Highlights
-
•
Longitudinal study investigating the relationship between development of cortical thickness and effortful control during adolescence.
-
•
Examined whether change in effortful control mediated the relationship between cortical development and change in socioemotional functioning, including psychopathology symptoms.
-
•
Greater cortical thinning of the left ACC was associated with superior development of effortful control.
-
•
Changes in effortful control mediated the relationship between greater thinning of the left ACC and improvements in socioemotional functioning.
Keywords: Self-regulation, Effortful control, Cortical development, Adolescence, Longitudinal study
Abstract
This study investigated the relationship between the development of effortful control (EC), a temperamental measure of self-regulation, and concurrent development of three regions of the prefrontal cortex (anterior cingulate cortex, ACC; dorsolateral prefrontal cortex, dlPFC; ventrolateral prefrontal cortex, vlPFC) between early- and mid-adolescence. It also examined whether development of EC mediated the relationship between cortical maturation and emotional and behavioral symptoms. Ninety-two adolescents underwent baseline assessments when they were approximately 12 years old and follow-up assessments approximately 4 years later. At each assessment, participants had MRI scans and completed the Early Adolescent Temperament Questionnaire-Revised, as well as measures of depressive and anxious symptoms, and aggressive and risk taking behavior. Cortical thicknesses of the ACC, dlPFC and vlPFC, estimated using the FreeSurfer software, were found to decrease over time. EC also decreased over time in females. Greater thinning of the left ACC was associated with less reduction in EC. Furthermore, change in effortful control mediated the relationship between greater thinning of the left ACC and improvements in socioemotional functioning, including reductions in psychopathological symptoms. These findings highlight the dynamic association between EC and the maturation of the anterior cingulate cortex, and the importance of this relationship for socioemotional functioning during adolescence.
1. Introduction
Self-regulation refers to internal and/or transactional processes that regulate emotion, cognition and behavior (Karoly et al., 2005, Steinberg, 2005). It plays a crucial role in the development of various aspects of functioning, and has been associated with increased social competence (Spinrad et al., 2006), superior coping abilities (Eisenberg et al., 1997), and academic success (Duckworth and Seligman, 2005, Eisenberg et al., 2010). Poorer regulatory capabilities, on the other hand, are related to problems with attention and hyperactivity, sexual risk taking, and addiction (Crockett et al., 2006, Quinn and Fromme, 2010), as well as other externalizing and internalizing problems (Eisenberg et al., 2010, Valiente et al., 2004).
Self-regulation has often been studied by developmental researchers within a temperamental framework (Bridgett et al., 2013, Zhou et al., 2012). Effortful control (EC), a construct that consistently arises from factorial analyses of temperament questionnaires, describes the ability to shift and focus attention, and inhibit a dominant response and/or activate a subdominant response (Rothbart and Rueda, 2005). The current study examined EC using a self-report measure of temperament specifically designed for adolescents: the Early Adolescent Temperament Questionnaire-Revised (EATQ-R; Capaldi and Rothbart, 1992, Ellis and Rothbart, 2001). EC is composed of three subscales within the EATQ-R; activity control, inhibitory control, and attentional control. Individuals with high levels of EC have been found to exhibit greater regulation of their attention, emotions and behavior, which is thought to have positive effects for their social and emotional functioning (Eisenberg and Spinrad, 2004).
Rothbart et al. (2003) propose that individual differences in EC are determined by the functioning of the executive attention network (Posner, 2012, Rothbart et al., 2007), which is thought to comprise the anterior cingulate cortex (ACC) and lateral prefrontal cortices (Posner, 2012, Rothbart and Rueda, 2005). Specifically, the ACC is thought to monitor competition between conflicting processes and signal this information to the lateral PFC (Botvinick et al., 1999, Casey et al., 2001). Within the lateral PFC, the dorsolateral prefrontal cortex (dlPFC) is involved in keeping goals active within working memory and directing attention, and the ventrolateral prefrontal cortex (vlPFC) is implicated in selection of goal-appropriate responses based on information from semantic memory, such as the causes, significance and potential outcomes of the situation (Kalisch, 2009, Ochsner and Gross, 2008, Ochsner et al., 2012).
1.1. Adolescent development
Adolescence is characterized by significant improvements in self-regulatory abilities. Although the development of EC has not been examined during this period, numerous studies have identified improvements in related constructs. For example, performance on tasks of cognitive control that tap into the same executive attention network that is implicated in EC has been found to improve into early adulthood (Leon-carrion et al., 2004, Luna et al., 2004). Similarly, emotion regulatory abilities have been found to continue to develop through adolescence (Garnefski and Kraaij, 2006, McRae et al., 2012). Furthermore, there is also marked cortical maturation during adolescence, including post-pubertal reductions in gray matter thickness (Brown et al., 2012, Mills et al., 2012, Mutlu et al., 2013, Shaw et al., 2008, Tamnes et al., 2010a). Specifically, higher-order association cortices within the prefrontal region, such as those underlying EC, have been found to exhibit protracted development into the early adult years (Gogtay et al., 2004).
However, little is known about the development of EC in relation to structural maturation of its presumed neurobiological substrates during adolescence. Although investigators have postulated that the protracted development of self-regulation may be attributed to delayed prefrontal cortical maturation (Nelson et al., 2005, Sowell, 2004), limited research has specifically investigated this relationship. To our knowledge, only one longitudinal study has directly examined within-subject change in gray matter structure in relation to change in self-regulatory abilities. Tamnes et al. (2013) found that improvements in working memory were related to reductions in cortical volume of the prefrontal and posterior parietal regions between 9 and 20 years of age. Cross-sectional studies are also limited, with inconsistent results to date (Fjell et al., 2012, Tamnes et al., 2010b, Østby et al., 2011). Therefore, further research is needed to examine the relationship between cortical maturation and development of self-regulatory processes.
Adolescence is also a period of increasing prevalence of various forms of psychopathology, such as depression, anxiety, substance use, and conduct problems (Paus et al., 2008). There is now a well-established role of regulatory processes in the development of psychopathology. Low levels of EC have consistently been related to the presence of internalizing symptoms, such as depression and anxiety, as well as externalizing symptoms, such as aggression, conduct problems, risk taking and hyperactivity (Eisenberg et al., 2001, Muris and Meesters, 2009, Oldehinkel et al., 2004, Valiente et al., 2004). Therefore, EC may be one potential indirect pathway through which neurobiological changes in the prefrontal regulatory regions impact on behavioral outcomes.
1.2. The current study
Given the paucity of research examining concurrent development of neurobiology and behavior, the current study investigated the relationship between development of EC and concurrent maturation of three hypothesized neural substrates (ACC, dlPFC, and vlPFC), in a community sample of adolescents. The study also investigated whether change in EC mediated the relationship between cortical maturation and the development of internalizing and externalizing symptoms. Sex effects in these relationships were also explored as there is some evidence of differences between males and females in cortical maturation (Mutlu et al., 2013, Raznahan et al., 2010) and in EC (Else-Quest et al., 2006).
A longitudinal design was employed to assess within-subject change between early- and mid-adolescence, with the age range of the sample being strictly constrained at both periods of assessment. To our knowledge, no longitudinal research has been conducted in this area using self-reported termperamental assessment of self-regulation, or using measures of psychopathology symptoms. Given that cortical maturation is characterized by reductions in thickness during adolescence, and more rapid thinning has been associated with higher levels of cognitive ability (Tamnes et al., 2013), it was hypothesized that greater thinning of the lateral PFC and ACC would be associated with an increase in EC, which would in turn be associated with improvements in socioemotional functioning and reductions in symptoms of psychopathology between early- and mid-adolescence.
2. Methodology
2.1. Participants
The sample described in the current study was derived from the Orygen Adolescent Development Study (OADS) cohort, a large longitudinal study of risk and resilience for the development of psychopathology, conducted in Melbourne, Australia.
This project was approved by the Human Research Ethics Committee at the University of Melbourne, Australia. Consent to participate in the study was obtained from both the child and at least one parent at all time points.
The OADS recruited 2453 students in their final year of primary school from schools across metropolitan Melbourne to participate in an initial school-screening phase, which involved completion of the Early Adolescent Temperament Questionnaire-Revised (see below EATQ-R; Capaldi and Rothbart, 1992, Ellis and Rothbart, 2001). Selection into the OADS was based on scores on two temperamental dimensions, Negative Emotionality and EC, based on their hypothesized role as risk factors for emotional and behavioral disorders. Accordingly, equal numbers of male and female students were selected from each of the following ranges of scores (i.e. “bins”) on each dimension: 0–1, 1–2, 2–2.5, and greater than 2.5 SD above and below the mean. This produced a smaller risk-enriched sample of 425 (16%) that showed a relatively even distribution across EC and Negative Emotionality, while maintaining the range of temperament scores evident in the initial school screening sample (Yap et al., 2008a).
Of the selected adolescents, 245 (58%) agreed to participate in further research. These participants were screened for DSM-IV Axis-I disorders using the Schedule for Affective Disorder and Schizophrenia for School-Aged Children: Present and Lifetime Version (Kaufman et al., 1997) and those who met the criteria for current or past MDD were excluded due to the broader aims of the study. Remaining participants were invited to take part in brain Magnetic Resonance Imaging (MRI) and temperament assessments (see below) at two time points, when they were aged approximately 12 and 16, respectively. A number of adolescents declined participation in the MRI assessments, resulting in 120 participants completing all assessments at both baseline and follow-up. Based on visual inspection of processed MRI data (i.e., FreeSurfer cortical parcellation, see below) by researchers trained in neuroanatomy, 17 of these participants were excluded due to poor MRI image quality and parcellation. Only 11 participants were predominantly left handed, as assessed by the Edinburgh Handedness Inventory (Oldfield, 1971). Given that this number was too small to examine handedness effects, left-handers were excluded due to potential laterality effects (Good et al., 2001).
Following MRI and handedness exclusions, 92 participants (47 = females) were available for analysis. Males and females did not differ on the demographic and cognitive variables listed in Table 1 (all p values > 0.05). IQ was assessed at baseline using a short form of the Weschler Intelligence Scale for Children, Fourth Version (Wechsler, 2003) and socioeconomic status (SES) was based on the Australian National University Four (ANU4) 100-point scale (Jones and McMillan, 2001). The final sample did not differ from the initial school screening sample (N = 2453) on socioeconomic disadvantage (t(2439) = 0.598; p = 0.550), EC (t(96) = −0.592; p = 0.555) or gender (Pearson's χ2 = 0.799; p = 0.939). The observed frequency of participants within each temperament “bin” in the final sample did not differ from that of the original selected sample (N = 415). Fifteen participants of the final sample met the criteria for past or current psychiatric disorder at baseline and twenty-six participants met the criteria for a psychiatric diagnosis between baseline and follow-up (of which seventeen had not met any criteria at baseline). Refer to table S1 for further detail on psychiatric diagnoses.
