Highlights
-
•
Adolescence is a critical period for the development of self-regulatory abilities.
-
•
The cultivation of mindfulness has been associated with improved self-regulation.
-
•
We examined brain development and dispositional mindfulness in adolescence.
-
•
Adolescents with higher levels of mindfulness demonstrated less thinning in the left anterior insula.
Keywords: Adolescence, Brain development, Mindfulness, Insula, Self-regulation, Intelligence
Abstract
Adolescence is a critical period of development, in which the increasing social and cognitive demands of independence need to be met by enhanced self-regulatory abilities. The cultivation of mindfulness has been associated with improved self-regulation in adult populations, and it is theorized that one neurodevelopmental mechanism that supports this capacity is the development of the prefrontal cortex. The current study examined the neurodevelopmental mechanisms associated with dispositional mindfulness in adolescence. Using a longitudinal within-persons design, 82 participants underwent structural magnetic resonance imaging (MRI) assessments at approximately ages 16 and 19, and also completed self-reported measurements of mindfulness at age 19. It was hypothesized that adolescents who demonstrated greater thinning of frontal cortical regions between the age of 16 and 19 would exhibit higher dispositional mindfulness levels at age 19. Results indicated that, contrary to predictions, adolescents with higher levels of mindfulness demonstrated less thinning in the left anterior insula. By contrast, higher IQ was associated with greater thinning of the right caudal middle frontal and right superior frontal regions. The involvement of insula development in mindfulness is consistent with a direct role for this structure in managing self-regulation, and in doing so concords with recent models of self-referential interoceptive awareness.
Over the adolescent period, teens spend increasingly less time under direct adult supervision (Turner et al., 1993). Given this burgeoning independence, an essential ability to be learnt is self-regulation – a skill set that involves the cultivation of behavioural self-control, the capacity to cope with negative emotions, and the ability to maintain goal-directed behaviour (Blakemore and Robbins, 2012, Spear, 2010). Failure of self-regulation during the adolescent period can lead to maladaptive behaviours such as excessive risk-taking, and poor mental health outcomes such as depression (Abela and Hankin, 2011, Casey et al., 2008).
The dispositional trait of mindfulness, which reflects the tendency towards present-moment awareness, has been associated with positive psychological functioning, including enhanced self-regulation (Brown and Ryan, 2003, Raes and Williams, 2010). Based on the theoretical accounts introduced by Kabat-Zinn (1982), a two-construct model of dispositional mindfulness has been proposed, capturing individual differences in (1) attention and awareness and (2) acceptance and non-reactivity (Bishop et al., 2006). However, Brown and Ryan (2003) have suggested attention to the moment to be the single most critical construct of mindfulness, subsuming acceptance and non-reactivity. Higher levels of dispositional mindfulness are thought to facilitate self-regulation, because they are permissive of present-moment focussed states of consciousness that allow the mind to break free from maladaptive patterns of thinking and behaviour (Chambers et al., 2009, Kumar et al., 2008). Further, dispositional mindfulness in adolescents has been shown to predict recovery from depression (Chambers et al., 2014). Higher levels of dispositional mindfulness have been measured as a positive outcome of mindfulness-based intervention (MBI) participation – which often involves mindfulness-based meditation practice (Baer, 2003). However, given the complexity of such interventions, the exact mechanisms that produce this outcome are still not understood (Nyklíček and Kuijpers, 2008, Shapiro et al., 2008). Given that dispositional mindfulness can be assessed as a trait within normal populations without specific training, including adolescent populations, research into the emergence of mindfulness over development provides an alternative perspective from which to investigate the mechanisms that determine dispositional mindfulness.
The adolescent period is a unique time for the development of the brain, and specific patterns of maladaptive behaviour that reflect poor self-regulation during the adolescent period have been attributed to a normative imbalance between executive frontal and subcortical development. For example, risk-taking behaviours, such as dangerous driving, unsafe sex and drug abuse can have severe health consequences for teens (Arnett, 1992, Erickson and Chambers, 2007). “Dual systems” models of adolescent development have suggested that risk-taking behaviour becomes more prevalent when subcortical reward and affective systems such as the nucleus accumbens and amygdala develop quickly and early over the adolescent period, temporarily dominating the more slowly developing prefrontal executive systems (Casey et al., 2005, Gladwin et al., 2011, Somerville et al., 2010, Steinberg, 2010). The relative over-activity of the subcortical systems is thought to lead to increased sensation-seeking, impulsivity and emotional reactivity (Carlo et al., 2012, Cook et al., 2013, Pearson et al., 2013).
One of the ways in which mindfulness is theorized to aid self-regulation is via interactions that involve both frontal executive and subcortical brain systems. Creswell et al. (2007), for example, examined the link between mindfulness, executive function and subcortical brain region activity. Using a cross-sectional design, dispositional mindfulness was measured along with individual differences in neural response to affect-labelling during functional magnetic resonance imaging (fMRI). When other measures of psychological distress were controlled for, higher levels of dispositional mindfulness were associated with widespread PFC activation and left insula activation in synchrony with attenuated amygdala responses during affect-labelling. This apparent synergy between frontal cortical and subcortical regions was interpreted to reflect a potential neurobiological pathway through which higher levels of mindfulness could promote adaptation and improve frontal regulation of subcortical activity. However, without a longitudinal examination, such adaptive changes over time could not be confirmed. Further, this study was performed using a young adult, rather than an adolescent, sample, and analysis was restricted to fMRI.
Given the marked, and well-documented developmental changes in brain structure during adolescence, investigation of structural brain development, and its relation to the emergence of dispositional mindfulness during adolescence, is of interest. Cortical grey matter has been documented to follow an inverted U-shaped trajectory, with cortical thickness and volume peaking during childhood and then thinning over the adolescent period to early adulthood (Giedd et al., 2009, Shaw et al., 2006, Shaw et al., 2008). While recent evidence points towards a normative peak in cortical thickness occurring well before the adolescent period (Tamnes et al., 2010a, Tamnes et al., 2010b), examination of cortical development in relation to cognitive ability suggests that some individuals have delayed thickening. For example, in a cohort-sequential study, it was observed that the cortices of those with superior IQ underwent protracted and increased thickening into early adolescence, followed by greater thinning from mid- to late adolescence (Shaw et al., 2006). The delayed peaking in thickness and subsequently greater thinning within the higher IQ group were identified in the frontal cortical regions of the right frontal superior cortex and right medial prefrontal cortex as well as left temporal gyrus. Moreover, greater cortical thinning has also been associated with superior working memory capacity and inhibitory control in adolescents (Tamnes et al., 2010a, Tamnes et al., 2010b, Tamnes et al., 2013). Given the association of superior cognitive ability with better functional outcomes, greater thinning over late adolescence could be considered to reflect more adaptive abilities. The above quasi-longitudinal designs reveal macro changes that would not necessarily be observed within a cross-sectional analysis. In particular, Shaw and colleagues’ (2006) finding that the pattern of normative cortical thinning in the frontal cortices corresponds to quantifiable cognitive outcomes is both an example of a longitudinal design revealing a trend, as well as an invitation to examine how patterns of frontal cortical development over adolescence could vary with other constructs.
While the relationship between structural brain development during adolescence and the emergence of individual differences in dispositional mindfulness has not yet been examined in adolescent populations, MRI studies have examined related issues in adults subsequent to the developmental cortical thinning of adolescence. Increases in cortical thickness have been noted amongst long term meditators throughout frontal brain areas, in particular within the medial PFC, inferior PFC, superior frontal cortex, (Kang et al., 2013, Lazar et al., 2005, Vestergaard-Poulsen et al., 2009) and right orbito-frontal cortex (Luders et al., 2009). The insula has a particularly salient history in morphological studies of brain-mindfulness associations, with increased thickness of the right anterior insula associated with both increased number of years of meditation (Lazar et al., 2005), as well as dispositional mindfulness (Murakami et al., 2012). Similarly, increased grey matter concentration within the right anterior insula has been associated with greater meditation experience (Hölzel et al., 2008).
