Abstract
Endocardial LAAO has been increasingly utilized in atrial fibrillation (AF) patients who are not suitable for long term oral anticoagulation. While overall procedural complications have decreased, rare complications like contiguous vessel and valve injury may be more frequently seen in the future with increase in the procedure volume. We performed a systematic search using predefined terms which reviewed all cases published in literature of contiguous vessel (pulmonary artery, pulmonary vein and left circumflex artery) and mitral valve injury caused by LAAO devices. Our results showed that Amplatzer Cardiac Plug (ACP) and Amplatzer Amulet devices were the most commonly used devices. Pulmonary artery perforation was the most commonly seen collateral vessel injury associated with LAAO. Close proximity of left atrial appendage to pulmonary artery was noted in all cases of pulmonary artery injury. Pulmonary artery injury commonly manifests as pericardial tamponade with hemodynamic collapse and is often fatal. Most common denominator of all the reviewed cases was the presence of an oversized LAAO device. In conclusion, collateral vessels and valve injury can be seen after LAAO mostly with double lobe devices such as ACP or Amulet. Increased awareness by the operators along with proper imaging and investigations could potentially mitigate such rare complications associated with LAAO.
Keywords: Left atrial appendage occlusion, Amplatzer Cardiac Plug, Amplatzer Amulet, Collateral injury, Complications
Introduction
Left atrial appendage occlusion (LAAO) has emerged as an appealing alternative to stroke prophylaxis in patients with non-valvular atrial fibrillation (AF) who are poor candidates for anticoagulation [1]. While there are several available devices for LAAO, the Watchman (Boston Scientific Corp, Minneapolis, MN) and the Amulet (Abbott Medical, Chicago, IL) are the most commonly implanted devices for catheter-based endocardial LAAO, with a greater percentage of the Amulet device being used within Europe compared to non-European geographies [2]. With the prevalence of non-valvular AF estimated to increase across the globe [3], utilization of LAAO is likely to increase in the future. Since the sharp rise in post market release complications of Watchman, there has been a steady decline in reported rate of common procedure-related complications [4]. A few rare complications of LAAO are linked to the close anatomical proximity of the left atrial appendage (LAA) to adjacent vessels and valve in the heart. LAA lies close to the pulmonary artery anterosuperiorly and left superior pulmonary vein posteriorly, mitral valve inferiorly and the LAA covers an area over the left atrioventricular groove which contains the left circumflex artery [5]. Although rare, contiguous vessel or valve injury with LAAO devices is more likely to be seen in the future with an increase in the utilization of these procedures. In this paper, we aim to review all contiguous vessel and valve injuries associated with LAAO that have been published to date and also aim to understand the pathophysiology of these complications.
Methods
We searched PubMed, EMBASE, CINAHL and Google Scholar from January 1, 2000 till March 15, 2019 using the following key words: “left atrial appendage closure,” “Watchman,” “Amplatzer Cardiac plug,” “Amulet,” “pulmonary vein,” “pulmonary artery,” “left circumflex artery” and “mitral valve”. The goal of this systematic review was to collect all the cases of collateral vessel and valve injuries that occur as complications from LAAO devices. The flow chart of study selection is elucidated in [Figure 1].
Figure 1. Flow chart of study selection.

Results
Comprehensive search revealed 12 publications from Asia, Europe, and Australia with description of 13 cases of contiguous vessels and mitral valve injury after LAAO [6-17]. The average age of the patients was 71.4 ± 8.2 years; 92% were Caucasians. Majority had persistent AF (62%). The most common type of injury after LAAO procedure was pulmonary artery injury. Most cases of pulmonary artery (PA) perforation occurred within 24 hours (62%) and were caused by Amplatzer Cardiac Plug and Amplatzer Amulet devices (92%). A case of delayed presentation after 6 months was also described where chronic pressure from the Amulet was found to kink the PA leading to occlusion of vasa vasorum and ischemic necrosis and perforation [13]. Stabilizing hooks of ACP/Amulet or metallic struts of Watchman were seen to cause the perforation of LAA and PA. In all cases, close proximity of LAA with PA was noted. PA perforation had a high mortality rate of 40%. The common presentation was sudden hemodynamic collapse with evidence of pericardial tamponade [Figure 2]. Less common contiguous vessel injuries included left inferior pulmonary vein compression due to atrial disc portion of ACP (10%) [6] as well as left circumflex coronary artery (LCX) compression by the lobe portion of the oversized ACP (10%) causing ST-segment elevation [9]. Pulmonary vein compression by ACP was diagnosed during a follow-up radiofrequency pulmonary vein isolation by low impedance in the ridge between left inferior pulmonary vein and LAA suggesting catheter contact with metal device. In the case of LCX compression, retrieval and repositioning of the device resulted in disappearance of ST elevation. There were three cases of mitral valve impingement and all were due to impingement of mitral leaflet by the outer disc of LAAO devices [15-17]. While two cases manifested as asymptomatic mitral regurgitation detected by imaging [15,17], one patient had recurrent syncope from possible dynamic obstruction of the valve by ACP [16]. The case of recurrent syncope required surgical removal of the device and LAA resection on the 4th post-operative day. Removal and reimplantation of a downsized device was required in the other case [17] while no information on the management was reported regarding the last case [15]. Detailed description of these cases is present in [Table 1].
