Abstract
Background
Closure of traumatic macular hole (TMH) can be achieved spontaneously or by surgical intervention. Thus far, there exist no prospective comparative studies that have analyzed the difference between the two modalities. This study aimed to compare the anatomical and visual recovery of eyes with TMH following either an immediate vitrectomy or six-month observation.
Methods
This was a multicenter prospective comparative study. Eight centers participated in the study. Patient data from 40 eyes with a recent history of blunt ocular trauma and newly formed full-thickness TMH were recruited in this study. The participating patients selected between an early vitrectomy or a six-month observation after a doctor explained the potential benefits and risks of both strategies in an unbiased manner. Twenty-five patients underwent an immediate vitrectomy, and 15 patients received six-month observation. Patients were assessed by spectral-domain optical coherence tomography (SD-OCT) and best-corrected visual acuity (BCVA).
Results
Closure rates were 66.7% for the observational group, and 100% for the surgical group (P=0.002). There were no vision-threatening ocular complications in both groups. For the observational group, the mean closure time was 2.5±1.6 months, and 80% of the hole closure occurred within 3 months; cystic edema on the edge of the hole at baseline was significantly more frequent in the non-closed subgroup than in the closed subgroup (P=0.03). There were no significant differences in the foveal microstructure and in the final visual outcome between the spontaneously closed cases and the surgically closed cases.
Conclusions
TMH had a moderately high incidence of spontaneous closure, but an immediate vitrectomy achieved an even higher closure rate. Vitrectomy was effective and safe to treat TMH, while a 3-month observation for spontaneous closure may be an alternative modality for TMH management. Cystic edema on the edge of the hole may be an unfavorable factor for the spontaneous closure of TMH.
Keywords: Traumatic macular hole (TMH), vitrectomy, observation
Introduction
Traumatic macular hole (TMH) is a well-recognized condition arising from blunt ocular trauma, and TMH occurs in 1.4% of closed-globe injuries and 0.15% of open globe injuries (1). Unlike an idiopathic macular hole’s gradual progression, TMH is caused by sudden eyeball compression and usually occurs immediately after trauma.
Therefore, the need for vitrectomy to treat TMH treatment is less clear than idiopathic macular hole treatment (2,3). Some researchers reported favorable results for vitrectomy surgery (4-9), while others reported that TMH could close without surgery (10-14). To date, no prospective studies have compared the two treatment approaches. The objectives of the current study were to analyze the closure rate difference between immediate vitrectomy and observation for a period of time, along with the foveal microstructure and visual recovery differences between the surgical closure and spontaneous closure of TMH.
Methods
Patients’ eligibility
The local ethics committees gave approval for this prospective multicenter comparative study. From January 2013 to June 2017, patients were recruited from eight specialized vitreoretinal units. Inclusion criteria were eyes with a recent history (within one month) of blunt ocular trauma, immediate vision decrease after trauma, and occurrence of full-thickness MH, which was confirmed by ophthalmoscopy and spectral-domain optical coherence tomography (SD-OCT). Exclusion criteria were eyes with open globe injuries, severe cataract or vitreous hemorrhage, retinal detachment, a refractive error greater than −6.00 diopters, subfoveal or juxtafoveal hemorrhage or choroidal rupture (r<200 µm), and patients older than 60 years old (as idiopathic MH cannot be excluded at this age). The grouping of patients was performed in all centers as outlined below. After thoroughly explaining the possible benefits and risks of early vitrectomy and observation, the patients were asked to choose one of the strategies depending on their judgement. All doctors involved in the study must remain neutral and unbiased, and were not allowed to give their own opinions or preferences to the patients. Written informed consent was obtained. For the surgical group, vitrectomy was performed as soon as the diagnosis of TMH was confirmed, while the injured eyes of the patients under the observational group were observed for 6 months to see if the TMH could close spontaneously.
