Highlights
-
•
Reappraisal of emotional faces in children was examined with magnetoencephalography.
-
•
Neural activity in the LPP time-interval was modulated in frontoparietal networks.
-
•
Occipital activity was reduced with increasing age, indexing neural maturation.
-
•
A similar reduction was linked to better emotion regulation and less anxiety.
-
•
This may indicate an age-independent advance in neural maturation.
Keywords: Emotion regulation, Reappraisal, Children, Emotional faces, Magnetoencephalography, LPP
Abstract
Emotion regulation has an important role in child development and psychopathology. Reappraisal as cognitive regulation technique can be used effectively by children. Moreover, an ERP component known to reflect emotional processing called late positive potential (LPP) can be modulated by children using reappraisal and this modulation is also related to children's emotional adjustment. The present study seeks to elucidate the neural generators of such LPP effects. To this end, children aged 8–14 years reappraised emotional faces, while neural activity in an LPP time window was estimated using magnetoencephalography-based source localization. Additionally, neural activity was correlated with two indexes of emotional adjustment and age. Reappraisal reduced activity in the left dorsolateral prefrontal cortex during down-regulation and enhanced activity in the right parietal cortex during up-regulation. Activity in the visual cortex decreased with increasing age, more adaptive emotion regulation and less anxiety. Results demonstrate that reappraisal changed activity within a frontoparietal network in children. Decreasing activity in the visual cortex with increasing age is suggested to reflect neural maturation. A similar decrease with adaptive emotion regulation and less anxiety implies that better emotional adjustment may be associated with an advance in neural maturation.
1. Introduction
The attainment of flexible and effective emotion regulation skills during childhood is considered a key developmental task in support of psychological adjustment (Cole and Deater-Deckert, 2009, Eisenberg et al., 2010). Much evidence documents the relevance of poor emotion regulation for a wide range of mental health problems in children and adolescents, including anxiety and major depression (e.g. Kovacs et al., 2008, Weems and Silverman, 2006). A frequently applied treatment for these disorders in children is cognitive behavioral therapy (Muñoz-Solomando et al., 2008), which seeks to reduce symptoms of emotional dysregulation via alterations of depressogenic or anxiety-provoking cognitions quite effectively (e.g. James et al., 2015, Weersing et al., 2009). Despite cognitive emotion regulation playing a key role in developmental psychopathology, to date only few studies elucidate its neural mechanisms in children.
A technique frequently used to study cognitive emotion regulation is reappraisal, which involves changing the emotional impact of a stimulus through reinterpretation (Gross and John, 2003). In experiments with adults, reappraisal is shown to modulate a variety of emotion indicators, including self-reports, facial electromyography, startle response, or electrodermal activity (Ray et al., 2010, Urry, 2009). Moreover, adult self-reports associate habitual use of reappraisal with greater psychological well-being (John and Gross, 2004) and less psychopathology (Garnefski et al., 2002). In experiments with children, successful implementation of reappraisals given by the experimenter (directed reappraisal, Dennis and Hajcak, 2009) is reported to start between the ages 5 and 7 while self-generated reappraisals reduce emotional responses to threatening pictures in children of age 10 and older (Carthy et al., 2010). Self-reports of the use of reappraisal are applicable by 9 years (Gullone et al., 2010), and adolescents’ self-reports confirm an association of reappraisal with mental health (Garnefski et al., 2002). Taken together, these findings suggest that both experimental instructions and self-reports of reappraisal are suitable to investigate cognitive emotion regulation in children from around the age of 8.
In adults, there is a relative consensus about relevant neural structures mediating reappraisal, based on convergent findings of fMRI studies. Both up- and down-regulation via reappraisal increased neural activity in regions associated with cognitive control, such as the prefrontal cortex, while neural activity in regions associated with emotion generation, such as the amygdala and insula, was increased during up-regulation and reduced during down-regulation (Ochsner and Gross, 2005, Ochsner and Gross, 2008). Previous fMRI experiments in children and adolescents converge by showing increased activity in prefrontal and increased or decreased activity in emotion generating regions during reappraisal. Moreover, these studies consistently demonstrate age-related changes particularly in prefrontal structures (Lévesque et al., 2004, McRae et al., 2012, Pitskel et al., 2011), although the detailed pattern of results is quite inconsistent. So, children's neural networks underlying reappraisal overlap with those of adults, but data of children are still rare and age-related changes remain to be specified.
Reappraisal has also been investigated by event-related potential (ERP) studies, which have focused on the late positive potential (LPP). The LPP is a visual ERP component occurring around 300 ms that is typically enhanced by emotional compared to neutral stimulus content in adults (Schupp et al., 2006, Olofsson et al., 2008) and children (Hajcak and Dennis, 2009, Wessing et al., 2011) and is believed to reflect facilitated attention to and enhanced processing of emotional stimuli (Lang and Bradley, 2010). In adults, ERP studies documented that the LPP can be modulated by reappraisal, with enhanced amplitudes during up-regulation (Moser et al., 2009, Moser et al., 2010) and reduced amplitudes during down-regulation (Hajcak and Nieuwenhuis, 2006, Moser et al., 2009). In children, an effective reduction of the LPP amplitude by reappraisal was observed in 7–10-year-olds (Dennis and Hajcak, 2009), while in 5–7-year-olds this effect could not yet be shown reliably (DeCicco et al., 2012, Dennis and Hajcak, 2009). Beyond that, these studies revealed positive correlations of the LPP with symptoms of anxiety and negative correlations with emotion regulation capacities. The neural generators of these effects are currently unknown.
The aim of the present study was to investigate neural mechanisms underlying cognitive emotion regulation in children using magnetoencephalography (MEG) based source localization. MEG offers a high time resolution and a fairly good localization of LPP-related cortical activity, which allows filling the gap between existing fMRI and ERP findings. Moreover, this non-invasive and easily tolerated method is perfectly suited for the investigation of children. As children were shown to effectively use reappraisal and give self-reports on emotion regulation with about 8 years, this determined the lower age limit. To further allow for an examination of age-related changes – as an approximation to development – within one experimental set-up the upper age limit was set to 14 years.
An experimental design for the regulation of emotions recently used in adults (Wessing et al., 2013) was adapted to children. Children saw pictures of faces with angry or neutral expressions, while MEG was recorded. Prior to picture presentation, children were instructed to use reappraisal, which involved imagination of the depicted persons in different social situations. To up-regulate threat (threat-up), children imagined they were confronted with an angry, scolding neighbor. To down-regulate threat (threat-down), children imagined to evaluate the performance of an actor. In a control condition, children attentively viewed the pictures (view). Following picture presentation, children performed a threat rating of each presented face and completed questionnaires on emotion regulation and anxiety.
Based on the precursor study with adult participants (Wessing et al., 2013), the LPP was examined in an interval between 280 and 680 ms and it was hypothesized that (1) an emotion effect should evoke enhanced neural activity in response to angry versus neutral faces in visual sensory and prefrontal cortex (PFC) regions, (2) reappraisal should increase activity in the PFC, and (3) down-regulation should reduce and up-regulation should enhance the emotion effect. In addition to experimental manipulations, correlation analyses were conducted to analyze changes of neural activity with age and two indexes of emotional adjustment. Based on previous findings (Dennis and Hajcak, 2009, DeCicco et al., 2012) it was hypothesized that LPP-related neural activity should show (4) negative correlations with emotion regulation and (5) positive correlations with trait anxiety.
