Highlights
-
•
First DTI-study in pre-readers with a family risk for dyslexia (FRD+).
-
•
Bilateral ventral and dorsal pathways sustain phonological awareness.
-
•
FRD+ group displayed lower FA in left ventral pathway.
-
•
No neural specialization for phonological processing in pre-reading stage.
-
•
Anomalies related to family risk of dyslexia are present prior to reading onset.
Keywords: Dyslexia, Preschool children, Reading network, Diffusion weighted imaging, Developmental neuroscience
Abstract
In adults and school-aged children, phonological aspects of reading seem to be sustained by left dorsal regions, while ventral regions seem to be involved in orthographic word recognition. Yet, given that the brain reorganises during reading acquisition, it is unknown when and how these reading routes emerge and whether neural deficits in dyslexia predate reading onset. Using diffusion MRI in 36 pre-readers with a family risk for dyslexia (FRD+) and 35 well matched pre-readers without a family risk (FRD−), our results show that phonological predictors of reading are sustained bilaterally by both ventral and dorsal tracts. This suggests that a dorsal and left-hemispheric specialisation for phonological aspects of reading, as observed in adults, is presumably gradually formed throughout reading development. Second, our results indicate that FRD+ pre-readers display mainly white matter differences in left ventral tracts. This suggests that atypical white matter organisation previously found in dyslexic adults may be causal rather than resulting from a lifetime of reading difficulties, and that the location of such a deficit may vary throughout development. While this study forms an important starting point, longitudinal follow-up of these children will allow further investigation of the dynamics between emerging literacy development and white matter connections.
1. Introduction
The ability to read and write constitutes a significant milestone in child development, and is the result of a long-term learning process. During reading development, neural connections between the visual and the spoken language system need to be modified in order to reorganise our brain into a reading network (Dehaene, 2009). Although most children eventually succeed in mastering fluent reading skills, 5–10% are diagnosed with developmental dyslexia, a learning disability characterised by severe and persistent reading and/or spelling impairments in the absence of intellectual and sensory deficits (Shaywitz, 1998, Vellutino et al., 2004). In the present study, we examine white matter connections in the pre-reading brain of children at risk for dyslexia, thereby excluding confounding effects of neural reorganization that arise throughout reading development. This approach allows us to search for an anatomical foundation of beginning reading as well as for a potential biomarker of dyslexia.
A large number of neuroimaging studies in adults and school-aged children show that reading involves a left lateralised neural network, with a dorsal interaction between left frontal (in and around Broca) and temporoparietal regions to sustain phonological processing and grapheme-phoneme decoding (Shaywitz, 1998, Price et al., 1997, Jobard et al., 2003, Rumsey et al., 1997, Vigneau et al., 2006, Taylor et al., 2014) and a ventral circuit, especially the Visual Word Form area (Dehaene and Cohen, 2011), to sustain orthographic aspects of reading (Jobard et al., 2003, Taylor et al., 2014, McCandliss and Noble, 2003). Although this is the standard neuroanatomical model on reading (Pugh et al., 2001), note that some studies point to a different functional organisation (for a review see Richlan, 2012). Regarding dyslexia, it is established that dyslexic adults exhibit a left neural deficit in dorsal (i.e. temporoparietal) and ventral (i.e. occipitotemporal) grey matter regions as well as in dorsal white matter connections (i.e. arcuate fasciculus) (Richlan, 2012, Vandermosten et al., 2012a, Vandermosten et al., 2012b, Turkeltaub et al., 2003).
Linking these findings to reading development allows us to make two predictions on neurodevelopment. More specifically, a beginning reader, who uses phonological resources to decodes a word by linking its component letters to the corresponding speech sounds, is assumed to rely on a left dorsal fronto-to-temporoparietal circuit. On the other hand, the more advanced reader, who has gradually learned to directly recognise (chunks of) words via orthographic word recognition, is assumed to increasingly rely on a left ventral occipitotemporal ‘word form area’ (Pugh et al., 2001, Turkeltaub et al., 2003, Shaywitz et al., 2002, Simos et al., 2002). Second, since a phonological deficit is considered as proximal cause of dyslexia (Vellutino et al., 2004, Snowling, 2000), the standard neuroanatomical model predicts that this primary phonological deficit is reflected in pre-reading left dorsal temporoparietal anomalies. Ventral differences found in dyslexic adults would reflect a secondary later-developed deficit in building up orthographic word representations due to insufficient reading input (Pugh et al., 2001, Richlan, 2012, Turkeltaub et al., 2003, Shaywitz et al., 2002).
However, evidence for this standard neuroanatomical model is limited since most neuroimaging studies on reading involve adults or school-aged children with several years of reading experience, and the few functional and structural studies that currently exist in pre- to beginning readers are inconclusive (Raschle et al., 2011, Raschle et al., 2012, Yamada et al., 2011, Brem et al., 2010, Maurer et al., 2006, Specht et al., 2009, Black et al., 2012, Hosseini et al., 2013). In addition, they need to be complemented by studies on the development of white matter connections during literacy emergence, especially since white matter organization remains plastic during the school years due to age-related maturation (Lebel et al., 2008) and experience-induced processes such as learning to read (see Carreiras et al., 2009, Thiebaut de Schotten et al., 2012 for studies in ex-illiterate adults). Diffusion Tensor images (DTI) coupled with tractography algorithms, provide a way to reconstruct the structural white matter connections in the living human brain and to quantify, via fractional anisotropy (FA), their microstructural properties such as myelination, axon packing and axon homogeneity (Beaulieu, 2002).
