Highlights
-
•
We assessed intrinsic connectivity of networks underlying cognition in youth.
-
•
Evidence of age effects on intrinsic connectivity was found across all networks.
-
•
Intrinsic connectivity showed no effect of sex, nor age by sex interactions.
Keywords: Functional magnetic resonance imaging (fMRI), Resting-state networks (RSN), Development, Independent component analysis (ICA)
Abstract
There is limited evidence on the effects of age and sex on intrinsic connectivity of networks underlying cognition during childhood and adolescence. Independent component analysis was conducted in 113 subjects aged 7–18; the default mode, executive control, anterior salience, basal ganglia, language and visuospatial networks were identified. The effect of age was examined with multiple regression, while sex and ‘age × sex’ interactions were assessed by dividing the sample according to age (7–12 and 13–18 years). As age increased, connectivity in the dorsal and ventral default mode network became more anterior and posterior, respectively, while in the executive control network, connectivity increased within frontoparietal regions. The basal ganglia network showed increased engagement of striatum, thalami and precuneus. The anterior salience network showed greater connectivity in frontal areas and anterior cingulate, and less connectivity of orbitofrontal, middle cingulate and temporoparietal regions. The language network presented increased connectivity of inferior frontal and decreased connectivity within the right middle frontal and left inferior parietal cortices. The visuospatial network showed greater engagement of inferior parietal and frontal cortices. No effect of sex, nor age by sex interactions was observed. These findings provide evidence of strengthening of cortico-cortical and cortico-subcortical networks across childhood and adolescence.
1. Introduction
Brain functional activation during cognitive tasks requiring executive control, attention, timing and motivation increases over development (for review see Rubia, 2013). Less is known, however, regarding functional connectivity of resting-state networks (RSN) associated with these cognitive functions. Given that cognitive and motivational strategies undergo significant changes across childhood and adolescence (Berl et al., 2010, Blakemore and Choudhury, 2006, Steinberg, 2005) the study of RSN underlying memory, executive, language, emotional and higher order visuospatial processes during this age period is of particular interest. However, the assessment of cognitive RSN in children and/or adolescents has only been partially addressed so far, by studies that either focus on selected brain networks, namely frontoparietal networks such as the default mode and executive control networks (Sato et al., 2014, Supekar et al., 2010, Gao et al., 2009, Fair et al., 2008, Thomason et al., 2008) or on a limited age range (de Bie et al., 2012, Thomason et al., 2011, Jolles et al., 2011, Uddin et al., 2010, Stevens et al., 2009), with few exceptions. Two recent studies have assessed samples with a wider age range (van Duijvenvoorde et al., 2015, Fareri et al., 2015), although both have focused on subcortical connectivity. We are aware of no study so far assessing RSN such as the default mode, executive control, salience, cortico-subcortical, language and visuospatial networks, including subjects from childhood to late adolescence.
The default mode network (DMN) is the most widely investigated RSN to date. It includes a set of brain regions involved in both cognitive (Buckner et al., 2008) and social cognition processes (Li et al., 2014), and in adult subjects it encompasses the posterior cingulate, precuneus, medial temporal lobe and medial prefrontal cortex. Two weeks after birth, a primitive DMN can be identified, while the posterior nodes of a more adult-like DMN can be distinguished by age two (Fransson et al., 2007, Gao et al., 2009). Several studies have suggested that the strength of connectivity between core regions of the DMN increases from childhood to late adolescence (Rubia, 2013, Fair et al., 2008, Power et al., 2010). The executive control network (ECN) includes the dorsolateral prefrontal cortex and the lateral posterior parietal cortex and is involved in cognitive flexibility and decision making (Seeley et al., 2007). In relation to the DMN, findings regarding age-related development of executive networks are less consistent, with some studies reporting different connectivity in children relative to adults (Barber et al., 2013, Jolles et al., 2011) and others finding little or no evidence of age effects (Vinette and Bray, 2015, Sato et al., 2014). De Bie and colleagues reported that in early childhood, components corresponding to the DMN and ECN appeared incomplete and fragmented (de Bie et al., 2012) in contrast to those related to basic motor function and sensory related processing, which had a functional organisation that was similar to mature adult patterns.
Another RSN identified in adults, which has been subject to more limited research in childhood and adolescence, is the anterior salience network, which encompasses the fronto-insular cortex and dorsal anterior cingulate, and has a role in emotional processing, homeostatic regulation and reward (Seeley et al., 2007). Fair and colleagues, employing graph-based measures, found that, in relation to adults, children showed incomplete connectivity between the dorsal anterior cingulate and medial superior frontal cortex, while connectivity was intermediate in adolescents (Fair et al., 2007). In addition, they demonstrated that during childhood, connectivity of the cingulo-opercular network was more closely connected to the frontoparietal network, and became segregated from this network with age. A later study by Uddin and colleagues also demonstrated differences in both structural and functional connectivity of the right fronto-insular cortex between children and adults (Uddin et al., 2011). A cortico-subcortical network involving the basal ganglia, engaging the inferior frontal gyri, striatum and thalami, has also been suggested to have a role in processing cognitive and motor information (Shirer et al., 2012, Tisch et al., 2004). van Duijvenvoorde and colleagues explored intrinsic connectivity in a sample of subjects from 8 to 25 years old in two main components: one frontoparietal and one subcortical component including the striatum, hipopocampus and the medial prefrontal cortex. In this latter component, the authors observed an age-related increase in connectivity in the anterior cingulate cortex but a decrease in the ventromedial prefrontal cortex (van Duijvenvoorde et al., 2015). Conversely, for the frontoparietal component they found an adolescent-specific increase in connectivity in the right anterior prefrontal cortex.
With regards language-related processing, two RSN have been previously identified in children aged 5–8: one including the auditory and opercular cortices, and the other encompassing fronto-temporal cortices and the posterior cingulate/precuneus (de Bie et al., 2012). The auditory network is considered to be involved in higher order functions related to language (Smith et al., 2009), and therefore has been studied as a component underlying language processing (Jolles et al., 2011). In a comparative study between children and young adults, Jolles and colleagues found greater functional connectivity of the left middle and inferior frontal cortices in younger subjects, denoting a more diffuse pattern of functional connectivity of this network in children (Jolles et al., 2011). To our knowledge, only one study so far, which was conducted in children, has identified a specific language network, separate from the auditory network and associated with a ventral attentional system (de Bie et al., 2012). However, this study did not examine age effects as it focused on a limited age range. Despite the fact that networks underlying the visual system have been characterised (de Bie et al., 2012, Jolles et al., 2011), to the best of our knowledge no study so far has explored the presence of a cognitive component underlying visuospatial processing, which has been suggested to rely on lateral frontal, inferior temporal and inferior parietal cortices (Shirer et al., 2012).
A source of discrepancy between studies is the distinct methods available to post-process resting-state functional magnetic resonance imaging (fMRI) (for review, see Margulies et al., 2010). For instance, both seed-based and graph-theoretical approaches require prior definition of regions of interest (ROI), and hence, decisions regarding specific ROI location, size and shape may influence results (Margulies et al., 2010). Conversely, ICA is an exploratory, data-driven approach that enables decomposition of the haemodynamic signal obtained with fMRI into brain regions showing synchronised patterns of signal fluctuation (Calhoun et al., 2008, Beckmann et al., 2005). This allows for the identification of spatial maps or components that are later studied independently. Furthermore, ICA is particularly good at removing motion artefacts, which can be particularly problematic in child fMRI studies (Uddin et al., 2010). It has been suggested that when motion is properly controlled, RSN of children and adolescents are robust and reliable, and may provide an accurate reflection of underlying neurobiology (Thomason et al., 2011).
