Highlights
-
•
Does music training shape the development of neural mechanisms of auditory attention?
-
•
We compared cortical responses to attended speech in child and adult musicians and nonmusicians.
-
•
Musician children and adults had less prefrontal auditory response variability during attention.
Keywords: Cortical, Response Variability, Language, Musicians, Children
Abstract
Selective attention decreases trial-to-trial variability in cortical auditory-evoked activity. This effect increases over the course of maturation, potentially reflecting the gradual development of selective attention and inhibitory control. Work in adults indicates that music training may alter the development of this neural response characteristic, especially over brain regions associated with executive control: in adult musicians, attention decreases variability in auditory-evoked responses recorded over prefrontal cortex to a greater extent than in nonmusicians. We aimed to determine whether this musician-associated effect emerges during childhood, when selective attention and inhibitory control are under development. We compared cortical auditory-evoked variability to attended and ignored speech streams in musicians and nonmusicians across three age groups: preschoolers, school-aged children and young adults. Results reveal that childhood music training is associated with reduced auditory-evoked response variability recorded over prefrontal cortex during selective auditory attention in school-aged child and adult musicians. Preschoolers, on the other hand, demonstrate no impact of selective attention on cortical response variability and no musician distinctions. This finding is consistent with the gradual emergence of attention during this period and may suggest no pre-existing differences in this attention-related cortical metric between children who undergo music training and those who do not.
1. Introduction
Sensory function involves the neuronal filtering of a signal of interest from competing sources of stimulation, often occurring within the same sensory domain. This filtering can be guided by selective attention, which plays a dynamic gatekeeping role by modulating neural responses to sensory input to bring about awareness of the most behaviorally-relevant environmental elements and the suppression of others. While both cellular approaches in animal models and far-field recordings in humans yield insights into how neural activity can be modified by selective attention, we cannot yet model all components involved in this filtering process. Influences of life factors such as maturation and sensory enrichment on attention's underlying biology provide additional factors that must be incorporated into a reliable model (e.g., Booth et al., 2003, Coch et al., 2005, Patston et al., 2007, Stevens et al., 2009, Strait and Kraus, 2011a, Strait et al., 2014a).
Most commonly, electrophysiological studies of selective attention, at both single-cell and population levels, have considered averaged sensory-evoked activity, comparing averaged responses comprising hundreds of trials to attended and concurrently ignored inputs. This approach emphasizes those aspects of the response that occur consistently but limits the assessment of attention's effects on aspects of the response that vary from trial to trial. Consideration of response variability in itself may provide insights into how the brain responds to differing sensory demands (Reich et al., 1997, Steinmetz et al., 2000), maturational changes (Gogtay et al., 2004, Li et al., 2001), and neuromodulatory influences (Jacob et al., 2013)—moving us toward a more comprehensive model of the attentive brain.
We previously assessed the variability of scalp-recorded auditory-evoked activity during a selective attention task in adults and reported that, across the scalp, evoked responses to attended speech demonstrate less between-trial variability than responses to ignored speech (Strait and Kraus, 2011a). A reduction in response variability with attention had previously been reported in other domains, such as the somatosensory (Steinmetz et al., 2000) and visual systems (Fries et al., 2001, Fries et al., 2008), and more recently within auditory cortex during an interval discrimination task (Abolafia et al., 2013). Rather than “turning up the volume” of neural responses to attended input by increasing the size of the recruited neural population, selective attention fine-tunes the encoding of a target signal by synchronizing brain activity and reducing its variability over time, effectively increasing its signal-to-noise ratio.
The application of this same paradigm to children revealed that attention's effect on response variability increases with age (Strait et al., 2014a), from ages three to 35, and may provide an objective index of the development of selective attention and inhibitory control. The development of this effect may be shaped by training and sensory enrichment, such as that associated with music training: in adults, the degree to which attention decreases prefrontal response variability relates to musicianship (Strait and Kraus, 2011a). Whereas musicians and nonmusicians demonstrate equivalent variability in responses across the majority of the scalp, only musically-trained adults demonstrate decreases in prefrontal response variability with attention (Strait and Kraus, 2011a). Attention-related enhancements in musicians’ auditory-evoked activity have also been reported by other laboratories using alternate cortical metrics, including mismatch negativity (Besson et al., 2011, Putkinen et al., 2013b, Tervaniemi et al., 2009) and the magnitude of late cortical auditory-evoked responses (Zendel and Alain, 2011).
Here we aimed to determine whether the auditory expertise engendered by music training during early childhood alters the development of this cortical index of selective auditory attention. To this end we assessed the between-trial variability of scalp-recorded auditory-evoked activity in 77 musicians and nonmusicians between the ages of three to 35. We hypothesized that music training during early childhood is associated with the development of strengthened neural networks underlying auditory attention during mid-childhood, following the stabilization of attention ability (∼age seven; Booth et al., 2003, Tipper et al., 1989). Supposing that differences between musicians and nonmusicians reflect their training, at least in part, we further predicted that: (1) young children just initiating music training would not yet demonstrate musician-associated enhancements and (2) the extent to which prefrontal response variability decreases with selective attention would be greater in children and adults with more years of musical practice relative to peers with less training.
2. Methods
2.1. Participants
All experimental procedures were approved by the Northwestern University Institutional Review Board. Seventy-eight normal hearing children and adults (<20 dB pure tone thresholds at octave frequencies from 125 to 8000 Hz) between the ages of three and 35 years participated in this study and were grouped according to three age categories: preschoolers (3–5 year olds, N = 26), school-aged children (7–13 year olds, N = 29) and adults (18–35 year olds, N = 23). Subjects were recruited through various mechanisms, including but not limited to flyers, advertisements in school newsletters, relationships with area music teachers, and presentations given to early childhood music programs. The subject population overlapped with the cohort of a previously published report demonstrating interacting effects of age and attention on cortical response variability (Strait et al., 2014a) with the addition of preschool (N = 2), school-aged child (N = 1) and adult (N = 2) subjects and the omission of one adult who fit into neither the musician nor nonmusician categories. Participants and, in the case of minors, legal guardians provided informed consent and assent. Participants were monetarily compensated for their time. No participant reported a history of neurological or learning abnormalities.
Subjects within each age group were further categorized as musicians (Mus) or nonmusicians (NonMus). Mus and NonMus did not differ according to age, sex or IQ in any of the three age groups (Table 1). Musicians were currently undergoing private or, in the case of some preschoolers, group music training (e.g., Kindermusik, Orff music classes). Adult musicians (N = 13) began music training by age 10 (M = 5.6 years, SD = 1.63; years practiced M = 16.6, SD = 5.54) and had no significant lapses in their practice histories. School-aged child musicians (N = 17) began music training by age six (M = 4.3 years, SD = 1.69; years practiced: M = 7.8, SD = 2.11) and had consistently practiced for a minimum of twelve consecutive months leading up to the date of test. Adult musicians practiced a minimum of three days per week for ≥1 h per session whereas school-aged child musicians practiced for a minimum of 20 min per day five days per week. Preschool musicians (N = 14) had consistently practiced for a minimum of twelve consecutive months leading up to the date of test (years practiced M = 2.7, SD = 1.08). Although the style of training varied across preschoolers (e.g., with respect to group or private lessons, focus on tonal or percussive instruments, and extent of incorporated song and dance), all musicians received weekly instruction for a minimum of 45 min, were receiving training in instrumental music production (rather than “being around music,” as in a music-themed program), and used at-home practice materials at least four days per week. Preschool nonmusicians had no music training during the year leading up to the test and ≤six months over the course of their lives. In fact, only two preschool nonmusicians had any degree of music training (group music classes for three and six months, respectively). Child and adult nonmusicians had no music training during the year leading up to the test; child nonmusicians had <4 years of accumulated musical experience (N = 6; M = 1.0 years, SD = 1.65) and adult nonmusicians had <5 years of accumulated musical experience (N = 4; adults: M = 1.1 years, SD = 1.83).
