Stable |
| 3 | Lapland (Fi) | 1.55 | 0.05 | 1.64 | 0.098 | 14.2 | 38 | Stable |
| 4 | Kola Peninsula (Ru) | 1.70 | 0.13 | 2.39 | 0.042 | 52.4 | 15 | Increase |
| 8 | Yamal Peninsula (Ru) | – | – | – | – | – | – | |
| 13 | Colville River (US) | 1.25 | 0.06 | 1.35 | 0.023 | 26.0 | 27 | Decrease |
| 14 | Yukon River (US) | 1.48 | 0.05 | 3.93 | 0.001 | 39.8 | 47 | Stable |
| 15 | Yukon/Peel Rivers (Ca) | 1.15 | 0.04 | 2.75 | < 0.001 | 84.2 | 24 | Increase |
| 16 | North Slope (Ca) | – | – | – | – | – | – | – |
| 17 | Mackenzie River (Ca) | 1.44 | 0.12 | 1.72 | 0.318 | 33.9 | 10 | Stable |
| 19 | Rankin Inlet (Ca) | 1.10 | 0.08 | 1.77 | 0.045 | 20.2 | 35 | Decrease |
| 20 | Mary River (Ca) | – | – | – | – | – | – | – |
| 21 | Northwest Greenland | – | – | – | – | – | – | – |
| 22 | Central West Greenland | 2.31 | 0.05 | 3.82 | 0.043 | 36.1 | 33 | Stable |
| 23 | South Greenland | 1.85 | 0.06 | 5.28 | < 0.001 | 62.8 | 35 | Decrease |
Increasing trends in peregrine falcon occupancy were noted for the Mackenzie River (n = 10 years), Yukon River (n = 47 years), North Slope (n = 17 years), and Colville River, (n = 27 years; Fig. 2, Table 3). GAM results from the Mackenzie River indicate a shallow but steady increase over time (significant p value and low edf score). Occupancy on the Colville River increased from early 1980 through early 1990 (significant p value and high edf score), and stabilized thereafter (Fig. 2, Table 3). On the Yukon River in Alaska, results indicate a steady increase throughout the monitoring period (significant p value associated with non-overlapping confidence intervals). Results from the North Slope indicate a non-linear trend over the course of the monitoring period (high edf score, significant p value, and non-overlapping confidence intervals). Inspection of the graphical output indicates occupancy declined through the 1980s, increased through the 1990s, and stabilized thereafter.
Decreasing trends in peregrine falcon occupancy were observed for Norrbotten (n = 19 years), Lapland (n = 38 years), and Yamal Peninsula (n = 11 years). However, the validity of these trends is uncertain, and it is possible that they are confounded with survey methods (see Discussion for details).
For the gyrfalcon, stable trends in occupancy were identified (non-significant p values and low edf scores) in Denali National Park (n = 29 years), Colville River (n = 22 years), Northwest Greenland (n = 10 years), and Schuchya River (n = 11) throughout their respective monitoring periods (Fig. 3, Table 4). Despite an apparent non-linear trend for occupancy on the Kola Peninsula (n = 10), GAM results reveal (Fig. 3, Table 4) that occupancy has also remained constant through time (non-significant p value, low edf score, and overlapping confidence intervals). Although stable over the long-term, gyrfalcon occupancy in Iceland (n = 37) exhibited regular cycles (significant p value combined with high edf score). Trends for Finnmark (n = 19), Norrbotten (n = 16), North Slope (n = 15), and Lapland (n = 24) all exhibited non-linear patterns (increasing trend followed by a period of decreasing occupancy) which are supported by GAM results (significant p value, high edf score, and non-overlapping confidence intervals). A similar non-linear trend (significant p value, high edf score, and non-overlapping confidence intervals) is apparent for the North Slope where occupancy initially decreased in the 1980s followed by an increase in the 1990s (Fig. 3, Table 4).
Fig. 3.
Trends (black lines) in gyrfalcon occupancy (black circles) and productivity (open circles) estimated using general additive models (see Table 4 numerical outputs) for monitoring sites distributed throughout the circumpolar Arctic. Gray bands represent 95 % confidence intervals. See Methods for definitions of occupancy and productivity. Light purple refers to Sub-Arctic, medium purple to Low Arctic, and dark purple to High Arctic
Table 4.
Model structure, smoothing terms, and overall trends for gyrfalcon occupancy (top panel) and productivity (bottom panel) for monitoring sites distributed throughout the circumpolar Arctic. The intercept estimates the grand mean. Effective degrees of freedom (edf) were estimated using restricted maximum likelihood, where higher values indicate less smoothing and lower value indicate more smoothing. D % equals the proportion of the null deviance explained by the model and p is the significance level (p < 0.005 in bold) n = number of years in which 10 or more nesting territory visits were completed. Trend indicates whether the time series remained stable, or had decreased or increased over the course of the monitoring period
| Site# | Site | Occupancy s(year) | Smoothing terms | Trend | ||||
|---|---|---|---|---|---|---|---|---|
| Intercept | SE | edf | p value | D (%) | N | |||
| 1 | Norrbotten (Se) | 0.41 | 0.02 | 5.57 | < 0.001 | 90.4 | 16 | Stable |
| 2 | Finnmark (No) | 0.44 | 0.02 | 5.03 | 0.001 | 77.9 | 19 | Stable |
| 3 | Lapland (Fi) | 0.51 | 0.02 | 5.18 | < 0.001 | 74.8 | 24 | Stable |
| 4 | Kola Peninsula (Ru) | 0.50 | 0.06 | 1.71 | 0.563 | 25.7 | 10 | Stable |
| 7 | Schuchya River (Ru) | 0.44 | 0.04 | 1.72 | 0.175 | 39.6 | 11 | Stable |
| 10 | Seward Peninsula (US) | 0.33 | 0.01 | 0.92 | 0.004 | 51.0 | 14 | Decrease |
| 11 | Denali National Park (US) | 0.71 | 0.01 | 1.00 | 0.686 | 0.62 | 29 | Stable |
| 12 | South Yukon (Ca) | 0.78 | 0.01 | 7.34 | < 0.001 | 78.6 | 35 | Decrease |
| 13 | Colville River (US) | 0.36 | 0.03 | 1.00 | 0.938 | 0.03 | 22 | Stable |
| 16 | North Slope (Ca) | 0.75 | 0.02 | 3.42 | 0.028 | 63.8 | 15 | Stable |
| 21 | Northwest Greenland | 0.45 | 0.04 | 1.92 | 0.464 | 31.9 | 10 | Stable |
| 24 | Northeast Iceland | 0.62 | 0.01 | 7.83 | < 0.001 | 82.0 | 37 | Stable |
| Site# | Site | Productivity ~ s(year) | Smoothing terms | Trend | ||||
|---|---|---|---|---|---|---|---|---|
| Intercept | SE | edf | p value | D (%) | N | |||
| 1 | Norrbotten (Se) | 2.13 | 0.15 | 1.62 | 0.303 | 19.8 | 16 | Stable |
| 2 | Finnmark (No) | 1.36 | 0.14 | 1.54 | 0.205 | 19.9 | 19 | Stable |
| 3 | Lapland (Fi) | 1.09 | 0.09 | 3.34 | 0.035 | 45.4 | 24 | Stable |
| 4 | Kola Peninsula (Ru) | – | – | – | – | – | – | – |
| 7 | Schuchya River (Ru) | 1.75 | 0.13 | 1.00 | 0.304 | 11.6 | 11 | Stable |
| 10 | Seward Peninsula (US) | 1.50 | 0.11 | 1.32 | 0.739 | 7.24 | 14 | Stable |
| 11 | Denali National Park (US) | 1.45 | 0.10 | 1.00 | 0.481 | 1.85 | 29 | Stable |
| 12 | South Yukon (Ca) | 1.12 | 0.08 | 1.88 | 0.007 | 30.1 | 35 | Decrease |
| 13 | Colville River (US) | 1.20 | 0.09 | 5.84 | 0.007 | 74.14 | 20 | Decrease |
| 16 | North Slope (Ca) | 2.59 | 0.10 | 1.00 | 0.473 | 4.03 | 15 | Stable |
| 21 | Northwest Greenland | 2.75 | 0.14 | 1.00 | 0.236 | 17.0 | 10 | Stable |
| 24 | Northeast Iceland | 1.31 | 0.06 | 1.00 | 0.516 | 1.22 | 37 | Stable |
Decreasing trends in gyrfalcon occupancy were observed for the Seward Peninsula (n = 14 years) and South Yukon (n = 35 years). The apparent decline on the Seward Peninsula has been constant (significant p value, low edf score, and non-overlapping confidence intervals), whereas the overall decline in occupancy for the South Yukon exhibits non-linear trends (Fig. 3, Table 4). None of the 11 monitoring sites recorded trends indicative of increasing occupancy in gyrfalcon populations.
Trends in productivity
For the peregrine falcon, stable trends in productivity were noted for Norrbotten (n = 21 years), Lapland (n = 38 years), and Mackenzie River (n = 10 years). All exhibit non-significant p values, low edf scores, and overlapping confidence intervals consistent with constant productivity through time (Fig. 2, Table 3). GAM results for the Yukon River (n = 47 years) and central West Greenland (n = 33 years) indicate that peregrine falcon productivity was non-linear (moderately high edf, significant p value, and non-overlapping confidence intervals); however, inspection of the trend lines for each population shows overall long-term stability (Fig. 2, Table 3).
