Highlights
-
•
No age effects in direction of speech lateralisation or handedness.
-
•
Greater between-hand performance differences evident in younger children.
-
•
Atypical speech laterality links to increased motor performance differences.
Keywords: Speech production, Lateralization, Transcranial Doppler, Motor control, Development
Abstract
Commonly displayed functional asymmetries such as hand dominance and hemispheric speech lateralisation are well researched in adults. However there is debate about when such functions become lateralised in the typically developing brain. This study examined whether patterns of speech laterality and hand dominance were related and whether they varied with age in typically developing children. 148 children aged 3–10 years performed an electronic pegboard task to determine hand dominance; a subset of 38 of these children also underwent functional Transcranial Doppler (fTCD) imaging to derive a lateralisation index (LI) for hemispheric activation during speech production using an animation description paradigm. There was no main effect of age in the speech laterality scores, however, younger children showed a greater difference in performance between their hands on the motor task. Furthermore, this between-hand performance difference significantly interacted with direction of speech laterality, with a smaller between-hand difference relating to increased left hemisphere activation. This data shows that both handedness and speech lateralisation appear relatively determined by age 3, but that atypical cerebral lateralisation is linked to greater performance differences in hand skill, irrespective of age. Results are discussed in terms of the common neural systems underpinning handedness and speech lateralisation.
1. Introduction
Functional asymmetries in hand skill and hemispheric speech lateralisation are well researched in adults. However, there is debate about when such functions become lateralised in the typically developing brain. The majority of adults demonstrate a typical pattern of right handedness and left hemispheric dominance for speech production (e.g. Knecht et al., 2000), but evidence for the neural development of motor skill and speech is more varied. Studies of language lateralisation in children show that speech is clearly lateralised to the left hemisphere at around 6 or 7 years of age (Groen et al., 2012, Gaillard et al., 2003) and evidence from neuroimaging of pre-verbal infants demonstrates an early left hemisphere dominance for processing of speech sounds (Dehaene-Lambertz et al., 2002). However it has also been suggested that younger children exhibit more bi-lateral activation during speech production compared to adults (e.g. Holland et al., 2001). Similarly, research has suggested hand preference in adulthood may be predicted from lateralized motor behaviour in early gestation, comparing ultrasound observation of thumb sucking (Hepper et al., 1991), and neonate palmar grasp reflex strength (Tan and Tan, 1999). However, varying observations of hand preference in early childhood reveal that no general consensus exists for when adult-like handedness occurs. Some studies indicate that direction of hand preference is attained by age 3 (e.g. Archer et al., 1988, McManus et al., 1988), with others reporting shifting hand usage and increased variability on manual tasks up until age 6, suggesting this is a more likely reflection of later handedness (Bryden et al., 2000).
There is evidence that task proficiency is related to increased laterality (Groen et al., 2012, Sheehan and Mills, 2008), suggesting that very young children, who are not yet competent in either speech or motor control, may display more varied patterns of hemispheric lateralisation for these functions. Current thinking proposes that whilst the direction of cerebral lateralisation for language and motor functions may be genetically predisposed, it is in fact a complex interaction of environmental and genetic factors which mediate the individual profile of cerebral lateralisation during development (e.g. Bishop, 2013). Therefore it is crucial to understand the extent to which an individual’s laterality profile changes through development. If lateralisation shifts with age and task proficiency then it suggests that the underlying functional and structural neural architecture may also be changing and shifting in this period and is therefore potentially vulnerable to factors affecting this developmental trajectory.
Few studies have examined speech lateralisation in children below age 6, predominately due to methodological difficulties in measuring language performance in pre-verbal children. Speech paradigms designed for adults tend not to produce a reliable enough stream of speech in children, either due to task difficulty, the requirement for literacy or complex instructions not easily understood, especially by very young children. However, notable recent exceptions have been able to demonstrate that typically developing 4 year old children show predominately left hemisphere lateralised speech (Bishop et al., 2014), and that no age effects in overall laterality profile could be found in preschool children aged between 1 and 5 years (Kohler et al., 2015). That study did however find an effect of age in variability of the lateralisation measurement, which became more reliable with age. An emerging methodology known as functional transcranial Doppler (fTCD) ultrasound has been shown to be effective in overcoming the issue of problematic measurement in children, as it is non-invasive and can be performed in relative comfort, unlike other neuro-imaging techniques. Furthermore, specific speech production paradigms have been developed which allow assessment of lateralisation in pre-literate children, and which have been validated against standard word generation paradigms used in adult language lateralisation research (e.g. Bishop et al., 2009).
Research into the use of handedness as an indirect measure for speech laterality has formerly proved weak and inconclusive (Groen et al., 2013), predominately due to the variability of methodologies, and hand preference and skill definitions being highly dependent on the measurement and classification used (Groen et al., 2013). However, speech and motor control are said to share a common developmental trajectory (Iverson, 2010), sub served by overlapping neural pathways predominantly situated in the left hemisphere (see Binkofski and Buccino, 2004). Converging evidence underlines the relationship between language and motor function. For example, it has been shown that brain regions typically associated with movement (pre-motor cortex, supplementary motor area and cerebellum) are also activated by language tasks (e.g. Tremblay and Gracco, 2009, Petersen et al., 1989) and that classic speech production areas (i.e. Broca’s area/Brodmann areas 44 and 45) show increased activation during the execution of sequenced hand movements (Erhard et al., 1996). In addition, individuals with aphasia (Pedelty, 1987) and children with specific language impairments (Hill, 2001) frequently display co-occurring motor deficits.
Flowers and Hudson (2013) propose that motor and speech laterality are related where they involve a common feature of motor output, namely the co-ordination of sequences of movements or utterances to execute a plan or intention so as to achieve a goal, either limb movement or expression of an idea (Grimme et al., 2011). This rationale has demonstrated that measures of performance based hand skill are better at revealing the underlying commonalities between the two functions, and thus are more effective at informing on their neurological relationship (Flowers and Hudson, 2013, Groen et al., 2013). The present study investigated the speech and motor lateralisation profiles of children aged 3–10 years to determine whether the two functions develop in parallel and, specifically, whether younger children would show more variable laterality across these functions. It focussed on a direct measure of language lateralisation (fTCD) and a handedness task (electronic pegboard) which relies on the same concept of motor sequencing suggested to underlie speech and motor action. Specifically the research questions posed were as follows: 1. does age affect motor skill performance on the pegboard task? 2. Do speech lateralisation profiles vary with age? 3. Can skilled motor performance predict direction of hemispheric speech laterality?
