Highlights
-
•
Adolescents with a CUD had an attentional but no approach bias towards cannabis.
-
•
Cannabis craving significantly predicted cannabis use 6 months later.
-
•
These findings identify craving as a predictor of treatment outcome.
-
•
This study is among the first to investigate neuropsychological mechanisms underlying adolescent CUDs.
Keywords: Cannabis use disorder, Attentional bias, Approach bias, Craving, Cognitive control, Adolescence, Treatment progression
Abstract
Cannabis use disorders (CUDs) are the most prevalent substance use disorders among adolescents in treatment. Yet, little is known about the neuropsychological mechanisms underlying adolescent CUDs. Studies in adult cannabis users suggest a significant role for cognitive control and cannabis-oriented motivational processes, such as attentional bias, approach bias, and craving in CUDs. The current 6-month prospective study investigated the relationships between attentional bias, approach bias, craving, cognitive control, and cannabis use in adolescent patients in treatment for a primary or secondary CUD. Moreover, we investigated if these motivational processes and cognitive control could predict treatment progression after 6 months. Adolescents with a CUD had an attentional but no approach bias towards cannabis. In contrast to adult findings on the role of attentional bias, approach bias and cognitive control, only cannabis craving significantly correlated with current cannabis use and predicted cannabis use-related problems and abstinence from cannabis 6 months later. These findings identify craving as a predictor of treatment outcome, thereby supporting an important role for craving in the course of adolescent cannabis use and dependence. This prospective study is among the first to investigate neuropsychological mechanisms underlying adolescent CUDs, warranting future longitudinal studies.
1. Introduction
Among adolescents in treatment for a substance use disorder (SUD), cannabis use disorders (CUDs) are the most prevalent SUDs (SAMHSA, 2010, Wisselink et al., 2014). Adolescent compared to adult onset of cannabis use is associated with greater cognitive deficits, poorer socio-economic status, poorer educational achievement and more chronic CUD trajectories (Meier et al., 2012, Perkonigg et al., 2008, Stinson et al., 2006, Swift et al., 2001). Unfortunately, only a minority of individuals with a CUD enter treatment (Agosti and Levin, 2004) and post-treatment relapse rates remain high (52–70%; Budney et al., 2008, Chauchard et al., 2013, Zumdick et al., 2006). These high relapse rates and the significant personal and societal harms associated with adolescent CUDs warrant the development of new treatment strategies. Knowledge of the neuropsychological processes associated with adolescent CUDs may help to identify new treatment targets, however, little is known about these mechanisms in adolescents.
The imbalance between strong drug-oriented motivational processes and compromised control processes is thought to play a significant role in the development and maintenance of SUDs (Everitt and Robbins, 2005, Koob and Volkow, 2010, Wiers et al., 2007). Strong motivational processes may develop over the course of repeated substance use through processes such as sensitization and conditioning. Encounters with cues (e.g., certain emotional states, objects, contexts) that have previously been associated with substance use may bias behaviour towards substance use in a relatively automatic way. More specifically, substance-related cues can grab attention (attentional bias), activate approach action tendencies (approach bias) and increase craving (Wiers et al., 2007). Cognitive control appears to be compromised in individuals with a SUD and has been found to moderate the relation between biased motivational processes and substance use (Grenard et al., 2008, Houben and Wiers, 2009, Peeters et al., 2012, Sharbanee et al., 2013, Thush et al., 2008). A relatively poor capacity to regulate motivational processes (pre-existent or compromised by substance use) may thereby further support continued substance use and relapse.
In line with previous findings on other SUDs, evidence is emerging that biased motivational processes are present in adult heavy cannabis users and individuals with a CUD. For example, cannabis cues can induce craving in adults with a CUD (Lundahl and Johanson, 2011). Moreover, an attentional bias towards cannabis cues has repeatedly been established in heavy cannabis users (Cousijn et al., 2013a, Field, 2005, Field et al., 2004, Field et al., 2006) and individuals with a CUD (Asmaro et al., 2014, Cousijn et al., 2013a). Furthermore, dependent cannabis users showed a stronger attentional bias than non-dependent cannabis users (Cousijn et al., 2013a). The approach bias towards cannabis cues has been observed in heavy cannabis users (Cousijn et al., 2011, Field et al., 2006) and was found to be predictive of an increase in cannabis use six months later (Cousijn et al., 2011). Regarding cognitive control, the current literature provides preliminary evidence for a bidirectional relationship with CUDs: Long-term cannabis use may (temporarily) compromise cognitive control (Crean et al., 2011), whereas individuals with relative poor levels of cognitive control may have an increased risk of developing cannabis dependence (Cousijn et al., 2013b, Cousijn et al., 2014a, Cousijn et al., 2014b). Cognitive control may only moderate the relationship between motivational processes and cannabis use in more severe and chronic cannabis users, not in all heavy users (Cousijn et al., 2013a, Cousijn et al., 2013c). Despite these limited data on neuropsychological mechanisms underlying CUDs, the available studies in adult cannabis users suggest an important role for both cognitive control and motivational processes, such as attentional bias, approach bias, and craving in the development and maintenance of CUDs.
According to the literature on neurocognitive development, adolescence is marked by an increase in reward sensitivity and a not fully developed cognitive control system, putting adolescents at an increased risk to develop a SUD (Crone and Dahl, 2012, Gladwin et al., 2011). Indeed, prevalence of CUDs are highest during adolescence and young adulthood (SAMHSA, 2010, Wisselink et al., 2014). Similarly as in adults with a CUD, cannabis cues can induce craving in cannabis dependent adolescents (Gray et al., 2011, Nickerson et al., 2011). To the best of our knowledge, there are no published studies yet on attentional and approach bias in adolescent cannabis users. Cognitive control appears to be compromised in a substantial part of adolescent cannabis users (Dougherty et al., 2013, Hanson et al., 2010, Hanson et al., 2014, Harvey et al., 2007), however, the relationship between motivational processes and cognitive control in adolescents with a CUD remains unclear. To bridge this gap and to extend adult findings on the importance of these processes in the course of CUDs, we investigated the relationships between attentional bias, approach bias, craving, cognitive control, and cannabis use in adolescent patients in treatment for a primary or secondary CUD (n = 57). Moreover, in a subset of the adolescents (n = 46) we investigated if these motivational processes and cognitive control could predict treatment progression after 6 months. Based on previous findings on motivational processes in adult heavy cannabis users (Cousijn et al., 2011, Cousijn et al., 2013a, Field, 2005, Field et al., 2004), we expected attentional bias, approach bias and craving in response to cannabis-related stimuli to covary with amount of cannabis use and severity of cannabis-related problems. Moreover, we hypothesized that individual differences in cognitive control would moderate the relationship between motivational processes (attentional bias, approach bias, craving) and amount of cannabis use and severity of cannabis-related problems. Finally, we examined whether both motivational processes and cognitive control were related to treatment progression, such that a stronger attentional bias, approach bias, and craving for cannabis, but lower levels of cognitive control would predict early dropout or lack of progress in CUD related treatment objectives.
2. Materials and methods
The Ethics Committee of the University of Amsterdam approved the study.
2.1. Participants
Study participants were 57 adolescent patients (15–22 years) who received outpatient treatment for CUDs at Brijder Addiction Care, a large addiction care facility in the western part of the Netherlands. See Table 1 for sample characteristics. This study combined data from (1) a test session in which motivational and control processes were assessed, (2) clinical evaluations on treatment progress by the therapist and (3) detailed information of substance use history and problems as part of baseline and 6 month follow-up Routine Outcome Monitoring (ROM) assessments of Brijder Addiction Care. For the majority of these patients data were available on drug-related motivational and control processes (n = 54) and on treatment progress at 6-month follow-up (n = 55). Data on substance use history and related problems were retrieved from ROM for 48 patients at baseline and 33 patients at 6-month follow-up. Participants either had a primary or secondary CUD diagnosis (see Table 1). One participant had no formal CUD diagnosis but a Cannabis Use Disorder Identification Test (CUDIT, see Section 2.2) score of 10, which is indicative of a cannabis use disorder (Adamson and Sellman, 2003). Participants were excluded if they had any other SUD.
Table 1.
