Abstract
Background and Purpose
Marijuana is the illicit drug most commonly used among pregnant and breastfeeding women. Different studies reported long‐term adverse effects induced by in utero exposure to the main component of marijuana, Δ9‐tetrahydrocannabinol (THC), both in rodents and in humans. However, little is known about any potential sex‐dependent effects of marijuana consumption during pregnancy on newborns at early developmental ages.
Experimental Approach
We studied the effects of prenatal exposure to the cannabinoid receptor agonist WIN55,212‐2 (WIN; 0.5 mg·kg−1 from GD5 to GD20) on the emotional reactivity and cognitive performance of male and female rat offspring from infancy through adolescence and tested the role of mGlu5 receptor signalling in the observed effects.
Key Results
Prenatally WIN‐exposed male infant pups emitted less isolation‐induced ultrasonic vocalizations compared with male control pups, when separated from the dam and siblings and showed increased locomotor activity while females were spared. These effects were normalized when male pups were treated with the positive allosteric modulator of mGlu5 receptor CDPPB. When tested at the prepubertal and pubertal periods, WIN‐prenatally exposed rats of both sexes did not show any difference in social play behaviour, anxiety and temporal order memory.
Conclusions and Implications
We reveal a previously undisclosed sexual divergence in the consequences of fetal cannabinoids on newborns at early developmental ages, which is dependent on mGlu5 receptor signalling. These results provide new impetus for the urgent need to investigate the functional and behavioural substrates of prenatal cannabinoid exposure in both the male offspring and the female offspring.
What is already known
Long‐term adverse effects induced by in utero cannabis have been described in rodents and humans.
However, the majority of these studies have been conducted exclusively in the male progeny.
What this study adds
Cannabinoid fetal exposure causes sex‐specific, mGlu5‐related behavioural alterations in early developmental periods of the progeny.
The mechanisms by which prenatal cannabinoid exposure affect both male and female offspring remain unknown.
What is the clinical significance
Dissemination of our results adds further to preventative education of pregnant women using marijuana.
Abbreviations
- % OE
percentage of open arm entries
- % TO
percentage of time spent in the open arms
- CDPPB
the positive allosteric modulator of mGlu5 receptors
- CTRL
control
- GD
gestational day
- HDIPS
number of exploratory head dips
- PND
postnatal day
- SAP
number of stretched‐attend postures
- USVs
isolation‐induced ultrasonic vocalizations
- VEH
vehicle
- WIN
the cannabinoid receptor agonist WIN 55,212‐2
1. INTRODUCTION
Marijuana (produced from Cannabis sativa) is the illicit drug most commonly used among pregnant and breastfeeding women (Brown, 2017; Scheyer, 2019; Substance Abuse and Mental Health Services Administration, 2013). The main active principle of cannabis, Δ9‐tetrahydrocannabinol (https://www.guidetopharmacology.org/GRAC/LigandDisplayForward?ligandId=2424), enters maternal circulation and readily crosses the placenta (Hutchings, Gamagaris, Miller, & Fico, 1989). Thus, prenatal cannabis exposure might exert deleterious effects on the fetus. Nowadays, the legalization of medical and recreational cannabis is increasing throughout the United States and many other countries debate on its possible legalization. In this context, rigorous scientific research about the impact of cannabis use on health and well‐being becomes more important than ever.
Human studies have provided invaluable information on the detrimental effects of prenatal cannabis exposure on the offspring from the neonatal period through to early adulthood (Crume et al., 2018; El Marroun et al., 2018; Huizink, 2014; Ryan, Ammerman, & O'Connor, 2018), revealing increased tremors, startle, altered sleep patterns at birth (Calvigioni, Hurd, Harkany, & Keimpema, 2014; Volkow, Compton, & Wargo, 2017) and significant impairment of higher cognitive functions beyond infancy (Fried, 2002; Fried, Watkinson, & Gray, 1998; Grant, Campbell, & Beckert, 2018; Huizink & Mulder, 2006; Leech, Richardson, Goldschmidt, & Day, 1999; Passey, Sanson‐Fisher, D'Este, & Stirling, 2014; Smith, Fried, Hogan, & Cameron, 2006). However, one weakness of human studies is that they cannot control for environmental and genetic factors. Therefore, a wide array of animal studies has been performed to better evaluate the contribution of prenatal cannabis to adverse, even subtle neurodevelopmental consequences in the offspring (Grant et al., 2018; Trezza et al., 2012).
The endocannabinoid system plays a relevant role in a broad spectrum of neurodevelopmental processes: Notably, https://www.guidetopharmacology.org/GRAC/ObjectDisplayForward?objectId=56 already functional around gestational days (GD) 11–14 in rats, are involved in embryonal implantation, neural development and control of synaptic communication (Berghuis et al., 2007; Harkany et al., 2007). Pioneering animal studies have demonstrated specific deficits in prenatally cannabis‐exposed rodent offspring at different developmental periods (Grant et al., 2018; Richardson, Hester, & McLemore, 2016; Trezza et al., 2012). Interestingly, some of the behavioural deficits displayed by rodents prenatally exposed to cannabinoids have been related to changes in brain glutamatergic neurotransmission (Antonelli et al., 2004; Antonelli et al., 2005; Castaldo et al., 2007; Mereu et al., 2003).
Noteworthy, was that the majority of these studies were conducted exclusively in the male progeny. Therefore, an urgent need exists to understand the effects of prenatal cannabis exposure also in female progeny. Pioneering preclinical and clinical studies reported sexually dimorphic responses to cannabinoids, when administered during the gestational and/or early postnatal periods (Navarro, Rubio, & de Fonseca, 1995; Vela et al., 1998; Wang, Dow‐Edwards, Anderson, Minkoff, & Hurd, 2004; Wang, Dow‐Edwards, Anderson, Minkoff, & Hurd, 2006). Recently, our laboratories also revealed a previously undisclosed sexual divergence in the consequences of fetal cannabinoids at adulthood. Prenatal exposure to the cannabinoid receptor agonist https://www.guidetopharmacology.org/GRAC/LigandDisplayForward?ligandId=733 (WIN) reduced social interactions in adult male but not female rats and altered neuronal excitability and synaptic plasticity in the prefrontal cortex of male rats only. These deficits were paralleled by decreased levels of https://www.guidetopharmacology.org/GRAC/ObjectDisplayForward?objectId=293 mRNA. Amplifying mGlu5R signalling with a positive allosteric modulator for mGlu5R normalized the social and synaptic deficits displayed by male WIN‐exposed rats (Bara et al., 2018).
Based on these findings, this study follows up our recent report showing sex‐dependent effects of in utero cannabinoid exposure in rats at adulthood on their offspring and is aimed to test the effects of prenatal exposure to WIN in both male and female rats at infancy and at prepubertal and pubertal stages of development. This will allow the evaluation of the possible sex‐dependent effects induced by in utero WIN exposure on emotional reactivity and cognitive performance at different developmental ages before adulthood. The interaction between cannabinoids and mGlu5R has been extensively explored by using pharmacological, electrophysiological and anatomical approaches (Araque, Castillo, Manzoni, & Tonini, 2017; Jung et al., 2012; Katona & Freund, 2008; Lafourcade et al., 2007; Liang, Alger, & McCarthy, 2014; Won et al., 2012). Importantly, mGlu5R participate in the developmental regulation of the endocannabinoid system. Indeed, the developmentally dependent increase in endocannabinoid mobilization (that occurs between the neonatal and juvenile stages) correlates with increases in the levels of protein expression of mGlu5R (Liang et al., 2014). Based on this evidence and our recent findings on the interaction between cannabinoids and mGlu5R in modulating behavioural and synaptic states in the context of nutrition (Manduca et al., 2017) and social interaction in male offspring after in utero cannabinoid exposure (Bara et al., 2018), we here investigated whether the positive allosteric modulator of mGlu5R https://www.guidetopharmacology.org/GRAC/LigandDisplayForward?ligandId=1422 normalized the behavioural deficits induced by in utero cannabinoid exposure.
2. METHODS
2.1. Animals
Wistar (RGD_13508588) female rats (Charles River, Italy), weighing 250 ± 15 g, were mated overnight. The morning when spermatozoa were found was designated as gestational day 0 (GD0). Pregnant rats were singly housed in Macrolon cages (40 × 26 × 20 cm), under controlled conditions (temperature 20–21°C, 55–65% relative humidity and 12/12 hr light cycle with lights on at 07:00 a.m.). Food and water were available ad libitum. The synthetic cannabinoid receptor agonist WIN55,212‐2 (WIN, 0.5 mg·kg−1) was administered to the dams subcutaneously (s.c.) daily from GD 5 to GD 20 (WIN group, n = 27). Control dams (CTRL group, n = 26) received a similar volume injection of the vehicle solution. Newborn litters found up to 05:00 pm were considered to be born on that day (postnatal day [PND] 0). On PND 1, litters were culled to four males and four females. On PND 21, pups were weaned and housed in groups of three. The experiments were carried out on the male and female offspring at three different developmental ages: (a) infancy (PNDs 10 and 13); (b) prepubertal period (PND 28–35 for males and PND 22–28 for females) and (c) puberty (PND 50–60 for males and PND 30–40 for females offspring). Puberty corresponds to vaginal opening and first ovulation (i.e. around 5 weeks) for female and preputial separation for male rats (Beckman & Feuston, 2003; Korenbrot, Huhtaniemi, & Weiner, 1977; Schneider, 2013).
To avoid the so‐called “litter effects” one pup of both sexes per litter from different litters per treatment group was randomly used in each experiment (CTRL = 118 males and 89 females or WIN = 123 males and 87 females) as described in the Handbook of Behavioral Teratology (CV Vorhees); Developmental and Reproductive Toxicology: A Practical Approach (RD Hood). For power analysis, sample size (n) was based on our previous experiments and power analysis with the software GPower. Potential outliers within each data set were calculated using the GraphPad software. Sample size is indicated in the figure legends and represented in the figures as scatter dot plot. All behavioural tests were assessed by a trained observer who was unaware of treatment condition to reduce performance bias. Reproduction data including body weights of the dams (calculated from GD 1 to GD 21 and expressed as body weight gain in percentage) and the length of pregnancy, the litter size, weight gains of pup and postnatal viability (calculated as the number of live animals of both sexes at PND 21 [i.e. weaning]/the number of live animals of both sexes at PND 1 in percentage) were also measured.
Animal studies are reported in compliance with the ARRIVE guidelines (Kilkenny, Browne, Cuthill, Emerson, & Altman, 2010) and with the recommendations made by the British Journal of Pharmacology. The experiments were approved by the Italian Ministry of Health (Rome, Italy), with the guidelines released by the Italian Ministry of Health (D.L. 26/14) and the European Community Directive 2010/63/EU.
