Highlights
-
•
There are behavioural and physical parallels between adolescence in rats and humans.
-
•
Adolescent social instability stress in rats impairs adult social behaviour.
-
•
The developmental trajectory of the hippocampus is altered by adolescent stressors.
-
•
Age differences in stress-induced corticosterone release are stressor-specific.
-
•
Animal models provide insights into developmental stage-specific vulnerability.
Keywords: Adolescence, Rats, Social anxiety, Sexual behaviour, Hypothalamic–pituitary–adrenal axis, Neurogenesis
Abstract
Studies in animal models generate and test hypotheses regarding developmental stage-specific vulnerability that might inform research questions about human development. In both rats and humans, peer relationships are qualitatively different in adolescence than at other stages of development, and social experiences in adolescence are considered important determinants of adult social function. This review describes our adolescent rat social instability stress model and the long-lasting effects social instability has on social behaviour in adulthood as well as the possible neural underpinnings. Effects of other adolescent social stress experiences in rats on social behaviours in adulthood also are reviewed. We discuss the role of hypothalamic–pituitary–adrenal (HPA) function and glucocorticoid release in conferring differential susceptibility to social experiences in adolescents compared to adults. We propose that although differential perception of social experiences rather than immature HPA function may underlie the heightened vulnerability of adolescents to social instability, the changes in the trajectory of brain development and resultant social deficits likely are mediated by the heightened glucocorticoid release in response to repeated social stressors in adolescence compared to in adulthood.
1. Introduction
As outlined by Gottlieb and Lickliter (2004), the primary contribution of investigations in non-human animals for studies of people (and vice versa, as for comparative approaches in general) is to generate hypotheses and general principles of development that can then be tested. In the last ten or so years, there is increasing interest in understanding the neural plasticity of the adolescent period of development because of evidence that it may be a time of remediation (e.g., Bredy et al., 2004) for what were once thought to be relatively permanent programming effects of detrimental experiences in early life. The flip side is that this same plasticity may confer vulnerability in adolescence. Researches of the adolescent period in animal models may provide insights as to risk factors and the differential susceptibility at this stage of life.
Whether or not adolescence in humans is a bona fide developmental stage or a social construction that arose in modern history was a topic of debate as recently as the late 20th century (e.g., Fox, 1977, Schlegel and Barry, 1991). Thus it is not surprising that adolescence also has been considered unique to the human species. For example, Bogin and Smith (1996) argued that while adolescence is an evolved stage of life, it appeared relatively recently (after the appearance of Homo erectus). Adolescence was considered a specialization in humans, with non-human species transitioning from a juvenile to adult stage without an adolescent stage. Researchers’ acceptance nowadays of an adolescent period of development in non-human animals is exemplified in a figure by Brown and Spencer (2013) in which the developmental time course of circulating testosterone concentrations in males is illustrated in an altricial mammal (Norway rats), an altricial bird (zebra finches), a semi-precocial mammal (rhesus macaques), and a precocial bird (quail), all of which are depicted to show low testosterone concentrations during a juvenile period, a steep increase in an adolescent period, and high asymptotic concentrations in an adult period. The rise in testosterone, however, reflects the maturation of the hypothalamic–pituitary–gonadal axis that is a hallmark of puberty, and adolescence is not synonymous with puberty. Rather, adolescence extends beyond sexual maturation and is defined by the development of social and cognitive behaviour (Sisk and Foster, 2004).
Several parallels can be drawn between adolescence in humans and in rodent species, especially rats, for which the adolescent period has been investigated extensively in the laboratory. In both humans and rats, adolescence is a transitional period with no clear markers of onset or offset. A broad definition of adolescence in rats spans from about postnatal day 21 (approximately the time of weaning) to postnatal day 60 (approximately the time of sexual maturity), with physical indices of puberty evident at about postnatal day 35 in females and postnatal day 42 in males (reviewed in McCormick and Mathews, 2010). Changes in emotional and cognitive behaviour during adolescence are manifestations of ongoing brain development in both species (see reviews by Blakemore, 2012, Brenhouse and Andersen, 2011, Cooke and Shukla, 2011). Compared to adults, adolescents of both species show greater emotional reactivity, risk-taking, impulsivity, and novelty seeking (reviewed in Casey et al., 2010, Doremus-Fitzwater et al., 2010, Green and McCormick, 2013). In both humans and rats, there is extensive restructuring of social relationships in adolescence. For example, girls and boys switch their primary focus from relationships with the family to relationships with peers (Nelson et al., 2005). Adolescent rats spend more time in social interaction and social play, and find social interactions more rewarding than do adult rats (Spear, 2011, Trezza et al., 2010).
The importance of social learning in adolescence is underscored by the marked dysfunction that is evident when deprived of social interactions during that period of ontogeny. Social deprivation in rats typically involves housing the animals singly, and has been referred to as a form of “sociogenic brain damage”, or social malnourishment (Montagu, 1977), but is more widely known as social isolation. The effects of social isolation in rats, a highly social animal, have been considered to model psychopathologies such as schizophrenia in humans (Fone and Porkness, 2008). It has long been recognized that the effects of social isolation are greater when experienced in adolescence than in adulthood (Einon and Morgan, 1977, Panksepp and Beatty, 1980). Marked deficits in brain chemistry, cognitive and emotional function are evident in adulthood after social isolation in adolescence, even when the social isolation is limited to the prepubertal, early adolescent period (e.g., Baarendse et al., 2013). Whereas some studies find that the effects of social isolation persist even if followed by a period of social housing (e.g., Lukkes et al., 2009), others have shown that partial remediation is possible by providing environmental enrichment (Hellemans et al., 2004) or that remediation is possible through social re-housing when social isolation is limited to either the fourth or fifth week of life (Hol et al., 1999).
Less severe manipulations of social relationships than deprivation in adolescence also modify the trajectory of brain and behavioural development. In this review, we describe our adolescent social instability model, which we have shown to result in mild impairments in emotional and cognitive behaviour in adulthood when administered in adolescence, but not when administered in adulthood (reviewed in Green and McCormick, 2013, McCormick and Green, 2013). Here we focus on our more recent evidence of impairments in social behaviour in adulthood after social instability in adolescence as well as review the effects of other social manipulations in adolescence on adult social function. We then discuss factors, notably hypothalamic–pituitary–adrenal responses to stressors, that may underlie the differential susceptibility of adolescents and adults to social stressors.
