Abstract
Chronic low back pain has both substantial social and economic impacts on patients and healthcare budgets. Adding to the magnitude of the problem is the difficulty in identifying the exact causes of disc degeneration with modern day diagnostic and imaging techniques. With that said, current non-operative and surgical treatment modalities for discogenic low back pain fails to meet the expectations in many patients and hence the challenge. The objective for newly emerging stem cell regenerative therapy is to treat degenerative disc disease (DDD) by restoring the disc’s cellularity and modulating the inflammatory response. Appropriate patient selection is crucial for the success of stem cell therapy. Regenerative modalities for discogenic pain currently focus on the use of either primary cells harvested from the intervertebral discs or stem cells from other sources whether autogenic or allogenic. The microenvironment in which stem cells are being cultured has been recognized to play a crucial role in directing or maintaining the production of the desired phenotypes and may enhance their regenerative potential. This has led to a more specific focus on innovating more effective culturing techniques, delivery vehicles and scaffolds for stem cell application. Although stem cell therapy might offer an attractive alternative treatment option, more clinical studies are still needed to establish on the safety and feasibility of such therapy. In this literature review, we aim to present the most recent in vivo and in vitro studies related to the use of stem cell therapy in the treatment of discogenic low back pain.
Keywords: Stem cell transplantation, intervertebral disc degeneration, low back pain, regenerative medicine, tissue scaffolds
Introduction
Chronic low back pain affects approximately 632 million people worldwide with prevalence as high as 68% in adults older than 60 years of age (1,2). It has both substantial social and economic impacts on patients and healthcare budgets resulting in the highest economic burden among all musculoskeletal complaints (3). This tremendous impact was portrayed in a prospective multicenter study including 8 industrialized countries which found that patients with chronic spinal conditions have lower quality of life scores when compared with patients with other chronic conditions such as arthritis, chronic lung disease, congestive heart failure, and diabetes (4). Stem cell therapy for degenerative disc disease (DDD) is a relatively novel approach with promising results as an alternative to the conventional surgical and non-operative regimens (5). The purpose of this article is to review the current available evidence and provide a foundation for future research. In this article, we review the literature and present the stem cell regenerative therapies in DDD, different stem cell sources and their delivery mechanisms in the degenerated disc, as well as an overview of the challenges facing the implementation of this technique.
Methods
A comprehensive literature search was performed independently by two authors (AH Barakat, VA Elwell) and the results were collated and de-duplicated. The literature search was conducted on MEDLINE and EMBASE databases from database inception through on January 12th, 2019. The following Mesh terms were used (“Stem Cells”[Mesh]) AND (“Back Pain”[Mesh] OR “Low Back Pain”[Mesh]). Thesaurus terms were adapted for different databases. The search was limited to English language literature with all non-English papers excluded. A total of 286 papers were identified, reviewed and assessed. The studies cited here covers the most recent advances in this rapidly expanding field.
Pathogenesis of discogenic pain
The exact causes of disc degeneration are complex and difficult to pinpoint; they involve aging, genetic predispositions, nutritional factors as obesity, mechanical trauma, smoking and other related comorbidities (6-12).
The adult intervertebral disc (IVD) is an avascular organ that relies on passive diffusion from adjacent endplate vessels for nutrition, resulting in poor inherent healing potential (13,14). The centrally located gelatinous nucleus pulposus (NP) is a hypoxic, hydrophilic proteoglycan-rich structure consisting mainly of type II collagen, while the outer lamellated anulus fibrosus (AF) is a fibrous ring mostly of type I collagen (15-17). The extracellular matrix (ECM) of the disc is formed of water, proteoglycans and matrix proteins. The water content of the disc is dependent on both the proteoglycan and collagen contents, with the proteoglycan providing the swelling pressure through retaining water and the collagen providing the resistance to swelling. The proteoglycan and thus water content of the disc increases on progressing from the outer AF to the NP. Conversely, the collagen content of the disc decreases from the outward inward.
Aging is associated with lower water and proteoglycan content along with high collagen concentrations (18). Histologically, there is progressive loss of demarcation between the NP and AF with loss of the transition zone. This is due to a change in collagen synthesis by the NP cells from collagen II to collagen I with subsequent loss of proteoglycan and dehydration (19). The main proteoglycan of the IVD is aggrecan, it is present in both the NP and the AF and possesses both chondroitin sulfate and keratan sulfate chains bound to a protein core (20). With aging, there is a decrease in the chondroitin sulfate chain length and an increase in keratan sulfate chain lengths. This reciprocal change in chain lengths has been associated with the decreased oxygen supply to the disc with aging. The reason is that, oxidation is a prerequisite for the formation of the glucuronic acid needed for chondroitin sulfate synthesis however it is not for keratan sulfate synthesis. Aggrecan binds to hyaluronan through a link protein to form proteoglycan aggregates responsible for maintaining the swelling pressure. This binding capacity decreases with age leading to decreased aggregates and increased hyaluronan concentration in a degenerating disc (21).
Another constituent of the ECM is the matrix metalloproteinases (MMPs). MMPs play a major role in ECM degradation within the disc with aging as a result of an imbalance in their turnover and activation relative to loss of their inhibitors (22). These MMPs are primarily activated in acidic PH of the degenerated IVDs owing to their decreased nutrition and more anerobic metabolism with accumulation of lactate. This decreased nutrition is mainly due to growth of the disc size and calcification of the end plates.
Subsequent to the diminished disc nutrition, the cell density of the disc decreases with aging (23). During embryonic development, the notochord gives rise to the NP of the disc, whereas the surrounding AF is developed from the mesenchymal tissue. With the aging process, the notochordal cells (NC) in the NP are replaced by cells of mesenchymal origin, which have a chondrocyte and fibroblast-like appearance. This further explains the poor healing potential with aging as the NCs which have the regenerative capacity are depleted (24).
The above-mentioned changes including the decrease in water and proteoglycan aggregate content along with increasing fibrous nature of the NP causes the disc narrowing observed radiographically (25). Loss of the disc’s intrinsic hydrostatic pressure owing to its dehydration, affects the disc’s resilience to mechanical loading (12). The result is disc space narrowing, bulging and eventually osteophyte formation with end-plate sclerosis. This causes pressure on the nerve roots leading to back pain and eventually weakness and numbness. With the progression of IVD degeneration, the disc tends to be vascularized increasingly starting peripherally via angiogenesis. Vascular ingrowth eventually extends centrally into the NP and is associated with innervation of the disc causing discogenic pain (26).
Another important factor to be considered in the pathogenesis of DDD is biomechanical loading stresses. It is now appreciated that the metabolism of disc cells is enhanced by physiologic intermittent compressive loading which increases production of both proteoglycans and tissue inhibitors of MMPs (27). Genetic factors and familial predisposition are also recognized etiological factors for DDD. An immunogenetic epidemiologic study on 678 patients has strongly implicated both genetic and environmental factors in the etiology of DDD (28). This was supported by further epidemiologic studies which concluded a strong familial predisposition to DDD (29). It has been shown that DDD may be accelerated in some individuals because of genetic polymorphisms in genes such as the aggrecan or the MMPs genes (30). Obesity has been implicated in DDD owing to the biomechanical overloading as well as the metabolic element in these patients. A radiological MRI study on 129 middle-aged men showed a detrimental effect of obesity on DDD (31).
Both non-operative and surgical treatments have failed to meet many patient’s expectations in providing a satisfactory means for managing this condition. In a large-scale controlled study on 1,450 patients focusing on the outcome measure [return to work (RTW)], it was revealed that only 26% of patients RTW 2 years after fusion surgery, while 67% of non-operative controls had RTW within 2 years from the date of injury (32).
Initially, recruitment of conservative therapy including the use of nonsteroidal anti-inflammatory drugs (NSAIDs), muscle relaxants, opioids and physiotherapy leads to improvement in most patients. However, for non-responsive patients after exhausting conservative options, lumbar spinal fusion as the standard of surgical treatment can present with significant complications and morbidity (33).
Complications related to spine surgery include deep vein thrombosis, infection, and myocardial infarcts occurring in 6.6% of initial surgeries and in 6.3% of revisions (34,35). Adjacent segment disease and proximal junctional kyphosis are recognized as sequela of spinal fusions by comprising the mechanics of the spine at levels above and below the fusion and ensuing abnormal stresses during spinal motion (36). Other complications include pseudarthrosis and hardware complications. Stem cell-based therapies have advanced significantly over the past decade with numerous clinical trials aiming at providing a non-surgical biologic approach to improve pain and function in DDD. In this approach, the abnormal conditions of the diminished cellularity and altered cell phenotype are the targets for correction.
Patient selection
The objective for stem cell regenerative therapy is to treat DDD by restoring the disc’s cellularity and modulating the inflammatory response. Appropriate patient selection is crucial for the success of stem cell therapy. The optimum window for intervention is the following: early stages of degeneration, failure to respond to conservative treatment and prior to the onset of advanced disc height collapse and herniation (37). There is a consensus that the ideal candidates for such regenerative therapies should be limited for patients with moderate chronic back pain and disability as confirmed by functional scores, with affection of single-level disc as proven by radiological Pfirrmann grading of lumbosacral disc degeneration Grade III or IV on MRI (38). Advanced donor age for stem cells and co-morbidities are other factors that needs to be considered as MSCs may exhibit different phenotypic regenerative potentials (39,40).
Cell sources
Regenerative modalities for discogenic pain are currently focusing on the use of either primary cells harvested from the IVD or stem cells from other sources whether autogenic or allogenic.
Primary cells
Studies on primary and native stem cells for DDD have gained interest over the past decade. Several studies have confirmed the presence of NP and AF progenitor cells in IVD tissues possessing multipotential differentiation capabilities (41-44). These progenitor cells are thought to be responsible for regenerative capacity and homeostasis of IVD tissue, while their age-related depletion may be responsible for the loss of this regenerative and reparative capacity of IVDs. Mesenchymal stem cell (MSC) markers have been identified in both NP and AF.
Progenitor niches of importance are the notochord cells (NCs) known as NP progenitor cells (NPPCs). Sakai et al. identified NPPCs in the NP tissue via their tunica intima endothelial kinase (Tie2+) and disialoganglioside (GD2+) surface markers (45). Tie2 is a receptor tyrosine kinase receptor expressed in hematopoietic and neural stem cells while GD2 is a plasma membrane marker for bone marrow (BM) and umbilical cord MSCs (46-50). It was found that angiopoietin 1, which is a Tie2 ligand, plays a pivotal role in maintaining the NPPCs and protecting the cells from apoptosis. This might may lead to future research aiming to develop reliable methods with which to isolate, maintain, and expand these progenitor cells (51).
Regarding the AF progenitor cells, studies have demonstrated that AF-specific progenitor cells were present in both nondegenerative and degenerated IVDs (52). A unique feature of these cells is their potential to differentiate to different cell lineages including adipocytes, chondrocytes, osteoblasts, neural and endothelial cells.
Despite that the feasibility of isolating pure native disc progenitor cells without fibroblasts and macrophages was proven to be challenging, incorporation of IVD tissue-specific progenitors into tissue engineered scaffolds would significantly impact the regeneration potential and efficacy of tissue-engineered IVD constructs.
To overcome this difficulty and in resemblance to the autologous chondrocyte implantation techniques used in degenerated cartilage elsewhere, autologous isolated IVD disc cells were stimulated in conditioned media and re-implanted back into the same degenerated areas from where they were harvested. A canine model demonstrated after 2-year of follow-up, disc persistent cell viability, proliferative capacity, ECM synthetic ability and proteoglycan content (53).
The Euro disc randomized trial is a prospective, randomized, controlled, multicenter study comparing autologous disc chondrocyte transplantation plus discectomy versus discectomy alone in 112 patients (54). At the time of discectomy, autologous disc chondrocytes were sequestered and expanded in culture then reinjected into the disc after 12 weeks. This study demonstrated a clinically significant reduction in low back pain scores in the patients who received autologous disc cell transplantation after discectomy compared with those who had discectomy alone. Furthermore, the MRI of the treatment group revealed 41%-disc hydration when compared to 25% in the adjacent levels that had undergone discectomy without autologous disc chondrocyte transplantation. Mochida et al. (55) reported that such treatment has proven safety and efficacy in a 3-year follow-up with no major side effects and with good clinical results.
Owing to the practical and surgical risks in obtaining autologous primary NP tissue from either herniated or adjacent discs, motivation in identifying and characterizing alternative cell sources for disc regeneration has also been pursued (56,57).
Other accessible cell sources with reduced risk for donor site morbidity and relative ease of isolation, such as articular and nasal cartilage, have been investigated in vitro and in animal models for NP regeneration (58,59). These cell sources are still in the state of infancy and further research is required.
MSC
MSC transplantation has received considerable attention due to their versatility, and potential for stimulating a healthier host tissue microenvironment by their paracrine effects. MSCs are stem cells that have extensive proliferative capacity and multi-lineage potential in vitro and in vivo (60). The effects of MSCs in delaying and even reversing the degenerative cascade in IVDs has been well documented in many experimental studies including different animal models prior to being translated for clinical use (61-63). The transplanted cells not only restore the cell population in degenerated IVDs but also were capable of ECM and aggrecan production, leading to an increase in IVD height (64). Multiple tissue sources have been described including BM, adipose tissue, muscle and more recently from umbilical cord (65-68).