2.2. Drug treatment
WIN55,212‐2 mesylate ((R)‐(+)‐[2,3‐dihydro‐5‐methyl‐3‐(4‐morpholinyl‐methyl)pyrrolo[1,2,3‐de]‐1,4‐benzoxazin‐6‐yl]‐1‐naphthalenylmethanone) (WIN, Sigma, Italy and National Institute of Mental Health, USA) was suspended in 5% polyethylene glycol, 5% Tween 80 and 90% saline, and given subcutaneously (s.c.) at a volume of 1 ml·kg−1 to the gestating dams. The dose of WIN used in this study (0.5 mg·kg−1) has been estimated to correspond to a moderate or even to a low exposure to cannabis in humans (Antonelli et al., 2004; Compton, Johnson, Melvin, & Martin, 1992; French, Dillon, & Wu, 1997) and it does not induce any sign of toxicity and/or gross malformations in the rat offspring (Mereu et al., 2003). The positive allosteric modulator of mGlu5 receptors CDPPB (3‐cyano‐N‐(1,3‐diphenyl‐1H‐pyrazol‐5‐yl)benzamide) (National Institute of Mental Health, USA) was dissolved in 5% Tween 80/5% polyethylene glycol/saline and given intraperitoneally (i.p.) at the dose of 1.5 mg·kg−1 30 min before testing of offspring. Drug doses and pretreatment intervals were based on previous work and pilot experiments. Solutions were freshly prepared on the day of the experiment and were administered in a volume of 2.5 ml·kg−1 to offspring.
3. BEHAVIOURAL TESTS
3.1. Isolation‐induced ultrasonic vocalizations
Isolation‐induced ultrasonic vocalizations (USVs) are emitted by rodent pups when removed from the nest and play an important communicative role in mother–offspring interactions (Manduca, Campolongo, & Trezza, 2012). On PND 10, the isolation‐induced USVs emitted by CTRL‐ and WIN‐exposed pups were recorded as previously described (Antonelli et al., 2005; Melancia et al., 2018). Briefly, pups were individually removed from the nest and placed into a black Plexiglas arena (30 cm × 30 cm), located inside a sound‐attenuating and temperature‐controlled chamber. Pup USVs were detected for 15 s by an ultrasound microphone (Avisoft Bioacoustics, Berlin, Germany) sensitive to frequencies between 10 and 200 kHz and fixed at 15 cm above the arena and analysed quantitatively (numbers of calls/15 s).
3.2. Homing behaviour
The homing behaviour test exploits the tendency of immature rodent pups to maintain body contact with the dam and siblings, and to discriminate their own home cage odour from a neutral odour, which is an early indicator of social discrimination (Bignami, 1996). The homing behaviour test was performed as previously described (Servadio et al., 2018). Briefly, on PND 13, the litter was separated from the dam and kept for 30 min in a temperature‐controlled holding cage. Then each pup was placed into a Plexiglas box whose floor was covered for 1/3 with bedding from the pup's home cage and for 2/3 with clean bedding. The pup was located at the side of the box covered by clean bedding and its behaviour was video recorded for 4 min for subsequent analysis. The following parameters were scored by an observer, unaware of animal treatment, using the Observer 3.0 software (Noldus, The Netherlands), the latency (s) to reach the home‐cage bedding area; total time (s) spent by the pup in the nest bedding area, total number of entries into the nest bedding area and locomotor activity, expressed as the total number of crossings in the test box.
3.3. Social play behaviour
Social play behaviour is one of the earliest forms of non‐mother‐directed social behaviour very abundant during the juvenile phases of life in mammalian species, including rats (Vanderschuren, Achterberg, & Trezza, 2016). The test was performed as previously described (Manduca et al., 2016). Prepubertal and pubertal rats were individually habituated to the test cage for 10 min on each of the 2 days before testing. The test was performed between 9 am and 2 pm under low light condition and consisted of placing the animal together with a similarly treated partner into the test cage for 15 min. Behaviour was assessed per pair of animals and analysed by a trained observer, who was unaware of the treatment condition to reduce performance bias, using the Observer 3.0 software (Noldus Information Technology, The Netherlands). Both animals in a test pair had received the same treatment during gestation (CTRL‐ or WIN‐in utero). Animals in a test pair did not differ by >10 g in body weight and had no previous common social experience (i.e. they were not cage mates).
In rats, a bout of social play behaviour starts with one rat soliciting (“pouncing”) another animal by attempting to nose or rub the nape of its neck. If the animal that is pounced upon fully rotates to its dorsal surface “pinning” is the result (one animal lying with its dorsal surface on the floor with the other animal standing over it), which is considered the most characteristic posture of social play behaviour in rats (Pellis & Pellis, 2009).
We determine, (a) frequency of pinning, (b) frequency of pouncing and (c) time spent in social exploration (i.e. the total amount of time spent in non‐playful forms of social interaction, like sniffing any part of the body of the test partner, including the anogenital area or grooming any part of the partner body).
3.4. Elevated plus‐maze test
The elevated plus‐maze apparatus comprised two open (50 × 10 × 40 cm3; l × w × h) and two closed arms (50 × 10 × 40 cm3; l × w × h) that extended from a common central platform (10 × 10 cm2). The test was performed as previously described (Manduca et al., 2015; Trezza et al., 2008). Rats were individually placed on the central platform of the maze for 5 min. Each 5‐min session was recorded with a camera positioned above the apparatus for subsequent behavioural analysis carried out by an observer, unaware of animal treatment to reduce performance bias, using the Observer 3.0 software (Noldus, The Netherlands). The following parameters were analysed: ‐
% time spent in the open arms (% TO): (seconds spent on the open arms of the maze/seconds spent on the open + closed arms) × 100. Time on the open quadrants was timed from the moment all four paws of the rat were placed on an open section and ended when all four paws re‐entered a closed quadrant.
% open arm entries (% OE): (the number of entries into the open arms of the maze/number of entries into open + closed arms) × 100.
number of closed‐arm entries.
number of stretched‐attend postures (SAP) made from the exit of a “closed” quadrant towards an “open” quadrant. This exploratory posture can be described as a forward elongation of the body with static hind‐quarters followed by a retraction to the original position.
number of exploratory head dips (HDIPS) made over the edge of the platform, either from the exit of the “closed” quadrant or whilst on the “open” quadrant.
3.5. Temporal order memory test
Animals were habituated to the experimental arena (40 × 40 cm) without objects for 10 min daily for 2 days before testing. This task consisted of two sample phases and one test trial (Barker, Bird, Alexander, & Warburton, 2007; Manduca et al., 2017). In each sample phase, rats were allowed to explore two copies of an identical object for a total of 4 min. Different objects were used for sample Phases 1 and 2, with a delay between the sample phases of 1 hr. After 3 hr from sample Phase 2, rats performed the test trial (4 min duration) where a third copy of the objects from sample Phase 1 and a third copy of the objects from sample Phase 2 were used. The positions of the objects in the test and the objects used in sample Phase 1 and sample Phase 2 were counterbalanced between the rats. An intact temporal order memory requested the subjects to spend more time exploring the object from Sample 1 (i.e., the object presented less recently) compared with the object from Sample 2 (i.e., the “new” object). The discrimination ratio was calculated as the difference in time spent by each animal exploring the object from sample Phase 1 compared with the object from sample Phase 2, divided by the total time spent exploring both objects in the test phase. Negative discrimination means that animals investigated more the object in Phase 2 than the object in Phase 1. Each 4‐min session was recorded with a camera positioned above the apparatus for subsequent behavioural analysis carried out an observer, unaware of animal treatment to reduce performance bias, using the Observer 3.0 software (Noldus, The Netherlands).
3.6. Statistical analysis
The data and statistical analysis comply with the recommendations on experimental design and analysis in pharmacology (Curtis et al., 2018). Data are expressed as mean ± SEM. To assess the effects of the prenatal treatments (WIN or CTRL) in the male and female offspring, the behavioural data were analysed by two‐way ANOVA, with treatment and sex as factors. Two‐way ANOVA was also used to assess the effects of prenatal (WIN or CTRL) and postnatal (CDPPB or vehicle) treatments. Three‐way ANOVA was also used to assess the effects of prenatal (WIN or CTRL) treatments in both male and female offspring depending on the different developmental ages (prepubertal and pubertal periods) (see Supplementary Figure 1). To assess the effects on reproduction data, the data were analysed by using Student's t‐test (WIN or CTRL). Statistical significance was set at P < .05 with no further distinction made for P < .01 and P < .001. If main or interaction effects were found significant in the ANOVA analysis with no variance inhomogeneity, the Student–Newman–Keuls post hoc test was used for individual group comparisons. Sample sizes subjected to statistical analysis were at least 8 animal per group (n = 8), where n = number of independent values. The software Sigma Plot (RRID:SCR_003210) and GraphPad Prism (RRID:SCR_002798) were used for statistical analysis of the data. Random allocation of animals to treatment groups and to behavioural tasks and blinding of investigators assessing outcomes were adopted to reduce selection and detection bias in our trials (Curtis et al., 2018).
3.7. Nomenclature of targets and ligands
Key protein targets and ligands in this article are hyperlinked to corresponding entries in http://www.guidetopharmacology.org, the common portal for data from the IUPHAR/BPS Guide to PHARMACOLOGY (Harding et al., 2018), and are permanently archived in the Concise Guide to PHARMACOLOGY 2019/20 (Alexander et al., 2019).
4. RESULTS
4.1. Reproduction data
No differences in body weight gains were observed between WIN‐ and CTRL‐treated dams. Prenatal WIN exposure did not affect pregnancy length, litter size at birth, postnatal viability and pup weight gain at different developmental ages (Table 1).
Table 1.
Reproduction data following in utero exposure to WIN
| Treatment group | Dam weight gain (%) | Pregnancy length (days) | Litter size | Postnatal viability (%) | Pup weight (g) | |||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|
| PND10 | PND13 | PND25 | PND45 | |||||||||
| ♂ | ♀ | ♂ | ♀ | ♂ | ♀ | ♂ | ♀ | |||||
| CTRL | 34.3 ± 1.91 | 22.6 ± 0.29 | 12.9 ± 0.78 | 87.9 ± 1.048 | 24.6 ± 0.65 | 23.9 ± 0.67 | 30.3 ± 0.82 | 29.0 ± 1.02 | 71.7 ± 1.83 | 59.6 ± 1.55 | 162.8 ± 1.99 | 142.1 ± 3.10 |
| WIN | 33.1 ± 1.42 | 22.6 ± 0.17 | 12.7 ± 0.67 | 88.4 ± 1.352 | 23.2 ± 0.70 | 23.5 ± 0.46 | 30.5 ± 0.75 | 28.5 ± 0.87 | 68.7 ± 2.23 | 58.9 ± 1.33 | 158.7 ± 2.90 | 143.9 ± 3.71 |
Note. Dam weight gain was calculated from GD 1 to GD 21 for n = 10 dams per group (WIN vs. CTRL). Pup weight at different developmental ages was calculated for n = 9 CTRL‐ and n = 10 WIN‐exposed male and n = 8 CTRL‐ and WIN‐exposed female pups from different litters. Data represent mean values ± SEM.
