Abstract
Working memory (WM) – temporary storage and manipulation of information in the mind – is a key component of cognitive maturation, and structural brain changes throughout development are associated with refinements in WM. Recent functional neuroimaging studies have shown that there is greater activation in prefrontal and parietal brain regions with increasing age, with adults showing more refined, localized patterns of activations. However, few studies have investigated the neural basis of verbal WM development, as the majority of reports examine visuo-spatial WM.
We used fMRI and a 1-back verbal WM task with six levels of difficulty to examine the neurodevelopmental changes in WM function in 40 participants, twenty-four children (ages 9–15 yr) and sixteen young adults (ages 20–25 yr). Children and adults both demonstrated an opposing system of cognitive processes with increasing cognitive demand, where areas related to WM (frontal and parietal regions) increased in activity, and areas associated with the default mode network decreased in activity. Although there were many similarities in the neural activation patterns associated with increasing verbal WM capacity in children and adults, significant changes in the fMRI responses were seen with age. Adults showed greater load-dependent changes than children in WM in the bilateral superior parietal gyri, inferior frontal and left middle frontal gyri and right cerebellum. Compared to children, adults also showed greater decreasing activation across WM load in the bilateral anterior cingulate, anterior medial prefrontal gyrus, right superior lateral temporal gyrus and left posterior cingulate. These results demonstrate that while children and adults activate similar neural networks in response to verbal WM tasks, the extent to which they rely on these areas in response to increasing cognitive load evolves between childhood and adulthood.
Keywords: Verbal working memory, Functioning magnetic resonance imaging, Development
1. Introduction
Working memory (WM) allows for the temporary storage and manipulation of information (Baddeley, 1992). WM capacity, the number of items that can be held in WM at one time, plays an important role in the development of other complex cognitive skills, such as reading ability (Engle, 2002, Cain et al., 2004), math performance (Dumontheil and Klingberg, 2012), social ability (Dennis et al., 2009), as well as in general intelligence (Colom et al., 2007, Engle et al., 1999), overall learning (Gathercole and Alloway, 2004) and academic achievement (Gathercole et al., 2004a, Alloway, 2009). Impaired WM capacity has been linked to a number of neurodevelopmental disorders, such as Attention Deficit Hyperactivity Disorder (ADHD; see Martinussen et al., 2005) and Autism Spectrum Disorder (ASD; Southwick et al., 2011), and various learning (Hwang and Hosokawa, 2007, Wang and Liu, 2007) and language processing difficulties (see Wright and Fergadiotis, 2012).
Behavioral studies have documented improvements in WM ability throughout childhood to adulthood (e.g., Conklin et al., 2007, Gathercole et al., 2004b, Huizinga et al., 2006, Zald and Iacono, 1998). Whereas many other executive function components show development typically only up until mid-adolescence, WM continues to show protracted development well into young-adulthood (Huizinga et al., 2006), making it particularly susceptible to developmental disturbances. Structural brain changes throughout development are associated with refinements in various cognitive functions, including WM (Tamnes et al., 2013). Specifically, changes in structure and function of brain regions involved in WM, such as parietal and frontal regions, occur later than many regions, consistent with the protracted maturation of WM functions (Sowell et al., 1999). Given the key role WM plays in cognitive maturation, it is important to understand and characterize the neural basis of this development.
Recent functional neuroimaging studies that have examined the neural underpinnings of WM across development have shown that with increased age, children and adolescents exhibit greater activation in prefrontal (Klingberg et al., 2002, Scherf et al., 2006) and parietal (Nagel et al., 2013, Spencer-Smith et al., 2013, Klingberg et al., 2002, Scherf et al., 2006) regions on visuo-spatial WM tasks. Adults showed similar neural patterns as children and adolescents on these tasks, but with more refined, localized activation (Scherf et al., 2006), and some increased activity in “performance-enhancing” regions, such as the dorsolateral prefrontal cortex (DLPFC; Jolles et al., 2011, Scherf et al., 2006). For example, Scherf et al. (2006) found that children showed limited recruitment of critical WM substrates (DLPFC and parietal regions) during a visuo-spatial WM task, and instead relied mainly on ventromedial prefrontal regions. However, they observed more specialized networks (i.e., DLPFC, ventrolateral prefrontal cortex [VLPFC] and supramarginal gyrus) as adolescents moved into adulthood. This developmental pattern of brain activity has also been characterized as a shift from posterior to anterior activation, with adults showing increased activity in the DLPFC and VLPFC (Kwon et al., 2002; Scherf et al., 2006). Thus, previous literature suggests that the development of higher-level WM function involves a combination of increasing localization within core WM regions and their integration with performance-enhancing regions.
Fewer studies have examined the neural basis of verbal WM development, as the majority of studies utilized visuo-spatial or other nonverbal tasks. Verbal WM is particularly important, given its vital role in linguistic processes that are necessary for language and other higher-level cognitive functions (Smith et al., 1998). Brahmbhatt et al. (2008) found similar activation patterns between adolescents and adults during an n-back visual word task, with both groups showing activation in the bilateral fusiform gyrus, anterior cingulate, left precentral gyrus, left superior anterior temporal gyrus, left DLPFC, premotor cortex and left thalamus. Age-related changes were evident in the left parietal lobe, in which adults showed significantly greater activity than adolescents. In addition to the nature of tasks, the pattern of brain activation also depends on the amount of information (i.e., load) that needs to be maintained in WM. Previous literature exploring age-related changes in brain activity associated with verbal WM under conditions of increasing load found that adolescents and adults showed a greater increase in activation across load in left parietal (O’Hare et al., 2008, Thomason et al., 2009), left lateral prefrontal (Thomason et al., 2009) and right cerebellar (O’Hare et al., 2008) regions than children. In contrast, Jolles et al. (2011) did not find age-related load sensitivity in children and adults. Also, previous reports used only up to three levels of difficulty.
The present study examined the effects of increasing load, with six difficulty levels in a verbal WM task, and how load-dependent change in brain function differed in children and adults. Prior developmental visual verbal WM studies that included load as a manipulation used complex tasks that required maintenance and reordering (Jolles et al., 2011) or changed stimuli appearance and size (O’Hare et al., 2008, Thomason et al., 2009) as difficulty level increased. In a typical n-back task, participants view a series of stimuli and indicate whether the currently presented stimulus matches one presented ‘n’ (e.g., 0, 1, 2 or 3) trials prior. As difficulty level increases, the number of interfering stimuli between the target and relevant stimulus increases, requiring the utilization of different mental strategies at each level (e.g., 0-back: recognition; 1-back: maintenance; 2-back: maintenance and monitoring). These manipulations increase both memory load and executive function demands (i.e., strategy needed to complete the task) in a non-linear fashion from each level to the next, making function-specific alterations difficult to quantify and relate with specific brain regions. To avoid these confounds, we used a 1-back letter matching task (LMT) which manipulated memory load while keeping executive function uniform across the difficulty levels, allowing us to investigate directly the impact of cognitive load on verbal WM. The executive demands (i.e., procedural strategies for solving the task) were constant across all levels of the LMT; what varied with each level was the number of items (letters) that had to be remembered. A visuo-spatial analogue of LMT has been used successfully to explore WM in functional neuroimaging studies of adults (Arsalidou et al., 2013) and children with and without ASD (Vogan et al., 2014). Observations from these studies point to a linear pattern of WM function across load. Our task can capture neural correlates associated with this linear pattern of activation with cognitive load, and our objective is to determine whether patterns of activation in WM processing change across development.