Figure 2. CT scan demonstrating contrast extravasation from main pulmonary artery with pericardial tamponade.
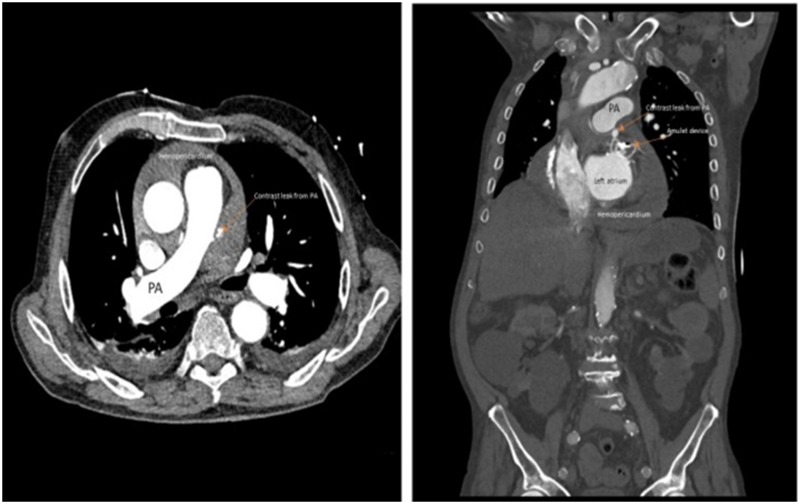
Table 1. Study and patient characteristics .
*all were Caucasian, yr=year,parox=paroxysmal,persis=persistent,LAA=left atrial appendage LAAO=left atrial appendage occlusion, ACP=Amplatzer cardiac plug,mm=millimeter,GI=gastrointestinal, PCI=percutaneous coronary intervention, MI=myocardial infarction, PEA=pulseless electrical activity, STEMI=ST elevation myocardial infarction, LZ=landing zone,MPA=main pulmonary artery,LIPV=left inferior pulmonary vein, PVI=pulmonary vein isolation, MR=mitral regurgitation, STE=ST elevation
| Study | Country | Age (yr)* | Sex | AF type | LAAO type | LAAO size (mm) | CHA2DS2VASC | Reason for LAAO | Implantation | Diagnosed | Presenting Symptoms/Signs | LAA characteristics | Injury | LAA and vessel relation | Management | Outcome |
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Pulmonary artery Injury | ||||||||||||||||
| Scislo et al 2018 (1st case) | Poland | 67 | F | Parox | Amulet | 25 | 9 | GI and intracranial bleeding | No issue | 17 hrs post procedure | Chest pain, dyspnea, hemodynamic collapse | Winsock type, LZ-20mm | 3mm posterolateral tear to MPA by anchoring hook | 2mm groove between MPA and LAA | Thoracotomy and repair | Discharged alive |
| Scislo et al 2018 (2nd case) | Poland | 62 | M | Parox | Amulet | 28 | 3 | GI bleeding | No issue | 3hrs post-procedure | Cardiac tamponade | Winsock type, LZ 23mm | 3mm posterolateral tear of MPA by anchoring hook | No groove between MPA and LAA | Thoracotomy and repair | Discharged alive |
| Wang et al 2018 | Australia | 87 | M | Persis | Amulet | 31 | 6 | Hemorrhagic stroke | No issue | 6months post-procedure | Cardiac tamponade and collapse | LZ 25mm | 2mm posterolateral tear from chronic pressure ofanchoring hook without erosion of LAA | - | Thoracotomy and repair | Discharged alive |
| Suwalski et al 2016 | Poland | 66 | M | Persis | ACP | 22 | - | Intracranial hemorrhage | No issue | 17 days post-procedure | Cardiac Tamponade | LAA origin 18mm, depth 24mm | 2mm lateral surface tear by anchoring hook | - | Thoracotomy and repair | Discharged alive |
| Bianchi et al 2013 | Italy | 76 | M | Persis | ACP | 22 | 3 | Intracranial hemorrhage | No Issue | 3hrs post procedure | Cardiac tamponade and collapse | - | 2mm tear by anchoring hook | - | Thoracotomy and repair | Discharged alive |
| Sepahpour et al 2013 | Australia | 72 | F | Parox | Watchman | 24 | 6 | Complicated PCI and need for lifelong dual antiplatelet | Transient inf STEMI, no apparent reason and resolved by reimplantation with 2nddevice | 16 days post procedure | Shock, PEA, could not be resuscitated | LAA orifice diameter 17mm, depth 35mm | 10mm tear on superior and left aspect of MPA by a metallic strut of Watchman | - | Died | Died |
| Zwirner et al 2016 | Germany | 71 | F | Persis | Amulet | - | - | Traumatic subdural hemorrhage | No issue | 8hrs post-procedure | Patient was found pulseless, failed resuscitation | - | 2mm tear on the pulmonary artery by a hook of amulet with punctiform tear of LAA | - | Died | Died |