BM and adipose derived (AD) MSCs are the most frequently utilized sources for this purpose. However, these are usually associated with procedures for harvesting the cells such as BM aspiration or liposuction. As a result, studies on using MSCs derived from umbilical cord Wharton’s jelly have been conducted and may prove to be a potentially favorable alternative (16). A case study on two patients was conducted using human umbilical cord MSCs (HUC-MSCs) with favorable outcomes regarding pain and disability as surveyed by the visual analogue score (VAS) and the Oswestry functional score (69).
BM derived MSCs are of particular interest since they are easily accessible and isolated. Thus, they have been extensively studied as a prime site and working horse for MSC isolation for the purposes of treatment of DDD (70). Intradiscal BM derived allogeneic MSCs are currently being explored in a phase II randomized controlled study (71). Single-level mild degenerated lumbar IVDs were selected. Preliminary data show that a greater number of patients treated with intradiscal MSCs reported ≥50% reduction in low back pain compared with controls at 12 months after injection. Specifically, of the patients treated with intradiscal MSCs, 69% reported this successful outcome, compared with only 33% of control patients. Studies on autologous stem cells derived from BM have shown promise manifested by improvement in clinical outcomes in human trials. In a case series study by Orozco et al. (72) autologous BM MSCs were injected into the NP of 10 patients with chronic back pain and followed up for 1 year resulted in improvement of clinical symptoms with no reported adverse effects. The patients were functionally analyzed using the VAS, Oswestry Disability Index (ODI), and 36-Item Short Form Health Survey (SF-36). MRI measurements of disc height and fluid content were also done. There was a rapid initial improvement in pain and disability at 3 months followed by modest additional improvements at 12 months. Water content increased, but disc height did not recover.
In one prospective study, 33 patients with lower back pain and disc degeneration were treated with culture-expanded, autologous, BM-derived MSCs with follow-up period up to 6 years (73). The study has proven safety with only minor adverse events and significant improvements in pain, function, and overall subjective improvement as evidenced by numeric pain score (NPS), modified single assessment numeric evaluation (SANE) rating and functional rating index (FRI). Measurement of the intervertebral disc posterior dimension has shown that 85% of the patients who were evaluated by MRI demonstrated a reduction in disc bulge size, with an average reduction size of 23% post treatment.
In a pilot study by Pettine et al. (74) twenty-six patients with discogenic low back pain had autologous BM-MSCs percutaneous injections. There was a significant improvement in VAS and ODI after the 12-month study period with 8 patients having increased disc heights as evidenced by improvement in their Pfirrmann MRI grading. In another study by Yoshikawa et al. (75) similar outcomes were reported after 2-year follow-up.
However, the centrifugation process to obtain autologous stem cells from BM is limited by absence of a high concentration of pure homogeneous MSCs. There is adherence to plastic during the preparation (76,77). One of the emerging separation methods of stem cells other than centrifugation is the cell SELEX (systematic evolution of ligands by exponential enrichment) technique (78). SELEX uses aptamers to selectively capture target cells. Aptamers are modified nucleic acids identified from large nucleic acid libraries by their high affinity to target molecules and specific cells in similarity to antibodies targeting. The concept is still evolving and is now limited by specificity and insufficient collection of high purity stem cells.
Progenitor cells derived from other tissue sources such as adipose tissue have also been shown to possess significant potential for differentiation and tissue forming capabilities (79,80). The ease of harvesting of autologous adipose derived stem cells (AD-MSCs) can be performed as an outpatient setting with yields up to 25,000 MSCs per gram of tissue (81). This has led AD-MSCs to provide a better alternative and candidate for cell therapy and disc regeneration, due to their abundance and ease of isolation. Additionally, they have a lower inherent capacity for endochondral ossification than BM derived MSCs (82).
Furthermore, some studies suggested that AD-MSCs might be a more appropriate cell type than BM-MSCs for IVD regeneration because AD-MSCs could differentiate into cells with a more NP-like phenotype (83). An in vivo model of mice with severely degenerated IVDs treated with AD-MSCs intradiscally also found promising positive radiographic findings (84). This study showed survival of the injected AD-MSCs up to 12 weeks after implantation with significant increase of aggrecan tissue levels. Another in vivo study on a rabbit model showed that the AD-MSCs injected discs exhibited elevated ECM secretion as well as survival of the implanted cells 10 weeks after implantation (85). A canine model-controlled study showed significantly higher production of aggrecan, collagen type II and increased cell density in AD-MSCs treated discs (86). An in vitro Co-culture of AD-MSCs with NP cells in a type II collagen hydrogels caused upregulation of collagen type II and aggrecan gene expression (87).
More recently NCs as nucleus pulposus progenitor cells (NPPCs) for disc regeneration have received considerable interest. NCs are the progenitors of adult NP cells, and their loss in humans during postnatal growth is thought to contribute to the onset of degeneration later in life (88). NCs are crucial since they can generate NP cells and may potentially survive better in the unfavorable microenvironment post transplantation (89). Since NCs are scarcely present in adult human NP tissue and cannot be readily obtained as previously mentioned, most studies have focused on harnessing the therapeutic potential of NC secreted factors by co-culturing with MSCs directing them to a more NP like phenotype (90). This might guide future studies towards the importance of generation of high-quantity and functional NCs from induction of other pluripotent stem cell niches (91).
Recent focus on the isolation of pluripotent stem cells such as induced pluripotent stem cells (iPSCs) or embryonic stem cells (ESCs) could be a potentially promising source for NCs (Table 1). ESCs are pluripotent stem cells derived from the inner cell mass of embryonic blastocyst of a donated in vitro fertilization (IVF) embryo, while iPSCs are adult cells genetically reprogrammed to be pluripotent. These cells are different from the multipotent MSCs. Sheikh et al. (92) demonstrated that ESC-derived chondroprogenitors could potentially differentiate into NCs in a rabbit model of IVD degeneration. Compared with ESCs, use of iPSCs for disc repair may be more attractive due to patient specificity, less ethical concerns and less immune rejection. Liu et al. (89) showed certain effectiveness of using natural NP tissue matrix to direct NC differentiation of iPSCs. To promote differentiation of MSCs, culturing in NC-conditioned media (NCCM) has been reported to increase secretion of glycosaminoglycans (GAG) and type III collagen and led to the production of a more NP like phenotype (93).
Table 1. Comparison of different stem cell types and sources used in treatment of discogenic low back pain.
| Cell type | Cell source | Characteristics |
|---|---|---|
| Nucleus pulposus progenitor cells (NPPC) (45,88) | • Primary cells isolated from IVDs | • Difficulty in isolating pure native disc without fibroblasts and macrophages |
| Annulus fibrosus (AF)-specific progenitor cells (52) | • Possible disc damage during harvest | |
| • Exhaustion and decreased availability with aging | ||
| Autologous intervertebral disc (IVD) cells (53-55) | • Autologous isolated IVD cells stimulated in conditioned media then re-implanted | • Not pure progenitor cells |
| • Possible disc damage during harvest | ||
| Autologous chondrocytes (58,59) | • From more accessible sites as articular or nasal cartilage | • Differences between chondrocytes and NP cells |
| • Mainly in vitro studies | ||
| Mesenchymal stem cells (MSCs) | • Bone marrow (BM) derived (70-75) | • Need for bone marrow aspiration |
| • Absence of a high concentration of pure homogeneous MSCs | ||
| • Higher inherent capacity for endochondral ossification | ||
| • Adipose tissue derived (AD) (79-87) | • Need for adipose tissue liposuction | |
| • Higher abundance of MSCs | ||
| • Could differentiate into cells with a more NP-like phenotype compared to BM-MSCs | ||
| • Lower inherent capacity for endochondral ossification | ||
| • Umbilical cord Wharton’s jelly (16,69) | • More feasible MSC isolation and abundance | |
| • No need for invasive harvesting of stem cells | ||
| • Ethical issues | ||
| Embryonic stem cells (ESCs) (92,93) | • Inner cell mass of the blastocyst of a donated in vitro fertilization embryo | • Could be directed to notochord cells • Tumorigenesis potential due to pluripotency |
| • Ethical issues | ||
| • Allogenic source | ||
| Induced Pluripotent stem cells (iPSCs) (89) | • Specialized cells genetically reprogrammed | • Other source for production of notochord cells |
| • Tumorigenesis potential due to pluripotency | ||
| • More patient specific (Autogenic) |
One of the potential challenges in utilizing stem cells is that they are undifferentiated and are being transplanted into a harsh environment consisting of low cellularity, nutrients and acidic conditions (94). All of these factors could detrimentally impact the differentiation potential and viability of the implanted cells (95).
Effect of microenvironment
The microenvironment in which stem cells are being cultured has been recognized to play a crucial role in directing or maintaining the production of the desired phenotypes and may enhance their regenerative potential. Priming stem cells using growth factors or other environmental factors such as altered oxygen or glucose level has been a subject of rigorous research in vitro and in vivo studies (96-100).
Several studies have shown that exposure to low levels of oxygen enhances MSC differentiation towards NP phenotypes. An in vitro study by Kumar et al. (101) has demonstrated MSCs enhanced differentiation into NP phenotypes under hypoxic conditions by culturing in a biodegradable polymer hydrogel scaffold. This is consistent with other studies, indicating that differentiation of MSCs depends largely on the local microenvironment (102-104). In one small scale human study, the safety and feasibility of an intra-discal injection of autologous, hypoxic cultured BM derived MSCs in five patients with chronic lower back pain was evaluated (105). The follow-up was up to 6 years and utilized clinical examination, MRI radiological evaluation and quality of life questionnaire as an outcome measures for improvement. All patients reported overall improvement, there was improvement in strength with four out of five patient’s leg mobility. At the end of the follow-up period, there were no reported adverse effects or neoplastic transformation by radiological evaluation. The overall results are promising and might pave the way for a larger double-blind, controlled, randomized clinical study with significant number of patients and implementation of validated endpoint measurements as next steps in order to further validate the efficacy of this technique.
Another important factor is glucose levels used in the culture medium of stem cells. An in vitro study by Stolzing et al. (106) on rat BM-MSCs demonstrated that high glucose concentration has a negative effect on MSC colony formation and phenotypic differentiation. This was supported by other authors who have shown that hypoglycemic conditions on human AD-MSCs have a favorable effect on survival and biological behaviors of these cells (107). An in vitro on rat derived BM-MSC showed low glucose enhanced matrix biosynthesis and maintained cell proliferation whereas high osmolarity and low pH conditions reduced biosynthesis and proliferation of the MSCs (108).
High osmolarity have been shown to inhibit DNA damage response and arrest cell proliferation in bovine NP cells. For the MSCs, multiple studies have shown reduced cellular viability and matrix synthesis by disc-like high osmolarity (109-111). PH was also found to be detrimental to stem cell proliferation and ECM expression. An in vitro study on rat derived BM-MSC cultured in different PH levels showed that acidic conditions inhibited Aggrecan expression in the ECM as well as a decrease in proliferation and viability and was associated with a change in cell morphology (112).
Differentiation of stem cells is also affected by mechanical stresses depending on the loading pattern and intensity (113,114). It has been found that transition and phenotypic maturation of the resident NCs to NP-like cells, can be significantly enhanced by static compressive loadings or dynamic hydrostatic pressures (115,116). For example, in vitro studies have demonstrated that radial compressive loadings promoted the AF-like differentiation in BM-MSC (117), whereas the NP-like differentiation of AD-MSC can be significantly enhanced by dynamic compressions (118).
Despite that this review is mainly focusing on stem cell regenerative therapies, it is worthwhile mentioning the effect of growth factors as part of the microenvironment in enhancing phenotypic differentiation. Recent understanding of the cellular and molecular cascade of IVD homeostasis has engendered the hypothesis of priming the cultured stem cells or direct IVD injection with growth factors such as epidermal growth factor (EGF) and recombinant human growth and differentiation factor-5 (rhGDF-5) to enhance cell proliferation and matrix synthesis (119).
RhGDF-5 is a member of the transforming growth factor-b (TGF-b) superfamily and the bone morphogenetic (BMP) subfamily, and is known to influence the growth and differentiation of various tissues, including the intervertebral disc. Various in vitro studies on rhGDF-5 has demonstrated that it has a key role in suppressing ECM degradation by suppression of matrix metalloproteases and in enhancing the proliferation and matrix anabolism through increased production of aggrecan, GAG and collagen type II (120-122). Moreover, it was reported by one study that a combination of rhGDF-5 and hypoxia had a synergistic effect on NP-like differentiation of MSCs (123).
There are challenges in direct application of growth factors causing a sustainable effect on tissue remodeling to be unreliable. For example, the half-lives, interstitial solubility of these factors, the presence of other inhibiting factors and low numbers of viable cells available to be stimulated in already degenerated IVD disks (124). A different approach is to try to control the catabolic pathway leading to IVD degeneration. By antagonizing the transcription factors that activate the proteolytic genes contributing to IVD degeneration such as receptor activator of nuclear factor-k B ligand (RANKL), the degenerative cascade might be halted (125). Upregulation of MMP and ADAMTS expression has been implicated in disc ECM destruction, leading to the cascade of IVD degeneration (126). Exposure of MSCs to inflammatory factors (IL-1b and TNF-a) might negatively modulate the MSC differentiation potential by promoting osteogenic-like mineral deposition, which is not desirable for disk repair (127). These findings might provide alternative future therapeutics depending on administration of specific antagonists of these proteins directly into the disk to prevent pathological proteolysis of disc ECM.
