Highlights
-
•
Late childhood and adolescence are a sensitive juncture for social anxiety onset.
-
•
Cognitive biases linked to social anxiety manifest in differences in functional brain response.
-
•
Normative socio-affective developments may exacerbate biased processing.
-
•
Increased plasticity/learning may make adolescence an ideal time to intervene.
-
•
Modifying processing biases through cognitive training and neurofeedback represent two possible age-appropriate interventions.
Keywords: Social anxiety, SAD, Cognitive bias, Neurocognitive development, fMRI, Adolescence
Abstract
Social anxiety disorder represents a debilitating condition that has large adverse effects on the quality of social connections, educational achievement and wellbeing. Age-of-onset data suggests that early adolescence is a developmentally sensitive juncture for the onset of social anxiety. In this review, we highlight the potential of using a developmental cognitive neuroscience approach to understand (i) why there are normative increases in social worries in adolescence and (ii) how adolescence-associated changes may ‘bring out’ neuro-cognitive risk factors for social anxiety in a subset of individuals during this developmental period. We also speculate on how changes that occur in learning and plasticity may allow for optimal acquisition of more adaptive neurocognitive strategies through external interventions. Hence, for the minority of individuals who require external interventions to target their social fears, this enhanced flexibility could result in more powerful and longer-lasting therapeutic effects. We will review two novel interventions that target information-processing biases and their neural substrates via cognitive training and visual feedback of neural activity measured through functional magnetic resonance imaging.
1. Introduction
Adolescence is a transitional period beginning with the onset of puberty and culminating in the assumption of a stable adult role (Lerner and Steinberg, 2004). There is considerable individual variability in both its onset and length. While the onset of adolescence is likely to be dependent on the release of pubertal hormones which set in motion a cascade of physical developments (e.g., dimorphic secondary sexual characteristics, neural changes), the offset is more contingent on sociocultural norms (Blakemore and Mills, 2014, Forbes and Dahl, 2010, Goddings et al., 2014, Herting et al., 2014, Peper and Dahl, 2013; Sisk and Foster, 2004). Adolescence is characterized by changes in psychological make-up too, particularly in social-affective (the experience and regulation of emotion in response to social cues) and social-cognitive (the reasoning about the social world and others’ mental states) abilities. These changes are observed in and driven by maturing neural circuits (Nelson et al., 2005, Haller et al., 2014) and are likely to be adaptive as adolescents begin to engage with increasingly complex peer networks (Steinberg and Silverberg, 1986).
Adolescence is also a period of vulnerability for the emergence of many psychiatric conditions. One of these is social anxiety disorder (SAD), a debilitating condition characterized by a paralyzing fear of negative evaluation from others (Clark and Wells, 1995, Rapee and Spence, 2004). Since peer interactions carry important learning experiences for adolescents, avoidance of social exchanges, often used as a way to cope with social anxiety, is likely even more impairing and disruptive during this time (Miers et al., 2014). Social anxiety may be viewed as an ‘adolescent disorder’. Indeed, age-of-onset data show that around 75% of more extreme and persistent forms of social anxiety have their onset by mid-adolescence with a median age of onset of 13 years (Gregory et al., 2007, Kessler et al., 2005a, Kessler et al., 2005b, Wittchen et al., 1999). Although several neurobehavioral hypotheses have been proposed to account for the general vulnerability to psychiatric conditions in adolescence (e.g., Sturman and Moghaddam, 2011), there is less understanding of how typical age-associated changes in adolescence may serve as vehicles for the expression of social anxiety risks in particular. In this article, we consider whether and why SAD risk factors emerge at the adolescent juncture, with a focus on the underlying neuro-developmental mechanisms that enable risk factors to find expression. We will first review changes in the functional architecture of social-affective and -cognitive brain circuits in adolescence. Next, after reviewing the cognitive characteristics and neural correlates of adolescent SAD, we will suggest how adolescent changes may ‘bring out’ aspects of SAD-risk.
More recently, adolescence has also been suggested as a period of heightened learning and flexibility (Crone and Dahl, 2012). This raises the question of whether adolescence may be an optimal period for targeting risks associated with SAD through translational interventions. A second set of goals of this paper is to (a) highlight how typical neuro-developmental changes can allow flexible and adaptive long-term learning about social-emotional events and, (b) highlight how developmental cognitive neuroscience research can inform the timing of psychological treatments for SAD. We ask whether there is greater social-affective plasticity in adolescence and how this might enable age-appropriate interventions to bring stronger, longer-term benefits.
2. How does the importance of peers change in adolescence?
A pronounced change in adolescence is the preoccupation with peers and romantic interests. Compared to adults, adolescents are far more concerned about peer feedback and respond more negatively to peer exclusion (Coleman, 1974, Kloep, 1999, O’Brien and Bierman, 1988, Reijntjes et al., 2006, Westenberg et al., 2004). Adolescents more frequently experience self-consciousness and use social comparison as a method of self-evaluation compared to pre-adolescent children (Butler, 1998, Elkind, 1967, Elkind, 1985, Elkind and Bowen, 1979, Harter, 2006, Pfeifer et al., 2009). There is also an increased interest in understanding and tracking the mental states of peers, manifesting, for example, in increased awareness of their peers’ likes and dislikes (fashion, music, gadgets or language neologisms). Studies using behavioral paradigms show a growing understanding of another's mental state in terms of visual perspective-taking, feelings and motivations (‘mentalizing’; Dumontheil et al., 2010, Vetter et al., 2013) and more differentiated pro-social behavior (Güroğlu et al., 2014; Burnett Heyes et al., submitted for publication; Van den Bos et al., 2012).
These broad but pervasive adolescence-associated social-affective and cognitive changes may be mediated by prolonged maturation of functional (and structural) brain networks (Nelson et al., 2005). Indeed, over the last decade, there has been a surge in investigations of age- and/or puberty-related typical functional brain maturation of networks underpinning social-affective and cognitive processing (e.g., Blakemore, 2008, Blakemore, 2012). The networks of regions involve limbic and temporal areas and several functional sub-divisions of the pre-frontal cortex (PFC). Data suggest that these regions likely work in concert, as interactive networks enabling flexible responding to social-emotional cues.
Studies investigating developmental changes in the neural responses to social-affective stimuli can be divided into those investigating automatic regulatory responses to basic social and non-social threats and rewards, and those that probe more controlled regulatory responses. In the first category, complex, region-specific linear and quadratic trajectories across adolescence have been reported in the neural sensitivity of subcortical and cortical regions to social but also non-social threats and rewards. Broadly, these data suggest a peak in the neural responses of subcortical affect- and reward-processing regions such as the amygdalae and striatum to simple threatening or rewarding stimuli (e.g., monetary rewards and static, emotional faces) (e.g., Chein et al., 2012, Ernst et al., 2005, Hare et al., 2008, Pfeifer et al., 2011, Passarotti et al., 2009, Somerville et al., 2011, Van Leijenhorst et al., 2010). When peak sensitivities occur is not clear: while some researchers have documented curvilinear trends with a peak in mid-adolescence (Hare et al., 2008, Somerville et al., 2011), others have reported linear declines throughout adolescence with peaks in late childhood (Gee et al., 2013). Developmental trajectories of frontal areas in response to social-affective stimuli are equally, if not more, complex. Using basic inhibition-based paradigms (such as go/nogo tasks), which tap automatic regulatory responses to emotional face displays, studies have reported increased activity in functional portions of the PFC during response inhibition in adolescents compared to older age groups. These often occur in the presence of overall poorer behavioral performance (Dreyfuss et al., 2014, Somerville et al., 2011). Turning to paradigms that measure participants’ automatic regulatory responses to more complex socially provocative stimuli (e.g., tasks that simulate online social exclusion), these have found more extensive differences occurring in the insula, anterior cingulate cortex (ACC) and medial and lateral functional subdivisions of the PFC between children, adolescents and adults (e.g., Moor et al., 2010, Moor et al., 2012, Guyer et al., 2009, Lau et al., 2011a, Lau et al., 2011b, Masten et al., 2009, Sebastian et al., 2011). However, inconsistencies in the directionality of these differences across studies make drawing interpretations about developmental change difficult. The second category of studies investigating more effortful, controlled regulatory processing is more limited, but nonetheless show similarly inconsistent linear and quadratic trends in prefrontal activation (McRae et al., 2012, Monk et al., 2003, Pitskel et al., 2011). The inconsistency of findings across studies may stem from the use of different tasks tapping subtly different processes. Alternatively, variations may also arise from differences in the salience and relevance of the task context (Braams et al., 2014, Crone and Dahl, 2012).
As well as studying differences in individual regions, many studies have also explored adolescent changes in functional connectivity – the co-activation between different areas, either during ‘resting state’ or during a task. Resting state functional connectivity studies suggest that functional integration within networks increases across adolescence (Fair et al., 2007). A ‘switch’ from positive to negative connectivity in the amygdalae-medial prefrontal cortex (mPFC) network has also been reported across 4–22 year olds during the viewing of fearful emotional faces (Gee et al., 2013). Specifically, for children aged (4–9 years) increased amygdala activation was associated with increased mPFC activity, while from early adolescence (10–13 years) to adulthood higher mPFC activation was associated with lower amygdala activity. These changes in connectivity may reflect continuous maturation of top-down modulatory ability in frontal areas from childhood to adolescence to adulthood.
To investigate age trends in the neural substrates of social-cognitive processes, studies have investigated functional engagement of ‘social brain’ regions during perspective-taking. Early studies mostly compared brain activity during mental state attribution in a single adolescent group (often with a wide age range) with an adult group (e.g., Blakemore et al., 2007, Burnett et al., 2009, Moriguchi et al., 2007, Wang et al., 2006). However, more recent studies have used longitudinal designs or compared multiple adolescent age groups to delineate more continuous changes in networks across age. Exemplifying this, Moor and colleagues (2011) scanned two adolescent age groups (aged 10–12 and 14–15) and one young adult group (19–23 years of age) during the ‘Mind in the Eyes’ task (participants had to label emotional expressions as displayed in the eyes). While all age groups showed increased activation along the superior temporal sulcus during this task, only the youngest group exhibited additional engagement of the dorsal mPFC. In a two-year follow up, participants were re-tested with the same paradigm, corroborating decreases in pre-frontal activation with age in the same individuals. However, while linear trends were found for some frontal regions (e.g.?, the inferior frontal gyrus), there was also a quadratic pattern in the dorsal mPFC with a dip in mid-adolescence (Overgaauw et al., 2014). Studies using tasks that simulate more complex social exchanges have found similar patterns. For example, one study by Van den Bos et al. (2011) scanned pre-pubertal youth (12–14 years), post-pubertal youth (15–17 years) and young adults (18–22) during a task that probed fairness and trust during the assignment and sharing of monetary rewards. While only the youngest group showed additional mPFC activity, the researchers found a gradual age-related increase in activity of the left TPJ and the right dorsolateral PFC, which correlated with increased reciprocity in exchanges. Together, these studies suggest that with age, relative contributions of prefrontal and temporal areas to mental state processing change, possibly toward increased recruitment of temporal areas. Such findings have been suggested to reflect increased automaticity of engaging in mentalizing across adolescence (e.g., Blakemore, 2008, Van den Bos et al., 2011), perhaps through an increased reliance on social scripts.
