Highlights
-
•
Difficulties in cognitive control are related to several psychiatric conditions.
-
•
Inhibitory control (IC) of children predicts academic and professional successes.
-
•
ACC sulcal patterns at age 5 were related to IC efficiency at age 5 (Stroop scores).
-
•
ACC sulcal patterns at age 5 explained IC efficiency at age 9 (Stroop scores).
-
•
ACC sulcal patterns constrain IC efficiency during childhood.
Keywords: Cognitive control, Inhibitory control, Brain imaging, Anterior cingulate cortex, Sulcal pattern, Stroop
Abstract
Difficulties in cognitive control including inhibitory control (IC) are related to the pathophysiology of several psychiatric conditions. In healthy subjects, IC efficiency in childhood is a strong predictor of academic and professional successes later in life. The dorsal anterior cingulate cortex (ACC) is one of the core structures responsible for IC. Although quantitative structural characteristics of the ACC contribute to IC efficiency, the qualitative structural brain characteristics contributing to IC development are less-understood. Using anatomical magnetic resonance imaging, we investigated whether the ACC sulcal pattern at age 5, a stable qualitative characteristic of the brain determined in utero, explains IC at age 9. 18 children performed Stroop tasks at age 5 and age 9. Children with asymmetrical ACC sulcal patterns (n = 7) had better IC efficiency at age 5 and age 9 than children with symmetrical ACC sulcal patterns (n = 11). The ACC sulcal patterns appear to affect specifically IC efficiency given that the ACC sulcal patterns had no effect on verbal working memory. Our study provides the first evidence that the ACC sulcal pattern – a qualitative structural characteristic of the brain not affected by maturation and learning after birth – partially explains IC efficiency during childhood.
1. Introduction
Difficulties in the ability to control impulses and to inhibit a prepotent response (i.e., inhibitory control, hereafter referred to as IC) are related to various pathologic conditions, including Attention Deficit Hyperactivity Disorder (Willcutt et al., 2005), addiction (Goldstein and Volkow, 2011), risk behavior (Quinn and Fromme, 2010), conduct problems (Rothbart et al., 2011), schizophrenia (Insel, 2010) and poor academic performance (Diamond et al., 2007). In healthy subjects, cognitive control including IC efficiency in childhood is a strong predictor of academic and professional successes later in life (Moffitt et al., 2011). The dorsal anterior cingulate cortex (ACC, also labeled the midcingulate cortex, Vogt, 2009) is one of the core structures of the brain functional network responsible for the ability to control impulses and to resolve cognitive conflict (Petersen and Posner, 2012). Within the theoretical framework of the conflict-monitoring hypothesis (Botvinick, 2007, Botvinick et al., 2001), the role of the dorsal ACC is to signal conflict in information processing to the cognitive control system supported through dorsolateral prefrontal cortices. The cognitive control system will supposedly increase the activation of task-relevant information and inhibit task-irrelevant information to resolve the information processing conflict (see Egner and Hirsch, 2005).
The ability to control impulses and to inhibit a prepotent response is determined in part by the ACC structure, as suggested by the relationship between interindividual differences in adults’ cognitive control efficiency and differences in the cortical thickness (Westlye et al., 2011), surface area (Fjell et al., 2012) and the gray matter volume (Takeuchi et al., 2012) of the ACC. Moreover, the relationship between cognitive control efficiency and these quantitative structural characteristics of the ACC appears to vary during the course of the development (Giedd et al., 2009), with the relationship being stronger for younger children compared to older ones (Fjell et al., 2012). However, the variations in the quantitative characteristics of the brain structure are affected not only by brain maturation but also by learning. Indeed, previous studies have demonstrated that prolonged learning and specific trainings – leading to the improvement of cognitive efficiency – can modify the quantitative structural characteristics of the areas responsible for the trained processes (Draganski et al., 2004, Draganski et al., 2006, Hyde et al., 2009). For example, increases in gray matter volume have been reported in adults in response to 3 months of intense training in juggling (Draganski et al., 2004) and to intense learning for medical examinations (Draganski et al., 2006) and in children in response to 15 months of musical training – with a relationship between the quantitative structural brain changes induced by the training and behavioral improvements (Hyde et al., 2009).
Although quantitative characteristics of the brain structure reveal the dynamic interplay between brain maturation and learning/training on cognitive development, they provide no information on the early constraint imposed by the structure of the brain on cognitive development. The study of the sulcal pattern, a qualitative characteristic of the brain, might provide such information. Indeed, the sulcal pattern is a stable feature of the brain morphology determined in utero (Rakic, 2004, White et al., 2010), and it is not affected by maturation and learning occurring after birth (Sun et al., 2012). Specifically, asymmetry in the sulcal pattern of the ACC between the right and left hemispheres has been associated with increased IC efficiency in preschoolers (Cachia et al., 2014) and in adults (Fornito et al., 2004, Huster et al., 2009). However, there has not yet been evidence that the sulcal pattern of the ACC at a given age explains IC efficiency later in development.
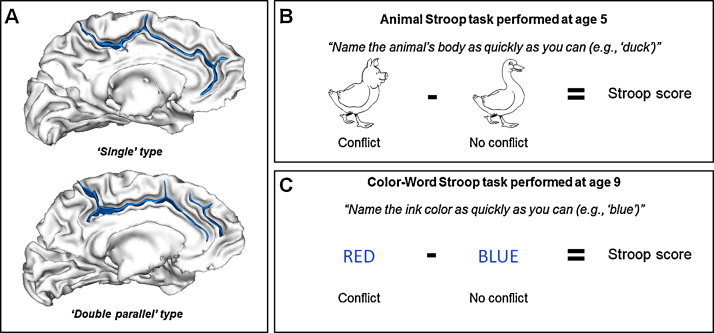
To provide such evidence, we asked the same 18 children to perform Stroop tasks at age 5 and at age 9 – a developmental window in which IC efficiency increases dramatically (Fjell et al., 2012) – and we studied whether a qualitative measure of the ACC morphology (i.e., the sulcal pattern of the ACC) at age 5 was associated with the children's behavioral performance on the Stroop tasks both at age 5 and at age 9. Consistent with previous studies (Cachia et al., 2014, Fornito et al., 2004, Huster et al., 2009), the sulcal pattern of the ACC was classified as a ‘single’ type when only the cingulate sulcus was present and as a ‘double parallel’ type when a paracingulate sulcus (PCS) ran parallel to the cingulate sulcus (Ono et al., 1990, Paus et al., 1996a, Paus et al., 1996b) (see Fig. 1A). Children performed the Color-Word Stroop task (Stroop, 1935) at age 9 and the Animal Stroop task (Wright et al., 2003) – an adaptation of the Color-Word Stroop Task for non-reading children – at age 5 (see Fig. 1B). In the Color-Word Stroop task, children named the color of the ink of printed words that denoted colors in a no-conflict condition, in which the ink colors matched the colors denoted by the words (e.g., ‘BLUE’ appeared in blue ink), and in a conflict condition, in which the colors denoted by the words interfered with the ink colors to be named (e.g., ‘RED’ appeared in blue ink). Similarly, in the Animal Stroop task, children named the body of animals in a no-conflict condition – i.e., the head matched the animal's body (e.g., a duck's head on a duck's body) – and in a conflict condition – i.e., the head of the animal was replaced by the head of a different animal (e.g., a pig's head on a duck's body). In both Stroop tasks, the difference in response times (i.e., the Stroop interference scores) between the no-conflict and conflict conditions typically reflects the ability to process conflicting information, drawing, in part, on IC efficiency. Critically, the ACC is consistently activated in the Stroop tasks (Petersen and Posner, 2012).