4.2. Prenatal exposure to the cannabinoid receptor agonist WIN caused sex‐dependent deficits in social communication and locomotion in the infant rat offspring
Prenatally WIN‐exposed male pups emitted less isolation‐induced ultrasonic vocalizations (USVs) at infancy (PND 10) when separated from the dam and siblings compared with male CTRL‐pups (Figure 1: [p (sex) = n.s.; p (treat) < .05; p (sex × treat) < .05]). Interestingly, the deleterious effects of in utero WIN exposure on USVs were specific to the male offspring. Post hoc analysis revealed that prenatally WIN‐exposed female pups showed no difference in the rate of USVs at PND 10 when compared to age‐matched females from CTRL group (Figure 1) suggesting that prenatal exposure to the cannabinoid WIN causes sex‐dependent deficits in early social communication of the offspring.
Figure 1.
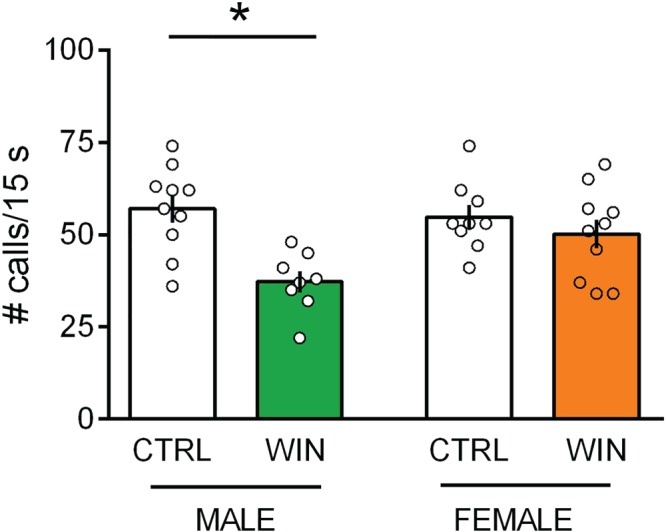
Prenatal WIN exposure induces sex‐specific communicative deficits in the infant rat offspring. At infancy (PND 10), male progeny from dams exposed during gestation to WIN vocalized significantly less compared to CTRL‐pups when separated from the dam and siblings. In contrast, the communicative profile of female littermates was normal (males: CTRL n = 10, WIN n = 8; females: CTRL n = 9, WIN n = 10). Specifically, prenatally WIN‐exposed male but not female showed a decrease in the rate of USVs/15 s compared to their age‐matched male progeny from CTRL group. Scatter dot plot represents each animal. Error bars indicate SEM. * P < .05. Student–Newman–Keuls test
When tested in the homing behaviour test at PND13, male and female pups prenatally exposed to WIN did not differ from control animals in the latency to reach the nest arena (Figure 2a: [p (sex) = n.s.; p (treat) < .05; p (sex × treat) = n.s.]), in the total time spent in the nest zone (Figure 2b: [p (sex) = n.s.; p (treat) = n.s.; p (sex × treat) = n.s.]) and in the number of entries in the nest zone (Figure 2c: [p (sex) = n.s.; p (treat) = n.s.; p (sex × treat) = n.s.]). However, the frequency of crossing in the test arena was increased specifically in the male WIN‐exposed offspring, while WIN‐exposed females were spared (Figure 2d: [p (sex) = n.s.; p (treat) < .05; p (sex × treat) < .05]), suggesting a sex‐dependent detrimental effects induced by prenatal cannabinoid exposure on early life locomotion.
Figure 2.
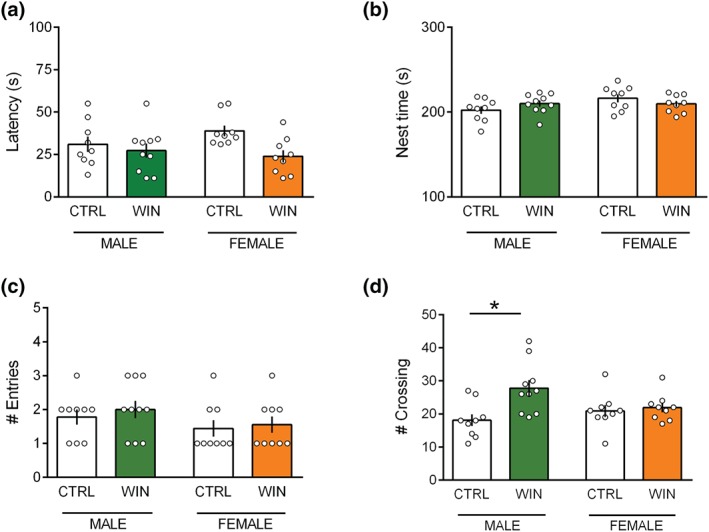
Prenatal WIN exposure induces sex‐specific deficits in locomotor activity in the infant rat offspring. (a–d) In male and female progeny at PND13, prenatal WIN exposure did not alter the latency to reach the nest arena (a) the total time spent in the nest zone (b) and the number of entries in the nest zone and (c) in the homing test. Conversely, the frequency of crossing in the test arena was increased only in male offspring exposed in utero to WIN, while female were spared (males: CTRL n = 9, WIN n = 10; females: CTRL n = 9, WIN n = 9) (d). Scatter dot plot represents each animal. Error bars indicate SEM. * P < .05. Student–Newman–Keuls test
4.3. Prenatal exposure to the cannabinoid receptor agonist WIN had no effect on social play, anxiety‐like behaviours and temporal order memory in the prepubertal progeny
When tested at prepubertal period, WIN and CTRL prenatally exposed rats did not show any difference in social play behaviour (Figure 3a,b). A detailed analysis of the various social play parameters revealed that pinning (Figure 3a: [p (sex) = n.s.; p (treat) = n.s.; p (sex × treat) = n.s.]) and pouncing (Figure 3b: [p (sex) = n.s.; p (treat) = n.s.; p (sex × treat) = n.s.]) frequencies were similar between WIN and CTRL animals of both sexes. Moreover, the time spent in general social exploration (including non‐playful forms of social interaction, like sniffing) was unchanged during the 15‐min session ([p (sex) = n.s.; p (treat) = n.s.; p (sex × treat) = n.s.], data not showed).
Figure 3.
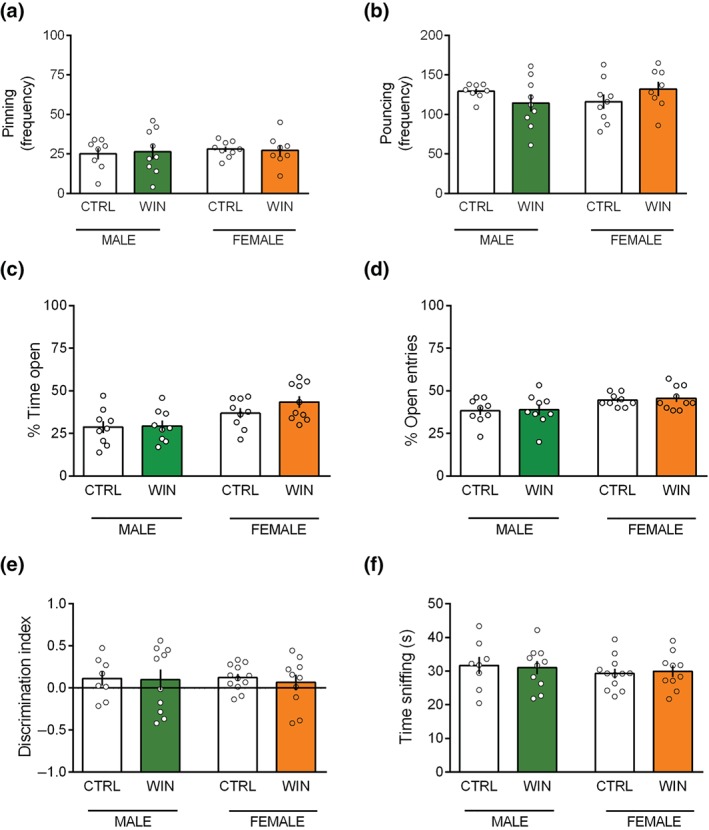
Prenatal WIN exposure does not affect social, anxious and cognitive behaviours in prepubescent male and female offspring. (a and b) No differences between WIN‐exposed and CTRL animals in both sexes were found in the social play behaviour in the prepubertal period as expressed in the frequency of pinning (a) and pouncing (males: CTRL n = 8, WIN n = 9; females: CTRL n = 9, WIN n = 8) (b). In the elevated plus‐maze test, prenatal cannabinoid exposure did not modify the percentage of time spent in the open arms (c) and the percentage of open arm entries (males: CTRL n = 9, WIN n = 9; females: CTRL n = 9, WIN n = 10) (d). (e and f) In the temporal order memory task, male and female prepubertal rats exposed in utero to the cannabinoid WIN showed no differences in their discrimination index (e) and in the total time exploring the objects during the test phase (males: CTRL n = 8, WIN n = 10; females: CTRL n = 12, WIN n = 10) (f). Scatter dot plot represents a pair of animals for social behaviour (a and b) and each animal for the elevated plus‐maze (c and d) and the temporal order task (e and f). Error bars indicate SEM
To test whether prenatal cannabinoid exposure induced deficits in emotional control and cognitive abilities, we tested WIN and CTRL offspring of both sexes for their anxiety‐like behaviour and temporal order memory. No differences between WIN and CTRL prenatally exposed prepubertal rats were found in the elevated plus‐maze. Specifically, there was no change in the percentage of time spent in the open arms of the maze (Figure 3c: [p (sex) = n.s.; p (treat) < .05; p (sex × treat) = n.s.]), in the percentage of open arm entries (Figure 3d: p (sex) = n.s.; p (treat) < .05; p (sex × treat) = n.s.]), and in the number of closed‐arm entries (considered as a measure of locomotion in the maze) ([p (sex) = n.s.; p (treat) = n.s.; p (sex × treat) = n.s.], data not shown). Also, the number of stretched‐attend postures (SAP) ([p (sex) = n.s.; p (treat) = n.s.; p (sex × treat) = n.s.], data not shown) and the number of exploratory head dips (HDIPS) ([p (sex) = n.s.; p (treat) < .05; p (sex × treat) = n.s.], data not shown) were not influenced by in utero WIN exposure in neither the male offspring nor the female offspring.
Regarding their cognitive abilities, prepubertal CTRL‐ and WIN‐exposed animals of both sexes displayed identical discrimination index (Figure 3e: [p (sex) = n.s.; p (treat) = n.s.; p (sex × treat) = n.s.]) and exploration time (Figure 3f: [p (sex) = n.s.; p (treat) = n.s.; p (sex × treat) = n.s.]) in the temporal order memory task. Thus, prenatal WIN exposure did not induce deficits in social play, anxiety‐like behaviours and temporal order memory in the progeny of both sexes at the prepubertal period.
4.4. Prenatal exposure to the cannabinoid receptor agonist WIN did not induce deficits in social play, anxiety‐like behaviours and changes in temporal order memory in the pubertal progeny
When tested in the pubertal period, no differences in social play behaviour were found between WIN and CTRL prenatally exposed rats. Thus, pinning (Figure 4a: [p (sex) < .05; p (treat) = n.s.; p (sex × treat) = n.s.]) and pouncing (Figure 4b: [p (sex) = n.s.; p (treat) = n.s.; p (sex × treat) = n.s.]) frequencies were similar in WIN and CTRL animals in both sexes, suggesting no main effects of in utero cannabinoid exposure on social play behaviour at pubescence. Moreover, the time spent in general social exploration (including non‐playful forms of social interaction, like sniffing) was unchanged during the 15‐min session ([p (sex) = n.s.; p (treat) = n.s.; p (sex × treat) = n.s.], data not showed).