Increasing trends in peregrine falcon productivity were observed for the Yukon/Peel River drainages (n = 24 years) and the Kola Peninsula (n = 15 years). Data exhibit weak non-linearity (relatively low edf scores accompanied by significant p values and non-overlapping confidence intervals), and inspection of the trend lines for each population supports the numerical evidence for increased productivity (Fig. 2, Table 3).
Decreasing trends in peregrine falcon productivity were recorded for Rankin Inlet (n = 35 years) and the Colville River (n = 27 years). GAM results exhibit low edf scores accompanied by significant p values and non-overlapping confidence intervals which indicate linear change through time (Fig. 2, Table 3). Inspection of the trend lines for each population indicates that productivity in both populations declined marginally throughout the monitoring period. South Greenland (n = 35 years) exhibited high edf scores accompanied by a significant p value and non-overlapping confidence intervals which indicate non-linear change through time. Inspection of the trend line indicates a period of relative stability followed by a decline over the last decade.
For gyrfalcons, stable trends in productivity were observed for Norrbotten (n = 16 years), Finnmark (n = 19 years), Schuchya River (n = 11 years), Seward Peninsula (n = 14 years), Denali (n = 29 years), North Slope (n = 15 years), Northwest Greenland (n = 10 years), Iceland (n = 37 years), and Lapland (n = 24 years). Other than Lapland, all exhibited non-significant p values, low edf scores, and overlapping confidence intervals associated with linear patterns through time. Inspection of trend lines for each of these populations is consistent with GAM results (Fig. 3, Table 4). Lapland exhibited a non-linear trend where productivity increased through the late 1990s/early 2000s, decreasing since.
None of the 11 monitoring sites recorded trends indicative of increasing productivity in gyrfalcon populations.
A Decreasing trend in gyrfalcon productivity is evident for South Yukon (n = 35 years), where data exhibited a low edf score accompanied by a significant p value and non-overlapping confidence intervals, all of which indicate linear change through time. Inspection of the trend line indicates that productivity declined throughout the monitoring period. However, for the Colville River (n = 20 years), GAM results indicate a non-linear trend (high edf score, significant p value, and non-overlapping confidence intervals). Inspection of the trend line reveals a cyclic patter with overall downward trend through the monitoring period (Fig. 3, Table 4).
Other FEC attributes
Although demographic parameters were reported regularly, results for other FEC attributes were not reported consistently among monitoring sites. Here we summarize information pertinent to FEC attributes other than demography.
Abundance
The current population of peregrine falcons in the Arctic exceeds 20 000 pairs (Table S4), which is likely an underestimate (Franke 2016). Throughout North America and Europe, the peregrine falcon experienced widespread population declines from the 1950s through 1970s due to organochlorine pesticides (Peakall and Kiff 1979; Peakall et al. 1983; Risebrough and Peakall 1988; Court et al. 1990; Henny et al. 1994; Johnstone et al. 1996; Franke et al. 2010). By the 1970s, the nominate race F. p. peregrinus in the Palearctic was virtually extirpated, including in northern Fennoscandia (Lindberg et al. 1988), where it is considered to be recovering. In North America, the species was extirpated over its range east of the Rocky Mountains and south of the boreal forest by 1975 (Fyfe et al. 1976). However, in most tundrius and calidus populations, the effects of organochlorine pesticides are thought to have been less severe compared to populations south of the Arctic biome (Burnham and Mattox 1984; Mattox and Seegar 1988; Falk et al. 2018). Most contemporary peregrine falcon populations are considered to be recovered (White et al. 2013a). For example, populations in Alaska and northern Fennoscandia have experienced considerable increases in abundance. In sub-arctic Fennoscandia, the peregrine population recovered from a low of 65 pairs in 1975 (Lindberg et al. 1988) to approximately 750 pairs by 2017 (Table S4). Increasing abundance of northern peregrine falcon populations is also supported by standardized autumn migration counts. For example, counts at Falsterbo, Sweden, increased from an annual average of 2.5 birds/year from 1973 to 1983 to 88 birds/year from 2005 to 2015 (Kjellén 2018). Similar data from eastern North America show annual average of nine peregrine falcons/year from 1972 to 1981, increasing to 63 birds/year from 2009 to 2018 (Hawk Mountain International 2019).
The total population (i.e., Arctic-wide) of gyrfalcons is estimated to be fewer than 11 000 pairs (Table S4). No long-term changes in population size/density have been reported for gyrfalcons (Barichello and Mossop 2011; Bente 2011; Koskimies 2011; Nielsen 2011). Johansen and Østlyngen (2011) reported no change in the number of gyrfalcon nesting attempts from two 11-year periods separated by 150 years. The gyrfalcon was not affected by pesticide residues that affected the peregrine (Booms et al. 2008).
Phenology
Breeding phenology (e.g., laying date, hatching date) summaries for each monitoring site are reported in Table S5. Although data describing breeding phenology are likely estimable for most monitoring sites, analyses of long-term trends are currently only available for two sites: for peregrine falcons breeding along the Mackenzie River, breeding advanced 1.5 to 3.6 days decade−1 depending on latitude, from 1985 to 2010 (Carrière and Matthews 2013). Similarly, hatching date in peregrine falcons in South Greenland advanced 0.9 days decade−1 during the period from 1981 to 2017 (Falk and Møller unpubl.). There are currently no data available on changes in gyrfalcon phenology, but the two species may respond differently: for example, although low spring temperatures are associated with later arrival of gyrfalcons at nesting territories in Nunavut, there was no effect on laying dates (Poole and Bromley 1988).
Spatial structure
Across the Palearctic tundra biome, the peregrine falcon generally breeds farther north than the gyrfalcon, whereas the opposite is true in the Nearctic (Pokrovsky and Lecomte 2011). In the Nearctic, tundrius breeds north of the tree-line, wherever suitable nesting habitat and sufficient prey are present, from Alaska throughout northern Canada, to Greenland, where breeding occurs to at least to latitude 77°N (Burnham et al. 2012; White et al. 2013a). Tundrius intergrades with Falco peregrinus anatum throughout the North American taiga, forming a cline of variation. Tundrius and calidus are long-distance migrants (Yates et al. 1988; White et al. 2013a). Tundrius winters throughout South and Central America, the Caribbean Islands, as well as the southern United States, and Mexico (Yates et al. 1988; Mattox and Restani 2014). In the Palearctic, calidus intergrades with the F.p. peregrinus towards the south and west, and with F.p. japonicus in NE Siberia (White et al. 2013a). Calidus winters mainly from the Mediterranean and eastwards across South and Southeast Asia (Dixon et al. 2017; Sokolov et al. 2018), but some individuals winter as far south as southern Africa (Meyburg et al. 2017). Yates et al. (1988) concluded that breeding populations originating in the western and eastern portions of the North American Arctic and sub-Arctic tend to separate longitudinally during outward migration. A similar migratory pattern exists for peregrine falcons breeding in northern Eurasia, which also separate longitudinally (Dixon et al. 2012; Sokolov et al. 2018). Peck et al. (2018) estimated the breeding distribution and habitat selection of the peregrine falcon in Nunavut, Canada, and indicated that peregrine falcons selected nesting territories in rugged terrain, in areas with higher than average summer temperatures, productive land cover types, lower mean elevations, and lower mean summer precipitation. Gyrfalcons inhabit tundra and taiga regions from 82°N in Greenland to the sub-Arctic as far south as 55°N in Alaska and Canada, and 51°N at Kamchatka in Russia (Pokrovsky and Lecomte 2011). The gyrfalcon is a year-round resident in Iceland, the only country where it is not sympatric with the peregrine falcon. The large islands distributed from Svalbard (Norway) to Severnaya Zemlya (Russia) have not been colonized by either species. In North America and Fennoscandia, the gyrfalcon is generally considered to be a northern resident (Booms et al. 2008) as many breeding-aged birds remain within the breeding range throughout the year (Cade 1982; Poole and Bromley 1988). Immature birds are much more likely to winter south of breeding range (Potapov and Sale 2005). Icelandic gyrfalcons are entirely resident (Nielsen and Cade 1990), whereas those in Russia are partly migratory, likely depending on food availability (Potapov and Sale 2005). Greenlandic gyrfalcons are generally short-distance migrants, but are also known to winter on the pack ice where seabirds are available (Burnham and Newton 2011). Although little information is available for assessing changes in the spatial extent of the breeding distribution of Arctic falcons, Burnham et al. (2012) attributed increased numbers of peregrine falcon nesting territories in Northwest Greenland to range expansion made possible by ameliorating climatic conditions (i.e., spring and autumn extensions). In northern Russia, gyrfalcon breeding distribution may have expanded due to availability of stick nests (i.e., built by ravens or rough-legged buzzards) on recently constructed anthropogenic features (e.g., railway bridges and oil rigs) in areas that were previously devoid of ravens (Morozov 2011, Appendix S1).