Fig. 1.
ACC sulcal pattern classification and assessment of IC efficiency. The figure depicts the two types of ACC sulcal patterns (sulci are depicted in blue on the gray/white interface): ‘single’ type when only the cingulate sulcus was present and ‘double parallel’ type when a paracingulate sulcus ran parallel to the cingulate sulcus (A). The figure also depicts examples of stimuli in the conflict and no-conflict conditions of the Animal Stroop task (B) and the Color-Word Stroop task (C). (For interpretation of the references to color in this figure legend, the reader is referred to the web version of this article.)
We reasoned that if the sulcal pattern of the ACC constrains IC efficiency during childhood, then the children with asymmetrical ACC sulcal patterns (i.e., the ‘single’ type in the left hemisphere and the ‘double parallel’ type in the right hemisphere or vice versa) should have lower Stroop interference scores (i.e., better IC efficiency) than children with symmetrical ACC sulcal patterns (i.e., the ‘single’ type or the ‘double parallel’ type in both hemispheres) not only at age 5 but also at age 9. In addition, if the ACC sulcal patterns not only partially explain IC efficiency during childhood but affect the development of IC efficiency per se, then the ACC sulcal patterns should have an effect on the difference in Stroop interference scores between age 5 and age 9.
To assess the specificity of the effect of the ACC sulcal pattern on the development of IC efficiency, we also investigated, in the same sample of children, whether the sulcal pattern of the ACC partially explained the development of their verbal working memory. This investigation was critical given that the Stroop task involves not only the ability to overcome perceptual and cognitive conflicts (MacLeod, 1991) but also a range of other cognitive processes, including working memory (MacLeod et al., 2003). To evaluate the verbal working memory efficiency of children, we asked children at age 5 and age 9 to perform, in addition to the Stroop tasks, the forward and backward digit span tasks from the Wechsler Intelligence Scale for Children (WISC-IV, Wechsler, 2003). Children were instructed to listen to a series of discrete digits, and they subsequently recalled the series of digits in the same (i.e., forward digit span task) or reverse (i.e., backward digit span task) order of presentation. We reasoned that if the sulcal patterns of the ACC constrain specifically IC efficiency during childhood and not other executive functions, then the sulcal pattern of the ACC should have no effect on the forward or backward digit spans at age 5 and age 9.
Finally, to determine whether a qualitative characteristic of the ACC (i.e., the sulcal patterns of the ACC) explain to a greater extent IC efficiency during childhood than quantitative characteristics of the ACC, we investigated the relationship between the thickness and surface area of the ACC at age 5 and the Stroop interference scores at age 5 and at age 9. Consistent with previous studies on adults (see e.g., Takeuchi et al., 2012, Westlye et al., 2011) and children (Fjell et al., 2012), we expected the thickness and the surface area of the right but not the left ACC at age 5 to be related to IC efficiency at the same age. In addition, given that quantitative structural characteristics of the ACC reflect the interplay of brain maturation and environment (including training and learning that might have been different for the children between 5- and 9-year-old), the thickness and the surface area of the right ACC at age 5 should not explain IC efficiency at age 9.
2. Materials and methods
All children were scanned before performing the Animal Stroop task. They were tested on the Color-Word Stroop task by a different experimenter than the one who tested them on the Animal Stroop task. The classification of the sulcal patterns was performed after the two testing sessions by three experimenters that did not participate in the behavioral testing. The experimenters that collected the behavioral data were blind to the classification of the sulcal patterns and the experimenters that performed the classification of the sulcal patterns were blind to the behavioral data.
2.1. Participants
Sample descriptive are provided in Table 1. Eighteen right-handed preschoolers (age at testing on the Animal Stroop task was 5.48 ± 0.18 years and age at testing on the Stroop Color-Word task was 9.47 ± 0.57 years; 10 females) were recruited from a public preschool in Caen (France). They had no history of neurological disease and no cerebral abnormalities. The children were tested in accordance with national and international norms that govern the use of human research participants. We obtained written informed consent from the children's parents that allowed us to enroll their children in the study. The ethics committee approved our study (CPP Nord-Ouest III, France).
Table 1.
Sample characteristics.
| Sym. n = 11 | Asym. n = 7 | Welch-t/χ2 | p-Value | |
|---|---|---|---|---|
| Age first Stroop testing (year) | 5.49 (0.18) | 5.46 (0.2) | t = .31 | .76 |
| Age second Stroop testing (year) | 9.31 (0.55) | 9.73 (0.51) | t = 1.64 | .12 |
| Gender (female/male) | 5/6 | 5/2 | χ2 = .35 | .55 |
| Household (average income) | 3136 (1125) | 2714 (983) | t = .84 | .42 |
| Handedness (Oldfield score) | 100 (0) | 87.3 (33.6) | t = 1 | .36 |
| Raven (raw score) | 28.6 (2.8) | 28.3 (2.6) | t = .20 | .84 |
Sym: symmetrical sulcal pattern; Asym: asymmetrical sulcal pattern. Standard deviations appear in parentheses.
2.2. Behavioral assessment
2.2.1. Inhibitory control
We used the Animal Stroop task (Wright et al., 2003) to assess IC efficiency at age 5 and the Color-Word Stroop task (Stroop, 1935) to assess IC efficiency at age 9. In the Animal Stroop task, the conflict and no-conflict conditions comprised 24 Animal Stroop stimuli printed on a sheet of paper. The stimuli were designed and based on the following four images of animals: a cow, a duck, a pig, and a sheep. Children were asked to name the animal's body. In the no-conflict condition, the head and the animal's body were matched. In the conflict condition, the head of the animal was substituted with the head of another animal. In the Color-Word Stroop task, participants were asked to name the ink color of 50 printed words in each condition. In the no-conflict condition, the colors denoted by the words were congruent with the ink color in which they were printed (e.g., BLUE printed in blue). In the conflict condition, the colors denoted by the words were incongruent with the ink color in which they were printed (e.g., RED printed in blue). In each Stroop task, the children started with the no-conflict condition. We recorded separately the response times and the number of errors in the conflict and no-conflict conditions. The Stroop interference scores were defined as the difference in response times or error rates between the conflict and no-conflict conditions. Thus, children with lower Stroop interference scores had higher IC efficiency.
2.2.2. Working memory
We used the forward and backward digit span tasks from the WISC-IV (Wechsler, 2003) to assess the verbal working memory efficiency of children. In these two tasks, series of discrete digits are presented and children are instructed to recall the series either in the same (i.e., forward digit span task) or reverse (i.e., backward digit span task) order of presentation. Each task started with series of two digits and then series were increased by one digit every two trials. We stopped the task when a child failed to recall two consecutive series with the same number of digits. The working memory span (or score) was the number of digits correctly recalled in the last series presented. The forward and backward digit span assessed children's ability to maintain (i.e., forward digit span task) or maintain and manipulate information (i.e., backward digit span task), respectively, in verbal working memory.