The insula is theorized to have a governing role in the maintenance of interoceptive awareness – the subjective awareness of internal body states (Bar-On et al., 2003, Craig, 2003). The anterior insula has been associated with subjective feeling states that may refer to interoceptive information; the posterior insula appears to be involved in conveying information of a homeostatic nature, including pressure and touch, and may also contain primary interoceptive representations (Craig, 2009). While interoceptive awareness is theorized to be associated with a largely automated regulatory mechanism, it may also inform higher-order cognitions that guide decision-making, particularly towards reduced-stress outcomes (Liotti et al., 2001, Craig, 2003, Critchley, 2005, Singer et al., 2009, Farb et al., 2013). Since it draws upon deliberate body scanning practices, mindfulness meditation may tap into the same (potentially) insular-centric mechanisms that underlie automated interoceptive awareness.
Given the cross-sectional nature of studies examining the association between mindfulness and brain structure to date, as well as the absence of standardized measurement of mindfulness across these studies, it is unclear to what extent brain structural change is associated with improved dispositional mindfulness levels, or indeed whether structural change may represent a developmental marker or precursor of trait mindfulness. Nevertheless, compelling meta-evidence is presented in these studies of the association between long-term practises that arguably enhance mindfulness (Nyklíček and Kuijpers, 2008), with changes in the structure of the PFC and insula cortex, which are areas of the brain implicated in capacities that undergo substantial development during adolescence. These capacities include emotional regulation, changes in self-perspective and conscious awareness (Creswell et al., 2007, Steinberg, 2005), which have been theorized to have corresponding neurological mechanisms that are related to mindful awareness (Hölzel et al., 2011, Modinos et al., 2010). Therefore, it is reasonable to speculate that development of the PFC and insula regions during adolescence might support the development of dispositional mindfulness.
Further, the unique neurobiological and developmental changes that occur over the adolescent period particularly mandate study of the relationship between brain development and mindfulness using a truly within-subjects longitudinal design. The current study examined the association between developmental brain change and dispositional mindfulness, with particular focus on the frontal cortices and insula due to the theorized connection of mindfulness with self-regulation and awareness. Based on the established research demonstrating that higher rates of cortical thinning during late adolescence occur in association with adaptive measures and outcomes, including increased intelligence and self-regulation (Shaw et al., 2006, Tamnes et al., 2010a, Tamnes et al., 2010b), it was predicted that cortical thinning over a 3-year developmental period between ages 16 and 19, would be positively associated with superior regulatory abilities as measured by dispositional mindfulness. Accordingly, it was hypothesized that higher levels of dispositional mindfulness at age 19 would be preceded by greater cortical thinning within the insula and regions of the PFC over the previous 3 years. Further, in order to evaluate the specificity of these effects to dispositional mindfulness, the relationship between cortical development and IQ was similarly examined in the frontal cortices. Given the evidence associating cortical thinning in the prefrontal cortex during the early adolescent period with self-regulatory outcomes (Vijayakumar et al., 2014a, Tamnes et al., 2010a, Tamnes et al., 2010b), additional analyses were undertaken to elucidate potential relationships between cortical thickness change in the frontal cortices from ages 12 to 16 and dispositional mindfulness outcomes at age 19.
1. Method
This study employed a longitudinal within-person design with adolescent data that was collected as part of the Orygen Adolescent Development Study conducted by the University of Melbourne. The final study sample consisted of 82 individuals who participated in two waves of MRI scanning at ages 16 and 19, respectively, in addition to completing questionnaires at the time of the second scanning.
1.1. Participants and recruitment procedure
From a sampling pool of 4587 participants, 2453 6th graders participated in an initial screening, which involved completion of the Early Adolescent Temperament Questionnaire – Revised (EATQ-R) (Capaldi and Rothbart, 1992). As described in Whittle and colleagues’ (2011) study, an equal sample of males and females were selected, with a range of EATQ-R scores that oversampled extreme scores for the four higher order factors of Effortful Control, Surgency, Affiliativeness, and Negative Affect. This process was designed to produce a sample that provided a maximized range of risk and resilience for psychopathology. Based on the EATQ-R scores, 415 (16%) participants were invited to participate in further research, of which 245 (58%) agreed. Socioeconomic status (SES) was also measured, based on the Australian National University Four (ANU4) 100-point scale (Jones and McMillan, 2001). Adolescents were invited to participate in MRI and questionnaire assessments at early (12 years), mid (16 years), and late (19 years) adolescence. The final sample used in this study comprised 82 participants who completed MRI at mid- (T1) and late adolescence (T2). In addition, secondary analyses were conducted on 77 participants who completed scans at both early (baseline) and mid-adolescence (T1). Please refer to the supplementary section for further information on the secondary analyses.
MRI scans used for the primary analyses (from T1 and T2) were acquired at the Royal Children's Hospital in Parkville, Melbourne. Baseline scans that were utilized in the secondary analyses were performed at the Austin and Repatriation Medical Centre, Melbourne. Informed consent was obtained from both the child and at least one parent/guardian (if under 18 years of age), consistent with the guidelines of the Human Research Ethics Committee at the University of Melbourne, Australia. On the day of T2 scanning procedures, participants had their IQ measured, and were also asked to fill out a number of questionnaires, including adolescent temperament scales. Dispositional mindfulness was assessed with an additional questionnaire, on the day of T2 scanning and IQ measurements for the current study were taken at the time of the T2 assessment.
1.2. Design and measures
1.2.1. Image acquisition and processing
MRI scans at T1 and T2 were performed on the identical 3 Tesla Siemens scanner at the Royal Children's Hospital, Melbourne, Australia. A T1-weighted sequence was obtained for high resolution structural images using the following sequence parameters: repetition time = 1900 ms; echo time = 2.24 ms; flip angle = 9°, field of view = 23 cm; 176 T1-weighted contiguous 0.9-mm thick slices (voxel dimensions = 0.9 mm3).
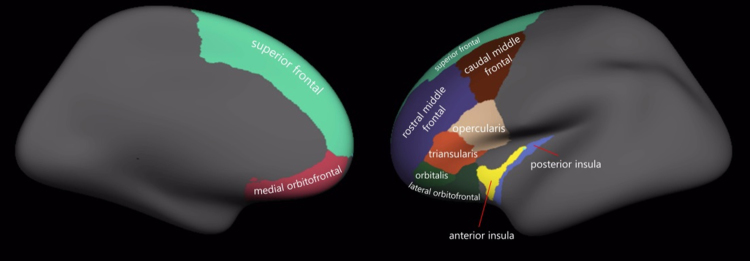
Cortical reconstruction of images was completed on an SGI/Linux workstation at the University of Melbourne, using FreeSurfer image analysis suite version 5.3 (http://surfer.nmr.mgh.harvard.edu/), which has recognized superior robustness with respect to noise, intensity normalization and outliers when compared to alternative registration tools (Jha et al., 2010). A template space and average image at T1 and T2 time points was created for participants using inverse consistent registration – a process that can deal with different intensity scales as well as detect outlier regions in the image (Reuter and Fischl, 2011) – thus significantly improving the repeatability and power of cortical thickness measurements. Images were also corrected for tissue signal heterogeneity using FreeSurfer's N3 correction (optimized for 3 Tesla images), an intensity normalization method, which improves accuracy during cortical segmentation (Zheng et al., 2009). The template was then segmented according to the Desikan–Killiany brain atlas, which is a standardized gyral-based atlas of well-recognised brain regions (Desikan et al., 2006). In the current study, the thickness of eight frontal ROIs was extracted in left and right hemispheres using the Desikan–Killiany atlas, including caudal middle frontal, medial orbitofrontal, lateral orbitofrontal, pars orbitalis, pars triangularis, pars opercularis, rostral middle frontal, and superior frontal regions. The thickness of anterior and posterior insula ROIs were likewise extracted according to the Destrieux atlas, which is an alternative atlas that demarcates anterior and posterior subdivisions for separate analysis (Destrieux et al., 2010). This presented a total of 20 ROIs for further analysis. Average cortical thickness at T1 was also calculated by averaging the thicknesses of both hemispheres. All cross-sectional, base, and longitudinal output images were manually inspected, and in most cases, pial surface edits and/or control points were used to correct for minor errors in cortical surface estimates.
