Highlights
-
•
Positive parenting predicts development of adolescent amygdala and prefrontal cortex.
-
•
Positive parenting has a unique influence on adolescent brain development.
-
•
Positive and negative parenting are not opposite sides of a continuum.
-
•
Parenting interventions may promote healthy adolescent brain development.
Keywords: Adolescence, Brain development, Environment, Parenting, Resilience, Positive
Abstract
Little work has been conducted that examines the effects of positive environmental experiences on brain development to date. The aim of this study was to prospectively investigate the effects of positive (warm and supportive) maternal behavior on structural brain development during adolescence, using longitudinal structural MRI. Participants were 188 (92 female) adolescents, who were part of a longitudinal adolescent development study that involved mother–adolescent interactions and MRI scans at approximately 12 years old, and follow-up MRI scans approximately 4 years later. FreeSurfer software was used to estimate the volume of limbic-striatal regions (amygdala, hippocampus, caudate, putamen, pallidum, and nucleus accumbens) and the thickness of prefrontal regions (anterior cingulate and orbitofrontal cortices) across both time points. Higher frequency of positive maternal behavior during the interactions predicted attenuated volumetric growth in the right amygdala, and accelerated cortical thinning in the right anterior cingulate (males only) and left and right orbitofrontal cortices, between baseline and follow up. These results have implications for understanding the biological mediators of risk and protective factors for mental disorders that have onset during adolescence.
1. Introduction
Adverse childhood environments represent an important risk factor for the development of psychopathology later in life (Heim and Nemeroff, 2001, Moran et al., 2004), and there is accumulating evidence that neurobiological changes (particularly with regard to brain structure) may mediate this relationship (Tupler and De Bellis, 2006). Indeed, there has been a recent surge of interest in the effects of adverse childhood environments on structural brain development, with a number of recent reviews highlighting the deleterious effects of adverse early environments on brain structure (Andersen and Teicher, 2008, Hart and Rubia, 2012, Lupien et al., 2009, McCrory et al., 2010).
Although a focus on the effects of adverse childhood environments on structural brain development is important and has implications for the development of targeted interventions for at-risk individuals, the influence of positive childhood environments on brain development is equally important to consider, given their importance for predicting positive life outcomes (e.g., Woolley and Grogan-Kaylor, 2006). In particular, positive (warm and supportive) parenting has been suggested as a critical environmental factor that has strong influences on child outcomes. Specifically, supportive parenting early in life has been shown to have positive effects on cognitive, behavioral, and psychological development throughout the lifespan (Beckwith et al., 1992, Eshel et al., 2006, Landry et al., 2008).
Despite this evidence, to date, little work has been conducted that examines the effects of positive parenting on brain structure in children and adolescents. Nonetheless, preliminary work has shown neurobiological effects of positive parenting, albeit with some inconsistent findings. For example, work that has shown that maternal support in early childhood predict larger hippocampal volumes in school-aged children (Luby et al., 2012). Other work, however, has found that higher-quality parental care early in life (i.e., age 4) predicted smaller hippocampal volumes during early-to-mid adolescence, as well as reduced gray matter in the anterior cingulate cortex (ACC) and thalamus (Rao et al., 2010). The latter study also found that parental nurturance later in childhood (i.e., age 8) predicted reduced gray matter in the orbitofrontal cortex (OFC) and fusiform cortex, and increased gray matter in the superior parietal and premotor cortices. Frye et al. (2010) found that adolescents who had experienced consistent responsive mothering during childhood had thinner cortex globally than those who had experienced inconsistent responsive mothering.
Though this research shows promise in elucidating the effects of positive parenting on brain structure, there are a number of gaps in the literature. First, the majority of research has focused on early (i.e., infancy and childhood) positive parenting factors, and has largely neglected the adolescent period. This is significant in that we and others have shown that positive parenting during the adolescent years is associated with favorable outcomes in terms of adjustment and mental health (Aquilino and Supple, 2001, Gaté et al., 2013, Schwarz et al., 2012). Further, the period of adolescence is characterized by marked neurodevelopment, second only to infancy in its extent (Andersen and Teicher, 2008). Thus, increased brain plasticity during this time may render the adolescent brain particularly sensitive to environmental influence (Bateson et al., 2004).
Second, to our knowledge, none of the existing research has assessed longitudinal measures of brain development. Such research is crucial for understanding how the neurobiological effects of positive parenting might change or unfold over time. This issue has been illustrated by findings that adverse environments have differential (and sometimes opposite) effects on brain structure when assessed in childhood versus adulthood (see McCrory et al., 2010, Tottenham and Sheridan, 2010), suggesting that the effects of parenting on the brain may not be static but are likely to change across the life span. A wealth of animal studies indicates that environmental enhancement has effects on brain development across the lifespan (Halperin and Healey, 2011). Further, other research has shown that development of cortical thickness during adolescence is related to cognitive and emotional functioning. For example, studies have found that accelerated cortical thinning in the ACC and OFC is associated with increased emotional and behavioral functioning (Ducharme et al., 2013, Shaw et al., 2006, Vijayakumar et al., in press-a, Vijayakumar et al., in press-b). It is of note that in these studies, exaggerations of the normative pattern of growth (i.e., cortical thinning, Shaw et al., 2006) appear to be ‘optimal’ (i.e., associated with superior functioning). Less research has investigated the relationship between volumetric change in subcortical structures and adolescent functioning. Our previous work has shown that decreased levels of psychopathology are associated with attenuated growth of the amygdala, and accelerated growth of the hippocampus, during adolescence (Whittle et al., 2013). For the hippocampus, this finding again appears to reflect exaggerated normative growth (Dennison et al., 2013, Ostby et al., 2009) as being ‘optimal’. Our amygdala finding suggests that attenuated growth of the amygdala may be ‘optimal’, however, relating this finding to normative development is difficult, given that descriptions of normative amygdala growth during adolescence have been inconsistent (Dennison et al., 2013, Ostby et al., 2009). In any case, studies of adolescent brain development appear to be important for understanding risk processes for adolescent emotional and behavioral outcomes.
The aim of the current study was to examine the effects of positive parenting during early adolescence (i.e., 11–13) on structural brain development from early to midadolescence. Positive parenting was operationalized as the frequency of positive maternal behaviors displayed during observed conflictual interactions with their adolescent children. The display of positive behaviors in such contexts is thought to be particularly important for influencing outcomes. For example, we have found low levels of positive maternal behaviors during conflictual interactions to prospectively predict the onset of depressive disorders during adolescence (Schwartz et al., in press). We focused on the structural development of a number of cortical and subcortical regions of interest (ROIs), which were chosen based on (a) the premise that an adolescent's experience of their mother's positive behavior would primarily engage, and hence influence, neural circuitry implicated in processing positive cues from the environment (i.e., reward processing and learning) and in regulating emotion, and (b) the involvement of these regions in the research to date (e.g., Ducharme et al., 2013, Rao et al., 2010) that has found brain structural associations with aspects of positive parenting and associated indices of adolescent functioning.
The structures investigated in this study include the amygdala, hippocampus, dorsal and ventral striatum, OFC, and ACC. The striatum is thought to play a central role in synchronizing different aspects of reward and learning (Everitt and Robbins, 2005). The OFC and ACC have been associated with a number of cognitive, social and emotional functions, including mediating different aspects of reward-based decision-making (Bush et al., 2002, Kringelbach, 2005), and regulating emotion and behavior (Ochsner and Gross, 2005). The amygdala and hippocampus are thought to be involved in the detection and representation of affectively salient stimuli, and in relaying information about emotional valence and salience for further processing (Habel et al., 2005, Haber et al., 2006).
Given the lack of longitudinal human research investigating the impact of positive environments on brain structure, hypotheses were based on other research showing associations between age-related change in the volume or thickness of ROIs and aspects of the environment and behavior (discussed above). Given that positive parenting is thought to be associated with positive adolescent outcomes, we hypothesized that high levels of positive maternal behavior would be associated with accelerated thinning of the ACC and OFC, accelerated growth of hippocampus volumes, and attenuated growth of amygdala volumes. Little is known about the normal developmental patterns of the volumes of striatal regions during adolescence (Dennison et al., 2013, Ostby et al., 2009), let alone, how these patterns are associated with environmental exposures or concurrent emotional or behavioral development. Thus, our analyses for striatal structure volumes were largely exploratory in nature. Given evidence for sex differences in both the development of reward-related brain regions during adolescence (Dennison et al., 2013), and the effects of positive parenting on outcomes (Schwartz et al., in press), we explored the moderating role of sex in all analyses.