| Hanazawa et al 2014 | Germany | 75 | F | Parox | ACP | 24 | 5 | Subdural hematoma | No issue | 24hrs post-procedure | Hypotensive, cardiac tamponade | LAA orifice diameter 18mm, depth 27mm | Perforation of LAA and leading to erosion of the bottom of the pulmonary artery | 3D reconstruction of cardiac CT showed one lobe of LAA touched the inferior pulmonary artery | Died | Died |
| Pulmonary Vein Compression | ||||||||||||||||
| Ayati et al 2014 | Germany | 76 | F | Persis | ACP | - | - | Risk of bleeding | No issue | 3 months post-procedure | Worsening exertional dyspnea | - | LIPV compression diagnosed on CT and during PVI, mapping at the ridge between LIPV and LAA showed decreased impedance suggesting catheter contact with metal device | LIPV was compressed with atrial part of ACP | Successful PVI, ACP was left in place | Discharged alive |
| Left Circumflex artery Compression | ||||||||||||||||
| Katona et al 2015 | Hungary | 59 | M | Persis | ACP | 23 | - | Recurrent head contusions | ST elevation in inferior leads | During procedure | ST elevation | LAA with huge ostium | After positioning of ACP inferior lead showing ST elevation | Coronary angiogram showing compression of proximal circumflex and device was seen sitting superficially. | After removal and repositioning of device STE disappeared. | Discharged |
| Mitral valve impingement | ||||||||||||||||
| Berrebi et al 2017 | France | 84 | F | Pesis | Amulet | 28 | 5 | Hemorrhagic shock due to GI bleeding | No issue. Amulet was in contact with ant. Mitral leaflet but mitral valve kinetics was normal | 6 weeks after procedure | Denovo mild mitral regurgitation on routine TEE at 6 weeks | LAA ostium 31 mm and neck 26mm | tear of A1 portion of anterior mitral leaflet | Progressive leaflet erosion by amulet outer disc | Not available | Not available |
| Cruz-Gonzalez et al 2014 | Spain | 72 | F | Parox | ACP | 24 | 5 | GI bleeding | Pericardial tamponade needing tube drainage after transseptal puncture. Following ACP deployment, inferior part of the external disc appeared over posterior leaflet without any mitral valve dysfunction | Few days after LAAO | Recurrent syncope | LAA neck by angiography 22mm, -by TEE 19mm | Possible dynamic obstruction of valve by device causing syncope | Compression of posterior mitral leaflet by ACP | Surgical removal of device and left atrial appendage with resolution of syncope | Discharged alive |
| Walia et al 2016 | Taiwan | 61 | M | Persis | ACP | 26 | 5 | Recurrent strokes and bleeding | Immediately post-implantation rhythmic movement of ACP disc edge and mild MR were noted | During the procedure | Rhythmic movement of disc edge and MR | Cauliflower morphology Base 21.8mm Depth 18.5mm | Disc impingement and MR | Outer disc of ACP causing mitral leaflet impingement | Removal of device and reimplantation of downsized ACP (24mm) | Discharged alive |
Discussion
Our review of LAAO related contiguous vessel and valve injury provides comprehensive evaluation of a rare complications associated with the procedure. Contiguous vessel and valve injury from LAAO are rare but can add significant morbidity and mortality. With increasing utilization of LAAO for stroke prophylaxis, such complications are likely to be encountered more frequently in the future. Therefore, operators performing LAAO procedures should be cognizant of this complication.
