Abstract
Background:
Though consensus guidelines on the management of cardiovascular disease (CVD) in pregnancy reserve cesarean delivery for obstetric indications, there is a paucity of data to support this approach.
Objectives:
To compare cardiovascular and obstetric morbidity in women with cardiovascular disease (CVD) according to plan for vaginal birth or cesarean delivery.
Study Design:
We assembled a prospective cohort of women delivering at an academic tertiary care center with a protocolized multidisciplinary approach to management of CVD between September 2011 and December 2016. Our practice is to encourage vaginal birth in women with CVD unless there is an obstetric indication for cesarean delivery. We allow women attempting vaginal birth a trial of Valsalva in the second stage with the ability to provide operative vaginal delivery if pushing leads to changes in hemodynamics or symptoms. Women were classified according to planned mode of delivery—either vaginal birth or cesarean delivery. We then used univariate analysis to compare adverse outcomes according to planned mode of delivery. The primary composite cardiac outcome of interest included sustained arrhythmia, heart failure, cardiac arrest, cerebral vascular accident, need for cardiac surgery or intervention, or death. Secondary obstetric and neonatal outcomes were also considered.
Results:
We included 276 consenting women with congenital heart disease (68.5%), arrhythmias (11.2%), connective tissue disease (9.1%), cardiomyopathy (8.0%), valvular disease (1.4%) or vascular heart disease (1.8%) at or beyond 24 weeks gestation. Seventy-six percent (n=210) planned vaginal birth and 24% (n=66) planned cesarean delivery. Women planning vaginal birth had lower rates of left ventricular outflow tract obstruction, multiparity, and preterm delivery. All women attempting vaginal birth were allowed to Valsalva. Among planned vaginal deliveries 86.2% (n=181) were successful with a 9.5% operative vaginal delivery rate. Five women underwent operative vaginal delivery for the indication of cardiovascular disease without another obstetric indication at the discretion of the delivering provider. Four of these patients tolerated trials of Valsalva ranging from 15 to 75 minutes prior to delivery. Adverse cardiac outcomes were similar between planned vaginal birth and cesarean delivery groups (4.3% v. 3.0%, p=1). Rates of postpartum hemorrhage (1.9% v. 10.6%, p<0.01) and transfusion (1.9% v. 9.1%, p=0.01) were lower in the planned vaginal birth group. There were no differences in adverse cardiac, obstetric or neonatal outcomes in the cohort overall or the subset of women with high-risk CVD or a high burden of obstetric comorbidity.
Conclusions:
These findings suggest that cesarean delivery does not reduce adverse cardiovascular outcomes and lend support to a planned vaginal birth for the majority of women with CVD including those with high-risk disease.
Keywords: Cardiovascular disease, maternal morbidity, operative vaginal delivery, vaginal delivery
INTRODUCTION
Improvements in cardiovascular care have led to an increased prevalence of cardiovascular disease (CVD) in pregnancy with a rising contribution of CVD to maternal mortality.1,2 Women with CVD in pregnancy are at an increased risk of adverse obstetric and cardiac events.3–7 While risk factors and scoring systems for adverse events in women with CVD have been described, most studies fail to explore the impact of management strategies on the rates of these outcomes.7–10
Planned mode of delivery is an important decision for pregnant women with CVD and their providers. Consensus guidelines typically reserve cesarean delivery for obstetric indications with specific exceptions for high risk disease.11,12 However, rates of cesarean delivery for women with CVD are higher than those in the general population with a 33% rate of primary cesarean delivery for cardiac indications in recent literature.10,14 For women attempting vaginal birth, the role of operative vaginal delivery to minimize Valsalva may actually increase maternal morbidity.11,14,15 These consensus guidelines are based primarily on expert opinion with a paucity of data to support the recommendations.
Though decreasing rates of primary cesarean delivery is a national priority, the impact of mode of delivery on current and subsequent pregnancies for women with CVD is largely unexplored.16–20 We undertook this study to examine the association between planned mode of delivery and maternal morbidity in a population of women with CVD. We hypothesize that in these women planned vaginal birth confers no increased risk of obstetric or cardiovascular morbidity compared to planned cesarean delivery.
MATERIALS AND METHODS
We prospectively enrolled pregnant women with CVD receiving care at the Brigham and Women’s Hospital from September 2011 through December 2016 in the Standardized Outcomes In Reproductive Cardiovascular Care (STORCC) initiative. The Institutional Review Boards of the Brigham and Women’s Hospital and Boston Children’s Hospital approved this protocol and each patient provided written informed consent. Data was collected prospectively at each clinic visit, during all pregnancy admissions and through delivery and postpartum care. We categorized women according to their underlying CVD into one of the following groups: congenital heart disease, connective tissue disease, cardiomyopathy, valvular disease, vascular disease, and arrhythmia. Women were classified as having high-risk CVD with a history of any of the following criteria or if they developed these high-risk features during pregnancy: New York Heart Association (NYHA) function Class > II, oxygen saturation <90%, systemic ejection fraction (EF) < 40%, left ventricular outflow tract (LVOT) peak gradient > 30 mmHg, subpulmonary EF <40%, or aortic conditions associated with connective tissue disease.
We discussed each woman at a monthly multidisciplinary conference that included representatives from maternal-fetal medicine, cardiology, obstetric anesthesia and nursing. Delivery discussions included location (labor and delivery, cardiac intensive care unit (ICU), cardiac catheterization laboratory, hybrid cardiac operating room), monitoring (telemetry, invasive hemodynamic monitoring, continuous pulse oximetry), timing, and mode of delivery (vaginal delivery, cesarean delivery, or vaginal delivery with assisted second stage with vacuum or forceps). Our practice is to encourage vaginal birth in women with CVD unless there is an obstetric indication for cesarean delivery. The ultimate mode of delivery was at the discretion of the delivering obstetrician.