Another issue that has received limited attention so far has been the investigation of sex effects on age-related changes in functional connectivity (Rubia, 2013). While sex-related differences have been observed in task-based fMRI studies undertaken in children and adolescents (for a review see Rubia, 2013), this evidence is more limited in studies assessing intrinsic connectivity. In a study using graph-based indices, boys showed greater global efficiency while girls presented a trend towards higher clustering in regions related to the default mode, language and vision systems (Wu et al., 2013). The authors concluded that boys may have a more optimal configuration for global processing, and girls for specialised processing; a distinction that could contribute to sex-related cognitive differences in children. However, we are unaware of any study so far examining the effect of sex on within-network connectivity during the resting state in youth.
Therefore, given the limited number of available studies examining a range of cognitive RSN in childhood and adolescence, we set out to assess six resting-state networks linked to cognitive and emotional processes: the default mode, executive control, anterior salience, cortico-subcortical or basal ganglia, language and visuospatial networks in a sample of healthy children and adolescents aged 7–18. In view of the abovementioned studies pointing to a progressive increase of within-network connectivity with age, and bearing in mind that performance in cognitive and emotional tasks underlying these networks shows age-related gains during childhood and adolescence, we expected to find age-related increases of intrinsic connectivity within the core regions of each network. A secondary objective was to study the effect of sex and the interaction between age and sex in intrinsic connectivity of these RSN. To this respect, and taking into account the following issues: first, cognitive performance differences have been observed between males and females (Blakemore, 2012), second, task-based fMRI studies have shown sex-related differences in tasks of executive function, language and visuospatial processing (Boghi et al., 2006, Bell et al., 2006, Halari et al., 2006) and third, previous literature also suggests sex-related differences in networks underlying default mode, language, and visual processes (Wu et al., 2013); we expected to observe different patterns of functional connectivity between males and females, particularly in executive, salience, language and visuospatial networks.
2. Materials and methods
2.1. Participants
137 right and left-handed children and adolescents aged 7–18 were recruited through advertisements posted in primary health care centres and other community locations within the area of Barcelona, Spain. Exclusion criteria were: intellectual disability according to the Diagnostic and Statistical Manual of Mental Disorders, Fourth Edition (American Psychiatric Association, APA, 1994) criteria (IQ below 70 and impaired functioning), severe medical conditions, history of brain traumatism with loss of consciousness, any history of psychotropic medications and presence of lifetime axis I psychopathology. The study was approved by the local ethics committee of the Hospital Clínic of Barcelona. For participants aged under 18, parents provided written informed consent and participants older than 12 provided assent. Participants aged 18 years old gave written informed consent.
All youth were assessed at the Child and Adolescent Psychiatry and Psychology Department by experienced child and adolescent psychiatrists and psychologists. Mental health conditions were evaluated using the Schedule for Affective Disorders and Schizophrenia for School-Age Children–Present and Lifetime version (K-SADS-PL, Geller et al., 2001). A measure of general cognitive capacity (IQ) was estimated using the Vocabulary, Similarities, Block Design and Matrix Reasoning subtests of the Wechsler Intelligence Scale for Children (WISC-III, Wechsler, 1994) or the Wechsler Adult Intelligence Scale (WAIS-III, Wechsler, 1999) (for participants aged 16 and over).
Mean age of the final sample (n = 113) was 13.42 (SD 3.1), including 55 boys (48.7%) and 58 girls (51.3%). IQ values ranged from 80 to 125 (mean IQ 107.64, SD 10.71). Distribution of handedness, which was available for 89.4% of the sample, was comparable between children and adolescents (χ2 = 0.691, p = 0.461), and between males and females (χ2 = 0.675, p = 0.489).
2.2. Imaging data acquisition
Scans were acquired on a 3 T Siemens Magnetom Trio Tim (Siemens Medical Systems, Germany) scanner at the Magnetic Resonance Image (MRI) Core Facility of IDIBAPS, Centre for Image Diagnosis, Hospital Clínic of Barcelona. Before entering the MRI scan, participants were instructed to remain as still as possible and soft pads were placed at the sides of their heads in order to help avoid further movement. A high-resolution T1-weighted 3-dimensional (3D) magnetisation-prepared rapid sequence was acquired with the following parameters: 240 sagital slices; TR = 2300 ms; TE = 3.01 ms; slice thickness = 1 mm; inversion time (TI) = 900; FOV = 394 × 240; matrix size = 256 × 256; and flip angle = 9°. An 8-min resting-state fMRI (rs-fMRI) sequence was also acquired, prior to which participants were instructed to keep their eyes closed and not fall asleep. Acquisition parameters were as follows: 240 volumes, TR = 2000 ms; TE = 29 ms; matrix size = 480 × 480; slice thickness = 4 mm, acquisition matrix = 80 × 80 mm, 32 slices, voxel size 3 mm × 3 mm × 4 mm.
2.3. Image preprocessing and independent component analysis (ICA)
rs-fMRI data preprocessing was carried out using the Data Processing Assistant for Resting-State fMRI (DPARSF) (Yan and Zang, 2010, http://rfmri.org/DPARSF) which is based on Statistical Parametric Mapping (SPM12) (http://www.fil.ion.ucl.ac.uk/spm) and the toolbox for Data Processing & Analysis of Brain Imaging (DPABI, http://rfmri.org/DPABI) running in Matlab (R2013a; www.mathworks.com). The following steps were conducted: reorientation of 3D and fMRI images along the anterior and posterior commissural line; realignment with motion correction and inspection of outputs following which, subjects with more than 1.5 mm translation and 1.5 degrees rotation in any of the x, y or z directions (Allen et al., 2014) were disregarded for further analyses (22 subjects were thus excluded; 17 subjects younger and 5 older than age 12). Corregistration of anatomical to functional data ensued; as well as normalisation into standard Montreal Neurological Institute (MNI) space (Burgund et al., 2002). Smoothing with an 8 mm full width at half-maximum Gaussian kernel was applied. Finally, to reduce potential micromotion artefacts, scrubbing was applied (Power et al., 2012) using a framewise displacement (FD) threshold of 0.2 mm where bad points were linearly interpolated.
Mean FD differed between groups (children 0.1271 (SD 0.0481), adolescents 0.0953 (SD 0.0299), Mann–Whitney U = 862, p < 0.0001) and therefore, the percentage of scans that were flagged for scrubbing was greater for children than for adolescents (14.15% vs. 5.40%, respectively, Mann–Whitney U = 803, p < 0.0001). No mean FD differences were seen between males and females (9.31% vs. 7.88%, respectively, U = 1.31, p = 0.1) and no outliers were observed (mean FD ranged from 0.4713 to 0.2251). Given the stringent threshold employed for scrubbing, no further subjects were excluded from subsequent analyses due to motion.
Two more subjects were disregarded due to other reasons: one presented an arachnoid cyst and in one case technical difficulties were experienced during fMRI acquisition. Thus, the final sample consisted of 113 participants (41 children aged 7–12 and 72 adolescents aged 13–18).