Sample characteristics and treatment progress at 6-month follow-up.
| Baseline |
6-Month follow-up |
|||
|---|---|---|---|---|
| N | %/M (SD; range) | N | %/M (SD; range) | |
| Demographic characteristics | ||||
| Sex (% female) | 57 | 24.6 | 33 | 30.3 |
| Age | 57 | 19.6 (2.0; 15.9–22.9) | 33 | 20.0 (2.3; 16.0–23.8) |
| Routine outcome monitoring: substance-use & related problems | ||||
| CUDIT | 47 | 21.8 (8.3; 5–39) | 32 | 12.8 (9.1; 0–31) |
| Cannabis past 30 days (# days) | 48 | 19.3 (10.7; 0–30) | 32 | 10.4 (11.9; 0–30) |
| Cannabis use in past 30 days (yes %) | 48 | 89.6 | 32 | 65.6 |
| Duration cannabis use (years) | 48 | 4.9 (2.0; 1–11) | – | – |
| Age first cannabis use | 48 | 15.1 (2.0; 10–19) | – | – |
| AUDIT | 48 | 9.3 (6.5; 0–29) | – | – |
| FTND | 48 | 3.1 (2.4; 0–9) | – | – |
| Illicit substance use (% ever) | 48 | 66.7 | – | – |
| Illicit substance use (% past 6 months) | 48 | 18.8 | – | – |
| BDI | 45 | 16.7 (9.7; 2–14) | – | – |
| BDI (% above cut-off score) | 45 | 64.4 | – | – |
| Assessments during test session | ||||
| Attentional bias cannabis | 57 | 2.4 (6.2; −17.1 to 21.0) | ||
| Attentional bias alcohol | 57 | −0.17 (4.8; −13.0 to 9.0) | ||
| Approach bias cannabis | 54 | 16.5 (88.3; −197.0 to 313.7) | ||
| Approach bias alcohol | 54 | −5.9 (96.9; −332.5 to 223.6) | ||
| Approach bias neutral | 54 | 7.0 (92.0; −309.0 to 243.8) | ||
| Approach bias appetitive | 54 | 4.8 (89.3; −222.1 to 219.0) | ||
| Craving cannabis | 57 | 3.9 (2.8; 0–9.6) | ||
| Craving alcohol | 57 | 1.5 (1.9; 0–7.6) | ||
| Craving tobacco | 57 | 5.3 (3.3; 0–10.0) | ||
| Classical Stroop performance | 57 | 36.0 (13.1; 14.2–79.4) | ||
| Cannabis use past 14 days (# joints) | 57 | 15.6 (17.2; 0–64) | ||
| Alcohol use past 14 days (# drinks) | 57 | 16.6 (23.0; 0–116) | ||
| Clinical evaluations by therapist | ||||
| Primary CUD diagnosis (%) | 57 | 89.5 | – | – |
| Secondary CUD diagnosis (%) | 57 | 8.8 | – | – |
| Treatment evaluation (% successful) | – | – | 55 | 63.6 |
CUD: Cannabis Use Disorder; CUDIT: Cannabis Use Disorder Identification Test (8 = cut-off at-risk use); AUDIT: Alcohol Use Disorder Identification Test (8 = cut-off hazardous use); Fagerstrom Test for Nicotine Dependence; BDI: Beck Depression Inventory. Successful treatment evaluation refers to successful treatment completion, abstinence or reduced cannabis use.
All participants received cognitive behavioural treatment. The exact approach and duration of treatment highly varied between participants. A team of more than 30 therapists were involved in the treatment of this sample. Participants were not financially compensated for their participation. However, a single voucher of 50 Euros was raffled among them.
2.2. Questionnaires on substance use, craving and psychological functioning
As part of the ROM baseline and 6-month follow-up, the 10-item CUDIT was used to measure severity of cannabis use and related problems during the past 6 months. The CUDIT contains items relating to consumption, symptoms of dependence, and other cannabis-related problems. Scores can range from 0 to 40 with a discriminant validity of 0.93 to detect a current CUD and a score of 8 or higher is considered indicative of at-risk cannabis use (Adamson and Sellman, 2003). Moreover, patients were asked on how many days in the past 30 days they had used cannabis. Due to lack of variance in the baseline assessment of this measure, only data of the follow-up assessment were used.
As part of the ROM baseline, the Alcohol Use Disorder Identification Test (AUDIT; Saunders et al., 1993), the Fagerstrom Test for Nicotine Dependence (FTND; Heatherton et al., 1991) and the Beck Depression Inventory-II (BDI-II; Beck et al., 1996) were administered. The AUDIT measures severity of alcohol use and related problems during the past 6 months and consists of 10 items assessing consumption and alcohol-related problems. Scores range between 0 and 40, with a cut-off score of 8 for hazardous drinking (Saunders et al., 1993). The FTND contains 6 items assessing severity of nicotine use and dependence during the past six months (Heatherton et al., 1991). Scores range between 0 and 10 and a score of 6 or higher is indicative of severe nicotine dependence. Moreover, the BDI contains 21 items assessing physical and psychological symptoms of depression (e.g., fatigue or suicidal thoughts). Scores range from 0 to 63 and test–retest reliability and internal consistency are high (Beck et al., 1996). A cut-off score on the BDI-II of ≥12 is suggested to be optimal to screen for depression in a clinical sample of adolescents with substance abuse problems (Subramaniam et al., 2009). For descriptive purposes, we presented both the mean score and this cut-off score to distinguish between study participants with low and participants with high levels of depressive symptoms (see Table 1).
During the test session, a self-report Timeline Follow-Back (TLFB) was used to measure cannabis and alcohol use during the last two weeks prior to the test session (adapted from Sobell et al., 1996). Using a calendar, participants reported their cannabis use in joints per day and alcohol use in standard units, starting with yesterday going back to 14 days earlier. Moreover, craving for cannabis, alcohol, and nicotine was assessed at the start of the test session. On three separate Visual Analogue Scales (VAS) ranging from ‘not at all’ to ‘very much’ participants indicated how much they currently craved for cannabis, alcohol, and nicotine. Scores ranged from 0 to 10.
2.3. Classical Stroop
Similarly as in Cousijn et al. (2013a), cognitive control was measured with the validated Dutch paper version of the Classical Stroop Task (Hammes, 1971). The task consists of three subtasks. The material for each subtask was a single sheet of grey paper upon which 100 words or solid colour patches were printed (in random order). For the first subtask, the printed words pertained to four colours (blue, green, red, yellow) and were printed in black ink. Participants had to read the words aloud as quickly as possible. In the second subtask, participants saw solid coloured patches (either blue, green, red or yellow) and were asked to name the colour. In the third subtask, the printed words pertained to the same four colours, but were printed in an incongruent colour (e.g., the word ‘blue’ printed in yellow ink). The total time in seconds taken to complete each of the three subtasks were measured with a stopwatch. The difference between the congruent (first two) subtasks and incongruent (last) subtask is thought to be a measure of cognitive control, with high scores indicating more interference and therefore lower cognitive control.
2.4. Cannabis and alcohol Stroop
The paper version of the cannabis Stroop task (Cousijn et al., 2013a) was used to measure attentional bias for cannabis-related words. A total of 14 words related to cannabis were matched for length, number of syllabi and frequency to 14 neutral words related to office stationary. The task consisted of a cannabis and neutral subtask. Each task contained a sheet of paper with either the cannabis or neutral words printed on it four times in different ink colours (blue, red, yellow, blue) in random order. For each subtask participants had to read aloud the colour of the words as fast as possible. Total time in seconds needed to complete each subtask was recorded with a stopwatch. The order of the subtasks was counterbalanced across participants and the time taken to complete each subtask was recorded. The paper version of the alcohol Stroop was used to measure the attentional bias for alcohol-related words. The alcohol Stroop task included 14 words related to alcohol, matched for length, number of syllabi and frequency to 14 neutral words related to office stationary. The alcohol Stroop task procedure was identical to the cannabis Stroop procedure.
2.5. Cannabis-Alcohol Approach Avoidance Task (CA-AAT)
The computerized Cannabis-Alcohol Approach Avoidance Task (CA-AAT; adapted from the cannabis AAT, see Cousijn et al., 2011, Cousijn et al., 2013c) was used to measure approach-bias towards cannabis and alcohol-related stimuli. It consisted of one cannabis and one alcohol block, of which the order was counterbalanced over participants. Heavy cannabis and alcohol use are often comorbid, approach and avoidance action tendencies were therefore measured towards both cannabis and alcohol. The cannabis block contained 12 different cannabis-related images (i.e., objects associated with cannabis use or someone using cannabis), whereas the alcohol block contained 12 different alcohol related images (i.e., glass of beer or someone drinking beer). Across the two blocks, two control image categories were presented: 12 neutral images (i.e., stationeries) and 12 appetitive images (i.e., water or someone drinking water). The images were rotated 3° to the left or right, and participants had to pull (approach) or push (avoid) a joystick in response to the rotation direction, as fast as possible. Half of the participants had to push images rotated to the left, and pull images rotated to the right, while the other half of the participants were given opposite instructions. Pulling gradually increased image-size, whereas pushing decreased it (zooming feature). Each image was presented twice in pull and twice in push format, resulting in 98 trials per block.