Understanding the effect of age on brain regions implicated in WM and WM capacity can provide insight into the developmental trajectory of verbal WM networks, and enhance our ability to determine optimal timing for interventions in paediatric populations with WM difficulties. Given previous literature and findings from our recent work using the visuo-spatial version of LMT, we expected frontal and parietal cortical areas associated with WM would be under-recruited in children compared to adults, and these differences would increase with increasing cognitive load. Further, neural activation in both groups would be left-hemisphere dominant, given the verbal nature of the task (e.g., Brahmbhatt et al., 2008), and this localized pattern would be less evident in children who are more likely to demonstrate diffuse activation (Scherf et al., 2006).
2. Methods
2.1. Participants
Twenty-four typically developing children aged 9–15 (M = 12.8, SD = 1.7; 7 female) and 16 young adults aged 20–25 (M = 22.7, SD = 1.5; 8 female) were included in the analyses. Sixteen other children (i.e., 40 were tested in total) were excluded from the analyses due to excessive movement (n = 1), inadequate task performance (n = 13) or a combination of these factors (n = 2). A chi-square analysis confirmed that sex distribution did not differ between age groups, χ2(1) = 1.78, p = 0.18). Cognitive function of child participants was assessed using the Wechsler Abbreviated Scale of Intelligence-II (Wechsler, 2003) to ensure IQ > 80 (M = 114.6, SD = 8.1). All subjects were screened and not included on the basis of any current significant psychiatric comorbidities (APA, 2013), neurological disorders, medical illnesses, prematurity, uncorrected vision, as well as standard MRI contraindicators (e.g., ferromagnetic implants). A history of developmental disorders, learning disability or ADHD was also used to exclude participants. Participants were recruited through email lists, posters in the hospital and community, private schools and word of mouth. Informed consent and MRI scanning were performed at the Hospital for Sick Children in Toronto. Experimental procedures were approved by the Research Ethics Board at the Hospital for Sick Children, and all participants provided informed consent; for the children; parents gave written consent while children gave verbal assent.
2.2. The letter matching task (LMT)
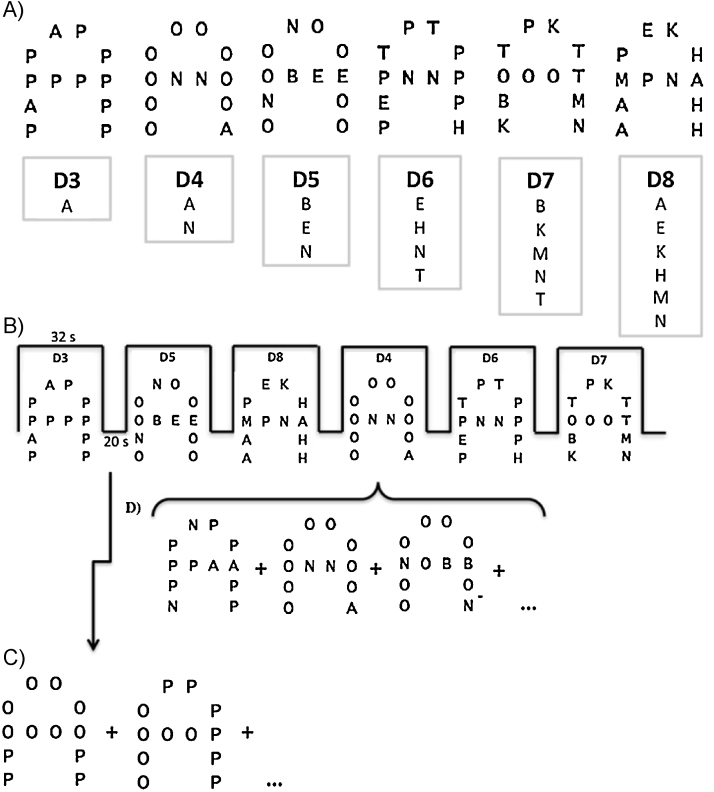
In the current study, LMT is considered a verbal WM task; although it is not auditory in nature, it uses completely verbal (i.e., linguistic) stimuli. Participants were required to attend to letters embedded in a figural letter ‘A’. Participants were taught to ignore the global figure ‘A’ and two of the embedded letters (‘O’ and ‘P’) that were defined as irrelevant, and focus on the other letters, defined as relevant. The task included eight relevant letters (A, B, E, H, K, M, N, T), all were presented in upper case. We included relevant and irrelevant letters, as tasks that contain misleading or irrelevant factors cause interference and increased cognitive control; these types of tasks have been shown to provide more reliable measures of WM capacity and estimates of development (Arsalidou et al., 2010, Powell et al., 2014). In addition to irrelevant letters, the large figural ‘A’ was also used to evoke interference and elicit cognitive control. The number of ‘n’ relevant letters (capacity) in the figures was increased by one for each increase in level of difficulty. LMT has two functions that each require attention: participants must first identify the relevant letter(s) embedded in the ‘A’ figure and second, remember if they are the same or different letters from the previous stimulus. As such, items with one relevant letter (e.g. n = 1) have a difficulty level of n + 2 (e.g., 3; Fig. 1A) to account for the additional functions mentioned above. The stimuli were presented one at a time. Participants indicated after each stimulus whether the relevant letters of the current figure matched those from the immediately preceding figure (i.e. 1-back), disregarding letter location and repetition. Using a dual-key MRI compatible keypad with the right hand, participants responded in the scanner by pushing one button for ‘same’ when the stimulus contained the same embedded letters as the immediately previous stimulus and another button for ‘different’ when the stimulus had different embedded letters. Non-matching stimuli (i.e., ‘different’ stimuli) differed from the previous stimuli by only one letter for all levels. Prior to scanning, all participants were trained and successfully completed practice trials on a computer outside the scanner with accuracy of 80% or greater.
Fig. 1.
Protocol description of the Letter Matching Task (LMT). (A) There were 6 levels of difficulty where the number of relevant letters (A, B, E, K, N, M, N, T) increased by one to increase the difficulty level. Difficulty = (# of relevant letters) + 2. Participants were asked to ignore the global “A” figure, letter location, letter repetition and irrelevant letters (O and P). (B) It was a block design task, where each run consisted of six 32s task blocks (for each difficulty level) followed by 20s baseline blocks where figures are presented with only O and P (irrelevant letters). Task blocks were presented pseudo-randomly within each run. (C) Example of part of a sequence in a baseline block. Participants were instructed not to respond. (D) Example of part of a sequence in a task block; participants indicated if the current figure “A” contained of the same or different letters as the previous figure. All stimuli were presented for 3s followed by a 1s inter-stimulus fixation cross.
Twenty-four task blocks (with a total of 168 task trials) and 24 baseline blocks were presented across four runs. Each run included six 32-s blocks, one for each of the six difficulty levels; each task block consisted of eight stimuli of the same difficulty level and the six difficulty levels were randomized within each run (Fig. 1B). Task blocks alternated with 20-s baseline blocks (Fig. 1C), where the ‘A’ figures contained only ‘O’ and ‘P’, the irrelevant letters, and participants were instructed to look at the figures but not respond. The same four runs were presented to all participants in the same order. Participants had 3 s to view a stimulus and respond, followed by a 1-s inter-stimulus interval where a fixation cross was presented (Fig. 1D). The fMRI task took approximately 22 min of scan time.
