Abstract
Background
Pulmonary hypertension (PH) is a multi-causal disease and no satisfactory therapeutic strategies for it. Statins have been suggested as potential drugs in PH, whose effects in different clinic types of PH have not been conclusive. In this study, we included randomized controlled clinical trials (RCTs) evaluating the efficacy and safety of statins therapy in PH.
Methods
We searched databases including Medline, Embase, Cochrane, PubMed and Web of science, with time up to January 1, 2019. With 95% confidence interval (CI), weighted mean difference (WMD) or standardized mean difference (SMD) was pooled and calculated in a random or fixed effect model according to I2 statistic.
Results
A total of nine RCTs with 657 patients were included. Four types of statins (atorvastatin, pravastatin, rosuvastatin and simvastatin) were used at different doses (10–80 mg daily) for up to 6 months. In the pooled-data analysis, compared with placebo, there were significant improvements in pulmonary arterial pressure (PAP), in addition to low-density lipoprotein (LDL) in patients treated with statins, but not in 6-minute walking distance (6MWD), cardiac index (CDI). No more adverse events and all-cause mortality were revealed. Subgroup analysis indicated that statins could decrease PAP in the subtype of PH due to chronic obstructive pulmonary disease (COPD), but not pulmonary arterial hypertension (PAH).
Conclusions
This study indicates that statins can efficiently and safely reduce PAP in PH, especially in the subtype due to COPD. Further RCTs are needed to focus on the efficacy and safety of statin therapy in different subtypes of PH.
Keywords: Pulmonary hypertension (PH), chronic obstructive pulmonary disease (COPD), pulmonary arterial hypertension (PAH), statins, meta-analysis
Introduction
Pulmonary hypertension (PH) is a fatal disorder characterized by persistently increased pulmonary arterial pressure (PAP) of various causes, which is defined as an increase of the mean PAP above 25 mmHg at rest (1). According to primary causes, PH is categorized into five clinic subtypes (2): (I) pulmonary arterial hypertension (PAH), including idiopathic, heritable forms and PAH associated with connective tissue disease and congenital heart disease, etc.; (II) PH due to left heat disease; (III) PH due to lung diseases and/or hypoxia [chronic obstructive pulmonary disease (COPD), etc.]; (IV) chronic thromboembolic PH (CTPH) and other pulmonary artery obstructions; (V) PH with unclear and/or multifactorial mechanisms. These subtypes give rise to mechanisms regarding vasoconstriction, vascular wall thickening, stenosis, along with remodeling, thrombosis and blood viscosity and volume increasing caused by chronic hypoxia. The long-term progression would produce aggravated cardiac load, ultimately, cor pulmonale which severely affects the respiratory and cardiovascular systems. Current routine medications for PH include vasodilators, such as endothelin receptor antagonists, prostacyclin analogues (prostanoids) and their likes (3,4), anticoagulants, diuretics, cardiotonic agents and oxygen, etc. However, the prognosis has been always unsatisfactory since the lack of mechanism-targeted therapy. Besides, given the treatments of PH differing with respect to their types, more effective and targeted therapy is urgently needed.
Statins, as HMG-CoA reductase inhibitors, have pleiotropic effects, such as lipid-lowering, anti-proliferative, antioxidant, anti-inflammatory and endothelial cell functions maintaining properties (5-7), which may be helpful in attenuating the progression of PH. It was reported that statins can prevent and reverse pulmonary vascular remodeling in several animal models of PH (8). An observational study showed there were significant improvements in 6-minute walking distance (6MWD), cardiac output and right ventricular systolic pressures in PH treated with simvastatin (9). However, Anand et al. found that there was no significant improvement in 6MWD and mortality with statin therapy, compared to placebo (10). Some meta-analyses showed that statins treatment had no effect of PAP in PH patients (11-13). An increasing number of studies have been focused on statin therapy in PH these years, even though the results were controversial (14-16). And statins have been suggested as novel and effective drugs for PH in some vivo and vitro studies (5-7).
Given the undetermined situation, we performed this systematic review and meta-analysis to evaluate the efficacy and safety of statin therapy in randomized controlled trials (RCTs) of PH and a subgroup analysis concerning PAH and PH due to COPD, with the expectation to provide evidence regarding the role of statin therapy in PH, especially those due to PAH and COPD.
Methods
Search strategies
We searched Medline (1946 to December week 4, 2018), Embase (1974 to January 2019), Cochrane controlled trials register (The Cochrane Library Issue 1, 2019), and PubMed (updated to January, 2019), Web of science (1990–2019) for eligible articles, using the terms: “pulmonary hypertension”, “pulmonary arterial hypertension”, and “PAH”, combined with the following individual search terms: “HMG-CoA reductase inhibitor”, “statin”, “statins”; “atorvastatin”, “cerivastatin”, “fluvastatin”, “lovastatin”, “pravastatin”, “pitavastatin”, “rosuvastatin”, “simvastatin”. Studies were included without any restrictions on language, sex, age or publication date. These studies and relevant references cited therein were reviewed. Both abstracts and full manuscripts were considered. Moreover, no patient and public involvement should be ethically stated in this meta-analysis.
Inclusion and exclusion criteria
Studies were eligible for inclusion as the following criteria: (I) they were RCTs; (II) they evaluated the clinical efficiency of statin therapy in patients with PH; (III) the trials provided data on at least one outcome of interest: PAP, exercise tolerance (6MWD), cardiac index (CDI), low-density lipoprotein (LDL), all-cause mortality and adverse events; (IV) if the same patient group appeared in other publications, only the latest or complete report was incorporated.
