Highlights
-
•
We studied gender effect on grey matter volumes in children with ADHD.
-
•
A gender-by-diagnosis interaction was found in the ventral Anterior Cingulate Gyrus.
-
•
This finding may underlie emotion dysregulation symptoms in ADHD.
-
•
Contribute to differences in symptoms profiles between boys and girls with ADHD.
Keywords: Attention deficit/hyperactivity disorder (ADHD), Anterior cingulate cortex, Gender, MRI, Emotion regulation
Abstract
Female participants have been underrepresented in previous structural magnetic resonance imaging reports on attention-deficit/hyperactivity disorder (ADHD). In this study, we used optimized voxel-based morphometry to examine grey matter volumes in a sample of 33 never-medicated children with combined-type ADHD and 27 typically developing (TD) children. We found a gender-by-diagnosis interaction effect in the ventral anterior cingulate cortex (ACC), whereby boys with ADHD exhibited reduced volumes compared with TD boys, while girls with ADHD showed increased volumes when compared with TD girls. Considering the key role played by the ventral ACC in emotional regulation, we discuss the potential contribution of these alterations to gender-specific symptoms’ profiles in ADHD.
1. Introduction
Attention-deficit/hyperactivity disorder (ADHD) is the most common neurodevelopmental disorder in children and adolescent, with a worldwide prevalence rate between 5.3% and 7.1% (Polanczyk et al., 2007). Research on gender differences in ADHD suggests a male-to-female ratio of 3:1 in population-based studies (Gaub and Carlson, 1997, Barkley, 2006), and between 5:1–9:1 in clinical samples (Gaub and Carlson, 1997, Sandberg, 2002). Girls have been underrepresented in past studies on ADHD (Mahone and Wodka, 2008), probably due to the predominance of male subjects in clinical settings (Ramtekkar et al., 2010). Females with ADHD have fewer hyperactive/impulsive symptoms, more inattentive symptoms, present more commonly with the predominantly inattentive subtype and tend to be underdiagnosed when compared to boys with ADHD (Gaub and Carlson, 1997, Gershon, 2002, Hinshaw et al., 2006). Higher rates of anxiety, as well as lower rates of physical aggression and externalizing behaviors have also been found in girls when compared to boys with ADHD (Levy et al., 2005, Rucklidge, 2010, Skogli et al., 2013).
Multiple structural magnetic resonance imaging (sMRI) studies have examined the structural brain correlates of childhood ADHD, but most of these studies included primarily or exclusively male participants. Early region-of interest (ROI) sMRI studies conducted in primarily male samples have revealed multiple regional grey matter (GM) volume abnormalities in children with ADHD, with the most consistent findings located in the prefrontal cortex, the right caudate and the cerebellum (Valera et al., 2007). More recent sMRI studies have relied on voxel-based morphometry (VBM), a whole brain, fully automated technique for characterizing regional brain volume on a voxel-wise basis (Good et al., 2001). In a meta-analysis encompassing seven pediatric VBM studies, as well as previous ROI studies examining the caudate nuclei volumes, children with ADHD presented with reduced right globus pallidus, caudate and putamen volumes when compared with typically developing children (Frodl and Skokauskas, 2012).
The generalizability of these sMRI findings to girls with ADHD remains to be established. Only one VBM study to date included a sufficient number of boys and girls to examine gender effects in ADHD (Yang et al., 2008). Authors reported no interaction between diagnosis and gender, but several potential confounding factors were present: the study sample was characterized by a large age range (7–17 years), participants presented with various comorbidities, and most patients were receiving medication treatment. Three previous ROI structural studies also examined gender-by-diagnosis interactions in childhood ADHD (Mahone et al., 2011, Qiu et al., 2009, Dirlikov et al., 2014). Two of these studies were restricted to the frontal lobe, manually or automatically delimiting functionally relevant sub regions such as the primary motor cortex, the anterior cingulate cortex, the premotor region, the orbitofrontal cortex or the inferior prefrontal cortex (Mahone et al., 2011, Dirlikov et al., 2014). Mahone et al. (2011) reported significantly smaller left lateral premotor cortices in girls (but not boys) with ADHD when compared with TD participants (Mahone et al., 2011). Dirlikov et al. (2014) found widely distributed reductions of surface area in girls with ADHD in the bilateral dorsolateral prefrontal cortex, the left inferior lateral prefrontal cortex, the right medial prefrontal cortex and the right orbitofrontal cortex, while boys with ADHD showed reduced surface area in the right anterior cingulate cortex and in the left medial prefrontal cortex, when compared with TD children (Dirlikov et al., 2014). The third study examined the volumes of the caudate, putamen and globus pallidus, and reported significantly smaller basal ganglia volumes in boys with ADHD when compared with TD boys. No volume differences were reported in girls with ADHD when compared with TD girls (Qiu et al., 2009). Finally, in one previous study restricted to female participants, Castellanos et al. (2001) found no significant differences between girls with ADHD and TD girls when measuring the volumes of the caudate nucleus, globus pallidus, frontal lobe (total volume)) and cerebellum, while girls with ADHD exhibited decreased grey matter volumes of the posterior inferior lobule in the cerebellar vermis when compared to TD girls (Castellanos et al., 2001). This limited number of studies suggest that girls with ADHD may not exhibit the structural abnormalities of the basal ganglia consistently reported in predominantly male ADHD samples (Valera et al., 2007, Nakao et al., 2011, Frodl and Skokauskas, 2012).