Table 1.
Sample characteristics.
| Sex |
Total | ||
|---|---|---|---|
| Male | Female | ||
| Sample size | 45 | 47 | 92 |
| Age at baseline (years) | 12.67; 0.422; 11.37–13.61 | 12.65; 0.457; 11.95–14.08 | 12.67; 0.438; 11.37–14.08 |
| Age at follow up (years) | 16.42; 0.464; 14.96–17.27 | 16.44; 0.54; 15.28–17.49 | 16.43; 0.503; 14.96–17.69 |
| Time interval (years)a | 3.76; 0.166; 3.48–4.12 | 3.78; 0.295; 2.69–4.56 | 3.77; 0.240; 2.69–4.56 |
| Estimated full scale IQ | 107.06; 11.023; 79–128 | 104.07; 10.924; 87–123 | 105.53; 11.015; 79–128 |
| SESb | 58.98; 20.22; 14.4–96.0 | 58.53; 22.13; 14.0–100.0 | 58.76; 21.220; 14.0–100.0 |
NB: Values represent Mean; standard deviation; range.
Time interval between baseline and follow-up MRI scan.
Data missing for one female participant.
2.2. MRI acquisition and analysis
2.2.1. Image acquisition
At baseline, MRI scans were performed on a 3 Tesla GE scanner at the Brain Research Institute, Austin and Repatriation Medical Centre, Melbourne, Australia, with the following parameters: repetition time = 36 ms; echo time = 9 ms; flip angle = 35°, field of view = 20 cm2, 124 T1-weighted contiguous slices (voxel dimensions = 0.4883 × 0.4883 × 1.5 mm). MRI scans at follow-up were performed on a 3 Tesla Siemens scanner at the Royal Children's Hospital, Melbourne, Australia, with the following parameters: repetition time = 1900 milliseconds; echo time = 2.24 milliseconds; flip angle = 9°, field of view = 23 cm; 176 T1-weighted contiguous 0.9-mm thick slices (voxel dimensions = 0.9 mm3).
2.2.2. Processing
Images were transferred to an SGI/Linux workstation for morphometric analysis. Cortical reconstruction was performed using the FreeSurfer image analysis suite (http://surfer.nmr.mgh.harvard.edu/). In order to address issues arising from the longitudinal and multisite MRI locations (such as geometric distortion and voxel dimension drift), images were processed through the longitudinal stream of FreeSurfer (Reuter et al., 2012), which creates a within-unbiased subject template space and average image from both time points using robust, inverse consistent registration (Reuter and Fischl, 2011, Reuter et al., 2010). The template is used as an estimate to initialize subsequent segmentation processes in the longitudinal stream for each time point, providing common information regarding anatomical structures. This process can deal with different intensity scales, guarantees inverse consistency (i.e., symmetry), and automatically estimates a sensitivity parameter to detect outlier regions in the image. It significantly improves the repeatability and power of cortical thickness measurements, having superior robustness with respect to noise, intensity scaling and outliers when compared to alternate registration tools (Reuter et al., 2010). All images were also corrected for tissue signal inhomogeneity using FreeSurfer's N3 correction (optimized for 3 Tesla images), a non-parametric non-uniformity intensity normalization method, which reduces sensitivity to changes in scanner platform and improves accuracy and robustness during cortical segmentation (Boyes et al., 2008, Han and Fischl, 2007, Zheng et al., 2009). All FreeSurfer image processing was conducted on a high performance computing facility at the Melbourne Neuropsychiatry Centre, Melbourne, Australia.
2.2.3. ROI delineation
A customized ACC ROI was created by combining the rostral and caudal ACC labels defined by FreeSurfer's automated cortical parcellation procedure (Desikan et al., 2006, Fischl, 2004). The dlPFC ROI was created by combining the superior frontal, rostral middle frontal and caudal middle frontal gyri, while the vlPFC ROI was created by combining the pars opercularis, pars triangularis and pars orbitalis, as labeled by FreeSurfer. A coronal cut was applied at Talairach coordinate y = 26 to the dlPFC and vlPFC ROIs so that only prefrontal regions were included (in order to conform to the conservative Talairach criteria described by Rajkowska and Goldman-Rakic (1995). In addition, another cut was made along the superior edge of medial wall of the brain for the dlPFC ROI, in order to exclude the medial surface of the brain.
2.2.4. Interscanner reliability
Given that different scanners were used at time 1 and 2, a reliability analysis was undertaken to address concerns that changes in cortical thickness over time may be due to measurement bias from the different scanner platforms and acquisition parameters. Four individuals, aged 23, 28, 35 and 36 were each scanned at RCH and BRI within a two-week period. The same acquisition parameters were used at each location to those described above, as well as the same semi-automated methods of processing to extract ROI thickness.
Based on mean absolute thickness difference, inter-scanner variability was found to vary from 0.04 mm to 0.09 mm across ROIs, as shown in Table 2. These variations are consistent with within scanner estimates previously reported (Han et al., 2006). Table 2 also contains test-retest reproducibility errors and 95% confidence intervals for ROIs. The reproducibility errors for each subject were calculated as the absolute test–retest percent change relative to the mean test–retest value. These values were then averaged across subjects. These results did not reveal a systematic bias due to changing scanners, as the confidence intervals for reproducibility errors contained zero for all ROIs apart from the right dlPFC. Given the importance of this issue, the data from the inter-scanner reliability analysis was applied to the current sample using the descriptive procedure proposed by Lebel and Beaulieu (2011). Details of the inter-scanner reliability study are presented in the Supplementary section. In summary, the results indicated that, for all ROIs, the majority of individuals (>50%) experienced greater change over time, in the predicted direction, than would be attributed to inter-scanner variance alone based on the reliability estimates.
Table 2.
Reproducibility errors (%) and confidence interval of the reproducibility errors (%) of ROI thickness for individuals scanned at both sites for reliability analysis.
| Structure | Mean absolute difference (mm) | Reproducibility error (%) | 95% CI for reproducibility Error (%) |
|---|---|---|---|
| Left ACC | 0.043 | 1.400 | −1.151, 3.952 |
| Right ACC | 0.074 | 2.531 | −1.671, 6.734 |
| Left dlPFC | 0.090 | 3.450 | −0.316, 7.216 |
| Right dlPFC | 0.081 | 3.141 | 0.907, 5.376 |
| Left vlPFC | 0.087 | 3.221 | −0.383, 6.825 |
| Right vlPFC | 0.083 | 3.091 | −0.700, 6.251 |
NB: The group reproducibility error for each structure is derived averaging the reproducibility errors across subjects, where for each subject the error is estimated as the absolute test–retest thickness percent change relative to the mean test–retest thickness.
2.3. Effortful control
Participants completed the EATQ-R, which consists of 65 items used to derive ten subscales that load onto four higher order factors (EC, negative affectivity, surgency, and affiliation), and two additional behavioral scales to examine socioemotional functioning. Of interest to the current study is the EC factor, which is comprised of the activity control, inhibitory control, and attentional control subscales. Attentional control is the capacity to focus and shift attention as required, inhibitory control refers to the capacity to plan and suppress inappropriate behavior, and activity control is the ability to perform an action when there is a strong desire/tendency to avoid it. Higher scores reflect higher EC.
2.4. Socioemotional functioning
Participants completed a number of measures assessing socioemotional functioning. At baseline and follow-up, depressive symptoms were measured using the self-report Center for Epidemiological Studies-Depression Scale (CES-D; Radloff, 1977). The questionnaire contains 20 items that relate to mood, somatic complaints, relations with others, and motor functioning during the past week. The CES-D has been found to be a valid and reliable measure for use in adolescent populations (Garrison et al., 1991). Based on the recommended clinical cut-off, 28% of adolescents at baseline and 17% of adolescents at follow-up exhibited clinically significant levels of depressive symptoms. Anxiety symptoms were also measured at baseline and follow-up using the self-report Beck Anxiety Inventory (BAI). The questionnaire contains 21 items relating to anxiety symptoms in the past week. The BAI has been found to be valid and highly reliable (Beck et al., 1988). Based on the recommended clinical cut-off, 18% of adolescents at baseline and 11% of adolescents at follow-up experienced clinically significant levels of anxiety symptoms. Aggressive behavior was also measured at both time points using the EATQ-R. As mentioned above, the EATQ-R is a self-report questionnaire with strong validity and reliability. The aggressive behavior scale is one of the two behavioral scales within the EATQ-R that assess socioemotional functioning (Ellis & Rothbart, 2001). Risk taking behavior was measured at follow-up using the Youth Risk Behavior Scale (YRBS). This YRBS is a self-report questionnaire assessing engagement in substance use, risky sexual behavior, unhealthy dietary practices, violence, and other reckless behavior adolescents. It has been found to be reliable and valid (Brener et al., 1995).
2.5. Puberty
Pubertal development was assessed at baseline using the parent-report Pubertal Development Scale (Petersen et al., 1988). For females, this measure includes 8 items assessing the stage of breast development, hair growth, acne presence, hip width and menarcheal status. For males, this measure includes 11 items assessing genitalia development, hair growth, acne presence and voice change. Reliability and validity of the PDS has been well established (Brooks-Gunn et al., 1987, Petersen et al., 1988). For descriptive purposes, the PDS data was coded into a 5-point scale in accordance with the Tanner stages based on Shirtcliff et al. (2009). This information is presented in table S2.
2.6. Statistical analysis
All analyses were conducted using SPSS version 20 and results were considered significant at p < 0.05. Separate linear mixed models (LMMs) were used to investigate change in each ROI thickness (6 models), EC (1 model) and each behavior/psychopathology measure (5 models). All LMMs included the main effect of time (within-subjects factor) and sex (between-subjects factor), the interaction between time and sex, as well as baseline puberty and SES as covariates. All models were re-analyzed using baseline age, instead of baseline puberty, as a covariate.
Given that the time interval between baseline and follow-up varied between individuals (see Table 1), correlational analyses were conducted to examine whether differences in time interval between baseline and follow-up assessments (i.e., T2–T1 age) were associated with development (i.e., T2–T1 cortical thickness/behavioral scores). Statistically significant associations were identified between time interval and change in right dlPFC thickness (r = −0.216) and EC (r = −.207). When time interval was also included in the LMMs for right dlPFC development and EC development, however, the pattern of significant findings did not change. Thus, variation in time interval was deemed to be non-meaningful, and time interval was modeled as a categorical (rather than nuisance) variable.