Pre-processed fMRI images were then subjected to spatial independent component analysis (ICA), employing the Group ICA fMRI Toolbox (GIFT v3.0a; http://mialab.mrn.org/software/). Images from all subjects were concatenated and ICA was performed in 3 stages: (1) Principal component analyses was conducted twice: the dataset was first reduced to 30 components, and then was further limited to an output of 20 components (2) the Infomax algorithm was used to decompose the reduced dataset into maximally independent component images; and (3) components were then back-reconstructed using the Group ICA tool. Components which depicted connectivity in at least two distal brain regions (Stevens et al., 2009) were visually inspected and networks underlying the default mode network, executive control, cortico-striatal, salience, language and visuosptial processing were interpreted on the basis of their spatial scope. One sample t-tests were carried out for each component in order to determine anatomical regions within each network. For the purpose of describing each network we used a voxel-wise correction of p < 0.001 family wise error (FWE) corrected and a minimum extent cluster size of 500 voxels.
Those components related to either white matter, cerebrospinal fluid, cerebellar networks, and components matching primary areas such as a sensorimotor, auditory and primary visual regions were identified but not taken into account for further analyses. Selected cognitive components were exported to SPM12 for group analyses.
2.4. Analysis
Gender distribution and typified IQ scores were compared between age groups with the Statistical Package for Social Sciences (SPSS v.20) employing chi-square and ANOVA tests. IQ distribution was tested for normality with the Kolmogorov–Smirnov test.
To test the effects of age on brain connectivity during rest, multiple regression analyses were carried out for each RSN. Next, group differences in intrinsic connectivity as a function of age and sex were conducted with full factorial models controlling for IQ. Interaction between the two factors (age group: children vs. adolescents, and sex: male vs. female) yielded four cells: child male, child female, adolescent male and adolescent female. The group of children included subjects aged 7–12, and the group of adolescents were aged 13–18. Global effect of sex (male vs. female) was also explored for each RSN by comparing cells corresponding to males versus females. Finally, pair-wise comparisons (child male vs. child female and adolescent male vs. adolescent female) were also explored.
A grey matter mask was obtained from the smoothed, modulated and normalised grey matter images for the whole sample, which was used as an inclusive mask for all second-level analyses. Results were interpreted at a voxel-wise threshold of p < 0.001 uncorrected and a cluster-wise threshold of p < 0.05 FWE corrected, with a minimum cluster size of 50 contiguous voxels.
Signal intensity within global maxima clusters showing a significant effect of age and/or gender was extracted using a volume-of-interest (VOI) approach using spheres (3 mm radius) and exported to an SPSS database. Due to the presence of siblings in the sample (N = 27; 14 in the child group and 13 in the adolescent group), all significant group comparisons were subjected to confirmatory analyses using generalised linear mixed models in SPSS. A mixed model was conducted for each cluster showing a significant effect of age or sex on intrinsic connectivity, where mean connectivity values were included as dependent variables. Each subject was assigned a family number, which was shared by siblings, and was included as random effect. For replication of age effects, age was included as a fixed effect, and for the confirmation of putative sex or ‘age × sex’ effects, sex was included as a fixed factor and IQ as a covariate.
3. Results
3.1. ICA-derived resting-state networks
Spatial maps for each cognitive RSN for the whole sample resulting from the one-sample t tests are summarised in Table 1. We identified two components which coincided spatially with the DMN, one located in posterior and another in anterior brain regions (pDMN and aDMN, respectively). Executive control networks were also split into two components: left and right (lECN and rECN, respectively).
Table 1.
Description of main anatomic locations according to the Automated Anatomical Labelling software (AAL Atlas) and spatial group maps for each resting-state network (RSN). L, left hemisphere; R, right hemisphere. Images are displayed in neurological orientation.
| RSN | Main anatomical locations | Axial view |
|---|---|---|
| Posterior default mode network | R precuneus, L paracentral lobule, L precentral, R middle frontal/temporal, L & R inferior parietal, R middle occipital, L inferior frontal. | 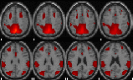 |
| Anterior default mode network | R medial frontal, L & R superior frontal, L medial orbitofrontal, R anterior cingulate, L inferior occipital, L middle/inferior temporal, R precuneus, R posterior cingulate, R calcarine. | 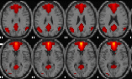 |
| Left executive control network | L inferior temporal, L supramarginal, L middle cingulate, L orbitofrontal, L superior/inferior frontal. | 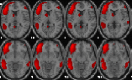 |
| Right executive control network | R orbitofrontal, R inferior temporal, L medial frontal, R angular, R supramarginal, R superior/inferior frontal. | 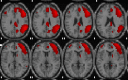 |
| Basal ganglia network | L orbitofrontal, R inferior temporal, R anterior cingulate, L and R putamen, L pallidum, R caudate, R thalamus, L Heschl. | 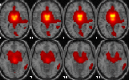 |
| Anterior salience network | L precentral, R middle/medial/inferior frontal, R parahippocampal, R supplementary motor area, R anterior/middle cingulated, R insula, L olfactory, L anterior cingulate. | 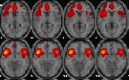 |
| Language network | L & R temporal pole, R operculum, L paracentral lobule, R precuneus, L & R angular, L superior temporal, L insula, L inferior frontal, R medial frontal, L anterior cingulate. | 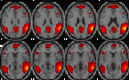 |
| Visuospatial network | L & R superior parietal, L & R superior frontal, L & R precentral, L & R inferior parietal, L & R precuneus, R middle occipital. | 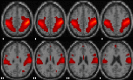 |
3.2. Functional connectivity: effects of age
The spatial location of brain regions showing an effect of age is depicted in Table 2. All networks presented positive correlations with age. In addition, the pDMN, lECN, anterior salience and language networks also exhibited regions of negative correlations with age. More specifically, the pDMN exhibited increased connectivity of frontal, parietal and subcortical regions and decreased connectivity of the anterior cingulate and left inferior parietal cortex. The aDMN showed higher connectivity of anterior regions, including the anterior cingulate extending to the medial prefrontal cortex, as well as the precuneus. The lECN engaged more regions of the left inferior parietal cortex and middle and orbitofrontal cortices with increasing age, accompanied by decreased connectivity in a cluster within the left inferior frontal cortex. The rECN showed stronger connectivity of right frontal and inferior parietal regions with age. The cortico-subcotrical network presented greater connectivity of the striatum, thalami, olfactory cortex and postcentral cortex. Furthermore, the anterior salience component showed stronger connectivity of bilateral anterior regions and the cerebellum, as well as weaker connectivity in the left orbitofrontal, middle cingulate and right inferior parietal cortices. The language network exhibited greater functional connectivity of bilateral inferior frontal, temporal cortices and the left precuneus, and decreased connectivity within the left inferior parietal and right middle frontal cortices. Finally, the visuospatial network showed increased connectivity of inferior frontal and parietal cortices, right middle occipital cortex extending to the precuneus, as well as the middle cingulate cortex.
Table 2.