2. Methods
2.1. Participants
Fifty-six children (28 female) aged 8–14 years (M = 132.43 months; SD = 21.82) participated in this study, with gender almost evenly distributed across ages (χ2(2) = 0.24; p = .94; see Table 1). All children had normal or corrected-to-normal vision, did not suffer from any child psychiatric disease (structured clinical interview: Kinder-DIPS; Unnewehr et al., 2009), and had normal to high intelligence (IQ: M = 113.71; SD = 16.33; up to 8; 5 years: CFT-1, 5th ed., Cattell et al., 1997; above 8; 5 years: CFT-20-R, Weiß, 2008). Participants were recruited via classroom information sessions. Each child obtained a cinema voucher for participation and parents received a compensation for their travel expenses. Children and parents were informed about the procedure in written and oral form, and both gave written informed consent. The study design was approved by the ethics committee of the Medical Faculty, University of Muenster, in accordance with the Declaration of Helsinki.
Table 1.
Distribution of age and gender.
| Gender | Age |
|||
|---|---|---|---|---|
| 8–9 | 10–11 | 12–14 | Total | |
| Male | 8 | 11 | 10 | 29 (51.8%) |
| Female | 9 | 9 | 9 | 27 (48.2%) |
| Total | 17 (30.4%) | 20 (35.7%) | 19 (33.9%) | 56 (100%) |
2.2. Self-report of emotion regulation
Children completed the Questionnaire for the Measurement of Emotion Regulation in Children and Adolescents (FEEL-KJ; Grob and Smolenski, 2005), which captures the use of adaptive and maladaptive emotion regulation strategies for anxiety, anger, and sadness. This German-language questionnaire consists of 90 items and is designed for the ages 10–20 years. An experimenter ensured the understanding of all items and response schemes also by younger children. T-scores were built for the use of adaptive and maladaptive strategies based on the T-norms for 10;0 to 15;11 years. A difference score was calculated by subtracting the T-scores for adaptive and maladaptive strategies, resulting in a single score with positive values for a predominant use of adaptive and negative values for a predominant use of maladaptive strategies.
2.3. Self-report of trait anxiety
Children's trait anxiety was measured using the T-Anxiety scale of the State-Trait Anxiety Inventory for Children (STAIC; Spielberger, 1973). This 20-item questionnaire was designed for elementary school children. The response to each item is scored with 1–3 points, with more points signaling increasing anxiety. Since there are no T-norms available for the German version (Weinbrenner, 2005), sum scores of all items were used as an index of anxiety.
2.4. Stimuli
Three sets of angry and neutral faces were created by selecting overall 60 different male or female adult individuals from the “NimStim set of facial expressions” (Tottenham et al., 2009) and the “Karolinska Directed Emotional Faces – KDEF” set (Lundqvist et al., 1998). Each face set comprised 20 different faces, including 5 male angry, 5 female angry, 5 male neutral, and 5 female neutral faces. All pictures were converted to gray scale and aligned in brightness and size. The face region was selected and overlaid on a gray background (see Fig. 1).
Fig. 1.
Schematic experimental run. Children were seated in the MEG scanner and one of three reappraisal instructions was given before 20 faces from one of overall three face sets were presented. The order of instructions and corresponding face sets were counterbalanced across participants. Then an overview screen appeared and children identified faces corresponding to the instruction. Finally, faces were rated for perceived threat.
2.5. Instructions
The experimental conditions were established by three different instructions (for literal instructions see Appendix 1). In an up-regulation condition (threat-up), the child was instructed to imagine playing ball on the street and accidentally smashing the neighbor's window. The neighbor (represented by the faces) approaches, while scolding at the child. The child should imagine each face to be the neighbor and decide which neighbor would be most threatening. In this threatening situation, the child faces a warrantably angry authority figure that may cause embarrassment or penalty. In a down-regulation condition (threat-down), the child was instructed to imagine being a member of a drama group. Different actors (represented by the faces) apply for the part of the villain and the child should decide which actor is best at expressing an angry face. This is a safe situation in which the displayed expression of anger is only pretended. The child was asked to imagine both reappraisal situations as lively as possible and to afterwards identify the best expressive or most threatening face, respectively. In a view control condition (view), the child was instructed to relax and attentively view the faces. To parallelize tasks and maintain the child's attention, the child was told that there will be a question afterwards and then asked to identify if two sample faces had been shown before.
2.6. Procedure
Children and their accompanying parent(s) were familiarized with the MEG chamber and introduced to the procedures. Children's head shape was registered using a 3D head digitisation system (Polhemus 3Space® Fasttrack). Next, children were seated in the MEG scanner. Their position was monitored using landmark coils that were attached to the two auditory canals and the nasion.
MEG data were recorded in three subsequent runs (Fig. 1). During each run one of the three face sets was presented. Within sets, faces were repeated 8 times in pseudorandom order, resulting in 160 trials per run. The probability of transition between face types (male angry, female angry, male neutral, and female neutral) was equated. The stimuli were presented for 750 ms with a jittered inter-stimulus interval (ISI) of 1250 ± 500 ms and displayed on a screen at 95 cm distance, covering a visual angle of approximately 14° vertically and 10° horizontally. Across runs, the face sets were presented in a fixed order, while the order of experimental conditions, i.e. the assignment of face set and instruction, were counterbalanced across participants. At the beginning of each run, one of the three instructions was given, followed by an approximate 5-min stimulus presentation. Next, children saw an overview screen depicting the presented face set and were asked to select one or two faces according to the instruction. At the end of each run, children performed a threat rating. All 20 faces of the just presented set were randomly shown for 750 ms each, along with the question “How threatening is this face?”, and children rated perceived threat on a 5-point scale ranging from 1 (not at all) to 5 (very). Picture presentation and rating were executed using Presentation software (version 13.0; Neurobehavioral Systems, Inc.; Albany, CA).
2.7. MEG data recording and processing
MEG data were acquired using a 275 MEG whole-head sensor system (Omega 275, CTF) in the frequency band between 0 and 150 Hz and a sampling rate of 600 Hz. Offline data were down-sampled to 300 Hz and band-pass filtered between 0.1 and 48 Hz. Data were averaged in epochs ranging from 200 ms before to 700 ms after stimulus presentation, using a 150 ms pre-stimulus interval for baseline-adjustment. Single-trial data editing and artifact rejection were conducted using the method for statistical control of artifacts in high-density EEG/MEG data (Junghöfer et al., 2000). This procedure (1) detects individual channel artifacts, (2) detects global artifacts, (3) replaces artifact-contaminated sensors with spline interpolation, statistically weighted on the basis of all remaining sensors, and (4) computes the variance of the signal across trials to document the stability of the averaged waveform. The rejection of artifact-contaminated trials and sensor epochs relies on the calculation of statistical parameters for the absolute measured magnetic field amplitudes over time, their standard deviation over time, the maximum of their gradient over time (first temporal derivative), and the determination of boundaries for each of these parameters. Epochs were averaged in correspondence to the experimental conditions. On the basis of the averaged responses, cortical sources of the event-related magnetic fields were estimated using the L2-Minimum-Norm-Estimates (L2-MNE) method (Hämäläinen and Ilmoniemi, 1994). A spherical shell consisting of 350 evenly distributed dipole pairs with azimuthal and polar orientation was used as source model. A source shell radius of around 90% of the individually fitted head radius was chosen, which roughly corresponds to the gray matter depth. A Tikhonov regularization parameter k of 0.2 was applied. Eventually, for each test dipole position the estimated neural activity was calculated as vector length of each dipole pair. Preprocessing and analysis of MEG data were conducted with the MATLAB-based EMEGS software (www.emegs.org; Peyk et al., 2011).