2. Materials and methods
2.1. Participants
Participants were a community sample of 188 adolescents (92 females) recruited from primary schools (year 6) across metropolitan Melbourne, Australia as part of a larger cohort study (the Orygen Adolescent Development Study [ADS]). The aim of the ADS was to prospectively examine biopsychosocial risk and protective factors for emotional and behavioral problems during adolescence. In order to maximize variability in such factors, the ADS screened a large number (i.e., 2453) of early adolescents, on key affective temperaments thought to promote risk and resilience for psychopathology (namely, Effortful Control and Negative Affectivity, Ellis and Rothbart, 2001, Mezulis et al., 2011, Muris et al., 2007), from primary schools in and around metropolitan Melbourne, Australia, and selected a smaller sample of adolescents (i.e., 415) to participate in further longitudinal assessments. Specifically, equal numbers of males and females were selected from each of the following ranges of scores on the Effortful Control and Negative Affectivity dimensions of the EATQ-R: 0–1, 1–2, 2–2.5, and greater than 2.5 SD above and below the mean. The selected 415 adolescents thus represented an oversampling of those with high and low temperamental risk for psychopathology, and an undersampling of those with an intermediate level of risk, resulting in a distribution that retained the variance associated with the larger screening sample but was still normally distributed. Selected participants who agreed to partake in further research (n = 245) were excluded from entering the study if, at an initial assessment, there was any evidence of current or past depressive, substance-use or eating disorder (n = 4) established using the Kiddie Schedule for Affective Disorders and Schizophrenia for school-age children – Present and Lifetime [K-SADS-PL] interview (Kaufman et al., 1997). Participants were excluded from neuroimaging if there was evidence of chronic illness, language or learning disabilities, or use of medicines known to affect nervous system functioning (n = 2). The 188 adolescents (92 females) in the current study represented those who, from the selected sample of 415, consented to participating in baseline mother–child interaction tasks (n = 163), and/or both a baseline and follow-up neuroimaging assessment (mean follow-up period = 3.80 years, SD = 0.20) and whose Magnetic Resonance Imaging (MRI) scans were of acceptable quality for image analysis (n = 102, see below).
Intelligence was assessed at baseline by a short form of the Wechsler Intelligence Scale for Children, Fourth Version (WISC-IV, Wechsler, 2003). Socioeconomic status (SES) of participants was estimated using the Australian National University Four (ANU4) Scale (Jones and McMillan, 2001), which has a range of 0–100. Handedness was assessed by the Edinburgh Handedness Inventory (Oldfield, 1971), and 88% of participants were right handed. After complete description of the study to the participants, written informed consent was obtained from all adolescents and their parent/guardian in accordance with the guidelines of the Human Research Ethics Committee of the University of Melbourne, Australia.
2.2. Procedure and measures
2.2.1. Neuroimaging
2.2.1.1. Acquisition and pre-processing
Brain imaging was conducted with 120 participants at both baseline and follow-up. At baseline, MRI scans were performed at the Brain Research Institute (BRI), Melbourne, Australia, on a 3 T GE (repetition time = 36 ms; echo time = 9 ms; flip angle = 35°, field-of-view = 20 cm2) to obtain 124 T1-weighted contiguous slices (i.e., voxel dimensions = 0.4883 mm × 0.4883 mm × 1.5 mm). Follow-up scans were conducted at the Royal Children's Hospital (RCH), Melbourne, Australia, on a 3-T Siemens (repetition time = 1900 ms; echo time = 2.24 ms; flip angle = 9°, field of view = 23 cm2), producing 176 T1-weighted contiguous slices (voxel dimensions = 0.9 mm3).
The stability of image acquisition in longitudinal and/or multi-site studies is critical but may be compromised in several ways, including instrument-related differences between sites (e.g., scanner manufacturer), and instrument/software upgrades within sites (Han et al., 2006). In the current study, steps were employed to address two main sources of error (i.e., geometric distortion and voxel dimension drifts, Wang and Doddrell, 2005) that are known to result from multi-site and/or longitudinal scanning. Firstly, images were corrected for tissue signal inhomogeneity, which has been shown to result from geometric distortion (Wang and Doddrell, 2005). This was achieved via a nonparametric non-uniformity intensity normalization method optimized for 3 T images (Zheng et al., 2009). Secondly, voxel dimension drift was corrected using linear registration procedures employed by the longitudinal processing stream in FreeSurfer (Version 5.1; http://surfer.nmr.mgh.harvard.edu/fswiki/LongitudinalProcessing), which involves the creation of an unbiased within-subject template space and average image using robust, inverse consistent registration (Reuter et al., 2010). Further, to investigate the possibility of inter-scanner bias, an inter-scanner reliability study was performed (see Dennison et al., 2013, Vijayakumar et al., in press-b, for full details). This reliability study indicated no systematic inter-scanner bias in regional volumetric or thickness measures.
2.3. ROI analysis
Subcortical (i.e., amygdala, hippocampus, caudate, nucleus accumbens, putamen, and pallidum) volumes and cortical ROI (i.e., ACC and OFC) thickness were estimated at baseline and follow-up using the longitudinal stream in FreeSurfer version 5.1. The automated subcortical segmentation procedure involves the assignment of a neuroanatomical label to each voxel in a MRI volume using a probabilistic atlas and Bayesian classification rule for label assignment. Subcortical segmentation output was visually inspected for accuracy by an individual trained in neuroanatomy. Cortical thickness values were automatically quantified within FreeSurfer on a vertex-by-vertex basis by computing the average shortest distance between the white matter boundary and the pial surface (Fischl and Dale, 2000). Surface boundaries were visually inspected by a trained rater and, if necessary, errors due to segmentation miss-classification were manually corrected and re-processed. Of the 120 longitudinal data sets, 18 images were discarded due to excessive artifact or poor segmentation. Within each hemisphere, 34 cortical parcellation units of the cortex were automatically identified and labeled according to the Desikan atlas of gyral-based definitions included within FreeSurfer's automatic cortical parcellation routine (Desikan et al., 2006). ACC and OFC ROI's for each hemisphere were created by combining the rostral and caudal ACC, and medial and lateral OFC parcellation units, respectively. Cortical thickness was averaged across all vertices within the ACC and OFC ROI's for each subject. Whole brain volume (WBV) was calculated at both time-points as the total of all gray and white matter as per the FreeSurfer segmentation. Average thickness was calculated for left and right hemispheres as the average thickness of all parcellations in the respective hemisphere.
Subcortical volumes were corrected for whole brain volume, and cortical ROI thickness measures were corrected for average hemispheric thickness, using a covariance procedure (Free et al., 1995).
2.3.1. Family interaction assessment
One hundred and sixty three adolescents completed a lab-based family interaction assessment with their mothers at baseline. Adolescent-mother dyads completed two 20-min interaction tasks that were videorecorded for subsequent coding. An event-planning interaction (EPI) was completed first, followed by a problem-solving interaction (PSI). The tasks were designed to differentially elicit positive and negative behavior, respectively. The ordering of tasks was fixed because of concern that negative affective states elicited by the problem-solving task had the potential to persist into the positive event-planning task if conducted subsequently (Gilboa and Revelle, 1994).
For the EPI, mothers and adolescents were instructed to plan one or more pleasant events to do together, with up to five events chosen based on items that both the mother and adolescent rated as being ‘very pleasant’ on the Pleasant Events Schedule (MacPhillamy and Lewinsohn, 1976). For the PSI, up to five issues for discussion were selected that both the mother and adolescent endorsed as occurring the most frequently and generating the highest intensity of anger on the Issues Checklist (Prinz et al., 1979).