To overcome the temporary effects and the obstacles of such factors when directly applied to IVDs, another strategy has been developed which is gene therapy aiming to silence catabolic or activating anabolic pathways in the degenerated IVDs. Gene therapy has advantages over direct delivery of proteins. For example, possibility of sustained long-term efficacy and maintained endogenous synthesis of growth factors or anti-inflammatory factors (128,129). The desired nucleic acids are being commonly delivered using a viral vector, so that the functional status of recipient cells can be modified. For example, an in vitro study has also been established using cultured human IVD cells transfected with adenoviral vectors carrying inhibitor to interleukin-1 (IL-1), an important cytokine in the inflammatory cascade, in degenerate IVD (130). The inhibitory effects on the production of IL-1 was maintained for the 2 weeks period of the study duration. Another alternative for the use of gene transfer is to stimulate the anabolic pathways. Various studies have demonstrated successful maintained regenerative effects after transfection of IVD cells. The transfected cells have shown improved ability of ECM production and collagen II synthesis through utilizing genes responsible for production of BMP-2, LIM mineralization protein-1 (LMP-1), chondroitinase ABC, TIMP (tissue inhibitor of metalloproteinases) and SOX9Jeny (131-133). Nonetheless, despite its potential safety concerns, immunogenicity and tumorigenesis potential for such modality needs further investigations.
Another alternative, other than direct injection of growth factors or their vector mediated delivery through gene therapy, is to simply prime and direct MSCs differentiation during the culturing process, into IVD-like phenotypes with induction by growth differentiation factor 6 (GDF6), bone morphogenic protein-2 (BMP-2) and transforming growth factor-beta (TGF-β) (134). Numerous in vitro and animal studies have portrayed that TGF-β signaling in growth plate chondrocytes and inner AF cells to be critical in growth and maintenance of matrix tissue and cellularity of endplate cartilage cells (135-138). It was shown by Clarke et al. that both BM-MSCs and AD-MSCs primed with GDF6 have demonstrated through identification of NP marker genes; increased phenotypic differentiation into NP cells with secretion of an ECM that is more proteoglycan rich and consistent with IVD micromechanical properties (100). BMP proteins family plays a pivotal role as increasing proteoglycan synthesis, upregulation of collagen type II mRNA (139). In vitro application of BMP-2 in a rat model increased cell proliferation and disc ECM production (140). Rabbit IVDs exposed to injections of BMP-7 also led to restoration of disc height and increased proteoglycan content (141). Table 2 summarizes the cited studies and shows the effect of different microenvironments on growth and survival of stem cells.
Table 2. A summary of the literature review as regards the effect of micro-environment on growth and survival of stem cells.
| Reference | Type of study | Cell source | Intervention | Outcome |
|---|---|---|---|---|
| Hudson et al. (98) | In vitro | hMSCs | Hypoxic (5% O2) versus normoxic (21% O2) conditions | Hypoxic expansion of human MSCs enhances 3D maturation of tissue engineered IVDs |
| Adesida et al. (99) | In vitro | hBM-MSCs | Hypoxic conditions (3% O2) versus normoxic conditions (21% O2) | Hypoxic conditions augment the chondrogenic potential of hBM-MSCs |
| Clarke et al. (100) | In vitro | hAD-MSCs and hBM-MSCs | Media supplemented with TGF-β3, GDF5, or GDF6 | GDF6 stimulation of AD-MSCs induces differentiation to an NP-like phenotype and results in a more proteoglycan-rich matrix with less stiff composition |
| Kumar et al. (101) | In vitro | hMSCs | Hypoxic (2% O2) chondrogenic media versus normoxic non chondrogenic media | MSCs enhanced differentiation into NP phenotypes with elevated expression levels of aggrecan and collagen II under hypoxic conditions |
| Elabd et al. (105) | Human in vivo study | hBM-MSCs | Hypoxic (5% O2) conditions | MRI radiological evaluation and quality of life questionnaire as an outcome measures showed significant improvement |
| Stolzing et al. (106) | In vitro | Rat nonadherent BM-MSCS | Different glucose levels in cultures | Culture in high-glucose-containing medium had a negative effect on colony formation and differentiation |
| Liang et al. (107) | In vitro | hAD-MSCs | Age (cells from mature and young male donors), glucose, acidity and osmolarity | Low glucose is a positive factor but high osmolarity and low pH are deleterious factors that affect the survival and biological behaviors of hAD-MSCs. Age did not affect the results |
| Wuertz et al. (108) | In vitro | Rat BM-MSCs | Age (cells from mature and young male donors), glucose, acidity, osmolarity and combined conditions | IVD-like glucose conditions stimulated aggrecan and collagen-1 expression. IVD-like osmolarity and pH strongly decreased proliferation and expression of matrix proteins. Osmolarity and pH dominated the effects of glucose |
| Mavrogonatou and Kletsas (109) | In vitro | Bovine-NP cells | High osmolality | |
| Liang et al. (110) | In vitro | hAD-MSCs | Low glucose, acidity, high osmolarity, and combined conditions | Low glucose is a positive factor but high osmolarity and low pH are deleterious factors that affect the survival and biological behaviors of AD-MSCs |
| Tao et al. (111) | In vitro | Rat NPCs, NP-MSCs and co-culture | High osmolality | High osmolarity inhibited cell viability and decreased the expression of aggrecan and collagen II at the mRNA and protein levels |
| Wuertz et al. (112) | In vitro | Rat BM-MSCs | Acidity | Acidity caused an inhibition of aggrecan, and collagen I, as well as a decrease in proliferation and viability and was associated with a change in cell morphology |
| Purmessur et al. (115) | In vitro | Porcine NCs | Dynamic pressurization | NP tissue maturation was induced from dynamic hydrostatic pressurization |
| Yurube et al. (116) | In vitro | Rat IVD cells | Static pressurization | Static compression-induced disc cell death and degeneration |
| See et al. (117) | In vitro | Rabbit BM-MSCs | Compressive mechanical stimulation | Extensive remodeling and ECM production occurred within the simulated IVD-like assembly |
| Dai et al. (118) | In vitro | Rats AD-MSCs | Dynamic compression and NPCs co-culture | Combination of dynamic compression and coculture showed an additive effect on NP-like cell differentiation |
| Li et al. (120) | In vitro | Rat NPCs | Priming with rGDF-5 | rGDF-5 treatment of disc cells from the GDF-5-deficient mice resulted in a dose-dependent upregulation of the aggrecan and type II collagen genes |
| Chujo et al. (121) | In vitro and animal in vivo study (rabbit model) | Bovine NP and AF cells, rat IVD model |
rhGDF-5 | In vitro, rhGDF-5 increased the DNA and proteoglycan contents. In vivo, the injection of rhGDF-5 improved disc height, MRI scores and histologic grading scores |
| Stoyanov et al. (123) | In vitro | hBM-MSCs | TGFß or GDF5 or coculture with bovine NPCs. All groups were incubated at low (2%) or normal (20%) oxygen | Hypoxia and GDF5 led to directing MSCs towards the IVD-like phenotype |
| Wehling et al. (127) | In vitro | hBM-MSCs | IL-1beta and TNF alpha | Both IL-1beta and TNF alpha inhibited chondrogenesis in a dose-dependent manner |
| Steck el al. (134) | In vitro | hBM-MSCs | TGF beta-3, dexamethasone, and ascorbate | After TGF beta mediated differentiation, MSC adopted a gene expression profile that resembled native IVD tissue more closely than native joint cartilage |
| Thompson et al. (137) | In vitro | Canine IVD disc | ILGF-1, EGF, FGF, or TGF beta-3 | TGF beta-3 and EGF elicited greater proliferative responses than FGF; ILGF-1 produced a marginally significant response in the nucleus and no response in the anulus and transition zone |
| Walsh et al. (138) | In vivo | Murine IVD disc | GDF-5, TGF beta-3, ILGF-1 or basic FGF | A statistically significant increase in disc height 4 weeks after GDF-5 treatment was measured |
| Li et al. (140) | In vitro | Rat AF cells | BMP-2 | BMP-2 increases aggrecan and collagen type II mRNA expression. BMP-2 also up-regulates mRNA expression for BMP-7 and TGF beta-3 |
Stem cell delivery
With respect to delivery of cell-based therapies, the traditional working horse has been image-assisted percutaneous injection through the AF. However, trans-annular injection has raised concerns regarding AF damage, leakage from the delivery site inducing osteophyte formation and diminished cell viability due to shear forces caused by small diameter needles (142-145).
To maximize both safety and efficacy of such modality the selection of needle size and the dose to be delivered should be optimized. Small needle diameters can hinder injection of viscous scaffolds as hydrogels while larger diameters might induce AF damage (146,147). In vivo animal study using a goat model by Gullbrand et al. (148) a 22G needle was found to be safe and feasible with no discernable degenerative changes on MRI or histology after 12 weeks. Since the degenerated IVD provides a very limited nutrient supply, the minimal effective dose of MSCs must be determined to maximize post-implantation survival. Data from larger model (canine, porcine, and ovine) studies showed that a dose of 106 BM-MSCs/per disk had the least number of MSC apoptosis (66).
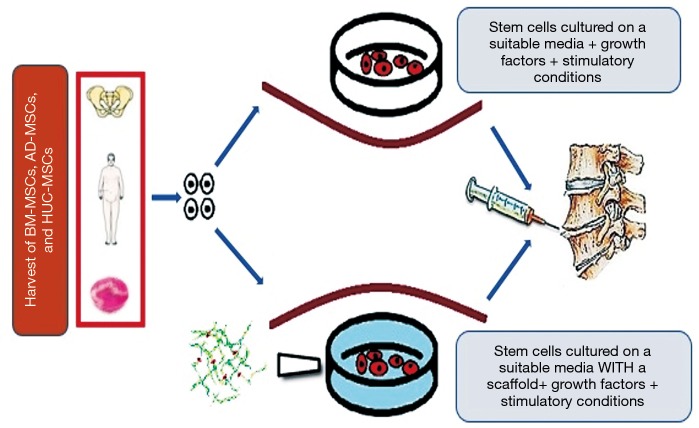
Current procedures often show backflow of cells through the injection site and a low survival rate of the implanted cells. In an attempt to avoid possible AF damage and to minimize extrusion of the injected material, an alternative delivery route that has been suggested which is through the pedicles (transpedicular approach) (149). Another alternative is the use of a delivery vehicle to act as retainers of injected suspensions. A human study where two patients with IVD degeneration had collagen porous sponges seeded with autologous BM-MSCs implanted through open approach and a collagen sponge was used as a sealant for the surgical hole in operated IVDs. In this study, both patients demonstrated promising results of improved outcome scores and increase in disc hydration as evidenced by MRI evaluation at 2 years after intervention (75). Other biomaterials as delivery vehicles, and in particular hydrogels, have been utilized to overcome retention issues and provide additional support for cell survival and phenotype retention (150-155) (Figure 1).
Figure 1.
An illustration showing the most common utilized sources for stem cells harvest. After harvesting the stem cells are cultured and primed with growth factors or cultured in a scaffold for additional support of cell survival.
Scaffolds
Various scaffolding materials as fibrin, hyaluronan, or atelocollagen have been devised in the past years in effort to maximize efficiency of stem cell delivery into degenerated IVDs (156-158). Ideally, biomaterial scaffolds should withstand physiological loads, possess adequate immune compatibility and ability to maintain the stem cells retained into their construct. A biomaterial scaffold may promote the viability and enhance differentiation of mesenchymal cells in the desired location by providing a three-dimensional (3D) microenvironment.
One unique difficulty in implantation and delivery of stem cells is leakage and inability to retain the implanted cells at site of interest. Injecting a cell suspension into the lumbar discs of rabbits has been shown to result in a 90% loss of the injected cells within the first 30 minutes post injection (159). Another concern is osteophyte formation as a result of ectopic osteoblast differentiation by way of leakage. Thus, application of a scaffolding materials has been strongly recommended to mitigate the risk of leakage and to act as a retainer of the transplanted cells (149). Additionally, animal models have established that autologous BM-MSCs have a relatively short survival time up to 48 weeks after transplantation (66).
Chitosan polysaccharide is a natural component of insect exoskeleton and is both PH and temperature responsive. An in vitro study showed chitosan nanoparticles have a potent anti-inflammatory effect on degenerated IVDs by down-regulating mediators as IL-6, IL-8, MMP1 and MMP3 while causing up-regulation of collagen type II and aggrecan production (160). Another in vitro study showed that a chitosan scaffold enhanced MSCs phenotypic differentiation to NP like cells with increased production of aggrecans and collagen II (161).
Atelocollagen, an injectable collagen hydrogel, has been studied in vitro and provide a biocompatible environment that augmented NP cell function (162-164). In vivo implantation of AF cells seeded in atelocollagen scaffolds in rabbit models prevented progression of IVD space narrowing and had viability and proliferative activity (163).
Gelatin is derived from thermo-treated collagen and as collagen, it induces cellular adhesion, proliferation and collagen II expression (165). Culture of AF cells in a gelatin scaffold demonstrated improved cellular adhesion, ECM protein expression and inhibition of MMPs (166). An in vivo study showed that transplantation of MSC-gelatin scaffold in punctured rabbit IVD inhibited cellular apoptosis and maintained disc height index (167).