Subsequently, in order to examine associations between change in each measure, change scores for each measure were calculated as standardized residuals, by regressing follow-up scores on baseline scores. These residual change scores were used in mediation analyses to examine the indirect effect of changes in cortical thickness on changes in behavior/psychopathology symptoms, through changes in EC. Mediation analyses were performed using macros developed by Preacher and Hayes (2004), implemented in SPSS 20. Mediation was tested by assessing the significance of the cross product of the coefficients for the IV (brain change) to mediator (EC change) relation (the a path), and the mediator to DV (change in behavior/psychopathology symptoms) relation, controlling for the IV (the b path). The a-b cross product tests the statistical significance of the difference between the total effect, or c path, and the direct effect, or c’ path, which is the impact of the IV on the DV adjusting for the effect of the mediator (see Fig. 1 for an example of the mediation analysis). Sex, SES and puberty at baseline were used as covariates in the mediation analyses. Finally, moderated-mediation analyses were used to assess whether the relationship between brain change and change in behavior/psychopathological symptoms, as mediated by change in EC, was moderated by sex. SES and puberty at baseline were used as covariates in these moderated-mediation analyses. Again, all mediation and moderated-mediation models were re-analyzed using baseline age, instead of baseline puberty, as a covariate.
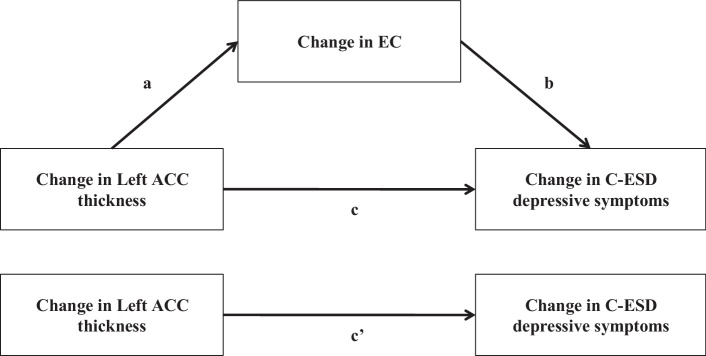
Fig. 1.
Mediation analysis of left ACC maturation on development of depressive symptoms through change in EC. Path a is the total effect of left ACC maturation (i.e. change in cortical thickness) on development of EC (i.e. change in EC), path b is the total effect of development of EC on development of depressive symptoms (i.e. change in depressive symptoms), path c is the total effect of left ACC maturation on development of depressive symptoms (i.e., not controlling for EC change), and path c’ is the direct effect of left ACC maturation on development of depressive symptoms (i.e. controlling for EC change).
A bootstrapping method was applied to test the significance of the mediation effect (Preacher and Hayes, 2004). This method generates an empirical approximation of the sampling distribution of a statistic by repeated random resampling from the available data, and uses this distribution to construct bias corrected and accelerated confidence intervals (Hayes, 2009). If the confidence intervals do not contain zero, the point estimate is significant at the level indicated.
2.7. Treatment of missing data
Six, seven and three participants in the final sample had missing EATQ-R (EC and Aggressive behavior scales), CESD and BAI data at baseline or follow-up, respectively. Ten participants had missing puberty data at T1, and one participant had missing YRBS data at T2. Little's MCAR test was found to be non-significant for all variables (p > .05), indicating that the data were missing completely at random, and thus, missing data was imputed using the Expectation Maximization procedure in SPSS version 20.
3. Results
3.1. Development of the cortex, EC, and socioemotional functioning
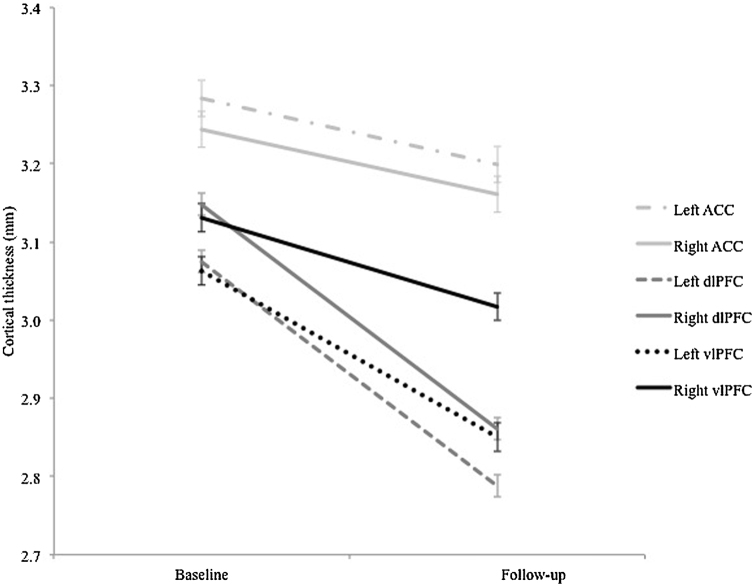
Descriptive statistics for ROI thickness, EC, behavior and psychopathology symptoms at T1 and T2 are reported in Table 3. Refer to table S4 for correlations between all variables of interest. LMMs revealed significant reductions in thickness between T1 and T2 for all six regions (see Fig. 2; left ACC: b = 0.048; t(91) = 2.710, p = 0.008; right ACC: b = 0.119; t(91) = 6.560, p < 0.001; left dlPFC: b = 0.270; t(91) = 14.769, p < 0.001; right dlPFC: b = 0.304; t(91) = 20.510, p < 0.001; left vlPFC: b = 0.212.; t(91) = 11.616, p < 0.001; right vlPFC: b = 0.113.; t(91) = 6.545, p < 0.001). There was also a significant main effect of sex on left dlPFC (b = 0.070; t(88) = 2.791, p = 0.006), right dlPFC (b =0.093; t(88) = 3.791, p < 0.001), left vlPFC thickness (b = 0.063; t(88) = 1.995, p = 0.049), and right vlPFC thickness (b = 0.120; t(88) = 3.680, p < 0.000), with males having thicker cortices across the lateral PFC compared to females. No other effects were significant.
Table 3.
Mean (SD) of ROI thickness, EC, behavior and psychopathology symptoms at baseline (T1) and follow-up (T2).
| Overall |
Males |
Females |
||||
|---|---|---|---|---|---|---|
| T1 | T2 | T1 | T2 | T1 | T2 | |
| Left ACC | 3.27 (0.25) | 3.22 (0.24) | 3.30 (0.21) | 3.24 (0.23) | 3.23 (0.28) | 3.20 (0.26) |
| Right ACC | 3.26 (0.23) | 3.14 (0.22) | 3.26 (0.21) | 3.14 (0.17) | 3.26 (0.25) | 3.15 (0.25) |
| Left dlPFC | 3.07 (0.15) | 2.80 (0.15) | 3.11 (0.15) | 2.82 (0.17) | 3.03 (0.15) | 2.77 (0.13) |
| Right dlPFC | 3.16 (0.15) | 2.82 (0.15) | 3.19 (0.15) | 2.91 (0.14) | 3.12 (0.13) | 2.80 (0.13) |
| Left vlPFC | 3.06 (0.18) | 2.85 (0.18) | 3.09 (0.18) | 2.89 (0.19) | 3.04 (0.17) | 2.82 (0.16) |
| Right vlPFC | 3.13 (0.19) | 3.02 (0.180 | 3.18 (0.19) | 3.09 (0.16) | 3.08 (0.18) | 2.94 (0.17) |
| EC | 3.53 (0.63) | 3.27 (0.67) | 3.41 (0.65) | 3.26 (0.64) | 3.66 (0.60) | 3.27 (0.71) |
| Depressive symptoms | 31.10 (8.25) | 30.05 (7.43) | 31.54 (8.92) | 28.77 (7.14) | 30.52 (7.61) | 31.28 (7.57) |
| Anxiety symptoms | 8.25 (7.72) | 7.66 (6.74) | 7.75 (7.33) | 5.86 (5.96) | 8.73 (8.13) | 9.38 (7.05) |
| Aggressive behavior | 2.15 (0.74) | 2.05 (0.65) | 2.29 (0.80) | 2.14 (0.60) | 2.02 (0.66) | 1.96 (0.69) |
| Risk taking | 7.08 (6.24) | 6.76 (6.59) | 7.58 (5.94) | |||
Fig. 2.
Change in cortical thickness (mm) of ROIs between baseline and follow-up assessments. Error bars represent standard error.
LMMs investigating change in behavior and psychopathology symptoms did not reveal any significant main effects or interactions.
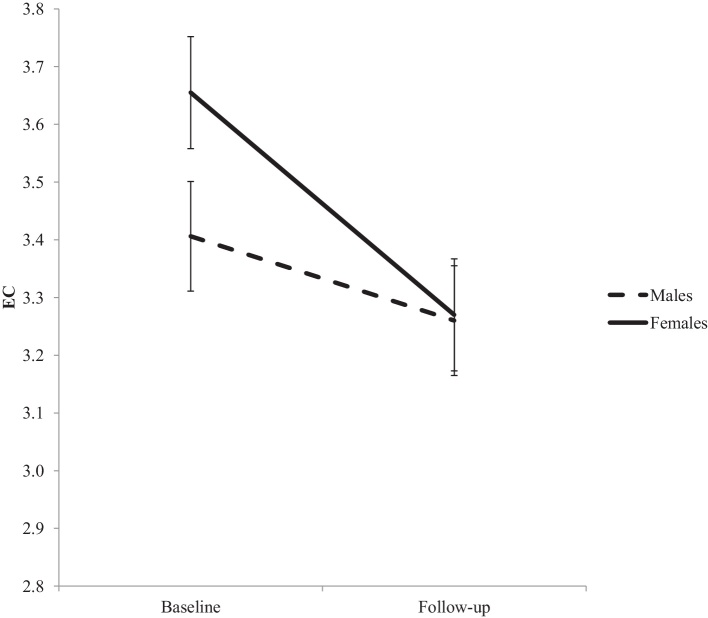
The LMM investigating EC development revealed a significant interaction between time and sex (b = −2.39; t(90) = −2.235, p = 0.028; see Fig. 3). Post-hoc analyses conducted separately for each sex showed significant reductions in EC between T1 and T2 in females (b = 0.386; t(46) = 6.325, p < 0.001), but no such effect in males (b = 0.146; t(44) = 1.642, p = 0.108). Females and males did not differ in EC scores at T1 or T2 (p > 0.05).