1.2.2. MAAS scale
The Mindfulness Attention Awareness Scale (MAAS) is a 15-item self-rated questionnaire designed to assess the theorized principal characteristic of dispositional mindfulness – the open, receptive awareness and attention to the present (Brown and Ryan, 2003), in which participants rate on a 6-point Likert scale the frequency with which they experience everyday accounts of mindfulness. The MAAS was introduced to capture Brown's one-construct model of dispositional mindfulness (Brown and Ryan, 2003) and has demonstrated high test–retest reliability, discriminant and convergent validity incremental validity, and criterion validity (Brown et al., 2011). Higher levels of dispositional mindfulness, as measured by MAAS, have been associated with positive measures of well-being, including self-control, life satisfaction and agreeableness (Brown et al., 2007, Thompson and Waltz, 2007), as well as significant negative correlations with both depression and neuroticism (Brown et al., 2011, Fetterman et al., 2010, Shapiro et al., 2005). The one-construct MAAS is the most widely used scale of dispositional mindfulness, with an extensive empirical track record in adult populations, as well as an established track record in adolescent populations (Black et al., 2012). An adolescent-specific MAAS-A questionnaire is available (Brown et al., 2011), which has a single driving-related item removed, however the (adult) MAAS questionnaire was administered as nearly all participants were at independent driving age at the time of testing.
1.2.3. Scales measuring temperament, emotion regulation and IQ
Given that a number of adolescent temperament scales were taken at the same time as MAAS, the opportunity was taken in the current study to further evaluate the validity of MAAS within an adolescent population. The Early Adolescent Temperament Questionnaire – Revised (EATQ-R)(Capaldi and Rothbart, 1992) is designed to specifically tap experiences common to adolescents, and was scored by 65 items on a 7-point Likert scale that loads onto a number of temperament scales, including attention, inhibitory control, frustration, as well as behavioural scales of aggression and depressive mood. The Emotional Regulation Questionnaire (ERQ) (Gross and John, 2003) was administered as a test of emotional self-regulation within adolescence. Self-reported levels of cognitive reappraisal – the deliberate reinterpretation of emotional material via cognitive means – and expressive suppression – the controlling of reactions to external events – were measured by scoring 10 items on a 7-point Likert scale. Full scale IQ was estimated using a short form of WISC-IV, based on three subtests (Vocabulary, Matric Reasoning, Symbol Search) (O’Donnell et al., 2009).
1.3. Data analysis
1.3.1. Final sample
Three participants were excluded based on poor quality cortical segmentation. In order to eliminate the possibility of systematic hemispheric differences, a further nine participants were excluded on the basis of being left-handed. Following exclusions, the total number of participants with matched cortical thickness data and mindfulness measures was 82 (female = 43). In the final sample, outlined in Table 1, there were no gender differences noted for MAAS and IQ (all p values > 0.05), and the sample did not differ from the initial school screening sample (N = 2453) on socioeconomic disadvantage (t(2439) = 0.592; p = 0.550) (Jones and McMillan, 2001) or gender (Pearson's χ2 = 0.006; p = 0.937).
Table 1.
Sample descriptives.
| Measure | Totala |
|---|---|
| Sample size | 82 (43 female) |
| Age at T1 (years) | 16.45; 0.53; 14.95–17.94 |
| Age at T2 (years) | 18.79; 0.47; 17.28–19.96 |
| Interscan interval T1–T2 (years)b | 2.34; 0.21; 1.67–2.88 |
| Estimate Full Scale IQ | 112.10; 11. 53; 82–136 |
| MAAS score | 4.30; 0.807; 2.40–5.93 |
| Percentage Low SESc | 58.76 |
Note: IQ and MAAS scores taken at T2.
Values represent mean; standard deviation; range.
Delay between baseline and follow-up MRI scan.
Data missing for one female participant.
1.4. Statistical analysis
Repeated measures analysis of variance (ANOVA) and hierarchical regression analysis were undertaken within IBM Statistical Package for the Social Sciences (SPSS), Version 21 (SPSS Inc., Chicago, IL) in order to examine how the rate of cortical thinning in frontal brain regions between 16 and 19 years of age influenced levels of mindfulness measured at age 19. Thickness change (i.e. thinning) scores were calculated as difference between T1 and T2, then standardized as change scores per year in order to factor in discrepancies in interscan interval (T1–T2/Interscan Interval). Average brain thickness at T1 and gender were used as covariates in the hierarchical regression analysis to control for physical differences between participant's brain sizes.
2. Results
2.1. Investigation of MAAS Scale
While criterion validity of MAAS has been verified in several large adolescent population studies, discriminant/convergent validity appears to have only been systematically confirmed with one adolescent-specific personality scale (Black et al., 2012, Brown et al., 2011). In order to further substantiate the validity of MAAS within the current adolescent sample, MAAS scores were correlated with the adolescent-specific descriptive measures from EATQ-R and ERQ, as well as overall IQ. After controlling for age and gender, Table 2 shows that MAAS scores had a significant positive correlation with self-reported measures of cognitive reappraisal, attention and inhibitory control, and significant negative correlation with self-reported frustration, aggression and depressive mood. The correlation between MAAS and IQ observed in the sample was non-significant (r = −0.84, n = 82). Further, high internal consistency and reliability of MAAS was demonstrated (15 items; α = .86).
Table 2.
Correlation of MAAS score with other measures.
| Measure | Correlation score |
|---|---|
| ERQ Cognitive Reappraisal | .303** |
| ERQ Expressive Suppression | −.245* |
| EATQ Attention | .432** |
| EATQ Inhibitory Control | .288* |
| EATQ Frustration | −.352** |
| EATQ Aggression | −.300** |
| EATQ Depressive Mood | −.568** |
| Full Scale IQ | −.084 |
p < .05.
p < 0.01.
2.2. Cortical thinning between T1 and T2 in frontal ROIs
In order to verify normative developmental thinning over time within the frontal regions, including PFC and insula, the following frontal brain regions were subjected to two-way repeated measures ANOVAs (with time and hemisphere as within-subject variables): caudal middle frontal, medial orbitofrontal, lateral orbitofrontal, pars orbitalis, pars triangularis, pars opercularis, rostral middle frontal, superior frontal, anterior and posterior insula (see Fig. 1). As indicated in Table 3, most of these brain regions demonstrated thinner cortices at T2, compared with T1, with the exception of pars orbitalis and rostral middle frontal.
Fig. 1.
Visualization of prefrontal regions of interest.
Table 3.
Cortical thickness change over time across brain regions.
| Frontal region | Average thickness at T1 | Average thickness at T2 | Average thickness change between T1 and T2 | Significance of change over time |
|---|---|---|---|---|
| Caudal Middle Frontal | 2.838; .131 | 2.826; .127 | −.012 | .018* |
| Medial Orbitofrontal | 2.695; .144 | 2.636; .140 | −.059 | <.000** |
| Lateral Orbitofrontal | 2.893; .134 | 2.861; .143 | −.032 | .005** |
| Pars Orbitalis | 3.006; .047 | 3.006; .201 | .000 | .951 |
| Pars Triangularis | 2.741; .126 | 2.707; .133 | −.034 | <.000** |
| Pars Opercularis | 2.877; .144 | 2.850; .135 | −.027 | .002** |
| Rostral Middle Frontal | 2.620; .128 | 2.607; .125 | −.013 | .149 |
| Superior Frontal | 3.088; .123 | 3.051; .117 | −.037 | <.000** |
| Anterior Insula | 3.847; .135 | 3.782; .132 | −.065 | <.000** |
| Posterior Insula | 3.575; .221 | 3.527; .222 | −.048 | <0.000** |
Note: Values represent mean (mm); standard deviation.
p < .05.
p < .0.01.