Hyaluronan as a scaffold material was also studied as a potential biomaterial to address difficulties in stem cell delivery. Subhan et al. (168) in a controlled study on a rabbit model have demonstrated significant improvement in radiological and histological outcomes in the cell loaded scaffold group in comparison to other control groups. In another controlled study on an ovine model of damaged IVDs, Cell‐free, freeze‐dried resorbable polyglycolic acid-hyaluronan implants were implanted after nucleotomy of the IVD. Implantation of this cell‐free polymer‐based implant induced NPs tissue regeneration and improved disc water content both radiologically and histologically (169).
An in vivo sheep model showed increased NPs proteoglycan synthesis in IVDs injected with MSCs combined embedded in a fibrin scaffold (170). After 6 months, biochemical and histological markers showed better disc hydration and cellularity in the discs with scaffolding and MSCs than the discs injected with scaffolding alone. Also, radiological assessment revealed better Pfirmann scores compared to the control group. In a prospective study on 15 patients diagnosed with single-level lumbar spondylosis were treated via a percutaneous transplantation of allogenic juvenile chondrocytes encapsulated in a fibrin matrix (38). Six months follow-up revealed improvement in disc hydration by MRI evaluation and improvement in pain and disability scores with no reported adverse effects. Porous silk scaffolds have been also studied and shown to support AF cell attachment and ECM accumulation (171).
Alginate is another natural polysaccharide polymer derived from algae. Studies on alginate as scaffold showed induced MSCs differentiation to NP-like cells as well as increased ECM protein expressions (172,173). Synthetic polymers have shown promise as a scaffold material. Examples include, Polyethylene glycol (174), Polycaprolactone (175), Polyurethane (176) and Polylactic acid (177).
Restoration of disc height is necessary to ensure functionality of the IVD. Thus, a multifunctional therapeutic modality has been derived. A Recent advance in the field of bioengineering was the combination of such materials to create biphasic scaffolds to engineer the whole IVD by recapitulating the unique structures and functions of both NP and AF (178,179). For instance, a cell loaded biphasic scaffold was fabricated in which silk was used for the AF while fibrin and hyaluronan for the NP thus a whole IVD was generated in vitro (180). An in vivo animal study, utilized a novel cell loaded biphasic IVD by integrating a freeze-dried, cross-linked porcine bone matrix gelatin for the AF and porcine acellular cartilage for the NP (181).
Another innovative approach to scaffold development that is emerging is based on the inherent ability of cells to form their own matrix, much like that of the destination tissue (182). This approach involves culturing cells to produce ECM that will ultimately serve as the IVD implant with similar structural and compositional features of native tissue (183,184).
Some researchers also paid attention to natural biologic materials such as decellularized matrix from IVD to act as a natural scaffold for implanted cells. An in vitro study showed potential for the use of decellularized bovine IVD as a xenogenic scaffold (185). The included studies as regards the used scaffolds and delivery vehicles for stem cells in discogenic low back pain are summarized in Table 3.
Table 3. A summary of the literature review as regards the used scaffolds and delivery vehicles for stem cells in discogenic low back pain.
| Reference | Type of study | Cell source | Scaffold/delivery vehicle | Outcome |
|---|---|---|---|---|
| Bertram et al. (159) | In vitro and animal in vivo study (Rabbit model) | Rabbit NPCs (cultured in fibrin matrix) | Injectable cell fibrin gel | Up to 50% matrix injected cells remained in the nucleus and transition zone in contrast to a rapid loss of medium-injected cells. Enhanced survival of cells cultured with fibrin matrix |
| Teixeira et al. (160) | In vitro | Bovine IVD | Chitosan-Diclofenac nanoparticles | Chitosan-Diclofenac nanoparticles reduces inflammation and also decreases ECM degradation |
| Richardson et al. (161) | In vitro | hMSCs | Chitosan-glycerophosphate | Chitosan directs MSCs phenotypic differentiation to more NP like cells |
| Sato et al. (163) | In vitro | Rabbit AF cells (cultured in atelocollagen) | Atelocollagen honeycomb-shaped scaffold with a membrane seal | The amount of type II collagen and its mRNA expression, GAG and proteoglycans in the scaffold cultured cells remained at a higher level than in the monolayer cultured cells |
| Sakai et al. (164) | In vitro | Human NPCs (cultured in atelocollagen) | Atelocollagen | Results showed that both DNA synthesis and content is significantly greater when cultured in Atelocollagen than in alginate |
| Yang et al. (167) | Animal in vivo study (Rabbit model) | Rat IVD MSCs | Pure fibrinous gelatin (PFG) | The transplanted MSCs in PFG inhibited apoptosis and slowed the rate of decrease in disc height index |
| Subhan et al. (168) | Animal in vivo study (Rabbit model) | Rabbit BM-MSCs (weren’t scaffold cultured but were delivered with hyaluronan hydrogel) | Hyaluronan hydrogel | Better MRI scores for MSCs delivered with the hydrogel. Immunohistochemistry staining for collagen type II and aggrecan staining were also higher |
| Woiciechowsky et al. (169) | Animal in vivo study (Ovine model) | Acellular | Polyglycolic acid (PGA) | Histological analysis showed ingrowth of cells with typical chondrocytic morphology, even cell distribution, and ECM rich in proteoglycan |
| Chang et al. (171) | In vitro | Bovine AF cells (Cultured in porous silk scaffolds) | porous silk scaffolds and grown in either dynamic or static flow conditions | Dynamic flow conditions and scaffold pore size can affect the formation of engineered AF tissue |
| Yang et al. (172) | In vivo | Rat NP and AF cells | Alginate hydrogel seeded with NPCs, Polycaprolactone (PCL) seeded with AF cells | Long term implantation in rats generated highly hydrated soft tissues and well-integrated into the adjacent vertebrae |
| Zeng et al. (173) | In vivo and in vitro | hAD-MSCs | Polyacrylate microcryogels (PM) | Enhanced cell retention of PMs assisted cell delivery to a load bearing environment of rat IVD |
| Benz et al. (174) | In vivo and in vitro | Human IVD tissue | Hydrogel composed of chemically activated albumin crosslinked by polyethylene glycol | The expression of cartilage- and disc-specific mRNAs was maintained in hydrogels in vitro and in vivo |
| Hu et al. (176) | In vitro | Rabbit BM-MSCs | Silk fibroin/ polyurethane (SF/PU) composite hydrogel | The composite hydrogel exhibited suitable physical-mechanical properties as prosthetic biomaterial for NP replacement |
| Kim et al. (177) | In vivo | Rat NPCs | polylactic-co-glycolic acid | As the pores became smaller, the value of the compressive strength of the scaffold was increased |
| Choy et al. (178) | Mechanical vitro study | Acellular | Biphasic scaffold fabricated with collagen and glycosaminoglycans (GAGs) | Biphasic scaffolds comprised of 10 annulus fibrosus-like lamellae had the best overall mechanical performance among the various designs |
| Elsaadany et al. (179) | Mechanical in vitro study | hAD-MSCs | Biphasic mechanically conditioned scaffold encapsulated with hAD-MSCs | Equiaxial loading increased secretion of ECM proteins and gene expression of AF markers compared to unstrained samples |
| Park et al. (180) | In vitro | Porcine AF cells (cultured in silk scaffolds) | Silk scaffold (lamellar versus porous) | Histology, biochemical assays, mechanical testing and gene expression indicated that the lamellar scaffold generated results that were more favorable in terms of ECM expression and tissue function than the porous scaffold for AF tissue |
| Xu et al. (186) | Animal In vivo study (Rat model) | Rat NP and AF cells (cultured in the biphasic scaffold in vitro) | Freeze-dried, cross-linked biphasic scaffold of pig bone matrix gelatin for the outer AF and pig acellular cartilage ECM for the NP | IVD like tissue formed in mice as confirmed by histology after subcutaneous implantation of cell-scaffold constructs |
| Illien et al. (183) | In vitro | Human NPC and MSCs (cultured with the scaffold) | Decellularized injectable bovine ECM material | The scaffold maintained native NP tissue structure and composition closest to natural ECM and promoted cellular adaptation of NP cells and MSCs |
Current challenges
Stem cell therapy for discogenic low back pain is still in infancy and will need to demonstrate pre-clinical efficacy and safety using in vitro and in vivo model systems before being translated to wide clinical use. One of the greatest challenges facing in vivo animal models is the need to replicate the size and height of the human disc in the pre-clinical trials. For example, lumbar disc height in sheep is about 4 mm compared to about 11 mm in humans (187).
The replication and testing of the regenerative potential of stem cells under conditions which mimics the complex micro-environment in the IVD is another challenge. Not only biochemical conditions but also physiological loading conditions should be replicated to allow reproducible and reliable results after transplantation. The physical environment can be mimicked through culturing in soft 3D scaffolds such as hydrogels and mechanical stresses such as dynamic compressive loads or hydrostatic pressures applied via bioreactors (188-191).
The high cost of clinical trials and ethical concerns regarding the use of embryonic and umbilical stem cells is another obstacle. A recent systematic review showed that most of the literature available on the topic is methodologically poor with small sample sizes, high risk of bias and lack of a control intervention (192). Equally important, current patient reported outcome measure are influenced by significant placebo effects (191). Also ensuring safety and tolerability mandates that uncontrolled differentiation of stem cells to be checked and devising more efficient delivery strategies.
Another challenge is the correct targeting of the painful degenerated discs rather than painless degeneration. Except in cases with nerve root compression or central stenosis, conventional MRI studies do not distinguish effectively between painful and nonpainful degenerating disks (193). Also, to support clinical application and efficacy of stem cell therapy, there is a critical need for new techniques to quantify the therapeutic effects on treated levels.
More recent MRI techniques such as T1ρ and T2 relaxation times, and chemical exchange saturation transfer (CEST) provides a quantitative analysis for disc composition indicating more accurately DDD (194,195). Painful disks are characterized by hypoxia and inflammation which leads to accumulation of certain metabolites such as lactate, alanine, and lipids (196). These metabolites may serve as biomarkers that are detectable by means of MR spectroscopy (197). A recent imaging technique is quantitative sodium MRI which detects sodium levels in the IVD and thus the hydration status. A recent study suggests that this technique may differentiate discs between asymptomatic and symptomatic patients (198). However, sodium MRI is in limited use because of the requirement for special hardware modifications to the conventional MRI scanner. Fluorine 18 fluorodeoxyglucose positron emission tomography (FDG-PET) has been shown to identify inflamed degenerated discs and may be a beneficial diagnostic tool (199). As previously stated, inflammation is a central feature of painful disks. PET/CT may be useful in selection of patients and localizing the spinal levels.
Invasive provocative discography, which includes disc stimulation and morphological evaluation, is often used to distinguish a painful disc from other potential sources of pain such as facet joint pain. A recent systematic review supported the use of provocative lumbar discography as an accurate diagnostic tool (200).
Conclusions
Novel stem cell regenerative therapy for discogenic low back pain is a promising alternative to the conventional surgical management and other non-operative alternatives. Patient selection as well as accurate localization of pain generators is a pre-requisite for successful effective treatment. The utilized stem cells are broadly either derived from the IVD itself (resident stem cells or primary cells) or derived from other pluripotent sources as BM or adipose tissue. Many challenges face this relatively infant field, ranging from isolation, culture and host delivery. The complex and harsh microenvironment of the degenerated IVD plays a detrimental role in multiplication and survival of the transplanted stem cells. More basic science and clinical studies are needed to establish the clinical efficacy of such treatments.
Limitations
This is a literature review on the current concepts and advances in the field of stem cell regenerative therapy for discogenic back pain. Most of the available studies are animal or in vitro studies with relative scarcity of human trials. Nevertheless, a strength of our review is that we conducted a comprehensive search of multiple databases. Independent reviewers performed the search, selected and appraised the included studies.
Acknowledgments
None.
Ethical Statement: The authors are accountable for all aspects of the work in ensuring that questions related to the accuracy or integrity of any part of the work are appropriately investigated and resolved.
Footnotes
Conflicts of Interest: The authors have no conflicts of interest to declare.