Behavioral data were recorded for accuracy (proportion correct) and reaction time; items were correct if subjects responded correctly within 3-s of stimulus onset. Participants were excluded if they did not reach at least 70% accuracy (averaged across four runs) on the two easiest levels, and were also excluded if they did not have at least two out of the four runs where 50% or more of the blocks were acceptable in terms of performance (70% accuracy) and motion. A 70% accuracy criterion was chosen to ensure that participants were performing better than chance (50%). Motion was considered acceptable if participants moved less than 1.5 mm from their median head position in at least 60% of the volumes within a task block. See Section 2.5 for a description of displacement calculations.
2.3. Image acquisition
All imaging data were acquired using a 3T Siemens Trio MRI scanner with a 12-channel head coil. Motion restriction and head stabilization were achieved with foam padding. A high-resolution T1-weighted 3D MP-RAGE structural scan (Sagittal; FOV = 192 × 240 × 256 mm; 1 mm isometric voxels; TR/TE/TI/FA = 2300/2.96/900/9), was used as an individual anatomical reference for the fMRI images. During structural MRI acquisition, participants watched their choice of movie using MR compatible goggles and earphones. Functional images were acquired with a single-shot echo planar imaging sequence (Axial; FOV = 192 × 192; Res = 64 × 64; 30 slices 5 mm thick; 3 × 3 × 5 mm voxels; TR/TE/FA = 2000/30/70). Visual stimuli for the LMT task were displayed on the MR compatible goggles. Stimuli were displayed and behavioural performance was recorded using Presentation (Neurobehavioral Systems Inc., Berkeley, CA, USA).
2.4. Behavioral data analyses
Behavioral accuracy (% correct) and reaction times were calculated for each difficulty level, averaging across runs for each individual. Children showed poor accuracy on difficulty levels 7 and 8 (D7: M = 0.64, SD = 0.12; D8: M = 0.57, SD = 0.13), where they performed only marginally above chance (50%). Thus analyses of only the first four difficulty levels (D3 to D6) were completed to eliminate performance as a confounding factor in brain activation. The data were analyzed using repeated measures analyses of variance (ANOVA), with a between-subject factor of group (children and adults) and a within-subject factor of difficulty level (i.e., load; D3, D4, D5, D6).
2.5. fMRI data analyses
Image preprocessing of fMRI data was completed using a combination of tools from AFNI (Cox, 1996) and FMRIB's Software Library (FSL; Worsley, 2001). We discarded the first three volumes of each run for scanner stabilization. After slice-timing and motion correction (using 3dvolreg), the data were smoothed in-plane using a FWHM Gaussian kernel (6 mm), temporally filtered (lower and upper cut-off frequencies of 0.01 Hz & 0.2 Hz), and then converted to percent signal change from the baseline volumes. The maximum Euclidean displacement (MD) that any voxel moved within the brain was calculated using 3dvolreg from AFNI, and was performed during the motion correction stage of preprocessing. We used the MD metric to flag those volumes with unacceptable motion (greater than 1.5 mm from the median head position). If more than 1/3 of volumes exceeded these criteria within a task block, the block was considered to have excessive motion. We explored group differences in head motion using the average MD for each subject. Although more motion was found in children (M = 0.32 mm, SD = 0.21 mm) than adults (M = 0.14 mm, SD = 0.11 mm), t(38) = 3.50, p = 0.001, both groups had minimal average motion of under 0.35 mm. To further control for motion, the MD signal was included as a no-interest covariate in the GLM. Finally, prior to group-level analyses, the images were registered to the MNI-152 template.
Data were analyzed with the FSL fMRI Expert Analysis Tool (FEAT; Worsley, 2001). Data were fit first to a block-design general linear model convolved with a gamma function to model hemodynamic response, using the task parameters (D3 to D6). To examine areas that showed increasing activation with increasing load in each group, linear trend analyses were conducted from D3 to D6 using fixed-effects higher level modeling for each group separately. Individual results were then averaged across runs for each subject in a second level analysis. To examine group differences in load-dependent growths of activation, a mixed-design ANOVA was used, testing for a Group × Load interaction. Between-group comparisons were carried out using FMRIB's Local Analysis of Mixed Effects-1 (FLAME 1; Worsley, 2001) to obtain an accurate between-subject variance estimation, which increased our ability to detect real activation (Woolrich et al., 2009). Significant activations were reported using cluster-based thresholding determined by Z > |2.3|, and a corrected cluster significance threshold of p < 0.05.
2.6. Laterality index calculation
Laterality indices (LI) were calculated between all bilateral regions on the AAL 90-region atlas. A 5 mm radius sphere was drawn around each coordinate in the atlas and was transformed to each subjects’ functional space. The average statistical value within each sphere was calculated and used to calculate LI as follows:
A negative index represents right-lateralized activation whereas positive LI represents left-lateralized activation. False discovery rate (FDR) was used to correct for multiple comparisons.
2.7. Brain-behavior exploratory analyses
An exploratory analysis was conducted to understand the link between LMT performance (i.e., accuracy) and neural activation. Pearson's r was computed to explore whether increasing activation from D3 to D6 (i.e., D6 > D3 contrast) was related to performance at the most difficult level (D6) in areas that demonstrated a significant Load × Group interaction in the fMRI analysis above. An FDR threshold of p= 0.05 was used to correct for multiple comparisons.
3. Results
3.1. Behavioral data
To ensure that there was no sex distribution difference in our sample, we analyzed behavioral performance across difficulty for males and females. Findings demonstrated that there was no sex × load interaction (F(1) = 0.691, p = 0.495); both males and females were showing similar performance trends across difficulty, regardless of their group.