2.8. Statistical analyses
Threat rating and MEG data were analyzed by two-way repeated-measures analyses of variance (ANOVAs) including the factors emotion (angry, neutral) and instruction (threat-up, threat-down, view). Distinct contributions of the three instruction conditions were further specified by post hoc T-tests. For all ANOVAs and T-tests, an alpha level of p < .05 was used as a significance criterion. Violation of the sphericity assumption was controlled using Mauchly's tests and did not occur in any of the reported effects.
Statistical analyses of MEG data were conducted in the a priori defined LPP time interval between 280 and 680 ms, corresponding to the MEG equivalent of the LPP component found in the companion study with adults using identical stimuli and stimulus presentation. This time window had been selected to begin with the onset of LPP emotion effects and to exclude effects of stimulus off-set expectancy (Wessing et al., 2013). Given the good accordance with children's data at visual inspection, this definition maximized comparability of adult and child data. Hence, estimated neural activity (L2-MNE; below called neural activity) was averaged across all time points in the LPP time interval and the above specified ANOVA was calculated. As result, F-values for main and interaction effects were assigned to each cortical test source. Next, clusters of cortical sources were determined that showed significant effects after correction for multiple comparisons using non-parametric cluster permutation tests (Maris and Oostenveld, 2007). To this end, F-values of a minimum of ten adjacent sources all exceeding a sensor-level significance criterion of p < .05 (sig. cluster) were averaged. Identical first-level clusters were calculated for 1000 permutations of the complete data set (participants and experimental conditions) which served as non-parametric F-distribution. Clusters exceeding a cluster-level criterion of p < .05 were regarded as significant and – for illustrative purposes – projected onto a standard brain. For the calculation of post hoc T-tests, neural activity was averaged across all cortical sources within these clusters and LPP time interval.
In addition, the relationship between neural activity in the LPP time interval, age and self-report data was explored by correlation analyses. To this end, scores reflecting mean and difference activities were calculated for each cortical source. For mean activity neural activity was averaged across all experimental conditions (emotion, instruction). Difference activity was calculated by averaging activity across instruction conditions, but separately for angry and neutral faces, and then building a difference score (angry minus neutral). Interrelations of mean and difference activity with age (in months) and self-report data (emotion regulation, trait anxiety) were analyzed using Pearson's correlation coefficients. As a result, r-values representing these correlations were assigned to each cortical source. Convergent to the non-parametric analysis described above, clusters of cortical sources were determined that showed significant correlations after correction for multiple comparisons (first-level criterion: p < .05; cluster-level criterion: p < .01; cluster of at least 10 neighboring test sources). For illustration purposes, topographic maps of significant clusters were projected onto a standard brain. Additionally, Pearson's correlation coefficients also analyzed the relationship between age and self-report data.
Statistical analyses and source localizations of MEG data were performed using EMEGS software (www.emegs.org; Peyk et al., 2011). All other statistical analyses were conducted using PASW 22 (SPSS Inc.).
3. Results
3.1. Threat rating
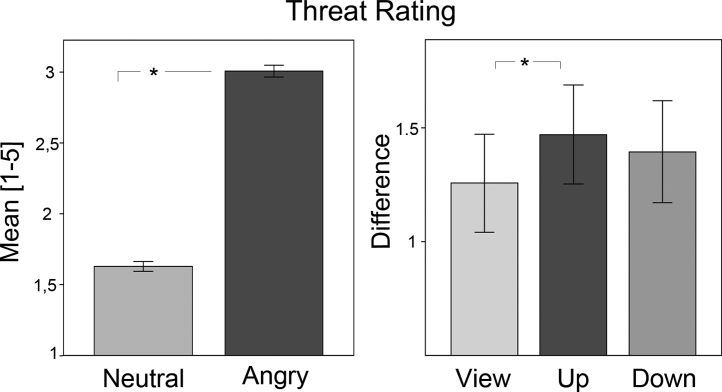
Confirming the experimental setup, children rated angry faces as more threatening than neutral faces (emotion: F(1, 541 ) = 190.69; p < .001; see Fig. 2, left). Moreover, children's differential threat rating was modulated by reappraisal (emotion × instruction: F(1, 54) = 3.66; p < .05). The difference between angry and neutral faces was enhanced in the threat-up vs. view condition (T(54) = 2.6; p < .05). Hence, compared to attentive viewing, threat appraisal was not reduced in the threat-down, but enhanced in the threat-up condition.
Fig. 2.
Threat rating. Mean threat ratings for neutral and angry faces (left) and difference (angry minus neutral faces, right) in the view, threat-up and threat-down conditions.
3.2. Source localization of experimental effects
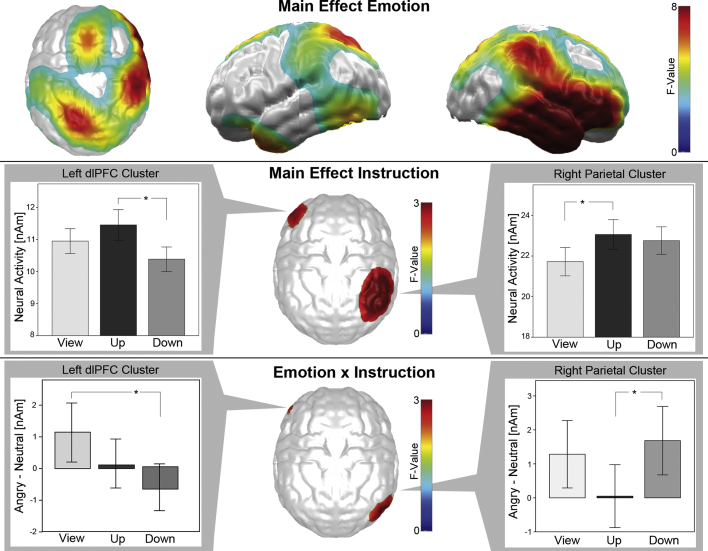
Clusters of neural sources showing a significant main effect of emotion (p < .05, below called emotion effect), with enhanced activity in response to angry compared to neutral faces, were localized in a widespread cortical network (see Fig. 3, top). The emotion effect was strongest in the right hemisphere, where it involved the whole temporal and ventrolateral prefrontal cortex and also a large part of the parietal cortex, spreading into occipital regions. In the left hemisphere, the emotion effect was less strong and involved parietal and temporo-occipital regions, the temporal pole, as well as part of the left ventrolateral prefrontal cortex. Finally, a dorsal frontal region was involved that spread across both hemispheres.
Fig. 3.
Topography of experimental effects. Topographic maps of cluster-level significant F-values projected on a standard brain for the main effect of emotion (top), the main effect of instruction (middle) and the interaction of emotion and instruction (bottom). Bargraphs show the mean (middle) and difference (angry minus neutral faces, bottom) of neural activity in the view, threat-up and threat-down conditions, averaged in the respective clusters.
This strong emotion effect was accompanied by comparably smaller, but significant changes induced by reappraisal. Clusters of neural sources showing a main effect of instruction and an interaction of emotion × instruction (p < .05) were each localized in overlapping regions in the right parietal and left dorsolateral prefrontal cortex (dlPFC; see Fig. 3, middle and bottom).