In the present study, we used VBM to compare GM volumes between non-comorbid and never-medicated boys and girls with ADHD combined-type and TD boys and girls who did not differ in terms of age and intellectual quotient (IQ). We hypothesized that boys with ADHD would exhibit decreased GM volumes in the basal ganglia when compared with TD boys, while girls with ADHD would show increased GM volumes in the left lateral premotor cortex and decreased GM volumes in the posterior inferior lobule of the cerebellar vermis when compared to TD girls.
2. Material and methods
2.1. Participants
Participants were 33 children with combined-type ADHD (18 boys) and 27 typically developing children (13 boys) aged 7.9–12.9 years (mean (M) = 10.1 years, standard deviation (SD) = 1.3). Children with ADHD were recruited from the outpatient clinic in Erasme Hospital, Université libre de Bruxelles, Belgium. TD participants were recruited from local schools in Brussels or via personal request to professionals working at Erasme Hospital. The two groups were comparable on age and IQ estimate, as measured by the age-appropriate Wechsler Abbreviated Scale of Intelligence (WASI; Wechsler, 1999) (Table 1). Diagnosis for ADHD was based on clinical features including typical history and behavioural report. The Kiddie Schedule for Affective Disorders and Schizophrenia for School Aged Children-Present and Lifetime Version (K-SADS-PL; Endicott and Spitzer, 1978) was completed at screening for each participant to establish the diagnosis according to DSM-IV-R criteria in children with ADHD and to ensure that TD children presented no psychiatric condition. Symptoms’ severity in children with ADHD was measured using the ADHD rating scale parent form (DuPaul et al., 1998).
Table 1.
Characteristics of the male and female participants.
| Measure | TD Boys (n = 13) |
TD Girls (n = 14) |
ADHD Boys (n = 18) |
ADHD Girls (n = 15) |
p-value | ||||
|---|---|---|---|---|---|---|---|---|---|
| Mean | SD | Mean | SD | Mean | SD | Mean | SD | ||
| Age in years | 9.9 | 1.2 | 9.7 | 1.2 | 10.4 | 1.6 | 10.2 | 1.2 | .45 |
| IQa | 109.1 | 10.9 | 112.7 | 9.5 | 107.4 | 9.3 | 103.8 | 12.1 | .15 |
| ADHD scoresb | N/A | N/A | 39.0 | 1.3 | 36.9 | 1.4 | .28 | ||
Abbreviations: ADHD, attention-deficit/hyperactivity disorder; IQ, intelligence quotient; N/A, non applicable; SD, standard deviation; TD, typically developing; Data were analyzed using one-way ANOVAs.
One data missing for each group except ADHD girls.
ADHD symptoms’ severity as assessed through parents’ ratings with the ADHD Rating Scale-IV home version.
All participants were right-handed and medication-naïve (had never taken psychotropic drugs). Exclusion criteria for children with ADHD and TD children were presence of a psychiatric condition other than ADHD (as assessed by the K-SADS-PL), history of prematurity, current or past medical or neurological disorder, contraindication to MRI, learning disorder and IQ estimate under 85. Screening for learning disorder was based on an interview with parents, history reports and school reports. In suspicious cases, personal calls were made to teachers and an evaluation was conducted by a speech therapist. All subjects lived with their family and were attending normal primary schools. The investigation was carried out in accordance with the Declaration of Helsinki (2013). Each child and her/his parents gave their written consent to participate in this study approved by the Ethics Committee of the Erasme University Hospital (reference: P2007/332/B40620072950).
2.2. Image acquisition
Participants were scanned using a 3 Tesla Philips Achieva MRI scanner (Philips Healthcare, Best, The Netherlands) with an 8 channel SENSE head coil. A high-resolution, 3D T1-weighted structural scan was acquired using a sagittal turbo field equo sequence with the following parameters: 160 slices; TR = 1960 ms; TE = 4.60 ms; TI = 1040 ms; flip angle = 8°; field of view = 250 mm × 250 mm; matrix size = 320 × 320; reconstruction interpolated voxel size = 0.87 × 0.87 × 1.0 mm.
2.3. Behavioural data analyses
For continuous demographic and extracted brain volume data, groups were compared using independent-sample t-tests and univariate analysis of variance (ANOVA) using pair-wise comparisons with Fisher's LSD procedure. Demographic data that were categorical were analysed using Chi-Square tests. All analyses were carried out using the Statistical Package for Social Sciences version 17.0 (SPSS Inc., Chicago IL, USA).