2. The social instability stress model
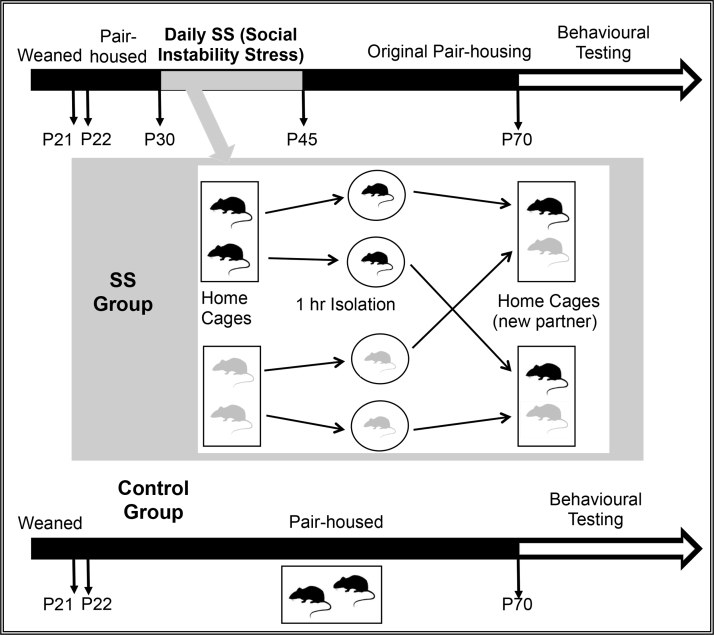
The social instability stress (SS) procedure involves pair-housed rats (Long Evans rats) that are removed from the colony room for one hour, after which rats are returned to the colony but to a new cage in which they are paired with a new cage partner of the same age that is also undergoing the SS procedure (see Fig. 1). The one hour isolation and pairings with new cage partners occur every day for 15 days. On the 16th day, after one hour isolation, all are returned to their original cage partner, at which time the SS procedure ends and rats are left undisturbed except for cage maintenance until time of behavioural testing. Control (non-stressed) rats are left undisturbed except for cage maintenance until time of behavioural testing throughout, except on postnatal days 30 and 45 when rats in both groups are weighed. When investigating the lasting effects of SS in adulthood, behavioural tests typically begin after postnatal day 70. In our initial studies, we applied the SS procedure in adolescence from postnatal day 33 to postnatal day 45 (McCormick et al., 2004, McCormick et al., 2005), but then changed the age range to between postnatal days 30 and 45 to capture a pre- and post-pubertal period in both sexes. Nevertheless, the use of the same ages for manipulation in both males and females means the sexes are at different developmental stages during the SS procedure. This problem is not easily resolved; although females attain pubertal milestones earlier than males, not all developmental milestones are attained earlier in females. For example, microglial colonization of the hippocampus, cortex, and amygdala (Schwarz et al., 2012) and neuronal numbers in the locus coeruleus (Pinos et al., 2001) reach plateaus earlier in males than in females. Thus, we focus more on the sex-specificity of our SS procedure rather than on sex differences per se. In this review, we describe our results for males only.
Fig. 1.
Timeline for the adolescent social instability stress experimental procedures.
The daily one hour isolation part of the SS procedure involves confining the adolescents in small containers (approximately 10 cm in height, 14 cm in diameter) in a room separate from the colony. Such one hour isolation was known to initiate a stress response involving activation of the hypothalamic–pituitary–adrenal (HPA) axis and elevation of blood concentrations of circulating glucocorticoids (primarily corticosterone in rats) in adolescents (McCormick et al., 2001, McCormick et al., 2002). When developing our procedure, we did not know whether adolescents would show potentiation of glucocorticoid release to a repeated stressor as do neonates (Knuth and Etgen, 2005, McCormick et al., 1998), or habituation (reduction) of glucocorticoid release to a repeated stressor as do adults (Grissom and Bhatnagar, 2009). Nevertheless, we hypothesized that returning to an unfamiliar cage partner would prolong glucocorticoid release, and we describe recent experiments investigating HPA function and behaviour in the home cage after isolation in a later section. Although initially we focused on how adolescent social instability altered behavioural responses to drugs of abuse (reviewed in McCormick, 2010), our approach is neuropsychological in that we are interested in characterizing a broad range of behavioural dimensions to help target the possible underlying neural substrates affected by adolescent social instability.
3. Effects on social behaviour in adulthood
The Social Interaction Test is a widely used measure of social anxiety in rodents (reviewed in File and Seth, 2003), and involves habituating a rat (test rat) to an arena and introducing a novel conspecific (stimulus rat). The main measure in the task is the amount of time spent in social interaction (e.g., play behaviours, sniffing, grooming). As adults, rats that underwent the social instability procedure in adolescence (SS rats) initiated fewer social interactions with the novel stimulus rat compared to control rats, irrespective of whether the stimulus rat was a control rat or another SS rat (Green et al., 2013). The same pattern was evident for stimulus rats; SS rats initiated fewer social interactions than did control rats, resulting in a greater time spent in social interactions in pairs in which both test and stimulus rats were control rats and fewest for pairs in which both test and stimulus rats were SS rats. The reduced initiation of social interactions by SS rats did not appear to be the result of social avoidance; the mean distance between test and stimulus rats in a test session was the same for all pairings of rats. Further, in a separate test in which novel stimulus rats were kept behind wire mesh at one end of a chamber, SS rats showed a strong preference for the side of the chamber near the stimulus rat, and did not differ from control rats in the amount of time spent near the stimulus rat. Thus, the unfamiliar conspecific may be more anxiety provoking when not confined and may be unpredictable in its movements. Further, SS male rats show more anxiety-like behaviours than control rats in other tests of anxiety that do not have social factors, such as willingness to venture out into unprotected spaces rather than remain close to walls or in enclosed spaces (Green et al., 2013, McCormick et al., 2008). It is also possible that the reduced time in social interactions of SS rats reflects an impoverished social repertoire compared to control rats.
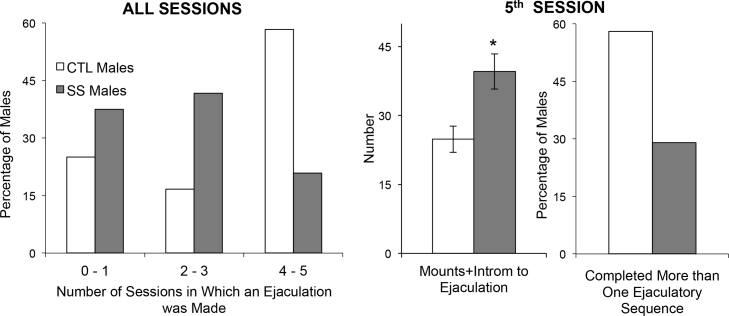
SS rats also display impaired social behaviour compared to control rats in tests of mating behaviour (McCormick et al., 2013). As in humans, sexual behaviour in rats involves learned components and practice effects in addition to unlearned components. For example, there is evidence that the incentive properties of females are learned by males and that the performance of males improves with experience (reviewed in Agmo, 1999, Pfaus et al., 2012). In a standard mating chamber in which a male is paired with a receptive female, latency to mount the female is used as a measure of motivation. The three main copulatory performance measures of male sexual behaviour in rats are mounts, intromissions, and ejaculations, with several mounts and intromissions preceding an ejaculation, although fewer are required in sexually experienced males. Thus, number of mounts and intromissions preceding an ejaculation is used as an index of copulatory efficiency (lower number = greater copulatory efficiency). Across five test sessions, SS rats decreased their latencies to mount the female and increased the number of ejaculations made, as did control rats (McCormick et al., 2013). SS rats, however, showed evidence of deficits in sexual behaviour (see Fig. 2). Compared to control males, individual SS males were less likely to complete an ejaculatory sequence in the majority (>3) of the sessions. In the fifth session, the SS males displayed reduced copulatory efficiency, and control males were twice as likely to ejaculate more than once in a session (McCormick et al., 2013). These results suggest that SS males’ sexual behaviour would be particularly compromised under naturalistic mating conditions, which for rats involves several males competing for females and in larger spaces that allow females to set the pace of mating. Although the sexual performance of the SS males fits the definition of “sexually sluggish” in the literature, neither the basis of SS males deficits or of sexually sluggish males is well understood (e.g., Antonio-Cabrera and Paredes, 2012). Although it is possible that social instability altered the ongoing development of limbic and cortical regions that facilitate male sexual behaviour, it may be that the deficits in sexual behaviour of male SS rats are secondary to increased social anxiety and/or an impoverished social repertoire.
Fig. 2.
As adults, adolescents that underwent adolescent social instability stress (SS) had impaired sexual behaviour compared to control rats (CTL). Across all sessions, a smaller proportion of SS than CTL rats made an ejaculation in the majority of sessions. In the 5th session, the higher mean (±S.E.M.) number of mounts and intromissions before ejaculation of SS rats compared to CTL rats is indicative of reduced copulatory efficiency (*p = 0.004), and a smaller percentage of SS than CTL males completed more than one ejaculatory sequence.