2. Method and materials
2.1. Participants
Participants were 153 children aged between 3yrs and 10yrs (74 males; mean age = 5.9yrs, SD age = 2.02yrs). All children were reported by parental report to be typically developing. Parents were asked to report any reading, language or motor impairments or concerns, as well as any developmental disorders such as Autism or ADHD; any children with such conditions were excluded. All participating children had normal, or corrected to normal, vision and none had a history of neurological injury or disease or were on medication known to affect the central nervous system, or cardiovascular system. All participants were British and had English as a first and only language; 4 of the 153 children tested were of African ethnicity, and the remaining 149 children were Caucasian, which is representative of the local population. Participants were recruited through local schools, parent/toddler groups and via the University of Lincoln's science outreach events. The investigation was approved by the ethics committee of the School of Psychology, University of Lincoln. Parental consent was obtained in writing at least 48 h prior to the testing session following acknowledge receipt of detailed study information sheets and briefing on the study via phone/email contact. Children were also required to assent to participation at the time of testing. Failure on behalf of the child to assent super ceded the parental consent, such that those children did not continue with the study. During testing participants were accompanied by a female experimenter sitting beside them to ensure they were happy to continue. Children were free to withdraw at any time without prejudice, and this right was clearly explained to them and they were asked to practise saying they wanted to stop. In addition, silence, lack of response, changes in demeanour and eye contact, were all taken as signs from the child of disinclination to continue, thus triggering the cessation of testing. Only one instance occurred of a child asking to withdraw before the testing had started.
2.2. Behavioural assessments
Participants completed a series of assessments to ascertain their levels of motor and language abilities.
2.2.1. Handedness assessment
All participants underwent assessments of their hand preference via completion of 5 manual tasks selected as reliable indicators of manual preference. The tasks were selected from a group of manual actions usually found on handedness questionnaires (e.g Flowers and Hudson, 2013, Annett, 2002). This approach was taken due to the range of ages in the sample, where it was considered a standard handedness inventory would be inappropriate due to the literacy skills required to complete such a questionnaire. Similarly reliance on self-reported writing hand was not considered a robust enough approach given the age range of the youngest participants.
The 5 manual tasks used to assess hand preference were as follows: 1. Underarm throw of a soft ball to the experimenter; 2. Eat with a spoon from a bowl of imaginary cereal; 3. Sharpen a pencil; 4. Unscrew a lid from a jar; 5. Draw a circle with a pencil. Each task was performed 3 times by the child and the hand used was recorded by the experimenter. The circle drawing task always went last, as research has shown the act of writing can influence subsequent hand use (Annett, 2002). The tasks were not demonstrated by the experimenter, only described verbally, to avoid direct copying. The number of items performed with each hand was calculated into a handedness quotient using the following formula:
| [(R-L)/(R + L)]*100, | (1) |
where positive values indicate right hand preference and negative values left handedness.
This 5-item hand preference scale appeared to have good internal consistency, α = 0.93. All items appeared worthy of retention in this measure, with the biggest increase in alpha coming from the deletion of the ‘lid unscrewing’ task, however removal of this item only increased alpha by 0.03.
2.2.2. Motor assessment
A sub set of 65 participants completed the Movement Assessment Battery of Children 2nd Edition (MABC-2; Henderson et al., 2007). This test battery assesses a range of gross and fine motor skills, including balance, dexterity and hand-eye coordination, and provides a standardised score of motor development. These scores can then be measured against sets of normalised performance scores which determine whether a child is typically developing in motor skills for their age. The MABC-2 was included to ensure all children met the criteria of having typical motor development for their age.
2.2.3. Vocabulary assessment
In addition to the motor assessments 83 of the participants also completed the British Picture Vocabulary Scale-II (BPVS-II; Dunn & Dunn, 2009) to assess language ability. The BPVS-II is a test of receptive vocabulary consisting of 168 items arranged in 14 sets of 12 items, each becoming progressively more difficult. The BPVS-II requires children to select which picture out of four possible options best fits the word read aloud by the experimenter. This test was selected as it has normalised scores for children aged 3 and above, and because it does not require reading and literacy skills to complete, both factors which suited our sample of participants. The BPVS-II produces a raw score, which, following conversion to a standardised score, can then be compared to normalised scores by age. Split-half analysis were used to assess internal consistency of the items presented and the results showed these items were highly reliable (r = 0.93, p < 0.001).
2.3. Experimental procedure
2.3.1. Motor skill assessment
To give a more accurate measure of hand skill and motor dexterity, all the participants carried out an electronic, 4 trial version of the pegboard task described by Flowers and Hudson (2013). Briefly, this consisted of a 280 × 100 × 20 mm board with two rows of 20 holes (7 mm diameter) drilled 13 mm apart along the length. The distance between the two lines of holes was 70 mm. The Fitts’ (1954) Index of Difficulty (Id) measurement for this board was Id = 7.6, making it unlikely that the task can be performed by pre-programmed aimed movements, and must involve some “online” movement control where handedness differences are most consistently found (Annett et al., 1979, Flowers and Hudson, 2013). To improve timing accuracy the board was constructed to allow detection of peg lifting and placing via an electrical circuit in the board. This was connected to the PC’s Parallel Port, where a Visual Basic programme continuously monitored and recorded the times at which pegs were removed from or inserted into the holes. Cloaked standard electrical fuses (6 mm diameter × 24 mm long) were used as pegs, the metal caps of which allowed conduction between the wire contacts when the pegs were inserted in the holes.
Pegs were moved either away from the body, that is, from the near row of holes to the far one (‘Out’ condition) or in reverse direction toward the body (‘In’ condition) on successive trials, which were ordered as follows: 1. Preferred Hand Out; 2. Non-Preferred Hand Out; 3. Non-Preferred Hand In; 4. Preferred Hand In. Scores on this task were also used to confirm hand preference as measured by the 5 item task.