References
- 1.Mathew J, Singh SB, Garis S, et al. Backing up the stories: the psychological and social costs of chronic low-back pain. Int J Spine Surg 2013;7:e29-38. 10.1016/j.ijsp.2013.02.001 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Schwab F, Dubey A, Gamez L, et al. Adult scoliosis: prevalence, SF-36, and nutritional parameters in an elderly volunteer population. Spine (Phila Pa 1976) 2005;30:1082-5. 10.1097/01.brs.0000160842.43482.cd [DOI] [PubMed] [Google Scholar]
- 3.Manchikanti L, Singh V, Datta S, et al. Comprehensive review of epidemiology, scope, and impact of spinal pain. Pain Physician 2009;12:E35-70. [PubMed] [Google Scholar]
- 4.Pellisé F, Vila-Casademunt A, Ferrer M, et al. European Spine Study Group Impact on health related quality of life of adult spinal deformity (ASD) compared with other chronic conditions. Eur Spine J 2015;24:3-11. 10.1007/s00586-014-3542-1 [DOI] [PubMed] [Google Scholar]
- 5.Sakai D, Andersson GB. Stem cell therapy for intervertebral disc regeneration: obstacles and solutions. Nat Rev Rheumatol 2015;11:243-56. 10.1038/nrrheum.2015.13 [DOI] [PubMed] [Google Scholar]
- 6.Urban JP, Roberts S. Degeneration of the intervertebral disc. Arthritis Res Ther 2003;5:120-30. 10.1186/ar629 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Ashley JW, Enomoto-Iwamoto M, Smith LJ, et al. Intervertebral disc development and disease-related genetic polymorphisms. Genes Dis 2016;3:171-7. 10.1016/j.gendis.2016.04.006 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Vo NV, Hartman RA, Patil PR, et al. Molecular mechanisms of biological aging in intervertebral discs. J Orthop Res 2016;34:1289-306. 10.1002/jor.23195 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Dario AB, Ferreira ML, Refshauge KM, et al. The relationship between obesity, low back pain, and lumbar disc degeneration when genetics and the environment are considered: a systematic review of twin studies. Spine J 2015;15:1106-17. 10.1016/j.spinee.2015.02.001 [DOI] [PubMed] [Google Scholar]
- 10.Vieira LA, dos Santos AA, Peluso C, et al. Influence of lifestyle characteristics and VDR polymorphisms as risk factors for intervertebral disc degeneration: a case-control study. Eur J Med Res 2018;23:11. 10.1186/s40001-018-0309-x [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Teraguchi M, Yoshimura N, Hashizume H, et al. Progression, incidence, and risk factors for intervertebral disc degeneration in a longitudinal population based cohort: the Wakayama Spine Study. Osteoarthritis Cartilage 2017;25:1122-31. 10.1016/j.joca.2017.01.001 [DOI] [PubMed] [Google Scholar]
- 12.Bogduk N. Clinical anatomy of the lumbar spine and sacrum. 4th edition. New York: Elsevier, 2005:147-8. [Google Scholar]
- 13.Roberts S, Evans H, Trivedi J, et al. Histology and pathology of the human intervertebral disc. J Bone Joint Surg Am 2006;88:10-4. [DOI] [PubMed] [Google Scholar]
- 14.Melrose J, Smith SM, Little CB, et al. Recent advances in annular pathobiology provide insights into rimlesion mediated intervertebral disc degeneration and potential new approaches to annular repair strategies. Eur Spine J 2008;17:1131-48. 10.1007/s00586-008-0712-z [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Roughley PJ. Biology of intervertebral disc aging and degeneration: involvement of the extracellular matrix. Spine 2004;29:2691-9. 10.1097/01.brs.0000146101.53784.b1 [DOI] [PubMed] [Google Scholar]
- 16.Richardson SM, Kalamegam G, Pushparaj P, et al. Mesenchymal stem cells in regenerative medicine: focus on articular cartilage and intervertebral disc regeneration. Methods 2016;99:69-80. 10.1016/j.ymeth.2015.09.015 [DOI] [PubMed] [Google Scholar]
- 17.Cornefjord M, Olmarker K, Rydevik R, et al. Mechanical and biochemical injury of spinal nerve roots: a morphological and neurophysiological study. Eur Spine J 1996;5:187-92. 10.1007/BF00395512 [DOI] [PubMed] [Google Scholar]
- 18.Pearce RH, Grimmer BJ, Adams ME. Degeneration and the chemical composition of the human lumbar intervertebral disc. J Orthop Res 1987;5:198-205. 10.1002/jor.1100050206 [DOI] [PubMed] [Google Scholar]
- 19.Richardson SM, Freemont AJ, Hoyland JA. Pathogenesis of Intervertebral Disc Degeneration. The Intervertebral Disc 2013;177-200. [Google Scholar]
- 20.Bushell GR, Ghosh P, Taylor TF, et al. Proteoglycan chemistry of intervertebral disks. Clin Orthop 1977;129:115-23. 10.1097/00003086-197711000-00013 [DOI] [PubMed] [Google Scholar]
- 21.Oegema TR, Bradford DS, Cooper KM. Aggregated proteoglycan synthesis in organ culture of human nucleus pulposus. J Biol Chem 1979;254:10579-81. [PubMed] [Google Scholar]
- 22.Goupille P, Jayson MIV, Valat JP, et al. Matrix metalloproteinases: the clue to intervertebral disc degeneration? Spine 1998;23:1612-26. 10.1097/00007632-199807150-00021 [DOI] [PubMed] [Google Scholar]
- 23.Roughley PJ. Biology of Intervertebral Disc Aging and Degeneration. Spine 2004;29:2691-9. 10.1097/01.brs.0000146101.53784.b1 [DOI] [PubMed] [Google Scholar]
- 24.Hwang PY, Chen J, Jing L, et al. The Role of Extracellular Matrix Elasticity and Composition In Regulating the Nucleus Pulposus Cell Phenotype in the Intervertebral Disc: A Narrative Review. J Biomech Eng 2014;136:021010. 10.1115/1.4026360 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Pfirrmann CW, Metzdorf A, Zanetti M, et al. Magnetic resonance classification of lumbar intervertebral disc degeneration. Spine 2001;26:1873-8. 10.1097/00007632-200109010-00011 [DOI] [PubMed] [Google Scholar]
- 26.Freemont AJ, Peacock TE, Goupille P, et al. Nerve ingrowth into diseased intervertebral disc in chronic back pain. Lancet 1997;350:178-81. 10.1016/S0140-6736(97)02135-1 [DOI] [PubMed] [Google Scholar]
- 27.Hutton WC, Elmer WA, Boden SD, et al. The effect of hydrostatic pressure on intervertebral disc metabolism. Spine 1999;24:1507-15. 10.1097/00007632-199908010-00002 [DOI] [PubMed] [Google Scholar]
- 28.Postacchini F, Lami R, Pugliese O. Familial Predisposition to Discogenic Low-Back Pain. Spine 1988;13:1403-6. 10.1097/00007632-198812000-00012 [DOI] [PubMed] [Google Scholar]
- 29.Matsui H, Kanamori M, Ishihara H, et al. Familial Predisposition for Lumbar Degenerative Disc Disease. Spine 1998;23:1029-34. 10.1097/00007632-199805010-00013 [DOI] [PubMed] [Google Scholar]
- 30.Doege KJ, Coulter SN, Meek LM, et al. A human-specific polymorphism in the coding region of the aggrecan gene: variable number of tandem repeats produce a range of core protein sizes in the general population. J Biol Chem 1997;272:13974-9. 10.1074/jbc.272.21.13974 [DOI] [PubMed] [Google Scholar]
- 31.Liuke M, Solovieva S, Lamminen A, et al. Disc degeneration of the lumbar spine in relation to overweight. Int J Obes (Lond) 2005;29:903-8. 10.1038/sj.ijo.0802974 [DOI] [PubMed] [Google Scholar]
- 32.Nguyen TH, Randolph DC, Talmage J, et al. Long-term outcomes of lumbar fusion among workers' compensation subjects: a historical cohort study. Spine 2011;36:320-31. 10.1097/BRS.0b013e3181ccc220 [DOI] [PubMed] [Google Scholar]
- 33.Baliga S, Treon K, Craig NJ. Low Back Pain: current surgical approaches. Asian Spine J 2015;9:645-57. 10.4184/asj.2015.9.4.645 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Basques BA, Diaz-Collado PJ, Geddes BJ, et al. Primary and revision posterior lumbar fusion have similar short-term complication rates. Spine 2016;41:E101-6. 10.1097/BRS.0000000000001094 [DOI] [PubMed] [Google Scholar]
- 35.Ma Y, Passias P, Gaber-Baylis LK, et al. Comparative in-hospital morbidity and mortality after revision versus primary thoracic and lumbar spine fusion. Spine J 2010;10:881-9. 10.1016/j.spinee.2010.07.391 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Lee JC, Choi SW. Adjacent segment pathology after lumbar spinal fusion. Asian Spine J 2015;9:807-17. 10.4184/asj.2015.9.5.807 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Sakai D. Future perspectives of cell-based therapy for intervertebral disc disease. Eur Spine J 2008;17:452-8. 10.1007/s00586-008-0743-5 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Coric D, Pettine K, Sumich A, et al. Prospective study of disc repair with allogeneic chondrocytes. In: Vaccaro AR editor. Chart showing the intradiscal surgical treatment options. J Neurosurg Spine 2013;18:85-95. 10.3171/2012.10.SPINE12512 [DOI] [PubMed] [Google Scholar]
- 39.Choudhery MS, Badowski M, Muise A, et al. Donor age negatively impacts adipose tissue-derived mesenchymal stem cell expansion and differentiation. J Transl Med 2014;12:8. 10.1186/1479-5876-12-8 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Kim M, Erickson IE, Huang AH, et al. Donor Variation and Optimization of Human Mesenchymal Stem Cell Chondrogenesis in Hyaluronic Acid. Tissue Eng Part A 2018;24:1693-703. 10.1089/ten.tea.2017.0520 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Risbud MV, Guttapalli A, Tsai TT, et al. Evidence for skeletal progenitor cells in the degenerate human intervertebral disc. Spine 2007;32:2537-44. 10.1097/BRS.0b013e318158dea6 [DOI] [PubMed] [Google Scholar]
- 42.Feng G, Yang X, Shang H, et al. Multipotential differentiation of human anulus fibrosus cells: an in vitro study. J Bone Joint Surg Am 2010;92:675-85. 10.2106/JBJS.H.01672 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Liu LT, Huang B, Li CQ, et al. Characteristics of stem cells derived from the degenerated human intervertebral disc cartilage endplate. PLoS One 2011;6:e26285. 10.1371/journal.pone.0026285 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Henriksson H, Thornemo M, Karlsson C, et al. Identification of cell proliferation zones, progenitor cells and a potential stem cell niche in the intervertebral disc region: a study in four species. Spine 2009;34:2278-87. 10.1097/BRS.0b013e3181a95ad2 [DOI] [PubMed] [Google Scholar]
- 45.Sakai D, Nakamura Y, Nakai T, et al. Exhaustion of nucleus pulposus progenitor cells with ageing and degeneration of the intervertebral disc. Nat Commun 2012;3:1264. 10.1038/ncomms2226 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Arai F, Hirao A, Ohmura M, et al. Tie2/angiopoietin-1 signaling regulates hematopoietic stem cell quiescence in the bone marrow niche. Cell 2004;118:149-61. 10.1016/j.cell.2004.07.004 [DOI] [PubMed] [Google Scholar]
- 47.Koblizek TI, Runting AS, Stacker SA, et al. Tie2 receptor expression and phosphorylation in cultured cells and mouse tissues. Eur J Biochem 1997;244:774-9. 10.1111/j.1432-1033.1997.00774.x [DOI] [PubMed] [Google Scholar]
- 48.Suri C, Jones PF, Patan S, et al. Requisite role of angiopoietin- 1, a ligand for the Tie2 receptor, during embryonic angiogenesis. Cell 1996;87:1171-80. 10.1016/S0092-8674(00)81813-9 [DOI] [PubMed] [Google Scholar]
- 49.Martinez C, Hofmann TJ, Marino R, et al. Human bone marrow mesenchymal stromal cells express the neural ganglioside GD2: a novel surface marker for the identification of MSCs. Blood 2007;109:4245-8. 10.1182/blood-2006-08-039347 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.Xu J, Liao W, Gu D, et al. Neural ganglioside GD2 identifies a subpopulation of mesenchymal stem cells in umbilical cord. Cell Physiol Biochem 2009;23:415-24. 10.1159/000218188 [DOI] [PubMed] [Google Scholar]
- 51.Tekari A, Chan SC, Sakai D, et al. Angiopoietin-1 receptor Tie2 distinguishes multipotent differentiation capability in bovine coccygeal nucleus pulposus cells. Stem Cell Res Ther 2016;7:75. 10.1186/s13287-016-0337-9 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52.Gruber HE, Riley FE, Hoelscher GL, et al. Human annulus progenitor cells: analyses of this viable endogenous cell population. J Orthop Res 2016;34:1351-60. 10.1002/jor.23319 [DOI] [PubMed] [Google Scholar]
- 53.Meisel HJ, Siodla V, Ganey T, et al. Clinical experience in cell-based therapeutics: disc chondrocyte transplantation. A treatment for degenerated or damaged intervertebral discs. Biomol Eng 2007;24:5-21. 10.1016/j.bioeng.2006.07.002 [DOI] [PubMed] [Google Scholar]
- 54.Hohaus C, Ganey TM, Minkus Y, et al. Cell transplantation in lumbar spine disc degeneration disease. Eur Spine J 2008;17:492-503. 10.1007/s00586-008-0750-6 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 55.Mochida J, Sakai D, Nakamura Y, et al. Intervertebral disc repair with activated nucleus pulposus cell transplantation: a three-year, prospective clinical study of its safety. Eur Cell Mater 2015;29:202-12. 10.22203/eCM.v029a15 [DOI] [PubMed] [Google Scholar]