In summary, some of the most documented adolescence-associated behavioral adaptions are an increased emotional reactivity and more sophisticated social understanding. These are paralleled by changes in the functioning of PFC-amygdalae-temporal circuits. Presumably these neurocognitive changes prepare the adolescent for navigation in a novel and more complex social world. However these adaptive neural changes may also have negative ‘side effects’. Specifically, these developments may lead to increased social concerns and susceptibility to peer conformity and influence. In the next section, we suggest that the protracted maturational changes associated with social and affective functioning explain why adolescence is a peak period for the emergence of more distressing social worries in some individuals. We suggest that the as-yet poorer emotion regulation abilities, and the evolving ability to take another's perspective, mean that certain neuro-cognitive risk factors for social anxiety are more likely to cascade into socially anxious behavior in this developmental period. However, before considering this hypothesis further, we first review the cognitive characteristics of adolescent SAD and their neural bases.
3. How does social anxiety affect information processing?
Dominant cognitive models of SAD highlight how differences in the processing of social information in socially anxious individuals may serve to maintain the disorder. Specifically, cognitive biases – maladaptive systematic distortions in information processing – have been reported in individuals with SAD at various processing stages including attention, interpretation and judgment or expectation (Clark and Wells, 1995, Jarcho et al., 2013a, Jarcho et al., 2013b, Muris and Field, 2008).
A handful of studies have found maladaptive allocation of attention toward threatening social cues in adolescents with SAD (Roy et al., 2009, Stirling et al., 2006), and also in behaviorally inhibited (BI) youths (Reeb-Sutherland et al., 2009, Perez-Edgar et al., 2010a). BI is a temperamental factor that shows clear parallels with social anxiety in the wariness, fear and avoidance of novel social situations, although there is contention over the degree to which BI is an early marker for SAD. While these findings on attention biases are promising in pinpointing cognitive underpinnings of SAD, it is important to highlight that a small body of studies have also found no group differences in attentional deployment (Bar-Haim et al., 2007, Kindt et al., 2003). Biased interpretations, that is, a tendency to interpret ambiguous social situations as threatening, have also been studied in adolescents with SAD. As social information is particularly ambiguous – we constantly have to interpret and track complex bodily, verbal and facial cues throughout interactions – biases in interpretation may be specifically relevant for social anxiety (relative to other types of anxiety symptoms). A few studies (e.g., Miers et al., 2008, Haller et al., 2014) have found that adolescents with increased social anxiety were more likely to endorse negative interpretations of ambiguous situations. Interestingly, while links between biased interpretations and social anxiety symptoms are fairly consistent in adolescence, findings in children are mixed. Some studies have documented biases (Bögels and Zigterman, 2000, Muris et al., 2000) while others report no biases in socially anxious children (Creswell et al., 2013, In-Albon et al., 2009). It may either be that current measurement tools are less suitable to detect interpretation biases in younger populations or that interpretation biases do not mature as risk factors for social anxiety until adolescence. Finally, biases in expectations of performance in social-evaluative contexts also characterize socially anxious children, teens and at-risk populations. Individuals with SAD expect to perform worse and expect to show visible signs of nervousness in social and performance situations (Alfano et al., 2006, Morgan and Banerjee, 2006, Pass et al., 2012, Rheingold et al., 2003, Spence et al., 1999). Findings as to whether socially anxious youths actually perform worse or whether these expectations are only existent in their own perception of their social competence are, however, mixed (e.g., Cartwright-Hatton et al., 2003, Cartwright-Hatton et al., 2005, Erath et al., 2007, Miers et al., 2010).
These cognitive biases have been shown to manifest in differential engagement of fronto-limbic circuits during the anticipation of, during, and post social-event processing. Interestingly, the networks implicated in aberrant processing in SAD are those documented to undergo prolonged functional restructuring in adolescence. There are now a few studies investigating the neural substrates of information processing in adolescents with social concerns. Increased affective responses to negative social cues (e.g., negative faces) have been linked to increased amygdalae sensitivity as well as differential responses in the ACC, striatum, mPFC, ventrolateral PFC (vlPFC) and insula in adolescents with SAD or increased social worries (Battaglia et al., 2012, Killgore and Deborah, 2005, McClure et al., 2007). Tasks that present more dynamic social exchanges have replicated and extended these data (e.g., Jarcho et al., 2013a, Jarcho et al., 2013b). For example, studies using the ‘Chat Room Task’ (e.g., Guyer et al., 2009), which presents participants with photographs of peers that the participant either ‘accepted’ or ‘rejected’ for online interactions in a previous session, have found differential responses in both anticipation and post-event processing. When anticipating feedback from the previously ‘rejected’ peers, adolescents with social concerns manifested greater amygdala activity relative to non-anxious adolescents. Socially anxious participants also displayed aberrant functional connectivity between the vlPFC and amygdalae compared to the control participants (Guyer et al., 2008, Lau et al., 2011a, Lau et al., 2011b). Another study of the same sample found that prior to receiving peer feedback, amygdala activity was heightened in both groups of adolescents. However, after receiving feedback, this activity declined in healthy but not in participants with more social concerns. FMRI studies that have included behaviorally inhibited youth find similar fronto-limbic perturbations as those described in youth with SAD. Notably, studies with adolescents with BI have also reported increased responses in the amygdalae to negative emotional expressions, differential striatal responses to the anticipation of monetary and social rewards, and during conflict adaptation (when individuals habituate to successively presented contradictory emotional information (Etkin et al., 2006, Bar-Haim et al., 2009, Guyer et al., 2014, Hardee et al., 2013, Helfinstein et al., 2011, Jarcho et al., 2013a, Jarcho et al., 2013b, Jarcho et al., 2014, Pérez-edgar et al., 2007).
While these neurocognitive differences may explain why some socially anxious (or behaviorally inhibited) young people develop and maintain impairing social concerns, questions remain over why these fears emerge at the adolescent juncture. In the last section, we speculated that adolescence-related changes in neural circuits and associated information processing underscore the salience of social situations for all teenagers. Here, we further suggest that for a subgroup of youngsters, these adolescence-associated changes might magnify pre-existing cognitive biases and/or trigger the emergence of new biases. Speculatively, the protracted maturation of circuits involved in regulating reactivity to social-affective information might exaggerate pre-existing attention biases for threatening social cues. This, in turn, could reciprocally affect trajectories of functional brain development by biasing both the nature of incoming information and the ways in which this information is processed and subsequently consolidated. Another possibility is that developments in mentalizing underpinned by trajectories of the ‘social brain’ may enable the expression of interpretation biases. As mentalizing abilities become increasingly sophisticated across adolescence, the teenager is able to generate more ‘mental explanations’ for others’ behavior. This may result in an increase in perceived complexity and ambiguity of daily social interactions. As there are parallel increases in time spent interacting with peers within larger social networks, these developments in the perception of social interactions may ‘expose’ in some individuals the tendency to decode ambiguous social cues in a negative manner. This could potentially explain why interpretation biases are not consistently found in younger populations: biases in interpretation may only manifest once these advanced social-cognitive capacities are attained.
In summary, maladaptive cognitive biases characterize adolescents with SAD and may contribute to the maintenance of the disorder. A growing body of data also suggests that these biases may be rooted in the perturbed functioning of key fronto-limbic circuits dedicated to the processing of emotional and social stimuli. While normative changes in those neural circuits may explain why there are heightened social concerns during adolescence, these same changes may also push a subset of adolescents with cognitive vulnerabilities toward more extreme social fears and worries. By biasing individual differences in social-affective networks further, brain maturational changes in adolescence may, in a minority of youth who already fall at the end of the continuous distribution, result in a shift further toward the extreme end.
4. Are there unique learning opportunities during adolescence?
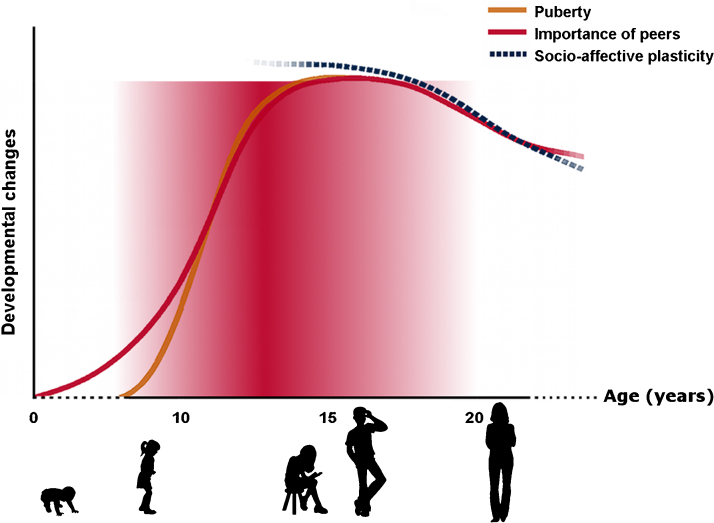
Alongside the relatively bleak picture of a period where preexisting vulnerabilities find expression, it may also be the case that the adolescent-typical neurodevelopmental changes we have described enhance plasticity to positive environmental experiences (see Fig. 1). These experiences may often center around positive peer interactions and academic achievements, but could also include early interventions. Indeed, research on normative changes suggests that adolescence is a period of opportunity. Recent conceptual frameworks view the adolescence-associated increase in ‘emotionality’ and ‘sociality’ as facilitating learning for specific, relevant (social-emotional) experiences. Protracted maturation of neurocognitive abilities may enable individuals to be more flexible in their behavioral responses and adapt rapidly to changing social contextual demands (e.g., Crone and Dahl, 2012, Nelson et al., 2014). Following this line of thought, it has been suggested that interventions implemented at developmentally-sensitive junctures may yield more powerful and sustained therapeutic benefits (Cohen Kadosh et al., 2013). This may be particularly so for interventions which target neurocognitive processes that are undergoing maturational change in adolescence. Thus, the benefits of early treatment may not simply be limited to a timely amelioration of symptoms to avoid long-term negative outcomes. Instead, administering interventions early may take advantage of developmentally sensitive plasticity and learning. Here, we first consider how neurodevelopmental changes may enhance learning and plasticity, and next, which interventions may be most appropriate.
