Abstract
The individual tendency to interpret ambiguous situations negatively is associated with mental disorders. Interpretation biases are already evident during adolescence and due to the greater plasticity of the developing brain it may be easier to change biases during this time. We investigated in healthy adolescents and adults whether stabilizing memories of positive or negative scenes modulates the later interpretation of similar scenes. In the evening, participants learnt associations between ambiguous pictures and words that disambiguate the valence of the pictures in a positive or negative direction. Half of the words were acoustically presented (i.e. cued) during post-learning sleep which is known to benefit memory consolidation by inducing reactivation of learned information. Cued compared to un-cued stimuli were remembered better the next morning. Importantly, cueing positively disambiguated pictures resulted in more positive interpretations whereas cueing negatively disambiguated pictures led to less positive interpretations of new ambiguous pictures with similar contents the next morning. These effects were not modulated by participants’ age indicating that memory cueing was as efficient in adolescents as in adults. Our findings suggest that memory cueing during sleep can modify interpretation biases by benefitting memory stabilization and generalization. Implications for clinical settings are discussed.
Keywords: Development, Memory, Sleep, Plasticity, Reactivation, Interpretation bias
1. Introduction
There are innumerable situations in life that can be interpreted in more than one way. People differ greatly with regard to the way they interpret such ambiguous situations. Consider the example of greeting a colleague when seeing him walking on campus, but the colleague does not respond to your greeting. To some people, this behaviour signals dislike or ignorance, whereas for others it simply indicates that he is deeply in his thoughts or even highly stressed. An “interpretation bias” is defined as an individual tendency to frequently classify ambiguous situations in a particular valenced direction, e.g. as threatening or pleasant. There is a reciprocal relationship between a person's schema about the self, world and future and an interpretation bias (Bartlett, 1932, Hertel et al., 2008, Tran et al., 2011, Field and Field, 2013). More specifically, on the one hand constantly interpreting ambiguous situations in a positive or negative way can form and strengthen such schema, but on the other hand a specific interpretive bias can be an expression of an existing schema. Leading cognitive psychotherapy techniques aim to modify negative biases by either challenging the validity of the negative interpretation and/or supporting the acquisition of a positive interpretation (Beck, 1976, Mathews and MacLeod, 2005, Holmes et al., 2009). The generalization of these learned interpretations to new situations is a key goal of cognitive therapy. Negative interpretation biases are already observable in adolescents suffering from anxiety disorders and such early emerging psychopathology can have long-term detrimental outcomes (Salemink and Wiers, 2011, Lau et al., 2012). Due to the high plasticity of the developing brain, it has been hypothesized that the chance to modify an existing interpretation bias is greater during early as compared to later developmental stages (Lau, 2013). However, empirical evidence for this is rather scarce.
The interpretation of ambiguous situations essentially depends on our memory about previous outcomes of similar situations. Thus, strengthening the consolidation of such situations might change the interpretation of future situations. There is now extensive evidence supporting the idea that sleep and here mainly slow wave sleep (SWS, that is characterized by EEG activity at a frequency of 0.5–4 Hz) strongly benefits the consolidation of newly acquired memories thereby enhancing performance at a later recall (Diekelmann and Born, 2010, Inostroza and Born, 2013). Besides the mere stabilization, sleep also contributes to the transformation of memories. More specifically, it has been argued that sleep-dependent memory consolidation results in the abstraction of a context-free, schema-like mental representation that enables the generalization of knowledge (Inostroza and Born, 2013, Lewis and Durrant, 2011). In a recent experiment, we demonstrated that sleep in children as compared to adults is more efficient in supporting the transformation of newly acquired memories. This superior ability was associated with the high amount of SWS in this age group and with hippocampal activation at memory recall (Wilhelm et al., 2013). The consolidation of memories during sleep can be improved by re-exposing context cues. More specifically, the re-exposure of an odour, words or tones that are associated with the newly learnt memories during sleep benefits later recall of these memories (Rasch et al., 2007, Rudoy et al., 2009, Schreiner and Rasch, 2014). The memory enhancing effect of cueing has been attributed to the facilitation of endogenous memory reactivations during SWS which is proposed to be an underlying mechanism of memory consolidation (Diekelmann and Born, 2010).
In the present experiment, we aimed to investigate in adolescents and adults whether sleep-related cueing of ambiguous pictures that were disambiguated in an either positive or negative direction (i) improves later retention of these stimuli and (ii) modifies the interpretation of new ambiguous pictures of similar content with this being considered a measure of generalization. We used a learning task that includes mental imagery from a field perspective (i.e. mental imagery in the first person). This innovative method is assumed to mimic the way memories about real events are processed by inducing vivid memories of high personal relevance (Kosslyn et al., 2001, Holmes et al., 2008).
2. Materials and methods
2.1. Participants
Twenty-one adolescents between 9 and 16 yrs (M = 12.34, SEM = 0.50 yrs; 6 females, 15 males) and nineteen adults between 18 and 25 yrs (M = 22.22, SEM = 0.61 yrs; 14 females, 5 males) participated in the study. Participants were recruited via advertisements placed at the university, at the children's hospital and in local newspapers. Interviews with the participants and their parents (for adolescents) as well as standardized questionnaires ensured that the participants had no behavioural problems, cognitive impairments or sleep disorders. More specifically, we assessed in a telephone screening interview (for recruitment) and in a more elaborate interview (at the beginning of the adaption night) the information of life-time and current irregularities in sleeping behaviour, learning behaviour and general diseases. Moreover, prior to the experiment, the participants’ intelligence quotient (IQ) was assessed by a short form of the Hamburg-Wechsler Intelligence test for children (HAWIK-IV; Waldmann, 2008) which is the German adaptation of the Wechsler Intelligence Scales for Children in fourth revision (WISC-IV; Wechsler, 2003). The four-subtest short form of the HAWIK-IV encompasses the subtests of ‘Vocabulary’, ‘Letter-Number-Sequencing’, ‘Matrix reasoning’ and ‘Symbol Search’ corresponding to the cognitive abilities ‘verbal comprehension’, ‘perceptual reasoning’, ‘working memory’ and ‘processing speed’, respectively. All participants included in the sample were of normal intelligence (mean: 108.50, SD: 13.20, range: 87–129). Neurological and psychiatric disorder were tested in a structured diagnostic interview, i.e. the Mini International Neuropsychiatric Interview for Children and Adolescents (MINI-KID; Sheehan et al., 1998) performed by a trained research clinician. Additionally, an inventory for the assessment of social anxiety in children was filled out by participants (SPAIK, Melfsen et al., 2001) since we intended to carefully control for one of the most common mental disorders during childhood. All participants were asked for individual sleep habits, i.e. usual time to go to bed, time getting up, etc. in order to schedule both nights in the sleep laboratory in accordance with their usual sleep habits. Also, participants took notes on their daily activities, sleep time and time to get up in a sleep diary for the seven days prior to the experiment which was additionally compared and supplemented by actigraphical monitoring. Participants did not take any medication at the time of the experiment, and the ingestion of caffeine or alcohol was not allowed on experimental days. The study was approved by the local ethics committee, and participants gave written informed consent before participating. For the adolescents this was accomplished by a parent. Additionally, all adolescents provided verbal assent. One participant from the younger age-group did not enter the sleep EEG analyses because of technical problems, and data was lacking from two further participants from the younger age-group for the subjective ratings and generalization task.