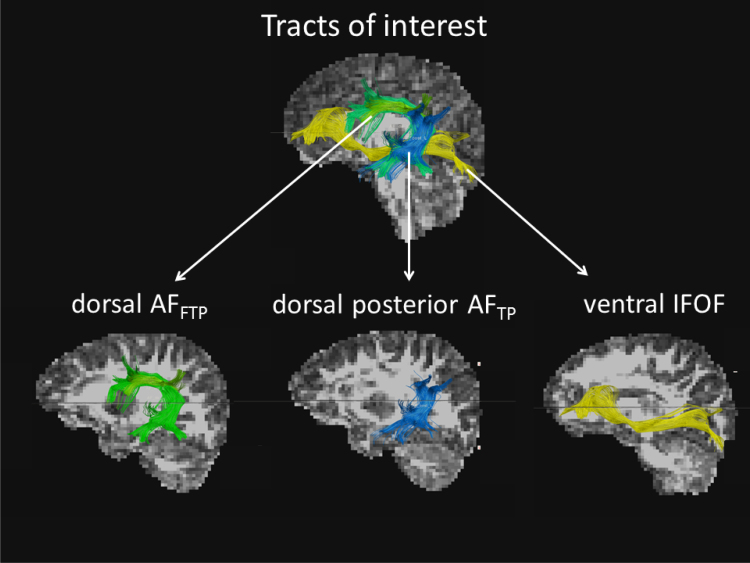
The aim of the present study in pre-readers is to inspect white matter organization in dorsal and ventral tracts on (1) their specific reading-related function at that young age, and on (2) the presence of pre-reading anomalies related to family-risk factors of dyslexia. First, in order to investigate whether a dorsal specialization for phonological versus a ventral specialization for orthographic aspects, as observed in adults (Vandermosten et al., 2012b), is already present in pre-readers, we will relate dorsal and ventral white matter organization to pre-school performance on phonological awareness (PA), rapid automatised naming (RAN) and letter knowledge (LK). These cognitive skills are known to be the best predictors of later literacy performance (Puolakanaho et al., 2007), with PA contributing to phonological aspects of reading and RAN and LK contributing relatively more to the orthographic aspects (Boets et al., 2010, Verhagen et al., 2008, Savage and Frederickson, 2005, Allor, 2002). Second, potential dyslexia-associated white matter anomalies will be investigated by comparing white matter tracts of pre-readers with a family risk for dyslexia (FRD+, i.e. first degree relative diagnosed with dyslexia) and closely matched pre-readers without a family risk for dyslexia (FRD−, i.e. no first degree relative with reading problems). FRD+-pre-readers have a 40–60% chance to develop dyslexia whereas this is only 5–10% in the FRD−-pre-readers (Gilger et al., 1991, Grigorenko, 2001). The selection of the tracts was based on their position and their function. As ventrally running tract, we selected the IFOF (Fig. 1) given its orthographic function in adult readers (Vandermosten et al., 2012b) and since it passes along anterior (such as pars triangularis) and posterior (such as the Visual Word Form Area) regions that are activated when adults directly recognise visual word patterns (Jobard et al., 2003). As dorsal tract, we selected the arcuate fasciculus which connects frontal regions (such as pars opercularis) to two posterior (inferior parietal and temporal) regions, here referred to as AFFTP (see Fig. 1). These regions are activated in adults during phonological and grapheme-phoneme coupling tasks (Jobard et al., 2003, van Atteveldt et al., 2009) and white matter organization in this tract has been repetitively demonstrated to relate to phonological processing (Vandermosten et al., 2012b, Yeatman et al., 2011, Saygin et al., 2013). We also examined white matter organization in a second dorsal tract that connects posterior temporal and inferior parietal regions, here referred to as ‘posterior AFTP’ (Fig. 1). This tract may be particularly interesting at the initial phase of reading since it exhibits FA-differences in ex-illiterates who learned to read (Thiebaut de Schotten et al., 2012). Finally, taking into account that pre- and beginning readers do not display a clear left lateralised pattern of reading-related activity (Yamada et al., 2011), we also examined the right homologues of the three tracts of interest.
Fig. 1.
shows the left tracts of interest; (1) dorsal AFFTP, depicted in green, (2) dorsal posterior AFTP, depicted in blue, and (3) ventral IFOF, depicted in yellow. Right homologues are not presented in the figure but were also delineated and included in the analyses. (For interpretation of the references to colour in this figure legend, the reader is referred to the web version of the article.)
2. Methods
2.1. Participants
The present DTI-study is part of an on-going longitudinal project which includes the collection of multiple behavioural, EEG and MRI measures before and after learning to read. It is approved by the local Ethical Board and an informed consent was obtained from the parents of the participants according to the Declaration of Helsinki. In total, 87 Dutch-speaking children were selected for this longitudinal project and every FRD+-child was individually matched to a FRD−-child based on five criteria: (1) educational environment, i.e. same class, (2) gender, (3) age, (4) non-verbal IQ, based on the Coloured Progressive Matrices (Raven et al., 1984), and (5) parent's socio-economic status (SES), based on the Family Affluence Scale (Boudreau and Poulin, 2009, Boyce et al., 2006). In addition, five exclusion criteria were taken into consideration: (1) a non-verbal IQ below 80, (2) a hearing loss, i.e. a Fletcher Index of 20 dB HL or more, (3) multilingualism, (4) a history of brain damage, vision deficits, or articulatory problems, and (5) a high risk for developing ADHD, i.e. based on the Strengths and Difficulties Questionnaire. EEG and behavioural data (such as PA and RAN) were collected in the total sample of 87 children at the beginning of the last year of kindergarten (Vanvooren et al., 2014). At the end of the last year of kindergarten a MRI-scan and a LK task were administered in a large subsample of these children, namely in the 75 children for whom parents gave informed consent to participate in the MRI-session. In both test sessions (at the start and end of the third year of kindergarten), data of all participants were collected within two months (with the majority of the children within one month) in order to exclude differences between participants in the amount of schooling. In line with the guidelines of the Flemish government (http://www.ond.vlaanderen.be/), none of the participating schools provided reading instruction in kindergarten, hence the participating children can be considered as pre-readers at the time of scanning. Four children had to be excluded from the DTI-analyses due to unsuccessful DTI-acquisition (i.e. incorrect slice orientation which resulted in unreliable tensor estimation). Hence, the DTI-results reported in the present paper include data of 71 Dutch-speaking pre-reading children, of whom 36 belong to the FRD+-group and 35 to the FRD− group.
2.2. Behavioural testing
The most important phonological and orthographic predictors of literacy were assessed, namely PA, RAN and LK (Puolakanaho et al., 2007). In contrast to PA, it is not straightforward to test orthographic knowledge in pre-readers because visual word representations have not been acquired yet. Therefore, we tested LK and RAN which provide a rough early index of orthographic representations despite also partly relying on phonological abilities (Boets et al., 2010, Verhagen et al., 2008, Allor, 2002, Savage et al., 2005). PA was tested by an end phoneme and end rhyme identification task. RAN was assessed by naming as fast and accurately as possible objects and colours (Boets et al., 2010). Letter knowledge (LK) was assessed via a productive and a receptive test (Boets et al., 2008). More details on these cognitive tests are provided in Boets et al., 2008, Boets et al., 2010. For the correlational analyses with DTI-measures, standardised scores on the two tasks of each cognitive predictor were averaged into one composite score.
2.3. DTI
2.3.1. MRI procedure and DTI acquisition
Given the young age of the participants, a detailed protocol was used to prepare the children for their MRI-scan (for more information on the procedure and motion parameters see Theys et al., 2014). All children underwent MRI examination on a 3 T Philips system (Best, The Netherlands) with 32-channel head coil. The total duration of the MRI-scan session was half an hour, of which 10 min 32 sec for the DTI-scan. The DTI data were acquired using a single-spin shot echo-planar image (SE-EPI) with SENSE (sensitivity encoding) acquisition. DTI images covering the entire brain and the brainstem were acquired with the following parameters: 58 continuous sagittal slices, slice thickness = 2.5 mm, repetition time = 7600 ms (TR), echo time = 65 ms (TE), field of view (FOV) = 200 × 240, voxel size = 2.5 mm × 2.5 mm. Diffusion gradients were applied in 60 noncollinear directions (b = 1300 s/mm2) and 6 nondiffusion-weighted images were acquired.
