Highlights
-
•
There have now been several longitudinal studies of structural brain development.
-
•
We discuss current methods and analysis techniques in longitudinal MRI.
-
•
We relate MRI measures to possible underlying physiological mechanisms.
-
•
We encourage more open discussion amongst researchers regarding best practices.
Keywords: Adolescence, Childhood, DTI, Maturation, MRI, Morphometry
Abstract
Magnetic resonance imaging (MRI) has allowed the unprecedented capability to measure the human brain in vivo. This technique has paved the way for longitudinal studies exploring brain changes across the entire life span. Results from these studies have given us a glimpse into the remarkably extended and multifaceted development of our brain, converging with evidence from anatomical and histological studies. Ever-evolving techniques and analytical methods provide new avenues to explore and questions to consider, requiring researchers to balance excitement with caution. This review addresses what MRI studies of structural brain development in children and adolescents typically measure and how. We focus on measurements of brain morphometry (e.g., volume, cortical thickness, surface area, folding patterns), as well as measurements derived from diffusion tensor imaging (DTI). By integrating finding from multiple longitudinal investigations, we give an update on current knowledge of structural brain development and how it relates to other aspects of biological development and possible underlying physiological mechanisms. Further, we review and discuss current strategies in image processing, analysis techniques and modeling of brain development. We hope this review will aid current and future longitudinal investigations of brain development, as well as evoke a discussion amongst researchers regarding best practices.
1. Introduction
The human brain undergoes profound changes in structure across development. Several longitudinal magnetic resonance imaging (MRI) investigations of brain development in childhood and/or adolescence are currently underway, with more beginning each year (see Table 1). The questions addressed by these investigations are diverse, with some exploring genetic and experience-dependent changes, and others relating changes in brain structure to well-being, behavior, or cognitive development. Despite the diversity of topics for investigation, each of these studies utilizes methods to process and analyze brain images acquired longitudinally. This review discusses the most commonly used MRI measures and our current knowledge of brain development through longitudinal investigations (Section 2), the biological validity of interpretations derived from these investigations (Sections 3, 4), as well as the variety of methods to process, analyze and model longitudinal changes in brain structure (Sections 5, 6). We conclude with a discussion on the benefits of longitudinal designs (Section 7). It is our hope that this review will stimulate further discussion amongst researchers regarding best practices in longitudinal studies of brain development.
Table 1.
A non-exhaustive list of longitudinal MRI projects examining developmental changes in brain structure in childhood and/or adolescence.
| Dataset | Participants (age range) | Location | Years | Website |
|---|---|---|---|---|
| NIMH Child Psychiatry Branch | ∼2000 participants (5–60 years) | NIMH; Bethesda, MD | 1991–2011 | http://www.intramural.nimh.nih.gov/chp/index.html |
| Leonard Florida sample | 45 participants (5–12 years) | Alachua County, FL, USA | 1999–2004 | http://www.kidsbrains.org/index.php |
| NIH MRI Study of Normal Brain Development | 433 participants (4–18 years) | Six Locations; USA | 2001–2007 | http://www.pediatricmri.nih.gov/ |
| Department of Psychology, University of Minnesota | 191 participants (9–24 years) | Twin Cities, MN, USA | 2004– | http://www.psych.umn.edu |
| The Netherlands Twin Register BRAINSCALE | 190 participants (9–12 years) | Utrecht, The Netherlands | 2005– | http://www.tweelingenregister.org/onderzoek/lopend-onderzoek/brainscale/ |
| NICHE | 147 participants (7–23 years) | Utrecht, The Netherlands | 2006–2011 | http://www.niche-lab.nl/ |
| Neurocognitive Development | ∼200 participants (8–25 years) | Oslo, Norway | 2008– | http://www.oslobrains.no |
| PLING | 105 participants (5–8 years) | San Diego, CA, USA | 2010– | http://www.chd.ucsd.edu/research/pling-study.html |
| Laboratory of Neurocognitive Development | 129 participants (8–28 years) | Pittsburgh, PA, USA | 2010– | https://www.lncd.pitt.edu/wp/ |
| Mother-Child Cohort Study | ∼350 participants (4–10 years) | Oslo & Trondheim, Norway | 2011– | http://www.oslobrains.no |
| BRAINTIME | 299 participants (8–25 years) | Leiden, The Netherlands | 2011– | http://www.juniorhersenen.nl/braintime |
| U-Change | 300 participants (14–24 years) | Cambridge & London, UK | 2012– | http://www.nspn.org.uk/ |
| Tokyo Teen Cohort Project | 300–400 pairs (9–12 years) | Tokyo, Japan | 2012– | http://www.ttcp.umin.jp/about/ |
1.1. Histological discoveries and post-mortem work
Research in the 1960s and 1970s provided the first anatomical evidence that the human brain continues to develop beyond childhood (Dekaban and Sadowsky, 1978, Huttenlocher, 1979, Yakovlev and Lecours, 1967). By quantifying the synaptic profiles of layer III in the middle frontal gyrus, Huttenlocher showed that synaptic density in this area is greater in childhood than in adulthood. At age 7 years, synaptic density in this portion of the prefrontal cortex was 36% greater than the adult mean, and remained relatively stable between ages 16 and 72 years (Huttenlocher, 1979). Separate post-mortem work looking at myelination cycles throughout the brain provided support that association cortices continue to gain myelin into the second and third decades (Benes, 1989, Yakovlev and Lecours, 1967). These results prompted Yakovlev and Lecours to theorize that these protracted changes in white matter development paralleled behavioral changes occurring at this time, with special emphasis on social navigation.
“[T]he exponential myelination of the supralimbic division of the hemisphere and cerebral cortex correlates with the exponential maturation of the behavioural patterns in the sphere of motility of effective societal transactions – symbolized thought, language and manufacture, and of learning from individual experience.”
-Yakovlev and Lecours, 1967, p. 63.
Although constrained by small sample sizes that almost entirely exclude the teenage years, these studies challenged prevailing ideas that brain development was complete by early childhood and spurred subsequent work investigating structural brain changes beyond the first decade of life.
Autopsy reports from hospitals in and around the Washington, DC area dating between 1964 and 1973 were pooled together to extract brain weight and other physical data from 4736 individuals representing ages across the entire life span (Dekaban and Sadowsky, 1978). Brain weight was measured separately for females and males and compared against age, body height, and body weight, demonstrating the relatively dramatic changes in brain weight that occur within the first three years of life as well as the relatively protracted climb to maximum brain weight obtained in the late teen years. The now ubiquitous average sex difference of ∼9% greater brain weight in males than females was observed in this study, crucially relating these differences to measures of body size (Dekaban and Sadowsky, 1978).
1.2. Magnetic resonance imaging
Although post-mortem work paved much of the way in our understanding of both the microscopic and macroscopic changes occurring in the brain across development, MRI has quickly become the instrument of choice to measure changes in brain structure. Without the need for ionizing radiation, MRI is both safe for children and allows for imaging the same individual multiple times. Although an MRI machine can seem intimidating to young participants, planning visits to the MRI through videos (e.g., http://vimeo.com/32255381) or mock-scanning visits, and friendly scanning operators help to alleviate the anxieties of participants. The main limiting factors to developmental MRI appear to be minimizing the amount of noise introduced to the images by factors such as scanner artifacts and participant motion.
2. What MRI studies measure
MRI is an imaging technique based on the principles of nuclear magnetic resonance that detects proton signals from water molecules and that allows us to produce high quality images of the internal structure of the living brain. MRI differentiates between tissue types, and protocols designed to create anatomical images of the brain can distinguish between gray matter, white matter, and cerebrospinal fluid (CSF). By providing the contrast needed to distinguish these, MRI allows researchers to measure the sizes as well as various other properties of different parts of the brain (Fig. 1).
Fig. 1.
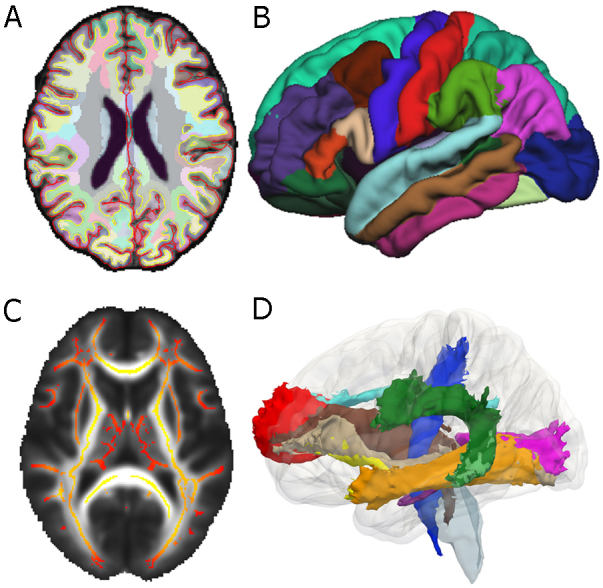
Illustration of key MRI methods discussed in this review. (A) Horizontal slice of T1 image showing a whole brain segmentation used for volumetric analyses and (B) a left lateral view of an averaged parcellated cerebral cortex used for surface-based analyses, both from FreeSurfer. (C) Horizontal slice of TBSS mean FA white matter skeleton overlaid on a mean FA map and (D) a left lateral view of a 3D rendering of probabilistic fiber tracts from the Mori atlas.
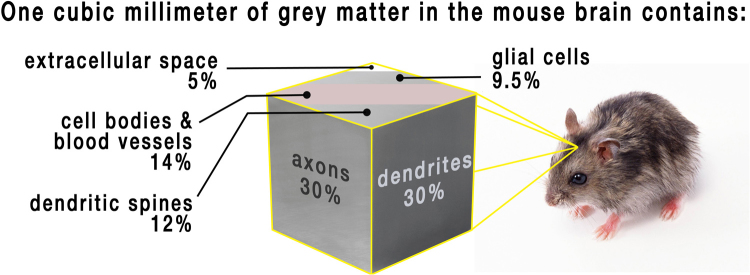
Typical current anatomical images of the brain captured by MRI have a spatial resolution of approximately 1 cubic millimeter (mm3). The volume of an adult human brain is, on average, between 1,131,000–1,273,000 mm3, with substantial variation between individuals (Allen et al., 2002). Although the spatial resolution of modern MRI protocols is very high, 1 mm3 of cortical gray matter can contain between 10,000 and 60,000 neurons, up to four times as many glial cells per neuron (Ribeiro et al., 2013), as well as neuronal processes, blood vessels, intracortical myelin and dendritic spines. As displayed in Fig. 2, one study calculated that 1 mm3 of gray matter in the cortex of a mouse consists mostly of dendrites and axons (Braitenberg, 2001). However, primate and rodent brains differ on a number of levels, including neuronal size and packing density (Herculano-Houzel, 2009). Currently, we cannot be certain of the microscopic processes that underlie the gross changes in human brain structure captured by MRI, but we integrate evidence from post-mortem and animal work to hypothesize what may underlie the signal changes (see Section 4).
Fig. 2.
The composition of a cubic millimeter of gray matter in the mouse cortex.
Data derived from Braitenberg (2001).
2.1. Volume
One of the first introduced and most popular structural measurements of the brain is volume. Before MRI, researchers would measure the intracranial volume of skulls to infer the brain size of individuals (Harper et al., 1984). However, given the nature of this method, longitudinal analyses were impossible to conduct. With the advent of brain imaging, we can now measure the volume of an individual's brain as well as the volumes of different tissue types or specific structures, multiple times in the same individual.
2.1.1. Whole brain volume
Whole brain volume, sometimes referred to as total brain volume, is typically measured by summing the gray and white matter volumes, excluding the brainstem. However, sometimes whole brain volumes also include non-brain matter such as CSF, ventricles and the choroid plexus. There have been a number of longitudinal imaging studies examining developmental changes in whole brain volume, several of which were included in a recent meta-analysis (Hedman et al., 2012). Results from this meta-analysis showed whole brain volume increases throughout childhood and early adolescence, until around the age 13 years. After this age, whole brain volume slightly decreases, remaining roughly stable until the mid-thirties. However, changes occurring between mid-adolescence and mid-adulthood might be biased due to the age ranges of the studies included. Furthermore, recent studies that were not included in the meta-analysis provide somewhat mixed results. A study of 103 individuals scanned at least twice across ages 5–32 years did not find any significant overall whole brain volume changes, although a proportion of participants showed volume increases between ages 5–11 years (∼50%) and 8–14 years (∼35%), and a smaller proportion of participants showed volume decreases between ages 11–19 years (∼30%) and 15–22 years (∼30%) (Lebel and Beaulieu, 2011). A recent study of 292 individuals (882 scans) scanned between two and four times across ages 4.5–22 years revealed an increase in whole brain volume until mid to late adolescence (Aubert-Broche et al., 2013). These studies call into question the assumption that whole brain volume development is complete in late childhood.
2.1.2. Gray matter and white matter volumes
Gray matter is composed of neuronal bodies, glial cells, dendrites, blood vessels, extracellular space and both unmyelinated and myelinated axons. The gray matter that forms the outer ∼4 mm of the cerebrum is called the cerebral cortex, although gray matter is also found subcortically and in the cerebellum. White matter is composed of myelinated axons, glial cells, and extracellular space. These are the two major components measured by structural MRI, and each has a distinctive developmental trajectory.
Cortical gray matter volume is greatest in childhood, generally decreases throughout adolescence, and begins to decelerate in volume loss around the early to mid-twenties (Aubert-Broche et al., 2013, Tamnes et al., 2013a). Studies using the National Institute of Mental Health (NIMH) Child Psychiatry Branch dataset have reported inverted-U shaped patterns of cortical gray matter volume development, with “peak” gray matter volumes attained in late childhood or early adolescence (Giedd et al., 1999, Lenroot et al., 2007, Raznahan et al., 2011), but this finding has not been replicated in other samples (Aubert-Broche et al., 2013, Lebel and Beaulieu, 2011, Tamnes et al., 2013a, Wierenga et al., 2014).