Temporal cycles
Apart from the widespread pesticide-induced population decline and subsequent recovery of peregrine falcons, breeding populations are known to be remarkably stable with very little among-year variation in breeding density and occupancy (Newton 1988). Although breeding success (productivity) can be highly variable, it does not manifest as cyclic patterns associated with prey, but rather is likely due to effects of weather (Anctil et al. 2014; Carlzon et al. 2018). Icelandic gyrfalcons exhibit cyclic trends where occupancy lags spring density of ptarmigan by four years (Nielsen 2011). Falkdalen et al. (2011) indicated that reproductive rates of gyrfalcons in central Sweden followed a three-year cycle where the count of breeding pairs was related to the count of nestlings produced 3 years earlier, and that the best predictor of reproductive success was the production of willow ptarmigan chicks in the prior year. In Finland (with some nests bordering on Sweden and Norway), Koskimies (2011) indicated that ptarmigan species overwhelmingly dominated the annual diet of gyrfalcons, and that fluctuations in ptarmigan had a marked effect on reproductive success. Mossop (2011) indicated the presence of stable, regular, synchronous, 10-year cycles in gyrfalcons and ptarmigan in Yukon, and (Barichello and Mossop 2011) reported higher reproductive success in gyrfalcons (young fledged per nest) when ptarmigan were abundant than when ptarmigan were scarce.
Health
The negative health effects and associated global population declines due to contamination from organochlorine pesticides in peregrine falcons are well established (see Hickey 1969; Cade et al. 1988). However, over the past decades pesticide loads have declined to levels that do not cause population effects (Wegner et al. 2005; Vorkamp et al. 2009; Franke et al. 2010; Andreasen et al. 2018; Falk et al. 2018).
Recent studies have shown that peregrine falcons are exposed to several other contaminants. Brominated flame retardants (BFRs) are compounds that, in some cases, are persistent in the environment, bioaccumulative, and can cause endocrine disruption (Darnerud 2008). Concentrations of the brominated flame retardant, BDE-209, have increased in peregrine falcon eggs from Greenland, and peaked in eggs from Europe, likely due to regulation of the compound (Vorkamp et al. 2018). Despite the potential negative effects of these compounds, and their presence in peregrine falcon populations in Europe and North America (Guerra et al. 2012), there is currently no evidence indicating detrimental health effects at the population level in peregrine falcons. In addition to BFRs, peregrine falcon eggs collected in South Greenland from 1986 to 2014 also showed variable, but ongoing exposure to perfluoroalkyl substances and polychlorinated naphthalenes (Vorkamp et al. 2019). Barnes et al. (2019) documented widespread, but low levels of mercury exposure in peregrines migrating from the northern latitudes of North America. Although the concentration associated with toxic effects in peregrine falcons is unknown, recent population growth of northern peregrine falcon populations suggests that health effects due to mercury exposure are currently of no consequence. Analyses of contaminants in gyrfalcons indicate low concentrations of organochlorines and heavy metals. Where gyrfalcons prey primarily on ptarmigan, they have low, non-toxic body burdens of persistent contaminants; however, a shift in diet that incorporates migratory waterfowl can result in higher concentrations of contaminants, particularly for mercury (Lindberg 1984; Jarman et al. 1994; Matz et al. 2011). As part of a study to assess mercury concentrations in marine and terrestrial birds, Burnham et al. (2018) indicated that some peregrine falcons exhibited mercury concentrations suggestive of medium risk for toxicity (i.e., between 1000 and 3000 ng g−1 wet weight). Burnham et al. (2018) did not quantify mercury concentrations in adult gyrfalcons, and it is unknown whether gyrfalcons have accumulated mercury to the same degree that peregrine falcons have, although in museum specimens from Greenland (1880–2000) the levels were roughly comparable between the two species over time (Dietz et al. 2006).
Diversity
Despite considerable morphological variation among peregrine falcon subspecies, White et al. (2013b) indicated that haplotypes among 12 of the 19 recognized subspecies (including tundrius, but not calidus) were broadly shared. Using North American tissue samples collected pre-collapse, Brown (2007) indicated that anatum and tundrius were genetically indistinguishable from one another, but could be differentiated from F. p. pealei. However, post-recovery samples indicated that tundrius and anatum could be differentiated due to increased genetic diversity within southern anatum populations, likely due to use of exotic subspecies for captive breeding during the recovery phase (Brown 2007). Similarly, using Alaskan samples, Talbot et al. (2017) indicated that although pealei could be genetically differentiated from tundrius and anatum, the latter two subspecies could not be distinguished genetically. Using samples collected during migration (1985 to 2007) at Padre Island, Texas, Johnson et al. (2010) found little difference between tundrius and anatum, and suggested that delineation between the two subspecies breeding at northern latitudes was not justified. For gyrfalcons, Johnson et al. (2007) indicated little genetic structure among populations throughout a large portion of their circumpolar distribution. Greenlandic and Icelandic populations were considered separate, whereas Norway, Alaska, and Canada were identified as a single population consistent with contemporary gene flow across Russia.
Discussion
The work undertaken here represents the first formal collaboration among scientists involved in the Arctic Falcon Specialist Group. Although disparate application of survey methods among research groups currently hampers absolute comparison of occupancy and productivity among monitoring sites (see below for additional discussion), it does not preclude relative comparison of trends at the scale of the circumpolar Arctic, or regionally within the Nearctic and Palearctic. From the standpoint of occupancy and productivity, our results indicate that most peregrine falcon and gyrfalcon populations are generally stable, and assuming that these patterns hold beyond the temporal and spatial extents of the monitoring sites we report on here, it is reasonable to suggest that breeding populations at broader scales are similarly stable.
At the scale of the circumpolar Arctic, occupancy trends for peregrine falcons and gyrfalcons were available from 12 monitoring sites meeting the criteria for inclusion in analyses, whereas productivity trends were available from 10 sites monitoring peregrine falcons and from 11 sites monitoring gyrfalcons. For peregrine falcons, nine of 12 monitoring sites indicated that occupancy was either stable or had increased over the course of monitoring, and three monitoring sites resulted in trends that indicate occupancy had declined. Seven of 10 peregrine falcon monitoring sites presented productivity trends that were either stable or increasing, and three resulted in trends that had declined. For gyrfalcons at the circumpolar scale, occupancy trends at 10 of 12 monitoring sites were found to be stable or had increased, whereas occupancy at the two remaining monitoring sites had declined. Productivity trends for gyrfalcons at nine of 11 monitoring were stable, none showed evidence of increased productivity, and two presented trends that declined over the course of the monitoring period.
Within the Nearctic only, peregrine falcon monitoring has occurred at 12 monitoring sites, and eight had data sets that involved monitoring at 10 or more nesting territories in 10 or more years. Occupancy trends at all of the peregrine falcon monitoring sites in the Nearctic were either stable or had increased. Productivity trends were available for seven Nearctic monitoring sites, of which four were considered to be stable or to have increased and three presented productivity trends that had decreased. In the Palearctic, peregrine falcon monitoring has occurred at nine monitoring sites, and four had data sets that involved annual monitoring at 10 or more nesting territories in 10 or more years. Occupancy trends were considered to be stable at one monitoring site, and three monitoring sited presented trends that have declined. Two of the monitoring sites for which declines were evident (Norrbotten and Lapland) are located in close proximity to one another, and based on these results, it may be tempting to suggest that a relatively local decline in occupancy has occurred. However, in Lapland, occupancy is considered to be biased high during the 1980s as a result of failure to report nesting territories that had been occupied, but did not produce young. We attempted to account for this by excluding the data from the 1980s (i.e., truncating early years); however, this had no effect on the downward trend. In Norrbotten, it is unclear whether the apparent decline in occupancy is due to limitations in survey methods, or if it reflects the demography of a recovering population (Lindberg et al. 1988). More specifically, it is important to note that declining occupancy that is evident in Norrbotten and Lapland is at odds with abundance estimates, which clearly show that counts have increased by 5–10 % annually since the 1990s (Lindberg 2008). Peregrine falcon productivity trends in the Palearctic were only available for three monitoring sites, all of which were considered to be stable, or to have increased.
Within the Nearctic only, gyrfalcon monitoring has occurred at eight monitoring sites, of which six had data sets that involved monitoring at 10 or more nesting territories in 10 or more years. Occupancy trends for gyrfalcons in the Nearctic were stable at four monitoring sites, and decreased at the remaining two, both of which were in the western Nearctic. Productivity trends for gyrfalcons in the Nearctic were available for six monitoring sites, four of which were stable and two showed declines. Only the South Yukon population in Canada had declines in both occupancy and productivity, and it is interesting to note that this population is most southernly located relative to any other Nearctic monitoring sites. Within the Palearctic, monitoring has occurred at six gyrfalcon monitoring sites, all of which had data sets that involved monitoring at 10 or more nesting territories in 10 or more years. Trends for both occupancy and productivity were stable in all six monitoring sites.