2.3.2. DTI processing
Pre-processing of DTI-data was performed by using the software program ExploreDTI (Leemans et al., 2009). This consists of visual quality assurance and rigorous motion and eddy current correction with the required reorientation of the b-matrix (Leemans and Jones, 2009). To acquire a summary measure of head motion for each child, the root-mean-square of the 6 parameters describing rigid movement (3 translation, 3 rotation) was calculated for each volume relative to the preceding volume (Theys et al., 2014). The median displacement for the individual DTI scans varied from 0.03 mm to 0.61 mm over the 35 FRD− children (mean = 0.11 mm), and varied from 0.03 mm to 0.51 mm over the 36 FRD+ children (mean = 0.10 mm). Although there was no significant group difference [t(69) = 0.56, p = .58], we included this motion index as covariate in the statistical analyses in order to exclude spurious group differences or relationships (Yendiki et al., 2013). To avoid artefacts due to normalization – given the anatomical differences between a toddler's brain and the standard template of an adult brain – the DTI data of each child were processed in native space. The diffusion tensors were estimated using non-linear least square fitting, and the resulting eigenvalues were used to compute fractional anisotropy (FA) (FA; Basser and Pierpaoli, 1996). Finally, whole brain tractography of each individual DTI dataset was performed using a seed point resolution of (Shaywitz, 1998, Shaywitz, 1998, Shaywitz, 1998), FA threshold of 0.2 to seed and end tracking, angle threshold of 40°, and fibre length range of 50–500 mm. In order to allow a direct comparison with our previous DTI study in adults (Vandermosten et al., 2012), fibre tracts were estimated using a deterministic streamline tracking algorithm with a 1 mm fixed step size.
2.3.3. Manual delineation of specific white matter tracts
In order to delineate specific white matter tracts, the reconstructed fibres obtained via whole brain tractography were imported into TrackVis (http://www.trackvis.org; Wedeen et al., 2008). For each child we manually delineated IFOF, AFFTP and posterior AFTP in the left and right hemisphere (see Fig. 1). An average FA-value along each tract was calculated. More information and additional analyses on the different segments of the arcuate fasciculus is provided in the Supplementary Information (SI). More details on delineating IFOF, and the different segments of AF is provided in Vandermosten et al. (2012b).
For each of the pre-readers, we were able to reconstruct bilateral AFFTP, posterior AFTP and IFOF, except for one pre-reader for whom we did not find a right posterior AFTP. Although manually defining the ROIs for each individual in native space avoids normalization artefact, inter-rater variability might be induced as ROIs need to be defined for each subject individually. To assess the robustness of the obtained results, each tract in the left hemisphere was delineated by two raters and Concordance Correlation Coefficients (CCC) (Crawford et al., 2007) were calculated for the extracted FA-values. For each of the left hemispheric tracts the correspondence between the two raters was very high, with CCC equal to .981, .977 and .964 for IFOF, AFFTP and posterior AFTP, respectively. In the analyses reported, the FA-values of the left hemisphere tracts were averaged across rater 1 and rater 2 and the FA-values of the right hemisphere tracts were taken from rater 1. The robustness of our results was confirmed since the same significant group differences and correlations occurred when FA-values of the left hemispheric tracts were either taken from rater 1 or from rater 2.
3. Results and discussion
3.1. Behavioural data
Table 1 provides test statistics for the two groups on the matching criteria, handedness and the cognitive measures. As expected from matching, no significant differences were observed between FRD+ and FRD− pre-readers on gender, SES, ADHD-risk score, age and non-verbal IQ. Handedness was not an a priori matching criteria but information was gathered after participant selection through the Edinburgh Handedness Inventory (Oldfield, 1971). There was no significant difference in the number of left-handed participants in the FRD+-group (n = 7) versus the FRD− group (n = 2). With regard to the cognitive measures, no group difference was observed in PA, whereas LK and RAN approached significance. Children of the FRD−-group had on average productive knowledge of 10 letters and receptive knowledge of 11 letters whereas this was, respectively, 8 and 9 letters in the children of the FRD+-group.
Table 1.
Characteristics of the participants.
| FRD+ (n = 36) |
FRD− (n = 35) |
Test statistics | |
|---|---|---|---|
| Demographic data | |||
| Gender (boy/girl) | 23/13 | 18/17 | Fisher's exact test: p = .34 |
| SESa | 5.3 (1.6) | 5.6 (1.6) | Fisher's exact test: p = .18 |
| ADHDa | 2.5 (2.2) | 1.5 (1.5) | Fisher's exact test: p = .40 |
| Age in monthsb | 61.4 (3.1) | 61.7 (3.0) | t(69) = 0.37, p = .71 |
| Non-verbal IQc | 109.9 (13.2) | 110.4 (10.0) | t(69) = 0.18, p = .85 |
| Handedness | |||
| (left/right) | 7/29 | 2/32 | Fisher's exact test: p = .15 |
| Cognitive predictors (composite scored) | |||
| Phonological Awareness (PA) | −0.06 (1.28) | 0 (1) | t(69) = 0.17, p = .86 |
| Rapid Automatised Naming (RAN) | −0.46 (1.08) | 0 (1) | t(69) = 1.77, p = .08 |
| Letter Knowledge (LK) | −0.51 (1.25) | 0 (1) | t(69) = 1.85, p = .07 |
SES and ADHD scores were calculated from ordinal data.
Age at start of data collection, i.e. beginning of the last year of kindergarten.
Standardised scores with population average M = 100 and SD = 15.
To assist in the interpretation of the results, composite scores of the three phonological abilities were transferred to effect sizes relatively to the mean and standard deviation of the FRD− group.
3.2. DTI-data: link with cognitive risk factor of dyslexia
The first aim of our study was to test the function of the tracts of interest in pre-readers. As a first exploratory step, Pearson correlation coefficients between FA in the white matter tracts and the cognitive measures were calculated across all 71 participants. Analyses showed a significant relationship between PA and FA in bilateral IFOF (left: r = .362, p = .0019; right: r = .375, p = .0013), bilateral AFFTP (left: r = 0.302, p = .010; right: r = .276, p = .0201) and left posterior AFTP (r = .268, p = .0236). In addition, LK and RAN correlated significantly with FA in right IFOF (LK: r = .261, p = .0278; RAN: r = .311, p = .0083). Fisher z-tests indicated that the correlations within the FRD+ and FRD− group did not differ from each other (p > .05), suggesting a similar correlational pattern between groups. Note that after applying a False Discovery Rate correction for multiple testing, only the relationship between PA and FA in bilateral IFOF and left AF remained significant as well as the relationship between RAN and FA in right IFOF.