- 56.Vedicherla S, Buckley CT. Cell-based therapies for intervertebral disc and cartilage regeneration – current concepts, parallels, and perspectives. J Orthop Res 2017;35:8-22. 10.1002/jor.23268 [DOI] [PubMed] [Google Scholar]
- 57.Chen J, Yan W, Setton LA. Molecular phenotypes of notochordal cells purified from immature nucleus pulposus. Eur Spine J 2006;15:S303-11. 10.1007/s00586-006-0088-x [DOI] [PMC free article] [PubMed] [Google Scholar]
- 58.Acosta F, Jr, Metz L, Liu J, et al. Juvenile chondrocytes are superior to undifferentiated mesenchymal stem cells for porcine intervertebral disc repair. Spine J 2008;50S:8. [Google Scholar]
- 59.Vedicherla S, Buckley CT. In vitro extracellular matrix accumulation of nasal and articular chondrocytes for intervertebral disc repair. Tissue Cell 2017;49:503-13. 10.1016/j.tice.2017.05.002 [DOI] [PubMed] [Google Scholar]
- 60.Tsaryk R, Silva-Correia J, Oliveira JM, et al. Biological performance of cell-encapsulated methacrylated gellan gum-based hydrogels for nucleus pulposus regeneration. J Tissue Eng Regen Med 2017;11:637-48. 10.1002/term.1959 [DOI] [PubMed] [Google Scholar]
- 61.Noriega DC, Ardura F, Hernandez-Ramajo R, et al. Intervertebral disc repair by allogeneic mesenchymal bone marrow cells: a randomized controlled trial. Transplantation 2017;101:1945-51. 10.1097/TP.0000000000001484 [DOI] [PubMed] [Google Scholar]
- 62.Kim DH, Kim SH, Heo SJ, et al. Enhanced differentiation of mesenchymal stem cells into NP-like cells via 3D co-culturing with mechanical stimulation. J Biosci Bioeng 2009;108:63-7. 10.1016/j.jbiosc.2009.02.008 [DOI] [PubMed] [Google Scholar]
- 63.Wei A, Chung SA, Tao H, et al. Differentiation of rodent bone marrow mesenchymal stem cells into intervertebral disc-like cells following coculture with rat disc tissue. Tissue Eng Part A 2009;15:2581-95. 10.1089/ten.tea.2008.0458 [DOI] [PubMed] [Google Scholar]
- 64.Daly C, Ghosh P, Jenkin G, et al. A review of animal models of intervertebral disc degeneration: pathophysiology, regeneration, and translation to the clinic. Biomed Res Int 2016;2016:5952165. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 65.Salzig D, Schmiermund A, Gebauer E, et al. Influence of porcine intervertebral disc matrix on stem cell differentiation. J Funct Biomater 2011;2:155-72. 10.3390/jfb2030155 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 66.Yim RL, Lee JT, Bow CH, et al. A systematic review of the safety and efficacy of mesenchymal stem cells for disc degeneration: Insights and future directions for regenerative therapeutics. Stem Cells Dev 2014;23:2553-67. 10.1089/scd.2014.0203 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 67.Li H, Ghazanfari R, Zacharaki D, et al. Isolation and characterization of primary bone marrow mesenchymal stromal cells. Ann NY Acad Sci 2016;1370:109-18. 10.1111/nyas.13102 [DOI] [PubMed] [Google Scholar]
- 68.Rodriguez AM, Elabd C, Amri EZ, et al. The human adipose tissue is a source of multipotent stem cells. Biochimie 2005;87:125-8. 10.1016/j.biochi.2004.11.007 [DOI] [PubMed] [Google Scholar]
- 69.Pang X, Yang H, Peng B. Human umbilical cord mesenchymal stem cell transplantation for the treatment of chronic discogenic low back pain. Pain Physician 2014;17:E525-30. [PubMed] [Google Scholar]
- 70.Oehme D, Goldschlager T, Ghosh P, et al. Cell-based therapies used to treat lumbar degenerative disc disease: a systematic review of animal studies and human clinical trials. Stem Cells Int 2015;2015:946031. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 71.DePalma MJ. International Spine Intervention Society. Annual Scientific Meeting, Orlando (FL), July 30, 2014. [Google Scholar]
- 72.Orozco L, Soler R, Morera C, et al. Intervertebral disc repair by autologous mesenchymal bone marrow cells: a pilot study. Transplantation 2011;92:822-8. 10.1097/TP.0b013e3182298a15 [DOI] [PubMed] [Google Scholar]
- 73.Centeno C, Markle J, Dodson E, et al. Treatment of lumbar degenerative disc diseaseassociated radicular pain with culture-expanded autologous mesenchymal stem cells: a pilot study on safety and efficacy. J Transl Med 2017;15:197. 10.1186/s12967-017-1300-y [DOI] [PMC free article] [PubMed] [Google Scholar]
- 74.Pettine KA, Murphy MB, Suzuki RK, et al. Percutaneous lumbar intradiscal injection of autologous bone marrow concentrated cells significantly reduces discogenic pain through 12 months. Stem Cells 2015;33:146-56. 10.1002/stem.1845 [DOI] [PubMed] [Google Scholar]
- 75.Yoshikawa T, Ueda Y, Miyazaki K, et al. Disc regeneration therapy using marrow mesenchymal cell transplantation: a report of two case studies. Spine 2010;35:E475-80. 10.1097/BRS.0b013e3181cd2cf4 [DOI] [PubMed] [Google Scholar]
- 76.DePalma MJ, Gasper JJ. Cellular supplementation technologies for painful spine disorders. PM R 2015;7:S19-25. 10.1016/j.pmrj.2015.01.022 [DOI] [PubMed] [Google Scholar]
- 77.Blanco JF, Graciani IF, Sanchez-Guijo FM, et al. Isolation and characterization of mesenchymal stromal cells from human degenerated nucleus pulposus: comparison with bone marrow mesenchymal stromal cells from the same subjects. Spine 2010;35:2259-65. 10.1097/BRS.0b013e3181cb8828 [DOI] [PubMed] [Google Scholar]
- 78.Shamah SM, Healy JM, Cload ST. Complex target SELEX. Acc Chem Res 2008;41:130-8. 10.1021/ar700142z [DOI] [PubMed] [Google Scholar]
- 79.Guilak F, Estes BT, Diekman BO, et al. 2010 Nicolas Andry Award: multipotent adult stem cells from adipose tissue for musculoskeletal tissue engineering. Clin Orthop Relat Res 2010;468:2530-40. 10.1007/s11999-010-1410-9 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 80.Jurgens WJ, Oedayrajsingh-Varma MJ, Helder MN, et al. Effect of tissue-harvesting site on yield of stem cells derived from adipose tissue: implications for cell-based therapies. Cell Tissue Res 2008;332:415-26. 10.1007/s00441-007-0555-7 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 81.Yang X, Li X. Nucleus pulposus tissue engineering: a brief review. Eur Spine J 2009;18:1564-72. 10.1007/s00586-009-1092-8 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 82.Diekman BO, Rowland CR, Lennon DP, et al. Chondrogenesis of adult stem cells from adipose tissue and bone marrow: induction by growth factors and cartilage-derived matrix. Tissue Eng Part A 2010;16:523-33. 10.1089/ten.tea.2009.0398 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 83.Vasiliadis ES, Pneumaticos SG, Evangelopoulos DS, et al. Biological treatment of mild and moderate intervertebral disc degeneration. Mol Med 2014;20:400-9. 10.2119/molmed.2014.00145 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 84.Marfia G, Campanella R, Navone SE, et al. Potential use of human adipose mesenchymal stromal cells for intervertebral disc regeneration: a preliminary study on biglycan-deficient murine model of chronic disc degeneration. Arthritis Res Ther 2014;16:457. 10.1186/s13075-014-0457-5 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 85.Chun HJ, Kim YS, Kim BK, et al. Transplantation of Human Adipose-Derived Stem Cells in a Rabbit Model of Traumatic Degeneration of Lumbar Discs. World Neurosurg 2012;78:364-71. 10.1016/j.wneu.2011.12.084 [DOI] [PubMed] [Google Scholar]
- 86.Ganey T, Hutton WC, Moseley T, et al. Intervertebral Disc Repair Using Adipose Tissue-Derived Stem and Regenerative Cells. Spine 2009;34:2297-304. 10.1097/BRS.0b013e3181a54157 [DOI] [PubMed] [Google Scholar]
- 87.Lu ZF, Doulabi BZ, Wuisman PI, et al. Influence of collagen type II and nucleus pulposus cells on aggregation and differentiation of adipose tissue-derived stem cells. J Cell Mol Med 2008;12:2812-22. 10.1111/j.1582-4934.2008.00278.x [DOI] [PMC free article] [PubMed] [Google Scholar]
- 88.Smith LJ, Nerurkar NL, Choi KS, et al. Degeneration and regeneration of the intervertebral disc: lessons from development. Dis Model Mech 2011;4:31-41. 10.1242/dmm.006403 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 89.Liu Y, Rahaman MN, Bal BS. Modulating notochordal differentiation of human induced pluripotent stem cells using natural nucleus pulposus tissue matrix. PLoS One 2014;9:e100885. 10.1371/journal.pone.0100885 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 90.Purmessur D, Schek RM, Abbott RD, et al. Notochordal conditioned media from tissue increases proteoglycan accumulation and promotes a healthy nucleus pulposus phenotype in human mesenchymal stem cells. Arthritis Res Ther 2011;13:R81. 10.1186/ar3344 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 91.Gantenbein B, Calandriello E, Wuertz-Kozak K, et al. Activation of intervertebral disc cells by co-culture with notochordal cells, conditioned medium and hypoxia. BMC Musculoskelet Disord 2014;15:422. 10.1186/1471-2474-15-422 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 92.Sheikh H, Zakharian K, De La Torre RP, et al. In vivo intervertebral disc regeneration using stem cell-derived chondroprogenitors. J Neurosurg Spine 2009;10:265-72. 10.3171/2008.12.SPINE0835 [DOI] [PubMed] [Google Scholar]
- 93.Korecki CL, Taboas JM, Tuan RS, et al. Notochordal cell conditioned medium stimulates mesenchymal stem cell differentiation toward a young nucleus pulposus phenotype. Stem Cell Res Ther 2010;1:18. 10.1186/scrt18 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 94.Holm S, Maroudas A, Urban JP, et al. Nutrition of the intervertebral disc: Solute transport and metabolism. Connect Tissue Res 1981;8:101-19. 10.3109/03008208109152130 [DOI] [PubMed] [Google Scholar]
- 95.Krock E, Rosenzweig DH, Haglund L. The inflammatory milieu of the degenerate disc: Is mesenchymal stem cell-based therapy for intervertebral disc repair a feasible approach? Curr Stem Cell Res Ther 2015;10:317-28. 10.2174/1574888X10666150211161956 [DOI] [PubMed] [Google Scholar]
- 96.Pei M. Environmental preconditioning rejuvenates adult stem cells proliferation and chondrogenic potential. Biomaterials 2017;117:10-23. 10.1016/j.biomaterials.2016.11.049 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 97.Naqvi SM, Gansau J, Buckley CT. Priming and cryopreservation of microencapsulated marrow stromal cells as a strategy for intervertebral disc regeneration. Biomed Mater 2018;13:034106. 10.1088/1748-605X/aaab7f [DOI] [PubMed] [Google Scholar]
- 98.Hudson KD, Bonassar LJ. Hypoxic expansion of human mesenchymal stem cells enhances three-dimensional maturation of tissue-engineered intervertebral discs. Tissue Eng Part A 2017;23:293-300. 10.1089/ten.tea.2016.0270 [DOI] [PubMed] [Google Scholar]
- 99.Adesida AB, Mulet-Sierra A, Jomha NM. Hypoxia mediated isolation and expansion enhances the chondrogenic capacity of bone marrow mesenchymal stromal cells. Stem Cell Res Ther 2012;3:9. 10.1186/scrt100 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 100.Clarke LE, McConnell JC, Sherratt MJ, et al. Growth differentiation factor 6 and transforming growth factor-beta differentially mediate mesenchymal stem cell differentiation, composition, and micromechanical properties of nucleus pulposus constructs. Arthritis Res Ther 2014;16:R67. 10.1186/ar4505 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 101.Kumar D, Gerges I, Tamplenizza M, et al. Three-dimensional hypoxic culture of human mesenchymal stem cells encapsulated in a photocurable, biodegradable polymer hydrogel: a potential injectable cellular product for nucleus pulposus regeneration. Acta Biomater 2014;10:3463-74. 10.1016/j.actbio.2014.04.027 [DOI] [PubMed] [Google Scholar]
- 102.Chen AK, Reuveny S, Oh SK. Application of human mesenchymal and pluripotent stem cell microcarrier cultures in cellular therapy: achievements and future direction. Biotechnol Adv 2013;31:1032-46. 10.1016/j.biotechadv.2013.03.006 [DOI] [PubMed] [Google Scholar]
- 103.Zhang YG, Guo X, Xu P, et al. Bone mesenchymal stem cells transplanted into rabbit intervertebral discs can increase proteoglycans. Clin Orthop Relat Res 2005;430:219-26. 10.1097/01.blo.0000146534.31120.cf [DOI] [PubMed] [Google Scholar]
- 104.Crevensten G, Walsh AJ, Ananthakrishnan D, et al. Intervertebral disc cell therapy for regeneration: mesenchymal stem cell implantation in rat intervertebral discs. Ann Biomed Eng 2004;32:430-4. 10.1023/B:ABME.0000017545.84833.7c [DOI] [PubMed] [Google Scholar]