Fig. 1.
Adolescence as a period of vulnerability for SAD. Red shaded region highlights risk for onset or amplification of neurocognitive risk factors through interplay of adolescence-associated changes. As socio-affective networks are thought to develop across adolescence with complex linear and quadratic changes we suggest greater plasticity to socio-affective stimuli across the teenage years (relative to adults). (For interpretation of the references to color in this figure legend, the reader is referred to the web version of the article.)
Across adolescence, changes in brain networks may result in: (i) greater affective responding to (socially) threatening and rewarding feedback and (ii) a greater engagement with, and understanding of, complex interpersonal situations. These changes may focus learning on ‘developmentally-appropriate’ cues such as peer feedback when seeking out new group membership. Differential reactivity both in amygdalae and striatal function, which have been shown to support basic learning processes of threat and reward (Cohen et al., 2010, Gallagher and Hollandt, 1994), may also guide and enhance emotional learning in adolescence. Protracted maturation of individual regions and connectivity of prefrontal-limbic-temporal networks may allow for these abilities to emerge gradually across adolescence. But is there evidence that (social and non-social) emotional learning is enhanced in adolescence?
Learning from (both positive and negative) feedback in changing environments appears to develop until early adulthood. Behavioral data from several experiments suggests that youngsters are more influenced by irrelevant negative feedback, experience more arousal in response to anticipated loss, and exhibit an increased learning rate from events that are worse than expected compared to adults (Crone and van der Molen, 2007, Hauser et al., 2015, Hooper et al., 2004). A few imaging studies have revealed increasing engagement of dorsolateral PFC and parietal cortex to negative feedback with age (Van Duijvenvoorde et al., 2008, Crone et al., 2008). Two more recent studies have assessed neuro-developmental differences in more complex, probabilistic learning environments, by looking at the neural underpinnings of prediction error in development. In brief, prediction error refers to the difference between expected and actual/experienced (positive or negative) outcome. A study by Cohen and colleagues (2010) found that a neural representation of prediction error in the ventral striatum only emerged in the mid- to late-adolescent group, with heightened sensitivity to positive predictions errors in adolescence. Van den Bos and colleagues (2012) further found that ventral striatum-mPFC connectivity during prediction error learning changed from stronger connectivity after negative feedback to stronger connectivity after positive feedback (though they did not find striatal differences per se in response to prediction errors). A further cross-sectional study by Jones and colleagues (2014) probed neural responses during positive social reinforcement learning. The authors reported that teenagers, in comparison to both children and adults, exhibited greater insular activity during positive prediction error learning. Together, these studies suggest that adaptive developmental learning during this period is aided by a unique reactivity/receptiveness to certain (social and non-social) learning signals and changing mechanisms by which these signals guide and inform behavior.
Changes in experience-dependent learning also emerge in animal models of adolescence. While most animal literature has focused on facilitated learning in early prenatal periods as sensitive windows for cognitive and social learning (e.g., Pascalis et al., 2005, Nelson et al., 2007), there are some studies that provide evidence for a unique developmental window in adolescence for relevant stimuli classes. These include reproductive and social cues (Romeo et al., 2002, Scherf et al., 2012) and faster, more flexible learning of new reward associations (Johnson and Wilbrecht, 2011). Lesion studies of non-human primates have further shown that amygdalae lesions at different developmental periods have different effects on social behavior. For example, monkeys that received lesions as neonates exhibited an increased fear response, were less dominant and of lower rank in the social hierarchy when interacting with same-aged monkeys during adolescence (Bauman et al., 2004). In contrast, monkeys that received lesions as young adults (i.e., after their experiences of adolescent social interactions) displayed reduced levels of fear and retained pre-lesion social dominance and their rank in the hierarchy (Machado and Bachevalier, 2006, Bauman et al., 2006). Rodent research has also demonstrated that social deprivation/isolation during puberty/adolescence has long lasting effects on adult rat behavior and physiology (Romeo et al., 2006a, Romeo et al., 2006b). There is some evidence that these effects may be mediated through aberrant structural development (synaptic density) of the infralimbic cortex and the cingulate gyrus (Leussis et al., 2008), and reduced prefrontal myelination in male rodents (Leussis and Andersen, 2008). Lastly, there is also evidence for resilience and rapid learning for positive social experiences. While exposure to stress during adolescence has been shown to exacerbate stress responses in adult rats (Avital and Richter-Levin, 2005), conversely, rats reared with consistent exposure to social contact during adolescence were found to exhibit attenuated pituitary-adrenocortical stress response in adulthood (Kaiser et al., 2007). Complementing these findings, studies on rodent models of (social) stress have demonstrated, for instance, that less socially skilled (subordinate) adolescent rats can rapidly increase social skills when co-housed with more dominant, wild type rats (Buwalda et al., 2011).
In summary, recent theoretical models of adolescent social-affective brain development no longer conceptualize the unique time course of maturation as an obstacle toward optimal interpersonal functioning. Instead, most recent syntheses of the data on brain–behavior relationships in adolescence describe this period as a time of flexibility and adaptation during which the teenage brain is specifically receptive to certain socially relevant experiences (e.g., Crone and Dahl, 2012, Nelson et al., 2014). The unique maturational schedule and sensitivities motivate and enable the adolescents to learn about social interactions and further develop competencies needed to navigate the world independently. These unique characteristics of adolescent brain development may render this period suitable for early, neurocognitive interventions with potentially increased and long-lasting benefits.
5. Age-appropriate SAD interventions to capitalize on plasticity
Given some evidence that adolescent-specific developmental changes may facilitate emotional and social learning, developmentally appropriate interventions could harness this knowledge to make most of the unique response profile of adolescence. If specific interventions administered in adolescence could yield greater and longer-lasting therapeutic effects, what possible interventive tools are there? Ideally interventions would target neurocognitive processes that are still changing across the adolescent period. Here we suggest that (i) modification of attention and interpretation biases through cognitive training and (ii) modulation of underlying neural circuits through fMRI-based neurofeedback (NF) might be especially promising options for teenage participants.
Cognitive bias modification of attention (CBM-A) and interpretation (CBM-I) training programs have recently been implicated in the treatment of adult anxiety and mood conditions. Both types of training capitalize on basic cognitive science findings suggesting that information-processing biases (such as an in attention or interpretation) may be causally linked to anxiety (and depressive symptoms). Thus, ‘correcting’ these or encouraging more adaptive forms of information-processing styles could attenuate symptoms. The process of teaching new styles of attentional orienting and interpretation occurs through repeated learning, in which the adaptive but not maladaptive form of responding to a stimulus is reinforced through feedback. Modifying attention biases (i.e., CBM-A) has most commonly been achieved through the dot-probe task. The dot probe task (often used to assess selective attention-orienting biases) presents participants with a pair of stimuli (usually one threatening and the other non-threatening i.e., neutral or positive) in different locations on a computer screen. This is followed by a ‘probe’ stimulus replacing one of the two stimuli. Unlike the standard dot-probe, training versions of the task always present the probe to which the participant responds after the non-threatening target. Thus, over trials, the participant learns to automatically direct his/her attention away from the threatening target and toward the non-threatening stimulus. Another version of CBM-A tasks has relied on visual search paradigms (e.g., Waters et al., 2013). Participants view a grid of faces one of which is positive (wearing a smile) while the others are negative (wearing frowns). The participant must select the positive expression as quickly as possible. Again, the principle is that the participant learns over trials to disengage attention from threatening stimuli and instead direct attention to a benign stimulus. As only a subset of anxious children manifest attention biases, training attention not only away from threat but also toward positive stimuli has the advantage to minimize any potentially negative effects for those teens who do not present with biases and instead may even have unique benefits (Eldar et al., 2012, Cowart and Ollendick, 2011, Waters et al., 2013). CBM of interpretations (CBM-I) operates via similar principles of repetitive learning and feedback, but encourages benign interpretative styles. Most commonly, participants are presented with an incomplete ambiguous passage, which can be completed by filling in the missing letter of a word fragment, presented at the end of the passage. The word fragment is selected such that correct completion always resolves the passage in a benign or positive direction. Thus, again after many trials, participants learn to endorse more adaptive interpretations of ambiguous scenarios.
Both CBM-A and CBM-I techniques rely on associative learning principles to reinforce the acquisition of more adaptive processing styles (attention and interpretation). As they are thought to involve automatic, implicit learning through repeated training, they may present complementary methods for modifying cognitive biases to traditional effortful and explicit top-down strategies, such as those used in cognitive behavioral therapy (CBT) (Mathews and Mackintosh, 2000). These training methods are thought to be similar to the processes by which children and young people acquire anxiety-associated processing styles as these are also thought to involve associative learning. More specifically, by modeling those of their care-givers or even peers through observation (vicarious learning) or being verbally instructed (verbal learning) about the appropriate adoption of threat-congruent processing styles across daily situations (see Field, 2006 for review), certain anxiety processing styles become associated with particular situations. Thus, retraining processing styles through CBM-I and CBM-A, which use similar associative learning methods, speculatively, may be more age-appropriate than CBT.