For this study we classified women according to their planned mode of delivery into one of two groups: planned vaginal birth or planned cesarean delivery. Patients in the planned cesarean delivery group were further classified by obstetric eligibility for planned vaginal birth. Women with placenta previa, malpresentation, prior uterine surgery, or maternal or fetal instability prohibiting labor were considered ineligible for planned vaginal birth. Peripartum records were independently reviewed by two obstetricians (SRE, CER) with complete ascertainment as to both the planned and actual modes of delivery. Actual mode of delivery was defined as vaginal or cesarean delivery. Vaginal delivery was divided into spontaneous vaginal delivery or operative vaginal delivery (either vacuum- or forceps-assisted vaginal delivery). The indication for planned cesarean deliveries, operative vaginal deliveries, and unplanned cesarean deliveries in the attempted vaginal birth group were noted.
Maternal cardiac, obstetric, and neonatal covariates were collected using standard obstetric definitions unless otherwise noted.21 We noted a history of an adverse event in the pregnancy of interest based on our primary outcome definition below. To better characterize the burden of medical comorbidity in the cohort, patient comorbidities were collected and tallied using the obstetric comorbidity index developed by Bateman and colleagues.22 This validated index accounts for medical and obstetric comorbidities with higher scores predictive of admission to the ICU and adverse maternal outcomes.
For the present analysis, the primary outcome of interest was a composite outcome of peripartum cardiovascular morbidity. The composite cardiac outcome consisted of one or more of the following: congestive heart failure (diagnosed by physical examination and requiring diuresis), sustained symptomatic arrhythmia (>30 seconds in duration or requiring therapy), cerebral vascular event, new or worsening valvar dysfunction, endocarditis, aortic dissection, need for cardiac intervention, cardiac arrest, and cardiac death.9 The objective of the present study was to determine the impact of attempted mode of delivery on this primary composite cardiovascular outcome. Therefore, the primary outcome was considered present only if it occurred in the peripartum or 6 week postpartum period and was not present at the time of admission for delivery.
Secondary outcomes of interest included mode of delivery, a composite outcome of obstetric morbidity, maternal ICU admission, the occurrence of severe maternal morbidity, admission to the neonatal intensive-care unit (NICU), and a composite outcome of neonatal morbidity. The composite obstetric outcome was based on the primary outcome set forth in the Maternal-Fetal Medicine University Networks Assessment of Perinatal Excellence (APEX) study with some modifications.23 The composite obstetric outcome included venous thromboembolism (including deep venous thrombosis), postpartum hemorrhage, and peripartum infection during the first 6 weeks postpartum.23 Severe maternal morbidity was defined using the recent consensus guidelines set forth by the American College of Obstetricians and Gynecologists (ACOG) and the Society for Maternal-Fetal Medicine.24 As with the primary outcome, both severe maternal morbidity and maternal ICU admission were considered present only if they occurred in the peripartum period and were absent at the time of admission for delivery. We did not include third or fourth degree perineal lacerations involving the anal sphincter in the composite obstetric outcome despite their association with short and long-term morbidity in line with ACOG recommendations and the APEX composite outcome definition.23,25,26 These rates are reported separately. The composite neonatal outcome was similarly based on the work of the APEX study and, along with NICU admission, limited to term, singleton, nonanomalous fetuses.23
The associations between intended mode of delivery and maternal, fetal, or obstetric characteristics were evaluated with the Chi Square test or Fishers Exact test for categorical variables or the Wilcoxon rank sum test for continuous variables. Statistical significance was defined with a two-tailed p-value <0.05. The heterogeneity of CVD and maternal comorbidity in the cohort coupled with the low rate of adverse outcomes limited our statistical power to perform adjusted analyses controlling for multiple covariates. We therefore conducted multiple preplanned sensitivity analyses examining outcomes for the subset of women with CVD and for those with an obstetric comorbidity index above 7. All analyses were performed with Statistical Analysis Software (SAS), version 9.4 (Copyright 2013, SAS Institute, Inc. Cary North Carolina).
RESULTS
Figure 1 shows the identification and categorization of patients for the current study. Two-hundred seventy-six women met inclusion criteria and delivered in the time period of interest. Of these women, 76.1% (n=210) planned a vaginal birth and 23.9% (n=66) planned cesarean delivery. As shown in the Figure, the majority of patients in the cohort had congenital heart disease (68.5%), followed by arrhythmias (11.2%), connective tissue disease (9.1%), cardiomyopathy (8.0%), valvular disease (1.4%) or vascular heart disease (1.8%) The specific cardiac diagnoses are detailed in Supplemental Table 1. There was no difference in the type of heart disease between women attempting vaginal birth and those planning cesarean delivery (p=0.69). After reviewing each patient in our multidisciplinary conference, no patients had a plan for cesarean delivery specifically for the indication of cardiac disease.
Figure 1: Planned mode of delivery and type of cardiac disease for women in the cohort.