Data extraction and management
Two independent reviewers (F Chen and M Yang) separately screened the titles and abstracts, performed duplicate checking, and reviewed full articles that met the inclusion criteria. Data were independently abstracted from each identified reference with a predesigned review form. Disagreement was resolved by consensus with the third author (C Wan). The details from each study included general characteristics of the study (publication year, area, study design), participants (age, gender, number of patients in treatment and control group, diagnosis, PAP and LDL before treatment) and intervention (statin types, dose, background treatment and duration). We extracted data on the following outcomes: PAP, exercise tolerance (6MWD), CDI, LDL, adverse events and all-cause mortality.
Quality assessment
We assessed the risk of bias of each fully published trial according to the Cochrane risk of bias tool. The main domains were checked, including random sequence generation (selection bias), allocation concealment (selection bias), blinding of participants and personnel (performance bias), blinding of outcome assessment (detection bias), incomplete outcome data (attrition bias), selective reporting (reporting bias), and other biases. The judgments were expressed as “low risk”, “high risk”, or “unclear risk” of bias. Any disagreements were resolved by discussion and consensus.
Statistical analysis
The data analysis was performed with Stata 12.0 (StataCorp, College Station, TX, USA). We used the random-effect model to conduct the meta-analysis and assessed heterogeneity with the I2 statistic and the fixed-effect model was used when the value of P>0.1. The weighted mean difference (WMD) and 95% confidence intervals (CIs) were calculated with continuous data. When the different measurements became variable, standardized mean difference (SMD) was used. We performed subgroup analysis to estimate the clinical efficacy of statins in trials enrolling patients with concomitant COPD and patients diagnosed with PAH. When assessment of the influences of individual studies on the pooled effects was necessary, sensitivity analysis was conducted by withdrawing trials one by one. P value less than 0.05 was considered statistical significance.
Results
Search results
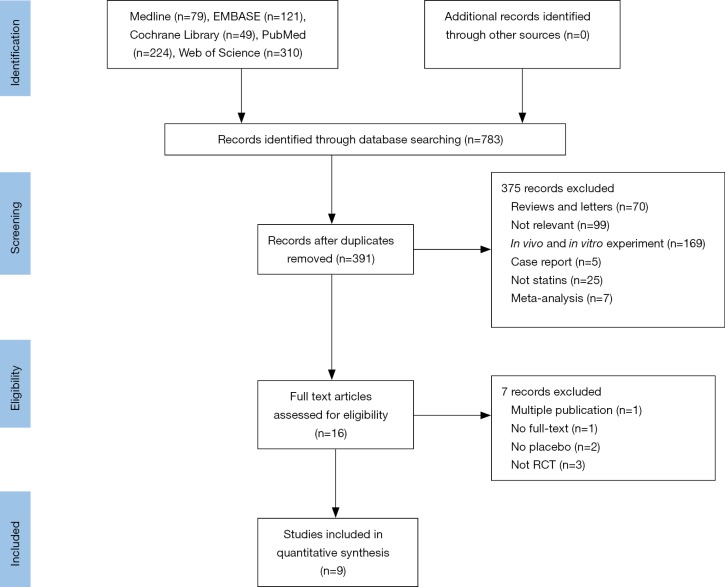
Our literature search found 783 potential relevant publications and 391 were removed because of duplication. Of the remains, 16 clinic trials comparing the treatment effects of statins with placebo in PH patients were identified. Ultimately, nine RCTs completely fulfilled the criteria for consideration and the data in which were extracted to further analysis (17-25). The search process is shown in Figure 1. All the selected studies were shown in English and published during 2008–2017. And publishing areas were as follows: Brazil, Taiwan, Germany, United States, China, Iran and India. The clinical features of included studies are shown in Table 1.
Figure 1.
PRISMA flowchart outlining the literature search process.
Table 1. Clinical features of included studies.