If effective, CBM-A and CBM-I could be used at different stages of intervention: as a therapeutic intervention for current symptoms or an ‘add on’ intervention to enhance effectiveness of current treatments. They could even serve as a preventative intervention to target vulnerability in at-risk individuals. However, empirical studies are quite mixed in their assessment of efficacy. First, clinical trials of CBM-A in pediatric patients with anxiety disorders have reported promising but also inconsistent results (Bar-Haim et al., 2011, Shechner et al., 2014, Eldar et al., 2012, Waters et al., 2013). There have been no clinical trials of CBM-I yet; community samples report some positive findings in attenuating anxious responses after a single session of training but as effects on symptoms in non-clinical groups are suspected to be rather weak, this has resulted in inconsistent findings across studies (Lau, 2013). The one study applying CBM-I to patients (including but not limited to patients with SAD) showed that, while biases could be altered through training, effects on symptoms were again small and insignificant (Fu et al., 2013). These initially disappointing results could be due to only single training sessions being administered. Interestingly, a recent study looking specifically at adolescents with high levels of social concerns showed that a combination of CBM-A and CBM-I was effective (Sportel et al., 2013), but these effects were less persistent than those of other frontline treatments such as CBT. One strategy to improve effectiveness may be to increase user-engagement of these computerized training programs. The design of this interface could draw on findings from developmental cognitive neuroscience highlighting the motivational salience of social stimuli to facilitate greater learning. More particularly, one could use as training materials, descriptions or even pictures of social scenes where the participant has to seek out friendly individuals over unfriendly individuals (attention training) or attribute the cause of an ambiguous social scene (a frowning individual) to a benign reason (the individual is feeling unwell).
FMRI-based neurofeedback (“NF”) is a newly emerging technique that utilizes the latest developments of real-time data processing and pattern analysis in order to train participants in the self-modulation of neural networks. In fMRI-based NF studies, participants are presented with real-time brain activation in specific regions (or circuits) of interest (for example through a visually-presented thermometer), and they are trained to reliably regulate their online brain response with high spatial precision (Weiskopf et al., 2004a, Weiskopf et al., 2004b, deCharms et al., 2005, deCharms, 2007, Johnston et al., 2010). FMRI-based NF has proven useful for up- or down-regulating the brain regions involved in healthy adults’ emotional responses (Johnston et al., 2010, Johnston et al., 2011, Zotev et al., 2011). A recent study of adult patients with depression, for example, found that fMRI-based NF was associated with significant improvements in symptoms, whereas cognitive training alone was not (Linden et al., 2012; see also Linden, 2014, for a review). Studies extending fMRI-based NF to pediatric populations are currently underway with promising first findings (Cohen Kadosh et al., submitted for publication). As with CBM methods, NF may be especially age-appropriate as it could directly modulate brain networks which are undergoing change – and which may be more responsive to external interventions. Like CBM, the effectiveness of NF for adolescents could be enhanced by delivering feedback in the form of socially salient tasks. As an example, adolescent participants might be asked to regulate particular brain regions during the simulation of stressful social situations (e.g., giving a speech in front of peers). Rather than presenting participants with a thermometer representing changes in levels of brain activity, participants could view signs of approval from a virtual audience as indices of effective up/down regulation.
It is worth pointing out the case for needing new interventive tools. First, although frontline psychological treatments such as CBT are effective (when compared to no treatment) in treating anxiety conditions in pediatric samples (Cartwright-Hatton et al., 2004; James et al., 2005; James et al., 2013), analysis of ‘number needed to treat’ data indicates that for every one young person who successfully attains remission from psychotherapy, six are required to be treated (James et al., 2013). This variability in outcome appears to be particularly apparent in social anxiety disorder (Hudson et al., submitted for publication; Knight et al., 2014). Pharmacological treatments can work, too, but concerns have been raised over long-term use in this age range, including concerns about increased suicidality following some antidepressant usage (Muris, 2012). In general, both psychological and pharmacological treatments can be difficult to access and costly to administer. Thus, novel, targeted interventions that are engaging and easy to access are much needed. A second consideration is the suitability of current interventions for pediatric samples. Both psychological and pharmacological treatments were developed primarily for adults. Although the same can be said for cognitive training and neurofeedback techniques, if effective, these tools could be argued to reflect more age-appropriate interventions. As reviewed in earlier sections, the neural substrates of emotion regulation are still undergoing protracted maturation during adolescence. Therefore, one might expect targeted NF intervention on increasingly specialized social brain networks to be most effective at the adolescent juncture. In turn, complementary cognitive and neurofeedback training could yield more positive trajectories of change at the behavioral but also neurocognitive levels of functioning. Of course these suggestions on beneficial developmental timing of interventions may be applicable to other traditional interventions that target cognitive biases as well.
We would like to suggest CBM methods and fMRI-based NF as non-invasive and easily acquired techniques that can target maturing neurocognitive circuitry involved in SAD directly. There is still a lot to be learnt about the mechanisms by which these methods could achieve therapeutic benefits. It remains to be seen whether CBM methods can impact brain functioning both in terms of neural patterns of activation during emotion-processing, but also in terms of more long-term indices of functioning such as the reorganization of neural connections and circuits. While there is little research to support these speculations, it is notable that in adults, other psychological interventions such as CBT have been reported to alter indices of neural vulnerability for mood and anxiety disorders (e.g., Lipka et al., 2013, Porto et al., 2009, Straube et al., 2006). By timing these interventive approaches to coincide with a period of substantial brain and cognitive development, such as adolescence, it is likely that intervention-induced changes to neurocognitive circuitry will have knock-on effects on behavior that are stronger and more persistent than at other developmental stages (Cohen Kadosh et al., 2013).
6. Concluding remarks and future directions
In this review, we have summarized neurocognitive risk factors associated with adolescent SAD and delineated ways in which normative neurodevelopmental progressions may contribute to adolescence-associated increases in social pre-occupation and affective responding. Some of the unique maturational changes in adolescence may serve to exacerbate individual differences in SAD vulnerability. But, by the same token, we have argued that these normative changes may also allow for the adolescent's development into a socially competent, independent individual and facilitate developmentally-sensitive learning. We have therefore discussed adolescence as an optimal time for administering neurocognitive interventions. Recently, attention has been drawn to the need for greater crosstalk between neuroscience and clinical science to accelerate progress toward better comprehension and improvement of evidence-based mental health treatments (Holmes et al., 2014). Understanding the mechanisms by which normative neurodevelopmental changes underpin the expression of SAD-linked risk factors in adolescence can advance current theoretical models of SAD and, in parallel, inform when early interventions should be administered to maximize benefits.
As some of the material in this review is speculative, it is worth being more concrete about specific lines of research to follow up on our suggestions. First, it would be important to establish whether there is indeed a developmental moderation of risk for SAD. More precisely, here, we make the suggestion that certain cognitive risk factors for social anxiety, such as interpretation biases, only emerge at particular stages of development. The presence of these biases would be contingent on a more sophisticated understanding of social situations and increased complexity of social interactions. There is some suggestion that interpretation biases only influence social anxiety during the adolescent years, through cross-sectional comparison of findings. However, more systematic, longitudinal investigations in the same sample could be conducted, assessing whether interpretation biases only contribute toward social anxiety at particular stages of development. An even better strategy would be to derive indices of social-cognitive development or underlying brain maturation as an index of development rather than chronological age. A second exciting area of research is to establish whether plasticity and specifically responsiveness to particular external interventions are in fact greater in adolescence compared to children and adults – and moreover, whether within adolescence there are particular ‘hotspots’. In this review, we have discussed growing evidence that learning and flexibility may be greater during adolescence, but this data has yet to be extended to responsiveness to particular anxiety-reduction techniques or interventions. These data would carry important implications for when to administer interventions. Finally, as mentioned at the end of Section 4, developing versions of CBM and NF training (separately or in combined form) that are optimally effective, for example by using task stimuli that are motivationally salient for adolescents should be a priority.
Conflict of interest
The authors declare that they have no conflict of interest.
Acknowledgements
S. H. is supported by a studentship from the Medical Research Council. J. L. has received funding from the ESRC, Calleva Research Centre and British Academy to conduct research in this area. The work on NF was supported by the European Commission FP7 Braintrain grant (602186). G. S. is funded by a James S. McDonnell Foundation (Understanding Human Cognition) Scholar Award.