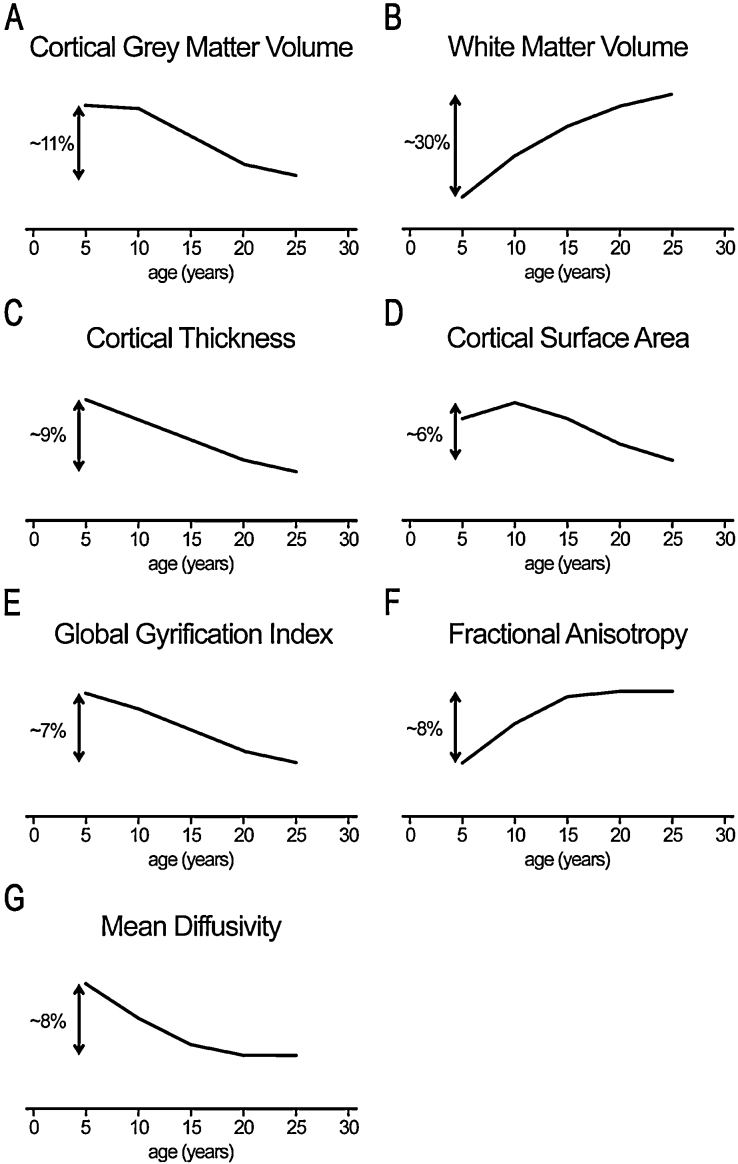
The previously mentioned study of 103 individuals scanned at least twice across ages 5–32 years reported a linearly decrease in total gray matter volume across this age span (Lebel and Beaulieu, 2011). Of the participants scanned between ages 5 and 11 years, over 50% showed a decrease in gray matter volume. This proportion increased to over 80% for 8–14 year olds, 90% for 11–19 year olds, and 80% for 15–22 year olds. The majority of participants scanned between 22–32 years showed no change in gray matter volume. In the previously mentioned study of 292 individuals scanned between two and four times across ages 4.5–22 years, gray matter volume showed relative stability between ages 5 and 10 years, and decreased between ages 10 and 20 years, with similar trajectories for both female and male participants (Aubert-Broche et al., 2013). Cortical gray matter volume decreased across ages 8–22 years in a sample of 85 participants scanned twice, with the steepest decrease roughly coinciding with the teen years (Tamnes et al., 2013a). Taken together, this study reported a 1.15% mean annual decrease in cortical gray matter volume between late childhood and the early twenties. A similar pattern was found in study of 135 individuals (201 scans) aged 7–23.3 years (Wierenga et al., 2014). These four separate longitudinal studies suggest that gray matter volume does not “peak” at a specific age in late childhood or adolescence. Instead, it appears that gray matter is at its highest volume during mid-to-late childhood, and decreases across the second decade (illustrated in Fig. 3a).
Fig. 3.
Schematic illustrations of developmental MRI findings discussed in this review. Each schematic drawing is an estimate of the typical developmental trajectory of the measurement of interest, based on longitudinal studies discussed in the review. The trajectories are broken into five-year increments, and percent changes are estimates based on available data. The measurements include (A) cortical gray matter volume, (B) white matter volume, (C) cortical thickness, (D) cortical surface area, (E) global gyrification index, (F) fractional anisotropy, and (G) mean diffusivity. It is important to note that these schematic drawings represent global brain measures, and that specific brain regions or fiber tracts could show different developmental trajectories.
Longitudinal studies consistently report an increase in white matter volume across childhood and adolescence (Fig. 3b). The Lebel and Beaulieu (2011) study reported an increase in white matter volume between ages 5 and 25 years, with white matter volume stabilizing around this time. Over 90% of participants scanned between 5–11 years, 8–14 years and 11–19 years showed an increase in white matter volume. Only half of the sample scanned between 22 and 32 years showed an increase in white matter volume, with the other half showing no change. The Aubert-Broche et al. (2013) study reported an increase in white matter volume across ages 5–20 years, with males showing an almost linear increase and females starting to stabilize in white matter volume in the late teens.
2.1.3. Regional volumes
While it is possible to describe the overall developmental pattern of cortical gray matter and white matter volumes, there exists substantial heterogeneity in developmental timing between different cortical and subcortical brain regions. Many studies have examined the developmental trajectories of specific lobar volumes, often divided into the frontal, temporal, parietal, and occipital lobes. Some studies have examined cortical regions defined by more fine-grained parcellation methods (Tamnes et al., 2013a), or a priori regions of interest (Mills et al., 2013). Depending on the software and parcellation scheme used, it is possible to measure gray matter and white matter for regional volumes (e.g., FreeSurfer). The Aubert-Broche et al. (2013) study reported similar developmental trajectories for frontal and temporal gray matter volume, which largely resembled the overall pattern for total gray matter volume. However, parietal and occipital gray matter volumes showed an almost linear decline across ages 5–20 years, with the parietal cortex showing a greater decrease than the occipital cortex, in contrast with earlier reports (Giedd et al., 1999, Lenroot et al., 2007). Two separate longitudinal samples have shown that the developmental trajectories for frontal, temporal, parietal, and occipital white matter volumes do not appear to differ much from the whole brain white matter volume trajectory, although they show some variability in the magnitude of change occurring across childhood and adolescence (Aubert-Broche et al., 2013, Lenroot et al., 2007).
2.1.4. Subcortical volumes
Subcortical brain structures were some of the first brain structures examined in developmental MRI studies, and their measurement and development continue to be of great interest to developmental neuroscience. Commonly measured subcortical structures include the hippocampus, amygdala, thalamus, globus pallidus, putamen, nucleus accumbens, and caudate. Several longitudinal studies have now investigated the developmental trajectories of these structures, revealing substantial heterogeneity in subcortical brain development. An analysis of 60 individuals, scanned twice across ages 11–18 years, found significant hemisphere and gender effects for several subcortical structures, as well as interindividual variability (Dennison et al., 2013). For example, the right nucleus accumbens decreased in volume for ∼50% of participants, while the left nucleus accumbens increased in volume for ∼55% of participants. Overall, the study found increased volume in the hippocampus, pallidum, and left accumbens, decreased volume in the putamen, caudate, thalamus and right accumbens, and no change in amygdala volume across adolescence (Dennison et al., 2013).
These results were overall similar to those found in a sample of 85 individuals scanned twice across ages 8–22 years (Tamnes et al., 2013a). Across this age range, the caudate, putamen, and nucleus accumbens decreased in volume, whereas the amygdala and hippocampus showed little or no change. The pallidum and thalamus showed a slight decrease in volume, with the largest changes occurring between mid- and late adolescence. In a sample of 275 individuals (711 scans) spanning ages 7–20 years, many subcortical structures showed both age-related and puberty-related changes in volume (Goddings et al., 2013). Across this period of adolescence, the hippocampus and amygdala increased in volume, whereas the nucleus accumbens, caudate, globus pallidus, and putamen decreased. Overall, it thus seems that the medial temporal lobe structures follow different developmental trajectories than most of the other subcortical structures.
2.2. Surface-based measures
By identifying the borders between tissue types, surface-based cortical reconstruction software allows for the ability to measure not only gray matter volume, but also cortical thickness, surface area, and gyrification and folding patterns. We briefly outline these measures and their developmental trajectories below.
2.2.1. Cortical thickness
Cortical thickness is typically calculated by measuring the distance between the boundary between white matter and cortical gray matter, and gray matter and the pia mater. The thickness of the cerebral cortex varies roughly between 2 mm and 4 mm, with the thinnest cortical regions found in the frontal and occipital poles and the thickest regions found in temporal and insular cortices (Ribeiro et al., 2013).
Different samples have revealed different developmental trajectories for cortical thickness (Fig. 3c). The first longitudinal study of developmental changes in cortical thickness (n = 45, scanned twice) showed widespread cortical thinning between mid to late childhood – with the exception of classical language areas, which showed cortical thickening during this short developmental window (Sowell et al., 2004). The NIMH Child Psychiatry Branch sample of 647 participants (1274 scans) showed that global cortical thickness followed a cubic trajectory, decreasing ∼9% between late childhood and the early twenties (Raznahan et al., 2011). Linear global cortical thinning was reported between ages 7 and 23.3 years in recent study of 135 individuals (201 scans) (Wierenga et al., 2014). A vertex-based analysis of 137 participants (209 scans) found the majority of the cortex thinning linearly between ages 6 and 29 years, with regions in the lateral prefrontal cortex, medial prefrontal cortex, medial posterior parietal cortex, and temporal–parietal–occipital junction following a quadratic trajectory (Mutlu et al., 2013). Direct comparisons and testing of the effects of different cortical thickness estimation procedures are likely needed to resolve this inconsistency across studies.
2.2.2. Surface area
Cortical surface area can be defined using surface-based measurements in a number of ways. Most commonly, it is calculated as the area of the boundary between the white matter and gray matter – often termed the white matter surface. However, cortical surface area has also been defined as the area of the boundary between gray matter and pia mater (called the pial surface), or it has been calculated as the average of the white matter surface and pial surface. The multiple ways in which cortical surface area can be defined limits how easily one may reproduce or compare values across studies. It is also unclear if these different measures of cortical surface reflect the same underlying processes. Therefore, it is crucial to specify how cortical surface area is calculated to promote greater transparency and potential for replication. The average cortical surface area (white matter surface) of an adult human brain is 154,700 ± 14,600 mm2 (Winkler et al., 2010).
In the NIMH Child Psychiatry Branch sample mentioned above, total cortical surface area followed a cubic trajectory, decreasing ∼7% between late childhood and the early twenties (Raznahan et al., 2011). A similar pattern was found in smaller study of 135 individuals (201 scans) aged 7–23.3 years (Wierenga et al., 2014) (Fig. 3d). However, certain areas of the cortex expand more than others during development (Fjell et al., 2013, Hill et al., 2010). In a cross-sectional sample of 331 individuals aged 4–20 years, several regions of the cortex showed more expansion than the mean expansion of the total cortical surface, including the lateral and medial temporal cortex, cingulate cortex, retrosplenial cortex, lateral orbitofrontal cortex, superior frontal gyrus, inferior frontal gyrus, insula, temporo-parietal junction, cuneus, and lingual gyrus (Fjell et al., 2013).
2.2.3. Gyrification and folding patterns
The human cortex is highly convoluted, with approximately one third of the cortical surface exposed on gyri, and two-thirds buried within sulci. The complexity of cerebral folding patterns has been of great interest to brain imaging research, and recent methods have made the quantification of these folding patterns easier. The gyrification index of the whole brain is defined as the ratio of the total folded cortical surface over the total perimeter of the brain (Zilles et al., 1988), whereas the local gyrification index measures the degree of cortical folding at specific points of the cortical surface (Schaer et al., 2008). The gyrification index of the human brain decreases between childhood and young adulthood, whereas the amount of exposed cortical surface increases from childhood to mid-adolescence (Raznahan et al., 2011), and between middle and late adolescence (Alemán-Gómez et al., 2013). A study of 52 adolescents scanned twice between ages 11 and 17 years showed an overall flattening of the cortex during adolescence, related to decreases in sulcal depth and increases in sulcal width (Alemán-Gómez et al., 2013). Similar to other structural measurements, the development changes in local gyrification varies across the cortex, with regions in medial prefrontal cortex, occipital cortex, and temporal cortex undergoing little to no change between ages 6 and 29 years (Mutlu et al., 2013). However, similar to what has been found in whole brain (Raznahan et al., 2011) and lobar-level (Alemán-Gómez et al., 2013) analyses, Mutlu et al. (2013) observed linear decreases in local gyrification index across the majority of the cortex (Fig. 3e).
2.3. Diffusion tensor imaging
In addition to morphometric approaches, diffusion MRI has over the last two decades become a standard modality in neuroimaging (for in-depth introductions, see: Johansen-Berg and Behrens, 2009, Mori, 2007). Diffusion MRI is a noninvasive in vivo method that is sensitive to the natural displacement of water molecules that occur as part of the physical diffusion process. Water diffusion in biological tissue is however not free and uniform (isotropic diffusion), but reflects interactions with obstacles, such as membranes, cytoskeleton and macromolecules and is as a result of this not necessarily the same in all direction (anisotropic diffusion). The diffusion patterns can therefore indirectly reveal details about tissue architecture at a micrometer scale well beyond the usual millimetric resolution of MRI. A common use of diffusion MRI referred to as diffusion tensor imaging (DTI) involves fitting a tensor, for each voxel, that estimates diffusion in three dimensions (Le Bihan and Johansen-Berg, 2012). The tensor is a mathematical description of an ellipsoid and the volume, shape and orientation of the ellipsoid can be considered. The length of the ellipsoid axes are called eigenvalues (λ1, λ2, λ3), while their orientations are called eigenvectors (V1, V2, V3). Various quantitative indices can be derived from the estimated diffusion tensor, with the two primary DTI outcome variables being mean diffusivity (MD), reflecting overall magnitude of water diffusion (mean of all three eigenvalues), and fractional anisotropy (FA), which indexes degree of net directionality in water diffusion in the tissue and theoretically ranges from 0 when the diffusion is isotropic to 1 when diffusion occurs only along one axis. Additionally, diffusion along [axial diffusivity (AD): λ1] and across [radial diffusivity (RD): mean of λ2 and λ3] the main axis of the diffusion tensor can be estimated.