2.3. MRI acquisition
Anatomical magnetic resonance images (MRIs) were acquired on a 3 T MRI scanner (Achieva, Philips Medical System, The Netherlands) from the Cyceron biomedical imaging platform (Caen, France, www.cyceron.fr) using 3D T1-weighted spoiled gradient images (FOV: 256 mm; slice thickness: 1.33 mm; 128 slices; matrix size: 192 × 192 voxels). The children passively watched a cartoon on an MRI-compatible screen during MRI acquisitions to reduce motion, provide a positive experience and decrease wait times (Lemaire et al., 2009).
2.4. MRI analysis
An automated pre-processing step skull-stripped the T1 MRIs and segmented the brain tissues. MRIs were not spatially normalized to overcome the potential bias that may have resulted from the sulcus shape deformations induced by the warping process. The cortical folds were automatically segmented throughout the cortex from the skeleton of the gray matter/cerebrospinal fluid mask. This procedure provided a stable and robust sulcal surface definition that was not affected by variations in the cortical thickness or the gray matter/white matter contrast (Mangin et al., 2004). We visually checked the images at each processing step for each MRI. No motion artifacts and no segmentation errors were detected. Image analysis was performed with the Morphologist toolbox using BrainVISA 4.2 software (http://brainvisa.info/).
2.5. ACC classification
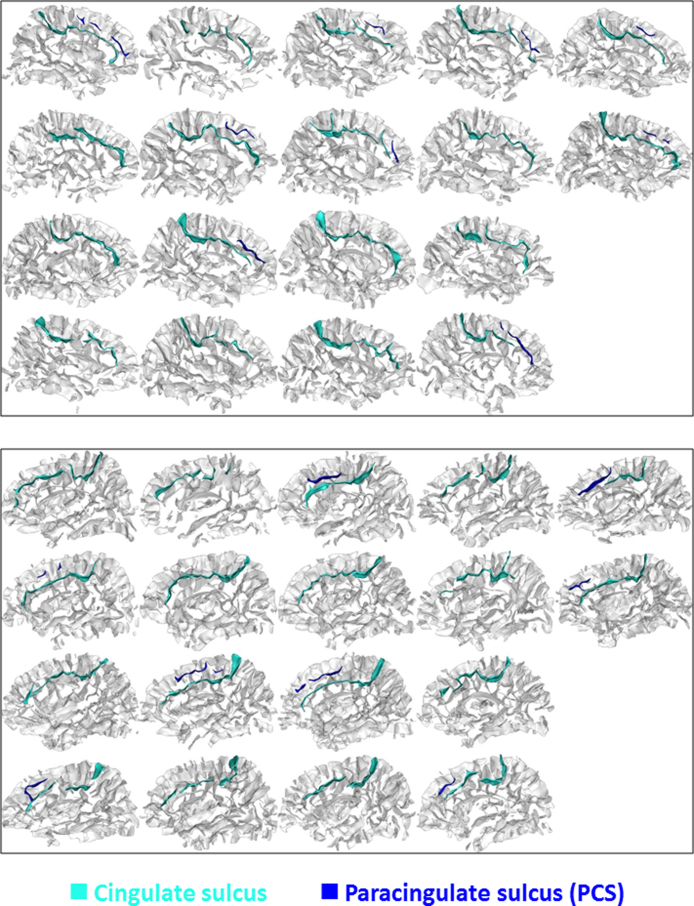
The identification of the sulcal pattern of the ACC was based on a visual inspection of a three-dimensional, mesh-based reconstruction of the cortical folds (Cachia et al., 2014, Huster et al., 2007, Leonard et al., 2009, Yucel et al., 2001). We classified the ACC sulcal pattern as ‘single’ or ‘double parallel’ type (Fornito et al., 2004) depending on the presence or absence of a paracingulate sulcus (PCS) (see Fig. 1A). The PCS is a sulcus located dorsal to the cingulate sulcus with a course parallel to the cingulate sulcus (Huster et al., 2009, Yucel et al., 2001). The anterior limit of the PCS was defined as the point at which the sulcus extended posteriorly from an imaginary vertical line running perpendicular to the line passing through the anterior and posterior commissures (AC–PC) and parallel to the anterior commissure (Huster et al., 2007, Yucel et al., 2001). The PCS was considered present if there were clear developed horizontal sulcus elements parallel to the cingulate sulcus with a total length of at least 20 mm. Interruptions or gaps between the PCS elements were not included in the total length of the PCS. The sulcal patterns of the ACC were independently classified by three of the co-authors (Arnaud Cachia, Grégoire Borst & Julie Vidal). Reliability for the left hemisphere was 100% and 94.7% for the right hemisphere (i.e., one sulcal pattern out of 18 classified differently by one of the three co-authors). We classified this sulcal pattern as classified by the majority of the three co-authors (see Fig. 2 for a depiction of the ACC sulcal patterns of each child in the left and right hemispheres). In the present study, we did not adopt the finer distinction between ‘present’ and ‘prominent’ PCS – i.e., prominent PCS (Leonard et al., 2009) defined as a PCS greater than 20 mm (Huster et al., 2009) or greater than 40 mm (Yucel et al., 2001) – or 5 categories of PCS length with a 15-mm step (Huster et al., 2007). Given that these criteria are based on adult brains and that the brain size and PCS length increases with age, these classifications are not suitable for characterizing the ACC sulcal patterns of developing brains. Critically, the classification of the ACC sulcal pattern (‘single’/‘double parallel’ type) adopted here has been used previously to study the effect of the ACC sulcal pattern on IC efficiency in schizophrenia (Fornito et al., 2006a, Fornito et al., 2006b).
Fig. 2.
ACC sulcal pattern of each child. 3-D mesh-based reconstructions of the cingulate sulcus (turquoise) and PCS (blue) of each child at age 5 in the left (top) and the right (bottom) hemispheres. Other sulci of the cortex are depicted in light gray. (For interpretation of the references to color in this figure legend, the reader is referred to the web version of this article.)
2.6. Thickness and surface area estimation
We calculated the cortical thickness and surface area estimates with a fully automated set of tools available in the FreeSurfer software suite (http://surfer.nmr.mgh.harvard.edu/). T1-weighted structural images were corrected for distortions caused by gradient nonlinearities, coregistered, averaged, and rigidly resampled into alignment with an atlas brain. Details regarding the surface based cortical reconstruction and subcortical volumetric segmentation can be found elsewhere (Dale et al., 1999, Fischl et al., 1999, Fischl et al., 2002, Fischl et al., 2004, Fischl and Dale, 2000). Note that these morphometric procedures have been validated for children as young as 4-years old (Ghosh et al., 2010). Based on the MRI scans of each child a 3D model of the cortical surface was constructed. This 3D model included the segmentation of the white matter, the tessellation of the gray/white matter boundary, the inflation of the folded, tessellated surface, and the correction of topological defects. Cortical thicknesses were calculated from this cortical surface reconstruction by estimating and then refining the gray/white boundary, deforming the surface out to the pial surface, and measuring the distances from each point on the white matter surface to the pial surface (Fischl and Dale, 2000). Cortical surface area was calculated at the pial level and represents the area of vertex on the gray matter surface, calculated as the average of the area of the tessellated triangles touching that vertex. We restricted our analysis to the thickness and surface area estimates of the right and left ACC because (a) we investigated the sulcal morphology of this region and (b) previous studies have demonstrated that quantitative structural characteristics of this region are associated with IC efficiency in both adults (Takeuchi et al., 2012, Westlye et al., 2011) and children (Fjell et al., 2012, Khornitova et al., 2013).