2.3.1.1. Living in family environments (LIFE) coding system
The LIFE (Hops et al., 1995a) is an observational, microsocial coding system that enables a detailed analysis of individual family members’ behaviors and interactive family behaviors. It consists of 10 nonverbal affect codes (e.g., anger, dysphoria, happy) and 27 verbal content codes (e.g., validation, complaint, provoke). Coding of videorecorded interactions used an event-based protocol in which new codes were entered each time the affect or content of one of the interactants changed. The 10 affect and 27 verbal content codes were used to develop composite behavior constructs. In this study, the construct of interest was Positive Behavior, but we also used Aggressive Behavior to examine the specificity of findings to Positive Behavior. The Positive construct included all behaviors with happy or caring affect as well as approving, validating, affectionate or humorous comments made with neutral affect. The Aggressive construct included all behaviors with contemptuous, angry, or belligerent affect, as well as cruel, provocative, annoying/disruptive, or argumentative verbal statements made with neutral affect.
LIFE data was used to construct a measure of behavioral frequency for each construct, calculated as the rate per minute (rpm) of a particular behavior (i.e., the average number of times a mother expressed a behavior type [i.e., Aggressive or Positive] per minute). Our previous research using this paradigm has indicated that cross-context effects are critical in predicting adolecent outcomes (Schwartz et al., in press). For example, adolescents whose mothers behave aggressively when asked to discuss something pleasant with their child, and whose mothers find it relatively difficult to generate positivity when discussing a conflictual topic, may be at particular risk for depression. Thus, we used the frequency of positive maternal behavior from the PSI, and the frequency of aggressive maternal behavior from the EPI, in all analyses.
Coders were extensively trained; approximately 20% of the interactions were coded by a second observer to provide an estimate of observer agreement. Random pairs of observers were assigned to the interactions to minimize ‘drift’ between any two observers. Inter-observer agreement was assessed using Kappa, a conservative index that controls for chance agreement (Hops et al., 1995b). Kappa coefficients for the Aggressive and Positive behavior constructs were .70 and .86, respectively, across interactants. The validity of the LIFE system as a measure of family processes has been established in numerous studies (e.g., Katz and Hunter, 2007, Sheeber et al., 2007).
2.3.2. Self-report questionnaires
Given that the frequency of maternal emotional behaviors was linked to adolescent depressive and anxiety symptoms in the current cohort (Schwartz et al., 2012), we wished to take account of the possibility that adolescent symptoms at baseline might account for some of the effects of maternal positive behavior on adolescent reward-related neural circuitry development. As such, depressive and anxiety symptoms at baseline were assessed using the Center for Epidemiological Studies-Depression Scale (CES-D, Radloff, 1977), and the Beck Anxiety Inventory (Beck and Steer, 1993), respectively.
2.4. Treatment of missing data
Of the 188 adolescents with available MRI and/or mother–child interaction data, 86 (46%) had missing MRI and IQ data, 25 (13%) had missing mother–child interaction data, 16 (9%) had missing CESD data, and 7 (4%) had missing BAI data. Those with and without MRI data did not differ on gender, SES, symptoms or any EATQ-R temperament variable (p′ > 0.12). Those with and without mother–child interaction also data did not differ on any of these variables (p′s > 0.17). Missing data were handled with full information maximum likelihood (FIML) procedures. FIML is a direct model-based method for estimating parameters in the presence of missing data (Olinsky et al., 2003), and involves estimation of parameters on the basis of the available complete data as well as the implied values of the missing data given the observed data. It has been suggested that FIML is a suitable method for taking account of missing data, even when up to 50% of data is missing, as long as auxiliary variables are used to improve the missing data model and subsequent analyses (Schlomer et al., 2010). Temperament variables from the EATQ-R (administered at baseline to all participants) were used as auxiliary variables in the FIML estimation. Although FIML was deemed a suitable method for taking account of missing data in the sample, given the large amount of missing MRI data, we also reran analyses using data only from those participants with complete MRI and mother–child interaction data (n = 77).
2.5. Data analysis
To measure brain ‘development’, residual change scores were calculated for each ROI such that the resulting ROI change score was a measure of deviation from the predicted pattern of volume/thickness change over time (based on the average of all participants), whereby positive scores indicated greater change as compared to the average pattern, and negative scores indicated less change as compared to the average pattern. Residualized rather than arithmetic change scores were used because they account for the correlation between baseline and change, and are thought to be less prone to measurement error (Bergh and Fairbank, 2002). A series of hierarchical linear regression analyses were performed to assess the effects of positive maternal behavior on change in volume or thickness of each ROI. The change measure was the dependent variable in each analysis. Age at baseline MRI, SES, IQ, depressive and anxiety symptoms were included as covariates in the first step of the regression. Rate of aggressive maternal behavior during the EPI was also entered as a covariate in this step so as to ensure that the effects observed for positive maternal behavior were not better explained by aggressive maternal behavior. Rate of positive maternal behavior and sex were added as predictors in the second step of the regression, and a rate of positive maternal behavior by sex interaction term was added as a predictor in the third and final step of the regression. The rate of positive maternal behavior was centered prior to creating the interaction term. All analyses were conducted using Mplus software (Muthén and Muthén, 1998–2012). Note that associations between positive maternal behavior and brain structure at both baseline and follow-up were investigated (using the same regression procedure described above) in order to provide a full picture of associations in the data. Given that brain development was the primary focus of the study, the findings regarding associations between baseline maternal behavior, and baseline and follow-up ROI structure, are reported as Supplementary data. All regression coefficients reported below are standardized beta estimates.
3. Results
Demographic data at baseline and follow-up are presented in Table 1, and subcortical brain volumes and cortical thicknesses for ROIs for each hemisphere at baseline and follow up assessments are presented in Table 2.
Table 1.
Demographic information at baseline.
| Measure | n | Mean | SD |
|---|---|---|---|
| Age at FI assessment | 163 | 12.65 | 0.46 |
| Age baseline MRI | 102 | 12.62 | 0.44 |
| Age follow-up MRI | 102 | 16.42 | 0.50 |
| IQ | 102 | 106.68 | 11.03 |
| SES | 188 | 66.90 | 20.27 |
| CESD | 172 | 11.76 | 9.53 |
| BAI | 181 | 8.77 | 9.17 |
| Maternal positive (rpm) | 163 | 1.66 | 0.70 |
| Maternal aggressive (rpm) | 163 | 0.61 | 0.43 |
| Years to follow up | 102 | 3.78 | 0.20 |
Abbreviations: FI, family interaction; rpm, rate per minute.
Table 2.
Subcortical brain volumes and cortical thicknesses for regions of interest for each hemisphere at baseline and follow up assessments. The mean whole brain volume was 1,344,847.69 mm3 (SD = 114,763.72) at baseline, and 1,377,601.97 mm3 (SD = 133,588.44) at follow up.
| Baseline MRI |
Follow Up MRI |
|||||||
|---|---|---|---|---|---|---|---|---|
| LH |
RH |
LH |
RH |
|||||
| Mean | SD | Mean | SD | Mean | SD | Mean | SD | |
| Subcortical volume (mm3) | ||||||||
| Amygdala | 1621.84 | 208.70 | 1621.90 | 205.17 | 1694.39 | 214.63 | 1665.39 | 209.51 |
| Hippocampus | 4514.11 | 489.78 | 4555.26 | 454.89 | 4584.58 | 476.30 | 4730.37 | 449.78 |
| Caudate | 4458.87 | 555.18 | 4427.59 | 567.35 | 4244.23 | 571.40 | 4240.84 | 599.15 |
| Nucleus accumbens | 496.59 | 90.79 | 716.76 | 104.82 | 528.25 | 83.37 | 675.53 | 91.93 |
| Putamen | 7427.99 | 742.76 | 7323.56 | 788.01 | 7080.24 | 780.31 | 7158.75 | 869.11 |
| Pallidum | 2163.60 | 263.08 | 1942.75 | 233.53 | 2354.31 | 329.97 | 2015.12 | 256.24 |
| Cortical thickness (mm) | ||||||||
| Whole brain average | 2.93 | 0.12 | 2.94 | 0.12 | 2.92 | 0.12 | 2.95 | 0.12 |
| Anterior cingulate (ACC) | 3.27 | 0.24 | 3.23 | 0.23 | 3.25 | 0.23 | 3.15 | 0.20 |
| Orbitofrontal (OFC) | 3.01 | 0.15 | 3.00 | 0.17 | 2.88 | 0.18 | 2.94 | 0.17 |
Abbreviations: LH, left hemisphere; RH, right hemisphere.