- 105.Elabd C, Centeno CJ, Schultz JR, et al. Intra-discal injection of autologous, hypoxic cultured bone marrow-derived mesenchymal stem cells in five patients with chronic lower back pain: a long-term safety and feasibility study. J Transl Med 2016;14:253. 10.1186/s12967-016-1015-5 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 106.Stolzing A, Bauer E, Scutt A. Suspension cultures of bone-marrow-derived mesenchymal stem cells: effects of donor age and glucose level. Stem Cells Dev 2012;21:2718-23. 10.1089/scd.2011.0406 [DOI] [PubMed] [Google Scholar]
- 107.Liang C, et al. Responses of human adipose-derived mesenchymal stem cells to chemical microenvironment of the intervertebral disc. J Transl Med 2012;10:49. 10.1186/1479-5876-10-49 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 108.Wuertz K, Godburn K, Neidlinger-Wilke C, et al. Behavior of Mesenchymal Stem Cells in the Chemical Microenvironment of the Intervertebral Disc. Spine 2008;33:1843-9. 10.1097/BRS.0b013e31817b8f53 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 109.Mavrogonatou E, Kletsas D. High osmolality activates the G1 and G2 cell cycle checkpoints and affects the DNA integrity of nucleus pulposus intervertebral disc cells triggering an enhanced DNA repair response. DNA Repair (Amst) 2009;8:930-43. 10.1016/j.dnarep.2009.05.005 [DOI] [PubMed] [Google Scholar]
- 110.Liang C, Li H, Tao Y, et al. Responses of human adipose-derived mesenchymal stem cells to chemical microenvironment of the intervertebral disc. J Transl Med 2012;10:49. 10.1186/1479-5876-10-49 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 111.Tao YQ, Liang CZ, Li H, et al. Potential of co-culture of nucleus pulposus mesenchymal stem cells and nucleus pulposus cells in hyperosmotic microenvironment for intervertebral disc regeneration. Cell Biol Int 2013;37:826-34. 10.1002/cbin.10110 [DOI] [PubMed] [Google Scholar]
- 112.Wuertz K, Godburn K, Iatridis JC. MSC response to pH levels found in degenerating intervertebral discs. Biochem Biophys Res Commun 2009;379:824-9. 10.1016/j.bbrc.2008.12.145 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 113.Vergroesen PP, Kingma I, Emanuel KS, et al. Mechanics and biology in intervertebral disc degeneration: a vicious circle. Osteoarthr Cartil 2015;23:1057-70. 10.1016/j.joca.2015.03.028 [DOI] [PubMed] [Google Scholar]
- 114.Neidlinger-Wilke C, Galbusera F, Pratsinis H, et al. Mechanical loading of the intervertebral disc: from the macroscopic to the cellular level. Eur Spine J 2014;23 Suppl 3:S333-43. 10.1007/s00586-013-2855-9 [DOI] [PubMed] [Google Scholar]
- 115.Purmessur D, Guterl CC, Cho SK, et al. Dynamic pressurization induces transition of notochordal cells to a mature phenotype while retaining production of important patterning ligands from development. Arthritis Res Ther 2013;15:R122. 10.1186/ar4302 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 116.Yurube T, Hirata H, Kakutani K, et al. Notochordal cell disappearance and modes of apoptotic cell death in a rat tail static compression-induced disc degeneration model. Arthritis Res Ther 2014;16:R31. 10.1186/ar4460 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 117.See EY, Toh SL, Goh JC. Effects of radial compression on a novel simulated intervertebral disc-like assembly using bone marrow-derived mesenchymal stem cell cell-sheets for annulus fibrosus regeneration. Spine (Phila Pa 1976) 2011;36:1744-51. 10.1097/BRS.0b013e31821986b3 [DOI] [PubMed] [Google Scholar]
- 118.Dai J, Wang H, Liu G, et al. Dynamic compression and co-culture with nucleus pulposus cells promotes proliferation and differentiation of adipose-derived mesenchymal stem cells. J Biomech 2014;47:966-72. 10.1016/j.jbiomech.2014.01.023 [DOI] [PubMed] [Google Scholar]
- 119.Masuda K. Biological repair of the degenerated intervertebral disc by the injection of growth factors. Eur Spine J 2008;17:441-51. 10.1007/s00586-008-0749-z [DOI] [PMC free article] [PubMed] [Google Scholar]
- 120.Li X, Leo BM, Beck G, et al. Collagen and proteoglycan abnormalities in the gdf-5-deficient mice and molecular changes when treating disk cells with recombinant growth factor. Spine (Phila Pa 1976) 2004;29:2229-34. 10.1097/01.brs.0000142427.82605.fb [DOI] [PubMed] [Google Scholar]
- 121.Chujo T, An HS, Akeda K, et al. Effects of growth differentiation factor-5 on the intervertebral disc-in vitro bovine study and in vivo rabbit disc degeneration model study. Spine (Phila Pa 1976) 2006;31:2909-17. 10.1097/01.brs.0000248428.22823.86 [DOI] [PubMed] [Google Scholar]
- 122.Kim NK, Shin DA, Han IB, et al. The association of aggrecan gene polymorphism with the risk of intervertebral disc degeneration. Acta Neurochir (Wien) 2011;153:129-33. 10.1007/s00701-010-0831-2 [DOI] [PubMed] [Google Scholar]
- 123.Stoyanov JV, Gantenbein-Ritter B, Bertolo A, et al. Role of hypoxia and growth and differentiation factor-5 on differentiation of human mesenchymal stem cells towards intervertebral nucleus pulposus-like cells. Eur Cell Mater 2011;21:533-47. 10.22203/eCM.v021a40 [DOI] [PubMed] [Google Scholar]
- 124.Kepler CK, Ponnappan RK, Tannoury CA, et al. The molecular basis of intervertebral disc degeneration. Spine J 2013;13:318-30. 10.1016/j.spinee.2012.12.003 [DOI] [PubMed] [Google Scholar]
- 125.Mackiewicz Z, Salo J, Konttinen YT, et al. Receptor activator of nuclear factor kappa B ligand in an experimental intervertebral disc degeneration. Clin Exp Rheumatol 2009;27:299-306. [PubMed] [Google Scholar]
- 126.Vo NV, Hartman RA, Yurube T, et al. Expression and regulation of metalloproteinases and their inhibitors in intervertebral disc aging and degeneration. Spine J 2013;13:331-41. 10.1016/j.spinee.2012.02.027 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 127.Wehling N, Palmer GD, Pilapil C, et al. Interleukin-1beta and tumor necrosis factor alpha inhibit chondrogenesis by human mesenchymal stem cells through NF-kappaBdependent pathways. Arthritis Rheum 2009;60:801-12. 10.1002/art.24352 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 128.Evans CH, Huard J. Gene therapy approaches to regenerating the musculoskeletal system. Nat Rev Rheumatol 2015;11:234-42. 10.1038/nrrheum.2015.28 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 129.Zhang Y, Chee A, Thonar EJ, et al. Intervertebral disk repair by protein, gene, or cell injection: A framework for rehabilitation-focused biologics in the spine. PM R 2011;3:S88-94. 10.1016/j.pmrj.2011.04.020 [DOI] [PubMed] [Google Scholar]
- 130.Le Maitre CL, Freemont AJ, Hoyland JA. A preliminary in vitro study into the use of IL-1Ra gene therapy for the inhibition of intervertebral disc degeneration. Int J Exp Pathol 2006;87:17-28. 10.1111/j.0959-9673.2006.00449.x [DOI] [PMC free article] [PubMed] [Google Scholar]
- 131.Paul R, Haydon RC, Cheng H, et al. Potential use of SOX9 gene therapy for intervertebral degenerative disc disease. Spine 2003;28:755-63. 10.1097/01.BRS.0000058946.64222.92 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 132.Leckie SK, Bechara BP, Hartman RA, et al. Injection of AAV2-BMP2 and AAV2-TIMP1 into the nucleus pulposus slows the course of intervertebral disc degeneration in an in vivo rabbit model. Spine J 2012;12:7-20. 10.1016/j.spinee.2011.09.011 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 133.Kuh SU, Zhu Y, Li J, et al. The AdLMP-1 transfection in two different cells; AF cells, chondrocytes as potential cell therapy candidates for disc degeneration. Acta Neurochir 2008;150:803-10. 10.1007/s00701-008-1617-7 [DOI] [PubMed] [Google Scholar]
- 134.Steck E, Bertram H, Abel R, et al. Induction of intervertebral disc-like cells from adult mesenchymal stem cells. Stem Cells 2005;23:403-11. 10.1634/stemcells.2004-0107 [DOI] [PubMed] [Google Scholar]
- 135.Jin H, Shen J, Wang B, et al. TGF-beta signaling plays an essential role in the growth and maintenance of intervertebral disc tissue. FEBS Lett 2011;585:1209-15. 10.1016/j.febslet.2011.03.034 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 136.Konttinen YT, Kemppinen P, Li TF, et al. Transforming and epidermal growth factors in degenerated intervertebral discs. J Bone Joint Surg Br 1999;81:1058-63. 10.1302/0301-620X.81B6.0811058 [DOI] [PubMed] [Google Scholar]
- 137.Thompson JP, Oegema TR, Jr, Bradford DS. Stimulation of mature canine intervertebral disc by growth factors. Spine 1991;16:253-60. 10.1097/00007632-199103000-00001 [DOI] [PubMed] [Google Scholar]
- 138.Walsh AJ, Bradford DS, Lotz JC. In vivo growth factor treatment of degenerated intervertebral discs. Spine 2004;29:156-63. 10.1097/01.BRS.0000107231.67854.9F [DOI] [PubMed] [Google Scholar]
- 139.Le Maitre CL, Richardson SM, Baird P, et al. Expression of receptors for putative anabolic growth factors in human intervertebral disc: implications for repair and regeneration of the disc. J Pathol 2005;207:445-52. 10.1002/path.1862 [DOI] [PubMed] [Google Scholar]
- 140.Li J, Yoon ST, Hutton WC. Effect of bone morphogenetic protein-2 (BMP-2) on matrix production, other BMPs, and BMP receptors in rat intervertebral disc cells. J Spinal Disord Tech 2004;17:423-8. 10.1097/01.bsd.0000112084.85112.5d [DOI] [PubMed] [Google Scholar]
- 141.Masuda K, Pfister BE, Sah RL, et al. Osteogenic protein-1 promotes the formation of tissue-engineered cartilage using the alginate-recovered-chondrocyte method. Osteoarthritis Cartilage 2006;14:384-91. 10.1016/j.joca.2005.10.006 [DOI] [PubMed] [Google Scholar]
- 142.Michalek AJ, Funabashi KL, Iatridis JC. Needle puncture injury of the rat intervertebral disc affects torsional and compressive biomechanics differently. Eur Spine J 2010;19:2110-6. 10.1007/s00586-010-1473-z [DOI] [PMC free article] [PubMed] [Google Scholar]
- 143.Vadalà G, Sowa G, Hubert M, et al. Mesenchymal stem cells injection in degenerated intervertebral disc: cell leakage may induce osteophyte formation. J Tissue Eng Regen Med 2012;6:348-55. 10.1002/term.433 [DOI] [PubMed] [Google Scholar]
- 144.Li YY, Diao HJ, Chik TK, et al. Delivering mesenchymal stem cells in collagen microsphere carriers to rabbit degenerative disc: reduced risk of osteophyte formation. Tissue Eng Part A 2014;20:1379-91. 10.1089/ten.tea.2013.0498 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 145.Moyer HR, Kinney RC, Singh KA, et al. Alginate microencapsulation technology for the percutaneous delivery of adipose-derived stem cells. Ann Plast Surg 2010;65:497-503. 10.1097/SAP.0b013e3181d37713 [DOI] [PubMed] [Google Scholar]
- 146.Amer MH, White LJ, Shakesheff KM. The effect of injection using narrow-bore needles on mammalian cells: administration and formulation considerations for cell therapies. J Pharm Pharmacol 2015;67:640-50. 10.1111/jphp.12362 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 147.Aguado BA, Mulyasasmita W, Su J, et al. Improving Viability of Stem Cells During Syringe Needle Flow Through the Design of Hydrogel Cell Carriers. Tissue Engineering Part A 2012;18:806-15. 10.1089/ten.tea.2011.0391 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 148.Gullbrand SE, Malhotra NR, Schaer TP, et al. A large animal model that recapitulates the spectrum of human intervertebral disc degeneration. Osteoarthritis Cartilage 2017;25:146-56. 10.1016/j.joca.2016.08.006 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 149.Vadalà G, De Strobel F, Bernardini M, et al. The transpedicular approach for the study of intervertebral disc regeneration strategies: in vivo characterization. Eur Spine J 2013;22:S972-8. 10.1007/s00586-013-3007-y [DOI] [PMC free article] [PubMed] [Google Scholar]
- 150.Thorpe AA, Dougill G, Vickers L, et al. Thermally triggered hydrogel injection into bovine intervertebral disc tissue explants induces differentiation of mesenchymal stem cells and restores mechanical function. Acta Biomater 2017;54:212-26. 10.1016/j.actbio.2017.03.010 [DOI] [PubMed] [Google Scholar]