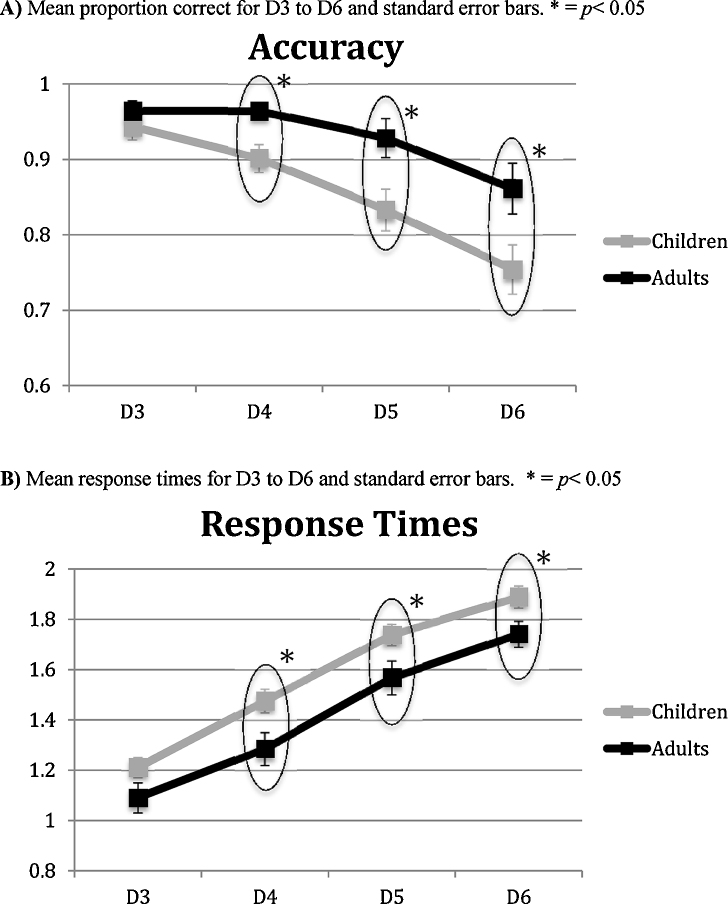
Due to violations of sphericity, we only report significant results that also survived Greenhouse–Geisser correction. To test age and load effects on LMT accuracy, a repeated measures ANOVA was performed with load (D3–D6) as within-subjects variable and with group (children and adults) as a between-subjects factor. The ANOVA demonstrated a main effect of load on accuracy, F(3) = 24.84, p < 0.001, ηp2 = 0.40, with accuracy decreasing with increasing load in both children and adults. Post hoc pairwise comparisons, adjusted for multiple comparisons using Bonferroni, revealed that accuracy significantly decreased with increasing load (p < 0.05), except between the two easiest and two most difficult levels in children. In adults, accuracy significantly decreased between D3 and D6 and between D4 and D6 (p < 0.05). There was also a significant effect of group on accuracy, F(1) = 6.66, p = 0.01, ηp2 = 0.15, with adults performing significantly better on D4, D5 and D6 (see Table 1 and Fig. 2A). However, children performed well above-chance (>75%) on all difficulty levels, and between group differences in brain activity were not confounded by inadequate task performance for either group. A Group × Load interaction was not found in accuracy scores, F(3) = 2.20, p = 0.12.
Table 1.
LMT behavioral performance: accuracy and response times.
| Difficulty level | Children |
Adults |
||
|---|---|---|---|---|
| Accuracy (%) | S.D. (%) | Accuracy (%) | S.D. (%) | |
| D3 | 0.94 | 0.08 | 0.96 | 0.06 |
| D4 | 0.90 | 0.09 | 0.96 | 0.04 |
| D5 | 0.83 | 0.14 | 0.92 | 0.10 |
| D6 | 0.75 | 0.16 | 0.86 | 0.13 |
|
RT (s) |
S.D. (s) |
RT (s) |
S.D. (s) |
|
| D3 | 1.21 | 0.20 | 1.09 | 0.24 |
| D4 | 1.48 | 0.23 | 1.29 | 0.25 |
| D5 | 1.74 | 0.21 | 1.57 | 0.27 |
| D6 | 1.89 | 0.22 | 1.74 | 0.21 |
Fig. 2.
LMT behavioral performance. (A) Mean proportion correct for D3 to D6 and standard error bars. * p < 0.05. (B) Mean response times for D3 to D6 and standard error bars. * p < 0.05.
A similar ANOVA was conducted to test age and load effects on LMT response times. Overall, there was a main effect of load on response times, F(3) = 182.90, p < 0.001, ηp2 = 0.83, with response times increasing as function of load for both children and adults. Post hoc comparisons confirmed that response times increased significantly at each difficulty level in children (p < 0.01) and adults (p < 0.05). There was also a significant main effect of group on response times, F(1) = 6.28, p < 0.05, ηp2 = 0.14, with children showing longer response time than adults at D4, D5 and D6 (see Table 1 and Fig. 2B). A Group × Load interaction was not found in response times.
3.2. Functional imaging results
The fMRI analyses determined if the pattern of brain activity differed as a function of the WM load (i.e., difficulty level) and between children and adults. Linear trend analyses (D3 to D6) showed that some brain areas showed increases in activity with difficulty level, while others decreased. We use ‘increasing activation’ to refer to an increase in the BOLD signal with an increase in WM load (i.e., a positive linear relation between BOLD activity and task difficulty level) and ‘decreasing activation’ to refer to a decrease in the BOLD signal with an increase in load (i.e., a negative linear relation between BOLD activity and task difficulty).
3.2.1. Task-related activation within group: Children
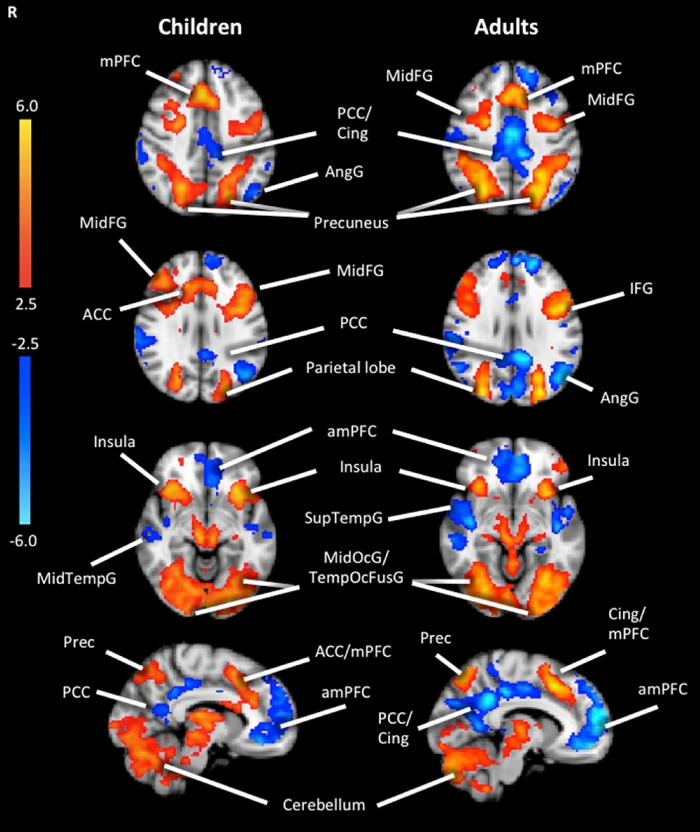
The influence of load on functional activity was first examined separately in children and adults. As shown in Fig. 3, areas that exhibited increasing activation (i.e., showed a positive linear relation) under conditions of increasing WM load among children were: bilateral insula, anterior cingulate extending to the medial prefrontal cortex, middle frontal gyrus, precuneus, middle occipital gyrus, temporal occipital fusiform cortex, cerebellum and left medial frontal gyrus extending to the cingulate. Areas that exhibited decreasing activation (i.e., showed a negative linear relation) under conditions of increasing WM load were: left anterior medial frontal cortex, right middle temporal gyrus, left angular gyrus and left posterior cingulate (see Supplementary material Table 1).
Fig. 3.
Group activation maps for the linear trend analysis in children and adults during LMT. Significant activations using cluster-based thresholding determined by Z > |2.3| and a corrected cluster significant threshold of p = 0.05 (using the FEAT toolbox of FSL). Areas in red depict regions of increasing activation as a function of difficulty level, and areas in blue depict regions of decreasing activation as a function of difficulty level. mPFC = medial prefrontal cortex, MidFG = middle frontal gyrus, PCC = posterior cingulate cortex, Cing = cingulate, AngG = Angular Gyrus; Prec = precuneus, ACC = anterior cingulate cortex, IFG = inferior frontal gyrus, amPFC = anterior medial prefrontal cortex, SupTempG = superior temporal gyrus, MidTempG = middle temporal gyrus, TempOcFusG = temporal occipital fusiform gyrus. (For interpretation of the references to color in this figure legend, the reader is referred to the web version of this article.)