The main effect of instruction in the left dlPFC was driven by a significant difference between the threat-down and threat-up conditions (T(55) = 2.70; p < .05), with stronger activity during the threat-up and less activity during the threat-down condition. Activity in the view condition was in between both other conditions and differences were insignificant in post hoc tests (Fig. 3, middle left). The main effect of instruction in the right parietal cortex was due to enhanced activity during the threat-up compared to the view condition (T(55) = 2.78; p < .01). In this case, activity in the threat-down condition lay in between, showing no significant differences to both other conditions in post hoc tests (Fig. 3, middle right).
The emotion × instruction interaction in the dlPFC was a result of a reduced emotion effect in the threat-down compared to the view condition (T(55) = −2.66; p < .05), driven by reduced activity in response to angry faces in the threat-down condition (Fig. 3, bottom left; angry faces: threat-down vs. view T(55) = −3.47; p < .01; threat-up vs. view: n.s., threat-down vs. threat-up T(55) = −2.96; p < .01; neutral faces: all n.s.). The emotion × instruction interaction in the right parietal cortex was based on a reduced emotion effect in the threat-up compared to the view (T(55) = −2.75; p < .01) and threat-down conditions (T(55) = −3.14; p < .01), driven by enhanced processing of neutral faces in the threat-up condition (Fig. 3, bottom right; angry faces: all n.s.; neutral faces: threat-up vs. view T(55) = 3.07; p < .01; threat-down vs. view n.s.; threat-up vs. threat-down T(55) = 2.08; p < .05).
3.3. Source localization of correlations
Clusters of significant correlations (p < .01) of mean activity (averaged across all experimental conditions) were found with age, emotion regulation, and trait anxiety. Clusters of significant correlations (p < .01) of difference activity (angry minus neutral faces, averaged across instruction conditions) were evident with age and emotion regulation, but not trait anxiety.
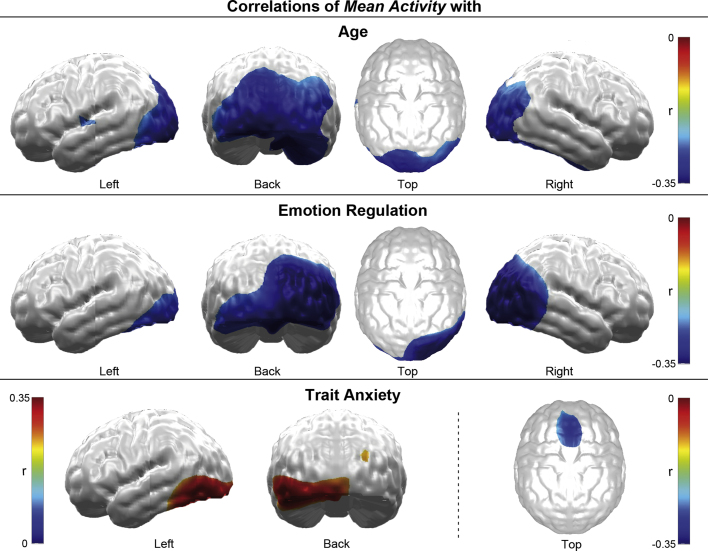
Mean activity was reduced with increasing age in the bilateral occipital cortex, the right ventral temporal cortex, and a small left ventral frontal/superior temporal region (Fig. 4, top). Moreover, mean activity in the (more right-hemispheric) occipital cortex was reduced with increasingly adaptive emotion regulation (Fig. 4, middle). Finally, mean activity in the (more left-hemispheric) occipital cortex was also associated with trait anxiety, but in this case activity increased with increasing anxiety (Fig. 4, bottom left). In addition, increasing trait anxiety was associated with reduced mean activity in the dorsal PFC (Fig. 4, bottom right).
Fig. 4.
Topography of correlations of mean activity. Topographic maps of cluster-level significant r-values projected on a standard brain for the correlations of mean activity with age (top), emotion regulation (middle) and trait anxiety (bottom). Negative correlations are depicted in blue and positive correlations in red shadings.
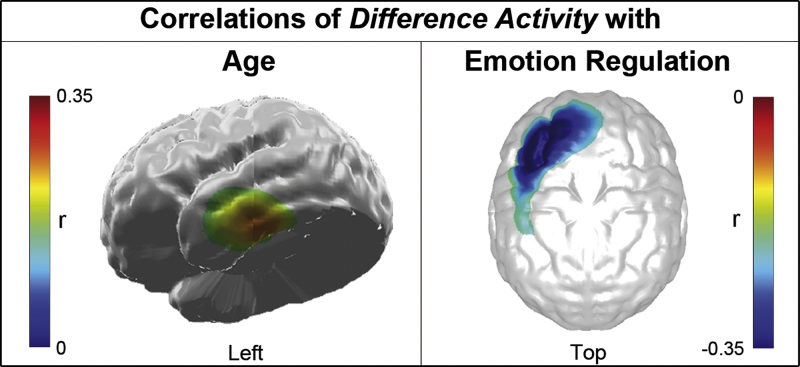
Regarding difference activity (angry minus neutral faces), a positive correlation with age was found in the left ventral temporal cortex (Fig. 5, left). Moreover, difference activity was reduced with more adaptive emotion regulation in the left dlPFC (Fig. 5, right).
Fig. 5.
Topography of correlations of difference activity. Topographic maps of cluster-level significant r-values projected on a standard brain for the correlations of difference activity (angry minus neutral faces) with age (left) and emotion regulation (right). Negative correlations are depicted in blue and positive correlations in red shadings.
Finally, correlation analyses of age and self-report data revealed a negative correlation of emotion regulation with trait anxiety (r = −.56; p < .001), which may correspond to their opposing correlations with visual cortical activity. However, both indexes of emotional adjustment were clearly uncorrelated with age (emotion regulation × age: r = .16; n.s.; trait anxiety × age: r = .01; n.s.).
4. Discussion
The results of the present study demonstrate neural changes via cognitive emotion regulation in 8–14 year old children and relations of neural functioning with age and day-to-day emotional adjustment.
Reappraisal of faces as threatening (threat-up) led to enhanced threat ratings and enhanced processing of all, but especially neutral faces in the right parietal cortex. On the contrary, reappraisal of faces as non-threatening (threat-down) did not decrease subjective threat ratings, but reduced activity in the left dlPFC particularly in response to angry faces. The timing of reappraisal effects, occurring in the LPP time interval, is consistent with prior studies in adults (Wessing et al., 2013, Moser et al., 2009) and children (Dennis and Hajcak, 2009). Also the localization of reappraisal effects in parietal and dlPFC regions (see Fig. 3) is consistent with results in adults (Wessing et al., 2013, Ochsner and Gross, 2008).
Corroborating hypothesis (1), an LPP emotion effect with enhanced activity in response to angry versus neutral faces was localized in visual and prefrontal cortical regions. This is in line with prior MEG and combined EEG/fMRI source localizations using emotional scenes (Liu et al., 2012, Moratti et al., 2011, Sabatinelli et al., 2013) and faces (Wessing et al., 2013). The observed right lateralization is consistent with a right-hemispheric advantage for face processing in general (Rossion et al., 2003), albeit regarding a lateralization of facial emotion processing results are rather mixed (Bayle and Taylor, 2010, Hung et al., 2010).