Footnotes
Available online 28 February 2015
References
- Alfano C.A., Beidel D.C., Turner S.M. Cognitive correlates of social phobia among children and adolescents. J. Abnorm. Child Psychol. 2006;34:189–201. doi: 10.1007/s10802-005-9012-9. [DOI] [PubMed] [Google Scholar]
- Avital A., Richter-Levin G. Exposure to juvenile stress exacerbates the behavioural consequences of exposure to stress in the adult rat. Int. J. Neuropsychopharmacol. 2005;8:163–173. doi: 10.1017/S1461145704004808. [DOI] [PubMed] [Google Scholar]
- Bauman M.D., Lavenex P., Mason W.A., Capitanio J.P., Amaral D.G. The development of mother-infant interactions after neonatal amygdala lesions in rhesus monkeys. J. Neurosci. 2004;24(3):711–721. doi: 10.1523/JNEUROSCI.3263-03.2004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bauman M.D., Toscano J.E., Mason W.A., Lavenex P., Amaral D.G. The expression of social dominance following neonatal lesions of the amygdala or hippocampus in rhesus monkeys (Macaca mulatta) Behav. Neurosci. 2006;120:749–760. doi: 10.1037/0735-7044.120.4.749. [DOI] [PubMed] [Google Scholar]
- Bar-Haim Y., Fox N.A., Benson B., Guyer A.E., Williams A., Nelson E.E., Ernst M. Neural correlates of reward processing in adolescents with a history of inhibited temperament. Psychol Sci. 2009;20(8):1009–1018. doi: 10.1111/j.1467-9280.2009.02401.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bar-Haim Y., Lamy D., Pergamin L., Bakermans-Kranenburg M.J., van IJzendoorn M.H. Threat-related attentional bias in anxious and nonanxious individuals: a meta-analytic study. Psychol. Bull. 2007;133(1):1–24. doi: 10.1037/0033-2909.133.1.1. [DOI] [PubMed] [Google Scholar]
- Bar-Haim Y., Morag I., Glickman S. Training anxious children to disengage attention from threat: a randomized controlled trial. J. Child Psychol. Psychiatry Allied Discip. 2011;52(8):861–869. doi: 10.1111/j.1469-7610.2011.02368.x. [DOI] [PubMed] [Google Scholar]
- Battaglia M., Zanoni A., Taddei M., Giorda R., Bertoletti E., Lampis V., Tettamanti M. Cerebral responses to emotional expressions and the development of social anxiety disorder: a preliminary longitudinal study. Depress. Anxiety. 2012;29(1):54–61. doi: 10.1002/da.20896. [DOI] [PubMed] [Google Scholar]
- Blakemore S.-J. The social brain in adolescence. Nat. Rev. Neurosci. 2008;9(4):267–277. doi: 10.1038/nrn2353. [DOI] [PubMed] [Google Scholar]
- Blakemore S.J. Imaging brain development: the adolescent brain. Neuroimage. 2012;61(2):397–406. doi: 10.1016/j.neuroimage.2011.11.080. [DOI] [PubMed] [Google Scholar]
- Blakemore S.-J., Mills K.L. Is adolescence a sensitive period for sociocultural processing? Annu. Rev. Psychol. 2014;65:187–207. doi: 10.1146/annurev-psych-010213-115202. [DOI] [PubMed] [Google Scholar]
- Blakemore S.-J., den Ouden H., Choudhury S., Frith C. Adolescent development of the neural circuitry for thinking about intentions. Soc. Cogn. Affect. Neurosci. 2007;2(2):130–139. doi: 10.1093/scan/nsm009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bögels S.M., Zigterman D. Dysfunctional cognitions in children with social phobia, separation anxiety disorder, and generalized anxiety disorder. J. Abnorm. Child Psychol. 2000;28:205–211. doi: 10.1023/a:1005179032470. [DOI] [PubMed] [Google Scholar]
- Braams B.R., Peters S., Peper J.S., Güroğlu B., Crone E.A. Gambling for self, friends, and antagonists: differential contributions of affective and social brain regions on adolescent reward processing. Neuroimage. 2014;100:281–289. doi: 10.1016/j.neuroimage.2014.06.020. [DOI] [PubMed] [Google Scholar]
- Buwalda B., Geerdink M., Vidal J., Koolhaas J.M. Social behavior and social stress in adolescence: a focus on animal models. Neurosci. Biobehav. Rev. 2011;35(8):1713–1721. doi: 10.1016/j.neubiorev.2010.10.004. [DOI] [PubMed] [Google Scholar]
- Burnett S., Bird G., Moll J., Frith C., Blakemore S.-J. Development during adolescence of the neural processing of social emotion. J. Cogn. Neurosci. 2009;21(9):1736–1750. doi: 10.1162/jocn.2009.21121. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Butler R. Age trends in the use of social and temporal comparison for self-evaluation: examination of a novel developmental hypothesis. Child Dev. 1998;69(4):1054–1073. [PubMed] [Google Scholar]
- Cartwright-Hatton S., Hodges L., Porter J. Social anxiety in childhood: the relationship with self and observer rated social skills. J. Child Psychol. Psychiatry. 2003;44(5):737–742. doi: 10.1111/1469-7610.00159. [DOI] [PubMed] [Google Scholar]
- Cartwright-Hatton S., Roberts C., Chitsabesan P., Fothergill C., Harrington R. Systematic review of the efficacy of cognitive behaviour therapies for childhood and adolescent anxiety disorders. Br. J. Clin. Psychol. 2004;43:421–436. doi: 10.1348/0144665042388928. [DOI] [PubMed] [Google Scholar]
- Cartwright-Hatton S., Tschernitz N., Gomersall H. Social anxiety in children: social skills deficit, or cognitive distortion? Behav. Res. Ther. 2005;43(1):131–141. doi: 10.1016/j.brat.2003.12.003. [DOI] [PubMed] [Google Scholar]
- Chein J., Albert D., Brien L.O., Uckert K., Steinberg L. Peers increase adolescent risk taking by enhancing activity in the brain's reward circuitry. Dev. Sci. 2012;14(2):1–16. doi: 10.1111/j.1467-7687.2010.01035.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Clark D.M., Wells A. A cognitive model of social phobia. In: Heimberg R.G., Liebowitz M.R., Hope D., editors. Social Phobia – Diagnosis, Assessment, and Treatment. Guilford; New York: 1995. pp. 69–93. [Google Scholar]
- Cohen J.R., Asarnow R.F., Sabb F.W., Bilder R.M., Bookheimer S.Y., Knowlton B.J., Poldrack R.A. A unique adolescent response to reward prediction errors. Nat. Neurosci. 2010;13(6):669–671. doi: 10.1038/nn.2558. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cohen Kadosh K., Linden D.E.J., Lau J.Y. Plasticity during childhood and adolescence: innovative approaches to investigating neurocognitive development. Dev. Sci. 2013;16(4):574–583. doi: 10.1111/desc.12054. [DOI] [PubMed] [Google Scholar]
- Coleman J.C. Routledge & Kegan Paul; London: 1974. Relationships in Adolescence. [Google Scholar]
- Cowart M.J.W., Ollendick T.H. Attention training in socially anxious children: a multiple baseline design analysis. J. Anxiety Disord. 2011;25:972–977. doi: 10.1016/j.janxdis.2011.06.005. [DOI] [PubMed] [Google Scholar]
- Creswell C., Murray L., Cooper P. Interpretation and expectation in childhood anxiety disorders: age effects and social specificity. J. Abnorm. Child Psychol. 2013;42(3):453–465. doi: 10.1007/s10802-013-9795-z. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Crone E.A., Dahl R.E. Understanding adolescence as a period of social-affective engagement and goal flexibility. Nat. Rev. Neurosci. 2012;13(9):636–650. doi: 10.1038/nrn3313. [DOI] [PubMed] [Google Scholar]
- Crone E.A., van der Molen M.W. Development of decision making in school-aged children and adolescents: evidence from heart rate and skin conductance analysis. Child Dev. 2007;78:1288–1301. doi: 10.1111/j.1467-8624.2007.01066.x. [DOI] [PubMed] [Google Scholar]
- Crone E.A., Zanolie K., van Leijenhorst L., Westenberg P., Rombouts S.A. Neural mechanisms supporting flexible performance adjustment during development. Cogn. Affect. Behav. Neurosci. 2008;8:165–177. doi: 10.3758/cabn.8.2.165. [DOI] [PubMed] [Google Scholar]
- deCharms R.C. Reading and controlling human brain activation using real-time functional magnetic resonance imaging. Trends Cogn. Sci. 2007;11:473–481. doi: 10.1016/j.tics.2007.08.014. [DOI] [PubMed] [Google Scholar]
- deCharms R.C., Maeda F., Glover G.H., Ludlow D., Pauly J.M., Soneji D., Gabrieli J.D.E., Mackey S.C. Control over brain activation and pain learned by using real-time functional MRI. Proc. Natl. Acad. Sci. 2005;102:18626–18631. doi: 10.1073/pnas.0505210102. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dumontheil I., Apperly I.A., Blakemore S.-J. Online usage of theory of mind continues to develop in late adolescence. Dev. Sci. 2010;13(2):331–338. doi: 10.1111/j.1467-7687.2009.00888.x. [DOI] [PubMed] [Google Scholar]
- Dreyfuss M., Caudle K., Drysdale A.T., Johnston N.E., Cohen A.O., Somerville L.H., Casey B.J. Teens impulsively react rather than retreat from threat. Dev. Neurosci. 2014;36(3–4):220–227. doi: 10.1159/000357755. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Eldar S., Apter A. Attention bias modification treatment for pediatric anxiety disorders: a randomized controlled trial. Am. J. Psychiatry. 2012;169(2):213–220. doi: 10.1176/appi.ajp.2011.11060886. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Elkind D. Egocentrism in adolescence. Child Dev. 1967;38:1025–1034. [PubMed] [Google Scholar]
- Elkind D. Egocentrism redux. Dev. Rev. 1985;5(3):218–226. [Google Scholar]
- Elkind D., Bowen R. Imaginary audience behavior in children and adolescents. Dev. Psychol. 1979;15(1):38–44. [Google Scholar]
- Erath S.A., Flanagan K.S., Bierman K.L. Social anxiety and peer relations in early adolescence: behavioral and cognitive factors. J. Abnorm. Child Psychol. 2007;35:405–416. doi: 10.1007/s10802-007-9099-2. [DOI] [PubMed] [Google Scholar]
- Ernst M., Nelson E.E., Jazbec S., McClure E.B., Monk C.S., Leibenluft E., Pine D.S. Amygdala and nucleus accumbens in responses to receipt and omission of gains in adults and adolescents. Neuroimage. 2005;25(4):1279–1291. doi: 10.1016/j.neuroimage.2004.12.038. [DOI] [PubMed] [Google Scholar]
- Etkin A., Egner T., Peraza D.M., Kandel E.R., Hirsch J. Resolving emotional conflict: a role for the rostral anterior cingulate cortex in modulating activity in the amygdala. Neuron. 2006;51:871–882. doi: 10.1016/j.neuron.2006.07.029. [DOI] [PubMed] [Google Scholar]