| Study | Year | Area | Study design | Age (statins/control) (years) | Patients (statins/control) | Control treatment | Disease | PH group | PAP (mmHg) | LDL | Gender (M/F) | Statins | Dose (mg/day) | Treatment time (months) | Follow-up time (months) |
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Barreto | 2008 | Brazil | Randomized, double-blind, placebo-controlled trial | 34.6±12.3/33.7±11.1 | 30/30 | Placebo | IPAH or PAH-CHD | 1 | 53±16 | NA | 24/36 | Rosuvastatin | 10 | 6 | 6 |
| Lee | 2009 | Taiwan (China) | Randomized, double-blind, placebo-controlled trial | 71±8/72±6 | 27/26 | Placebo | COPD + PH | 3 | >25 | 145±46/148±50 mg/dL | 39/14 | Pravastatin | 40 | 6 | 6 |
| Wilkins | 2010 | Germany | Randomized, double-blind, placebo-controlled trial | 43.2 [19–67]/49.1 [24–73] | 19/23 | Placebo | PAH | 1 | 55.8±10.3/55.7±12.5 | NA | 10/32 | Simvastatin | 80 | 6 | 12 |
| Kawut | 2011 | United States | Randomized, double-blind, placebo-controlled trial | 50.5±14.3/51.0±13.6 | 32/33 | Placebo | PAH | 1 | NA | 110±22/104±21 mg/dL | 9/56 | Simvastatin | 40 | 6 | 6 |
| Zeng | 2012 | China | Randomized, double-blind, placebo-controlled trial | 35±13/37±13 | 112/108 | Placebo | PAH or CTPH | 1 or 4 | 69±19/66±20 | 2.3±0.7/2.3±0.8 mmol/L | 76/144 | Atorvastatin | 20 | 6 | 6 |
| Liu | 2013 | China | Randomized, controlled trial | 66.2±7.4/64.9±8.2 | 33/35 | No statin | PHD | 3 | 52.7±8.1/51.7±7.9 | NA | 43/25 | Atorvastatin | 20 | 6 | 6 |
| Moosavi | 2013 | Iran | Randomized, triple-blind, parallel-group trial | 65±11/68±14 | 24/21 | Placebo | COPD + PH | 3 | >40 | 109±33/104±31 mg/dL | 28/17 | Atorvastatin | 40 | 6 | 6 |
| Chogtu | 2016 | India | Randomized, double-blind, placebo-controlled trial | 61.4±8.4/65.9±9.7 | 32/30 | Placebo | COPD + PH | 3 | 30< sPAP <75 | NA | NA | Rosuvastatin | 20 | 3 | 3 |
| Arian | 2017 | Iran | Randomized, double-blind, controlled trial | 65.8±11.5/63.7±7.6 | 21/21 | No statin | COPD + PH | 3 | 47.9±15.4/49.2±16.3 | 133.4±27.8/111.1±27.3 mg/dL | 11/23 | Atorvastatin | 40 | 6 | 6 |
PH, pulmonary hypertension; PAP, pulmonary arterial pressure; LDL, low-density lipoprotein; M, male; F, female; IPAH, idiopathic pulmonary arterial hypertension; PAH, pulmonary arterial hypertension; CHD, congenital heart disease; NA, not available; COPD, chronic obstructive pulmonary disease; CTPH, chronic thromboembolic pulmonary hypertension; PHD, pulmonary heart disease; sPAP, systolic pulmonary arterial pressure.
Patients
A total of 657 PH patients were recruited to these 9 trials; 330 were randomized to the statin group and 327 to the control group. Mean age ranged from 33 to 72 years. Study duration ranged from 12 weeks to 6 months. The subjects were permitted to receive basic treatment such as diuretics, long-acting muscarinic antagonists, long-acting β2-agonists, oxygen supplement and anticoagulant therapy. Four types of statins were investigated: atorvastatin, pravastatin, rosuvastatin and simvastatin.
Trial quality
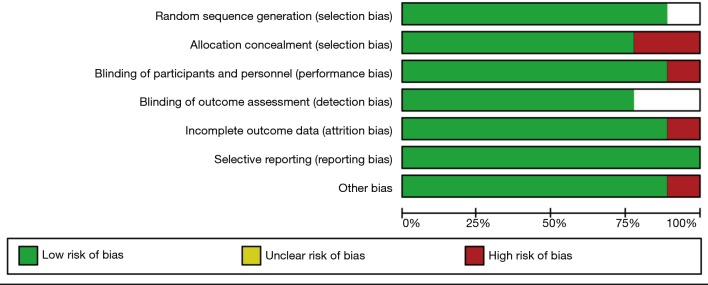
The quality of the included studies was generally high. Methods of randomization and allocation concealment were adequately addressed in the majority of trials. Among the 9 included studies, 7 were double-blind, 1 was triple-blind and the remaining 1 was unclear. The blinding of participants and outcome assessors was reported in all studies, reflecting an overall low risk of detection bias. A summary of the “Risk of bias” assessment is presented in Figure 2.
Figure 2.
Risk of bias graph.
Clinic outcomes
PAP
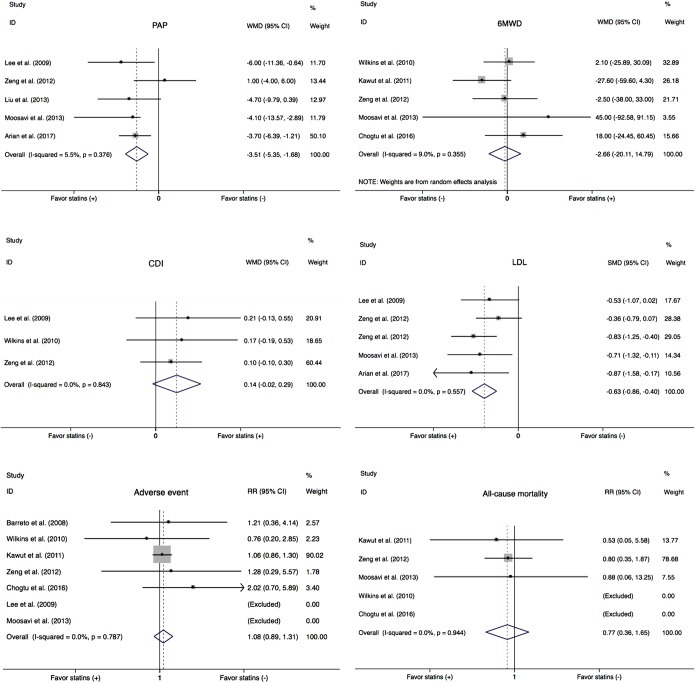
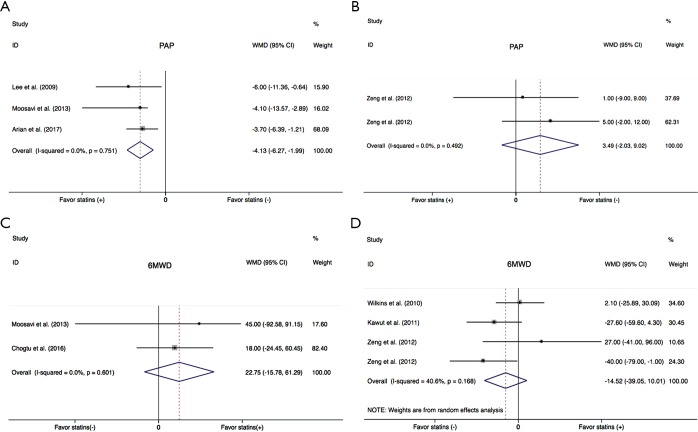
Data on PAP were available in five studies (18,21-23,25). Overall there was a significant decrease of PAP in PH patients treated with statins compared with placebo group (WMD =−3.51, 95% CI: −5.35 to −1.68; P<0.001) (Figure 3). There was a small heterogeneity among studies (I2=5.5%). In the subgroup analysis, there was a significant improvement of PAP in COPD patients (WMD =−4.13, 95% CI: −6.27 to −1.99; P<0.001) (Figure 4A), but not in PAH (WMD =3.49, 95% CI: −2.03 to 9.02; P=0.215) (Figure 4B).
