Abstract
Background and Purpose
Fentanyl overdose deaths have reached “epidemic” levels in North America. Death in opioid overdose invariably results from respiratory depression. In the present work, we have characterized how fentanyl depresses respiration, and by comparing fentanyl with heroin and morphine, the active breakdown product of heroin, we have sought to determine the factors, in addition to high potency, that contribute to the lethality of fentanyl.
Experimental Approach
Respiration (rate and tidal volume) was measured in awake, freely moving mice by whole body plethysmography.
Key Results
Intravenously administered fentanyl produced more rapid depression of respiration than equipotent doses of heroin or morphine. Fentanyl depressed both respiratory rate and tidal volume. Fentanyl did not depress respiration in μ‐opioid receptor knockout mice. Naloxone, the opioid antagonist widely used to treat opioid overdose, reversed the depression of respiration by morphine more readily than that by fentanyl, whereas diprenorphine, a more lipophilic antagonist, was equipotent in reversing fentanyl and morphine depression of respiration. Prolonged treatment with morphine induced tolerance to respiratory depression, but the degree of cross tolerance to fentanyl was less than the tolerance to morphine itself.
Conclusion and Implications
We propose that several factors (potency, rate of onset, lowered sensitivity to naloxone, and lowered cross tolerance to heroin) combine to make fentanyl more likely to cause opioid overdose deaths than other commonly abused opioids. Lipophilic antagonists such as diprenorphine may be better antidotes than naloxone to treat fentanyl overdose.
What is already known
Fentanyls are potent opioids responsible for many overdose deaths in North America..
What this study adds
Fentanyl is faster in onset than heroin and depresses both respiratory rate and tidal volume.
Fentanyl respiratory depression shows reduced cross tolerance and is resistant to reversal by naloxone.
What is the clinical significance
Fentanyl is hazardous due to potency, fast on rate, lower cross tolerance and naloxone resistance.
Abbreviation
- NorBNI
nor‐binaltorphimine
1. INTRODUCTION
Since 2013, there has been a dramatic rise in acute opioid overdose deaths involving new synthetic opioids, primarily the fentanyls (https://www.guidetopharmacology.org/GRAC/LigandDisplayForward?ligandId=1626 and structurally related medicinal and illicit drugs), in North America (NIH, 2019). Of the over 60,000 opioid overdose deaths in the United States in 2017, almost 30,000 involved fentanyls, exceeding those involving https://www.guidetopharmacology.org/GRAC/LigandDisplayForward?ligandId=9082 or prescription opioids such as https://www.guidetopharmacology.org/GRAC/LigandDisplayForward?ligandId=7093 and https://www.guidetopharmacology.org/GRAC/LigandDisplayForward?ligandId=7081. Elsewhere, in Europe, fentanyl deaths have been recorded in Estonia (for some time, fentanyls were the main street opioids available in that country), and there have been sporadic outbreaks of fentanyl‐related deaths in the United Kingdom, Germany, and Finland (EMCDDA, 2018). Ease of synthesis (cf. the need to grow swathes of opium poppies to produce heroin), high potency (smaller quantities need to be shipped by comparison with heroin), and ease of purchase on the dark web make the fentanyls attractive to suppliers of illicit opioids (Fairbairn, Coffin, & Walley, 2017). Fentanyls are frequently mixed with heroin to increase its potency (Griswold et al., 2017; Marinetti & Ehlers, 2014). A recent development is the addition of fentanyls to cocaine products and to illicit prescription opioid and benzodiazepine tablets (Green & Gilbert, 2016; Sutter et al., 2017). Death in opioid overdose results primarily from depression of respiration (Mathers et al., 2013; Pierce, Bird, Hickman, & Millar, 2015). Fentanyls and other opioid agonists depress respiration by acting on https://www.guidetopharmacology.org/GRAC/ObjectDisplayForward?objectId=319 at various sites to reduce the response to raised pCO2 and lowered pO2 and thus reduce the drive to breathe (Pattinson, 2008). This reduction in respiratory drive results in a decrease in the rate of breathing and in periods of apnoea (cessation of breathing) which in extremis results in death.
A number of factors may contribute to why the fentanyls are so deadly. Their high potency means that only small amounts are required to produce profound effects and thus even a small error in weighing out the drug can result in too much being taken. Rapid penetration into the brain can result in overdose levels being reached more quickly than with heroin. Deaths in heroin overdose may take more than 30 min to occur after injection (Darke & Duflou, 2016), providing a window of opportunity for intervention (administration of the opioid antagonist https://www.guidetopharmacology.org/GRAC/LigandDisplayForward?ligandId=1638). In contrast, fentanyl overdose deaths can occur very quickly before there is an opportunity to administer naloxone (Burns, DeRienz, Baker, Casavant, & Spiller, 2016). Fentanyls induce muscle stiffness (Benthuysen, Smith, Sanford, Head, & Dec‐Silver, 1986; Streisand et al., 1993) including in intercostal and diaphragm muscles, often referred to as “wooden chest,” and this is likely to make it harder to breathe. There have been several reports suggesting that depression of respiration by fentanyls shows reduced sensitivity to reversal by naloxone (Fairbairn et al., 2017; Lynn & Galinkin, 2018; Peterson et al., 2016; Schumann, Erickson, Thompson, Zautcke, & Denton, 2008). In one report, several cases were recorded in which multiple doses of naloxone were required before recovery of respiration following a fentanyl overdose (Somerville et al., 2017). Furthermore, a low level of cross tolerance between heroin and fentanyls may allow the fentanyls to retain potency to depress respiration in individuals tolerant to heroin‐induced respiratory depression. In the present paper, we have characterized how fentanyl depresses respiration in mice and have sought to determine how the factors described above may contribute to fentanyl overdose.
2. METHODS
2.1. Animals
Male CD‐1 mice weighing approximately 30 g were obtained from Charles River (UK). Breeding pairs of μ‐opioid receptor knockout mice (Oprm1 tm1Kff, RRID:IMSR_JAX:007559) were obtained from the Jackson Laboratory (USA) and offspring bred in house at the University of Bristol. Both male and female μ‐opioid receptor knockout mice weighing between 25 and 30 g were used in this study. Male and female wild‐type C57BL/J mice weighing approximately 30 g were purchased from Charles River. All mice were maintained at 22°C on a reversed 12‐hr dark–light cycle with food and water available ad libitum. All experiments were performed in the dark (active) phase. Mice were randomly ascribed to treatment groups with the experimenter blinded to the drug treatment or knockout/wild‐type phenotype as appropriate.
All procedures were performed in accordance with the UK Animals (Scientific Procedures) Act 1986, the European Communities Council Directive (2010/63/EU) and the University of Bristol ethical review document. Animal studies are reported in compliance with the ARRIVE guidelines (Kilkenny, Browne, Cuthill, Emerson, & Altman, 2010) and with the recommendations made by the British Journal of Pharmacology.
2.2. Measurement of respiration
Respiration was measured in freely moving mice using plethysmography chambers (EMKA Technologies, France) supplied with a 5% CO2 in air mixture (BOC Gas Supplies, UK) as described previously (Hill et al., 2016; Hill, Dewey, Kelly, & Henderson, 2018). The day prior to experimentation, mice were habituated to plethysmograph chambers for 30 min while breathing air. On the day of experimentation, a 20‐min baseline respiration period was recorded prior to administration of an opioid agonist challenge. Rate and depth of respiration were recorded and averaged over 1‐ or 5‐min periods (except immediately after drug injection when the time period was 0.5 or 3 min respectively) and converted to minute volume (rate × tidal volume). Tidal volume was calculated from the raw inspiration and expiration data in concert with compensation accounting for internal plethysmograph chamber variations in temperature and humidity as well as reference atmospheric pressure according to the Drorbaugh and Fenn (1955) formula. Drugs were administered i.v. or i.p. as indicated. Changes in body temperature induced by opioid agonists were not monitored during the plethysmography experiments.
2.3. Measurement of locomotor activity
A beam break rig (Linton Instrumentation, UK) was used to assess the locomotor activity of mice. An automated data logging suite (AMON Lite, Linton Instrumentation) was used to track the movement of mice throughout the experimental session. On the day prior to locomotor assessment, each mouse was placed in a fresh cage and allowed to explore the cage for 30 min. On the experimental day, the mouse was again allowed to explore the cage for 30 min before drug administration. Locomotor activity was then measured for 30 min following drug administration. Mice had access to water ad libitum but had no access to food in either session in order to dissuade rearing and climbing behaviour.
2.4. Drug administration
In most experiments, we have used i.p. injection as this is a simple and relatively non‐stressful means of drug administration to mice. However, in those experiments designed to determine rate of onset of effect, opioid agonist drugs were administered i.v. with mice restrained in a clear plastic tube, while opioid or vehicle was administered by tail vein injection in a 0.1 ml volume.