Figure 4.
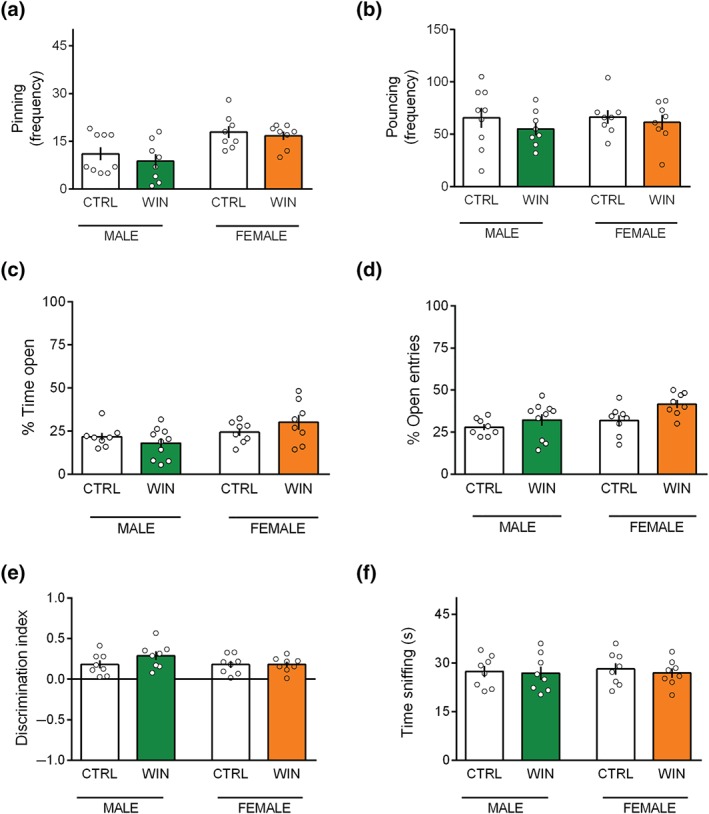
Prenatal WIN exposure does not affect social, cognitive, and anxious behaviours in pubescent offspring of both sexes. (a and b) Prenatal cannabinoid exposure did not alter social behaviour at puberty in WIN‐exposed and CTRL animals as expressed in the frequency of pinning (a) and pouncing (b) (males: CTRL n = 9, WIN n = 8; females: CTRL n = 8, WIN n = 8). In the elevated plus‐maze test, prenatal cannabinoid exposure did not modify the percentage of time spent in the open arms (c) and the percentage of open arm entries (males: CTRL n = 8, WIN n = 10; females: CTRL n = 8, WIN n = 8) (d). (e and f) In the temporal order memory task, male and female pubertal rats exposed to the cannabinoid WIN in utero showed no differences in their discrimination index (e) and in the total time exploring the objects during the test phase (males: CTRL n = 8, WIN n = 8; females: CTRL n = 8, WIN n = 8) (f). Scatter dot plot represents a pair of animals for social behaviour (a and b) and each animal for the elevated plus‐maze (c and d) and the temporal order task (e and f). Error bars indicate SEM
Further, no differences between WIN and CTRL prenatally exposed rats were found in the elevated plus‐maze. Specifically, there was no change in the percentage of time spent in the open arms of the maze (Figure 4c: [p (sex) < .05; p (treat) = n.s.; p (sex × treat) = n.s.]), in the percentage of open arm entries (Figure 4d: [p (sex) < .05; p (treat) = n.s.; p (sex × treat) = n.s.]), in the number of closed‐arm entries ([p (sex) = n.s.; p (treat) = n.s.; p (sex × treat) = n.s.], data not shown), in SAP ([p (sex) = n.s.; p (treat) = n.s.; p (sex × treat) = n.s.], data not shown), and HDIPS ([p (sex) = n.s.; p (treat) = n.s.; p (sex × treat) = n.s.], data not shown). When tested in the temporal order memory task, pubertal animals displayed identical discrimination index (Figure 4e: [p (sex) = n.s.; p (treat) = n.s.; p (sex × treat) = n.s.]) and exploration time (Figure 4f: [p (sex) = n.s.; p (treat) = n.s.; p (sex × treat) = n.s.]), suggesting an intact temporal order memory. Collectively, these results show that prenatal WIN exposure did not induce deficits in social play, anxiety‐like behaviours, and temporal order memory in the progeny at pubescence.
4.5. Positive allosteric modulation of mGlu5 receptors corrected the behavioural deficits induced by prenatal exposure to the cannabinoid receptor agonist WIN in the male offspring at infancy
Our previous works demonstrated the ability of mGlu5 positive allosteric modulation to correct synaptic and behavioural deficits induced by prenatal WIN exposure at adulthood (Bara et al., 2018). Along this line, we found that systemic treatment with the positive allosteric modulator of mGlu5 receptors CDPPB (1.5 mg, i.p.) at PND 10 normalized the altered USV profile displayed by WIN‐exposed pups but remained without effect in the CTRL group, indicating selectivity of the drug effects to the disease‐state (Figure 5a: [p (WIN in utero) = n.s.; p (CDPPB) = n.s.; p (WIN in utero × CDPPB < .05]). Specifically, post hoc analysis revealed that CDPPB rescued the decrease of USVs induced by in utero WIN treatment at PND10 in the male offspring. Furthermore, we found that potentiating mGlu5 signalling by CDPPB administration normalized the hyperlocomotion induced by prenatal exposure to WIN in the male offspring tested in the homing behaviour test at PND 13 (Figure 5b: [p (WIN in utero) < .05; p (CDPPB) = n.s.; p (WIN in utero × CDPPB) < .05) without affecting the number of crossing in the CTRL group. Overall, these data show that potentiating mGlu5 signalling normalized the behavioural deficits induced by prenatal exposure to cannabinoids in the infant offspring.
Figure 5.
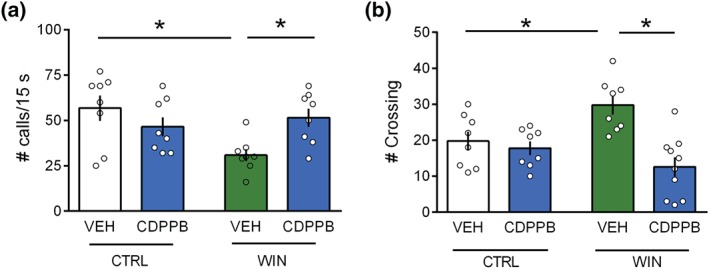
Positive allosteric modulation of mGlu5 receptors normalizes the communicative and locomotor deficits displayed by male pups prenatally exposed to WIN. (a) Systemic administration of CDPPB (1.5 mg·kg−1, i.p.) at PND 10 rescued the decrease in the rate of USVs in male rats prenatally exposed to WIN without affecting the USV frequency in the CTRL group (males CTRL: VEH n = 8, CDPPB n = 8; males WIN: VEH n = 8, CDPPB n = 8). (b) In the homing behaviour, treatment with CDPPB (1.5 mg·kg−1, i.p.) at PND 13 corrects the increase in the frequency of crossing in male rats prenatally exposed to WIN without affecting the number of crossing in the CTRL group (males CTRL: VEH n = 8, CDPPB n = 8; males WIN: VEH n = 8, CDPPB n = 10). Scatter dot plot represents each animal. Error bars indicate SEM. * P < .05. Student–Newman–Keuls test
5. DISCUSSION
In the present study, we have demonstrated for the first time that fetal exposure to cannabinoids, in the present case the synthetic cannabinoid WIN55,212‐2, causes sex‐specific mGlu5‐related behavioural alterations in the progeny at early developmental periods. Specifically, we found that prenatal exposure to WIN altered isolation‐induced USVs and locomotor activity in the male but not female infant rat offspring. Conversely, the social, emotional and cognitive profile was spared in the offspring of both sexes tested at the prepubertal and pubertal periods. Interestingly, potentiating mGlu5R signalling reverted the early behavioural deficits displayed by WIN‐exposed infant male rats. Infant rodents produce USVs in response to separation from the mother and the nest, and USVs are a potent tool to detect subtle effects of adverse events during development (Branchi, Santucci, & Alleva, 2001; Branchi, Santucci, & Alleva, 2006; Cuomo, De Salvia, Maselli, Santo, & Cagiano, 1987; Insel, Hill, & Mayor, 1986). It has previously been shown that cannabinoid exposure during pregnancy and/or lactation alters isolation‐induced USVs (Antonelli et al., 2005; Trezza et al., 2008). These early studies, however, were only performed in the male offspring, while the consequences induced by developmental cannabinoid exposure in the female offspring were not investigated. Here, we report that male but not female WIN‐exposed pups display a decreased rate of isolation‐induced USVs compared to control rats. Whether the decreased USV emission displayed by WIN‐exposed male pups could be the consequence of an altered maternal responsiveness, which is one of the factors tuning the rate of USV emission of the offspring (D'Amato, Scalera, Sarli, & Moles, 2005), is an interesting issue that deserves further investigation. Related to this, previous studies reported disrupted maternal behaviour in lactating rats exposed to very high doses of THC (Bromley, Rabii, Gordon, & Zimmerman, 1978; Navarro et al., 1995). Conversely, other authors failed to detect changes in maternal care in rhesus monkeys exposed to low doses of THC during pregnancy and lactation (Golub, Sassenrath, & Chapman, 1981). Recently, it has been shown that THC administered to pregnant mice (GD 5.5–GD 17.5) at a “non‐intoxicating” daily dose (3 mg·kg−1, i.p.) did not alter maternal behaviour or physical measures (Tortoriello et al., 2014), suggesting that moderate doses of cannabinoid should not alter maternal behaviour and in turn influence mum–pup interaction. By this evidence, we cannot certainly exclude that prenatal exposure to low doses of WIN (0.5 mg·kg−1, s.c.) may induce any alteration in maternal behaviour which in turn may contribute to the altered pattern of emotionality displayed by WIN‐exposed male pups. Therefore, this issue still remains unresolved.
The synthetic cannabinoid receptor agonist used in this study (i.e. WIN) has effects that are highly comparable to those of the main active principle of cannabis THC, regardless that they differ in affinity at CB1 receptors and profile of action (Compton et al., 1992; Wiley & Martin, 2002). Therefore, we surmise that the sex‐specific behavioural deficits we here observed at early life stages after prenatal WIN exposure could be similar to those obtained by administering THC during the prenatal period. In support of this hypothesis, we recently demonstrated that prenatal THC administration (from GD 5 to GD 20) induced similar sex‐specific synaptic deficits in the prefrontal cortex of adult rats, without any sign of toxicity and/or gross malformations in the rat offspring as WIN did (Bara et al., 2018). However, in a recent inhalation mouse study, a dose of ~0.5 mg·kg−1·day−1 THC smoke from GD 5.5 to GD 17.5 produced deficits in fetal growth and reduced birth weights in cannabis‐exposed male offspring suggesting that low‐dose exposure to THC via inhalation can compromise fetal development (Benevenuto et al., 2017). This highlights that differences in the treatment schedule, routes of administration, doses and animal strain may account for different results following in utero cannabinoid exposure.