2.3.2. Speech laterality
All children were invited to take part in the imaging section of the study, with deliberate focus on encouraging the left handed participants (as defined by the 5-item preference test) to increase the likelihood of recruiting atypically lateralised individuals. Thirty eight of the children (22 males; aged between 3 and 10 yrs, mean age = 6.5yrs, SD age = 1.92yrs) responded to this invitation and underwent functional transcranial Doppler (fTCD) imaging to determine their language lateralisation profile. Language lateralisation was determined by measuring hemispheric changes in cerebral blood flow volume (CBFV) with fTCD during an animation description task. The Animation description (AD) task was developed as an effective neuroimaging paradigm to elicit speech lateralisation in pre-literate children (Bishop et al., 2009). To date the paradigm has been used specifically within fTCD and it has been validated against the standard word generation paradigm used in adult participants to determine speech laterality. The paradigm is described in detail by Bishop et al. (2010). In brief, participants were seated in front of a computer screen with the fTCD headset fitted. Each trial consisted of a watch phase, a report phase and rest phase. Initially a silent animation was presented in the centre of a computer screen for 12 s, during this time participants were required to sit silently and watch; the ‘watch’ phase. At the onset of the trial a 500 ms epoch marker was simultaneously sent to the Doppler. Participants were then required to describe aloud details of the cartoon for 10 s; the ‘report’ phase. The trial concluded with the ‘rest’ phase, which was an 8 s period of relaxation to allow CBFV to return to baseline before the onset of the next trial. The AD paradigm consisted of 20 trials in total, each lasting 30 s. Animation presentation was randomised and none were presented more than once to any given participant. Although the clips do have a chronological sequence, the ‘story’ they tell is very basic, consisting mainly of characters playing/doing activities either inside or outside. Therefore randomisation of the clips would not cause confusion by putting events out of order; as there was no obvious sequential element. The ‘watch’ phase also served as the pre-speaking baseline period, following previous research showing no evidence of lateralised activation while participants passively watched these video clips (Bishop et al., 2009). The responses to each animation were audio recorded to enable subsequent analysis of fluency.
2.4. Data analysis
2.4.1. Pegboard performance
Performance on the electronic Pegboard task was measured by the speed with which the rows of pegs were completed. Mean movement times were calculated for the preferred and non-preferred hands, and a measurement of between-hand performance difference was calculated by subtracting the non-preferred hand mean time from the preferred hand mean time. To allow for more reliable comparison between individuals the between-hand difference measurement was transposed into an adapted version of the laterality quotient score, as described by Annett (2002). In this study the quotient score was derived to indicate the degree of relative hand skill on this task, rather than handedness direction, and was calculated by the following formula: [(preferred hand mean score − non preferred hand mean score)/(preferred hand mean score + non preferred hand mean score)]*100. Higher positive quotient scores indicate greater proficiency with the preferred hand, and higher negative quotient scores indicate grater proficiency with the non-preferred hand. Hand preference was used as opposed to right vs left as the hypothesis concerns the relative performance differences between the hands, and not the direction of preference per se (see Flowers and Hudson, 2013).
2.4.2. fTCD
Relative changes in CBFV within the left and right Middle Cerebral Arteries (MCAs) were assessed using bilateral fTCD monitoring from a commercially available system (DWL Doppler-Box™X: manufacturer, DWL Compumedics Germany GmbH). A 2-MHz transducer probe attached to an adjustable headset was positioned over each temporal acoustic window bilaterally. PsychoPy Software (Peirce, 2007) controlled the animation description experiment and sent marker pulses to the Doppler system to denote the onset of a trial. Data were analysed off-line with a MATLAB (Mathworks Inc., Sherborn, MA, USA) based software package called dopOSCCI (see Badcock et al., 2012 for a detailed description). DopOSCCI makes a number of computations in order to summarize the fTCD data and advance the validity of measuring hemispheric differences in CBFV. First, the numbers of samples were reduced by downsampling the data from ∼ 100 Hz to 25 Hz. Second, variations in cardiac cycle which may contaminate task-related signals were corrected using a cardiac cycle integration technique (Deppe et al., 1997). Third, data contaminated by movement or ‘drift’ were removed prior to normalisation, using the ‘activation rejection’ (whereby epochs with activation of less than 70% or greater than 130% of the average blood flow velocity were excluded from the analysis) and ‘epoch normalisation’ settings in dopOSCCI respectively (Badcock et al., 2012). Trials where the experimenter had noted occurrence of large body movements, talking during the baseline or coughing were manually excluded from the analysis. Normalised epochs were subsequently screened and excluded as measurement artefacts if activation values exceeded the acceptable range (± 40% mean CBFV). Fourth, to control for physiological processes that can influence CBFV (e.g. breathing rate; arousal), the mean activation of the baseline period (taken from the ‘watch’ phase, between 0–10s) was subtracted from each individual epoch. Deviations in left versus right activity were therefore baseline corrected and reflect relative changes in CBFV. A laterality index (LI) was derived for each participant based on the difference between left and right sided activity within a 2 s window. The activation window was centralised to the time point at which the left-right deviation was greatest within the period of interest (POI). In the present paradigm the POI was taken from the ‘report’ phase of the paradigm and ranged from 12 to 22 s following onset of the trial (Bishop et al., 2009).
Speech laterality was assumed to be clear in all cases in which the LI deviated by > 2 SE from 0 (Knecht et al., 2001; Hudson and Hodgson, 2016). Left-hemisphere or right-hemisphere speech dominance was indicated by positive or negative indices respectively. Cases with an LI < 2 SE from 0 were categorised as having bilateral speech representation. Individuals were categorised as having ‘Typical’ speech representation if they displayed a clear LI score which was positive, alternatively individuals with a bilateral LI score or a clear LI score which was negative were categorised as having ‘Atypical’ speech representation. Participants required a minimum of 10 acceptable trials from the total of 20 presented to be included in the analysis (i.e. 50%); all 38 participants reached this threshold, and 23 of them achieved 100% acceptable trials (mean number of trials accepted = 18.44; SD = 2.3).
To ensure high reliability within the LI scores derived from the speech task, split half reliability estimates were calculated from Pearson correlations of the odd and even epochs for each individual. For the group as a whole correlations indicate a high level of internal consistency between the readings (r = 0.62, p = 0.001), meaning that the fTCD measurements were reliable.