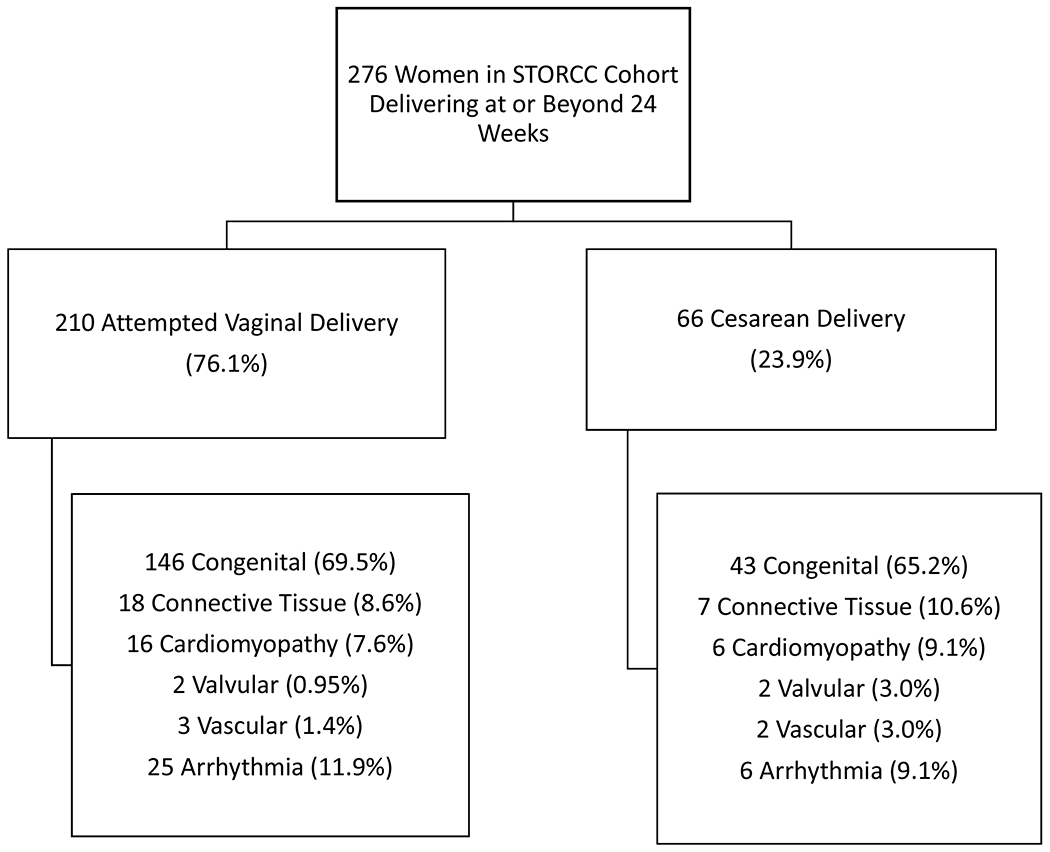
Description: Seventy-six percent of women in the cohort attempted vaginal delivery compared to 24% of women planning cesarean delivery. Congenital heart disease was the most common type of cardiovascular disease in both groups.
The demographic and clinical characteristics of eligible women according to planned mode of delivery are shown in Table 1. Women attempting vaginal birth had lower rates of elevated left ventricular outflow track (LVOT) peak gradients compared to those with planning cesarean delivery (4.8% v. 13.6%, p=0.02). Five of the 9 women in the group of patients with elevated LVOT peak gradients planning cesarean delivery were elective repeat cesarean deliveries after being denied a trial of labor at other institutions during their first pregnancies. Rates of adverse cardiac events before and during pregnancy were similar between groups.
Table 1:
Demographic and clinical characteristics according to planned mode of delivery
| Characteristic | Total (N=276) | Planned Vaginal Birth (n=210) | Planned Cesarean Delivery (n=66) | p-valuea |
|---|---|---|---|---|
| Maternal age (years) b | 32.6 (29.2-35.4) | 32.4 (29.2-35.0) | 33.5 (29.4-37.5) | 0.06 |
| Race/Ethnicity | 0.34 | |||
| White, Not Hispanic | 193 (69.9) | 142 (67.6) | 51 (77.3) | |
| Black | 21 (7.6) | 17 (8.1) | 4 (6.1) | |
| Hispanic, White | 30 (10.9) | 23 (10.9) | 7 (10.6) | |
| Hispanic, Nonwhite | 3 (1.1) | 3 (1.4) | 0 (0) | |
| Asian | 12 (4.4) | 12 (5.7) | 0 (0) | |
| Other/Declined | 17 (6.2) | 13 (6.2) | 4 (6.1) | |
| Smoking | 20 (7.3) | 14 (6.7) | 6 (9.1) | 0.59 |
| Prepregnancy BMI ≥ 30 kg/m2 | 62 (22.5) | 44 (21.0) | 18 (27.3) | 0.28 |
| Chronic Hypertension | 12 (4.4) | 7 (3.3) | 5 (7.6) | 0.17 |
| Pregestational Diabetes | 3 (1.1) | 2 (0.95) | 1 (1.5) | 0.56 |
| Prior Adverse Event | 39 (14.1) | 30 (14.3) | 9 (13.6) | 0.89 |
| High-Risk Cardiac Diseasec | ||||
| Connective Tissue Disease | 25 (9.1) | 18 (8.6) | 7 (10.6) | 0.62 |
| NYHA Class > II | 6 (2.2) | 3 (1.4) | 3 (4.5) | 0.15 |
| Oxygen Saturation < 90% | 1 (0.37) | 0 (0) | 1 (1.5) | 0.24 |
| Systemic EF < 40% | 8 (2.9) | 5 (2.4) | 3 (4.6) | 0.40 |
| LVOT Peak Gradient > 30 mm/Hg | 19 (7.0) | 10 (4.8) | 9 (13.6) | 0.02 |
| Adverse Event in Pregnancyd | 15 (5.4) | 8 (3.8) | 7 (10.6) | 0.06 |
p-value calculated by Chi-Square test or Fisher exact test for categorical variables unless otherwise noted.
Continuous variables presented as median (interquartile range) with p-value calculated by Wilcoxon Rank Sum test.
High risk CVD defined as one or more of the following risk factors: prior adverse event, New York Heart Association (NYHA) Class > II, oxygen saturation < 90%, systemic ejection fraction (EF) < 40%, and left ventricular outflow tract (LVOT) peak gradient >30 mmHg, subpulmonary EF <40% or connective tissue disease.
Adverse event in pregnancy includes heart failure, sustained symptomatic arrhythmia, new or worsening valvar dysfunction, endocarditis, aortic dissection, need for cardiac intervention, cardiac arrest, and cardiac death not associated with delivery.
Table 2 demonstrates the obstetric and intrapartum characteristics for women in the cohort. Rates of nulliparity (52.9% v. 28.8%, p<0.01) were higher in the vaginal delivery group compared to the cesarean delivery group. Women attempting vaginal birth were less likely to be preterm (11.9% v. 28.8%, p<0.01) compared to their cesarean delivery counterparts. Other obstetric features including rates of preeclampsia or gestational hypertension, gestational diabetes, fetal growth restriction, and fetal anomalies were similar between the two groups. The planned cesarean delivery group had a higher median obstetric comorbidity index score with similar rates of obstetric comorbidity indices greater than seven.