During the early phases of postnatal life, olfaction, and in particular the learned association between maternal odours and maternal stimulation, is crucial for the development of social behaviour and social recognition (Terry & Johanson, 1996). Therefore, we tested the infant offspring in the homing behaviour test, which requires intact sensory, olfactory and motor capabilities that allow the pup to recognize the mother's odour among others (Bignami, 1996). Both WIN‐exposed male and female pups were able to use olfactory cues to discriminate between a neutral odour and their own home cage odour. Interestingly, however, locomotor activity in the test arena was increased specifically in prenatally WIN‐exposed male rats, while females were spared, suggesting a sex‐dependent detrimental effects of prenatal WIN exposure on early life locomotor activity. Maternal exposure to cannabinoid drugs during pregnancy and/or lactation might particularly affect the ontogeny of motor behaviours: an age‐dependent hyperlocomotion has been reported in the lactating offspring of mothers receiving THC (during GD 6–12; Borgen, Lott, & Davis, 1973). Other studies demonstrated that rats prenatally and/or postnatally exposed to cannabinoids displayed motor hyperactivity at infancy and adolescence but not at adulthood (Bara et al., 2018; Mereu et al., 2003; Navarro et al., 1995; Silva, Zhao, Popp, & Dow‐Edwards, 2012). These preclinical studies are in line with human data showing that children of both sexes prenatally exposed to cannabis are hyperactive and impulsive starting around age 6 (Fried & Smith, 2001; Goldschmidt, Richardson, Cornelius, & Day, 2004; Sharapova et al., 2018). Altogether, the abnormal USV profile and locomotor activity displayed by WIN‐exposed male pups indicate the presence of sex‐specific deficits in social communication and locomotion at early life stages. Previous evidence suggested that prenatal exposure to WIN permanently alters https://www.guidetopharmacology.org/GRAC/LigandDisplayForward?ligandId=1067 and glutamate circuits in the prefrontal cortex and hippocampus of the offspring (Antonelli et al., 2004; Antonelli et al., 2005; Mereu et al., 2003; Saez, Aronne, Caltana, & Brusco, 2014). Notably, a reduction in cortical glutamatergic neurotransmission and NMDA receptor activity has been reported (Antonelli et al., 2005; Mereu et al., 2003). These alterations might result in an inappropriate assembly of neuronal network that could represent a substrate for the observed emotional and locomotor dysfunctions displayed by the WIN‐exposed male offspring. Based on this experimental evidence and the prominent role of mGlu5R in synaptic endocannabinoid‐mediated signalling (Araque et al., 2017), we tested the ability of CDPPB, a well‐described positive allosteric modulator of mGlu5Rs, to rescue the behavioural deficits displayed by WIN‐exposed male pups. We found that systemic administration of CDPPB normalized the altered USVs profile and the increased locomotion induced in male pups by prenatal WIN exposure. This finding extends our previous data demonstrating the ability of mGlu5 positive allosteric modulation to correct synaptic and behavioural deficits induced by prenatal cannabinoid exposure at adulthood (Bara et al., 2018). Female did not show the behavioural deficits displayed by the male offspring at infancy, however we cannot exclude that the administration of CDPPB per se could affect their USVs and homing performances since CDPPB is known to affect cognitive and operant responding tasks in rodents (Cleva & Olive, 2012; Fowler et al., 2013; Lee, Coelho, Class, & Szumlinski, 2018). In the present study, we used a dose of CDPPB (1.5 mg·kg−1) that did not affect early life behavioural parameters (i.e. USVs and homing behaviour) in the CTRL male progeny, therefore we hypothesize that the same dose would not have an effect per se in the female progeny. Related to this, it should be considered that prenatal exposure to WIN induced sex‐related differences in the postsynaptic mGluR proteins at adulthood (Bara et al., 2018) and that mGlu5R modulate spine plasticity in the nucleus accumbens of female mice depending on oestrogen receptors (Peterson, Mermelstein, & Meisel, 2015), suggesting the importance of sex‐dependent specificity of the mGluR signalling in the brain.
Moreover, it remains to clarify how prenatal WIN exposure induces sex‐specific detrimental behavioural effects at early life stages. Different studies have focused on the sexual dimorphism of the endocannabinoid system, which could explain at least in part the sex dissimilarities in the consequences induced by in utero cannabinoid exposure. Beside molecular and structural differences (Castelli et al., 2014; Garrett & Wellman, 2009; Rodriguez de Fonseca, Cebeira, Ramos, Martin, & Fernandez‐Ruiz, 1994), prenatal exposure to cannabinoids throughout gestation induces sex‐specific effects on dopaminergic neurotransmission in the limbic forebrain (Alpar, Di Marzo, & Harkany, 2016; Navarro et al., 1995; Rodriguez de Fonseca, Cebeira, Fernandez‐Ruiz, Navarro, & Ramos, 1991) and also changes in the ontogenetic expression of TH gene (Navarro et al., 1995). Moreover, sex‐differences in mRNA expression levels for https://www.guidetopharmacology.org/GRAC/ObjectDisplayForward?objectId=289 have been reported in the prefrontal cortex of adult rats prenatally exposed to WIN, with an increase in mGlu1R mRNA levels exclusively in the male progeny (Bara et al., 2018). In humans, impaired https://www.guidetopharmacology.org/GRAC/ObjectDisplayForward?objectId=215 expression in amygdala is most evident in males in association with prenatal cannabis exposure suggesting a potential pathway for altered emotional regulation (Wang et al., 2004). Interestingly, 10‐year‐old boys prenatally exposed to marijuana are more susceptible to behavioural problems than girls (Goldschmidt et al., 2004). However, the neurobiological mechanisms underlying maternal exposure to cannabinoids still remain complex. Changes in the epigenetic role of steroid hormones (both sex‐steroids and glucocorticoids) on brain development induced by prenatal cannabinoid exposure could be responsible for some specific behavioural effects that we here found at early life stages. Indeed, it has been proposed that the epigenetic effects of abused drugs including marijuana on brain development might be the result of both drug mimicking or modification of the action of natural hormones, which play a very important role in neuronal phase during early stages of brain development and cortical organization during perinatal ages in rodents (Navarro et al., 1995). Moreover, marijuana exposure in early fetal life also decreases the expression of genes (through histone lysine methylation) for dopamine D2 receptors in brain areas mediating rewarding processes (i.e. nucleus accumbens) which may explain higher rates of drug addiction in adults exposed prenatally to marijuana (DiNieri et al., 2011). Prenatal exposure to THC also causes substantial changes in gene expression levels of several other significant systems in the brain that are linked to the endocannabinoid signalosome such as the https://www.guidetopharmacology.org/GRAC/FamilyDisplayForward?familyId=50, glutamate and GABA systems, which may persist well into adulthood (Jutras‐Aswad, DiNieri, Harkany, & Hurd, 2009; Navarro et al., 1995) and sex‐dependently affect behavioural outcomes since early life stages.
Profound changes in behavioural repertoire and physiological status occur between weaning and puberty; it is during this stage that mammals progressively achieve sexual maturation and establish a sense of independence from their primary caregivers (Spear, 2000; Vanderschuren et al., 2016). This process of development involves several behavioural processes influenced by endocannabinoid signalling (Hill & Tasker, 2012; Solinas, Goldberg, & Piomelli, 2008; Zanettini et al., 2011). We here showed that prenatally WIN‐exposed animals of both sexes did not exhibit deficits in social play, neither anxious‐like behaviours in the elevated plus‐maze test nor cognitive deficits in temporal order memory at the prepubertal and pubertal periods. The endocannabinoid system has a strong interaction with different neurotransmitters present from early stages of brain development (Alpar et al., 2016). It could be that in utero WIN administration induced mGluR sex‐mediated deficits at early life stages (as we found in the present study) and then the reorganization of this system occurs and different targets (such as dopamine or opioids) become predominant in mediating specific motivational, rewarding and emotional processes that we here did not explore. For instance, prenatal THC‐induced reorganization of the dopamine system occurs within this sensitive period and might disrupt reward circuits by genetic and epigenetic modifications (DiNieri et al., 2011; Spano, Ellgren, Wang, & Hurd, 2007).
Previous findings from our group demonstrated that perinatal exposure to THC (GD 15 to PND 9) altered social play and induced anxiety‐like behaviours in the male rat offspring (Trezza et al., 2008). Moreover, it has been recently shown that the postnatal exposure to the cannabinoid receptor agonist CP 55,940 from PND 4 to PND 10, a period of brain development equivalent to the third trimester in human, increased the time spent in the open arms of the elevated plus‐maze in offspring of both sexes at prepubertal period (Breit, Zamudio, & Thomas, 2019). We here showed that prenatally WIN‐exposed animals of both sexes did not exhibit anxious‐like behaviours in the elevated plus‐maze. The discrepancy with our present findings may depend on the different cannabinoid agonists used (THC or CP 55,940 vs. WIN) and the treatment schedule (perinatal or postnatal vs. prenatal exposure). Moreover, it is possible that a longer activation of endocannabinoid neurotransmission that extends beyond birth till after the early postnatal period may be required to disrupt social play behaviour and to induce an anxious‐like phenotype in the elevated plus‐maze.
Furthermore, social play behaviour (van Kerkhof, Damsteegt, Trezza, Voorn, & Vanderschuren, 2013) and temporal order memory (Barker et al., 2007) are mediated by functional activity in the prefrontal cortex and certain levels of regional frontal specificity to the effects of prenatal cannabinoid exposure have been demonstrated (Bara et al., 2018). The fact that the prefrontal cortex develops late in to postnatal life (i.e. late adolescence/early adulthood; Arain et al., 2013; Kolb et al., 2012) and that temporal order memory requires cortical more than hippocampal integrity (Barker et al., 2017) may explain the normal behaviour of (pre)pubertal WIN‐exposed rats in these tasks compared to their impaired performance when tested for other forms of memory (Antonelli et al., 2005; Castaldo et al., 2007; Drazanova et al., 2019; Ferraro et al., 2009; Mereu et al., 2003). Further, it can be hypothesized that perturbations of the fetal endocannabinoid system induced by in utero exposure to WIN predisposed the offspring to abnormalities in memory and altered emotionality later in life (Richardson et al., 2016): Thus, WIN in utero induced an imbalanced brain circuit at sub‐threshold levels (non‐manifested during prepubescent and pubescent period) that can precipitate neurodevelopmental disease by otherwise sub‐threshold stimuli later in life (Bara et al., 2018; Campolongo et al., 2007; Mereu et al., 2003; Tortoriello et al., 2014; Trezza et al., 2008; Vargish et al., 2017).
Overall, our results clearly show previously undisclosed sexual divergence in the consequences of fetal cannabinoids at early stages providing new impetus for the urgent need to investigate the functional and behavioural substrates of prenatal cannabinoid exposure in both male and female offspring.