2.6. Treatment evaluation at 6 months follow-up
The therapist evaluated the patients’ treatment progress after 6 months and reported in the Electronic Patient Record (EPD) whether the patient had successfully completed treatment, was still in treatment and had stopped or reduced his/her cannabis use, was still in treatment but had not stopped his/her cannabis use, had relapsed or had quitted treatment against the advice of and/or without informing the therapist. This information was categorized into a dichotomous outcome variable. Successful treatment completion and abstinence or reduced cannabis use were defined as “treatment progression”. No reduction in cannabis use, relapse in cannabis use or quitting treatment against the advice of or without informing the therapist were defined as “no treatment progression”.
2.7. Procedure
Data collection took place between April 2012 and March 2014. All patients who entered the youth department of Brijder Addiction Care received an information leaflet about the study. Patients who were assigned to the outpatient treatment programme, who had a primary or secondary CUD and did not have any other SUD were invited by the therapist to take part in the study. After eligible patients had provided written informed consent (and their parents/guardian as well if participants were younger than 18 years), a research assistant contacted them to schedule the test session. Test sessions took place during office hours at the addiction care facility and were usually planned immediately before or after the therapy session. Although we aimed for conducting the test-sessions shortly after the start of the treatment programme, this was not always feasible due to numerous cancellations or no-shows by patients. Hence, in practice test sessions took place at various moments during the course of treatment. Time between actual start of treatment and the test session was on average 75 days (range between −88 and 225 days) and 4 participants completed the test session before treatment onset. During the test-session, participants first indicated their level of cannabis, alcohol and cigarette craving. Next, participants performed the CA-AAT, followed by the Cannabis and Alcohol Stroop. Order of the Cannabis and Alcohol Stroop was counterbalanced over participants. The Classical Stroop was always performed after the Cannabis and Alcohol Stroop to prevent practice effects to carry over to the cannabis and alcohol attentional bias measures (Cox et al., 2006). Finally, participants filled out the TLFB, after which the participants was debriefed and thanked.
ROM assessments including the CUDIT, AUDIT, FTND, BDI and information on cannabis use in the past 30 days took place at the start and at 6-month follow-up. Participants’ treatment progress at 6-month follow-up were retrieved from EPD at the end of the data collection.
2.8. Data preparation and statistical analyses
For the Classical Stroop, the mean time required to complete the first two (congruent) subtasks was subtracted from the time taken to complete the third (incongruent subtask). The attentional bias for cannabis and alcohol was separately calculated by subtracting time taken to name the colour of the cannabis or alcohol words from the time taken to name the colour of the neural words. A positive score therefore indicates an attentional bias for cannabis or alcohol words. Reaction time data in the CA-AAT were corrected for outliers by removing reaction times below 200 ms, above 2000 ms, and reacting times deviating more than 3 standard deviations from the individual mean per-participant. Error trials were also removed. For each participant, cannabis, alcohol, appetitive, and neutral bias-scores were calculated by subtracting the mean approach reaction time from the mean avoid reaction time for that specific picture category. This way a positive score indicated faster approach compared to avoidance (approach-bias). Reliability of the CA-AAT was investigated by calculating Cronbach's alpha for each bias-score with the individual bias-scores per picture. Internal reliability of the cannabis bias (12 items, Cronbach's α = 0.25), alcohol bias (12 items, Cronbach's α = 0.45), neutral bias (12 items, Cronbach's α = 0.60), and appetitive bias (12 items, Cronbach's α = 0.50) was fairly poor, but not unusual for reaction time tests (Ataya et al., 2012).
To test if adolescents with a CUD have an attentional bias and approach bias towards cannabis and alcohol stimuli, one-sample t-tests were computed (testing against zero). Moreover, to investigate if the cannabis attentional bias deviated from the alcohol attentional bias, a paired t-test was performed. To investigate if the cannabis approach bias deviated from the alcohol approach, appetitive and neutral approach bias, a repeated measures ANOVA was performed with bias type (i.e., cannabis, alcohol, appetitive, neutral) as within subject factor. Correlational analyses were used to investigate the univariate association between attentional biases, approach biases, craving, and Classical Stroop performance and the substance use measures. Moreover, linear regression analyses were used to investigate if cognitive control moderated the association between motivational measures and cannabis use. With CUDIT scores as the dependent variable, the motivational measure (either cannabis attentional bias, cannabis approach bias corrected for the appetitive approach bias or craving), Classical Stroop performance and the interaction term between the motivational measure and Classical Stroop performance were entered in the model. These analyses were repeated a second time with cannabis use during the past 2 weeks (number of joints) as dependent variable.
The predictive value of cognitive control and motivational measures for patients’ treatment outcomes at six months follow-up was subsequently examined by conducting hierarchical linear and logistic regression analyses. To comprehensively capture progress after treatment, it was assessed at three levels: (1) CUDIT-scores from the ROM at six-month follow-up (continuously measured), (2) cannabis use in the past 30 days (yes/no) from the ROM, and (3) treatment progress reported by the therapists at six-month follow-up (yes/no). Given the very skewed distribution of cannabis use in the past 30 days at follow-up (50% used cannabis on less than two occasions), this variable was dichotomized into yes/no. In a hierarchical linear regression analysis, CUDIT at 6-month follow-up was predicted by CUDIT at baseline (Step 1), motivational measures (i.e., cannabis attentional bias, approach bias, and craving; Step 2) and cognitive control (i.e., Classical Stroop performance; Step 3). In addition, hierarchical logistic regression analyses were performed predicting either patients’ evaluated treatment progress at 6-month follow-up (0 = no treatment progress and 1 = treatment progress) or cannabis abstinence over past 30 days (0 = abstinent and 1 = not abstinent) at six-month follow-up by the CUDIT at baseline (Step 1), motivational measures (Step 2) and cognitive control (Step 3).
3. Results
3.1. Attentional bias and approach bias at baseline
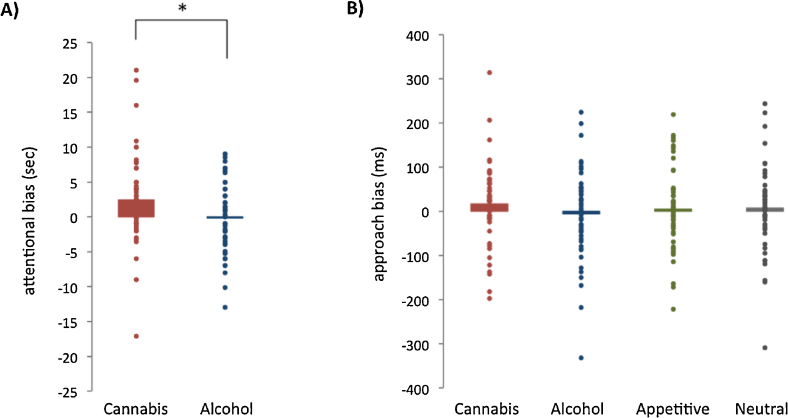
The CUD adolescents displayed a significant attentional bias for cannabis words (t56 = 2.94, p = 0.005, d = 0.38), not for alcohol words (t56 = 0.26, p = 0.79, d = 0.04). This cannabis bias-score was significantly larger than the alcohol bias-score (t56 = 2.74, p = 0.008, d = 0.36, mean difference = 2.59 s, see Fig. 1A). Regarding the CA-AAT, data from three participants was missing due to a technical error. The remaining sample of 54 CUD adolescents did not display a significant approach bias for cannabis (t53 = 1.37, p = 0.18, d = 0.19), alcohol (t53 = 0.45, p = 0.65, d = 0.06), appetitive (t53 = 0.39, p = 0.70, d = 0.05), and neutral pictures (t53 = 0.56, p = 0.58, d = 0.08). Moreover, the approach bias-scores did not significantly differ from each other (F3,51 = 1.10, p = 0.36, see Fig. 1B).
Fig. 1.
Attentional and approach biases in adolescents with a cannabis use disorder. (A) Attentional bias (in seconds) for cannabis words significantly differed from attentional bias for alcohol words (p = 0.008). A positive score indicates that participants were slower in naming the colour of substance-related words compared to neutral words. (B) Approach bias (in milliseconds) for cannabis, alcohol, appetitive and neutral pictures did not significantly differ. A positive score indicates that participants were faster in pulling (approach) compared to pushing (avoidance) the joystick for that picture category. Average (bar) and individual (dots) bias scores are depicted in both figures.