2.5. Reversal of opioid respiratory depression by opioid antagonists
Respiration was recorded for 20 min followed by an acute i.p. injection of opioid or vehicle. Respiration was then recorded for 20 min following opioid/vehicle administration, allowing maximal depression of respiration to occur. Naloxone or https://www.guidetopharmacology.org/GRAC/LigandDisplayForward?ligandId=1617 was then administered (20 min after opioid/vehicle) by i.p. injection on the opposite side of the peritoneal cavity to the opioid/vehicle injection.
2.6. Induction of morphine tolerance
Tolerance to morphine respiratory depression was induced by 3 × 100 mg·kg−1 morphine injections, administered 12 hr apart followed by subcutaneous implantation of morphine‐filled osmotic minipumps delivering 45 mg·kg−1·day−1 morphine for a total of 6 days as described previously (Hill et al., 2016; Hill et al., 2018). Control mice were injected with saline and implanted with minipumps that were filled with saline.
2.7. Experimental design and data analysis
Data from previous experiments where respiratory depression and locomotor activity were measured either following acute opioid administration in naïve mice or following pump implantation were subjected to post hoc power analyses using G*Power (Version 3.1.9; RRID:SCR_002798). Our calculations indicated that for depression of respiration, n = 6 (acute experiments) or n = 7 (pump experiments) and for locomotor activity, n = 8 for each individual group would produce a significant result if an actual effect occurred.
For each mouse, the change in each behavioural parameter (respiratory rate, tidal volume, minute volume, and locomotor activity) following acute drug administration has been calculated as the percentage of the pre‐drug baseline as described previously (Hill et al., 2016; Hill et al., 2018; Withey et al., 2017). Area under the response versus time curve (AUC) was determined using a 100% baseline as described previously (Hill et al., 2016). Overall changes from a single factor were analysed using a one‐way ANOVA with Bonferroni's posttest. Statistical significance is assumed when P < .05. Only where P < .05 in one‐ or two‐way ANOVA analyses were post hoc comparisons made between parametric variables. Data were excluded only where values were different by greater than two SDs from the mean (as outlined in the appropriate figure legends). GraphPad Prism 5 (RRID:SCR_013726) was used for all statistical analyses. All data are displayed as mean ± SEM. The data and statistical analysis (on group sizes of n > 5 where each n is an independent value) comply with the recommendations of the British Journal of Pharmacology on experimental design and analysis in pharmacology (Curtis et al., 2018).
2.8. Materials
Heroin hydrochloride (diacetyl morphine hydrochloride), https://www.guidetopharmacology.org/GRAC/LigandDisplayForward?ligandId=1627 hydrochloride (both from Macfarlane Smith, UK), fentanyl citrate, naloxone hydrochloride, https://www.guidetopharmacology.org/GRAC/LigandDisplayForward?ligandId=1641 hydrochloride, and https://www.guidetopharmacology.org/GRAC/LigandDisplayForward?ligandId=1642 (NorBNI) dihydrochloride (all from Sigma Aldrich, UK) were dissolved in sterile saline. Diprenorphine (Tocris, UK) was dissolved in water by adding an equivalent amount of hydrochloric acid and subsequently diluted in sterile saline.
2.9. Nomenclature of targets and ligands
Key protein targets and ligands in this article are hyperlinked to corresponding entries in http://www.guidetopharmacology.org/, the common portal for data from the IUPHAR/BPS Guide to PHARMACOLOGY (Harding et al., 2018), and are permanently archived in the Concise Guide to PHARMACOLOGY 2017/18 (Alexander et al., 2017).
3. RESULTS
3.1. Depression of respiration by fentanyl, heroin, and morphine
To study the rate of onset of respiratory depression, fentanyl, heroin, and morphine were administered i.v. to mice. Morphine (7.5 mg·kg−1), heroin (7.5 mg·kg−1), and fentanyl (112 μg·kg−1) each depressed minute volume significantly, reaching approximately the same degree of respiratory depression 10–15 min after injection (Figure 1a). Saline injection did not significantly alter respiration. The half‐time to reach maximum depression of minute volume was determined for each agonist by fitting a single exponential curve (Figure 1a). Fentanyl had the fastest rate of onset and morphine the slowest (Table 1). The rate of onset of respiratory depression correlated with the lipophilicity of each drug (Table 1).
Figure 1.
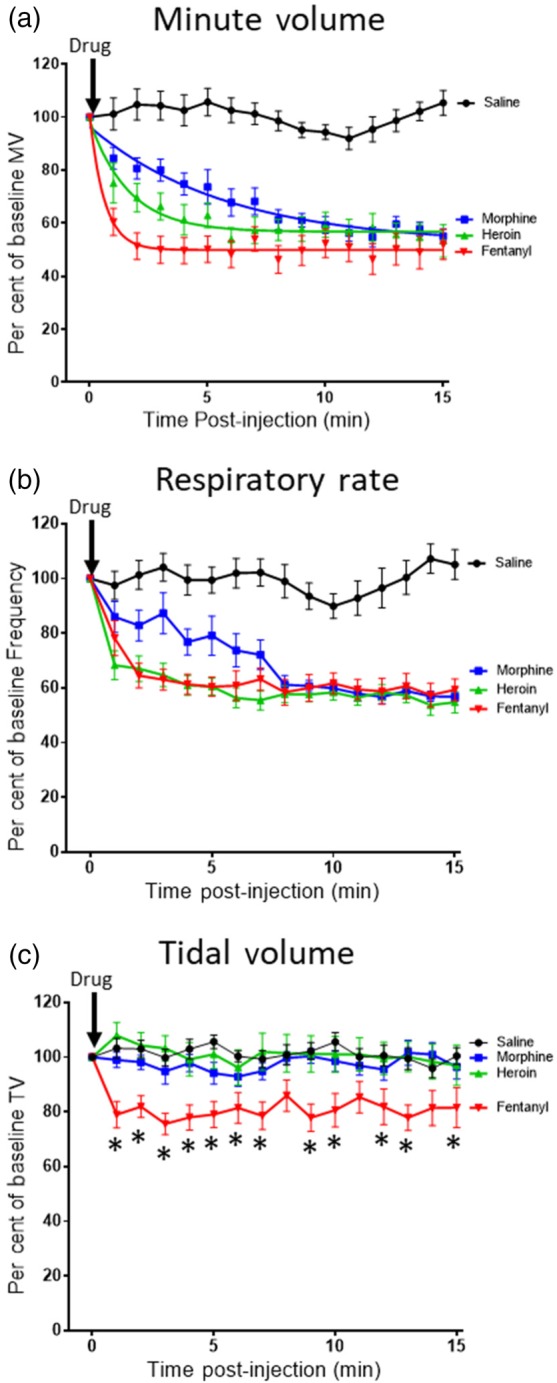
Rate of onset of opioid respiratory depression. Respiratory parameters were monitored in mice receiving i.v. injection of fentanyl (112 μg·kg−1), heroin (7.5 mg·kg−1) morphine (7.5 mg·kg−1), or saline. (a) Fentanyl, heroin, and morphine rapidly depressed minute volume (MV), the effect of the drugs reaching a similar steady state 10–15 min post‐administration. Data for each drug are fitted to a single exponential. (b) Fentanyl, heroin, and morphine depressed respiratory rate. (c) Heroin and morphine had no effect on tidal volume (TV), whereas fentanyl significantly depressed tidal volume [F = 65.05 (dfn = 3, dfd = 704)]. (a–c) Saline injection did not alter any of the respiratory parameters. All data presented as mean ± SEM. Statistical comparison in (c) was made by two‐way ANOVA with Bonferroni's comparison. * indicates P < .05 compared to saline. n = 12 for each group
Table 1.
Rate of onset of opioid depression of respiration following i.v. administration and calculated lipid solubility values (ClogP) for opioid agonists
| Opioid | Rate of onset (t 1/2, min) | ClogP |
|---|---|---|
| Fentanyl | 0.54 ± 0.09*, ¶ | 3.62 |
| Heroin | 1.70 ± 0.68* | 1.48 |
| Morphine | 4.64 ± 1.62 | 0.57 |
Note. The data for the onset of respiratory depression for opioid agonists were fitted to a single exponential (see Figure 1a) to obtain the t 1/2 value for each opioid drug. Data are presented as the mean ± SEM; n = 12 for each drug. Statistical comparison was made by one‐way ANOVA with Bonferroni's comparison. The ClogP value for each drug was calculated using Chem3D (PerkinElmer).
Indicates statistical difference (P < .05) from morphine.
Indicates statistical difference (P < .05) from heroin [F = 5.093 (dfn = 2, dfd = 36)].