ACKNOWLEDGEMENTS
This study was supported by Marie Curie Career Reintegration Grant PCIG09‐GA‐2011‐293589 (V.T.), Jerome Lejeune Foundation Research Grant 1674 (V.T.), and by L'Oreal‐UNESCO Pour les Femmes et la Science Individual Fellowship (A.M.).
AUTHOR CONTRIBUTIONS
A.M., F.M., M.S., and S.S. performed, analysed, and contributed to the design of the behavioural experiments. O.M. and V.T. contributed to the design of the experiments and edited the manuscript. A.M., O.M., and V.T. supervised the project, designed the experiments and wrote the manuscript.
CONFLICT OF INTEREST
The authors declare that, except for income received from their primary employers, no financial support or compensation has been received from any individual or corporate entity over the past five years for research or professional service and there are no personal financial holdings that could be perceived as constituting a potential conflict of interest.
DECLARATION OF TRANSPARENCY AND SCIENTIFIC RIGOUR
This Declaration acknowledges that this paper adheres to the principles for transparent reporting and scientific rigour of preclinical research as stated in the BJP guidelines for https://bpspubs.onlinelibrary.wiley.com/doi/full/10.1111/bph.14207, and https://bpspubs.onlinelibrary.wiley.com/doi/full/10.1111/bph.14206, and as recommended by funding agencies, publisher and other organisations engaged with supporting research.
Supporting information
Figure S1. Age‐dependent effects of prenatal exposure to the cannabinoid receptor agonist WIN in social, anxiety and cognitive tasks.
Manduca A, Servadio M, Melancia F, Schiavi S, Manzoni OJ, Trezza V. Sex‐specific behavioural deficits induced at early life by prenatal exposure to the cannabinoid receptor agonist WIN55, 212‐2 depend on mGlu5 receptor signalling. Br J Pharmacol. 2020;177:449–463. 10.1111/bph.14879
REFERENCES
- Alexander, S. P. , Christopoulos, A. , Davenport, A. P. , Kelly, E. , Mathie, A. , Peters, J. A. , et al. (2019). The Concise Guide to PHARMACOLOGY 2018/19: G protein‐coupled receptors. British Journal of Pharmacology, 176, S21–S141. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Alpar, A. , Di Marzo, V. , & Harkany, T. (2016). At the tip of an iceberg: Prenatal marijuana and its possible relation to neuropsychiatric outcome in the offspring. Biological Psychiatry, 79(7), e33–e45. [DOI] [PubMed] [Google Scholar]
- Antonelli, T. , Tanganelli, S. , Tomasini, M. C. , Finetti, S. , Trabace, L. , Steardo, L. , … Ferraro, L. (2004). Long‐term effects on cortical glutamate release induced by prenatal exposure to the cannabinoid receptor agonist (R)‐(+)‐[2,3‐dihydro‐5‐methyl‐3‐(4‐morpholinyl‐methyl)pyrrolo[1,2,3‐de]‐1,4‐benzo xazin‐6‐yl]‐1‐naphthalenylmethanone: An in vivo microdialysis study in the awake rat. Neuroscience, 124(2), 367–375. 10.1016/j.neuroscience.2003.10.034 [DOI] [PubMed] [Google Scholar]
- Antonelli, T. , Tomasini, M. C. , Tattoli, M. , Cassano, T. , Tanganelli, S. , Finetti, S. , … Ferraro, L. (2005). Prenatal exposure to the CB1 receptor agonist WIN 55,212‐2 causes learning disruption associated with impaired cortical NMDA receptor function and emotional reactivity changes in rat offspring. Cerebral Cortex, 15(12), 2013–2020. 10.1093/cercor/bhi076 [DOI] [PubMed] [Google Scholar]
- Arain, M. , Haque, M. , Johal, L. , Mathur, P. , Nel, W. , Rais, A. , … Sharma, S. (2013). Maturation of the adolescent brain. Neuropsychiatric Disease and Treatment, 9, 449–461. 10.2147/NDT.S39776 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Araque, A. , Castillo, P. E. , Manzoni, O. J. , & Tonini, R. (2017). Synaptic functions of endocannabinoid signaling in health and disease. Neuropharmacology, 124, 13–24. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bara, A. , Manduca, A. , Bernabeu, A. , Borsoi, M. , Serviado, M. , Lassalle, O. , … Manzoni, O. J. (2018). Sex‐dependent effects of in utero cannabinoid exposure on cortical function. eLife, 7, 1–31. 10.7554/eLife.36234 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Barker, G. R. , Banks, P. J. , Scott, H. , Ralph, G. S. , Mitrophanous, K. A. , Wong, L. F. , … Warburton, E. C. (2017). Separate elements of episodic memory subserved by distinct hippocampal‐prefrontal connections. Nature Neuroscience, 20(2), 242–250. 10.1038/nn.4472 [DOI] [PubMed] [Google Scholar]
- Barker, G. R. , Bird, F. , Alexander, V. , & Warburton, E. C. (2007). Recognition memory for objects, place, and temporal order: A disconnection analysis of the role of the medial prefrontal cortex and perirhinal cortex. The Journal of Neuroscience: The Official Journal of the Society for Neuroscience, 27(11), 2948–2957. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Beckman, D. A. , & Feuston, M. (2003). Landmarks in the development of the female reproductive system. Birth Defects Research. Part B, Developmental and Reproductive Toxicology, 68(2), 137–143. [DOI] [PubMed] [Google Scholar]
- Benevenuto, S. G. , Domenico, M. D. , Martins, M. A. , Costa, N. S. , de Souza, A. R. , Costa, J. L. , … Veras, M. M. (2017). Recreational use of marijuana during pregnancy and negative gestational and fetal outcomes: An experimental study in mice. Toxicology, 376, 94–101. 10.1016/j.tox.2016.05.020 [DOI] [PubMed] [Google Scholar]
- Berghuis, P. , Rajnicek, A. M. , Morozov, Y. M. , Ross, R. A. , Mulder, J. , Urban, G. M. , … Harkany, T. (2007). Hardwiring the brain: Endocannabinoids shape neuronal connectivity. Science, 316(5828), 1212–1216. 10.1126/science.1137406 [DOI] [PubMed] [Google Scholar]
- Bignami, G. (1996). Economical test methods for developmental neurobehavioral toxicity. Environmental Health Perspectives, 104(Suppl 2), 285–298. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Borgen, L. A. , Lott, G. C. , & Davis, W. M. (1973). Cannabis‐induced hypothermia: A dose‐effect comparison of crude marihuana extract and synthetic 9‐tetrahydrocannabinol in male and female rats. Research Communications in Chemical Pathology and Pharmacology, 5(3), 621–626. [PubMed] [Google Scholar]
- Branchi, I. , Santucci, D. , & Alleva, E. (2001). Ultrasonic vocalisation emitted by infant rodents: A tool for assessment of neurobehavioural development. Behavioural Brain Research, 125(1‐2), 49–56. [DOI] [PubMed] [Google Scholar]
- Branchi, I. , Santucci, D. , & Alleva, E. (2006). Analysis of ultrasonic vocalizations emitted by infant rodents. Current Protocols in Toxicology, Chapter 13 Unit13 12, 1–14. [DOI] [PubMed] [Google Scholar]
- Breit, K. R. , Zamudio, B. , & Thomas, J. D. (2019). The effects of alcohol and cannabinoid exposure during the brain growth spurt on behavioral development in rats. Birth Defects Research, 111, 760–774. 10.1002/bdr2.1487 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bromley, B. L. , Rabii, J. , Gordon, J. H. , & Zimmerman, E. (1978). Δ9‐tetrahydrocannabinol inhibition of suckling‐induced prolactin release in the lactating rat. Endocrine Research Communications, 5(4), 271–278. [DOI] [PubMed] [Google Scholar]
- Brown, A. (2017). Breastfeeding as a public health responsibility: A review of the evidence. Journal of Human Nutrition and Dietetics : The Official Journal of the British Dietetic Association, 30(6), 759–770. [DOI] [PubMed] [Google Scholar]
- Calvigioni, D. , Hurd, Y. L. , Harkany, T. , & Keimpema, E. (2014). Neuronal substrates and functional consequences of prenatal cannabis exposure. European Child & Adolescent Psychiatry, 23(10), 931–941. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Campolongo, P. , Trezza, V. , Cassano, T. , Gaetani, S. , Morgese, M. G. , Ubaldi, M. , … Cuomo, V. (2007). Perinatal exposure to Δ9‐tetrahydrocannabinol causes enduring cognitive deficits associated with alteration of cortical gene expression and neurotransmission in rats. Addiction Biology, 12(3‐4), 485–495. [DOI] [PubMed] [Google Scholar]
- Castaldo, P. , Magi, S. , Gaetani, S. , Cassano, T. , Ferraro, L. , Antonelli, T. , … Cuomo, V. (2007). Prenatal exposure to the cannabinoid receptor agonist WIN 55,212‐2 increases glutamate uptake through overexpression of GLT1 and EAAC1 glutamate transporter subtypes in rat frontal cerebral cortex. Neuropharmacology, 53(3), 369–378. 10.1016/j.neuropharm.2007.05.019 [DOI] [PubMed] [Google Scholar]
- Castelli, M. P. , Fadda, P. , Casu, A. , Spano, M. S. , Casti, A. , Fratta, W. , & Fattore, L. (2014). Male and female rats differ in brain cannabinoid CB1 receptor density and function and in behavioural traits predisposing to drug addiction: effect of ovarian hormones. Current Pharmaceutical Design, 20(13), 2100–2113. 10.2174/13816128113199990430 [DOI] [PubMed] [Google Scholar]
- Cleva, R. M. , & Olive, M. F. (2012). mGlu receptors and drug addiction. Wiley Interdisciplinary Reviews. Membrane Transport and Signaling, 1(3), 281–295. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Compton, D. R. , Johnson, M. R. , Melvin, L. S. , & Martin, B. R. (1992). Pharmacological profile of a series of bicyclic cannabinoid analogs: Classification as cannabimimetic agents. The Journal of Pharmacology and Experimental Therapeutics, 260(1), 201–209. [PubMed] [Google Scholar]
- Crume, T. L. , Juhl, A. L. , Brooks‐Russell, A. , Hall, K. E. , Wymore, E. , & Borgelt, L. M. (2018). Cannabis use during the perinatal period in a state with legalized recreational and medical marijuana: The association between maternal characteristics, breastfeeding patterns, and neonatal outcomes. The Journal of Pediatrics, 197, 90–96. [DOI] [PubMed] [Google Scholar]
- Cuomo, V. , De Salvia, M. A. , Maselli, M. A. , Santo, L. , & Cagiano, R. (1987). Ultrasonic calling in rodents: A new experimental approach in behavioural toxicology. Neurotoxicology and Teratology, 9(2), 157–160. [DOI] [PubMed] [Google Scholar]