3.2. Univariate association between motivational processes, cognitive control and cannabis use at baseline
The attentional bias score for cannabis and alcohol were not related (r = 0.19, p = 0.16), whereas the approach bias scores for cannabis, alcohol, appetitive, and neutral stimuli were all significantly related (all rs > 0.33, all ps < 0.015). Craving for cannabis was not significantly related to craving for alcohol (r = 0.03, p = 0.85) and nicotine (r = 0.01, p = 0.92). Regarding the relationship between different cannabis-related motivational measures, the attentional bias, approach bias, and craving were not significantly related (attentional bias – approach bias: r = 0.24, p = 0.08; attentional bias – craving: r = 0.01, p = 0.98; approach bias – craving: r = −0.01, p = 0.96). Similarly, the different alcohol-related motivational measures were not significantly related (attentional bias – approach bias: r = 0.01, p = 0.93; attentional bias – craving: r = 0.12, p = 0.38; approach bias – craving: r = 0.11, p = 0.43). The Classical Stroop performance was not significantly related to any of the substance-related motivational measures (i.e., attentional bias, approach bias, craving; r < 0.22, p > 0.10).
Next, correlations between substance-related motivational measures, Classical Stroop performance, and substance use measures were computed. Regarding the alcohol and cannabis approach bias, given the shared variance between the different AAT measures, we controlled for general appetitive approach and avoidance action tendencies by performing partial correlations and regression analyses with the appetitive bias score as covariate in all subsequent analyses (see also Cousijn et al., 2011). Attentional bias for cannabis and alcohol did not significantly correlate with any of the substance use measures (all rs < 0.20, all ps > 0.10). The approach bias for cannabis and alcohol (corrected for the appetitive bias) also did not significantly correlate with any of the substance use measures (all rs < 0.26, all ps > 0.06). In contrast, cannabis craving significantly correlated with number of joints smoked during the last two weeks (r = 0.36, p = 0.006), alcohol craving significantly correlated with number of drinks during the last two weeks (r = 0.33, p = 0.01) and cigarette craving significantly correlated with the FTND (r = 0.49, p < 0.001). Moreover, Classical Stroop performance significantly correlated with number of drinks during the last two weeks (r = 0.27, p = 0.04). All other correlations were non-significant.
3.3. Cognitive control as moderator between motivational processes and substance use at baseline
Cognitive control as indexed by Classical Stroop performance did not significantly moderate the associations between any of the motivational measures and cannabis use related problems (CUDIT) or cannabis use during the past two weeks.
3.4. Prediction of treatment progress at follow-up1
As indicators of treatment progress, we used the CUDIT-scores and cannabis use in the past 30 days from the ROM at six-month follow-up (n = 32), and treatment progress reported by the therapists at six-month follow-up (n = 55). A Little's Missing Completely At Random test (MCAR; Little, 1988) with all the study variables included in the regression analyses below indicated that the individuals who did not complete the follow-ups were missing at random (X2 = 29.1, df = 23, p = 0.18). Moreover, t-test comparing completers with non-completed revealed no significant differences between these groups on any of these variables (all ps > 0.11). CUDIT scores significantly decreased (t33 = 4.08, p < 0.001) over time, indicating a decrease in cannabis use related problems over the course of treatment. Furthermore, at six-month follow-up 35.4% had not used cannabis in the past 30 days and 63.6% received a positive evaluation regarding treatment progress.
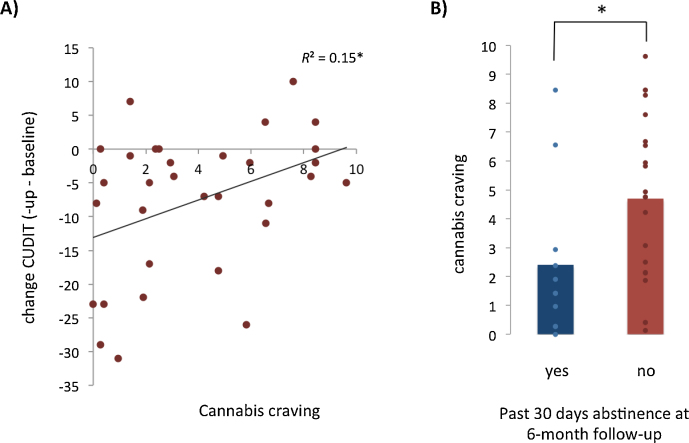
Results of the hierarchical linear regression analysis testing the predictive value of cannabis-related motivational processes and cognitive control on CUDIT scores at 6-month follow-up, while controlling for CUDIT at baseline, are depicted in Table 2. Preliminary analyses indicated no violation of the assumption of normality, linearity, multicollinearity and homoscedasticity (maximum Cook's distance = 0.20, maximum standardized residual = 2.68). The final model explained 32% of the variance in CUDIT scores at follow-up (F6,24 = 1.87, p = 0.13), with a significant contribution of cannabis craving only (p = 0.01). The positive association between cannabis craving and CUDIT at follow-up implied that higher levels of craving predicted less decrease in cannabis use-related problems at 6-month follow-up. Fig. 2A depicts the positive univariate association between cannabis craving and change in CUDIT scores (R2 = 0.15, p = 0.026). Cannabis attentional bias, cannabis approach bias and cognitive control were not significantly predictive of CUDIT at 6-month follow-up (see Table 2).
Table 2.
Hierarchical linear regression analysis predicting CUDIT at follow-up by motivational and control processes during the baseline test-session.
| CUDIT at 6-month follow-up (n = 31) |
|||||
|---|---|---|---|---|---|
| B | SE B | β | p-Value | R2 change | |
| Step 1 | |||||
| CUDIT baseline | 0.26 | 0.19 | 0.24 | 0.19 | 0.06 |
| Step 2 | |||||
| Cannabis attentional bias | −0.14 | 0.22 | −0.10 | 0.54 | 0.26† |
| Cannabis approach bias | 0.01 | 0.02 | 0.08 | 0.39 | |
| Appetitive approach bias | 0.02 | 0.02 | 0.15 | 0.46 | |
| Cannabis craving | 1.39 | 0.51 | 0.47 | 0.01 | |
| Step 3 | |||||
| Classical Stroop | −0.04 | 0.12 | −0.05 | 0.76 | 0.10 |
CUDIT; Cannabis Use Disorder Identification Test. Significant effect are depicted in bold.
p = 0.08 for R2-change.
Fig. 2.
Association cannabis craving and cannabis use at 6-month follow-up. (A) Higher cannabis craving was associated with less change in cannabis use-related problems 6 months after the start of treatment, as measured with the Cannabis Use Disorder Identification Test (p = 0.026). (B) Cannabis craving was significantly higher in patients that used cannabis during the past 30 days at 6 month follow-up (p = 0.044). Average (bar) and individual (dot) craving scores are depicted.
Results of the hierarchical logistic regression analyses predicting treatment progress at 6-month follow-up are presented in Table 3. As shown, the first model including only CUDIT at baseline was statistically significant (χ21 = 5.89, p = 0.015). Higher levels of CUDIT at baseline were associated with a decreased likelihood of treatment progress at 6-month follow-up (B = −0.10, p = 0.027; OR = 0.90). Adding motivational measures as predictors (Model 2) increased the explained variance from 18% to 36% (Nagelkerke R2) but reduced the number of correctly classified cases from 77.3% to 75%. Both baseline CUDIT and cannabis craving showed marginally significant associations with treatment progress (p < 0.10). The cannabis attentional and approach bias were not significantly associated with treatment progress. Adding the Classical Stroop performance as a predictor (Model 3) only slightly increased the explained variance (38%) and number of correctly classified cases (79.5%). However, Classical Stroop performance was not significantly associated with treatment progress and all other associations remained similar to those found in Model 2. Finally, Table 4 presents the results of the hierarchical logistic regression analyses predicting cannabis abstinence at 6-month follow-up. As can be seen, including baseline CUDIT as predictor (Model 1) did not result in a significant model compared to the constant-only model (χ21 = 0.51, ns). Adding motivational measures in the second step (Model 2) resulted in a marginally significant model (χ25 = 9.30, p = 0.10) increasing the explained variance from 2% to 36% and the number of correctly predicted cases from 64.5% to 80.6%. Cannabis craving was significantly associated with abstinence at 6-month follow-up (B = 0.43, p = 0.023; OR = 1.53) indicating that higher levels of cannabis craving were associated with a decreased likelihood of cannabis abstinence in the past 30 days at 6-month follow-up. Indeed, a post hoc t-test showed that craving was significantly higher in patients that were using cannabis at 6-month follow-up (t30 = 2.10, p = 0.044, see Fig. 2B). Including Classical Stroop performance as an additional predictor (Model 3) did not increase the explained variance nor the number of correctly predicted cases. Moreover, Classical Stroop performance was not significantly related to cannabis abstention at 6-month follow-up and all other associations remained similar to those found in the previous model.