Although directional changes in occupancy over time can serve as a metric of population status (MacKenzie et al. 2003), we caution against conflating counts of occupied nesting territories with counts of individual animals (or in this case, with breeding pairs), and concluding that the count of occupied nesting territories reflects local abundance. Although exceptions exist, it is generally inappropriate to equate the status and count of nesting territories (i.e., occupied or unoccupied) with the existence (or count) of individuals capable of occupying them. In any given year, it is not unusual for nesting territories to be unoccupied despite the fact that individuals exist, and possess the potential to occupy them. Thus, a low occupancy rate does not necessarily imply local depletion in numbers of animals, and a high occupancy rate does not necessarily imply local gain in the number of animals. Exceptions to this general pattern exist for surveys that have been conducted on rivers only, where the entire river (i.e., all habitat) has been regularly surveyed throughout the monitoring period, and where previously unoccupied nesting territories (i.e., during the monitoring period) are then assumed to have been occupied at some historical point prior to discovery. By back-casting the total count of known territories to year one of the monitoring period (regardless of the year in which the territory was first deemed occupied), the denominator in the occupancy equation becomes invariant. Under these conditions, occupancy can serve as a proxy for local annual abundance. In our study, only the Colville River and Yukon River (USA) fit these criteria.
Multiple surveys within breeding seasons explicitly account for detection error, and when combined with census-like sampling designs are known to reduce bias due to detection error (Kéry and Schmidt 2008). Although detection of nesting territories occupied by successful pairs is straightforward, bias in estimates of occupancy can result from non-detection of failed breeding pairs (i.e., failed nesting attempt), non-laying pairs, and individuals that remain cryptic during pre-laying and incubation. Failed nesting attempts can easily go undetected, particularly when nesting territories are visited only once per breeding season, usually late in the breeding season when nest visits are timed to coincide with the period when nestlings are of an age for fitting leg bands. Bias can also be exacerbated by lower survey effort during the initial few survey years, and is further challenged by the presence of ephemeral nesting territories that may be encountered in the first few years, but which remain unoccupied in later years. These factors drive occupancy towards 1.0 in the early years of the monitoring, and the problem usually remains unresolved until sufficient time has elapsed to account for irregularly occupied nesting territories. Thus, a decline in occupancy can represent an artifact of insufficient survey coverage, and in these cases should not be interpreted as representative of a true decline in occupancy. Excluding the initial few years of monitoring from a time series to account for this is reasonable.
Detection is always imperfect, and estimating the proportion of occupied sites without accounting for detection error invariably leads to an underestimation of occupancy (Kéry and Schmidt 2008). Detection of occupied nesting territories can be influenced by the type of observation platform. For example, surveys conducted by fixed-wing aircraft preclude detailed inspection of nesting sites for evidence of a nesting attempt (e.g., egg shell fragments, molted feathers or down, recent excrement, fresh prey remains) that is possible during ground-based and, to some degree, helicopter surveys. However, aerial surveys allow for rapid coverage of large spatial extents in remote areas that can be challenging with ground-based surveys. Detection probability of cliff-nesting raptors during aerial surveys likely varies by observer experience and the number of independent observers, seating arrangement in the aircraft, platform type (fixed-wing vs. rotary-wing), species, and location, but can be as high as 80 % (Booms et al. 2010). Detection probability for well-designed ground-based surveys is likely high and may approach 100 %, though this can also vary with variables such as terrain ruggedness, timing of the visits, number and duration of visits, weather, time of day, and observer experience. However, it is relatively straightforward to include these variables as part data collection process, and then use them as covariates in the detection modeling process.
Survey effort and design typically varies according to research priorities and capacity. Thus, we caution against comparing estimates among monitoring sites that use widely differing approaches to calculate estimates. Within study populations, it is typical for a sub-set of all known nesting territories to be regularly occupied, while others are occupied only in certain years out of many. A stratified sampling approach usually samples regularly occupied territories, whereas census-like sampling designs typically survey all known nesting territories (and intervening habitat that may harbor breeding birds) regardless of occupancy frequency. Logistics, funding limitations, and research priorities have resulted in partial surveys, where data collection was typically limited to only the brood-rearing period resulting in underestimates of occupancy and overestimates of productivity per occupied territory. Furthermore, many researchers record the number of young before nestlings have reached the recommended minimum acceptable age, which can result in further overestimation of productivity regardless of whether reproductive success is measured per territorial pair or per occupied territory. In reality, rather than estimating productivity per se (number of young hatched from a single nesting attempt by a pair of birds), many researchers actually report mean brood size for nestlings aged 10 days or more, or alternately for nestlings less than 10 days of age (see Franke et al. 2017 for distinction). These are important methodological problems that most likely cannot be corrected retrospectively, but could be accounted for in future monitoring.
Climate change has been identified as a major driver affecting the biodiversity of Arctic ecosystems (Christensen et al. (2013). In this regard, warmer Arctic temperatures have facilitated range expansion of pathogens and parasites (Loiseau et al. 2012; Kutz et al. 2013; Van Hemert et al. 2014), and Arctic-nesting falcons may experience negative impacts from novel pathogens and parasites. For example, Franke et al. (2016) reported the first observations of nestling mortality due to biting black flies in peregrines (F. p. tundrius), and suggested that ongoing annual monitoring will be required to determine whether hematophagous black flies (and other parasites and pathogens) are to become a regular and frequently occurring challenge for avian species raising altricial young in the arctic (Lamarre et al. 2018). Similarly, shifts in plant assemblages (Wheeler et al. 2015) may influence the distribution and demography of tundra-obligate species such as the gyrfalcon. Species associated with dense shrubs and taiga forest may benefit from range expansion, while tundra obligates will potentially experience climate-induced habitat loss and extirpation from historically occupied areas. Increases in density, height, and distribution of shrubs on tundra landscapes have already been reported in Arctic and sub-Arctic biomes, and are predicted to continue (Myers-Smith et al. 2015). Indeed, Johansen and Østlyngen (2011) indicated that despite regular occupancy at 60 % of historically used nesting territories, evidence of human disturbance or environmental change including expansion of birch (Betula pubescens) forests was evident at nesting territories that had become irregularly used or experienced long-term vacancy. Expansion of birch forest may benefit willow ptarmigan, while simultaneously negatively affecting rock ptarmigan, which prefer open habitat (Myers-Smith et al. 2015; Fuglei et al. 2019). Booms et al. (2011) used fundamental niche to estimate backward and forward projections of gyrfalcon distribution in Alaska. Although results are entirely predictive and should be interpreted cautiously, forward-models projected spatial contraction of gyrfalcon distribution was likely. Backward projections similarly suggested that gyrfalcon distribution in Alaska had experienced climate-related spatial contraction in the past.
Conclusion
In general, organization of activities among Arctic scientists actively involved in research of large falcons in the Nearctic and Palearctic has been poorly coordinated. However, ample opportunity exists to establish a coordinated circum-Arctic monitoring program for both falcon species (and potentially other raptors, e.g., rough-legged hawks) that supports CBMP goals (Christensen et al. 2013) including results shared directly with the CBMP Terrestrial Bird Expert Network (see Box 1 for specific recommendations).
Box 1.
Recommendations for future monitoring of Arctic falcons
We have highlighted several challenges that preclude direct comparisons of FEC attributes among monitoring sites, and have offered straightforward explanations for existing deficiencies (see Box 1). Notwithstanding the limitations associated with individual programs, we recommend that researchers conduct ‘full’ surveys that can explicitly account for detection error, and combine these repeated visits with census-like sampling designs which are most robust. Second, we recommend that researchers carefully consider whether their data reflect estimates of productivity or mean brood size. These are critical issues that most likely cannot be corrected retrospectively, but we recommend that researcher explicitly account for this detail in future monitoring.
Although we have offered discussion regarding underlying mechanisms (i.e., drivers of observed trends), analyses that have been included here are limited to presentation of temporal trends related to priority FEC attributes (i.e., time is the only covariate in all models). We therefore recommend that, in the short-term, a retrospective analysis that involves correlating FEC attributes with other covariates that are readily available (e.g., distance to disturbance, temperature, and precipitation), be conducted among monitoring sites. Considering evidence that shows early lay-dates are associated with increased nestling survival for Arctic-nesting raptors (Anctil et al. 2014), and that early lay-dates are associated with pre-laying body condition (Lamarre et al. 2017), we recommend investigating the broad scale relationship between lay-date (FEC attribute ‘phenology’) and productivity. This would contribute to an understanding of mechanisms affecting individual reproductive success, particularly if these covariates are used in conjunction with biotic (e.g., food supply) and abiotic (e.g., temperature and precipitation) variables.
Electronic supplementary material
Below is the link to the electronic supplementary material.
Acknowledgements
Support to coordinate data acquisition and compilation, and manuscript preparation was provided to AF by Environment and Climate Change Canada, and to KF by the Danish Cooperation for Environment in the Arctic (DANCEA) of the Danish Environmental Protection Agency (MST-112-00276). We thank all members of the Arctic Falcon Specialist Group for freely sharing their data, and for contributing to the development of this manuscript. We are particularly grateful to the volunteer field staff. AS, VS, and IF were supported by Russian Fund for Basic Research and Yamal Government; other funding agencies and permitting authorities of each of the monitoring sites are too numerous to name; none of the work presented here would be possible without their support.
Biographies
Alastair Franke
is Principle Investigator of the Arctic Raptors Project, a research group working in Nunavut, Canada focused on studying the ecology of Arctic-nesting raptors and their prey within the context of climate change. He holds an adjunct professorship in the Department of Biological Sciences at the University of Alberta, Edmonton, Alberta, Canada.
Knud Falk
is an independent researcher and consultant on Arctic biodiversity monitoring and management; since 1981, together with Søren Møller, he has been leading the peregrine falcon monitoring project in South Greenland.