Adapted from McCormick et al. (2013).
To date, however, we have evidence only for altered hippocampal plasticity after the social instability stress procedure. During the first few days of the social instability procedure, there is increased proliferation in the granule layer of the dentate gyrus of the hippocampal formation, based on measurement of Ki67 immunoreactive cell numbers (McCormick et al., 2012) (Ki67 is an endogenous marker of cell proliferation that has a short window of expression, limited to active stages of the cell cycle, Kee et al., 2002). On postnatal day 46 (one day after day the last day of the social instability procedure), there was no difference in cell proliferation between SS and control rats, nor was there a difference in proliferation in adulthood. Neurogenesis, however, appeared to be permanently altered after social instability stress. Using an endogenous, neuron-specific marker of cell proliferation and survival, doublecortin, a protein that is expressed for longer (about three weeks, while neurons are immature, Brown et al., 2003), there were higher numbers of immature neurons at postnatal day 46 and several weeks after the social instability procedure in adulthood in SS rats compared to control rats (McCormick et al., 2012). These data suggest that proliferating neurons in the hippocampal formation survive longer in SS rats than in control rats. Nevertheless, we do not know whether these neurons continue to mature to be incorporated functionally in to the hippocampus. If they are incorporated functionally into the hippocampus, then the incorporation is likely aberrant; SS rats have reduced performance on hippocampal-dependent memory tests and lower expression of the phosphorylated calcium/calmodulin-dependent kinase II subunit threonine 286, a marker of synaptic plasticity compared with controls (McCormick et al., 2012). Thus, the developmental trajectory of the hippocampus appears to be altered after social instability stress in adolescence.
The evidence of higher survival of immature neurons after social instability stress in adolescence is contrary to the typical finding of reduced neurogenesis after chronic stress when administered in adulthood (see review by Balu and Lucki, 2009), and may be unique to the social instability and/or stress of the procedure in the adolescent period of life. A study in adolescent rats similar to ours (they did not manipulate cage partners but their 12 one hour sessions of restraint between postnatal days 30 and 52 are similar to our 16 sessions of one hour isolation) also reported higher survival of neurons without any difference in proliferation in adulthood in stressed male rats compared to control rats (Barha et al., 2011). Further, in keeping with our results, mice that showed social avoidance four weeks after a chronic stress exposure had higher survival of neurons generated soon after the termination of the stress exposure than non-avoidant mice (Lagace et al., 2010). The hippocampus is implicated in depressive and anxious behaviour (Bannerman et al., 2014), and, of relevance for our findings described earlier, the hippocampus has been implicated in anxiety in the social interaction test (File et al., 1998, Hollis et al., 2010, Marco et al., 2011). Further, in addition to effects on approach-avoidance conflict, lesions of the hippocampus impaired behavioural sequencing in male rats (the predictability of behaviour of one rat by the behaviour of its partner) (Maaswinkel et al., 1997); thus it may be that the deficits in social behaviour in SS rats also involve deficits in social sequencing.
4. Other adolescent social stress models and effects on social behaviour in adulthood
Although there are several different stress procedures that have been used in research of adolescents, few involve social stressors and few involve measures of social behaviour in adulthood (see reviews by Green and McCormick, 2013, McCormick and Green, 2013). Some chronic variable stress procedures involve social components as part of the procedure (some isolation), and have long-lasting effects (e.g., Chaby et al., 2013), and one study reported decreased social interactions after such varied prepubertal stress exposures in rats (Toth et al., 2008). Exposure to stressors in adolescence without specific social disruption, such as predator stress, also have been found to decrease social interactions in adulthood (e.g., predator stress Wright et al., 2008, Wright et al., 2012), although social context may moderate the influence of such stressors (e.g., Kendig et al., 2011). Social defeat is an effective social stressor that involves introducing the adolescent to the cage of much larger aggressive male (see review by Buwalda et al., 2011). Adolescent male Wistar rats exposed to five sessions (postnatal days 45–57) of social defeat spent less time as adults (postnatal day 78) in interaction with an unfamiliar conspecific compared to control adult males (Vidal et al., 2007). Socially-defeated males also had increased latencies to enter the interaction zone of the arena compared to control males, but no differences were found in number of entries into the interaction zone, or distance travelled (Vidal et al., 2007). Thus, the effects appeared specific to social anxiety. In another study, adolescent male Sprague-Dawley rats were exposed to repeated social defeat (daily for 5 days beginning at postnatal day 35), then tested at postnatal day 56 for anxiety behaviours in the context where the defeat took place, as well as in the Elevated Plus Maze and Open Field tests (Watt et al., 2009). When returned to the defeat context, socially-defeated rats had decreased active exploration and increased risk-assessment behaviour compared to control rats. Nevertheless, in the elevated plus maze test, socially-defeated rats showed less anxiety-like behaviour compared to control rats (Watt et al., 2009). Further, the effects of social defeat may be stronger in some strains of rats than others (e.g., lasting effects on social anxiety were found in Wistar rats and not in Wild-type Groningen rats, Vidal et al., 2011). Lasting changes in the nervous system are found in socially-defeated rats, such as changes to the dopaminergic system in the medial prefrontal cortex (Novick et al., 2008, Watt et al., 2009). Of relevance to our finding of lasting changes in the hippocampus after social instability stress, social defeat in male rats alters structural and electrophysiological properties of the hippocampus (Buwalda et al., 2005). Other adolescent stress procedures also alter the ongoing development of the hippocampus (e.g., chronic variable physical stressors, Isgor et al., 2004). Many of the negative consequences of social defeat, however, require that the rats be housed singly after the social defeat (Buwalda et al., 2013, de Jong et al., 2005), which also highlights the importance of social buffering in rats.
Might adolescent social instability be a form of enrichment rather than a stressor per se? This possibility is suggested by evidence that rats undergoing environmental enrichment from postnatal day 25 (7 rats per cage equipped with horizontal platforms and toys) had impaired sexual behaviour and increased anxiety compared to rats reared in a standard cage (2–3) cage (Urakawa et al., 2014), similar to our effects for social instability stress. Alternatively, such enriched group housing may be similar to the visible burrow group-housing context in which subordinates display reductions in reproductive hormones and increased stress because of agonistic interactions when forming dominance hierarchies (Hardy et al., 2002).
5. Basis for the lasting effects of social instability stress in adolescence
Stressors, largely through the high, prolonged release of glucocorticoids, are known to be important moderators of neural plasticity and important mediators of the effects of life experiences on the nervous system (McEwen, 2010). The release of glucocorticoids in response to a stressor is the endpoint of activation of the hypothalamic–pituitary–adrenal (HPA) axis; the perception of a stressor initiates signals that are integrated in the paraventricular nucleus of the hypothalamus causing the release of corticotrophin releasing hormone to act on the pituitary to release adrenocorticotrophic hormone, which then acts on the adrenal to release glucocorticoids (primarily corticosterone in rats) into the circulatory system. There is a high density of glucocorticoid receptors in brain regions such as the hippocampus, medial prefrontal cortex, and amygdala, all of which continue to develop throughout adolescence (McCormick et al., 2010). Further, increased vulnerability during times of biological transitions is proposed to involve change in the reactivity of the stress systems (Dorn and Chrousos, 1997).