In a second step, we performed multiple regression analyses to control for potential confounding effects and to test the specificity of the functional relationship. More specifically, two control variables (motion index and group membership) were included to rule out potential influences of individual differences in head motion (Yendiki et al., 2013) and to ensure that the pattern of correlations was not inflated by group differences. In addition, all three cognitive variables (PA, LK and RAN) were included simultaneously to investigate to what extent their relationship with FA in the delineated tracts was driven by orthographic rather than phonological awareness aspects. Simple correlations cannot inform us on the specificity of phonological versus orthographic contributions since LK and RAN are not a pure orthographic measure but also depend on phonological processes. Multiple regression analyses showed no unique contributions of LK and RAN to white matter organisation in the delineated tracts when PA and control variables were included. Yet, PA remained a significant predictor of FA in bilateral IFOF (left: t = 2.55, p = .0132; right: t = 2.08, p = .0412) and left AFFTP (left: t = 1.98, p = .052), which confirms the significant correlation after correction for multiple comparisons. The scatterplots of these three correlations are provided in Fig. 2.
Fig. 2.
depicts the significant correlations between phonological awareness (PA) and fractional anisotropy (FA) in left AFFTP and bilateral IFOF. Data of FRD− group are depicted in blue diamonds and data of FRD+ group in red circles. The reported Pearson correlation values are calculated across the whole sample. (For interpretation of the references to colour in this figure legend, the reader is referred to the web version of the article.)
The dorsal correlations we observed with PA are in full agreement with what Saygin et al. (2013) found in a sample of pre- to beginning readers. They showed that FA in the left AFFTP and in a region located on the left posterior AFTP1 was correlated with PA but not with RAN and LK. In combination with studies in primary school children (Yeatman et al., 2011) and adults (Vandermosten et al., 2012b), this suggests that the relationship between phonological awareness and FA in the arcuate fasciculus is present across development. Yet in contrast to adults (Vandermosten et al., 2012b), PA in our pre-readers additionally showed a correlation with left ventral IFOF and its right homologue. Hence, our results support a ventral involvement in the phonological aspects of reading at an early age (for similar findings see Raschle et al., 2012, Yamada et al., 2011, Brem et al., 2010; but see Saygin et al., 2013). Given that dorsal white matter fibres are among the latest to fully mature in human ontogeny (Giorgio et al., 2008), they might be insufficiently developed in young children to sustain phonological and language processing, and therefore make additional use of the ventral fibres which mature earlier on (Brauer et al., 2011). The absence of a left-hemispheric specialization confirms previous cross-sectional studies in children (Turkeltaub et al., 2003, Shaywitz et al., 2002) and suggests that right hemispheric regions are recruited during the early stage of literacy development and are only later disengaged, eventually resulting in a left lateralised matured reading network. Hence, the correlational analyses suggest, together with several other studies, that a left-lateralised and dorsal specialisation for phonological aspects of reading is not established before reading onset, but presumably arises throughout reading development. Note that advanced diffusion MRI-techniques indicate the existence of short U-shaped fibres between neighbouring regions and tracts, hence continuous interaction might be an important feature to develop specialisation within each tract.
3.3. DTI data: the link to familial risk factor of dyslexia
The second aim was to examine where FA-anomalies in FRD+ children, if any, are specifically located. To this end, FA-values are analysed by means of a 2 (Group: FRD+ vs. FRD−) × 2 (Hemisphere: left vs. right) × 3 (tract: ventral IFOF vs. dorsal AFFTP vs. dorsal posterior AFTP) full factorial model. We used mixed model analysis (Littell et al., 2002), with group as a between-subject variable, tract and hemisphere as within-subject variable and motion index as covariate. Fig. 3 depicts the mean FA and standard error of each tract for the FRD+ and FRD− pre-readers. Results showed no main Group effect [F(1,344) = 2.58, p = .109] nor a significant two-way interaction involving Group (Group × Hemisphere: F(1,344) = 1.05, p = .307; Group × Tract: F(2,344) = 1.19, p = .307). However, there was a significant Group × Hemisphere × Tract interaction [F(2,344) = 3.45, p = .033]. Examining each tract individually revealed that FA was significantly lower in FRD+ pre-readers than in FRD− pre-readers for left IFOF [F(1,68) = 6.36, p = .014] and that the FA-difference was close to significance for left posterior AFTP [F(1,68) = 3.71, p = .058]. In contrast, no significant group differences were found in left AFFTP [F(1,68) = 0.01, p = .932] and the right homologues (AFFTP: F(1,68) = 1.08, p = .302, posterior AFTP: F(1,68) = 2.73, p = .103, IFOF: F(1,68) = 0.02, p = .887). Note that although the group differences in left IFOF did not reach the corrected significance level of 0.008 (=0.05/6 for 6 post hoc comparisons), the three-way-interaction indicate that group differences are predominantly present in the left ventral tract relative to left dorsal or right-hemispheric tracts.
Fig. 3.
Average fractional anisotropy of bilateral AFFTP, posterior AFTP and IFOF for FRD+ pre-readers (dark grey bar) and FRD— pre-readers (light grey bar). Error bars indicate ± 1 SEM per group. *p < 0.05 (uncorrected p-values).
Although previous EEG and MRI-studies in kindergartners and new-borns with a family risk for dyslexia have already suggested a neural origin for reading problems (Raschle et al., 2011, Raschle et al., 2012, Specht et al., 2009, Black et al., 2012, Hosseini et al., 2013, Guttorm et al., 2001, Guttorm et al., 2005, Molfese, 2000), the present study is the first to provide preliminary evidence for early white matter anomalies. Our results suggest that white matter differences associated with dyslexia do not just arise as a consequence of failed reading development but seem to restrict reading acquisition from the very start. Based on DTI-studies in adults and school-aged children with dyslexia (Vandermosten et al., 2012b, Deutsch et al., 2005, Beaulieu et al., 2005) and in ex-illiterates (Thiebaut de Schotten et al., 2012), we expected an altered FA in the dorsal connections, AFFTP and posterior AFTP. Instead, our results revealed that especially the ventral IFOF was distorted. This seems to oppose the standard neurodevelopmental model on dyslexia which predicts a primary deficit specifically located in left dorsal tracts, yet our study is not alone in finding ventral anomalies in young children with (a family risk for) dyslexia (Raschle et al., 2011, Raschle et al., 2012, Specht et al., 2009, Richlan et al., 2011, Bach et al., 2010). Although this suggests that the standard model might need rethinking, an alternative interpretation for the unexpected observation of an intact dorsal AFFTP could be the lack of group differences on PA between our FRD+ and FRD− pre-readers. Since AFFTP sustains PA in adults (Vandermosten et al., 2012b), in children (Yeatman et al., 2011, Yeatman et al., 2012) and – as the present results show- also in pre-readers (see also Saygin et al., 2013), white matter anomalies in AFFTP might currently be obscured by the fact that our FRD+ sample contains relatively few children with PA problems Finally, in contrast to a bilateral correlation pattern, right hemispheric FA-differences between FRD+ and FRD− are not present before reading onset but seem to be restricted to the left hemisphere. Hence, a primary deficit in left white matter tracts might hinder a gradual development from bilateral recruitment towards a left lateralization for reading.