2.4. Voxel-based morphometry analysis
Data were processed using the Statistical Parametric Mapping Software version 8 (SPM8; http://www.fil.ion.ucl.ac.uk/spm/software/spm8; Wellcome Department of Imaging Neuroscience, London, UK) and the VBM8 Toolbox (http://dbm.neuro.uni-jeda.de/vbm.html) implemented in MATLAB 7.8 (The MathWorks, Natick, MA, USA). Because the participants included in this study consisted of children, customised tissue probability maps were created in the Montreal Neurological Institute (MNI) space for use with the VBM8 Toolbox. These customised tissue probability maps were produced using the matched template approach of the Template-O-Matic Toolbox for SPM8 with each participant's age and gender as defining variables (Wilke et al., 2008). First, all T1-weighted images were checked for scanner- and individual-based artefacts (e.g. extreme motion). Next, the anterior commissure was manually indicated on all structural images as the [0, 0, 0 mm] origin in the MNI spatial coordinate system. Individual images were then corrected for bias-field inhomogeneities, segmented and spatially normalised (affine-only transformation) with reference to customised tissue probability maps. Segmentation accuracy was visually checked for each participant. Based on individual registered grey matter (GM) and white matter (WM) segmentations, an average DARTEL (Diffeomorphic Anatomical Registration Through Exponentiated Lie Algebra) template of all participants was created in the MNI space (Ashburner and Friston, 2000). The affine-registered GM segments were then warped to this average template using the high-dimensional DARTEL approach and modulated. Crucially, the voxel's signal intensity values in the grey matter segments were only multiplied by the non-linear component of the registration to account for individual differences in brain size (Kurth et al., 2010). Finally, the GM segments were smoothed using a 12 × 12 × 12 mm3 full-width-at-half maximal Gaussian kernel (Ashburner and Friston, 2000).
For statistics, we examined gender by diagnosis interactions on a voxel-by-voxel basis through a two-way analysis of variance, with diagnosis and gender as between subject factors and age and IQ as covariates of no interest. Cluster-based statistics were used to locate significant regions based on both their peak value and spatial extent after applying an initial cluster defining threshold of p < .001. Due to structural images displaying local variation in smoothness, a non-stationary cluster extent correction was then applied when calculating family-wise error (FWE-cluster) corrected p values (p < .05) using the NS toolbox (Hayasaka et al., 2004, Meisenzahl et al., 2008, Silver et al., 2011, Cullen et al., 2013). For completeness, we also report the p-value for a family-wise error correction at the voxel level (FWE-voxel) (Bennett et al., 2009). Finally, post hoc tests (TD boys vs. boys with ADHD; TD girls vs. girls with ADHD) were conducted to assess direction of change in regions where significant interaction effects were detected (p < .05 with a family-wise ‘small volume’ correction, in a 20 mm radius sphere centered on the coordinate displaying a significant gender-by-diagnosis interaction).
3. Results
3.1. Demographic characteristics
Gender-based subgroups of children with ADHD and TD children did not differ significantly for age or IQ (Table 1). Analyses revealed no significant differences between children with ADHD and TD children for age (TD: M = 9.82, SD = 1.2; ADHD: M = 10.3, SD = 1.4; t = −1.5, p = .13) or IQ (TD: M = 111, SD = 10.3; ADHD: M = 105.7, SD = 10.7; t = 1.9, p = .07).
3.2. Voxel-based morphometry
3.2.1. Main effect-diagnosis
No significant differences were found when comparing children with ADHD and TD children.
3.2.2. Interaction effects
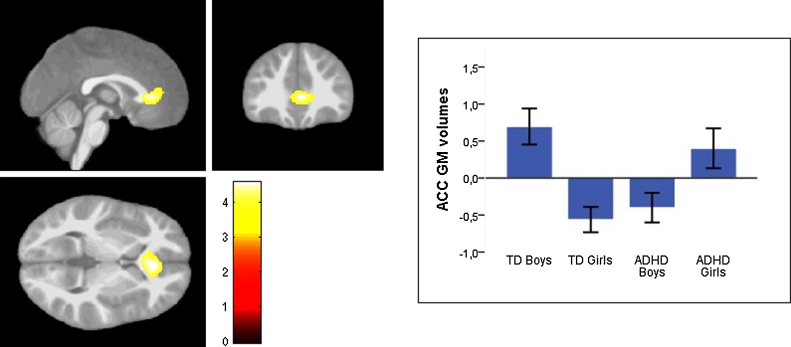
We observed a gender-by-diagnosis interaction in the anterior cingulate cortex (ACC) (ventral subdivision, rostral part) (MNI coordinates: x = 3; y = 35; z = 4; Z-score = 4.18; cluster size = 2613; p = .04 FWE-cluster; p = .09 FWE-voxel; Fig. 1). Underlying this interaction, girls with ADHD showed increased GM volumes when compared to TD girls (MNI coordinates: x = 5; y = 39; z = 7; Z-score = 3.24; cluster size = 148; p = .04 – small volume correction), while boys with ADHD exhibited decreased volumes when compared to TD boys (MNI coordinates: x = −2; y = 27; z = 3; Z-score = 3.53; cluster size = 185; p = .02 – small volume correction).
Fig. 1.
Statistical Parametric Map (SPM) showing foci of significant interactions between diagnosis and gender (overlaid on a mean structural scan from the 60 participants and thresholded at p < .001 uncorrected), with groups’ mean grey matter volume parameter estimate (age regressed out, standard-error displayed) within our significant cluster in the ACC (MNI coordinates: x = 3; y = 35; z = 4) (TD Boys, n = 13; TD Girls, n = 14; Boys with ADHD, n = 18; Girls with ADHD, n = 15). Abbreviations: ACC, anterior cingulate cortex; ADHD, attention-deficit/hyperactivity disorder; GM, grey matter; TD, typically developing.