Positive and negative correlations of functional connectivity within the studies RSN. k, cluster size in voxels; MNI, Montreal Neurological Institute coordinates [x,y,z]; L, left, R, right. Brain images were visualised with xjview toolbox (http://www.alivelearn.net/xjview) using neurological display.
| RSN | Age-related positive correlations | Age-related negative correlations | Positive correlations (red)/negative correlations (blue) |
|---|---|---|---|
| Posterior default mode network | • L superior/middle frontal cortex: pFWE = 0.004, k = 100, MNI [−30,−3,60]. L cuneus and precuneus: pFWE = 0.022, k = 69, MNI [6,−66,21]. • R pallidum and putamen: pFWE = 0.036, k = 60, MNI [30,6,3]. |
• Anterior cingulate extending to the R medial frontal cortex: pFWE = 0.006, t = 5.15, k = 92, MNI [3,39,21]. • L inferior parietal cortex: pFWE = 0.004, t = 4.74, k = 101, MNI [−66,−18,21]. |
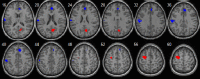 |
| Anterior default mode network | • Anterior cingulate extending to the medial frontal cortex: pFWE < 0.0001, k = 656, MNI [−27,36,42]. • R middle frontal extending to the medial frontal cortex: pFWE < 0.0001, k = 272, MNI [21,45,36]. • Precuneus: pFWE = 0.004, k = 102, MNI [0,−63,30]. |
– | 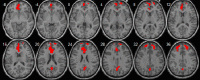 |
| Left executive control network | • L supramarginal extending to the precuneus: pFWE < 0.0001, k = 461, MNI [−36,−69,45]. • L middle frontal cortex: pFWE < 0.0001, k = 295, MNI [−39,3,54]. • L orbitofrontal: pFWE = 0.039, k = 59, MNI [−45,9,30]. |
• L inferior frontal: pFWE = 0.042, k = 58, MNI [−30,39,27] | 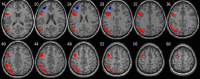 |
| Right executive control network | • R orbitofrontal: pFWE < 0.0001, t = 5.72, k = 282, MNI [39,45,18]. • R superior occipital extending to the precuneus: pFWE = 0.006, k = 92, MNI [9,−72,48]. • R inferior frontal/middle cortex: pFWE = 0.001, k = 132, MNI [30,3,57]. • R medial frontal extending to the middle cingulate cortex: pFWE = 0.025, k = 67, MNI [3,33,33]. • R angular gyrus: pFWE = 0.008, k = 88, MNI [48,−51,51]. |
– | 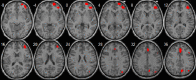 |
| Basal ganglia network | • R postcentral cortex extending to the R precuneus: pFWE = 0.001, k = 127, MNI [27,−30,66]. • L caudate and thalami: pFWE < 0.0001, k = 157, MNI [9,−6,12]. • L olfactory extending to the L putamen and amygdala: pFWE = 0.008, k = 84, MNI [−27,3,−15]. |
– | 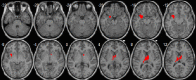 |
| Anterior salience network | • L superior frontal cortex: pFWE = 0.004, k = 99, MNI [−6,0,54]. • R anterior cingulate: pFWE < 0.0001, k = 183, MNI [−9,33,18]. • R inferior frontal cortex: pFWE < 0.0001, k = 148, MNI [24,42,33]. • L inferior frontal cortex: pFWE < 0.0001, k = 175, MNI [−24,45,15]. • L middle/superior temporal extending to the insula: pFWE = 0.029, k = 63, MNI [−45,12,−12]. • Cerebellum: pFWE = 0.047, k = 55, MNI [3,−81,−27]. |
• L orbitofrontal extending to the medial frontal cortex: pFWE = 0.032, k = 61, MNI [9,69,0]. • R middle cingulate: pFWE = 0.019, k = 70, MNI [12,30,36]. • R angular gyrus: pFWE = 0.024, k = 66, MNI [48,−63,27]. |
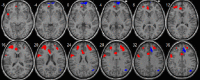 |
| Language network | • L inferior/superior temporal cortex: pFWE = 0.001, k = 132, MNI [−60,−57,9]. • L inferior frontal cortex: pFWE = 0.041, k = 58, MNI [−48,15,18]. • R inferior frontal cortex: pFWE = 0.001, k = 126, MNI [51,21,21]. • L precuneus: pFWE = 0.049, k = 55, MNI [−6,−54,42]. • R medial frontal extending to the anterior cingulate: pFWE = 0.002, k = 119, MNI [0,54,12]. • R middle temporal cortex: pFWE = 0.032, k = 62, MNI [57,−42,−3]. |
• L inferior parietal cortex: pFWE = 0.003, k = 105, MNI [−39,−45,33]. • R middle frontal cortex: pFWE = 0.041, k = 58, MNI [48,42,18]. |
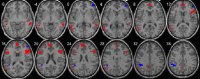 |
| Visuospatial network | • L inferior frontal cortex: pFWE = 0.001, k = 121, MNI [−45,−3,27]. • L inferior parietal cortex: pFWE < 0.0001, k = 212, MNI [−54,−27,33]. • R middle occipital cortex extending to the precuneus: pFWE = 0.001, k = 130, MNI [30,−69,30]. • R inferior parietal cortex: pFWE = 0.001, k = 132, MNI [39,−36,39]. • Middle cingulate extending to the R paracentral lobule: pFWE = 0.008, k = 84, MNI [9,−21,66]. |
– | 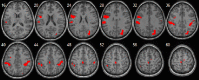 |
3.3. Functional connectivity: ‘age × sex’ and sex effects
Child and adolescent groups did not differ in terms of gender distribution (male/female: 22/19 for children and 33/39 for adolescents; χ2 = 0.64, p = 0.273) or IQ scores (mean IQ for children 108.71 (SD 12.49); adolescents 107.03 (9.51), Mann–Whitney U = 1256.5, p = 0.19). However, differences in IQ emerged when comparing sex groups within the child subgroup: child males had significantly higher IQ than child females (child male: mean IQ = 113.73 (SD 10.13); child female, mean IQ = 102.89 (SD 12.88), Mann–Whitney U = 106, p = 0.007). No differences in IQ were seen between males and females within the adolescent group (adolescent males, mean IQ = 108.82 (9.09); adolescent females, mean IQ = 105.51 (9.71), Mann–Whitney U = 511, p = 0.134).
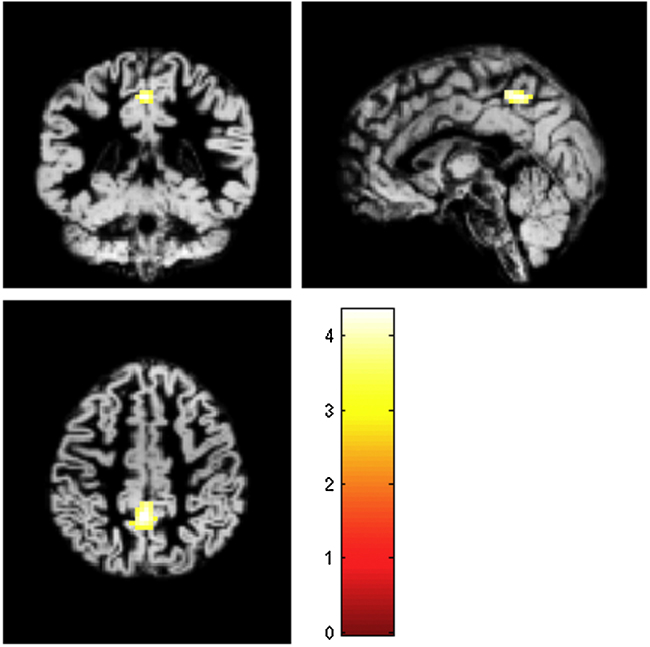
Analyses resulting from the full factorial design, which were controlled for IQ, yielded no regions of interaction between the two factors (age and sex). Contrasts for male versus female or vice versa did not show any significant differences, irrespective of age. Nonetheless, t-tests derived from this approach revealed sex-related differences within age subgroups for the visuospatial network (VSN). Specifically, for the adolescent group, males exhibited increased intrinsic connectivity within the precuneus relative to females (pFWE = 0.045, t = 4.35, k = 54, MNI [0,−48,48] (see Fig. 1).