Both morphine and heroin depressed respiration by decreasing the rate of respiration, having no effect on tidal volume (Figure 1b,c). As previously reported (Hill et al., 2016; Hill et al., 2018), tidal volume was maintained due to the prolongation of inspiration by apneustic compensation. However, fentanyl depression of respiration resulted from both a decrease in the rate of respiration (Figure 1b) and a decrease in tidal volume (Figure 1c). As the rate of onset of the decrease in rate of respiration was the same for both heroin and fentanyl (Figure 1b), then the faster depression of minute volume by fentanyl (see Figure 1a) seems likely to be due to the rapid decrease in tidal volume (Figure 1c).
We have previously demonstrated the dose dependency of depression of respiration by morphine and oxycodone in mice breathing 5% CO2 in air (Hill et al., 2016; Hill et al., 2018). Heroin (1–90 mg·kg−1 i.p.) produced a dose‐dependent depression of minute volume and respiratory rate (Figure 2a,e,g) but only slightly decreased tidal volume at the highest dose of 90 mg·kg−1 (Figure 2c,f,g). The potency of heroin to depress minute volume was similar to that previously reported for morphine (Hill et al., 2016). Fentanyl (0.05–1.35 mg·kg−1 i.p.) produced a dose‐dependent depression of tidal volume, respiratory rate, and minute volume (Figure 2b,d,e,f,g). To depress minute volume, fentanyl was approximately 70‐fold more potent than heroin.
Figure 2.
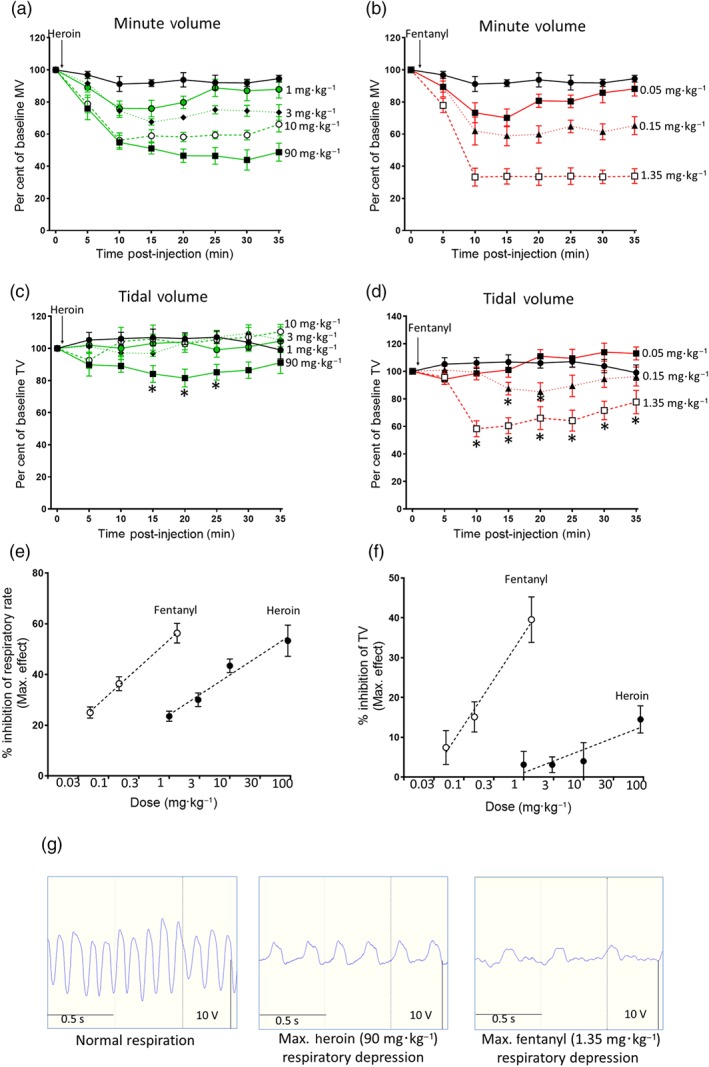
Effect of heroin or fentanyl on mouse respiration. (a) Heroin (1–90 mg·kg−1, i.p.) dose‐dependently depressed minute volume (MV). (b) Fentanyl (0.05–1.35 mg·kg−1, i.p.) dose‐dependently depressed minute volume. (c) Heroin only slightly depressed tidal volume (TV) at the highest dose tested (data from one mouse have been excluded from all heroin 90 mg·kg−1 results due to the TV values being two SDs higher than the group mean). (d) Fentanyl dose‐dependently depressed tidal volume. In (a–d), the saline control is shown in black. In (c) [F = 13.07 (dfn = 4, dfd = 192)] and (d) [F = 58.33 (dfn = 3, dfd = 160)], statistical comparison was made by two‐way ANOVA with Bonferroni's comparison. * indicates P < .05 compared to saline pretreated mice. n = 6 for each group. In (a and b), statistical significance was observed for most time points for each dose of heroin and fentanyl, but *s have been omitted for clarity as the size of effect of each dose is clear from the dose–response graphs in (e) and (f). (e) Dose–response curves for fentanyl and heroin depression of respiratory rate (data presented as peak depression of respiratory rate as calculated from experiments shown in a and b). (f) Dose–response curves for fentanyl and heroin inhibition of tidal volume (data presented as peak depression of tidal volume calculated from experiments shown in c and d). (g) Left‐hand trace—control respiratory trace in the absence of opioid. Middle trace—at maximum respiratory depression by heroin 90 mg·kg−1. Right‐hand trace—at maximum respiratory depression by fentanyl 1.35 mg·kg−1
3.2. Effects of fentanyl and heroin on locomotor activity
In mice, at high doses, morphine and other μ‐opioid receptor agonists increase locomotor activity (Hill et al., 2016; Lessov & Phillips, 2003; Valjent et al., 2010). In the present study, we examined the effect on locomotor activity of doses of heroin and fentanyl that produced similar levels of depression of respiratory rate (Figure 2e) and observed that heroin (90 mg·kg−1 i.p.) increased locomotor activity, whereas fentanyl (1.35 mg·kg−1 i.p.) decreased locomotor activity (Figure 3).
Figure 3.
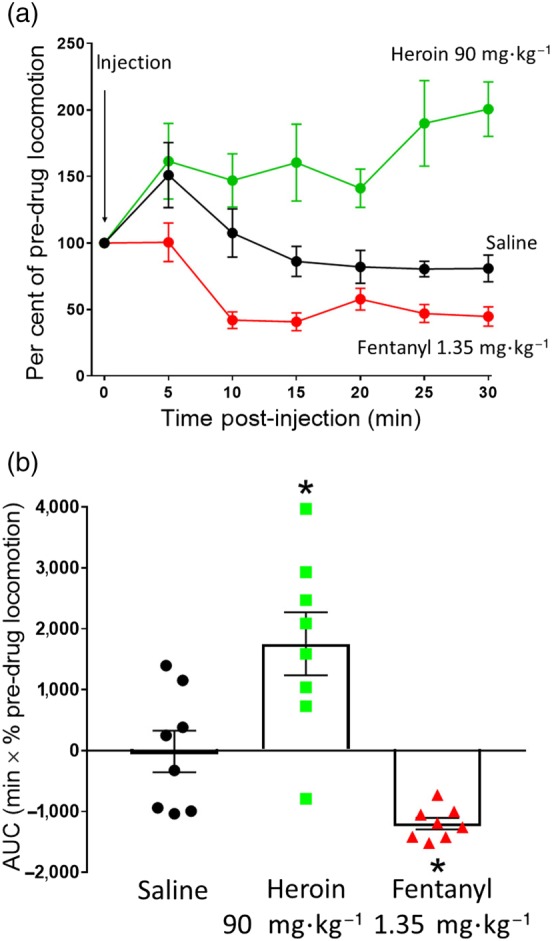
Change in mouse locomotor activity following heroin or fentanyl administration. (a) Saline (i.p.), heroin (90 mg·kg−1 i.p.), or fentanyl (1.35 mg·kg−1 i.p.) were administered to mice and locomotor activity measured. Heroin caused a sustained increase in locomotor activity compared to saline, whereas fentanyl caused a decrease in locomotor activity compared to saline. (b) AUC analysis of data in (a). Statistical comparison in (b) made using one‐way ANOVA with Bonferroni's comparison. * indicates P < .05 compared to saline. n = 8 for each group
3.3. Antagonism of fentanyl depression of respiration
It has been suggested that in overdose in humans, the depression of respiration by fentanyl is less effectively reversed by naloxone compared to that by heroin (Peterson et al., 2016; Schumann et al., 2008; Somerville et al., 2017). To investigate antagonist reversal of opioid respiratory depression, we administered equipotent doses of morphine, the main active breakdown product of heroin, and fentanyl (10 and 0.15 mg·kg−1 i.p. respectively) to mice, allowed maximal depression of respiration to develop over 20 min, and then administered naloxone or diprenorphine. Naloxone (0.3 mg·kg−1 i.p.) rapidly antagonized the depression of respiration induced by morphine, with full reversal being apparent 5 min after naloxone administration (Figure 4a). In contrast, the same dose of naloxone did not fully reverse the respiratory depression induced by fentanyl (Figure 4a). Naloxone (1 mg·kg−1 i.p.) also did not fully reverse the respiratory depression induced by fentanyl (Figure 4b) but did fully reverse respiratory depression induced by morphine. Only when naloxone (3 mg·kg−1 i.p.) was administered did full reversal of the respiratory depression induced by fentanyl occur (Figure 4c), that is, a 10‐fold greater dose of naloxone was required to reverse fentanyl respiratory depression compared to that by an equipotent dose of morphine. On the other hand, when naloxone (0.3 mg·kg−1 i.p.) was administered 20 min prior to fentanyl or morphine, the response to either opioid was attenuated although the fentanyl response was less affected than that of morphine (Figure 4f).