Fig. 3.
Change in EC between baseline and follow-up assessments, for males and females. Error bars represent standard error.
When these models were re-analyzed with baseline age as a covariate, as opposed to puberty, the pattern of significant and non-significant effects remained the same. Baseline age had a significant main effect on right vlPFC thickness alone (b = −0.104; t(88) = −2.896, p = 0.005), with older age related to thinner cortices.
3.2. The effect of cortical development on change in socioemotional functioning through EC development
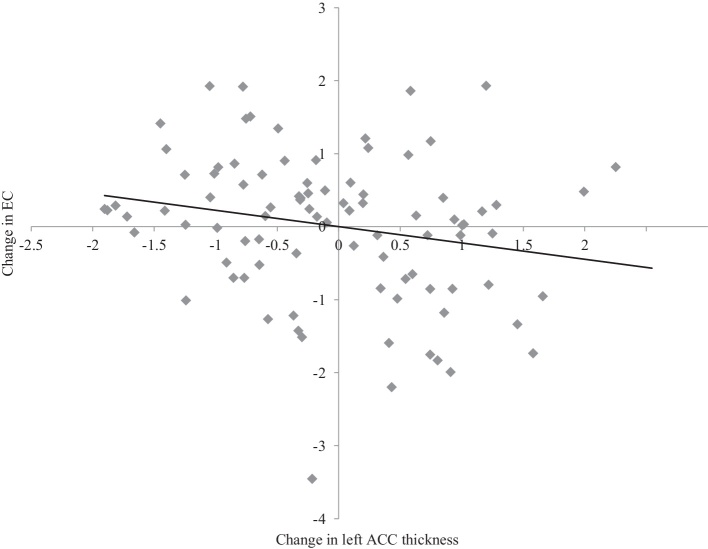
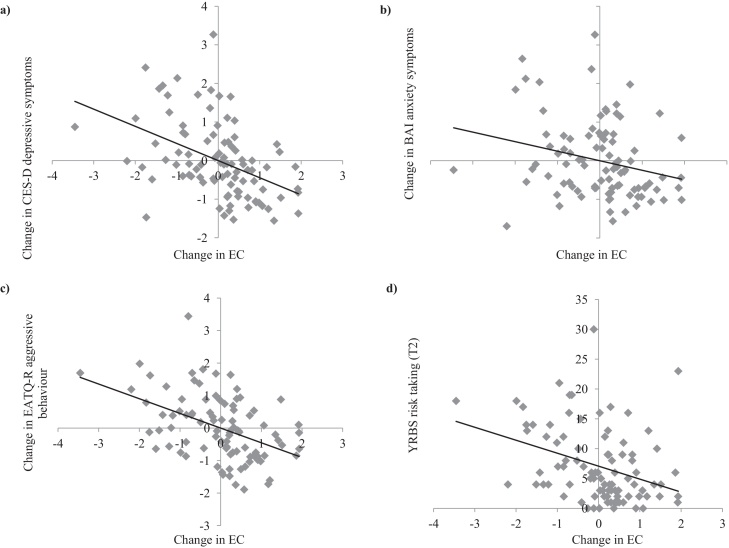
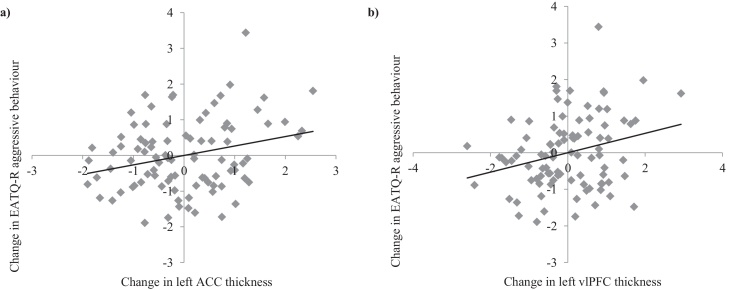
Mediation models examined the indirect effect of change in cortical thickness on changes in behavior and psychopathology symptoms, through change in EC (change for each variable of interest was calculated as standardized residual scores). The results for all mediation models involving the left ACC are reported in Table 4. Results revealed that greater thinning of left ACC thickness was associated with less reductions in EC (path a, see Fig. 4), and that less reductions in EC were related to reductions in depression, anxiety, and aggressive behavior, as well as lower risk taking at T2 (path b, see Fig. 5). There was no significant direct effect of left ACC thinning (path c’, i.e., controlling for EC change) or total effect (path c, i.e., not controlling for EC change) on depression, anxiety and risk taking. However, there was a significant direct and total effect of left ACC thinning of aggressive behavior, with greater thinning related to reductions in aggression (see Fig. 6a). Furthermore, bias-corrected 95% confidence intervals showed that less reduction in EC significantly mediated the relationship between greater left ACC thinning and reductions in depression, anxiety and aggressive behavior, as well as lower risk taking at T2. Sex had a significant main effect on change in anxiety (b = 0.44, SE = 0.20; t = 2.14, p = 0.035), with females exhibiting an increase in symptoms compared to males.
Table 4.
Mediation analyses: the indirect effect of left ACC development on behavior and psychopathology symptoms through EC development.
| Path a | Path b | Path c’: Direct effect | Path c: Total effect | Indirect effect | |
|---|---|---|---|---|---|
| Depressive symptoms | b = 0.22, SE = 0.10, t = −2.08, p = 0.041 | b = −0.43, SE = 0.10, t = −4.33, p < 0.001 | b = −0.03, SE = 0.10, t = −0.29, p = 0.773 | b = 0.06, SE = 0.11, t = 0.61, p = 0.544 | CI: 0.018—0.195, M = 0.09, SE = 0.04 |
| Anxiety symptoms | b = 0.22, SE = 0.10, t = −2.08, p = 0.041 | b = −0.21, SE = 0.10, t = −2.04, p = 0.044 | b = −0.01, SE = 0.10, t = −0.14, p = 0.886 | b = 0.03, SE = 0.10, t = 0.30, p = 0.762 | CI: 0.003—0.143, M = 0.05, SE = 0.03 |
| Aggressive behavior | b = −0.22, SE = 0.10, t = −2.08, p = 0.041 | b = −0.44, SE = 0.10, t = −4.67, p < 0.001 | b = 0.19, SE = 0.10, t = 2.01, p = 0.047 | b = 0.28, SE = 0.10, t = 2.79, p = 0.007 | CI: 0.022—0.19, M = 0.10, SE = 0.04 |
| Risk taking | b = −0.22, SE = 0.10, t = −2.08, p = 0.041 | b = −2.13, SE = 0.66, t = −3.23, p = 0.002 | b = 0.22, SE = 0.65, t = 0.33, p = 0.742 | b = 0.67, SE = 0.67, t = 1.01, p = 0.318 | CI: 0.060—1.215,
M = 0.46, SE = 0.28 |
Fig. 4.
Change in left ACC thickness in relation to change in EC. Change scores represent standardized residuals from regression follow-up scores from baseline scores. Negative scores represent greater reductions, while positive scores represent less reduction.
Fig. 5.
Change in EC in relation to (a) change in C-ESD depressive symptoms, (b) change in BAI anxiety symptoms and (c) change in EATQ-R aggressive behavior, and (d) YRBS risk taking at T2. Change scores represent standardized residuals from regression follow-up scores from baseline scores. Negative change scores on the x-axis represent greater reductions, while positive change scores represent less reduction. Negative change scores on the y-axis represent reductions, while positive change scores represent increase.
Fig. 6.
Change in (a) left ACC and (b) left vlPFC thickness in relation to change in EATQ-R aggressive behavior. Change scores represent standardized residuals from regression follow-up scores from baseline scores. Negative change scores on the x-axis represent greater reductions, while positive change scores represent less reduction. Negative change scores on the y-axis represent reductions, while positive change scores represent increase.
Mediation models analyzing other ROIs did not identify any significant effects on symptoms, apart from a significant direct effect of left vlPFC thickness on aggressive behavior (b = 0.22, SE = 0.10; t = 2.29, p = 0.025). As shown in Fig. 6b, greater left vlPFC thinning was related to reductions in aggressive behavior.
As moderated mediation is possible in the absence of simple mediation (Preacher and Hayes, 2004), we tested the moderating role of sex in the above analyses (using model 58 of macro). The interaction between cortical development and sex in the mediator model (i.e. predicting EC) was not found to be significant for any of the ROIs. However, there was a significant interaction between EC and sex in predicting anxiety (i.e. the dependent variable model). Running analyses separately by sex revealed that less reduction in EC was significantly related to reductions in anxiety symptoms in females (b = −0.47, SE = 0.16; t = −3.05, p = 0.04), but not in males (b = -0.03, SE = 0.14; t = −0.22, p = 0.83). However, indirect effects of cortical development on anxiety symptoms through EC were not present when examining the two sexes separately (males: CI = −0.054—0.079, M = 0.01, SE = 0.03; females: CI = −0.010—0.391, M = 0.12, SE = 0.10). No such significant interaction effects were identified in relation to depression, aggressive behavior or risk taking.
Mediation and moderated-mediation analyses controlling for baseline age, as opposed to puberty, largely resulted in the same pattern of significant and non-significant results. The direct effect of left vlPFC thickness on aggressive behavior was now trending towards significance (b = 0.17, SE = 0.10; t = 1.78, p = 0.078). There was also a significant effect of age on depression (b = 0.43, SE = 0.21; t = 2.01, p = 0.047), with older participants experiencing increased depressive symptoms over time.
4. Discussion
This study is the first to describe the relationship between maturation of the prefrontal cortex and temperamental EC during adolescence using a within-subjects longitudinal design. A reduction in thickness of the ACC, dlPFC and vlPFC was identified between early- and mid-adolescence, as well as a reduction in EC in females over the same period. Change in EC over time was found to mediate the relationship between thinning of the left ACC and reductions in internalizing and externalizing symptoms for the overall sample. Sex was not found to moderate this mediation effect.
Reductions in thickness of the dlPFC, vlPFC and ACC identified between early- and mid-adolescence are consistent with cross-sectional and longitudinal MRI studies of brain development (Brown et al., 2012, Mills et al., 2012, Mutlu et al., 2013, Shaw et al., 2008, Sowell, 2004, Tamnes et al., 2010a). It has been hypothesized that these changes reflect neurobiological processes that improve neuronal efficiency, stability and temporal precision of neuronal firing patterns (Lewis, 1997, Rutherford et al., 1998). Synaptic pruning, changes in axonal calibre and proliferation of glial cells have all been postulated to account for grey matter reductions (Bourgeois and Rakic, 1993, Huttenlocher and Dabholkar, 1997, Paus et al., 2008). Additionally, increased myelination during this time period may encroach on previously unmyelinated tissue at the periphery of the brain, thus decreasing the amount of tissue appearing as grey, compare to white, on MRI (Sowell, 2004). Consistent with these changes, activity in the prefrontal cortex has been found to become less diffuse and more focal over adolescence (Durston and Casey, 2006), suggesting that concurrent development of self-regulation is highly likely during this period.