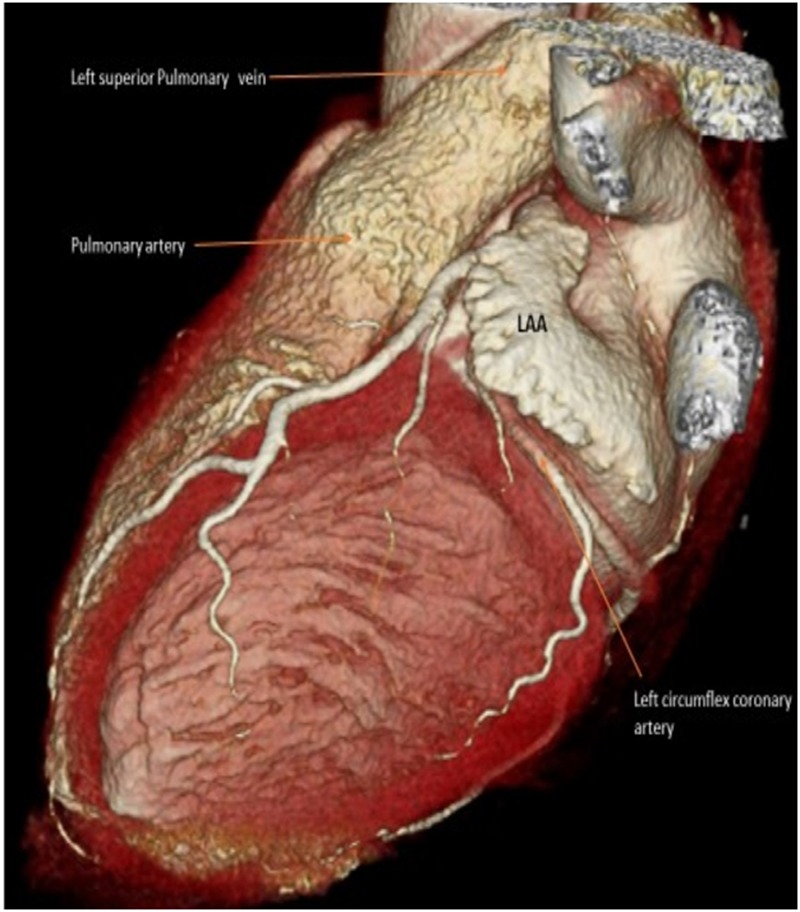
The topographic relationship of LAA with neighboring structures is well known. However, the effect of LAAO devices on potentially causing injury to neighboring vessels and mitral valve is based on sporadic case reports. Furthermore, due to interindividual variation in LAA morphologies and diverse types and sizes of LAAO devices used, ‘one size fits all’ recommendation cannot be made for preventing such adverse outcomes. Therefore, prevention and management of collateral injury related to LAAO should be individually addressed on a case by case basis. However, there may be certain anatomical and imaging characteristics that could enable providers to increase vigilance about the possibility of such complications. Anatomical proximity between LAA and vessels is a prerequisite for such complications. In fact, LAA may get in direct contact with main pulmonary artery in a significant number of patients undergoing LAAO [Figure 3]. In a review of 100 AF patients by cardiac-gated computed tomography angiogram of LAA, Halkin et al found 28% of patients had contact between LAA and PA in the proximal LAA (proximal 15mm extending into LAA from ostium or LAA before 1st major bend that is <15mm from ostium) and 65% had contact involving the distal LAA [18]. Proximal LAA contact poses increased vulnerability to injury where the anchoring hook of LAAO devices are usually situated after deployment. In a vast majority of cases, the landing zone of the lobe or disc-lobe devices is immediately distal to the LCX area and is away from the pulmonary artery or the main LCX trunk. This perhaps explains the rare incidence of these complications even though the ostial and distal portions of the LAA seem to be in closer proximity to the PA. This is again primarily determined by the shape of the LAA. Similarly, a recently published cardiac computed tomography (CT) evaluation in 48 patients with LAAO devices after 6 months of implantation revealed that the distance between occluder device and left upper pulmonary vein was affected by LAA morphology with cauliflower type having the closest proximity [19].