Table 1.
Musicians and nonmusicians did not differ by age, sex or IQ in any age group. Means and standard deviations (in parentheses) are presented for musicians and nonmusicians in each age group denoting age in years, sex and abbreviated IQ, as well as appropriate group comparison statistics. All P > 0.25.
| Pre-schoolers | School-aged children | Adults | |
|---|---|---|---|
| Age (years) | |||
| Mus | 4.9 (0.80) | 10.4 (1.55) | 24.2 (4.12) |
| NonMus | 4.6 (0.82) | 10.5 (1.55) | 23.4 (4.12) |
| Group comparison | t = 0.91 | t = 0.31 | t = 0.45 |
| Sex | |||
| Mus | 5 M, 9 F | 8 M, 9 F | 7 M, 6 F |
| NonMus | 7 M, 5 F | 7 M, 5 F | 5 M, 5 F |
| Group comparison | X2 = 1.33 | X2 = 0.36 | X2 = 0.35 |
| IQa(%) | |||
| Mus | 82 (8.3) | 58 (8.5) | 62 (5.2) |
| NonMus | 73 (16.1) | 68 (20.5) | 60 (8.4 |
| Group comparison | U = 52.5 | U = 85.5 | t = 0.61 |
Approximated by the Peabody Picture Vocabulary Test (preschoolers) or the Matrix Reasoning subtest of the Weschler Abbreviated Scale of Intelligence (school-aged children and adults); values represent age-normed percentile ranks.
2.2. Electrophysiological recording
The electrophysiological recording paradigm and adjustments made for each age group have been detailed in Strait et al., 2013a, Strait et al., 2014a and will be more briefly described here. The evoking stimulus was a six-formant, 170 ms speech syllable [da] synthesized using a Klatt-based synthesizer, with a 5 ms voice onset time and a level fundamental frequency (100 Hz). It was presented using NeuroScan Stim2 (Compumedics, Charlotte, NC, USA). Auditory-evoked potentials were recorded to this syllable using a 31-channel tin-electrode cap (Electrocap International, Eaton, OH, USA) in NeuroScan Acquire 4.3 (Compumedics) while participants were seated in a sound-attenuated booth. Only 13 of the 31 channels were applied in preschoolers to limit testing time (see Fig. 1B for channels employed). Single electrodes were placed on the earlobes and on the superior and outer canthi of the left eye, thereby acting as reference and eye-blink monitors, respectively. Contact impedances for all electrodes were under 5 kΩ for school-aged children and adults and under 20 kΩ for preschoolers with less than 5 kΩ difference across channels. Neural recordings were digitally sampled at a rate of 500 Hz and filtered online from 0.1 to 100 Hz.
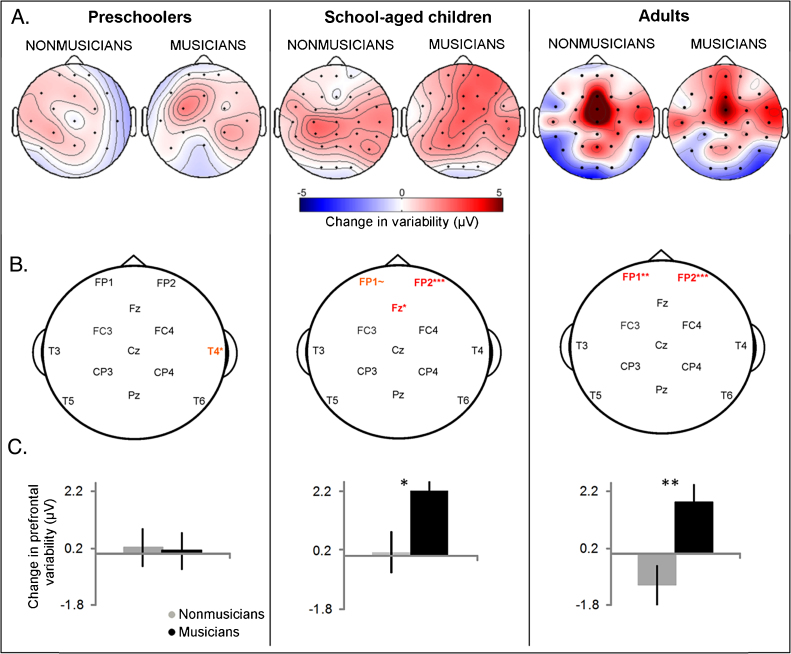
Fig. 1.
Effects of attention and musicianship on the variability of cortical auditory-evoked responses. (A) Differences in response variability to ignored and attended stimuli in musicians and nonmusicians are plotted at each recording site. Because differences were calculated by subtracting attend from ignore variability, positive values (red) indicate a decrease in variability in responses to the attended relative to the ignored stimuli. (B) Electrode sites shared across all age groups are labeled. Red denotes sites for which musicians and nonmusicians differed with regard to response variability with attention. (C) The mean change in variability over FP1 and FP2 recording sites with attention is plotted for each age group (ignore variability minus attend variability). School-aged child and adult musicians demonstrate more distinct prefrontal response variability with attention than nonmusicians. ∼P < 0.10; *P < 0.05; **P < 0.01; ***P < 0.001. (For interpretation of the references to color in this figure legend, the reader is referred to the web version of the article.)
The stimulus was presented in the context of two simultaneously-presented short stories played through two wall-mounted loudspeakers to the left and right of participants, separated by 180°. Children heard stories that were recorded from fictional picture books while adults listened to recorded autobiographical nonfiction. Participants were asked to attend to one of the two stories and to direct their gaze at a wall-mounted screen located 1.5 m ahead. The stories differed in direction (left/right), voice (male/female) and content. Instructions also described both the direction of the attended story and its speaker's sex. This procedure was adapted from Coch et al. (2005), who subsequently established its viability in children as young as age three (Sanders et al., 2006). The attended voice and its initial direction were randomized across participants to control for potential advantages or disadvantages of attending to one voice over the other. Although adults listened to the stories without visual stimulation, children viewed projected still images that corresponded to the attended story (as in Sanders et al., 2006, but using different audio materials).
The evoking stimulus was presented randomly through the left or right speakers with randomized inter-stimulus intervals (ISIs) that were either 600, 900, or 1200 ms for stimuli directed at each side. Although the evoking stimulus was presented in the context of both the attended and ignored acoustic streams, it was never presented on both sides concurrently; each stimulus presentation was to one side or the other, within the attended or the ignored story. The stories and the stimulus were presented with a 10 dB difference (stories: 65 dB SPL; stimulus: 75 dB SPL). For older children and adults, the recording took place over four eight-minute (adults) or four-minute (school-aged children) blocks. For preschoolers, only two five-minute blocks were presented. After each block, participants were questioned on the content of the attended story; then they were issued new directions to attend to the opposite side (left/right) in order to continue with the same voice and story. The entire recording session yielded 550 (adults), 350 (school-aged children) or 250 (preschoolers) responses recorded in each condition (i.e., attended and ignored).
All participants were able to perform the task as indicated by performance on quizzes that addressed story content. The quizzes were presented to screen participants for task compliance, not to measure sensitive between-subject differences in attention performance. Adults correctly answered ≥four out of five questions on a written multiple-choice quiz after each block. School-aged children and preschoolers correctly answered ≥two out of three orally-administered free-answer questions on the attended story after each block.
2.3. Offline data processing
The removal of eye-blink artifacts was conducted using the spatial filtering algorithm in Neuroscan Edit 4.3 (Compumedics). Continuous neural data were then epoched from −100 to 500 ms, referenced to the presentation of the stimulus (0 ms); responses were baseline-corrected and epochs demonstrating amplitudes beyond 100 μV were rejected as muscular artifact.