2.4.3. Statistical analysis
The research questions outlined in the introduction were assessed by a series of regression analyses. Firstly, to assess whether skilled motor performance changes with age, a multiple linear regression was conducted with the time difference (in s) between the non-preferred hand mean time and the preferred hand mean time derived from the pegboard trials as the dependent variable, and age (in years and whole months) and the behavioural assessment scores (MABC-2, BPVS-II and hand preference quotients derived from the 5-item task) as predictor variables. To probe the pegboard performance more closely a series of paired samples t-tests were used to determine differences between the 4 trials on the pegboard.
A second multiple linear regression was conducted on the sub-group of children who underwent fTCD during speech production, to assess whether speech laterality profile varied significantly with age and motor skill. The model treated speech LI as the dependent variable and had 6 predictor variables as follows: Age (in years and months); the behavioural assessment scores (MABC-2, BPVS-II and hand preference quotients derived from the 5-item task); the number of words produced during the speech task and mean between-hand difference scores from the pegboard trials. Bootstrapping was applied to this analysis to accommodate the increased number of predictor variables and the reduced sample size arising from the sub-group.
Finally a binary logistic regression was performed to test the assumption that atypical speech lateralisation could be predicted from motor performance, by treating laterality group (typical vs. atypical) as the dependent variable and mean between-hand difference scores from the pegboard trials as an independent predictor variable.
3. Results
3.1. Behavioural assessments
Table 1 shows the correlation matrix of the participant’s performance across each of the behavioural tests by age. This indicates that there were no significant relationships between age of the participants and any of the behavioural measures, meaning that participants were similarly matched for hand preference, motor and vocabulary ability; furthermore all participants fell within normal ranges for their age on these measures (see Table 2). Participants did not differ significantly on handedness quotients as derived from the 5-item preference task; there were 26 participants with a handedness quotient at or below zero, denoting left-handedness.
Table 1.
Pearson’s r values for the behavioural assessments across the whole sample (n = 148). * indicates p < 0.05; ** indicates p < 0.001.
| Age | Handedness Quotient | BPVS-II | MABC-2 | Pegboard PH mean | ||
|---|---|---|---|---|---|---|
| Whole Sample (n = 148) | Handedness Quotient | −0.01 | ||||
| BPVS-II | −0.09 | −0.08 | ||||
| MABC-2 | −0.07 | 0.07 | 0.23 | |||
| Pegboard PH mean | −0.83** | −0.06 | −0.06 | −0.12 | ||
| Pegboard NPH mean | −0.82** | −0.03 | −0.02 | −0.13 | 0.95** | |
| Speech Laterality sub-group (n = 38) | Handedness Quotient | −0.04 | ||||
| BPVS-II | 0.39* | −0.32 | ||||
| MABC-2 | −0.003 | −0.03 | 0.15 | |||
| Pegboard PH mean | −0.82** | −0.08 | −0.32 | −0.12 | ||
| Pegboard NPH mean | −0.87** | −0.01 | −0.41* | −0.15 | 0.91** | |
Table 2.
Performance scores on the behavioural assessments for the motor laterality analysis and the speech laterality sub-group.
| Motor Laterality (whole sample) | Speech Laterality (sub-group, n = 38) |
|||
|---|---|---|---|---|
| Atypical speech | Typical speech | |||
| n | 148 | 13 | 25 | |
| Sex: M:F | 73:75 | 7:6 | 15:10 | |
| Age: mean (SD) | 6.41 (2.05) | 7.1 (2.02) | 6.9 (1.9) | |
| 5-item task score (max = 5): mean (SD) | Right Left Either |
4.02 (1.77) 0.97 (1.76) 0.02 (0.14) |
3.54 (2.29) 1.5 (2.29) n/a |
4.4 (1.44) 0.6 (1.44) n/a |
| Handedness Quotient: mean (SE) | 61.1 (5.71) | 41.5 (25.4) | 76.0 (11.54) | |
| BPVS-II Score: mean (SD) | n = 83 | 98.3 (13.5) | 97.2 (10.9) | 96.5 (14.4) |
| MABC-2 Score: mean (SD) | n = 65 | 8.4 (1.8) | 7.8 (2.3) | 8.7 (1.7) |
| LI: mean (SE) | n/a | −2.01 (0.40) | 2.8 (1.9) | |
| Words produced: mean (SD) | n/a | 17.3 (4.3) | 14.5 (3.3) | |
3.2. Does age affect motor skill performance?
Data from 5 of the original 153 participants was incomplete, due to too few trials performed or failure to complete the task at all, meaning that adequate data was available for a total of 148 children. The excluded children were aged as follows: 2 × 3yrs, 1 × 4yrs, 1 × 5yrs and 1 × 8 yrs.
A multiple linear regression was conducted with mean between-hand difference score from the pegboard trials as the dependent variable. Age, BPVS-II score, MABC-2 score and handedness quotient derived from the 5-item preference test were all entered as predictor variables. Intercorrelations between the regression variables were reported in Table 1 and the regression statistics are in Table 3 a). The analysis revealed that Age was the only variable that contributed significantly to the regression model, and it accounted for 49% of the variation in between-hand difference scores on the pegboard. This indicates that younger children had greater performance differences between their preferred and non-preferred hands, whereas older children performed similarly with both of their hands.
Table 3.
Summary of regression analysis for variables predicting a) between-hand difference scores and b) speech lateralisation indices.
| (a) | |||
|---|---|---|---|
| B | SE B | β | |
| Constant | 15.34 | 4.93 | |
| Age | −1.13 | 0.32 | −0.49* |
| Handedness Quotient | −0.01 | 0.01 | −0.06 |
| BPVS-II score | −0.01 | 0.05 | −0.03 |
| MABC-2 score | −0.14 | 0.34 | −0.05 |
| Note: R2 =. 27 (ps < 0.001). | |||
| *p < 0.001. | |||
| (b) | |||
|---|---|---|---|
| B. | SE B. | β | |
| Constant | 7.26. | 2.36. | |
| Age | −0.35. | 0.26. | −0.26. |
| Handedness Quotient | 0.01. | 0.01. | .07. |
| Mean Words Produced | −0.15. | 0.13. | −0.22. |
| Between-hand difference | −0.31 | 0.11 | −0.48* |
| Note: Bootstrapping applied, R2 = 0.28 (ps < 0.01). | |||
| *p < 0.01. Excluded variables = MABC-2 scores and BPVS-II scores. | |||
Paired samples t-tests were used to determine differences in preferred and non-preferred hand performance on the pegboard across all participants irrespective of age; a significant difference was found, where the mean preferred hand (PH) movement times were lower, thus indicating faster performance, than non-preferred hand (NPH) movement times, t (147) = −14.49, p< 0.001 (PH mean time = 38.94s, SD = 11.1; NPH mean time = 44.13s, SD = 13.4, d = 0.42). T-tests revealed practise effects within the pegboard task, with later trials being performed significantly faster than earlier trials, t (147) = 4.76, p< 0.001 (Trial 1 mean time = 41.91s, SD = 14.3; Trial 4 mean time = 38.52s, SD = 11.9, d = .25, see Table 4).