Clinical data analysis
In the initial examination and during each follow-up visit, all patients underwent a complete ophthalmic examination, including best-corrected visual acuity (BCVA), biomicroscopy, indirect ophthalmoscopy, and SD-OCT. BCVA was measured using a decimal visual acuity chart, and converted to the logarithm of the minimum angle of resolution (logMAR) equivalent for statistical analysis. Baseline information included the patient’s age and gender; the interval between ocular trauma and the onset of visual disturbances; the interval between ocular trauma and the time of confirmed diagnosis; posttraumatic BCVA; indirect ophthalmoscopy records including the shape of the hole, presence of Weiss ring, presence of Berlin edema, and presence of extrafoveal hemorrhage or choroidal rupture (r>200 µm); SD-OCT records including the minimum and base diameter of the hole, presence of cystic edema on the edge of the hole, and presence of perifoveal posterior vitreous detachment (PVD). The baseline characteristics of the two groups were compared.
Outcome measures
The primary outcome measurement was the closure rate of the macular hole, which was assessed 6 months after the primary injury by an SD-OCT. The secondary outcome measures consisted of surgical complications and observation complications, microstructure of the fovea, and the visual outcome at the endpoint of the study. The endpoint of the study was defined as 6 months after vitrectomy for the surgical group, and 6 months after the hole closure for the observational group. The microstructure assessment of the fovea included the closure type of the hole, length of the photoreceptor inner segment/outer segment (IS/OS) junction defect, and the central foveal thickness (CFT) measured by SD-OCT. The visual outcome included the final logMAR BCVA and the rate of visual improvement (≥0.3 logMAR equivalent).
SD-OCT
Four types of SD-OCT were used for this study (Heidelberg OCT, Heidelberg Engineering, Heidelberg, Germany; Stratus OCT, Carl Zeiss Meditec, Dublin, CA, USA; Topcon 3D-OCT 2000, Topcon Medical Systems, Inc., Japan; RTVue-100, Optovue Inc., Fremont, CA, USA). In all SD-OCT recordings, particular care was taken, to pass through the center of the hole during both horizontal and vertical scans. Serial SD-OCT was performed regularly during each revisit follow-up. All SD-OCT images were transferred to PUEC and interpreted by two independent and masked investigators (HJ Chen and Y Jin). If a disagreement between examiners occurred for the interpretation of one specific OCT image, a third investigator (ZZ Ma) would be consulted for the final decision. The diameter of the hole was measured at the baseline by SD-OCT; measurements were taken on every follow-up revisit as long as the hole remained open. The minimum diameter of the hole was measured as the minimum inner diameter of the hole, and the base diameter of the hole was measured as the hole’s diameter at the level of the retinal pigment epithelium (RPE) (Figure 1). Hole closure was defined as the flattening of the elevated edges of the hole with the resolution of the surrounding subretinal fluid cuff (Figure 2). For eyes with holes that were anatomically closed, the closure type of the hole, length of the IS-OS junction defect, and CFT were analyzed. Closure type of the hole was classified into type 1 (Figure 2A) and type 2 (Figure 2B), according to the previous literature (15). The length of the IS/OS junction defect was measured as the length of loss of the hyper-reflective line corresponding to the IS/OS junction above the RPE (Figure 3A). CFT was measured as the minimum height from the vitreoretinal interface to the RPE at the fovea (Figure 3B). By using the caliper function of the software package, parameters that included the minimum and base diameter of the hole, the length of the IS-OS junction defect, and CFT, were measured. All parameters were determined by averaging the values measured from the vertical and horizontal SD-OCT images through the fovea.
Figure 1.
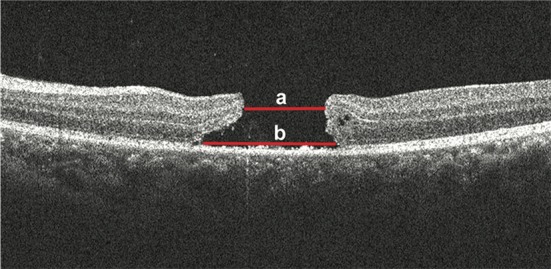
Measurements of the macular hole diameter by spectral domain-optical coherence tomography. The hole minimum diameter (a) was measured as the minimum inner diameter of the hole. The hole base diameter (b) was measured as the hole diameter at the level of the retinal pigment epithelium.