Figure 3.
Summary effects of statins in PH. PAP, pulmonary arterial pressure; WMD, weighted mean difference; CI, confidence interval; 6MWD, 6-minute walking distance; CDI, cardiac index; LDL, low-density lipoprotein; SMD, standardized mean difference; RR, risk ratio; PH, pulmonary hypertension.
Figure 4.
Summary effects of statins on PAP (A,B) and 6-minute walk distance (C,D) in COPD (A,C) and PAH (B,D). PAP, pulmonary arterial pressure; WMD, weighted mean difference; CI, confidence interval; 6MWD, 6-minute walking distance; PAH, pulmonary arterial hypertension; COPD, chronic obstructive pulmonary disease.
Exercise capacity
Most of included studies reported 6MWD, an indicator of exercise capacity. In this study, 6MWD were extracted from five trials (19-21,23,24). There was no significant benefit in 6MWD with statins in PH subjects (WMD =−2.66, 95% CI: −20.11 to 14.79; P=0.765) (Figure 3). Statistical heterogeneity was small (I2=9.0%). In subgroup analysis, statins neither improved 6MWD in the COPD (WMD =22.75, 95% CI: −15.78 to 61.29; P=0.247) (Figure 4C), nor in PAH (WMD =−14.52, 95% CI −39.05 to 10.01; P=0.246) (Figure 4D).
Cardiac index
Only three trails evaluated the effect of statin therapy on CDI (18,19,21). There was no improvement with statins, compared to placebo (WMD =0.14; 95% CI: −0.02 to 0.29; P=0.086) (Figure 3), the statistical heterogeneity was not significant (I2=0%).
Serum LDL
The effect of statins on serum LDL was determined in four studies. As expected, there was a significant decrease in the level of LDL (SMD =−0.63, 95% CI: −0.86 to −0.40; P<0.001) (Figure 3). No observed heterogeneity among studies (I2=0%).
Adverse events
A total of 7 eligible trials including 550 individuals reported adverse events (17-21,23,24). In this meta-analysis, the adverse events contained clinical deterioration, hemoptysis, syncope, skeletal muscle pain, liver function abnormalities, bleeding, insomnia, etc. There was no obvious discrepancy in adverse events between the placebo and statin groups [risk ratio (RR) =1.08, 95% CI: 0.89 to 1.31; P=0.444] (Figure 3) and no significant heterogeneity among the studies (I2=0.0%).
All-cause mortality
Five eligible trials reported all-cause mortality (19-21,23,24). There were 11 deaths among 219 patients in the statin groups and 14 deaths among 215 patients in the control groups. There was no statistically significant difference in all-cause mortality (RR =0.77, 95% CI: 0.36 to 1.65; P=0.514) (Figure 3). The heterogeneity among trials was not significant (I2=0.0%).
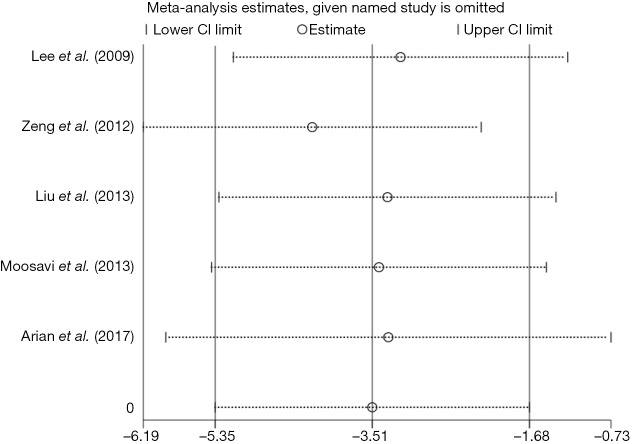
Sensitivity analysis
The leave-one-out sensitivity analysis for the effect of statins on PAP in PH patients that was performed by using both random and fixed effect models, and practically the same outcomes were found. No pooled results or heterogeneity of this meta-analysis was significantly altered (Figure 5).
Figure 5.
The leave-one-out sensitivity analysis for the effect of statins on PAP in PH patients. CI, confidence interval; PAP, pulmonary arterial pressure; PH, pulmonary hypertension.