2.2. Design and procedure
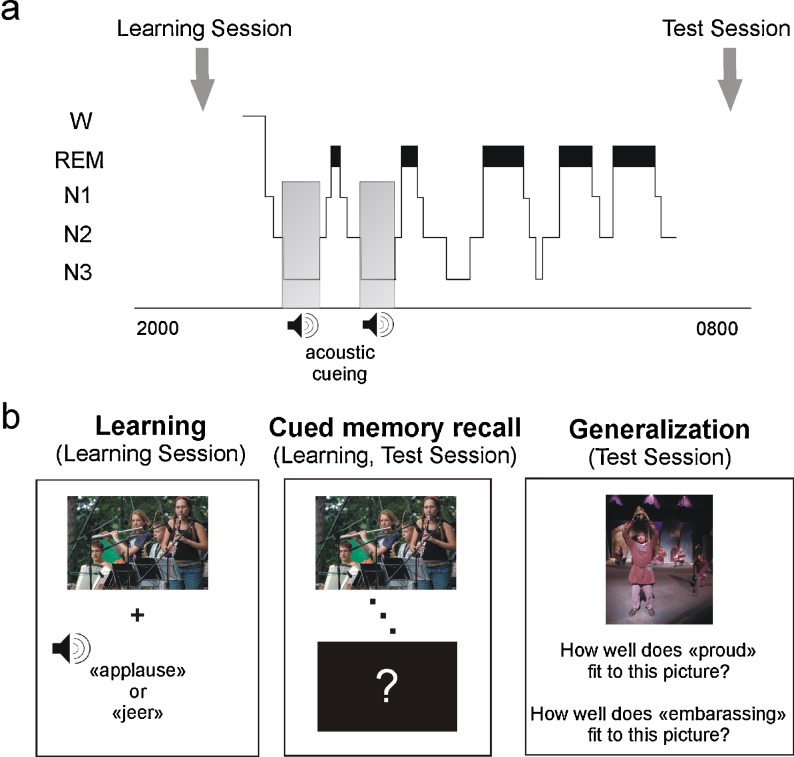
Participants were adapted to polysomnographic recordings on a night preceding the experimental night. Experimental and adaptation night in the sleep lab were separated by at least one night at home to exclude possible effects of bad sleep quality in the adaptation night on sleep in the experimental night. In the experimental night, participants came to the sleep lab around 2.5 hrs before participants’ habitual bedtime. First, the electrode net was placed. Immediately thereafter, participants performed the picture-word association task (including learning and immediate recall of picture-word associations) and went to bed afterwards. During sleep stage N3, half of the learnt words were presented via loudspeaker placed behind the participants’ head to stimulate reactivation of the respective picture-word associations. The next morning, participants were awakened at their habitual wake time. Testing started ∼45 min later to avoid any modulating effect of sleep inertia on recall performance (see Fig. 1a for the experimental procedure). This Test Session included the cued memory recall of picture-word associations, the subjective ratings of arousal and valence of pictures and, 1–2 h later, the generalization task. In the Learning and the Test Session, participants first rated their tiredness, concentration and motivation with a subjective visual analogue scale.
Fig. 1.
Experimental procedure and picture-word association task. (a) After placement of the electrode net participants completed a learning phase followed by immediate recall testing taking place between ∼20:00 and 22:00 h. A typical polysomnogram visualizes the proportion of sleep stages during the nocturnal retention interval (wake (W), non-rapid eye movement (Non-REM) sleep stages 1–3 (N1–N3), REM sleep) and speakers indicate that acoustic cueing was performed during N3 in the post-learning night for a maximum number of 12 times per word. The next morning, participants performed on a cued memory recall and on the generalization task. (b) Learning the picture-word association task required participants to associate ambiguous pictures with acoustically presented positive or negative words that disambiguate the pictures. Participants had to vividly imagine themselves in the situation shown on the picture. In a cued memory recall immediately after learning and in the Test Session after sleep pictures from the Learning Session were shown again one after another and participants had to recall the corresponding word. In the generalization task, a list of new ambiguous pictures (with each of the pictures corresponding to one of the old pictures with regard to its content) was presented to the participants and they were required to indicate on a 9-point scale how well a new positive and a new negative related word fits to this picture.