Kevin Hawkshaw
is a Ph.D. candidate at the University of Alberta studying predator–prey interactions in peregrine falcons as part of the Arctic Raptors Project based in Rankin Inlet, Nunavut, Canada.
Skip Ambrose
is a retired U.S. Fish and Wildlife Service wildlife biologist, working primarily on Falco peregrinus anatum in interior Alaska. He has surveyed the upper Yukon River for peregrine falcons annually since 1973, and continues this effort after retirement.
David L. Anderson
is Director and founder of The Peregrine Fund’s Gyrfalcon and Tundra Conservation Program and Adjunct Professor in the Department of Biology at Boise State University. His research interests include the ecology of forest canopies and canopy access methods for science.
Peter J. Bente
is a wildlife biologist with research interests focused on the distribution, abundance, population trends, and ecological relationships of cliff-nesting raptor communities in Boreal and Arctic zones of Alaska.
Travis Booms
is a regional wildlife biologist for the Alaska Department of Fish and Game conducting research on northern raptors, songbirds, and mammals of conservation concern.
Kurt K. Burnham
is President and founder of the High Arctic Institute and has worked throughout Greenland for the past 28 years studying peregrine falcons and gyrfalcons and their prey. Primary research interests are in High Arctic ecosystems with a focus on migratory strategies, pollutants, and population biology.
Johan Ekenstedt
is an independent researcher that has studied the ecology of gyrfalcons in Norrbotten County in Sweden since 1998 through a long-term monitoring project.
Ivan Fufachev
is a researcher at the Arctic Research Station of Institute of Plant and Animal Ecology of Ural branch of Russian Academy of Sciences. He has studied birds of prey in Yamal Peninsula since 2012.
Sergey Ganusevich
is an independent researcher. In 1977, he discovered the diverse raptor community on the Kola Peninsula and since then has monitored the populations annually. His work as a director of the Center for Rescue of Wild Animals focuses on addressing the illegal trade of raptors and rehabilitation of poached falcons back to the wild.
Kenneth Johansen
is an independent researcher, who for the last three decades have studied the ecology of gyrfalcons in Finnmark County, Norway, through a long-term monitoring project.
Jeff A. Johnson
is an associate professor in the Department of Biological Sciences and Advanced Environmental Research Institute at University of North Texas. His primary research interests are in conservation and evolution biology focused on raptors and grouse.
Sergey Kharitonov
is a leading research biologist of the Bird Ringing Centre of Russia, A.N. Severtsov Institute of Ecology and Evolution RAS, Ph.D., DSc.
Pertti Koskimies
worked at Zoological Museum, University of Helsinki, for planning and running the monitoring program of Finnish avifauna in the 1980s and 1990s. He is currently an independent biologist specialized on gyrfalcon and osprey biology since the early 1990s.
Olga Kulikova
has been studying Arctic raptor breeding and migration in North-West Russia for the last decade and is a doctoral candidate in The University of Konstanz (Germany) making a dissertation on movement ecology of the rough-legged buzzard. She is also currently employed in the Institute of Biological Problems of the North in Magadan (Russia).
Peter Lindberg
is a senior researcher at the Department of Biological and Environmental Sciences at Göteborg University. He has conducted a monitoring and conservation project for the peregrine falcon since 1972. Main topics are population dynamics, reproduction, and effects of contaminants. He has also studied raptors and rodent cycles in Swedish Arctic Lapland.
Berth-Ove Lindström
is an independent researcher that has studied the ecology of gyrfalcons in Norrbotten County in Sweden since 1998 through a long-term monitoring project.
William G. Mattox
(MA, Ph.D.) in 1972 initiated the first long-term peregrine monitoring in central West Greenland and led it for 26 years.
Carol L. McIntyre
is a wildlife biologist at Denali National Park and Preserve, Alaska, USA. Among her main interests is the ecology of northern breeding raptors and their prey.
Svetlana Mechnikova
has monitored the raptors in southern Yamal peninsula since 1986, and defended a doctoral thesis “Raptors of the Southern Yamal: breeding and population dynamic.” She is also interested in the site fidelity of raptors, especially rough-legged buzzard.
Dave Mossop
is a professor emeritus and research scientist at the Yukon Research Center of Yukon College, in Whitehorse, Canada. His prime research interest is in understanding tundra community, focusing mostly on willow ptarmigan and waterbirds as key-stone species; gyrfalcon and peregrine falcon, top predators.
Søren Møller
is an associate professor at Roskilde University, Denmark. Since 1981, together with Knud Falk, he has been leading the peregrine falcon monitoring project in South Greenland.
Ólafur K. Nielsen
is a wildlife ecologist at the Icelandic Institute for Natural History. He is responsible for the monitoring of the Icelandic rock ptarmigan population. His main research interests relate to population dynamics of the rock ptarmigan and the role of food-web connections including herbivore–plant, predator–prey, and parasite–host interactions.
Tuomo Ollila
is senior advisor at the Metsähallitus, Parks and Wildlife. He is responsible for the monitoring of gyrfalcon and peregrine falcon in Finland.
Arve Østlyngen
is current leader of the Raptor Group Finnmark, responsible for long-term census program of gyrfalcons in western Finnmark. He is also employed in monitoring golden eagles, peregrines, and rough-legged buzzards in sub-arctic Finnmark.
Ivan Pokrovsky
is a postdoc at the Max-Planck Institute for Ornithology (Germany) and senior researcher at the Institute of Biological Problems of the North (Russia). His research interests include bird migration and ecology of the northern populations and ecosystems.
Kim Poole
is an independent researcher who has studied and surveyed raptors, with an emphasis on gyrfalcons, across the Canadian Arctic for over 30 years.
Marco Restani
is a former professor of wildlife ecology. He currently consults on a range of environmental issues affecting wildlife in the US Rocky Mountain Region.
Bryce W. Robinson
is the director of Ornithologi: a Studio for Bird Study, which aims to integrate visual media with research to better communicate topics in ornithology. His research interests range broadly within the study of avian life histories.
Robert Rosenfield
is a professor of biology at the University of Wisconsin – Stevens Point. He has conducted population surveys and ecological studies of breeding peregrine falcons in central west Greenland with UWSP undergraduate students for over 30 years. He has also investigated the population and behavioral ecology of nesting Cooper’s hawks in Wisconsin, USA, for 39 years.
Aleksandr Sokolov
is a researcher at the Arctic Research Station of Institute of Plant and Animal Ecology of Ural Branch of Russian Academy of Sciences. He has studied terrestrial ecosystems, mainly mammals and birds in Yamal Peninsula and the rest of Russian Arctic since 1998.
Vasiliy Sokolov
is a deputy director of the Institute of Plant and Animal Ecology. His research interests focus on study of structure and dynamic of Arctic bird species and their communities on Yamal Peninsula, as well as the migration strategy of Arctic peregrines breeding in northern Eurasia.
Ted Swem
is a biologist with the Fish and Wildlife Service in Fairbanks, Alaska, where he works in endangered species conservation and raptor population monitoring.
Katrin Vorkamp
is a senior scientist in environmental chemistry at Aarhus University, Denmark. She works with Knud Falk and Søren Møller on the contaminant exposure of the South Greenland peregrine falcons.
Footnotes
Publisher's Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Change history
1/21/2020
While collating contributions and comments from 36 researchers, the coordinating authors accidentally omitted Dr. Suzanne Carrière from the list of contributing co-authors. Dr. Carrière’s data are described in Tables 1 and 3, Figure 2 and several places in the narrative.
The new author list is thus updated in this article.
Contributor Information
Alastair Franke, Email: alastair.franke@ualberta.ca.
Knud Falk, Email: knudfalk@hotmail.com, https://www.vandrefalk.dk.
Kevin Hawkshaw, Email: hawkshaw@ualberta.ca.
Skip Ambrose, Email: skipambrose@frontiernet.net.
David L. Anderson, Email: danderson@peregrinefund.org
Peter J. Bente, Email: pjbente@cox.net
Travis Booms, Email: travis.booms@alaska.gov.
Kurt K. Burnham, Email: kburnham@higharctic.org
Johan Ekenstedt, Email: jekenstedt@gmail.com.
Ivan Fufachev, Email: fufa4ew@yandex.ru.
Sergey Ganusevich, Email: sganusevich@mail.ru.
Kenneth Johansen, Email: kennethalta@hotmail.com.
Jeff A. Johnson, Email: jeff.johnson@unt.edu
Sergey Kharitonov, Email: serpkh@gmail.com.
Pertti Koskimies, Email: pertti.koskimies@kolumbus.fi.
Olga Kulikova, Email: gaerlach@gmail.com.
Peter Lindberg, Email: peter.lindberg@bioenv.gu.se.
Berth-Ove Lindström, Email: berthove.lindstrom@gmail.com.
William G. Mattox, Email: wgmattox2@earthlink.net
Carol L. McIntyre, Email: carol_mcintyre@nps.gov
Svetlana Mechnikova, Email: mechnikova@yandex.ru.
Dave Mossop, Email: dmossop@yukoncollege.yk.ca.
Søren Møller, Email: moller@ruc.dk, https://www.vandrefalk.dk.
Ólafur K. Nielsen, Email: okn@ni.is
Tuomo Ollila, Email: tuomo.ollila@metsa.fi.
Arve Østlyngen, Email: aoestly@online.no.
Ivan Pokrovsky, Email: ivanpok@mail.ru.
