Abstract
Investigating how brain development during adolescence and early adulthood underlies guilt- and shame-proneness may be important for understanding risk processes for mental disorders. The aim of this study was to investigate the neurodevelopmental correlates of interpersonal guilt- and shame-proneness in healthy adolescents and young adults using structural magnetic resonance imaging (sMRI). Sixty participants (age range: 15–25) completed sMRI and self-report measures of interpersonal guilt- and shame-proneness. Independent of interpersonal guilt, higher levels of shame-proneness were associated with thinner posterior cingulate cortex (PCC) thickness and smaller amygdala volume. Higher levels of shame-proneness were also associated with attenuated age-related reductions in thickness of lateral orbitofrontal cortex (lOFC). Our findings highlight the complexities in understanding brain–behavior relationships during the adolescent/young adult period. Results were consistent with growing evidence that accelerated cortical thinning during adolescence may be associated with superior socioemotional functioning. Further research is required to understand the implications of these findings for mental disorders characterized by higher levels of guilt and shame.
Keywords: Brain development, Cortical thickness, MRI, Moral emotions, Social
1. Introduction
Adolescence is a dynamic period of life characterized by marked changes across a number of domains, including physical maturation, drive for independence, increased salience of social and peer interactions, and brain development. There is an increasing recognition that changes in relational and social processes are key for adaptive functioning during adolescence and the emerging adult period (Smetana et al., 2006). The prosocial, and particularly negative moral emotions, guilt and shame, are thought to be of particular importance for the maturing adolescent, serving to maintain attachments, and acting as ‘social regulators’ that encourage a balance between one's self-interested motivations and the rights and needs of others. It has been suggested that increases in self-consciousness and concern with others’ opinions during adolescence results in an increase in the frequency and intensity of the experience of negative moral emotions such as guilt and shame (Zeman et al., 2006). While developmental increases in the experience of guilt and shame may be normative, the tendency to experience guilt and shame inappropriately or excessively may be maladaptive and related to the increased incidence of psychopathology during adolescence and young adulthood (Muris and Meesters, 2014).
While we have learned a great deal about the developing brain during adolescence over the past two decades (particularly with regards to neuroanatomical development) (Giedd et al., 1999, Lenroot et al., 2007), we know very little about how adolescent brain development underlies, or is associated with, social functioning, and in particular, the propensity to experience negative moral emotions. Existing functional magnetic resonance imaging (fMRI) work has identified the experience of guilt to be associated with broad engagement of prefrontal cortical regions, including the ventral medial prefrontal cortex (vmPFC; extending to subgenual cingulate cortex [SGC]) (Basile et al., 2011a, Moll et al., 2011, Zahn et al., 2009a, Zahn et al., 2009b), dmPFC (Basile et al., 2011a, Michl et al., 2012, Morey et al., 2012), and lOFC (Wagner et al., 2011); insular cortex (Basile et al., 2011b, Zahn et al., 2009b); and posterior medial wall regions, including PCC and precuneus (Basile et al., 2011a, Kedia et al., 2008). Far less research has been devoted to the neural correlates of shame. Two studies in healthy population, however, have found shame to be associated with activation of the anterior cingulate cortex (ACC) and PCC (Michl et al., 2012, Roth et al., 2014), dorsolateral PFC, dmPFC, and insula (Roth et al., 2014).
Structural brain imaging studies have been less informative. Only two studies have investigated the neuroanatomical correlates of guilt; one found a significant negative association between trait guilt and insula cortex volume (Belden et al., 2015), while a second study found a positive association between a behavioral measure of guilt (compensatory behavior during a pain administration task) and ACC volume (Yu et al., 2013). However, neither study investigated the specificity of their findings to guilt. Indeed, across the functional and structural literature, few studies have investigated the unique correlates of guilt and shame, an important endeavor given that the two emotions, although sharing common features, are notably distinct (Tangney et al., 1996). Namely, it has been suggested that guilt is associated with self-blame related to one's own behavior, whereas shame is associated with self-blame at a deeper level where the individual sees their global self as ‘faulty’ (Barr, 2004). Guilt is thought to be associated with feelings of regret and remorse, and is the counterpart to prosocial tendencies associated with empathy. Conversely, shame is thought to be associated with feelings of helplessness, and a desire to hide or escape (Barr, 2004), in addition to a preoccupation with worry about negative social-evaluation (Tangney et al., 1996). Further, few studies have investigated trait measures of guilt and shame (i.e., guilt- and shame-proneness), which may be more relevant for understanding risk for mental illness than state/behavioral measures (Ghatavi et al., 2002). Finally, no study to our knowledge has investigated the neuroanatomical correlates of guilt and shame using a developmental framework. Such work will be important for better understanding the neurodevelopmental mechanisms underlying guilt- and shame-propensity during the adolescent and young adult period. Measures of brain development have been shown to contribute unique information about psychological functioning during adolescence, often uncovering informative associations that would have been obscured if development had not been taken into account. Brain development during adolescence is characterized by, among other changes, cortical thinning that starts in primary sensory and motor areas and proceeds to association cortex (Shaw et al., 2008). Importantly, while studies of early onset psychopathology (e.g., childhood onset depression, schizophrenia and bipolar disorder) show exaggerated cortical thinning with age (Gogtay and Thompson, 2010, Luby et al., 2016), an attenuation of the normal pattern of cortical thinning during adolescence has been associated with indices of poor emotional and cognitive functioning, including relatively low IQ, attention problems, increased internalizing symptoms, depression risk, and lower temperamental effortful control (Ducharme et al., 2012, Ducharme et al., 2014, Papmeyer et al., 2014, Shaw et al., 2006, Vijayakumar et al., 2014).
In the current study, we sought to investigate the unique structural neurodevelopmental correlates of interpersonal guilt- and shame-proneness in a sample of healthy young people. Interpersonal types of guilt were the focus given the centrality of social relationships to adolescent development and functioning. We hypothesized that guilt and shame could be dissociated in terms of distinct neurodevelopmental correlates within social brain regions. Specifically, we hypothesized that guilt would be uniquely associated with the development of SGC/vmPFC, by virtue of the role of these regions in empathy (Zahn et al., 2009a), and anticipation of the social–emotional consequences (e.g., feeling guilty) in social decision-making (Grossman et al., 2010, Harrison et al., 2012, Moll et al., 2011). We also hypothesized that guilt would be associated with the development of the lOFC, given evidence for activation of this region when social cues initiate a change of current behavior (Blair and Cipolotti, 2000). Conversely, we hypothesized that shame would be uniquely associated with the development of the dmPFC, PCC and precuneus. We made this prediction based on the contribution of these regions to social-oriented self-referential processing (Harrison et al., 2008), which have direct links to theoretical definitions of shame as involving thinking about the self in relation to others. We also hypothesized that shame would be uniquely associated with development of the insula and amygdala, given evidence for their involvement in the experience of aversive feeling states (Craig, 2009) and social threat appraisal (Wolfgang and Miltnera, 2005). Given that excessive propensity to experience guilt and shame may be maladaptive, it was hypothesized that higher trait levels of these emotions would be associated with less an attenuation of the normal pattern of brain development (e.g., attenuated or reduced cortical thinning with age).