Table 3.
Logistic regression analyses predicting treatment progress at 6-month follow-up by motivational and control processes during the baseline test-session.
| Treatment progress (0 = no; 1 = yes) at 6-month follow-up (n = 44) |
|||||||||
|---|---|---|---|---|---|---|---|---|---|
| Model 1 |
Model 2 |
Model 3 |
|||||||
| B | Exp (B) | 95% CI | B | Exp (B) | 95% CI | B | Exp (B) | 95% CI | |
| Constant | 3.16** | – | – | 3.80** | – | – | 5.13* | – | – |
| CUDIT baseline | −0.10* | 0.90 | 0.82–0.99 | −0.10† | 0.91 | 0.82–1.00 | −0.09† | 0.90 | 0.83–1.01 |
| Cannabis attentional bias | 0.11 | 1.12 | 0.95–1.31 | 0.11 | 1.12 | 0.95–1.31 | |||
| Cannabis approach bias | 0.00 | 1.00 | 0.99–1.01 | 0.02 | 1.00 | 0.99–101 | |||
| Appetitive approach bias | −0.01 | 0.99 | 0.98–1.00 | −0.01 | 0.99 | 0.98–1.00 | |||
| Cannabis craving | −0.23† | 0.79 | 0.60–1.04 | −0.26† | 0.77 | 0.58–1.02 | |||
| Classical Stroop | −0.04 | 0.96 | 0.90–1.03 | ||||||
| Model chi-square [df] | 5.89* [1] | 12.88* [5] | 14.08* [6] | ||||||
| Block chi-square [df] | 5.89* [1] | 6.00 [4] | 1.20 [1] | ||||||
| % Correct predictions | 77.3 | 75.0 | 79.5 | ||||||
| Nagelkerke R2 | 0.18 | 0.36 | 0.38 | ||||||
p < 0.01.
p < 0.05.
p < 0.10.
Table 4.
Logistic regression analyses predicting abstention from cannabis use in past 30 days at 6-month follow-up by motivational and control processes during the baseline test-session.
| Abstention in past 30 days (0 = yes; 1 = no) at 6-month follow-up (n = 44) |
|||||||||
|---|---|---|---|---|---|---|---|---|---|
| Model 1 |
Model 2 |
Model 3 |
|||||||
| B | Exp (B) | 95% CI | B | Exp (B) | 95% CI | B | Exp (B) | 95% CI | |
| Constant | −0.04 | – | – | −1.67 | – | – | −1.67 | – | – |
| CUDIT baseline | 0.03 | 1.03 | 0.94–1.13 | 0.04 | 1.04 | 0.94–1.16 | 0.04 | 1.04 | 0.94–1.16 |
| Cannabis attentional bias | 0.06 | 1.06 | 0.93–1.21 | 0.06 | 1.06 | 0.93–1.21 | |||
| Cannabis approach bias | −0.01 | 0.99 | 0.98–1.00 | −0.01 | 0.99 | 0.98–1.00 | |||
| Appetitive approach bias | 0.01 | 1.01 | 1.00–1.02 | 0.01 | 1.01 | 1.00–1.02 | |||
| Cannabis craving | 0.43* | 1.53 | 1.06–2.21 | 0.43* | 1.53 | 1.06–2.21 | |||
| Classical Stroop | −0.00 | 1.00 | 0.93–1.07 | ||||||
| Model chi-square [df] | 0.51 [1] | 9.30† [5] | 9.31 [6] | ||||||
| Block chi-square [df] | 0.51 [1] | 8.79† [4] | 0.00 [1] | ||||||
| % correct predictions | 64.5 | 80.6 | 80.6 | ||||||
| Nagelkerke R2 | 0.02 | 0.36 | 0.36 | ||||||
p < 0.05.
p < 0.10.
4. Discussion
We investigated the role of cognitive control and cannabis-oriented motivational processes in the course of cannabis use and cannabis use-related problems in adolescent patients with a primary or secondary CUD. Adolescents with a CUD had an attentional but no approach bias towards cannabis. Only cannabis craving was consistently associated with current and future levels of cannabis use, contrasting adult findings on the role of attentional and approach bias in cannabis use (Cousijn et al., 2011, Cousijn et al., 2013a). Cannabis craving significantly correlated with cannabis use during the past two weeks and significantly predicted cannabis use-related problems and abstinence from cannabis 6 months later. In contrast to our hypothesis, cognitive control did not significantly moderate the relationship between motivational processes and amount of cannabis use and severity of cannabis-related problems, nor did it predict treatment progress at 6-month follow-up.
Higher craving at the start of the test-session related to higher cannabis use in the past two weeks prior to the test session. Cannabis use-related problems as measured with the CUDIT significantly decreased during treatment and higher craving predicted less change in problems over the course of 6 months. Few studies investigated the role of motivational processes like craving in adolescent cannabis users in treatment for a CUD. Nonetheless, our findings are in line with another study in adolescents with a CUD that showed that cannabis withdrawal symptoms including craving predicted rapid relapse after the start of treatment (Cornelius et al., 2008) and severity of cannabis use-related problems at one year follow-up (Chung et al., 2008). Craving reflects a powerful urge to use and it plays an important role in the course of substance use and dependence (Verdejo-Garcia et al., 2012). Similarly as in other SUDs, cannabis craving in young adults is associated with functioning of fronto-limbic brain areas, including the striatum, orbitofrontal cortex and anterior cingulate cortex (Cousijn et al., 2013d, Filbey et al., 2009). Moreover, subjective craving correlated negatively with activity in the dorsolateral prefrontal cortex (central area in cognitive control) activity when young adult cannabis users viewed cannabis versus neutral pictures (Cousijn et al., 2013d). The current findings suggest that craving plays an equally important role in adolescent CUDs compared to adult CUDs and other SUDs, implicating a crucial role for the aforementioned brain areas in the course of adolescent CUDs. One could argue, however, that substance-related brain changes are accelerated in adolescents compared to adults (Chambers et al., 2014). Adolescence is marked by an increase in reward sensitivity, protracted development of cognitive control and high flexibility to learn from and adapt to changing environments (Crone and Dahl, 2012). Adolescent onset of SUDs are associated with more severe clinical outcomes (Meier et al., 2012, Perkonigg et al., 2008, Stinson et al., 2006, Swift et al., 2001). Substance use and other life experiences, but also interventions, may be especially prone to induce (long-lasting) brain changes in adolescents compared to adults (Gulley and Juraska, 2013). From a clinical perspective it is therefore important to investigate the neurocognitive mechanisms underlying CUDs in adolescents compared to adults. To the best of our knowledge there are no studies directly comparing the mechanisms underlying craving in adolescents compared to adults. To enhance fundamental knowledge as well as treatment and prevention, these are important issues to be considered in future studies.
Higher baseline cannabis use-related problems were associated with a decreased likelihood of treatment progress at 6-month follow-up. A post hoc t-test indicated that those who made progress had lower problems at follow-up (t30 = 2.28, p = 0.03) than those who did not (t30 = 2.28, p = 0.03). This implies that initial problem level is a good indicator of the remaining problems after treatment. Counterintuitively, baseline problems did not significantly predict follow-up problems. This may be a power issue as there were fewer participants included in the latter analyses. Careful inspection of the CUDIT scores over time does suggest that higher CUDIT scores at baseline relate to higher CUDIT scores at follow-up. Yet, about seven initially high scoring participants showed a strong decrease towards almost no use-related problem. Speculatively, there may be different groups in treatment response, warranting studies with large sample sizes.