2.3. Picture-word association task
The picture-word association task used in our study is a modification of a task previously used in studies on mental imagery (Holmes et al., 2008, Pictet et al., 2011, see Fig. 1b for an illustration of the task). The task includes a set of 120 (for participants older than 12.5 yrs) or 102 (for participants younger than 12.5 yrs) ambiguously valenced photographs of common everyday life objects and scenes. A different number of stimuli in younger and older participants were chosen to ensure comparable encoding level in all participants. This is considered to be relevant because it is well known that memory capacity increases with age until early adulthood and that the level of encoding is associated with the efficacy of memory cueing (Creery et al., 2015). In a pilot experiment we had determined that 102 stimuli for the younger half (i.e. 9–12.5) and 120 stimuli for the older half of the age-range (12.5–16 yrs) led to encoding levels of 50–80% of the presented stimuli. The pictures used here were either taken from previous studies (Holmes et al., 2008, Pictet et al., 2011) or were downloaded from the Internet (non-copyrighted). Pictures were paired with words that had been chosen so that their pairing with the picture (e.g. a picture showing a person acting in a play in front of an audience, see Fig. 1b) either resolved ambiguity of the scene shown on the picture in a positive (half of the pictures; e.g. “applause”) or in a negative way (e.g. “jeer”). Words were spoken by a male voice and recorded at a sampling rate of 96 kHz. During learning, normal speech volume (70 dB sound pressure level) was used. Three different sets of words and pictures (set 1 for participants < 12.5 yrs, set 2 for participants > 12.5 and <16; set 3 for participants > 16) were used so that they are suitable for each of the tested participants (e.g. by showing people at the participants’ age and by showing scenes typically encountered by participants of the respective age). Before starting the learning procedure, all participants were given an imagery generation instruction similar to that used in Holmes et al. (2008) as well as two practice trials. More specifically, participants were first informed about the concept of mental imagery from a field perspective (i.e. seeing the situation through their own eyes, as if they were actively involved) and then were instructed to generate vivid mental imageries in response to picture-word associations by imagining the scenes from a field perspective. In case they were not able to do so in a sufficient manner in the two practice trials, they received another round of the training. Nevertheless, we cannot exclude that there is some variability in the extent to which participant had been engaged in mental imagery, especially, since we did not ask for it. Future studies need to test for each of the stimuli the extent to which participants were engaged in mental imagery. We chose here mental imagery instead of merely presenting the stimuli because this is reported to elicit a much stronger emotional response due to the greater personal relevance (Holmes et al., 2008, Mathews et al., 2013). Participants were also instructed to memorize the picture-word associations because memory performance would be tested after learning and again after the retention interval. During learning, each of the picture-word associations was presented for 1500 ms. A black screen then appeared for 3000 ms during which the participants were asked to shut their eyes and to generate a mental image about the imagined scene. Thereafter, a 1000 ms beep informed participants to open their eyes and the next picture-word association was presented. Memory performance was tested for all picture-word associations in a cued recall procedure immediately after learning to assess baseline performance, and with the same procedure again the next morning. In these two cued recall tests, all pictures were presented one after another in a random order for a duration of 1500 ms and the participants were required to recall the associated word (by speaking out loud the remembered word to the experimenter who stood next to them and recorded memory performance). The time to generate a response was not limited. All words that were identical with the learnt word or included the word-stem of the learnt word were counted as correct. We calculated the relative difference between correctly recalled words before and after the retention interval for the category of cued and un-cued picture-word associations as a measure of retention performance (i.e. 100% means the same amount of words being correctly recalled before and after sleep).
2.4. Valence and arousal ratings
The valence and arousal ratings were obtained for all pictures in the Test Session with the computer-based version of the Self-Assessment Manikin (SAM) rating system (Bradley and Lang, 1994). For each picture, participants were instructed to rate spontaneously and quickly how emotional they perceived the picture on the dimensions of valence (i.e. pleasantness) and arousal (i.e. excitement) on a 9-point dimensional scale (valence: 1 = very unpleasant, 5 = neutral, 9 = very pleasant; arousal: 1 = not arousing at all, and 9 = very arousing).
2.5. Generalization task
In order to test whether disambiguating pictures in a positive or a negative way during encoding can be generalized, the interpretation of new ambiguous pictures was assessed. We presented 83 (for the younger age-group) or 90 (for the older age-group) new ambiguous pictures during the Test Session, with each of these pictures corresponding to one of the pictures from the Learning session in terms of the content (see Fig. 1b for an example). Participants were required to rate for each of these pictures on a 9-point scale how well a new semantically related word that either disambiguates the picture in a positive or a negative way “fits” with the presented picture. Both semantically related words were presented one after the other on a screen together with the rating scale. The order of both valence categories was balanced insofar as for half of the trials participants first had to make their rating for “fit” of the positive word, and for the other half of trials participants first rated “fit” for the negative word.
2.6. Tiredness, concentration and motivation
The extent of tiredness, concentration and motivation was assessed in the evening before learning and in the morning before retrieval testing by using a visual analogue scale (VAS) in order to exclude possible age-dependent differences that might have impacted task performance. Participants responded to a VAS item by indicating a position along a continuous line between two end-points (i.e.: tiredness – from ‘not tired at all/fully awake’ until ‘very tired/close to falling asleep’, concentration – from ‘very un-concentrated’ until ‘very concentrated’ and motivation – from ‘very motivated’ until ‘not motivated at all’). Marked positions in the VAS items were measured and calculated as the relative amount of tiredness, concentration and motivation in percent (e.g. 80% tired). The age-groups did not differ in their subjective ratings of tiredness, concentration and motivation in the Learning and the Test Session (all p > 0.29) suggesting that these variables did not affect task performance differentially in the two age-groups.
2.7. Memory cueing during sleep
During post-learning periods of N2 and N3 sleep, emotional words that had been paired with a picture during the learning phase were presented again aurally via loudspeaker (with a 55 dB sound pressure level). Half of the learnt words were cued, whereas the other half of the learnt words were not cued. Half of the cued words were randomly and individually chosen from the group of words that had been correctly remembered by a participant during the learning phase (i.e. hits), whereas the other half were taken from unknown words (i.e. misses). Half of the cued words were positive and half of them were negative. For example, given that a participant correctly remembered half of the 120 picture-word associations—with half of them being negative and half of them being positive—this would result in cueing of 15 positive and 15 negative hits and misses. Words were presented with an inter-stimulus-interval of 6 s with a random jitter of 0–0.4 s. Each of the chosen words was cued at a maximum of 12 times. An experienced experimenter inspected the EEG in real-time to determine sleep stages and to detect any indicator of an arousal. Word cueing was started when a participant had spent more than 10 min in non-REM stage N3, and it was immediately stopped whenever any sign of an arousal, waking up or REM-sleep was observed by the experimenter.
2.8. Sleep EEG
Sleep in both the experimental and the adaptation night was recorded using high-density sleep EEG (Electrical Geodesics Sensor Net for long-term monitoring, 128 channels) including electroencephalographic (EEG), electromyographic and electrooculographic recordings. Data were sampled at 500 Hz (0.01–200 Hz). Offline, the EEG was bandpass filtered (0.5–50 Hz) and downsampled to 128 Hz. The EEG was visually scored for sleep stages Wake, N1, N2, N3 and REM at frontal, central and occipital electrodes (20 s epochs) based on American Academy of Sleep Medicine standard criteria (Iber et al., 2007). As expected, relative to adults, the adolescents slept longer and sleep in this group was characterized by a higher percentage of N3, lower percentage of lighter sleep stages N1 and N2, and a shorter sleep latency (see Table 1 for all sleep parameters and statistics). Cueing had no substantial impact on sleep in this experiment as all relevant sleep parameters (i.e. sleep latency, total sleep time, wake time after sleep onset and sleep efficacy) in the current sample were comparable with those from a previous high-density EEG experiment in adolescents (N = 27) and adults (N = 19) that did not include cueing during the night (Wilhelm et al., 2014).
Table 1.