2.3. Testing the association between cortical thickness change and MAAS scores
Cortical thickness change scores between T1 and T2 were created for the 10 frontal regions of caudal middle frontal, medial orbitofrontal, lateral orbitofrontal, pars orbitalis, pars triangularis, pars opercularis, rostral middle frontal, superior frontal, anterior and posterior insula, resulting in 20 variables in total for each region in the left and right hemispheres. Hierarchical regression was used to test whether cortical thickness change predicted MAAS scores after controlling for extraneous variables. After controlling for gender and average whole brain cortical thickness at T1, less thinning in the left anterior insula cortex (R2 (R square change) = .067, F(1, 78) = 5.91, b = −.263, p = 0.017) significantly predicted higher MAAS scores. Associations between change in other PFC regions and MAAS were non-significant. IQ scores were regressed onto cortical thickness change using the same analytic strategy as above. Greater thinning in right caudal middle frontal (R2 = .072, F(1, 78) = 6.972, b = .277, p = .010) and right superior frontal cortical regions (R2 = .053, F(1, 78) = 4.973, b = .237, p = .029) significantly predicted higher IQ scores.
Given the close theoretical and empirical connection of dispositional mindfulness with self-regulation and attention (Brown and Ryan, 2003, Chambers et al., 2009), further testing was undertaken to determine whether the above result remained significant when related attentional and self-regulatory constructs were controlled for. Attention and inhibitory control were introduced into the above analysis as covariates (in addition to gender and whole brain thickness at T1) in two separate analyses. After controlling for EATQ Attention, less thinning in the left anterior insula cortex (R2 = .065, F(1, 77) = 5.31, b = −.284, p = 0.024) significantly predicted higher MAAS scores; similarly, after controlling for EATQ Inhibitory Control, less thinning in the left anterior insula cortex (R2 = .055, F(1, 77) = 4.46, b = −.247, p = 0.038) significantly predicted higher MAAS scores.
Secondary analyses were conducted to examine whether cortical development during the early adolescent period predicted mindfulness outcomes at age 19, by comparing the data for the age 16 scan with a baseline scan obtained on the sample at age 12 (Whittle et al., 2014). No significant relationships were found between dispositional mindfulness levels at age 19 and prefrontal cortical thinning trajectories between the ages of 12 and 16. However, due to the lack of proximity between brain scan times and late adolescent MAAS scores, as well as the fact that a different scanner was used at baseline, these analyses are considered secondary to the main analyses. Please refer to the supplementary section for detailed participant characteristics and methodology relevant to these analyses.
2.4. Exploring the effects of cortical development on mindfulness
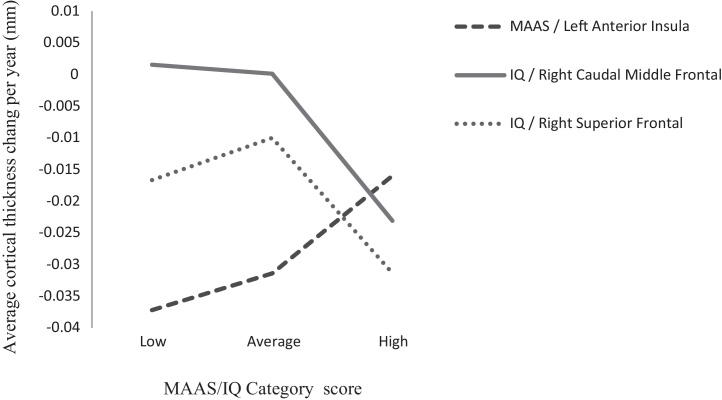
In order to describe the nature of the association between cortical thickness change in the left anterior insula and MAAS score, the main sample was split into three equally sized groups of low, average and high mindfulness based on the distribution of MAAS scores. IQ score was similarly treated in the significant frontal ROIs for IQ. Score categories were plotted against average thickness change per year to allow for comparison of differing thinning rates according to both construct type and construct category score (see Fig. 2).
Fig. 2.
MAAS and IQ scores vs. annualized cortical thickness change.
Participants categorized as high in MAAS score demonstrated less thinning over time within the left anterior insula, when compared to participants who scored average or low in MAAS score. In a reversed trend to mindfulness, participants categorized as high in IQ score demonstrated greater thinning over time within both the right caudal middle frontal and superior frontal region, when compared to subjects who scored average and low in IQ.
3. Discussion
The current study is the first to examine the association between cortical development and levels of dispositional mindfulness over adolescence. On the basis of previous research on adolescent cortical thinning and cognitive functioning, it was hypothesized that higher levels of dispositional mindfulness at age 19 would be preceded by greater cortical thinning in the insula and other regions of the PFC over the previous 3 years. However, this hypothesis was not supported. While there was a significant association between change in cortical thickness in the left anterior insula and mindfulness levels, the pattern of thinning was opposite to that predicted. Participants with higher levels of dispositional mindfulness had less prior cortical thinning in the left anterior insula when compared to participants with relatively lower levels of mindfulness. Further, no significant relationship was found between levels of dispositional mindfulness and development within areas of the PFC. As part of an additional analysis, no significant relationships were found between dispositional mindfulness and cortical thinning trajectories during the earlier developmental period of 12–16 years, suggesting that the relationship between these patterns of brain development and dispositional mindfulness in late adolescence are specific to the mid- to late adolescent years.
The insula has an established empirical relationship with neurobiological mechanisms that are theorized to underlie mindfulness. Functional imaging studies show that higher activation levels in the left insula are associated with greater dispositional mindfulness (Creswell et al., 2007), and structurally, greater thickness (and grey matter density) in the right anterior insula has been associated with both long-term meditation and dispositional mindfulness (Hölzel et al., 2008, Lazar et al., 2005, Murakami et al., 2012). Although a number of methodological issues weaken the interpretation of these findings, including small sample sizes and lack of longitudinal within-group measurements, they are consistent with the current result.
The present study, with a large sample and within-groups longitudinal design, adds significant empirical weight to the previous evidence for the anterior insula as a potential element of the neural substrate of mindfulness abilities. Further, with systematic measures of dispositional mindfulness it expands the literature to date that has examined cortical change and mindful awareness in adult meditating populations by adding evidence from the adolescent period and non-meditating populations.
While evidence for anterior insula involvement in adult long-term meditators has been interpreted to indicate an effect of mindfulness meditation on insula structure and function, the current results suggest that structural development of the anterior insula may contribute to the development of dispositional mindfulness (although as noted below, methodological limitations prevent us from making any strong causal inferences). Thus, we have evidence of a mindfulness-insula association demonstrated from both adult and adolescent-specific developmental perspectives, as well as an association that is in accord with empirical evidence implicating the insula's role in interoceptive awareness – a form of attention that mindfulness meditation deliberately cultivates (Farb et al., 2013, Hölzel et al., 2011).
A further complicating factor in the meta-analysis of mindfulness-insula associations is the opposing laterality of the outcome of the studies. The current study's result of less left anterior insula thinning over the late adolescent period being associated with higher mindfulness levels contrasts with previous research demonstrating altered right anterior insula morphology in association with long-term adult meditation (Hölzel et al., 2008, Lazar et al., 2005, Murakami et al., 2012). However, key methodological dissimilarities limit inference as to the cause of this observed contrast in laterality. These include differing sample characteristics between studies, and the absence of dispositional mindfulness measures in the adult structural studies. Furthermore, research has not revealed a clear and systematic relationship between laterality in the insula and the mechanisms that facilitate interoceptive awareness. For example, both left and right anterior insula have been implicated in interoceptive decision-making and the monitoring of subjective feeling-states (Critchley et al., 2004, Giuliani et al., 2011, Goldin et al., 2008, Terasawa et al., 2013). So, although there is now good evidence of a mindfulness-anterior insula association, further empirical investigation is needed to understand the laterality differences that characterize this body of research.
The results from the current study stimulate the need for further investigation into the neurobiological substructures that govern different forms of self-regulation in adolescence. Dual systems models have attributed a lack of coordination between frontal executive and subcortical centres to self-regulatory failure (Casey et al., 2005, Gladwin et al., 2011, Somerville et al., 2010, Steinberg, 2010). However, through its connection with subjective bodily feelings – which are thought to underlie emotional states – the insula is theorized to play a greater role that extends beyond basic homeostatic processing of bodily states to include decision-making and affective and behavioural control (Craig, 2003, Naqvi and Bechara, 2010, Singer et al., 2009). Given that dispositional mindfulness has been associated with enhanced self-regulation (Brown and Ryan, 2003, Lakey et al., 2007), our results are congruent with such a conceptualization, and suggest a more vital role for the insula in the maintenance of self-regulation during the adolescent period than has been suggested so far.