We have previously described the change in subcortical volumes from early to midadolescence in this sample (Dennison et al., 2013). Briefly, we found the caudate, thalamus and putamen to decline in volume over time. The pallidum and hippocampus increased in volume over time. While the left nucleus accumbens increased in size, the right accumbens decreased in size over the follow-up period. While amygdala volume did not change over time, there was a main effect of hemisphere such that the left amygdala was larger than the right. For the OFC, a repeated measures ANOVA with time and hemisphere as within-subjects variables and sex as the between subjects variable, revealed significant main effects of time (p < 0.001) and gender (p < 0.001), and a significant time by hemisphere interaction (p < 0.001). Males had thicker whole brain corrected OFC's than females. There was significant thinning in the OFC over time, with greater thinning in the left compared to the right hemisphere. For the ACC, a repeated measures ANOVA revealed significant main effects of time (p = 0.001) and hemisphere (p = 0.004), and a significant time by hemisphere interaction (p < 0.001). Whole brain corrected thickness was greater in the left compared to the right ACC. There was significant thinning in the ACC over time, with greater in the right compared to the left hemisphere.
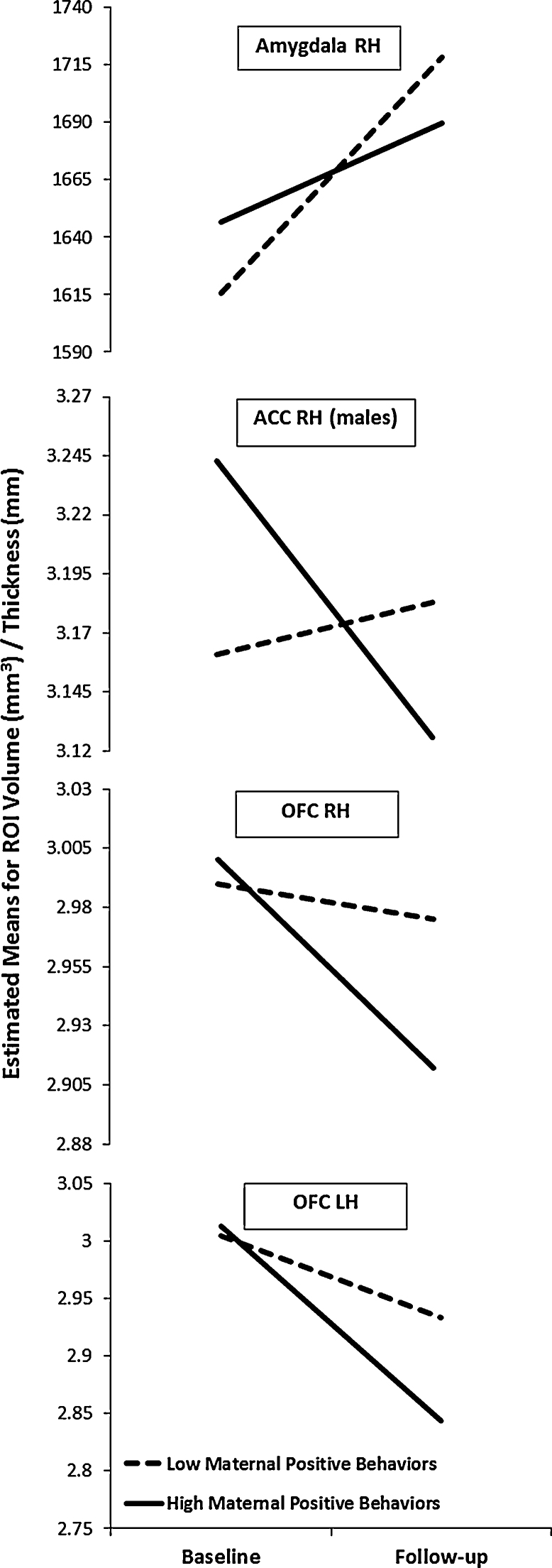
Regarding the effects of positive maternal behavior on brain change, there were significant effects for the right amygdala (β = −0.284, p = 0.014), and left and right OFC (β = −0.262, p = 0.024; β = −0.258, p = 0.022, respectively). Higher rate of positive maternal behavior was associated with reduced growth of the right amygdala from early- to mid-adolescence, and was also associated with greater thinning of the left and right OFC (see Fig. 1). Positive maternal behavior also interacted with sex to predict right ACC change (β = −0.311, p = 0.045). Follow-up analyses showed that positive maternal behavior was associated with greater thinning of the right ACC for males (β = −0.525, p = 0.007; see Fig. 1), but showed no association with thinning for females (β = 0.042, p = 0.789, respectively). Aggressive maternal behavior was associated with change in only one ROI, the right putamen; specifically, it predicted increased growth of this structure over time (β = 0.306, p = 0.012).1
Fig. 1.
Effects of positive high and low maternal behavior (based on median split) on development of right amygdala volume, left and right orbitofrontal cortex (OFC) thickness, and male right anterior cingulate cortex (ACC) thickness, from baseline (early adolescence) to follow-up (midadolescence). Plotted values take into account covariates (including whole brain volume for the amygdala and average hemispheric thickness for the OFC and ACC). LH, left hemisphere; RH, right hemisphere.
4. Discussion
To our knowledge, this is the first human study to investigate the effect of variations in positive family environments during early adolescence on the structural development of the brain over time using a prospective, longitudinal design. We found that the frequency of positive maternal behavior during early adolescence was associated with structural development of regions implicated in reward processes, emotional reactivity and regulation, with some sex differences also being observed. While maternal aggressive behavior predicted increased growth of the right putamen, none of the effects observed for positive maternal behavior were better explained by rate of aggressive maternal behavior, suggesting some specificity of the effects of positive environments on adolescent brain development.
While we found that positive parenting was associated with development in a number of regions, it was not associated with the structure of the majority of these regions at baseline or follow-up (see Supplementary data). These findings suggest that for some brain regions, measures of developmental change may be more sensitive to, or may reveal more complex associations with, positive environments than do measures of absolute volume or thickness. Our findings also highlight the fact that associations in cross-sectional studies may differ markedly depending on the age at which brain structure is measured, which has significant implications for interpretations of findings. Further, cross-sectional studies that include adolescents spanning wide age ranges may produce anomalous findings because they have not considered inter-individual differences in the marked development of the brain during the adolescent period.
We found that higher frequency of positive maternal behavior was associated with reduced growth of the right amygdala, and accelerated thinning of the left and right OFC and right ACC (males only) from early to midadolescence. The results for the ACC and OFC are consistent with hypotheses, and with work showing that similar developmental trajectories of these structures are associated superior cognitive and emotional functioning. Specifically, Shaw et al. (2006), using a cohort-sequential design, found that adolescents with superior intellectual abilities exhibited greater cortical thinning in a number of cortical areas, including the OFC, into adulthood. Ducharme et al. (2013), also using a cohort-sequential design, found that lower levels of internalizing symptoms were associated with greater thinning in the OFC and ACC. Finally, in other work with the current sample, we have found that greater thinning in the ACC was associated with higher temperamental effortful control (Vijayakumar et al., in press-b). Given these findings, and given that positive maternal behaviors are thought to shape the development of their children's ability to regulate emotion and behaviors (Yap et al., 2010), it is possible that the effects of positive maternal behaviors on the development of the ACC and OFC during adolescence, in turn, have effects on adolescents’ regulation abilities. Further research is needed to investigate this possibility.
Our finding regarding the amygdala was also consistent with hypotheses, which were based on our previous finding that low levels of psychopathology were associated with attenuated amygdala development from early to midadolescence (Whittle et al., 2013). Although no other human study to date has reported an effect of positive parenting on amygdala structure, there is some evidence from animal studies that environmental enrichment has an effect on amygdala development (Okuda et al., 2009). The amygdala is thought to be primarily involved in detection of, and reactivity to, emotionally salient stimuli (Habel et al., 2005). There is also evidence that the interaction between prefrontal structures and the amygdala is critical to emotion regulation; specifically prefrontal down-regulation of amygdala activity has been associated with the attenuation of negative affect during emotion regulation (Banks et al., 2007). As such, the association between maternal positive behavior and attenuated growth of amygdala volume across adolescence may reflect a decrease in emotional reactivity and an increase in emotion regulation abilities. Again, further researcher is required to examine this possibility.