4. Discussion
To our knowledge, our study is the first VBM study to report a gender-by-diagnosis interaction in individuals with ADHD. The interaction effect was found in the ventral ACC, where girls with ADHD showed increased GM volumes when compared to TD girls, while boys with ADHD exhibited decreased volumes when compared to TD boys. In the only previous VBM study considering potential gender effects, no interaction between gender and diagnosis of ADHD were found, possibly due to the large age range (7–17 years) of the participants included, spanning different brain maturational stages (Yang et al., 2008). Our results are, however, consistent with previous evidence of gender-by-diagnosis interaction in structural brain imaging (Mahone et al., 2011, Dirlikov et al., 2014), electroencephalography (Clarke et al., 2001, Hermens et al., 2004) and functional brain imaging studies of children with ADHD (Ernst et al., 1994, Valera et al., 2010). Thus, our data adds to an increasing number of neuroimaging studies by documenting opposite alterations in brain structure in boys and girls with ADHD, possibly underlying gender-related differences in symptomatology (Hinshaw et al., 2006, Skogli et al., 2013).
Our interaction finding was located in a large cluster centered in the ventral ACC (Brodmann areas (b.a.): 24 and 32; pregenual and subgenual parts). Characterized by a strong anatomical connectivity with core emotion-processing regions such as the amygdala, the periaqueductal grey matter and the hippocampus, the ventral ACC is known to play a key role in top-down emotional regulation (Etkin et al., 2011). It is involved in the inhibition of conditioned fear through extinction, in the automatic regulation of emotional conflict, or when self-distracting from a fear-conditioned stimulus (Bush et al., 2000, Etkin et al., 2011). It is also implicated in the production of positive emotions, which can serve to regulate and diminish negative emotions (Etkin et al., 2011).
Dysregulation of both positive and negative emotions is recognized as an important feature of ADHD (Sjowall et al., 2013, Shaw et al., 2014, Villemonteix et al., 2014). However, to date, few sMRI studies of childhood ADHD have reported GM volumes alterations in brain regions supporting emotion processing or its integration with cognitive control processes (Carmona et al., 2005, Plessen et al., 2006, Frodl et al., 2010, Sasayama et al., 2010). In particular, meta-analyses of VBM studies in children with ADHD did not detect disorder related- structural abnormalities in the ACC (Nakao et al., 2011, Frodl and Skokauskas, 2012). Interestingly, though, the majority of these studies were carried out in children who received medication treatment for ADHD (Nakao et al., 2011, Frodl and Skokauskas, 2012). When treatment was considered as a covariate, studies with more untreated children were associated with decreased GM volumes in the right ACC (Frodl and Skokauskas, 2012). One ROI study also reported that treatment-naïve children with ADHD exhibit smaller right ACC volumes compared with controls, whereby this is not the case for treated children (Semrud-Clikeman et al., 2006). Exposure to psychostimulants may therefore represent a confounding factor when investigating ADHD related- GM volume alterations in this brain region.
Here, never-medicated girls with ADHD showed increased GM volumes when compared to TD girls in the ventral ACC, while never-medicated boys with ADHD exhibited decreased GM volumes when compared to TD boys. Decreased GM volumes in the right ACC have been found in healthy boys exhibiting aggression and defiance (Boes et al., 2008), and decreased cortical thickness in the ventral ACC has been associated with increased levels of aggression in children close to pathological levels of impulsive aggression (Ducharme et al., 2011). A known genetic risk factor for impulsive aggression, the low expression variant of the X-linked monoamine oxidase A (MAOA) gene, is also known to be associated with decreased GM volumes in the ventral ACC (Meyer-Lindenberg et al., 2006). Based on these findings, we hypothesize that decreased GM volumes in boys with ADHD may represent a risk factor for developing externalizing symptoms such as anger outbursts and impulsive reactive aggressions (Skogli et al., 2013).
On the other hand, larger volumes in the right ACC have been linked to harm avoidance, a temperamental disposition characterized by excessive worrying, pessimism and shyness, in both genders (Pujol et al., 2002). Women, who are known to be at higher risk for internalizing disorders (McLean et al., 2011), tend to exhibit larger volumes in the right ACC compared to men (Mann et al., 2011, Ruigrok et al., 2014). They also recruit more the right ventral ACC during emotional processing (Wrase et al., 2003); a finding confirmed in a quantitative meta-analysis of 65 neuroimaging studies of emotional processing (Wager et al., 2003). Based on these findings, it could be hypothesized that increased GM volumes in the right ventral ACC in girls with ADHD represent a risk factor for developing internalizing symptoms (Skogli et al., 2013). However, one must also note that reduced grey matter volumes in the ventral ACC have been consistently reported in patients suffering from internalizing disorders such as major depressive disorder or anxiety disorders, in both genders (Drevets et al., 2008, Van Tol et al., 2010). More studies in healthy children and adults are therefore needed to disentangle the relationships between grey matter volumes in the ventral ACC, pathological anxiety and temperamental anxiety. It may be that increased vs. decreased GM volumes in the ventral ACC represent both risk factors for anxiety symptoms, through different pathways (subtending, for example, a tendency to overthink vs. a lack of conscious integration of negative feelings (Bassett et al., 2015)); or that decreased GM volumes in the ventral ACC only appear in adult patients following a long-term history of depression or anxiety disorder, which would be consistent with the cortisol neurotoxicity hypothesis (Treadway et al., 2009).