Table 2:
Obstetric and intrapartum characteristics according to planned mode of delivery
| Characteristic | Total (N=276) | Planned Vaginal Birth (n=210) | Planned Cesarean Delivery (n=66) | p-valuea |
|---|---|---|---|---|
| Nulliparous | 130 (47.1) | 111 (52.9) | 19 (28.8) | <0.01 |
| Gestational Age < 37 Weeks | 44 (15.9) | 25 (11.9) | 19 (28.8) | <0.01 |
| Spontaneous Conception | 247 (89.5) | 189 (90.0) | 58 (87.9) | 0.62 |
| Preeclampsia or Gestational Hypertension | 25 (9.1) | 18 (8.6) | 7 (10.6) | 0.62 |
| Gestational Diabetes | 19 (6.9) | 14 (6.7) | 5 (7.6) | 0.78 |
| Intrauterine Growth Restriction | 38 (13.8) | 28 (13.3) | 10 (15.2) | 0.69 |
| Fetal Anomalies | 21 (7.6) | 16 (7.6) | 5 (7.6) | 1 |
| Multiple Gestation | 3 (1.1) | 2 (0.95) | 1 (1.5) | 0.56 |
| Neuraxial Analgesia | 261 (94.6) | 199 (94.8) | 62 (93.9) | 0.76 |
| Obstetric Comorbidity Indexb | 4 (3-5) | 4(3-5) | 5 (3-6) | <0.01 |
| Obstetric Comorbidity Index > 7 | 11 (4.0) | 6 (2.9) | 5 (7.6) | 0.14 |
p-value calculated by Chi-Square test or Fisher exact test for categorical variables unless otherwise noted.
Continuous variables presented as median (interquartile range) with p-value calculated by Wilcoxon Rank Sum test.
The mode of delivery with associated indications are displayed in Figure 2. Eighty-six percent of the women (n=181) attempting vaginal delivery achieved vaginal birth. The majority of these (n=155) were spontaneous vaginal deliveries or vaginal births after cesarean deliveries (n=6) with a 9.5% rate of operative vaginal delivery (n=20) in the planned vaginal delivery group. All women attempting vaginal birth were allowed to Valsalva.
Figure 2: Actual mode of delivery and associated indications according to planned mode of delivery.
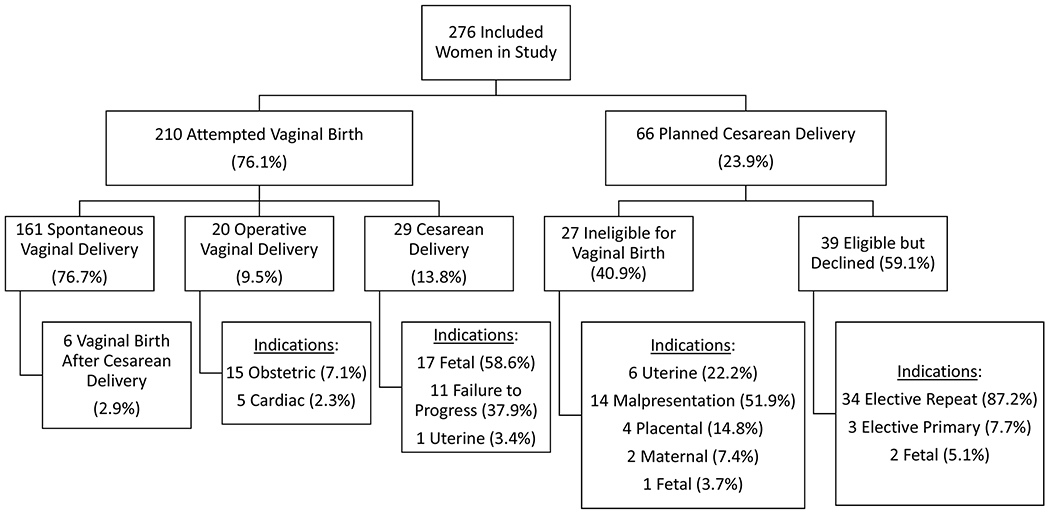
Description: Among planned vaginal deliveries 86.2% (n=181) were successful with a 9.5% operative vaginal delivery rate. Fifty-nine percent of women planning cesarean delivery had no contraindication to attempted vaginal birth with elective repeat cesarean delivery as the the most common indication for cesarean in this group.
Table 3 presents the details of the five (2.4%) operative vaginal deliveries performed for the indication of CVD without another obstetric indication. Although all five women had a plan for a trial of Valsalva with no recommendation for assisted second stage from the multidisciplinary review, they ultimately underwent operative deliveries at the discretion of the delivering provider. Three of the patients had aortic stenosis while one patient representing two deliveries had Marfan syndrome with a strong family history of aortic dissection. One patient with aortic stenosis was prohibited from a trial of Valsalva at the discretion of the delivering provider. The remainder of the women in the cohort tolerated Valsalva without hemodynamic instability.
Table 3:
Details of patients with assisted second stage for cardiac disease.
| Case | Disease | Details of Cardiac Diseasea | Minutes of Valsalva | Outcome |
|---|---|---|---|---|
| 1 | Marfan Syndrome | Aortic root 3.3 cm with strong family history of dissection | 75 | Uncomplicated vacuum-assisted delivery |
| 2 | Marfan Syndrome | Aortic root 3.3 cm with strong family history of dissection | 30 | Uncomplicated forceps-assisted delivery |
| 3 | Aortic Stenosis | Subaortic stenosis s/p repair and resultant moderate stenosis and severe regurgitation | 15 | Uncomplicated vacuum-assisted delivery |
| 4 | Aortic Stenosis | Severe aortic stenosis s/p aortic valve replacement with development of ascending aortic aneurysm (peak gradient 79 mmHg mean gradient 47 mmHg) | 75 | Uncomplicated vacuum-assisted vaginal delivery; postpartum aortic valve replacement |
| 5 | Aortic Stenosis | Severe but stable aortic stenosis (peak gradient 79 mmHg mean gradient 47 mmHg) | 0 | Forceps-assisted vaginal delivery complicated by fourth degree perineal laceration and breakdown requiring multiple reoperations |
Aortic root area and peak and mean aortic valve gradient listed for patients on the transthoracic echocardiogram closest to delivery.