Table 4.
Pegboard performance for preferred (PH) and non-preferred hands (NPH), classifed by hand preference category (derived from the 5-item task score), across the whole sample and for the speech laterality sub-group.
| Mean (SD) pegboard movement times per trial (seconds) | |||||
|---|---|---|---|---|---|
| Whole Group (n = 148) | n | 1st PH out | 2nd NPH out | 3rd NPH in | 4th PH in |
| Right-handed | 123 | 41.6 (14.4) | 42.4 (13.1) | 44.4 (13.3) | 38.1 (12.1) |
| Left-handed | 25 | 44.2 (13.5) | 43.6 (12.7) | 45.8 (17.6) | 40.5 (11.1) |
| p | 0.403 | 0.690 | 0.656 | 0.386 | |
| Speech Laterality sub-group (n = 38) | n | 1st PH out | 2nd NPH out | 3rd NPH in | 4th PH in |
| Right-handed | 31 | 36.6 (9.4) | 39.4 (9.9) | 43.3 (14.4) | 35.6 (10.7) |
| Left-handed | 7 | 42.2 (12.5) | 40.7 (14.0) | 43.5 (15.5) | 41.1 (16.4) |
| p | 0.194 | 0.774 | 0.980 | 0.272 | |
3.3. Do speech lateralisation profiles vary with age?
As expected, across the whole sample there was an overall bias towards activation in the left hemisphere during speech production, with the combined mean LI = 1.17 (SD = 2.59). Thirty seven of the participants had LIs > 2 SE from 0, denoting clear lateralisation. Twelve of the cases had a negative LI score denoting right hemisphere biased activation, and 25 cases had left hemisphere dominant activation. The remaining case was classed as bi-lateral due to LI < 2 SE from 0, (mean LI score = 0.56).
A bootstrapped multiple linear regression was conducted with mean speech lateralisation index as the dependent variable. Age, mean between-hand difference score, mean number of words produced, BPVS-II score, MABC-2 score and handedness quotient derived from the 5-item preference test were all entered as predictor variables. Intercorrelations between the regression variables were reported in Table 1 and the regression statistics are in Table 3 b). The analysis revealed that mean between-hand difference on the pegboard was the only variable that contributed significantly to the regression model, and it accounted for 48% of the variation in mean speech LI scores. Notably, age did not significantly predict speech LI score (see Fig. 3 ), indicating that younger children had a similar pattern of left hemispheric speech representation as older children.
Fig. 1.
Between-hand difference scores on the pegboard task by age for the whole sample, with regression line fitted.
Fig. 2.
Mean movement times for all pegboard trials combined, split by preferred hand (PH) and non-preferred hand (NPH), across participant age. The solid regression line represents the NPH fit and the dashed line is the pH fit.
Fig. 3.
Scatterplot of the mean speech lateralisation indices across age of the participants. The dotted line denotes the separation between left hemisphere lateralisation (denoted by positive LI values) and right hemisphere lateralisation (denoted by negative LI values).
3.4. Can motor skill performance predict speech lateralisation?
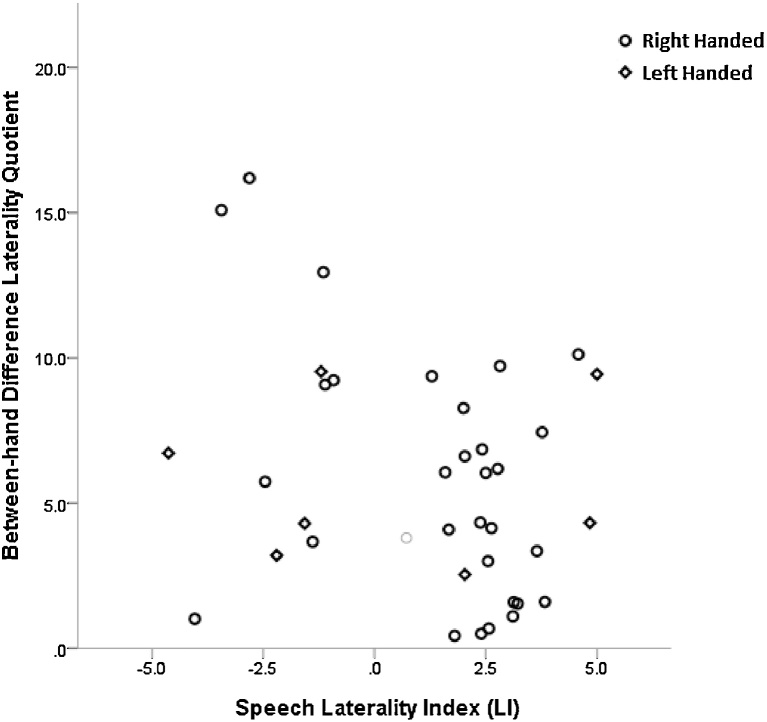
Finally, a suggestion from research into neurodevelopmental disorders affecting speech and motor control indicate that atypical hemispheric speech activation could be representative of an immature, or impaired, neural speech network (Hodgson and Hudson, 2016, Hsu and Bishop, 2014, Bishop, 2013). To examine whether atypical speech representation was reflected in the motor performance scores, the data was divided into two groups to represent; 1. Typical left hemisphere activation profiles and 2. Atypical activation profiles, denoted by right hemisphere or bi-lateral activation. Thirteen children were classed as having atypical lateralisation and 25 with typical. A stepwise binary logistic regression model was used to assess whether pegboard performance was an accurate predictor of speech laterality. Group (typical or atypical) was entered as the dependent variable and the independent predictor was the time difference (in s) between the non-preferred hand mean time and the preferred hand mean time derived from the pegboard trials. The model showed that between hand difference on the mean pegboard performance scores is a significant, albeit weak, indicator of speech lateralisation, R2 = 0.16 (Nagelkerke) [χ2 (1) = 4.61, Exp β = 1.171] (95% CI = 1.003–1.386, p < 0.05; see Fig. 4). This indicates that greater performance differences between the hands significantly predicts atypical speech lateralisation profiles.