Figure 2.

Closure of the hole was defined as the flattening of the elevated edges of the hole with resolution of the surrounding subretinal fluid cuff by spectral domain-optical coherence tomography. (A) Representing type 1 closure; indicating no interruptions in the continuity of the foveal tissue above the retinal pigment epithelium (RPE). The normal foveal contour is usually encountered in type 1 closures. (B) Representing type 2 closure; indicating an interruption in the continuity of the foveal tissue. Thus, the RPE is denuded. The hole edge is attached to the underlying RPE.
Figure 3.

Spectral domain-optical coherence tomography (A) shows the length of the inner segment/outer segment (IS/OS) junction defect, which was measured as the length of loss of the hyper-reflective line corresponding to the IS/OS junction above the retinal pigment epithelium (RPE); (B) shows the central foveal thickness (CFT), which was measured as the minimum height from the vitreoretinal interface to the top of RPE at the fovea.
Surgery vs. observation
In the surgical group, all eyes underwent pars plana vitrectomy, internal limiting membrane (ILM) peeling, fluid-air exchange, and perflouropropane (C3F8) or air filling. No other techniques, including inverted ILM flap, ILM insertion, or autologous blood, were used in this study. The intraoperative staining of ILM, gas concentration, and the postoperative face-down position duration were determined based on each surgeon’s preference. Ten surgeons took part in the study. Patients revisited 1, 3, and 6 months after the surgery.
No surgery was performed for the observational group. Patients were asked to revisit promptly, if a decrease or increase in vision was felt. Otherwise, the patient’s revisit was conducted at one-month intervals for 6 months until the hole was closed. After hole closure, patients were asked to revisit after 1, 3, and 6 months. If the TMH did not close within 6 months, patients were given a choice to continue undergoing observation or change to surgical intervention. Observation by the investigators were to cease before the 6-month period, should one of the following situations develop: occurrence of RD, enlargement of the hole (≥20% increase of the minimum diameter or ≥50% increase of the base diameter), and decrease of the BCVA (≥0.3 logMAR equivalent). The observational group was further divided into two subgroups: those with holes that closed within 6 months, and those with holes that did not close within 6 months. The baseline data were compared between the two subgroups to investigate possible unfavorable factors for the spontaneous closure of TMH.
Statistical methods
Sample size was calculated using Stata V10.1 (College Station, TX, USA). An Independent t-test was used to compare the differences between the means for continuous data. A Chi-square test was used to compare the percentage differences. The value of P<0.05 was considered to denote statistical significance. The statistical analysis of the data was performed by using the SPSS V20.0 software for Windows (SPSS, Chicago, IL, USA).
Results
Patients and baseline data
From January 2013 to June 2017, 44 eyes from 44 patients fulfilled the inclusion criteria (Figure 4). From these cases, 4 were excluded due to the following reasons: 1 patient was older than 60 years old, for whom idiopathic MH could not be excluded, and 3 eyes had juxtafoveal (r<200 µm) hemorrhage and choroidal rupture. Ultimately, 40 patients were enrolled in the study. Among them, 25 patients chose to receive early vitrectomy, and 15 patients chose to receive observation. All patients completed the study. The baseline clinical characteristics are summarized in Table 1. All variables were balanced between the two groups. In the observational group, the baseline data were further compared between the two subgroups (TMH closed within 6 months vs. TMH non-closed within 6 months). We found that there were no significant differences in all baseline parameters, except for the cystic edema on the edge of the hole (Table 2).
Figure 4.

Flowchart showing patient selection of the study.