Beyond the very rapid changes seen in DTI indices in infancy (Dubois et al., 2008, Geng et al., 2012, Hermoye et al., 2006), cross-sectional studies have consistently documented age-related differences in structural connectivity in childhood and adolescence in the form of FA increases and overall diffusivity decreases with increasing age in most white matter regions (Lebel et al., 2008, Peters et al., 2012, Schmithorst and Yuan, 2010, Tamnes et al., 2010). Studies with very wide age-ranges have further extended these findings, indicating non-monotonic lifespan age trajectories of FA, MD and RD characterized by three phases: (1) initially fast, but decelerating changes through childhood and adolescence and into early adulthood followed by (2) relative stability in mid-adulthood with subsequent (3) accelerating changes in senescence (Lebel et al., 2012, Westlye et al., 2010). Longitudinal developmental studies are now also confirming widespread white matter FA increases (Fig. 3f), and MD and RD decreases through childhood and adolescence (Fig. 3g), but the results for AD are less consistent (Bava et al., 2010, Brouwer et al., 2012, Lebel and Beaulieu, 2011). Importantly, as for cortical development, the rates and timing of the DTI changes vary regionally in the brain and a pattern of maturation in which major white matter tracts with fronto-temporal connections develop more slowly than other tracts has emerged (Colby et al., 2011, Lebel and Beaulieu, 2011, Tamnes et al., 2010). Of the major fiber bundles, the cingulum which is implicated in e.g. cognitive control has been shown to have a particularly prolonged development (Lebel et al., 2012, Westlye et al., 2010). Crucially, individual- and age-related differences in DTI measures of white matter microstructure have also been linked to a range of behavioral measures, documenting their functional consequences (Johansen-Berg, 2010).
3. Relating biological development to brain development
It is essential to relate developmental trajectories to an appropriate scale. Across fields, most developmental literature uses chronological age to quantify development. However, there are also other developmental processes that occur during the first two decades of human development which likely impact on brain development, such as body growth and puberty.
3.1. Age
Age is easily quantified with high reliability and validity and therefore allows easy comparison across studies and investigative techniques. Furthermore, it provides a linear scale throughout the human life cycle, allowing studies to compare absolute and relative growth at different stages of life (Tamnes et al., 2013a). Multi-model brain imaging pattern analysis techniques show that aspects of brain structure can predict age with high accuracy (Brown et al., 2012). Many of the longitudinal studies described in the previous sections have modeled brain development during childhood and adolescence against age, and it remains the most popular measure of biological development.
Whilst many brain imaging studies have related brain development to age (as reviewed in previous sections), considerable individual variability exists, and age only explains a certain proportion of the variance in modeled trajectories. One limitation of using age as a measure against which to judge brain development is that it provides little information on the underlying physiological mechanisms. During late childhood and adolescence, individuals undergo physical changes such as a height growth spurt and puberty, which happen at different ages across individuals. These other physical measures are discussed in the following sections.
3.2. Body size
A linear relationship between brain size and body size exists for the primate species, with human brains deviating by only ∼10% from its expected size (Azevedo et al., 2009, Herculano-Houzel et al., 2007). New evidence has prompted researchers to suppose that humans do not have a larger brain than would be expected for a primate of our body size, but instead that other primates, such as orangutans and gorillas, have larger bodies than would be expected (Herculano-Houzel et al., 2007). Further, these researchers suggest that brain mass and body mass are only correlated, and that brain size is not determined by body mass (Herculano-Houzel, 2009). Specific genes are related to both brain size and body size (Silver et al., 2010), however the mechanisms linking the two remain largely unknown. Recent anthropological research suggests that the increase in brain size in the genus Homo around 300,000–138,000 years ago occurred independently of increases in body mass (Gallagher, 2013). To date, there have been few studies examining brain size against body size within a large group of humans, or using a longitudinal design.
The relationship between body size and brain size continues to influence the debate regarding sex differences in the brain. Although the, on average, larger brain size of human males compared to human females is often attributed to the, on average, larger body size of males, it is not certain if empirical studies support this notion. One controversial analysis of over 1000 post-mortem human brains found that males were still about 100 g heavier than female brains after correcting for body height or body surface area (Ankney, 1992). However, the validity of the statistical methods used in this study have been questioned (Forstmeier, 2011).
3.3. Puberty
Changes in brain structure during adolescence can also be related to the hormonal changes underlying the onset of and progression through puberty. Pubertal onset varies by as much as 4–5 years across individuals (Parent et al., 2003), which can introduce substantial variation into studies of brain development between childhood and adolescence if only age is measured. A recent review highlighted several genetic mechanisms that interact with the neuroendocrine system to initiate puberty (Ojeda and Lomniczi, 2013).
Previous structural brain imaging studies have attributed developmental trajectory characteristics to pubertal onset (Giedd et al., 1999, Lenroot et al., 2007). Gender differences in reported gray matter volume “peaks” have been attributed to the discrepancy in pubertal timing between females and males. For example, in studies using the NIMH Child Psychiatry Branch dataset, the 1–2 year difference in “peak” cortical gray matter volumes of females compared to males is described as “corresponding to the average age difference at puberty” (p. 1071, Lenroot et al., 2007). Indeed, clinical endocrinology studies suggest that pubertal development in females is, on average, 1–2 years earlier than in males (Bordini and Rosenfield, 2011, Sun et al., 2002). However, although the age of onset for pubertal milestones is different, the age at which females and males attain the final milestones of puberty (menarche and testes development, respectively) overlap substantially (11.0–14.1 years for females, 11.5–16.5 years for males) (Bordini and Rosenfield, 2011). Furthermore, multiple studies have failed to find gender differences in cortical gray matter volume trajectory shape (Aubert-Broche et al., 2013, Wierenga et al., 2014), suggesting that if a relationship between cortical gray matter volume and puberty does exist, age alone might not be sensitive enough to detect it. Future work combining different measures of physical maturity, such as height, pubertal status and hormones, may help elucidate the different mechanisms underlying brain development during the transition from childhood to adolescence.
4. Physiological mechanisms underlying structural changes
What does a reduction in gray matter volume, assessed by a T1 weighted MRI, reflect on a cellular level? Do changes in brain structure during development reflect the same processes as changes observed during adulthood? What are the limitations in MRI, and can we extrapolate microcellular mechanisms underlying these macrostructural changes? Although these questions remain unanswered, they are crucial to developmental neuroscientists, as they link efforts in neuroimaging to neurophysiological and anatomical research.
4.1. In development
The underlying mechanisms of developmental changes in structural MRI measures are still debated (see Paus, 2013, Paus et al., 2008). To date, there are no studies that have directly tested the relationship between developmental changes in morphometric MRI measures to changes in cellular or synaptic anatomy. However, many studies propose that reductions in gray matter volume during adolescence partly reflect synaptic pruning (Blakemore, 2008, Giedd et al., 1999). While synaptic densities in selected regions of the prefrontal cortex are at their greatest levels at some point during the first two decades, and appear to decrease throughout the second and third decade (Huttenlocher and Dabholkar, 1997, Petanjek et al., 2011), we cannot directly relate these data to findings in MRI. For one, synaptic boutons (also known as synaptopil) are incredibly small, comprising only a fraction of gray matter volume (Bourgeois and Rakic, 1993). Even when synapses are particularly dense, they are estimated to represent only 2% of a cubic millimeter of neuropil or less than 1.5% cortical volume (Bourgeois and Rakic, 1993). Given this relatively small percentage, it is unlikely that the marked decreases in cortical volume observed across adolescence are purely reflective of synaptic pruning. The reduction in number of synapses might, however, in addition to a reduction in neuropil, also be accompanied by a reduction in the number of cortical glial cells and these events could together account for more of the cortical structural changes observed during development, although this remains purely a speculation. Other processes, such as the encroachment of subcortical white matter, and continued intracortical myelination, also likely impact on measurements of cortical gray matter, by changing the signal intensity values and contrasts such that the boundary between white and gray matter is moved outwards with increasing age. Undoubtedly, there is a myriad of both parallel and interacting neurobiological processes underlying the macrostructural changes observed during childhood and adolescence in MRI studies.
Developmental changes in DTI indices in white matter are mainly thought to relate to processes including increased relative axon caliber and myelin content, as well as changes in fiber packing density (Paus, 2010). Overall, animal studies have shown that axonal membranes are the primary determinants of diffusion anisotropy in both peripheral nerves and central nervous system white matter, while myelin can modulate anisotropy (Beaulieu, 2009, Concha et al., 2010). For instance, rodent dysmyelination models show that FA values still indicate anisotropy and reduce only by ∼15% in the complete absence of myelin (Beaulieu, 2009). Further, a rare study comparing human in vivo DTI with subsequent microscopy of the fimbria-fornix in patients with temporal lobe epilepsy has documented a robust positive correlation between FA and axonal membranes (Concha et al., 2010). However, animal studies also consistently indicate that RD is particularly sensitive by de- and dysmyelination (Song et al., 2005, Song et al., 2002) and correlations between DTI and myelin content and to a lesser degree axon count have also been shown in postmortem human brain of patients with multiple sclerosis (Schmierer et al., 2008, Schmierer et al., 2007). The myelin content interpretation has because of such and other findings often been stressed both in developmental studies and in other contexts. Although myelination, a process than begins between weeks 20 and 28 of gestation, has been shown to continue throughout childhood and adolescence (Benes, 1989, Benes et al., 1994, Tau and Peterson, 2009, Yakovlev and Lecours, 1967), it does not logically follow from the above mentioned rodent and postmortem studies that age-related differences in RD in healthy humans reliably indicates differences in myelination. It has therefore been argued that hypothesized differences in myelination perhaps too hastily has been considered as a main explanation for differences in DTI parameters, to the exclusion of other possible neurobiological factors (Paus, 2010). In general, a number of factors, including axon caliber, myelin content and fiber density, as mentioned above, as well as brain water content, crossing or diverging fibers and partial voluming, influence DTI indices (Beaulieu, 2009, Beaulieu, 2002). The relative roles of the various factors in development may likely also be age-dependent. Importantly, DTI parameters are sensitive to general diffusion properties of brain tissue and are not selective markers of specific neurobiological properties. Precise interpretations of the underlying tissue alterations of DTI changes are thus challenging and should be done with great caution. However, investigating multiple DTI indices, including RD and AD, yields additional information to better characterize tissue microstructure, and future multimodal imaging studies and studies combining imaging and histology can hopefully be informative in untangling the factors influencing DTI indices and their changes during development.
4.2. Pre- and post-intervention
While the high degree of plasticity in childhood and adolescence allows for a lot of experience dependent structural change, the neuroanatomical and physiological changes that underlie many of the common MRI changes in the developing brain are likely at least partly different from those underlying experience or training-induced changes in the adult brain (Zatorre et al., 2012). Although changes in MRI measurements should be similarly reflective of changes in the various neuroanatomical components that make up the measure of interest (e.g., gray matter volume), the mechanisms and specific changes could be different. For example, synaptic changes in adulthood are not nearly as large as the changes observed during the first two decades. The neuroanatomical pioneer Peter Huttenlocher stated that “there is no evidence for any large net increase in synapses in the cerebral cortex during the adult years” (p.173, 2002). Huttenlocher supposed that any new synapses formed in adulthood are “likely to be balanced by loss of other synaptic connections.” Similarly, the majority of stable dendritic spines are formed during development. One rodent study showed that only a small percentage of dendritic spines formed by learning (or novel experiences) in the adult mouse are retained (Yang et al., 2009). Still, changes in synapses and dendritic spines continue to be a popular explanation for MRI volume changes observed in adult training studies. Animal studies that combine MRI and histological measures can contribute to our understanding to some extent. One such study in rodents suggests that training induced changes in MRI volumes are more reflective of changes in neural processes rather than an increase in neural cell size or number (Lerch et al., 2011). As reviewed extensively in Zatorre et al. (2012), multiple neuroanatomical processes could underlie training or experience-induced changes in structural MRI volumes, but many of these possibilities have yet to be investigated.
A recent large multi-generation family study suggests that regional measures of brain morphometry (e.g., cortical and subcortical volumes, cortical thickness, surface area) are under strong genetic control (McKay et al., 2013). Twin studies indicate that DTI indices of white matter microstructure are highly heritable and even that relationships between DTI and cognitive function to a substantial degree are mediated by genetic factors (Blokland et al., 2012, Chiang et al., 2009). Environmental and experiential variables do however also influence brain morphometry and white matter microstructure across the lifespan. Longitudinal studies measuring the effects of training interventions on brain morphometry have been reviewed in depth elsewhere (Kanai and Rees, 2011, Valkanova et al., 2014). Longitudinal DTI studies document effects of sensorimotor and cognitive training in the form of FA increases and MD decreases following intervention (Engvig et al., 2012, Keller and Just, 2009, Lövdén et al., 2010, Scholz et al., 2009, Takeuchi et al., 2010). As in the case of brain development, ascribing training related changes to specific underlying cellular and molecular level events is challenging as there are multiple possibly coordinated candidate mechanisms. Likely mechanisms also depend on whether the sample includes children, adults or elderly, and whether healthy or clinical groups are investigated. We hope that this brief overview will stimulate future discussion about the neurophysiological mechanisms underlying structural brain changes.
5. Methods of processing structural brain images
Like with functional MRI, there are several ways to process structural brain images. First, in structural MRI studies it is relatively common to acquire multiple T1 weighted sequences from each individual at each time-point. One of the first choices during processing is thus whether to combine these in order to increase the signal-to-noise ratio, or to only include the highest quality sequence from each scan sessions (see discussion about quality control below in Section 5.4). Although averaging sequences will usually increase the signal-to-noise ratio, the effects of data averaging on various structural measures are not well investigated (Han et al., 2006, Jovicich et al., 2009) and this might not be the best approach if the number of available high-quality sequences varies across individuals or time points. A general recommendation is thus to stick to one of these approaches consistently within a study, and avoid mixing averaged and single acquisitions if possible. Second, while many early studies on structural brain development used hand-tracing methods, there now exists several automated programs that researchers can use to segment the entire brain in a fraction of the time needed to hand-trace individual brain structures. However, some automated software is available for public use, whereas other programs are limited to collaborators, and quality control is always a concern for automated methods. We briefly detail several methods that are in current use for processing structural MRI and DTI, as well as essential quality control procedures, below.