Figure 4.
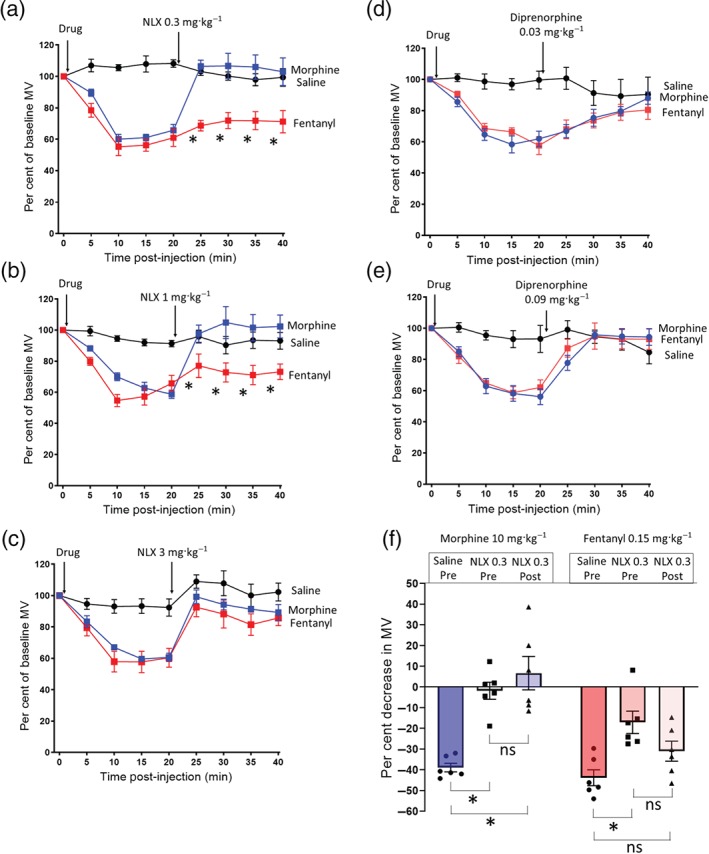
Reversal of morphine and fentanyl respiratory depression by naloxone and diprenorphine. (a–c) Equipotent respiratory depressant doses of morphine (10 mg·kg−1 i.p.) and fentanyl (0.15 mg·kg−1 i.p.) were administered to mice before naloxone administration 20 min after saline or opioid agonist. (a) Naloxone 0.3 mg·kg−1 i.p. fully reversed morphine respiratory depression, but fentanyl respiratory depression was unaffected (−43.8 ± 4.8% pre‐naloxone vs. −28.1 ± 7.8% post‐naloxone P > .05) [F = 55.3 (dfn = 2, dfd = 15)]. (b) Naloxone 1 mg·kg−1 i.p. fully reversed morphine respiratory depression, whereas fentanyl was not reversed (−42.7 ± 5.4% pre‐naloxone vs. −28.9 ± 6.2% post‐naloxone P > .05) [F = 14.98 (dfn = 2, dfd = 15)]. (c) Naloxone 3 mg·kg−1 i.p. fully reversed both morphine and fentanyl respiratory depression. (d) Diprenorphine 0.03 mg·kg−1 i.p. reversed morphine and fentanyl respiratory depression to the same degree. (e) Diprenorphine 0.09 mg·kg−1 i.p. rapidly reversed both morphine and fentanyl respiratory depression back to baseline levels. All data presented as mean ± SEM. Statistical comparison of minute volume following naloxone administration made by two‐way ANOVA with Bonferroni's comparison. * indicates P < .05 compared to saline. n = 6 for each group
In contrast to naloxone, diprenorphine (0.03 mg·kg−1 i.p.) reversed equipotent doses of morphine and fentanyl to the same degree (Figure 4d). However, administration of a higher dose of diprenorphine (0.09 mg·kg−1 i.p.) more rapidly reversed morphine and fentanyl depression of respiration (Figure 4e).
Fentanyl has been reported to be a relatively selective agonist at μ‐opioid receptors showing 100‐fold and 400‐fold higher affinity for the μ‐opioid receptor over https://www.guidetopharmacology.org/GRAC/ObjectDisplayForward?objectId=318 and https://www.guidetopharmacology.org/GRAC/ObjectDisplayForward?objectId=317 respectively (Toll et al., 1998). To examine the possibility that there may be a δ opioid receptor component to fentanyl's respiratory depressant activity, we have assessed the ability of the δ opioid antagonist naltrindole to prevent the effect of fentanyl on minute volume, respiratory rate, and tidal volume in CD‐1 mice. Pretreatment with naltrindole (10 mg·kg−1) failed to prevent the depression of minute volume, respiratory rate, and tidal volume produced by fentanyl (1.35 mg·kg−1; Figure 5); indeed, naltrindole slightly potentiated the effect of fentanyl on tidal volume (Figure 5c) but not on respiratory rate (Figure 5b). Furthermore, pretreatment of CD‐1 mice with the κ opioid receptor antagonist NorBNI (10 mg·kg−1 i.p.) 24 hr prior to fentanyl administration did not affect the respiratory depressant effects of fentanyl (1.35 mg·kg−1; Figure 5d–f). We have previously reported that this treatment with NorBNI prevented the antinociceptive response to the κ opioid receptor agonist https://www.guidetopharmacology.org/GRAC/LigandDisplayForward?ligandId=1655 in the tail flick latency assay and that U69593 alone did not depress respiration (Hill et al., 2018). Treatment of mice with naltrindole or NorBNI alone did not alter respiratory parameters (Table S1).
Figure 5.
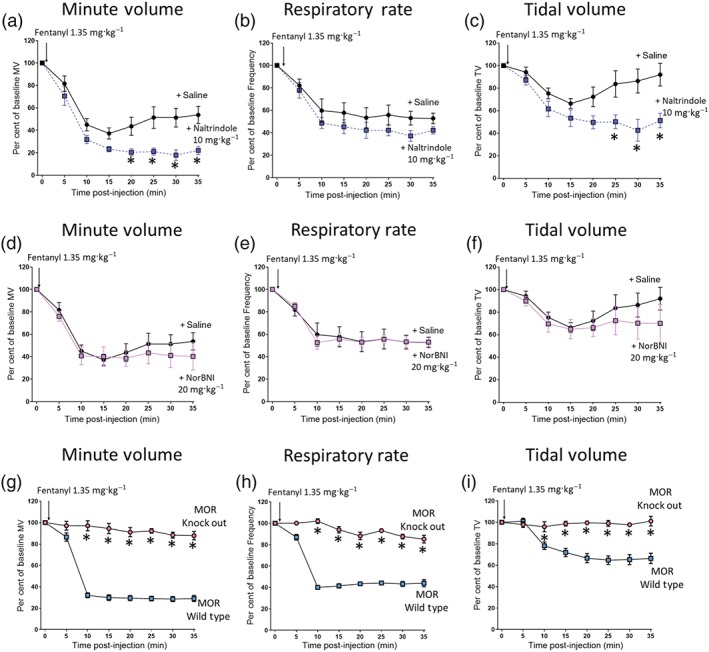
Fentanyl depression of respiration results from activation of μ‐opioid receptors. (a, b, and c) Pretreatment with naltrindole (10 mg·kg−1 i.p.) 20 min prior to fentanyl (1.35 mg·kg−1 i.p.) injection did not prevent fentanyl depression of minute volume (a), respiratory rate (b), or tidal volume (c). Instead, naltrindole pretreatment significantly enhanced fentanyl depression of both minute volume and tidal volume. (d, e, and f) Pretreatment with NorBNI (20 mg·kg−1 i.p.) 24 hr prior to administration of fentanyl did not alter fentanyl‐induced depression of minute volume (d), respiratory rate (e), or tidal volume (f). (g) Administration of fentanyl (1.35 mg·kg−1 i.p.) significantly depressed minute volume in wild‐type background strain mice (MOR Wild Type), but there was no effect in μ‐opioid receptor knockout mice (MOR Knock Out) [F = 933 (dfn = 1, dfd = 80)]. (h) Fentanyl (1.35 mg·kg−1 i.p.) also depressed respiratory rate in wild‐type mice, but there was no effect in μ‐opioid receptor knockout mice [F = 414.1 (dfn = 1, dfd = 80)]. (i) Fentanyl (1.35 mg·kg−1 i.p.) depressed tidal volume in wild‐type mice, but there was no effect in μ‐opioid receptor knockout mice [F = 170.9 (dfn = 1, dfd = 80)]. All data presented as mean ± SEM. Statistical comparison made by two‐way ANOVA with Bonferroni's comparison. * indicates P < .05 compared to saline pretreated mice. n = 6 for each group
Finally, we examined the ability of fentanyl to depress respiration in μ‐opioid receptor knockout mice (C57BL/J background strain). Wild‐type and μ‐opioid receptor knockout mice exhibited similar respiratory parameters prior to drug administration (Table S2). Administration of fentanyl (1.35 mg·kg−1 i.p.) to the wild‐type mice depressed minute volume by ~80%, respiratory rate by ~60%, and depressed tidal volume by ~30% (Figure 5g–i). Administration of the same dose of fentanyl to μ‐opioid receptor knockout mice produced no depression of either minute or tidal volume (Figure 5e,f).