Prior to assessing response variability, we visually inspected the averaged evoked responses in attended and ignored conditions for each age group. The objective was to confirm that the scalp recordings in each age group reflected well-established developmental characteristics (e.g., a broad positivity in children versus the emergence of the P1–N1 complex in adults). For the generation of averaged responses, continuous recordings were first bandpass-filtered from 0.1 to 40 Hz (12 dB/octave, zero phase shift), prior to the eye-blink removal stage described above. Following the epoching and artifact rejection procedures, responses were subjected to a noise reduction algorithm described in Abrams, et al. (2008). The algorithm computes the degree of similarity between each epoch and the average of all epochs using Pearson's correlations. Individual responses were ranked according to their Pearson's r-values and the most poorly correlated 30% were discarded. The remaining 70% were averaged, making up the final averaged evoked response for each subject in each condition.
In addition to generating averaged responses, evoked response variability was computed for each subject in each condition. Subaverages of 25 (adults and school-aged children) or 15 (preschoolers) individual responses were generated for each condition. Response variability over the first 300 ms post-stimulus onset was determined through calculation of amplitude variances across subaverages within each condition using a technique adapted from Smith and Goffman (1998). Rather than comparing amplitudes on a point-by-point basis, we averaged amplitudes across 50 adjacent 6-ms increments in each subaverage, computed the variances across the subaverages for each of the 50 increments, and then summed the 50 variances. This generated a single index of variability for each subject in each condition to facilitate the assessment of variance across the cortical evoked response, including early evoked potentials that are not observable in single-trial evoked responses (i.e., P1/N1). All data processing was executed with scripts generated in Matlab 7.5.0 (The Mathworks, Natick, MA, USA).
2.4. Statistical analyses
Differences in response variability between attend and ignore conditions were compared for the thirteen electrode sites that were employed across all subjects using a mixed design, or “split-plot,” ANOVA with condition (attend/ignore) and electrode site as within-subjects factors and age (preschool/school-aged/adult) and musician groups as between-subjects factors. Following a four-way interaction between age, musicianship, condition and electrode site, we assessed effects of attention and musicianship in each age group separately using paired and independent samples t-tests. Relations to extent of music training were examined with Pearson's correlations. All results reported reflect two-tailed values with α = 0.05; normality for all data was confirmed using the Kolmogorov–Smirnov test for equality. Non-parametric tests were used for group comparisons in conditions of incomparable variances.
3. Results
Music training during early childhood was associated with the variability of prefrontal auditory-evoked responses recorded over the course of the selective auditory attention task. Like adults, school-aged musically-trained children had greater attentional effects on prefrontal response variability than their nonmusician peers. This effect of musicianship was not observed in preschool-aged musicians just initiating music training and may emerge with continued training and/or development. Furthermore, the extent to which prefrontal response variability decreased to attended relative to ignored speech increased with more years of musical practice in both school-aged children and, as reported in Strait and Kraus (2011a), adults.
3.1. Averaged evoked responses
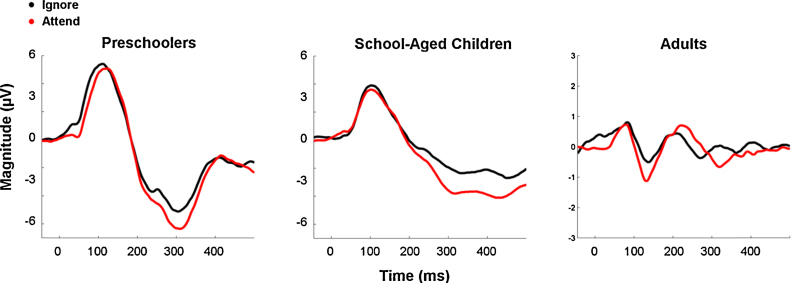
Prior to assessing response variability, we visually inspected the averaged evoked responses in attended and ignored conditions. The objective was to confirm that the scalp recordings in each age group reflected well-established developmental characteristics. Accordingly, we confirmed that the averaged evoked potentials in all three age groups demonstrated characteristic maturational changes, with smaller amplitudes and earlier latencies visible with development (Fig. 2). Furthermore, whereas children demonstrate a broad positivity (the P1) followed by a broad negativity (the N2), the distinct P1–N1–P2 complex emerges during adolescence and is observed in adults (Cunningham et al., 2000, Pang and Taylor, 2000, Ponton et al., 2000).
Fig. 2.
Averaged auditory-evoked potentials recorded from Cz. Average waveforms demonstrate characteristic morphologies and maturational effects, such as the emergence of the P1/N1/P2 complex with increasing age.
3.2. Selective auditory attention decreases cortical response variability in older children and adults
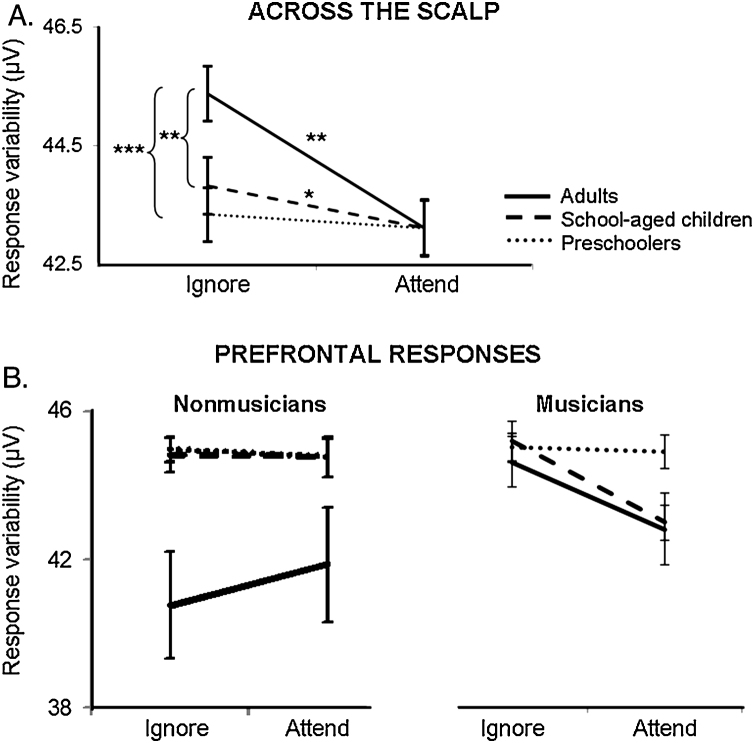
Consistent with our previous report, a mixed design ANOVA with attention and electrode site as within-subject factors and age and musicianship as between-subject factors revealed main effects of condition and age on response variability, indicating that responses to attended input were generally less variable than responses to ignored input and that overall cortical response variability decreased across the scalp with age (condition: F(1,71) = 26.24, P < 0.001; age: F(2,71) = 3.61, P = 0.03). Also consistent with our previous report, we observed a two-way interaction between attention and age (F(2,71) = 3.23, P = 0.04) and a three-way interaction between attention, age and electrode site (F(24,122) = 3.25, P < 0.001), indicating that the extent to which attention decreases cortical response variability to speech increases with development across the scalp except for over prefrontal recording sites (Fig. 3). This interaction retained significance even when musicians and nonmusicians were considered separately (NonMus: F(12,122) = 5.14, P < 0.001; Mus: F(13,122) = 3.51, P < 0.001). Additionally, we observed a three-way interaction between attention, musicianship, and electrode site (F(12,60) = 2.00, P = 0.04) and two-way interactions between electrode site and age group (F(12,60) = 2.65, P < 0.001) and attention and electrode site (F(12,60) = 4.34, P < 0.001). No further significant interactions were observed.