Fig. 1.
Image depicts the region showing significant differences in the ‘adolescent males > adolescent females’ contrast within the visuospatial network (precuneus, peak MNI coordinates x, y, z [0,−48,48]).
3.4. Sibling random effect
All results described above were confirmed when the effect of siblings in the sample was taken into account (see Supplementary table).
4. Discussion
The results of the present study indicate that connectivity within the default, executive control, anterior salience, basal ganglia, language and visuospatial networks present progressive engagement of brain regions suggestive of maturation throughout childhood and adolescence. We failed to find any effect of sex or any interaction between age and sex.
4.1. Functional connectivity: effects of age
We identified two separate components corresponding to the anterior and posterior DMN. Such fragmentation has been found in studies using paediatric samples and has been interpreted as poor maturation of the network (de Bie et al., 2012, Fair et al., 2008). Positive correlations with age were observed for both anterior and posterior components of the DMN and included the left superior and middle prefrontal cortex, the medial prefrontal cortex, the precuneus, as well as the anterior cingulate and right putamen and pallidum. Interestingly, the relationship between connectivity in the anterior cingulate cortex and age was found to be positive for the aDMN but negative for the pDMN, suggesting that, with increasing age, the former component may become more anterior, while the latter may develop a more refined engagement of posterior regions. Somewhat unexpectedly, the left inferior parietal cortex presented a negative association with age. Since this region overlaps with the lECN in our sample, this may indicate that, over time, this region may become increasingly involved in the lECN, and more segregated from the DMN, as discussed below. Taken together, our findings provide support to the notion that, as age increases, connectivity is enhanced between regions that typically belong to the DMN, while connectivity with other areas is weakened (Power et al., 2010). Such progressive shift may be related to greater involvement of episodic and autobiographical memory functions in the DMN over development.
For the ECN, we found evidence of increased age-related connectivity in the inferior parietal lobule, left middle frontal, right medial frontal cortices extending to the middle cingulate, the precuneus and bilateral orbitofrontal cortex, while age-related connectivity decreased within the left inferior frontal cortex. The left inferior parietal cluster presented similar MNI coordinates to those described by Dosenbach et al. (2007). These authors highlighted the role of the left intraparietal sulcus, lateral frontal cortex, middle cingulate and precuneus in the top-down control of attention. A recent study failed to observe an age-related gain in connectivity between the intraparietal sulcus and the prefrontal cortex, while it found an unexpected increase in connectivity between the anterior intraparietal sulcus and visual fusiform regions over the course of adolescence (Vinette and Bray, 2015). In our rECN component, age appeared to be associated with increased connectivity in a posterior cluster including the right superior occipital cortex. The involvement of visual regions in this frontoparietal network could be associated with its role in visual attention (Vinette and Bray, 2015).
We also observed age-related increases in the right precuneus, striatum, thalami, olfactory cortex and amygdala within the basal ganglia network, which are suggestive of a strengthening of cortico-subcortical connections with age. A recent study described connectivity between the amygdala and the striatum across a wide age range (Fareri et al., 2015); we expand on these findings demonstrating that connectivity between these brain regions is likely to show age-related gains. Our observation of increased connectivity of the superior parietal cortex within the basal ganglia network also concurs with the recent demonstration of structural and functional connections between the striatum and the parietal cortex by Jarbo and Verstynen (2015), which is likely to reflect the increasing complexity of connectivity of the caudate and the putamen nuclei during adolescence, underlying cognitive and motor maturation characteristic of this age period.
The anterior salience network was found to engage more anterior regions with increasing age, including the inferior and superior frontal cortices, the right anterior cingulate and left insula; but also one cluster belonging to the cerebellum. Negative correlations were seen for the orbitofrontal cortex, right middle cingulate cortex and right angular gyrus. This brain system has a role in detecting relevant stimuli, a process that can be either unconscious (Critchley and Harrison, 2013) or dependent on cognitive control processes (Corbetta et al., 2008). The finding of an age-related increase in connectivity in the anterior cingulate and insula, key nodes of the salience network (Menon and Uddin, 2010), is in line with previous reports (van Duijvenvoorde et al., 2015, Cauda et al., 2011). The insula has positive correlations with the frontal gyri, temporoparietal junction, anterior cingulate, cuneus and precuneus and superior temporal gyri (Cauda et al., 2011). Interestingly, these regions were also found to be positively correlated with age in our study. Cauda and colleagues also found positive correlations between connectivity in the insula and the basal ganglia, thalamus and cerebellar cortices (Cauda et al., 2011), which may correspond to our basal ganglia component, more likely to be implicated in motor control and executive function (Gordon et al., 2015). On the other hand, we failed to find any differences regarding laterality in the fronto-insular cortex, which contrasts with findings from a previous study using graph-based measures in a small sample (Uddin et al., 2011). These authors suggested that the right front-insular cortex is likely to undergo critical maturation with age, providing access to attentional and working memory resources (Uddin et al., 2011). In our substantially larger study we have found age related gains in intrinsic connectivity in regions relevant to the anterior salience network located in both brain hemispheres. Furthermore, the decrease in connectivity in the right middle cingulate and angular gyrus, regions which are typically involved in frontoparietal control networks (Fair et al., 2007), are also indicative of refinement of the anterior salience network, insofar as it shows a better delineation from the frontoparietal, executive control network.
The language-related network also presented increased intrinsic connectivity of bilateral frontotemporal cortices and left precuneus with age, which are regions that have also been associated with a language network in a child sample (de Bie et al., 2012). However, the left inferior parietal cortex showed the inverse pattern, which is a somewhat unexpected finding. Since the left inferior parietal cortex is particularly relevant for language processing (Booth et al., 2004, McDermott et al., 2003), the negative age effects may indicate that during younger ages connectivity in this region may be aberrant, although this would require further examination in future studies. Besides, variability of RSN appears to be greatest for language, executive control and attention networks (Mueller et al., 2013).
Finally, the visuospatial network presented an age-related increase in connectivity in frontoparietal, cingulate and occipital cortices, while there was no region showing age-negative effects. This concurs with findings from a previous study which found an interaction between activity in the inferior parietal cortex and age during the execution of a visuospatial working memory task (Klingberg et al., 2002). Interestingly, development in visuospatial skills may be related to language development (Bavin et al., 2005) and therefore, refinement of functional connectivity within the left inferior parietal may be underlying maturation in these two cognitive domains.
4.2. Functional connectivity: effects of sex and ‘age × sex’
Although there is evidence of developmental sex-related structural differences (Raznahan et al., 2010, Zielinski et al., 2010), sex differences in fMRI patterns have not been thoroughly explored in child and adolescent samples (Rubia, 2013). In adult subjects, Weissman-Fogel and colleagues failed to find significant sex-related differences in the executive control, anterior salience and default mode networks (Weissman-Fogel et al., 2010), while a more recent study revealed sex differences in spontaneous brain activity in the DMN, attention, executive and primary visual and sensorimotor networks (Xu et al., 2015). In the only study so far assessing the effects of sex on resting-state connectivity in youth, Wu and colleagues reported that boys and girls may have distinct distribution of functional networks, particularly in the DMN, language and visual systems (Wu et al., 2013). In our analyses using factorial design, we did not find an effect of sex on any of the RSN assessed. It may be possible that the methodological approach used here was too conservative to detect more subtle sex-related differences in our sample. We also failed to observe any ‘age × sex’ interactions. However, when focusing on the adolescent group, we found that boys presented increased connectivity within the precuneus relative to girls, within the visuospatial network. The precuneus is a functionally and anatomically well-connected structure involved in a range of cognitive processes, including visuospatial processing (Wenderoth et al., 2005). The fact that we did not observe these differences in children suggests that the sex-dimorphic pattern in this intrinsic network may emerge during or after puberty. Poorer performance in adult females on tasks requiring visuospatial processing has been reported in relation to males (Schöning et al., 2007, Weiss et al., 2003). However, given that we only had limited measures of cognitive function, we are unable to comment on whether the reported sex-related difference in functional connectivity within the precuneus may be translated into changes in cognitive performance. We therefore interpret our findings of sex-related differences in rs-fMRI as a manifestation of the different developmental processes taking place in boys and girls throughout childhood and adolescence. Nonetheless, this particular result remains tentative, since the sample size was more limited in this secondary analysis.