Fig. 4.
Scatterplot of the between-hand difference laterality quotient scores across two classifications of speech laterality; typical and atypical. Higher hand laterality quotients reflect greater discrepancy in performance between the dominant and the non-dominant hands. The grey circle denotes the one bi-lateral case.
4. Discussion
4.1. Does speech lateralisation vary with age?
The aim of this study was to assess the speech and motor lateralisation profiles of children aged 3–10 years to determine whether the two functions developed in parallel and whether younger children would demonstrate more variable laterality. Results showed that mean speech lateralisation scores showed a significant leftwards bias across all ages tested, giving clear indication that speech lateralisation is biased to the left hemisphere by 3 years of age. This is in line with other recent neuroimaging data showing that even very young children display the expected pattern of left hemisphere language dominance (Bishop et al., 2014, Kohler et al., 2015). The data also revealed that hand preference was similarly well established by age 3, with all the children in this study showing a clear hand dominance effect on the 5-item preference score and the motor skill task. This provides confirmatory evidence, from a large sample, in line with previous research suggesting that direction of handedness is established early on in motor development (for review see Scharoun and Bryden, 2014).
4.2. Does age affect motor skill performance?
Motor performance was significantly affected by age, with younger children showing a stronger performance preference for their dominant hand on the pegboard task, a difference which narrowed during development. This finding is relatively rare, but has been observed previously in studies also using a pegboard paradigm (e.g. Kilshaw and Annett, 1983, Roy et al., 2003), and represents the developmental trajectory of bi-manual proficiency. It also demonstrates that a skill based performance measure is more sensitive to assessing handedness development, than inventories based preference tools. The motor skill data also indicates that the performance differences younger children display are mediated by the proficiency, or lack thereof, of the non-preferred hand (NPH). This is confirmed within our testing by the finding that children showed significantly longer latencies for pegboard trials requiring a change in direction when moving the NPH. This is something not seen in previous adult pegboard data (e.g. Flowers and Hudson, 2013), but is in accordance with previous evidence that children find it easier to perform away-from body manual actions, rather than those towards the body (e.g. Boessenkool et al., 1999). Evidence shows that specialist areas of the left hemisphere play a greater role in the control of complex, fine motor tasks for control of both the right and left hand. This ipsilateral control network for the left hand is in contrast to the typical contralateral cortico-motor control networks which govern motor actions (Serrien et al., 2006, Haarland et al., 2004). Therefore the finding that NPH proficiency underlies this difference in pegboard performance suggests that it is specifically the development of ipsilateral pathway, from left hemisphere to left hand, which is key to understanding the neural profile of motor skill development. This finding is in line with recent work showing that adults with developmental motor coordination impairments, such as Developmental Coordination Disorder, perform more poorly on fine motor tasks with their non-dominant hand (Debrabant et al., 2013, Hodgson and Hudson, 2016) and that apraxic patients with left hemisphere damage show deficits performing heterogeneous motor sequences. Taken together these findings indicate that the ipsilateral pathway controlling the non-dominant hand from the language dominant hemisphere (typically the left), may take longer to develop to functional maturity, and that individuals with deficits in motor coordination are actually displaying performance of an immature ipsilateral control pathway.
4.3. Can motor skill performance predict speech lateralisation?
A further key finding from this data was the relationship between direction of hemispheric speech representation and extent of performance difference between the hands, a finding which was independent of age or hand preference. Individuals who display atypical speech lateralisation show greater performance differences between their hands on the motor skill task. These results support the theory that action involving fine motor sequencing and speech production engage a common cognitive-motor neural network, and that these networks develop in parallel for the dominant hand/hemisphere mapping. The data indicates that this relationship between speech laterality and motor skill was irrespective of direction of hand preference, and thus given the left hemisphere’s specialism for sequential response ordering in both the left and right hands (Serrien and Sovijarvi-Spape, 2015, Kotz and Schwartze, 2016), it is possible to conclude that relative performance differences between the hands are more informative about the relationship between cerebral lateralisation and handedness, than is the direction of hand preference per se. Furthermore, this data suggest that the performance of non-dominant hand throughout development, and particularly whether this performance difference reduces with age, may be key to identifying those with atypical speech lateralisation, who are therefore potentially more likely to have difficulties with motor/language tasks. Although, it should be stressed that atypical lateralisation does not necessitate language/motor deficits, in fact little evidence exists to support this (Bishop, 2013), but rather that those who do have developmental difficulties may be detected through simple motor skill tasks. Causality cannot be inferred from this data, but the finding that atypical speech representation is linked to hand skill is in line with evidence from neurodevelopmental disorders showing atypical patterns of speech laterality in individuals with developmental motor coordination impairments (Hodgson and Hudson, 2016), indicating shifting functional organisation in speech networks as a result of impaired motor pathways.
4.4. Behavioural assessments
One unexpected outcome from this study was that performance on the behavioural assessments of vocabulary and motor ability did not correlate with pegboard performance or speech laterality. It has been shown previously that there are links between task proficiency and degree of lateralisation (Groen et al., 2012), but our data did not replicate this. A possible explanation for that failure is that the types of behavioural assessment we used (BPVS-II and MABC-2) lacked sensitivity to the particular functions we were assessing. The BPVS-II does not contain tasks or performance measurements related to sequencing or motor response timing, and so could easily be argued not to tap into the type of phonological processing. Furthermore, the test battery does not require a verbal response to be made, but merely provides a score of vocabulary ability based upon recognition only, nevertheless due to the age ranges in our sample, it was necessary to use an assessment tool which did not rely on literacy ability. Future work should investigate the component processes involved in speech production and measure relative lateralisation profiles across development. The lack of sensitivity in the MABC-2 was more surprising, as this test battery does indeed contain several tasks directly related to sequencing, motor timing and co-ordination, all components thought to form the basis of the speech-motor system (Kotz and Schwartze, 2016). However the scoring system employed by this battery makes it difficult to detect subtle and nuanced motor deficits as results are drawn from sub-sections of grouped tests, where some may have been performed well but others less well, resulting in an average score indicating typical development, but not an in-depth profile of differing aspects of motor development. However, as with the BPVS-II, the MABC-2 was useful in confirming typicality of our sample, although future work relating speech and motor ability should focus on a range of behavioural proficiency measures.