These findings are interesting to consider in the context of the normative development of these structures during adolescence. We and others have found evidence for normative thinning in the OFC and ACC across adolescence (Shaw et al., 2006, Vijayakumar et al., in press-a, Vijayakumar et al., in press-b). Thus, our findings suggest that positive maternal behavior is associated with an augmentation of the ‘normal’ pattern of development of these regions during adolescence. Cortical thinning during adolescence is thought to reflect some combination of synaptic pruning, changes in axonal caliber, proliferation of glial cells and increased myelination of previously unmyelinated tissue at the periphery of the brain (Bourgeois and Rakic, 1993, Huttenlocher and Dabholkar, 1997, Paus et al., 2008, Sowell et al., 2004). These neurobiological processes are thought to improve neuronal efficiency, stability and temporal precision of neuronal firing patterns (Lewis, 1997, Rutherford et al., 1998). While cortical thinning during adolescence is thus thought to be adaptive, adult studies report thicker prefrontal cortex to be associated with superior functioning, including enhanced fear regulation (Rauch et al., 2005) and executive control (Westlye et al., 2011). Further research is required to examine the mechanisms underlying the development of cortical thickness from late adolescence to early adulthood, and to investigate the point at which thinning presumably becomes no longer adaptive.
There is inconsistent evidence regarding the normal pattern of amygdala development during adolescence. Although we found no evidence for significant development of the amygdala from early- to mid-adolescence in this sample (Dennison et al., 2013), others have found normative increases in the volume of this structure over the adolescent years (e.g., Ostby et al., 2009). In any case, our findings suggest that relatively reduced amygdala growth during early- to mid-adolescence may be developmentally optimal. Environmental stress has been suggested as key in shaping amygdala development across the lifespan (Lupien et al., 2009), largely due to the key role of the amygdala in modulating hypothalamic–pituitary–adrenal (HPA) axis function (Dedovic et al., 2009). As such, attenuated amygdala development associated with positive maternal behavior may be due to altered HPA axis function. This speculation is consistent with animal research showing increased maternal care to be associated with reduced HPA axis responsiveness and stress susceptibility (Plotsky and Meaney, 1993), and also with human research showing a longitudinal association between reduced perceived stress and reductions in amygdala volume in adults (Hölzel et al., 2010).
Our findings may have implications for understanding the mechanisms underlying the relationship between positive parenting and reduced risk for a range of negative outcomes. Given that the specific measure of positive maternal behavior utilized in the current study has been particularly implicated in protection against the development of depression (Schwartz et al., in press), our findings may be particularly relevant for understanding the mechanisms conferring resilience for this disorder. Abnormalities in the structure and function of all of the regions associated with positive parenting in the current study (i.e., amygdala, OFC, ACC) have been found in both adolescent and adult depression. For example, while structural abnormalities in the amygdala have been inconsistent in adolescent depression, there is evidence that larger amygdala volumes might be a risk factor for depression onset during adulthood (Lorenzetti et al., 2009b). Given that in the current study, positive maternal behavior was associated with reduced growth of the right amygdala such that those experiencing high levels of positive maternal behavior had relatively smaller right amygdala at follow-up (approximately age 16), it may be that reduced growth of the amygdala during adolescence might be a protective factor mediating the link between high levels of positive maternal behavior and resilience to developing depression. Reductions in gray matter volume and cortical thickness in the OFC and ACC (among other prefrontal regions) have been consistently reported in adult depression (Drevets, 2007, Lorenzetti et al., 2009a, Price and Drevets, 2010). Less work has been done in adolescent depression, and the existing research has been inconsistent (Botteron et al., 2002, Nolan et al., 2002, Shad et al., 2012, Steingard et al., 2002). Although it is unclear how the trajectories of thinning in the OFC and ACC during early to midadolescence might extrapolate into late adolescence and adulthood, one can only presume that the increased cognitive and behavioral functioning associated with increased thinning during this age period are protective against the development of depression. Whether the structural findings associated with increased positive parenting in our data represent a marker of protection against the development of depression, or other negative outcomes, should be the subject of future investigation.
Although negative environmental factors have been found to influence many of the structures assessed in the current study (Lupien et al., 2009), it is noteworthy that aggressive maternal behavior was controlled for in all analyses, and as such, we can be confident that the brain regions we found to be associated with positive parenting are likely to have been specifically influenced by positive, as opposed to negative, aspects of parenting. These results suggest that positive and aggressive maternal behavior do not exist on a continuum of positive to negative, but rather may reflect two different aspects of parenting behaviors that have distinct effects on children's brain development.
There are a number of limitations to this study that should be kept in mind when interpreting results. First, although this study is unique in that it utilized a within-subjects repeated measures design, the availability of imaging data from only two time points during early- and mid-adolescence limited modeling to linear patterns of brain development, and as such, we cannot comment on whether positive parenting has non-linear effects on brain development, or whether the effects may extend into late adolescence and adulthood. Second, although the use of observed (as opposed to self-report) measures of maternal behavior is a strength of the study, as was the decision to focus on a measure of positive maternal behavior that has been specifically linked to protection against depression, we did not assess other (or more specific) aspects of positive parenting (such as warmth, responsivity, and support) that may be important protective factors for other deleterious outcomes. Future research should utilize other measures of positive parenting (and positive environments more broadly) to examine the specificity versus generalizability of the present results. Third, we were unable to include fathers in analyses, due to the low number of participating fathers in the study. Fathers play a significant role in the socialization of emotion in their children, although this may differ to that of mothers (Cassano et al., 2006, Jacob and Johnson, 2001, Sheeber et al., 2007). Forth, as MRI scans were acquired multisite, with different imaging sequences acquired at each site, there is a possibility of scanner/sequence bias affecting volumetric and thickness estimates. However, post-acquisition procedures were adopted to minimize, as much as possible, any scanner/sequence effects on the acquired images. An inter-scanner reliability assessment also indicated no systematic inter-scanner bias. Further, it is very unlikely that the measure of positive maternal behavior interacted with scanner or sequence type in any way that might bias the reported results. Fifth, it has been suggested that reliability for some FreeSurfer delineated structures (particularly the amygdala and nucleus accumbens) is relatively low, and as such, the results for these structures should be interpreted with caution. However, it is of note that sample sizes of approximately 80 and above are suggested to be adequately powered to detect small to medium effect sizes in these structures (Morey et al., 2010). Sixth, given our temperamental sampling strategy, our results may have restricted generalizability. Seventh, although we have suggested that parenting behaviors during adolescence may be particularly likely to influence adolescent brain structure due to brain plasticity, it may be the case that parenting behaviors during childhood may be equally as predictive. Indeed, there is evidence for stability over childhood and adolescence when one examines measures of parent–child relationship closeness, conflict, and autonomy (Loeber et al., 2000, Sheeber et al., 1998). Finally, we did not control for multiple comparisons, and hence, findings should be interpreted with caution (particularly those with small effect sizes), and should be replicated before any firm conclusions can be made.
In conclusion, we have found, for the first time, longitudinal effects of positive parenting on structural development of the brain during adolescence. Given that positive parenting has been found to be associated with increased positive adolescent outcomes, and resilience against negative adolescent outcomes, our results have implications for understanding the mechanisms underlying these relationships.
Conflict of interest statement
All authors are free of any actual or potential conflict of interest including any financial, personal or other relationships with other people or organizations within three years of beginning the submitted work that would inappropriately influence, or be perceived to influence, their work.
Acknowledgements
This research was supported by grants from the Colonial Foundation, the National Health and Medical Research Council (NHMRC; Australia; Program Grant 350241), the Australian Research Council (ARC; Discovery Grant DP0878136), and the University of Melbourne. Dr. Whittle is supported by an NHMRC Career Development Fellowship (ID: 1007716).
Neuroimaging analysis was facilitated by the Neuropsychiatry Imaging Laboratory at the Melbourne Neuropsychiatry Centre and supported by Neurosciences Victoria. The authors would like to thank the Brain Research Institute and the Murdoch Childrens Research Institute, Royal Childrens Hospital, for support in acquiring the neuroimaging data, Oregon Research Institute for its role in the coding of family interaction data, and the families who participated in the study.
Footnotes
Analyses using only participants with complete MRI and maternal behavior data (n = 77) yielded the same pattern of results. That is, all results remained significant at the p < 0.05 level.
Supplementary data associated with this article can be found, in the online version, at http://dx.doi.org/10.1016/j.dcn.2013.10.006.