Kim Poole, Email: kpoole@aurorawildlife.com.
Marco Restani, Email: Restani@stcloudstate.edu.
Bryce W. Robinson, Email: bryce@ornithologi.com
Robert Rosenfield, Email: rrosenfi@uwsp.edu.
Aleksandr Sokolov, Email: sokhol@yandex.ru.
Vasiliy Sokolov, Email: vsokolov@inbox.ru.
Ted Swem, Email: ted_swem@fws.gov.
Katrin Vorkamp, Email: kvo@envs.au.dk.
References
- Anctil A, Franke A, Bêty J. Heavy rainfall increases nestling mortality of an arctic top predator: experimental evidence and long-term trend in peregrine falcons. Oecologia. 2014;174:1033–1043. doi: 10.1007/s00442-013-2800-y. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Andreasen NP, Falk K, Møller S. The Danish peregrine falcon population: reestablishment and eggshell thinning. Ornis Hungarica. 2018;26:159–163. [Google Scholar]
- Barichello N, Mossop D. The overwhelming influence of ptarmigan abundance on gyrfalcon reproductive success in the central Yukon, Canada. In: Watson RT, Cade TJ, Fuller M, Hunt G, Potapov E, editors. Gyrfalcons and ptarmigan in a changing world. Boise: The Peregrine Fund; 2011. pp. 307–322. [Google Scholar]
- Barnes JG, Doney GE, Yates MA, Seegar WS, Gerstenberger SL. A Broadscale Assessment of mercury contamination in peregrine falcons across the northern latitudes of North America. Journal of Raptor Research. 2019;53:1–13. [Google Scholar]
- Bente PJ. Abundance and multi-year occupancy of gyrfalcons Falco rusticolus on the Seward Peninsula, Alaska. In: Watson RT, Cade TJ, Fuller M, Hunt G, Potapov E, editors. Gyrfalcons and ptarmigan in a changing world. Boise: The Peregrine Fund; 2011. pp. 295–305. [Google Scholar]
- Booms TL, Cade TJ, Clum NJ. Gyrfalcon (Falco rusticolus), version 2.0. In: Poole AF, editor. The birds of North America. Ithaca: Cornell Lab of Ornithology; 2008. [Google Scholar]
- Booms T, Lindgren M, Huettmann F. Linking Alaska’s predicted climate, gyrfalcon, and ptarmigan distributions in space and time: a unique 200-year perspective. In: Watson RT, Cade TJ, Fuller M, Hunt G, Potapov E, editors. Gyrfalcons and ptarmigan in a changing world. Boise: The Peregrine Fund; 2011. pp. 177–190. [Google Scholar]
- Booms TL, Schempf PF, McCaffery BJ, Lindberg MS, Fuller MR. Detection probability of cliff-nesting raptors during helicopter and fixed-wing aircraft surveys in western Alaska. Journal of Raptor Research. 2010;44:175–187. [Google Scholar]
- Bradley M, Oliphant LW. The diet of peregrine falcons in Rankin Inlet, Northwest Territories—an unusually high proportion of mammalian prey. Condor. 1991;93:193–197. [Google Scholar]
- Brown JW. Appraisal of the consequences of the DDT-induced bottleneck on the level and geographic distribution of neutral genetic variation in Canadian peregrine falcons, Falco peregrinus. Molecular Ecology. 2007;16:327–343. doi: 10.1111/j.1365-294X.2007.03151.x. [DOI] [PubMed] [Google Scholar]
- Burnham JH, Burnham KK, Chumchal MM, Welker JM, Johnson JA. Correspondence between mercury and stable isotopes in high Arctic marine and terrestrial avian species from Northwest Greenland. Polar Biology. 2018;41:1475–1491. [Google Scholar]
- Burnham KK, Burnham WA, Newton I, Johnson JA, Gosler AG. The history and range expansion of peregrine falcons in the Thule area, Northwest Greenland. Monographs on Greenland Bioscience. 2012;60:1–106. [Google Scholar]
- Burnham KK, Newton I. Seasonal movements of gyrfalcons Falco rusticolus include extensive periods at sea. Ibis. 2011;153:468–484. [Google Scholar]
- Burnham WA, Mattox WG. Biology of the peregrine and gyrfalcon in Greenland. Monographs on Greenland Bioscience. 1984;14:1–25. [Google Scholar]
- Cade TJ. Falcons of the world. Ithaca: Comstock/Cornell University Press; 1982. [Google Scholar]
- Cade TJ, Enderson JH, Thelander CG, White CM, editors. Peregrine falcon populations; their management and recovery. Boise: The Peregrine Fund; 1988. [Google Scholar]
- Carlzon L, Karlsson A, Falk K, Liess A, Møller S. Extreme weather affects peregrine falcon (Falco peregrinus tundrius) breeding success in South Greenland. Ornis Hungarica. 2018;26:38–50. [Google Scholar]
- Carrière, S., and S. Matthews. 2013. Peregrine falcon surveys along the Mackenzie River, Northwest Territories, Canada. File Report No. 140, Environment and Natural Resources Government of the Northwest Territories, Yellowknife, NT.
- Christensen, T., S. Longan, T. Barry, C. Price, and K. F. Lárusson. 2018. Circumpolar biodiversity monitoring program strategic plan 2018–2021. CAFF Monitoring Series Report No. 29. Conservation of Arctic Flora and Fauna, Akureyri, Iceland.
- Christensen, T., J. Payne, M. Doyle, G. Ibarguchi, J. Taylor, N. M. Schmidt, M. Gill, M. Svoboda, et al. 2013. The Arctic terrestrial biodiversity monitoring plan. CAFF Monitoring Series Report Nr. 7. CAFF International Secretariat, Akureyri, Iceland.
- Court GS, Gates CC, Boag DA. Natural-history of the peregrine falcon in the Keewatin district of the Northwest Territories. Arctic. 1988;41:17–30. [Google Scholar]
- Court GS, Gates CC, Boag DA, Macneil JD, Bradley DM, Fesser AC, Patterson JR, Stenhouse GB, et al. A toxicological assessment of peregrine falcons Falco peregrinus tundrius breeding in the Keewatin district of the Northwest Territories, Canada. Canadian Field-Naturalist. 1990;104:255–272. [Google Scholar]
- Darnerud PO. Brominated flame retardants as possible endocrine disrupters. International Journal of Andrology. 2008;31:152–160. doi: 10.1111/j.1365-2605.2008.00869.x. [DOI] [PubMed] [Google Scholar]
- Dawson RD, Mossop DH, Boukall B. Prey use and selection in relation to reproduction by peregrine falcons breeding along the Yukon River, Canada. Journal of Raptor Research. 2011;45:27–37. [Google Scholar]
- Dietz R, Riget FF, Boertmann D, Sonne C, Olsen MT, Fjeldså J, Falk K, Kirkegaard M, et al. Time trends of mercury in feathers of West Greenland birds of prey during 1851–2003. Environmental Science and Technology. 2006;40:5911–5916. doi: 10.1021/es0609856. [DOI] [PubMed] [Google Scholar]
- Dixon A, Rahman L, Sokolov A, Sokolov V. Peregrine falcons crossing the roof of the world. In: Prins H, Namgail T, editors. Bird migration across the Himalayas: wetland functioning amidst mountains and glaciers. Cambridge: Cambridge University Press; 2017. pp. 128–142. [Google Scholar]
- Dixon A, Sokolov A, Sokolov V. The subspecies and migration of breeding peregrines in northern Eurasia. Falco. 2012;39:4–9. [Google Scholar]
- Falk K, Møller S, Rigét FF, Sørensen PB, Vorkamp K. Raptors are still affected by environmental pollutants: Greenlandic peregrines will not have normal eggshell thickness until 2034. Ornis Hungarica. 2018;26:171–176. [Google Scholar]
- Falkdalen U, Hörnell-Willebrand M, Nygård T, Bergström T, Lind G, Nordin A, Warensjö B. Relations between willow ptarmigan (Lagopus lagopus) density and gyrfalcon (Falco rusticolus) breeding performance in Sweden. In: Watson RT, Cade TJ, Fuller M, Hunt G, Potapov E, editors. Gyrfalcons and ptarmigan in a changing world. Boise: The Peregrine Fund; 2011. pp. 171–176. [Google Scholar]
- Ferguson-Lees J, Christie DA, Ferguson-Lees J, Christie DA. Raptors of the world. Boston: Houghton Mifflin Company; 2001. [Google Scholar]
- Franke A. Population estimates for northern juvenile peregrine falcons with implications for harvest levels in North America. Journal of Fish and Wildlife Management. 2016;7:36–45. [Google Scholar]
- Franke A, Lamarre V, Hedlin E. Rapid nestling mortality in Arctic peregrine falcons due to the biting effects of black flies. Arctic. 2016;69:281–285. [Google Scholar]
- Franke A, Setterington M, Court G, Birkholz D. Long-term trends of persistent organochlorine pollutants, occupancy and reproductive success in peregrine falcons (Falco peregrinus tundrius) breeding near Rankin Inlet, Nunavut, Canada. Arctic. 2010;63:442–450. [Google Scholar]
- Franke A, Steenhoff K, McIntyre CL. Terminology. In: Anderson DL, McClure CM, Franke A, editors. Applied raptor ecology: essentials from gyrfalcon research. Boise: The Peregrine Fund; 2017. pp. 33–42. [Google Scholar]
- Fuglei E, Henden JA, Callahan CT, Gilg O, Hansen J, Ims RA, Isaev AP, Lang J, et al. Circumpolar status of Arctic ptarmigan: population dynamics and trends. Ambio. 2019 doi: 10.1007/s13280-019-01191-0. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fyfe RW, Temple SA, Cade TJ. The 1975 North American peregrine falcon survey. Canadian Field Naturalist. 1976;90:228–273. [Google Scholar]
- Ganusevich, S. A. 2006. Status and monitoring of the peregrine and gyrfalcon in the Kola Peninsula, Russia. In Status of raptor populations in eastern Fennoscandia. Proceedings of the workshop, Kostomuksha, Karelia, Russia, November 8–10, 2005., ed. P. Koskimies and N. V. Lapshin, 30–36. Petrozavodsk, Russia: Karelian Research Centre of the Russian Academy of Science and Finnish-Russia Working Group on Nature Conservation.