5. Conclusions
This study set out to answer three specific research questions regarding the effect of age on motor skill performance and speech lateralisation indices, as well as on the interaction between motor performance and speech lateralisation. The data showed that, 1) motor skill does vary with age, whereby younger children show significantly larger performance differences between their preferred and non-preferred hands; 2) Speech lateralisation indices do not vary with age in a sample of 3–10 year old typically developing children; 3) greater performance differences between the hands significantly predicts atypical speech lateralisation, regardless of participant age or hand preference.
In conclusion these data suggest that lateralisation of language and motor control is a process which begins very early in development, before the child is proficient at manual coordination or speech. Evidence from early lateralisation of auditory processing (Dehaene-Lambertz et al., 2002) may indicate the start of this hemispheric specialisation seen in later childhood; perhaps most critical is the period in which speech sound and motor output mappings are beginning to be formed and rehearsed. The specialisation of the left hemisphere for control of response sequences and timing integration also accounts for the patterns observed between speech laterality and motor performance (Serrien and Sovijarvi-Spape, 2015). Future work needs to focus on isolating the common components of the speech and motor tasks which may be driving this relationship and will also look at the performance of individuals with motor impairments.
References
- Annett J., Annett M., Hudson P.T.W., Turner A. The control of movement in the preferred and non-preferred hands. Q. J. Exp. Psychol. 1979;31:641–652. doi: 10.1080/14640747908400755. [DOI] [PubMed] [Google Scholar]
- Annett M. In: Handedness and Brain Asymmetry; the right shift theory. Hove E., editor. Psychology Press; Sussex: 2002. [Google Scholar]
- Archer L.A., Campbell D., Segalowitz S.J. A prospective study of hand preference and language development in 18 to 30-month olds: hand preference. Dev. Neuropsychol. 1988;4:85–92. [Google Scholar]
- Badcock N.A., Holt G., Holden A., Bishop D.V. dopOSCCI: A functional transcranial doppler ultrasonography summary suite for the assessment of cerebral lateralization of cognitive function. J. Neurosci. Methods. 2012;204(2):383–388. doi: 10.1016/j.jneumeth.2011.11.018. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Binkofski F., Buccino G. Motor functions of the Broca's region. Brain Lang. 2004;89:362–369. doi: 10.1016/S0093-934X(03)00358-4. [DOI] [PubMed] [Google Scholar]
- Bishop D., Watt H., Papadatou-Pastou M. An efficient and reliable method for measuring cerebral lateralization during speech with functional Transcranial Doppler ultrasound. Neuropsychologia. 2009;47:587–590. doi: 10.1016/j.neuropsychologia.2008.09.013. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bishop D., Badcock N., Holt G. Assessment of cerebral lateralisation in children using functional transcranial doppler ultrasound (fTCD) J. Vis. Exp. 2010;43:2161. doi: 10.3791/2161. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bishop D., Holt G., Whitehouse A., Groen M. No population bias to left-hemisphere language in 4-year-olds with language impairment. Peer J. 2014;2:e507. doi: 10.7717/peerj.507. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bishop D. Cerebral asymmetry and language development: cause, correlate, or consequence? Science. 2013;340:1230531. doi: 10.1126/science.1230531. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Boessenkool J.J., Nijhof E., Erkelens C.J. Variability and correlations in bi-manual pointing movements. Hum. Mov. Sci. 1999;41(4):525–552. [Google Scholar]
- Bryden P.J., Pryde K.M., Roy E.A. A developmental analysis of the relationship between hand preference and performance: II. A performance-based method of measuring hand preference in children. Brain Cognit. 2000;43:60–64. [PubMed] [Google Scholar]
- Debrabant J., Gheysen F., Caeyenberghs K., Van Waelvelde H., Vingerhoets G. Neural underpinnings of impaired predictive motor timing in children with Developmental Coordination Disorder. Res. Dev. Disabil. 2013;34:1478–1487. doi: 10.1016/j.ridd.2013.02.008. [DOI] [PubMed] [Google Scholar]
- Dehaene-Lambertz G., Dehaene S., Hertz-Pannier L. Functional neuroimaging of speech perception in infants. Science. 2002;298:2013–2015. doi: 10.1126/science.1077066. [DOI] [PubMed] [Google Scholar]
- Deppe M., Knecht S., Henningsen H., Ringelstein E. AVERAGE: A windows® program for automated analysis of event related cerebral blood flow. J. Neurosci. Methods. 1997;75(2):147–154. doi: 10.1016/s0165-0270(97)00067-8. [DOI] [PubMed] [Google Scholar]
- Dunn L., Dunn D. 3rd Ed. GL Assessment; London, UK: 2009. The British Picture Vocabulary Scale Manual. [Google Scholar]
- Erhard P., Kato T., Strupp J., Andersen P., Adriany G., Strick P., Ugurbil K. Functional mapping of motor in and near broca's area. Neuroimage. 1996;3(3):S367. [Google Scholar]
- Fitts P.M. The information capacity of the human motor system in controlling the amplitude of movement. J. Exp. Psychol. 1954;47:381–391. [PubMed] [Google Scholar]
- Flowers K., Hudson J. Motor laterality as an indicator of speech laterality. Neuropsychology. 2013;27:256–265. doi: 10.1037/a0031664. [DOI] [PubMed] [Google Scholar]
- Gaillard W., Sachs B.C., Whitnah J.R., Ahmad Z., Balsamo L.M., Petrella J.R. Developmental aspects of language processing: fMRI of verbal fluency in children and adults. Human Brain Mapp. 2003;18:176–185. doi: 10.1002/hbm.10091. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Grimme B., Fuchs S., Perrier P., Schöner G. Limb versus speech motor control: A conceptual review. Motor Control. 2011;15(1):5–33. doi: 10.1123/mcj.15.1.5. [DOI] [PubMed] [Google Scholar]