Twenty-nine women (13.8%) attempting vaginal birth required cesarean delivery for routine obstetric indications. For the women planning cesarean delivery 40.9% (n=27) had an obstetric indication. The remaining 59.1% (n=39) had no contraindication to attempted vaginal birth. Obstetric contraindications to vaginal birth included history of uterine surgery, malpresentation of the fetus, placental indications such as previa or abruption, and one cesarean delivery for a fetus with anomalies precluding vaginal birth. There were two patients with maternal contraindications to vaginal delivery. One of these women had severe hemolysis elevated liver function tests and low platelets (HELLP) syndrome and the other woman had Marfan syndrome with an acute aortic dissection. Thirty-four of the 39 cesarean deliveries performed without obstetric contraindications to labor were elective repeat cesarean deliveries.
The primary and secondary outcomes according to planned mode of delivery are presented in Table 4. Women attempting vaginal birth had a similar rate of the primary cardiac outcome (4.3% v. 3.0%, p=1) compared to their planned cesarean delivery counterparts. One patient experienced cardiac arrest at 20 weeks gestation and one patient had a cerebrovascular accident in the setting of a mechanical heart valve in the second trimester. Neither of these serious adverse events was attributable to delivery. There were no significant differences in the rates of secondary maternal outcomes including the composite obstetric outcome, severe maternal morbidity, and maternal ICU admission. Women planning vaginal birth had lower rates of postpartum hemorrhage (1.9% v. 10.6%, p < 0.01) with a lower rate of blood transfusion (1.9% v. 9.1%, p=0.01). Six women (2.9%) in the planned vaginal birth group experienced a shoulder dystocia without any neonatal complications. Three women (1.4%) in the planned vaginal birth group had a third or fourth degree perineal laceration. Rates of admission to the neonatal intensive care unit for singleton, term nonanomalous fetuses were low and similar between groups. No neonates in either arm experienced the composite neonatal outcome.
Table 4:
Obstetric and cardiovascular outcomes according to planned mode of delivery
| Outcome | Total (N=276) | Planned Vaginal Birth (n=210) | Planned Cesarean Delivery (n=66) | p-valuea |
|---|---|---|---|---|
| Composite Cardiac Outcomeb | 11 (4.0) | 9 (4.3) | 2 (3.0) | 1 |
| Sustained Arrhythmia | 1 (0.36) | 1 (0.48) | 0 (0) | 1 |
| Heart Failure | 9 (3.3) | 7 (3.3) | 2 (3.0) | 1 |
| Composite Obstetric Outcomec | 33 (12.0) | 21 (10.0) | 11 (18.2) | 0.08 |
| Postpartum Hemorrhage | 11 (4.0) | 4 (1.9) | 7 (10.6) | <0.01 |
| Blood Transfusion | 10 (3.6) | 4 (1.9) | 6 (9.1) | 0.01 |
| Estimated Blood Loss ≥ 1500 cc | 8 (2.9) | 3 (1.4) | 5 (7.6) | 0.02 |
| Hysterectomy | 2 (0.72) | 1 (0.48) | 1 (1.5) | 0.42 |
| Peripartum Infection | 24 (8.8) | 19 (9.1) | 5 (7.6) | 0.81 |
| Chorioamnionitis | 11 (4.0) | 11 (5.2) | 0 (0) | 0.07 |
| Endometritis | 7 (2.5) | 4 (1.9) | 3 (4.6) | 0.36 |
| Wound Cellulitis | 4 (1.5) | 3 (1.4) | 1 (1.5) | 1 |
| Wound Reopening | 2 (0.72) | 1 (0.48) | 1 (1.5) | 0.42 |
| Venous Thromboembolism | 3 (1.1) | 0 (0) | 3 (4.6) | 0.01 |
| Severe Maternal Morbidity | 17 (6.2) | 9 (4.3) | 8 (12.1) | 0.04 |
| Maternal ICU Admission | 3 (1.1) | 1 (0.48) | 2 (3.0) | 0.14 |
| NICU Admissiond | 5 (2.4) | 4 (2.3) | 1 (2.4) | 1 |
| Composite Neonatal Outcomed | 0 (0) | 0 (0) | 0 (0) | 1 |
p-value calculated by Chi-Square test or Fisher exact for categorical variables.
None of the cases of cerebral vascular accidents (n=1), cardiac arrest (n=1), endocarditis, percutaneous intervention (n=5), aortic dissection (n=1), or cardiac surgery (n=6) were attributable to delivery. There were no maternal deaths.
Composite obstetric outcome consisting of postpartum hemorrhage, peripartum infection, venous thromboembolism.
Neonatal ICU (NICU) admission and composite neonatal outcome limited to 37 week, singleton, nonanomalous fetuses. Composite neonatal outcome includes 5 minute Apgar < 4, skeletal fracture, nerve palsy, subgaleal hemorrhage, intubation within the first 24 hours for at least two days, hypoxic ischemic encephalopathy, and neonatal death.
In the sensitivity analysis according to the presence of high-risk CVD, women planning vaginal birth had similar rates of the primary cardiac outcome (2.8% v. 4.8%, p=1) and the composite obstetric outcome (11.1% v. 28.6%, p=0.15) to those planning cesarean delivery. Rates of the primary cardiac outcome were similar between those planning vaginal birth and those planning cesarean delivery after excluding women with connective tissue disease (5.6% v. 7.1%, p=1). When comparing planned vaginal birth to cesarean delivery in the 265 women with obstetric comorbidity indices less than seven, rates of the cardiac outcome (3.4% v. 3.3%, p=1) and the composite maternal outcome (8.8% v. 16.4%, p=0.09) were similar.