Contrary to our hypotheses, we did not report decreased GM volumes in boys with ADHD when compared with TD boys in the basal ganglia. ADHD is a heterogeneous condition, involving multiple causal pathways (Sonuga-Barke and Halperin, 2010). Notably, findings from individual sMRI studies have been inconsistent, probably reflecting the neuropsychological and etiological heterogeneity of the disorder itself (Castellanos et al., 2006). Reduced GM volume in children with ADHD in the basal ganglia are one of the most replicated findings in sMRI studies (Nakao et al., 2011, Frodl and Skokauskas, 2012), but structural deficits in this region are not expected to be found in all subgroups of children with ADHD. Here, the lack of significant finding in the basal ganglia may also be related to the characteristics of our ADHD sample, which included boys with no psychiatric comorbidity, no learning disorder and a mean IQ of 105.7. Indeed, high-functioning samples presumably exhibit more subtle brain alterations that may be difficult to detect at a corrected statistical thresholding (Seidman et al., 2011).
Similarly, we did not replicate previous findings from ROI structural studies reporting increased GM volumes in the left lateral premotor cortex, as well as decreased GM volumes in the posterior inferior lobule of the cerebellar vermis in girls with ADHD when compared to TD girls. Comparison between ROI and VBM findings can prove to be difficult. Indeed, ROI methods yield a single value for the volume of the region examined, obtained after averaging signal over the ROI. This signal averaging can cause a dilution of the measure of the volume difference, especially when this difference is only present in a limited part of the ROI (Voormolen et al., 2010). For this reason, VBM has been shown to outperform ROI methods when detecting focal differences in morphology (Voormolen et al., 2010). However, theoretically, ROI methods remain superior when between-group differences are distributed uniformly over a small ROI, since the ROI analysis at this spatial scale benefits from substantial signal averaging (Voormolen et al., 2010). Both methods can therefore provide different types of information and are considered as complementary (Giuliani et al., 2005).
A limitation of the present study was the sample size of our TD groups. Findings should therefore be considered preliminary until replicated in larger samples. On the other hand, our ADHD sample presented with a narrow age range and was homogenous, both medication-naïve and presenting with no comorbidities.
In conclusion, our study provides novel evidence indicating an interaction between diagnosis and gender in ADHD in the ventral ACC. Interestingly, ADHD related-structural alterations in this brain region were found in a sample non-comorbid for other psychiatric disorders, indicating that changes in regions subserving emotional regulation can be found in ADHD even without any concurrent diagnosed emotional disorder. We suggest that the decreased GM volumes found in boys with ADHD in the ventral ACC may represent a risk factor for developing externalizing symptoms, whereas increased GM volumes in girls with ADHD may be related to a temperamental disposition to experience internalizing symptoms. Despite well characterized differences in symptom's profiles between boys and girls with ADHD, neuroimaging research on gender differences in emotional regulation in ADHD is still lacking. Studies relying both on sMRI and fMRI are needed to clarify the role of the ventral ACC in ADHD related – emotional dysregulation symptomatology.
Conflict of interest
The authors declare no competing financial interests.
Acknowledgements
This work was supported by a grant from the Belgian National Fund for Scientific Research (FNRS 3.4.516.08.F). Stéphane A. De Brito was supported by a research fellowship from the Swiss National Science Foundation (SNSF PA00P1_139586).
References
- Ashburner J., Friston K.J. Voxel-based morphometry – the methods. NeuroImage. 2000;11:805–821. doi: 10.1006/nimg.2000.0582. [DOI] [PubMed] [Google Scholar]
- Barkley R.A. Guilford Press; New York: 2006. Attention-Deficit Hyperactivity Disorder: A Handbook for Diagnosis and Treatment. [Google Scholar]
- Bassett D.S., Yang M., Wymbs N.F., Grafton S.T. Learning-induce autonomy of sensorimotor systems. Nat. Neurosci. 2015;18:744–751. doi: 10.1038/nn.3993. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bennett C.M., Wolford G.L., Miller M.B. The principled control of false positives in neuroimaging. Soc. Cogn. Affect. Neurosci. 2009;4(4):417–422. doi: 10.1093/scan/nsp053. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Boes A.D., Tranel D., Anderson S.W., Nopoulos P. Right anterior cingulate: a neuroanatomical correlate of aggression and defiance in boys. Behav. Neurosci. 2008;122:677–684. doi: 10.1037/0735-7044.122.3.677. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bush G., Luu P., Posner M.I. Cognitive and emotional influences in anterior cingulate cortex. Trends Cogn. Sci. 2000;4:215–222. doi: 10.1016/s1364-6613(00)01483-2. [DOI] [PubMed] [Google Scholar]