- Fair D.A., Schlaggar B.L., Cohen A.L., Miezin F.M., Dosenbach N.U., Wenger K.K., Fox M.D., Snyder A.Z., Raichle M.E., Petersen S.E. A method for using blocked and event-related fMRI data to study resting state functional connectivity. Neuroimage. 2007;35:396–405. doi: 10.1016/j.neuroimage.2006.11.051. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Field A.P. The behavioral inhibition system and the verbal information pathway to children's fears. J. Abnorm. Psychol. 2006;115(4):742. doi: 10.1037/0021-843X.115.4.742. [DOI] [PubMed] [Google Scholar]
- Forbes E.E., Dahl R.E. Pubertal development and behavior: hormonal activation of social and motivational tendencies. Brain Cogn. 2010;72(1):66–72. doi: 10.1016/j.bandc.2009.10.007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fu X., Du Y., Au S., Lau J.Y.F. Reducing negative interpretations in adolescents with anxiety disorders: a preliminary study investigating the effects of a single session of cognitive bias modification training. Dev. Cogn. Neurosci. 2013;4:29–37. doi: 10.1016/j.dcn.2012.11.003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gallagher M., Hollandt P.C. The amygdala complex: Multiple roles in associative learning and attention. Proc. Natl. Acad. Sci. U. S. A. 1994;91:11771–11776. doi: 10.1073/pnas.91.25.11771. (Review) [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gee D.G., Humphreys K.L., Flannery J., Goff B., Telzer E.H., Shapiro M., Tottenham N. A developmental shift from positive to negative connectivity in human amygdala-prefrontal circuitry. J. Neurosci. 2013;33(10):4584–4593. doi: 10.1523/JNEUROSCI.3446-12.2013. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Goddings A.L., Mills K.L., Clasen L.S., Giedd J.N., Viner R.M., Blakemore S.J. The influence of puberty on subcortical brain development. Neuroimage. 2014;88:242–251. doi: 10.1016/j.neuroimage.2013.09.073. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gregory A.M., Ph D., Caspi A., Moffitt T.E., Koenen K., Eley T.C., Poulton R. Juvenile mental health histories of adults with anxiety disorders. Am. J. Psychiatry. 2007;164(2):301–308. doi: 10.1176/ajp.2007.164.2.301. [DOI] [PubMed] [Google Scholar]
- Moor B.G., Güroğlu B., Op de Macks Z.A., Rombouts S.A.R.B., Van der Molen M.W., Crone E.A. Social exclusion and punishment of excluders: neural correlates and developmental trajectories. Neuroimage. 2012;59(1):708–717. doi: 10.1016/j.neuroimage.2011.07.028. [DOI] [PubMed] [Google Scholar]
- Moor B.G., Macks Z.A., Güroglu B., Rombouts S.A., Molen M.W., Crone E.A. Neurodevelopmental changes of reading the mind in the eyes. Soc. Cogn. Affect. Neurosci. 2011;7(1):44–52. doi: 10.1093/scan/nsr020. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Moor B.G., van Leijenhorst L., Rombouts S.A., Crone E.A., van der Molen M.W. Do you like me? Neural correlates of social evaluation and developmental trajectories. Soc. Neurosci. 2010;5(5–6):461–482. doi: 10.1080/17470910903526155. [DOI] [PubMed] [Google Scholar]
- Güroğlu B., van Den Bos W., Crone E.A. Sharing and giving across adolescence: an experimental study examining the development of prosocial behavior. Front. Psychol. 2014;5 doi: 10.3389/fpsyg.2014.00291. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Guyer A.E., Benson B., Choate V.R., Bar-Haim Y., Perez-Edgar K., Jarcho J.M., Pine D.S., Ernst M., Fox N.A., Nelson E.E. Lasting associations between early childhood temperament and late-adolescent reward-circuitry response to peer feedback. Dev. Psychopathol. 2014;26(01):229–243. doi: 10.1017/S0954579413000941. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Guyer A.E., Lau J.Y.F., Mcclure-tone E.B., Parrish J., Fox N.A., Ernst M., Nelson E.E. Amygdala and ventrolateral prefrontal cortex function during anticipated peer evaluation in pediatric social anxiety. Arch. Gen. Psychiatry. 2008;65(11):1303–1312. doi: 10.1001/archpsyc.65.11.1303. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Guyer A.E., Mcclure-tone E.B., Shiffrin N.D., Nelson E.E. Probing the neural correlates of anticipated peer evaluation in adolescence. Child Dev. 2009;80(4):1000–1015. doi: 10.1111/j.1467-8624.2009.01313.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Haller S.P.W., Kadosh K.C., Lau J.Y.F. A developmental angle to understanding the mechanisms of biased cognitions in social anxiety. Front. Hum. Neurosci. 2014;7:846. doi: 10.3389/fnhum.2013.00846. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hauser T.U., Iannaccone R., Walitza S., Brandeis D., Brem S. Cognitive flexibility in adolescence: neural and behavioral mechanisms of reward prediction error processing in adaptive decision making during development. Neuroimage. 2015;104:347–354. doi: 10.1016/j.neuroimage.2014.09.018. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hardee J.E., Benson B.E., Bar-Haim Y., Mogg K., Bradley B.P., Chen G. Patterns of neural connectivity during an attention bias task moderate associations between early childhood temperament and internalizing symptoms in young adulthood. Biol. Psychiatry. 2013;74:273–279. doi: 10.1016/j.biopsych.2013.01.036. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hare T.A., Tottenham N., Galvan A., Voss H.U., Glover G.H., Casey B.J. Biological substrates of emotional reactivity and regulation in adolescence during an emotional go-nogo task. Biol. Psychiatry. 2008;63(10):927–934. doi: 10.1016/j.biopsych.2008.03.015015. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Harter S. Handbook of Child Psychology. Wiley & Sons; 2006. The self. [Google Scholar]
- Helfinstein S.M., Benson B., Perez-Edgar K., Bar-Haim Y., Detloff A., Pine D.S., Ernst M. Striatal responses to negative monetary outcomes differ between temperamentally inhibited and non-inhibited adolescents. Neuropsychologia. 2011;49(3):479–485. doi: 10.1016/j.neuropsychologia.2010.12.015. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Holmes E.A., Craske M.G., Graybiel A.M. Psychological treatments: a call for mental-health science. Nature. 2014;511(7509):287–289. doi: 10.1038/511287a. [DOI] [PubMed] [Google Scholar]
- Hooper C.J., Luciana M., Conklin H.M., Yarger R.S. Adolescents’ performance on the Iowa gambling task: implications for the development of decision making and ventromedial prefrontal cortex. Dev. Psychol. 2004;40:1148–1158. doi: 10.1037/0012-1649.40.6.1148. [DOI] [PubMed] [Google Scholar]
- Herting M.M., Gautam P., Spielberg J.M., Kan E., Dahl R.E., Sowell E.R. The role of testosterone and estradiol in brain volume changes across adolescence: a longitudinal structural MRI study. Hum. Brain Mapp. 2014;35:5633–5645. doi: 10.1002/hbm.22575. [DOI] [PMC free article] [PubMed] [Google Scholar]
- In-Albon T., Dubi K., Rapee R.M., Schneider S. Forced choice reaction time paradigm in children with separation anxiety disorder, social phobia, and nonanxious controls. Behav. Res. Ther. 2009;47(12):1058–1065. doi: 10.1016/j.brat.2009.08.003. [DOI] [PubMed] [Google Scholar]
- Jarcho J.M., Fox N.A., Pine D.S., Etkin A., Leibenluft E., Shechner T. The neural correlates of emotion-based cognitive control in adults with early childhood behavioral inhibition. Biol. Psychol. 2013;92:306–314. doi: 10.1016/j.biopsycho.2012.09.008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jarcho J.M., Fox N.A., Pine D.S., Leibenluft E., Shechner T., Degnan K.A. Enduring influence of early temperament on neural mechanisms mediating attention–emotion conflict in adults. Depress. Anxiety. 2014;31:53–62. doi: 10.1002/da.22140. [DOI] [PMC free article] [PubMed] [Google Scholar]
- James A.A.C.J., Soler A., Weatherall R.R.W. Cognitive behavioural therapy for anxiety disorders in children and adolescents. Cochrane Database Syst. Rev. 2005;4 doi: 10.1002/14651858.CD004690.pub2. [DOI] [PubMed] [Google Scholar]
- James A.C., James G., Cowdrey F.A., Soler A., Choke A. Cognitive behavioural therapy for anxiety disorders in children and adolescents. Cochrane Database Syst. Rev. 2013;6 doi: 10.1002/14651858.CD004690.pub3. [DOI] [PubMed] [Google Scholar]
- Jarcho J.M., Leibenluft E., Walker O.L., Fox N.A., Pine D.S., Nelson E.E. Neuroimaging studies of pediatric social anxiety: paradigms, pitfalls and a new direction for investigating the neural mechanisms. Biol. Mood Anxiety Disord. 2013;3(1):14. doi: 10.1186/2045-5380-3-14. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Johnston S., Boehm S., Healy D., Goebel R., Linden D. Neurofeedback: a promising tool for the self-regulation of emotion networks. Neuroimage. 2010;49:1066–1072. doi: 10.1016/j.neuroimage.2009.07.056. [DOI] [PubMed] [Google Scholar]
- Johnston S., Linden D.E.J., Healy D., Goebel R., Habes I., Boehm S.G. Upregulation of emotion areas through neurofeedback with a focus on positive mood. Cogn. Affect. Behav. Neurosci. 2011;11:44–51. doi: 10.3758/s13415-010-0010-1. [DOI] [PubMed] [Google Scholar]
- Jones R.M., Somerville L.H., Li J., Ruberry E.J., Powers A., Mehta N., Casey B.J. Adolescent-specific patterns of behavior and neural activity during social reinforcement learning. Cogn. Affect. Behav. Neurosci. 2014:1–15. doi: 10.3758/s13415-014-0257-z. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Johnson C., Wilbrecht L. Juvenile mice show greater flexibility in multiple choice reversal learning than adults. Dev. Cogn. Neurosci. 2011;1:540–551. doi: 10.1016/j.dcn.2011.05.008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kaiser S., Harderthauer S., Sachser N., Hennessy M.B. Social housing conditions around puberty determine later changes in plasma cortisol levels and behavior. Physiol. Behav. 2007;90:405–411. doi: 10.1016/j.physbeh.2006.10.002. [DOI] [PubMed] [Google Scholar]