The current study also examined how cortical thinning in frontal brain regions related to intelligence. Results were consistent with those reported in Shaw and colleagues’ (2006) study, with greater cortical thinning across late adolescence observed in the right superior cortex and the adjacent right caudal middle cortex in association with higher IQ. These congruent findings support models of structural change that associate greater thinning over the adolescent period with more adaptive cognitive traits (Shaw et al., 2006, Tamnes et al., 2010a, Tamnes et al., 2010b, Vijayakumar et al., 2014b).
In examining how patterns of cortical thinning occur in relation to both dispositional mindfulness and intelligence, the current study allows for the comparison of patterns of cortical change associated with different adaptive measures with a high certainty that the dissimilarities observed are due to the different adaptive qualities themselves rather than methodological inconsistencies between studies. In this light, the current study's finding of lesser cortical thinning over time in association with higher levels of dispositional mindfulness, when contrasted with intelligence findings, suggests that greater cortical thinning in later adolescence may not always be adaptive for all individual qualities (or may not be adaptive in all brain regions). Indeed, evidence from adult studies suggests that larger and/or thicker cortices can be adaptive. For example, enlargement of the hippocampus has been associated with extra demand for visual-spatial learning and memory (Biegler et al., 2001, Maguire et al., 2003), and as noted above, meditation in adults has been associated with thicker insula cortex. Discrepancies have also been reported in previous research on adolescent cortical thinning, with reduced thinning associated with better performance on attentional, working memory tasks and assessments of cognitive control (Tamnes et al., 2010a, Tamnes et al., 2010b, Tamnes et al., 2013, Vijayakumar et al., 2014b). While greater cortical thinning has been associated with some poor mental health outcomes, as demonstrated by evidence within clinical populations relating prefrontal cortical thinning with attention deficits and schizophrenia (Kuperberg et al., 2003, Narr et al., 2009).
Different patterns of thinning associated with mindfulness and intelligence suggest that different brain centres and modes of neural connectivity underlie these qualities. One explanation that could integrate the experimental results presented here with the previous evidence is that two competing neurological processes could influence cortical thickness change over the adolescent period – a normative developmental thinning process occurring at a macro level, driven by the unfoldment of genetically determined developmental sequence, and a secondary experience-dependent process modulated by individual behaviour and environmental stimulus within select cortical areas. Based on this model, it is possible to conjecture that a process of neuroplastic change occurs to meet the demands of higher levels of mindfulness (i.e., cortical thickening in the insula) that in highly mindful individuals acts to balance out the normative developmental thinning process. Viewed at a micro-level, this process could reflect the retention of useful circuits related to mindfulness against a backdrop of selective developmental synaptic pruning that occurs over time – a process that has been shown to be a widespread phenomenon within the adolescent brain (Bourgeois et al., 1989, Bourgeois and Rakic, 1993).
However, the association observed between higher intelligence and greater cortical thinning in the superior frontal regions may reflect an increase in neuronal efficiency associated with thinning (Giedd et al., 1999). Superior cognition as an adaptive outcome of more efficient connectivity is likely to facilitate general intelligence, given that IQ assessments consist predominantly of cognitive, working memory and performance-based tasks that place a consistent emphasis on processing speed (Ackerman et al., 2005, Colom, 2007). In contrast to IQ assessments, measures of mindfulness, such as the MAAS, assess the ability to be present-centred, which is conceptualized as an experiential quality of consciousness with self-regulatory, rather than performance-based, consequences (Baer, 2003, Brown and Ryan, 2003, Kabat-Zinn, 1982, Kabat-Zinn, 2003). Empirical evidence of the benefits of mindfulness to date supports this regulatory connection (Baer et al., 2006, Grossman et al., 2010, Nyklíček and Kuijpers, 2008, van de Weijer-Bergsma et al., 2012). Therefore, the development of dispositional mindfulness as an adaptive individual difference could be expected to be associated with a quite different set of brain changes in different brain centres during late adolescence than those that are associated with intelligence – a conclusion that is further supported by the non-significant correlation between IQ and MAAS in the current study.
A number of limitations should be borne in mind with respect to the current study. The first limitation is the absence of correction for multiple comparisons. Although the analysis involved the examination of 20 discrete frontal brain regions, since most frontal regions were examined as part of an exploratory investigation, with little expectation of a significant relationship, it was deemed less essential to employ correction. Second, the MAAS scale may not have captured all facets of dispositional mindfulness. While the evidence presented in this study supports the validity of MAAS for the use for which it was intended – as a measure of present-centred awareness (Brown et al., 2007), alternate scales such as the Toronto Mindfulness scale (Bishop et al., 2006, Lau et al., 2006) could have targeted assessment of individual differences in the theorized acceptance and non-reactivity aspect of mindfulness more efficiently. Another potential – and related – limitation is the fact that the MAAS measure inattentiveness, and then reverse-scores to produce a measure of attentiveness; this measurement process could be viewed as incongruent to the construct of mindfulness. However, inattentiveness is thought to be more accessible to most individuals (Brown and Ryan, 2003); thus assessing inattentiveness may provide the best means to measure the diversity of dispositional mindfulness levels within populations. Other measurements have been proposed, which include qualitative assessments comparing mindfulness practioners and nonpractitioners (Grossman, 2008). However, improving and testing the validity of self-report scales of dispositional mindfulness is also a vital endeavour, given the practical utility of such scales. Third, although the current study focussed on the frontal cortices for theoretical reasons, a number of other brain regions could potentially have a detectable relationship with mindful awareness. Future research could employ whole brain analysis to detect other potentially important neurodevelopmental predictors of mindfulness. Lastly, measures of mindfulness were only available in conjunction with the second assessment at approximately age 19. As such, it is not possible to ascertain whether cortical change was causally related to levels of dispositional mindfulness – it could just as easily reflect a developmental process typical of people with that trait; a longitudinal design with mindfulness measures taken at several time point could elucidate with greater specificity how dispositional mindfulness levels might alter over time in relation to cortical developmental trajectories. It could also allow a more accurate examination of the relationship between mindfulness and cortical change over early adolescence. In the current study, no significant relationship was found between dispositional mindfulness levels at age 19 and cortical development trajectories during the developmental period between age 12 and 16. However, the lack of proximity between scanning times and the time that dispositional mindfulness measures were taken could be a potential confound in this result.
This was the first study to investigate the association between dispositional mindfulness and cortical development in adolescence using a longitudinal within-subjects design, and was also the first to compare patterns of thinning that occur in association with the markedly differing adaptive qualities of mindfulness and intelligence. The finding that higher mindfulness levels were significantly associated with lesser cortical thinning trajectories over the adolescent period within the insula, a brain region that has associations with interoceptive awareness and self-regulatory functions, has further evinced dispositional mindfulness as a distinct construct with a measurable neurobiological imprint and self-regulatory implications. Further, the finding that lesser rather than (the expected) greater cortical thinning between mid- and late adolescence is associated with higher levels of dispositional mindfulness, when contrasted with the greater thinning observed in association with higher intelligence (albeit in distinct brain regions), suggests a more complex picture of cortical change during adolescence than might be revealed by the study of a single trait alone. In light of the complex findings of this study, it is conjectured that cortical development over the late adolescent period may be under the influence of both normative-developmental and experience-adaptive processes, resulting in a variety of observed relationships between cortical thinning and various adaptive qualities.
Conflict of interest
None declared.
Acknowledgements
This research was supported by grants from the Colonial Foundation, the National Health and Medical Research Council (NHMRC; Australia; Programme Grant 350241) and the Australian Research Council (ARC; Discovery Grant DP0878136). Dr. Vijayakumar is supported by a Melbourne International Research Scholarship. Dr. Whittle is supported by a NHMRC Career Development Fellowship (ID: 1007716).