- Carmona S., Vilarroya O., Bielsa A., Trèmols V., Soliva J.C., Rovira M. Global and regional gray matter reductions in ADHD: a voxel-based morphometric study. Neurosci. Lett. 2005;389:88–93. doi: 10.1016/j.neulet.2005.07.020. [DOI] [PubMed] [Google Scholar]
- Castellanos F.X., Giedd J.N., Berquin P.C., Walter J.M., Sharp W., Tran T. Quantitative brain magnetic resonance imaging in girls with attention-deficit/hyperactivity disorder. Arch. Gen. Psychiatry. 2001;58:289–295. doi: 10.1001/archpsyc.58.3.289. [DOI] [PubMed] [Google Scholar]
- Castellanos F.X., Sonuga-Barke E.J.S., Milham M.P., Tannock R. Characterizing cognition in ADHD: beyond executive dysfunction. Trends Cogn. Sci. 2006;10:117–123. doi: 10.1016/j.tics.2006.01.011. [DOI] [PubMed] [Google Scholar]
- Clarke A.R., Barry R.J., McCarthy R., Selikowitz M. Electroencephalogram differences in two subtypes of attention-deficit/hyperactivity disorder. Psychophysiology. 2001;38:212–221. [PubMed] [Google Scholar]
- Cullen A.E., De Brito S.A., Gregory S.L., Murray R.M., Williams S.C., Hodgins S. Temporal lobe volume abnormalities precede the prodrome: a study of children presenting antecedents of schizophrenia. Schizophr. Bull. 2013;39(6):1318–1327. doi: 10.1093/schbul/sbs128. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dirlikov B., Shiels Rosch K., Crocetti D., Denckla M.B., Mahone E.M., Mostofsky S.H. Distinct frontal lobe morphology in girls and boys with ADHD. Neuroimage Clin. 2014;7:222–229. doi: 10.1016/j.nicl.2014.12.010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Drevets W.C., Savitz J., Trimble M. The subgenual anterior cingulate cortex in mood disorders. CNS Spectr. 2008;13:663–681. doi: 10.1017/s1092852900013754. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ducharme S., Hudziak J.J., Botteron K.N., Ganjavi H., Lepage C., Collins D.L. Right anterior cingulate cortical thickness and bilateral striatal volume correlate with child behavior checklist aggressive behavior scores in healthy children. Biol. Psychiatry. 2011;70:283–290. doi: 10.1016/j.biopsych.2011.03.015. [DOI] [PMC free article] [PubMed] [Google Scholar]
- DuPaul G.J., Power T.J., Anastopoulos A.D., Reid R. Guilford Press; New York: 1998. ADHD Rating Scale-IV: Checklists, Norms, and Clinical Interpretation. [Google Scholar]
- Endicott J., Spitzer R.L. A diagnostic interview: the schedule for affective disorders and schizophrenia. Arch. Gen. Psychiatry. 1978;35:837–844. doi: 10.1001/archpsyc.1978.01770310043002. [DOI] [PubMed] [Google Scholar]
- Ernst M., Liebenauer L.L., King A.C., Fitzgerald G.A., Cohen R.M., Zametkin A.J. Reduced brain metabolism in hyperactive girls. J. Am. Acad. Child Adolesc. Psychiatry. 1994;33:858–868. doi: 10.1097/00004583-199407000-00012. [DOI] [PubMed] [Google Scholar]
- Etkin A., Egner T., Kalisch R. Emotional processing in anterior cingulate and medial prefrontal cortex. Trends Cogn. Sci. 2011;15:85–93. doi: 10.1016/j.tics.2010.11.004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Frodl T., Stauber J., Schaaff N., Koutsouleris N., Scheuerecker J., Ewers M. Amygdala reduction in patients with ADHD compared with major depression and healthy volunteers. Acta Psychiatr. Scand. 2010;121:111–118. doi: 10.1111/j.1600-0447.2009.01489.x. [DOI] [PubMed] [Google Scholar]
- Frodl T., Skokauskas N. Meta-analysis of structural MRI studies in children and adults with attention deficit hyperactivity disorder indicates treatment effects. Acta Psychiatr. Scand. 2012;125:114–126. doi: 10.1111/j.1600-0447.2011.01786.x. [DOI] [PubMed] [Google Scholar]
- Gaub M., Carlson C.L. Gender differences in ADHD: a meta-analysis and critical review. J. Am. Acad. Child Adolesc. Psychiatry. 1997;36:1783. doi: 10.1097/00004583-199708000-00011. (Original work published vol. 36, p. 1036, 1997) [DOI] [PubMed] [Google Scholar]
- Gershon J. A meta-analytic review of gender differences in ADHD. J. Atten. Disord. 2002;5:143–154. doi: 10.1177/108705470200500302. [DOI] [PubMed] [Google Scholar]
- Giuliani N.R., Calhoun V.D., Pearlson G.D., Francis A., Buchanan R.W. Voxel-based morphometry versus region of interest: a comparison of two methods for analyzing gray matter differences in schizophrenia. Schizophr. Res. 2005;74:135–147. doi: 10.1016/j.schres.2004.08.019. [DOI] [PubMed] [Google Scholar]
- Good C.D., Ashburner J., Frackowiak R.S.J. Computational neuroanatomy: new perspectives for neuroradiology. Rev. Neurol. 2001;157:797–805. [PubMed] [Google Scholar]
- Hayasaka S., Phan K.L., Liberzon I., Worsley K.J., Nichols T.E. Nonstationary cluster-size inference with random field and permutation methods. Neuroimage. 2004;22:676–687. doi: 10.1016/j.neuroimage.2004.01.041. [DOI] [PubMed] [Google Scholar]