Figure 3. CT scan showing posteriorly directed LAA tucked underneath the pulmonary artery.
In the post-FDA approval experience of Watchman, pericardial tamponade occurred in about 1% of patients [4]. Similarly, in a multicenter study of ACP involving 1047 patients, pericardial tamponade was noted in 1.2% (13/1047) with 1 case reported as being caused by pulmonary artery tear (0.09%) [20]. Majority of the bleeding complications after LAAO were able to be treated percutaneously without the need for cardiac surgery. Even though most of these bleeding complications were probably related to micro-perforation of the LAA from the hooks or struts, contiguous vessel injury remains a concern.
Our review has some important clinical implications. First, it underscores the importance of proper preoperative imaging study to define the relationship of LAA with surrounding structures. While transesophageal echocardiography is commonly used as standard modality, additional imaging such as CT scan needs to be considered in select cases. Second, it reinforces the importance of proper device sizing. Based on our cases, from mechanistic perspective, oversized LAA device can erode the vessel after perforating LAA or compress from outside without perforation. Similarly, the outer disc of larger devices can impinge on mitral valve leaflet and the left superior pulmonary vein ostium. Selecting a larger device is associated with risk of LAA perforation and cardiac tamponade in previous studies [21,22]. As a corollary of this, larger devices may be associated with contiguous vessel and valve injury. Sometimes the LAA may be behind the pulmonary artery especially the landing zone of the LAA where an oversized device could potentially exert significant radial forces leading to perforation through the anchors.
Study limitations
Our study has all the potential limitations of a systematic review. The data is retrospectively pooled and many anatomical and intraprocedural details are not readily available. The exact details of the degree of oversizing and degree of the compression of the lobe (ACP/Amulet) and or the main body (Watchman) are largely unknown. Oversizing and compression are almost always thought to be the underlying etiology in most of these cases. Whether it is truly the case or not cannot be accurately verified. But it is logical to hypothesize that significant oversizing can result in over compression with higher radial forces on the walls of the LAA and the contiguous vessels. Oftentimes, operators err on the side of oversizing to accomplish tighter seal of the LAA. This approach has to be reviewed with caution in light of the results of our study. Finally, the true incidence of the contiguous vessel or valve injury in the general population cannot be estimated from our study.
Funding
This research did not receive any specific grant from funding agencies in the public, commercial, or not-for-profit sectors.
Acknowledgements
None
Conclusions
Thorough architectural knowledge of pulmonary artery, pulmonary vein, left circumflex artery and mitral valve leaflets in relation to LAA can help guide pre-operative and post-operative management, as well as anticipation of the rare complication of injury to the contiguous vessels and valve during LAAO procedures. Given increasing use of LAAO devices, we anticipate that these rare complications have a potential to increase in frequency in the future. Increased operator awareness along with proper preoperative imaging can potentially mitigate these rare complications.