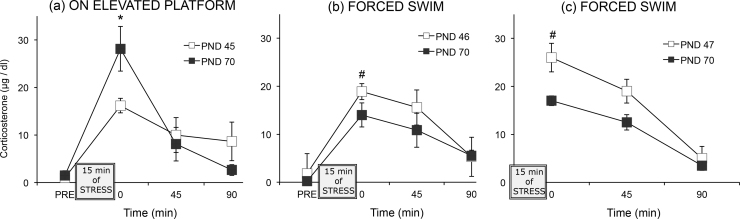
Prolonged release of glucocorticoids in response to an acute stressor in adolescents relative to adults is one possible mechanism underlying the vulnerability to such stressors in adolescents (Eiland and Romeo, 2013). While this age difference may be true for some acute stressors, such as restraint in pre-pubertal adolescents (postnatal days 23–32) compared to in adults (Bingham et al., 2011, Doremus-Fitzwater et al., 2009, Foilb et al., 2011, Lui et al., 2012, Romeo et al., 2006a, Romeo et al., 2006b, Romeo et al., 2004a, Romeo et al., 2004b) corticosterone release is greater to nicotine (Cao et al., 2010) and more prolonged after injection of lipopolysaccharide (Goble et al., 2011), in adults than in pre-pubertal adolescents, and corticosterone release did not differ for adolescents and adults to novelty stress (Goldman et al., 1973) or after injection of nicotine in a different study (Cruz et al., 2008). We have found for mid-adolescents (postnatal days 45–47) that the direction of difference in pattern of glucocorticoid release compared to adults depends on the stressor (see Fig. 3). Whereas mid-adolescent rats had higher corticosterone concentrations than did adult rats after 15 min of forced swimming (Mathews et al., 2008, Waters and McCormick, 2011), they had lower corticosterone concentrations than did adult rats after 15 min confined to an elevated platform (confined to the open arm of the Elevated Plus Maze) (McCormick et al., 2008). The reduced corticosterone release of adolescent males on the elevated platform is likely a reflection of their greater willingness to venture out onto an open, elevated platform in the Elevated Plus Maze test of anxiety than are adult males (McCormick et al., 2008), whereas the higher release of adolescents in the forced swim test may reflect greater energy expenditure because of their reduced buoyancy compared to adults. Adolescent and adults do not differ in glucocorticoid receptor expression in the brain (Romeo et al., 2008, Vazquez, 1998), although the adrenal gland (Romeo et al., 2014) and the neural structures that either directly or indirectly innervate the paraventricular nucleus (Eiland and Romeo, 2013) are developing throughout adolescence. Thus, it is difficult to ascertain the extent to which adolescents have an immature HPA axis compared to adults versus the extent to which the perception of/experience of stressors differs at the two phases of ontogeny.
Fig. 3.
Data from three separate studies portraying mean (±S.E.M.) plasma corticosterone concentrations for mid-adolescent and adult male rats before and after a 15 min stress exposure. *Higher than adolescent rats, p < 0.05; #Higher than adult males, p < 0.05.
Data are adapted from (a) McCormick et al. (2008), (b) Mathews et al. (2008), and (c) Waters and McCormick (2011)
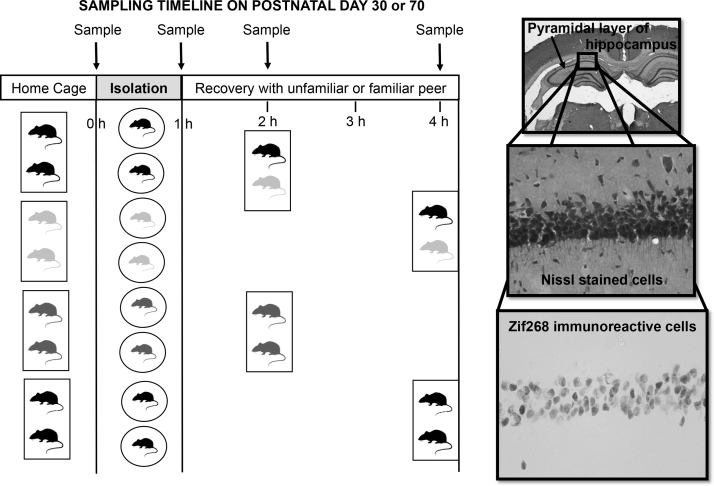
We recently compared how prepubertal (postnatal day 30) adolescents and adults responded to one hour isolation and a return to an unfamiliar cage partner (as in our social instability stress procedure) or to a familiar cage partner by measuring plasma corticosterone concentrations and protein expression of the immediate early gene, zif268, to gauge neuronal activation in response to isolation and partner familiarity at both ages (see Fig. 4) (Hodges et al., 2014). Adolescent and adult males did not differ in corticosterone concentrations at any sampling time. Both age groups showed an effect of social buffering, in that those returning to a familiar cage partner had lower corticosterone concentrations than did those with an unfamiliar cage partner. Although effects of isolation and of partner familiarity were found in Zif268-immunoreactive cell counts in several neural regions, the only effect of age was found in the paraventricular nucleus, whereby after one hour of recovery in the colony after isolation, adolescents had higher Zif268-immunoreactive cell counts than did adults (Hodges et al., 2014). Thus, these results are consistent with more prolonged activation of the HPA axis in response to stressors in adolescents than adults, although no differences in corticosterone release were observed.
Fig. 4.
Experimental design and sampling timeline for experiment by Hodges et al. (2014) for measurement of plasma corticosterone and Zif268 immunoreactive cell counts in specific brain regions before and after one hour isolated and recovery in home cage with either a familiar or unfamiliar peer. The figure shows coronal sections of the rat hippocampus pyramidal layer stained for Nissl bodies (40× and 400× magnification) and Zif268 immunoreactive cells (400× magnification).
Greater age differences might emerge under conditions of repeated stress exposures, and we are currently investigating corticosterone release and Zif268 expression at time points on the last day of the social instability stress procedure administered to both adolescents (postnatal days 30–45) and adults (postnatal days 70–115). There are few comparisons of HPA function of adolescents and adults to repeated stressors, although the typical result is that whereas adults show evidence of habituation to stressors (reduced corticosterone release to a repeated stressor), adolescents do not (Doremus-Fitzwater et al., 2009, Lui et al., 2012, Romeo et al., 2006b). These studies, however, involved the pre-pubertal period and a shorter time frame than the 16 days of the social instability stress procedure, which extends to the post-pubertal period. We previously found that corticosterone release to the 16th episode of isolation on postnatal day 45 in SS male rats was lower than control rats undergoing their first isolation on day 45, indicating habituation of corticosterone release in SS rats, but there were no adult comparison groups and the study involved only a baseline and immediately after isolation time points (McCormick et al., 2007). There were no differences among the groups in baseline expression of CRH mRNA in the central nucleus of the amygdala. Higher CRH mRNA expression in the central nucleus, however, was observed after isolation in those that had undergone unfamiliar partner pairings (social instability) compared to those returning to a familiar cage partner after isolation. Regulation of CRH by glucocorticoids differs for the central nucleus of the amygdala compared to the paraventricular nucleus of the hypothalamus. For example, glucocorticoids decrease CRH mRNA expression in the paraventricular nucleus and increase expression in the central nucleus, whereas adrenalectomy increases CRH mRNA expression in the paraventricular nucleus and decreases expression in the central nucleus (Schulkin et al., 2005). Because the amygdala is implicated in fear and anxiety behaviours, perhaps by increasing the saliency of sensory cues (Merali et al., 2004), we hypothesized that isolation differs for SS rats because they have learned that isolation predicts the pairing with a new cage partner, and thus isolation remains a highly salient stimulus for SS rats and not for rats that return to a familiar partner after isolation (McCormick et al., 2007).