- 151.Gullbrand SE, Schaer TP, Agarwal P, et al. Translation of an injectable triple-interpenetrating-network hydrogel for intervertebral disc regeneration in a goat model. Acta Biomater 2017;60:201-9. 10.1016/j.actbio.2017.07.025 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 152.Zhou X, Wang JK, Fang WJ, et al. Genipin cross-linked type II collagen/ chondroitin sulfate composite hydrogel-like cell delivery system induces differentiation of adipose-derived stem cells and regenerates degenerated nucleus pulposus. Acta Biomater 2018;71:496-509. 10.1016/j.actbio.2018.03.019 [DOI] [PubMed] [Google Scholar]
- 153.Zeng Y, Chen C, Liu W, et al. Injectable microcryogels reinforced alginate encapsulation of mesenchymal stromal cells for leak-proof delivery and alleviation of canine disc degeneration. Biomaterials 2015;59:53-65. 10.1016/j.biomaterials.2015.04.029 [DOI] [PubMed] [Google Scholar]
- 154.Frith JE, Cameron AR, Menzies DJ, et al. An injectable hydrogel incorporating mesenchymal precursor cells and pentosan polysulphate for intervertebral disc regeneration. Biomaterials 2013;34:9430-40. 10.1016/j.biomaterials.2013.08.072 [DOI] [PubMed] [Google Scholar]
- 155.Smith LJ, Gorth DJ, Showalter BL, et al. In vitro characterization of a stem cell-seeded triple interpenetrating network hydrogel for functional regeneration of the nucleus pulposus. Tissue Eng Part A 2014;20:1841-9. 10.1089/ten.tea.2013.0516 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 156.Blanquer SB, Grijpma DW, Poot AA. Delivery systems for the treatment of degenerated intervertebral discs. Adv Drug Deliv Rev 2015;84:172-87. 10.1016/j.addr.2014.10.024 [DOI] [PubMed] [Google Scholar]
- 157.Malafaya PB, Silva GA, Reis RL. Natural–origin polymers as carriers and scaffolds for biomolecules and cell delivery in tissue engineering applications. Adv Drug Deliv Rev 2007;59:207-33. 10.1016/j.addr.2007.03.012 [DOI] [PubMed] [Google Scholar]
- 158.Pereira DR, Silva-Correia J, Oliveira JM, et al. Hydrogels in acellular and cellular strategies for intervertebral disc regeneration. J Tissue Eng Regen Med 2013;7:85-98. 10.1002/term.500 [DOI] [PubMed] [Google Scholar]
- 159.Bertram H, Kroeber M, Wang H, et al. Matrix-assisted cell transfer for intervertebral disc cell therapy. Biochem Biophys Res Commun 2005;331:1185-92. 10.1016/j.bbrc.2005.04.034 [DOI] [PubMed] [Google Scholar]
- 160.Teixeira GQ, Leite Pereira C, Castro F, et al. Anti-inflammatory Chitosan/Poly-gamma-glutamic acid nanoparticles control inflammation while remodeling extracellular matrix in degenerated intervertebral disc. Acta Biomater 2016;42:168-79. 10.1016/j.actbio.2016.06.013 [DOI] [PubMed] [Google Scholar]
- 161.Richardson SM, Hughes N, Hunt JA, et al. Human mesenchymal stem cell differentiation to NP-like cells in chitosan-glycerophosphate hydrogels. Biomaterials 2008;29:85-93. 10.1016/j.biomaterials.2007.09.018 [DOI] [PubMed] [Google Scholar]
- 162.Teng YD, Lavik EB, Qu X, et al. Functional recovery following traumatic spinal cord injury mediated by a unique polymer scaffold seeded with neural stem cells. Proc Natl Acad Sci U S A 2002;99:3024-9. 10.1073/pnas.052678899 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 163.Sato M, Kikuchi M, Ishihara M, et al. Tissue engineering of the intervertebral disc with cultured annulus fibrosus cells using atelocollagen honeycomb-shaped scaffold with a membrane seal (ACHMS scaffold). Med Biol Eng Comput 2003;41:365-71. 10.1007/BF02348444 [DOI] [PubMed] [Google Scholar]
- 164.Sakai D, Mochida J, Iwashina T, et al. Atelocollagen for culture of human nucleus pulposus cells forming nucleus pulposus-like tissue in vitro: influence on the proliferation and proteoglycan production of HNPSV-1 cells. Biomaterials 2006;27:346-53. 10.1016/j.biomaterials.2005.06.040 [DOI] [PubMed] [Google Scholar]
- 165.Aldana AA, Abraham GA. Current advances in electrospun gelatin-based scaffolds for tissue engineering applications. Int J Pharm 2017;523:441-53. 10.1016/j.ijpharm.2016.09.044 [DOI] [PubMed] [Google Scholar]
- 166.Xiao L, Ding MM, Saadoon O, et al. A novel culture platform for fast proliferation of human annulus fibrosus cells. Cell Tissue Res 2017;367:339-50. 10.1007/s00441-016-2497-4 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 167.Yang H, Wu JA, Liu JY, et al. Transplanted mesenchymal stem cells with pure fibrinous gelatin-transforming growth factor-beta 1 decrease rabbit intervertebral disc degeneration. Spine J 2010;10:802-10. 10.1016/j.spinee.2010.06.019 [DOI] [PubMed] [Google Scholar]
- 168.Subhan RA, Puvanan K, Murali MR, et al. Fluoroscopy assisted minimally invasive transplantation of allogenic mesenchymal stromal cells embedded in HyStem reduces the progression of nucleus pulposus degeneration in the damaged intervertebral disc: a preliminary study in rabbits. ScientificWorldJournal 2014;2014:818502. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 169.Woiciechowsky C, Abbushi A, Zenclussen ML, et al. Regeneration of nucleus pulposus tissue in an ovine intervertebral disc degeneration model by cell-free resorbable polymer scaffolds. J Tissue Eng Regen Med 2014;8:811-20. 10.1002/term.1582 [DOI] [PubMed] [Google Scholar]
- 170.Oehme D, Ghosh P, Shimmon S, et al. Mesenchymal progenitor cells combined with pentosane polysulfate mediating disc regeneration at the time of microdiscectomy: a preliminary study in an ovine model. J Neurosurg Spine 2014;20:657-69. 10.3171/2014.2.SPINE13760 [DOI] [PubMed] [Google Scholar]
- 171.Chang G, Kim HJ, Vunjak-Novakovic G, et al. Enhancing annulus fibrosus tissue formation in porous silk scaffolds. J Biomed Mater Res A 2010;92:43-51. 10.1002/jbm.a.32326 [DOI] [PubMed] [Google Scholar]
- 172.Yang J, Yang XL, Wang L, et al. Biomimetic nanofibers can construct effective tissue-engineered intervertebral discs for therapeutic implantation. Nanoscale 2017;9:13095-103. 10.1039/C7NR03944A [DOI] [PubMed] [Google Scholar]
- 173.Zeng Y, Feng SY, Liu W, et al. Preconditioning of mesenchymal stromal cells toward nucleus pulposus-like cells by microcryogels-based 3D cell culture and syringe-based pressure loading system. J Biomed Mater Res B Appl Biomater 2017;105:507-20. 10.1002/jbm.b.33509 [DOI] [PubMed] [Google Scholar]
- 174.Benz K, Stippich C, Osswald C, et al. Rheological and biological properties of a hydrogel support for cells intended for intervertebral disc repair. BMC Musculoskelet Disord 2012;13:54. 10.1186/1471-2474-13-54 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 175.Kook YM, Kang YM, Moon SH, et al. Bi-compartmental 3D scaffolds for the co-culture of intervertebral disk cells and mesenchymal stem cells. J Ind Eng Chem 2016;38:113-22. 10.1016/j.jiec.2016.04.013 [DOI] [Google Scholar]
- 176.Hu J, Chen B, Guo F, et al. Injectable silk fibroin/polyurethane composite hydrogel for nucleus pulposus replacement. J Mater Sci Mater Med 2012;23:711-22. 10.1007/s10856-011-4533-y [DOI] [PubMed] [Google Scholar]
- 177.Kim HY, Kim HN, Lee SJ, et al. Effect of pore sizes of PLGA scaffolds on mechanical properties and cell behaviour for nucleus pulposus regeneration in vivo. J Tissue Eng Regen Med 2017;11:44-57. 10.1002/term.1856 [DOI] [PubMed] [Google Scholar]
- 178.Choy AT, Chan BP. A structurally and functionally biomimetic biphasic scaffold for intervertebral disc tissue engineering. PLoS One 2015;10:e0131827. 10.1371/journal.pone.0131827 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 179.Elsaadany M, Winters K, Adams S, et al. Equiaxial strain modulates adipose-derived stem cell differentiation within 3D biphasic scaffolds towards annulus fibrosus. Sci Rep 2017;7:12868. 10.1038/s41598-017-13240-3 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 180.Park SH, Gil ES, Mandal BB, et al. Annulus fibrosus tissue engineering using lamellar silk scaffolds. J Tissue Eng Regen Med 2012;6:s24-33. 10.1002/term.541 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 181.Ikada Y. Challenges in tissue engineering. J R Soc Interface 2006;3:589-601. 10.1098/rsif.2006.0124 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 182.Hamilton DJ, Seguin CA, Wang J, et al. Formation of a nucleus pulposus-cartilage endplate construct in vitro. Biomaterials 2006;27:397-405. 10.1016/j.biomaterials.2005.07.007 [DOI] [PubMed] [Google Scholar]
- 183.Illien-Jünger S, Sedaghatpour DD, Laudier DM, et al. Development of a bovine decellularized extracellular matrix biomaterial for nucleus pulposus regeneration. J Orthop Res 2016;34:876-88. 10.1002/jor.23088 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 184.O'Connell GD, Vresilovic EJ, Elliott DM. Comparison of animals used in disc research to human lumbar disc geometry. Spine (Phila Pa 1976) 2007;32:328-33. 10.1097/01.brs.0000253961.40910.c1 [DOI] [PubMed] [Google Scholar]
- 185.Chan LK, Leung VY, Tam V, et al. Decellularized bovine intervertebral disc as a natural scaffold for xenogenic cell studies. Acta Biomaterialia 2013;9:5262-72. 10.1016/j.actbio.2012.09.005 [DOI] [PubMed] [Google Scholar]
- 186.Xu B, Xu H, Wu Y, et al. Intervertebral disc tissue engineering with natural extracellular matrix-derived biphasic composite scaffolds. PLoS One 2015;10:e0124774. 10.1371/journal.pone.0124774 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 187.Chan SC, Ferguson SJ, Wuertz K, et al. Biological response of the intervertebral disc to repetitive short-term cyclic torsion. Spine (PhilaPa 1976) 2011;36:2021-30. [DOI] [PubMed]
- 188.Chan SC, Walser J, Käppeli P, et al. Region specific response of intervertebral disc cells to complex dynamic loading: an organ culture study using a dynamic torsion-compression bioreactor. PLoS One 2013;8:e72489. 10.1371/journal.pone.0072489 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 189.Walter BA, Korecki CL, Purmessur D, et al. Complex loading affects intervertebral disc mechanics and biology. Osteoarthritis Cartilage 2011;19:1011-8. 10.1016/j.joca.2011.04.005 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 190.Shah BS, Chahine NO. Dynamic hydrostatic pressure regulates nucleus pulposus phenotypic expression and metabolism in a cell density-dependent manner. J Biomech Eng 2018;140:021003. 10.1115/1.4038758 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 191.Harden RN, Saracoglu M, Connolly S, et al. "Managing" the placebo effect: the single-blind placebo lead-in response in two pain models. Pain Med 2016;17:2305-10. 10.1093/pm/pnv109 [DOI] [PubMed] [Google Scholar]
- 192.Meisel HJ, Agarwal N, Hsieh PC, et al. Cell Therapy for Treatment of Intervertebral Disc Degeneration: A Systematic Review. Global Spine J 2019;9:39S-52S. 10.1177/2192568219829024 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 193.Sheehan NJ. Magnetic resonance imaging for low back pain: indications and limitations. Ann Rheum Dis 2010;69:7-11. 10.1136/ard.2009.110973 [DOI] [PubMed] [Google Scholar]
- 194.Samartzis D, Borthakur A, Belfer I, et al. Novel diagnostic and prognostic methods for disc degeneration and low back pain. Spine J 2015;15:1919-32. 10.1016/j.spinee.2014.09.010 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 195.Lotz JC, Haughton V, Boden SD, et al. New treatments and imaging strategies in degenerative disease of the intervertebral disks. Radiology 2012;264:6-19. 10.1148/radiol.12110339 [DOI] [PubMed] [Google Scholar]
- 196.Keshari KR, Lotz JC, Link TM, et al. Lactic acid and proteoglycans as metabolic markers for discogenic back pain. Spine (Phila Pa 1976) 2008;33:312-7. 10.1097/BRS.0b013e31816201c3 [DOI] [PubMed] [Google Scholar]
- 197.Hu S, Peacock J, Claude J, et al. Modified magnetic resonance spectroscopy diagnosis of painful and non-painful lumbar intervertebral discs. Presented at the 2010 Annual Meeting of the Orthopaedic Research Society, 2010. [Google Scholar]
- 198.Wang C, McArdle E, Fenty M, et al. Validation of sodium magnetic resonance imaging of intervertebral disc. Spine 2010;35:505-10. 10.1097/BRS.0b013e3181b32d3b [DOI] [PMC free article] [PubMed] [Google Scholar]
- 199.Basu S, Zaidi H, Houseni M, et al. Novel quantitative techniques for assessing regional and global function and structure based on modern imaging modalities: implications for normal variation, aging and diseased states. Semin Nucl Med 2007;37:223-39. 10.1053/j.semnuclmed.2007.01.005 [DOI] [PubMed] [Google Scholar]
- 200.Manchikanti L, Soin A, Benyamin RM, et al. An Update of the Systematic Appraisal of the Accuracy and Utility of Discography in Chronic Spinal Pain. Pain Physician 2018;21:91-110. 10.36076/ppj.2018.2.91 [DOI] [PubMed] [Google Scholar]