- Hermens D.F., Williams L.M., Lazzaro I., Whitmont S., Melkonian D., Gordon E. Sex differences in adult ADHD: a double dissociation in brain activity and autonomic arousal. Biol. Psychol. 2004;66:221–233. doi: 10.1016/j.biopsycho.2003.10.006. [DOI] [PubMed] [Google Scholar]
- Hinshaw S.P., Owens E.B., Sami N., Fargeon S. Prospective follow-up of girls with attention-deficit/hyperactivity disorder into adolescence: evidence for continuing cross-domain impairment. J. Consult. Clin. Psychol. 2006;74:489–499. doi: 10.1037/0022-006X.74.3.489. [DOI] [PubMed] [Google Scholar]
- Kurth F., Luders E., Gaser C. 2010. VBM8-Toolbox Manual. [Google Scholar]
- Levy F., Hay D.A., Bennett K.S., McStephen M. Gender differences in ADHD subtype comorbidity. J. Am. Acad. Child Adolesc. Psychiatry. 2005;44:368–376. doi: 10.1097/01.chi.0000153232.64968.c1. [DOI] [PubMed] [Google Scholar]
- Mahone E.M., Wodka E.L. The neurobiological profile of girls with ADHD. Dev. Disabil. Res. Rev. 2008;14:276–284. doi: 10.1002/ddrr.41. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mahone E.M., Ranta M.E., Crocetti D., O’Brien J., Kaufmann W.E., Denckla M.B. Comprehensive examination of frontal regions in boys and girls with attention-deficit/hyperactivity disorder. J. Int. Neuropsychol. Soc. 2011;17:1047–1057. doi: 10.1017/S1355617711001056. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mann S.L., Hazlett E.A., Byne W., Hof P.R., Buchsbaum M.S., Cohen B.H. Anterior and posterior cingulate cortex volume in healthy adults: effects of aging and gender differences. Brain Res. 2011;1401:18–29. doi: 10.1016/j.brainres.2011.05.050. [DOI] [PMC free article] [PubMed] [Google Scholar]
- McLean C.P., Asnaani A., Litz B.T., Hofmann S.G. Gender differences in anxiety disorders: prevalence, course of illness, comorbidity and burden of illness. J. Psychiatr. Res. 2011;45:1027–1035. doi: 10.1016/j.jpsychires.2011.03.006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Meisenzahl E.M., Koutsouleris N., Gaser C. Structural brain alterations in subjects at high-risk of psychosis: a voxelbased morphometric study. Schizophr. Res. 2008;102:105–162. doi: 10.1016/j.schres.2008.02.023. [DOI] [PubMed] [Google Scholar]
- Meyer-Lindenberg A., Buckholtz J.W., Kolachana B.R., Hariri A., Pezawas L., Blasi G. Neural mechanisms of genetic risk for impulsivity and violence in humans. Proc. Natl. Acad. Sci. U. S. A. 2006;103:6269–6274. doi: 10.1073/pnas.0511311103. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Nakao T., Radua J., Rubia K., Mataix-Cols D. Gray matter volume abnormalities in ADHD: voxel-based meta-analysis exploring the effects of age and stimulant medication. Am. J. Psychiatry. 2011;168:1154–1163. doi: 10.1176/appi.ajp.2011.11020281. [DOI] [PubMed] [Google Scholar]
- Plessen K.J., Bansal R., Zhu H., Whiteman R., Amat J., Quackenbush G.A. Hippocampus and amygdala morphology in attention-deficit/hyperactivity disorder. Arch. Gen. Psychiatry. 2006;63:795–807. doi: 10.1001/archpsyc.63.7.795. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Polanczyk G., De Lima M.S., Horta B.L., Biederman J., Rohde L.A. The worldwide prevalence of ADHD: a systematic review and metaregression analysis. Am. J. Psychiatry. 2007;164:942–948. doi: 10.1176/ajp.2007.164.6.942. [DOI] [PubMed] [Google Scholar]
- Pujol J., Lopez A., Deus J., Cardoner N., Vallejo J., Capdevila A. Anatomical variability of the anterior cingulate gyrus and basic dimensions of human personality. Neuroimage. 2002;15:847–855. doi: 10.1006/nimg.2001.1004. [DOI] [PubMed] [Google Scholar]
- Qiu A., Crocetti D., Adler M., Mahone E.M., Denckla M.B., Miller M.I. Basal ganglia volume and shape in children with attention deficit hyperactivity disorder. Am. J. Psychiatry. 2009;166:74–82. doi: 10.1176/appi.ajp.2008.08030426. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ramtekkar U.P., Reiersen A.M., Todorov A.A., Todd R.D. Sex and age differences in attention-deficit/hyperactivity disorder symptoms and diagnoses: implications for DSM-V and ICD-11. J. Am. Acad. Child Adolesc. Psychiatry. 2010;49:217–228. [PMC free article] [PubMed] [Google Scholar]
- Rucklidge J.J. Gender differences in attention-deficit/hyperactivity disorder. Psychiatr. Clin. N. Am. 2010;33:357–373. doi: 10.1016/j.psc.2010.01.006. [DOI] [PubMed] [Google Scholar]
- Ruigrok A.N., Salimi-Khorshidi G., Lai M.C., Baron-Cohen S., Lombardo M.V., Tait R.J. A meta analysis of sex differences in human brain structure. Neurosci. Biobehav. Rev. 2014;39:34–50. doi: 10.1016/j.neubiorev.2013.12.004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sandberg S. 2nd ed. Cambridge University Press; Cambridge, England: 2002. Hyperactivity and Attention Disorders of Childhood. [Google Scholar]