- Groen M., Whitehouse A., Badcock N., Bishop D. Does cerebral lateralization develop? A study using functional transcranial Doppler ultrasound assessing lateralization for language production and visuospatial memory. Brain Behav. 2012;2(3):256–269. doi: 10.1002/brb3.56. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Groen M., Whitehouse A., Badcock N., Bishop D. Associations between handedness and cerebral lateralisation for language: a comparison of three measures in children. PLoS One. 2013;8(5):e64876. doi: 10.1371/journal.pone.0064876. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Haarland K., Elsinger C., Mayer A., Durgerian S., Rao S. Motor sequence complexity and performing hand produce differential patterns of hemispheric lateralization. J. Cogn. Neurosci. 2004;16:621–636. doi: 10.1162/089892904323057344. [DOI] [PubMed] [Google Scholar]
- Hepper P.G., Shahidullah S., White R. Handedness in the human fetus. Neuropsychologia. 1991;29:1107–1111. doi: 10.1016/0028-3932(91)90080-r. [DOI] [PubMed] [Google Scholar]
- Henderson S.E., Sugden D.A., Barnett A.L. 2nd ed. Pearson Education Inc.; London, UK: 2007. The movement assessment battery for children (movement ABC-2) [Google Scholar]
- Hill E.L. Non-specific nature of specific language impairment: a review of the literature with regard to concomitant motor impairments. Int. J. Lang. Comm. Dis. 2001;36:149–171. doi: 10.1080/13682820010019874. [DOI] [PubMed] [Google Scholar]
- Hodgson J.C., Hudson J.M. Atypical speech lateralization in adults with developmental coordination disorder demonstrated using functional transcranial Doppler ultrasound. J. Neuropsychol. 2016 doi: 10.1111/jnp.12102. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Holland S., Plante E., Weber Byars A., Strawsburg R.H., Schmithorst V.J., Ball W.S. Jr. Normal fMRI brain activation patterns in children performing a verb generation task. NeuroImage. 2001;14:837–843. doi: 10.1006/nimg.2001.0875. [DOI] [PubMed] [Google Scholar]
- Hsu H.J., Bishop D.V. Sequence-specific procedural learning deficits in children with specific language impairment. Dev. Sci. 2014;17:352–365. doi: 10.1111/desc.12125. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Iverson J. Developing language in a developing body: the relationship between motor development and language development. J. Child Lang. 2010;37:229–261. doi: 10.1017/S0305000909990432. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kilshaw D., Annett M. Right- and left-hand skill: effects of age: sex and hand preference showing superior skill in left-handers. Br. J. Psychol. 1983;74:253–268. doi: 10.1111/j.2044-8295.1983.tb01861.x. [DOI] [PubMed] [Google Scholar]
- Knecht S., Deppe M., Dräger B., Bobe L., Lohmann H., Ringelstein E., Henningsen H. Language lateralization in healthy right-handers. Brain. 2000;123:74–81. doi: 10.1093/brain/123.1.74. [DOI] [PubMed] [Google Scholar]
- Knecht S., Dräger B., Flöel A., Lohmann H., Breitenstein C., Deppe M., Ringelstein E. Behavioural relevance of atypical language lateralization in healthy subjects. Brain. 2001;124(8):1657–1665. doi: 10.1093/brain/124.8.1657. [DOI] [PubMed] [Google Scholar]
- Kohler M., Keage H., Spooner R., Flitton A., Hofmann J., Churches O., Elliott S., Badcock N.A. Variability in lateralised blood flow response to language is associated with language development in children aged 1–5 years. Brain and Language. 2015;145-146:34–41. doi: 10.1016/j.bandl.2015.04.004. [DOI] [PubMed] [Google Scholar]
- Kotz S., Schwartze M. Motor timing and sequencing in speech production. In: Hickok G., Small S., editors. Neurobiology of Language. Elsevier; London: 2016. pp. 717–724. [Google Scholar]
- McManus I.C., Sik G., Cole D.R., Mellon A.F., Wong J., Kloss J. The development of handedness in children. Br. J. Dev. Psychol. 1988;6:257–273. [Google Scholar]
- Pedelty L.L. The University of Chicago; 1987. Gesture in Aphasia: Unpublished Doctoral Dissertation. [Google Scholar]
- Peirce J.W. PsychoPy—psychophysics software in python. J. Neurosci. Methods. 2007;162(1):8–13. doi: 10.1016/j.jneumeth.2006.11.017. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Petersen S.E., Fox P.T., Posner M.I., Mintun M., Raichle M.E. Positron emission tomographic studies of the processing of singe words. J. Cognit. Neurosci. 1989;1(2):153–170. doi: 10.1162/jocn.1989.1.2.153. [DOI] [PubMed] [Google Scholar]
- Roy E., Bryden P., Cavill S. Hand differences in pegboard performance through development. Brain Cogn. 2003;53:315–317. doi: 10.1016/s0278-2626(03)00133-7. [DOI] [PubMed] [Google Scholar]
- Scharoun S., Bryden P. Hand preference, performance abilities, and hand selection in children. Front. Psychol. 2014;5:82. doi: 10.3389/fpsyg.2014.00082. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Serrien D., Sovijarvi-Spape M. Hemispheric asymmetries and the control of motor sequences. Behav. Brain Res. 2015;283:30–36. doi: 10.1016/j.bbr.2015.01.021. [DOI] [PubMed] [Google Scholar]
- Serrien D., Ivry R., Swinnen S. Dynamics of hemispheric specialization and integration in the context of motor control. Nat. Rev. Neurosci. 2006;7:160–166. doi: 10.1038/nrn1849. [DOI] [PubMed] [Google Scholar]
- Sheehan E., Mills D. The effect of early word learning on brain development. In: Friederici A., Thierry G., editors. Early Language Development Bridging brain and behaviour. John Benjamins Publishing; Philadelphia: 2008. pp. 161–190. [Google Scholar]
- Tan U., Tan M. Incidence of asymmetries for the palmar grasp reflex in neonates and hand preference in adults. Neuroreport. 1999;10:3253–3256. doi: 10.1097/00001756-199911080-00001. [DOI] [PubMed] [Google Scholar]
- Tremblay P., Gracco V. Contribution of the pre-SMA to the production of words and non-speech oral motor gestures, as revealed by rTMS. Brain Res. 2009;1268:112–124. doi: 10.1016/j.brainres.2009.02.076. [DOI] [PubMed] [Google Scholar]