- Curtis, M. J. , Alexander, S. , Cirino, G. , Docherty, J. R. , George, G. H. , Giembycz, M. A. , … Ahluwalia, A. (2018). Experimental design and analysis and their reporting II: updated and simplified guidance for authors and peer reviewers. British Journal of Pharmacology, 175(7), 987–993. 10.1111/bph.14153 [DOI] [PMC free article] [PubMed] [Google Scholar]
- D'Amato, F. R. , Scalera, E. , Sarli, C. , & Moles, A. (2005). Pups call, mothers rush: Does maternal responsiveness affect the amount of ultrasonic vocalizations in mouse pups? Behavior Genetics, 35(1), 103–112. [DOI] [PubMed] [Google Scholar]
- DiNieri, J. A. , Wang, X. , Szutorisz, H. , Spano, S. M. , Kaur, J. , Casaccia, P. , … Hurd, Y. L. (2011). Maternal cannabis use alters ventral striatal dopamine D2 gene regulation in the offspring. Biological Psychiatry, 70(8), 763–769. 10.1016/j.biopsych.2011.06.027 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Drazanova, E. , Ruda‐Kucerova, J. , Kratka, L. , Stark, T. , Kuchar, M. , Maryska, M. , … Micale, V. (2019). Different effects of prenatal MAM vs. perinatal THC exposure on regional cerebral blood perfusion detected by arterial spin labelling MRI in rats. Scientific Reports, 9(1), 10 6062 10.1038/s41598-019-42532-z [DOI] [PMC free article] [PubMed] [Google Scholar]
- El Marroun, H. , Brown, Q. L. , Lund, I. O. , Coleman‐Cowger, V. H. , Loree, A. M. , Chawla, D. , & Washio, Y. (2018). An epidemiological, developmental and clinical overview of cannabis use during pregnancy. Preventive Medicine, 116, 1–5. 10.1016/j.ypmed.2018.08.036 [DOI] [PubMed] [Google Scholar]
- Ferraro, L. , Tomasini, M. C. , Beggiato, S. , Gaetani, S. , Cassano, T. , Cuomo, V. , … Antonelli, T. (2009). Short‐ and long‐term consequences of prenatal exposure to the cannabinoid agonist WIN55,212‐2 on rat glutamate transmission and cognitive functions. Journal of Neural Transmission (Vienna), 116(8), 1017–1027. 10.1007/s00702-009-0230-0 [DOI] [PubMed] [Google Scholar]
- Fowler, S. W. , Walker, J. M. , Klakotskaia, D. , Will, M. J. , Serfozo, P. , Simonyi, A. , & Schachtman, T. R. (2013). Effects of a metabotropic glutamate receptor 5 positive allosteric modulator, CDPPB, on spatial learning task performance in rodents. Neurobiology of Learning and Memory, 99, 25–31. 10.1016/j.nlm.2012.10.010 [DOI] [PubMed] [Google Scholar]
- French, E. D. , Dillon, K. , & Wu, X. (1997). Cannabinoids excite dopamine neurons in the ventral tegmentum and substantia nigra. Neuroreport, 8(3), 649–652. [DOI] [PubMed] [Google Scholar]
- Fried, P. A. (2002). Conceptual issues in behavioral teratology and their application in determining long‐term sequelae of prenatal marihuana exposure. Journal of Child Psychology and Psychiatry, and Allied Disciplines, 43(1), 81–102. [DOI] [PubMed] [Google Scholar]
- Fried, P. A. , & Smith, A. M. (2001). A literature review of the consequences of prenatal marihuana exposure. An emerging theme of a deficiency in aspects of executive function. Neurotoxicology and Teratology, 23(1), 1–11. [DOI] [PubMed] [Google Scholar]
- Fried, P. A. , Watkinson, B. , & Gray, R. (1998). Differential effects on cognitive functioning in 9‐ to 12‐year olds prenatally exposed to cigarettes and marihuana. Neurotoxicology and Teratology, 20(3), 293–306. [DOI] [PubMed] [Google Scholar]
- Garrett, J. E. , & Wellman, C. L. (2009). Chronic stress effects on dendritic morphology in medial prefrontal cortex: sex differences and estrogen dependence. Neuroscience, 162(1), 195–207. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Goldschmidt, L. , Richardson, G. A. , Cornelius, M. D. , & Day, N. L. (2004). Prenatal marijuana and alcohol exposure and academic achievement at age 10. Neurotoxicology and Teratology, 26(4), 521–532. [DOI] [PubMed] [Google Scholar]
- Golub, M. S. , Sassenrath, E. N. , & Chapman, L. F. (1981). Mother‐infant interaction in rhesus monkeys treated clinically with Δ9‐tetrahydrocannabinol. Child Development, 52(1), 389–392. [PubMed] [Google Scholar]
- Grant, K. , Campbell, V. , & Beckert, L. (2018). Cannabis‐don't smoke it! Four cannabis‐related pathologies in one radiograph. The New Zealand Medical Journal, 131(1471), 84–85. [PubMed] [Google Scholar]
- Harding, S. D. , Sharman, J. L. , Faccenda, E. , Southan, C. , Pawson, A. J. , Ireland, S. , … NC‐IUPHAR (2018). The IUPHAR/BPS Guide to PHARMACOLOGY in 2018: Updates and expansion to encompass the new guide to immunopharmacology. Nucleic Acids Research, 46(D1), D1091–D1106. 10.1093/nar/gkx1121 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Harkany, T. , Guzman, M. , Galve‐Roperh, I. , Berghuis, P. , Devi, L. A. , & Mackie, K. (2007). The emerging functions of endocannabinoid signaling during CNS development. Trends in Pharmacological Sciences, 28(2), 83–92. [DOI] [PubMed] [Google Scholar]
- Hill, M. N. , & Tasker, J. G. (2012). Endocannabinoid signaling, glucocorticoid‐mediated negative feedback, and regulation of the hypothalamic‐pituitary‐adrenal axis. Neuroscience, 204, 5–16. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Huizink, A. C. (2014). Prenatal cannabis exposure and infant outcomes: Overview of studies. Progress in Neuro‐Psychopharmacology & Biological Psychiatry, 52, 45–52. [DOI] [PubMed] [Google Scholar]
- Huizink, A. C. , & Mulder, E. J. (2006). Maternal smoking, drinking or cannabis use during pregnancy and neurobehavioral and cognitive functioning in human offspring. Neuroscience and Biobehavioral Reviews, 30(1), 24–41. [DOI] [PubMed] [Google Scholar]
- Hutchings, D. E. , Gamagaris, Z. , Miller, N. , & Fico, T. A. (1989). The effects of prenatal exposure to Δ9‐tetrahydrocannabinol on the rest‐activity cycle of the preweanling rat. Neurotoxicology and Teratology, 11(4), 353–356. [DOI] [PubMed] [Google Scholar]
- Insel, T. R. , Hill, J. L. , & Mayor, R. B. (1986). Rat pup ultrasonic isolation calls: Possible mediation by the benzodiazepine receptor complex. Pharmacology, Biochemistry, and Behavior, 24(5), 1263–1267. [DOI] [PubMed] [Google Scholar]
- Jung, K. M. , Sepers, M. , Henstridge, C. M. , Lassalle, O. , Neuhofer, D. , Martin, H. , … Manzoni, O. J. (2012). Uncoupling of the endocannabinoid signalling complex in a mouse model of fragile X syndrome. Nature Communications, 3, 11 1080 10.1038/ncomms2045 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jutras‐Aswad, D. , DiNieri, J. A. , Harkany, T. , & Hurd, Y. L. (2009). Neurobiological consequences of maternal cannabis on human fetal development and its neuropsychiatric outcome. European Archives of Psychiatry and Clinical Neuroscience, 259(7), 395–412. [DOI] [PubMed] [Google Scholar]
- Katona, I. , & Freund, T. F. (2008). Endocannabinoid signaling as a synaptic circuit breaker in neurological disease. Nature Medicine, 14(9), 923–930. [DOI] [PubMed] [Google Scholar]
- Kilkenny, C. , Browne, W. J. , Cuthill, I. C. , Emerson, M. , & Altman, D. G. (2010). Improving bioscience research reporting: The ARRIVE guidelines for reporting animal research. PLoS Biology, 8(6), 1–5. e1000412. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kolb, B. , Mychasiuk, R. , Muhammad, A. , Li, Y. , Frost, D. O. , & Gibb, R. (2012). Experience and the developing prefrontal cortex. Proceedings of the National Academy of Sciences of the United States of America, 109(Suppl 2), 17186–17193. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Korenbrot, C. C. , Huhtaniemi, I. T. , & Weiner, R. I. (1977). Preputial separation as an external sign of pubertal development in the male rat. Biology of Reproduction, 17(2), 298–303. [DOI] [PubMed] [Google Scholar]
- Lafourcade, M. , Elezgarai, I. , Mato, S. , Bakiri, Y. , Grandes, P. , & Manzoni, O. J. (2007). Molecular components and functions of the endocannabinoid system in mouse prefrontal cortex. PLoS ONE, 2(8), 1–11. e709. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lee, K. M. , Coelho, M. A. , Class, M. A. , & Szumlinski, K. K. (2018). mGlu5‐dependent modulation of anxiety during early withdrawal from binge‐drinking in adult and adolescent male mice. Drug and Alcohol Dependence, 184, 1–11. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Leech, S. L. , Richardson, G. A. , Goldschmidt, L. , & Day, N. L. (1999). Prenatal substance exposure: Effects on attention and impulsivity of 6‐year‐olds. Neurotoxicology and Teratology, 21(2), 109–118. [DOI] [PubMed] [Google Scholar]
- Liang, S. L. , Alger, B. E. , & McCarthy, M. M. (2014). Developmental increase in hippocampal endocannabinoid mobilization: Role of metabotropic glutamate receptor subtype 5 and phospholipase C. Journal of Neurophysiology, 112(10), 2605–2615. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Manduca, A. , Bara, A. , Larrieu, T. , Lassalle, O. , Joffre, C. , Laye, S. , & Manzoni, O. J. (2017). Amplification of mGlu5‐endocannabinoid signaling rescues behavioral and synaptic deficits in a mouse model of adolescent and adult dietary polyunsaturated fatty acid imbalance. The Journal of Neuroscience: The Official Journal of the Society for Neuroscience, 37(29), 6851–6868. 10.1523/JNEUROSCI.3516-16.2017 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Manduca, A. , Campolongo, P. , & Trezza, V. (2012). Cannabinoid modulation of mother‐infant interaction: Is it just about milk? Reviews in the Neurosciences, 23(5‐6), 707–722. [DOI] [PubMed] [Google Scholar]
- Manduca, A. , Lassalle, O. , Sepers, M. , Campolongo, P. , Cuomo, V. , Marsicano, G. , … Manzoni, O. J. (2016). Interacting cannabinoid and opioid receptors in the nucleus accumbens core control adolescent social play. Frontiers in Behavioral Neuroscience, 10, 211–217. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Manduca, A. , Morena, M. , Campolongo, P. , Servadio, M. , Palmery, M. , Trabace, L. , … Trezza, V. (2015). Distinct roles of the endocannabinoids anandamide and 2‐arachidonoylglycerol in social behavior and emotionality at different developmental ages in rats. European Neuropsychopharmacology: The Journal of the European College of Neuropsychopharmacology, 25(8), 1362–1374. 10.1016/j.euroneuro.2015.04.005 [DOI] [PubMed] [Google Scholar]