5.1. Manual tracing
Early MRI studies relied on trained individuals to hand trace major brain divisions and structures. This technique is still used today, but remains less practical as datasets grow larger. However, some investigators still prefer to use manual tracing methods for structures that are particularly difficult to segment using automated procedures, such as the amygdala (Morey et al., 2009). The cost for this preference is high, as it is estimated that the amygdala takes around 2 h for a trained expert to trace by hand (Hanson et al., 2012), and reliability and reproducibility is always a concern when relying on manual tracing. To overcome the persistent challenges of segmenting the amygdala, Hanson et al. (2012) developed a method that requires a small proportion of hand-traced scans (∼20) to train an automated machine learning-based segmentation procedure to accurately segment the amygdala in large samples.
5.2. Automated software
Many automated programs have emerged over the past 20 years. We have included a list of selected automated software methods in Table 2. The reliability of automated methods is likely to vary amongst programs and across brain structures, but there have been a few studies comparing estimates obtained by automated methods with those obtained through manual tracing. For example, FreeSurfer's estimates for cortical thickness have been validated against both histological analysis (Rosas et al., 2002), and manual tracing (Kuperberg et al., 2003, Salat et al., 2004). Automated subcortical segmentation of the amygdala and hippocampus has also been compared against manual tracing efforts (Morey et al., 2009). Subcortical structures show variable scan-rescan reliability when segmented with automated methods, with some subcortical structures showing higher reliability (e.g., thalamus) than others (Morey et al., 2010). An analysis of 31 children (4–11 years) found that automated software using surface-based registration was more accurate than volume-based registration methods, and that registering these still-developing brains to a common space did not introduce age-related biases (Ghosh et al., 2010). The variety of programs and techniques available to define brain measurements introduces challenges to replication efforts. While there have been studies comparing brain measurements estimated by voxel-based and surface-based programs (Winkler et al., 2010), there have been relatively few studies comparing measurements between specific programs (e.g., FreeSurfer, FSL, CIVET).
Table 2.
A non-exhaustive list of automated software used to process structural MRI and DTI data.
| Software | Description | Methods Papers | Measurements | Notes | Base | Website |
|---|---|---|---|---|---|---|
| 3D Slicer | Software package for visualization and image analysis. | Fedorov et al. (2012) | Cortical volume, Subcortical volume, DTI | Freely available; Open Source; Ongoing updates | Surgical Planning Lab, Boston, USA | www.slicer.org/ |
| AFNI | Software package for mapping human brain activity, with add-on programs and toolboxes that allow for cortical surface-based analysis (SUMA), and DTI tractography analysis (FATCAT). | Cox (1996), Cox and Hyde (1997) and Taylor and Saad (2013) | Cortical volume, Subcortical volume, DTI | Freely available; Open Source; Ongoing updates; Interacts with FreeSurfer and FSL | National Institute of Mental Health, Bethesda, USA | www.afni.nimh.nih.gov/ |
| Caret | Surface and volume-based software package for structural and functional analyses of the cerebral and cerebellar cortex. | Van Essen and Dierker (2007) and Van Essen (2012) | Surface measures, Myelin mapping, Cortical depth | Freely available; Open Source; Ongoing updates; Interacts with FreeSurfer | Van Essen Lab, St. Louis, USA | www.brainvis.wustl.edu/wiki/index.php |
| CIVET | Surface-based human brain image-processing pipeline for corticometric, morphometric and volumetric analyses. | Zijdenbos et al. (2002) | Cortical thickness, Surfrace area, Mean curvature, Gyrification index | Freely available; Ongoing updates | McConnell Brain Imaging Center, Montreal, Canada | www.bic.mni.mcgill.ca/ServicesSoftware/CIVET/ |
| ExploreDTI | Toolbox for exploratory diffusion tensor MRI and fiber tractography | Leemans et al. (2009) | DTI | Freely available; Ongoing updates | Image Sciences Institute, University Medical Center Utrecht, Netherlands | www.exploredti.com/ |
| FreeSurfer | Surface- and volume-based software suite for processing and analyzing brain MR images. FreeSurfer reconstructs the cortical surface, segments subcortical structures, and provides a number of labeling and statistical analysis options. | Dale et al. (1999), Fischl et al. (1999) and Reuter et al. (2012) | Cortical volume, Subcortical volume, Cortical thickness, DTI, Surfrace area, Mean curvature, Gyrification index | Freely available; Open Source; Ongoing updates; Interacts with Caret and FSL; Calculates DTI metrics via TRACULA; Has longitudinal pipeline | Laboratory for Computational Neuroimaging, Boston, USA | www.surfer.nmr.mgh.harvard.edu/ |
| FSL | Comprehensive library of analysis tools for fMRI, MRI and DTI brain imaging data. | Jenkinson et al. (2012) and Smith et al. (2004) | Gray matter concentration, volumetry, DTI, fMRI | Freely available; Ongoing updates; Interacts with FreeSurfer | Analysis Group, FMRIB, Oxford, UK | www.fsl.fmrib.ox.ac.uk/fsl/fslwiki/ |
| LL method | The Longitudinal registration and Longitudinal classification (LL) method measures structural volume changes in longitudinal MRI scans in which participant-specific information is used for both registration and segmentation. | Aubert-Broche et al. (2013) | Cortical volume, Subcortical volume | Not available for public use; Specific to longitudinal designs | McConnell Brain Imaging Center, Montreal, Canada | N/A |
| QUARC | Quantitative anatomical regional change (QUARC) is a nonlinear registration method that measures longitudinal change on a voxel-wise basis. | Holland and Dale (2011) | Cortical volume, Subcortical volume | Not available for public use; Specific to longitudinal designs; Interacts with FreeSurfer | Multimodal Imaging Laboratory, San Diego, USA | N/A |
| VBM | Voxel-based morphometry (VBM) is a technique that measures the concentration of gray matter within each voxel of the brain, and provides voxel-wise comparisons of local tissue volumes within a group or across groups. | Ashburner and Friston (2000) | Gray matter concentration or signal intensity | Freely available; Ongoing updates; Has longitudinal pipeline; Interacts with SPM | Functional Imaging Laboratory, London, UK | www.fil.ion.ucl.ac.uk/spm/dbm.neuro.uni-jena.de/vbm/ |
Reliability across scanner manufacturers and field strengths has been assessed as high e.g. for FreeSurfer (Han et al., 2006, Jovicich et al., 2009, Reuter et al., 2012). Nonetheless, a general recommendation that holds across software methods is to, if possible, avoid mixing scans from different scanners, field strengths, protocols and scanner software versions in the same study, and furthermore to avoid mixing different processing software versions during analyses. This is especially important in longitudinal studies and if these potentially confounding variables are related to age, time point, sex or other study parameters. However, large-scale multi-site studies are a possible exception, given that they provide larger samples than usually possible in single-site studies and, therefore, the possibility to investigate the consistency of effects, and the ability to statistically control for site, hardware or software related variables. But even in such large-scale studies, effort should be devoted to evaluating and adjusting the scanners and sequences used, as for instance done in the Alzheimer's Disease Neuroimaging Initiative (Jack et al., 2008), and extensive analyses of the effects of potentially confounding factors are recommended.
5.3. DTI analysis
DTI indices can be evaluated and analyzed on a voxel-wise basis, across voxels within manually or automatically outlined regions or tracts of interest or e.g. within the white matter skeleton using tract-based spatial statistics (Smith et al., 2006). DTI is most commonly used to investigate white matter in the nervous system. White matter consists largely of organized myelinated neuronal axons necessary for fast, consistent and synchronized flow of information in neural networks and DTI provides information about microstructural properties of these fibers. Further, tractography algorithms can be used to determine whether adjacent voxels are likely to be connected and to find paths through which diffusion is least hindered (Behrens and Jbabdi, 2009). Putative major brain fiber tracts can then be visualized, yielding new possibilities for inferring patterns of anatomical connectivity. Current large scale projects, such as the Human Connectome Project (www.humanconnectome.org), aim to provide comprehensive open-access maps of system-level brain connectivity (Van Essen et al., 2013). Because DTI indices and white matter volumes are only weakly to moderately related, these measures are believed to be differentially sensitive to tissue characteristics and to provide complimentary information (Fjell et al., 2008, Tamnes et al., 2010). Importantly, this means that even though regional DTI measures and white matter volume to some degree are related, great caution should be taken when comparing results from DTI- and volumetric studies, and furthermore that multimodal studies are warranted when investigating e.g. developmental or lifespan changes in white matter. Notably, DTI can also be used to investigate tissue properties in subcortical gray matter structures or the cerebral cortex (see for instance: Douaud et al., 2013, Grydeland et al., 2013, Lebel et al., 2008).
5.4. Quality control
Like all data, structural MRI data requires quality control procedures to reduce noise and guard against spurious findings. One aspect of quality control can occur right after scans are acquired, through visual inspection of the raw images. Visual inspection for gross abnormalities by a trained individual or radiologist is standard for many protocols, and most studies of typical development will remove individuals with any neurological issues. However, visual inspection should also be conducted to identify and document any artifacts due to head motion or scanner peculiarities. Systematic artifacts can bias data and affect results, even in large datasets.
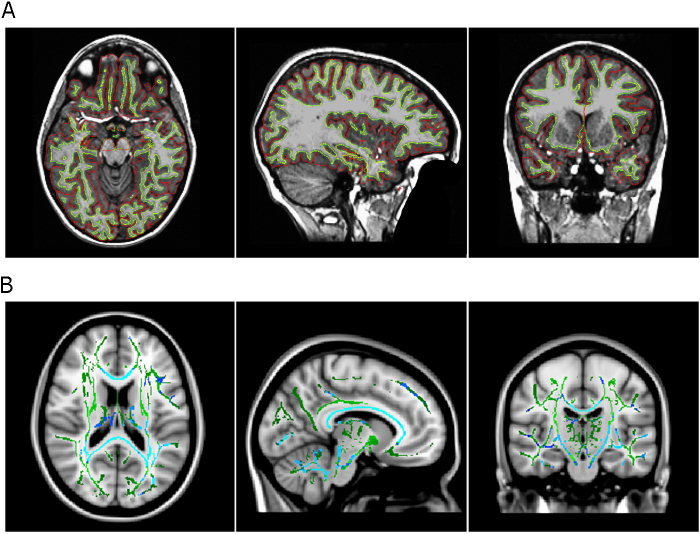
Visual inspection procedures should also be implemented after scans have been processed to ensure that there were no errors in the segmentation or reconstruction processes. Automated methods are susceptible to biases introduced by motion artifact, which for instance might make gray matter volumes appear smaller (Blumenthal et al., 2002). Detailed quality control processes can be found in the documentation of many software programs listed in Table 2. Solely controlling for the quality of images does not guarantee a sample of flawless post-processed brain segmentations or reconstructions. For example, one report indicated that, in a sample of 857 scans that had been rated as high quality from T1 image inspection, 48% were inadequately reconstructed by the imaging analysis software around the anterior temporal lobe, which was detected by post-processing visual inspection (Mills et al., 2013; illustrated in Fig. 4a). Two recent conference presentations addressed potential biases in developmental trajectories due to excessive head motion in younger participants (Alexander-Bloch et al., 2012, Stockman et al., 2012), but this concern has yet to be addressed in detail in published reports. Many studies provide descriptive accounts of their quality control procedures (Dennison et al., 2013, Nguyen et al., 2013, Wierenga et al., 2014), which are becoming increasingly important given the variety of methodologies used in longitudinal brain development studies. Moreover, the field would also benefit from an increased focus on quantitative head motion detection and measurement (Fig. 4b), as well increased use of both prospective and retrospective motion compensation procedures, and the inclusion of such measurement and procedures in commonly used software packages (Yendiki et al., 2014). Motion-related artifacts in developmental studies of structural MRI likely requires a similar level of awareness and consideration as has been shown for functional MRI in the past few years (Fair et al., 2012, Power et al., 2012, Yendiki et al., 2014).
Fig. 4.
Examples of quality control considerations in structural MRI. (A) An illustration of one participant's surface-based cortical reconstruction (using FreeSurfer 5.3). The yellow line indicates the boundary between the white matter and gray matter and the red line indicates the boundary between gray matter and pia mater. This T1 image passed visual inspection with no visible motion artifact, and the majority of the cortex was adequately reconstructed. However, the automated program failed to reconstruct the anterior temporal cortex. (B) Voxel-based analysis of FA vs. motion in a cohort of children 5–12 years old. The white matter skeleton is shown in green, while blue voxels indicate a significant association of FA and rotational motion. Increased motion was associated with decreased FA, and the corpus callosum showed the strongest association. Reprinted with permission from Yendiki et al. (2014).
6. Modeling brain development
In longitudinal designs with multiple time points, simple regression analyses cannot be used because brain measures taken from the same individual across time are not independent of each other. Therefore, statistical methods that take into account the effects of continuous dependent (within-participant) and independent (between-participant) variables are necessary. Complexity is added when we consider the non-linearity of brain development. There are a variety of analysis techniques commonly used for developmental trajectory analysis – some of which go by multiple names, which we discuss briefly below.
Multilevel modeling (also known as: mixed models, mixed-effects models, hierarchical linear models): Multilevel models estimate the fixed effects of a chosen variable (e.g., age, pubertal status) on a measure of interest (e.g., gray matter volume, cortical thickness), while also taking into account the within-participant dependence of observations. This technique has the flexibility to model data that has been collected at uneven intervals, and does not require all participants to have the same number of data points. The models can have fixed or variable intercepts and slopes, depending on the hypothesis. Multilevel models are often used to generate population-level trajectories, but can also be used to compare the developmental trajectories between groups and to examine individual differences. These individual differences can be modeled by including random effects for the intercept and slope of the time variable. In non-linear models, the independent variable is often centered to reduce correlations between the different terms. There have been many books written on this topic, but Singer and Willett's Applied Longitudinal Data Analysis is a particularly relevant guide for multilevel analysis of longitudinal brain imaging data (Singer and Willett, 2003). Various methods exist that perform multilevel modeling, and we have highlighted a few of these in Table 3.