Appendix A. Supplementary data
The following are the supplementary data to this article:
References
- Andersen S.L., Teicher M.H. Stress, sensitive periods and maturational events in adolescent depression. Trends Neurosci. 2008;31:183–191. doi: 10.1016/j.tins.2008.01.004. [DOI] [PubMed] [Google Scholar]
- Aquilino W.S., Supple A.J. Long-term effects of parenting practices during adolescence on well-being outcomes in young adulthood. J. Fam. Issues. 2001;22:289–308. [Google Scholar]
- Banks S.J., Eddy K.T., Angstadt M., Nathan P.J., Phan K.L. Amygdala - frontal connectivity during emotion regulation. Social Cognitive and Affective Neuroscience. 2007;2:303–312. doi: 10.1093/scan/nsm029. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bateson P., Barker D., Clutton-Brock T., Deb D., D’Udine B., Foley R.A., Gluckman P., Godfrey K., Kirkwood T., Lahr M.M., McNamara J., Metcalfe N.B., Monaghan P., Spencer H.G., Sultan S.E. Developmental plasticity and human health. Nature. 2004;430:419–421. doi: 10.1038/nature02725. [DOI] [PubMed] [Google Scholar]
- Beck A.T., Steer R.A. Psychological Corporation; San Antonio, TX: 1993. Beck Anxiety Inventory Manual. [Google Scholar]
- Beckwith L., Rodning C., Cohen S. Preterm children at early adolescence and continuity and discontinuity in maternal responsiveness from infancy. Child Dev. 1992;63:1198–1208. doi: 10.1111/j.1467-8624.1992.tb01689.x. [DOI] [PubMed] [Google Scholar]
- Bergh D.D., Fairbank J.F. Measuring and testing change in strategic management research. Strateg. Manag. J. 2002;23:359–366. [Google Scholar]
- Botteron K.N., Raichle M.E., Drevets W.C., Heath A.C., Todd R.D. Volumetric reduction in left subgenual prefrontal cortex in early onset depression. Biol. Psychiatry. 2002;51:342–344. doi: 10.1016/s0006-3223(01)01280-x. [DOI] [PubMed] [Google Scholar]
- Bourgeois J.P., Rakic P. Changes of synaptic density in the primary visual cortex of the macaque monkey from fetal to adult stage. The Journal of neuroscience. 1993;13(7):2801–2820. doi: 10.1523/JNEUROSCI.13-07-02801.1993. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bush G., Vogt B.A., Holmes J., Dale A.M., Greve D., Jenike M.A., Rosen B.R. Dorsal anterior cingulate cortex: a role in reward-based decision making. Proc. Natl. Acad. Sci. U.S.A. 2002;99:523–528. doi: 10.1073/pnas.012470999. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cassano M., Adrian M., Veits G., Zeman J. The inclusion of fathers in the empirical investigation of child psychopathology: an update. J. Clin. Child Adolesc. Psychol. 2006;35:583–589. doi: 10.1207/s15374424jccp3504_10. [DOI] [PubMed] [Google Scholar]
- Dedovic K., Duchesne A., Andrews J., Engert V., Pruessner J.C. The brain and the stress axis: the neural correlates of cortisol regulation in response to stress. Neuroimage. 2009;47:864–871. doi: 10.1016/j.neuroimage.2009.05.074. [DOI] [PubMed] [Google Scholar]
- Dennison M., Whittle S., Yucel M., Vijayakumar N., Kline A., Simmons J.G., Allen N.B. Mapping subcortical brain maturation during adolescence: evidence of hemisphere- and sex-specific longitudinal changes. Dev. Sci. 2013 doi: 10.1111/desc.12057. [DOI] [PubMed] [Google Scholar]
- Desikan R.S., Segonne F., Fischl B., Quinn B.T., Dickerson B.C., Blacker D., Buckner R.L., Dale A.M., Maguire R.P., Hyman B.T., Albert M.S., Killiany R.J. An automated labeling system for subdividing the human cerebral cortex on MRI scans into gyral based regions of interest. Neuroimage. 2006;31:968–980. doi: 10.1016/j.neuroimage.2006.01.021. [DOI] [PubMed] [Google Scholar]
- Drevets W.C. Orbitofrontal cortex function and structure in depression. Ann. N.Y. Acad. Sci. 2007;1121:499–527. doi: 10.1196/annals.1401.029. [DOI] [PubMed] [Google Scholar]
- Ducharme S., Albaugh M.D., Hudziak J.J., Botteron K.N., Nguyen T.-V., Truong C., Evans A.C., Karama S., For the Brain Development Cooperative Group Anxious/depressed symptoms are linked to right ventromedial prefrontal cortical thickness maturation in healthy children and young adults. Cereb. Cortex. 2013 doi: 10.1093/cercor/bht151. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ellis L.K., Rothbart M.K. Revision of the Early Adolescent Temperament Questionnaire. 2001 Biennial Meeting of the Society for Research in Child Development; Minneapolis. Minnesota. 2001 [Google Scholar]
- Eshel N., Daelmans B., de Mello M.C., Martines J. Responsive parenting: interventions and outcomes. Bull. World Health Organ. 2006;84:991–998. doi: 10.2471/blt.06.030163. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Everitt B.J., Robbins T.W. Neural systems of reinforcement for drug addiction: from actions to habits to compulsion. Nat. Neurosci. 2005;8:1481–1489. doi: 10.1038/nn1579. [DOI] [PubMed] [Google Scholar]
- Fischl B., Dale A.M. Measuring the thickness of the human cerebral cortex from magnetic resonance images. Proc. Natl. Acad. Sci. U.S.A. 2000;97:11044–11049. doi: 10.1073/pnas.200033797. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Free S.L., Bergin P.S., Fish D.R., Cook M.J., Shorvon S.D., Stevens J.M. Methods for normalization of hippocapal volumes measured with MR. Am. J. Neuroradiol. 1995;16:637–643. [PMC free article] [PubMed] [Google Scholar]
- Frye R.E., Malmberg B., Swank P., Smith K., Landry S. Preterm birth and maternal responsiveness during childhood are associated with brain morphology in adolescence. J. Int. Neuropsychol. Soc. 2010;16:784–794. doi: 10.1017/S1355617710000585. [DOI] [PubMed] [Google Scholar]
- Gaté M.A., Watkins E.R., Simmons J.G., Byrne M.L., Schwartz O.S., Whittle S., Sheeber L.B., Allen N.B. Maternal parenting behaviors and adolescent depression: the mediating role of rumination. J. Clin. Child Adolesc. Psychol. 2013:1–10. doi: 10.1080/15374416.2012.755927. [DOI] [PubMed] [Google Scholar]
- Gilboa E., Revelle W. Personality and the structure of affective responses. In: van Goozen S.H.M., Van de Poll N.E., Sergeant J.A., editors. Emotions: Essays on emotion theory Hillsdale. Lawrence Earlbaum Associates, Inc; New Jersey: 1994. [Google Scholar]
- Habel U., Klein M., Kellermann T., Shah N.J., Schneider F. Same or different? Neural correlates of happy and sad mood in healthy males. Neuroimage. 2005;26:206–214. doi: 10.1016/j.neuroimage.2005.01.014. [DOI] [PubMed] [Google Scholar]
- Haber S.N., Kim K.-S., Mailly P., Calzavara R. Reward-related cortical inputs define a large striatal region in primates that interface with associative cortical connections, providing a substrate for incentive-based learning. J. Neurosci. 2006;26:8368–8376. doi: 10.1523/JNEUROSCI.0271-06.2006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Halperin J.M., Healey D.M. The influences of environmental enrichment, cognitive enhancement, and physical exercise on brain development: can we alter the developmental trajectory of ADHD? Neurosci. Biobehav. Rev. 2011;35:621–634. doi: 10.1016/j.neubiorev.2010.07.006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Han X., Jovicich J., Salat D., van der Kouwe A., Quinn B., Czanner S., Busa E., Pacheco J., Albert M., Killiany R., Maguire P., Rosas D., Makris N., Dale A., Dickerson B., Fischl B. Reliability of MRI-derived measurements of human cerebral cortical thickness: the effects of field strength, scanner upgrade and manufacturer. Neuroimage. 2006;32:180–194. doi: 10.1016/j.neuroimage.2006.02.051. [DOI] [PubMed] [Google Scholar]