3. Results
Based on the three-dimensional mesh-based reconstruction of cortical folds at age 5, we identified 11 right-handed children with symmetrical ACC sulcal patterns – ‘single’ (n = 6) or ‘double parallel’ (n = 5) type in both hemispheres – and 7 right-handed children with asymmetrical ACC sulcal patterns – ‘single’ type in the left hemisphere and ‘double parallel’ type in the right hemisphere (n = 3) or vice versa (n = 4). Age, gender, household income as a proxy indicator for socioeconomic status, and raw scores on the colored progressive matrices of Raven as a proxy indicator for general intelligence (Raven et al., 1998) did not differ between the two groups (see Table 1).
At a behavioral level, we found classical Stroop interference effects (M ± SD) in the Animal Stroop task at age 5: children were slower and less accurate in the conflict condition (M = 64.84 ± 29.12 s and M = 8.1 ± 4.84%) than in the no-conflict condition (M = 37.4 ± 10.77 s and M = 3.24 ± 3.66%), respectively t(17) = 5.15, p = .00004, d = 1.84, for the response times and t(17) = 4.75, p = .0001, d = 1.16, for the error rates (see Table 2). Similarly, we found classical Stroop interference effects in the Color-Word Stroop task at age 9 on the responses times (M = 77.04 ± 17.47 s in the conflict condition and M = 27.82 ± 5.39 s in the no-conflict condition, t(17) = 13.28, p < .0001, d = 4.14) and on the error rates (M = 8.11 ± 4.47% in the conflict condition and M = 0% in the no-conflict condition, t(17) = 7.70, p < .0001, d = 1.28). A stem and leaf plot procedure revealed no outliers in the data.
Table 2.
Mean (M) and standard deviation (SD) of the response times (RTs) and error rates (ERs) in the no-conflict and conflict conditions of the Animal Stroop task and the Stroop Color-Word task for children with symmetrical (Sym.) and asymmetrical (Asym.) sulcal patterns of the ACC.
| Animal Stroop task |
Color-Word Stroop task |
|||
|---|---|---|---|---|
| Sym. n = 11 | Asym. n = 7 | Sym. n = 11 | Asym. n = 7 | |
| RTs (s) | ||||
| No-conflict | 40.91 (11.65) | 31.89 (6.69) | 28.01 (3.74) | 27.51 (7.68) |
| Conflict | 78.16 (30.11) | 43.90 (7.37) | 83.8 (15.96) | 66.43 (15.01) |
| Stroop score | 37.26 (22.59) | 12.01 (12.01) | 55.79 (15.63) | 38.91 (9.48) |
| ERs (%) | ||||
| No-conflict | 3.41 (4.09) | 2.98 (3.15) | 0 (0) | 0 (0) |
| Conflict | 9.47 (4.20) | 5.95 (5.30) | 8.91 (3.94) | 6.86 (5.27) |
| Stroop score | 6.06 (3.42) | 2.98 (5.22) | 8.91 (3.94) | 6.86 (5.27) |
Standard deviations appear in parentheses.
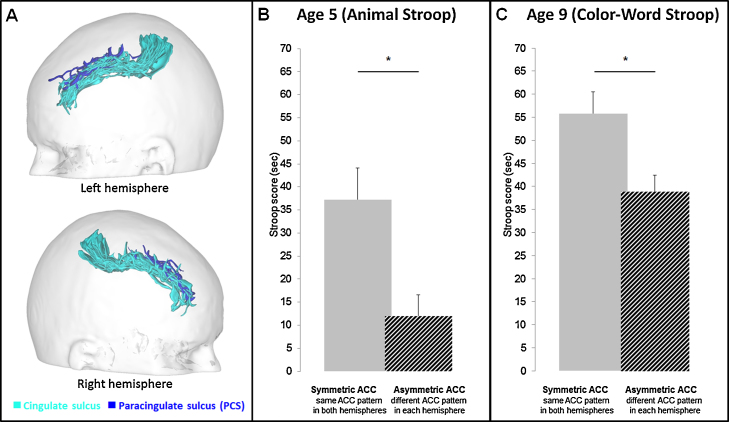
At a neurocognitive level, a 2 (age of testing: 5 vs. 9 years old) × 2 (sulcal pattern: asymmetrical vs. symmetrical) mixed-design repeated measures ANOVA with age of testing as a within-subject factor and sulcal pattern as a between-subject factor revealed that the Stroop interference scores based on the response times differed between children with symmetrical and asymmetrical ACC sulcal patterns (i.e., significant main effect of the sulcal pattern), F(1, 15) = 4.67, p = .047, ηp2 = .23, and this effect did not vary with age, as witnessed by the lack of a significant interaction between the age of testing and the sulcal pattern of the ACC in two different Stroop tasks adapted for the age of the children (i.e., the Animal Stroop task at age 5 and the Color-Word Stroop task at age 9), F(1, 16) = 1.55, p = .23.2 As shown in Fig. 3B, the Stroop interference scores were lower (and thus IC efficiency was higher) in children at age 5 with asymmetrical ACC (12.01 ± 12.01 s) than in children with symmetrical ACC (37.26 ± 22.59 s), t(16) = 3.07, p = .0004, d = 1.49. Critically, at age 9, children with an asymmetrical ACC sulcal pattern at age 5 (M = 38.91 ± 9.48 s) continued to have lower Stroop interference scores than children with a symmetrical ACC sulcal pattern at age 5 (M = 55.79 ± 15.63 s), t(16) = 2.85, p = .006, d = 1.383 (see Fig. 3C). Note that a stem and leaf plot procedure revealed no outliers in the data. Two separate one-way ANOVA with the sulcal patterns of the ACC as a between-subject factor on the Stroop interference scores at age 5 and age 9 demonstrated that ACC asymmetry at age 5 explained 27% of the Stroop interference score variability at age 5 and 25% of the Stroop interference score variability at age 9. Given that we found no effect of the ACC sulcal pattern on the Stroop interference scores based on the error at age 5 (p = .15) or at age 9 (p = .36), the effect reported on the Stroop interference scores based on the response times was unlikely to reflect a speed accuracy trade-off. In addition, to determine whether the sulcal patterns of the ACC constrain the development of IC efficiency per se, we normalized the Animal Stroop and the Color-Word Stroop interference scores and then computed, for each child, the difference between the normalized Stroop interference scores at age 9 and age 5. The difference in the normalized Stroop interference scores reflects the development of IC efficiency per se between 5 and 9 years of age. A one way ANOVA on these difference scores with the ACC sulcal patterns as a between-subject factor revealed no main effect of the ACC sulcal patterns, F(1, 16) = 1.59, p = .22.
Fig. 3.