In line with previous findings in adults (Asmaro et al., 2014, Cousijn et al., 2013a, Field, 2005, Field et al., 2004, Field et al., 2006), adolescents with a CUD displayed an attentional bias towards cannabis-related words but not towards alcohol-related words during the cannabis and alcohol Stroop. A clear limitation of the current study is the lack of a control group. However, the cannabis bias was significantly higher than the alcohol bias, suggesting a cannabis specific attentional bias. Two previous studies showed that adult dependent users had a stronger attentional bias than non-dependent users (Cousijn et al., 2013a, Field, 2005). In the current study, the attentional bias was not significantly correlated with cannabis use, problems or craving. Even though various studies reported a positive association between attentional bias and measures of cannabis use, the results appear inconsistent. Including the current study, 2 out of 5 studies reported a correlation with craving (Field, 2005, Field et al., 2004), 1 out of 5 reported a correlation with cannabis use-related problems (Cousijn et al., 2013a) and 1 out of 5 reported a correlation with frequency of cannabis use (Field et al., 2004). These discrepant findings may relate to differences in sample characteristics, the severity of cannabis use-related problems or power issues. In sum, both adults and adolescents with a CUD appear to have an attentional bias towards cannabis. Further studies should elucidate the clinical value of the attentional bias on the group as well as on the individual level.
The current sample of adolescents in treatment for a CUD did not display an approach bias towards cannabis. Thus far, the approach bias has only been observed in heavy cannabis users with moderate cannabis use-related problems (Cousijn et al., 2011, Field et al., 2006). Within heavy users, the approach bias predicted changes in cannabis use six months later (Cousijn et al., 2011). One could speculate that the approach bias may only be evident in early stages of cannabis abuse, predicting escalation of cannabis use rather than problem severity. This is in line with addiction models that suggest that motivational processes, like attentional and approach bias may play a role when substance use is still under voluntary control (Di Chiara, 2000, Everitt and Robbins, 2005). Yet, craving, but not cognitive control, predicted treatment progress, contradicting these same models. Moreover, approach bias retraining is capable to improve treatment outcome in adults with alcohol addiction (Eberl et al., 2013, Wiers et al., 2011), supporting an important role for the approach bias during compulsive substance use as well. Alternatively, the relatively poor (but similar to other studies; Cousijn et al., 2011, Cousijn et al., 2013c, Cousijn et al., 2014b) reliability of the CA-AAT may explain the lack of significant approach bias effect. Previous AAT studies showed substantial evidence of the validity of the approach bias as a measure related to substance use and dependence; it has been found to correlate with substance use (e.g., Sharbanee et al., 2013), predict future substance use (e.g., Cousijn et al., 2011) and retraining improves treatment outcome (Eberl et al., 2013, Wiers et al., 2011). Yet, the generally poor reliability is a limitation of the measure and an important point of discussion (see also Ataya et al., 2012, Field and Christiansen, 2012). The use of relatively complex visual scenes of different lexical and visual categories potentially explains the poor reliability (Ataya et al., 2012). The promising initial studies on approach bias in relationship to substance dependence but the poor reliability of the task warrant the development of more reliable approach bias measures.
The relationship between cognitive control and cannabis use is thought to be bidirectional: Chronic cannabis use may impair cognitive control, but relative poor levels of cognitive control may also predispose individuals to CUDs (Cousijn et al., 2013b, Cousijn et al., 2014a; Crean et al., 2011). A recent study in adult treatment seeking cannabis users compared to healthy matched controls showed that lower brain activations during the Stroop task in brain areas involved in the regulation of behaviour (anterior cingulate cortex) and reward processing (ventral striatum) predicted more cannabis use one year later (Kober et al., 2014). Moreover, functioning of the brain network involved in cognitive control during a working memory task predicted cannabis use six months later in non-treatment seeking adult heavy cannabis users (Cousijn et al., 2014a, Cousijn et al., 2014b). Similar findings for a link between neural activity during tasks assessing cognitive control and drug use or treatment outcomes at follow-up have been shown in cocaine-dependent patients (Marhe et al., 2013, Mitchell et al., 2013). In the current study, cognitive control as measured with the Classical Stroop task was not associated with self-reported cannabis use at baseline and follow-up and treatment progression. Due to the lack of an age-matched control group, we cannot infer whether cognitive control was actually impaired in our patients. Yet, similarly as in two previous studies from our lab (Cousijn et al., 2013a, Cousijn et al., 2013c), cognitive control did not moderate relations between cannabis-oriented motivational processes and cannabis use. This contrasts behavioural studies in drinkers and smokers (Grenard et al., 2008, Thush et al., 2008). Neural indexes of cognitive control may therefore be more indicative of the course of cannabis use than behavioural indexes. Yet, a direct comparison between the predictive power of these two indexes of behavioural control in cannabis dependent individuals is currently missing.
The prospective design of our study is a strength, however, some limitations must be considered. First, the varying time differences between the ROM assessments including the CUDIT and the cognitive assessments may have influenced our results. Conducting a study in outpatient adolescents with a CUD proved to be very difficult. The number of no-shows resulted in a suboptimal timing of the test session with regard to the baseline and 6-month follow-up ROM assessments. Second, the number of treatment sessions before the test session varied. Craving, attentional bias and approach bias may differ within individuals depending of factors like the context of the test session, current concerns and current satiation levels (Cousijn et al., 2013c, Cousijn et al., 2014b, Field et al., 2009, Watson et al., 2012). Also, varying durations of abstinence during the cognitive measurements could have influenced our results. That is, cognitive deficits seen in cannabis users appear to (almost fully) improve after abstinence, also in adolescent samples (Schulte et al., 2014). In addition, cannabis intoxication slows down cannabis avoidance (Cousijn et al., 2013c) and reduces cognitive control (Crean et al., 2011). To minimize potential effects of abstinence, context, and treatment progress on the cognitive measurements, future studies could benefit from including precisely timed multiple assessments over the course of treatment (including a pre-treatment assessment) and more objective measures of cannabis exposure. Third, as discussed above, the reliability and validity of the CA-AAT remains an important issue that should addressed in future studies. Fourth, the current study included a relatively small sample of adolescents with a CUD only. Comorbid SUDs are common in CUDs and may be as high as 50% (Stinson et al., 2006). Our findings may not generalize to all adolescents with a CUD. Finally, given the current sample size, we dichotomized treatment progress as evaluated by the therapist. Ideally, further studies should include a better operationalized assessment of treatment progress by the therapist, with clear stages of recovery.
In conclusion, cannabis craving, but not attentional bias, approach bias and cognitive control, significantly correlated with current cannabis use and predicted cannabis use-related problems and abstinence from cannabis 6 months later in adolescent patient with a CUD. These findings support an important role for craving in the course of adolescent cannabis use and dependence. This prospective study is among the first to investigate neuropsychological mechanisms underlying cannabis use in adolescents in treatment for a CUDs, warranting future longitudinal studies in large samples of cannabis dependent adolescents compared to matched controls.
Conflict of interest statement
All authors report no conflict of interest.
Acknowledgements
This study was conducted without external funding. We like to thank Daan Siderius, Marcel Corbijn, and Jessica Ziebula for their help with the data collection. Moreover, we like to thank the mental health practitioners of the youth department of Brijder Addiction Care for facilitating patient inclusions.
Footnotes
All analyses were run a second time with time between start of treatment and the ROM assessments as additional covariate, a third time including only those participants that completed all assessments (n = 31), and a fourth time including age and gender as additional covariates. All results and interpretations remain similar.