Fig. 3.
Musicianship and development interact with effects of attention on cortical response variability. (A) Mean response variability in all subjects across the scalp in all but prefrontal channels. Adults and school-aged children demonstrate increased cortical response variability to the ignored compared to the attended stimuli. (B) Mean response variability averaged across the two prefrontal channels. Only musician school-aged children and adults demonstrate impacts of attention and development on prefrontal response variability. *P < 0.05; **P < 0.01; ***P < 0.001.
3.3. Effects of musicianship on auditory-evoked prefrontal response variability with attention
In addition to the main effects and interactions detailed above, we observed a four-way interaction between age, electrode site, attention and musicianship (F(24,122) = 1.76, P = 0.02). Post-hoc independent samples t-tests revealed that school-aged child and adult musicians had a greater impact of attention on prefrontal responses to speech than age-matched nonmusicians (Fig. 1, Fig. 3B). While differences between prefrontal variability in musician and nonmusician adults were not significant (attend and ignore condition group comparisons both P > 0.15), nonmusician adults were unique in demonstrating diminished overall prefrontal response variability relative to other nonmusician age groups (adult-child: attend n.s., ignore t = 2.09, P = 0.05; adult-preschooler: attend t = 1.96, P = 0.05, ignore t = 2.30, P = 0.04). No distinctions were observed between preschool musicians and nonmusicians except for over one right temporal recording site (T4; Fig. 1).
Procedural differences between preschoolers and older children/adults related to stimulus presentation could not account for the different effects of musicianship observed between age groups. This is because group distinctions in older children and adults held when we constrained these analyses to match preschoolers’ data collection parameters, considering only the first 90 artifact-free sweeps in each of the first two blocks for a total of 180 individual responses. Musicians and nonmusician group differences with regard to the effect of attention on prefrontal response variability remained, with musicians demonstrating less variable attend relative to ignore responses than nonmusicians (children: F(1,28) = 4.55, P = 0.04; adults: F(1,22) = 10.20, P = 0.004). As observed in our initial analyses, older child musicians further demonstrated a greater effect of attention on response variability at a frontocentral site (Fz) relative to nonmusicians (Fig. 1B; F(1,28) = 6.08, P = 0.02). No group differences in either age group were observed at other electrode sites.
3.4. Extent of music training relates to effects of selective attention on prefrontal auditory-evoked response variability
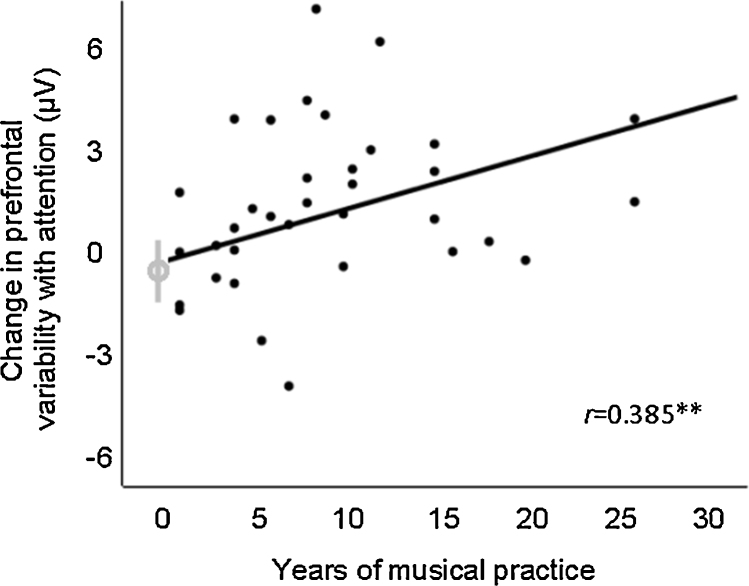
Across all school-aged child and adult participants with some degree of music training, the number of years of music training correlated with the extent to which response variability decreased in response to the attended speech (Fig. 4; collapsed across right and left prefrontal sites; adults: Pearson's r = 0.55, P = 0.006; children: Pearson's r = 0.42, P = 0.03). Specifically, more years of music training related to a greater impact of attention on cortical response variability. These relationships lost significance at P < 0.05 when considering musicians alone, potentially due to the loss of spread in the “years of practice” variable, although positive trends remained: (adult musicians: r = 0.46, P = 0.08; child musicians: r = 0.41, P = 0.07).
Fig. 4.
Prefrontal attention effects relate to music training. Years of musical practice related to the extent to which prefrontal evoked responses became less variable with attention across school-aged children and adults. Nonmusicians’ mean and ±/−1 standard error is indicated in gray. Within age-group correlations were also significant. Adults: r = 0.55*; Children: r = 0.42*. *P < 0.05; **P < 0.01.
4. Discussion
Our results reveal that the development of cortical mechanisms underlying selective auditory attention is associated with childhood music training. This work adds to rapidly accruing evidence for neurodevelopmental distinctions in children undergoing music training, who demonstrate heightened neural sensitivity to acoustic input (Chobert et al., 2011, Putkinen et al., 2014b) and more robust neural encoding of acoustic input (for review see Putkinen et al., 2013a, Strait and Kraus, 2014); longitudinal and cross-sectional efforts indicate that many of these effects may be training-dependent (Chobert et al., 2014, Kraus et al., 2014, Moreno and Besson, 2005, Moreno et al., 2009, Putkinen et al., 2014a, Strait et al., 2013a). More specifically, the present results extend previous observations in musician adults to school-aged children, establishing that heightened attentional effects on auditory-evoked response variability over prefrontal cortex emerge in musicians during early childhood. That these effects are not observed in preschoolers just initiating music training may suggest no pre-existing differences in selective attention, as indexed by prefrontal response variability, between children who undergo music training from those who do not. Alternative explanations may be that innate predispositions are not yet apparent in preschoolers given their relatively undeveloped prefrontal cortex, or that distinctions were not observed in musician preschoolers because a large portion of this population received group music lessons rather than private instruction. Furthermore, it is notable that preschool musicians demonstrated decreased variability over one right temporal electrode site relative to preschool nonmusicians. Subsequent studies are necessary to determine whether this finding is replicable and, if so, whether it reflects innate predispositions or training-related malleability. Here we discuss developmental and practical implications of these data in the context of the neurobiological generators of response variability.
A previous report using this same approach defined cortical response variability to attended and ignored speech as a developmental index for selective attention and inhibitory control (Strait et al., 2014a). Outcomes indicated that the maturation of neural mechanisms involved in suppressing ignored input is a key component of attention development: although preschoolers, school-aged children and young adults have equivalent response variability to attended speech, only school-aged children and adults have more variable evoked activity in response to ignored relative to attended speech streams (the same effect observed in the present data is illustrated in Fig. 3A). This relationship was observed across the scalp except for over prefrontal electrode sites. The effects of musicianship reported herein are specific to these prefrontal sites and, as in Strait et al., 2013a, Strait et al., 2014a, do not relate to the variability observed to attended and ignored input across the remainder of the scalp. Furthermore, musicianship does not relate to increased variability to ignored input relative to nonmusicians but, rather, decreased variability to attended relative to ignored input. This may indicate that, rather than changing the normal developmental trajectory of selective attention and inhibitory control, music training strengthens a unique neuronal mechanism to promote selective attention, observed through response variability over executive control regions. This may reflect enhanced top–down attentional control for auditory processing in musicians relative to nonmusicians, potentially accounting for their more proficient performance on attention-themed tasks (Parbery-Clark et al., 2011, Strait et al., 2010, Strait et al., 2012, Strait et al., 2014b, Tervaniemi et al., 2009, Zendel and Alain, 2011). Such effects may not be specific to music training, however. Future investigations should compare these outcomes to active control groups, such as speakers of multiple languages or auditory-motor experts (e.g., dancers).