Discussion
This systematic review and meta-analysis assessed the efficacy and safety of statins in PH with nine RCTs, which yielded the following outcomes. First, statin therapy could significantly reduce PAP, as well as LDL level in patients with PH, whereas 6MWD and CDI showed no statistical amelioration. Second, there were nonsignificant trends toward rise of the incidence rate of adverse events and all-cause mortality in a short term. Third, according to the subgroup analysis, there was an obvious reduction of PAP in PH due to COPD, but not PAH, treated with statins.
PH, means an abnormal augment of PAP due to elevated pulmonary vascular resistance, whose main vascular changes are vasoconstriction, thrombosis and vascular remodeling associated with smooth-muscle cell and endothelial-cell proliferation (4). Since the pleiotropic properties with regard to cardiovascular system of statin drugs, a couple of researches have paid attentions to statin therapy in PH. The outcomes concerning PAP in this study were in agreement with that of several studies applying animal models (26-28). Carlin et al. revealed that some sorts of statins such as Fluvastatin could reduce PAP in PH rats (26). The finding of Li et al. showed that rosuvastatin administration could ameliorate mean PAP in Monocrotaline-induced PAH in rats, which might result from the regulation of Rho-associated coiled-coil-containing kinase 1 (ROCK-1), proliferating cell nuclear antigen (PCNA), and endothelial nitric oxide synthase (eNOS) expression (27). That is to say, the present outcomes confirm and extend the previous outcomes regarding the favorable impacts of statins on PAP. Also, the potential mechanisms deserve attention, in the included RCTs of this meta-analysis, Lee et al. inferred that statins inhibiting the generation of endothelin 1 (ET-1) was one of the possible reasons, as urinary ET-1 levels were significantly reduced in pravastatin-treated patients (18). Combined with analyses of antecedent researches, it is reasonable to infer that statins decrease PAP by prompting apoptosis, an important physiological process to reverse pulmonary artery remodeling, inhibiting smooth-muscle cell proliferation, promoting secretion of vasodilators such as prostacyclin and nitric oxide, inhibiting endothelin generation and anti-inflammatory, etc. (29-32).
However, not all the related researches favor statins’ improving PAP, making us consider possible reasons. In the RCT conducted by Moosavi et al., Atorvastatin therapy did not notably ameliorate the systolic PAH (SPAH) in PH and they got an idea that, small sample size could be a main account for it (23). Given the discordant results, we can make a speculation that results might be affected by the sorts and dosages of statins, clinic types of PH, background diseases, other drugs, duration and sample size, etc. For instance, previous study showed that pravastatin, instead of simvastatin, slowed down the progression of PH (33). Moreover, Carlin et al. reported that some sorts of statins like fluvastatin may be more effective in PAP (26). Statins differ in metabolism, pharmacological lipophilicity, structure and solubility, which may give rise to different efficacy in PH. In the five included RCTs concerning PAP, four applying atorvastatin showed improvements in PAP except Zeng et al., who used atorvastatin at 10 mg/day whereas others used at 20 or 40 mg per day. This might indicate that atorvastatin plays a protective part in PH, and the dosage of significant effects should not be lower than 20 mg (18,21-23). In addition, there would be biases because of PH patients’ older ages and background diseases. This study excluded background diseases, since severe gastrointestinal disorders and liver dysfunction could undermine the absorption and metabolism of drugs. Although it was suggested that patients with COPD at elevated cardiovascular risk might be the appropriate subgroup for statins (34,35), potential impacts of cardiovascular diseases remain controversial. Of course, drug interaction cannot be neglected, such as the interplay between statins and clopidogrel, being used in cardiovascular diseases frequently, consequently promoting platelet aggregation, which does harm to cardiovascular system, as well as weakens beneficial effects of statins. Potential influencing factors above could also explain the unexpected outcomes of 6MWD, which correlates with markers of disease severity in PH, such as pulmonary haemodynamics (36). According to the pathogenesis, the improvement of 6MWD may be later than that of PAP, indicating a longer duration is needed for the change. And another plausible explanation for it was that 6MWD depends largely on motivation and encouragement, making it difficult to standardize (37). On basis of information above, a well-conducted study is necessary.
PAH and PH due to COPD, as two clinic subtypes of PH, differ in primary causes and pathogenesis. Previous studies have indicated the effects of statin therapy in PAH and COPD were inconsistent (10,17,24,38). In our meta-analysis, PH due to COPD patients appeared to benefit more than the PAH from statins. There were possible reasons. First, ever-increasing researches have proved that COPD is a chronic systemic inflammation (35,39). Inflammation and hypoxia impair pulmonary blood vessels, ultimately leading to PH. It is noticeable, COPD patients conspicuous changes in pulmonary vascular remodeling (40). Moreover, anti-inflammation and maintaining endothelial cell function properties make statins favourable to PH due to COPD (5,7). Actually, statin therapy has played a major role in the prevention and reversal of PH secondary to hypoxia in studies. Second, PAH as the group 1 of PH, which comprises various causes of diseases, such as idiopathic (IPAH), heritable forms, congenital systemic-to-pulmonary shunts and other conditions (20). It is worth nothing that, the nine included RCTs, only one (Zeng et al.) reported the change value of PAP in whole PH patients and the subgroups of PAP in PAH patients (connective tissue disorder and congenital heart disease), combined with the research of Rysz-Górzynska et al. showing that statins treatment had nonsignificant change of PAP among PAH patients (11). It is possible that these mixed diseases included in PAH group limit the overall efficacy of statins. However, the direct pulmonary vascular effect of statin in PAH is unclear. Therefore, it is advisable to explore the causes of PAH simultaneously. On basis of the ever-growing incidence rate of COPD, it would be promising to get more efficient and valuable results if future studies recruit more PH combined with COPD patients to determine the role of statin therapy.