Several concerns arise from this study. First, in order to fully understand development of RSN, future reports should ideally be designed longitudinally, as some indexes of functional maturation specifically those linked to the DMN, may have a non-linear development (Dosenbach et al., 2010). A recent report with wider age ranges (8–25) has assessed age-related linear and non-linear changes in functional connectivity (van Duijvenvoorde et al., 2015). Due to the age range of our sample (7–18) and given the fact that during this period changes may be of a more linear nature (Rubia, 2013), we decided to focus our analyses on linear changes. However, by doing so we are aware that we may only be capturing a portion of developmental changes in intrinsic functional connectivity. Another issue concerns interpretation of results when dealing with ICA, as findings of reduced/increased connectivity in a particular spatial component may imply changes in temporal coherence, in magnitude of fluctuation or rather reflect a different relationship with other components (Fox and Raichle, 2007). A further limitation is the somewhat arbitrary division of the sample by age, whereas a clinical measure of pubertal status or determination of sex hormones would have proven a more accurate measure with which to distinguish “childhood” from “adolescence”. Level of pubertal hormones may influence functional connectivity as has been demonstrated in a previous study, in which increasing oestradiol concentrations in girls were associated with enhanced functional connectivity in a network linked to social emotion processing (Klapwijk et al., 2013).
5. Conclusions
Our study provides evidence of within-network strengthening over age, indicating progressive synchronisation of cognitive RSN within the DMN, executive control, cortico-subcortical, anterior salience, language and visuospatial networks, denoting potential maturation of these networks throughout development.
Footnotes
Supplementary data associated with this article can be found, in the online version, at http://dx.doi.org/10.1016/j.dcn.2015.11.004.
Appendix A. Supplementary data
The following are the supplementary data to this article:
References
- Allen E.A., Damaraju E., Plis S.M., Erhardt E.B., Eichele T., Calhoun V.D. Tracking whole-brain connectivity dynamics in the resting state. Cereb. Cortex. 2014;24:663–676. doi: 10.1093/cercor/bhs352. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Barber A.D., Caffo B.S., Pekar J.J., Mostofsky S.H. Developmental changes in within- and between-network connectivity between late childhood and adulthood. Neuropsychologia. 2013;51:156–167. doi: 10.1016/j.neuropsychologia.2012.11.011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bavin E.L., Wilson P.H., Maruff P., Sleeman F. Spatio-visual memory of children with specific language impairment: evidence for generalized processing problems. Int. J. Lang. Commun. Disord. 2005;40:319–332. doi: 10.1080/13682820400027750. [DOI] [PubMed] [Google Scholar]
- Beckmann C.F., de Luca M., Devlin J.T., Smith S.M. Investigations into resting-state connectivity using independent component analysis. Philos. Trans. R. Soc. Lond. B: Biol. Sci. 2005;360:1001–1013. doi: 10.1098/rstb.2005.1634. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bell E.C., Willson M.C., Wilman A.H., Dave S., Silverstone P.H. Males and females differ in brain activation during cognitive tasks. Neuroimage. 2006;30:529–538. doi: 10.1016/j.neuroimage.2005.09.049. [DOI] [PubMed] [Google Scholar]
- Berl M.M., Duke E.S., Mayo J., Rosenberger L.R., Moore E.N., VanMeter J., Ratner N.B., Vaidya C.J., Gaillard W.D. Functional anatomy of listening and reading comprehension during development. Brain Lang. 2010;114:115–125. doi: 10.1016/j.bandl.2010.06.002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Blakemore S.J. Imaging brain development: the adolescent brain. Neuroimage. 2012;61:397–406. doi: 10.1016/j.neuroimage.2011.11.080. [DOI] [PubMed] [Google Scholar]
- Blakemore S.J., Choudhury S. Development of the adolescent brain: implications for executive function and social cognition. J. Child Psychol. Psychiatry. 2006;47:296–312. doi: 10.1111/j.1469-7610.2006.01611.x. [DOI] [PubMed] [Google Scholar]
- Boghi A., Rasetti R., Avidano F., Manzone C., Orsi L., D’Agata F., Caroppo P., Bergui M., Rocca P., Pulvirenti L., Bradac G.B., Bogetto F., Mutani R., Mortara P. The effect of gender on planning: an fMRI study using the Tower of London task. Neuroimage. 2006;33:999–1010. doi: 10.1016/j.neuroimage.2006.07.022. [DOI] [PubMed] [Google Scholar]
- Booth J.R., Burman D.D., Meyer J.R., Gitelman D.R., Parrish T.B., Mesulam M.M. Development of brain mechanisms for processing orthographic and phonologic representations. J. Cogn. Neurosci. 2004;16:1234–1249. doi: 10.1162/0898929041920496. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Buckner R.L., Andrews-Hanna J.R., Schacter D.L. The brain's default network: anatomy, function, and relevance to disease. Ann. N. Y. Acad. Sci. 2008;1124:1–38. doi: 10.1196/annals.1440.011. [DOI] [PubMed] [Google Scholar]
- Burgund E.D., Kang H.C., Kelly J.E., Buckner R.L., Snyder A.Z., Petersen S.E., Schlaggar B.L. The feasibility of a common stereotactic space for children and adults in fMRI studies of development. Neuroimage. 2002;17:184–200. doi: 10.1006/nimg.2002.1174. [DOI] [PubMed] [Google Scholar]
- Calhoun V.D., Kiehl K.A., Pearlson G.D. Modulation of temporally coherent brain networks estimated using ICA at rest and during cognitive tasks. Hum. Brain. Mapp. 2008;29:828–838. doi: 10.1002/hbm.20581. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cauda F., D’Agata F., Sacco K., Duca S., Geminiani G., Vercelli A. Functional connectivity of the insula in the resting brain. Neuroimage. 2011;55:8–23. doi: 10.1016/j.neuroimage.2010.11.049. [DOI] [PubMed] [Google Scholar]
- Corbetta M., Patel G., Shulman G.L. The reorienting system of the human brain: from environment to theory of mind. Neuron. 2008;8:306–324. doi: 10.1016/j.neuron.2008.04.017. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Critchley H.D., Harrison N.A. Visceral influences on brain and behavior. Neuron. 2013;77:624–638. doi: 10.1016/j.neuron.2013.02.008. [DOI] [PubMed] [Google Scholar]