Preliminary evidence supports this hypothesis. Rats that underwent 16 episodes of isolation and then placed for the 16th time with an unfamiliar cage partner on postnatal day 45 had higher corticosterone concentrations compared to rats undergoing a first day of isolation and then placed for one hour with an unfamiliar partner (Hodges and McCormick, in preparation). These data indicate that although adolescent male rats habituate to daily isolation, they show a sensitized response to repeated pairings with a new cage partner. The same comparisons in adult rats (the isolation and change of partners occurred in adulthood) found no evidence for sensitization; adult rats showed evidence of habituation to both isolation and to unfamiliar partners (Hodges and McCormick, in preparation). Thus, it may be that adolescents code the repeated pairings with an unfamiliar partner as a heterotypic event, whereas adults code the repeated pairings as a homotypic event; habituation is more likely to occur to homotypic stressors than to heterotypic stressors (Grissom and Bhatnagar, 2009). The sensitized corticosterone response of adolescent, and habituated response of adult, rats to unfamiliar conspecifics may seem at odds with the greater reward value of social interactions in adolescents than adults. An important consideration, however, is that although high corticosterone concentrations are used as an indication of stress, such concentrations per se do not indicate valence; rats show similar elevations in glucocorticoid release to both aversive and rewarding experiences (e.g., Buwalda et al., 2012).
Behaviour in the home cage does not differ markedly between rats returning to an unfamiliar cage partner or to a familiar cage partner. We have not found any significant aggression in observations in the home cage, and, although SS rats stay somewhat more active after isolation, within about 30 min SS rats typically are snuggled up next to their cage partner as are rats returned to their familiar partner (McCormick et al., 2007). Preliminary evidence, though, suggests that dominance relationships within pairs of cage partners might differ between SS rats and control rats. Under conditions of food competition, SS pairs displayed higher aggressive behaviour than did control rats (Cummings, Thompson, and McCormick, in preparation). These results are another indication of difference in adult social behaviour after social instability stress in adolescence.
6. Conclusions
One of the main challenges of adolescence is learning to navigate the social world beyond the family. There is growing evidence that the quality of social experiences, experiences to which the adolescent brain appears particularly tuned, shapes social function in adulthood. The differential effects of stressors and activation of the hypothalamic–pituitary–axis in adolescents compared to adults is likely based on their different neural substrates. Social experiences are not the same event for adolescents and adults, hence elicit different activation of the HPA axis. In turn, the effects of prolonged exposure to glucocorticoids differ at the two ages because they act on different substrates. To unravel developmental-stage-specific plasticity will require greater understanding of how social relationships are perceived and negotiated in both studies with animal models and humans.
Conflict of interest
The authors have no conflict of interest to declare.
Acknowledgements
CMM holds a Natural Sciences and Engineering Research Council of Canada (NSERC) Discovery Grant that supported the research.
Footnotes
Available online 21 April 2014
References
- Agmo A. Sexual motivation – an inquiry into events determining the occurrence of sexual behavior. Behav. Brain Res. 1999;105:129–150. doi: 10.1016/s0166-4328(99)00088-1. [DOI] [PubMed] [Google Scholar]
- Antonio-Cabrera E., Paredes R.G. Effects of chronic estradiol or testosterone treatment upon sexual behavior in sexually sluggish male rats. Pharmacol. Biochem. Behav. 2012;101:336–341. doi: 10.1016/j.pbb.2012.01.021. [DOI] [PubMed] [Google Scholar]
- Baarendse P.J.J., Counotte D.S., O’Donnell P., Vanderschuren L. Early social experience is critical for the development of cognitive control and dopamine modulation of prefrontal cortex function. Neuropsychopharmacology. 2013;38:1485–1494. doi: 10.1038/npp.2013.47. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Balu D.T., Lucki I. Adult hippocampal neurogenesis, regulation, functional implications, and contribution to disease pathology. Neurosci. Biobehav. Rev. 2009;33:232–252. doi: 10.1016/j.neubiorev.2008.08.007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bannerman D.M., Sprengel R., Sanderson D.J., McHugh S.B., Rawlins J.N., Monyer H., Seeburg P.H. Hippocampal synaptic plasticity, spatial memory and anxiety. Nat. Rev. Neurosci. 2014;15:181–192. doi: 10.1038/nrn3677. [DOI] [PubMed] [Google Scholar]
- Barha C., Brummelte S., Lieblich S.E., Galea L.A.M. Chronic restraint stress in adolescence differentially influences hypothalamic–pituitary–adrenal axis function and adult hippocampal neurogenesis in male and female rats. Hippocampus. 2011;21:1216–1227. doi: 10.1002/hipo.20829. [DOI] [PubMed] [Google Scholar]
- Bingham B., McFadden K., Zhang X.Y., Bhatnagar S., Beck S., Valentino R. Early adolescence as a critical window during which social stress distinctly alters behavior and brain norepinephrine activity. Neuropsychopharmacology. 2011;36:896–909. doi: 10.1038/npp.2010.229. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Blakemore S.J. Development of the social brain in adolescence. J. R. Soc. Med. 2012;105:111–116. doi: 10.1258/jrsm.2011.110221. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bogin B.A., Smith H.B. Evolution of the human life cycle. Am. J. Human Biol. 1996;8:703–716. doi: 10.1002/(SICI)1520-6300(1996)8:6<703::AID-AJHB2>3.0.CO;2-U. [DOI] [PubMed] [Google Scholar]
- Bredy T.W., Zhang T.Y., Grant R.J., Diorio J., Meaney M.J. Peripubertal environmental enrichment reverses the effects of maternal care on hippocampal development and glutamate receptor subunit expression. Eur. J. Neurosci. 2004;20:1355–1362. doi: 10.1111/j.1460-9568.2004.03599.x. [DOI] [PubMed] [Google Scholar]
- Brenhouse H.C., Andersen S.L. Developmental trajectories during adolescence in males and females: a cross-species understanding of underlying brain changes. Neurosci. Biobehav. Rev. 2011;35:1687–1703. doi: 10.1016/j.neubiorev.2011.04.013. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Brown G.R., Spencer K.A. Steroid hormones, stress and the adolescent brain: a comparative perspective. Neuroscience. 2013;249:115–128. doi: 10.1016/j.neuroscience.2012.12.016. [DOI] [PubMed] [Google Scholar]
- Brown J.P., Couillard-Despres S., Cooper-Kuhn C.M., Winkler J., Aigner L., Kuhn H.G. Transient expression of doublecortin during adult neurogenesis. J. Comparative Neurol. 2003;467:1–10. doi: 10.1002/cne.10874. [DOI] [PubMed] [Google Scholar]