In terms of safety, common statin-induced adverse effects containing liver impairing, bleeding, irregular menstrual cycles, abdominal distention and their likes (10). Several rare but serious, such as rhabdomyolysis, new-onset diabetes, and possibly acute kidney injury make it necessary to determine the safety of statin therapy in PH concerning does, type and time (41). Nakano et al. suggested statin therapy was relatively safe among PH patients, which was similar to this study’s (42). Further, there was a research revealed statins’ significant chemopreventive effect to reduce cancer risk in COPD (42,43). However, in view of the short-term duration our finding, while PH requiring long-term drug therapy, we should consider the results dialectically. Liang et al. made a subgroup analysis showing that fluvastatin had a significant impact on liver, and the incidence rate of adverse events increased when the dose of statins more than 40 mg. They also indicated side effects associated with statin therapy were more obvious in the first 2 years (44). It is worth nothing that this study only assessed the liver function, with the recruited patients were not all suffering PH. Nonetheless, future researches can still base on it, further optimize the inclusion and exclusion criteria, increase indicators to better assess the safety of statins, especially long-term safety.
Limitation
This systematic review and meta-analysis has limitations. First, the overall scale of the contributing studies was small and the confidence intervals were wide, which are limited by the source data. Second, several important specific outcomes of COPD and PAH including adverse events and all-cause mortality could not be analyzed in subgroups, due to lack of reported outcomes in trials. Third, despite generally similar demographic characteristics and minimal evidence of heterogeneity among these RCTs, some confounding factors such as different types and doses of statins, which might have caused potential biases. Fourth, our study only includes three subtypes of PH patients (types 1, 3 and 4), therefore the outcomes may not suitable for all groups of the PH patients. Future clinic trials with rigorously predefined patient inclusion criteria are warranted to better research the potential role of statin therapy in PH.
Conclusions
This systematic review and meta-analysis showed that with routine treatment, adding statins could be beneficial for PAP, but not 6MWD and CDI in PH. And during short-term therapy, adverse events and mortality were not apparent, suggesting a relative safety. It is advisable that more future researches should focus on the efficacy and safety of statin therapy in different subtypes of PH.
Acknowledgments
Funding: This study was supported in part by grant 18PJ410 from the Health and Family Planning Commission of Sichuan Province and grant 2014SZ0220 from the Science and Technology Support Program of Sichuan Province and grant 2016YFC0901100 from the National Key Research and Development Program of China.
Ethical Statement: The authors are accountable for all aspects of the work in ensuring that questions related to the accuracy or integrity of any part of the work are appropriately investigated and resolved.
Footnotes
Conflicts of Interest: The authors declare they have no conflict of interests.
References
- 1."2015 ESC/ERS Guidelines for the diagnosis and treatment of pulmonary hypertension. The Joint Task Force for the Diagnosis and Treatment of Pulmonary Hypertension of the European Society of Cardiology (ESC) and the European Respiratory Society (ERS)." Nazzareno Galie, Marc Humbert, Jean-Luc Vachiery, Simon Gibbs, Irene Lang, Adam Torbicki, Gerald Simonneau, Andrew Peacock, Anton Vonk Noordegraaf, Maurice Beghetti, Ardeschir Ghofrani, Miguel Angel Gomez Sanchez, Georg Hansmann, Walter Klepetko, Patrizio Lancellotti, Marco Matucci, Theresa McDonagh, Luc A. Pierard, Pedro T. Trindade, Maurizio Zompatori and Marius Hoeper. Eur Respir J 2015; 46: 903-975. Eur Respir J 2015;46:1855-6. [DOI] [PubMed] [Google Scholar]
- 2.Kovacs G, Dumitrescu D, Barner A, et al. Definition, clinical classification and initial diagnosis of pulmonary hypertension: Updated recommendations from the Cologne Consensus Conference 2018. Int J Cardiol 2018;272S:11-9. 10.1016/j.ijcard.2018.08.083 [DOI] [PubMed] [Google Scholar]
- 3.Sharma M, Pinnamaneni S, Aronow WS, et al. Existing drugs and agents under investigation for pulmonary arterial hypertension. Cardiol Rev 2014;22:297-305. 10.1097/CRD.0000000000000035 [DOI] [PubMed] [Google Scholar]
- 4.Hoeper MM, Ghofrani HA, Grunig E, et al. Pulmonary Hypertension. Dtsch Arztebl Int 2017;114:73-84. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Bradbury P, Traini D, Ammit AJ, et al. Repurposing of statins via inhalation to treat lung inflammatory conditions. Adv Drug Deliv Rev 2018;133:93-106. 10.1016/j.addr.2018.06.005 [DOI] [PubMed] [Google Scholar]