2. Materials and methods
2.1. Participants
Sixty-five healthy young people aged between 15 and 25 years participated in the study. They were confirmed to be without current or past diagnosis of a psychiatric or neurological disorder using a structured clinical interview (SCID-I non-patient version (First and Spitzer)). All participants (and their parents if <18 years of age) provided written, informed consent to complete this study after a complete description of its protocol, which was approved by the Melbourne Health Human Research Ethics Committee. Participants were excluded if they: had a medical or neurological condition; were being treated with psychoactive medication; had any history of Axis I psychopathology; were taking antidepressants (previously or currently); had experienced loss of consciousness for 5 min or more as a result of serious head injury; were pregnant; or had any other contraindications to MRI. After excluding participants for whom brain image segmentation was poor (see below), the final sample consisted of 60 participants (33 Female, M age 20.51, SD 2.99).
2.2. Measures and procedures
All participants completed an assessment that determined eligibility for the study, recorded details on demographics, medical and family history, and that included an MRI scan. Participants also completed the Quick Inventory of Depressive Symptoms-Self Report (QIDS-SR, Rush et al., 2003). In a follow-up assessment approximately 0.77 years (SD 0.36 years) post MRI scan, participants completed the Interpersonal Guilt Questionnaire-67 (IGQ-67) (O’Connor et al., 1997), and the Experience of Shame Scale (ESS) (Andrews et al., 2002).
The IGQ-67 was designed to measure trait levels of irrational guilt related to concerns about harming others. It comprises 67 statements that the participant must either agree or disagree with using a 5-point Likert scale (e.g., “I worry about hurting other people's feelings if I turn down an invitation from somebody who is eager for me to accept”). Fifteen items associated with ‘self-hate’ were excluded given the stronger theoretical association between self-hate and shame as opposed to guilt (Gibson, 2013). The ESS is a 25-item questionnaire that measures experiential, cognitive and behavioral components of trait shame, with questions aimed to uncover personal levels of shame experienced over the past year. Each item is rated on a 4-point Likert scale, from 1—not at all to 4—very much (e.g., “Have you worried about what other people think of you when you said something stupid?”). Total trait guilt and shame scores were obtained by summing scores on all items from the IGQ-67 (excluding self-hate items) and EES, respectively. These total scores were used as continuous interval-type data for all analyses as per prior literature (e.g., Andrews et al., 2002, O’Connor et al., 1997). In the current study, internal consistencies (Cronbach's alpha coefficients) for total guilt and shame scores were 0.77 and 0.92, respectively.
2.3. Neuroimaging
2.3.1. Image acquisition
A 3 T Signa Excite system (General Electric) equipped with an 8-channel phased-array head coil was used in combination with ASSET parallel imaging (Sunshine Hospital, Western Health, Melbourne). A high-resolution T1-weighted anatomical image was acquired using the following 3D BRAVO sequence: 140 contiguous slices; repetition time, 7900 ms; echo time, 3000 ms; flip angle, 13°; in a 25.6-cm field of view, with a 256 × 256 pixel matrix and a slice thickness of 1 mm (1 mm gap).
2.3.2. Image processing and analysis
Reconstruction of the cortical surfaces and measurement of cortical thickness was performed using FreeSurfer v5.3 (http://surfer.nmr.mgh.harvard.edu/). FreeSurfer provides a set of tools to reconstruct topologically correct and geometrically accurate surface models of the inner and outer cortical boundaries. Automated processing includes intensity normalization, Talairach transformation, removal of non-brain tissue, segmentation of the subcortical white matter (WM) and deep gray matter (GM) volumetric structures, tessellation of the boundary between the WM and GM, smoothing of the tessellated surface and automated topology correction (Dale et al., 1999). A deformable surface algorithm works on the tessellated surface to optimally locate the inner and outer cortical boundaries. Once the cortical model has been constructed, a variety of deformable procedures enable further data processing and analysis. This latter step includes registration of the constructed surface to a predefined cortical surface-based atlas, thus allowing each voxel to receive a neuroanatomical label. FreeSurfer's cortical maps are not restricted to the voxel resolution of original data, and can therefore measure thickness at sub-millimeter accuracy.
Any topological errors that occur after processing such as inaccuracies in the skull strip or poor separation between GM and WM were inspected and manually corrected as necessary. During this process, 5 of the 65 original participants were excluded due to inaccuracies in segmentation that could not be corrected with manual intervention. To measure cortical thickness, the shortest distance between the GM/WM boundaries and the pial surface at each point on the cortical mantle were calculated (Fischl and Dale, 2000).