Table 3.
Statistical methods used to analyze longitudinal structural MRI data in a selected group of studies.
| Study | Journal | Method | Software | Measure |
|---|---|---|---|---|
| Giedd et al. (1999) | Prog in Neuro-Psychopharm and Bio Psychiatry. | NLMM | PROC MIXED in SAS | Subcortical volumes |
| Lenroot et al. (2007) | Neuroimage | NLMM | SPSS | Cortical and subcortical volumes |
| Lebel and Beaulieu (2011) | J Neuroscience | LMM | ExploreDTI and Freesurfer | Whole brain, white and gray matter volumes |
| Raznahan et al. (2011) | J Neuroscience | NLMM | nlme in R | Cortical volume, thickness, surface area and gyrification |
| Mills et al. (2013) | SCAN | NLMM | nlme in R | Cortical volume, thickness and surface area |
| Nguyen et al. (2013) | Cereb Cortex | LMM | SurfStat, SPSS | Cortical thickness |
| Mutlu et al. (2013) | Neuroimage | NLMM | Matlab R2012a | Cortical thickness and gyrification |
| Dennison et al. (2013) | Dev Science | HLM | Stata | Subcortical volumes |
| Aubert-Broche et al. (2013) | Neuroimage | NLMM | nlme in R | Cortical and subcortical volumes |
| Ordaz et al. (2013) | J Neuroscience | HLM | HLM Version 6 | fMRI |
| Goddings et al. (2013) | Neuroimage | NLMM | nlme in R | Subcortical volumes |
HLM, Hierarchical Linear Models; LMM, Linear Mixed Models; NLMM, Nonlinear Mixed Models.
Latent Growth Modeling (also known as: latent growth curve analysis, growth mixture modeling, latent variable analysis) is another method used to analyze developmental trajectories with longitudinally acquired data, but is distinguishable from multilevel modeling because it involves structural equation modeling (Hox and Stoel, 2005). Structural equation modeling uses latent variables – unobserved variables that are inferred from measured variables – to account for relationships between observed variables. Unlike multilevel models, latent growth models require participants to have been measured at similar time intervals. This makes latent growth modeling problematic for unstructured longitudinal designs, where participants are scanned at different ages or developmental milestones.
6.1. Physiological plausibility
When choosing growth models for structural brain measures, it is essential to consider the physiological plausibility of the model. This depends on the developmental period, the age-span covered, and the brain measure being examined. For example, it might be physiologically plausible for cortical thickness to decrease almost linearly across adolescence, whereas it might not be plausible across the first decade of life or across adulthood and senescence. The cubic age model that has often been fitted to various cortical brain measures (e.g., gray matter volume) is physiologically plausible in an age range that spans childhood, adolescence and young adulthood; given that cortical gray matter volume tends to be greater in childhood than in adulthood. Quadratic models should work for shorter age spans, where it is not expected for one end of the age span to show relative stability. Linear models might be the best fit for age ranges where steady change is likely. However, if one is interested in more precisely mapping and describing developmental trajectories and in interpreting e.g. exact peaks or break-points, nonparametric local smoothing techniques (e.g. the smoothing spline) are likely to be more accurate, since global fits such as quadratic models may be affected by irrelevant factors, as discussed below.
6.2. Comparing brain developmental trajectories
Previous studies have used a variety of strategies in deciding the best fitting model of a brain measure. Early studies using the NIMH Child Psychiatry Branch sample adopted a step-down model selection procedure to determine if cubic, quadratic and linear age effects best fit the data (for description, see Shaw et al., 2008). Using this technique, the most complex model (i.e., cubic model) is selected if it is a significant fit at p < 0.05. Current statistical procedures suggest using the heuristic of parsimony (i.e., Occam's Razor) in selecting the best model, which means finding a model that explains the most amount of variance using the least number of parameters. This is often achieved by likelihood ratio tests or comparing the Akaike Information Criteria (AIC) values of different models. AIC can be used to compare models that are not nested because it is a standardized measure of the goodness of fit of a chosen model, while penalizing the model for complexity. A lower AIC value reflects a better fit to the data. Final reported values should take into account how much better the selected model is over the null, or baseline, model. For example, a previous study found that cortical thickness in specific areas of the cortex (e.g., temporal pole, occipital pole), did not significantly change between ages 6 and 29 years (Mutlu et al., 2013). Without taking the null model into consideration, a study could potentially report an erroneous developmental effect.
Comparing the developmental trajectories of different brain structures, or the same brain structures between groups, can be difficult. Brain structures that follow different non-linear developmental trajectories are particularly difficult to compare to one another. One strategy is to calculate annualized rate of change or standardized rate of change across brain regions (Goddings et al., 2013, Lenroot et al., 2007, Tamnes et al., 2013a). Using this strategy one can compare the amount of change occurring at different age periods for different brain regions.
Many studies are interested in comparing developmental trajectories between groups, such as females and males or clinical groups and controls. However, if the compared groups follow different developmental patterns (e.g., quadratic and cubic) for the measurement of interest then it is not easy to statistically determine the difference. Some studies reporting inverted-U shaped trajectories have calculated the age at reaching the “peak” of a certain measure by solving the first-order derivative of the growth trajectory equation. While this is an attractive method of calculating a comparable value, the inflection point (or “peak”) of a growth model is sensitive to potential biases, including the age range of sample, the selected model, and any measurement error (Fjell et al., 2010). In addition, these peak ages have often been reported without confidence intervals, without which small differences might be exaggerated.
6.3. Correcting brain measures
Some developmental studies comparing brain structure differences longitudinally have corrected or controlled for total brain size (Dennison et al., 2013, Nguyen et al., 2013), whereas others have not performed any type of such correction (Aubert-Broche et al., 2013, Goddings et al., 2013, Mills et al., 2013, Wierenga et al., 2014). Rather than correcting or controlling for individual variability in intracranial volume – a composite of not only brain tissue (∼80%), but also blood (∼10%) and CSF (∼10%) (Rengachary and Ellenbogen, 2005), many have opted to correct for whole brain volume. However, such correction in developmental samples might be problematic given that whole brain volume increases until around the age of 13 (Hedman et al., 2012). In addition, given that different components of the brain develop at different rates, controlling for whole brain volume could potentially bias results (Barnes et al., 2010). The impact of correcting or controlling for whole brain volume in longitudinal developmental samples has not yet been systematically studied. If corrections are performed, it is therefore useful to also report the uncorrected results and the developmental effects on the measure used for correction.
7. The benefits of longitudinal designs
In this review, we focus on the methods and results from longitudinal analyses of structural MRI data. Note however that most of the reviewed studies have employed accelerated longitudinal designs to allow for investigation of wider age ranges. There have also been a number of cross-sectional studies investigating structural brain development that use large samples (Brain Development Cooperative Group, 2012, Koolschijn and Crone, 2013, Østby et al., 2009). However, longitudinal samples require far fewer participants than cross-sectional studies in order to detect small differences in brain structure (Steen et al., 2007). For example, a sample size of at least 146 participants is necessary to have adequate power to detect a 5% difference in whole brain volume between groups in a cross-sectional design, whereas only 4 participants are required to detect changes of similar magnitude in a longitudinal design (Steen et al., 2007). Cross-sectional studies require many more participants because comparative differences are affected by both measurement precision and natural variation in brain sizes – a proportion of which will not likely be relevant. However, measurement precision is the only factor that can affect the required sample size necessary to detect subtle differences in longitudinal studies. Furthermore, there are also a number of other challenges involved in drawing inferences about developmental processes from cross-sectional studies (Kraemer et al., 2000).
7.1. Inter-individual variability
Individuals vary substantially in brain size. Studies of adults have reported wide variability in whole brain volume, ranging from 974.9 to 1498.5 cm3 (Allen et al., 2002), and 783 to 1414 mL (Steen et al., 2007). These figures suggest that whole brain volume can vary by up to 81% across adults. As would be expected, this variability in whole brain volume is also observable for specific tissue types and other measures of brain morphometry. A study of 486 individuals aged 26–85 years showed wide variability between participants for both gray matter volume and surface area (Winkler et al., 2010). Similarly wide ranges can also be observed for regional volumes and in developmental samples where the raw data are either reported or visualized. It is this degree of individual variability that makes longitudinal designs imperative for describing developmental trajectories. Articles that only report group averages or graph best-fitting models miss the opportunity to reveal this incredible diversity. Presenting the raw values of individual brain measurements is crucial to convey the degree of overlap that can exist between groups and across development.
7.2. Intra-individual variability
How much can we reasonably expect an individual's brain structure to change? The answer to this question will depend on the age period studied, the time interval between scans, as well as what is being measured. Most longitudinal studies that describe the amount of structural change that has occurred over a period of time will do so only on a group level, and some of the few that report change across individuals are studies comparing developmental changes in brain structure with cognitive performance (Schnack et al., 2014, Shaw et al., 2006, Tamnes et al., 2013b, Urošević et al., 2012, Vijayakumar et al., 2013; for a recent review see Walhovd et al., 2014). These studies reveal substantial individual variability in change in brain structures across development. However, studies that correlate developmental changes in brain structure with developmental changes in cognitive performance are unable to quantify what amount of structural change is due to developmental events unrelated to cognitive capacities. However, it might be that we will never be able to fully disentangle these factors. One recent functional MRI study has gone further than reporting individual differences in rates of change by comparing individual differences in longitudinal growth curves with performance on an inhibitory control task (Ordaz et al., 2013). This technique (extensively described in their methods section) will become more feasible for future investigations as datasets grow larger and gain more waves of data.
7.3. Considerations for large datasets
Longitudinal studies are costly, and often involve the collection of large amounts of non-imaging data to relate to brain measures. These rich datasets have the capability to describe how brain development relates to biological measures such as genes, hormones, and prenatal measurements, as well as to measures of behavior, cognitive development, and well-being. However, the large number of possible tests that could be run in large datasets make them vulnerable to practices that could bias our knowledge (Ioannidis et al., 2014). We can guard against this possibility by reporting all tests that were conducted, including tests that failed to find a relationships between measures (Simmons et al., 2011). Researchers in epidemiology have emphasized the need for specific hypotheses even in large datasets: “Developing large national cohorts without attention to specific hypotheses is inefficient, will fail to address many associations with high-quality data, and may well produce spurious results” (Kuller et al., 2013). It is therefore important for future studies to make transparent which tests are exploratory, and which are hypothesis driven (Miguel et al., 2014).
8. Conclusion
In this review, we have highlighted a number of potential issues and choices that researchers examining structural brain development using longitudinal designs might encounter. Choices in regard to measurements, processing, analysis and modeling may affect results and interpretations of longitudinal brain imaging studies. We hope that this review will help guide future studies and open the discussion amongst researchers regarding best practices.
Conflict of interest statement
There are no conflicts of interest to report.
Funding
KLM is supported by the National Institute of Mental Health Intramural Research program and the NIH Graduate Partnership Program. CKT is supported by the Department of Psychology, University of Oslo.
Acknowledgement
We thank Dr. Anne-Lise Goddings for providing the initial inspiration for this review, as well as for many helpful conversations on the topic.