- Kessler R.C., Berglund P., Demler O., Jin R., Merikangas K.R., Walters E.E. Lifetime prevalence and age-of-onset distributions of DSM-IV disorders in the National Comorbidity Survey Replication. Arch. Gen. Psychiatry. 2005;62:593–602. doi: 10.1001/archpsyc.62.6.593. [DOI] [PubMed] [Google Scholar]
- Kessler R.C., Chiu W.T., Demler O., Merikangas K.R., Walters E.E. Prevalence, severity, and comorbidity of 12-month DSM-IV disorders in the National Comorbidity Survey Replication. Arch. Gen. Psychiatry. 2005;62(6):617–627. doi: 10.1001/archpsyc.62.6.617. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Killgore W.D.S., Deborah A.Y. Social anxiety predicts amygdala activation in adolescents viewing fearful faces. Neuroreport. 2005;16(15):1671–1675. doi: 10.1097/01.wnr.0000180143.99267.bd. [DOI] [PubMed] [Google Scholar]
- Kindt M., Bögels S., Morren M. Processing bias in children with separation anxiety disorder, social phobia and generalised anxiety disorder. Behav. Change. 2003;20:143–150. [Google Scholar]
- Kloep M. Love is all you need? Focusing on adolescents’ life concerns from an ecological point of view. J. Adolesc. 1999;22:49–63. doi: 10.1006/jado.1998.0200. [DOI] [PubMed] [Google Scholar]
- Knight A., McLellan L., Jones M., Hudson J. Pre-treatment predictors of outcome in childhood anxiety disorders: a systematic review. Psychopathol. Rev. 2014;1(1):77–129. [Google Scholar]
- Lau J.Y. Cognitive bias modification of interpretations: a viable treatment for child and adolescent anxiety? Behav. Res. Ther. 2013;51(10):614–622. doi: 10.1016/j.brat.2013.07.001. [DOI] [PubMed] [Google Scholar]
- Lau J.Y., Britton J.C., Nelson E.E., Angold A., Ernst M., Goldwin M., Pine D.S. Distinct neural signatures of threat learning in adolescents and adults. Proc. Natl. Acad. Sci. U. S. A. 2011;108(11):4500–4505. doi: 10.1073/pnas.1005494108. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lau J.Y.F., Guyer A.E., Tone E.B., Jenness J., Parrish J.M., Pine D.S., Nelson E.E. Neural responses to peer rejection in anxious adolescents: contributions from the amygdala-hippocampal complex. Int. J. Behav. Dev. 2011;36(1):36–44. doi: 10.1177/0165025411406854. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lerner R.M., Steinberg L. John Wiley & Sons; New Jersey: 2004. Handbook of Adolescent Psychology. [Google Scholar]
- Linden D.E., Habes I., Johnston S.J., Linden S., Tatineni R., Subramanian L., Sorger B., Healy D., Goebel R. Real-time self-regulation of emotion networks in patients with depression. PLoS ONE. 2012;7:e38115. doi: 10.1371/journal.pone.0038115. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Linden D.E. Neurofeedback and networks of depression. Dialogues clin. neurosci. 2014;16(1):103. doi: 10.31887/DCNS.2014.16.1/dlinden. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lipka J., Hoffmann M., Miltner W.H., Straube T. Effects of cognitive-behavioral therapy on brain responses to subliminal and supraliminal threat and their functional significance in specific phobia. Biol. Psychiatry. 2013;14 doi: 10.1016/j.biopsych.2013.11.008. [DOI] [PubMed] [Google Scholar]
- Leussis M.P., Andersen S.L. Is adolescence a sensitive period for depression? Behavioral and neuroanatomical findings from a social stress model. Synapse. 2008;62:22–30. doi: 10.1002/syn.20462. [DOI] [PubMed] [Google Scholar]
- Leussis M.P., Lawson K., Stone K., Andersen S.L. The enduring effects of an adolescent social stressor on synaptic density, part II: Poststress reversal of synaptic loss in the cortex by adinazolam and MK-801. Synapse. 2008;62:185–192. doi: 10.1002/syn.20483. [DOI] [PubMed] [Google Scholar]
- Mathews A., Mackintosh B. Induced emotional interpretation bias and anxiety. J. Abnorm. Psychol. 2000;109(4):602. [PubMed] [Google Scholar]
- Machado C.J., Bachevalier J. The impact of selective amygdala, orbital frontal cortex, or hippocampal formation lesions on established social relationships in rhesus monkeys (Macaca mulatta) Behav. Neurosci. 2006;120:761–786. doi: 10.1037/0735-7044.120.4.761. [DOI] [PubMed] [Google Scholar]
- Masten C.L., Eisenberger N.I., Borofsky L.A., Pfeifer J.H., McNealy K., Mazziotta J.C., Dapretto M. Neural correlates of social exclusion during adolescence: understanding the distress of peer rejection. Soc. Cogn. Affect. Neurosci. 2009;4(2):143–157. doi: 10.1093/scan/nsp007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- McClure E.B., Monk C.S., Nelson E.E., Parrish J.M., Adler A., Blair R.J.R., Pine D.S. Abnormal attention modulation of fear circuit function in pediatric generalized anxiety disorder. Arch. Gen. Psychiatry. 2007;64(1):97–106. doi: 10.1001/archpsyc.64.1.97. [DOI] [PubMed] [Google Scholar]
- McRae K., Gross J.J., Weber J., Robertson E.R., Sokol-Hessner P., Ray R.D., Gabrieli J.D.E., Ochsner K.N. The development of emotion regulation: an fMRI study of cognitive reappraisal in children, adolescents and young adults. Soc. Cogn. Affect. Neurosci. 2012;7(1):11–22. doi: 10.1093/scan/nsr093. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Miers A.C., Blöte A.W., Bögels S.M., Westenberg P.M. Interpretation bias and social anxiety in adolescents. J. Anxiety Disord. 2008;22:1462–1471. doi: 10.1016/j.janxdis.2008.02.010. [DOI] [PubMed] [Google Scholar]
- Miers A.C., Bloete A.W., Westenberg P.M. Peer perceptions of social skills in socially anxious and nonanxious adolescents. J. Abnorm. Child Psychol. 2010;38:33–41. doi: 10.1007/s10802-009-9345-x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Miers A.C., Blöte A.W., Heyne D.A., Westenberg P.M. Developmental pathways of social avoidance across adolescence: the role of social anxiety and negative cognition. J. Anxiety Disord. 2014;28:787–794. doi: 10.1016/j.janxdis.2014.09.008. [DOI] [PubMed] [Google Scholar]
- Monk C.S., McClure E.B., Nelson E.E., Zarahn E., Bilder R.M., Leibenluft E., Pine D.S. Adolescent immaturity in attention-related brain engagement to emotional facial expressions. Neuroimage. 2003;20(1):420–428. doi: 10.1016/s1053-8119(03)00355-0. [DOI] [PubMed] [Google Scholar]
- Moriguchi Y., Ohnishi T., Mori T., Matsuda H., Komaki G. Changes of brain activity in the neural substrates for theory of mind during childhood and adolescence. Psychiatry Clin. Neurosci. 2007;61(4):355–363. doi: 10.1111/j.1440-1819.2007.01687.x. [DOI] [PubMed] [Google Scholar]
- Morgan J., Banerjee R. Social anxiety and self-evaluation of social performance in a nonclinical sample of children. J. Clin. Child Adolesc. Psychol. 2006;35:292–301. doi: 10.1207/s15374424jccp3502_13. [DOI] [PubMed] [Google Scholar]
- Muris P. Treatment of childhood anxiety disorders: what is the place for antidepressants? Expert Opin. Pharmacother. 2012;13(1):43–64. doi: 10.1517/14656566.2012.642864. [DOI] [PubMed] [Google Scholar]
- Muris P., Field A.P. Distorted cognition and pathological anxiety in children and adolescents. Cogn. Emotion. 2008;22(3):395–421. [Google Scholar]
- Muris P., Merckelbach H., Damsma E. Threat perception bias in nonreferred, socially anxious children. J. Clin. Child Psychol. 2000;29:348–359. doi: 10.1207/S15374424JCCP2903_6. [DOI] [PubMed] [Google Scholar]
- Nelson E.E., Leibenluft E., McClure E.B., Pine D.S. The social re-orientation of adolescence: a neuroscience perspective on the process and its relation to psychopathology. Psychol. Med. 2005;35(2):163–174. doi: 10.1017/s0033291704003915. [DOI] [PubMed] [Google Scholar]
- Nelson E.E., Lau J.Y., Jarcho J.M. Growing pains and pleasures: how emotional learning guides development. Trends Cogn. Sci. 2014;18(2):99–108. doi: 10.1016/j.tics.2013.11.003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Nelson C.A., Zeanah C.H., Fox N.A., Marshall P.J., Smyke A., Guthrie D. Cognitive recovery in socially deprived young children: the Bucharest Early Intervention Project. Science. 2007;318:1937–1940. doi: 10.1126/science.1143921. [DOI] [PubMed] [Google Scholar]
- O’Brien S.F., Bierman K.L. Conception and perceived influence of peer groups – interviews with preadolescents and adolescents. Child Dev. 1988;59:1360–1365. doi: 10.1111/j.1467-8624.1988.tb01504.x. [DOI] [PubMed] [Google Scholar]
- Overgaauw S., van Duijvenvoorde A.C., Moor B.G., Crone E.A. A longitudinal analysis of neural regions involved in reading the mind in the eyes. Soc. Cogn. Affect. Neurosci. 2014 doi: 10.1093/scan/nsu095. (in press) [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pascalis O., Scott L.S., Kelly D.J., Shannon R.W., Nicholson E., Coleman M., Nelson C.A. Plasticity of face processing in infancy. Proc. Natl. Acad. Sci. U. S. A. 2005;102(14):5297–5300. doi: 10.1073/pnas.0406627102. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Passarotti A.M., Sweeney J.a., Pavuluri M.N. Neural correlates of incidental and directed facial emotion processing in adolescents and adults. Soc. Cogn. Affect. Neurosci. 2009;4(4):387–398. doi: 10.1093/scan/nsp029. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pass L., Arteche A., Cooper P., Creswell C., Murray L. Doll Play narratives about starting school in children of socially anxious mothers, and their relation to subsequent child school-based anxiety. J. Abnorm. Child Psychol. 2012;40(8):1375–1384. doi: 10.1007/s10802-012-9645-4. [DOI] [PubMed] [Google Scholar]