- Buwalda B., Geerdink M., Vidal J., Koolhaas J.M. Social behavior and social stress in adolescence: a focus on animal models. Neurosci. Biobehav. Rev. 2011;32:1713–1721. doi: 10.1016/j.neubiorev.2010.10.004. [DOI] [PubMed] [Google Scholar]
- Buwalda B., Kole M.H., Veenema A.H., Huininga M., de Boer S.F., Korte S.M., Koolhaas J.M. Long-term effects of social stress on brain and behavior: a focus on hippocampal functioning. Neurosci. Biobehav. Rev. 2005;29:83–97. doi: 10.1016/j.neubiorev.2004.05.005. [DOI] [PubMed] [Google Scholar]
- Buwalda B., Scholte J., de Boer S.F., Coppens C.M., Koolhaas J.M. The acute glucocorticoid stress response does not differentiate between rewarding and aversive social stimuli in rats. Horm. Behav. 2012;61:218–226. doi: 10.1016/j.yhbeh.2011.12.012. [DOI] [PubMed] [Google Scholar]
- Buwalda B., Stubbendorff C., Zickert N., Koolhaas J.M. Adolescent social stress does not necessarily lead to a compromised adaptive capacity during adulthood: a study on the consequences of social stress in rats. Neuroscience. 2013;249:258–270. doi: 10.1016/j.neuroscience.2012.12.050. [DOI] [PubMed] [Google Scholar]
- Cao J.R., Belluzzi J.D., Loughlin S.E., Dao J.M., Chen Y.L., Leslie F.M. Locomotor and stress responses to nicotine differ in adolescent and adult rats. Pharmacol. Biochem. Behav. 2010;96:82–90. doi: 10.1016/j.pbb.2010.04.010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Casey B.J., Jones R.N., Levita L., Libby V., Pattwell S.S., Ruberry E.J., Soliman F., Somerville L.H. The storm and stress of adolescence: insights from human imaging and mouse genetics. Dev. Psychobiol. 2010;52:225–235. doi: 10.1002/dev.20447. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chaby L.E., Cavigelli S.A., White A., Wang K., Brathwaite V.A. Long-term changes in cognitive bias and coping response as a result of chronic unpredictable stress during adolescence. Front. Human Neurosci. 2013;7:1–10. doi: 10.3389/fnhum.2013.00328. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cooke B.M., Shukla D. Double helix, reciprocity between juvenile play and brain development. Dev. Cogn. Neurosci. 2011;1:459–470. doi: 10.1016/j.dcn.2011.07.001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cruz F., DeLucia R., Planeta C. Effects of chronic stress on nicotine-induced locomotor activity and corticosterone release in adult and adolescent rats. Addict. Biol. 2008;13:63–69. doi: 10.1111/j.1369-1600.2007.00080.x. [DOI] [PubMed] [Google Scholar]
- de Jong J.G., van der Vegt B.J., Buwalda B., Koolhaas J.M. Social environment determines the long-term effects of social defeat. Physiol. Behav. 2005;84:87–95. doi: 10.1016/j.physbeh.2004.10.013. [DOI] [PubMed] [Google Scholar]
- Doremus-Fitzwater T.L., Varlinskaya E.I., Spear L.P. Social and non-social anxiety in adolescent and adult rats after repeated restraint. Physiol. Behav. 2009;97:484–494. doi: 10.1016/j.physbeh.2009.03.025. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Doremus-Fitzwater T.L., Varlinskaya E.L., Spear L.P. Motivational systems in adolescence: possible implications for age differences in substance abuse and other risk-taking behaviors. Brain Cogn. 2010;72:114–123. doi: 10.1016/j.bandc.2009.08.008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dorn L.D., Chrousos G.P. The neurobiology of stress: understanding regulation of affect during female biological transitions. Semin. Reprod. Endocrinol. 1997;15:19–35. doi: 10.1055/s-2008-1067965. [DOI] [PubMed] [Google Scholar]
- Eiland L., Romeo R.D. Stress and the developing adolescent brain. Neuroscience. 2013;249:162–171. doi: 10.1016/j.neuroscience.2012.10.048. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Einon D.F., Morgan M.J. A critical period for social isolation in the rat. Dev. Psychobiol. 1977;10:123–132. doi: 10.1002/dev.420100205. [DOI] [PubMed] [Google Scholar]
- File S.E., Kenny P.J., Ouagazzal A. Bimodal modulation by nicotine of anxiety in the social interaction test: role of the dorsal hippocampus. Behav. Neurosci. 1998;112:1423–1429. doi: 10.1037//0735-7044.112.6.1423. [DOI] [PubMed] [Google Scholar]
- File S.E., Seth P. A review of 25 years of the social interaction test. Eur. J. Pharmacol. 2003;463:35–53. doi: 10.1016/s0014-2999(03)01273-1. [DOI] [PubMed] [Google Scholar]
- Foilb A.R., Lui P., Romeo R.D. The transformation of hormonal stress responses throughout puberty and adolescence. J. Endocrinol. 2011;210:391–398. doi: 10.1530/JOE-11-0206. [DOI] [PubMed] [Google Scholar]
- Fone K.C., Porkness M.V. Behavioural and neurochemical effects of post-weaning social isolation in rodents-relevance to developmental neuropsychiatric disorders. Neurosci. Biobehav. Rev. 2008;32:1087–1102. doi: 10.1016/j.neubiorev.2008.03.003. [DOI] [PubMed] [Google Scholar]
- Fox V.C. Is adolescence a phenomenon of modern times? J. Psychohist. 1977;5:271–290. [Google Scholar]
- Goble K.H., Bain Z.A., Padow V.A., Lui P., Klein Z.A., Romeo R.D. Pubertal-related changes in hypothalamic–pituitary–adrenal axis reactivity and cytokine secretion in response to an immunological stressor. J. Neuroendocrinol. 2011;23:129–135. doi: 10.1111/j.1365-2826.2010.02085.x. [DOI] [PubMed] [Google Scholar]
- Goldman L., Winget C., Hollingshead G.W., Levine S. Postweaning development of negative feedback in the pituitary–adrenal system of the rat. Neuroendocrinology. 1973;12:199–211. doi: 10.1159/000122169. [DOI] [PubMed] [Google Scholar]
- Gottlieb G., Lickliter R. The various roles of animal models in understanding human development. Social Dev. 2004;13:311–325. [Google Scholar]
- Green M.R., Barnes B., McCormick C.M. Social instability stress in adolescence increases anxiety and reduces social interactions in adulthood in male Long-Evans rats. Dev. Psychobiol. 2013;55:849–859. doi: 10.1002/dev.21077. [DOI] [PubMed] [Google Scholar]
- Green M.R., McCormick C.M. Effects of stressors in adolescence on learning and memory in rodent models. Horm. Behav. 2013;64:364–379. doi: 10.1016/j.yhbeh.2012.09.012. [DOI] [PubMed] [Google Scholar]
- Grissom N., Bhatnagar S. Habituation to repeated stress. Get used to it. Neurobiol. Learn. Mem. 2009;92:215–224. doi: 10.1016/j.nlm.2008.07.001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hardy M.P., Sottas C.M., Ge R., McKittrick C.R., Tamashiro K.L., McEwen B.S., Haider S.G., Markham C.M., Blanchard R.J., Blanchard D.C., Sakai R.R. Trends of reproductive hormones in male rats during psychosocial stress: role of glucocorticoid metabolism in behavioral dominance. Biol Reprod. 2002;67:1750–1755. doi: 10.1095/biolreprod.102.006312. [DOI] [PubMed] [Google Scholar]
- Hellemans K.G.C., Benge L.C., Olmstead M.C. Adolescent enrichment partially reverses the social isolation syndrome. Dev. Brain Res. 2004;150:103–115. doi: 10.1016/j.devbrainres.2004.03.003. [DOI] [PubMed] [Google Scholar]
- Hodges T.E., Green M.R., Simone J.J., McCormick C.M. Effects of social context on endocrine function and Zif268 expression in response to an acute stressor in adolescent and adult rats. Int. J. Dev. Neurosci. 2014;35:25–34. doi: 10.1016/j.ijdevneu.2014.03.001. [DOI] [PubMed] [Google Scholar]