- Hart H., Rubia K. Neuroimaging of child abuse: a critical review. Front. Hum. Neurosci. 2012;6 doi: 10.3389/fnhum.2012.00052. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Heim C., Nemeroff C.B. The role of childhood trauma in the neurobiology of mood and anxiety disorders: preclinical and clinical studies. Biol. Psychiatry. 2001;49:1023–1039. doi: 10.1016/s0006-3223(01)01157-x. [DOI] [PubMed] [Google Scholar]
- Hölzel B.K., Carmody J., Evans K.C., Hoge E.A., Dusek J.A., Morgan L., Pitman R.K., Lazar S.W. Stress reduction correlates with structural changes in the amygdala. Soc. Cogn. Affect. Neurosci. 2010;5:11–17. doi: 10.1093/scan/nsp034. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hops H., Davis B., Longoria N. Methodological issues in direct observation: Illustration with the Living in Familial Environments (LIFE) coding system. Journal of Clinical Child Psychology. 1995;24(2):193–203. [Google Scholar]
- Hops H., Davis B., Longoria N. Methodological issues in direct observation: illustration with the living in familial environments (life) coding system. J. Clin. Child Psychol. 1995;24:193–203. [Google Scholar]
- Huttenlocher P.R., Dabholkar A.S. Regional differences in synaptogenesis in human cerebral cortex. The Journal of Comparative Neurology. 1997;387(2):167–178. doi: 10.1002/(sici)1096-9861(19971020)387:2<167::aid-cne1>3.0.co;2-z. [DOI] [PubMed] [Google Scholar]
- Jacob T., Johnson S.L. Sequential interactions in the parent–child communications of depressed fathers and depressed mothers. J. Fam. Psychol. 2001;15:38–52. [PubMed] [Google Scholar]
- Jones F.L., McMillan J. Scoring occupational categories for social research: a review of current practice, with Australian examples. Work Employ. Soc. 2001;15:539–563. [Google Scholar]
- Katz L.F., Hunter E.C. Maternal meta-emotion philosophy and adolescent depressive symptomatology. Soc. Dev. 2007;16:343–360. [Google Scholar]
- Kaufman J., Birmaher B., Brent D., Rao U., Flynn C., Moreci P., Williamson D., Ryan N. Schedule for affective disorders and schizophrenia for school-age children present and lifetime version (k-sads-pl): initial reliability and validity data. J. Am. Acad. Child Adolesc. Psychiatry. 1997;36:980–988. doi: 10.1097/00004583-199707000-00021. [DOI] [PubMed] [Google Scholar]
- Kringelbach M.L. The human orbitofrontal cortex: linking reward to hedonic experience. Nat. Rev. Neurosci. 2005;6:691–702. doi: 10.1038/nrn1747. [DOI] [PubMed] [Google Scholar]
- Landry S.H., Smith K.E., Swank P.R., Guttentag C. A responsive parenting intervention: the optimal timing across early childhood for impacting maternal behaviors and child outcomes. Dev. Psychol. 2008;44:1335–1353. doi: 10.1037/a0013030. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lewis D.A. Development of the prefrontal cortex during adolescence: insights into vulnerable neural circuits in schizophrenia. Neuropsychopharmacology. 1997;16:385–398. doi: 10.1016/S0893-133X(96)00277-1. [DOI] [PubMed] [Google Scholar]
- Loeber R., Drinkwater M., Yin Y., Anderson S., Schmidt L., Crawford A. Stability of family interaction from ages 6 to 18. J. Abnorm. Child Psychol. 2000;28:353–369. doi: 10.1023/a:1005169026208. [DOI] [PubMed] [Google Scholar]
- Lorenzetti V., Allen N.B., Fornito A., Yucel M. Structural brain abnormalities in major depressive disorder: a selective review of recent MRI studies. J. Affect. Disord. 2009;117:1–17. doi: 10.1016/j.jad.2008.11.021. [DOI] [PubMed] [Google Scholar]
- Lorenzetti V., Allen N.B., Fornito A., Yücel M. Structural brain abnormalities in major depressive disorder: a selective review of recent MRI studies. J. Affect. Disord. 2009;117:1–17. doi: 10.1016/j.jad.2008.11.021. [DOI] [PubMed] [Google Scholar]
- Luby J.L., Barch D.M., Belden A., Gaffrey M.S., Tillman R., Babb C., Nishino T., Suzuki H., Botteron K.N. Maternal support in early childhood predicts larger hippocampal volumes at school age. Proc. Natl. Acad. Sci. U.S.A. 2012;109(8):2854–2859. doi: 10.1073/pnas.1118003109. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lupien S.J., McEwen B.S., Gunnar M.R., Heim C. Effects of stress throughout the lifespan on the brain, behaviour and cognition. Nat. Rev. Neurosci. 2009;10:434–445. doi: 10.1038/nrn2639. [DOI] [PubMed] [Google Scholar]
- MacPhillamy D.J., Lewinsohn P.M. University of Oregon; Eugene, Oregon: 1976. Manual for the Pleasant Events Schedule. [Google Scholar]
- McCrory E.J., De Brito S.A., Viding E. Research review: the neurobiology and genetics of maltreatment and adversity. J. Child Psychol. Psychiatry. 2010;51:1079–1095. doi: 10.1111/j.1469-7610.2010.02271.x. [DOI] [PubMed] [Google Scholar]
- Mezulis A., Simonson J., McCauley E., Vander Stoep A. The association between temperament and depressive symptoms in adolescence: brooding and reflection as potential mediators. Cogn. Emotion. 2011;25:1460–1470. doi: 10.1080/02699931.2010.543642. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Moran P.B., Vuchinich S., Hall N.K. Associations between types of maltreatment and substance use during adolescence. Child Abuse Negl. 2004;28:565–574. doi: 10.1016/j.chiabu.2003.12.002. [DOI] [PubMed] [Google Scholar]
- Morey R.A., Selgrade E.S., Wagner H.R., Huettel S.A., Wang L., McCarthy G. Scan–rescan reliability of subcortical brain volumes derived from automated segmentation. Hum. Brain Mapp. 2010;31:1751–1762. doi: 10.1002/hbm.20973. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Muthén L.K., Muthén B.O. Seventh Edition. Muthén & Muthén; Los Angeles, CA: 1998-2012. Mplus User's Guide. [Google Scholar]
- Muris P., Meesters C., Blijlevens P. Self-reported reactive and regulative temperament in early adolescence: relations to internalizing and externalizing problem behavior and big three personality factors. J. Adolesc. 2007;30:1035–1049. doi: 10.1016/j.adolescence.2007.03.003. [DOI] [PubMed] [Google Scholar]
- Nolan C.L., Moore G.J., Madden R., Farchione T., Bartoi M., Lorch E., Stewart C.M., Rosenberg D.R. Prefrontal cortical volume in childhood-onset major depression. Arch. Gen. Psychiatry. 2002;59:173–179. doi: 10.1001/archpsyc.59.2.173. [DOI] [PubMed] [Google Scholar]
- Ochsner K.N., Gross J.J. The cognitive control of emotion. Trends Cogn. Sci. 2005;9:242–249. doi: 10.1016/j.tics.2005.03.010. [DOI] [PubMed] [Google Scholar]
- Okuda H., Tatsumi K., Makinodan M., Yamauchi T., Kishimoto T., Wanaka A. Environmental enrichment stimulates progenitor cell proliferation in the amygdala. J. Neurosci. Res. 2009;87:3546–3553. doi: 10.1002/jnr.22160. [DOI] [PubMed] [Google Scholar]
- Oldfield R.C. The assessment and analysis of handedness: the Edinburgh handedness inventory. Neuropsychologia. 1971;9:97–114. doi: 10.1016/0028-3932(71)90067-4. [DOI] [PubMed] [Google Scholar]
- Olinsky A., Chen S., Harlow L. The comparative efficacy of imputation methods for missing data in structural equation modeling. Eur. J. Operat. Res. 2003;151:53–79. [Google Scholar]