Contrary to hypothesis (2), reappraisal compared to attentive viewing did not evoke enhanced activity in the PFC, which was evident in previous fMRI studies (Ochsner and Gross, 2008) and was associated with the execution of cognitive control. Instead, activity differed between up- and down-regulation in the left dlPFC, with less activity during down-regulation. Moreover, up-regulation compared to passive viewing enhanced activity in the right parietal cortex. Taken together, these opposing effects with reduced activity during down-regulation and enhanced activity during up-regulation suggest an interpretation as being a result of emotion regulation, rather than reflecting the execution of cognitive control.
The lack of enhanced PFC activity during reappraisal may be based on methodical and/or developmental aspects. Methodically, the rather early LPP time window used may be relevant. Using markedly longer LPP time windows (several seconds), Parvaz et al. (2012) showed enhanced PFC activity during reappraisal, as indexed by reduced frontal alpha power. Hence, PFC activity associated with cognitive control may be captured only at later stages of processing. Moreover, in the study at hand reappraisal instructions were given for a whole block of stimulus presentations. Therefore, the execution of reappraisal was not necessarily stimulus-locked, which makes it difficult to capture. Indeed, also the precursor adult study, using the same block design, revealed enhanced PFC activity at trend level only (Wessing et al., 2013).
Considering developmental aspects, children may have had more difficulties to implement reappraisal as adults, constricting their ability to stay on task during the whole block. Moreover, during childhood, cognitive control-related PFC activity is known to undergo substantial developmental changes (Durston and Casey, 2006, Rubia, 2012). In fact, prior fMRI reappraisal studies in children and adolescents found both enhanced and reduced PFC activity (Lévesque et al., 2004, McRae et al., 2012, Pitskel et al., 2011) and reappraisal-related activity in the left inferior frontal gyrus was reported to increase with age, whereby children aged 10–13 years did not yet show enhanced activity (McRae et al., 2012). Based on these developmental changes, it is conceivable that some results are confounded by analyses across a rather broad age range.
Regarding hypothesis (3), results indeed showed effective modulation of the neural emotion effect by reappraisal, but they also deviated from expectations. In the case of down-regulation (threat-down), processing of angry (vs. neutral) faces was reduced, in line with hypothesis (3). The localization of this effect in the left dlPFC is consistent with two recent meta-analyses linking the left lateral PFC particularly to the perception of anger (Lindquist et al., 2012, Vytal and Hamann, 2010). So, indeed, processing of angry faces seemed to be down-regulated on a neural level, but this down-regulation was not reflected on a behavioral level by the threat ratings. Considering the effects of up-regulation (threat-up), it was not the difference between angry and neutral faces that was enhanced (as expected and found in adults), but rather the response to neutral faces, which might be explained by developmental aspects. The ability to recognize emotional expressions is still developing in the examined age range and has not yet reached adult levels (Thomas et al., 2007, Johnston et al., 2011). Moreover, children may perceive neutral faces as more ambiguous than emotional faces, as indexed by enhanced amygdala responding (Thomas et al., 2001). Hence, during up-regulation, children may have appraised faces as threatening despite their neutral expression, and thus processing was enhanced. Of course, such a post hoc interpretation requires further validation, especially since threat ratings were rather enhanced for angry faces.
Taken together, 8–14 year old children were able to effectively regulate neural responses via reappraisal. On the whole, this speaks for the usefulness of cognitive emotion regulation techniques in children of this age, e.g. in therapeutic settings. However, children's results also deviated from expectations and from adults’ results, e.g. in showing divergent effects in the behavioral and neural domain. These deviations possibly reflect child-specific characteristics regarding the implementation of reappraisal, the relationship between conscious choices (rating) and intuitive emotional face perception (neural responses), and the localization of neural functioning. Hence, children's capability to reappraise may not yet be fully developed and its effects not yet fully accessible to conscious awareness. With respect to clinical applications, this stresses the importance of well-considered, child-oriented tutorial assistance and practice.
In addition to the effects of reappraisal, correlation analyses explored the relationship of neural activity (mean and difference activity) with age and two indexes of emotional adjustment. Correlations of neural activity with age may be interpreted as an approximation of functional neural development (McRae et al., 2012, Pitskel et al., 2011). With increasing age, mean activity was reduced in the visual cortex (Fig. 4, top), which is consistent with age-related decreases in the amplitudes of visual ERPs (Taylor et al., 2004, Kuefner et al., 2010, Sumich et al., 2012). Moreover, an age-related reduction in EEG power was linked to a decrease in gray matter volume (Whitford et al., 2007). Based on these findings, the observed reduction of visual cortical activity with age may be interpreted as an index of neural maturation. Additionally, increasing age was related to increased difference activity in the left ventral temporal cortex (Fig. 5, left). The ventral temporal cortex and specifically the fusiform face area exhibit increasing specialization for faces in both hemispheres during development (Joseph et al., 2011). Based on the observation that the emotion effect occurred in the temporal cortices of both hemispheres in adults (Wessing et al., 2013), it is suggested that the additional recruitment of the left ventral temporal cortex with increasing age reflects increasing regional specialization for the processing of emotion in faces.
Correlations of mean activity with day-to-day emotional adjustment were found in strikingly similar visual cortical regions as with age. More precisely, corroborating hypothesis (4), visual cortical activity was reduced with increasingly adaptive emotion regulation (Fig. 4, middle). Moreover, corroborating hypothesis (5), it was enhanced with increasing trait anxiety (Fig. 4, bottom left). This is consistent with previous reappraisal studies in children, showing that specific LPP amplitudes were reduced with more adaptive emotion regulation and enhanced with increasing anxious-depressed symptoms (Dennis and Hajcak, 2009), and that the LPP difference between unpleasant and neutral stimuli was enhanced with higher anxiety and more fearful behavior (DeCicco et al., 2012). Although involving somewhat different indicators of LPP-related neural processing, these results converge in showing that less intense neural responses were associated with better emotional adjustment.
What is more, contrary to hypothesis (5), mean activity was also negatively correlated with trait anxiety in the superior PFC (Fig. 4, bottom right). So, trait anxiety was negatively correlated with prefrontal and positively with visual cortical activity. Consistently, in adolescents suffering from anxiety disorders, symptom severity was negatively correlated with prefrontal and positively with amygdala activity (Pine et al., 2008) and, during emotional processing, the superior PFC was inversely connected with the amygdala (Blair et al., 2007). The amygdala is proposed to assess the motivational relevance of stimuli and to evoke enhanced processing thereof in the visual cortex (Lang and Bradley, 2010). Reduced prefrontal inhibition, and thus enhanced activity of the amygdala, may have evoked enhanced visual cortical activity during emotional processing. Therefore, the present inverse correlations may reflect reduced prefrontal inhibition of the amygdala in more anxious children.
Finally, difference activity was reduced during more adaptive emotion regulation in the left dlPFC (Fig. 5, right). This fits with the assumption that this region mediates anger processing, which is reduced during down-regulation. A reduced differentiation of expressions in this region may indicate a more effective reduction of angry face processing during down-regulation in adaptively regulating children. However, the present correlations reflect difference activity across all instruction conditions, so adaptively regulating children might also show less difference activity in this region in general.