3.2.2. Task-related activation within group: Adults
Among adults, areas that exhibited increasing activation as a function of WM load were: bilateral insula, inferior frontal gyrus, precuneus, middle occipital gyrus, cerebellum, middle frontal gyrus and left superior frontal gyrus extending to the cingulate. Areas that displayed decreasing activation as a function of WM load were: bilateral anterior medial prefrontal cortex, superior temporal gyrus, left angular gyus and left posterior cingulate (see Supplementary material Table 2).
3.2.3. Group differences in WM load-dependent activation
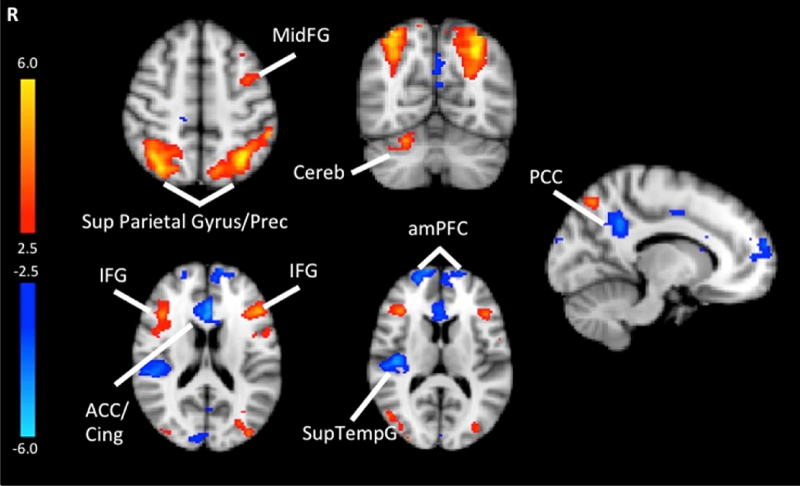
A Group × Load analysis tested for age effects in WM load-dependent activation. Group differences were observed for both negative and positive linear load-dependent relations. As shown in Fig. 4, with increasing WM load, BOLD activity was significantly greater in adults than children in the bilateral superior parietal gyrus extending to the precuneus, inferior frontal gyrus, left middle fontal gyrus and right cerebellum. Compared to children, adults showed significant decreases in activation across WM load in the bilateral anterior cingulate, anterior medial prefrontal gyrus, right superior lateral temporal gyrus and left posterior cingulate (Table 2).
Fig. 4.
Results from the Group × Load interaction analysis. Significant activations using cluster-based thresholding determined by Z > |2.3| and a corrected cluster significant threshold of p = 0.05. Areas in red depict regions where adults showed greater increasing activation as a function of difficulty level compared to children. Areas in blue depict regions of where adults showed greater decreasing activation as a function of difficulty level compared to children. MidFG = middle frontal gyrus, PCC = posterior cingulate cortex, Cing = cingulate, ACC = anterior cingulate cortex, IFG = inferior frontal gyrus, amPFC = anterior medial prefrontal cortex, Sup Parietal Gyrus = superior parietal gyrus; Prec = precuneus, SupTempG = superior temporal gyrus, Cereb = cerebellum. (For interpretation of the references to color in this figure legend, the reader is referred to the web version of this article.)
Table 2.
Areas that showed a significant group × load interaction.
| Regions where adults showed greater activation as a function of difficulty level than children | |||||||
|---|---|---|---|---|---|---|---|
| Region | Hem | MNI Coordinates |
Cluster Size (voxels) | Z value | P-value | ||
| x | y | z | |||||
| Superior Parietal Gyrus extending to Precuneus | L | −30 | −64 | 46 | 2962 | 6.35 | 2.96 × 10−10 |
| Superior Parietal Gyrus extending to Precuneus | R | 36 | −60 | 54 | 2595 | 6.19 | 2.68 × 10−9 |
| Inferior Frontal Gyrus | L | −48 | 26 | 18 | 924 | 5.05 | 3.94 × 10−4 |
| Inferior Frontal Gyrus | R | 38 | 30 | 14 | 682 | 4.64 | 3.56 × 10−3 |
| Cerebellum | R | 24 | −64 | −24 | 634 | 4.54 | 5.66 × 10−3 |
| Middle Frontal Gyrus | L | −34 | 6 | 58 | 456 | 4.65 | 0.04 |
| Regions where adults showed less activation as a function of difficulty level than children | |||||||
|---|---|---|---|---|---|---|---|
| Region | Hem | MNI Coordinates |
Cluster size (Voxels) | Z value | p-Value | ||
| x | y | z | |||||
| Anterior medial prefrontal cortex | L | −4 | 62 | 22 | 3155 | 6.49 | 9.62 × 10−11 |
| Anterior cingulate/cingulate gyrus | L | −4 | 18 | 24 | X | 5.51 | |
| Anterior cingulate/cingulate gyrus | R | 4 | 14 | 26 | X | 4.69 | |
| Anterior medial prefrontal cortex | R | 12 | 68 | 10 | X | 5.05 | |
| Heschl's gyrus | R | 42 | −20 | 14 | 1707 | 4.88 | 8.94 × 10−7 |
| Posterior cingulate cortex | L | −12 | −50 | 36 | 1094 | 4.24 | 9.42 × 10−5 |
Note: the p-value shown for each cluster represents the estimated significance as determined by the FEAT toolbox of FSL; X = peak local maximas within cluster.
3.2.4. Laterality analyses
For both children and adults, none of the 45 bilateral AAL regions tested exhibited an LI that was significantly different from 0 (using a 2-tailed t-test), suggesting that BOLD activity was symmetric across hemispheres.
3.2.5. Brain-behavior correlations
Brain-behavior relations were explored in frontal and parietal areas that demonstrated significant Load × Group interactions. When collapsing adult and child groups, accuracy at D6 (highest load) was significantly positively correlated with the D6 > D3 contrast of brain activation in the bilateral superior parietal gyrus (Left: r(38) = 0.42, p = 0.006; Right: r(38) = 0.44, p = 0.004), left inferior frontal gyrus (r(38) = 0.51, p = 0.001) and left middle frontal gyrus (r(38) = 0.40, p = 0.011), FDR corrected for multiple comparisons using a threshold of p = 0.05. In other words, as D6 performance increased, the change in activation from D3 to D6 also increased in these frontal and parietal areas. However, when exploring within group brain-behavior correlations at D6, children showed no regions in which D6 performance was correlated with neural activation. However, adults showed a significant correlation between D6 accuracy and change in activation from D3 to D6 in the left inferior frontal gyrus (r(14) = 0.67, p = 0.005), FDR-corrected for multiple comparisons with a threshold of p = 0.05.
4. Discussion
The present study examined age-related differences in the neural correlates of verbal working memory, and the impact of increasing cognitive load, using a task that isolated WM from executive function demands. Although adults performed better than children on the three higher difficultly levels of the task (LMT), differences were only moderate and both groups had at least a 75% accuracy rate on all levels. Therefore, functional neural activation in response to LMT was not confounded by inadequate task performance for children or adults. Neuroimaging results demonstrate many similarities in children and adults in the neural activation patterns associated with increasing verbal WM capacity. Both age groups also demonstrated an opposing system of cognitive processes where areas related to WM (frontal and parietal regions) increased in activity, and areas associated with the default mode network (DMN) decreased in activity with increasing cognitive demand. However, there were age group differences observed for linear-load dependent functional activation.