4. Conclusion
Despite the methodological challenges to collect MRI-data in young children, a lot can be learned from developmental imaging that would be missed when simply examining the adult brain. The present study provides DTI-data before reading onset in a group at high and low hereditary risk for dyslexia. This allows to investigate white matter pathways before reading-induced neural reorganisation has taken place and before brain anomalies playing a causal role in dyslexia are obscured by brain irregularities that arise as a consequence of impoverished reading input or compensational mechanisms. Our findings confirm an important role for left AFFTP in phonological awareness, but they also show that ventral and right homologues additionally sustain this crucial pre-reading function. This suggests that a left-lateralised and dorsal specialization for phonological aspects of reading presumably arises throughout reading development but is not yet present at the start of reading acquisition. Second, our results reveal that family risk pre-readers display white matter anomalies in left IFOF, but not (yet) in left AFFTP nor in right homologues. This seems to oppose the standard neuroanatomical model, expecting a primary dorsal deficit associated with dyslexics’ core phonological problems, but it might also reflect the lack of PA-difficulties in our FRD+ pre-readers. Longitudinal follow-up of our sample throughout reading development and regrouping them according to a formal dyslexia diagnosis instead of family risk will aid in unravelling a gradual neural specialization for reading and in finding potential pre-diagnostic neural markers of dyslexia.
Supplementary information
For the dorsal tract, we selected the perisylvian tract which connects the inferior frontal with the inferior parietal and with the temporal lobe, i.e. AFFTP (Fig. 1). This dorsal perisylvian tract is frequently described in literature, though its terminology and its exact projection points are not without disagreement (Dick and Tremblay, 2012). In the fibre tracking atlas of Wakana et al. (2007) this tract is referred to as the superior longitudinal fasciculus, though tract-tracer studies in non-human primates suggest that Superior Longitudinal Fasciculus additionally consists of branches lying superior of the perisylvian language tract (Petrides and Pandya, 2006, Petrides and Pandya, 2009, Schmahmann and Pandya, 2006). Therefore, we refer to this perisylvian tract as AFFTP, yet note that in literature ‘arcuate fasciculus’ is often used to refer to the frontal-to-temporal fibres only (Duffau, 2008). We have addressed this issue by additional analyses in which AFFTP was split up in its frontal-to-temporal (long AFFT) and frontal-to-parietal (anterior AFFP) fibres (see below). We also delineated a second dorsal tract, which connects posterior temporal and inferior parietal fibres, posterior AFTP. We decided to keep this tract separate from the other dorsal tract, AFFTP, since tractography atlases are inconsistent in considering ‘posterior AFTP’ as part of arcuate fasciculus/superior longitudinal fasciculus or as a distinct tract (e.g. Catani and Thiebaut de Schotten, 2008 versus Wakana et al., 2004, Glasser and Rilling, 2008).
When splitting up AFFTP into a long AFFT and anterior AFFP, a missing left anterior AFFP was observed in 2 pre-readers, but to a much greater extent, a missing right long AFFT was observed in 21 pre-readers (no significant difference between FRD+ and FRD− children, Fisher's exact test: p = .12). The inability to reconstruct the right long AFFT in a substantial portion of the subjects is consistent with previous literature (Catani et al., 2007), though it is debated to what extent this actually represents a missing tract or whether this is explained by limitations inherent to DTI fibre tracking methods (Yeatman et al., 2011).
Given the evidence that AFFTP may consist of two separate segments, one connecting fronto-to-parietal (anterior AFFP) and one connecting fronto-to-temporal (long AFFT) (Catani et al., 2005), we re-analysed group differences and correlations with AFFTP split up in its two branches. Since especially left long AFFT seems to be involved in reading and dyslexia (Saygin et al., 2013), combining the two segments might have obscured significant findings. Results showed that – in line with AFFTP – group differences were neither present for long AFFT (left: [F(1,68) = 0.27, p = .607]; right: [F(1,47) = 2.76, p = .103]) nor in anterior AFFP (left: F(1,67) = 0.79, p = .377; right: F(1,68) = 0.00, p = .983). With regard to brain–behaviour relationships, the correlation with phonological awareness was present in left long AFFT (r = .277, p = .021) but did not reach significance in bilateral anterior AFFP (left: r = .051, p = .678; right: r = .146, p = .231) and in right long AFFT (r = .239, p = .101). The latter might be due to a lack of power, since delineation of the right long AFFT was only possible in 71% of the subjects. These results show that correlations between phonological awareness and AFFTP seem to be driven more by the long AFFT than the anterior AFFP.
Conflict of interest
There is no conflict of interest; no financial, personal interest or belief that could affect the objectivity.
Acknowledgements
This research was funded by the Research Council of KU Leuven (OT/12/044) and the Research Foundation Flanders (G0920.12). Maaike Vandermosten is postdoctoral fellow of the Research Foundation Flanders. Sophie Dandache is gratefully acknowledged for participant selection and behavioural data collection. We also like to thank Ron Peeters, Alexander Leemans and Flavio Del’Aqua for technical assistance of DTI-processing.
Footnotes
Saygin et al. (2013) and Vandermosten et al. (2012b) closer examination of the cluster found by whole-brain analyses (voxel coordinates: −42, −54, 28) in five randomly selected pre-readers of our sample indicates that the cluster belongs to left posterior AFTP rather than to long AFFTP as interpreted by Saygin et al. This is also confirmed when the coordinates are compared with a white matter probability atlas based on adult data (Catani and Thiebaut de Schotten, 2008).
Supplementary data associated with this article can be found, in the online version, at http://dx.doi.org/10.1016/j.dcn.2015.05.006.