Table 1. Comparison of baseline characteristics between patients with TMH who received early vitrectomy surgery and patients who received 6-month observation.
| Group | Surgical (n=25) | Observational (n=15) | P value |
|---|---|---|---|
| Age (in years), mean ± SD | 31.0±12.5 | 33.1±11.6 | 0.60 |
| Gender, n (%) | |||
| Male | 22 (88.0) | 14 (93.3) | 0.58 |
| Female | 3 (12.0) | 1 (6.7) | 0.58 |
| Duration between injury and vision decrease (in days), mean ± SD | 0.32±1.4 | 0.87±3.1 | 0.45 |
| Duration between injury and confirmed diagnosis of TMH (in days), mean ± SD | 12±8.1 | 9.8±9.1 | 0.43 |
| Posttraumatic BCVA (logMAR) | 1.00±0.35 | 1.11±0.48 | 0.46 |
| Mean diameter of the hole (μm), mean ± SD | |||
| Minimum diameter | 512.4±315.1 | 423.2±242.9 | 0.35 |
| Base diameter | 1,356.6±579.4 | 1,763.1±891.1 | 0.08 |
| Weiss ring, n (%) | 0 (0) | 0 (0) | 1.0 |
| Perifoveal PVD, n (%) | 0 (0) | 0 (0) | 1.0 |
| Cystic edema of the hole edge, n (%) | 11 (44.0) | 4 (26.7) | 0.27 |
| Presence of Berlin edema, n (%) | 9 (36.0) | 6 (40.0) | 0.60 |
| Presence of extrafoveal hemorrhage or choroidal rupture, n (%) | 4 (16.0) | 4 (26.7) | 0.20 |
| Shape of the TMH, n (%) | |||
| Round | 14 (56.0) | 8 (53.3) | 0.90 |
| Elliptic | 11 (44.0) | 7 (46.7) | 0.87 |
TMH, traumatic macular hole; SD, standard deviation; BCVA, best corrected visual acuity; logMAR, logarithm of the minimum angle of resolution; PVD, posterior vitreous detachment.
Table 2. Comparison of baseline characteristics in the observational group between patients whose TMH closed and patients whose TMH did not close.
| Subgroups | Closed (n=10) | Non-closed (n=5) | P value |
|---|---|---|---|
| Age (in years), mean ± SD | 35.6±12.4 | 26.3±5.3 | 0.06 |
| Gender, n (%) | |||
| Male | 10 (100.0) | 4 (80.0) | 0.09 |
| Female | 0 (0.0) | 1 (20.0) | 0.09 |
| Duration between injury and vision decrease (in days), mean ± SD | 0.00±0.00 | 3.25±5.85 | 0.35 |
| Duration between injury and confirmed diagnosis of TMH (in days), mean ± SD | 9.45±10.45 | 10.75±4.92 | 0.82 |
| Posttraumatic BCVA (logMAR) | 1.75±1.09 | 1.53±1.16 | 0.74 |
| Mean diameter of the hole, mean ± SD | |||
| Minimal diameter | 404.9±277.1 | 473.5±122.1 | 0.65 |
| Basal diameter | 1,927.3±903.6 | 1,311.8±783.4 | 0.25 |
| Weiss ring, n (%) | 0 (0.0) | 0 (0.0) | – |
| Perifoveal PVD, n (%) | 0 (0.0) | 0 (0.0) | – |
| Cystic edema of the hole edge, n (%) | 1 (10.0) | 3 (60.0) | 0.03 |
| Presence of Berlin edema, n (%) | 5 (50.0) | 1 (20.0) | 0.31 |
| Presence of extrafoveal hemorrhage or choroidal rupture, n (%) | 3 (30.0) | 1 (20.0) | 0.68 |
| Shape of the TMH, n (%) | |||
| Round | 5 (50.0) | 3 (60.0) | |
| Elliptic | 5 (50.0) | 2 (40.0) | 0.71 |
TMH, traumatic macular hole; SD, standard deviation; BCVA, best corrected visual acuity; logMAR, logarithm of the minimum angle of resolution; PVD, posterior vitreous detachment.
Anatomical outcome
In the surgical group, the mean interval between the injury and the operation was 20.8±8.8 days (range, 8–30 days). The primary closure was achieved by single intervention in all 25 (100%) cases. No vision-threatening intraoperative or postoperative complications were observed in any of the cases. Six months after surgery, type 1 closure was observed in 23 (96%) eyes. The length of the photoreceptor IS/OS junction defect was 1,717.6±1,259.3 µm, while CFT was 159.2±97.2 µm.