- Ganusevich SA, Maechtle TL, Seegar WS, Yates MA, McGrady MJ, Fuller M, Schueck L, Dayton J, et al. Autumn migration and wintering areas of peregrine falcons Falco peregrinus nesting on the Kola Peninsula, northern Russia. Ibis. 2004;146:291–297. [Google Scholar]
- Guerra P, Alaee M, Jimenez B, Pacepavicius G, Marvin C, MacInnis G, Eljarrat E, Barcelo D, et al. Emerging and historical brominated flame retardants in peregrine falcon (Falco peregrinus) eggs from Canada and Spain. Environment International. 2012;40:179–186. doi: 10.1016/j.envint.2011.07.014. [DOI] [PubMed] [Google Scholar]
- Hawk Mountain International. 2019. Peregrine falcon. Retrieved 15 August, 2019, from https://www.hawkmountain.org/raptorpedia/hawks-at-hawk-mountain/hawk-species-at-hawk-mountain/peregrine-falcon/page.aspx?id=648.
- Henny, C. J., S. A. Ganusevich, F. P. Ward, and T. R. Schwartz. 1994. Organochlorine pesticides, chlorinated dioxins and furans, and PCBs in peregrine falcon Falco peregrinus eggs from the Kola Peninsula, Russia. In Raptor Conservation Today, Proceedings of the IV World Conference on Birds of Prey and Owls, Berlin, Germany, 10–17 May 1992, ed. B-U. Meyburg and R.D. Chancellor, 739–749. London: Columbia Environmental Research Center, Patuxent Wildlife Research Center.
- Hickey JJ. Peregrine falcon populations: their biology and decline. Madison: University of Wisconsin Press; 1969. [Google Scholar]
- Jarman WM, Burns SA, Mattox WG, Seegar WS. Organochlorine compounds in the plasma of peregrine falcons and gyrfalcons nesting in Greenland. Arctic. 1994;47:334–340. [Google Scholar]
- Johansen K, Østlyngen A. Ecology of the gyrfalcon in Finnmark based on data from two 11-year periods 150 years apart. In: Watson RT, Cade TJ, Fuller M, Hunt G, Potapov E, editors. Gyrfalcons and ptarmigan in a changing world. Boise: The Peregrine Fund; 2011. pp. 141–160. [Google Scholar]
- Johnson JA, Burnham KK, Burnham WA, Mindell DP. Genetic structure among continental and island populations of gyrfalcons. Molecular Ecology. 2007;16:3145–3160. doi: 10.1111/j.1365-294X.2007.03373.x. [DOI] [PubMed] [Google Scholar]
- Johnson JA, Talbot SL, Sage GK, Burnham KK, Brown JW, Maechtle TL, Seegar WS, Yates MA, et al. The use of genetics for the management of a recovering population: temporal assessment of migratory peregrine falcons in North America. PLoS ONE. 2010;5:e14042. doi: 10.1371/journal.pone.0014042. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Johnstone RM, Court GS, Fesser AC, Bradley DM, Oliphant LW, Macneil JD. Long-term trends and sources of organochlorine contamination in Canadian tundra peregrine falcons, Falco peregrinus tundrius. Environmental Pollution. 1996;93:109–120. doi: 10.1016/0269-7491(96)00037-1. [DOI] [PubMed] [Google Scholar]
- Kéry M, Schmidt BR. Imperfect detection and its consequences for monitoring for conservation. Community Ecology. 2008;9:207–216. [Google Scholar]
- Kjellén, N. 2018. Migration counts at Falsterbo, SW Sweden. Retrieved 7 May, 2018, from https://www.falsterbofagelstation.se/arkiv/strack/migr_frame.htm.
- Koskimies P. Conservation biology of the gyrfalcon (Falco rusticolus) in northern Fennoscandia. In: Watson RT, Cade TJ, Fuller M, Hunt G, Potapov E, editors. Gyrfalcons and ptarmigan in a changing world. Boise: The Peregrine Fund; 2011. pp. 95–124. [Google Scholar]
- Krebs CJ. Ecology: the experimental analysis of distribution and abundance. San Francisco: Benjamin Cummings; 2001. [Google Scholar]
- Kutz SJ, Checkley S, Verocai GG, Dumond M, Hoberg EP, Peacock R, Wu JP, Orsel K, Seegers K, Warren AL, Abrams A. Invasion, establishment, and range expansion of two parasitic nematodes in the Canadian Arctic. Global Change Biology. 2013;19:3254–3262. doi: 10.1111/gcb.12315. [DOI] [PubMed] [Google Scholar]
- Lamarre V, Franke A, Legagneux P, Love O, Bety J. Linking pre-laying energy allocation and timing of breeding in a migratory arctic raptor. Oecoligia. 2017;183:653–666. doi: 10.1007/s00442-016-3797-9. [DOI] [PubMed] [Google Scholar]
- Lamarre V, Legagneux P, Franke A, Casajus N, Currie DC, Berteaux D, Bêty J. Precipitation and ectoparasitism reduce reproductive success in an arctic-nesting top-predator. Scientific Reports. 2018;8:8530. doi: 10.1038/s41598-018-26131-y. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lindberg, P. 1983. Relations between the diet of Fennoscandian peregrines Falco peregrinus and organochlorines and mercury in their eggs and feathers, with a comparison to the gyrfalcon Falco rusticolus. PhD Thesis. Gothenburg, Sweden: University of Göteborg. 10.13140/RG.2.1.1686.5362.
- Lindberg P. Mercury in feathers of Swedish gyrfalcons, Falco rusticolus, in relation to diet. Bulletin of Environmental Contamination and Toxicology. 1984;32:453–459. doi: 10.1007/BF01607522. [DOI] [PubMed] [Google Scholar]
- Lindberg P. The fall and the rise of the Swedish peregrine falcon population. In: Sielicki J, Mizera T, editors. Peregrine Falcon populations—status and perspectives in the 21st century. Poznań: Warsaw and University of Life Sciences; 2008. pp. 125–132. [Google Scholar]
- Lindberg P, Schei PJ, Wikman M. The peregrine falcon in Fennoscandia In Peregrine falcon populations. In: Cade TJ, Enderson JH, White CM, editors. their management and recovery. Boise: The Peregrine Fund; 1988. pp. 159–172. [Google Scholar]
- Loiseau C, Harrigan RJ, Cornel AJ, Guers SL, Dodge M, Marzec T, Carlson JS, Seppi B, Sehgal RNM. First evidence and predictions of plasmodium transmission in Alaskan bird populations. PLoS ONE. 2012;7:e44729. doi: 10.1371/journal.pone.0044729. [DOI] [PMC free article] [PubMed] [Google Scholar]
- MacKenzie DI, Nichols JD, Hines JE, Knutson MG, Franklin AB. Estimating site occupancy, colonization, and local extinction when a species is detected imperfectly. Ecology. 2003;84:2200–2207. [Google Scholar]
- Mattox WG, Restani M. Migratory movements and mortality of peregrine falcons banded in Greenland, 1972–97. Arctic. 2014;67:433–440. [Google Scholar]
- Mattox WG, Seegar WS. The Greenland peregrine falcon survey, 1972-1985, with emphasis on recent population status. In: Cade TJ, Enderson JH, White CM, editors. Peregrine falcon populations; their management and recovery. Boise: The Peregrine Fund; 1988. pp. 27–36. [Google Scholar]
- Matz A, Swem T, Johnson P, Booms T, White C. Potential for climate change to increase contaminants exposure and effects in gyrfalcons. In: Watson RT, Cade TJ, Fuller M, Hunt G, Potapov E, editors. Gyrfalcons and ptarmigan in a changing world. Boise: The Peregrine Fund; 2011. pp. 161–176. [Google Scholar]
- Meyburg B-U, Paillat P, Meyburg C, McGrady M. Migration of a peregrine falcon Falco peregrinus calidus from Saudi Arabia to Cape Town as revealed by satellite telemetry. Ostrich. 2017;89:93–96. [Google Scholar]
- Millsap BA. Demography and metapopulation dynamics of an urban Cooper’s hawk subpopulation. Condor. 2018;120:63–80. [Google Scholar]
- Morozov VV. Ecological basis for the distribution and breeding of gyrfalcons in the tundra of European Russia and preconditions for spreading to new grounds. In: Watson RT, Cade TJ, Fuller M, Hunt G, Potapov E, editors. Gyrfalcons and ptarmigan in a changing world. Boise: The Peregrine Fund; 2011. pp. 229–238. [Google Scholar]
- Mossop DH. Long-term studies of willow ptarmigan and gyrfalcon in the Yukon Territory: a collapsing 10-year cycle and its apparent effect on the top predator. In: Watson RT, Cade TJ, Fuller M, Hunt G, Potapov E, editors. Gyrfalcons and ptarmigan in a changing world. Boise: The Peregrine Fund; 2011. pp. 323–336. [Google Scholar]