Appendix A. Supplementary data
The following are the supplementary data to this article:
References
- Dehaene S. Penguin Viking; New York: 2009. Reading in the Brain. [Google Scholar]
- Shaywitz B.A. Functional organization of the brain of reading and dyslexia. Biol. Psychiatry. 1998;43:210. [Google Scholar]
- Vellutino F.R., Fletcher J.M., Snowling M.J., Scanlon D.M. Specific reading disability (dyslexia): what have we learned in the past four decades? J. Child Psychol. Psychiatr. 2004;45:2–40. doi: 10.1046/j.0021-9630.2003.00305.x. [DOI] [PubMed] [Google Scholar]
- Price C.J., Moore C.J., Humphreys G.W., Wise R.J.S. Segregating semantic from phonological processes during reading. J. Cogn. Neurosci. 1997;9:727–733. doi: 10.1162/jocn.1997.9.6.727. [DOI] [PubMed] [Google Scholar]
- Jobard G., Crivello F., Tzourio-Mazoyer N. Evaluation of the dual route theory of reading: a metanalysis of 35 neuroimaging studies. Neuroimage. 2003;20:693–712. doi: 10.1016/S1053-8119(03)00343-4. [DOI] [PubMed] [Google Scholar]
- Rumsey J.M., Nace K., Donohue B., Wise D., Maisog J.M., Andreason P. A positron emission tomographic study of impaired word recognition and phonological processing in dyslexic men. Archiv. Neurol. 1997;54:562–573. doi: 10.1001/archneur.1997.00550170042013. [DOI] [PubMed] [Google Scholar]
- Vigneau M., Beaucousin V., Herve P.Y., Duffau H., Crivello F., Houde O., Mazoyer B., Tzourio-Mazoyer N. Meta-analyzing left hemisphere language areas: phonology, semantics, and sentence processing. Neuroimage. 2006;30:1414–1432. doi: 10.1016/j.neuroimage.2005.11.002. [DOI] [PubMed] [Google Scholar]
- Taylor J., Castle K., Davis M. Can cognitive models explain brain activation during word and pseudoword reading? A meta-analysis of 36 neuroimaging studies. Psychol. Bull. 2014;139:766–791. doi: 10.1037/a0030266. [DOI] [PubMed] [Google Scholar]
- Dehaene S., Cohen L. The unique role of the visual word form area in reading. Trends Cogn. Sci. 2011;15:254–262. doi: 10.1016/j.tics.2011.04.003. [DOI] [PubMed] [Google Scholar]
- McCandliss B.D., Noble K.G. The development of reading impairment: a cognitive neuroscience model. Ment. Retard. Dev. Disabil. Res. Rev. 2003;9:196–204. doi: 10.1002/mrdd.10080. [DOI] [PubMed] [Google Scholar]
- Pugh K., Mencl W., Jenner A., Katz L., Frost S., Lee J. Neurobiological studies of reading and reading disability. J. Commun. Disord. 2001;34:479–492. doi: 10.1016/s0021-9924(01)00060-0. [DOI] [PubMed] [Google Scholar]
- Richlan F. Developmental dyslexia: dysfunction of a left hemisphere reading network. Front. Hum. Neurosci. 2012;6 doi: 10.3389/fnhum.2012.00120. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Vandermosten M., Boets B., Wouters J., Ghesquiere P. A qualitative and quantitative review of diffusion tensor imaging studies in reading and dyslexia. Neurosci. Biobehav. Rev. 2012;36:1532–1552. doi: 10.1016/j.neubiorev.2012.04.002. [DOI] [PubMed] [Google Scholar]
- Turkeltaub P.E., Gareau L., Flowers D.L., Zeffiro T.A., Eden G.F. Development of neural mechanisms for reading. Nat. Neurosci. 2003;6:767–773. doi: 10.1038/nn1065. [DOI] [PubMed] [Google Scholar]
- Vandermosten M., Boets B., Poelmans H., Sunaert S., Wouters J., Ghesquiere P. A tractography study in dyslexia: neuroanatomic correlates of orthographic, phonological and speech processing. Brain. 2012;135:935–948. doi: 10.1093/brain/awr363. [DOI] [PubMed] [Google Scholar]
- Shaywitz B.A., Shaywitz S.E., Pugh K.R., Mencl W.E., Fulbright R.K., Skudlarski P., Constable R.T., Marchione K.E., Fletcher J.M., Lyon G.R., Gore J.C. Disruption of posterior brain systems for reading in children with developmental dyslexia. Biol. Psychiatry. 2002;52:101–110. doi: 10.1016/s0006-3223(02)01365-3. [DOI] [PubMed] [Google Scholar]
- Simos P.G., Breier J.I., Fletcher J.M., Foorman B.R., Castillo E.M., Papanicolaou A.C. Brain mechanisms for reading words and pseudowords: an integrated approach. Cereb. Cortex. 2002;12:297–305. doi: 10.1093/cercor/12.3.297. [DOI] [PubMed] [Google Scholar]
- Snowling M. Blackwell; Oxford, UK: 2000. Dyslexia. [Google Scholar]
- Raschle N.M., Zuk J., Gaab N. Functional characteristics of developmental dyslexia in left-hemispheric posterior brain regions predate reading onsety. Proc. Natl. Acad. Sci. U.S.A. 2012;109:2156–2161. doi: 10.1073/pnas.1107721109. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yamada Y., Stevens C., Dow M., Harn B.A., Chard D.J., Neville H.J. Emergence of the neural network for reading in five-year-old beginning readers of different levels of pre-literacy abilities: an fMRI study. Neuroimage. 2011;57:704–713. doi: 10.1016/j.neuroimage.2010.10.057. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Brem S., Bach S., Kucian K., Guttorm T.K., Martin E., Lyytinen H., Brandeis D., Richardson U. Brain sensitivity to print emerges when children learn letter-speech sound correspondences. Proc. Natl. Acad. Sci. U.S.A. 2010;107:7939–7944. doi: 10.1073/pnas.0904402107. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Maurer U., Brem S., Kranz F., Bucher K., Benz R., Halder P., Steinhausen H.C., Brandeis D. Coarse neural tuning for print peaks when children learn to read. Neuroimage. 2006;33:749–758. doi: 10.1016/j.neuroimage.2006.06.025. [DOI] [PubMed] [Google Scholar]
- Specht K., Hugdahl K., Ofte S., Nygard M., Bjornerud A., Plante E., Helland T. Brain activation on pre-reading tasks reveals at-risk status for dyslexia in 6-year-old children. Scand. J. Psychol. 2009;50:79–91. doi: 10.1111/j.1467-9450.2008.00688.x. [DOI] [PubMed] [Google Scholar]