For the observational group, the hole closed spontaneously within 6 months in 10 out of 15 (66.7%) cases. The mean closure time was 2.5±1.6 months (range, 0.5–5 months; median, 1.5 months), and 80% of the hole closure occurred within three months. Six months after hole closure, type 1 closure was observed in 10 out of 10 (100%) eyes. The length of the photoreceptor IS/OS junction defect was 2,601.5±1,981.5 µm, and CFT was 134.8±86.9 µm. For eyes with holes that did not close, none of them experienced any obvious enlargement of the hole or occurrence of RD within 6 months.
The closure rate was significantly higher in the surgical group than in the observational group (P=0.002). However, there were no significant differences in the rate of type 1 closure, the length of the photoreceptor IS/OS junction defect, and CFT between the surgically closed cases and spontaneously closed cases (P=0.37, 0.20, 0.48, respectively).
For the subgroup analysis in the observational group, the cystic edema on the edge of the hole at baseline was significantly more frequent in the non-closed subgroup than in the closed subgroup (P=0.03).
Functional outcome
In the surgical group, the mean BCVA at the baseline was 1.00±0.35 logMAR, and the final mean BCVA was 0.56±0.36 logMAR. The visual acuity of 22 out of 25 (88%) patients increased by 0.3 logMAR or more.
In the observational group, the mean BCVA at the baseline was 1.11±0.48 logMAR, and the final mean BCVA was 0.75±0.47 logMAR. For the10 eyes with TMH that closed within 6 months, the final mean BCVA was 0.78±0.54 logMAR. The visual acuity of 7 out of 10 (70%) eyes improved by 0.3 logMAR or more.
There were no significant differences in the final logMAR BCVA and the rate of eyes that had a visual improvement of 0.3 logMAR or more, between the surgically closed cases and the spontaneously closed cases (P=0.14, 0.09, respectively).
Discussion
TMH management is a controversial problem. Thus far, no clinical guidelines for this low-incidence disorder have been established. Several retrospective studies have reported varied success rates of vitrectomy surgery (4-9). However, the necessity of vitrectomy has also been questioned because spontaneous hole closure is not uncommon (10-14). Earlier studies regarding TMH management have several limitations. Many reports were based on small case series, and comparative studies have never been performed. It is still not known whether a significant difference in anatomical and functional outcomes exists between eyes that undergo vitrectomy and patients who are under observation for a period of time after injury. Moreover, most of the relevant studies have been published before the advent of SD-OCT or even before the widespread use of OCT; therefore, the visualization of the vitreoretinal interface at the hole and the analysis of a foveal microstructure after anatomical hole closure have been fairly limited.
Our prospective, multicenter, comparative study was designed to solve these problems. This is a prospective multicenter comparative study. In this study, patients in all centers were grouped in the following fashion: after thoroughly explaining the possible benefits and risks of early vitrectomy and observation, the patients were asked to choose one of the strategies according to their own judgement. All doctors involved in this study remained neutral and unbiased, and they were not allowed to express their own opinions or preferences to the patients. Table 1 shows the balanced baseline variables between the two groups.
In our study, the anatomic closure rate of TMH in the surgical group was 100%, even though no adjunctive therapy was used, such as inverted ILM flap, ILM insertion, and autologous blood. No vision-threatening intraoperative and postoperative surgical complications were observed. These positive results may partly be attributable to the surgical technique advances of modern vitreoretinal surgery. Furthermore, in all surgical cases, vitrectomy was performed early in this study.
According to the literatures, spontaneous TMH closure usually occurs between 1 to 6 months (2,3); for this reason, the observation time of our study was designed to be 6 months. Within this period, the spontaneous closure of the hole occurred in 10 out of 15 (66.7%) eyes. The closure rates reported in the literature vary (10-14). The retrospective nature of these studies may be the reason for these discrepancies. In this study, the mean time for the spontaneous closure of the hole was 2.5±1.6 months (range, 0.5–5 months; median, 1.5 months), and 80% of the hole closure occurred within three months. This result is in line with previous publications (10-13). In addition, no patients developed obvious hole enlargement or occurrence of RD during the 6-month observation period, and no significant differences were found in the final BCVA and the restoration of the foveal microstructure, between the spontaneous closure group and the surgical closure group. These results demonstrated that a 3-month observation period after injury may be an alternative modality for TMH management.