- Myers-Smith IH, Elmendorf SC, Beck PSA, Wilmking M, Hallinger M, Blok D, Tape KD, Rayback SA, et al. Climate sensitivity of shrub growth across the tundra biome. Nature Climate Change. 2015;5:887–891. [Google Scholar]
- Newton I. Population ecology of raptors. Berkhamsted: T & A. D. Poyser; 1979. [Google Scholar]
- Newton I. Population regulation in peregrines: an overview. In: Cade TJ, Enderson JH, Thelander CG, White CM, editors. Peregrine falcon populations; their management and recovery. Boise: The Peregrine Fund; 1988. pp. 761–770. [Google Scholar]
- Nielsen OK. Gyrfalcon population and reproduction in relation to rock ptarmigan numbers in Iceland. In: Watson RT, Cade TJ, Fuller M, Hunt G, Potapov E, editors. Gyrfalcons and ptarmigan in a changing world. Boise: The Peregrine Fund; 2011. pp. 21–48. [Google Scholar]
- Nielsen OK, Cade TJ. Annual cycle of the gyrfalcon in Iceland. National Geographic Research. 1990;6:41–62. [Google Scholar]
- Peakall DB, Kiff LF. Eggshell thinning and DDE residue levels among peregrine falcons Falco peregrinus—global perspectives. Ibis. 1979;121:200–203. [Google Scholar]
- Peakall DB, Lew TS, Springer AM, Walker W, Risebrough RW, Monk JG, Jarman WM, Walton BJ, et al. Determination of the DDE and PCB contents of peregrine falcon eggs—a comparison of whole egg measurements and estimates derived from eggshell membranes. Archives of Environmental Contamination and Toxicology. 1983;12:523–528. doi: 10.1007/BF01056547. [DOI] [PubMed] [Google Scholar]
- Peck K, Franke A, Lecomte N, Bêty J. Nesting habitat selection and distribution of an avian top predator in the Canadian Arctic. Arctic Science. 2018;4:499–512. doi: 10.1139/as-2017-0048. [DOI] [Google Scholar]
- Pokrovsky I, Lecomte N. Comparison of gyrfalcon distributions between the Palearctic and Nearctic. In: Watson RT, Cade TJ, Fuller M, Hunt G, Potapov E, editors. Gyrfalcons and ptarmigan in a changing world. Boise: The Peregrine Fund; 2011. pp. 65–70. [Google Scholar]
- Poole KG. Aspects of the ecology, food habits and foraging characteristics of gyrfalcons in the central Canadian Arctic. Raptor Research. 1987;21:80–80. [Google Scholar]
- Poole KG, Bromley RG. Natural-history of the gyrfalcon in the central Canadian Arctic. Arctic. 1988;41:31–38. [Google Scholar]
- Potapov E, Sale R. The gyrfalcon. Yale: Yale University Press; 2005. [Google Scholar]
- R Development Core Team. 2017. R: A language and environment for statistical computing. R Foundation for Statistical Programming, Vienna, Austria.
- Risebrough RW, Peakall DB. The relative importance of several organochlorines in the decline of peregrine falcon populations. In: Cade TJ, Enderson JH, Thelander CG, White CM, editors. Peregrine Falcon populations; their management and recovery. Boise: The Peregrine Fund; 1988. pp. 449–462. [Google Scholar]
- Ritchie R. Raptor surveys at lakes in the foothill-coastal plain transition, Colville to Kuk rivers, NPR-A, Alaska, July 2012 and 2013. Fairbanks: Bureau of Land Management; 2014. [Google Scholar]
- Robinson BW, Booms TL, Bechard MJ, Anderson DL. Dietary plasticity in a specialist predator, the gyrfalcon (Falco rusticolus): New insights into diet during breeding. Journal of Raptor Research. 2019;53:1–12. [Google Scholar]
- Saurola P, Valkama J, Velmala W. The Finnish Bird Ringing Atlas. Helsinki: Finnish Museum of Natural History and Ministry of Environment, Finland; 2013. [Google Scholar]
- Shapiro SS, Wilk MB. An analysis of variance test for normality. Biometrika. 1965;52:591–611. [Google Scholar]
- Sokolov V, Sokolov A, Dixon A. Migratory movements of peregrine falcons Falco peregrinus, breeding on the Yamal Peninsula, Russia. Ornis Hungarica. 2018;26:222–231. [Google Scholar]
- Steenhof K, Newton I. Assessing nesting success and productivity. In: Bird DM, Bildstein KL, editors. Raptor research and management techniques. Blaine: Hancock House; 2007. pp. 181–192. [Google Scholar]
- Steenhof K, Kochert MN, McIntyre CL, Brown JL. Coming to terms about describing golden eagle reproduction. Journal of Raptor Research. 2017;51:378–390. [Google Scholar]
- Talbot SL, Sage GK, Sonsthagen SA, Gravley MC, Swem T, Williams JC, Longmire JL, Ambrose S, et al. Intraspecific evolutionary relationships among peregrine falcons in western North American high latitudes. PLoS ONE. 2017;12:e0188185. doi: 10.1371/journal.pone.0188185. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tucker MA, Alexandrou O, Bierregaard RO, Jr, Bildstein KL, Böhning-Gaese K, Bracis C, Brzorad JN, Buechley ER, et al. Large birds travel farther in homogeneous environments. Global Ecology and Biogeography. 2019;28:576–587. [Google Scholar]
- Van Hemert C, Pearce JM, Handel CM. Wildlife health in a rapidly changing North: focus on avian disease. Frontiers in Ecology and the Environment. 2014;12:548–556. doi: 10.1890/130291. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Vorkamp K, Falk K, Møller S, Bossi R, Riget FF, Sørensen PB. Perfluoroalkyl substances (PFASs) and polychlorinated naphthalenes (PCNs) add to the chemical cocktail in peregrine falcon eggs. Science of the Total Environment. 2019;648:894–901. doi: 10.1016/j.scitotenv.2018.08.090. [DOI] [PubMed] [Google Scholar]
- Vorkamp K, Falk K, Møller S, Riget FF, Sørensen PB. Regulated and unregulated halogenated flame retardants in peregrine falcon eggs from Greenland. Environmental Science and Technology. 2018;52:474–483. doi: 10.1021/acs.est.7b04866. [DOI] [PubMed] [Google Scholar]
- Vorkamp K, Thomsen M, Møller S, Falk K, Sørensen PB. Persistent organochlorine compounds in peregrine falcon (Falco peregrinus) eggs from South Greenland: Levels and temporal changes between 1986 and 2003. Environment International. 2009;35:336–341. doi: 10.1016/j.envint.2008.08.008. [DOI] [PubMed] [Google Scholar]
- Wegner P, Kleinstäuber G, Baum F, Schilling F. Long-term investigation of the degree of exposure of German peregrine falcons (Falco peregrinus) to damaging chemicals from the environment. Journal of Ornithology. 2005;146:34–54. [Google Scholar]
- Wheeler HC, Chipperfield JD, Roland C, Svenning JC. How will the greening of the Arctic affect an important prey species and disturbance agent? Vegetation effects on arctic ground squirrels. Oecologia. 2015;178:915–929. doi: 10.1007/s00442-015-3240-7. [DOI] [PubMed] [Google Scholar]
- Wheeler HC, Hoye TT, Svenning JC. Wildlife species benefitting from a greener Arctic are most sensitive to shrub cover at leading range edges. Global Change Biology. 2018;24:212–223. doi: 10.1111/gcb.13837. [DOI] [PubMed] [Google Scholar]
- White CM. Diagnosis and relationships of North American tundra inhabiting peregrine falcons. Auk. 1968;85:179–191. [Google Scholar]
- White CM, Cade TJ, Enderson JH. Peregrine falcons of the world. Barcelona: Lynx Edicions; 2013. [Google Scholar]
- White CM, Sonsthagen SA, Sage GK, Anderson C, Talbot SL. Genetic relationships among some subspecies of the peregrine falcon (Falco peregrinus), inferred from mitochondrial DNA control-region sequences. Auk. 2013;130:78–87. [Google Scholar]
- Wood, S. 2016. Generalized Additive Mixed Models using ‘mgcv’ and ‘lme4’. Comprehensive R Archive Network.
- Yates MA, Riddle KE, Ward FP. Recoveries of peregrine falcons migrating through the eastern and central United States. In: Cade TD, Enderson JH, White CM, editors. Peregrine falcon populations; their management and recovery. Boise: The Peregrine Fund; 1988. [Google Scholar]
- Zuur AF, Ieno EN, Elphick CS. A protocol for data exploration to avoid common statistical problems. Methods in Ecology and Evolution. 2010;1:3–14. [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.