3.4. Interactions between heroin, morphine, and fentanyl
Opioid users are not thought to use fentanyl as their primary drug of choice; rather, they predominantly use heroin to which fentanyl has been added to enhance the “quality” of a given batch of heroin (Ciccarone, 2009; Dasgupta et al., 2013). They are therefore likely to have already developed some degree of tolerance to heroin. We therefore investigated the degree of cross tolerance to fentanyl produced by prolonged pretreatment with morphine, the main active breakdown product of heroin. In control mice that were implanted with a pump containing saline, an acute challenge with morphine (10 or 90 mg·kg−1 i.p.) produced respiratory depression of 40% and 60%, respectively, whereas in mice that had received prolonged pretreatment with morphine, the response to the 10 mg·kg−1 challenge dose of morphine was completely abolished and that to 90 mg·kg−1 markedly attenuated (Figure 6a,b). These data demonstrate that the morphine pretreatment had produced significant tolerance. In contrast, morphine pretreated mice showed significantly less cross tolerance when challenged with fentanyl (Figure 6c–f). At the lower challenge dose of fentanyl (0.15 mg·kg−1), the depression of respiration was partially reduced but to a lesser extent than the equipotent challenge dose of morphine (Figure 6c,e). At the higher challenge dose of fentanyl (1.35 mg·kg−1), respiratory depression was the same as in non‐morphine‐treated animals (Figure 6d,f).
Figure 6.
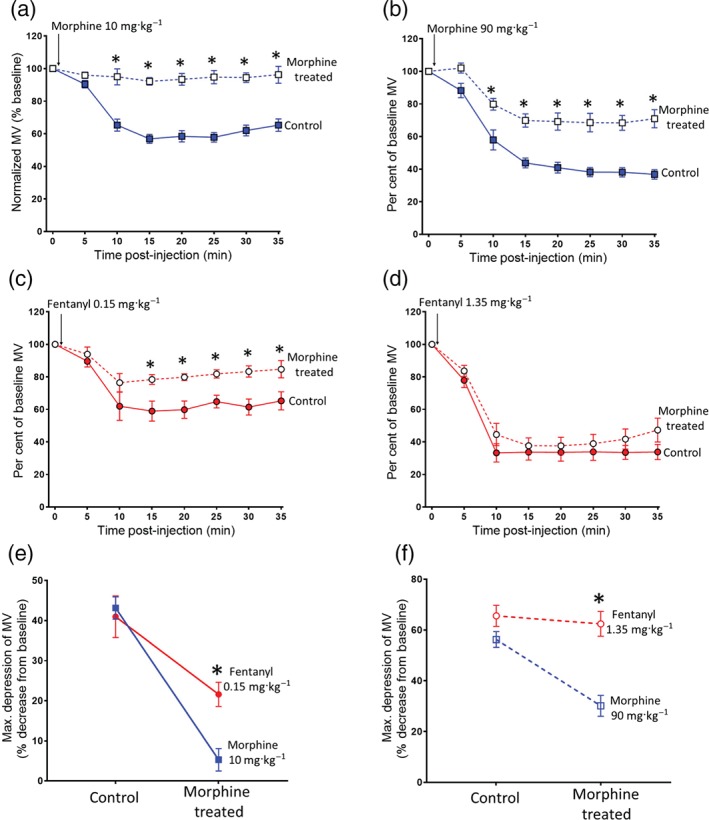
Reduced cross tolerance to fentanyl following prolonged morphine administration. (a–b) Acute morphine challenge (10 or 90 mg·kg−1 i.p.) induced less respiratory depression in morphine‐treated mice compared to saline‐treated controls. (c) Acute fentanyl challenge (0.15 mg·kg−1 i.p.) induced less respiratory depression in morphine‐treated mice compared to saline‐treated controls. (d) Acute fentanyl challenge (1.35 mg·kg−1 i.p.) induced the same level of respiratory depression in morphine‐treated mice as was observed in saline‐treated controls. (e–f) The depression of respiration by fentanyl challenge (0.15 and 1.35 mg·kg−1) was not reduced to the same extent as that by morphine challenge (10 or 90 mg·kg−1) in morphine‐treated animals. All data presented as mean ± SEM. Statistical comparison made by two‐way ANOVA with Bonferroni's comparison. (e) [F = 3.86 (dfn = 1, dfd = 24)]. (f) [F = 25.7 (dfn = 1, dfd = 24)]. * indicates P < .05 compared to morphine. n = 7 for each group
4. DISCUSSION
In the present study, we observed that in the mouse, fentanyl more rapidly depressed respiration than heroin and that with fentanyl, the depression of respiration involved both a decrease in the frequency of breathing and a decrease in tidal volume, whereas with heroin, only at the highest dose was a small effect on tidal volume observed. Fentanyl was approximately 70× more potent that heroin or morphine in depressing respiratory rate; we observed a similar relative potency of fentanyl and morphine to produce antinociception in the same mouse strain (Hill, 2019). We also observed that the depression of respiration by fentanyl required higher doses of the opioid antagonist naloxone to be reversed than did the depression induced by morphine, the active breakdown product of heroin. In contrast, diprenorphine reversed fentanyl and morphine depression of respiration to the same extent. The depression of respiration by fentanyl is mediated by the μ‐opioid receptor because it was not observed in μ‐opioid receptor knockout mice (see also Schmid et al., 2017). Finally, prolonged pretreatment of mice with morphine produced less cross tolerance to fentanyl than the tolerance to morphine itself.
The higher in vivo potency of fentanyls compared to other opioids is well documented as a factor in their lethality. However, we observed in this study that fentanyl was not only approximately 70× more potent than morphine and heroin when depressing mouse respiration but it also had a significantly faster rate of onset to depress respiration. A fast rate of onset has been reported in fentanyl‐using human populations with fentanyl suggested to produce lethal respiratory depression as quickly as 2 min following injection (Burns et al., 2016; Green & Gilbert, 2016). This would make effective intervention with naloxone in fentanyl overdose more difficult to achieve.
Schmid et al. (2017) have proposed that fentanyl's ability to depress respiration results from it being an “arrestin‐biased” μ‐opioid receptor agonist (i.e., it is better at recruiting and signalling through arrestin than activating G protein signalling) and that opioid depression of respiration is mediated by arrestin signalling as proposed by Raehal, Walker, and Bohn (2005). We have previously reported that fentanyl does not exhibit arrestin bias in arrestin translocation and GTPγS binding assays (McPherson et al., 2010; Rivero et al., 2012). Furthermore, in transgenic mice in which all the phosphorylation sites on the C tail of the μ‐opioid receptor had been mutated to alanine and which are therefore not phosphorylated by G protein receptor kinases (GRKs) and do not recruit and bind arrestins, fentanyl still depressed respiration (Kliewer et al., 2019). This observation brings into question the concept that opioid depression of respiration is a function of arrestin signalling (Montandon & Slutsky, 2019).