The mechanism of spontaneous TMH closure is a special issue of interest. Why does TMH have more frequent spontaneous closure than its idiopathic counterpart? The different status of the vitreous and vitreo-retinal interface in these two disease entities may account for the discrepancy of the spontaneous closure rate. After the advent of OCT, it came to be known that the evolution of the idiopathic macular hole is strongly associated with age-related perifoveal PVD (16-20). Antero-posterior vitreoretinal traction on the edge of up drawn fovea is usually seen in a stage II idiopathic macular hole (Figure 5A). This antero-posterior vitreoretinal traction plays a key role in preventing the hole from spontaneously closing. Whereas, most TMH cases involve young patients with a healthy vitreous gel and a firm vitreofoveal attachment. A sudden antero-posterior compression of the globe results in the expansion of the equator and flattening of the posterior sclera, producing significant stress on the retina at points of the firm vitreoretinal attachment, and thus, splitting the fovea (2). After the impact force ends, the transient traction disappears, and the young vitreous still tamponade the TMH. It has been demonstrated that PVD is seen less in eyes with TMH than in eyes with idiopathic macular hole (21). Johnson et al. observed that, during vitrectomy surgery, the posterior hyaloid was detached in 16% of TMH cases (2). Yanagiya et al. found that PVD was present in 15% of TMH eyes (22). Meanwhile, Arevalo et al., using biomicroscopic fundus examination that was later confirmed by OCT, found that complete PVD occurred in 50% of their TMH cases (23). However, Huang et al. found in their study that none of the 73 TMH eyes had clinical or OCT-detectable PVD (21,24). These inconsistencies likely owe to the differing inclusion criteria of patients across the various studies. When patients with old age and/or a long-time interval between the injury and presentation were included, the occurrence of PVD increased. In all cases in our series, TMH was diagnosed within one month after the trauma, and patients older than 60 years or with a refractive error of more than -6.00 diopters were excluded. We found that no patients in our series showed perifoveal PVD and Weiss ring upon presentation or during the 6-months observation period (Figure 5B). Our findings suggest that continuous vitreous traction rarely occurs in TMH cases with young healthy vitreous; and therefore, the role of vitrectomy in TMH may be less important than in its idiopathic counterpart.
Figure 5.

Spectral domain-optical coherence tomography (A) shows the perifoveal posterior vitreous detachment (PVD) and antero-posterior vitreous traction on the edge of the up drawn fovea is usually seen in a stage II idiopathic macular hole. This antero-posterior vitreoretinal traction prevents the hole from spontaneously closing. However, in a traumatic macular hole (B), perifoveal PVD and the continuous vitreous traction at the edge of the hole are seldom seen, due to the young healthy vitreous gel.
The factors that influence spontaneous TMH closure also need to be investigated. Young age, small hole size, and no PVD have been recognized as possible features for cases with spontaneous TMH closure (11). In our study, we further analyzed the baseline differences between eyes with TMH that closed within 6 months without surgical intervention and those that did not close within 6 months. Among all baseline clinical characteristics, we found no significant differences in age and diameter of the hole between the two subgroups; only the cystic edema on the edge of the hole was significantly frequent in eyes with TMH that did not close within 6 months, suggesting cystic edema may be an unfavorable predictor for spontaneous THM closure. This finding was in accordance with the result of a retrospective study in the literature (14).
There were some limitations to this study. Firstly, this was a non-randomized prospective study: receiving early vitrectomy or proceeding with observation was based on the patients’ choice after they were fully aware of the potential benefits and risks of each modality. Although the doctors involved in the study remained neutral and were not allowed to express their own opinion or preference to the patients, and the baseline data were comparable between the two groups, the possibility of selection bias has to be considered in interpreting our results. Secondly, due to the low prevalence of TMH, the sample size of this comparative study was limited; therefore, some confounding factors might have caused bias due to the limited sample size. Nevertheless, the sample size of this study is larger than other earlier longitudinal studies on TMH, and this is the first prospective comparative study to investigate both the anatomical and the visual differences between early surgical intervention and observation on TMH. The preliminary results obtained from this study may shed new lights on future studies and give some guidance to clinical decision-making for TMH.