- de Bie H.M., Boersma M., Adriaanse S., Veltman D.J., Wink A.M., Roosendaal S.D., Barkhof F., Stam C.J., Oostrom K.J., Delemarre-van de Waal H.A., Sanz-Arigita E.J. Resting-state networks in awake five- to eight-year old children. Hum. Brain Mapp. 2012;33:1189–1201. doi: 10.1002/hbm.21280. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dosenbach N.U., Fair D.A., Miezin F.M., Cohen A.L., Wenger K.K., Dosenbach R.A., Fox M.D., Snyder A.Z., Vincent J.L., Raichle M.E., Schlaggar B.L., Petersen S.E. Distinct brain networks for adaptive and stable task control in humans. Proc. Natl. Acad. Sci. U. S. A. 2007;104:11073–11078. doi: 10.1073/pnas.0704320104. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dosenbach N.U., Nardos B., Cohen A.L., Fair D.A., Power J.D., Church J.A., Nelson S.M., Wig G.S., Vogel A.C., Lessov-Schlaggar C.N., Barnes K.A., Dubis J.W., Feczko E., Coalson R.S., Pruett J.R., Jr., Barch D.M., Petersen S.E., Schlaggar B.L. Prediction of individual brain maturity using fMRI. Science. 2010;329:1358–1361. doi: 10.1126/science.1194144. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fair D.A., Dosenbach N.U., Church J.A., Cohen A.L., Brahmbhatt S., Miezin M., Barch D.M., Raichle M.E., Petersen S.E., Schlaggar B.L. Development of distinct control networks through segregation and integration. Proc. Natl. Acad. Sci. U. S. A. 2007;14:13507–13512. [Google Scholar]
- Fair D.A., Cohen A.L., Dosenbach N.U., Church J.A., Miezin F.M., Barch D.M., Raichle M.E., Petersen S.E., Schlaggar B.L. The maturing architecture of the brain's default network. Proc. Natl. Acad. Sci. U. S. A. 2008;105:4028–4032. doi: 10.1073/pnas.0800376105. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fareri D.S., Gabard-Durnam L., Goff B., Flannery J., Gee D.G., Lumian D.S., Caldera C., Tottenham N. Normative development of ventral striatal resting state connectivity in humans. Neuroimage. 2015;118:422–437. doi: 10.1016/j.neuroimage.2015.06.022. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fox M.D., Raichle M.E. Spontaneous fluctuations in brain activity observed with functional magnetic resonance imaging. Nat. Rev. Neurosci. 2007;8:700–711. doi: 10.1038/nrn2201. [DOI] [PubMed] [Google Scholar]
- Fransson P., Skiöld B., Horsch S., Nordell A., Blennow M., Lagercrantz H., Aden U. Resting-state networks in the infant brain. Proc. Natl. Acad. Sci. U. S. A. 2007;104:15531–15536. doi: 10.1073/pnas.0704380104. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gao W., Zhu H., Giovanello K.S., Smith J.K., Shen D., Gilmore J.H., Lin W. Evidence on the emergence of the brain's default network from 2-week-old to 2-year-old healthy pediatric subjects. Proc. Natl. Acad. Sci. U. S. A. 2009;106:6790–6795. doi: 10.1073/pnas.0811221106. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Geller B., Zimerman B., Williams M., Bolhofner K., Craney J.L., DelBello M.P., Soutullo C. Reliability of the Washington University in St, Louis Kiddie Schedule for Affective Disorders and Schizophrenia (WASH-U-KSADS) mania and rapid cycling sections. J. Am. Acad. Child Adolesc. Psychiatry. 2001;40:450–455. doi: 10.1097/00004583-200104000-00014. [DOI] [PubMed] [Google Scholar]
- Gordon E.M., Devaney J.M., Bean S., Vaidya C.J. Resting-state striato-frontal functional connectivity is sensitive to DAT1 genotype and predicts executive function. Cereb. Cortex. 2015;25:336–345. doi: 10.1093/cercor/bht229. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Halari R., Sharma T., Hines M., Andrew C., Simmons A., Kumari V. Comparable fMRI activity with differential behavioural performance on mental rotation and overt verbal fluency tasks in healthy men and women. Exp. Brain Res. 2006;169:1–14. doi: 10.1007/s00221-005-0118-7. [DOI] [PubMed] [Google Scholar]
- Jarbo K., Verstynen T.D. Converging structural and functional connectivity of orbitofrontal, dorsolateral prefrontal, and posterior parietal cortex in the human striatum. J. Neurosci. 2015;35:3865–3878. doi: 10.1523/JNEUROSCI.2636-14.2015. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jolles D.D., van Buchem M.A., Crone E.A., Rombouts S.A. A comprehensive study of whole-brain functional connectivity in children and young adults. Cereb. Cortex. 2011;21:385–391. doi: 10.1093/cercor/bhq104. [DOI] [PubMed] [Google Scholar]
- Klapwijk E.T., Goddings A.L., Burnett Heyes S., Bird G., Viner R.M., Blakemore S.J. Increased functional connectivity with puberty in the mentalising network involved in social emotion processing. Horm. Behav. 2013;64:314–322. doi: 10.1016/j.yhbeh.2013.03.012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Klingberg T., Forssberg H., Westerberg H. Increased brain activity in frontal and parietal cortex underlies the development of visuospatial working memory capacity during childhood. J. Cogn. Neurosci. 2002;14:1–10. doi: 10.1162/089892902317205276. [DOI] [PubMed] [Google Scholar]
- Li W., Mai X., Liu C. The default mode network and social understanding of others: what do brain connectivity studies tell us. Front. Hum. Neurosci. 2014;4(8):74. doi: 10.3389/fnhum.2014.00074. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Margulies D.S., Böttger J., Long X., Lv Y., Kelly C., Schäfer A., Goldhahn D., Abbushi A., Milham M.P., Lohmann G., Villringer A. Resting developments: a review of fMRI post-processing methodologies for spontaneous brain activity. MAGMA. 2010;23:289–307. doi: 10.1007/s10334-010-0228-5. [DOI] [PubMed] [Google Scholar]
- McDermott K.B., Petersen S.E., Watson J.M., Ojemann J.G. A procedure for identifying regions preferentially activated by attention to semantic and phonological relations using functional magnetic resonance imaging. Neuropsychologia. 2003;41:293–303. doi: 10.1016/s0028-3932(02)00162-8. [DOI] [PubMed] [Google Scholar]
- Menon V., Uddin L.Q. Saliency, switching, attention and control: a network model of insula function. Brain Struct. Funct. 2010;214:655–667. doi: 10.1007/s00429-010-0262-0. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mueller S., Wang D., Fox M.D., Yeo B.T., Sepulcre J., Sabuncu M.R., Shafee R., Lu J., Liu H. Individual variability in functional connectivity architecture of the human brain. Neuron. 2013;77:586–595. doi: 10.1016/j.neuron.2012.12.028. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Power J.D., Fair D.A., Schlaggar B.L., Petersen S.E. The development of human functional brain networks. Neuron. 2010;67:735–748. doi: 10.1016/j.neuron.2010.08.017. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Power J.D., Barnes K.A., Snyder A.Z., Schlaggar B.L., Petersen S.E. Spurious but systematic correlations in functional connectivity MRI networks arise from subject motion. Neuroimage. 2012;59:2142–2154. doi: 10.1016/j.neuroimage.2011.10.018. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Raznahan A., Lee Y., Stidd R., Long R., Greenstein D., Clasen L., Addington A., Gogtay N., Rapoport J.L., Giedd J.N. Longitudinally mapping the influence of sex and androgen signaling on the dynamics of human cortical maturation in adolescence. Proc. Natl. Acad. Sci. U. S. A. 2010;107:16988–16993. doi: 10.1073/pnas.1006025107. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rubia K. Functional brain imaging across development. Eur. Child Adolesc. Psychiatry. 2013;22:719–731. doi: 10.1007/s00787-012-0291-8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sato J.R., Salum G.A., Gadelha A., Picon F.A., Pan P.M., Vieira G., Zugman A., Hoexter M.Q., Anés M., Moura L.M., Gomes Del’Aquilla M.A., Amaro E., Jr., McGuire P., Crossley N., Lacerda A., Rohde L.A., Miguel E.C., Bressan R.A., Jackowski A.P. Age effects on the default mode and control networks in typically developing children. J. Psychiatr. Res. 2014;58:89–95. doi: 10.1016/j.jpsychires.2014.07.004. [DOI] [PubMed] [Google Scholar]