It is notable that nonmusician adults demonstrated decreased variability to both attended and ignored stimuli relative to nonmusician preschool- and school-aged children. This effect of age was not observed for musician adults, who maintained higher baseline variability, equivalent with musician children. Decreased overall variability in adults, regardless of condition, may have been reasonably predicted from known characteristics of brain maturation, which include extensive neural pruning over development that results in decreased synaptic density and gray matter volume (Huttenlocher, 1979, O’Leary, 1992, Pfefferbaum et al., 1994). From a network dynamics perspective, decreased overall cortical response variability could reflect a less functionally adaptive nervous system (Fingelkurts, 2004, McIntosh et al., 2008). It is possible that our results indicate the preservation of a more functionally adaptive auditory system in musicians into adulthood.
4.1. Propagating factors of intra-individual cortical response variability: potential roles of the dopaminergic system and ongoing oscillatory dynamics
Inter-trial fluctuations in neuronal firing are familiar among cellular physiologists, serving not as the exception but, rather, the rule for temporal aspects of cellular responses. Accordingly, Shafi and colleagues posit that stable and persistent activity is often an artifact that results from data processing techniques that obscure inter-trial variability through averaging (Shafi et al., 2007). This disregarded “noise,” or variability, may reveal biological factors underlying behavioral and conscious states, including attention (for further discussion see Arieli et al., 1996).
A number of sources of neural response variability have been identified in recent years. They include maturational changes in brain structure (Gogtay et al., 2004, Li et al., 2001), the diffusivity of cortical activation (Winterer et al., 2004), resting state oscillatory dynamics (Curto et al., 2009) and neuromodulatory influences (Jacob et al., 2013), most of which are state-dependent but all of which may interact to bring about trial-to-trial variability. We speculate that it is possible that distinctions in prefrontal response variability between musicians and nonmusicians relate to differences in the activation of the dopaminergic system, which heavily modulates neuronal responses in prefrontal cortex, during auditory processing. It is thought that dopamine prepares prefrontal circuitry to process sensory input by increasing the effective stimulus-to-noise ratio through a reduction in inter-trial variability (Jacob et al., 2013). This effect is principally achieved by D1- and D2-receptor-mediated effects on pyramidal and local circuit neurons, which contribute to the stability of neuronal representations of sensory input by mediating excitability and recurrent inhibition. In fact, decreased dopamine increases trial-to-trial response variability (e.g., Ikegaya et al., 2004), with parallel increases in behavioral variability (e.g., Pesek-Cotton et al., 2011). While we cannot definitely state that the neurogenic activity we recorded from the scalp originated in prefrontal cortex, a subset of prefrontal neurons are auditory-responsive, especially during attention, and demonstrate onset latencies that overlap with responses originating in auditory cortex. Prefrontal cortex would at the very least contribute to the scalp-recorded response to attended sound, and would be generated by neurons most proximal to prefrontal recording sites.
In addition to its direct effects on neuronal responses to sensory input, dopamine may indirectly modulate trial-to-trial response variability by affecting ongoing oscillatory networks. Resting-state oscillatory dynamics have been previously associated with evoked response variability, which can be predicted by deterministic interactions of sensory responses with ongoing spontaneous activity (Arieli et al., 1996, Curto et al., 2009). Changes in oscillatory networks within prefrontal cortex are profoundly controlled by neuromodulators such as dopamine and are thought to facilitate the formation of cell assemblies for information encoding during attention (for review see Benchenane et al., 2011). Musicians’ decreased prefrontal evoked response variability to the attended speech stream demonstrated here may indicate heightened attention-induced changes in ongoing oscillatory activity that are induced in part by dopamine release.
Although music listening is known to activate the dopaminergic system (Chanda and Levitin, 2013, Polston et al., 2011, Salimpoor et al., 2011, Zatorre and Salimpoor, 2013), dopamine is also an integral neurotransmitter underlying auditory learning and associated neuroplasticity (Bakin and Weinberger, 1996, Kilgard and Merzenich, 1998), which are implicit in music training. Dopamine may be involved in the strengthening of the auditory system during music training, mediating the system's maturation and/or auditory-evoked activation. The outcomes presented here may reflect earlier development of the mesolimbic dopamine pathway in musicians during auditory processing and its strengthened auditory-evoked activation into adulthood. This effect would account for musicians’ consistently enhanced auditory-cognitive performance (for review see Besson et al., 2011, Kraus et al., 2012, Strait and Kraus, 2011b) given the pivotal role of the dopaminergic system in the development of cognitive skills (Naneix et al., 2012).
4.2. Attention reflects intersecting innate and experience-related factors
There are clear genetic predispositions determining one's base attentional capacity: individual differences in attention have been related to heritable factors including temperament (Chang and Burns, 2005, Gerardi-Caulton, 2000, Rueda et al., 2005) and genotype (e.g., the dopamine transporter gene DAT1; Cook et al., 1995, Fossella et al., 2002, Rueda et al., 2005). Even in light of its hard-wired characteristics, attentional capacity is further subject to improvement with educational interventions, especially during sensitive developmental periods. Both short-term training (Rueda et al., 2005, Stevens et al., 2008) and enriched home environments (Neville et al., 2013), for example, strengthen attention ability in young children. Musical practice has been associated with strengthened auditory-specific attention ability, observed in both school-aged children (Strait et al., 2012, Strait et al., 2014b and adults with extensive music training beginning prior to age seven (Strait et al., 2010, Strait et al., 2014b). No musician-associated enhancements were observed in parallel visual tasks. The specificity of musicians’ attentional advantages to the auditory domain may reflect heightened top–down gating of sensory processing in the domain that music exercises most. Although we observed no pre-existing differences in neural selective-attention mechanisms in preschool musicians just initiating music training, controlled longitudinal studies are necessary to directly define music training's effects on attention above and beyond genetic predispositions.
While exposure to activities such as music that impact attention during early childhood may confer developmental benefits, strengthening the acquisition of auditory skills that depend on focused learning (e.g., reading), continued musical practice across the lifespan may promote further plastic changes in auditory attention networks. For example, musical practice during early development may induce prolonged maturation of auditory attention and its subcomponents (e.g., inhibitory control) well into middle-adulthood. This claim can be supported by our observed relation between auditory attention's effects on response variability over prefrontal cortex and extent of music practice, with musicians who had practiced for more years demonstrating greater attention effects than less-practiced peers. This outcome implies that early-onset music training continues to strengthen neural attention mechanisms even into adulthood, potentially conferring lifelong attentional advantages for auditory processing (Hanna-Pladdy and Gajewski, 2012, Parbery-Clark et al., 2011). Further work in older adult musicians might determine the point at which attention abilities stabilize and whether music training-related modifications mitigate aging-related cognitive declines. Furthermore, it remains to be determined whether music training initiated during adulthood grants cognitive benefits, well past sensitive developmental years.
4.3. Practical implications
Selective attention facilitates all aspects of learning and memory and even predicts the capacity for scholastic achievement (Razza et al., 2010, Rueda et al., 2010, Stevens and Bavelier, 2012). Interventions that target any aspect of attention may be especially useful for children from low-socioeconomic backgrounds, who are at-risk for attention deficits (Froehlich et al., 2007) and scholastically under-perform relative to mid- to high-SES peers (e.g., Sirin, 2005, Walker et al., 1994). Although private music instruction may not be a viable approach due to financial constraints, a number of not-for-profit classroom-based music training programs available to at-risk youth have shown great success (e.g., the Harmony Project, www.harmony-project.org). Incorporation of music training into early childhood education programs (e.g., Head Start) may provide preventative effects for disabilities such as attention-deficit and hyperactivity disorder (ADHD) that are associated with increased moment-to-moment variability in behavioral performance (Mullins et al., 2005, Vaurio et al., 2009) and neuronal activity (Depue et al., 2010).