2.3.3. Selection of regions of interest (ROIs)
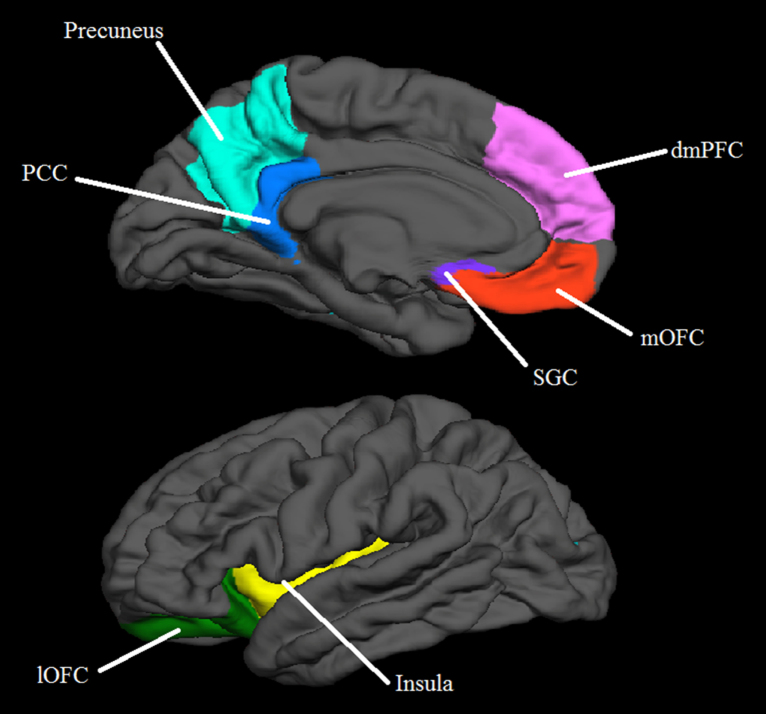
An ROI approach was used for hypothesis testing. ROIs were generated based on the subcortical (Fischl et al., 2004), Desikan (Desikan et al., 2006) and Destrieux (Destrieux et al., 2010) atlases as implemented in FreeSurfer, and included regions relevant to hypotheses: the amygdala, vmPFC (SGC and medial orbitofrontal cortex [mOFC]), PCC, precuneus, lOFC, insula and dmPFC. The dmPFC was created from the superior frontal gyrus region as delineated by the Desikan atlas. A coronal cut was applied at Talairach coordinate y = 26 so that only the prefrontal aspect of the dmPFC was included (in order to conform to the conservative Talairach criteria described by Rajkowska and Goldman-Rakic (1995)). In addition, another cut was made along the superior edge of the medial wall of the brain in order to exclude the lateral surface of the brain. The majority of regions came from the Desikan parcellation. Only the SCG came from the Destrieux parcellation. There was some overlap between the mOFC and SGC. We chose not to alter these labels to exclude overlap so that boundaries remained consistent with the published respective parcellations. See Fig. 1 for an illustration of ROIs.
Fig. 1.
Regions of interest (ROI) utilized in analyses. PCC = posterior cingulate cortex, dmPFC = dorsomedial prefrontal cortex, SGC = subgenual cingulate cortex, mOFC = medial orbitofrontal cortex, lOFC = lateral orbitofrontal cortex.
2.4. Statistical analysis
The unique effects of trait guilt and shame, and their interaction with age, on thickness in each cortical ROI, and amygdala volume, were examined via regression analysis in SPSS (SPSS Inc., Chicago, IL). For each regression for guilt, ROI structure was entered as the dependent variable. Age, sex, time between MRI scan and questionnaire completion, and trait shame were entered in the first block, two-way interactions were entered in the second block (age × sex, age × guilt and sex × guilt), and the three-way interaction between age, sex and guilt was entered in the third block. Each regression for shame was set-up similarly. As is standard in studies of ROI volumes (due to the association between ROI volumes and overall head size), estimated total intracranial volume was included as a covariate in amygdala analyses. Trait guilt and shame and age were centered prior to calculating interaction terms. The significance level was corrected using a false discovery rate (FDR) (Benjamini and Yekutieli, 2001) method for the 16 ROIs (eight in each hemisphere) yielding a significance level of 0.018 (Narum, 2006). Unlike Bonferroni corrections, which are considered to be too conservative in dependent data sets (Armitage et al., 2002), the FDR method used in the current study was designed to accommodate dependence among the hypothesis tests while providing a more conservative Type I error rate than earlier FDR approaches (Narum, 2006). Due to the novel nature of the study, however, uncorrected results (p < 0.05) are also reported.
Secondary (exploratory) analyses were performed to examine whether guilt and shame were associated with any quadratic age effects. Regression analyses similar to those described above were performed with the inclusion of an age × age term in the second block, age × age × sex and age × age × [guilt or shame] in the third block, and age × age × sex × [guilt or shame] in a fourth block. All results significant at the level of p < 0.05 are reported.
Finally, an exploratory whole brain vertex-wise analysis was performed in FreeSurfer to complement the ROI analyses, and to assess for potential further associations outside of our hypothesized regions of interest (p < 0.001, uncorrected). For completeness, results are also reported using significance thresholds of p < 0.005, and p < 0.01, uncorrected.
3. Results
Descriptive data are presented in Table 1. There were no sex differences in age, trait guilt or shame (all p's > 0.5). Table 2 shows correlations between age and all behavioral data for males and females. Of note, age was negatively associated with shame for the whole sample (r = 0.33, p = 0.011).
Table 1.
Descriptive data.
| Whole sample (N = 60) |
Males (n = 28) |
Females (n = 32) |
|
|---|---|---|---|
| Mean (SD) | Mean (SD) | Mean (SD) | |
| Age | 20.3 (3.0) | 20.5 (3.0) | 20.2 (2.9) |
| Guilt (IGQ-67) | 147.0 (19.3) | 141.8 (18.6) | 151.5 (19.1) |
| Shame (ESS) | 55.0 (13.8) | 53.5 (13.6) | 56.4 (14.0) |
Table 2.
Pearson's bivariate correlations (and p values) between age and behavioral data for males (below diagonal) and females (above diagonal).
| Age | Guilt (IGQ-67) | Shame (ESS) | |
|---|---|---|---|
| Age | 1 | −0.20 (0.28) | −0.31 (.08) |
| Guilt (IGQ-67) | −0.20 (0.31) | 1 | 0.53 (0.002) |
| Shame (ESS) | −0.33 (0.083) | 0.36 (0.06) | 1 |
3.1. FDR-corrected results
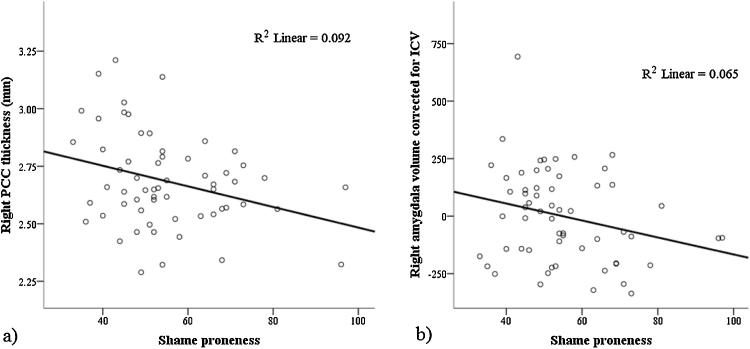
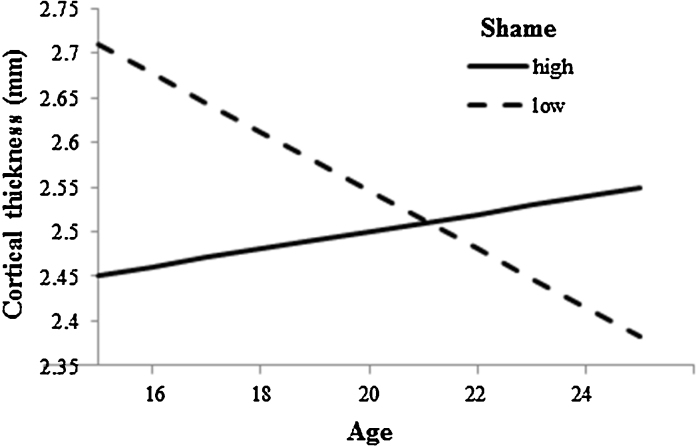
After correcting for multiple comparisons, there were no significant associations between guilt and brain structure, or any moderating effects of age. Higher trait shame was associated with thinner right (β = −0.39, t = −2.71, p = 0.009, partial r = −0.35, f2 = 0.14) and left PCC (β = −0.35, t = −2.44, p = 0.018, partial r = −0.32, f2 = 0.11), and smaller right amygdala volume (β = −0.29, t = −2.78, p = 0.008, partial r = −0.36, f2 = 0.15) (see Fig. 2). Age interacted with shame to predict left lOFC thickness (β = 0.38, t = 2.66, p = 0.010, partial r = −0.35, f2 = 0.14). Fig. 3 shows that higher levels of trait shame were associated with reduced lOFC thinning with age.