- Schöning S., Engelien A., Kugel H., Schäfer S., Schiffbauer H., Zwitserlood P., Pletziger E., Beizai P., Kersting A., Ohrmann P., Greb R.R., Lehmann W., Heindel W., Arolt V., Konrad C. Functional anatomy of visuo-spatial working memory during mental rotation is influenced by sex, menstrual cycle, and sex steroid hormones. Neuropsychologia. 2007;5:3203–3214. doi: 10.1016/j.neuropsychologia.2007.06.011. [DOI] [PubMed] [Google Scholar]
- Seeley W.W., Menon V., Schatzberg A.F., Keller J., Glover G.H., Kenna H., Reiss A.L., Greicius M.D. Dissociable intrinsic connectivity networks for salience processing and executive control. J. Neurosci. 2007;27:2349–2356. doi: 10.1523/JNEUROSCI.5587-06.2007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Shirer W.R., Ryali S., Rykhlevskaia E., Menon V., Greicius M.D. Decoding subject-driven cognitive states with whole-brain connectivity patterns. Cereb. Cortex. 2012;22:158–165. doi: 10.1093/cercor/bhr099. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Smith S.M., Fox P.T., Miller K.L., Glahn D.C., Fox P.M., Mackay C.E., Filippini N., Watkins K.E., Toro R., Laird A.R., Beckmann C.F. Correspondence of the brain's functional architecture during activation and rest. Proc. Natl. Acad. Sci. U. S. A. 2009;106:13040–13045. doi: 10.1073/pnas.0905267106. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Steinberg L. Cognitive and affective development in adolescence. Trends Cogn. Sci. 2005;9:69–74. doi: 10.1016/j.tics.2004.12.005. [DOI] [PubMed] [Google Scholar]
- Stevens M.C., Pearlson G.D., Calhoun V.D. Changes in the interaction of resting-state neural networks from adolescence to adulthood. Hum. Brain Mapp. 2009;30:2356–2366. doi: 10.1002/hbm.20673. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Supekar K., Uddin L.Q., Prater K., Amin H., Greicius M.D., Menon V. Development of functional and structural connectivity within the default mode network in young children. Neuroimage. 2010;52:290–301. doi: 10.1016/j.neuroimage.2010.04.009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Thomason M.E., Chang C.E., Glover G.H., Gabrieli J.D., Greicius M.D., Gotlib I.H. Default-mode function and task-induced deactivation have overlapping brain substrates in children. Neuroimage. 2008;41:1493–1503. doi: 10.1016/j.neuroimage.2008.03.029. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Thomason M.E., Dennis E.L., Joshi A.A., Joshi S.H., Dinov I.D., Chang C., Henry M.L., Johnson R.F., Thompson P.M., Toga A.W., Glover G.H., Van Horn J.D., Gotlib I.H. Resting-state fMRI can reliably map neural networks in children. Neuroimage. 2011;55:165–175. doi: 10.1016/j.neuroimage.2010.11.080. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tisch S., Silberstein P., Limousin-Dowsey P., Jahanshahi M. The basal ganglia: anatomy, physiology, and pharmacology. Psychiatr. Clin. N. Am. 2004;27:757–799. doi: 10.1016/j.psc.2004.06.004. [DOI] [PubMed] [Google Scholar]
- Uddin L.Q., Supekar K., Menon V. Typical and atypical development of functional human brain networks: insights from resting-state FMRI. Front. Syst. Neurosci. 2010;4:21. doi: 10.3389/fnsys.2010.00021. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Uddin L.Q., Supekar K.S., Ryali S., Menon V. Dynamic reconfiguration of structural and functional connectivity across core neurocognitive brain networks with development. J. Neurosci. 2011;31:18578–18789. doi: 10.1523/JNEUROSCI.4465-11.2011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- van Duijvenvoorde A.C., Achterberg M., Braams B.R., Peters S., Crone E.A. Testing a dual-systems model of adolescent brain development using resting-state connectivity analyses. Neuroimage. 2015;10:409–420. doi: 10.1016/j.neuroimage.2015.04.069. [DOI] [PubMed] [Google Scholar]
- Vinette S.A., Bray S. Variation in functional connectivity along anterior-to-posterior intraparietal sulcus, and relationship with age across late childhood and adolescence. Dev. Cogn. Neurosci. 2015;13:32–42. doi: 10.1016/j.dcn.2015.04.004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wechsler D. Paidós; Buenos Aires: 1994. The Wechsler Intelligence Scale for Children III. [Google Scholar]
- Wechsler D. TEA ediciones; Madrid: 1999. Wechlser Adult Intelligence Scale III. [Google Scholar]
- Weiss E., Siedentopf C.M., Hofer A., Deisenhammer E.A., Hoptman M.J., Kremser C., Golaszewski S., Felber S., Fleischhacker W.W., Delazer M. Sex differences in brain activation pattern during a visuospatial cognitive task: a functional magnetic resonance imaging study in healthy volunteers. Neurosci. Lett. 2003;344:169–172. doi: 10.1016/s0304-3940(03)00406-3. [DOI] [PubMed] [Google Scholar]
- Weissman-Fogel I., Moayedi M., Taylor K.S., Pope G., Davis K.D. Cognitive and default-mode resting state networks: do male and female brains “rest” differently? Hum. Brain Mapp. 2010;31:1713–1726. doi: 10.1002/hbm.20968. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wenderoth N., Debaere F., Sunaert S., Swinnen S.P. The role of anterior cingulate cortex and precuneus in the coordination of motor behaviour. Eur. J. Neurosci. 2005;22:235–246. doi: 10.1111/j.1460-9568.2005.04176.x. [DOI] [PubMed] [Google Scholar]
- Wu K., Taki Y., Sato K., Hashizume H., Sassa Y., Takeuchi H., Thyreau B., He Y., Evans A.C., Li X., Kawashima R., Fukuda H. Topological organization of functional brain networks in healthy children: differences in relation to age, sex, and intelligence. PLOS ONE. 2013;8:e55347. doi: 10.1371/journal.pone.0055347. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Xu C., Li C., Wu H., Wu Y., Hu S., Zhu Y., Zhang W., Wang L., Zhu S., Liu J., Zhang Q., Yang J., Zhang X. Gender differences in cerebral regional homogeneity of adult healthy volunteers: a resting-state FMRI study. Biomed. Res. Int. 2015;183074 doi: 10.1155/2015/183074. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yan C., Zang Y. DPARSF: a MATLAB toolbox for “pipeline” data analysis of resting-state fMRI. Front. Syst. Neurosci. 2010;4:13. doi: 10.3389/fnsys.2010.00013. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zielinski B.A., Gennatas E.D., Zhou J., Seeley W.W. Network-level structural covariance in the developing brain. Proc. Natl. Acad. Sci. U. S. A. 2010;107:18191–18196. doi: 10.1073/pnas.1003109107. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.