Interindividual variability of the ACC sulcal pattern of the children (A) and IC efficiency in the children with asymmetrical and symmetrical ACC sulcal patterns at age 5 (B) and at age 9 (C). (A) 3-D mesh-based reconstructions of the cingulate sulcus (turquoise) and PCS (blue) of each child at age 5 are depicted on the same gray/white interface after being linearly aligned in a common referential (MNI-space). (B) and (C) Mean Stroop interference scores at age 5 (on the Animal Stroop task) and at age 9 (on the Color-Word Stroop task) in children with symmetrical (single or double parallel type in both hemispheres; n = 11) and asymmetrical (single type in the right hemisphere and double type in the left hemisphere or vice versa; n = 7) ACC sulcal patterns. Children with asymmetrical ACC sulcal patterns at age 5 had lower Stroop interference scores than children with symmetrical ACC sulcal patterns at age 5, t(16) = 3.07, p = .0004, d = 1.49, and at age 9, t(16) = 2.85, p = .006, d = 1.38. Error bar denotes standard error of the mean (SEM); *p < .05. (For interpretation of the references to color in this figure legend, the reader is referred to the web version of this article.)
We used linear regressions to determine the association between the thickness and the surface area of the ACC at age 5 and the Stroop interference scores at age 5 and age 9. We found that the right ACC thickness at age 5 was marginally related to the Stroop interference scores at age 5 (B = 24.39, p = .067) but not at age 9 (B = 12.49, p = .19). Note that the positive relationship between the thickness of the right ACC and the Stroop interference score at age 5 suggests that a thinner right ACC was associated with a lower Stroop interference score (i.e., better IC efficiency) which is consistent with previous studies on children (see e.g., Khornitova et al., 2013). We found no association with the cortical thickness of the left ACC and the Stroop interference score at age 5 (B = 9.23, p = .51) and age 9 (B = −.99, p = .92). The surface area of the right and the left ACC at age 5 was neither associated with the Stroop interference score at age 5 (respectively, B = .008, p = .72 and B = .002, p = .94) nor with the Stroop interference score at age 9 (respectively, B = .012, p = .42 and B = .003, p = .87). Note that we found no association between the asymmetry in the cortical thickness and the surface area of the ACC at age 5 and the Stroop interference score at age 5 (respectively, B = −118.6, p = .20 and B = 80.89, p = .49) and age 9 (respectively, B = −97.99, p = .12 and B = −99.56, p = .18).
Finally, as expected if the sulcal pattern of the ACC partially explained specifically IC efficiency during childhood, we found no effect of the sulcal pattern of the ACC at age 5 and age 9 on the forward or backward digit spans (all ps > .16, see Table 3).
Table 3.
Mean forward and backward digit spans for children with symmetrical (Sym.) and asymmetrical (Asym.) sulcal patterns of the ACC.
| Sym. n = 11 | Asym. n = 7 | Welch-t | p-Value | |
|---|---|---|---|---|
| Forward digit span (age 5) | 3.90 (0.83) | 3.71 (1.11) | .40 | .70 |
| Backward digit span (age 5) | 2.09 (0.30) | 2.43 (0.54) | 1.52 | .16 |
| Forward digit span (age 9) | 5.09 (0.70) | 4.71 (0.76) | 1.06 | .31 |
| Backward digit span (age 9) | 3.64 (0.81) | 4 (0.58) | 1.11 | .28 |
Standard deviations appear in parentheses.
4. Discussion
As expected, children with asymmetrical ACC sulcal patterns were more efficient at resisting interference (i.e., naming the body of the animal in the Animal Stroop task) at age 5 than children with symmetrical ACC sulcal patterns. Critically, we provided evidence for the first time that the sulcal pattern of the ACC at age 5 partially explained IC efficiency at age 9; children with asymmetrical ACC sulcal patterns continued 4 years after (at age 9) to be more efficient at resisting interference than children with symmetrical ACC sulcal patterns in a different Stroop task (i.e., the Color-Word Stroop task), in which children had to inhibit their prepotency to read the word to identify the color of the ink in which the word was printed. Given that we found no difference in the rate at which IC efficiency developed during childhood between children with asymmetrical ACC sulcal patterns and children with symmetrical ACC sulcal patterns, then these ACC patterns might not constrain the development of IC efficiency per se but more likely correspond to a qualitative structural characteristic of the ACC which puts an early constrain on IC efficiency that is not compensated for at least throughout childhood. In addition, we found that IC efficiency at age 5 was related to the thickness of the right but not the left ACC at age 5 which is consistent with previous studies that reported a relationship between quantitative characteristics of the right ACC and IC efficiency in adults (see e.g., Takeuchi et al., 2012, Westlye et al., 2011). Moreover, as opposed to a previous study (Fjell et al., 2012), we found no association between the surface area of the right and left ACC at age 5 and IC efficiency at age 5 probably due to the limited sample size. The different patterns of association between two complementary quantitative characteristics of the ACC and IC efficiency at age 5 might be a consequence of the fact that the cortical thickness and the cortical surface area are influenced by different genetic (Panizzon et al., 2009) and developmental (Raznahan et al., 2011) factors. Critically, we found no relationship between the thickness and surface area of the ACC at age 5 and the Stroop interference score 4 years later (at age 9) which suggest that this quantitative structural characteristic of the ACC (i.e., the thickness) does not explain IC efficiency during childhood as efficiently as a qualitative structural characteristic of the ACC (i.e., the sulcal patterns). Finally, the ACC sulcal pattern at age 5 did not explain the ability to maintain and manipulate information in verbal working memory which provided evidence that the sulcal pattern of the ACC specifically explained IC efficiency.
Given that different sulcal patterns have been associated with white matter connectivity (Hilgetag and Barbas, 2006, Van Essen, 1997) and that cognitive processes involved in the Stroop tasks appear to be highly lateralized in the brain (Brown et al., 2001), we suspect that the higher IC efficiency of children with asymmetrical patterns of the ACC could stem from a greater asymmetry of the underlying white matter connectivity of the ACC. Finally, the sulcal pattern of the ACC, by affecting the white matter connectivity, might constrain the development of the connectivity of the ACC to other areas of the brain, enabling this structure and other prefrontal structures to take control over these areas (Posner, 2012), which could explain why this qualitative structural characteristic of the brain partially explained the IC efficiency during childhood. Alternatively, given that previous studies have demonstrated that the sulcal pattern of the ACC have likely an impact on ACC activation (Amiez et al., 2013, Artiges et al., 2006, Crosson et al., 1999, Paus et al., 1998) and on quantitative characteristics of the structure of the ACC (e.g., cortical thickness, gray and white matter volumes, and surface area, see Paus et al., 1996a, Paus et al., 1996b, Fornito et al., 2006a, Fornito et al., 2006b, Huster et al., 2007, Fornito et al., 2008), the constrain of the sulcal pattern of the ACC on IC efficiency reported in the present study could be mediated by the effect of the sulcal pattern of the ACC on the level of activation and/or quantitative characteristics of the structure of this region. However, we note that IC efficiency at age 5 and at age 9 were not associated with asymmetries in the thickness and surface area of the ACC at age 5.
A potential limitation of the present study is the small sample size, reflecting the difficulty to conduct brain imaging studies and longitudinal follow-up in young children. Hence, we could not statistically determine whether children with a leftward asymmetry of the sulcal pattern of the ACC (i.e., PCS in the left but not in the right hemispheres) have greater IC efficiency than children with a rightward asymmetry (i.e., PCS in the right but not in the left hemispheres) as in adults (Fornito et al., 2004, Huster et al., 2009, Whittle et al., 2009). However, our analyses revealed a similar pattern of results at two different ages using different behavioral tasks to assess IC, providing evidence of the robustness and replicability of our findings.