Neuroimaging analysis was facilitated by the Neuropsychiatry Imaging Laboratory at the Melbourne Neuropsychiatry Centre. The authors would like to thank the Brain Research Institute and Royal Children's Hospital for support in acquiring the neuroimaging data, and the families who participated in the study.
Footnotes
Supplementary data associated with this article can be found, in the online version, at http://dx.doi.org/10.1016/j.dcn.2015.07.001.
Appendix A. Supplementary data
The following are the supplementary data to this article:
References
- Abela J.R.Z., Hankin B.L. Rumination as a vulnerability factor to depression during the transition from early to middle adolescence: a multiwave longitudinal study. J. Abnorm. Psychol. 2011;120(2):259–271. doi: 10.1037/a0022796. [DOI] [PubMed] [Google Scholar]
- Ackerman P.L., Beier M.E., Boyle M.O. Working memory and intelligence: the same or different constructs? Psychol. Bull. 2005;131(1):30–60. doi: 10.1037/0033-2909.131.1.30. [DOI] [PubMed] [Google Scholar]
- Arnett J. Reckless behavior in adolescence: a developmental perspective. Dev. Rev. 1992;12(4):339–373. [Google Scholar]
- Baer R.A. Mindfulness training as a clinical intervention: a conceptual and empirical review. Clin. Psychol. Sci. Pract. 2003;10(2):125. [Google Scholar]
- Baer R.A., Smith G.T., Hopkins J., Krietemeyer J., Toney L. Using self-report assessment methods to explore facets of mindfulness. Assessment. 2006;13(1):27–45. doi: 10.1177/1073191105283504. [DOI] [PubMed] [Google Scholar]
- Bar-On R., Tranel D., Denburg N.L., Bechara A. Exploring the neurological substrate of emotional and social intelligence. Brain. 2003;126(8):1790–1800. doi: 10.1093/brain/awg177. [DOI] [PubMed] [Google Scholar]
- Biegler R., McGregor A., Krebs J.R., Healy S.D. A larger hippocampus is associated with longer-lasting spatial memory. Proc. Natl. Acad. Sci. U. S. A. 2001;98(12):6941–6944. doi: 10.1073/pnas.121034798. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bishop S.R., Lau M., Shapiro S., Carlson L., Anderson N.D., Carmody J., Devins G. Mindfulness: a proposed operational definition. Clin. Psychol. Sci. Pract. 2006;11(3):230–241. [Google Scholar]
- Black D.S., Sussman S., Johnson C.A., Milam J. Psychometric assessment of the Mindful Attention Awareness Scale (MAAS) among Chinese adolescents. Assessment. 2012;19(1):42–52. doi: 10.1177/1073191111415365. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Blakemore S.J., Robbins T.W. Decision-making in the adolescent brain. Nat. Neurosci. 2012;15(9):1184–1191. doi: 10.1038/nn.3177. (Research Support, Non-U.S. Gov’t Review) [DOI] [PubMed] [Google Scholar]
- Bourgeois J.P., Jastreboff P.J., Rakic P. Synaptogenesis in visual cortex of normal and preterm monkeys: evidence for intrinsic regulation of synaptic overproduction. Proc. Natl. Acad. Sci. U. S. A. 1989;86(11):4297–4301. doi: 10.1073/pnas.86.11.4297. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bourgeois J.P., Rakic P. Changes of synaptic density in the primary visual cortex of the macaque monkey from fetal to adult stage. J. Neurosci. 1993;13(7):2801–2820. doi: 10.1523/JNEUROSCI.13-07-02801.1993. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Brown K.W., Ryan R.M. The benefits of being present: mindfulness and its role in psychological well-being. J. Pers. Soc. Psychol. 2003;84(4):822–848. doi: 10.1037/0022-3514.84.4.822. [DOI] [PubMed] [Google Scholar]
- Brown K.W., Ryan R.M., Creswell J.D. Mindfulness: theoretical foundations and evidence for its salutary effects. Psychol. Inq. 2007;18(4):211–237. [Google Scholar]
- Brown K.W., West A.M., Loverich T.M., Biegel G.M. Assessing adolescent mindfulness: validation of an Adapted Mindful Attention Awareness Scale in adolescent normative and psychiatric populations. Psychol. Assess. 2011;23(4):1023–1033. doi: 10.1037/a0021338. [DOI] [PubMed] [Google Scholar]
- Capaldi D.M., Rothbart M.K. Development and validation of an early adolescent temperament measure. J. Early Adolesc. 1992;12(2):153–173. [Google Scholar]
- Carlo G., Crockett L.J., Wolff J.M., Beal S.J. The role of emotional reactivity, self-regulation, and puberty in adolescents’ prosocial behaviors. Soc. Dev. 2012;21(4):667–685. doi: 10.1111/j.1467-9507.2012.00660.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Casey B.J., Galvan A., Hare T.A. Changes in cerebral functional organization during cognitive development. Curr. Opin. Neurobiol. 2005;15(2):239–244. doi: 10.1016/j.conb.2005.03.012. [DOI] [PubMed] [Google Scholar]
- Casey B.J., Jones R.M., Hare T.A. The adolescent brain. Ann. N. Y. Acad. Sci. 2008;1124:111–126. doi: 10.1196/annals.1440.010. (Research Support, N.I.H., Extramural Review) [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chambers R., Gullone E., Allen N.B. Mindful emotion regulation: an integrative review. Clin. Psychol. Rev. 2009;29(6):560–572. doi: 10.1016/j.cpr.2009.06.005. (Review) [DOI] [PubMed] [Google Scholar]
- Chambers R., Gullone E., Hassed C., Knight W., Garvin T., Allen N. Mindful emotion regulation predicts recovery in depressed youth. Mindfulness. 2014;6(3):523–534. [Google Scholar]
- Colom R. Intelligence? What intelligence? Behav. Brain Sci. 2007;30(2):155–156. [Google Scholar]
- Cook E.C., Buehler C., Blair B.L. Adolescents’ emotional reactivity across relationship contexts. Dev. Psychol. 2013;49(2):341–352. doi: 10.1037/a0028342. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Craig A. Interoception: the sense of the physiological condition of the body. Curr. Opin. Neurobiol. 2003;13(4):500–505. doi: 10.1016/s0959-4388(03)00090-4. [DOI] [PubMed] [Google Scholar]
- Craig A.D. How do you feel – now? The anterior insula and human awareness. Nat. Rev. Neurosci. 2009;10(1):59–70. doi: 10.1038/nrn2555. [DOI] [PubMed] [Google Scholar]
- Creswell J.D., Way B.M., Eisenberger N.I., Lieberman M.D. Neural correlates of dispositional mindfulness during affect labeling. Psychosom. Med. 2007;69(6):560–565. doi: 10.1097/PSY.0b013e3180f6171f. (Controlled Clinical Trial Research Support, N.I.H., Extramural) [DOI] [PubMed] [Google Scholar]
- Critchley H.D. Neural mechanisms of autonomic, affective, and cognitive integration. J. Comp. Neurol. 2005;493(1):154–166. doi: 10.1002/cne.20749. [DOI] [PubMed] [Google Scholar]
- Critchley H.D., Wiens S., Rotshtein P., Arne m., Dolan R.J. Neural systems supporting interoceptive awareness. Nat. Neurosci. 2004;7(2):189–195. doi: 10.1038/nn1176. [DOI] [PubMed] [Google Scholar]
- Desikan R.S., Segonne F., Fischl B., Quinn B.T., Dickerson B.C., Blacker D., Killiany R.J. An automated labeling system for subdividing the human cerebral cortex on MRI scans into gyral based regions of interest. Neuroimage. 2006;31(3):968–980. doi: 10.1016/j.neuroimage.2006.01.021. [DOI] [PubMed] [Google Scholar]
- Destrieux C., Fischl B., Dale A., Halgren E. Automatic parcellation of human cortical gyri and sulci using standard anatomical nomenclature. Neuroimage. 2010;53(1):1–15. doi: 10.1016/j.neuroimage.2010.06.010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Erickson C.A., Chambers R.A. American Psychiatric Publishing, Inc.; Arlington, VA: 2007. Adolescence: Neurodevelopment and Behavioral Impulsivity Textbook of Men's Mental Health; pp. 23–46. [Google Scholar]