Fig. 2.
Association between trait shame and a) right posterior cingulate cortex (PCC) thickness, and b) right amygdala volume.
Fig. 3.
Association between age and left lOFC thickness for high (+1 SD) and low (−1 SD) levels of shame-proneness.
3.2. Uncorrected results
At an uncorrected level, there was a significant association between higher levels of guilt and thicker right dmPFC (β = 0.33, t = 2.15, p = 0.037, partial r = 0.29, f2 = 0.09). Uncorrected, age also interacted with guilt to predict thickness of the left dmPFC (β = 0.33, t = 2.15, p = 0.036, partial r = 0.30, f2 = 0.10). Higher levels of trait guilt were associated with reduced dmPFC thinning with age. Uncorrected, age and shame also interacted to predict left dmPFC thickness (β = 0.35, t = 2.37, p = 0.022, partial r = 0.32, f2 = 0.11). Similarly to guilt, higher levels of trait shame were associated with reduced dmPFC thinning with age. Uncorrected, there was an interaction between age, sex and shame in the prediction of left precuneus thickness (β = 0.45, t = 2.20, p = 0.032, partial r = 0.30, f2 = 0.10) and right PCC thickness (β = 0.42, t = 2.20, p = 0.032, partial r = 0.30, f2 = 0.10). Analyses performed separately for males and females revealed that age interacted with shame to predict thickness in both regions in males (left precuneus: β = 0.54, t = 2.23, p = 0.038, partial r = 0.43, f2 = 0.23; right PCC: β = 0.44, t = 2.36, p = 0.028, partial r = 0.45, f2 = 0.25) but not females. In males, higher levels of trait shame were associated with reduced cortical thinning with age in both regions.
3.3. Non-linear age effects
Shame interacted with quadratic age to predict left lOFC thickness (β = 0.53, t = 2.60, p = 0.012, partial r = 0.36, f2 = 0.14). Lower levels of shame were associated with a U-shaped decrease in thickness with age, whereas higher levels of shame were associated with a slight U-shaped increase with age. Guilt interacted with quadratic age to predict right precuneus thickness (β = 0.50, t = 2.23, p = 0.003, partial r = 0.31, f2 = 0.11). Lower levels of guilt were associated with U-shaped change in thickness with age, whereas higher levels of guilt were associated with an inverted-U shaped change in thickness with age.
Note that for both guilt- and shame-proneness, excluding the respective trait as a covariate had no impact on the pattern of significant and non-significant findings reported for any analysis described in the above sections. Further, results did not change when including QIDS-SR depressive symptoms as a covariate. This is notable given that shame-proneness was significantly associated with QIDS-SR depressive symptoms (shame: r = 0.37, p = 0.003; guilt: r = 0.20, p = 0.118).
3.4. Exploratory whole brain analyses
Whole brain vertex-wise analyses replicated the negative association between shame-proneness and right PCC thickness (p < 0.001), and the interaction between shame and age in predicting shame-proneness in the left lOFC (cluster in neighboring parsorbitalis region, p < 0.01). Results from the vertex-wise analysis can be found in Supplementary Material.
4. Discussion
We have identified specific developmental associations between the propensity to experience feelings of interpersonal guilt and shame, and brain structure during the mid-adolescent to early adulthood period. While our hypotheses regarding guilt were not supported, shame-proneness was associated with the size of the PCC and amygdala as hypothesized, and with age-related changes in the thickness of the left lOFC. Consistent with hypotheses regarding age-moderated associations, higher levels of shame-proneness were associated with attenuated age-related reductions in left lOFC thickness. Although not surviving correction for multiple comparisons, shame- and guilt-proneness were also associated with age-related changes in the thickness of hypothesized ROIs, including the dmPFC, PCC and precuneus (the latter two for males only). Importantly, controlling for the respective trait in analyses allowed us to infer neurodevelopmental correlates of guilt- and shame-proneness with some specificity.
While we did not make hypotheses regarding age differences in guilt- and shame-proneness, it is of note that a significant negative association was found between age and shame-proneness in the whole sample. Although the propensity to experience adaptive levels of negative moral emotions might increase during adolescence and young adulthood, supporting maturation of relational and social processes, our finding might reflect maladaptive levels of shame decreasing over this period as a result of increases in emotion regulatory abilities (Eisenberg, 2000, Zimmermann and Iwanski, 2014).
Independent of guilt-proneness, higher levels of shame-proneness were associated with thinner right PCC. The involvement of the PCC in shame is consistent with hypotheses, and also with fMRI studies showing shame to be associated with activation in this region (Michl et al., 2012, Roth et al., 2014). The PCC is involved in self-reflection, particularly in relation to outward-directed, social or contextual focus (Johnson et al., 2006). It has also been proposed to be involved in encoding and retrieving autobiographical memory (Svoboda et al., 2006), and particularly in on-line re-experiencing of past events (Maddock et al., 2003). As such, our finding might reflect an association between shame-proneness and such functions, consistent with the suggestion that shame is particularly associated with self-reflection in social contexts (Tangney et al., 1996) and rumination (Orth et al., 2006). A negative association between trait shame and PCC thickness is also consistent with structural MRI studies of disorders associated with elevated propensity to experience shame. For example, thinner PCC has been found in depression (Hulvershorn et al., 2011, Järnum et al., 2011) and social anxiety disorder (Syal et al., 2012).