To conclude, we provided evidence for the first time that the sulcal pattern of the ACC – a qualitative structural characteristic of the brain determined in utero that is not affected by maturation and learning after birth – partially explained the IC efficiency during childhood. Therefore, it appears that the sulcal pattern of the ACC constrains IC efficiency, at least in children aged 5–9 years. This result suggests an intriguing hypothesis that qualitative structural characteristics of the brain might contribute to the risk of developing impaired IC and thus neurodevelopmental disorders, in line with previous works reporting abnormal ACC sulcal pattern in patients with established schizophrenia (Fornito et al., 2006a, Fornito et al., 2006b, Yucel et al., 2002) as well as in subjects highly vulnerable to develop schizophrenia (Yucel et al., 2003). Further longitudinal studies with larger samples are needed to investigate the interaction between the effects of qualitative structural characteristics of the brain (e.g., sulcal patterns) and of quantitative characteristics of the structure of the brain (e.g., cortical thickness, gray and white matter volumes, surface area, and white matter connectivity) on IC efficiency in normal and pathological children.
Conflict of interest statement
We wish to confirm that there are no known conflict of interest associated with this publication and there has been no significant financial support for this work that could have influenced its outcome.
Acknowledgements
We thank the CNRS for financial support and the French Board of Education for their collaboration.
Footnotes
Note that a 2 (age of testing: 5 vs. 9 years old) × 2 (condition: congruent vs. incongruent) × 2 (sulcal pattern: asymmetrical vs. symmetrical) mixed-design repeated measures ANOVA on the RTs revealed a similar pattern of results to the one reported on the Stroop score. We found significant main effects of the condition, F (1, 16) = 111.97, p = .0001, ηp2 = .88, and of the sulcal pattern, F (1, 16) = 7.81, p = .013, ηp2 = .33, a significant interaction between the sulcal pattern and the condition, F(1, 16) = 9.59, p = .007, ηp2 = .38, but no interaction between the age of testing, the condition and the sulcal pattern of the ACC, F < 1. The same pattern of results was found when normalized RTs (i.e., z-transformed RTs) were considered except for the main effect of the condition that was not significant, F < 1.
Note that the main effect of the sulcal patterns of the ACC on the Stroop scores at age 9 was still significant, F (1, 15) = 5.87, p = .03, ηp2 = .28, when children's age at the time they were tested on the Color-Word Stroop task was entered as a covariate in a one-way ANOVA with the sulcal patterns as a between-subject factor.
References
- Amiez C., Neveu R., Warrot D., Petrides M., Knoblauch K., Procyk E. The location of feedback-related activity in the midcingulate cortex is predicted by local morphology. J. Neurosci. 2013;33(5):2217–2228. doi: 10.1523/JNEUROSCI.2779-12.2013. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Artiges E., Martelli C., Naccache L., Bartres-Faz D., Leprovost J.B., Viard A., Paillere-Martinot M.L., Dehaene S., Martinot J.L. Paracingulate sulcus morphology and fMRI activation detection in schizophrenia patients. Schizophr. Res. 2006;82:143–151. doi: 10.1016/j.schres.2005.10.022. [DOI] [PubMed] [Google Scholar]
- Botvinick M.M. Conflict monitoring and decision making: reconciling two perspectives on anterior cingulate function. Cogn. Affect. Behav. Neurosci. 2007;7:356–366. doi: 10.3758/cabn.7.4.356. [DOI] [PubMed] [Google Scholar]
- Botvinick M.M., Braver T.S., Barch D.M., Carter C.S., Cohen J.D. Conflict monitoring and cognitive control. Psychol. Rev. 2001;108:624–652. doi: 10.1037/0033-295x.108.3.624. [DOI] [PubMed] [Google Scholar]
- Brown W.S., Thrasher E.D., Paul L.K. Interhemispheric Stroop effects in partial and complete agenesis of the corpus callosum. J. Int. Neuropsychol. Soc. 2001;7:302–311. doi: 10.1017/s1355617701733048. [DOI] [PubMed] [Google Scholar]
- Cachia A., Borst G., Vidal J., Pineau A., Fisher C., Mangin J.-F., Houdé O. The shape of the ACC contributes to cognitive control efficiency in preschoolers. J. Cogn. Neurosci. 2014;26:96–106. doi: 10.1162/jocn_a_00459. [DOI] [PubMed] [Google Scholar]
- Crosson B., Sadek J.R., Bobholz J.A., Gökçay D., Mohr C.M., Leonard C.M., Maron L., Auerbach E.J., Browd S.R., Freeman A.J., Briggs R.W. Activity in the paracingulate and cingulate sulci during word generation: an fMRI study of functional anatomy. Cereb. Cortex. 1999;9:307–316. doi: 10.1093/cercor/9.4.307. [DOI] [PubMed] [Google Scholar]
- Dale A.M., Fischl B., Sereno M.I. Cortical surface-based analysis. I. Segmentation and surface reconstruction. Neuroimage. 1999;9:179–194. doi: 10.1006/nimg.1998.0395. [DOI] [PubMed] [Google Scholar]
- Diamond A., Barnett W.S., Thomas J., Munro S. Preschool program improves cognitive control. Science. 2007;318:1387–1388. doi: 10.1126/science.1151148. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Draganski B., Gaser C., Busch V., Schuierer G., Bogdahn U., May A. Neuroplasticity: changes in grey matter induced by training. Nature. 2004;427:311–312. doi: 10.1038/427311a. [DOI] [PubMed] [Google Scholar]
- Draganski B., Gaser C., Kempermann G., Kuhn H.G., Winkler J., Buchel C., May A. Temporal and spatial dynamics of brain structure changes during extensive learning. J. Neurosci. 2006;26:6314–6317. doi: 10.1523/JNEUROSCI.4628-05.2006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Egner T., Hirsch J. Cognitive control mechanisms resolve conflict through cortical amplification of task-relevant information. Nat. Neurosci. 2005;8:1784–1790. doi: 10.1038/nn1594. [DOI] [PubMed] [Google Scholar]
- Fischl B., Dale A.M. Measuring the thickness of the human cerebral cortex from magnetic resonance images. Proc. Natl. Acad. Sci. U.S.A. 2000;97:11050–11055. doi: 10.1073/pnas.200033797. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fischl B., Sereno M.I., Dale A.M. Cortical surface-based analysis. II: Inflation, flattening, and a surface-based coordinate system. Neuroimage. 1999;9:195–207. doi: 10.1006/nimg.1998.0396. [DOI] [PubMed] [Google Scholar]