- Farb N.A.S., Segal Z.V., Anderson A.K. Mindfulness meditation training alters cortical representations of interoceptive attention. Soc. Cogn. Affect. Neurosci. 2013;8(1):15–26. doi: 10.1093/scan/nss066. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fetterman A.K., Robinson M.D., Ode S., Gordon K.H. Neuroticism as a risk factor for behavioral dysregulation: a mindfulness-mediation perspective. J. Soc. Clin. Psychol. 2010;29(3):301–321. [Google Scholar]
- Giedd J.N., Blumenthal J., Jeffries N.O., Castellanos F.X., Liu H., Zijdenbos A., Rapoport J.L. Brain development during childhood and adolescence: a longitudinal MRI study. Nat. Neurosci. 1999;2(10):861–863. doi: 10.1038/13158. [DOI] [PubMed] [Google Scholar]
- Giedd J.N., Lalonde F.M., Celano M.J., White S.L., Wallace G.L., Lee N.R., Lenroot R.K. Anatomical brain magnetic resonance imaging of typically developing children and adolescents. J. Am. Acad. Child Adolesc. Psychiatry. 2009;48(5):465–470. doi: 10.1097/CHI.0b013e31819f2715. (Research Support, N.I.H., Intramural Review) [DOI] [PMC free article] [PubMed] [Google Scholar]
- Giuliani N.R., Drabant E.M., Bhatnagar R., Gross J.J. Emotion regulation and brain plasticity: expressive suppression use predicts anterior insula volume. Neuroimage. 2011;58(1):10–15. doi: 10.1016/j.neuroimage.2011.06.028. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gladwin T.E., Figner B., Crone E.A., Wiers R.W. Addiction, adolescence, and the integration of control and motivation. Dev. Cogn. Neurosci. 2011;1(4):364–376. doi: 10.1016/j.dcn.2011.06.008. (Research Support, Non-U.S. Gov’t Research Support, U.S. Gov’t, Non-P.H.S. Review) [DOI] [PMC free article] [PubMed] [Google Scholar]
- Goldin P.R., McRae K., Ramel W., Gross J.J. The neural bases of emotion regulation: reappraisal and suppression of negative emotion. Biol. Psychiatry. 2008;63(6):577–586. doi: 10.1016/j.biopsych.2007.05.031. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gross J.J., John O.P. Individual differences in two emotion regulation processes: implications for affect, relationships, and well-being. J. Pers. Soc. Psychol. 2003;85(2):348–362. doi: 10.1037/0022-3514.85.2.348. [DOI] [PubMed] [Google Scholar]
- Grossman P. On measuring mindfulness in psychosomatic and psychological research. J. Psychosom. Res. 2008;64(4):405–408. doi: 10.1016/j.jpsychores.2008.02.001. [DOI] [PubMed] [Google Scholar]
- Grossman P., Kappos L., Gensicke H., D'Souza M., Mohr D.C., Penner I.K., Steiner C. MS quality of life, depression, and fatigue improve after mindfulness training: a randomized trial. Neurology. 2010;75(13):1141–1149. doi: 10.1212/WNL.0b013e3181f4d80d. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hölzel B.K., Lazar S.W., Gard T., Schuman-Olivier Z., Vago D.R., Ott U. How does mindfulness meditation work? Proposing mechanisms of action from a conceptual and neural perspective. Perspect. Psychol. Sci. 2011;6(6):537–559. doi: 10.1177/1745691611419671. [DOI] [PubMed] [Google Scholar]
- Hölzel B.K., Ott U., Gard T., Hempel H., Weygandt M., Morgen K., Vaitl D. Investigation of mindfulness meditation practitioners with voxel-based morphometry. Soc. Cogn. Affect. Neurosci. 2008;3(1):55–61. doi: 10.1093/scan/nsm038. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jha A.P., Stanley E.A., Kiyonaga A., Wong L., Gelfand L. Examining the protective effects of mindfulness training on working memory capacity and affective experience. Emotion. 2010;10(1):54–64. doi: 10.1037/a0018438. [DOI] [PubMed] [Google Scholar]
- Jones F.L., McMillan J. Scoring occupational categories for social research: a review of current practice, with Australian examples. Work Employ. Soc. 2001;15(3):539–563. [Google Scholar]
- Kabat-Zinn J. An outpatient program in behavioral medicine for chronic pain patients based on the practice of mindfulness meditation: theoretical considerations and preliminary results. Gen. Hosp. Psychiatry. 1982;4(1):33–47. doi: 10.1016/0163-8343(82)90026-3. [DOI] [PubMed] [Google Scholar]
- Kabat-Zinn J. Mindfulness-based interventions in context: past, present, and future. Clin. Psychol.: Sci. Pract. 2003;10(2):144. [Google Scholar]
- Kang D.-H., Jo H.J., Jung W.H., Kim S.H., Jung Y.-H., Choi C.-H., Kwon J.S. The effect of meditation on brain structure: cortical thickness mapping and diffusion tensor imaging. Soc. Cogn. Affect. Neurosci. 2013;8(1):27–33. doi: 10.1093/scan/nss056. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kumar S., Feldman G., Hayes A. Changes in mindfulness and emotion regulation in an exposure-based cognitive therapy for depression. Cogn. Ther. Res. 2008;32(6):734–744. [Google Scholar]
- Kuperberg G.R., Broome M.R., McGuire P.K. Regionally localized thinning of the cerebral cortex in schizophrenia. Arch. Gen. Psychiatry. 2003;60(9):878–888. doi: 10.1001/archpsyc.60.9.878. [DOI] [PubMed] [Google Scholar]
- Lakey C.E., Campbell W.K., Brown K.W., Goodie A.S. Dispositional mindfulness as a predictor of the severity of gambling outcomes. Personal. Individ. Differ. 2007;43(7):1698–1710. doi: 10.1016/j.paid.2007.05.007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lau M.A., Bishop S.R., Segal Z.V., Buis T., Anderson N.D., Carlson L., Devins G. The Toronto Mindfulness Scale: development and validation. J. Clin. Psychol. 2006;62(12):1445–1467. doi: 10.1002/jclp.20326. [DOI] [PubMed] [Google Scholar]
- Lazar S.W., Kerr C.E., Wasserman R.H., Gray J.R., Greve D.N., Treadway M.T., Fischl B. Meditation experience is associated with increased cortical thickness. NeuroReport. 2005;16(17):1893–1897. doi: 10.1097/01.wnr.0000186598.66243.19. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Liotti M., Brannan S., Egan G., Shade R., Madden L., Abplanalp B., Denton D. Brain responses associated with consciousness of breathlessness (air hunger) Proc. Natl. Acad. Sci. U. S. A. 2001;98(4):2035–2040. doi: 10.1073/pnas.98.4.2035. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Luders E., Toga A.W., Lepore N., Gaser C. The underlying anatomical correlates of long-term meditation: larger hippocampal and frontal volumes of gray matter. Neuroimage. 2009;45(3):672–678. doi: 10.1016/j.neuroimage.2008.12.061. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Maguire E.A., Spiers H.J., Good C.D., Hartley T., Frackowiak R.S.J., Burgess N. Navigation expertise and the human hippocampus: a structural brain imaging analysis. Hippocampus. 2003;13(2):250–259. doi: 10.1002/hipo.10087. [DOI] [PubMed] [Google Scholar]
- Modinos G., Ormel J., Aleman A. Individual differences in dispositional mindfulness and brain activity involved in reappraisal of emotion. Soc. Cogn. Affect. Neurosci. 2010;5(4):369–377. doi: 10.1093/scan/nsq006. (Research Support, Non-U.S. Gov’t) [DOI] [PMC free article] [PubMed] [Google Scholar]