- Black J.M., Tanaka H., Stanley L., Nagamine M., Zakerani N., Thurston A., Kesler S., Hulme C., Lyytinen H., Glover G.H., Serrone C., Raman M.M., Reiss A.L., Hoeft F. Maternal history of reading difficulty is associated with reduced language-related gray matter in beginning readers. Neuroimage. 2012;59:3021–3032. doi: 10.1016/j.neuroimage.2011.10.024. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hosseini S.M.H., Black J.M., Soriano T., Bugescu N., Martinez R., Raman M.M., Kesler S.R., Hoeft F. Topological properties of large-scale structural brain networks in children with familial risk for reading difficulties. Neuroimage. 2013;71:260–274. doi: 10.1016/j.neuroimage.2013.01.013. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Raschle N.M., Chang M., Gaab N. Structural brain alterations associated with dyslexia predate reading onset. Neuroimage. 2011;57:742–749. doi: 10.1016/j.neuroimage.2010.09.055. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lebel C., Walker L., Leemans A., Phillips L., Beaulieu C. Microstructural maturation of the human brain from childhood to adulthood. Neuroimage. 2008;40:1044–1055. doi: 10.1016/j.neuroimage.2007.12.053. [DOI] [PubMed] [Google Scholar]
- Carreiras M., Seghier M.L., Baquero S., Estevez A., Lozano A., Devlin J.T., Price C.J. An anatomical signature for literacy. Nature. 2009;461 doi: 10.1038/nature08461. 983-U245. [DOI] [PubMed] [Google Scholar]
- Thiebaut de Schotten M., Cohen L., Amemiya E., Brada L.W., Dehaene S. Learning to read improves the structure of the arcuate fasciculus. Cereb. Cortex. 2012;24(4):989–995. doi: 10.1093/cercor/bhs383. [DOI] [PubMed] [Google Scholar]
- Beaulieu C. The basis of anisotropic water diffusion in the nervous system – a technical review. NMR Biomed. 2002;15:435–455. doi: 10.1002/nbm.782. [DOI] [PubMed] [Google Scholar]
- Puolakanaho A., Ahonen T., Aro M., Eklund K., Leppanen P.H.T., Poikkeus A.M., Tolvanen A., Torppa M., Lyytinen H. Very early phonological and language skills: estimating individual risk of reading disability. J. Child Psychol. Psychiatry. 2007;48:923–931. doi: 10.1111/j.1469-7610.2007.01763.x. [DOI] [PubMed] [Google Scholar]
- Boets B., De Smedt B., Cleuren L., Vandewalle E., Wouters J., Ghesquière P. Towards a further characterization of phonological and literacy problems in Dutch-speaking children with dyslexia. Br. J. Dev. Psychol. 2010;28:5–31. doi: 10.1348/026151010x485223. [DOI] [PubMed] [Google Scholar]
- Verhagen W., Aarnoutse C., van Leeuwe J. Phonological awareness and naming speed in the prediction of dutch children's word recognition. Sci. Stud. Reading. 2008;12:301–324. [Google Scholar]
- Savage R., Frederickson N. Evidence of a highly specific relationship between rapid automatic naming of digits and text-reading speed. Brain Language. 2005;93:152–159. doi: 10.1016/j.bandl.2004.09.005. [DOI] [PubMed] [Google Scholar]
- Allor J.H. The relationships of phonemic awareness and rapid naming to reading development. Learn. Disabil. Q. 2002;25:47–57. [Google Scholar]
- Gilger J.W., Pennington B.F., Defries J.C. Risk for reading-disability as a function of parental history in 3 family studies. Reading Writing. 1991;3:205–217. [Google Scholar]
- Grigorenko E.L. Developmental dyslexia: an update on genes, brains, and environments. J. Child Psychol. Psychiatry. 2001;42:91–125. [PubMed] [Google Scholar]
- van Atteveldt N., Roebroeck A., Goebel R. Interaction of speech and script in human auditory cortex: insights from neuro-imaging and effective connectivity. Hearing Res. 2009;258:152–164. doi: 10.1016/j.heares.2009.05.007. [DOI] [PubMed] [Google Scholar]
- Yeatman J.D., Dougherty R.F., Rykhlevskaia E., Sherbondy A.J., Deutsch G.K., Wandell B.A., Ben-Shachar M. Anatomical properties of the arcuate fasciculus predict phonological and reading skills in children. J. Cogn. Neurosci. 2011;23:3304–3317. doi: 10.1162/jocn_a_00061. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Saygin Z.M., Norton E.S., Osher D.E., Beach S.D., Cyr A.B., Ozernov-Palchik O., Yendiki A., Fischl B., Gaab N., Gabrieli J.D.E. Tracking the roots of reading ability: white matter volume and integrity correlate with phonological awareness in prereading and early-reading kindergarten children. J. Neurosci. 2013;33:13251–13258. doi: 10.1523/JNEUROSCI.4383-12.2013. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Raven J.C., Court J.H., Raven J. Lewis; London, United Kingdom: 1984. Manual for Raven's Progressive Matrices and Vocabulary Scales. [Google Scholar]
- Boudreau B., Poulin C. An examination of the validity of the Family Affluence Scale II (FAS II) in a general adolescent population of Canada. Soc. Indic. Res. 2009;94:29–42. [Google Scholar]
- Boyce W., Torsheim T., Currie C., Zambon A. The family affluence scale as a measure of national wealth: validation of an adolescent self-report measure. Soc. Indic. Res. 2006;78:473–487. [Google Scholar]
- Vanvooren S., Poelmans H., Hofmann M., Ghesquiere P., Wouters J. Hemispheric asymmetry in auditory processing of speech envelope modulations in pre-reading children. J. Neurosci. 2014;34:1523–1529. doi: 10.1523/JNEUROSCI.3209-13.2014. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Savage R., Frederickson N., Goodwin R., Patni U., Smith N., Tuersley L. Evaluating current deficit theories of poor reading: Role of phonological processing, naming speed, balance automaticity, rapid verbal perception and working memory. Percept. Motor Skills. 2005;101:345–361. doi: 10.2466/pms.101.2.345-361. [DOI] [PubMed] [Google Scholar]
- Boets B., Wouters J., van Wieringen A., De Smedt B., Ghesquière P. Modelling relations between sensory processing, speech perception, orthographic and phonological ability, and literacy achievement. Brain Lang. 2008;106:29–40. doi: 10.1016/j.bandl.2007.12.004. [DOI] [PubMed] [Google Scholar]
- Theys C., Wouters J., Ghesquière P. Diffusion tensor imaging and resting-state functional MRI-scanning in 5- and 6-year-old children: training protocol and motion assessment. PLoS ONE. 2014;9:e94019. doi: 10.1371/journal.pone.0094019. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Leemans, A., Jeurissen, B., Sijbers, J., Jones, D., 2009. ExploreDTI: A Graphical Toolbox for Processing Analyzing, and Visualizing Diffusion MR Data.