This study failed to identify any sex differences in cortical maturation (i.e., interactions between sex and time). While there is some evidence of females maturing earlier than males (Lenroot et al., 2007, Mutlu et al., 2013), one study has found greater cortical thinning in males (Raznahan et al., 2010), and others have failed to identify any sex differences (Koolschijn and Crone, 2013, Sowell et al., 2007, van Soelen et al., 2012). The lack of significant sex differences in cortical maturation in the current study may also be due to the availability of data from only two time points, which limited modeling to linear patterns of change, or due to increased noise in the data from changing scanners and coils between the two time points. Therefore, further research is needed to fully understand sex differences in cortical maturation during adolescence.
Our findings also indicate that effortful control remains stable between early- and mid-adolescence in males, but decreases over time in females. This is inconsistent with the hypothesis of improved self-regulatory abilities during adolescence. Most existing research focuses on EC development during infancy and childhood (Rothbart and Rueda, 2005, Zhou et al., 2012), with a lack of longitudinal studies examining adolescent EC development using temperament questionnaires. One potential explanation for our finding is that females may struggle to modulate their behavior early in adolescence due to the dramatic rise in emotional reactivity that occurs around the onset of puberty (Collins, 2003). Indeed, females have been found to be exhibit greater emotional reactivity and responding during adolescence compared to males (Hampel and Petermann, 2006, Hankin et al., 2007, Rudolph, 2002). Our mediation analyses also indicated that females experienced increasing anxiety symptoms compared to males, which is consistent with past findings of greater increase in psychopathological symptoms associated with emotion regulation in females than males during this period (Costello et al., 2003, Hayward and Sanborn, 2002, Lewinsohn et al., 1993). Another potential explanation for the reduction in EC relate to the sampling strategy used in this study, which over-sampled adolescents in the extreme ends of temperamental distributions. However, reductions in EC were correlated with higher risk taking and increases in aggressive behavior and depressive symptoms across the sample, as well as increases in anxiety symptoms in females alone. This is consistent with past research that has identified a significant negative relationship between EC and socioemotional functioning, including psychopathology (Eisenberg et al., 2001, Muris and Meesters, 2009, Oldehinkel et al., 2004, Valiente et al., 2004), and also supports the convergent validity of EC measured using the EATQ-R.
As hypothesized, greater cortical thinning of the left vlPFC and left ACC was associated with reductions in aggressive behavior. Although, to our knowledge, no prior research has examined brain development predicting psychopathology during adolescence, the findings are consistent with past research that has identified less cortical thinning in typically developing 6–18 year olds with higher levels of inattention problems, and depression/anxiety symptoms, as assessed by parent-reported Child Behavior Checklist at baseline (Ducharme et al., 2013, Ducharme et al., 2012). Similarly, Shaw et al. (2011) also identified slower cortical thinning in typically developing children with higher levels of hyperactivity and impulsivity during baseline assessments, as well as in children with ADHD. Interestingly, Whittle et al., 2013a, Whittle et al., 2013b, using the same sample of adolescents as the current study, found that the development of psychopathology between early and mid-adolescence was associated with greater thickening of the left superior parietal cortex. However, Whittle et al., 2013a, Whittle et al., 2013b examined the development of case-level psychopathology (combining externalizing and internalizing disorders) as a predictor of brain change, while the current study examined externalizing behaviors and internalizing symptoms as an outcome.
Furthermore, normative thinning of the cortex was associated with maturation of regulatory abilities. Specifically, greater thinning of the left ACC was associated with less reduction in EC for the overall sample. This finding is consistent with past research that has identified a positive relationship between prefrontal and posterior cortical thinning and cognitive control abilities (Tamnes et al., 2013, Tamnes et al., 2010b). Greater thinning of the left lateral dorsal frontal and left lateral parietal regions has also been associated with improved vocabulary abilities in a longitudinal study of children aged 5–11 (Sowell, 2004). This area of research is, however, in its infancy, and is characterized by inconsistent findings, which are likely due to differences in age groups, methodology and sample sizes. Indeed, Tamnes et al. (2010b) also found that less cortical thinning was related to superior Stroop performance, Fjell et al. (2012) and Østby et al. (2011) failed to identify any relationships between cortical development of cognitive control in adolescents. Whether this relates to task-specific effects or the cross-sectional nature of some studies remains to be discovered, but our findings highlight the importance of conducting longitudinal research on development.
Finally, this study revealed that the relationship between cortical thinning and change in socioemotional functioning during adolescence was mediated by concurrent change in EC. Specifically, less reduction in EC was found to mediate the relationship between greater thinning of the left ACC and reductions in depression, anxiety and aggressive behavior, as well as lower risk taking, across the sample. EC is a particularly suited to mediate this relationship given that is thought to play a central role in the etiology and maintenance of internalizing and externalising disorder (Muris and Ollendick, 2005). It is postulated that higher levels of EC allow individuals to restrain their impulses and undesirable urges, thus preventing externalizing problems. Higher levels of EC enable the restraint of impulses and undesirable urges, as well as regulate emotions through appropriate deployment of attentional resources. As mentioned above, numerous studies have also found that lower levels of EC are related to the presence of internalizing symptoms, such as depression and anxiety, as well as externalizing symptoms, such as aggression, conduct problems and risk taking (Eisenberg et al., 2001, Muris and Meesters, 2009, Oldehinkel et al., 2004, Valiente et al., 2004). These relationships have been found to remain significant after accounting for the effects of emotionality (Muris and Ollendick, 2005, Muris et al., 2007, Olson et al., 2005). There is also evidence that EC moderates the relationship between emotionality and psychopathology, with low levels of EC in combination with high levels of emotionality being associated with psychopathology (Lonigan and Vasey, 2009). Therefore, our findings provide valuable insight into one psychological factor that mediates the relationship between neurobiological development and mental health outcomes during adolescence.
4.1. Limitations
These findings must be considered within the context of several limitations. The availability of data from only two time points limited modeling to linear patterns development between early- and mid-adolescence, thus we cannot comment on non-linear trajectories of brain development or changes occurring during the late adolescent period.
Another limitation of the study was that scans at baseline and follow-up were acquired from different scanners, with different head coils and sequences. The inter-scanner reliability study, however, suggested that the significant change in cortical thickness observed in our sample of adolescents was unlikely to be due to inter-scanner bias, and thus reflected true developmental thinning.
It is important to keep in mind that a selective sampling bias was introduced in the study, whereby a greater proportion of participants in the extreme ends of the distribution of temperamental factors EC and Negative Affectivity were recruited. As mentioned above, this may affect the overall pattern of change in EC and explain discrepancies between our findings and the hypothesized results. However, the sampling strategy should not have influenced the relationship between cortical development and EC, but rather provided greater variance to model this relationship.
Alternatively, our findings of EC reductions in females may reflect a lack of relevance of the questionnaire items to older adolescents. That is, certain items on the EATQ-R may become less relevant with increasing age, such as completion of homework or concentrating on studies (functions tapped by the instrument). Given these issues, future research may benefit from incorporating other measures of self-regulation, such as tasks of cognitive control or emotion regulation that have been found to be correlated with EC. Indeed, there is no current consensus on how to best measure self-regulation and there is a general lack of research investigating associations between different measures of self-regulation (Bridgett et al., 2013, Zhou et al., 2012). Consequently, future studies should employ a multi-method approach to cover different aspects of self-regulation.
While the localized ROI approach employed by the current study provides valuable knowledge about the ACC, dlPFC, and vlPFC, three regions integral to the executive attention network, future studies should employ whole brain analyses to explore other cortical regions and networks underlying self-regulatory abilities. Indeed, previous research with this sample has shown that the development of psychopathology is related to thickness development in the parietal cortex, and volumetric development of the hippocampus and amygdala (Whittle et al., 2013a, Whittle et al., 2013b).
4.2. Conclusions
The findings from this study shed light on the relationship between cortical maturation and temperamental EC in adolescents. While previous research has focused on the neural correlates of EC using cross-sectional analyses, our findings revealed a dynamic relationship between the development of the ACC, EC and socioemotional behavior and psychopathology symptoms during adolescence. This highlights the importance of studying neural correlates of self-regulation using a longitudinal framework during this developmental period.
Conflict of interest
Drs Whittle, Dennison, Yücel, Simmons, and Allen, and Ms Vijayakumar report no biomedical financial interests or potential conflicts of interest.
Note
For further information on studies using neuroimaging data from the Orygen Adolescent Development Study, see Cheetham et al. (2012), Dennison et al. (2013), Kaess et al. (2013), Visser et al. (2013), Whittle et al., 2009a, Whittle et al., 2009b, Whittle et al., 2012a, Whittle et al., 2012b, Whittle et al., 2012c, Whittle et al., 2011a, Whittle et al., 2011b, Whittle et al., 2008a, Whittle et al., 2008b, Whittle et al., 2013a, Whittle et al., 2013b, Yap et al. (2008b), Zipursky et al. (2011).
Acknowledgments
This research was supported by grants from the Colonial Foundation, the National Health and Medical Research Council (NHMRC; Australia; Program Grant 350241 ) and the Australian Research Council (ARC; Discovery Grant DP0878136 ). Ms. Vijayakumar is supported by a Melbourne International Research Scholarship . Dr. Whittle is supported by a NHMRC Career Development Fellowship ( ID: 1007716 ). Ms Dennison is supported by an Australian Postgraduate Award . Prof. Yücel is supported by an NHMRC Fellowship ( ID: 1021973 ).
Neuroimaging analysis was facilitated by the Neuropsychiatry Imaging Laboratory at the Melbourne Neuropsychiatry Centre. The authors would like to thank the Brain Research Institute and Royal Children's Hospital for support in acquiring the neuroimaging data, and the families who participated in the study.
Footnotes
Supplementary material related to this article can be found, in the online version, at http://dx.doi.org/10.1016/j.dcn.2013.12.002.