- Hol T., Van Den Berg C.L., van Ree J.M., Spruijt B.M. Isolation during the play period in infancy decreases adult social interactions in rats. Behav. Brain Res. 1999;100:91–97. doi: 10.1016/s0166-4328(98)00116-8. [DOI] [PubMed] [Google Scholar]
- Hollis F., Wang H., Dietz D., Gunjan A., Kabbaj M. The effects of repeated social defeat on long-term depressive-like behavior and short-term histone modifications in the hippocampus in male Sprague-Dawley rats. Psychopharmacology (Berl) 2010;211:69–77. doi: 10.1007/s00213-010-1869-9. [DOI] [PubMed] [Google Scholar]
- Isgor C., Kabbaj M., Akil H., Watson S.J. Delayed effects of chronic variable stress during peripubertal-juvenile period on hippocampal morphology and on cognitive and stress axis functions in rats. Hippocampus. 2004;14:636–648. doi: 10.1002/hipo.10207. [DOI] [PubMed] [Google Scholar]
- Kee N., Sivalngam S., Bonnstra R., Wojtowicz J.M. The utility of Ki-67 and BrdU as proliferative markers of adult neurogenesis. J. Neurosci. Methods. 2002;115:97–105. doi: 10.1016/s0165-0270(02)00007-9. [DOI] [PubMed] [Google Scholar]
- Kendig M.D., Bowen M.T., Kemp A.H., McGregor I.S. Predatory threat induces huddling in adolescent rats and residual changes in early adulthood suggestive of increased resilience. Behav. Brain Res. 2011;225:405–414. doi: 10.1016/j.bbr.2011.07.058. [DOI] [PubMed] [Google Scholar]
- Knuth E.D., Etgen A.M. Corticosterone secretion induced by chronic isolation in neonatal rats is sexually dimorphic and accompanied by elevated ACTH. Horm. Behav. 2005;47:65–75. doi: 10.1016/j.yhbeh.2004.08.011. [DOI] [PubMed] [Google Scholar]
- Lagace D.C., Donovan M.H., DeCarolis N.A., Farnbauch L.A., Malhotra S., Berton O., Nestler E.J., Krishnan V., Eisch A.J. Adult hippocampal neurogenesis is functionally important for stress-induced social avoidance. Proc. Natl. Acad. Sci. U.S.A. 2010;107:4436–4444. doi: 10.1073/pnas.0910072107. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lui P., Padow V.A., Franco D., Hall B.S., Park B., Klein Z.A., Romeo R.D. Divergent stress-induced neuroendocrine and behavioral responses prior to puberty. Physiol. Behav. 2012;107:104–111. doi: 10.1016/j.physbeh.2012.06.011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lukkes J.L., Mokin M.V., Scholl J.L., Forster G.L. Adult rats exposed to early-life social isolation exhibit increased anxiety and conditioned fear behavior and altered hormonal stress responses. Horm. Behav. 2009;55:248–256. doi: 10.1016/j.yhbeh.2008.10.014. [DOI] [PubMed] [Google Scholar]
- Maaswinkel H., Gispen W.H., Spruijt B.M. Executive function of the hippocampus in social behavior in the rat. Behav. Neurosci. 1997;111:777–784. doi: 10.1037//0735-7044.111.4.777. [DOI] [PubMed] [Google Scholar]
- Marco E.M., Rapino C., Caprioli A., Borsini F., Maccarrone M., Lavioli G. Social encounter with a novel partner in adolescent rats, Activation of the central endocannabinoid system. Behav. Brain Res. 2011;220:140–145. doi: 10.1016/j.bbr.2011.01.044. [DOI] [PubMed] [Google Scholar]
- Mathews I.Z., Wilton A., Styles A., McCormick C.M. Heightened neuroendocrine function in males to a heterotypic stressor and increased depressive behaviour in females after adolescent social stress in rats. Behav. Brain Res. 2008;190:33–40. doi: 10.1016/j.bbr.2008.02.004. [DOI] [PubMed] [Google Scholar]
- McCormick C.M. An animal model of social instability stress in adolescence and risk for drugs of abuse. Physiol. Behav. 2010;99:194–203. doi: 10.1016/j.physbeh.2009.01.014. [DOI] [PubMed] [Google Scholar]
- McCormick C.M., Green M.R. From the stressed adolescent to the anxious and depressed adult: investigations in rodent models. Neuroscience. 2013;249:242–257. doi: 10.1016/j.neuroscience.2012.08.063. [DOI] [PubMed] [Google Scholar]
- McCormick C.M., Green M.R., Cameron N.M., Nixon F., Levy M.J., Clark R.A. Deficits in male sexual behavior in adulthood after social instability stress in adolescence in rats. Horm. Behav. 2013;63:5–12. doi: 10.1016/j.yhbeh.2012.11.009. [DOI] [PubMed] [Google Scholar]
- McCormick C.M., Kehoe P., Kovacs S. Corticosterone release in response to repeated, short episodes of neonatal isolation: evidence of sensitization. Int. J. Dev. Neurosci. 1998;16:175–185. doi: 10.1016/s0736-5748(98)00026-4. [DOI] [PubMed] [Google Scholar]
- McCormick C.M., Kehoe P., Mallinson K., Cecchi L., Frye C.A. Neonatal isolation alters stress hormone and mesolimbic dopamine release in juvenile rats. Pharmacol. Biochem. Behav. 2002;73:77–85. doi: 10.1016/s0091-3057(02)00758-x. [DOI] [PubMed] [Google Scholar]
- McCormick C.M., Mathews I.Z. Adolescent development, hypothalamic–pituitary–adrenal function, and programming of adult learning and memory. Prog. Neuropsychopharmacol. Biol. Psychiatry. 2010;34:756–765. doi: 10.1016/j.pnpbp.2009.09.019. [DOI] [PubMed] [Google Scholar]
- McCormick C.M., Mathews I.Z., Thomas C., Waters P. Investigations of HPA function and the enduring consequences of stressors in adolescence in animal models. Brain Cogn. 2010;72:73–85. doi: 10.1016/j.bandc.2009.06.003. [DOI] [PubMed] [Google Scholar]
- McCormick C.M., Merrick A., Secen J., Helmreich D.L. Social instability in adolescence alters the central and peripheral hypothalamic–pituitary–adrenal responses to a repeated homotypic stressor in male and female rats. J. Neuroendocrinol. 2007;19:116–126. doi: 10.1111/j.1365-2826.2006.01515.x. [DOI] [PubMed] [Google Scholar]
- McCormick C.M., Rioux T., Fisher R., Lang K., MacLaury K., Teillon S.M. Effects of neonatal corticosterone treatment on maze performance and HPA axis in juvenile rats. Physiol. Behav. 2001;74:371–379. doi: 10.1016/s0031-9384(01)00574-1. [DOI] [PubMed] [Google Scholar]
- McCormick C.M., Robarts D., Gleason E., Kelsey J.E. Stress during adolescence enhances locomotor sensitization to nicotine in adulthood in female, but not male, rats. Horm. Behav. 2004;46:458–466. doi: 10.1016/j.yhbeh.2004.05.004. [DOI] [PubMed] [Google Scholar]
- McCormick C.M., Robarts D., Kopeikina K., Kelsey J.E. Long-lasting, sex-and age-specific effects of social stress on corticosterone responses to restraint and locomotor responses to psychostimulants in rats. Horm. Behav. 2005;48:64–74. doi: 10.1016/j.yhbeh.2005.01.008. [DOI] [PubMed] [Google Scholar]
- McCormick C.M., Smith C., Mathews I.Z. Effects of chronic social stress in adolescence on anxiety and neuroendocrine response to mild stress in male and female rats. Behav. Brain Res. 2008;187:228–238. doi: 10.1016/j.bbr.2007.09.005. [DOI] [PubMed] [Google Scholar]
- McCormick C.M., Thomas C.M., Sheridan C.S., Nixon F., Flynn J.A., Mathews I.Z. Social instability stress in adolescent male rats alters hippocampal neurogenesis and produces a deficit in spatial location memory in adulthood. Hippocampus. 2012;22:1300–1312. doi: 10.1002/hipo.20966. [DOI] [PubMed] [Google Scholar]
- McEwen B.S. Stress, sex and neural adaptation to a changing environments: mechanisms of neuronal remodeling. Ann. N.Y. Acad. Sci. 2010;1204:E38–E59. doi: 10.1111/j.1749-6632.2010.05568.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Merali Z., Khan S., Michaud D.S., Shippy S.A., Anisman H. Does amygdaloid corticotropin-releasing hormone (CRH) mediate anxiety-like behaviors? Dissociation of anxiogenic effects and CRH release. Eur. J. Neurosci. 2004;20:229–239. doi: 10.1111/j.1460-9568.2004.03468.x. [DOI] [PubMed] [Google Scholar]