Higher levels of shame-proneness were associated with smaller right amygdala volumes, consistent with hypotheses, and potentially indicative of the role of the amygdala in processing aversive emotion and specifically, social threat (Wolfgang and Miltnera, 2005). A negative association between trait shame and amygdala volume is consistent with findings of smaller amygdala volume in patients with disorders marked by elevated shame experiences, such as depression (Hulvershorn et al., 2011) and anxiety (Milham et al., 2005). However, it is of note that increased amygdala volumes have also been found in these patient groups (Lange and Irle, 2004, Redlich et al., 2014), although, at least in the case of depression, volumetric enlargement has been suggested to result from antidepressant medication use (Hamilton et al., 2008). These conflicting findings suggest that a complex set of factors likely determine amygdala volume; while higher trait shame was associated with smaller amygdala volume in the current study, further research is required to elucidate the meaning of this finding for behavioral function and risk for psychopathology.
While there was no main effect of shame on thickness in the lOFC, age moderated the effect of shame in this region such that higher levels of shame were associated with thinner cortex in younger participants and thicker cortex in older participants. While this study was cross-sectional, we interpret this finding as reflecting an association between high shame proneness and an attenuation of the normal pattern of cortical thinning across age. If higher levels of trait shame are considered to be maladaptive, these findings are consistent with studies showing attenuated cortical thinning to be associated with inferior emotional and cognitive functioning (Ducharme et al., 2012, Ducharme et al., 2014, Papmeyer et al., 2014, Shaw et al., 2006, Vijayakumar et al., 2014). Although involvement of the lOFC in shame was not hypothesized, it is consistent with fMRI study findings of lOFC involvement in embarrassment (Finger et al., 2006), a closely-related construct to shame. The role of lOFC development in shame may reflect the role for this region in socially-motivated response reversal (Kringelbach and Rolls, 2003). Although the experience of guilt is commonly associated with reparative action, shame is also commonly associated with behavioral change (e.g., social retreat) in response to perceived social punishment. Shame-related lOFC thinning might reflect maturation of neural circuits associated with responding to social punishment, consistent with behavioral evidence for the development of punishment reversal learning from adolescence to early adulthood (van der Schaaf et al., 2011).
Although not surviving correction for multiple comparisons, high levels of both guilt and shame-proneness were associated with attenuated dmPFC cortical thinning with age. The involvement of the dmPFC in shame is consistent with hypotheses and with previous fMRI study findings (Roth et al., 2014). The role of dmPFC development in both guilt and shame may reflect maturation of other-relevant social-cognitive processes like perspective-taking, which have been ascribed to functioning of this region (Bzdok et al., 2013). High levels of shame-proneness were also associated with attenuated PCC and precuneus thickness with age in males, which might suggest a gender-specific association with shame-proneness and development of self-referential processing (Harrison et al., 2008). Given that these results did not survive correction for multiple comparisons, their interpretation warrants caution and replication in future work.
Our findings of main and age-moderated effects of shame-proneness on thickness in different cortical regions highlights the complexities in understanding brain–behavior relationships during adolescence and emerging adulthood, and suggests that two competing neurological processes likely influence cortical thickness over this period: (a) a normative developmental thinning process, which may occur later or in an attenuated fashion in some regions (e.g., lOFC) for high shame-prone individuals, potentially reflecting reduced neuronal efficiency; and (b) a secondary experience-dependent process modulated by individual behavior and environmental stimulus. In the case of the PCC (where age did not moderate the effects of shame-proneness on thickness for the whole sample), this process could reflect the retention of useful circuits related to low shame-proneness, or conversely, a general reduction in thickness in high shame-prone individuals might be due to excessive hyper- or hypo-activity in this region.
Further, our findings suggest that for some brain regions, measures of developmental change may be more sensitive to, or may reveal more complex associations with, guilt- and shame-proneness than do measures of absolute brain structure. Our findings highlight the fact that cross-sectional studies that include adolescents and young adults spanning wide age ranges may produce anomalous findings if they do not consider the marked development of the brain during this period. While we have provided evidence for the importance of brain development in understanding the propensity to experience guilt and shame, an important limitation of this work is that it is not longitudinal, and thus, we can only make inferences about developmental processes based on age differences. Future longitudinal work is needed in order to make inferences about intra-individual change. Questionnaire measures of guilt- and shame-proneness were collected on average 9 months after MRI scans, however, these measures are designed to assess stable traits, and time between scan and questionnaire completion was not significantly correlated with guilt- or shame-proneness or any ROI measure (all p's > 0.23), suggesting that this time difference did not introduce any measureable bias. Non-interpersonal types of guilt (e.g., existential guilt, societal guilt) were not investigated and thus, we cannot comment on neurodevelopmental correlates of guilt more broadly. While results for shame were found to be independent of depressive symptoms, we did not investigate other factors (e.g., temperamental shyness or inhibition) that may better explain the associations between shame and brain structure. Finally, the study may have been under-powered to see significant effects in some hypothesized ROIs. Further, exclusion of individuals with lifetime psychiatric disorder likely reduced variance and hence power. It is of note that most of the effects identified (corrected and uncorrected) can be classified as approximately medium in size (Cohen, 1992). Further research with larger, and more representative sample sizes is warranted.
In conclusion, we have provided new insight into how neurodevelopment during adolescence and young adulthood underlies individual differences in the propensity to experience shame, a moral emotion that is important for social development, and potentially, risk for psychopathology. Future investigation of the associations between brain development and the propensity to experience pathological levels of guilt and shame could enable a better understanding of the etiology and pathophysiology of disorders such as depression and social anxiety disorder.
Acknowledgments
This work was supported by a National Health and Medical Research Council of Australia (NHMRC) Project Grant to BJH (ID: 1064643). BJH was supported by a NHMRC Clinical Career Development Fellowship (ID: 628509). SW was supported by a NHMRC Biomedical Career Development Fellowship (ID: 1007716). We thank Dr. Rebecca Kerestes and Ms Katerina Stephanou for their assistance with data collection. We also thank staff from the Sunshine Hospital Medical Imaging Department (Western Health, Melbourne) for their contribution to this work.
Footnotes
Supplementary data associated with this article can be found, in the online version, at http://dx.doi.org/10.1016/j.dcn.2016.02.001.