Although children exhibited task-related activity in many of the same brain regions as adults, they failed to exhibit the same degree of increasing activation across cognitive load as adults in multiple frontal and parietal cortical regions, consistent with previous studies (Thomason et al., 2009, O’Hare et al., 2008). These areas included the bilateral superior pariety gyrus extending to the precuneus, bilateral inferior frontal gyrus, left middle frontal gyrus and right cerebellum. Thus, although the number of brain regions showing load-dependent activation did not change across age, the extent to which participants relied on these areas in response to increasing cognitive load changed between childhood and adulthood.
Increased recruitment of frontal and parietal areas during working memory tasks has been shown to underlie improvements in working memory and cognitive control over the course of development (see Bunge and Wright, 2007, for a review). The DLPFC (i.e., middle frontal gyrus) is referred to as a core “performance-enhancing” region believed to play a critical role in holding information ‘online’ (Powell and Voeller, 2004) and mediating strategic organization and data compression processes (Rypma et al., 2002, Bor et al., 2003). This is consistent with the dorsolateral prefrontal cortex being sensitive to increasing cognitive demand in ours and other studies (Scherf et al., 2006, Thomason et al., 2009). The inferior frontal gyrus has also been identified as an area involved in higher cognitive monitoring (e.g. choosing, comparing, judging and retrieving), as well as language processing, specifically in the left hemisphere (see Liakakis et al., 2011 for review; Strand et al., 2008). Findings from our study suggest that adults are increasingly recruiting these regions, more so than children, to adjust for increasing task demand.
In contrast with some developmental neuroimaging studies of verbal WM, (Thomason et al., 2009, Nagel et al., 2013), we did not find the expected left hemisphere lateralization of neural activation. Similar to Tamnes et al. (2013) who also did not find left lateralization in a verbal WM ‘Keep Track Task’, we used a complex task with visually presented stimuli and visual-search component that might depend on a wider, more general neural network. Participants may not have transformed visually presented verbal stimuli (i.e., letters) into a phonological code, and instead may have relied on visual-search strategies. Adults may have been more likely than children to rely on such visual-search strategies as cognitive demand increased, given the greater reliance on the precuneus with increasing cognitive load – an area responsive to spatial visual processing (Ungerleider and Mishkin, 1982) and widely implicated as a hub region in the brain (e.g., Mishkin and Ungerleider, 1982; Smith et al., 1998; van den Heuvel and Sporns, 2011) – as task difficulty increased.
In addition to the increased activation with task difficulty, we also found the inverse, with decreases as a function of load in areas typically associated with the default-mode network (Raichle et al., 2001), including the medial prefrontal, precuneus and posterior cingulate. This pattern of decreases was also seen in the visual-spatial analogue of this task in adults (Arsalidou et al., 2013) and children (Vogan et al., 2014). In the current study, both children and adults showed these decreases with increasing load, but the effects were greater in the adults, suggesting that they are better able to modulate mind-wandering (Buckner et al., 2008), during performance of a demanding task. Both groups showed decreases in the left angular gyrus, close to Wernicke's area, involved in language function. This area is implicated in reading and suggested that both children and adults, needed to supress the activity in this area, to stop reading the letters, to complete the harder levels of this task.
Cerebellar activation was seen in both children and adults, with the interaction showing greater right cerebellar involvement in adults with increasing memory load. Many studies have shown the cerebellum is implicated in cognitive and executive functions (for reviews see De Smet et al., 2013, Strick et al., 2009), and deficits associated with cerebellar dysfunction are often reported in working memory and verbal fluency in adults and children (Bellebaum and Daum, 2007, Gottwald et al., 2004, Levisohn et al., 2000). Consistent with our findings, particularly right cerebellar activations are seen in adults in verbal tasks (Chen and Desmond, 2005, Kirschen et al., 2005, O’Hare et al., 2008). The specific role of the cerebellum in cognitive functions is still to be delineated (Timmann and Daum, 2007, Stoodley, 2012); however, our findings of increasing cerebellar activation with increasing verbal WM load, with age-related differences, aligns with the model of its role in executive functions which show marked development between mid-childhood and adulthood.
To avoid performance being a confounding factor in the neural activation during LMT, individuals with inadequate task performance were excluded from the present study. Consequently, our sample may be less representative of children with lower WM capacity. Further, due to the complex nature of LMT, we could not analyze the highest two task difficulty levels (D7 and D8) where children performed only slightly above chance. Understanding WM processing at very high cognitive demands is important in our understanding the development of WM capacity. In addition, as many cognitive tasks, selective attention is required when completing LMT. However, we did not administer an independent measure of selective attention, and therefore its specific influence on this task cannot be analyzed. The current study extends knowledge of verbal WM development by using a novel task that isolated cognitive load on WM, holding executive function constant, and utilizing multiple difficulty levels to examine the impact of cognitive load. Performance on this complex task depended on a general neural network rather than a left lateralized neural system, and our findings demonstrate that with development, the degree to which individuals rely on frontal and parietal regions increases with cognitive demands. Future studies will compare normative developmental patterns of verbal WM processing to clinical populations who have cognitive and memory dysfunction.
Acknowledgements
The authors would like to thank all of the families and children for their support and participation. We would also like to thank Rachel Leung and Becky Baatjes for administering the ADOS, and Dr. Jessica Brian for reviewing all assessments. Sincere thanks to our MRI technicians, Ruth Weiss and Tammy Rayner, for all their support in data acquisition. The authors would also like to thank Marie Arsalidou for developing the LMT task, and allowing us to use it. Lastly, thanks Chiara Borrelli in her assistance with data analysis. Many thanks to Rina Goukon for her help with participant recruitment and data collection. This research was funded by Canadian Institutes of Health Research (MOP-106582).
Footnotes
Supplementary data associated with this article can be found, in the online version, at http://dx.doi.org/10.1016/j.dcn.2015.10.008.