4.4. Conclusions
Selective attention acts as a mediator between the world around us and our experience of that world. It provides an essential foundation for almost every aspect of our lives, including how we learn, how we act, and the selection of thoughts and perceptions to meld into memories. For young children, the development of strong auditory attention skills is critical to successful learning and language development. Our findings provide the first direct evidence of a biological index for enhanced selective auditory attention in young musicians and suggest that music training can support the maturation of auditory attention during pivotal developmental years. Our lack of an active control group, however, prevents us from addressing the exclusivity of these results to musicians; it is possible that auditory expertise developed through other activities such as foreign language-learning and dance yields similar effects. A more thorough definition of attention's underlying mechanisms has the potential to improve the categorization and identification of attention deficits and for more targeted training strategies for attention's habilitation and, in the case of impairment, rehabilitation. Furthermore, an awareness of music training-associated benefits for the development of attention and its underlying biology provides important considerations for educators and educational policy-makers involved in curriculum design.
Conflict of interest statement
The authors confirm that there are no known conflicts of interest associated with this publication and there has been no financial support for this work that could have influenced its outcome. The manuscript has been read and approved by all named authors and that there are no other persons who satisfied the criteria for authorship but are not listed. We further confirm that the order of authors listed in the manuscript has been approved by all authors.
Acknowledgements
The authors wish to thank Victor Abecassis, Karen Chan, Jennifer Krizman and Dr. Samira Anderson for their assistance with testing and Carolyn Hsu for her contributions to stimulus design. This work was funded by the National Science Foundation grant BCS-0921275 and a grant from the Grammy Foundation.
Footnotes
Available online 13 January 2015
References
- Abolafia J.M., Martinez-Garcia M., Deco G., Sanchez-Vives M.V. Variability and information content in auditory cortex spike trains during an interval-discrimination task. J. Neurophysiol. 2013;110(9):2163–2174. doi: 10.1152/jn.00381.2013. [DOI] [PubMed] [Google Scholar]
- Abrams D., Nicol T., Zecker S., Kraus N. Right-hemisphere auditory cortex is dominant for coding syllable patterns in speech. J. Neurosci. 2008;28(15):3958–3965. doi: 10.1523/JNEUROSCI.0187-08.2008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Arieli A., Sterkin A., Grinvald A., Aertsen A. Dynamics of ongoing activity: explanation of the large variability in evoked cortical responses. Science. 1996;273(5283):1868–1871. doi: 10.1126/science.273.5283.1868. [DOI] [PubMed] [Google Scholar]
- Bakin J.S., Weinberger N.M. Induction of a physiological memory in the cerebral cortex by stimulation of the nucleus basalis. Proc. Natl. Acad. Sci. USA. 1996;93(20):11219–11224. doi: 10.1073/pnas.93.20.11219. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Benchenane K., Tiesinga P.H., Battaglia F.P. Oscillations in the prefrontal cortex: a gateway to memory and attention. Curr. Opin. Neurobiol. 2011;21(3):475–485. doi: 10.1016/j.conb.2011.01.004. [DOI] [PubMed] [Google Scholar]
- Besson M., Chobert J., Marie C. Transfer of training between music and speech: common processing, attention, and memory. Front. Psychol. 2011;2:94. doi: 10.3389/fpsyg.2011.00094. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Booth J.R., Burman D.D., Meyer J.R., Lei Z., Trommer B.L., Davenport N.D., Mesulam M.M. Neural development of selective attention and response inhibition. Neuroimage. 2003;20(2):737–751. doi: 10.1016/S1053-8119(03)00404-X. [DOI] [PubMed] [Google Scholar]
- Chanda M.L., Levitin D.J. The neurochemistry of music. Trends Cogn. Sci. 2013;17(4):179–193. doi: 10.1016/j.tics.2013.02.007. [DOI] [PubMed] [Google Scholar]
- Chang F., Burns B.M. Attention in preschoolers: associations with effortful control and motivation. Child Dev. 2005;76(1):247–263. doi: 10.1111/j.1467-8624.2005.00842.x. [DOI] [PubMed] [Google Scholar]
- Chobert J., Francois C., Velay J.L., Besson M. Twelve months of active musical training in 8- to 10-year-old children enhances the preattentive processing of syllabic duration and voice onset time. Cereb. Cortex. 2014;24(4):956–967. doi: 10.1093/cercor/bhs377. [DOI] [PubMed] [Google Scholar]
- Chobert J., Marie C., Francois C., Schon D., Besson M. Enhanced passive and active processing of syllables in musician children. J. Cogn. Neurosci. 2011;23(12):3874–3887. doi: 10.1162/jocn_a_00088. [DOI] [PubMed] [Google Scholar]
- Coch D., Sanders L.D., Neville H.J. An event-related potential study of selective auditory attention in children and adults. J. Cogn. Neurosci. 2005;17(4):605–622. doi: 10.1162/0898929053467631. [DOI] [PubMed] [Google Scholar]
- Cook E.H., Jr., Stein M.A., Krasowski M.D., Cox N.J., Olkon D.M., Kieffer J.E., Leventhal B.L. Association of attention-deficit disorder and the dopamine transporter gene. Am. J. Hum. Genet. 1995;56(4):993–998. [PMC free article] [PubMed] [Google Scholar]
- Cunningham J., Nicol T., Zecker S., Kraus N. Speech-evoked neurophysiologic responses in children with learning problems: development and behavioral correlates of perception. Ear Hear. 2000;21(6):554–568. doi: 10.1097/00003446-200012000-00003. [DOI] [PubMed] [Google Scholar]
- Curto C., Sakata S., Marguet S., Itskov V., Harris K.D. A simple model of cortical dynamics explains variability and state dependence of sensory responses in urethane-anesthetized auditory cortex. J. Neurosci. 2009;29(34):10600–10612. doi: 10.1523/JNEUROSCI.2053-09.2009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Depue B.E., Burgess G.C., Willcutt E.G., Bidwell L.C., Ruzic L., Banich M.T. Symptom-correlated brain regions in young adults with combined-type ADHD: their organization, variability, and relation to behavioral performance. Psychiatry Res. 2010;182(2):96–102. doi: 10.1016/j.pscychresns.2009.11.011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fingelkurts A.A. Making complexity simpler: multivariability and metastability in the brain. Int. J. Neurosci. 2004;114(7):843–862. doi: 10.1080/00207450490450046. [DOI] [PubMed] [Google Scholar]
- Fossella J., Sommer T., Fan J., Wu Y., Swanson J.M., Pfaff D.W., Posner M.I. Assessing the molecular genetics of attention networks. BMC Neurosci. 2002;3:14. doi: 10.1186/1471-2202-3-14. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fries P., Reynolds J.H., Rorie A.E., Desimone R. Modulation of oscillatory neuronal synchronization by selective visual attention. Science. 2001;291(5508):1560–1563. doi: 10.1126/science.1055465. [DOI] [PubMed] [Google Scholar]