- Fischl B., van der Kouwe A., Destrieux C., Halgren E., Segonne F., Salat D.H., Busa E., Seidman L.J., Goldstein J., Kennedy D., Caviness V., Makris N., Rosen B., Dale A.M. Automatically parcellating the human cerebral cortex. Cereb. Cortex. 2004;14:11–22. doi: 10.1093/cercor/bhg087. [DOI] [PubMed] [Google Scholar]
- Fischl B., Salat D.H., Busa E., Albert M., Dieterich M., Haselgrove C., van der Kouwe A., Killiany R., Kennedy D., Klaveness S., Montillo A., Makris N., Rosen B., Dale A.M. Whole brain segmentation: automated labeling of neuroanatomical structures in the human brain. Neuron. 2002;33:341–355. doi: 10.1016/s0896-6273(02)00569-x. [DOI] [PubMed] [Google Scholar]
- Fjell A.M., Walhovd K.B., Brown T.T., Kuperman J.M., Chung Y., Hagler D.J., Jr., Venkatraman V., Roddey J.C., Erhart M., McCabe C., Akshoomoff N., Amaral D.G., Bloss C.S., Libiger O., Darst B.F., Schork N.J., Casey B.J., Chang L., Ernst T.M., Gruen J.R., Kaufmann W.E., Kenet T., Frazier J., Murray S.S., Sowell E.R., van Zijl P., Mostofsky S., Jernigan T.L., Dale A.M. Multimodal imaging of the self-regulating developing brain. Proc. Natl. Acad. Sci. U. S. A. 2012;109:19620–19625. doi: 10.1073/pnas.1208243109. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fornito A., Whittle S., Wood S.J., Velakoulis D., Pantelis C., Yucel M. The influence of sulcal variability on morphometry of the human anterior cingulate and paracingulate cortex. Neuroimage. 2006;33:843–854. doi: 10.1016/j.neuroimage.2006.06.061. [DOI] [PubMed] [Google Scholar]
- Fornito A., Wood S.J., Whittle S., Fuller J., Adamson C., Saling M.M., Velakoulis D., Pantelis C., Yucel M. Variability of the paracingulate sulcus and morphometry of the medial frontal cortex: associations with cortical thickness, surface area, volume, and sulcal depth. Hum. Brain Mapp. 2008;29:222–236. doi: 10.1002/hbm.20381. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fornito A., Yucel M., Wood S., Stuart G.W., Buchanan J.A., Proffitt T., Anderson V., Velakoulis D., Pantelis C. Individual differences in anterior cingulate/paracingulate morphology are related to executive functions in healthy males. Cereb. Cortex. 2004;14:424–431. doi: 10.1093/cercor/bhh004. [DOI] [PubMed] [Google Scholar]
- Fornito A., Yucel M., Wood S.J., Proffitt T., McGorry P.D., Velakoulis D., Pantelis C. Morphology of the paracingulate sulcus and executive cognition in schizophrenia. Schizophr. Res. 2006;88:192–197. doi: 10.1016/j.schres.2006.06.034. [DOI] [PubMed] [Google Scholar]
- Giedd J.N., Lalonde F.M., Celano M.J., White S.L., Wallace G.L., Lee N.R., Lenroot R.K. Anatomical brain magnetic resonance imaging of typically developing children and adolescents. J. Am. Acad. Child Adolesc. Psychiatry. 2009;48:465–470. doi: 10.1097/CHI.0b013e31819f2715. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Goldstein R.Z., Volkow N.D. Dysfunction of the prefrontal cortex in addiction: neuroimaging findings and clinical implications. Nat. Rev. Neurosci. 2011;12:652–669. doi: 10.1038/nrn3119. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ghosh S.S., Kakunoori S., Augustinack J., Nieto-Castanon A., Kovel-man I., Gaab N., Christodoulou J.A. Evaluating the validity of volume-based and surface-based brain image registration for developmental cognitive neuroscience studies in children 4 to 11 years of age. Neuroimage. 2010;53:85–93. doi: 10.1016/j.neuroimage.2010.05.075. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hilgetag C.C., Barbas H. Role of mechanical factors in the morphology of the primate cerebral cortex. PLoS Comput Biol. 2006;2:e22. doi: 10.1371/journal.pcbi.0020022. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Huster R.J., Westerhausen R., Kreuder F., Schweiger E., Wittling W. Morphologic asymmetry of the human anterior cingulate cortex. Neuroimage. 2007;34:888–895. doi: 10.1016/j.neuroimage.2006.10.023. [DOI] [PubMed] [Google Scholar]
- Huster R.J., Wolters C., Wollbrink A., Schweiger E., Wittling W., Pantev C., Junghofer M. Effects of anterior cingulate fissurization on cognitive control during stroop interference. Hum. Brain Mapp. 2009;30:1279–1289. doi: 10.1002/hbm.20594. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hyde K.L., Lerch J., Norton A., Forgeard M., Winner E., Evans A.C., Schlaug G. Musical training shapes structural brain development. J. Neurosci. 2009;29:3019–3025. doi: 10.1523/JNEUROSCI.5118-08.2009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Insel T.R. Rethinking schizophrenia. Nature. 2010;468:187–193. doi: 10.1038/nature09552. [DOI] [PubMed] [Google Scholar]
- Khornitova M., Martin R.E., Gabrieli J.D.E., Sheridan M.A. Cortical gray-matter thinning is associated with age-related improvements on executive function tasks. Dev. Cogn. Neurosci. 2013;6:61–71. doi: 10.1016/j.dcn.2013.07.002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lemaire C., Moran G.R., Swan H. Impact of audio/visual systems on pediatric sedation in magnetic resonance imaging. J. Magn. Reson. Imaging. 2009;30:649–655. doi: 10.1002/jmri.21870. [DOI] [PubMed] [Google Scholar]
- Leonard C.M., Towler S., Welcome S., Chiarello C. Paracingulate asymmetry in anterior and midcingulate cortex: sex differences and the effect of measurement technique. Brain Struct. Funct. 2009;213:553–569. doi: 10.1007/s00429-009-0210-z. [DOI] [PubMed] [Google Scholar]
- MacLeod C.M. Half a century of research on the Stroop effect: an integrative review. Psychol. Bull. 1991;109:163–203. doi: 10.1037/0033-2909.109.2.163. [DOI] [PubMed] [Google Scholar]
- MacLeod C.M., Dodd M.D., Sheard E.D., Wilson D.E., Bibi U. In opposition to inhibition. In: Ross B.H., editor. The Psychology of Learning and Motivation. Academic Press; San Diego: 2003. pp. 163–214. [Google Scholar]
- Mangin J.F., Riviere D., Cachia A., Duchesnay E., Cointepas Y., Papadopoulos-Orfanos D., Scifo P., Ochiai T., Brunelle F., Regis J. A framework to study the cortical folding patterns. Neuroimage. 2004;23(Suppl. 1):S129–S138. doi: 10.1016/j.neuroimage.2004.07.019. [DOI] [PubMed] [Google Scholar]
- Moffitt T.E., Arseneault L., Belsky D., Dickson N., Hancox R.J., Harrington H., Houts R., Poulton R., Roberts B.W., Ross S., Sears M.R., Thomson W.M., Caspi A. A gradient of childhood self-control predicts health, wealth, and public safety. Proc. Natl. Acad. Sci. U. S. A. 2011;108:2693–2698. doi: 10.1073/pnas.1010076108. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ono M., Kubik S., Abarnathey C.D. Georg Thieme; New York: 1990. Atlas of the Cerebral Sulci. [Google Scholar]