Appendix A. Supplementary data
The following are Supplementary data to this article:
References
- Beck A.T., Epstein N., Brown G., Steer R.A. An inventory for measuring clinical anxiety: psychometric properties. J. Consult. Clin. Psychol. 1988;56(6):893–897. doi: 10.1037//0022-006x.56.6.893. [DOI] [PubMed] [Google Scholar]
- Botvinick M., Nystrom L.E., Fissell K., Carter C.S., Cohen J.D. Conflict monitoring versus selection-for-action in anterior cingulate cortex. Nature. 1999;402(6758):179–181. doi: 10.1038/46035. doi 10.1038/46035. [DOI] [PubMed] [Google Scholar]
- Bourgeois J.P., Rakic P. Changes of synaptic density in the primary visual cortex of the macaque monkey from fetal to adult stage. J. Neurosci. 1993;13(7):2801–2820. doi: 10.1523/JNEUROSCI.13-07-02801.1993. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Boyes R.G., Gunter J.L., Frost C., Janke A.L., Yeatman T., Hill D.L.G. Intensity non-uniformity correction using N3 on 3-T scanners with multichannel phased array coils. NeuroImage. 2008;39(4):1752–1762. doi: 10.1016/j.neuroimage.2007.10.026. doi 10.1016/j.neuroimage.2007.10.026. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Brener N.D., Collins J.L., Kann L., Warren C.W., Williams B.I. Reliability of the youth risk behavior survey questionnaire. Am. J. Epidemiol. 1995;141(6):575–580. doi: 10.1093/oxfordjournals.aje.a117473. [DOI] [PubMed] [Google Scholar]
- Bridgett D.J., Oddi K.B., Laake L.M., Murdock K.W., Bachmann M.N. Integrating and differentiating aspects of self-regulation: effortful control, executive functioning, and links to negative affectivity. Emotion. 2013;13(1):47–63. doi: 10.1037/a0029536. DOI 10.1037/a0029536. [DOI] [PubMed] [Google Scholar]
- Brooks-Gunn J., Warren M.P., Rosso J., Gargiulo J. Validity of self-report measures of girls’ pubertal status. Child Dev. 1987;58(3):829–841. [PubMed] [Google Scholar]
- Brown T.T., Kuperman J.M., Chung Y., Erhart M., McCabe C., Hagler D.J. Neuroanatomical assessment of biological maturity. Curr. Biol. 2012;22(18):1693–1698. doi: 10.1016/j.cub.2012.07.002. doi 10.1016/j.cub.2012.07.002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Capaldi D.M., Rothbart M.K. Development and validation of an early adolescent temperament measure. J. Early Adolescence. 1992;12(2):153–173. [Google Scholar]
- Casey B.J., Durston S., Fossella J.A. Evidence for a mechanistic model of cognitive control. Clin. Neurosci. Res. 2001;1(4):267–282. [Google Scholar]
- Cheetham A., Allen N.B., Whittle S., Simmons J.G., Yücel M., Lubman D.I. Orbitofrontal volumes in early adolescence predict initiation of cannabis use: a 4-year longitudinal and prospective study. Biol. Psychiatry. 2012;71(8):684–692. doi: 10.1016/j.biopsych.2011.10.029. [DOI] [PubMed] [Google Scholar]
- Collins W.A. More than myth: the developmental significance of romantic relationships during adolescence. J. Res. Adolescence. 2003;13(1):1–24. [Google Scholar]
- Costello E.J., Mustillo S., Erkanli A., Keeler G., Angold A. Prevalence and development of psychiatric disorders in childhood and adolescence. Arch. Gen. Psychiatry. 2003;60(8):837–844. doi: 10.1001/archpsyc.60.8.837. [DOI] [PubMed] [Google Scholar]
- Crockett L.J., Raffaelli M., Shen Y.-L. Linking self-regulation and risk proneness to risky sexual behavior: pathways through peer pressure and early substance use. J. Res. Adolescence. 2006;16(4):503–525. [Google Scholar]
- Dennison M., Whittle S., Yücel M., Vijayakumar N., Kline A., SIMMONS J., Allen N.B. Mapping subcortical brain maturation during adolescence: evidence of hemisphere- and sex-specific longitudinal changes. Dev. Sci. 2013 doi: 10.1111/desc.12057. [DOI] [PubMed] [Google Scholar]
- Desikan R.S., Ségonne F., Fischl B., Quinn B.T., Dickerson B.C., Blacker D. An automated labeling system for subdividing the human cerebral cortex on MRI scans into gyral based regions of interest. NeuroImage. 2006;31(3):968–980. doi: 10.1016/j.neuroimage.2006.01.021. [DOI] [PubMed] [Google Scholar]
- Ducharme S., Albaugh M.D., Hudziak J.J., Botteron K.N., Nguyen T.-V., Truong C. Anxious/depressed symptoms are linked to right ventromedial prefrontal cortical thickness maturation in healthy children and young adults. Cerebr. Cortex. 2013 doi: 10.1093/cercor/bht151. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ducharme S., Hudziak J.J., Botteron K.N., Albaugh M.D., Nguyen T.-V., Karama S., Evans A.C. Decreased regional cortical thickness and thinning rate are associated with inattention symptoms in healthy children. JAAC. 2012;51(1):18–27. doi: 10.1016/j.jaac.2011.09.022. e2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Duckworth A.L., Seligman M.E.P. Self-discipline outdoes IQ in predicting academic performance of adolescents. Psychol. Sci. 2005;16(12):939–944. doi: 10.1111/j.1467-9280.2005.01641.x. [DOI] [PubMed] [Google Scholar]
- Durston, S., Casey, B.J., 2006. What have we learned about cognitive development from neuroimaging? Neuropsychologia 44 (11), 2149–2157, 10.1016/j.neuropsychologia.2005.10.010. [DOI] [PubMed]
- Eisenberg N., Spinrad T.L. Emotion-related regulation: sharpening the definition. Child Dev. 2004;75(2):334–339. doi: 10.1111/j.1467-8624.2004.00674.x. [DOI] [PubMed] [Google Scholar]
- Eisenberg N., Cumberland A., Spinrad T.L., Fabes R.A., Shepard S.A., Reiser M. The relations of regulation and emotionality to children's externalizing and internalizing problem behavior. Child Dev. 2001;72(4):1112–1134. doi: 10.1111/1467-8624.00337. [DOI] [PubMed] [Google Scholar]
- Eisenberg N., Fabes R.A., Shepard S.A., Murphy B.C., Guthrie I.K., Jones S. Contemporaneous and longitudinal prediction of children's social functioning from regulation and emotionality. Child Dev. 1997;68(4):642–664. [PubMed] [Google Scholar]
- Eisenberg N., Spinrad T.L., Eggum N.D. Emotion-related self-regulation and its relation to children's maladjustment. Annual Rev. Clin. Psychol. 2010;6(1):495–525. doi: 10.1146/annurev.clinpsy.121208.131208. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ellis L.K., Rothbart M.K. Minnesota; Minneapolis: 2001. Revision of the Early Adolescent Temperament Questionnaire. Biennial Meeting of the Society for Research in Child Development. [Google Scholar]
- Else-Quest N.M., Hyde J.S., Goldsmith H.H., Van Hulle C.A. Gender differences in temperament: a meta-analysis. Psychol. Bull. 2006;132(1):33. doi: 10.1037/0033-2909.132.1.33. [DOI] [PubMed] [Google Scholar]
- Fischl B. Automatically parcellating the human cerebral cortex. Cerebr. Cortex. 2004;14(1):11–22. doi: 10.1093/cercor/bhg087. [DOI] [PubMed] [Google Scholar]
- Fjell A.M., Walhovd K.B., Brown T.T., Kuperman J.M., Chung Y., Hagler D.J. Multimodal imaging of the self-regulating developing brain. Proc. Natl. Acad. Sci. U. S. A. 2012;109(48):19620–19625. doi: 10.1073/pnas.1208243109. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Garnefski N., Kraaij V. Relationships between cognitive emotion regulation strategies and depressive symptoms: a comparative study of five specific samples. Personality Individual Diff. 2006;40(8):1659–1669. [Google Scholar]
- Garrison C.Z., Addy C.L., Jackson K.L., McKeown R.E., Waller J.L. The CES-D as a screen for depression and other psychiatric disorders in adolescents. J. Am. Acad. Child Adolescent Psychiatry. 1991;30(4):636–641. doi: 10.1097/00004583-199107000-00017. [DOI] [PubMed] [Google Scholar]
- Gogtay N., Giedd J.N., Lusk L., Hayashi K.M., Greenstein D., Vaituzis A.C. Dynamic mapping of human cortical development during childhood through early adulthood. Proc. Natl. Acad. Sci. 2004;101(21):8174–8179. doi: 10.1073/pnas.0402680101. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Good C.D., Johnsrude I., Ashburner J., Henson R.N.A., Friston K.J., Frackowiak R.S.J. Cerebral asymmetry and the effects of sex and handedness on brain structure: a voxel-based morphometric analysis of 465 normal adult human brains. NeuroImage. 2001;14(3):685–700. doi: 10.1006/nimg.2001.0857. [DOI] [PubMed] [Google Scholar]
- Hampel P., Petermann F. Perceived stress, coping, and adjustment in adolescents. J. Adolescent Health. 2006;38(4):409–415. doi: 10.1016/j.jadohealth.2005.02.014. [DOI] [PubMed] [Google Scholar]
- Han, X., Fischl, B., 2007. Atlas renormalization for improved brain MR image. Segmentation across scanner platforms. IEEE Trans. Med. Imag. 26 (4), 479–486. 10.1109/TMI.2007.893282. [DOI] [PubMed]
- Han X., Jovicich J., Salat D., van der Kouwe A., Quinn B., Czanner S. Reliability of MRI-derived measurements of human cerebral cortical thickness: the effects of field strength, scanner upgrade and manufacturer. NeuroImage. 2006;32(1):180–194. doi: 10.1016/j.neuroimage.2006.02.051. [DOI] [PubMed] [Google Scholar]
- Hankin B.L., Mermelstein R., Roesch L. Sex differences in adolescent depression: stress exposure and reactivity models. Child Dev. 2007;78(1):279–295. doi: 10.1111/j.1467-8624.2007.00997.x. [DOI] [PubMed] [Google Scholar]
- Hayes A.F. Beyond Baron and Kenny: statistical mediation analysis in the new millennium. Commun. Monogr. 2009;76(4):408–420. [Google Scholar]
- Hayward C., Sanborn K. Puberty and the emergence of gender differences in psychopathology. J. Adolescent Health. 2002;30(4 Suppl.):49L 58. doi: 10.1016/s1054-139x(02)00336-1. [DOI] [PubMed] [Google Scholar]
- Huttenlocher P.R., Dabholkar A.S. Regional differences in synaptogenesis in human cerebral cortex. J. Comp. Neurol. 1997;387(2):167–178. doi: 10.1002/(sici)1096-9861(19971020)387:2<167::aid-cne1>3.0.co;2-z. doi 10.1002/(SICI)1096-9861(19971020)387:2<167::AID-CNE1>3.0.CO;2-Z. [DOI] [PubMed] [Google Scholar]