We have previously reported that in the mouse, the depression of respiration by several μ‐opioid receptor agonists, morphine, oxycodone, and https://www.guidetopharmacology.org/GRAC/LigandDisplayForward?ligandId=5458, results from a reduction in respiratory rate and at the doses tested did not involve a decrease in tidal volume (depth of breathing; Hill et al., 2016; Hill et al., 2018; Withey et al., 2017). In the present study, however, we observed that fentanyl depressed both respiratory rate and tidal volume, while heroin was only observed to reduce tidal volume slightly at the highest dose tested. The decrease in tidal volume produced by fentanyl may result from an increase in respiratory muscle stiffness and/or changes in phrenic motor activity during inspiration expiration (Campbell, Weinger, & Quinn, 1995). Both fentanyl and https://www.guidetopharmacology.org/GRAC/LigandDisplayForward?ligandId=7108 have been reported to cause profound muscle stiffness in rodents by an action on μ‐opioid receptors in the CNS (Lui, Chang, Lee, & Chan, 1993; Weinger, Smith, Blasco, & Koob, 1991). Conversely, the hyper locomotor activity induced by heroin in mice may stimulate respiration and obscure any decrease in tidal volume. This would not occur with the high dose of fentanyl we tested as it reduced locomotor activity but may occur at lower doses which do enhance locomotor activity (Kliewer et al., 2019; Varshneya et al., 2019).
The δ opioid antagonist naltrindole potentiated the fentanyl depression of tidal volume and thus also minute volume, without affecting the depression of respiratory rate. We have previously reported that naltrindole does not alter the depression of minute volume by morphine or oxycodone (Withey et al., 2017). This would imply that the effect of naltrindole on fentanyl depression of tidal volume is not an off‐target effect as any such off‐target effect would be expected to be observed against all the opioid agonists. Rather, it implies that the fentanyl depression of tidal volume involves the release of endogenous opioid(s) on to δ opioid receptors. Given the possible role of muscle stiffness in the depression of tidal volume, it is interesting to note that δ opioid agonists have been shown to inhibit alfentanil‐induced muscle stiffness (Vankova et al., 1996)
In man, i.v. administration of high doses of fentanyl and alfentanil produces skeletal muscle rigidity resulting in stiffness of the chest wall (Benthuysen et al., 1986; Streisand et al., 1993; Waller, Hug, Nagle, & Craver, 1981). Brain microinjection studies in rats have implicated several brain regions—locus coeruleus, basal ganglia, nucleus raphe pontis, and periaqueductal grey—as sites of action of fentanyls to induce muscle rigidity (Blasco et al., 1986; Lui, Lee, & Chan, 1989; Lui, Lee, & Chan, 1990; Slater & Starkie, 1987; Weinger et al., 1991; Widdowson, Griffiths, & Slater, 1986) and have shown that it is mediated by activation of μ, and not δ or κ opioid receptors (Vankova et al., 1996). It is likely therefore that in humans, i.v. injection of fentanyl results in both a decreased drive to breathe and a mechanical resistance to breathing both of which would contribute to overdose death (Burns et al., 2016). Signs of muscle rigidity have been observed in opioid injectors who have presumably injected illicit fentanyls at a supervised drug injection facility in Vancouver (Kinshella, Gauthier, & Lysyshyn, 2018).
Our finding that naloxone less readily reversed respiratory depression by fentanyl compared with morphine confirms reports from studies in humans that more naloxone may be required to reverse a fentanyl overdose compared to a heroin overdose (Fairbairn et al., 2017; Lynn & Galinkin, 2018; Peterson et al., 2016; Schumann et al., 2008; Somerville et al., 2017). In our study, we allowed the depression of respiration by each agonist to reach maximum before naloxone was administered to mimic how the drugs would be administered in an overdose situation. Lower sensitivity of fentanyl to antagonism by naloxone cannot be explained simply by fentanyl having high affinity for the μ‐opioid receptor given that under competitive conditions, the degree of antagonism does not depend on the affinity of the agonist but only upon the affinity and concentration of the antagonist (Rang, Ritter, Flower, & Henderson, 2016). Furthermore, the lipophilic opioid antagonist, diprenorphine, a non‐selective opioid antagonist (Corbett, Paterson, & Kosterlitz, 1993) used in veterinary medicine to reverse the tranquilizing effects of https://www.guidetopharmacology.org/GRAC/LigandDisplayForward?ligandId=1625 and https://www.guidetopharmacology.org/GRAC/LigandDisplayForward?ligandId=10040 in large animals, reversed both fentanyl and morphine depression of respiration equally. Why diprenorphine is better at antagonizing fentanyl than naloxone is unknown at present. We can speculate that, due to its higher lipophilicity, diprenorphine may exhibit a differential drug concentration effect in the vicinity of the plasma membrane as described previously for dopamine antagonists (Gherbi, Briddon, & Charlton, 2018). Alternatively, fentanyl and diprenorphine may bind to the μ‐opioid receptor in a manner distinct from morphine and naloxone, as reported for certain ligands at M2, M3, and M4 muscarinic receptors (Chan et al., 2018; Dror et al., 2013).
We have previously demonstrated that tolerance develops to the respiratory depressant effects of μ‐opioid receptor agonists such as morphine, oxycodone, and methadone (Hill et al., 2016; Hill et al., 2018). In the present study, we observed that when morphine pretreated mice were challenged with either fentanyl or morphine, the degree of cross tolerance to fentanyl was less than the tolerance to morphine itself. A similar reduced level of cross tolerance between fentanyl and morphine has been reported in studies of rodent locomotor activity (Brase, 1986) and antinociception (Bobeck, Schoo, Ingram, & Morgan, 2019; Paronis & Holzman, 1992), although one study has reported equal cross tolerance between morphine and fentanyl on antinociception (Romero, Miranda, & Puig, 2010). Cross tolerance develops between drugs acting at the same receptor, but the degree of cross tolerance will depend upon agonist intrinsic efficacy. Fentanyl has higher intrinsic efficacy (McPherson et al., 2010) and so needs to occupy a smaller proportion of the available receptors to produce its response. It will therefore be less affected by the loss of μ‐opioid receptor function—from either receptor desensitization, internalization, or degradation—that underlies tolerance. The implication being that with opioid drug users, fentanyl will be able to “break through” tolerance induced by heroin, oxycodone, and methadone and produce respiratory depression.
5. CONCLUSIONS
Our studies in mice indicate that in overdose by humans, a number of factors may contribute to the high lethality of fentanyl. These include its high potency, rapidity of onset of action, depression of rate and depth of respiration, lower sensitivity to reversal by naloxone, and reduced cross tolerance to other abused opioids.
AUTHOR CONTRIBUTIONS
R.H., W.L.D., E.K., and G.H. participated in research design. R.H. and R.S. performed the experiments. R.H., R.S., and G.H. analysed the data. R.H., W.L.D., E.K., and G.H. participated in writing the manuscript.
CONFLICT OF INTEREST
The authors declare no conflicts of interest.
DECLARATION OF TRANSPARENCY AND SCIENTIFIC RIGOUR
This Declaration acknowledges that this paper adheres to the principles for transparent reporting and scientific rigour of preclinical research as stated in the BJP guidelines for https://bpspubs.onlinelibrary.wiley.com/doi/full/10.1111/bph.14207, and https://bpspubs.onlinelibrary.wiley.com/doi/full/10.1111/bph.14206, and as recommended by funding agencies, publishers and other organisations engaged with supporting research.
Supporting information
Table S1.
Respiratory parameter values before and after opioid antagonist administration
Table S2. Baseline respiratory parameters in μ‐opioid receptor knock‐out and wild type mice.
ACKNOWLEDGEMENT
We thank Ruby Fletcher for assistance with respiratory data analysis.
The work described in this paper was supported by a grant from National Institute on Drug Addiction (Grant RO1DA036975) to W.L.D. and G.H. The funder of the study had no role in the study design, data collection, data analysis, data interpretation, or writing of the report.