Sleep parameters.
| Adolescents | Adults | |
|---|---|---|
| Mean ± SEM | Mean ± SEM | |
| Sleep latency | 17.00 ± 2.52 | 26.19 ± 3.97# |
| Wake after sleep onset (%) | 9.95 ± 2.01 | 5.70 ± 2.00 |
| Non-REM 1 (%) | 3.52 ± 0.55 | 6.02 ± 0.98* |
| Non-REM 2 (%) | 44.84 ± 1.97 | 49.73 ± 1.51# |
| Non-REM 3 (%) | 32.47 ± 2.07 | 24.77 ± 1.66** |
| REM (%) | 19.17 ± 0.72 | 19.49 ± 1.06 |
| TST | 520.88 ± 9.88 | 423.96 ± 8.92*** |
| Sleep efficacy | 89.03 ± 1.70 | 90.46 ± 1.50 |
Sleep parameters are given in Mean ± SEM of absolute time in minutes and percentage of total sleep time; REM = rapid eye movement sleep and TST = total sleep time.
p < 0.1 (statistical differences between age-groups are indicated).
p < 0.05 (statistical differences between age-groups are indicated).
p < 0.01 (statistical differences between age-groups are indicated).
p < 0.001 (statistical differences between age-groups are indicated).
2.9. Statistical analyses
Statistical analysis of memory retention and subjective ratings was based on 2 × 2 × 2 analyses of variance (ANOVA) including the repeated measures factors ‘valence at encoding’ (positive, negative) and ‘cueing’ (cued, un-cued) and the between-subjects factor ‘age-group’ (adolescents, adults). The generalization of the learnt disambiguation of pictures was first analyzed with a 2 × 2 × 2 × 2 ANOVA including the within-subjects factors ‘valence at encoding’, ‘cueing’, ‘target valence’ and the between-subjects factor ‘age-group’. Thereafter, we separately analyzed the subjectively rated fit of a positive and a negative word with a new ambiguous picture with a 2 × 2 × 2 ANOVA including the within-subjects factors ‘valence at encoding’ and ‘cueing’ and the between-subjects factor ‘age-group’. Post hoc comparisons as well as all statistical comparisons with only one level of two groups/conditions were performed using student t-tests. The level of significance was set to p < 0.05.
3. Results
3.1. Retention performance of picture-word associations (Learning and Test Session)
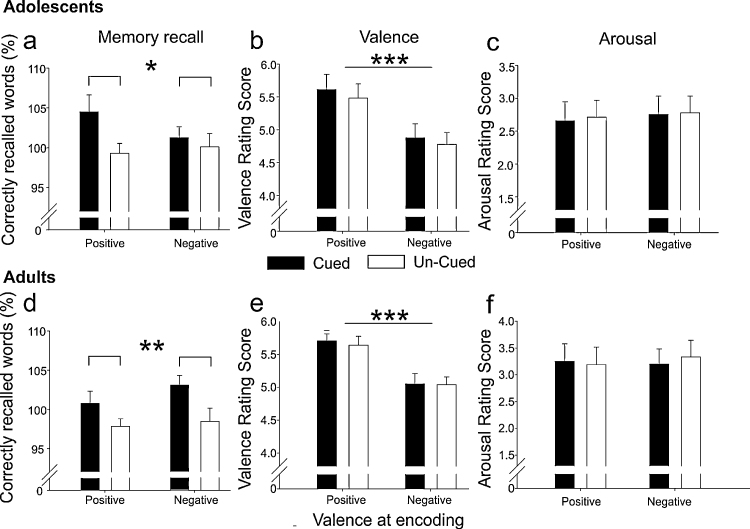
During post-learning SWS, half of the positive and negative words that had been associated with the ambiguous pictures in the Learning Session were acoustically presented again (being termed as “cued” associations), whereas the other half was not presented (“un-cued” associations). As a first step, we analyzed whether cueing did substantially strengthen memory for picture-word associations. Both age-groups indeed correctly remembered more picture-word associations that had been cued during post-learning sleep than those that had not been cued (main effect of ‘cueing’: F(1,38) = 12.70, p = 0.001; Fig. 2a and d). Importantly, the beneficial effect of memory cueing during sleep on retention performance did not differ between both age-groups (interaction ‘age-group’ × ‘cueing’: F(1,38) = 0.09, p > 0.76) and it was also not modulated by the valence of picture-word pairs (interaction ‘cueing’ × ‘valence at encoding’: F(1,38) = 0.33, p > 0.56). Memory cueing resulted in an increase in additionally remembered picture-word associations (i.e. words that were not remembered during encoding, but were remembered in the Test Session—these words are termed as “gains”; main effect of ‘cueing’ for gains F(1,38) = 12.06, p = 0.001) and a reduced forgetting of previously remembered words (main effect of ‘cueing’ for losses F(1,38) = 5.02, p = 0.03). Again, these effects were not modulated by the age of participants (interaction ‘age-group’ × ‘cueing’ for both gains and losses p > 0.20; see Table 2 for descriptives). Retention performance immediately after learning also was neither affected by the valence of picture-word associations nor by the age of participants (all p > 0.25, see Table 2 for descriptives).
Fig. 2.
Memory performance and subjective ratings of valence and arousal after post-learning sleep (in the Test Session) in adolescents (a–c) and adults (d–f). (a and d) Retention performance was increased for cued (black bars) as compared to un-cued (white bars) picture-word associations in the morning after cueing (the main effect of ‘cueing’ in a 2 (‘cueing’) × 2 (‘valence at encoding’) ANOVA is indicated). The relative difference in correctly recalled words before and after the retention interval for cued and un-cued picture-word associations with pre-sleep level being set to 100% is indicated as a measure of retention performance. (b and e) Disambiguating the pictures in a negative or positive way by associating a positive or negative word in the Learning Session modulated later ratings of pleasantness/valence of the pictures but cueing did not modulate the subjectively rated pleasantness. (c and f) The subjectively rated arousal of pictures was neither affected by the valence at encoding nor by cueing. Values are Mean ± SEM, *p ≤ 0.05, **p ≤ 0.01, ***p ≤ 0.001.
Table 2.