References
- 1.Onalan Orhan, Crystal Eugene. Left atrial appendage exclusion for stroke prevention in patients with nonrheumatic atrial fibrillation. Stroke. 2007 Feb;38 (2 Suppl):624–30. doi: 10.1161/01.STR.0000250166.06949.95. [DOI] [PubMed] [Google Scholar]
- 2.Santoro Gennaro, Meucci Francesco, Stolcova Miroslava, Rezzaghi Marco, Mori Fabio, Palmieri Cataldo, Paradossi Umberto, Pastormerlo Luigi Emilio, Rosso Gabriele, Berti Sergio. Percutaneous left atrial appendage occlusion in patients with non-valvular atrial fibrillation: implantation and up to four years follow-up of the AMPLATZER Cardiac Plug. EuroIntervention. 2016 Feb;11 (10):1188–94. doi: 10.4244/EIJY14M10_13. [DOI] [PubMed] [Google Scholar]
- 3.Savelieva Irina, Camm John. Update on atrial fibrillation: part I. Clin Cardiol. 2008 Feb;31 (2):55–62. doi: 10.1002/clc.20138. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Reddy Vivek Y, Gibson Douglas N, Kar Saibal, O'Neill William, Doshi Shephal K, Horton Rodney P, Buchbinder Maurice, Gordon Nicole T, Holmes David R. Post-Approval U.S. Experience With Left Atrial Appendage Closure for Stroke Prevention in Atrial Fibrillation. J. Am. Coll. Cardiol. 2017 Jan 24;69 (3):253–261. doi: 10.1016/j.jacc.2016.10.010. [DOI] [PubMed] [Google Scholar]
- 5.DeSimone Christopher V, Prakriti Bs Gaba, Tri Jason, Syed Faisal, Sm Amit Noheria, Asirvatham Samuel J. A Review Of The Relevant Embryology, Pathohistology, And Anatomy Of The Left Atrial Appendage For The Invasive Cardiac Electrophysiologist. J Atr Fibrillation. 2015 Aug 31;8 (2) doi: 10.4022/jafib.1129. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Ayati Maryam, Ouyang Feifan, Kuck Kh. Pulmonary vein compression after implantation of a left atrial appendage occluder: presentation and discussion of a case. Indian Pacing Electrophysiol J. 2014 Jul;14 (4):194–8. doi: 10.1016/s0972-6292(16)30775-6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Bianchi Giacomo, Solinas Marco, Gasbarri Tommaso, Bevilacqua Stefano, Tiwari Kaushal Kishore, Berti Sergio, Glauber Mattia. Pulmonary artery perforation by plug anchoring system after percutaneous closure of left appendage. Ann. Thorac. Surg. 2013 Jul;96 (1):e3–5. doi: 10.1016/j.athoracsur.2012.12.057. [DOI] [PubMed] [Google Scholar]
- 8.Hanazawa Koji, Brunelli Michele, Saenger Joerg, Große Anett, Raffa Santi, Lauer Bernward, Geller J Christoph. Close proximity between pulmonary artery and left atrial appendage leading to perforation of the artery, tamponade and death after appendage closure using cardiac plug device. Int. J. Cardiol. 2014 Aug 01;175 (2):e35–6. doi: 10.1016/j.ijcard.2014.04.260. [DOI] [PubMed] [Google Scholar]
- 9.Katona András, Temesvári András, Szatmári András, Nemes Attila, Forster Tamás, Fontos Géza. Left circumflex coronary artery occlusion due to a left atrial appendage closure device. Postepy Kardiol Interwencyjnej. 2015;11 (1):69–70. doi: 10.5114/pwki.2015.49192. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Scisło Piotr, Wilimski Radosław, Zbroński Karol, Huczek Zenon. Main pulmonary artery perforations after left atrial appendage occluder implantation. EuroIntervention. 2018 Oct 20;14 (8):894–895. doi: 10.4244/EIJ-D-18-00419. [DOI] [PubMed] [Google Scholar]
- 11.Sepahpour Ali, Ng Martin K C, Storey Philip, McGuire Mark A. Death from pulmonary artery erosion complicating implantation of percutaneous left atrial appendage occlusion device. Heart Rhythm. 2013 Dec;10 (12):1810–1. doi: 10.1016/j.hrthm.2013.07.046. [DOI] [PubMed] [Google Scholar]
- 12.Suwalski Grzegorz, Wojnowski Andrzej, Mizerski Jeremi, Gryszko Leszek. Delayed Pulmonary Artery Perforation With Left Atrial Appendage Occluder Hooks. Ann. Thorac. Surg. 2016 Feb;101 (2):e37–9. doi: 10.1016/j.athoracsur.2015.09.026. [DOI] [PubMed] [Google Scholar]