- Sasayama D., Hayashida A., Yamasue H., Harada Y., Kaneko T., Kasai K. Neuroanatomical correlates of attention-deficit-hyperactivity disorder accounting for comorbid oppositional defiant disorder and conduct disorder. Psychiatry Clin. Neurosci. 2010;64:394–402. doi: 10.1111/j.1440-1819.2010.02102.x. [DOI] [PubMed] [Google Scholar]
- Seidman L.J., Biederman J., Liang L., Valera E.M., Monuteaux M.C., Brown A. Gray matter alterations in adults with attention-deficit/hyperactivity disorder identified by voxel based morphometry. Biol. Psychiatry. 2011;69:857–866. doi: 10.1016/j.biopsych.2010.09.053. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Semrud-Clikeman M., Pliszka S.R., Lancaster J., Liotti M. Volumetric MRI differences in treatment-naïve vs. chronically treated children with ADHD. Neurology. 2006;67:1023–1027. doi: 10.1212/01.wnl.0000237385.84037.3c. [DOI] [PubMed] [Google Scholar]
- Shaw P., Stringaris A., Nigg J., Leibenluft E. Emotion dysregulation in attention deficit hyperactivity disorder. Am. J. Psychiatry. 2014;171:276–293. doi: 10.1176/appi.ajp.2013.13070966. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Silver M., Montana G., Nichols T.E. False positive in neuroimaging genetics using voxel-based morphometry data. Neuroimage. 2011;54(2):992–1000. doi: 10.1016/j.neuroimage.2010.08.049. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sjowall D., Roth L., Lindqvist S., Thorell L.B. Multiple deficits in ADHD: executive dysfunction, delay aversion, reaction time variability, and emotional deficits. J. Child Psychol. Psychiatry. 2013;54:619–627. doi: 10.1111/jcpp.12006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Skogli E.W., Teicher M.H., Andersen P.N., Hovik K.T., Oie M. ADHD in girls and boys – gender differences in co-existing symptoms and executive function measures. BMC Psychiatry. 2013:13. doi: 10.1186/1471-244X-13-298. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sonuga-Barke E.J.S., Halperin J.M. Developmental phenotypes and causal pathways in attention deficit/hyperactivity disorder: potential targets for early intervention? J. Child Psychol. Psychiatry. 2010;51:368–389. doi: 10.1111/j.1469-7610.2009.02195.x. [DOI] [PubMed] [Google Scholar]
- Treadway M.T., Grant M.M., Ding Z., Hollon S.D., Gore J.C., Shelton R.C. Early adverse events, HPA activity and rostral anterior cingulate volume in MDD. PLoS ONE. 2009;4:e4887. doi: 10.1371/journal.pone.0004887. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Valera E.M., Brown A., Biederman J., Faraone S.V., Makris N., Monuteaux M.C. Sex differences in the functional neuroanatomy of working memory in adults with ADHD. Am. J. Psychiatry. 2010;167:86–94. doi: 10.1176/appi.ajp.2009.09020249. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Valera E.M., Faraone S.V., Murray K.E., Seidman L.J. Meta-analysis of structural imaging findings in attention-deficit/hyperactivity disorder. Biol. Psychiatry. 2007;61:1361–1369. doi: 10.1016/j.biopsych.2006.06.011. [DOI] [PubMed] [Google Scholar]
- Van Tol M.J., Van der Wee N.J., Van den Heuvel O.A., Nielen M.M., Demenescu L.R., Aleman A. Regional brain volume in depression and anxiety disorders. Arch. Gen. Psychiatry. 2010;67:1002–1011. doi: 10.1001/archgenpsychiatry.2010.121. [DOI] [PubMed] [Google Scholar]
- Villemonteix T., Purper-Ouakil D., Romo L. Is emotional dysregulation a component of attention deficit/hyperactivity disorder (ADHD)? Encephale. 2014;41(2):108–114. doi: 10.1016/j.encep.2013.12.004. [DOI] [PubMed] [Google Scholar]
- Voormolen E.H.J., Wei C., Chow E.W.C., Bassett A.S., Mikulis D.J., Crawley A.P. Voxel-based morphometry and automated lobar volumetry: the trade-off between spatial scale and statistical correction. Neuroimage. 2010;49:587–596. doi: 10.1016/j.neuroimage.2009.07.018. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wager T.D., Phan K.L., Liberzon I., Taylor S.F. Valence, gender, and lateralization of functional brain anatomy in emotion: a meta-analysis of findings from neuroimaging. Neuroimage. 2003;19:513–531. doi: 10.1016/s1053-8119(03)00078-8. [DOI] [PubMed] [Google Scholar]
- Wechsler D. The Psychological Corporation; San Antonio: 1999. Wechsler Abbreviated Scale of Intelligence. [Google Scholar]
- Wilke M., Holland S.K., Altaye M., Gaser C. Template-O-Matic: a toolbox for creating customized pediatric templates. NeuroImage. 2008;41:903–913. doi: 10.1016/j.neuroimage.2008.02.056. [DOI] [PubMed] [Google Scholar]
- Wrase J., Klein S., Gruesser S.M., Hermann D., Flor H., Mann K. Gender differences in the processing of standardized emotional visual stimuli in humans: a functional magnetic resonance imaging study. Neurosci. Lett. 2003;348:41–45. doi: 10.1016/s0304-3940(03)00565-2. [DOI] [PubMed] [Google Scholar]
- Yang P., Wang P.-N., Chuang K.-H., Jong Y.-J., Chao T.-C., Wu M.-T. Absence of gender effect on children with attention-deficit/hyperactivity disorder as assessed by optimized voxel-based morphometry. Psychiatry Res. Neuroimaging. 2008;164:245–253. doi: 10.1016/j.pscychresns.2007.12.013. [DOI] [PubMed] [Google Scholar]