References
- Adamson S.J., Sellman J.D. A prototype screening instrument for cannabis use disorder: the Cannabis Use Disorders Identification Test (CUDIT) in an alcohol-dependent clinical sample. Drug Alcohol Rev. 2003;22:309–315. doi: 10.1080/0959523031000154454. [DOI] [PubMed] [Google Scholar]
- Agosti V., Levin F.R. Predictors of treatment contact among individuals with cannabis dependence. Am. J. Drug Alcohol Abuse. 2004;30:121–127. doi: 10.1081/ada-120029869. [DOI] [PubMed] [Google Scholar]
- Asmaro D., Carolan P.L., Liotti M. Electrophysiological evidence of early attentional bias to drug-related pictures in chronic cannabis users. Addict. Behav. 2014;39:114–121. doi: 10.1016/j.addbeh.2013.09.012. [DOI] [PubMed] [Google Scholar]
- Ataya A.F., Adams S., Mullings E., Cooper R.M., Attwood A.S., Munafo M.R. Internal reliability of measures of substance-related cognitive bias. Drug Alcohol Depend. 2012;121:148–151. doi: 10.1016/j.drugalcdep.2011.08.023. [DOI] [PubMed] [Google Scholar]
- Beck A.T., Steer R.A., Ball R., Ranieri W. Comparison of Beck Depression Inventories-IA and -II in psychiatric outpatients. J. Pers. Assess. 1996;67:588–597. doi: 10.1207/s15327752jpa6703_13. [DOI] [PubMed] [Google Scholar]
- Budney A.J., Vandrey R.G., Hughes J.R., Thostenson J.D., Bursac Z. Comparison of cannabis and tobacco withdrawal: severity and contribution to relapse. J. Subst. Abuse Treat. 2008;35:362–368. doi: 10.1016/j.jsat.2008.01.002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chambers R.A., Taylor J.R., Potenza M.N. Developmental neurocircuitry of motivation in adolescence: a critical period of addiction vulnerability. Am. J. Psychiatry. 2014;6:1041–1052. doi: 10.1176/appi.ajp.160.6.1041. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chauchard E., Septfons A., Chabrol H. Motivations for cannabis cessation, coping and adaptation strategies, and perceived benefits: impact on cannabis use relapse and abstinence. Encephale. 2013;39:385–392. doi: 10.1016/j.encep.2013.03.008. [DOI] [PubMed] [Google Scholar]
- Chung T., Martin C.S., Cornelius J.R., Clark D.B. Cannabis withdrawal predicts severity of cannabis involvement at 1-year follow-up among treated adolescents. Addiction. 2008;103:787–799. doi: 10.1111/j.1360-0443.2008.02158.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cornelius J.R., Chung T., Martin C., Wood D.S., Clark D.B. Cannabis withdrawal is common among treatment-seeking adolescents with cannabis dependence and major depression, and is associated with rapid relapse to dependence. Addict. Behav. 2008;33:1500–1505. doi: 10.1016/j.addbeh.2008.02.001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cousijn J., Goudriaan A.E., Wiers R.W. Reaching out towards cannabis: approach-bias in heavy cannabis users predicts changes in cannabis use. Addiction. 2011;106:1667–1674. doi: 10.1111/j.1360-0443.2011.03475.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cousijn J., Watson P., Koenders L., Vingerhoets W.A., Goudriaan A.E., Wiers R.W. Cannabis dependence, cognitive control and attentional bias for cannabis words. Addict. Behav. 2013;38:2825–2832. doi: 10.1016/j.addbeh.2013.08.011. [DOI] [PubMed] [Google Scholar]
- Cousijn J., Wiers R.W., Ridderinkhof K.R., van den Brink W., Veltman D.J., Porrino L.J. Individual differences in decision making and reward processing predict changes in cannabis use: a prospective functional magnetic resonance imaging study. Addict. Biol. 2013;18:1013–1023. doi: 10.1111/j.1369-1600.2012.00498.x. [DOI] [PubMed] [Google Scholar]
- Cousijn J., Snoek R.W., Wiers R.W. Cannabis intoxication inhibits avoidance action tendencies: a field study in the Amsterdam coffee shops. Psychopharmacology (Berl.) 2013;229:167–176. doi: 10.1007/s00213-013-3097-6. [DOI] [PubMed] [Google Scholar]
- Cousijn J., Goudriaan A.E., Ridderinkhof K.R., van Den Brink W., Veltman D.J., Wiers R.W. Neural responses associated with cue-reactivity in frequent cannabis users. Addict. Biol. 2013;18:570–580. doi: 10.1111/j.1369-1600.2011.00417.x. [DOI] [PubMed] [Google Scholar]
- Cousijn J., Wiers R.W., Ridderinkhof K.R., van den Brink W., Veltman D.J., Goudriaan A.E. Effect of baseline cannabis use and working-memory network function on changes in cannabis use in heavy cannabis users: a prospective fMRI study. Hum. Brain Mapp. 2014;35:2470–2482. doi: 10.1002/hbm.22342. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cousijn J., Luijten M., Wiers R.W. Mechanisms underlying alcohol-approach action tendencies: the role of emotional primes and drinking motives. Front. Psychiatry. 2014;5:44. doi: 10.3389/fpsyt.2014.00044. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cox W.M., Fadardi J.S., Pothos E.M. The addiction-Stroop test: theoretical considerations and procedural recommendations. Psychol. Bull. 2006;132:443–476. doi: 10.1037/0033-2909.132.3.443. [DOI] [PubMed] [Google Scholar]
- Crean R.D., Crane N.A., Mason B.J. An evidence based review of acute and long-term effects of cannabis use on executive cognitive functions. J. Addict Med. 2011;5:1–8. doi: 10.1097/ADM.0b013e31820c23fa. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Crone E.A., Dahl R.E. Understanding adolescence as a period of social-affective engagement and goal flexibility. Nat. Rev. Neurosci. 2012;13:636–650. doi: 10.1038/nrn3313. [DOI] [PubMed] [Google Scholar]
- Di Chiara G. Role of dopamine in the behavioural actions of nicotine related to addiction. Eur. J. Pharmacol. 2000;393:295–314. doi: 10.1016/s0014-2999(00)00122-9. [DOI] [PubMed] [Google Scholar]
- Dougherty D.M., Mathias C.W., Dawes M.A., Furr R.M., Charles N.E., Liguori A. Impulsivity, attention, memory, and decision-making among adolescent marijuana users. Psychopharmacology (Berl.) 2013;226:307–319. doi: 10.1007/s00213-012-2908-5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Eberl C., Wiers R.W., Pawelczack S., Rinck M., Becker E.S., Lindenmeyer J. Approach bias modification in alcohol dependence: do clinical effects replicate and for whom does it work best? Dev. Cogn. Neurosci. 2013;4:38–51. doi: 10.1016/j.dcn.2012.11.002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Everitt B.J., Robbins T.W. Neural systems of reinforcement for drug addiction: from actions to habits to compulsion. Nat. Neurosci. 2005;8:1481–1489. doi: 10.1038/nn1579. [DOI] [PubMed] [Google Scholar]
- Field M., Mogg K., Bradley B.P. Cognitive bias and drug craving in recreational cannabis users. Drug Alcohol Depend. 2004;74:105–111. doi: 10.1016/j.drugalcdep.2003.12.005. [DOI] [PubMed] [Google Scholar]
- Field M. Cannabis ‘dependence’ and attentional bias for cannabis-related words. Behav. Pharmacol. 2005;16:473–476. doi: 10.1097/00008877-200509000-00021. [DOI] [PubMed] [Google Scholar]
- Field M., Eastwood B., Bradley B.P., Mogg K. Selective processing of cannabis cues in regular cannabis users. Drug Alcohol Depend. 2006;85:75–82. doi: 10.1016/j.drugalcdep.2006.03.018. [DOI] [PubMed] [Google Scholar]
- Field M., Munafo M.R., Franken I.H. A meta-analytic investigation of the relationship between attentional bias and subjective craving in substance abuse. Psychol. Bull. 2009;135:589–607. doi: 10.1037/a0015843. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Field M., Christiansen P. Commentary on, ‘Internal reliability of measures of substance-related cognitive bias’. Drug Alcohol Depend. 2012;124:189–190. doi: 10.1016/j.drugalcdep.2012.02.009. [DOI] [PubMed] [Google Scholar]
- Filbey F.M., Schacht J.P., Myers U.S., Chavez R.S., Hutchison K.E. Marijuana craving in the brain. Proc. Natl. Acad. Sci. U. S. A. 2009;106:13016–13021. doi: 10.1073/pnas.0903863106. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gladwin T.E., Figner B., Crone E.A., Wiers R.W. Addiction, adolescence, and the integration of control and motivation. Dev. Cogn. Neurosci. 2011;1:364–376. doi: 10.1016/j.dcn.2011.06.008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gray K.M., LaRowe S.D., Watson N.L., Carpenter M.J. Reactivity to in vivo marijuana cues among cannabis-dependent adolescents. Addict. Behav. 2011;36:140–143. doi: 10.1016/j.addbeh.2010.08.021. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Grenard J.L., Ames S.L., Wiers R.W., Thush C., Sussman S., Stacy A.W. Working memory capacity moderates the predictive effects of drug-related associations on substance use. Psychol. Addict. Behav. 2008;22:426–432. doi: 10.1037/0893-164X.22.3.426. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gulley J.M., Juraska J.M. The effects of abused drugs on adolescent development of corticolimbic circuitry and behavior. Neuroscience. 2013;249:3–20. doi: 10.1016/j.neuroscience.2013.05.026. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hammes J.G.W. Swets and Zeitlinger; Lisse: 1971. De Stroop Kleur-Woord Test. Handleiding. [Google Scholar]