- Peper J.S., Dahl R.E. Surging hormones: brain–behavior interactions during puberty. Curr. Dir. Psychol. Sci. 2013;22(2):134–139. doi: 10.1177/0963721412473755. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pérez-edgar K., Roberson-nay R., Hardin M.G., Poeth K., Guyer A.E., Nelson E.E., McClure E.B., Henderson H.A., Fox N.A., Pine D.S., Ernst M. Attention alters neural responses to evocative faces in behaviorally inhibited adolescents. Neuroimage. 2007;35:1538–1546. doi: 10.1016/j.neuroimage.2007.02.006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Perez-Edgar K., Bar-Haim Y., McDermott J.M., Chronis-Tuscano A., Pine D.S., Fox N.A. Attention biases to threat and behavioral inhibition in early childhood shape adolescent social withdrawal. Emotion. 2010;10:349–357. doi: 10.1037/a0018486. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pfeifer J.H., Masten C.L., Borofsky L.A., Dapretto M., Fuligni A.J., Lieberman M.D. Neural correlates of direct and reflected self-appraisals in adolescents and adults: when social perspective-taking informs self-perception. Child Dev. 2009;80(4):1016–1038. doi: 10.1111/j.1467-8624.2009.01314.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pfeifer J.H., Masten C.L., Moore W.E., III, Oswald T.M., Mazziotta J.C., Iacoboni M., Dapretto M. Entering adolescence: resistance to peer influence, risky behavior, and neural changes in emotion reactivity. Neuron. 2011;69:1029–1036. doi: 10.1016/j.neuron.2011.02.019. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Porto P., Oliveira L., Mari J., Volchan E., Figueira I., Ventura P. Does cognitive behavioral therapy change the brain: a systematic review of neuroimaging in anxiety disorders. J. Neuropsychiatry Clin. Neurosci. 2009;21:114–125. doi: 10.1176/jnp.2009.21.2.114. [DOI] [PubMed] [Google Scholar]
- Pitskel N.B., Bolling D.Z., Kaiser M.D., Crowley M.J., Pelphrey K.A. How grossed out are you? The neural bases of emotion regulation from childhood to adolescence. Dev. Cogn. Neurosci. 2011;1(3):324–337. doi: 10.1016/j.dcn.2011.03.004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rapee R.M., Spence S.H. The etiology of social phobia: empirical evidence and an initial model. Clin. Psychol. Rev. 2004;24(7):737–767. doi: 10.1016/j.cpr.2004.06.004. [DOI] [PubMed] [Google Scholar]
- Reeb-Sutherland B.C., Vanderwert R.E., Degnan K.A., Marshall P.M., Perez Edgar K., Chronis-Tuscano A. Attention to novelty in behaviorally inhibited adolescents moderates risk for anxiety. J. Child Psychol. Psychiatry. 2009;50:1365–1372. doi: 10.1111/j.1469-7610.2009.02170.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Reijntjes A., Stegge H., Terwogt M.M., Kamphuis J.H., Telch M.J. Children's coping with in vivo peer rejection: an experimental investigation. J. Abnorm. Child Psychol. 2006;34:877–889. doi: 10.1007/s10802-006-9061-8. [DOI] [PubMed] [Google Scholar]
- Rheingold A.A., Herbert J.D., Franklin M.E. Cognitive bias in adolescents with social anxiety disorder. Cogn. Ther. Res. 2003;27:639–655. [Google Scholar]
- Romeo R.D., Richardson H.N., Sisk C.L. Puberty and the maturation of the male brain and sexual behavior: recasting a behavioral potential. Neurosci. Biobehav. Rev. 2002;26(2002):381–391. doi: 10.1016/s0149-7634(02)00009-x. [DOI] [PubMed] [Google Scholar]
- Romeo R.D., Karatsoreos I.N., McEwen B.S. Pubertal maturation and time of day differentially affect behavioral and neuroendocrine responses following an acute stressor. Horm. Behav. 2006;50:463–468. doi: 10.1016/j.yhbeh.2006.06.002. [DOI] [PubMed] [Google Scholar]
- Romeo R.D., Bellani R., Karatsoreos I.N., Chhua N., Vernov M., Conrad C.D., McEwen B.S. Stress history and pubertal development interact to shape hypothalamic–pituitary–adrenal axis plasticity. Endocrinology. 2006;147:1664–1674. doi: 10.1210/en.2005-1432. [DOI] [PubMed] [Google Scholar]
- Roy A.K., Ph D., Vasa R.A., Bruck M., Mogg K., Bradley P., CAMS Team Attention bias toward threat in pediatric anxiety disorders. J. Am. Acad. Child Adolesc. Psychiaty. 2009;47(10):1189–1196. doi: 10.1097/CHI.0b013e3181825ace. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Scherf K.S., Behrmann M., Dahl R.E. Facing changes and changing faces in adolescence: a new model for investigating adolescent-specific interactions between pubertal, brain and behavioral development. Dev. Cogn. Neurosci. 2012;2(2):199–219. doi: 10.1016/j.dcn.2011.07.016. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sebastian C.L., Tan G.C.Y., Roiser J.P., Viding E., Dumontheil I., Blakemore S.-J. Developmental influences on the neural bases of responses to social rejection: implications of social neuroscience for education. Neuroimage. 2011;57(3):686–694. doi: 10.1016/j.neuroimage.2010.09.063. [DOI] [PubMed] [Google Scholar]
- Shechner T., Rimon-Chakir A., Britton J.C., Lotan D., Apter A., Bliese P.D., Pine D.S., Bar-Haim Y. Attention bias modification treatment augmenting effects on cognitive behavioral therapy in children with anxiety: randomized controlled trial. J. Am. Acad. Child Adolesc. Psychiatry. 2014;53(1):61–71. doi: 10.1016/j.jaac.2013.09.016. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sisk C.L., Foster D.L. The neural basis of puberty and adolescence. Nat. Neurosci. 2004;7(10):1040–1047. doi: 10.1038/nn1326. [DOI] [PubMed] [Google Scholar]
- Somerville L.H., Hare T.A., Casey B.J. Fronto striatal maturation predicts behavioral regulation failures to appetitive cues in adolescence. J. Cogn. Neurosci. 2011;23(9):2123–2134. doi: 10.1162/jocn.2010.21572. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Straube T., Glauer M., Dilger S., Mentzel H.J., Miltner W.H. Effects of cognitive-behavioral therapy on brain activation in specific phobia. Neuroimage. 2006;29:125–135. doi: 10.1016/j.neuroimage.2005.07.007. [DOI] [PubMed] [Google Scholar]
- Spence S.H., Donovan C., Brechman-Toussaint M. Social skills, social outcomes, and cognitive features of childhood social phobia. J. Abnorm. Psychol. 1999;108:211–221. doi: 10.1037//0021-843x.108.2.211. [DOI] [PubMed] [Google Scholar]
- Sportel B.E., de Hullu E., de Jong P.J., Nauta M.H. Cognitive bias modification versus CBT in reducing adolescent social anxiety: a randomized controlled trial. PLOS ONE. 2013;8(5):e64355. doi: 10.1371/journal.pone.0064355. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Steinberg L., Silverberg S.B. The vicissitudes of autonomy in early adolescence. Child Dev. 1986;57(4):841–851. doi: 10.1111/j.1467-8624.1986.tb00250.x. [DOI] [PubMed] [Google Scholar]
- Stirling L.J., Eley T.C., Clark D.M. Preliminary evidence for an association between social anxiety symptoms and avoidance of negative faces in school-age children. J. Clin. Child Adolesc. Psychol. 2006;35(3):440–445. doi: 10.1207/s15374424jccp3503_9. [DOI] [PubMed] [Google Scholar]
- Sturman D.A., Moghaddam B. The neurobiology of adolescence: changes in brain architecture, functional dynamics, and behavioral tendencies. Neurosci. Behav. Rev. 2011;35:1704–1712. doi: 10.1016/j.neubiorev.2011.04.003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Van den Bos W., van Dijk E., Westenberg M., Rombouts S.A., Crone E.A. Changing brains, changing perspectives: the neurocognitive development of reciprocity. Psychol. Sci. 2011;22:60–70. doi: 10.1177/0956797610391102. [DOI] [PubMed] [Google Scholar]
- Van den Bos W., van Dijk E., Crone E.A. Learning whom to trust in repeated social interactions: a developmental perspective. Group Process. Intergroup Relat. 2012;15(2):243–256. [Google Scholar]
- Van Duijvenvoorde A.C., Zanolie K., Rombouts S.A., Raijmakers M.E., Crone E.A. Evaluating the negative or valuing the positive? Neural mechanisms supporting feedback-based learning across development. J. Neurosci. 2008;28(38):9495–9503. doi: 10.1523/JNEUROSCI.1485-08.2008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Van Leijenhorst L., Zanolie K., Van Meel C.S., Westenberg P.M., Rombouts S.A.R.B., Crone E.A. What motivates the adolescent? Brain regions mediating reward sensitivity across adolescence. Cereb. Cortex. 2010;20(1):61–69. doi: 10.1093/cercor/bhp078. [DOI] [PubMed] [Google Scholar]
- Vetter N.C., Leipold K., Kliegel M., Phillips L.H., Altgassen M. Ongoing development of social cognition in adolescence. Child Neuropsychol. 2013;19(6):615–629. doi: 10.1080/09297049.2012.718324. [DOI] [PubMed] [Google Scholar]
- Wang T., Lee S.S., Sigman M., Dapretto M. Developmental changes in the neural basis of interpreting communicative intent. Soc. Cogn. Affect. Neurosci. 2006;1(2):107–121. doi: 10.1093/scan/nsl018. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Waters A.M., Pittaway M., Mogg K., Bradley B.P., Pine D.S. Attention training towards positive stimuli in clinically anxious children. Dev. Cogn. Neurosci. 2013;4:77–84. doi: 10.1016/j.dcn.2012.09.004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Weiskopf N., Mathiak K., Bock S.W., Scharnowski F., Veit R., Grodd R., Goebel R., Birbaumer N. Principles of a brain–computer interface (BCI) based on real-time functional magnetic resonance imaging (fMRI) IEEE Trans. Biomed. Eng. 2004;51:966–970. doi: 10.1109/TBME.2004.827063. [DOI] [PubMed] [Google Scholar]
- Weiskopf N., Scharnowski F., Veit R., Goebel R., Birbaumer N., Mathias K. Self-regulation of local brain activity using real-time functional magnetic resonance imaging (fMRI) J. Physiol. Paris. 2004;98:357–373. doi: 10.1016/j.jphysparis.2005.09.019. [DOI] [PubMed] [Google Scholar]
- Westenberg P.M., Drewes M.J., Goedhart A.W., Siebelink B.M., Treffers P.D.A. A developmental analysis of self-reported fears in late childhood through mid-adolescence: social-evaluative fears on the rise? J. Child Psychol. Psychiatry. 2004;45:481–495. doi: 10.1111/j.1469-7610.2004.00239.x. [DOI] [PubMed] [Google Scholar]
- Wittchen H.U., Stein M.B., Kessler R.C. Social fears and social phobia in a community sample of adolescents and young adults: prevalence, risk factors and co-morbidity. Psychol. Med. 1999;29(2):309–323. doi: 10.1017/s0033291798008174. [DOI] [PubMed] [Google Scholar]
- Zotev V., Krueger F., Phillips R., Alvarez R.P., Simmons W.K., Bellgowan P., Drevets W.C., Bodurka J. Self-regulation of amygdala activation using real-time FMRI neurofeedback. PLoS ONE. 2011;6:e24522. doi: 10.1371/journal.pone.0024522. [DOI] [PMC free article] [PubMed] [Google Scholar]