- 6.Wilson SH, Herrmann J, Lerman LO, et al. Simvastatin preserves the structure of coronary adventitial vasa vasorum in experimental hypercholesterolemia independent of lipid lowering. Circulation 2002;105:415-8. 10.1161/hc0402.104119 [DOI] [PubMed] [Google Scholar]
- 7.Kwak B, Mulhaupt F, Myit S, et al. Statins as a newly recognized type of immunomodulator. Nat Med 2000;6:1399-402. 10.1038/82219 [DOI] [PubMed] [Google Scholar]
- 8.Ren J, Liu W, Li GC, et al. Atorvastatin Attenuates Myocardial Hypertrophy Induced by Chronic Intermittent Hypoxia In Vitro Partly through miR-31/PKC epsilon Pathway. Curr Med Sci 2018;38:405-12. 10.1007/s11596-018-1893-2 [DOI] [PubMed] [Google Scholar]
- 9.Kao PN. Simvastatin treatment of pulmonary hypertension: an observational case series. Chest 2005;127:1446-52. [DOI] [PubMed] [Google Scholar]
- 10.Anand V, Garg S, Duval S, et al. A systematic review and meta-analysis of trials using statins in pulmonary arterial hypertension. Pulm Circ 2016;6:295-301. 10.1086/687304 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Rysz-Górzynska M, Gluba-Brzózka A, Sahebkar A, et al. Efficacy of Statin Therapy in Pulmonary Arterial Hypertension: A Systematic Review and Meta-Analysis. Sci Rep 2016;6:30060. 10.1038/srep30060 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Wang L, Qu M, Chen Y, et al. Statins Have No Additional Benefit for Pulmonary Hypertension: A Meta-Analysis of Randomized Controlled Trials. PLoS One 2016;11:e0168101. 10.1371/journal.pone.0168101 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Zhang Y, Zeng W, Cheng S, et al. Efficacy and Safety of Statins for Pulmonary Hypertension: A Meta-Analysis of Randomised Controlled Trials. Heart Lung Circ 2017;26:425-32. 10.1016/j.hlc.2016.08.005 [DOI] [PubMed] [Google Scholar]
- 14.Holzhauser L, Hovnanians N, Eshtehardi P, et al. Statin therapy improves survival in patients with severe pulmonary hypertension: a propensity score matching study. Heart Vessels 2017;32:969-76. 10.1007/s00380-017-0957-8 [DOI] [PubMed] [Google Scholar]
- 15.King WT, Day RW. Treatment of pediatric pulmonary hypertension with simvastatin: an observational study. Pediatr Pulmonol 2011;46:261-5. 10.1002/ppul.21361 [DOI] [PubMed] [Google Scholar]
- 16.Wang P, Yang J, Yang Y, et al. Effect of azithromycin in combination with simvastatin in the treatment of chronic obstructive pulmonary disease complicated by pulmonary arterial hypertension. Pak J Med Sci 2017;33:260-4. 10.12669/pjms.332.11717 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Barreto AC, Maeda NY, Soares RP, et al. Rosuvastatin and vascular dysfunction markers in pulmonary arterial hypertension: a placebo-controlled study. Braz J Med Biol Res 2008;41:657-63. 10.1590/S0100-879X2008000800003 [DOI] [PubMed] [Google Scholar]
- 18.Lee TM, Chen CC, Shen HN, et al. Effects of pravastatin on functional capacity in patients with chronic obstructive pulmonary disease and pulmonary hypertension. Clin Sci (Lond) 2009;116:497-505. 10.1042/CS20080241 [DOI] [PubMed] [Google Scholar]
- 19.Wilkins MR, Ali O, Bradlow W, et al. Simvastatin as a treatment for pulmonary hypertension trial. Am J Respir Crit Care Med 2010;181:1106-13. 10.1164/rccm.2009111-699OC [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Kawut SM, Bagiella E, Lederer DJ, et al. Randomized clinical trial of aspirin and simvastatin for pulmonary arterial hypertension: ASA-STAT. Circulation 2011;123:2985-93. 10.1161/CIRCULATIONAHA.110.015693 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Zeng WJ, Xiong CM, Zhao L, et al. Atorvastatin in pulmonary arterial hypertension (APATH) study. Eur Respir J 2012;40:67-74. 10.1183/09031936.00149011 [DOI] [PubMed] [Google Scholar]
- 22.Liu HF, Qi XW, Ma LL, et al. Atorvastatin improves endothelial progenitor cell function and reduces pulmonary hypertension in patients with chronic pulmonary heart disease. Exp Clin Cardiol 2013;18:e40-3. [PMC free article] [PubMed] [Google Scholar]
- 23.Moosavi SA, Raji H, Faghankhani M, et al. Evaluation of the Effects of Atorvastatin on the Treatment of Secondary Pulmonary Hypertension due to Chronic Obstructive Pulmonary Diseases: A Randomized Controlled Trial. Iran Red Crescent Med J 2013;15:649-54. 10.5812/ircmj.8267 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Chogtu B, Kuriachan S, Magazine R, et al. A prospective, randomized study: Evaluation of the effect of rosuvastatin in patients with chronic obstructive pulmonary disease and pulmonary hypertension. Indian J Pharmacol 2016;48:503-8. 10.4103/0253-7613.190721 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Arian A, Moghadam SG, Kazemi T, et al. The Effects of Statins on Pulmonary Artery Pressure in Patients with Chronic Obstructive Pulmonary Disease: A Randomized Controlled Trial. J Res Pharm Pract 2017;6:27-30. 10.4103/2279-042X.200985 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Carlin CM, Celnik DF, Pak O, et al. Low-dose fluvastatin reverses the hypoxic pulmonary adventitial fibroblast phenotype in experimental pulmonary hypertension. Am J Respir Cell Mol Biol 2012;47:140-8. 10.1165/rcmb.2011-0411OC [DOI] [PubMed] [Google Scholar]