Taken together, correlation analyses confirmed that neural activity is reduced with more adaptive day-to-day emotion regulation (regarding both mean and difference activity) and enhanced with increasing trait anxiety (regarding mean activity), in line with hypotheses (4) and (5). Trait anxiety was furthermore associated with reduced mean activity in the superior PFC, possibly indicating reduced prefrontal inhibition. Moreover, correlation analyses show an intriguing overlap with age-related changes, as visual cortical activity was reduced with both increasing age and better emotional adjustment. Given that emotion regulation and trait anxiety were uncorrelated with age and in light of the hypothesis that reduced visual cortical activity reflects neural maturation, the present results suggest that children who reported better emotional adjustment showed an age-independent advance in neural maturation.
Specific methodical limitations are worth consideration. First, the minimum norm solution used here allows the localization of superficial cortical sources only, which is inferior to fMRI guided source localizations. However, MEG-based source localization offers a high time resolution and, with application of distributed source modeling, a fairly good assignment of LPP-related cortical sources to larger cortical structures, which allows filling the gap between existing fMRI (high spatial but minimal temporal resolution) and ERP findings without application of source modeling (high temporal but low spatial resolution). Compared to EEG, MEG source modeling is far less vulnerable to inaccurate estimates of geometry and conductivity properties of the head, which is – with respect to rapid age dependent changes of these – specifically relevant for studies with children (Lew et al., 2013). Last but not least, MEG is a completely non-invasive, non-radiative, silent and easily tolerated method with short set-up times and thus perfectly suited for the investigation of children. Nevertheless, the usage of realistic head models based on structural MRI scans could improve the quality of source localizations especially at regions such as the orbitofrontal cortex where a spherical shell model is a less good approximation of the human head compared to the fronto-parietal regions targeted here (Steinsträter et al., 2010).
Secondly, the rather broad age range of 8–14 years can be considered as a drawback since relevant results may be obscured by averaging across ages. The rationale behind the chosen age range was to allow analyses across a substantial period of development within one methodological framework. Nevertheless, cross-sectional analyses of age-related changes can only be an approximation to development and should be followed up by longitudinal studies. Future studies would also benefit from a tighter clustering of age and the introduction of puberty measures.
Finally, the experimental set-up deviates from prior studies in using emotional faces and a block design. This procedure reduces comparability with studies using emotional scenes. Moreover, imagination of the given situations across a whole block may have been challenging for children, which may have contributed to the fact that not all of the expected results were obtained. However, the experimental set-up was chosen as developmentally appropriate for the whole age range examined. Facial expressions, as compared to the commonly used emotional scenes, evoke less strong emotional responses (Britton et al., 2006), which makes them easily tolerable for children. Facial expressions are also less physically and semantically diverse than emotional scenes, providing good comparability across emotion categories and ages. Moreover, the present design allowed for the presentation of many stimuli within a short time, improving both tolerability and signal-to-noise-ratio.
In conclusion, LPP-related neural changes via reappraisal were observed in a frontoparietal network in children. The pattern of results still deviated from that of adults, presumably based on methodological and developmental aspects. During the LPP time interval, visual cortical activity was decreased with increasing age, which may be interpreted as index of neural maturation. A similar decrease of visual cortical activity with more adaptive emotion regulation and less trait anxiety might imply that better emotional adjustment is related to an age-independent advance in neural maturation.
Conflict of interest
None.
Acknowledgements
This work was funded by the Deutsche Forschungsgemeinschaft, project SFB TRR-58 C01, and the Department of Child and Adolescent Psychiatry, University Hospital Münster.
Footnotes
Available online 28 February 2015
The threat rating in the threat-up condition of 1 participant is missing.
Appendix 1.
A.1. Instructions
A.1.1. View
Imagine you are calm and relaxed while you are shown different faces on a screen. Please watch the following faces attentively. Afterwards, two sample faces will be selected and you will be asked if these persons were shown in the preceding session.
A.1.2. Threat-down
Imagine you act in a theater group. Together with your friends you have to decide who may play the part of the villain. Several actors and actresses apply for the role and demonstrate how well they can put on a vicious, angry face. Who is best at expressing an angry face?
A.1.3. Threat-up
Imagine you are playing ball on the street. Suddenly, you kick the ball through your neighbor's window and shattered pieces hit the ground. Somebody runs out of the house yelling and you hide quickly behind the next corner. But the neighbor has seen you and approaches you. Who are you most afraid of now?
References
- Bayle D.J., Taylor M.J. Attention inhibition of early activation to fearful faces. Brain Res. 2010;1313:113–123. doi: 10.1016/j.brainres.2009.11.060. [DOI] [PubMed] [Google Scholar]
- Blair K.S., Smith B.W., Mitchell D.G.V., Morton J., Vythilingam M., Pessoa L., Fridberg D., Zametkin A., Nelson E.E., Drevets W.C., Pine D.S., Matin A., Blair R.J.R. Modulation of emotion by cognition and cognition by emotion. Neuroimage. 2007;35(1):430–440. doi: 10.1016/j.neuroimage.2006.11.048. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Britton J.C., Taylor S.F., Sudheimer K.D., Liberzon I. Facial expressions and complex IAPS pictures: common and differential networks. Neuroimage. 2006;31(906):919. doi: 10.1016/j.neuroimage.2005.12.050. [DOI] [PubMed] [Google Scholar]
- Cattell R.B., Weiß R.H., Osterland J. 5 revidierte Auflage. Hogrefe; Göttingen: 1997. Grundintelligenztest Skala 1 (CFT-1) [Google Scholar]
- Carthy T., Horesh N., Apter A., Edge M.D., Gross J.J. Emotional reactivity and cognitive regulation in anxious children. Behav. Res. Ther. 2010;48:384–393. doi: 10.1016/j.brat.2009.12.013. [DOI] [PubMed] [Google Scholar]
- Cole P.M., Deater-Deckert K. Emotion regulation, risk, and psychopathology. J. Child Psychol. Psychiatry. 2009;50(11):1327–1330. doi: 10.1111/j.1469-7610.2009.02180.x. [DOI] [PubMed] [Google Scholar]