Behavioural data.
| Adolescents |
Adults |
|||
|---|---|---|---|---|
| Positive Mean ± SEM |
Negative Mean ± SEM |
Positive Mean ± SEM |
Negative Mean ± SEM |
|
| Immediate recall (absolute) | 41.10 ± 1.33 | 42.76 ± 1.23 | 45.37 ± 1.96 | 44.74 ± 1.94 |
| Immediate recall (relative) | 70.76 ± 2.25% | 72.73 ± 2.21% | 74.56 ± 3.23% | 75.61 ± 3.26% |
| Cued Mean ± SEM |
Un-cued Mean ± SEM |
Cued Mean ± SEM |
Un-cued Mean ± SEM |
|
|---|---|---|---|---|
| Gains | 2.57 ± 0.32 | 1.76 ± 0.28 | 1.89 ± 0.32 | 1.11 ± 0.27 |
| Losses | 1.57 ± 0.29 | 1.86 ± 0.33 | 1.00 ± 0.25 | 2.05 ± 0.39 |
| Rating of fit of positive words to new ambiguous pictures at test | ||||
| Pictures disambiguated positively at encoding | 6.58 ± 0.16 | 6.27 ± 0.21 | 6.47 ± 0.18 | 6.12 ± 0.16 |
| Pictures disambiguated negatively at encoding | 6.03 ± 0.19 | 6.43 ± 0.22 | 6.31 ± 0.16 | 6.42 ± 0.16 |
| Rating of fit of negative words to new ambiguous pictures at test | ||||
| Pictures disambiguated positively at encoding | 4.51 ± 0.31 | 4.63 ± 0.27 | 4.25 ± 0.17 | 4.25 ± 0.18 |
| Pictures disambiguated negatively at encoding | 4.92 ± 0.27 | 4.59 ± 0.26 | 4.10 ± 0.21 | 4.22 ± 0.28 |
It contains the behavioural data for encoding (immediate recall), the number of gains and losses after cueing as well as the rated fit of positive and negative words to new ambiguous pictures during the generalization task.
3.2. Subjective ratings of pictures
Please see Fig. 2 for results and statistics. The valence of words that disambiguates the pictures modulated later ratings of pleasantness/valence of the pictures (F = 77.58, p < 0.001) but cueing did not modulate the subjectively rated pleasantness (p > 0.21). The subjectively rated arousal of pictures was neither affected by the valence of the words nor by cueing (both p > 0.75).
3.3. Interpretation of new ambiguous pictures
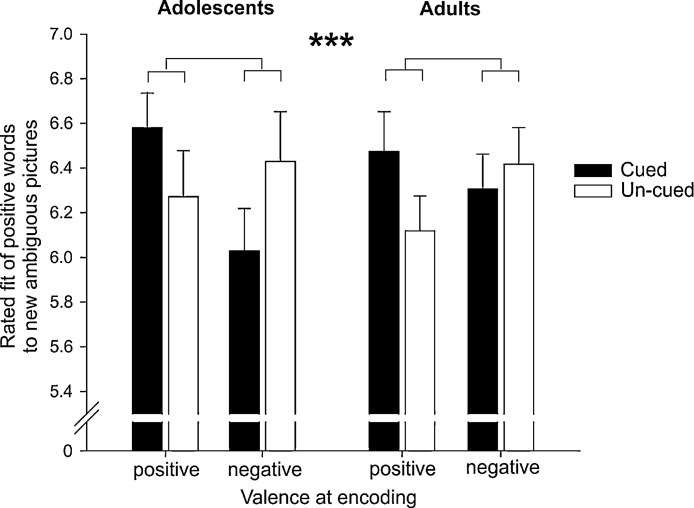
We further analyzed whether cueing during sleep supports the generalization of the positive or negative disambiguation of pictures in the Learning Session to the Test Session as indicated by the subjectively rated fit of a new positive or negative word to novel pictures being similar in content to the pictures presented in the Learning Session. We found that the effect of the valence at encoding on the rated fit of novel words to novel ambiguous pictures was strongly modulated by cueing during sleep (interaction between ‘cueing’, ‘valence at encoding’, ‘target valence’: F(1,36) = 11.38, p = 0.002 in 2 × 2 × 2 × 2 ANOVA). Neither the main effect nor any interaction including the factor ‘age-group’ reached significance (all p > 0.07). To explore the interaction effect in detail, we separately analyzed in two 2 (‘cueing’) × 2 (‘valence at encoding’) ANOVAs the fit of positive and the fit of negative words to new ambiguous pictures. Cueing did substantially modulate the subjectively rated fit of a positive word to new ambiguous pictures, with this critically depending on the valence of pictures at encoding (interaction ‘cueing’ × ‘valence at encoding’ F(1,37) = 13.14, p = 0.001; Fig. 3; see Table 2 for descriptives). More specifically, cueing pictures that had been disambiguated in a positive way led to a higher fit of positive new words to new ambiguous pictures (cued vs. un-cued pictures-word associations t(37) = 2.70, p = 0.01). Cueing pictures that were disambiguated in a negative way led to a lower fit of new positive words to new ambiguous pictures (t(37) = 2.30, p = 0.027). Moreover, a positive disambiguation during encoding did not per se lead to a greater fit of positive words at test, but it did so after cueing (positive vs. negative disambiguation – cued: t(39) = 2.61, p = 0.013; – uncued: t(39) = −1.18, p = 0.24). These findings indicate that cueing during sleep benefits the generalization of positively and negatively disambiguated scenes to new ambiguous scenes. The subjectively rated fit of a negative word to a new ambiguous picture was not affected by cueing (all main effects and interactions p > 0.20; see Table 2 for descriptives). The lack of effect of cueing on the subjective fit of negative words to novel pictures was coincided by a general tendency of participants to indicate a better fit of a positive as compared to a negative word to ambiguous pictures (main effect of valence of tested interpretation: F(1,37) = 157.84, p < 0.001).
Fig. 3.
Generalization of the positive and negative disambiguation of pictures to new ambiguous pictures. Cueing did substantially modulate positive interpretation of new ambiguous pictures in dependence of the learnt valence of the corresponding picture at encoding (interaction ‘cueing’ × ‘valence at encoding’ is indicated, cued interpretations: black bars; un-cued interpretations: white bars). In both age-groups, cued as compared to un-cued positively disambiguated pictures led to more positive interpretation of new ambiguous pictures as indicated by a higher fit of positive new words to the picture whereas cueing negatively disambiguated pictures led to a lower fit of positive new words to new ambiguous pictures. Word-picture fit is indicated on a 9-point scale (1 = very low fit and 9 = perfect fit). ***p ≤ 0.001.
4. Discussion
Here, we found in adolescents and adults that memory cueing during post-learning sleep facilitated the consolidation of newly acquired associations between ambiguous pictures and words that disambiguated the pictures in a positive or negative way. Importantly, cueing these picture-word associations during sleep modified the interpretation of similar pictures as indicated by the rated fit of positive words to new ambiguous pictures. More specifically, cueing positive picture-word associations resulted in a greater subjectively rated fit of a word that was chosen so that it disambiguates the scene presented on the picture in a positive direction. Cueing negative picture-word associations led to a smaller subjectively rated fit of a positive word to novel ambiguous pictures. The beneficial effects of memory cueing during sleep on the consolidation and generalization of newly acquired memories did not differ between adolescents and adults.