Hill R, Santhakumar R, Dewey W, Kelly E, Henderson G. Fentanyl depression of respiration: Comparison with heroin and morphine. Br J Pharmacol. 2020;177:254–266. 10.1111/bph.14860
REFERENCES
- Alexander, S. P. H. , Christopoulos, A. , Davenport, A. P. , Kelly, E. , Marrion, N. V. , Peters, J. A. , … CGTP Collaborators (2017). The concise guide to PHARMACOLOGY 2017/18: G protein‐coupled receptors. British Journal of Pharmacology, 174(Suppl 1), S17–S129. 10.1111/bph.13878 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Benthuysen, J. L. , Smith, N. T. , Sanford, T. J. , Head, N. , & Dec‐Silver, H. (1986). Physiology of alfentanil‐induced rigidity. Anesthesiology, 64, 440–446. 10.1097/00000542-198604000-00005 [DOI] [PubMed] [Google Scholar]
- Blasco, T. A. , Lee, D. , Amalric, M. , Swerdlow, N. R. , Smith, N. T. , & Koob, G. F. (1986). The role of the nucleus raphe pontis and the caudate nucleus in alfentanil rigidity in the rat. Brain Research, 386, 280–286. 10.1016/0006-8993(86)90164-2 [DOI] [PubMed] [Google Scholar]
- Bobeck, E. N. , Schoo, S. M. , Ingram, S. L. , & Morgan, M. M. (2019). Lack of antinociceptive cross‐tolerance with co‐administration of morphine and fentanyl Into the periaqueductal gray of male sprague‐dawley rats. The Journal of Pain [Epub ahead of print, 20, 1040–1047. 10.1016/j.jpain.2019.03.002 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Brase, D. A. (1986). Unequal opiate cross‐tolerance to morphine in the locomotor‐activation model in the mouse. Neuropharmacology, 25, 297–304. 10.1016/0028-3908(86)90255-8 [DOI] [PubMed] [Google Scholar]
- Burns, G. , DeRienz, R. T. , Baker, D. D. , Casavant, M. , & Spiller, H. A. (2016). Could chest wall rigidity be a factor in rapid death from illicit fentanyl abuse? Clinical Toxicology, 54, 420–423. 10.3109/15563650.2016.1157722 [DOI] [PubMed] [Google Scholar]
- Campbell, C. , Weinger, M. B. , & Quinn, M. (1995). Alterations in diaphragm EMG activity during opiate‐induced respiratory depression. Respiration Physiology, 100, 101–117. [DOI] [PubMed] [Google Scholar]
- Chan, H. C. S. , Wang, J. , Palczewski, K. , Filipek, S. , Vogel, H. , Liu, Z. J. , & Yuan, S. (2018). Exploring a new ligand binding site of G protein‐coupled receptors. Chemical Science, 9, 6480–6489. 10.1039/C8SC01680A [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ciccarone, D. (2009). Heroin in brown, black and white: Structural factors and medical consequences in the US heroin market. International Journal of Drug Policy, 20, 277–282. 10.1016/j.drugpo.2008.08.003 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Corbett, A. D. , Paterson, S. J. , & Kosterlitz, H. W. (1993). Selectivity of ligands for opioid receptors. Handbook of Experimental Pharmacology, 104, 645–680. [Google Scholar]
- Curtis, M. J. , Alexander, S. , Cirino, G. , Docherty, J. R. , George, C. H. , Giembycz, M. A. , … Ahluwalia, A. (2018). Experimental design and analysis and their reporting II: Updated and simplified guidance for authors and peer reviewers. British Journal of Pharmacology, 175, 987–993. 10.1111/bph.14153 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Darke, S. , & Duflou, J. (2016). The toxicology of heroin‐related death: Estimating survival times. Addiction, 111, 1607–1613. 10.1111/add.13429 [DOI] [PubMed] [Google Scholar]
- Dasgupta, N. , Freifeld, C. , Brownstein, J. S. , Menone, C. M. , Surratt, H. L. , Poppish, L. , … Dart, R. C. (2013). Crowdsourcing black market prices for prescription opioids. Journal of Medical Internet Research, 15, e178 10.2196/jmir.2810 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dror, R. O. , Green, H. F. , Valant, C. , Borhani, D. W. , Valcourt, J. R. , Pan, A. C. , … Shaw, D. E. (2013). Structural basis for modulation of a G‐protein‐coupled receptor by allosteric drugs. Nature, 503, 295–299. 10.1038/nature12595 [DOI] [PubMed] [Google Scholar]
- Drorbaugh, J. E. , & Fenn, W. O. (1955). A barometric method for measuring ventilation in newborn infants. Pediatrics, 16, 81–87. [PubMed] [Google Scholar]
- EMCDDA (2018). Fentanils and synthetic cannabinoids: Driving greater complexity into the drug situation. https://webarchiveorg/web/20190213103824/http://wwwemcddaeuropaeu/system/files/publications/8870/2018-2489-td0118414ennpdf Archived 13‐2‐19.
- Fairbairn, N. , Coffin, P. O. , & Walley, A. Y. (2017). Naloxone for heroin, prescription opioid, and illicitly made fentanyl overdoses: Challenges and innovations responding to a dynamic epidemic. International Journal of Drug Policy, 46, 172–179. 10.1016/j.drugpo.2017.06.005 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gherbi, K. , Briddon, S. J. , & Charlton, S. J. (2018). Micro‐pharmacokinetics: Quantifying local drug concentration at live cell membranes. Scientific Reports, 8, 3479 10.1038/s41598-018-21100-x [DOI] [PMC free article] [PubMed] [Google Scholar]
- Green, T. C. , & Gilbert, M. (2016). Counterfeit medications and fentanyl. JAMA Internal Medicine, 176, 1555–1557. 10.1001/jamainternmed.2016.4310 [DOI] [PubMed] [Google Scholar]
- Griswold, M. K. , Chai, P. R. , Krotulski, A. J. , Friscia, M. , Chapman, B. , Boyer, E. W. , … Babu, K. M. (2017). Self‐identification of nonpharmaceutical fentanyl exposure following heroin overdose. Clinical Toxicology (Philadelphia, Pa.), 56, 37–42. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Harding, S. D. , Sharman, J. L. , Faccenda, E. , Southan, C. , Pawson, A. J. , Ireland, S. , … NC‐IUPHAR (2018). The IUPHAR/BPS guide to PHARMACOLOGY in 2018: Updates and expansion to encompass the new guide to IMMUNOPHARMACOLOGY. Nucleic Acids Research, 46, D1091–D1106. 10.1093/nar/gkx1121 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hill R. (2019). Polydrug opioid abuse and mechanisms of tolerance to opioid respiratory depression. PhD thesis, University of Bristol.
- Hill, R. , Dewey, W. L. , Kelly, E. , & Henderson, G. (2018). Oxycodone‐induced tolerance to respiratory depression: Reversal by ethanol, pregabalin and protein kinase C inhibition. British Journal of Pharmacology, 175, 2492–2503. 10.1111/bph.14219 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hill, R. , Lyndon, A. , Withey, S. , Roberts, J. , Kershaw, Y. , MacLachlan, J. , … Henderson, G. (2016). Ethanol reversal of tolerance to the respiratory depressant effects of morphine. Neuropsychopharmacology, 41, 762–773. 10.1038/npp.2015.201 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kilkenny, C. , Browne, W. , Cuthill, I. C. , Emerson, M. , & Altman, D. G. (2010). Animal research: Reporting in vivo experiments: The ARRIVE guidelines. British Journal of Pharmacology, 160, 1577–1579. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kinshella, M. L. W. , Gauthier, T. , & Lysyshyn, M. (2018). Rigidity, dyskinesia and other atypical overdose presentations observed at a supervised injection site, Vancouver, Canada. Harm Reduction Journal, 15 10.1186/s12954-018-0271-5 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kliewer, A. , Schmiedel, F. , Sianati, S. , Bailey, A. , Bateman, J. T. , Levitt, E. S. , … Schulz, S. (2019). Phosphorylation‐deficient G‐protein‐biased μ‐opioid receptors improve analgesia and diminish tolerance but worsen opioid side effects. Nature Communications, 10, 367 10.1038/s41467-018-08162-1 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lessov, C. N. , & Phillips, T. J. (2003). Cross‐sensitization between the locomotor stimulant effects of ethanol and those of morphine and cocaine in mice. Alcoholism, Clinical and Experimental Research, 27, 616–627. 10.1111/j.1530-0277.2003.tb04398.x [DOI] [PubMed] [Google Scholar]
- Lui, P. W. , Chang, G. J. , Lee, T. Y. , & Chan, S. H. (1993). Antagonization of fentanyl‐induced muscular rigidity by denervation of the coerulospinal noradrenergic pathway in the rat. Neuroscience Letters, 157, 145–148. 10.1016/0304-3940(93)90723-X [DOI] [PubMed] [Google Scholar]
- Lui, P. W. , Lee, T. Y. , & Chan, S. H. (1989). Involvement of locus coeruleus and noradrenergic neurotransmission in fentanyl‐induced muscular rigidity in the rat. Neuroscience Letters, 96, 114–119. 10.1016/0304-3940(89)90252-8 [DOI] [PubMed] [Google Scholar]
- Lui, P. W. , Lee, T. Y. , & Chan, S. H. (1990). Involvement of coerulospinal noradrenergic pathway in fentanyl‐induced muscular rigidity in rats. Neuroscience Letters, 108, 183–188. 10.1016/0304-3940(90)90728-R [DOI] [PubMed] [Google Scholar]
- Lynn, R. R. , & Galinkin, J. L. (2018). Naloxone dosage for opioid reversal: Current evidence and clinical implications. Therapeutic Advances in Drug Safety, 9, 63–88. 10.1177/2042098617744161 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Marinetti, L. J. , & Ehlers, B. J. (2014). A series of forensic toxicology and drug seizure cases involving illicit fentanyl alone and in combination with heroin, cocaine or heroin and cocaine. Journal of Analytical Toxicology, 38, 592–598. 10.1093/jat/bku086 [DOI] [PubMed] [Google Scholar]
- Mathers, B. M. , Degenhardt, L. , Bucello, C. , Lemon, J. , Wiessing, L. , & Hickman, M. (2013). Mortality among people who inject drugs: A systematic review and meta‐analysis. Bulletin of the World Health Organization, 91, 102–123. 10.2471/BLT.12.108282 [DOI] [PMC free article] [PubMed] [Google Scholar]
- McPherson, J. , Rivero, G. , Baptist, M. , Llorente, J. , Al‐sabah, S. , Krasel, C. , … Kelly, E. (2010). μ‐Opioid receptors: correlation of agonist efficacy for signalling with ability to activate internalization. Molecular Pharmacology, 78, 756–766. 10.1124/mol.110.066613 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Montandon, G. , & Slutsky, A. S. (2019). Solving the opioid crisis: Respiratory depression by opioids as critical end point. Chest. [Epub ahead of print. 10.1016/j.chest.2019.05.015 [DOI] [PubMed] [Google Scholar]
- NIH (2019). National drug overdose deaths. https://webarchiveorg/web/20190213103737/https://wwwdrugabusegov/related-topics/trends-statistics/overdose-death-rates Archived 13–2‐19.