Appendix A. Supplementary data
Exploratory whole brain analyses were conducted to assess for potential further associations outside of our hypothesized regions of interest. Main effects of guilt- and shame-proneness, as well as interactions between guilt- and shame-proneness, and age and sex, were conducted within FreeSurfer (QDEC) using GLM whole brain vertex-wise analyses. The same nuisance variables used in ROI analyses were included in all models. Main effects of guilt- and shame-proneness are presented in Table S1 and Figs. S1 and S2. Interaction effects with age are presented in Table S2 and Figs. S3 and S4, and with age and sex are presented in Table S3 and Figs. S5 and S6.
References
- Andrews B., Qian M., Valentine J.D. Predicting depressive symptoms with a new measure of shame: the experience of Shame Scale. Br. J. Clin. Psychol. 2002;41(1):29–42. doi: 10.1348/014466502163778. [DOI] [PubMed] [Google Scholar]
- Armitage P., Berry G., Matthews J. Blackwell Science; Malden, MA: 2002. Statistical Methods in Medical Research. [Google Scholar]
- Barr P. Guilt and shame proneness and the grief of perinatal bereavement. Psychol. Psychother. 2004;77(4):493–510. doi: 10.1348/1476083042555442. [DOI] [PubMed] [Google Scholar]
- Basile B., Mancini F., Macaluso E., Caltagirone C., Frackowiak R.S., Bozzali M. Deontological and altruistic guilt: evidence for distinct neurobiological substrates. Hum. Brain Mapp. 2011;32(2):229–239. doi: 10.1002/hbm.21009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Basile B., Mancini F., Macaluso E., Caltagirone C., Frackowiak R.S.J., Bozzali M. Deontological and altruistic guilt: evidence for distinct neurobiological substrates. Hum. Brain Mapp. 2011;32(2):229–239. doi: 10.1002/hbm.21009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Belden A.C., Barch D.M., Oakberg T.J., April L.M., Harms M.P., Botteron K.N. Anterior insula volume and guilt: neurobehavioral markers of recurrence after early childhood major depressive disorder. JAMA Psychiatry. 2015;72(1):40–48. doi: 10.1001/jamapsychiatry.2014.1604. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Benjamini Y., Yekutieli D. The control of the false discovery rate in multiple testing under dependency. Ann. Stat. 2001;29(4):1165–1188. [Google Scholar]
- Blair R., Cipolotti L. Impaired social response reversal. A case of acquired sociopathy. Brain. 2000;123(6):1122–1141. doi: 10.1093/brain/123.6.1122. [DOI] [PubMed] [Google Scholar]
- Bzdok D., Langner R., Schilbach L., Engemann D.A., Laird A.R., Fox P.T. Segregation of the human medial prefrontal cortex in social cognition. Front. Hum. Neurosci. 2013;7:232. doi: 10.3389/fnhum.2013.00232. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cohen J. A power primer. Psychol. Bull. 1992;112(1):155. doi: 10.1037//0033-2909.112.1.155. [DOI] [PubMed] [Google Scholar]
- Craig A.D. The Anterior Insula and Human Awareness. 2009. How do you feel—now? [DOI] [PubMed] [Google Scholar]
- Dale A.M., Fischl B., Sereno M.I. Cortical surface-based analysis I. Segmentation and surface reconstruction. NeuroImage. 1999;9(2):179–194. doi: 10.1006/nimg.1998.0395. [DOI] [PubMed] [Google Scholar]
- Desikan R.S., Segonne F., Fischl B., Quinn B.T., Dickerson B.C., Blacker D. An automated labeling system for subdividing the human cerebral cortex on MRI scans into gyral based regions of interest. NeuroImage. 2006;31(3):968–980. doi: 10.1016/j.neuroimage.2006.01.021. [DOI] [PubMed] [Google Scholar]
- Destrieux C., Fischl B., Dale A., Halgren E. Automatic parcellation of human cortical gyri and sulci using standard anatomical nomenclature. NeuroImage. 2010;53(1):1–15. doi: 10.1016/j.neuroimage.2010.06.010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ducharme S., Hudziak J.J., Botteron K.N., Albaugh M.D., Nguyen T.V., Karama S. Decreased regional cortical thickness and thinning rate are associated with inattention symptoms in healthy children. J. Am. Acad. Child Adolesc. Psychiatry. 2012;51(1):18–27. doi: 10.1016/j.jaac.2011.09.022. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ducharme S., Albaugh M.D., Hudziak J.J., Botteron K.N., Nguyen T.-V., Truong C. Anxious/depressed symptoms are linked to right ventromedial prefrontal cortical thickness maturation in healthy children and young adults. Cereb. Cortex. 2014;24(11):2941–2950. doi: 10.1093/cercor/bht151. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Eisenberg N. Emotion, regulation, and moral development. Ann. Rev. Psychol. 2000;51(1):665–697. doi: 10.1146/annurev.psych.51.1.665. [DOI] [PubMed] [Google Scholar]
- Finger E.C., Marsh A.A., Kamel N., Mitchell D.G., Blair J.R. Caught in the act: the impact of audience on the neural response to morally and socially inappropriate behavior. NeuroImage. 2006;33(1):414–421. doi: 10.1016/j.neuroimage.2006.06.011. [DOI] [PubMed] [Google Scholar]
- First M.B., Spitzer R.L., Gibbon M., Williams J.B.W. Biometrics Research, New York State Psychiatric Institute; New York: 2002. Structured Clinical Interview for DSM-IV-TR Axis I Disorders, Research Version, Non-patient Edition: (SCID-I/NP) [Google Scholar]
- Fischl B., Dale A.M. Measuring the thickness of the human cerebral cortex from magnetic resonance images. Proc. Natl. Acad. Sci. U.S.A. 2000;97(20):11050–11055. doi: 10.1073/pnas.200033797. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fischl B., van der Kouwe A., Destrieux C., Halgren E., Segonne F., Salat D.H. Automatically parcellating the human cerebral cortex. Cereb. Cortex. 2004;14(1):11–22. doi: 10.1093/cercor/bhg087. [DOI] [PubMed] [Google Scholar]
- Ghatavi K., Nicolson R., MacDonald C., Osher S., Levitt A. Defining guilt in depression: a comparison of subjects with major depression, chronic medical illness and healthy controls. J. Affect. Disord. 2002;68(2):307–315. doi: 10.1016/s0165-0327(01)00335-4. [DOI] [PubMed] [Google Scholar]