Our findings are in line with a number of previous studies reporting a beneficial effect of memory cueing during periods of slow wave sleep on the stabilization of newly acquired memories (Rasch et al., 2007, Rudoy et al., 2009, Diekelmann et al., 2011, Schönauer et al., 2014). While most of these previous studies were conducted using odour or acoustic cues, here we show that more complex stimuli like words can also improve processes of memory consolidation. These findings are in line with a recent experiment reporting that the retention of newly acquired vocabularies can be improved by re-exposing them during post-learning sleep (Schreiner and Rasch, 2014). Our findings go beyond these earlier studies insofar as we demonstrate that cueing during sleep does not only enhance memory retention, but it benefits the generalization of newly acquired knowledge to new stimuli with similar characteristics. The ability to generalize acquired knowledge about the outcome of situations to new situations is essential as it thereby can change the interpretation of a wide range of different situations. The findings on memory cueing during sleep benefitting generalization are in line with theoretical assumptions on the interplay between sleep and memory. More specifically, it is argued that new memory representations are reactivated together with the overlapping features of associated memories during sleep, thereby abstracting schematic knowledge which in turn enables the generalization of new memories (Lewis and Durrant, 2011, Stickgold and Walker, 2013, Inostroza and Born, 2013). In two recent studies, sleep has been found to support the generalization from a specific target to similar stimuli in a fear-conditioning paradigm and language-learning task (Fenn et al., 2003, Pace-Schott et al., 2009). By using the method of memory cueing which has been assumed to increase the naturally occurring processes of memory reactivation, our findings are the first to indicate that reactivation of memories might indeed underlie the process of generalization during sleep. Importantly, because we only examined memory cueing during sleep, but not during wakefulness, we can not conclude that this effect is specific to sleep. On the background of a number of studies showing no or even detrimental effects of cueing on memory consolidation during wakefulness (Rasch et al., 2007, Diekelmann et al., 2011, Schreiner and Rasch, 2014) comparable effects after cueing during wakefulness appear to be unlikely.
This is the first study investigating the impact of memory cueing during sleep on the consolidation during adolescence, and also the first to directly compare the size of cueing effects during adolescence and adulthood. In a recent experiment in children and adults, we found that periods of sleep as compared to wakefulness after implicitly learning a motor sequence led to a greater amount of explicit knowledge about the sequence. This effect was greater in children and it was associated with their higher amount of slow wave sleep (Wilhelm et al., 2013). On this background, we had concluded that the developing brain extensively drives the abstraction of newly acquired memories with this critically depending on memory reactivation during slow wave sleep. However, in contrast to this initial hypothesis, we did not find any age-dependent differences in the beneficial effects of cueing neither for the consolidation of newly acquired picture-word associations nor for the generalization of the learnt information about the valence of the pictures. This lack of difference between both age-groups might be related to other parameters that are capable to modulate the effect of memory cueing such as the existence of a schema being related to the learning contents. In rats and humans, the process of consolidation is facilitated by the presence of an associative schema into which new information can be integrated (Tse et al., 2007, Tse et al., 2011, van Kesteren et al., 2010, Brod et al., 2015, van Buuren et al., 2014). And there is now evidence indicating that the beneficial effects of existing schema knowledge on memory consolidation and generalization critically depend on memory reactivation (Inostroza and Born, 2013, Preston and Eichenbaum, 2013). For example, it was reported that the reactivation of existing knowledge during learning of new related information is a necessary prerequisite for generalization to occur (Zeithamova et al., 2012). The more elaborated the schema into which a learning content can be integrated the higher the probability that reactivating these contents might result in the generalization to new situations. Adolescents due to fewer experiences with scenes as those presented on the pictures can be expected to have less elaborated schemas about this learning content. This might have hampered the possible effects of cueing in this age-group. Future research is needed to study in detail the role of schemas on the effect of cueing on memory consolidation and generalization by manipulating the complexity of learning-related schemas. Also, it is necessary to illuminate in more detail the extent of similarity between the learning and transfer situations that is necessary for generalization to occur.
Our findings indicate that memory cueing can modulate positive interpretations of new ambiguous scenes, while it did not impact negative interpretations. In line with previous studies, the absence of an effect of cueing on negative interpretations coincided with a general tendency of these participants to interpret ambiguous scenes more positively than negatively (Mathews and Mackintosh, 2000, Yiend et al., 2005, Mackintosh et al., 2006). We speculate that psychologically healthy individuals evaluate a negative outcome of an ambiguous situation as less likely due to their stable positive mental schemas. Furthermore, this tendency is stable and cannot be modified by a single intervention, such as cueing a negatively resolved scene during sleep. In depression and anxiety disorders, the interpretation and memory for ambiguous situations have been found to be negatively biased (Clark and Wells, 1995, Whitney et al., 2012, Dalgleish and Werner-Seidler, 2014). Because of more negative and less positive mental schemas in depressed and anxious patients we would expect that cueing positive experiences will not lead to generalization whereas this should be the case when cueing negative experiences. This hypothesis needs to be tested in future experiments.
Our finding that cueing can benefit the stabilization and generalization of newly acquired outcomes of ambiguous situations opens new research directions for clinical psychology and intervention development. One major goal in cognitive therapy is to reduce negative interpretations of ambiguous situations by challenging the interpretation (i.e. identifying distortions in the interpretation; cognitive restructuring of interpretation) (Beck, 1976). More recent intervention development includes training positive interpretations of ambiguous situations to over-ride negative interpretations, through computer-based platforms that can augment traditional cognitive therapies by offering repetitive training and habit building (Holmes et al., 2008, Mathews et al., 2013). Importantly, initial studies even point towards the great potential of CBM-I to alter negative interpretation biases in children and adolescents suffering from anxiety disorders. However, the importance for future research to identify how to maximize this potential has been clearly pointed out (Salemink and Wiers, 2011, Lau, 2013). Our findings indicate that memory for the trained items is an essential factor that might determine how efficient such training is in modulating individual biases. Further issues in CBM-I are related to the generalizability of the training-induced interpretation bias and the persistence of effects. Considering memory cueing during sleep in this context might not only help to increase our understanding of the psychopathological mechanisms of mental disorders but also give implications on how to support interventions targeting memory-related distortions. Future research should investigate whether cueing is capable of (1) changing mood as reported in previous studies on CBM-I (Holmes et al., 2008, Mathews et al., 2013), (2) modulating emotional processing of ambiguous situations as indicated by the heart rate response or subjective measures of stress and (3) altering how people respond to real-life ambiguous situations. Cueing the content of cognitive bias modification trainings during post-intervention periods of sleep with audio recordings (i.e. the phrases that disambiguate initally ambiguous scenes in a positive way) might enhance the effects of CBM-I as a clinical intervention. The method of post-interventional cueing is already applied in imagery rescripting techniques. Here, traumatized patients are guided to imagine problematic imagery of a scene from their lives, rescript the circumstances in a positive way during the therapy session and are advised to listen to a audio recording of that session at home (Holmes et al., 2007). To date, such cueing procedures are conducted during wakefulness but moving such components of interventions into sleep might be even more efficient. Future studies that examine these memory cueing and consolidation processes in clinical populations may reveal opportunities to translate advances in the neuroscience of sleep into models of developmental psychopathology that directly influence clinical practice.