Appendix A. Supplementary data
The following are the supplementary data to this article:
References
- Alloway T.P. Working memory, but not IQ, predicts subsequent learning in children with learning difficulties. Eur. J. Psychol. Assess. 2009;25(2):92–98. [Google Scholar]
- American Psychiatric Association . fifth ed. American Psychiatric Publishing; Arlington, VA: 2013. Diagnostic and Statistical Manual of Mental Disorders. [Google Scholar]
- Arsalidou M., Pascual-Leone J., Johnson J. Misleading cues improve developmental assessment of working memory capacity: the color matching tasks. Cogn. Dev. 2010;25(3):262–277. [Google Scholar]
- Arsalidou M., Pascual-Leone J., Johnson J., Morris D., Taylor M.J. A balancing act of the brain: activations and deactivations as a function of cognitive load. Brain Behav. 2013;3(3):273–285. doi: 10.1002/brb3.128. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Baddeley A. Working memory. Science. 1992;255(5044):556–559. doi: 10.1126/science.1736359. [DOI] [PubMed] [Google Scholar]
- Bellebaum C., Daum I. Cerebellar involvement in executive control. Cerebellum. 2007;6(3):184–192. doi: 10.1080/14734220601169707. [DOI] [PubMed] [Google Scholar]
- Bor D., Duncan J., Wiseman R.J., Owen A.M. Encoding strategies dissociate prefrontal activity from working memory demand. Neuron. 2003;37(2):361–367. doi: 10.1016/s0896-6273(02)01171-6. [DOI] [PubMed] [Google Scholar]
- Brahmbhatt S.B., McAuley T., Barch D.M. Functional developmental similarities and differences in the neural correlates of verbal and nonverbal working memory tasks. Neuropsychologia. 2008;46(4):1020–1031. doi: 10.1016/j.neuropsychologia.2007.11.010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Buckner R.L., Andrews-Hanna J.R., Schacter D.L. The brain's default network. Ann. N.Y. Acad. Sci. 2008;1124(1):1–38. doi: 10.1196/annals.1440.011. [DOI] [PubMed] [Google Scholar]
- Bunge S.A., Wright S.B. Neurodevelopmental changes in working memory and cognitive control. Curr. Opin. Neurobiol. 2007;17(2):243–250. doi: 10.1016/j.conb.2007.02.005. [DOI] [PubMed] [Google Scholar]
- Cain K., Oakhill J., Bryant P. Children's reading comprehension ability: concurrent prediction by working memory, verbal ability, and component skills. J. Educ. Psychol. 2004;96(1):31–42. [Google Scholar]
- Chen S.H.A., Desmond J.E. Temporal dynamics of cerebro-cerebellar network recruitment during a cognitive task. Neuropsychologia. 2005;43(9):1227–1237. doi: 10.1016/j.neuropsychologia.2004.12.015. [DOI] [PubMed] [Google Scholar]
- Colom R., Jung R.E., Haier R.J. General intelligence and memory span: evidence for a common neuroanatomic framework. Cogn. Neuropsychol. 2007;24(8):867–878. doi: 10.1080/02643290701781557. [DOI] [PubMed] [Google Scholar]
- Conklin H.M., Luciana M., Hooper C.J., Yarger R.S. Working memory performance in typically developing children and adolescents: behavioral evidence of protracted frontal lobe development. Dev. Neuropsychol. 2007;31(1):103–128. doi: 10.1207/s15326942dn3101_6. [DOI] [PubMed] [Google Scholar]
- Cox R.W. AFNI: Software for analysis and visulization of functional magnetic resonance neuroimages. Comput. Biomed. Res. 1996;29:162–173. doi: 10.1006/cbmr.1996.0014. [DOI] [PubMed] [Google Scholar]
- De Smet H.J., Paquier P., Verhoeven J., Mariën P. The cerebellum: its role in language and related cognitive and affective functions. Brain Lang. 2013;127(3):334–342. doi: 10.1016/j.bandl.2012.11.001. [DOI] [PubMed] [Google Scholar]
- Dennis M., Agostino A., Roncadin C., Levin H. Theory of mind depends on domain-general executive functions of working memory and cognitive inhibition in children with traumatic brain injury. J. Clin. Exp. Neuropsychol. 2009;31(7):835–847. doi: 10.1080/13803390802572419. [DOI] [PubMed] [Google Scholar]
- Dumontheil I., Klingberg T. Brain activity during a visuospatial working memory task predicts arithmetical performance 2 years later. Cerebral Cortex. 2012;22(5):1078–1085. doi: 10.1093/cercor/bhr175. [DOI] [PubMed] [Google Scholar]
- Engle R.W. Working memory capacity as executive attention. Curr. Dir. Psychol. Sci. 2002;11(1):19–23. [Google Scholar]
- Engle R.W., Tuholski S.W., Laughlin J.E., Conway A.R.A. Working memory, short-term memory, and general fluid intelligence: a latent-variable approach. J. Exp. Psychol.: Gen. 1999;128(3):309–331. doi: 10.1037//0096-3445.128.3.309. [DOI] [PubMed] [Google Scholar]
- Gathercole S.E., Alloway T.P. Working memory and classroom learning. J. Prof. Teach. Students Specific Learn. Difficulties. 2004;17:2–12. [Google Scholar]
- Gathercole S.E., Pickering S.J., Knight C., Stegmann Z. Working memory skills and educational attainment: evidence from national curriculum assessments at 7 and 14 years of age. Appl. Cogn. Psychol. 2004;18(1):1–16. [Google Scholar]
- Gathercole S.E., Pickering S.J., Ambridge B., Wearing H. The structure of working memory from 4 to 15 years of age. Dev. Psychol. 2004;40(2):177–190. doi: 10.1037/0012-1649.40.2.177. [DOI] [PubMed] [Google Scholar]
- Gottwald B., Wilde B., Mihajlovic Z., Mehdorn H.M. Evidence for distinct cognitive deficits after focal cerebellar lesions, Journal of Neurology. Neurosurg. Psychiatry. 2004;75(11):1524–1531. doi: 10.1136/jnnp.2003.018093. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Huizinga M., Dolan C.V., van d.M. Age-related change in executive function: developmental trends and a latent variable analysis. Neuropsychologia. 2006;44(11):2017–2036. doi: 10.1016/j.neuropsychologia.2006.01.010. [DOI] [PubMed] [Google Scholar]
- Hwang Y., Hosokawa T. Working memory in Japanese children with learning disabilities: assessment using the Japanese short form of the Swanson cognitive processing test. Jpn. J. Special Educ. 2007;44(6):463–472. [Google Scholar]
- Jolles D.D., Kleibeuker S.W., Rombouts S.A.R.B., Crone E.A. Developmental differences in prefrontal activation during working memory maintenance and manipulation for different memory loads. Dev. Sci. 2011;14(4):713–724. doi: 10.1111/j.1467-7687.2010.01016.x. [DOI] [PubMed] [Google Scholar]
- Kirschen M.P., Chen S.A., Schraedley-Desmond P., Desmond J.E. Load-and practice-dependent increases in cerebro-cerebellar activation in verbal working memory: an fMRI study. Neuroimage. 2005;24(2):462–472. doi: 10.1016/j.neuroimage.2004.08.036. [DOI] [PubMed] [Google Scholar]
- Klingberg T., Forssberg H., Westerberg H. Increased brain activity in frontal and parietal cortex underlies the development of visuospatial working memory capacity during childhood. J. Cogn. Neurosci. 2002;14(1):1–10. doi: 10.1162/089892902317205276. [DOI] [PubMed] [Google Scholar]
- Kwon H., Reiss A.L., Menon V. Neural basis of protracted developmental changes in visuo-spatial working memory. Proc. Natl. Acad. Sci. USA. 2002;99:13336–13341. doi: 10.1073/pnas.162486399. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Levisohn L., Cronin-Golomb A., Schmahmann J.D. Neuropsychological consequences of cerebellar tumour resection in children: cerebellar cognitive affective syndrome in a paediatric population. Brain: J. Neurol. 2000;123(5):1041–1050. doi: 10.1093/brain/123.5.1041. [DOI] [PubMed] [Google Scholar]