COMMENT
Principal Findings
This prospective single-center cohort study evaluated delivery associated cardiac and obstetric morbidity in women with CVD according to planned mode of delivery. Though women attempting cesarean delivery had higher risk obstetric and cardiac features, rates of delivery-associated obstetric and cardiac morbidity were low and similar between groups. Additionally, rates of delivery-associated cardiovascular morbidity were much lower than previously reported general rates of adverse outcomes for women with CVD in pregnancy.6,7,9,27,28 This data supports recent consensus guidelines discouraging primary cesarean delivery for cardiac indications and raises important questions regarding the recommendation to avoid Valsalva in the second stage of labor3,11
Results in Context
This study aims to explore a critical clinical management dilemma in the care of women with CVD in pregnancy. The investigators from the European Registry on Pregnancy and Heart Disease addressed this question using data from centers across 19 different countries and found higher rates of heart failure in women undergoing planned cesarean delivery (3.9% v. 8.4%, p<0.001).28 Given the wide variation between patient populations and healthcare utilization, our results offer important information for providers caring for parturients with CVD in a tertiary care center with a full complement of cardiac and obstetric services. Current literature regarding women with CVD in pregnancy largely evaluates adverse outcomes across the pregnancy—not outcomes attributable to delivery.5,8,9,27,29 The few studies focused on delivery-related outcomes for women with CVD in pregnancy have compared women with heart disease to a control population without cardiovascular comorbidities.10,13 Though these studies provide meaningful assessments of risk compared to a healthy population, they offer minimal guidance in clinical decision making towards choosing the optimal approach for the management of patients with CVD in pregnancy.
Strengths and Clinical Implications
Our work has several strengths over prior published literature. The prospective design allows for the evaluation of a variety of important covariates including functional status, history of adverse cardiac events, and echocardiographic parameters. These features are carefully evaluated in the clinical decision making for women with CVD in pregnancy, but are seldom considered in larger database-driven work.13,29 The granularity of data informing the more descriptive aspects of our study provide much needed evidence to the obstetricians and cardiologists making important clinical decisions about the mode of delivery and role of operative vaginal delivery in avoidance of Valsalva. Despite a high level of baseline cardiac comorbidity in our cohort, we reserved operative vaginal delivery for obstetric indications did not observe any hemodynamic changes or adverse outcomes attributed to Valsalva in the second stage of labor. Considering the obligatory increase in maternal morbidity and the potential for downstream lifelong consequences from obstetric anal sphincter injuries our experience supports liberalizing Valsalva for women with cardiac disease.15,26
Limitations and Research Implications
Despite these strengths, our study is not without limitations. While the single-center design minimizes the impact of the provider, it limits our sample size and may limit our generalizability given the diversity in management styles for women with CVD. One theoretical benefit of cesarean delivery is the opportunity to plan delivery at times when resources and personnel are available. Our academic tertiary care center has the ability to mobilize resources to support the delivery of women with cardiovascular disease in pregnancy at any time. The role of induction of labor to support the safety of planned vaginal birth—particularly in centers with more limited resources—is an important direction for future research.
There are baseline obstetric differences between women in the two groups, but the indication for cesarean delivery in those undergoing both intrapartum and planned cesarean delivery mirror national indications.32 Women with decompensated cardiac status, such as concurrent myocardial infarction or severe pulmonary hypertension, were rare in our high-risk group. Collaborative studies examining the safety of planned vaginal birth for this uncommon but most challenging subgroup of patients are needed. Given the low rate of adverse cardiac outcomes in our cohort we are admittedly underpowered to detect a difference between groups. With a composite morbidity rate of 4.3% compared to 3.0% and a two-sided alpha of 0.05 a minimum of 1614 women would be required in each arm to have even 50% power to detect a difference in the rate of cardiac morbidity for women attempting vaginal birth. The prevalence of CVD in pregnancy may be a limitation for a well-designed, adequately powered, contemporary study.
Conclusions
Even after acknowledging these limitations, our descriptive analysis represents a meaningful step to fill the void of evidence addressing mode of delivery for women with cardiac disease. The low and similar rate of cardiovascular morbidity according to planned mode of delivery offers reassurance to mothers with CVD in pregnancy about the safety of planned vaginal birth without an obligatory operative vaginal delivery.11 Future studies examining the safety of Valsalva for high risk women and the relative contribution of cesarean delivery to long-term morbidity in women with cardiovascular disease are warranted.33–35 Our data provide evidence to support contemporary guidelines and reassure clinicians about the safety of attempted vaginal birth for women with CVD in pregnancy.
Supplementary Material
AJOG AT A GLANCE:
A. Why was this study conducted?
To compare cardiovascular and obstetric morbidity in women with cardiovascular disease (CVD) according to plan for vaginal birth or cesarean delivery.
B. What are the key findings?
Adverse cardiac outcomes were similar between women planning vaginal birth and those planning cesarean delivery (4.3% v. 3.0%, p=1). Rates of postpartum hemorrhage (1.9% v. 10.6%, p<0.01) and transfusion (1.9% v. 9.1%, p=0.01) were lower in the planned vaginal birth group. All women tolerated a trial of Valsalva without hemodynamic compromise or adverse cardiac events.
C. What does this study add to what is already known?
This prospective cohort study from a contemporary patient population managed by a multidisciplinary team with a standardized approach to care demonstrates the safety of attempted vaginal birth including a trial of Valsalva for women with cardiac disease in pregnancy.
ACKNOWLEDGMENTS
The authors wish to acknowledge the other members of the STORCC team for contributing to the acquisition of data: Laith Alshawabkeh, MD1; Nancy Barker, PA-C1; Yoni Buber, MD1; Jean Marie Carabuena, MD2; Matthew R. Carazo, MD1; Emily Dollar, BS1; Sheila Drakeley, BS1; Gabriele Egidy-Assenza, MD1; Julia Graf, BS1; Kimberlee Gauvreau, ScD1; Michelle Gurvitz, MD1; Daniel Halpern, MD; Amy Harmon, PhD1; Kelsey Hickey, BS1; Caitlyn Joyce, PA-C1;; Roisin Morgan, MD1; Mary Mullen, MD1;; Sara Partington, MD1; Dorothy Pearson, PA-C1; Saraubh Rajpal, MD1; Keri Shafer, MD1; Shailendra, Upadhyay, MD1; Fred Wu, MD1
Affiliations:
1Department of Cardiology, Boston Children’s Hospital; Department of Medicine, Division of Cardiology, Brigham and Women’s Hospital, Harvard Medical School, Boston, MA, USA
2Division of Obstetric Anesthesia, Brigham and Women’s Hospital, Harvard Medical School, Boston, Massachusetts, USA
Financial Support: This study was funded by the Brigham and Women’s Hospital Watkins Discovery Award and The Weinberg and Barton Family Fund.