Conclusions
In conclusion, our study showed that 6-month observation after the occurrence of TMH can produce a moderately high rate (66.7%) of spontaneous closure, while early vitrectomy can result in an even higher closure rate (100%) than the 6-month observation. Both the early vitrectomy and the 6-month observation after injury are safe and have no vision-threatening ocular complications. The mean time for the spontaneous closure of the hole was 2.5±1.6 months, and 80% of the hole closure occurred within three months. Cystic edema on the edge of the hole may be an unfavorable factor for the spontaneous closure of TMH. There were no significant differences in the restoration of the foveal microstructure and the final visual acuity, between the spontaneous closure group and the surgical closure group. Therefore, vitrectomy was effective and safe to treat TMH, while a 3-month observation for spontaneous closure may be an alternative modality for TMH management.
Acknowledgments
This study was sponsored by Academic Group of Eye Trauma, Chinese Ophthalmological Society.
Ethical Statement: The authors are accountable for all aspects of the work in ensuring that questions related to the accuracy or integrity of any part of the work are appropriately investigated and resolved. The study was approved by the institutional review committee of Peking University Third Hospital (ID No. IRB00006761-2011100). The research followed the tenets of the Declaration of Helsinki. ChinaTrial registration number: NCT01509092.
Footnotes
Conflicts of Interest: The authors have no conflicts of interest to declare.
References
- 1.Kuhn F, Morris R, Witherspoon CD, et al. Epidemiology of blinding trauma in the United States Eye Injury Registry. Ophthalmic Epidemiol 2006;13:209-16. 10.1080/09286580600665886 [DOI] [PubMed] [Google Scholar]
- 2.Johnson RN, McDonald HR, Lewis H, et al. Traumatic macular hole: observations, pathogenesis, and results of vitrectomy surgery. Ophthalmology 2001;108:853-7. 10.1016/S0161-6420(00)00650-3 [DOI] [PubMed] [Google Scholar]
- 3.Miller JB, Yonekawa Y, Eliott D, et al. A review of traumatic macular hole: diagnosis and treatment. Int Ophthalmol Clin 2013;53:59-67. 10.1097/IIO.0b013e3182a26efe [DOI] [PubMed] [Google Scholar]
- 4.Rubin JS, Glaser BM, Thompson JT, et al. Vitrectomy, fluid-gas exchange and transforming growth factor--beta-2 for the treatment of traumatic macular holes. Ophthalmology 1995;102:1840-5. 10.1016/S0161-6420(95)30786-5 [DOI] [PubMed] [Google Scholar]
- 5.de Bustros S. Vitreous surgery for traumatic macular hole. Retina 1996;16:451-2. 10.1097/00006982-199616050-00018 [DOI] [PubMed] [Google Scholar]
- 6.García-Arumí J, Corcostegui B, Cavero L, et al. The role of vitreoretinal surgery in the treatment of posttraumatic macular hole. Retina 1997;17:372-7. 10.1097/00006982-199717050-00003 [DOI] [PubMed] [Google Scholar]
- 7.Margherio AR, Margherio RR, Hartzer M, et al. Plasmin enzyme-assisted vitrectomy in traumatic pediatric macular holes. Ophthalmology 1998;105:1617-20. 10.1016/S0161-6420(98)99027-3 [DOI] [PubMed] [Google Scholar]
- 8.Kuhn F, Morris R, Mester V, et al. Internal limiting membrane removal for traumatic macular holes. Ophthalmic Surg Lasers 2001;32:308-15. [PubMed] [Google Scholar]