- Gibson M. Shame and guilt in child protection social work: new interpretations and opportunities for practice. Child Fam. Soc. Work. 2013;20(3):333–343. [Google Scholar]
- Giedd J.N., Blumenthal J., Jeffries N.O., Castellanos F.X., Liu H., Zijdenbos A. Brain development during childhood and adolescence: a longitudinal MRI study. Nat. Neurosci. 1999;2(10):861–863. doi: 10.1038/13158. [DOI] [PubMed] [Google Scholar]
- Gogtay N., Thompson P.M. Mapping gray matter development: implications for typical development and vulnerability to psychopathology. Brain Cogn. 2010;72(1):6–15. doi: 10.1016/j.bandc.2009.08.009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Grossman M., Eslinger P.J., Troiani V., Anderson C., Avants B., Gee J.C. The role of ventral medial prefrontal cortex in social decisions: converging evidence from fMRI and frontotemporal lobar degeneration. Neuropsychologia. 2010;48(12):3505–3512. doi: 10.1016/j.neuropsychologia.2010.07.036. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hamilton J.P., Siemer M., Gotlib I.H. Amygdala volume in major depressive disorder: a meta-analysis of magnetic resonance imaging studies. Mol. Psychiatry. 2008;13(11):993–1000. doi: 10.1038/mp.2008.57. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Harrison B.J., Pujol J., López-Solà M., Hernández-Ribas R., Deus J., Ortiz H., Soriano-Mas C., Yücel M., Pantelis C., Cardoner N. Consistency and functional specialization in the default mode brain network. Proc. Natl. Acad. Sci. U.S.A. 2008;105(28):9781–9786. doi: 10.1073/pnas.0711791105. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Harrison B.J., Pujol J., Soriano-Mas C., Hernández-Ribas R., López-Solà M., Ortiz H. Neural correlates of moral sensitivity in obsessive-compulsive disorder. Arch. Gen. Psychiatry. 2012;69(7):741–749. doi: 10.1001/archgenpsychiatry.2011.2165. [DOI] [PubMed] [Google Scholar]
- Hulvershorn L.A., Cullen K., Anand A. Toward dysfunctional connectivity: a review of neuroimaging findings in pediatric major depressive disorder. Brain Imaging Behav. 2011;5(4):307–328. doi: 10.1007/s11682-011-9134-3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Järnum H., Eskildsen S.F., Steffensen E.G., Lundbye Christensen S., Simonsen C.W., Thomsen I.S. Longitudinal MRI, study of cortical thickness, perfusion, and metabolite levels in major depressive disorder. Acta Psychiatr. Scand. 2011;124(6):435–446. doi: 10.1111/j.1600-0447.2011.01766.x. [DOI] [PubMed] [Google Scholar]
- Johnson M.K., Raye C.L., Mitchell K.J., Touryan S.R., Greene E.J., Nolen-Hoeksema S. Dissociating medial frontal and posterior cingulate activity during self-reflection. Soc. Cogn. Affect. Neurosci. 2006;1(1):56–64. doi: 10.1093/scan/nsl004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kedia G., Berthoz S., Wessa M., Hilton D., Martinot J.-L. An agent harms a victim: a functional magnetic resonance imaging study on specific moral emotions. J. Cogn. Neurosci. 2008;20(10):1788–1798. doi: 10.1162/jocn.2008.20070. [DOI] [PubMed] [Google Scholar]
- Kringelbach M.L., Rolls E.T. Neural correlates of rapid reversal learning in a simple model of human social interaction. NeuroImage. 2003;20(2):1371–1383. doi: 10.1016/S1053-8119(03)00393-8. [DOI] [PubMed] [Google Scholar]
- Lange C., Irle E. Enlarged amygdala volume and reduced hippocampal volume in young women with major depression. Psychol. Med. 2004;34(06):1059–1064. doi: 10.1017/s0033291703001806. [DOI] [PubMed] [Google Scholar]
- Lenroot R.K., Gogtay N., Greenstein D.K., Wells E.M., Wallace G.L., Clasen L.S. Sexual dimorphism of brain developmental trajectories during childhood and adolescence. NeuroImage. 2007;36(4):1065–1073. doi: 10.1016/j.neuroimage.2007.03.053. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Luby J.L., Belden A.C., Jackson J.J., Lessov-Schlaggar C.N., Harms M.P., Tillman R. Early childhood depression and alterations in the trajectory of gray matter maturation in middle childhood and early adolescence. JAMA Psychiatry. 2016;73(1):31–38. doi: 10.1001/jamapsychiatry.2015.2356. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Maddock R.J., Garrett A.S., Buonocore M.H. Posterior cingulate cortex activation by emotional words: fMRI evidence from a valence decision task. Hum. Brain Mapping. 2003;18(1):30–41. doi: 10.1002/hbm.10075. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Michl P., Meindl T., Meister F., Born C., Engel R.R., Reiser M. Neurobiological underpinnings of shame and guilt: a pilot fMRI study. Soc. Cogn. Affect. Neurosci. 2012;9(2):150–157. doi: 10.1093/scan/nss114. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Milham M.P., Nugent A.C., Drevets W.C., Dickstein D.S., Leibenluft E., Ernst M. Selective reduction in amygdala volume in pediatric anxiety disorders: a voxel-based morphometry investigation. Biol. Psychiatry. 2005;57(9):961–966. doi: 10.1016/j.biopsych.2005.01.038. [DOI] [PubMed] [Google Scholar]
- Moll J., Zahn R., de Oliveira-Souza R., Bramati I.E., Krueger F., Tura B., Cavanagh A.L., Grafman J. Impairment of prosocial sentiments is associated with frontopolar and septal damage in frontotemporal dementia. NeuroImage. 2011;54(2):1735–1742. doi: 10.1016/j.neuroimage.2010.08.026. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Morey R.A., McCarthy G., Selgrade E.S., Seth S., Nasser J.D., LaBar K.S. Neural systems for guilt from actions affecting self versus others. NeuroImage. 2012;60(1):683–692. doi: 10.1016/j.neuroimage.2011.12.069. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Muris P., Meesters C. Small or big in the eyes of the other: on the developmental psychopathology of self-conscious emotions as shame, guilt, and pride. Clin. Child Fam. Psychol. Rev. 2014;17(1):19–40. doi: 10.1007/s10567-013-0137-z. [DOI] [PubMed] [Google Scholar]