Footnotes
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
Disclosure Statement: The authors report no conflict of interest.
Presentation: Presented as poster at the Society for Maternal-Fetal Medicine Annual Meeting, Dallas TX, January 29 to February 3, 2018.
CONDENSATION: Planned vaginal birth including a trial of Valsalva appears safe for women with cardiovascular disease in pregnancy with lower rates of morbidity than previously reported.
REFERENCES
- 1.Creanga AA, Berg CJ, Syverson C, Seed K, Bruce FC, Callaghan WM. Pregnancy-related mortality in the United States, 2006-2010. Obstet Gynecol. 2015. January;125(1):5–12. [DOI] [PubMed] [Google Scholar]
- 2.Thompson JL, Kuklina EV, Bateman BT, Callaghan WM, James AH, Grotegut CA. Medical and obstetric outcomes among pregnant women with congenital heart disease. Obstet Gynecol. 2015. August;126(2):346–54. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.European Society of Gynecology (ESG); Association for European Paediatric Cardiology (AEPC); German Society for Gender Medicine (DGesGM), Regizt-Zagrosek V, Blomstrom Lundqvist C, Borghi C, Cifkova R, Ferreira R, Foidart JM, Gibbs JS, Gohlke-Baerwolf C, Gorenek B, Iung B, Kirby M, Maas AH, Morais J, Nihoyannopoulos P, Pieper PG, Presbitero P, Roos-Hesselink JW, Schaufelberger M, Seeland U, Torracca L; ESC Committee for practice guidelines. ESC Guidelines on the management of cardiovascular diseases during pregnancy: The Task Force on the Management of Cardiovascular Diseases during Pregnancy of the European Society of Cardiology (ESC). Eur Heart J. 2011. December;32(24):3147–97. [DOI] [PubMed] [Google Scholar]
- 4.Balint OH, Siu SC, Mason J, Grewal J, Wald R, Oechslin EN, Kovacs B, Sermer M, Colman JM, Silversides CK. Cardiac outcomes after pregnancy in women with congenital heart disease. Heart. 2010. October;96(20):1656–61. [DOI] [PubMed] [Google Scholar]
- 5.Khairy P, Ouyang DW, Fernandes SM, Lee-Parritz A, Economy KE, Landzberg MJ. Pregnancy outcomes in women with congenital heart disease. Circulation. 2006. January 31;113(4):517–24. [DOI] [PubMed] [Google Scholar]
- 6.Opotowsky AR, Siddiqi OK, D’Souza B, Webb GD, Fernandes SM, Landzberg MJ. Maternal cardiovascular events during childbirth among women with congenital heart disease. Heart. 2012. January;98(2):145–51. [DOI] [PubMed] [Google Scholar]
- 7.Roos-Hesselink JW, Ruys TP, Stein JI, Thilen U, Webb GD, Niwa K, Kaemmerer H, Baumgartner H, Budts W, Maggioni AP, Tavazzi L, Taha N, Johnson MR, Hall R; ROPAC Investigators. Outcome of pregnancy in patients with structural or ischaemic heart disease: results of a registry of the European Society of Cardiology. Eur Heart J. 2013. March;34(9):657–65. [DOI] [PubMed] [Google Scholar]
- 8.Drenthen W, Boersma E, Balci A, Moons P, Roos-Hesselink JW, Mulder BJ, Vliegen HW, van Dijk AP, Voors AA, Yap SC, van Veldhuisen DJ, Pieper PG; ZAHARA Investigators. Predictors of pregnancy complications in women with congenital heart disease. Eur Heart J. 2010. September;31(17):2124–32. [DOI] [PubMed] [Google Scholar]
- 9.Silversides CK, Grewal J, Mason J, Sermer M, Kiess M, Rychel V, Wald RM, Colman JM, Siu SC. Pregnancy outcomes in women with heart disease: The CARPREG II Study. J Am Coll Cardiol. 2018. May 29;71(21):2419–30. [DOI] [PubMed] [Google Scholar]
- 10.Hrycyk J, Kaemmerer H, Nagdyman N, Hamann M, Schneider K, Kuschel B. Mode of delivery and pregnancy outcome in women with congenital heart disease. PLoS One. 2016. December 22;11(12):e0167820. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Canobbio MM, Warnes CA, Aboulhosn J, Connolly HM, Khanna A, Koos BJ, Mital S, Rose C, Silversides C, Stout K; American Heart Association Council on Cardiovascular and Stroke Nursing; Council on Clinical Cardiology; Council on Cardiovascular Disease in the Young; Council on Functional Genomics and Translational Biology; and Council on Quality of Care and Outcomes Research. Management of pregnancy in patients with complex congenital heart disease: A scientific statement for healthcare professionals from the American Heart Association. Circulation. 2017. February 21;135(8):e50–287. [DOI] [PubMed] [Google Scholar]
- 12.Regitz-Zagrosek V, Gohlke-Barwolf C, Iung B, Pieper PG. Management of cardiovascular diseases during pregnancy. Curr Probl Cardiol. 2014. Apr-May;39(4–5):85–151. [DOI] [PubMed] [Google Scholar]
- 13.Hayward RM, Foster E, Tseng ZH. Maternal and fetal outcomes of admission for delivery in women with cardiac disease. JAMA Cardiol. 2017. April 12 Epub ahead of print. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.D’Souza R, Sermer M, Silversides C. The management of the cardiac patient in labour: primum non nocere. BJOG. 2017. August;124(9):1310. [DOI] [PubMed] [Google Scholar]
- 15.Ouyang DW, Khairy P, Fernandes SM, Landzberg MJ, Economy KE. Obstetric outcomes in pregnant women with congenital heart disease. Int J Cardiol. 2010. October 8;144(2):195–9. [DOI] [PubMed] [Google Scholar]
- 16.Silver RM, Landon MB, Rouse DJ, Leveno KJ, Spong CY, Thom EA, et al. National Institute of Child Health and Human Development Maternal-Fetal Medicine Units Network. Maternal morbidity associated with multiple repeat cesarean deliveries. Obstet Gynecol. 2006; 107:1226–32. [DOI] [PubMed] [Google Scholar]