Role of the funding source
This work was supported by the Swiss National Science Foundation, by the Deutsche Forschungsgemeinschaft (Wi 4059/1-1), the Jacobs Foundation and the Child Research Centre of the Children's University Hospital, Zürich. The funding sources were not involved in data collection, analysis, interpretation or the decision to submit the article for publication.
References
- Bartlett F. Macmillan Co.; New York: 1932. Remembering. [Google Scholar]
- Beck A. International Universities Press; New York: 1976. Cognitive Therapy and the Emotional Disorders. [Google Scholar]
- Bradley M., Lang P. Measuring emotion: the self-assessment manikin and the semantic differential. J. Behav. Ther. Exp. Psychiatry. 1994;25(1):49–59. doi: 10.1016/0005-7916(94)90063-9. [DOI] [PubMed] [Google Scholar]
- Brod G., Lindenberger U., Werkle-Bergner M., Shing Y.L. Differences in the neural signature of remembering schema-congruent and schema-incongruent events. Neuroimage. 2015;117:358–366. doi: 10.1016/j.neuroimage.2015.05.086. [DOI] [PubMed] [Google Scholar]
- Clark D., Wells A. A cognitive model of social phobia. In: Heimberg R., Liebowitz M., Hope D., Scheier F., editors. Social Phobia: Diagnosis, Assessment, Treatment. 1st ed. Guilford; New York: 1995. pp. 69–93. [Google Scholar]
- Creery J.D., Oudiette D., Antony J.W., Paller K.A. Targeted memory reactivation during sleep depends on prior learning. Sleep. 2015;38(5):755–763. doi: 10.5665/sleep.4670. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dalgleish T., Werner-Seidler A. Disruptions in autobiographical memory processing in depression and the emergence of memory therapeutics. Trends Cogn. Sci. 2014;18(11):596–604. doi: 10.1016/j.tics.2014.06.010. [DOI] [PubMed] [Google Scholar]
- Diekelmann S., Born J. The memory function of sleep. Nat. Rev. Neurosci. 2010 doi: 10.1038/nrn2762. [DOI] [PubMed] [Google Scholar]
- Diekelmann S., Büchel C., Born J., Rasch B. Labile or stable: opposing consequences for memory when reactivated during waking and sleep. Nat. Neurosci. 2011;14(3):381–386. doi: 10.1038/nn.2744. [DOI] [PubMed] [Google Scholar]
- Fenn K., Nusbaum H., Margoliash D. Consolidation during sleep of perceptual learning of spoken language. Nature. 2003;425(6958):614–616. doi: 10.1038/nature01951. [DOI] [PubMed] [Google Scholar]
- Field Z., Field A. How trait anxiety, interpretation bias and memory affect acquired fear in children learning about new animals. Emotion. 2013;13(3):409–423. doi: 10.1037/a0031147. [DOI] [PubMed] [Google Scholar]
- Hertel P., Brozovich F., Joormann J., Gotlib I. Biases in interpretation and memory in generalized social phobia. J. Abnorm. Psychol. 2008;117(2):278–288. doi: 10.1037/0021-843X.117.2.278. [DOI] [PubMed] [Google Scholar]
- Holmes E.A., Arntz A., Smucker M.R. Imagery rescripting in cognitive behaviour therapy: images, treatment techniques and outcomes. J. Behav. Ther. Exp. Psychiatry. 2007;38(4):297–305. doi: 10.1016/j.jbtep.2007.10.007. [DOI] [PubMed] [Google Scholar]
- Holmes E., Lang T., Shah D. Developing interpretation bias modification as a “cognitive vaccine” for depressed mood: Imagining positive events makes you feel better than thinking about them verbally. J. Abnorm. Psychol. 2009;118(1):76–88. doi: 10.1037/a0012590. [DOI] [PubMed] [Google Scholar]
- Holmes E., Mathews A., Mackintosh B., Dalgleish T. The causal effect of mental imagery on emotion assessed using picture-word cues. Emotion. 2008;8(3):395–409. doi: 10.1037/1528-3542.8.3.395. [DOI] [PubMed] [Google Scholar]
- Iber C., Ancoli-Israel S., Chesson A.L., Quan S.F. American Academy of Sleep Medicine; Westchester, IL: 2007. The AASM Manual for the Scoring of Sleep and Associated Events: Rules, Terminology and Technical Specification. [Google Scholar]
- Inostroza M., Born J. Sleep for preserving and transforming episodic memory. Annu. Rev. Neurosci. 2013;36(1):79–102. doi: 10.1146/annurev-neuro-062012-170429. [DOI] [PubMed] [Google Scholar]
- Kosslyn S., Ganis G., Thompson W. Neural foundations of imagery. Nat. Rev. Neurosci. 2001;2(9):635–642. doi: 10.1038/35090055. [DOI] [PubMed] [Google Scholar]
- Lau J. Cognitive bias modification of interpretations: a viable treatment for child and adolescent anxiety? Behav. Res. Ther. 2013;51(10):614–622. doi: 10.1016/j.brat.2013.07.001. [DOI] [PubMed] [Google Scholar]
- Lau J., Hilbert K., Goodman R., Gregory A., Pine D., Viding E., Eley T. Investigating the genetic and environmental bases of biases in threat recognition and avoidance in children with anxiety problems. Biol. Mood Anxiety Disord. 2012;2(1):12. doi: 10.1186/2045-5380-2-12. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lewis P., Durrant S. Overlapping memory replay during sleep builds cognitive schemata. Trends Cogn. Sci. 2011;15(8):343–351. doi: 10.1016/j.tics.2011.06.004. [DOI] [PubMed] [Google Scholar]
- Mackintosh B., Mathews A., Yiend J., Ridgeway V., Cook E. Induced biases in emotional interpretation influence stress vulnerability and endure despite changes in context. Behav. Ther. 2006;37(3):209–222. doi: 10.1016/j.beth.2006.03.001. [DOI] [PubMed] [Google Scholar]
- Mathews A., Mackintosh B. Induced emotional interpretation bias and anxiety. J. Abnorm. Psychol. 2000;109(4):602–615. [PubMed] [Google Scholar]
- Mathews A., MacLeod C. Cognitive vulnerability to emotional disorders. Annu. Rev. Clin. Psychol. 2005;1(1):167–195. doi: 10.1146/annurev.clinpsy.1.102803.143916. [DOI] [PubMed] [Google Scholar]