- Narum S. Beyond Bonferroni: less conservative analyses for conservation genetics. Conserv. Genet. 2006;7(5):783–787. [Google Scholar]
- O’Connor L.E., Berry J.W., Weiss J., Bush M., Sampson H. Interpersonal guilt: the development of a new measure. J. Clin. Psychol. 1997;53(1):73–89. doi: 10.1002/(sici)1097-4679(199701)53:1<73::aid-jclp10>3.0.co;2-i. [DOI] [PubMed] [Google Scholar]
- Orth U., Berking M., Burkhardt S. Self-conscious emotions and depression: rumination explains why shame but not guilt is maladaptive. Pers. Soc. Psychol. Bull. 2006;32(12):1608–1619. doi: 10.1177/0146167206292958. [DOI] [PubMed] [Google Scholar]
- Papmeyer M., Giles S., Sussmann J.E., Kielty S., Stewart T., Lawrie S.M. Cortical thickness in individuals at high familial risk of mood disorders as they develop major depressive disorder. Biol. Psychiatry. 2014;78(1):58–66. doi: 10.1016/j.biopsych.2014.10.018. [DOI] [PubMed] [Google Scholar]
- Rajkowska G., Goldman-Rakic P.S. Cytoarchitectonic definition of prefrontal areas in the normal human cortex: I. Remapping of areas 9 and 46 using quantitative criteria. Cereb. Cortex. 1995;5(4):307–322. doi: 10.1093/cercor/5.4.307. [DOI] [PubMed] [Google Scholar]
- Redlich R., Grotegerd D., Opel N., Kaufmann C., Zwitserlood P., Kugel H. Are you gonna leave me? Separation anxiety is associated with increased amygdala responsiveness and volume. Soc. Cogn. Affect. Neurosci. 2014 doi: 10.1093/scan/nsu055. nsu055. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Roth L., Kaffenberger T., Herwig U., Bruehl A.B. Brain activation associated with pride and shame. Neuropsychobiology. 2014;69(2):95–106. doi: 10.1159/000358090. [DOI] [PubMed] [Google Scholar]
- Rush A.J., Trivedi M.H., Ibrahim H.M., Carmody T.J., Arnow B., Klein D.N. The 16-Item Quick Inventory of Depressive Symptomatology (QIDS), clinician rating (QIDS-C), and self-report (QIDS-SR): a psychometric evaluation in patients with chronic major depression. Biol. Psychiatry. 2003;54(5):573–583. doi: 10.1016/s0006-3223(02)01866-8. [DOI] [PubMed] [Google Scholar]
- Shaw P., Greenstein D., Lerch J., Clasen L., Lenroot R., Gogtay N. Intellectual ability and cortical development in children and adolescents. Nature. 2006;440(7084):676–679. doi: 10.1038/nature04513. [DOI] [PubMed] [Google Scholar]
- Shaw P., Kabani N.J., Lerch J.P., Eckstrand K., Lenroot R., Gogtay N. Neurodevelopmental trajectories of the human cerebral cortex. J. Neurosci. 2008;28(14):3586–3594. doi: 10.1523/JNEUROSCI.5309-07.2008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Smetana J.G., Campione-Barr N., Metzger A. Adolescent development in interpersonal and societal contexts. Annu. Rev. Psychol. 2006;57:255–284. doi: 10.1146/annurev.psych.57.102904.190124. [DOI] [PubMed] [Google Scholar]
- Svoboda E., McKinnon M.C., Levine B. The functional neuroanatomy of autobiographical memory: a meta-analysis. Neuropsychologia. 2006;44(12):2189–2208. doi: 10.1016/j.neuropsychologia.2006.05.023. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Syal S., Hattingh C.J., Fouché J.-P., Spottiswoode B., Carey P.D., Lochner C. Grey matter abnormalities in social anxiety disorder: a pilot study. Metab. Brain Dis. 2012;27(3):299–309. doi: 10.1007/s11011-012-9299-5. [DOI] [PubMed] [Google Scholar]
- Tangney J.P., Miller R.S., Flicker L., Barlow D.H. Are shame, guilt, and embarrassment distinct emotions? J. Pers. Soc. Psychol. 1996;70(6):1256. doi: 10.1037//0022-3514.70.6.1256. [DOI] [PubMed] [Google Scholar]
- van der Schaaf M.E., Warmerdam E., Crone E.A., Cools R. Distinct linear and non-linear trajectories of reward and punishment reversal learning during development: relevance for dopamine's role in adolescent decision making. Dev. Cogn. Neurosci. 2011;1(4):578–590. doi: 10.1016/j.dcn.2011.06.007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Vijayakumar N., Whittle S., Dennison M., Yuecel M., Simmons J., Allen N.B. Development of temperamental effortful control mediates the relationship between maturation of the prefrontal cortex and psychopathology during adolescence: a 4-year longitudinal study. Dev. Cogn. Neurosci. 2014;9:30–43. doi: 10.1016/j.dcn.2013.12.002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wagner U., N’Diaye K., Ethofer T., Vuilleumier P. Guilt-specific processing in the prefrontal cortex. Cereb. Cortex. 2011;21(11):2461–2470. doi: 10.1093/cercor/bhr016. [DOI] [PubMed] [Google Scholar]
- Wolfgang T.S.H.-J.M., Miltnera H. Common and distinct brain activation to threat and safety signals in social phobia. Neuropsychobiology. 2005;52:163–168. doi: 10.1159/000087987. [DOI] [PubMed] [Google Scholar]
- Yu H., Hu J., Hu L., Zhou X. The voice of conscience: neural bases of interpersonal guilt and compensation. Soc. Cogn. Affect. Neurosci. 2013;9(8):1150–1158. doi: 10.1093/scan/nst090. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zahn R., de Oliveira-Souza R., Bramati I., Garrido G., Moll J. Subgenual cingulate activity reflects individual differences in empathic concern. Neurosci. Lett. 2009;457(2):107–110. doi: 10.1016/j.neulet.2009.03.090. [DOI] [PubMed] [Google Scholar]
- Zahn R., Moll J., Paiva M., Garrido G., Krueger F., Huey E.D. The neural basis of human social values: evidence from functional MRI. Cereb. Cortex. 2009;19(2):276–283. doi: 10.1093/cercor/bhn080. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zeman J., Cassano M., Perry-Parrish C., Stegall S. Emotion regulation in children and adolescents. J. Dev. Behav. Pediatr. 2006;27(2):155–168. doi: 10.1097/00004703-200604000-00014. [DOI] [PubMed] [Google Scholar]
- Zimmermann P., Iwanski A. Emotion regulation from early adolescence to emerging adulthood and middle adulthood Age differences, gender differences, and emotion-specific developmental variations. Int. J. Behav. Dev. 2014;38(2):182–194. [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.