- 17.Blanchette Howard. The rising Cesarean delivery rate in America: What are the consequences? Obstet Gynecol 2011;118:687–690. [DOI] [PubMed] [Google Scholar]
- 18.Caughey AB, Cahill AG, Guise JM, Rouse DJ. Safe prevention of the primary cesarean delivery. Am J Obstet Gynecol. 2014. March;210(3):179–93. [DOI] [PubMed] [Google Scholar]
- 19.Lindquist SAI, Shah N, Overgaard C, Torp-Pedersen C, Glavind K, Larsen T, Plough A, Galvin G, Knudsen A. Association of previous cesarean delivery with surgical complications after a hysterectomy later in life. JAMA Surg. 2017. August 9 Epub ahead of print. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Liu S, Liston RM, Joseph KS, Heaman M, Sauve R, Kramer MS; Maternal Health Study Group of the Canadian Perinatal Surveillance System. Maternal mortality and severe morbidity associated with low-risk planned cesarean delivery versus planned vaginal delivery at term. CMAJ. 2007. February 13;176(4):455–60. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Menard MK, Main EK, Currigan SM. Executive summary of the revitalize initiative: standardizing obstetric data definitions. Obstet Gynecol. 2014. July;124(1):150–3. [DOI] [PubMed] [Google Scholar]
- 22.Bateman BT, Mhyre JM, Hernandez-Diaz S, Huybrechts KF, Fischer MA, Creanga AA, Callaghan WM, Gagne JJ. Development of a comorbidity index for use in obstetric patients. Obstet Gynecol. 2013. November;122(5):957–65. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Bailit JL, Grobman WA, Rice MM, Spong CY, Wapner RJ, Varner MW, Thorp JM, Leveno KJ, Caritis SN, Shubert PJ, Tita AT, Saade G, Sorokin Y, Rouse DJ, Blackwell SC, Tolosa JE, Van Dorsten JP. Risk-adjusted models for adverse obstetric outcomes and variation in risk-adjusted outcomes. Am J Obstet Gynecol. 2013. November;209(5):446.e1–446.e30. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.American College of Obstetricians and Gynecologists and the Society for Maternal-Fetal Medicine. Severe maternal morbidity: screening and review. Am J Obstet Gynecol. 2016. September;215(3):B17–22. [DOI] [PubMed] [Google Scholar]
- 25.American College of Obstetricians and Gynecologists. Practice Bulletin No. 165: Prevention and Management of Obstetric Lacerations at Vaginal Delivery. Obstet Gynecol. 2016. July;128(1):e1–215. [DOI] [PubMed] [Google Scholar]
- 26.Halle TK, Salvesen KA, Volloyhag I. Obstetric anal sphincter injury and incontinence 15–23 years after vaginal delivery. Acta Obstet Gynecol Scand. 2016. August;95(8):941–7. [DOI] [PubMed] [Google Scholar]
- 27.Ruys TP, Roos-Hesselink JW, Hall R, Subirana-Domenech MT, Grando-Ting J, Estensen M, Crepaz R, Fesslova V, Gurvitz M, De Backer J, Johnson MR, Pieper PG. Heart failure in pregnant women with cardiac disease: data from the ROPAC. Heart. 2014. February;100(3):231–8. [DOI] [PubMed] [Google Scholar]
- 28.Ruys TP, Roos-Hesselink JW, Pijuan-Domenech A, Vasario E, Gaisin IR, Iung B, Freeman LJ, Gordon EP, Pieper PG, Hall R, Boersma E, Johnson MR; ROPAC investigators. Is a planned caesarean section in women with cardiac disease beneficial? Heart. 2015. April;101(7):530–6. [DOI] [PubMed] [Google Scholar]
- 29.Kuklina E, Callaghan W. Chronic heart disease and severe obstetric morbidity among hospitalisations in the USA: 1995–2006. BJOG. 2011. February;118(3):345–52. [DOI] [PubMed] [Google Scholar]
- 30.Briller J, Koch AR, Geller SE. Maternal Cardiovascular Mortality in Illinois, 2002–2011. Obstet Gynecol. 2017. May;129(5):819–26. [DOI] [PubMed] [Google Scholar]
- 31.American College of Obstetricians and Gynecologists and Society for Maternal-Fetal Medicine, Menard MK, Kilpatrick S, Saade G, Hollier LM, Joseph GF Jr, Barfield W, Callaghan W, Jennings J, Conry J. Levels of maternal care. Am J Obstet Gynecol. 2015. March;212(3):259–71. [DOI] [PubMed] [Google Scholar]
- 32.Zhang J, Troendle J, Reddy UM, Laughon SK, Branch DW, Burkman R, Landy HJ, Hibbard JU, Haberman S, Ramirez MM, Bailit JL, Hoffman MK, Gregory KD, Gonzalez-Quintero VH, Kominiarek M, Learman LA, Hatjis CG, van Veldhuisen P; Consortion on Safe Labor. Contemporary cesarean delivery practice in the United States. Am J Obstet Gynecol. 2010. October;203(4):326.e1–326.e10. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Clark SL, Belfort MA, Dildy GA, Herbst MA, Meyers JA, Hankins GD. Maternal death in the 21st century: causes, prevention, and relationship to cesarean delivery. Am J Obstet Gynecol 2008;199:36.e1–36.35. [DOI] [PubMed] [Google Scholar]
- 34.Yee LM, Kaimal AJ, Houston KA, Wu E, Thiet MP, Nakagawa S, Caughey AB, Firouzian A, Kuppermann M. Mode of delivery preferences in a diverse population of pregnant women. Am J Obstet Gynecol. 2015. March;212(3):377.e–124. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Houston KA, Kaimal AJ, Nakagawa S, Gregorich SE, Yee LM, Kuppermann M. Mode of delivery and postpartum depression: the role of patient preferences. Am J Obstet Gynecol. 2015. February;212(2):299.e1–7. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.