- Liakakis G., Nickel J., Seitz R.J. Diversity of the inferior frontal gyrus—a meta-analysis of neuroimaging studies. Behav. Brain Res. 2011;225(1):341–347. doi: 10.1016/j.bbr.2011.06.022. [DOI] [PubMed] [Google Scholar]
- Martinussen R., Hayden J., Hogg-Johnson S., Tannock R. A meta-analysis of working memory impairments in children with attention-deficit/hyperactivity disorder. J. Am. Acad. Child Adolesc. Psychiatry. 2005;44(4):377–384. doi: 10.1097/01.chi.0000153228.72591.73. [DOI] [PubMed] [Google Scholar]
- Mishkin M., Ungerleider L.G. Contribution of striate inputs to the visuospatial functions of parieto-preoccipital cortex in monkeys. Behav. Brain Res. 1982;6(1):57–77. doi: 10.1016/0166-4328(82)90081-x. [DOI] [PubMed] [Google Scholar]
- Nagel B.J., Herting M.M., Maxwell E.C., Bruno R., Fair D. Hemispheric lateralization of verbal and spatial working memory during adolescence. Brain Cogn. 2013;82(1):58–68. doi: 10.1016/j.bandc.2013.02.007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- O’Hare E.D., Lu L.H., Houston S.M., Bookheimer S.Y., Sowell E.R. Neurodevelopmental changes in verbal working memory load-dependency: an fMRI investigation. Neuroimage. 2008;42(4):1678–1685. doi: 10.1016/j.neuroimage.2008.05.057. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Powell T.L., Arsalidou M., Vogan V., Taylor M.J. Letter and colour matching tasks: parametric measure of developmental working memory capacity. Child Dev. Res. 2014;2014 (Article ID 961781)9 pages. [Google Scholar]
- Powell K.B., Voeller K.K.S. Prefrontal executive function syndromes in children. J. Child Neurol. 2004;19(10):785–797. doi: 10.1177/08830738040190100801. [DOI] [PubMed] [Google Scholar]
- Raichle M.E., MacLeod A.M., Snyder A.Z., Powers W.J., Gusnard D.A., Shulman G.L. A default mode of brain function. Proc. Natl. Acad. Sci. U.S.A. 2001;98(2):676–682. doi: 10.1073/pnas.98.2.676. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rypma B., Berger J.S., D’Esposito M. The influence of working-memory demand and subject performance on prefrontal cortical activity. J. Cogn. Neurosci. 2002;14(5):721–731. doi: 10.1162/08989290260138627. [DOI] [PubMed] [Google Scholar]
- Scherf K.S., Sweeney J.A., Luna B. Brain basis of developmental change in visuospatial working memory. J. Cogn. Neurosci. 2006;18(7):1045–1058. doi: 10.1162/jocn.2006.18.7.1045. [DOI] [PubMed] [Google Scholar]
- Smith E.E., Jonides J., Marshuetz C., Koeppe R.A. Components of verbal working memory: evidence from neuroimaging. Proc. Natl. Acad. Sci. U.S.A. 1998;95:876–882. doi: 10.1073/pnas.95.3.876. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Southwick J.S., Bigler E.D., Froehlich A., DuBray M.B., Alexander A.L., Lange N., Lainhart J.E. Memory functioning in children and adolescents with autism. Neuropsychology. 2011;25(6):702. doi: 10.1037/a0024935. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sowell E.R., Thompson P.M., Holmes C.J., Jernigan T.L., Toga A.W. In vivo evidence for post-adolescent brain maturation in frontal and striatal regions. Nat. Neurosci. 1999;2(10):859–861. doi: 10.1038/13154. [DOI] [PubMed] [Google Scholar]
- Spencer-Smith M., Ritter B.C., Mürner-Lavanchy I., El-Koussy M., Steinlin M., Everts R. Age, sex, and performance influence the visuospatial working memory network in childhood. Dev. Neuropsychol. 2013;38(4):236–255. doi: 10.1080/87565641.2013.784321. [DOI] [PubMed] [Google Scholar]
- Stoodley C.J. The cerebellum and cognition: evidence from functional imaging studies. Cerebellum. 2012;11(2):352–365. doi: 10.1007/s12311-011-0260-7. [DOI] [PubMed] [Google Scholar]
- Strand F., Forssberg H., Klingberg T., Norrelgen F. Phonological working memory with auditory presentation of pseudo-words--an event related fMRI study. Brain Res. 2008;1212:48–54. doi: 10.1016/j.brainres.2008.02.097. [DOI] [PubMed] [Google Scholar]
- Strick P.L., Dum R.P., Fiez J.A. Cerebellum and nonmotor function. Annu. Rev. Neurosci. 2009;32:413–434. doi: 10.1146/annurev.neuro.31.060407.125606. [DOI] [PubMed] [Google Scholar]
- Tamnes C.K., Walhovd K.B., Grydeland H., Holland G., Ostby Y., Dale A.M. Longitudinal working memory developing is related to structural maturation of frontal and parietal cortices. J. Cogn. Neurosci. 2013;25:1611–1623. doi: 10.1162/jocn_a_00434. [DOI] [PubMed] [Google Scholar]
- Thomason M.E., Race E., Burrows B., Whitfield-Gabrieli S., Glover G.H., Gabrieli J.D.E. Development of spatial and verbal working memory capacity in the human brain. J. Cogn. Neurosci. 2009;21(2):316–332. doi: 10.1162/jocn.2008.21028. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Timmann D., Daum I. Cerebellar contributions to cognitive functions: a progress report after two decades of research. Cerebellum. 2007;6(3):159–162. doi: 10.1080/14734220701496448. [DOI] [PubMed] [Google Scholar]
- Ungerleider L.G., Mishkin M. Two cortical visual systems. In: Ingle D.J.I., Goodale M.A., Mansfield R.J.W., editors. Analysis of Visual Behavior. The MIT Press; Cambridge, MA: 1982. pp. 549–586. [Google Scholar]
- van den Heuvel M.P., Sporns O. Rich-club organization of the human connectome. J. Neurosci. 2011;31:15775–15786. doi: 10.1523/JNEUROSCI.3539-11.2011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Vogan V.M., Morgan B.R., Lee W., Powell T.L., Smith M.L., Taylor M.J. The neural correlates of visuo-spatial working memory in children with autism spectrum disorder: effects of cognitive load. J. Neurodev. Disord. 2014;6(1):1–15. doi: 10.1186/1866-1955-6-19. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wang E., Liu C. Working memory capacity of adolescents with learning disability. Chin. Ment. Health J. 2007;21:587–590. [Google Scholar]
- Wechsler D. Psychological Corporation; San Antonia, TX: 2003. Wechsler Intelligence Scale for Children. [Google Scholar]
- Woolrich M.W., Jbabdi S., Patenaude B., Chappell M., Makni S., Behrens T., Smith S.M. Bayesian analysis of neuroimaging data in FSL. NeuroImage. 2009;45(1):S173–S186. doi: 10.1016/j.neuroimage.2008.10.055. [DOI] [PubMed] [Google Scholar]
- Worsley K.J. Statistical analysis of activation impacts. In: Jezzard P., Matthews P.M., Smith S.M., editors. Functioning MRI: An Introduction to Methods. Oxford University Press; Oxford, UK: 2001. In Ch 14. [Google Scholar]
- Wright H.H., Fergadiotis G. Conceptualising and measuring working memory and its relationship to aphasia. Aphasiology. 2012;26(3–4):258–278. doi: 10.1080/02687038.2011.604304. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zald D.H., Iacono W.G. The development of spatial working memory abilities. Dev. Neuropsychol. 1998;14(4):563–578. [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.