- Hanson K.L., Thayer R.E., Tapert S.F. Adolescent marijuana users have elevated risk-taking on the balloon analog risk task. J. Psychopharmacol. 2014;8:1080–1087. doi: 10.1177/0269881114550352. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hanson K.L., Winward J.L., Schweinsburg A.D., Medina K.L., Brown S.A., Tapert S.F. Longitudinal study of cognition among adolescent marijuana users over three weeks of abstinence. Addict. Behav. 2010;35:970–976. doi: 10.1016/j.addbeh.2010.06.012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Harvey M.A., Sellman J.D., Porter R.J., Frampton C.M. The relationship between non-acute adolescent cannabis use and cognition. Drug Alcohol Rev. 2007;26:309–319. doi: 10.1080/09595230701247772. [DOI] [PubMed] [Google Scholar]
- Heatherton T.F., Kozlowski L.T., Frecker R.C., Fagerstrom K.O. The Fagerstrom Test for Nicotine Dependence: a revision of the Fagerstrom Tolerance Questionnaire. Br. J. Addict. 1991;86:1119–1127. doi: 10.1111/j.1360-0443.1991.tb01879.x. [DOI] [PubMed] [Google Scholar]
- Houben K., Wiers R.W. Response inhibition moderates the relationship between implicit associations and drinking behavior. Alcohol Clin. Exp. Res. 2009;33:626–633. doi: 10.1111/j.1530-0277.2008.00877.x. [DOI] [PubMed] [Google Scholar]
- Kober H., DeVito E.E., DeLeone C.M., Carroll K.M., Potenza M.N. Cannabis abstinence during treatment and one-year follow-up: relationship to neural activity in men. Neuropsychopharmacology. 2014;39:2288–2298. doi: 10.1038/npp.2014.82. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Koob G.F., Volkow N.D. Neurocircuitry of addiction. Neuropsychopharmacology. 2010;35:217–238. doi: 10.1038/npp.2009.110. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Little R.J.A. A test of missing completely at random for mulitivariate data with missing values. J. Am. Stat. Assoc. 1988;83:1198–1202. [Google Scholar]
- Lundahl L.H., Johanson C.E. Cue-induced craving for marijuana in cannabis-dependent adults. Exp. Clin. Psychopharmacol. 2011;19:224–230. doi: 10.1037/a0023030. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Marhe R., van de Wetering B.J., Franken I.H. Error-related brain activity predicts cocaine use after treatment at 3-month follow-up. Biol. Psychiatry. 2013;73:782–788. doi: 10.1016/j.biopsych.2012.12.016. [DOI] [PubMed] [Google Scholar]
- Meier M.H., Caspi A., Ambler A., Harrington H., Houts R., Keefe R.S. Persistent cannabis users show neuropsychological decline from childhood to midlife. Proc. Natl. Acad. Sci. U. S. A. 2012;109:E2657–E2664. doi: 10.1073/pnas.1206820109. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mitchell M.R., Balodis I.M., Devito E.E., Lacadie C.M., Yeston J., Scheinost D. A preliminary investigation of Stroop-related intrinsic connectivity in cocaine dependence: associations with treatment outcomes. Am. J. Drug Alcohol Abuse. 2013;39:392–402. doi: 10.3109/00952990.2013.841711. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Nickerson L.D., Ravichandran C., Lundahl L.H., Rodolico J., Dunlap S., Trksak G.H. Cue reactivity in cannabis-dependent adolescents. Psychol. Addict. Behav. 2011;25:168–173. doi: 10.1037/a0021117. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Peeters M., Wiers R.W., Monshouwer K., van de Schoot R., Janssen T., Vollebergh W.A. Automatic processes in at-risk adolescents: the role of alcohol-approach tendencies and response inhibition in drinking behavior. Addiction. 2012;107:1939–1946. doi: 10.1111/j.1360-0443.2012.03948.x. [DOI] [PubMed] [Google Scholar]
- Perkonigg A., Goodwin R.D., Fiedler A., Behrendt S., Beesdo K., Lieb R. The natural course of cannabis use, abuse and dependence during the first decades of life. Addiction. 2008;103:439–449. doi: 10.1111/j.1360-0443.2007.02064.x. [DOI] [PubMed] [Google Scholar]
- SAMHSA . Center for Behavioral Health Statistics and Quality; Rockville, MD: 2010. Results from the 2009 National Survey on Drug Use and Health: Mental Health Findings. NSDUH Series H-39. [Google Scholar]
- Saunders J.B., Aasland O.G., Babor T.F., de la Fuente J.R., Grant M. Development of the Alcohol Use Disorders Identification Test (AUDIT): WHO Collaborative Project on Early Detection of Persons with Harmful Alcohol Consumption – II. Addiction. 1993;88:791–804. doi: 10.1111/j.1360-0443.1993.tb02093.x. [DOI] [PubMed] [Google Scholar]
- Schulte M.H., Cousijn J., den Uyl T.E., Goudriaan A.E., van den Brink W., Veltman D.J. Recovery of neurocognitive functions following sustained abstinence after substance dependence and implications for treatment. Clin. Psychol. Rev. 2014;34:531–550. doi: 10.1016/j.cpr.2014.08.002. [DOI] [PubMed] [Google Scholar]
- Sharbanee J.M., Stritzke W.G., Wiers R.W., MacLeod C. Alcohol-related biases in selective attention and action tendency make distinct contributions to dysregulated drinking behaviour. Addiction. 2013;108:1758–1766. doi: 10.1111/add.12256. [DOI] [PubMed] [Google Scholar]
- Sobell L.C., Brown J., Leo G.I., Sobell M.B. The reliability of the Alcohol Timeline Followback when administered by telephone and by computer. Drug Alcohol Depend. 1996;42:49–54. doi: 10.1016/0376-8716(96)01263-x. [DOI] [PubMed] [Google Scholar]
- Stinson F.S., Ruan W.J., Pickering R., Grant B.F. Cannabis use disorders in the USA: prevalence, correlates and co-morbidity. Psychol. Med. 2006;36:1447–1460. doi: 10.1017/S0033291706008361. [DOI] [PubMed] [Google Scholar]
- Subramaniam G., Harrell P., Huntley E., Tracy M. Beck Depression Inventory for depression screening in substance-abusing adolescents. J. Subst. Abuse Treat. 2009;37:25–31. doi: 10.1016/j.jsat.2008.09.008. [DOI] [PubMed] [Google Scholar]
- Swift W., Hall W., Teesson M. Cannabis use and dependence among Australian adults: results from the National Survey of Mental Health and Wellbeing. Addiction. 2001;96:737–748. doi: 10.1046/j.1360-0443.2001.9657379.x. [DOI] [PubMed] [Google Scholar]
- Thush C., Wiers R.W., Ames S.L., Grenard J.L., Sussman S., Stacy A.W. Interactions between implicit and explicit cognition and working memory capacity in the prediction of alcohol use in at-risk adolescents. Drug Alcohol Depend. 2008;94:116–124. doi: 10.1016/j.drugalcdep.2007.10.019. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Verdejo-Garcia A., Clark L., Dunn B.D. The role of interoception in addiction: a critical review. Neurosci. Biobehav. Rev. 2012;36:1857–1869. doi: 10.1016/j.neubiorev.2012.05.007. [DOI] [PubMed] [Google Scholar]
- Watson P., de Wit S., Hommel B., Wiers R.W. Motivational mechanisms and outcome expectancies underlying the approach bias toward addictive substances. Front. Psychol. 2012;3:440. doi: 10.3389/fpsyg.2012.00440. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wiers R.W., Bartholow B.D., van den Wildenberg E., Thush C., Engels R.C., Sher K.J. Automatic and controlled processes and the development of addictive behaviors in adolescents: a review and a model. Pharmacol. Biochem. Behav. 2007;86:263–283. doi: 10.1016/j.pbb.2006.09.021. [DOI] [PubMed] [Google Scholar]
- Wiers R.W., Eberl C., Rinck M., Becker E.S., Lindenmeyer J. Retraining automatic action tendencies changes alcoholic patients’ approach bias for alcohol and improves treatment outcome. Psychol. Sci. 2011;22:490–497. doi: 10.1177/0956797611400615. [DOI] [PubMed] [Google Scholar]
- Wisselink D.J., Kuijpers W.G.T., Mol A. Houten; Stichting Informatie Voorziening Zorg (IVZ): 2014. Kerncijfers Verslavingszorg 2013: Landelijk Alcohol en Drugs Informatie Systeem (LADIS) [Google Scholar]
- Zumdick S., Schneider U., Leweke M., Julicher A., Tossmann P., Bonnet U. A survey on trials focussing on the treatment of cannabis-dependence. Fortschritte Neurologie-Psychiatrie. 2006;74:211–225. doi: 10.1055/s-2005-870974. [DOI] [PubMed] [Google Scholar]