- Jones F.L., McMillan J. Scoring occupational categories for social research: a review of current practice, with Australian examples. Work, Employment Soc. 2001;15(3):539–563. [Google Scholar]
- Kaess M., Simmons J.G., Whittle S., Jovev M., Chanen A.M., Yücel M. Sex-specific prediction of hypothalamic-pituitary-adrenal axis activity by pituitary volume during adolescence: a longitudinal study from 12 to 17 years of age. Psychoneuroendocrinology. 2013;38(11):2694–2704. doi: 10.1016/j.psyneuen.2013.06.028. [DOI] [PubMed] [Google Scholar]
- Kalisch R. The functional neuroanatomy of reappraisal: time matters. Neuroscience and Biobehavioral Reviews. 2009;33(8):1215–1226. doi: 10.1016/j.neubiorev.2009.06.003. [DOI] [PubMed] [Google Scholar]
- Karoly P., Boekaerts M., Maes S. Toward consensus in the psychology of self-regulation: How far have we come? How far do we have yet to travel? Appl. Psychol. 2005;54(2):300–311. [Google Scholar]
- Kaufman J., Birmaher B., Brent D., Rao U., Flynn C. Schedule for affective disorders and schizophrenia for school-age children—present and lifetime version (K-SADS-PL): initial reliability and validity data. J. Am. Acad. Child Adolescent Psychiatry. 1997;36(7):980–988. doi: 10.1097/00004583-199707000-00021. [DOI] [PubMed] [Google Scholar]
- Koolschijn P.C.M.P., Crone E.A. Sex differences and structural brain maturation from childhood to early adulthood. Dev. Cognit. Neurosci. 2013;5:106–118. doi: 10.1016/j.dcn.2013.02.003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lebel C., Beaulieu C. Longitudinal development of human brain wiring continues from childhood into adulthood. J. Neurosci. 2011;31(30):10937–10947. doi: 10.1523/JNEUROSCI.5302-10.2011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lenroot R.K., Gogtay N., Greenstein D.K., Wells E.M., Wallace G.L., Clasen L.S. Sexual dimorphism of brain developmental trajectories during childhood and adolescence. NeuroImage. 2007;36(4):1065–1073. doi: 10.1016/j.neuroimage.2007.03.053. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Leon-carrion J., Garcia-Orza J., Perez-Santamaria F.J. Development of the inhibitory component of the executive functions in children and adolescents. Int. J. Neurosci. 2004;114(10):1291–1311. doi: 10.1080/00207450490476066. [DOI] [PubMed] [Google Scholar]
- Lewinsohn P.M., Hops H., Roberts R.E., Seeley J.R., Andrews J.A. Adolescent psychopathology: I. Prevalence and incidence of depression and other DSM-III—R disorders in high school students. J. Abnorm. Psychol. 1993;102(1):133–144. doi: 10.1037//0021-843x.102.1.133. [DOI] [PubMed] [Google Scholar]
- Lewis D.A. Development of the prefrontal cortex during adolescence: insights into vulnerable neural circuits in schizophrenia. Neuropsychopharmacology. 1997;16(6):385–398. doi: 10.1016/S0893-133X(96)00277-1. [DOI] [PubMed] [Google Scholar]
- Lonigan C.J., Vasey M.W. Negative affectivity, effortful control, and attention to threat-relevant stimuli. J. Abnorm. Child Psychol. 2009;37(3):387–399. doi: 10.1007/s10802-008-9284-y. [DOI] [PubMed] [Google Scholar]
- Luna B., Garver K.E., Urban T.A., Lazar N.A., Sweeney J.A. Maturation of cognitive processes from late childhood to adulthood. Child Dev. 2004;75(5):1357–1372. doi: 10.1111/j.1467-8624.2004.00745.x. [DOI] [PubMed] [Google Scholar]
- McRae K., Gross J.J., Weber J., Robertson E.R., Sokol-Hessner P., Ray R.D. The development of emotion regulation: an fMRI study of cognitive reappraisal in children, adolescents and young adults. Soc. Cognit. Affect. Neurosci. 2012;7(1):11–22. doi: 10.1093/scan/nsr093. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mills K.L., Lalonde F., Clasen L.S., Giedd J.N., Blakemore S.-J. Developmental changes in the structure of the social brain in late childhood and adolescence. Soc. Cognit. Affect. Neurosci. 2012 doi: 10.1093/scan/nss113. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Muris P., Meesters C. Reactive and regulative temperament in youths: psychometric evaluation of the early adolescent temperament questionnaire-revised. J. Psychopathol. Behav. Assess. 2009;31(1):7–19. [Google Scholar]
- Muris P., Ollendick T.H. The role of temperament in the etiology of child psychopathology. Clin. Child Family Psychol. Rev. 2005;8(4):271–289. doi: 10.1007/s10567-005-8809-y. [DOI] [PubMed] [Google Scholar]
- Muris P., Meesters C., Blijlevens P. Self-reported reactive and regulative temperament in early adolescence: Relations to internalizing and externalizing problem behavior and Big Three personality factors. J. Adolescence. 2007;30(6):1035–1049. doi: 10.1016/j.adolescence.2007.03.003. [DOI] [PubMed] [Google Scholar]
- Mutlu A.K., Schneider M., Debbané M., Badoud D., Eliez S., Schaer M. Sex differences in thickness, and folding developments throughout the cortex. NeuroImage. 2013;82C:200–207. doi: 10.1016/j.neuroimage.2013.05.076. [DOI] [PubMed] [Google Scholar]
- Nelson E., Leibenluft E., McClure E., Pine D. The social re-orientation of adolescence: a neuroscience perspective on the process and its relation to psychopathology. Psychol. Med. 2005;35(2):163–174. doi: 10.1017/s0033291704003915. [DOI] [PubMed] [Google Scholar]
- Ochsner K.N., Gross J.J. Cognitive emotion regulation: insights from social cognitive and affective neuroscience. Curr. Direct. Psychol. Sci. 2008;17(2):153–158. doi: 10.1111/j.1467-8721.2008.00566.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ochsner K.N., Silvers J.A., Buhle J.T. Functional imaging studies of emotion regulation: a synthetic review and evolving model of the cognitive control of emotion. Ann. N. Y. Acad. Sci. 2012;1251(1):E1–E24. doi: 10.1111/j.1749-6632.2012.06751.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Oldehinkel A.J., Hartman C.A., De Winter A.F., Veenstra R., Ormel J. Temperament profiles associated with internalizing and externalizing problems in preadolescence. Dev. Psychopathol. 2004;16(2):421–440. doi: 10.1017/s0954579404044591. [DOI] [PubMed] [Google Scholar]
- Oldfield R.C. The assessment and analysis of handedness: the Edinburgh inventory. Neuropsychologia. 1971;9(1):97–113. doi: 10.1016/0028-3932(71)90067-4. [DOI] [PubMed] [Google Scholar]
- Olson S.L., Sameroff A.J., Kerr D.C., Lopez N.L., Wellman H.M. Developmental foundations of externalizing problems in young children: the role of effortful control. Dev. Psychopathol. 2005;17(1):25–45. doi: 10.1017/s0954579405050029. [DOI] [PubMed] [Google Scholar]
- Østby Y., Tamnes C.K., Fjell A.M., Walhovd K.B. Morphometry and connectivity of the fronto-parietal verbal working memory network in development. Neuropsychologia. 2011;49(14):3854–3862. doi: 10.1016/j.neuropsychologia.2011.10.001. [DOI] [PubMed] [Google Scholar]
- Paus T., Keshavan M., Giedd J.N. Why do many psychiatric disorders emerge during adolescence? Nat. Rev. Neurosci. 2008;9(12):947–957. doi: 10.1038/nrn2513. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Petersen A.C., Crockett L., Richards M., Boxer A. A self-report measure of pubertal status: reliability, validity, and initial norms. J. Youth Adolescence. 1988;17(2):117–133. doi: 10.1007/BF01537962. [DOI] [PubMed] [Google Scholar]
- Posner M.I. Imaging attention networks. NeuroImage. 2012;61(2):450–456. doi: 10.1016/j.neuroimage.2011.12.040. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Preacher K.J., Hayes A.F. SPSS and SAS procedures for estimating indirect effects in simple mediation models. Behav. Res. Methods, Instruments Comput. 2004;36(4):717–731. doi: 10.3758/bf03206553. [DOI] [PubMed] [Google Scholar]
- Quinn P.D., Fromme K. Self-regulation as a protective factor against risky drinking and sexual behavior. Psychol. Addict. Behav. 2010;24(3):376–385. doi: 10.1037/a0018547. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Radloff L.S. The CES-D scale: a self-report depression scale for research in the general population. Appl. Psychol. Measur. 1977;1(3):385–401. [Google Scholar]
- Rajkowska G., Goldman-Rakic P.S. Cytoarchitectonic definition of prefrontal areas in the normal human cortex: II. Variability in locations of areas 9 and 46 and relationship to the Talairach coordinate system. Cerebr. Cortex. 1995;5(4):323–337. doi: 10.1093/cercor/5.4.323. [DOI] [PubMed] [Google Scholar]
- Raznahan A., Lee Y., Stidd R., Long R., Greenstein D., Clasen L. Longitudinally mapping the influence of sex and androgen signaling on the dynamics of human cortical maturation in adolescence. Proc. Natl. Acad. Sci. 2010;107(39):16988–16993. doi: 10.1073/pnas.1006025107. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Reuter M., Fischl B. Avoiding asymmetry-induced bias in longitudinal image processing. NeuroImage. 2011;57(1):19–21. doi: 10.1016/j.neuroimage.2011.02.076. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Reuter M., Rosas H.D., Fischl B. Highly accurate inverse consistent registration: a robust approach. NeuroImage. 2010;53(4):1181–1196. doi: 10.1016/j.neuroimage.2010.07.020. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Reuter M., Schmansky N.J., Rosas H.D., Fischl B. Within-subject template estimation for unbiased longitudinal image analysis. NeuroImage. 2012;61(4):1402–1418. doi: 10.1016/j.neuroimage.2012.02.084. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rothbart, M.K., Rueda, M.R., 2005. The development of effortful control. In: Mayr, U., Awh, E., Keele, S. (Eds.), Developing Individuality in the Human Brain: A Tribute to Michael I. Posner. American Psychological Association, Washington DC, pp. 167–188.