- Panizzon M.S., Fennema-Notestine C., Eyler L.T., Jernigan T.L., Prom-Wormley E., Neale M., Jacobson K., Lyons M.J., Grant M.D., Franz C.E., Xian H., Tsuang M., Fischl B., Seidman L., Dale A., Kremen W.S. Distinct genetic influences on cortical surface area and cortical thickness. Cereb. Cortex. 2009;19:2728–2735. doi: 10.1093/cercor/bhp026. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Paus T., Koski L., Caramanos Z., Westbury C. Regional differences in the effects of task difficulty and motor output on blood flow response in the human anterior cingulate cortex: a review of 107 PET activation studies. Neuroreport. 1998;9:R37–R47. doi: 10.1097/00001756-199806220-00001. [DOI] [PubMed] [Google Scholar]
- Paus T., Otkay N., Caramanos Z., MacDonald D., Zijdenbos A., D‘Avirro D., Gutmans D., Holmes C., Tomaiuolo F., Evans A.C. In vivo morphometry of the intrasulcal gray matter in the human cingulate, paracingulate, and superior rostral sulci: hemispheric asymmetries, gender differences and probability maps. J. Comp. Neurol. 1996;376:664–673. doi: 10.1002/(SICI)1096-9861(19961223)376:4<664::AID-CNE12>3.0.CO;2-M. [DOI] [PubMed] [Google Scholar]
- Paus T., Tomaiuolo F., Otaky N., MacDonald D., Petrides M., Atlas J., Morris R., Evans A.C. Human cingulate and paracingulate sulci: pattern, variability, asymmetry, and probabilistic map. Cereb. Cortex. 1996;6:207–214. doi: 10.1093/cercor/6.2.207. [DOI] [PubMed] [Google Scholar]
- Petersen S.E., Posner M.I. The attention system of the human brain: 20 years after. Annu. Rev. Neurosci. 2012;35:73–89. doi: 10.1146/annurev-neuro-062111-150525. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Posner M.I. Imaging attention networks. Neuroimage. 2012;61:450–456. doi: 10.1016/j.neuroimage.2011.12.040. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Quinn P.D., Fromme K. Self-regulation as a protective factor against risky drinking and sexual behavior. Psychol. Addict. Behav. 2010;24:376–385. doi: 10.1037/a0018547. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rakic P. Neuroscience. Genetic control of cortical convolutions. Science. 2004;303:1983–1984. doi: 10.1126/science.1096414. [DOI] [PubMed] [Google Scholar]
- Raven J., Raven J.C., Court J.H. Harcourt Assessment; San Antonio: 1998. Manual for Raven's Progressive Matrices and Vocabulary Scales. Section 2: The Coloured Progressive Matrices. [Google Scholar]
- Raznahan A., Shaw P., Lalonde F., Stockman M., Wallace G.L., Greenstein D., Clasen L., Gogtay N., Giedd J.N. How does your cortex grow? J. Neurosci. 2011;31:7174–7177. doi: 10.1523/JNEUROSCI.0054-11.2011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rothbart M.K., Sheese B.E., Rueda M.R., Posner M.I. Developing mechanisms of self-regulation in early life. Emot. Rev. 2011;32:207–213. doi: 10.1177/1754073910387943. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Stroop J.R. Studies of interference in serial verbal reactions. J. Exp. Psychol. 1935;18:643–662. [Google Scholar]
- Sun Z.Y., Kloppel S., Riviere D., Perrot M., Frackowiak R., Siebner H., Mangin J.F. The effect of handedness on the shape of the central sulcus. Neuroimage. 2012;60:332–339. doi: 10.1016/j.neuroimage.2011.12.050. [DOI] [PubMed] [Google Scholar]
- Takeuchi H., Taki Y., Sassa Y., Hashizume H., Sekiguchi A., Nagase T., Nouchi R., Fukushima A., Kawashima R. Regional gray and white matter volume associated with Stroop interference: evidence from voxel-based morphometry. Neuroimage. 2012;59:2899–2907. doi: 10.1016/j.neuroimage.2011.09.064. [DOI] [PubMed] [Google Scholar]
- Van Essen D.C. A tension-based theory of morphogenesis and compact wiring in the central nervous system. Nature. 1997;385:313–318. doi: 10.1038/385313a0. [DOI] [PubMed] [Google Scholar]
- Vogt B. Oxford University Press; Oxford: 2009. Cingulate Neurobiology and Disease. [Google Scholar]
- Wechsler D. Harcourt Assessment; San Antonio: 2003. Wechsler Intelligence Scale for Children-Fourth Edition. Administration and Scoring Manual. [Google Scholar]
- Westlye L.T., Grydeland H., Walhovd K.B., Fjell A.M. Associations between regional cortical thickness and attentional networks as measured by the attention network test. Cereb. Cortex. 2011;21:345–356. doi: 10.1093/cercor/bhq101. [DOI] [PubMed] [Google Scholar]
- White T., Su S., Schmidt M., Kao C.Y., Sapiro G. The development of gyrification in childhood and adolescence. Brain Cogn. 2010;72:36–45. doi: 10.1016/j.bandc.2009.10.009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Whittle S., Allen N.B., Fornito A., Lubman D.I., Simmons J.G., Pantelis C., Yucel M. Variations in cortical folding patterns are related to individual differences in temperament. Psychiatry Res. 2009;172:68–74. doi: 10.1016/j.pscychresns.2008.06.005. [DOI] [PubMed] [Google Scholar]
- Willcutt E.G., Doyle A.E., Nigg J.T., Faraone S.V., Pennington B.F. Validity of the executive function theory of attention-deficit/hyperactivity disorder: a meta-analytic review. Biol. Psychiatry. 2005;57:1336–1346. doi: 10.1016/j.biopsych.2005.02.006. [DOI] [PubMed] [Google Scholar]
- Wright I., Waterman M., Prescott H., Murdoch-Eaton D. A new Stroop-like measure of inhibitory function development: typical developmental trends. J. Child Psychol. Psychiatry. 2003;44:561–575. doi: 10.1111/1469-7610.00145. [DOI] [PubMed] [Google Scholar]
- Yucel M., Stuart G.W., Maruff P., Velakoulis D., Crowe S.F., Savage G., Pantelis C. Hemispheric and gender-related differences in the gross morphology of the anterior cingulate/paracingulate cortex in normal volunteers: an MRI morphometric study. Cereb. Cortex. 2001;11:17–25. doi: 10.1093/cercor/11.1.17. [DOI] [PubMed] [Google Scholar]
- Yucel M., Stuart G.W., Maruff P., Wood S.J., Savage G.R., Smith D.J., Crowe S.F., Copolov D.L., Velakoulis D., Pantelis C. Paracingulate morphologic differences in males with established schizophrenia: a magnetic resonance imaging morphometric study. Biol. Psychiatry. 2002;52:15–23. doi: 10.1016/s0006-3223(02)01312-4. [DOI] [PubMed] [Google Scholar]
- Yucel M., Wood S.J., Phillips L.J., Stuart G.W., Smith D.J., Yung A., Velakoulis D., McGorry P.D., Pantelis C. Morphology of the anterior cingulate cortex in young men at ultra-high risk of developing a psychotic illness. Br. J. Psychiatry. 2003;182:518–524. doi: 10.1192/bjp.182.6.518. [DOI] [PubMed] [Google Scholar]