- 27.Li XL, Guan RJ, Li JJ. Attenuation of monocrotaline-induced pulmonary arterial hypertension in rats by rosuvastatin. J Cardiovasc Pharmacol 2012;60:219-26. 10.1097/FJC.0b013e31825cce63 [DOI] [PubMed] [Google Scholar]
- 28.Satoh M, Satoh A. 3-Hydroxy-3-methylglutaryl (HMG)-COA reductase inhibitors and phosphodiesterase type V inhibitors attenuate right ventricular pressure and remodeling in a rat model of pulmonary hypertension. J Pharm Pharm Sci 2009;11:118s-30s. 10.18433/J34K5Z [DOI] [PubMed] [Google Scholar]
- 29.Gurbanov E, Shiliang X. The key role of apoptosis in the pathogenesis and treatment of pulmonary hypertension. Eur J Cardiothorac Surg 2006;30:499-507. 10.1016/j.ejcts.2006.05.026 [DOI] [PubMed] [Google Scholar]
- 30.Taraseviciene-Stewart L, Scerbavicius R, Choe KH, et al. Simvastatin causes endothelial cell apoptosis and attenuates severe pulmonary hypertension. Am J Physiol Lung Cell Mol Physiol 2006;291:L668-76. 10.1152/ajplung.00491.2005 [DOI] [PubMed] [Google Scholar]
- 31.Guerard P, Rakotoniaina Z, Goirand F, et al. The HMG-CoA reductase inhibitor, pravastatin, prevents the development of monocrotaline-induced pulmonary hypertension in the rat through reduction of endothelial cell apoptosis and overexpression of eNOS. Naunyn Schmiedebergs Arch Pharmacol 2006;373:401-14. 10.1007/s00210-006-0082-1 [DOI] [PubMed] [Google Scholar]
- 32.Ali OF, Growcott EJ, Butrous GS, Wharton J. Pleiotropic effects of statins in distal human pulmonary artery smooth muscle cells. Respir Res 2011;12:137. 10.1186/1465-9921-12-137 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Rakotoniaina Z, Guerard P, Lirussi F, et al. The protective effect of HMG-CoA reductase inhibitors against monocrotaline-induced pulmonary hypertension in the rat might not be a class effect: comparison of pravastatin and atorvastatin. Naunyn Schmiedebergs Arch Pharmacol 2006;374:195-206. 10.1007/s00210-006-0112-z [DOI] [PubMed] [Google Scholar]
- 34.Young RP, Hopkins RJ, Agusti A. Statins as adjunct therapy in COPD: how do we cope after STATCOPE? Thorax 2014;69:891-4. 10.1136/thoraxjnl-2014-205814 [DOI] [PubMed] [Google Scholar]
- 35.Zhang W, Zhang Y, Li CW, et al. Effect of Statins on COPD A Meta-Analysis of Randomized Controlled Trials. Chest 2017;152:1159-68. 10.1016/j.chest.2017.08.015 [DOI] [PubMed] [Google Scholar]
- 36.Lee WT, Peacock AJ, Johnson MK. The role of per cent predicted 6-min walk distance in pulmonary arterial hypertension. Eur Respir J 2010;36:1294-301. 10.1183/09031936.00155009 [DOI] [PubMed] [Google Scholar]
- 37.Frost AE, Langleben D, Oudiz R, et al. The 6-min walk test (6MW) as an efficacy endpoint in pulmonary arterial hypertension clinical trials: demonstration of a ceiling effect. Vascul Pharmacol 2005;43:36-9. 10.1016/j.vph.2005.03.003 [DOI] [PubMed] [Google Scholar]
- 38.Reed RM, Iacono A, DeFilippis A, et al. Statin therapy is associated with decreased pulmonary vascular pressures in severe COPD. COPD 2011;8:96-102. 10.3109/15412555.2011.558545 [DOI] [PubMed] [Google Scholar]
- 39.Janda S, Park K, FitzGerald JM, et al. Statins in COPD: a systematic review. Chest 2009;136:734-43. 10.1378/chest.09-0194 [DOI] [PubMed] [Google Scholar]
- 40.Barberà JA. Mechanisms of development of chronic obstructive pulmonary disease-associated pulmonary hypertension. Pulm Circ 2013;3:160-4. 10.4103/2045-8932.109949 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Li DQ, Kim RB, McArthur E, et al. Statin Safety in Chinese: A Population-Based Study of Older Adults. PLoS One 2016;11:e0150990. 10.1371/journal.pone.0150990 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Nakano K, Matoba T, Koga JI, et al. Safety, Tolerability, and Pharmacokinetics of NK-104-NP A Multicenter, Randomized, Placebo-Controlled Phase I Investigator-Initiated Trial for Intravenous Administration of Pitavastatin-Loaded PLGA Nanoparticles (NK-104-NP) in Healthy Japanese Male Subjects. Int Heart J 2018;59:1015-25. 10.1536/ihj.17-555 [DOI] [PubMed] [Google Scholar]
- 43.Chen CC, Hsu YP, Liu JC, et al. Statins Dose-Dependently Exert Significant Chemopreventive Effects Against Various Cancers in Chronic Obstructive Pulmonary Disease Patients: A Population-Based Cohort Study. J Cancer 2016;7:1892-900. 10.7150/jca.15779 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Liang X, He Q, Zhao Q. Effect of Stains on LDL Reduction and Liver Safety: A Systematic Review and Meta-Analysis. Biomed Res Int 2018;2018:7092414. 10.1155/2018/7092414 [DOI] [PMC free article] [PubMed] [Google Scholar]