- 13.Wang Edward, Lin Wah Wah, Xu Xiao Fang, Merry Chris. Delayed presentation of pulmonary artery perforation by an Amulet left atrial appendage closure device. BMJ Case Rep. 2018 Nov 03;2018 () doi: 10.1136/bcr-2018-227098. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Zwirner J, Bayer R, Hädrich C, Bollmann A, Klein N, Dreßler J, Ondruschka B. Pulmonary artery perforation and coronary air embolism-two fatal outcomes in percutaneous left atrial appendage occlusion. Int. J. Legal Med. 2017 Jan;131 (1):191–197. doi: 10.1007/s00414-016-1486-1. [DOI] [PubMed] [Google Scholar]
- 15.Berrebi Alain, Sebag Frederic A, Diakov Christelle, Amabile Nicolas. Early Anterior Mitral Valve Leaflet Mechanical Erosion Following Left Atrial Appendage Occluder Implantation. JACC Cardiovasc Interv. 2017 Aug 28;10 (16):1708–1709. doi: 10.1016/j.jcin.2017.06.030. [DOI] [PubMed] [Google Scholar]
- 16.Cruz-Gonzalez Ignacio, Perez-Rivera Jose-Angel, Bethencourt Armando. Recurrent syncope after left atrial appendage occlusion. Catheter Cardiovasc Interv. 2015 Feb 01;85 (2):E58–62. doi: 10.1002/ccd.25608. [DOI] [PubMed] [Google Scholar]
- 17.Walia Rohit, Lo Li-Wei, Lam Yat-Yin, Yu Wen-Chung, Chen Shih-Ann. Disc movement sign: A clue to malpositioned Amplatzer cardiac plug impinging on mitral leaflet. Int. J. Cardiol. 2016 Dec 15;225 ():109–110. doi: 10.1016/j.ijcard.2016.09.027. [DOI] [PubMed] [Google Scholar]
- 18.Halkin Amir, Cohen Clara, Rosso Raphael, Chorin Ehud, Schnapper Michael, Biner Simon, Topilsky Yan, Shiran Avinoam, Shmilovich Haim, Cohen Dotan, Keren Gad, Banai Shmuel, Aviram Galit. Left atrial appendage and pulmonary artery anatomic relationship by cardiac-gated computed tomography: Implications for late pulmonary artery perforation by left atrial appendage closure devices. Heart Rhythm. 2016 Oct;13 (10):2064–9. doi: 10.1016/j.hrthm.2016.07.015. [DOI] [PubMed] [Google Scholar]
- 19.Lindner Simon, Behnes Michael, Wenke Annika, Sartorius Benjamin, Dieker Wulf, Ansari Uzair, Akin Muharrem, Bertsch Thomas, Mashayekhi Kambis, Vogler Nils, Haubenreisser Holger, Schoenberg Stefan O, Borggrefe Martin, Akin Ibrahim. Relation of left atrial appendage closure devices to topographic neighboring structures using standardized imaging by cardiac computed tomography angiography. Clin Cardiol. 2019 Feb;42 (2):264–269. doi: 10.1002/clc.23136. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Tzikas Apostolos, Shakir Samera, Gafoor Sameer, Omran Heyder, Berti Sergio, Santoro Gennaro, Kefer Joelle, Landmesser Ulf, Nielsen-Kudsk Jens Erik, Cruz-Gonzalez Ignacio, Sievert Horst, Tichelbäcker Tobias, Kanagaratnam Prapa, Nietlispach Fabian, Aminian Adel, Kasch Friederike, Freixa Xavier, Danna Paolo, Rezzaghi Marco, Vermeersch Paul, Stock Friederike, Stolcova Miroslava, Costa Marco, Ibrahim Reda, Schillinger Wolfgang, Meier Bernhard, Park Jai-Wun. Left atrial appendage occlusion for stroke prevention in atrial fibrillation: multicentre experience with the AMPLATZER Cardiac Plug. EuroIntervention. 2016 Feb;11 (10):1170–9. doi: 10.4244/EIJY15M01_06. [DOI] [PubMed] [Google Scholar]
- 21.Schmidt-Salzmann Maximilian, Meincke Felix, Kreidel Felix, Spangenberg Tobias, Ghanem Alexander, Kuck Karl-Heinz, Bergmann Martin W. Improved Algorithm for Ostium Size Assessment in Watchman Left Atrial Appendage Occlusion Using Three-Dimensional Echocardiography. J Invasive Cardiol. 2017 Jul;29 (7):232–238. [PubMed] [Google Scholar]
- 22.Zhou Qing, Song Hongning, Zhang Lan, Deng Qing, Chen Jinling, Hu Bo, Wang Yijia, Guo Ruiqiang. Roles of real-time three-dimensional transesophageal echocardiography in peri-operation of transcatheter left atrial appendage closure. Medicine (Baltimore) 2017 Jan;96 (4) doi: 10.1097/MD.0000000000005637. [DOI] [PMC free article] [PubMed] [Google Scholar]