- Paronis, C. A. , & Holzman, S. G. (1992). Development of tolerance to the analgesic activity of μ agonists after continuous infusion of morphine, meperidine or fentanyl in rats. The Journal of Pharmacology and Experimental Therapeutics, 262, 1–9. [PubMed] [Google Scholar]
- Pattinson, K. T. (2008). Opioids and the control of respiration. British Journal of Anaesthesia, 100, 747–758. 10.1093/bja/aen094 [DOI] [PubMed] [Google Scholar]
- Peterson, A. B. , Gladden, R. M. , Delcher, C. , Spies, E. , Garcia‐Williams, A. , Wang, Y. , … Goldberger, B. A. (2016). Increases in fentanyl‐related overdose deaths—Florida and Ohio, 2013‐2015. Morbidity and Mortality Weekly Report, 65, 844–849. 10.15585/mmwr.mm6533a3 [DOI] [PubMed] [Google Scholar]
- Pierce, M. , Bird, S. M. , Hickman, M. , & Millar, T. (2015). National record linkage study of mortality for a large cohort of opioid users ascertained by drug treatment or criminal justice sources in England, 2005‐2009 (vol 146, pg 17, 2015). Drug and Alcohol Dependence, 156, 315–315. 10.1016/j.drugalcdep.2015.09.016 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Raehal, K. M. , Walker, J. K. , & Bohn, L. M. (2005). Morphine side effects in β‐arrestin 2 knockout mice. The Journal of Pharmacology and Experimental Therapeutics, 314, 1195–1201. 10.1124/jpet.105.087254 [DOI] [PubMed] [Google Scholar]
- Rang, H. P. , Ritter, J. M. , Flower, R. J. , & Henderson, G. (2016). Rang and Dale's Pharmacology (8th ed.). London: Elsevier. [Google Scholar]
- Rivero, G. , Llorente, J. , McPherson, J. , Cooke, A. , Mundell, S. J. , McArdle, C. A. , … Kelly, E. (2012). Endomorphin‐2: A biased agonist at the μ‐opioid receptor. Molecular Pharmaceutics, 82(2), 178–188. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Romero, A. , Miranda, H. F. , & Puig, M. M. (2010). Antinociceptive effects of morphine, fentanyl, tramadol and their combination, in morphine‐tolerant mice. Pharmacology, Biochemistry, and Behavior, 97, 363–369. 10.1016/j.pbb.2010.09.005 [DOI] [PubMed] [Google Scholar]
- Schmid, C. L. , Kennedy, N. M. , Ross, N. C. , Lovell, K. M. , Yue, Z. , Morgenweck, J. , … Bohn, L. M. (2017). Bias factor and therapeutic window correlate to predict safer opioid analgesics. Cell, 171, 1165–1175. 10.1016/j.cell.2017.10.035 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Schumann, H. , Erickson, T. , Thompson, T. M. , Zautcke, J. L. , & Denton, J. S. (2008). Fentanyl epidemic in Chicago, Illinois and surrounding Cook County. Clinical Toxicology (Philadelphia, Pa.), 46, 501–506. 10.1080/15563650701877374 [DOI] [PubMed] [Google Scholar]
- Slater, P. , & Starkie, D. A. (1987). Changes in limb tone produced by regional injections of opiates into rat brain. Naunyn‐Schmiedeberg's Archives of Pharmacology, 335, 54–58. 10.1007/BF00165036 [DOI] [PubMed] [Google Scholar]
- Somerville, N. J. , O'Donnell, J. , Gladden, R. M. , Zibbell, J. E. , Green, T. C. , Younkin, M. , … Walley, A. Y. (2017). Characteristics of fentanyl overdose—Massachusetts, 2014‐2016. Morbidity and Mortality Weekly Report, 66, 382–386. 10.15585/mmwr.mm6614a2 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Streisand, J. B. , Bailey, P. L. , LeMaire, L. , Ashburn, M. A. , Tarver, S. D. , Varvel, J. , & Stanley, T. H. (1993). Fentanyl‐induced rigidity and unconsciousness in human volunteers. Incidence, duration, and plasma concentrations. Anesthesiology, 78, 629–634. 10.1097/00000542-199304000-00003 [DOI] [PubMed] [Google Scholar]
- Sutter, M. E. , Gerona, R. R. , Davis, M. T. , Roche, B. M. , Colby, D. K. , Chenoweth, J. A. , … Albertson, T. E. (2017). Fatal fentanyl: One pill can kill. Academic Emergency Medicine, 24, 106–113. 10.1111/acem.13034 [DOI] [PubMed] [Google Scholar]
- Toll, L. , Berzetei‐Gurske, I. P. , Polgar, W. E. , Brandt, S. R. , Adapa, I. D. , Rodriguez, L. , … Auh, J. S. (1998). Standard binding and functional assays related to medications development division testing for potential cocaine and opiate narcotic treatment medications. NIDA Research Monograph, 178, 440–466. [PubMed] [Google Scholar]
- Valjent, E. , Bertran‐Gonzalez, J. , Aubier, B. , Greengard, P. , Herve, D. , & Girault, J. A. (2010). Mechanisms of locomotor sensitization to drugs of abuse in a two‐injection protocol. Neuropsychopharmacology, 35, 401–415. 10.1038/npp.2009.143 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Vankova, M. E. , Weinger, M. B. , Chen, D. Y. , Bronson, J. B. , Motis, V. , & Koob, G. F. (1996). Role of central μ, δ‐1, and κ‐1 opioid receptors in opioid‐induced muscle rigidity in the rat. Anesthesiology, 85, 574–583. 10.1097/00000542-199609000-00017 [DOI] [PubMed] [Google Scholar]
- Varshneya, N. B. , Walentiny, D. M. , Moisa, L. T. , Walker, T. D. , Akinfiresoye, L. R. , & Beardsley, P. M. (2019). Neuropharmacology. 151, 171–179. https://doi/10.1016/j.neuropharm.2019.03.023 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Waller, J. L. , Hug, C. C. Jr. , Nagle, D. M. , & Craver, J. M. (1981). Hemodynamic changes during fentanyl–oxygen anesthesia for aortocoronary bypass operation. Anesthesiology, 55, 212–217. 10.1097/00000542-198109000-00005 [DOI] [PubMed] [Google Scholar]
- Weinger, M. B. , Smith, N. T. , Blasco, T. A. , & Koob, G. F. (1991). Brain sites mediating opiate‐induced muscle rigidity in the rat: Methylnaloxonium mapping study. Brain Research, 544, 181–190. 10.1016/0006-8993(91)90052-W [DOI] [PubMed] [Google Scholar]
- Widdowson, P. S. , Griffiths, E. C. , & Slater, P. (1986). The effects of opioids in the periaqueductal grey region of rat brain on hind‐limb muscle tone. Neuropeptides, 7, 251–258. 10.1016/0143-4179(86)90019-3 [DOI] [PubMed] [Google Scholar]
- Withey, S. L. , Hill, R. , Lyndon, A. , Dewey, W. L. , Kelly, E. , & Henderson, G. (2017). Effect of tamoxifen and brain‐penetrant protein kinase C and c‐Jun N‐terminal kinase inhibitors on tolerance to opioid‐induced respiratory depression in mice. The Journal of Pharmacology and Experimental Therapeutics, 361, 51–59. 10.1124/jpet.116.238329 [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Table S1.
Respiratory parameter values before and after opioid antagonist administration
Table S2. Baseline respiratory parameters in μ‐opioid receptor knock‐out and wild type mice.