References
- Alemán-Gómez Y., Janssen J., Schnack H., Balaban E., Pina-Camacho L., Alfaro-Almagro F., Castro-Fornieles J., Otero S., Baeza I., Moreno D., Bargalló N., Parellada M., Arango C., Desco M. The human cerebral cortex flattens during adolescence. J. Neurosci. Off. J. Soc. Neurosci. 2013;33:15004–15010. doi: 10.1523/JNEUROSCI.1459-13.2013. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Alexander-Bloch A., Stockman M., Clasen L.S., Lalonde F., Raznahan A., Giedd J.N. Society for Neuroscience Annual Meeting; New Orleans, LA: 2012. In-scanner Motion Biases Automated Measures of Structural MRI Brain Morphometry. [Google Scholar]
- Allen J.S., Damasio H., Grabowski T.J. Normal neuroanatomical variation in the human brain: an MRI-volumetric study. Am. J. Phys. Anthropol. 2002;118:341–358. doi: 10.1002/ajpa.10092. [DOI] [PubMed] [Google Scholar]
- Ankney C.D. Sex differences in relative brain size: the mismeasure of woman, too? Intelligence. 1992;16:329–336. [Google Scholar]
- Ashburner J., Friston K.J. Voxel-based morphometry—the methods. NeuroImage. 2000;11:805–821. doi: 10.1006/nimg.2000.0582. [DOI] [PubMed] [Google Scholar]
- Aubert-Broche B., Fonov V., García-Lorenzo D., Mouiha A., Guizard N., Coupé P., Eskildsen S.F., Collins D.L. A new method for structural volume analysis of longitudinal brain MRI data and its application in studying the growth trajectories of anatomical brain structures in childhood. NeuroImage. 2013 doi: 10.1016/j.neuroimage.2013.05.065. [DOI] [PubMed] [Google Scholar]
- Azevedo F.A.C., Carvalho L.R.B., Grinberg L.T., Farfel J.M., Ferretti R.E.L., Leite R.E.P., Jacob Filho W., Lent R., Herculano-Houzel S. Equal numbers of neuronal and nonneuronal cells make the human brain an isometrically scaled-up primate brain. J. Comp. Neurol. 2009;513:532–541. doi: 10.1002/cne.21974. [DOI] [PubMed] [Google Scholar]
- Barnes J., Ridgway G.R., Bartlett J., Henley S.M.D., Lehmann M., Hobbs N., Clarkson M.J., MacManus D.G., Ourselin S., Fox N.C. Head size, age and gender adjustment in MRI studies: a necessary nuisance? NeuroImage. 2010;53:1244–1255. doi: 10.1016/j.neuroimage.2010.06.025. [DOI] [PubMed] [Google Scholar]
- Bava S., Thayer R., Jacobus J., Ward M., Jernigan T.L., Tapert S.F. Longitudinal characterization of white matter maturation during adolescence. Brain Res. 2010;1327:38–46. doi: 10.1016/j.brainres.2010.02.066. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Beaulieu C. The basis of anisotropic water diffusion in the nervous system – a technical review. NMR Biomed. 2002;15:435–455. doi: 10.1002/nbm.782. [DOI] [PubMed] [Google Scholar]
- Beaulieu C. CHAPTER 6 – the biological basis of diffusion anisotropy. In: Heidi Johansen-Berg, Timothy E.J., Behrens, editors. Diffusion MRI. Academic Press; San Diego: 2009. pp. 105–126. [Google Scholar]
- Behrens T.E.J., Jbabdi S. Chapter 15 – MR diffusion tractography. In: Heidi Johansen-Berg, Timothy E.J., Behrens, editors. Diffusion MRI. Academic Press; San Diego: 2009. pp. 333–351. [Google Scholar]
- Benes F. Myelination of cortical-hippocampal relays during late adolescence. Schizophr. Bull. 1989;15:585–593. doi: 10.1093/schbul/15.4.585. [DOI] [PubMed] [Google Scholar]
- Benes F., Turtle M., Khan Y., Farol P. Myelination of a key relay zone in the hippocampal formation occurs in the human brain during childhood, adolescence, and adulthood. Arch. Gen. Psychiatry. 1994;51:477–484. doi: 10.1001/archpsyc.1994.03950060041004. [DOI] [PubMed] [Google Scholar]
- Blakemore S.-J. The social brain in adolescence. Nat. Rev. Neurosci. 2008;9:267–277. doi: 10.1038/nrn2353. [DOI] [PubMed] [Google Scholar]
- Blokland G.A.M., de Zubicaray G.I., McMahon K.L., Wright M.J. Genetic and environmental influences on neuroimaging phenotypes: a meta-analytical perspective on twin imaging studies. Twin Res. Hum. Genet. Off. J. Int. Soc. Twin Stud. 2012;15:351–371. doi: 10.1017/thg.2012.11. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Blumenthal J.D., Zijdenbos A., Molloy E., Giedd J.N. Motion artifact in magnetic resonance imaging: implications for automated analysis. NeuroImage. 2002;16:89–92. doi: 10.1006/nimg.2002.1076. [DOI] [PubMed] [Google Scholar]
- Bordini B., Rosenfield R.L. Normal pubertal development: Part II: Clinical aspects of puberty. Pediatr. Rev. 2011;32:281–292. doi: 10.1542/pir.32-7-281. [DOI] [PubMed] [Google Scholar]
- Bourgeois J.P., Rakic P. Changes of synaptic density in the primary visual cortex of the macaque monkey from fetal to adult stage. J. Neurosci. Off. J. Soc. Neurosci. 1993;13:2801–2820. doi: 10.1523/JNEUROSCI.13-07-02801.1993. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Brain Development Cooperative Group Total and regional brain volumes in a population-based normative sample from 4 to 18 years: the NIH MRI Study of Normal Brain Development. Cereb. Cortex N. Y. N 1991. 2012;22:1–12. doi: 10.1093/cercor/bhr018. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Braitenberg V. Brain size and number of neurons: an exercise in synthetic neuroanatomy. J. Comput. Neurosci. 2001;10:71–77. doi: 10.1023/a:1008920127052. [DOI] [PubMed] [Google Scholar]
- Brouwer R.M., Mandl R.C.W., Schnack H.G., van Soelen I.L.C., van Baal G.C., Peper J.S., Kahn R.S., Boomsma D.I., Hulshoff Pol H.E. White matter development in early puberty: a longitudinal volumetric and diffusion tensor imaging twin study. PloS ONE. 2012;7:e32316. doi: 10.1371/journal.pone.0032316. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Brown T.T., Kuperman J.M., Chung Y., Erhart M., McCabe C., Hagler D.J., Jr., Venkatraman V.K., Akshoomoff N., Amaral D.G., Bloss C.S., Casey B.J., Chang L., Ernst T.M., Frazier J.A., Gruen J.R., Kaufmann W.E., Kenet T., Kennedy D.N., Murray S.S., Sowell E.R., Jernigan T.L., Dale A.M. Neuroanatomical assessment of biological maturity. Curr. Biol. CB. 2012;22:1693–1698. doi: 10.1016/j.cub.2012.07.002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chiang M.-C., Barysheva M., Shattuck D.W., Lee A.D., Madsen S.K., Avedissian C., Klunder A.D., Toga A.W., McMahon K.L., de Zubicaray G.I., Wright M.J., Srivastava A., Balov N., Thompson P.M. Genetics of brain fiber architecture and intellectual performance. J. Neurosci. Off. J. Soc. Neurosci. 2009;29:2212–2224. doi: 10.1523/JNEUROSCI.4184-08.2009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Colby J.B., Van Horn J.D., Sowell E.R. Quantitative in vivo evidence for broad regional gradients in the timing of white matter maturation during adolescence. NeuroImage. 2011;54:25–31. doi: 10.1016/j.neuroimage.2010.08.014. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Concha L., Livy D.J., Beaulieu C., Wheatley B.M., Gross D.W. In vivo diffusion tensor imaging and histopathology of the fimbria-fornix in temporal lobe epilepsy. J. Neurosci. Off. J. Soc. Neurosci. 2010;30:996–1002. doi: 10.1523/JNEUROSCI.1619-09.2010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cox R.W. AFNI: software for analysis and visualization of functional magnetic resonance neuroimages. Comput. Biomed. Res, Int. J. 1996;29:162–173. doi: 10.1006/cbmr.1996.0014. [DOI] [PubMed] [Google Scholar]
- Cox R.W., Hyde J.S. Software tools for analysis and visualization of fMRI data. NMR in Biomedicine. 1997;10:171–178. doi: 10.1002/(sici)1099-1492(199706/08)10:4/5<171::aid-nbm453>3.0.co;2-l. [DOI] [PubMed] [Google Scholar]
- Dale A.M., Fischl B., Sereno M.I. Cortical Surface-Based Analysis: I. Segmentation and Surface Reconstruction. NeuroImage. 1999;9:179–194. doi: 10.1006/nimg.1998.0395. [DOI] [PubMed] [Google Scholar]
- Dekaban A.S., Sadowsky D. Changes in brain weights during the span of human life: relation of brain weights to body heights and body weights. Ann. Neurol. 1978;4:345–356. doi: 10.1002/ana.410040410. [DOI] [PubMed] [Google Scholar]
- Dennison M., Whittle S., Yücel M., Vijayakumar N., Kline A., Simmons J., Allen N.B. Mapping subcortical brain maturation during adolescence: evidence of hemisphere- and sex-specific longitudinal changes. Dev. Sci. 2013;16:772–791. doi: 10.1111/desc.12057. [DOI] [PubMed] [Google Scholar]
- Douaud G., Menke R.A.L., Gass A., Monsch A.U., Rao A., Whitcher B., Zamboni G., Matthews P.M., Sollberger M., Smith S. Brain microstructure reveals early abnormalities more than two years prior to clinical progression from mild cognitive impairment to Alzheimer's disease. J. Neurosci. Off. J. Soc. Neurosci. 2013;33:2147–2155. doi: 10.1523/JNEUROSCI.4437-12.2013. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dubois J., Dehaene-Lambertz G., Perrin M., Mangin J.-F., Cointepas Y., Duchesnay E., Le Bihan D., Hertz-Pannier L. Asynchrony of the early maturation of white matter bundles in healthy infants: quantitative landmarks revealed noninvasively by diffusion tensor imaging. Hum. Brain Mapp. 2008;29:14–27. doi: 10.1002/hbm.20363. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Engvig A., Fjell A.M., Westlye L.T., Moberget T., Sundseth Ø., Larsen V.A., Walhovd K.B. Memory training impacts short-term changes in aging white matter: a longitudinal diffusion tensor imaging study. Hum. Brain Mapp. 2012;33:2390–2406. doi: 10.1002/hbm.21370. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fair D.A., Nigg J.T., Iyer S., Bathula D., Mills K.L., Dosenbach N.U.F., Schlaggar B.L., Mennes M., Gutman D., Bangaru S., Buitelaar J.K., Dickstein D.P., Di Martino A., Kennedy D.N., Kelly C., Luna B., Schweitzer J.B., Velanova K., Wang Y.-F., Mostofsky S., Castellanos F.X., Milham M.P. Distinct neural signatures detected for ADHD subtypes after controlling for micro-movements in resting state functional connectivity MRI data. Front. Syst. Neurosci. 2012;6:80. doi: 10.3389/fnsys.2012.00080. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fedorov A., Beichel R., Kalpathy-Cramer J., Finet J., Fillion-Robin J.-C., Pujol S., Kikinis R. 3D Slicer as an image computing platform for the Quantitative Imaging Network. Magn. Reson. Imaging. 2012;30:1323–1341. doi: 10.1016/j.mri.2012.05.001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fischl B., Sereno M.I., Dale A.M. Cortical surface-based analysis II: Inflation, flattening, and a surface-based coordinate system. Neuroimage. 1999;9:195–207. doi: 10.1006/nimg.1998.0396. [DOI] [PubMed] [Google Scholar]
- Fjell A.M., Walhovd K.B., Westlye L.T., Østby Y., Tamnes C.K., Jernigan T.L., Gamst A., Dale A.M. When does brain aging accelerate? Dangers of quadratic fits in cross-sectional studies. NeuroImage. 2010;50:1376–1383. doi: 10.1016/j.neuroimage.2010.01.061. [DOI] [PubMed] [Google Scholar]
- Fjell A.M., Westlye L.T., Amlien I., Tamnes C.K., Grydeland H., Engvig A., Espeseth T., Reinvang I., Lundervold A.J., Lundervold A., Walhovd K.B. High-expanding cortical regions in human development and evolution are related to higher intellectual abilities. Cereb. Cortex N. Y. N 1991. 2013 doi: 10.1093/cercor/bht201. [DOI] [PubMed] [Google Scholar]
- Fjell A.M., Westlye L.T., Greve D.N., Fischl B., Benner T., van der Kouwe A.J.W., Salat D., Bjørnerud A., Due-Tønnessen P., Walhovd K.B. The relationship between diffusion tensor imaging and volumetry as measures of white matter properties. NeuroImage. 2008;42:1654–1668. doi: 10.1016/j.neuroimage.2008.06.005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Forstmeier W. Women have relatively larger brains than men: a comment on the misuse of general linear models in the study of sexual dimorphism. 2007. Anat. Rec. 2011;294:1856–1863. doi: 10.1002/ar.21423. [DOI] [PubMed] [Google Scholar]
- Gallagher A. Stature, body mass, and brain size: a two-million-year odyssey. Econ. Hum. Biol. 2013 doi: 10.1016/j.ehb.2012.12.003. [DOI] [PubMed] [Google Scholar]
- Geng X., Gouttard S., Sharma A., Gu H., Styner M., Lin W., Gerig G., Gilmore J.H. Quantitative tract-based white matter development from birth to age 2 years. NeuroImage. 2012;61:542–557. doi: 10.1016/j.neuroimage.2012.03.057. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ghosh S.S., Kakunoori S., Augustinack J., Nieto-Castanon A., Kovelman I., Gaab N., Christodoulou J.A., Triantafyllou C., Gabrieli J.D.E., Fischl B. Evaluating the validity of volume-based and surface-based brain image registration for developmental cognitive neuroscience studies in children 4 to 11 years of age. NeuroImage. 2010;53:85–93. doi: 10.1016/j.neuroimage.2010.05.075. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Giedd J.N., Blumenthal J., Jeffries N.O., Castellanos F.X., Liu H., Zijdenbos A., Paus T., Evans A.C., Rapoport J.L. Brain development during childhood and adolescence: a longitudinal MRI study. Nat. Neurosci. 1999;2:861–863. doi: 10.1038/13158. [DOI] [PubMed] [Google Scholar]
- Goddings A.-L., Mills K.L., Clasen L.S., Giedd J.N., Viner R.M., Blakemore S.-J. The influence of puberty on subcortical brain development. NeuroImage. 2013 doi: 10.1016/j.neuroimage.2013.09.073. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Grydeland H., Walhovd K.B., Tamnes C.K., Westlye L.T., Fjell A.M. Intracortical myelin links with performance variability across the human lifespan: results from T1- and T2-weighted MRI myelin mapping and diffusion tensor imaging. J. Neurosci. Off. J. Soc. Neurosci. 2013;33:18618–18630. doi: 10.1523/JNEUROSCI.2811-13.2013. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Han X., Jovicich J., Salat D., van der Kouwe A., Quinn B., Czanner S., Busa E., Pacheco J., Albert M., Killiany R., Maguire P., Rosas D., Makris N., Dale A., Dickerson B., Fischl B. Reliability of MRI-derived measurements of human cerebral cortical thickness: the effects of field strength, scanner upgrade and manufacturer. NeuroImage. 2006;32:180–194. doi: 10.1016/j.neuroimage.2006.02.051. [DOI] [PubMed] [Google Scholar]