- DeCicco J.M., Solomon B., Dennis T.A. Neural correlates of cognitive reappraisal in children: an ERP study. Dev. Cogn. Neurosci. 2012;2(1):79–80. doi: 10.1016/j.dcn.2011.05.009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dennis T.A., Hajcak G. The late positive potential: a neurophysiological marker for emotion regulation in children. J. Child Psychol. Psychiatry. 2009;50(11):1373–1383. doi: 10.1111/j.1469-7610.2009.02168.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Durston S., Casey B.J. What have we learned about cognitive development from neuroimaging? Neuropsychologia. 2006;44:2149–2157. doi: 10.1016/j.neuropsychologia.2005.10.010. [DOI] [PubMed] [Google Scholar]
- Eisenberg N., Spinrad T.L., Eggum N.D. Emotion-related self-regulation and its relation to children's maladjustment. Annu. Rev. Clin. Psychol. 2010;6:495–525. doi: 10.1146/annurev.clinpsy.121208.131208. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Garnefski N., Legerstee J., Kraaij V., van den Kommer T., Teerds J. Cognitive coping strategies and symptoms of depression and anxiety: a comparison between adolescents and adults. J. Adolesc. 2002;25:603–611. doi: 10.1006/jado.2002.0507. [DOI] [PubMed] [Google Scholar]
- Grob A., Smolenski C. Bern; Verlag Hans Huber: 2005. Fragebogen zur Erhebung der Emotionsregulation bei Kindern und Jugendlichen. [Google Scholar]
- Gross J.J., John O.P. Individual differences in two emotion regulation processes: implications for affect, relationships, and well-being. J. Pers. Soc. Psychol. 2003;85(2):348–362. doi: 10.1037/0022-3514.85.2.348. [DOI] [PubMed] [Google Scholar]
- Gullone E., Hughes E.K., King N.J., Tonge B. The normative development of emotion regulation strategy use in children and adolescents. A 2-year follow-up study. Child Psychol. Psychiatry. 2010;51(5):567–574. doi: 10.1111/j.1469-7610.2009.02183.x. [DOI] [PubMed] [Google Scholar]
- Hajcak G., Dennis T.A. Brain potentials during affective picture processing in children. Biol. Psychol. 2009;80:333–338. doi: 10.1016/j.biopsycho.2008.11.006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hajcak G., Nieuwenhuis S. Reappraisal modulates the electrocortical response to unpleasant pictures. Cogn. Affect. Behav. Neurosci. 2006;6(4):291–297. doi: 10.3758/cabn.6.4.291. [DOI] [PubMed] [Google Scholar]
- Hämäläinen M.S., Ilmoniemi R.J. Interpreting magnetic fields of the brain: minimum norm estimates. Med. Biol. Eng. Comput. 1994;32(1):35–42. doi: 10.1007/BF02512476. [DOI] [PubMed] [Google Scholar]
- Hung Y., Smith M.L., Bayle D.J., Mills T., Cheyne D., Taylor M.J. Unattendend emotional faces elicit early lateralized amygdala-frontal and fusiform activations. NeuroImage. 2010;50:727–733. doi: 10.1016/j.neuroimage.2009.12.093. [DOI] [PubMed] [Google Scholar]
- James A.A.C.J., Soler A., Weatherall R.R.W. Cognitive behavioural therapy for anxiety disorders in children and adolescents. Cochrane Database Syst. Rev. 2015;(4) doi: 10.1002/14651858.CD004690.pub2. Art. No.: CD004690. [DOI] [PubMed] [Google Scholar]
- John O.P., Gross J.J. Healthy and unhealthy emotion regulation: personality processes, individual differences, and life span development. J. Pers. 2004;76(6):1301–1334. doi: 10.1111/j.1467-6494.2004.00298.x. [DOI] [PubMed] [Google Scholar]
- Johnston P.J., Kaufman J., Bajic J., Sercombe A., Michie P.T., Karayanidis F. Facial emotion and identity processing development in 5- to 15-year-old children. Front. Psychol. 2011;2(26):1–9. doi: 10.3389/fpsyg.2011.00026. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Joseph J.E., Gathers A.D., Bhatt R.S. Progressive and regressive developmental changes in neural substrates for face processing: testing specific predictions of the interactive specialisation account. Dev. Sci. 2011;14(2):227–241. doi: 10.1111/j.1467-7687.2010.00963.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Junghöfer M., Elbert T., Tucker D.M., Rockstroh B. Statistical control of artifacts in dense array EEG/MEG studies. Psychophysiology. 2000;37:523–532. [PubMed] [Google Scholar]
- Kovacs M., Joormann J., Gotlib I.H. Emotion (dys)regulation and links to depressive disorders. Child Dev. Perspect. 2008;2(3):149–155. doi: 10.1111/j.1750-8606.2008.00057.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kuefner D., de Heering A., Jacques C., Palmero-Soler E., Rossion B. Early visually evoked electrophysiological responses over the human brain (P1, N170) show stable patterns of face-sensitivity from 4 years to adulthood. Front. Hum. Neurosci. 2010;3(67):1–22. doi: 10.3389/neuro.09.067.2009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lang P.J., Bradley M.M. Emotion and the motivational brain. Biol. Psychol. 2010;84:437–450. doi: 10.1016/j.biopsycho.2009.10.007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lévesque J., Joanette Y., Mensour B., Beaudoin G., Leroux J.M., Bourgouin P. Neural basis of emotional self-regulation in childhood. Neuroscience. 2004;129:361–369. doi: 10.1016/j.neuroscience.2004.07.032. [DOI] [PubMed] [Google Scholar]
- Lew S., Sliva D.D., Choe M.S., Grant P.E., Okada Y., Wolters C.H., Hämäläinen M.S. MEG and EEG source analysis in a realistic infant head model. NeuroImage. 2013;76:282–293. doi: 10.1016/j.neuroimage.2013.03.017. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lindquist K.A., Wager T.D., Kober H., Bliss-Moreau E., Feldman Barrett L. The brain basis of emotion: a meta-analytic review. Behav. Brain Sci. 2012;35:121–202. doi: 10.1017/S0140525X11000446. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Liu Y., Huang H., McGinnis-Deweese M., Keil A., Ding M. Neural substrate of the late positive potential in emotional processing. J. Neurosci. 2012;32(42):14563–14572. doi: 10.1523/JNEUROSCI.3109-12.2012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lundqvist D., Flykt A., Öhman A. Department of Clinical Neuroscience, Psychology Section, Karolinska Institute; 1998. The Karolinska Directed Emotional Faces – KDEF, CD ROM. ISBN 91-630-7164-9. [Google Scholar]
- Maris E., Oostenveld R. Nonparametric statistical testing of EEG- and MEG-data. J. Neurosci. Methods. 2007;164(1):177–190. doi: 10.1016/j.jneumeth.2007.03.024. [DOI] [PubMed] [Google Scholar]
- McRae K., Gross J.J., Weber J., Robertson E.R., Sokol-Hessner P., Ray R.D. The development of emotion regulation: an fMRI study of cognitive reappraisal in children, adolescents and young adults. Social Cogn. Affect. Neurosci.nce. 2012;7:11–22. doi: 10.1093/scan/nsr093. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Moratti S., Saugar C., Strange B.A. Prefontal–occipitoparietal coupling underlies late lantency human neuronal responses to emotion. J. Neurosci. 2011;31(47):17278–17286. doi: 10.1523/JNEUROSCI.2917-11.2011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Moser J.S., Krompinger J.W., Dietz J.D., Simons R.F. Electrophysiological correlates of decreasing and increasing emotional responses to unpleasant pictures. Psychophysiology. 2009;46:17–27. doi: 10.1111/j.1469-8986.2008.00721.x. [DOI] [PubMed] [Google Scholar]
- Moser J.S., Most S.B., Simons R. Increasing negative emotions by reappraisal enhances subsequent cognitive control: a combined behavioural and electrophysiological study. Cogn. Affect. Behav. Neurosci. 2010;10(2):195–207. doi: 10.3758/CABN.10.2.195. [DOI] [PubMed] [Google Scholar]