- Rothbart M.K., Ellis L.K., Rosario Rueda M., Posner M.I. Developing mechanisms of temperamental effortful control. J. Personal. 2003;71(6):1113–1144. doi: 10.1111/1467-6494.7106009. [DOI] [PubMed] [Google Scholar]
- Rothbart M.K., Sheese B.E., Posner M.I. Executive attention and effortful control: linking temperament, brain networks, and genes. Child Dev. Perspect. 2007;1(1):2–7. [Google Scholar]
- Rudolph K.D. Gender differences in emotional responses to interpersonal stress during adolescence. J. Adolescent Health. 2002;30(4 Suppl.):3–13. doi: 10.1016/s1054-139x(01)00383-4. [DOI] [PubMed] [Google Scholar]
- Rutherford L.C., Nelson S.B., Turrigiano G.G. BDNF has opposite effects on the quantal amplitude of pyramidal neuron and interneuron excitatory synapses. Neuron. 1998;21(3):521–530. doi: 10.1016/s0896-6273(00)80563-2. [DOI] [PubMed] [Google Scholar]
- Shaw P., Gilliam M., Liverpool M., Weddle C., Malek M., Sharp W. Cortical development in typically developing children with symptoms of hyperactivity and impulsivity: support for a dimensional view of attention deficit hyperactivity disorder. Am. J. Psychiatry. 2011;168(2):143–151. doi: 10.1176/appi.ajp.2010.10030385. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Shaw P., Kabani N.J., Lerch J.P., Eckstrand K., Lenroot R., Gogtay N. Neurodevelopmental trajectories of the human cerebral cortex. J. Neurosci. 2008;28(14):3586–3594. doi: 10.1523/JNEUROSCI.5309-07.2008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Shirtcliff E.A., Dahl R.E., Pollak S.D. Pubertal development: correspondence between hormonal and physical development. Child Dev. 2009;80(2):327–337. doi: 10.1111/j.1467-8624.2009.01263.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sowell E.R. Longitudinal mapping of cortical thickness and brain growth in normal children. J. Neurosci. 2004;24(38):8223–8231. doi: 10.1523/JNEUROSCI.1798-04.2004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sowell E.R., Peterson B.S., Kan E., Woods R.P., Yoshii J., Bansal R. Sex differences in cortical thickness mapped in 176 healthy individuals between 7 and 87 years of age. Cerebr. Cortex. 2007;17(7):1550–1560. doi: 10.1093/cercor/bhl066. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Spinrad T.L., Eisenberg N., Cumberland A., Fabes R.A., Valiente C., Shepard S.A. Relation of emotion-related regulation to children's social competence: a longitudinal study. Emotion. 2006;6(3):498–510. doi: 10.1037/1528-3542.6.3.498. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Steinberg L. Cognitive and affective development in adolescence. Trends Cognit. Sci. 2005;9(2):69–74. doi: 10.1016/j.tics.2004.12.005. [DOI] [PubMed] [Google Scholar]
- Tamnes, C.K., Walhovd, K.B., Grydeland, H., Holland, D., Østby, Y., Dale, A.M., Fjell, A.M., 2013. Longitudinal working memory development is related to structural maturation of frontal and parietal cortices. J. Cognit. Neurosci. 25 (10), 1611–1623, 10.1162/jocn_a_00434. [DOI] [PubMed]
- Tamnes C.K., Østby Y., Fjell A.M., Westlye L.T., Due-Tønnessen P., Walhovd K.B. Brain maturation in adolescence and young adulthood: regional age-related changes in cortical thickness and white matter volume and microstructure. Cerebr. Cortex. 2010;20(3):534–548. doi: 10.1093/cercor/bhp118. [DOI] [PubMed] [Google Scholar]
- Tamnes C.K., Østby Y., Walhovd K.B., Westlye L.T., Due-Tønnessen P., Fjell A.M. Neuroanatomical correlates of executive functions in children and adolescents: a magnetic resonance imaging (MRI) study of cortical thickness. Neuropsychologia. 2010;48(9):2496–2508. doi: 10.1016/j.neuropsychologia.2010.04.024. [DOI] [PubMed] [Google Scholar]
- Valiente C., Eisenberg N., Smith C.L., Reiser M., Fabes R.A., Losoya S. The relations of effortful control and reactive control to children's externalizing problems: a longitudinal assessment. J. Personal. 2004;71(6):1171–1196. doi: 10.1111/1467-6494.7106011. [DOI] [PubMed] [Google Scholar]
- van Soelen I., Brouwer R.M., Van Baal G.C.M., Schnack H.G., Peper J.S., Collins D.L. Genetic influences on thinning of the cerebral cortex during development. NeuroImage. 2012;59(4):3871–3880. doi: 10.1016/j.neuroimage.2011.11.044. [DOI] [PubMed] [Google Scholar]
- Visser T.A.W., Ohan J.L., Whittle S., Yücel M., Simmons J.G., Allen N.B. Sex differences in structural brain asymmetry predict overt aggression in early adolescents. Soc. Cognit. Affect. Neurosci. 2013 doi: 10.1093/scan/nst013. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wechsler D. Harcourt Assessment, Inc.; San Antonio, TX: 2003. Wechsler Intelligence Scale for Children-Fourth Edition. [Google Scholar]
- Whittle S., Allen N.B., Fornito A., Lubman D.I., Simmons J.G., PANTELIS C., Yücel M. Variations in cortical folding patterns are related to individual differences in temperament. Psychiatry Res. 2009;172(1):68–74. doi: 10.1016/j.pscychresns.2008.06.005. [DOI] [PubMed] [Google Scholar]
- Whittle S., Bartholomeusz C., Yücel M., Dennison M., Vijayakumar N., Allen N.B. Orbitofrontal sulcogyral patterns are related to temperamental risk for psychopathology. Soc. Cognit. Affect. Neurosci. 2012 doi: 10.1093/scan/nss126. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Whittle S., Dennison M., Vijayakumar N., Simmons J.G., Yücel M., Lubman D.I. Childhood maltreatment and psychopathology affect brain development during adolescence. J. Am. Acad. Child Adolescent Psychiatry. 2013;52(9):940–952. doi: 10.1016/j.jaac.2013.06.007. e1. [DOI] [PubMed] [Google Scholar]
- Whittle S., Simmons J.G., Dennison M., Vijayakumar N., Schwartz O., Yap M.B.H. Positive parenting predicts the development of adolescent brain structure: a longitudinal study. Dev. Cognit. Neurosci. 2013 doi: 10.1016/j.dcn.2013.10.006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Whittle S., Yap M.B.H., Sheeber L., Dudgeon P., Yücel M., PANTELIS C. Hippocampal volume and sensitivity to maternal aggressive behavior: a prospective study of adolescent depressive symptoms. Dev. Psychopathol. 2011;23(01):115–129. doi: 10.1017/S0954579410000684. [DOI] [PubMed] [Google Scholar]
- Whittle S., Yap M.B.H., Yücel M., Fornito A., Simmons J.G., Barrett A. Prefrontal and amygdala volumes are related to adolescents’ affective behaviors during parent-adolescent interactions. Proc. Natl. Acad. Sci. U. S. A. 2008;105(9):3652–3657. doi: 10.1073/pnas.0709815105. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Whittle S., Yap M.B.H., Yücel M., Sheeber L., Simmons J.G., Pantelis C., Allen N.B. Maternal responses to adolescent positive affect are associated with adolescents’ reward neuroanatomy. Soc. Cognit. Affect. Neurosci. 2009;4(3):247–256. doi: 10.1093/scan/nsp012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Whittle S., Yücel M., Forbes E.E., Davey C.G., Harding I.H., Sheeber L. Adolescents depressive symptoms moderate neural responses to their mothers positive behavior. Soc. Cognit. Affect. Neurosci. 2012;7(1):23–34. doi: 10.1093/scan/nsr049. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Whittle S., Yücel M., Fornito A., Barrett A., Wood S.J., Lubman D.I. Neuroanatomical correlates of temperament in early adolescents. J. Am. Acad. Child Adolescent Psychiatry. 2008;47(6):682–693. doi: 10.1097/CHI.0b013e31816bffca. [DOI] [PubMed] [Google Scholar]
- Whittle S., Yücel M., Lorenzetti V., Byrne M.L., Simmons J.G., Wood S.J. Pituitary volume mediates the relationship between pubertal timing and depressive symptoms during adolescence. Psychoneuroendocrinology. 2012;37(7):881–891. doi: 10.1016/j.psyneuen.2011.10.004. [DOI] [PubMed] [Google Scholar]
- Whittle S., Yücel M., Yap M.B.H., Allen N.B. Sex differences in the neural correlates of emotion: evidence from neuroimaging. Biol. Psychol. 2011;87(3):319–333. doi: 10.1016/j.biopsycho.2011.05.003. [DOI] [PubMed] [Google Scholar]
- Yap M.B.H., Allen N.B., Ladouceur C.D. Maternal socialization of positive affect: the impact of invalidation on adolescent emotion regulation and depressive symptomatology. Child Dev. 2008;79(5):1415–1431. doi: 10.1111/j.1467-8624.2008.01196.x. [DOI] [PubMed] [Google Scholar]
- Yap M.B., Whittle S., Yücel M., Sheeber L., Pantelis C., Simmons J.G., Allen N.B. Interaction of parenting experiences and brain structure in the prediction of depressive symptoms in adolescents. Arch. Gen. Psychiatry. 2008;65(12):1377. doi: 10.1001/archpsyc.65.12.1377. [DOI] [PubMed] [Google Scholar]
- Zheng W., Chee M.W.L., Zagorodnov V. Improvement of brain segmentation accuracy by optimizing non-uniformity correction using N3. NeuroImage. 2009;48(1):73–83. doi: 10.1016/j.neuroimage.2009.06.039. [DOI] [PubMed] [Google Scholar]
- Zhou Q., Chen S.H., Main A. Commonalities and differences in the research on children's effortful control and executive function: a call for an integrated model of self-regulation. Child Dev. Perspect. 2012;6(2):112–121. [Google Scholar]
- Zipursky A.R., Whittle S., Yücel M., Lorenzetti V., Wood S.J., Lubman D.I. Pituitary volume prospectively predicts internalizing symptoms in adolescence. J. Child Psychol. Psychiatry. 2011;52(3):315–323. doi: 10.1111/j.1469-7610.2010.02337.x. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.