- Hanson J.L., Suh J.W., Nacewicz B.M., Sutterer M.J., Cayo A.A., Stodola D.E., Burghy C.A., Wang H., Avants B.B., Yushkevich P.A., Essex M.J., Pollak S.D., Davidson R.J. Robust automated amygdala segmentation via multi-atlas diffeomorphic registration. Front. Neurosci. 2012;6:166. doi: 10.3389/fnins.2012.00166. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Harper C., Kril J., Raven D., Jones N. Intracranial cavity volumes: a new method and its potential applications. Neuropathol. Appl. Neurobiol. 1984;10:25–32. doi: 10.1111/j.1365-2990.1984.tb00337.x. [DOI] [PubMed] [Google Scholar]
- Hedman A.M., van Haren N.E.M., Schnack H.G., Kahn R.S., Hulshoff Pol H.E. Human brain changes across the life span: a review of 56 longitudinal magnetic resonance imaging studies. Hum. Brain Mapp. 2012;33:1987–2002. doi: 10.1002/hbm.21334. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Herculano-Houzel S. The human brain in numbers: a linearly scaled-up primate brain. Front. Hum. Neurosci. 2009;3:31. doi: 10.3389/neuro.09.031.2009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Herculano-Houzel S., Collins C.E., Wong P., Kaas J.H. Cellular scaling rules for primate brains. Proc. Natl. Acad. Sci. 2007;104:3562–3567. doi: 10.1073/pnas.0611396104. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hermoye L., Saint-Martin C., Cosnard G., Lee S.-K., Kim J., Nassogne M.-C., Menten R., Clapuyt P., Donohue P.K., Hua K., Wakana S., Jiang H., van Zijl P.C.M., Mori S. Pediatric diffusion tensor imaging: normal database and observation of the white matter maturation in early childhood. NeuroImage. 2006;29:493–504. doi: 10.1016/j.neuroimage.2005.08.017. [DOI] [PubMed] [Google Scholar]
- Hill J., Inder T., Neil J., Dierker D., Harwell J., Van Essen D. Similar patterns of cortical expansion during human development and evolution. Proc. Natl. Acad. Sci. U. S. A. 2010;107:13135–13140. doi: 10.1073/pnas.1001229107. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Holland D., Dale A.M. Nonlinear registration of longitudinal images and measurement of change in regions of interest. Med. Image Anal. 2011;15:489–497. doi: 10.1016/j.media.2011.02.005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hox J.J., Stoel R.D. Vol. 3. 2005. pp. 1296–1305. (Multilevel and sem approaches to growth curve modeling). [Google Scholar]
- Huttenlocher P.R. Synaptic density in human frontal cortex—developmental changes and effects of aging. Brain Res. 1979;163:195–205. doi: 10.1016/0006-8993(79)90349-4. [DOI] [PubMed] [Google Scholar]
- Huttenlocher P.R. Harvard University Press; USA: 2002. Neural Plasticity. [Google Scholar]
- Huttenlocher P.R., Dabholkar A.S. Regional differences in synaptogenesis in human cerebral cortex. J. Comp. Neurol. 1997;387:167–178. doi: 10.1002/(sici)1096-9861(19971020)387:2<167::aid-cne1>3.0.co;2-z. [DOI] [PubMed] [Google Scholar]
- Ioannidis J.P.A., Munafò M.R., Fusar-Poli P., Nosek B.A., David S.P. Publication and other reporting biases in cognitive sciences: detection, prevalence, and prevention. Trends Cogn. Sci. 2014 doi: 10.1016/j.tics.2014.02.010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jack C.R., Jr., Bernstein M.A., Fox N.C., Thompson P., Alexander G., Harvey D., Borowski B., Britson P.J.L., Whitwell J., Ward C., Dale A.M., Felmlee J.P., Gunter J.L., Hill D.L.G., Killiany R., Schuff N., Fox-Bosetti S., Lin C., Studholme C., DeCarli C.S., Krueger G., Ward H.A., Metzger G.J., Scott K.T., Mallozzi R., Blezek D., Levy J., Debbins J.P., Fleisher A.S., Albert M., Green R., Bartzokis G., Glover G., Mugler J., Weiner M.W. The Alzheimer's Disease Neuroimaging Initiative (ADNI): MRI methods. J. Magn. Reson. Imaging. 2008;27:685–691. doi: 10.1002/jmri.21049. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jenkinson M., Beckmann C.F., Behrens T.E.J., Woolrich M.W., Smith S.M. FSL. NeuroImage. 2012;62:782–790. doi: 10.1016/j.neuroimage.2011.09.015. [DOI] [PubMed] [Google Scholar]
- Johansen-Berg H. Behavioural relevance of variation in white matter microstructure. Curr. Opin. Neurol. 2010;23:351–358. doi: 10.1097/WCO.0b013e32833b7631. [DOI] [PubMed] [Google Scholar]
- Johansen-Berg H., Behrens T.E.J. Academic Press; USA: 2009. Diffusion MRI: From Quantitative Measurement to In-vivo Neuroanatomy. [Google Scholar]
- Jovicich J., Czanner S., Han X., Salat D., van der Kouwe A., Quinn B., Pacheco J., Albert M., Killiany R., Blacker D., Maguire P., Rosas D., Makris N., Gollub R., Dale A., Dickerson B.C., Fischl B. MRI-derived measurements of human subcortical, ventricular and intracranial brain volumes: reliability effects of scan sessions, acquisition sequences, data analyses, scanner upgrade, scanner vendors and field strengths. NeuroImage. 2009;46:177–192. doi: 10.1016/j.neuroimage.2009.02.010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kanai R., Rees G. The structural basis of inter-individual differences in human behaviour and cognition. Nat. Rev. Neurosci. 2011;12:231–242. doi: 10.1038/nrn3000. [DOI] [PubMed] [Google Scholar]
- Keller T.A., Just M.A. Altering cortical connectivity: remediation-induced changes in the white matter of poor readers. Neuron. 2009;64:624–631. doi: 10.1016/j.neuron.2009.10.018. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Koolschijn P.C.M.P., Crone E.A. Sex differences and structural brain maturation from childhood to early adulthood. Dev. Cogn. Neurosci. 2013;5:106–118. doi: 10.1016/j.dcn.2013.02.003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kraemer H.C., Yesavage J.A., Taylor J.L., Kupfer D. How can we learn about developmental processes from cross-sectional studies, or can we? Am. J. Psychiatry. 2000;157:163–171. doi: 10.1176/appi.ajp.157.2.163. [DOI] [PubMed] [Google Scholar]
- Kuller L.H., Bracken M.B., Ogino S., Prentice R.L., Tracy R.P. The role of epidemiology in the era of molecular epidemiology and genomics: summary of the 2013 AJE-sponsored Society of Epidemiologic Research Symposium. Am. J. Epidemiol. 2013;178:1350–1354. doi: 10.1093/aje/kwt239. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kuperberg G.R., Broome M.R., McGuire P.K., David A.S., Eddy M., Ozawa F., Goff D., West W.C., Williams S.C.R., van der Kouwe A.J.W., Salat D.H., Dale A.M., Fischl B. Regionally localized thinning of the cerebral cortex in schizophrenia. Arch. Gen. Psychiatry. 2003;60:878–888. doi: 10.1001/archpsyc.60.9.878. [DOI] [PubMed] [Google Scholar]
- Le Bihan D., Johansen-Berg H. Diffusion MRI at 25: exporing brain tissue structure and function. NeuroImage. 2012;61:324–341. doi: 10.1016/j.neuroimage.2011.11.006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lebel C., Beaulieu C. Longitudinal development of human brain wiring continues from childhood into adulthood. J. Neurosci. Off. J. Soc. Neurosci. 2011;31:10937–10947. doi: 10.1523/JNEUROSCI.5302-10.2011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lebel C., Gee M., Camicioli R., Wieler M., Martin W., Beaulieu C. Diffusion tensor imaging of white matter tract evolution over the lifespan. NeuroImage. 2012;60:340–352. doi: 10.1016/j.neuroimage.2011.11.094. [DOI] [PubMed] [Google Scholar]
- Lebel C., Walker L., Leemans A., Phillips L., Beaulieu C. Microstructural maturation of the human brain from childhood to adulthood. NeuroImage. 2008;40:1044–1055. doi: 10.1016/j.neuroimage.2007.12.053. [DOI] [PubMed] [Google Scholar]
- Leemans A., Jeurissen B., Sijbers J., Jones D. Presented at the Proc. of the 17th Annual Meeting of Intl Soc Mag Reson Med. 2009. ExploreDTI: A Graphical Toolbox for Processing Analyzing, and Visualizing Diffusion MR Data. [Google Scholar]
- Lenroot R.K., Gogtay N., Greenstein D.K., Wells E.M., Wallace G.L., Clasen L.S., Blumenthal J.D., Lerch J., Zijdenbos A.P., Evans A.C., Thompson P.M., Giedd J.N. Sexual dimorphism of brain developmental trajectories during childhood and adolescence. NeuroImage. 2007;36:1065–1073. doi: 10.1016/j.neuroimage.2007.03.053. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lerch J.P., Yiu A.P., Martinez-Canabal A., Pekar T., Bohbot V.D., Frankland P.W., Henkelman R.M., Josselyn S.A., Sled J.G. Maze training in mice induces MRI-detectable brain shape changes specific to the type of learning. NeuroImage. 2011;54:2086–2095. doi: 10.1016/j.neuroimage.2010.09.086. [DOI] [PubMed] [Google Scholar]
- Lövdén M., Bodammer N.C., Kühn S., Kaufmann J., Schütze H., Tempelmann C., Heinze H.-J., Düzel E., Schmiedek F., Lindenberger U. Experience-dependent plasticity of white-matter microstructure extends into old age. Neuropsychologia. 2010;48:3878–3883. doi: 10.1016/j.neuropsychologia.2010.08.026. [DOI] [PubMed] [Google Scholar]
- McKay D.R., Knowles E.E.M., Winkler A.A.M., Sprooten E., Kochunov P., Olvera R.L., Curran J.E., Kent J.W., Jr., Carless M.A., Göring H.H.H., Dyer T.D., Duggirala R., Almasy L., Fox P.T., Blangero J., Glahn D.C. Influence of age, sex and genetic factors on the human brain. Brain Imaging Behav. 2013:1–10. doi: 10.1007/s11682-013-9277-5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Miguel E., Camerer C., Casey K., Cohen J., Esterling K.M., Gerber A., Glennerster R., Green D.P., Humphreys M., Imbens G., Laitin D., Madon T., Nelson L., Nosek B.A., Petersen M., Sedlmayr R., Simmons J.P., Simonsohn U., Van der Laan M. Social science. Promoting transparency in social science research. Science. 2014;343:30–31. doi: 10.1126/science.1245317. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mills K.L., Lalonde F., Clasen L.S., Giedd J.N., Blakemore S.-J. Developmental changes in the structure of the social brain in late childhood and adolescence. Soc. Cogn. Affect. Neurosci. 2013 doi: 10.1093/scan/nss113. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Morey R.A., Petty C.M., Xu Y., Hayes J.P., Wagner H.R., 2nd, Lewis D.V., LaBar K.S., Styner M., McCarthy G. A comparison of automated segmentation and manual tracing for quantifying hippocampal and amygdala volumes. NeuroImage. 2009;45:855–866. doi: 10.1016/j.neuroimage.2008.12.033. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Morey R.A., Selgrade E.S., Wagner H.R., 2nd, Huettel S.A., Wang L., McCarthy G. Scan-rescan reliability of subcortical brain volumes derived from automated segmentation. Hum. Brain Mapp. 2010;31:1751–1762. doi: 10.1002/hbm.20973. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mori S. Elsevier Science; The Netherlands: 2007. Introduction to Diffusion Tensor Imaging. [Google Scholar]
- Mutlu A.K., Schneider M., Debbané M., Badoud D., Eliez S., Schaer M. Sex differences in thickness, and folding developments throughout the cortex. NeuroImage. 2013;82:200–207. doi: 10.1016/j.neuroimage.2013.05.076. [DOI] [PubMed] [Google Scholar]
- Nguyen T.-V., McCracken J., Ducharme S., Botteron K.N., Mahabir M., Johnson W., Israel M., Evans A.C., Karama S., Brain Development Cooperative Group Testosterone-related cortical maturation across childhood and adolescence. Cereb. Cortex N. Y. N 1991. 2013;23:1424–1432. doi: 10.1093/cercor/bhs125. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ojeda S.R., Lomniczi A. Puberty in 2013: unravelling the mystery of puberty. Nat. Rev. Endocrinol. 2013 doi: 10.1038/nrendo.2013.233. (advance online publication) [DOI] [PubMed] [Google Scholar]
- Ordaz S.J., Foran W., Velanova K., Luna B. Longitudinal growth curves of brain function underlying inhibitory control through adolescence. J. Neurosci. Off. J. Soc. Neurosci. 2013;33:18109–18124. doi: 10.1523/JNEUROSCI.1741-13.2013. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Østby Y., Tamnes C.K., Fjell A.M., Westlye L.T., Due-Tønnessen P., Walhovd K.B. Heterogeneity in subcortical brain development: a structural magnetic resonance imaging study of brain maturation from 8 to 30 years. J. Neurosci. Off. J. Soc. Neurosci. 2009;29:11772–11782. doi: 10.1523/JNEUROSCI.1242-09.2009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Parent A.-S., Teilmann G., Juul A., Skakkebaek N.E., Toppari J., Bourguignon J.-P. The timing of normal puberty and the age limits of sexual precocity: variations around the world, secular trends, and changes after migration. Endocr. Rev. 2003;24:668–693. doi: 10.1210/er.2002-0019. [DOI] [PubMed] [Google Scholar]
- Paus T. Growth of white matter in the adolescent brain: myelin or axon? Brain Cogn. 2010;72:26–35. doi: 10.1016/j.bandc.2009.06.002. [DOI] [PubMed] [Google Scholar]
- Paus T. How environment and genes shape the adolescent brain. Horm. Behav. 2013 doi: 10.1016/j.yhbeh.2013.04.004. [DOI] [PubMed] [Google Scholar]