- Fries P., Womelsdorf T., Oostenveld R., Desimone R. The effects of visual stimulation and selective visual attention on rhythmic neuronal synchronization in macaque area V4. J. Neurosci. 2008;28(18):4823–4835. doi: 10.1523/JNEUROSCI.4499-07.2008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Froehlich T.E., Lanphear B.P., Epstein J.N., Barbaresi W.J., Katusic S.K., Kahn R.S. Prevalence, recognition, and treatment of attention-deficit/hyperactivity disorder in a national sample of US children. Arch. Pediatr. Adolesc. Med. 2007;161(9):857–864. doi: 10.1001/archpedi.161.9.857. [DOI] [PubMed] [Google Scholar]
- Gerardi-Caulton G. Sensitivity to spatial conflict and the development of self-regulation in children 24–36 months of age. Dev. Sci. 2000;3(4):397–404. [Google Scholar]
- Gogtay N., Giedd J.N., Lusk L., Hayashi K.M., Greenstein D., Vaituzis A.C., Thompson P.M. Dynamic mapping of human cortical development during childhood through early adulthood. Proc. Natl. Acad. Sci. USA. 2004;101(21):8174–8179. doi: 10.1073/pnas.0402680101. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hanna-Pladdy B., Gajewski B. Recent and past musical activity predicts cognitive aging variability: direct comparison with general lifestyle activities. Front. Hum. Neurosci. 2012;6:198. doi: 10.3389/fnhum.2012.00198. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Huttenlocher P.R. Synaptic density in human frontal cortex—developmental changes and effects of aging. Brain Res. 1979;163(2):195–205. doi: 10.1016/0006-8993(79)90349-4. [DOI] [PubMed] [Google Scholar]
- Ikegaya Y., Aaron G., Cossart R., Aronov D., Lampl I., Ferster D., Yuste R. Synfire chains and cortical songs: temporal modules of cortical activity. Science. 2004;304(5670):559–564. doi: 10.1126/science.1093173. [DOI] [PubMed] [Google Scholar]
- Jacob S.N., Ott T., Nieder A. Dopamine regulates two classes of primate prefrontal neurons that represent sensory signals. J. Neurosci. 2013;33(34):13724–13734. doi: 10.1523/JNEUROSCI.0210-13.2013. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kilgard M.P., Merzenich M.M. Cortical map reorganization enabled by nucleus basalis activity. Science. 1998;279(5357):1714–1718. doi: 10.1126/science.279.5357.1714. [DOI] [PubMed] [Google Scholar]
- Kraus N., Slater J., Thompson E., Hornickel J., Strait D.L., Nicol T., White-Schwoch T. Music enrichment programs improve the neural encoding of speech in at-risk children. J. Neurosci. 2014;34(36):11913–11918. doi: 10.1523/JNEUROSCI.1881-14.2014. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kraus N., Strait D.L., Parbery-Clark A. Cognitive factors shape brain networks for auditory skills: spotlight on auditory working memory. Ann. N. Y. Acad. Sci. 2012;1252:100–107. doi: 10.1111/j.1749-6632.2012.06463.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Li S.C., Lindenberger U., Sikstrom S. Aging cognition: from neuromodulation to representation. Trends Cogn. Sci. 2001;5(11):479–486. doi: 10.1016/s1364-6613(00)01769-1. [DOI] [PubMed] [Google Scholar]
- McIntosh A.R., Kovacevic N., Itier R.J. Increased brain signal variability accompanies lower behavioral variability in development. PLoS Comput. Biol. 2008;4(7):e1000106. doi: 10.1371/journal.pcbi.1000106. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Moreno S., Besson M. Influence of musical training on pitch processing: event-related brain potential studies of adults and children. Ann. N. Y. Acad. Sci. 2005;1060:93–97. doi: 10.1196/annals.1360.054. [DOI] [PubMed] [Google Scholar]
- Moreno S., Marques C., Santos A., Santos M., Castro S.L., Besson M. Musical training influences linguistic abilities in 8-year-old children: more evidence for brain plasticity. Cereb. Cortex. 2009;19(3):712–723. doi: 10.1093/cercor/bhn120. [DOI] [PubMed] [Google Scholar]
- Mullins C., Bellgrove M.A., Gill M., Robertson I.H. Variability in time reproduction: difference in ADHD combined and inattentive subtypes. J. Am. Acad. Child Adolesc. Psychiatry. 2005;44(2):169–176. doi: 10.1097/00004583-200502000-00009. [DOI] [PubMed] [Google Scholar]
- Naneix F., Marchand A.R., Di Scala G., Pape J.R., Coutureau E. Parallel maturation of goal-directed behavior and dopaminergic systems during adolescence. J. Neurosci. 2012;32(46):16223–16232. doi: 10.1523/JNEUROSCI.3080-12.2012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Neville H.J., Stevens C., Pakulak E., Bell T.A., Fanning J., Klein S., Isbell E. Family-based training program improves brain function, cognition, and behavior in lower socioeconomic status preschoolers. Proc. Natl. Acad. Sci. USA. 2013;110(29):12138–12143. doi: 10.1073/pnas.1304437110. [DOI] [PMC free article] [PubMed] [Google Scholar]
- O’Leary D.D. Development of connectional diversity and specificity in the mammalian brain by the pruning of collateral projections. Curr. Opin. Neurobiol. 1992;2(1):70–77. doi: 10.1016/0959-4388(92)90165-h. [DOI] [PubMed] [Google Scholar]
- Pang E.W., Taylor M.J. Tracking the development of the N1 from age 3 to adulthood: an examination of speech and non-speech stimuli. Clin. Neurophysiol. 2000;111(3):388–397. doi: 10.1016/s1388-2457(99)00259-x. [DOI] [PubMed] [Google Scholar]
- Parbery-Clark A., Strait D.L., Anderson S., Hittner E., Kraus N. Musical experience and the aging auditory system: implications for cognitive abilities and hearing speech in noise. PLoS ONE. 2011;6(5):e18082. doi: 10.1371/journal.pone.0018082. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Patston L.L., Hogg S.L., Tippett L.J. Attention in musicians is more bilateral than in non-musicians. Laterality. 2007;12(3):262–272. doi: 10.1080/13576500701251981. [DOI] [PubMed] [Google Scholar]
- Pesek-Cotton E.F., Johnson J.E., Newland M.C. Reinforcing behavioral variability: an analysis of dopamine-receptor subtypes and intermittent reinforcement. Pharmacol. Biochem. Behav. 2011;97(3):551–559. doi: 10.1016/j.pbb.2010.10.011. [DOI] [PubMed] [Google Scholar]
- Pfefferbaum A., Mathalon D.H., Sullivan E.V., Rawles J.M., Zipursky R.B., Lim K.O. A quantitative magnetic resonance imaging study of changes in brain morphology from infancy to late adulthood. Arch. Neurol. 1994;51(9):874–887. doi: 10.1001/archneur.1994.00540210046012. [DOI] [PubMed] [Google Scholar]
- Polston J.E., Rubbinaccio H.Y., Morra J.T., Sell E.M., Glick S.D. Music and methamphetamine: conditioned cue-induced increases in locomotor activity and dopamine release in rats. Pharmacol. Biochem. Behav. 2011;98(1):54–61. doi: 10.1016/j.pbb.2010.11.024. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ponton C.W., Eggermont J.J., Kwong B., Don M. Maturation of human central auditory system activity: evidence from multi-channel evoked potentials. Clin. Neurophysiol. 2000;111(2):220–236. doi: 10.1016/s1388-2457(99)00236-9. [DOI] [PubMed] [Google Scholar]
- Putkinen V., Saarikivi K., Tervaniemi M. Do informal musical activities shape auditory skill development in preschool-age children? Front. Psychol. 2013;4:572. doi: 10.3389/fpsyg.2013.00572. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Putkinen V., Tervaniemi M., Huotilainen M. Informal musical activities are linked to auditory discrimination and attention in 2–3-year-old children: an event-related potential study. Eur. J. Neurosci. 2013;37(4):654–661. doi: 10.1111/ejn.12049. [DOI] [PubMed] [Google Scholar]
- Putkinen V., Tervaniemi M., Saarikivi K., de Vent N., Huotilainen M. Investigating the effects of musical training on functional brain development with a novel Melodic MMN paradigm. Neurobiol. Learn Mem. 2014;110:8–15. doi: 10.1016/j.nlm.2014.01.007. [DOI] [PubMed] [Google Scholar]