- Mathews A., Ridgeway V., Holmes E. Feels like the real thing: Imagery is both more realistic and emotional than verbal thought. Cogn. Emot. 2013;27(2):217–229. doi: 10.1080/02699931.2012.698252. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Melfsen S., Florin I., Warnke A. Hogrefe; Götting: 2001. Sozialphobie und -angstinventar für Kinder (SPAIK) [Google Scholar]
- Pace-Schott E., Milad M., Orr S., Rauch S., Stickgold R., Pitman R. Sleep promotes generalization of extinction of conditioned fear. Sleep. 2009;32:19–26. [PMC free article] [PubMed] [Google Scholar]
- Pictet A., Coughtrey A., Mathews A., Holmes E. Fishing for happiness: the effects of generating positive imagery on mood and behaviour. Behav. Res. Ther. 2011;49(12):885–891. doi: 10.1016/j.brat.2011.10.003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Preston A.R., Eichenbaum H. Interplay of hippocampus and prefrontal cortex in memory. Curr. Biol. 2013;23(17):R764–R773. doi: 10.1016/j.cub.2013.05.041. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rasch B., Büchel C., Gais S., Born J. Odor cues during slow-wave sleep prompt declarative memory consolidation. Science. 2007;315(5817):1426–1429. doi: 10.1126/science.1138581. [DOI] [PubMed] [Google Scholar]
- Rudoy J., Voss J., Westerberg C., Paller K. Strengthening individual memories by reactivating them during sleep. Science. 2009;326(5956):1079. doi: 10.1126/science.1179013. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Salemink E., Wiers R. Modifying threat-related interpretive bias in adolescents. J. Abnorm. Child Psychol. 2011;39(7):967–976. doi: 10.1007/s10802-011-9523-5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Schönauer M., Geisler T., Gais S. Strengthening procedural memories by reactivation in sleep. J. Cogn. Neurosci. 2014;26(1):143–153. doi: 10.1162/jocn_a_00471. [DOI] [PubMed] [Google Scholar]
- Schreiner T., Rasch B. Boosting vocabulary learning by verbal cueing during sleep. Cereb. Cortex. 2014 doi: 10.1093/cercor/bhu139. [DOI] [PubMed] [Google Scholar]
- Sheehan D.V. The Mini-International Neuropsychiatric Interview (M.I.N.I.): the development and validation of a structured diagnostic psychiatric interview for DSM-IV and ICD-10. J. Clin. Psychiatry. 1998;59(Suppl. 20):22–33. (quiz 34–57) [PubMed] [Google Scholar]
- Stickgold R., Walker M. Sleep-dependent memory triage: evolving generalization through selective processing. Nat. Neurosci. 2013;16(2):139–145. doi: 10.1038/nn.3303. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tran T., Siemer M., Joormann J. Implicit interpretation biases affect emotional vulnerability: a training study. Cogn. Emot. 2011;25(3):546–558. doi: 10.1080/02699931.2010.532393. [DOI] [PubMed] [Google Scholar]
- Tse D., Langston R., Kakeyama M., Bethus I., Spooner P., Wood E. Schemas and memory consolidation. Science. 2007;316(5821):76–82. doi: 10.1126/science.1135935. [DOI] [PubMed] [Google Scholar]
- Tse D., Takeuchi T., Kakeyama M., Kajii Y., Okuno H., Tohyama C. Schema-dependent gene activation and memory encoding in neocortex. Science. 2011;333(6044):891–895. doi: 10.1126/science.1205274. [DOI] [PubMed] [Google Scholar]
- van Buuren M., Kroes M.C., Wagner I.C., Genzel L., Morris R.G., Fernández G. Initial investigation of the effects of an experimentally learned schema on spatial associative memory in humans. J. Neurosci. 2014;34(50):16662–16670. doi: 10.1523/JNEUROSCI.2365-14.2014. [DOI] [PMC free article] [PubMed] [Google Scholar]
- van Kesteren M.T., Fernández G., Norris D.G., Hermans E.J. Persistent schema-dependent hippocampal-neocortical connectivity during memory encoding and postencoding rest in humans. Proc. Natl. Acad. Sci. U. S. A. 2010;107(16):7550–7555. doi: 10.1073/pnas.0914892107. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Waldmann H.-C. Kurzformen des HAWIK-IV: Statistische Bewertung in verschiedenen Auswertungsszenarien. Diagnostica. 2008;54:202–210. [Google Scholar]
- Wechsler D., editor. Wechsler Intelligence Scale for Children. fourth ed. Harcourt Assessment; San Antonio, TX: 2003. [Google Scholar]
- Whitney J., Joormann J., Gotlib I., Kelley R., Acquaye T., Howe M. Information processing in adolescents with bipolar I disorder. J. Child Psychol. Psychiatry. 2012;53(9):937–945. doi: 10.1111/j.1469-7610.2012.02543.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wilhelm I., Kurth S., Ringli M., Mouthon A., Buchmann A., Geiger A. Sleep slow-wave activity reveals developmental changes in experience-dependent plasticity. J. Neurosci. 2014;34(37):12568–12575. doi: 10.1523/JNEUROSCI.0962-14.2014. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wilhelm I., Rose M., Imhof K., Rasch B., Büchel C., Born J. The sleeping child outplays the adult's capacity to convert implicit into explicit knowledge. Nat. Neurosci. 2013;16(4):391–393. doi: 10.1038/nn.3343. [DOI] [PubMed] [Google Scholar]
- Yiend J., Mackintosh B., Mathews A. Enduring consequences of experimentally induced biases in interpretation. Behav. Res. Ther. 2005;43(6):779–797. doi: 10.1016/j.brat.2004.06.007. [DOI] [PubMed] [Google Scholar]
- Zeithamova D., Dominick A.L., Preston A.R. Hippocampal and ventral medial prefrontal activation during retrieval-mediated learning supports novel inference. Neuron. 2012;75(1):168–179. doi: 10.1016/j.neuron.2012.05.010. [DOI] [PMC free article] [PubMed] [Google Scholar]