- Muñoz-Solomando A., Kendall T., Whittington C.J. Cognitive behavioural therapy for children and adolescents. Curr. Opin. Psychiatry. 2008;21:332–337. doi: 10.1097/YCO.0b013e328305097c. [DOI] [PubMed] [Google Scholar]
- Ochsner K.N., Gross J.J. The cognitive control of emotion. Trends Cogn. Sci. 2005;9(5):242–249. doi: 10.1016/j.tics.2005.03.010. [DOI] [PubMed] [Google Scholar]
- Ochsner K.N., Gross J.J. Cognitive emotion regulation. Insights from social cognitive and affective neuroscience. Curr. Direct. Psychol. Sci. 2008;17(2):153–158. doi: 10.1111/j.1467-8721.2008.00566.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Olofsson J.K., Nordin S., Sequeira H., Polich J. Affective picture processing: an integrative review of ERP findings. Biol. Psychol. 2008;77:247–265. doi: 10.1016/j.biopsycho.2007.11.006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Parvaz M.A., MacNamara A., Goldstein R.Z., Hajcak G. Event-related induced frontal alpha as a marker of lateral prefrontal cortex activation during cognitive reappraisal. Cogn. Affect. Neurosci. 2012;12(4):730–740. doi: 10.3758/s13415-012-0107-9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Peyk P., De Cesarei A., Junghöfer M. ElectroMagnetoEncephaloGraphy Software (EMEGS): overview and integration with other EEG/MEG toolboxes. Comput. Intell. Neurosci. 2011 doi: 10.1155/2011/861705. Special issue: Academic Software Applications for Electromagnetic Brain Mapping Using MEG and EEG. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pine D.S., Guyer A.E., Leibenluft E. Functional magnetic resonance imaging and pediatric anxiety. J. Am. Acad. Child Adolesc. Psychiatry. 2008;47(11):1217–1221. doi: 10.1097/CHI.0b013e318185dad0. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pitskel N.B., Bolling D.Z., Kaiser M.D., Crowley M.J., Pelphrey K.A. How grossed out are you? The neural bases of emotion regulation from childhood to adolescence. Dev. Cogn. Neurosci. 2011;1(3):S324–S337. doi: 10.1016/j.dcn.2011.03.004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ray R.D., McRae K., Ochsner K.N., Gross J.J. Cognitive reappraisal of negative affect: converging evidence from EMG and self-report. Emotion. 2010;10(4):587–592. doi: 10.1037/a0019015. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rossion B., Joyce C.A., Cottrell G.W., Tarr M.J. Early lateralization and orientation tuning for face, word, and object processing in the visual cortex. NeuroImage. 2003;20:1609–1624. doi: 10.1016/j.neuroimage.2003.07.010. [DOI] [PubMed] [Google Scholar]
- Rubia K. Functional brain imaging across development. Eur. Child Adolesc. Psychiatry. 2012 doi: 10.1007/s00787-012-0291-8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sabatinelli D., Keil A., Frank D.W., Lang P.J. Emotional perception: correspondence of early and late event-related potentials with cortical and subcortical functional MRI. Biol. Psychol. 2013;92:513–519. doi: 10.1016/j.biopsycho.2012.04.005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Schupp H.T., Flaisch T., Stockburger J., Junghöfer M. Emotion and attention: event-related brain potential studies, in: Anders, Ende, Junghöfer, Kissler, Wildgruber (Eds.), Understanding Emotions. Prog. Brain Res. 2006;156:31–51. doi: 10.1016/S0079-6123(06)56002-9. [DOI] [PubMed] [Google Scholar]
- Spielberger C.D. Mind Garden Inc.; Redwood City, CA: 1973. State-Trait Anxiety Inventory for Children (STAIC), Professional Manual. [Google Scholar]
- Steinsträter O., Sillekens S., Junghöfer M., Burger M., Wolters C.H. Sensitivity of beamformer source analysis to deficiencies in forward modeling. Hum. Brain Mapp. 2010;31(12):1907–1927. doi: 10.1002/hbm.20986. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sumich A.L., Sarkar S., Hermens D.F., Ibrahimovic A., Kelesidi K., Wilson D. Sex differences in brain maturation as measured using event-related potentials. Dev. Neuropsychol. 2012;37(5):415–433. doi: 10.1080/87565641.2011.653461. [DOI] [PubMed] [Google Scholar]
- Taylor M.J., Batty M., Itier R.J. The faces of development: a review of early face processing over childhood. J. Cogn. Neurosci. 2004;16(8):1426–1442. doi: 10.1162/0898929042304732. [DOI] [PubMed] [Google Scholar]
- Thomas L.A., De Bellis M.D., Graham R., LaBar K.S. Development of emotional facial recognition in late childhood and adolescence. Dev. Sci. 2007;10(5):547–558. doi: 10.1111/j.1467-7687.2007.00614.x. [DOI] [PubMed] [Google Scholar]
- Thomas K.M., Drevets W.C., Whalen P.J., Eccard C.H., Dahl R.E., Ryan N.D., Casey B.J. Amygdala response to facial expressions in adults and children. Biol. Psychiatry. 2001;49:309–316. doi: 10.1016/s0006-3223(00)01066-0. [DOI] [PubMed] [Google Scholar]
- Tottenham N., Tanaka J.W., Leon A.C., McCarry T., Nurse M., Hare T.A., Marcus D.J., Westerlund A., Casey B.J., Nelson C. The NimStim set of facial expressions: judgments from untrained research participants. Psychiatry Res. 2009;168(3):242–249. doi: 10.1016/j.psychres.2008.05.006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Unnewehr S., Schneider S., Margraf J. 2nd ed. Springer; Heidelberg: 2009. Kinder-Dips–Diagnostisches Interview bei psychischen Störungen im Kindes- und Jugendalter. [Google Scholar]
- Urry H.L. Using reappraisal to regulate unpleasant emotional episodes: goals and timing matter. Emotion. 2009;9(6):782–797. doi: 10.1037/a0017109. [DOI] [PubMed] [Google Scholar]
- Vytal K., Hamann S. Neuroimaging support for discrete neural correlates of basic emotions: a voxel-based meta-analysis. J. Cogn. Neurosci. 2010;22(12):2864–2885. doi: 10.1162/jocn.2009.21366. [DOI] [PubMed] [Google Scholar]
- Weems C.F., Silverman W.K. An integrative model of control: implications for understanding emotion regulation and dysregulation in childhood anxiety. J. Affect. Disord. 2006;91:113–124. doi: 10.1016/j.jad.2006.01.009. [DOI] [PubMed] [Google Scholar]
- Weersing V.R., Rozenman M., Gonzalez A. Core components of therapy in youth: do we know what to disseminate. Behav. Modif. 2009;33(1):24–47. doi: 10.1177/0145445508322629. [DOI] [PubMed] [Google Scholar]
- Weiß R.H. Hogrefe; Göttingen: 2008. Grundintelligenztest Skala 2–Revision (CFT-20-R) [Google Scholar]
- Weinbrenner B. University of Bielefeld; 2005. Fremddiagnostik bei Ängsten im Kindes- und Jugendalter. Dissertation. [Google Scholar]
- Wessing I., Fürniss T., Zwitserlood P., Dobel C., Junghöfer M. Early emotion discrimination in 8- to 10-year-old children: magnetoencephalographic correlates. Biol. Psychol. 2011;88:161–169. doi: 10.1016/j.biopsycho.2011.07.004. [DOI] [PubMed] [Google Scholar]
- Wessing I., Rehbein M.A., Postert C., Fürniss T., Junghöfer M. The neural basis of cognitive change: reappraisal of emotional faces modulates neural source activity in a frontoparietal attention network. NeuroImage. 2013;81:15–25. doi: 10.1016/j.neuroimage.2013.04.117. [DOI] [PubMed] [Google Scholar]
- Whitford T.J., Rennie C.J., Grieve S.M., Clark C.R., Gordon E., Williams L.M. Brain maturation in adolescence: concurrent changes in neuroanatomy and neurophysiology. Hum. Brain Mapp. 2007;28:228–237. doi: 10.1002/hbm.20273. [DOI] [PMC free article] [PubMed] [Google Scholar]