- Melancia, F. , Schiavi, S. , Servadio, M. , Cartocci, V. , Campolongo, P. , Palmery, M. , … Trezza, V. (2018). Sex‐specific autistic endophenotypes induced by prenatal exposure to valproic acid involve anandamide signalling. British Journal of Pharmacology, 175(18), 3699–3712. 10.1111/bph.14435 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mereu, G. , Fa, M. , Ferraro, L. , Cagiano, R. , Antonelli, T. , Tattoli, M. , … Cuomo, V. (2003). Prenatal exposure to a cannabinoid agonist produces memory deficits linked to dysfunction in hippocampal long‐term potentiation and glutamate release. Proceedings of the National Academy of Sciences of the United States of America, 100(8), 4915–4920. 10.1073/pnas.0537849100 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Navarro, M. , Rubio, P. , & de Fonseca, F. R. (1995). Behavioural consequences of maternal exposure to natural cannabinoids in rats. Psychopharmacology, 122(1), 1–14. [DOI] [PubMed] [Google Scholar]
- Passey, M. E. , Sanson‐Fisher, R. W. , D'Este, C. A. , & Stirling, J. M. (2014). Tobacco, alcohol and cannabis use during pregnancy: Clustering of risks. Drug and Alcohol Dependence, 134, 44–50. [DOI] [PubMed] [Google Scholar]
- Pellis, S. M. , & Pellis, V. C. (2009). The playful brainedn. OneWorld Publications: Oxford UK. [Google Scholar]
- Peterson, B. M. , Mermelstein, P. G. , & Meisel, R. L. (2015). Estradiol mediates dendritic spine plasticity in the nucleus accumbens core through activation of mGluR5. Brain Structure & Function, 220(4), 2415–2422. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Richardson, K. A. , Hester, A. K. , & McLemore, G. L. (2016). Prenatal cannabis exposure—The “first hit” to the endocannabinoid system. Neurotoxicology and Teratology, 58, 5–14. [DOI] [PubMed] [Google Scholar]
- Rodriguez de Fonseca, F. , Cebeira, M. , Fernandez‐Ruiz, J. J. , Navarro, M. , & Ramos, J. A. (1991). Effects of pre‐ and perinatal exposure to hashish extracts on the ontogeny of brain dopaminergic neurons. Neuroscience, 43(2‐3), 713–723. [DOI] [PubMed] [Google Scholar]
- Rodriguez de Fonseca, F. , Cebeira, M. , Ramos, J. A. , Martin, M. , & Fernandez‐Ruiz, J. J. (1994). Cannabinoid receptors in rat brain areas: Sexual differences, fluctuations during estrous cycle and changes after gonadectomy and sex steroid replacement. Life Sciences, 54(3), 159–170. [DOI] [PubMed] [Google Scholar]
- Ryan, S. A. , Ammerman, S. D. , & O'Connor, M. E. (2018). Marijuana use during pregnancy and breastfeeding: Implications for neonatal and childhood outcomes. Pediatrics, 142(3), 1–15. [DOI] [PubMed] [Google Scholar]
- Saez, T. M. , Aronne, M. P. , Caltana, L. , & Brusco, A. H. (2014). Prenatal exposure to the CB1 and CB2 cannabinoid receptor agonist WIN 55,212‐2 alters migration of early‐born glutamatergic neurons and GABAergic interneurons in the rat cerebral cortex. Journal of Neurochemistry, 129(4), 637–648. [DOI] [PubMed] [Google Scholar]
- Scheyer A. (2019). Prenatal exposure to cannabis affects the developing brain. In: The Scientist.
- Schneider, M. (2013). Adolescence as a vulnerable period to alter rodent behavior. Cell and Tissue Research, 354(1), 99–106. [DOI] [PubMed] [Google Scholar]
- Servadio, M. , Manduca, A. , Melancia, F. , Leboffe, L. , Schiavi, S. , Campolongo, P. , … Trezza, V. (2018). Impaired repair of DNA damage is associated with autistic‐like traits in rats prenatally exposed to valproic acid. European Neuropsychopharmacology: The Journal of the European College of Neuropsychopharmacology, 28(1), 85–96. 10.1016/j.euroneuro.2017.11.014 [DOI] [PubMed] [Google Scholar]
- Sharapova, S. R. , Phillips, E. , Sirocco, K. , Kaminski, J. W. , Leeb, R. T. , & Rolle, I. (2018). Effects of prenatal marijuana exposure on neuropsychological outcomes in children aged 1‐11 years: A systematic review. Paediatric and Perinatal Epidemiology, 32(6), 512–532. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Silva, L. , Zhao, N. , Popp, S. , & Dow‐Edwards, D. (2012). Prenatal tetrahydrocannabinol (THC) alters cognitive function and amphetamine response from weaning to adulthood in the rat. Neurotoxicology and Teratology, 34(1), 63–71. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Smith, A. M. , Fried, P. A. , Hogan, M. J. , & Cameron, I. (2006). Effects of prenatal marijuana on visuospatial working memory: An fMRI study in young adults. Neurotoxicology and Teratology, 28(2), 286–295. [DOI] [PubMed] [Google Scholar]
- Solinas, M. , Goldberg, S. R. , & Piomelli, D. (2008). The endocannabinoid system in brain reward processes. British Journal of Pharmacology, 154(2), 369–383. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Spano, M. S. , Ellgren, M. , Wang, X. , & Hurd, Y. L. (2007). Prenatal cannabis exposure increases heroin seeking with allostatic changes in limbic enkephalin systems in adulthood. Biological Psychiatry, 61(4), 554–563. [DOI] [PubMed] [Google Scholar]
- Spear, L. (2000). Modeling adolescent development and alcohol use in animals. Alcohol Research & Health: The Journal of the National Institute on Alcohol Abuse and Alcoholism, 24(2), 115–123. [PMC free article] [PubMed] [Google Scholar]
- Substance Abuse and Mental Health Services Administration (2013). National survey on drug use and health: summary of national findings. Department of Health and Human Services. 2013. http://www.samhsa.gov/data/sites/default/files/NSDUHresultsPDFWHTML2013/Web/NSDUHresults2013.htm#ch2 [PubMed]
- Terry, L. M. , & Johanson, I. B. (1996). Effects of altered olfactory experiences on the development of infant rats' responses to odors. Developmental Psychobiology, 29(4), 353–377. [DOI] [PubMed] [Google Scholar]
- Tortoriello, G. , Morris, C. V. , Alpar, A. , Fuzik, J. , Shirran, S. L. , Calvigioni, D. , … Harkany, T. (2014). Miswiring the brain: Δ9‐tetrahydrocannabinol disrupts cortical development by inducing an SCG10/stathmin‐2 degradation pathway. The EMBO Journal, 33(7), 668–685. 10.1002/embj.201386035 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Trezza, V. , Campolongo, P. , Cassano, T. , Macheda, T. , Dipasquale, P. , Carratu, M. R. , … Cuomo, V. (2008). Effects of perinatal exposure to Δ9‐tetrahydrocannabinol on the emotional reactivity of the offspring: A longitudinal behavioral study in Wistar rats. Psychopharmacology, 198(4), 529–537. 10.1007/s00213-008-1162-3 [DOI] [PubMed] [Google Scholar]
- Trezza, V. , Campolongo, P. , Manduca, A. , Morena, M. , Palmery, M. , Vanderschuren, L. J. , & Cuomo, V. (2012). Altering endocannabinoid neurotransmission at critical developmental ages: Impact on rodent emotionality and cognitive performance. Frontiers in Behavioral Neuroscience, 6, 2–12. [DOI] [PMC free article] [PubMed] [Google Scholar]
- van Kerkhof, L. W. , Damsteegt, R. , Trezza, V. , Voorn, P. , & Vanderschuren, L. J. (2013). Social play behavior in adolescent rats is mediated by functional activity in medial prefrontal cortex and striatum. Neuropsychopharmacology: Official Publication of the American College of Neuropsychopharmacology, 38(10), 1899–1909. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Vanderschuren, L. J. , Achterberg, E. J. , & Trezza, V. (2016). The neurobiology of social play and its rewarding value in rats. Neuroscience and Biobehavioral Reviews, 70, 86–105. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Vargish, G. A. , Pelkey, K. A. , Yuan, X. , Chittajallu, R. , Collins, D. , Fang, C. , & McBain, C. J. (2017). Persistent inhibitory circuit defects and disrupted social behaviour following in utero exogenous cannabinoid exposure. Molecular Psychiatry, 22(1), 56–67. 10.1038/mp.2016.17 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Vela, G. , Martin, S. , Garcia‐Gil, L. , Crespo, J. A. , Ruiz‐Gayo, M. , Fernandez‐Ruiz, J. J. , … Ambrosio, E. (1998). Maternal exposure to Δ9‐tetrahydrocannabinol facilitates morphine self‐administration behavior and changes regional binding to central mu opioid receptors in adult offspring female rats. Brain Research, 807(1‐2), 101–109. 10.1016/S0006-8993(98)00766-5 [DOI] [PubMed] [Google Scholar]
- Volkow, N. D. , Compton, W. M. , & Wargo, E. M. (2017). The risks of marijuana use during pregnancy. JAMA, 317(2), 129–130. [DOI] [PubMed] [Google Scholar]
- Wang, X. , Dow‐Edwards, D. , Anderson, V. , Minkoff, H. , & Hurd, Y. L. (2004). In utero marijuana exposure associated with abnormal amygdala dopamine D2 gene expression in the human fetus. Biological Psychiatry, 56(12), 909–915. [DOI] [PubMed] [Google Scholar]
- Wang, X. , Dow‐Edwards, D. , Anderson, V. , Minkoff, H. , & Hurd, Y. L. (2006). Discrete opioid gene expression impairment in the human fetal brain associated with maternal marijuana use. The Pharmacogenomics Journal, 6(4), 255–264. [DOI] [PubMed] [Google Scholar]
- Wiley, J. L. , & Martin, B. R. (2002). Cannabinoid pharmacology: Implications for additional cannabinoid receptor subtypes. Chemistry and Physics of Lipids, 121(1‐2), 57–63. [DOI] [PubMed] [Google Scholar]
- Won, H. , Lee, H. R. , Gee, H. Y. , Mah, W. , Kim, J. I. , Lee, J. , … Kim, E. (2012). Autistic‐like social behaviour in Shank2‐mutant mice improved by restoring NMDA receptor function. Nature, 486(7402), 261–265. 10.1038/nature11208 [DOI] [PubMed] [Google Scholar]
- Zanettini, C. , Panlilio, L. V. , Alicki, M. , Goldberg, S. R. , Haller, J. , & Yasar, S. (2011). Effects of endocannabinoid system modulation on cognitive and emotional behavior. Frontiers in Behavioral Neuroscience, 5, 57. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Figure S1. Age‐dependent effects of prenatal exposure to the cannabinoid receptor agonist WIN in social, anxiety and cognitive tasks.