- Leemans A., Jones D.K. The B-matrix must be rotated when correcting for subject motion in DTI data. Magn. Reson. Med. 2009;61:1336–1349. doi: 10.1002/mrm.21890. [DOI] [PubMed] [Google Scholar]
- Yendiki A., Koldewyn K., Kakunoori S., Kanwisher N., Fischl B. Spurious group differences due to head motion in a diffusion MRI study. Neuroimage. 2013;88:79–90. doi: 10.1016/j.neuroimage.2013.11.027. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Basser P.J., Pierpaoli C. Microstructural and physiological features of tissues elucidated by quantitative-diffusion-tensor MRI. J. Magn. Reson. Ser. B. 1996;111:209–219. doi: 10.1006/jmrb.1996.0086. [DOI] [PubMed] [Google Scholar]
- Wedeen V.J., Wang R.P., Schmahmann J.D., Benner T., Tseng W.Y.I., Dai G., Pandya D.N., Hagmann P., D’Arceuil H., de Crespignya A.J. Diffusion spectrum magnetic resonance imaging (DSI) tractography of crossing fibers. Neuroimage. 2008;41:1267–1277. doi: 10.1016/j.neuroimage.2008.03.036. [DOI] [PubMed] [Google Scholar]
- Crawford S.B., Kosinski A.S., Lin H.M., Williamson J.M., Barnhart H.X. Computer programs for the concordance correlation coefficient. Comput. Methods Prog. Biomed. 2007;88:62–74. doi: 10.1016/j.cmpb.2007.07.003. [DOI] [PubMed] [Google Scholar]
- Oldfield R.C. The assessment and analysis of handedness: the edinburgh inventory. Neuropsychologia. 1971;9:97–113. doi: 10.1016/0028-3932(71)90067-4. [DOI] [PubMed] [Google Scholar]
- Giorgio A., Watkins K., Douaud G., James A., James S., De Stefano N., Matthews P., Smith S., Johansen-Berg H. Changes in white matter microstructure during adolescence. Neuroimage. 2008;39:52–61. doi: 10.1016/j.neuroimage.2007.07.043. [DOI] [PubMed] [Google Scholar]
- Brauer J., Anwander A., Friederici A.D. Neuroanatomical prerequisites for language functions in the maturing brain. Cereb. Cortex. 2011;21:459–466. doi: 10.1093/cercor/bhq108. [DOI] [PubMed] [Google Scholar]
- Littell R.C., Stroup W.W., Freund R.J. SAS Institute; Cary: 2002. SAS for Linear Models. [Google Scholar]
- Guttorm T.K., Leppanen P.H.T., Richardson U., Lyytinen H. Event-related potentials and consonant differentiation in newborns with familial risk for dyslexia. J. Learn. Disabil. 2001;34:534–544. doi: 10.1177/002221940103400606. [DOI] [PubMed] [Google Scholar]
- Guttorm T.K., Leppanen P.H.T., Poikkeus A.M., Eklund K.M., Lyytinen P., Lyytinen H. Brain event-related potentials (ERPs) measured at birth predict later language development in children with and without familial risk for dyslexia. Cortex. 2005;41:291–303. doi: 10.1016/s0010-9452(08)70267-3. [DOI] [PubMed] [Google Scholar]
- Molfese D.L. Predicting dyslexia at 8 years of age using neonatal brain responses. Brain Lang. 2000;72:238–245. doi: 10.1006/brln.2000.2287. [DOI] [PubMed] [Google Scholar]
- Deutsch G.K., Dougherty R.F., Bammer R., Siok W.T., Gabrieli J.D.E., Wandell B.A. Children's reading performance is correlated with white matter structure measured by diffusion tensor imaging. Cortex. 2005;41:354–363. doi: 10.1016/s0010-9452(08)70272-7. [DOI] [PubMed] [Google Scholar]
- Beaulieu C., Plewes C., Paulson L.A., Roy D., Snook L., Concha L., Phillips L. Imaging brain connectivity in children with diverse reading ability. Neuroimage. 2005;25:1266–1271. doi: 10.1016/j.neuroimage.2004.12.053. [DOI] [PubMed] [Google Scholar]
- Richlan F., Kronbichler M., Wimmer H. Meta-analyzing brain dysfunctions in dyslexic children and adults. Neuroimage. 2011;56:1735–1742. doi: 10.1016/j.neuroimage.2011.02.040. [DOI] [PubMed] [Google Scholar]
- Bach S., Brandeis D., Hofstetter C., Martin E., Richardson U., Brem S. Early emergence of deviant frontal fMRI activity for phonological processes in poor beginning readers. 1. Neuroimage. 2010;53:682–693. doi: 10.1016/j.neuroimage.2010.06.039. [DOI] [PubMed] [Google Scholar]
- Yeatman J.D., Dougherty R.F., Ben-Shachar M., Wandell B.A. Development of white matter and reading skills. Proc. Natl. Acad. Sci. U.S.A. 2012;109:E3045–E3053. doi: 10.1073/pnas.1206792109. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dick A.S., Tremblay P. Beyond the arcuate fasciculus: consensus and controversy in the connectional anatomy of language. Brain. 2012;135:3529–3550. doi: 10.1093/brain/aws222. [DOI] [PubMed] [Google Scholar]
- Wakana S., Caprihan A., Panzenboeck M.M., Fallon J.H., Perry M., Gollub R.L., Hua K.G., Zhang J.Y., Jiang H.Y., Dubey P., Blitz A., van Zijl P., Mori S. Reproducibility of quantitative tractography methods applied to cerebral white matter. Neuroimage. 2007;36:630–644. doi: 10.1016/j.neuroimage.2007.02.049. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Petrides M., Pandya D.N. Distinct parietal and temporal pathways to the homologues of broca's area in the monkey. PLoS Biol. 2009;7 doi: 10.1371/journal.pbio.1000170. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Petrides M., Pandya D.N. Efferent association pathways originating in the caudal prefrontal cortex in the macaque monkey. J. Comp. Neurol. 2006;498:227–251. doi: 10.1002/cne.21048. [DOI] [PubMed] [Google Scholar]
- Schmahmann J.D., Pandya D.N. Principles of organization of cerebral white matter tracts. Ann. Neurol. 2006;60:S50. [Google Scholar]
- Duffau H. The anatomo-functional connectivity of language revisited new insights provided by electrostimulation and tractography. Neuropsychologia. 2008;46:927–934. doi: 10.1016/j.neuropsychologia.2007.10.025. [DOI] [PubMed] [Google Scholar]
- Catani M., Thiebaut de Schotten M. A diffusion tensor imaging tractography atlas for virtual in vivo dissections. Cortex. 2008;44:1105–1132. doi: 10.1016/j.cortex.2008.05.004. [DOI] [PubMed] [Google Scholar]
- Wakana S., Jiang H.Y., Nagae-Poetscher L.M., van Zijl P.C.M., Mori S. Fiber tract-based atlas of human white matter anatomy. Radiology. 2004;230:77–87. doi: 10.1148/radiol.2301021640. [DOI] [PubMed] [Google Scholar]
- Glasser M.F., Rilling J.K. DTI tractography of the human brain's language pathways. Cereb. Cortex. 2008;18:2471–2482. doi: 10.1093/cercor/bhn011. [DOI] [PubMed] [Google Scholar]
- Catani M., Allin M.P.G., Husain M., Pugliese L., Mesulam M.M., Murray R.M., Jones D.K. Symmetries in human brain language pathways correlate with verbal recall. Proc. Natl. Acad. Sci. U.S.A. 2007;104:17163–17168. doi: 10.1073/pnas.0702116104. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Catani M., Jones D.K., Ffytche D.H. Perisylvian language networks of the human brain. Ann. Neurol. 2005;57:8–16. doi: 10.1002/ana.20319. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.