- Murakami H., Nakao T., Matsunaga M., Kasuya Y., Shinoda J., Yamada J., Ohira H. The structure of mindful brain. PLoS ONE. 2012;7(9) doi: 10.1371/journal.pone.0046377. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Naqvi N.H., Bechara A. The insula and drug addiction: an interoceptive view of pleasure, urges, and decision-making. Brain Struct. Funct. 2010;214(5–6):435–450. doi: 10.1007/s00429-010-0268-7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Narr K.L., Woods R.P., Lin J., Kim J., Phillips O.R., Del’Homme M., Levitt J.G. Widespread cortical thinning is a robust anatomical marker for attention-deficit/hyperactivity disorder. J. Am. Acad. Child Adolesc. Psychiatry. 2009;48(10):1014–1022. doi: 10.1097/CHI.0b013e3181b395c0. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Nyklíček I., Kuijpers K.F. Effects of mindfulness-based stress reduction intervention on psychological well-being and quality of life: is increased mindfulness indeed the mechanism? Ann. Behav. Med. 2008;35(3):331–340. doi: 10.1007/s12160-008-9030-2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- O’Donnell L., Naglieri J.A., Goldstein S. 4th ed. John Wiley & Sons Inc.; Hoboken, NJ: 2009. The Wechsler Intelligence Scale for Children. [Google Scholar]
- Pearson M.R., Murphy E.M., Doane A.N. Impulsivity-like traits and risky driving behaviors among college students. Accid. Anal. Prev. 2013;53:142–148. doi: 10.1016/j.aap.2013.01.009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Raes F., Williams J.M.G. The relationship between mindfulness and uncontrollability of ruminative thinking. Mindfulness. 2010;1(4):199–203. [Google Scholar]
- Reuter M., Fischl B. Avoiding asymmetry-induced bias in longitudinal image processing. Neuroimage. 2011;57(1):19–21. doi: 10.1016/j.neuroimage.2011.02.076. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Shapiro S.L., Astin J.A., Bishop S.R., Cordova M. Mindfulness-based stress reduction for health care professionals: results from a randomized trial. Int. J. Stress Manag. 2005;12(2):164–176. [Google Scholar]
- Shapiro S.L., Oman D., Thoresen C.E., Plante T.G., Flinders T. Cultivating mindfulness: effects on well-being. J. Clin. Psychol. 2008;64(7):840. doi: 10.1002/jclp.20491. [DOI] [PubMed] [Google Scholar]
- Shaw P., Greenstein D., Lerch J., Clasen L., Lenroot R., Gogtay N., Giedd J.N. Intellectual ability and cortical development in children and adolescents. Nature. 2006;440(7084):676–679. doi: 10.1038/nature04513. (Research Support, N.I.H., Intramural) [DOI] [PubMed] [Google Scholar]
- Shaw P., Kabani N.J., Lerch J.P., Eckstrand K., Lenroot R., Gogtay N., Wise S.P. Neurodevelopmental trajectories of the human cerebral cortex. J. Neurosci. 2008;28(14):3586–3594. doi: 10.1523/JNEUROSCI.5309-07.2008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Singer T., Critchley H.D., Preuschoff K. A common role of insula in feelings, empathy and uncertainty. Trends Cogn. Sci. 2009;13(8):334–340. doi: 10.1016/j.tics.2009.05.001. [DOI] [PubMed] [Google Scholar]
- Somerville L.H., Jones R.M., Casey B.J. A time of change: behavioral and neural correlates of adolescent sensitivity to appetitive and aversive environmental cues. Brain Cogn. 2010;72(1):124–133. doi: 10.1016/j.bandc.2009.07.003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Spear L.P. WW Norton & Co.; New York, NY: 2010. The Behavioral Neuroscience of Adolescence. [Google Scholar]
- Steinberg L. Cognitive and affective development in adolescence. Trends Cogn. Sci. 2005;9(2):69–74. doi: 10.1016/j.tics.2004.12.005. [DOI] [PubMed] [Google Scholar]
- Steinberg L. A dual systems model of adolescent risk-taking. Dev. Psychobiol. 2010;52(3):216–224. doi: 10.1002/dev.20445. [DOI] [PubMed] [Google Scholar]
- Tamnes C.K., Oestby Y., Fjell A.M., Westlye L.T., Due-Toennessen P., Walhovd K.B. Brain maturation in adolescence and young adulthood: regional age-related changes in cortical thickness and white matter volume and microstructure. Cereb. Cortex. 2010;20(3):534–548. doi: 10.1093/cercor/bhp118. [DOI] [PubMed] [Google Scholar]
- Tamnes C.K., Østby Y., Walhovd K.B., Westlye L.T., Due-Tønnessen P., Fjell A.M. Neuroanatomical correlates of executive functions in children and adolescents: a magnetic resonance imaging (MRI) study of cortical thickness. Neuropsychologia. 2010;48(9):2496–2508. doi: 10.1016/j.neuropsychologia.2010.04.024. [DOI] [PubMed] [Google Scholar]
- Tamnes C.K., Walhovd K.B., Grydeland H., Holland D., Østby Y., Dale A.M., Fjell A.M. Longitudinal working memory development is related to structural maturation of frontal and parietal cortices. J. Cogn. Neurosci. 2013;25(10):1611–1623. doi: 10.1162/jocn_a_00434. [DOI] [PubMed] [Google Scholar]
- Terasawa Y., Shibata M., Moriguchi Y., Umeda S. Anterior insular cortex mediates bodily sensibility and social anxiety. Soc. Cogn. Affect. Neurosci. 2013;8(3):259–266. doi: 10.1093/scan/nss108. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Thompson B.L., Waltz J. Everyday mindfulness and mindfulness meditation: overlapping constructs or not? Personal. Individ. Differ. 2007;43(7):1875–1885. [Google Scholar]
- Turner R.A., Irwin C.E., Tschann J.M., Millstein S.G. Autonomy, relatedness, and the initiation of health risk behaviors in early adolescence. Health Psychol. 1993;12(3):200–208. doi: 10.1037//0278-6133.12.3.200. [DOI] [PubMed] [Google Scholar]
- van de Weijer-Bergsma E., Formsma A.R., Bruin E.I., Bögels S.M. The effectiveness of mindfulness training on behavioral problems and attentional functioning in adolescents with ADHD. J. Child Fam. Stud. 2012;21(5):775–787. doi: 10.1007/s10826-011-9531-7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Vestergaard-Poulsen P., van Beek M., Skewes J., Bjarkam C.R., Stubberup M., Bertelsen J., Roepstorff A. Long-term meditation is associated with increased gray matter density in the brain stem. NeuroReport. 2009;20(2):170–174. doi: 10.1097/WNR.0b013e328320012a. [DOI] [PubMed] [Google Scholar]
- Vijayakumar N., Whittle S., Dennison M., Yücel M., Simmons J., Allen N.B. Development of temperamental effortful control mediates the relationship between maturation of the prefrontal cortex and psychopathology during adolescence: a 4-year longitudinal study. Dev. Cogn. Neurosci. 2014;9:30–43. doi: 10.1016/j.dcn.2013.12.002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Vijayakumar N., Whittle S., Yuecel M., Dennison M., Simmons J., Allen N.B. Prefrontal structural correlates of cognitive control during adolescent development: a 4-year longitudinal study. J. Cogn. Neurosci. 2014;26(5):1118. doi: 10.1162/jocn_a_00549. [DOI] [PubMed] [Google Scholar]
- Whittle S., Lichter R., Dennison M., Vijayakumar N., Schwartz O., Byrne M.L., Allen N.B. Structural brain development and depression onset during adolescence: a prospective longitudinal study. Am. J. Psychiatry. 2014;171(5):564–571. doi: 10.1176/appi.ajp.2013.13070920. [DOI] [PubMed] [Google Scholar]
- Whittle S., Yap M.B.H., Sheeber L., Dudgeon P., Yücel M., Pantelis C., Allen N.B. Hippocampal volume and sensitivity to maternal aggressive behavior: a prospective study of adolescent depressive symptoms. Dev. Psychopathol. 2011;23(1):115–129. doi: 10.1017/S0954579410000684. [DOI] [PubMed] [Google Scholar]
- Zheng W., Chee M.W.L., Zagorodnov V. Improvement of brain segmentation accuracy by optimizing non-uniformity correction using N3. Neuroimage. 2009;48(1):73–83. doi: 10.1016/j.neuroimage.2009.06.039. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.