- Montagu A. Sociogenic brain damage. In: Arieti S., Chrzanowski G., editors. New Dimensions in Psychiatry: A World View. Wiley; New York: 1977. pp. 4–25. [Google Scholar]
- Nelson E.E., Leibenluft E., McClure E.B., Pine D.S. The social re-orientation of adolescence: a neuroscience perspective on the process and its relation to psychopathology. Psychol. Med. 2005;35:163–174. doi: 10.1017/s0033291704003915. [DOI] [PubMed] [Google Scholar]
- Novick A.M., Burke A.R., Renner K.J., Forster G.L., Tejani-Butt S.M., Watt M.J. Society for Neuroscience; Washington DC: 2008. Adolescent social defeat alters markers of adult dopaminergic function. pp. 477.2/QQ1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Panksepp J., Beatty W.W. Social deprivation and play in rats. Behav. Neural Biol. 1980;30:197–206. doi: 10.1016/s0163-1047(80)91077-8. [DOI] [PubMed] [Google Scholar]
- Pfaus J.G., Kippin T.E., Coria-Avila G.A., Gelez H., Afonso V.M., Ismail N., Parada M. Who, what, where, when (and maybe even why)? How the experience of sexual reward connects sexual desire, preference, and performance. Arch. Sex. Behav. 2012;41:31–62. doi: 10.1007/s10508-012-9935-5. [DOI] [PubMed] [Google Scholar]
- Pinos H., Collado P., Rodríguez-Zafra M., Rodríguez C., Segovia S., Guillamón A. The development of sex differences in the locus coeruleus of the rat. Brain Res. Bull. 2001;56:73–78. doi: 10.1016/s0361-9230(01)00540-8. [DOI] [PubMed] [Google Scholar]
- Romeo R.D., Ali F.S., Karatsoreos I.N., Bellani R., Chhua N., Vernov M., McEwen B.S. Glucocorticoid receptor mRNA expression in the hippocampal formation of male rats before and after pubertal development in response to acute or repeated stress. Neuroendocrinology. 2008;87:160–167. doi: 10.1159/000109710. [DOI] [PubMed] [Google Scholar]
- Romeo R.D., Bellani R., Karatsoreos I.N., Chhua N., Vernov M., Conrad C.D., McEwen B.S. Stress history and pubertal development interact to shape hypothalamic–pituitary–adrenal axis plasticity. Endocrinology. 2006;147:1664–1674. doi: 10.1210/en.2005-1432. [DOI] [PubMed] [Google Scholar]
- Romeo R.D., Karatsoreos I.N., McEwen B.S. Pubertal maturation and time of day differentially affect behavioral and neuroendocrine responses following an acute stressor. Horm. Behav. 2006;50:463–468. doi: 10.1016/j.yhbeh.2006.06.002. [DOI] [PubMed] [Google Scholar]
- Romeo R.D., Lee S.J., Chhua N., McPherson C.R., McEwen B.S. Testosterone cannot activate an adult-like stress response in prepubertal male rats. Neuroendocrinology. 2004;79:125–132. doi: 10.1159/000077270. [DOI] [PubMed] [Google Scholar]
- Romeo R.D., Lee S.J., McEwen B.S. Differential stress reactivity in intact and ovariectomized prepubertal and adult female rats. Neuroendocrinology. 2004;80:387–393. doi: 10.1159/000084203. [DOI] [PubMed] [Google Scholar]
- Romeo R.D., Minhas S., Svirsky S.E., Hall B.S., Savenkova M., Karatsoreos I.N. Pubertal shifts in adrenal responsiveness to stress and adrenocorticotropic hormone in male rats. Psychoneuroendocrinology. 2014;42:146–152. doi: 10.1016/j.psyneuen.2014.01.016. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Schlegel A., Barry H., III . Free Press; New York: 1991. Adolescence: An Anthropological Inquiry. [Google Scholar]
- Schulkin J., Morgan M.A., Rosen J.B. A neuroendocrine mechanism for sustaining fear. Trends Neurosci. 2005;28:629–635. doi: 10.1016/j.tins.2005.09.009. [DOI] [PubMed] [Google Scholar]
- Schwarz J.M., Sholar P.W., Bilbo S.D. Sex differences in microglial colonization of the developing rat brain. J. Neurochem. 2012;120:948–963. doi: 10.1111/j.1471-4159.2011.07630.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sisk C.L., Foster D.L. The neural basis of puberty and adolescence. Nat. Neurosci. 2004;7:1040–1047. doi: 10.1038/nn1326. [DOI] [PubMed] [Google Scholar]
- Spear L.P. Rewards, aversions and affect in adolescence: emerging convergences across laboratory animal and human data. Dev. Cogn. Neurosci. 2011;1:390–403. doi: 10.1016/j.dcn.2011.08.001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Toth E., Avital A., Leshem M., Richter-Levin G., Braun K. Neonatal and juvenile stress induces changes in adult social behavior without affecting cognitive function. Behav. Brain Res. 2008;190:135–139. doi: 10.1016/j.bbr.2008.02.012. [DOI] [PubMed] [Google Scholar]
- Trezza V., Baarendse P.J.J., Vanderschuren L. The pleasures of play: pharmacological insights into social reward mechanisms. Trends Pharmacol. Sci. 2010;31:463–469. doi: 10.1016/j.tips.2010.06.008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Urakawa S., Mitsushima D., Shimozuru M., Sakuma Y., Kondo Y. An enriched rearing environment calms adult male rat sexual activity: implication for distinct serotonergic and hormonal responses to females. PLoS ONE. 2014;9:e87911. doi: 10.1371/journal.pone.0087911. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Vazquez D.M. Stress and the developing limbic–hypothalamic–pituitary–adrenal axis. Psychoneuroendocrinology. 1998;23:663–700. doi: 10.1016/s0306-4530(98)00029-8. [DOI] [PubMed] [Google Scholar]
- Vidal J., Buwalda B., Koolhaas J.M. Male Wistar rats are more susceptible to lasting social anxiety than Wild-type Groningen rats following social defeat stress during adolescence. Behav. Process. 2011;88:76–80. doi: 10.1016/j.beproc.2011.08.005. [DOI] [PubMed] [Google Scholar]
- Vidal J., de Bie J., Granneman R.A., Wallinga A.E., Koolhaas J.M., Buwalda B. Social stress during adolescence in Wistar rats induces social anxiety in adulthood without affecting brain monoaminergic content and activity. Physiol. Behav. 2007;92:824–830. doi: 10.1016/j.physbeh.2007.06.004. [DOI] [PubMed] [Google Scholar]
- Waters P., McCormick C.M. Caveats of chronic exogenous corticosterone treatments in adolescent rats and effects on anxiety-like and depressive behaviour and HPA function. Biol. Mood Anxiety Disord. 2011;1:1–13. doi: 10.1186/2045-5380-1-4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Watt M.J., Burke A.R., Renner K.J., Forster G.L. Adolescent male rats exposed to social defeat exhibit altered anxiety behavioral and limbic monoamines as adults. Behav. Neurosci. 2009;123:564–576. doi: 10.1037/a0015752. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wright L.D., Hebert K.E., Perrot-Sinal T.S. Periadolescent stress exposure exerts long-term effects on adult stress responding and expression of prefrontal dopamine receptors in male and female rats. Psychoneuroendocrinology. 2008;33:130–142. doi: 10.1016/j.psyneuen.2007.10.009. [DOI] [PubMed] [Google Scholar]
- Wright L.D., Muir K.E., Perrot T.S. Enhanced stress responses in adolescent versus adult rats exposed to cues of predation threat and peer interaction as a predictor of adult defensiveness. Dev. Psychobiol. 2012;54:47–69. doi: 10.1002/dev.20575. [DOI] [PubMed] [Google Scholar]