- Paus T., Keshavan M., Giedd J.N. Why do many psychiatric disorders emerge during adolescence? Nat. Rev. Neurosci. 2008;9:947–957. doi: 10.1038/nrn2513. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Petanjek Z., Judaš M., Šimic G., Rasin M.R., Uylings H.B.M., Rakic P., Kostovic I. Extraordinary neoteny of synaptic spines in the human prefrontal cortex. Proc. Natl. Acad. Sci. U. S. A. 2011;108:13281–13286. doi: 10.1073/pnas.1105108108. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Peters B.D., Szeszko P.R., Radua J., Ikuta T., Gruner P., DeRosse P., Zhang J.-P., Giorgio A., Qiu D., Tapert S.F., Brauer J., Asato M.R., Khong P.L., James A.C., Gallego J.A., Malhotra A.K. White matter development in adolescence: diffusion tensor imaging and meta-analytic results. Schizophr. Bull. 2012;38:1308–1317. doi: 10.1093/schbul/sbs054. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Power J.D., Barnes K.A., Snyder A.Z., Schlaggar B.L., Petersen S.E. Spurious but systematic correlations in functional connectivity MRI networks arise from subject motion. NeuroImage. 2012;59:2142–2154. doi: 10.1016/j.neuroimage.2011.10.018. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Raznahan A., Shaw P., Lalonde F., Stockman M., Wallace G.L., Greenstein D., Clasen L., Gogtay N., Giedd J.N. How does your cortex grow? J. Neurosci. 2011;31:7174–7177. doi: 10.1523/JNEUROSCI.0054-11.2011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rengachary S.S., Ellenbogen R.G. Elsevier Mosby; Edinburgh, NY: 2005. Principles of neurosurgery. [Google Scholar]
- Reuter M., Schmansky N.J., Rosas H.D., Fischl B. Within-subject template estimation for unbiased longitudinal image analysis. NeuroImage. 2012;61:1402–1418. doi: 10.1016/j.neuroimage.2012.02.084. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ribeiro P.F.M., Ventura-Antunes L., Gabi M., Mota B., Grinberg L.T., Farfel J.M., Ferretti-Rebustini R.E.L., Leite R.E.P., Herculano-Houzel S. The human cerebral cortex is neither one nor many: neuronal distribution reveals two quantitatively different zones in the gray matter, three in the white matter, and explains local variations in cortical folding. Front. Neuroanat. 2013;7:28. doi: 10.3389/fnana.2013.00028. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rosas H.D., Liu A.K., Hersch S., Glessner M., Ferrante R.J., Salat D.H., van der Kouwe A., Jenkins B.G., Dale A.M., Fischl B. Regional and progressive thinning of the cortical ribbon in Huntington's disease. Neurology. 2002;58:695–701. doi: 10.1212/wnl.58.5.695. [DOI] [PubMed] [Google Scholar]
- Salat D.H., Buckner R.L., Snyder A.Z., Greve D.N., Desikan R.S.R., Busa E., Morris J.C., Dale A.M., Fischl B. Thinning of the cerebral cortex in aging. Cereb. Cortex N. Y. N 1991. 2004;14:721–730. doi: 10.1093/cercor/bhh032. [DOI] [PubMed] [Google Scholar]
- Schaer M., Cuadra M.B., Tamarit L., Lazeyras F., Eliez S., Thiran J. A surface-based approach to quantify local cortical gyrification. IEEE Trans. Med. Imaging. 2008;27:161–170. doi: 10.1109/TMI.2007.903576. [DOI] [PubMed] [Google Scholar]
- Schmierer K., Wheeler-Kingshott C.A.M., Boulby P.A., Scaravilli F., Altmann D.R., Barker G.J., Tofts P.S., Miller D.H. Diffusion tensor imaging of post mortem multiple sclerosis brain. NeuroImage. 2007;35:467–477. doi: 10.1016/j.neuroimage.2006.12.010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Schmierer K., Wheeler-Kingshott C.A.M., Tozer D.J., Boulby P.A., Parkes H.G., Yousry T.A., Scaravilli F., Barker G.J., Tofts P.S., Miller D.H. Quantitative magnetic resonance of postmortem multiple sclerosis brain before and after fixation. Magn. Reson. Med. Off. J. Soc. Magn. Reson. Med. Soc. Magn. Reson. Med. 2008;59:268–277. doi: 10.1002/mrm.21487. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Schmithorst V.J., Yuan W. White matter development during adolescence as shown by diffusion MRI. Brain Cogn. 2010;72:16–25. doi: 10.1016/j.bandc.2009.06.005. [DOI] [PubMed] [Google Scholar]
- Schnack H.G., van Haren N.E.M., Brouwer R.M., Evans A., Durston S., Boomsma D.I., Kahn R.S., Hulshoff Pol H.E. Changes in thickness and surface area of the human cortex and their relationship with intelligence. Cereb. Cortex N. Y. N 1991. 2014 doi: 10.1093/cercor/bht357. [DOI] [PubMed] [Google Scholar]
- Scholz J., Klein M.C., Behrens T.E.J., Johansen-Berg H. Training induces changes in white-matter architecture. Nat. Neurosci. 2009;12:1370–1371. doi: 10.1038/nn.2412. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Shaw P., Greenstein D., Lerch J., Clasen L., Lenroot R., Gogtay N., Evans A., Rapoport J., Giedd J. Intellectual ability and cortical development in children and adolescents. Nature. 2006;440:676–679. doi: 10.1038/nature04513. [DOI] [PubMed] [Google Scholar]
- Shaw P., Kabani N.J., Lerch J.P., Eckstrand K., Lenroot R., Gogtay N., Greenstein D., Clasen L., Evans A., Rapoport J.L., Giedd J.N., Wise S.P. Neurodevelopmental trajectories of the human cerebral cortex. J. Neurosci. Off. J. Soc. Neurosci. 2008;28:3586–3594. doi: 10.1523/JNEUROSCI.5309-07.2008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Silver D.L., Watkins-Chow D.E., Schreck K.C., Pierfelice T.J., Larson D.M., Burnetti A.J., Liaw H.-J., Myung K., Walsh C.A., Gaiano N., Pavan W.J. The exon junction complex component Magoh controls brain size by regulating neural stem cell division. Nat. Neurosci. 2010;13:551–558. doi: 10.1038/nn.2527. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Simmons J.P., Nelson L.D., Simonsohn U. False-positive psychology: undisclosed flexibility in data collection and analysis allows presenting anything as significant. Psychol. Sci. 2011;22:1359–1366. doi: 10.1177/0956797611417632. [DOI] [PubMed] [Google Scholar]
- Singer J.D., Willett J.B. Oxford University Press; USA: 2003. Applied Longitudinal Data Analysis: Modeling Change and Event Occurrence. [Google Scholar]
- Smith S.M., Jenkinson M., Woolrich M.W., Beckmann C.F., Behrens T.E.J., Johansen-Berg H., Matthews P.M. Advances in functional and structural MR image analysis and implementation as FSL. NeuroImage. 2004;23(Suppl 1):S208–S219. doi: 10.1016/j.neuroimage.2004.07.051. [DOI] [PubMed] [Google Scholar]
- Smith S.M., Jenkinson M., Johansen-Berg H., Rueckert D., Nichols T.E., Mackay C.E., Watkins K.E., Ciccarelli O., Cader M.Z., Matthews P.M., Behrens T.E.J. Tract-based spatial statistics: voxelwise analysis of multi-subject diffusion data. NeuroImage. 2006;31:1487–1505. doi: 10.1016/j.neuroimage.2006.02.024. [DOI] [PubMed] [Google Scholar]
- Song S.-K., Sun S.-W., Ramsbottom M.J., Chang C., Russell J., Cross A.H. Dysmyelination revealed through MRI as increased radial (but unchanged axial) diffusion of water. NeuroImage. 2002;17:1429–1436. doi: 10.1006/nimg.2002.1267. [DOI] [PubMed] [Google Scholar]
- Song S.-K., Yoshino J., Le T.Q., Lin S.-J., Sun S.-W., Cross A.H., Armstrong R.C. Demyelination increases radial diffusivity in corpus callosum of mouse brain. NeuroImage. 2005;26:132–140. doi: 10.1016/j.neuroimage.2005.01.028. [DOI] [PubMed] [Google Scholar]
- Sowell E.R., Thompson P.M., Leonard C.M., Welcome S.E., Kan E., Toga A.W. Longitudinal mapping of cortical thickness and brain growth in normal children. J. Neurosci. Off. J. Soc. Neurosci. 2004;24:8223–8231. doi: 10.1523/JNEUROSCI.1798-04.2004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Steen R.G., Hamer R.M., Lieberman J.A. Measuring brain volume by MR imaging: impact of measurement precision and natural variation on sample size requirements. AJNR Am. J. Neuroradiol. 2007;28:1119–1125. doi: 10.3174/ajnr.A0537. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Stockman M., Alexander-Bloch A., Raznahan A., Giedd J.N. Organization for Human Brain Mapping (OHBM) Annual Meeting; Beijing, China: 2012. Effects of Mild Motion Artifact on Cortical Measures from Structural MRI. [Google Scholar]
- Sun S.S., Schubert C.M., Chumlea W.C., Roche A.F., Kulin H.E., Lee P.A., Himes J.H., Ryan A.S. National estimates of the timing of sexual maturation and racial differences among US children. Pediatrics. 2002;110:911–919. doi: 10.1542/peds.110.5.911. [DOI] [PubMed] [Google Scholar]
- Takeuchi H., Sekiguchi A., Taki Y., Yokoyama S., Yomogida Y., Komuro N., Yamanouchi T., Suzuki S., Kawashima R. Training of working memory impacts structural connectivity. J. Neurosci. Off. J. Soc. Neurosci. 2010;30:3297–3303. doi: 10.1523/JNEUROSCI.4611-09.2010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tamnes C.K., Ostby Y., Fjell A.M., Westlye L.T., Due-Tønnessen P., Walhovd K.B. Brain maturation in adolescence and young adulthood: regional age-related changes in cortical thickness and white matter volume and microstructure. Cereb. Cortex N. Y. N 1991. 2010;20:534–548. doi: 10.1093/cercor/bhp118. [DOI] [PubMed] [Google Scholar]
- Tamnes C.K., Walhovd K.B., Dale A.M., Ostby Y., Grydeland H., Richardson G., Westlye L.T., Roddey J.C., Hagler D.J., Jr., Due-Tønnessen P., Holland D., Fjell A.M. Brain development and aging: overlapping and unique patterns of change. NeuroImage. 2013;68:63–74. doi: 10.1016/j.neuroimage.2012.11.039. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tamnes C.K., Walhovd K.B., Grydeland H., Holland D., Østby Y., Dale A.M., Fjell A.M. Longitudinal working memory development is related to structural maturation of frontal and parietal cortices. J. Cogn. Neurosci. 2013;25:1611–1623. doi: 10.1162/jocn_a_00434. [DOI] [PubMed] [Google Scholar]
- Tau G.Z., Peterson B.S. Normal Development of Brain Circuits. Neuropsychopharmacology. 2009;35:147–168. doi: 10.1038/npp.2009.115. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Taylor P.A., Saad Z.S. FATCAT: (an efficient) Functional and Tractographic Connectivity Analysis Toolbox. Brain Connectivity. 2013;3:523–535. doi: 10.1089/brain.2013.0154. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Urošević S., Collins P., Muetzel R., Lim K., Luciana M. Longitudinal changes in behavioral approach system sensitivity and brain structures involved in reward processing during adolescence. Dev. Psychol. 2012;48:1488–1500. doi: 10.1037/a0027502. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Valkanova V., Eguia Rodriguez R., Ebmeier K.P. Mind over matter – what do we know about neuroplasticity in adults? Int. Psychogeriatr. IPA 1-19. 2014 doi: 10.1017/S1041610213002482. [DOI] [PubMed] [Google Scholar]
- Van Essen D.C., Dierker D.L. Surface-based and probabilistic atlases of primate cerebral cortex. Neuron. 2007;56:209–225. doi: 10.1016/j.neuron.2007.10.015. [DOI] [PubMed] [Google Scholar]
- Van Essen D.C. Cortical cartography and Caret software. NeuroImage. 2012;62:757–764. doi: 10.1016/j.neuroimage.2011.10.077. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Van Essen D.C., Smith S.M., Barch D.M., Behrens T.E.J., Yacoub E., Ugurbil K., WU-Minn H.C.P., Consortium The WU-Minn Human Connectome Project: an overview. NeuroImage. 2013;80:62–79. doi: 10.1016/j.neuroimage.2013.05.041. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Vijayakumar N., Whittle S., Yücel M., Dennison M., Simmons J., Allen N.B. Prefrontal structural correlates of cognitive control during adolescent development: a 4-year longitudinal study. J. Cogn. Neurosci. 2013:1–13. doi: 10.1162/jocn_a_00549. [DOI] [PubMed] [Google Scholar]
- Walhovd K.B., Tamnes C.K., Fjell A.M. Brain structural maturation and the foundations of cognitive behavioral development. Curr. Opin. Neurol. 2014;27:176–184. doi: 10.1097/WCO.0000000000000074. [DOI] [PubMed] [Google Scholar]
- Westlye L.T., Walhovd K.B., Dale A.M., Bjørnerud A., Due-Tønnessen P., Engvig A., Grydeland H., Tamnes C.K., Ostby Y., Fjell A.M. Life-span changes of the human brain white matter: diffusion tensor imaging (DTI) and volumetry. Cereb. Cortex N. Y. N 1991. 2010;20:2055–2068. doi: 10.1093/cercor/bhp280. [DOI] [PubMed] [Google Scholar]
- Wierenga L.M., Langen M., Oranje B., Durston S. Unique developmental trajectories of cortical thickness and surface area. NeuroImage. 2014;87:120–126. doi: 10.1016/j.neuroimage.2013.11.010. [DOI] [PubMed] [Google Scholar]
- Winkler A.M., Kochunov P., Blangero J., Almasy L., Zilles K., Fox P.T., Duggirala R., Glahn D.C. Cortical thickness or grey matter volume? The importance of selecting the phenotype for imaging genetics studies. NeuroImage. 2010;53:1135–1146. doi: 10.1016/j.neuroimage.2009.12.028. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yakovlev P.A., Lecours I.R. The myelogenetic cycles of regional maturation of the brain. In: Minkowski A., editor. Regional Development of the Brain in Early Life. Blackwell; Oxford: 1967. [Google Scholar]
- Yang G., Pan F., Gan W.-B. Stably maintained dendritic spines are associated with lifelong memories. Nature. 2009;462:920–924. doi: 10.1038/nature08577. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yendiki A., Koldewyn K., Kakunoori S., Kanwisher N., Fischl B. Spurious group differences due to head motion in a diffusion MRI study. NeuroImage. 2014 doi: 10.1016/j.neuroimage.2013.11.027. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zatorre R.J., Fields R.D., Johansen-Berg H. Plasticity in gray and white: neuroimaging changes in brain structure during learning. Nat. Neurosci. 2012;15:528–536. doi: 10.1038/nn.3045. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zijdenbos A.P., Forghani R., Evans A.C. Automatic “pipeline” analysis of 3-D MRI data for clinical trials: application to multiple sclerosis. IEEE Trans. Med Imaging. 2002;21:1280–1291. doi: 10.1109/TMI.2002.806283. [DOI] [PubMed] [Google Scholar]
- Zilles K., Armstrong E., Schleicher A., Kretschmann H.-J. The human pattern of gyrification in the cerebral cortex. Anat. Embryol. (Berl.) 1988;179:173–179. doi: 10.1007/BF00304699. [DOI] [PubMed] [Google Scholar]