- Ostby Y., Tamnes C.K., Fjell A.M., Westlye L.T., Due T.P., Walhovd K.B. Heterogeneity in subcortical brain development: a structural magnetic resonance imaging study of brain maturation from 8 to 30 years. J. Neurosci. 2009;29:11772–11782. doi: 10.1523/JNEUROSCI.1242-09.2009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Paus T., Keshavan M., Giedd J.N. Why do many psychiatric disorders emerge during adolescence? Nature Reviews Neuroscience. 2008;9(12):947–957. doi: 10.1038/nrn2513. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Plotsky P.M., Meaney M.J. Early, postnatal experience alters hypothalamic corticotropin-releasing factor (CRF) MRNA, median eminence CRF content and stress-induced release in adult rats. Mol. Brain Res. 1993;18:195–200. doi: 10.1016/0169-328x(93)90189-v. [DOI] [PubMed] [Google Scholar]
- Price J.L., Drevets W.C. Neurocircuitry of mood disorders. Neuropsychopharmacology. 2010;35:192–216. doi: 10.1038/npp.2009.104. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Prinz R.J., Foster S.L., Kent R.N., O’Leary K.D. Multivariate assessment of conflict in distressed and nondistressed mother–adolescent dyads. J. Appl. Behav. Anal. 1979;12:691–700. doi: 10.1901/jaba.1979.12-691. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Radloff L.S. The ces-d scale: a self-report depression scale for research in the general population. Appl. Psychol. Meas. 1977;1:385–401. [Google Scholar]
- Rao H., Betancourt L., Giannetta J.M., Brodsky N.L., Korczykowski M., Avants B.B., Gee J.C., Wang J., Hurt H., Detre J.A., Farah M.J. Early parental care is important for hippocampal maturation: evidence from brain morphology in humans. Neuroimage. 2010;49:1144–1150. doi: 10.1016/j.neuroimage.2009.07.003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rauch S.L., Milad M.R., Orr S.R., Quinn B.T., Fischl B., Pitman R.K. Orbitofrontal thickness, retention of fear extinction, and extraversion. Neuroreport. 2005;16:1909–1912. doi: 10.1097/01.wnr.0000186599.66243.50. [DOI] [PubMed] [Google Scholar]
- Reuter M., Rosas H.D., Fischl B. Highly accurate inverse consistent registration. A robust approach. Neuroimage. 2010;53:1181–1196. doi: 10.1016/j.neuroimage.2010.07.020. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rutherford L.C., Nelson S.B., Turrigiano G.G. BDNF has opposite effects on the quantal amplitude of pyramidal neuron and interneuron excitatory synapses. Neuron. 1998;21:521–530. doi: 10.1016/s0896-6273(00)80563-2. [DOI] [PubMed] [Google Scholar]
- Schlomer G.L., Bauman S., Card N.A. Best practices for missing data management in counseling psychology. J. Counsel. Psychol. 2010;57:1. doi: 10.1037/a0018082. [DOI] [PubMed] [Google Scholar]
- Schwartz O.S., Byrne M.L., Simmons J.G., Whittle S., Dudgeon P., Sheeber L.S., Allen N.B. Parenting during early adolescence and adolescent onset major depression: a six-year prospective longitudinal study. Clin. Psychol. Sci. 2013 in press. [Google Scholar]
- Schwartz O.S., Dudgeon P., Sheeber L.B., Yap M.B.H., Simmons J.G., Allen N.B. Parental behaviors during family interactions predict changes in depression and anxiety symptoms during adolescence. J. Abnorm. Child Psychol. 2012;40:59–71. doi: 10.1007/s10802-011-9542-2. [DOI] [PubMed] [Google Scholar]
- Schwarz O., Dudgeon P., Yap M.B.H., Sheeber L., Allen N.B. Parental behaviors during family interactions predict changes in depression and anxiety during adolescence. J. Abnorm. Psychol. 2012;40:59–71. doi: 10.1007/s10802-011-9542-2. [DOI] [PubMed] [Google Scholar]
- Shad M.U., Muddasani S., Rao U. Gray matter differences between healthy and depressed adolescents: a voxel-based morphometry study. J. Child Adolesc. Psychopharmacol. 2012;22:190–197. doi: 10.1089/cap.2011.0005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Shaw P., Greenstein D., Lerch J., Clasen L., Lenroot R., Gogtay N., Evans A., Rapoport J., Giedd J. Intellectual ability and cortical development in children and adolescents. Nature. 2006;440:676–679. doi: 10.1038/nature04513. [DOI] [PubMed] [Google Scholar]
- Sheeber L., Davis B., Leve C., Hops H., Tildesley E. Adolescents’ relationships with their mothers and fathers: associations with depressive disorder and subdiagnostic symptomatology. J. Abnorm. Psychol. 2007;116:114–154. doi: 10.1037/0021-843X.116.1.144. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sheeber L., Hops H., Andrews J., Alpert T., Davis B. Interactional processes in families with depressed and non-depressed adolescents: reinforcement of depressive behavior. Behav. Res. Ther. 1998;36:417–427. doi: 10.1016/s0005-7967(97)10030-4. [DOI] [PubMed] [Google Scholar]
- Sowell E.R., Thompson P.M., Leonard C.M., Welcome S.E., Kan E., Toga A.W. Longitudinal mapping of cortical thickness and brain growth in normal children. Journal of Neuroscience. 2004;24(38):8223–8231. doi: 10.1523/JNEUROSCI.1798-04.2004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Steingard R.J., Renshaw P.F., Hennen J., Lenox M., Cintron C.B., Young A.D., Connor D.F., Au T.H., Yurgelun-Todd D.A. Smaller frontal lobe white matter volumes in depressed adolescents. Biol. Psychiatry. 2002;52:413–417. doi: 10.1016/s0006-3223(02)01393-8. [DOI] [PubMed] [Google Scholar]
- Tottenham N., Sheridan M.A. A review of adversity, the amygdala and the hippocampus: a consideration of developmental timing. Front. Hum. Neurosci. 2010;3:18. doi: 10.3389/neuro.09.068.2009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tupler L.A., De Bellis M.D. Segmented hippocampal volume in children and adolescents with posttraumatic stress disorder. Biol. Psychiatry. 2006;59:523–529. doi: 10.1016/j.biopsych.2005.08.007. [DOI] [PubMed] [Google Scholar]
- Vijayakumar N., Whittle S., Dennison M., Yucel M., Simmons J.G., Allen N.B. 2013. The development of emotion regulation and the prefrontal cortex: structural correlates of cognitive reappraisal in adolescents. (in press-a) [Google Scholar]
- Vijayakumar N., Whittle S., Dennison M., Yucel M., Simmons J.G., Allen N.B. 2013. Development of the prefrontal cortex and self-regulation: structural correlates of effortful control in adolescents. (in press-b) [Google Scholar]
- Wang D., Doddrell D.M. Geometric distortion in structural magnetic resonance imaging. Curr. Med. Imag. Rev. 2005;1:49–60. [Google Scholar]
- Wechsler D. Fourth Edition. Harcourt Assessment, Inc; San Antonio, TX: 2003. Wechsler Intelligence Scale for Children. [Google Scholar]
- Westlye L.T., Grydeland H., Walhovd K.B., Fjell A.M. Associations between regional cortical thickness and attentional networks as measured by the attention network test. Cereb. Cortex. 2011;21:345–356. doi: 10.1093/cercor/bhq101. [DOI] [PubMed] [Google Scholar]
- Whittle S., Dennison M., Vijayakumar N., Simmons J.G., Yücel M., Lubman D.I., Pantelis C., Allen N.B. Childhood maltreatment and psychopathology affect brain development during adolescence. J. Am. Acad. Child Adolesc. Psychiatry. 2013;52 doi: 10.1016/j.jaac.2013.06.007. 940–952.e1. [DOI] [PubMed] [Google Scholar]
- Woolley M.E., Grogan-Kaylor A. Protective family factors in the context of neighborhood: promoting positive school outcomes. Fam. Relat. 2006;55:93–104. [Google Scholar]
- Yap M.B.H., Schwartz O.S., Byrne M.L., Simmons J.G., Allen N.B. Maternal positive and negative interaction behaviors and early adolescents’ depressive symptomatology: adolescent emotion regulation as a mediator. J. Res. Adolesc. 2010;20:1014–1043. [Google Scholar]
- Zheng W., Chee M.W.L., Zagorodnov V. Improvement of brain segmentation accuracy by optimizing non-uniformity correction using n3. Neuroimage. 2009;48:73–83. doi: 10.1016/j.neuroimage.2009.06.039. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.