- Putkinen V., Tervaniemi M., Saarikivi K., Ojala P., Huotilainen M. Enhanced development of auditory change detection in musically trained school-aged children: a longitudinal event-related potential study. Dev. Sci. 2014;17(2):282–297. doi: 10.1111/desc.12109. [DOI] [PubMed] [Google Scholar]
- Razza R.A., Martin A., Brooks-Gunn J. Associations among family environment, sustained attention, and school readiness for low-income children. Dev. Psychol. 2010;46(6):1528–1542. doi: 10.1037/a0020389. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Reich D.S., Victor J.D., Knight B.W., Ozaki T., Kaplan E. Response variability and timing precision of neuronal spike trains in vivo. J. Neurophysiol. 1997;77(5):2836–2841. doi: 10.1152/jn.1997.77.5.2836. [DOI] [PubMed] [Google Scholar]
- Rueda M.R., Checa P., Rothbart M.K. Contributions of attentional control to socioemotional and academic development. Early Educ. Dev. 2010;21(5):744–764. [Google Scholar]
- Rueda M.R., Rothbart M.K., McCandliss B.D., Saccomanno L., Posner M.I. Training, maturation, and genetic influences on the development of executive attention. Proc. Natl. Acad. Sci. USA. 2005;102(41):14931–14936. doi: 10.1073/pnas.0506897102. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Salimpoor V.N., Benovoy M., Larcher K., Dagher A., Zatorre R.J. Anatomically distinct dopamine release during anticipation and experience of peak emotion to music. Nat. Neurosci. 2011;14(2):257–262. doi: 10.1038/nn.2726. [DOI] [PubMed] [Google Scholar]
- Sanders L.D., Stevens C., Coch D., Neville H.J. Selective auditory attention in 3- to 5-year-old children: an event-related potential study. Neuropsychologia. 2006;44(11):2126–2138. doi: 10.1016/j.neuropsychologia.2005.10.007. [DOI] [PubMed] [Google Scholar]
- Shafi M., Zhou Y., Quintana J., Chow C., Fuster J., Bodner M. Variability in neuronal activity in primate cortex during working memory tasks. Neuroscience. 2007;146(3):1082–1108. doi: 10.1016/j.neuroscience.2006.12.072. [DOI] [PubMed] [Google Scholar]
- Sirin S.R. Socioeconomic status and academic achievement: A meta-analytic review of research. Rev. Educ. Res. 2005;75(3):417–453. [Google Scholar]
- Smith A., Goffman L. Stability and patterning of speech movement sequences in children and adults. J. Speech Lang. Hear. Res. 1998;41(1):18–30. doi: 10.1044/jslhr.4101.18. [DOI] [PubMed] [Google Scholar]
- Steinmetz P.N., Roy A., Fitzgerald P.J., Hsiao S.S., Johnson K.O., Niebur E. Attention modulates synchronized neuronal firing in primate somatosensory cortex. Nature. 2000;404(6774):187–190. doi: 10.1038/35004588. [DOI] [PubMed] [Google Scholar]
- Stevens C., Bavelier D. The role of selective attention on academic foundations: a cognitive neuroscience perspective. Dev. Cogn. Neurosci. 2012;2:S30–S48. doi: 10.1016/j.dcn.2011.11.001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Stevens C., Fanning J., Coch D., Sanders L., Neville H. Neural mechanisms of selective auditory attention are enhanced by computerized training: electrophysiological evidence from language-impaired and typically developing children. Brain Res. 2008;1205:55–69. doi: 10.1016/j.brainres.2007.10.108. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Stevens C., Lauinger B., Neville H. Differences in the neural mechanisms of selective attention in children from different socioeconomic backgrounds: an event-related brain potential study. Dev. Sci. 2009;12(4):634–646. doi: 10.1111/j.1467-7687.2009.00807.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Strait D.L., Kraus N. Can you hear me now? Musical training shapes functional brain networks for selective auditory attention and hearing speech in noise. Front. Psychol. 2011;2:113. doi: 10.3389/fpsyg.2011.00113. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Strait D.L., Kraus N. Playing music for a smarter ear: cognitive, perceptual and neurobiological evidence. Music Percep. 2011;29(2):133–147. doi: 10.1525/MP.2011.29.2.133. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Strait D.L., Kraus N. Biological impact of auditory expertise across the life span: musicians as a model of auditory learning. Hear. Res. 2014;308:109–121. doi: 10.1016/j.heares.2013.08.004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Strait D.L., Kraus N., Parbery-Clark A., Ashley R. Musical experience shapes top-down auditory mechanisms: evidence from masking and auditory attention performance. Hear. Res. 2010;261:22–29. doi: 10.1016/j.heares.2009.12.021. [DOI] [PubMed] [Google Scholar]
- Strait D.L., O’Connell S., Parbery-Clark A., Kraus N. Musicians’ enhanced neural differentiation of speech sounds arises early in life: developmental evidence from ages three to thirty. Cereb. Cortex. 2014;24(9):2512–2521. doi: 10.1093/cercor/bht103. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Strait D.L., Parbery-Clark A., Hittner E., Kraus N. Musical training during early childhood enhances the neural encoding of speech in noise. Brain Lang. 2012;123:191–201. doi: 10.1016/j.bandl.2012.09.001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Strait D.L., Parbery-Clark A., O’Connell S., Kraus N. Biological impact of preschool music classes on processing speech in noise. Dev. Cogn. Neurosci. 2013;6:51–60. doi: 10.1016/j.dcn.2013.06.003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Strait D.L., Slater J., Abecassis V., Kraus N. Cortical response variability as a developmental index of selective auditory attention. Dev. Sci. 2014;17(2):175–186. doi: 10.1111/desc.12107. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tervaniemi M., Kruck S., De Baene W., Schroger E., Alter K., Friederici A.D. Top-down modulation of auditory processing: effects of sound context, musical expertise and attentional focus. Eur. J. Neurosci. 2009;30(8):1636–1642. doi: 10.1111/j.1460-9568.2009.06955.x. [DOI] [PubMed] [Google Scholar]
- Tipper S.P., Bourque T.A., Anderson S.H., Brehaut J.C. Mechanisms of attention: a developmental study. J. Exp. Child Psychol. 1989;48(3):353–378. doi: 10.1016/0022-0965(89)90047-7. [DOI] [PubMed] [Google Scholar]
- Vaurio R.G., Simmonds D.J., Mostofsky S.H. Increased intra-individual reaction time variability in attention-deficit/hyperactivity disorder across response inhibition tasks with different cognitive demands. Neuropsychologia. 2009;47(12):2389–2396. doi: 10.1016/j.neuropsychologia.2009.01.022. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Walker D., Greenwood C., Hart B., Carta J. Prediction of school outcomes based on early language production and socioeconomic factors. Child Dev. 1994;65(2 Spec No):606L 621. [PubMed] [Google Scholar]
- Winterer G., Coppola R., Goldberg T.E., Egan M.F., Jones D.W., Sanchez C.E., Weinberger D.R. Prefrontal broadband noise, working memory, and genetic risk for schizophrenia. Am. J. Psychiatry. 2004;161(3):490–500. doi: 10.1176/appi.ajp.161.3.490. [DOI] [PubMed] [Google Scholar]
- Zatorre R.J., Salimpoor V.N. From perception to pleasure: music and its neural substrates. Proc. Natl. Acad. Sci. USA. 2013;110:10430–10437. doi: 10.1073/pnas.1301228110. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zendel B.R., Alain C. Musicians experience less age-related decline in central auditory processing. Psychol. Aging. 2011;27(2):410–417. doi: 10.1037/a0024816. [DOI] [PubMed] [Google Scholar]