- 9.Wu WC, Drenser KA, Trese MT, et al. Pediatric traumatic macular hole: results of autologous plasmin enzyme-assisted vitrectomy. Am J Ophthalmol 2007;144:668-72. 10.1016/j.ajo.2007.07.027 [DOI] [PubMed] [Google Scholar]
- 10.Kusaka S, Fujikado T, Ikeda T, et al. Spontaneous disappearance of traumatic macular holes in young patients. Am J Ophthalmol 1997;123:837-9. 10.1016/S0002-9394(14)71136-5 [DOI] [PubMed] [Google Scholar]
- 11.Mitamura Y, Saito W, Ishida M, et al. Spontaneous closure of traumatic macular hole. Retina 2001;21:385-9. 10.1097/00006982-200108000-00020 [DOI] [PubMed] [Google Scholar]
- 12.Yamashita T, Uemara A, Uchino E, et al. Spontaneous closure of traumatic macular hole. Am J Ophthalmol 2002;133:230-5. 10.1016/S0002-9394(01)01303-4 [DOI] [PubMed] [Google Scholar]
- 13.Yamada H, Sakai A, Yamada E, et al. Spontaneous closure of traumatic macular hole. Am J Ophthalmol 2002;134:340-7. 10.1016/S0002-9394(02)01535-0 [DOI] [PubMed] [Google Scholar]
- 14.Chen H, Chen W, Zheng K, et al. Prediction of spontaneous closure of traumatic macular hole with spectral domain optical coherence tomography. Sci Rep 2015;5:12343. 10.1038/srep12343 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Kang SW, Ahn K, Ham DI. Types of macular hole closure and their clinical implications. Br J Ophthalmol 2003;87:1015-9. 10.1136/bjo.87.8.1015 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Niwa H, Terasaki H, Ito Y, et al. Macular hole development in fellow eyes of patients with unilateral macular hole. Am J Ophthalmol 2005;140:370-5. 10.1016/j.ajo.2005.03.070 [DOI] [PubMed] [Google Scholar]
- 17.Michalewska Z, Michalewski J, Sikorski BL, et al. A study of macular hole formation by serial spectral optical coherence tomography. Clin Experiment Ophthalmol 2009;37:373-83. 10.1111/j.1442-9071.2009.02041.x [DOI] [PubMed] [Google Scholar]
- 18.Yeh PT, Chen TC, Yang CH, et al. Formation of idiopathic macular hole-reappraisal. Graefes Arch Clin Exp Ophthalmol 2010;248:793-8. 10.1007/s00417-009-1297-x [DOI] [PubMed] [Google Scholar]
- 19.Takahashi A, Yoshida A, Nagaoka T, et al. Macular Hole Formation in Fellow Eyes With a Perifoveal Posterior Vitreous Detachment of Patients With a Unilateral Macular Hole. Am J Ophthalmol 2011;151:981-9.e4. 10.1016/j.ajo.2011.01.011 [DOI] [PubMed] [Google Scholar]
- 20.Takezawa M, Toyoda F, Kambara C, et al. Clarifying the mechanism of idiopathic macular hole development in fellow eyes using spectral-domain optical coherence tomography. Clin Ophthalmol 2011;5:101-8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Huang J, Liu X, Wu Z, et al. Comparison of full-thickness traumatic macular holes and idiopathic macular holes by optical coherence tomography. Graefes Arch Clin Exp Ophthalmol 2010;248:1071-5. 10.1007/s00417-009-1226-z [DOI] [PubMed] [Google Scholar]
- 22.Yanagiya N, Akiba J, Takahashi M, et al. Clinical characteristics of traumatic macular holes. Jpn J Ophthalmol 1996;40:544-7. [PubMed] [Google Scholar]
- 23.Arevalo JF, Sanchez JG, Costa RA, et al. Optical coherence tomography characteristics of full-thickness traumatic macular holes. Eye (Lond) 2008;22:1436-41. 10.1038/sj.eye.6702975 [DOI] [PubMed] [Google Scholar]
- 24.Huang J, Liu X, Wu Z, et al. Classification of full-thickness traumatic macular holes by optical coherence tomography. Retina 2009;29:340-8. 10.1097/IAE.0b013e31819241d0 [DOI] [PMC free article] [PubMed] [Google Scholar]


