Abstract
Background:
There is a need for highly sensitive and specific tests and biomarkers that would allow preclinical diagnosis of mild cognitive impairment (MCI) and Alzheimer’s disease (AD), which would also enable timely intervention.
New method:
We have developed a new system (ALZENTIA) that helps detect early MCI, mainly caused by AD. The system is based on a hidden goal task (HGT) in which the human subject has to find a target that is not visible; as such, the navigation is based on a previously memorized target position, in relation to the starting position (egocentric variant) and/or other navigational landmarks (allocentric variant of the task). We present our preliminary results obtained in 33 patients with MCI and 91 healthy controls (HC).
Results and comparison with existing methods:
Between-group differences in the average error measured in allocentric, egocentric, and combined allocentric-egocentric subtests were statistically significant in MCI compared to HC. The high negative predictive values suggested high discriminative capacity and diagnostic potential for the HGT test as a tool to detect subjects in healthy population who will progress to MCI. Considering the low sensitivity of the Mini-Mental Status Examination and Montreal Cognitive Assessment tests, we believe that HGT can improve early identification of MCI patients who will progress to AD.
Conclusion:
The HGT carried out with the ALZENTIA system proved to be a reliable screening test to identify individuals with MCI from an aging cohort.
Keywords: Alzheimer’s disease, early diagnosis, hidden goal task, mild cognitive impairment, screening test, spatial orientation
1. Introduction
In patients with Alzheimer’s disease (AD), difficulties in spatial orientation and navigation first occur in unfamiliar environments, and leads, in parallel with the progression of the disease, to gradual disorientation in familiar environments as well. In the advanced stage of the disease, patients cannot manage in their own home environment either and become dependent on the care of others (Monacelli et al., 2003).
At least two interdependent systems, mediated by different parts of the cerebral cortex and subcortical structures, are responsible for orientation in space: the allocentric and egocentric orientation systems. The hippocampus is a center of the allocentric spatial orientation network (Burgess et al., 2002). Although the precise mechanisms of spatial orientation have not been fully elucidated yet, the hippocampus enables a cognitive map that contains representations of spatial orientation, interrelationships of objects in a surrounding environment, and distances from and among individual objects in the environment (O’Keefe and Nadel, 1978). As such, the structures of the medial part of the temporal lobe form a dynamic representation of one’s position within the environment (Aguirre and D’Esposito, 1999; Feigenbaum and Morris, 2004).
Egocentric orientation is mediated mainly by the inferior parietal and parahippocampal cerebral cortex, so damage to the posterior parietal cortex results in a neurological deficit, in terms of reduced ability to localize objects relative to the subject’s position (Bohbot et al., 1998; Weniger et al., 2010). In addition to the cerebral cortex, many participating subcortical areas, primarily the striatum, are active when performing egocentric orientation (Iaria et al., 2003). In sharp contrast to allocentric orientation, the position and direction information of a person or animal within the egocentric system are based mainly on vestibular and somatosensory information, and the distance and relationships among objects (personal egocentric space map) are then determined from these information and with respect to the eye-centered reference frame (Stěpánková et al., 2003; Filimon, 2015). While allocentric orientation depends on significant, prominent landmarks, the egocentric system involves sensory and motor representation of the whole body, head movement and gaze orientation, mental image of distance, and previous time spent on a certain path (Weniger et al., 2011). Thus, to find the right path in an environment, it is necessary to convert coordinate information from the allocentric map in the entorhinal cortex/hippocampus to the body-related egocentric representation in the posterior part of the parietal lobe of the right hemisphere that will then provide these information to the premotor cerebral cortex for locomotor-directed activity.
Knowledge of spatial orientation is based on studies in hippocampus-lesioned rodents using the Morris water-maze task (MWM) (Morris et al., 1982). Using landmarks located around the water tank, rats with an intact hippocampus quickly find and remember where the platform is immersed when performing the test. In a real-life version of the MWM task, patients with isolated lesions of the medial part of the temporal lobe were examined using sensors hidden under a carpet (Bobhot et al., 2002). Subjects were asked to find a sensor using a minimum of two landmarks located on the wall of the room. In this task, patients with damage to the right hippocampus could not remember the location of the sensor. A similar test was conducted on children in a pool full of plastic objects beneath which was a treasure box they needed to locate (Overman et al., 1996); a similar test was used to examine the spatial orientation of children of different ages (Lehnung et al., 1998). Specifically, testing was conducted in a round arena with the floor containing 20 magnetic motion detectors and light tags, some of which, when included, were the exact positions of the target to be found using landmarks (Lehnung et al., 1998). Significant reductions in spatial orientation in subjects with hippocampal or parietal lobe damage have been demonstrated in numerous virtual versions of the MWM task or similar computer-aided spatial orientation programs (Maguire et al., 1998; Astur et al., 2002; Parslow et al., 2005; Jheng and Pai, 2009).
Given the possibility of a more successful treatment in the earliest stages of the disease, diagnostic methods to identify mild cognitive impairment (MCI) that may progress to AD with the highest specificity and sensitivity possible are needed (Hampel et al., 2011). Unfortunately, the specificity and sensitivity of the existing biological markers for such a purpose rarely exceed 70-80% (Mondrego, 2006; Babić Leko et al., 2016; Kiđemet-Piskač et al., 2018). Spatial orientation tests that would detect early and subtle impairments in space orientation and navigation, are therefore among yet needed, but the most promising potential biomarkers of early AD (Coughlan et al., 2018). Such tests should also be able to differentiate AD from other primary causes of cognitive decline.
Real-life and virtual tests of spatial orientation have been used to test the ability of spatial orientation in healthy controls (HC) and patients with MCI and AD. Results compared with neuroimaging investigations of the structure and activity of the brain, as well as their association with genetic risk factors, revealed two mechanisms through which spatial orientation is impaired in patients with MCI and AD seem to emerge: a visual-perceptual mechanism, which is dependent on visual information perception and visual-spatial attention, and an impaired cognitive mapping mechanism (Vlček and Laczó, 2014; Howett et al., 2019). The problem of screening tools for identifying vulnerable people in population with MCI who will progress to AD has not been adequately addressed. We have designed a new apparatus based on the hidden-goal task (HGT) where the aim is to find a target that is not visible, but instead the navigation must be based on previously memorized target position in relation to the starting position or other navigation markers. This system consists of original software and hardware, and stems from the original efforts by the late Jan Bureš and his colleagues to develop a HGT test in which the human subject relies on a previously memorized target position, in relation to the starting position and/or other navigational landmarks (Hort et al., 2007). This system (ALZENTIA) is an integrated solution for all test-system steps in one place, which allows automatic setting, calibration, and sequence test management, including tracking and calculation orientation errors, as well as displaying and saving results. After designing the system, the aim of the present study was to assess the HGT as a potential screening tool for persons with MCI in an adult population.
2. Subjects and Methods
2.1. Subjects
The study included a total of 136 subjects (Table 1): 91 were HC, whereas the remaining 45 subjects were patients diagnosed with either MCI (n=33) or AD (n=8). All patients were recruited at the University Hospital Center Zagreb. All subjects were tested only after informed consent was signed by themselves or their caregivers. Patients’ cognitive status was tested using the Alzheimer’s Disease Assessment Scale-cognitive subscale (ADAS-Cog), Montreal Cognitive Assessment (MoCA), and Mini-Mental State Examination (MMSE). In addition to neuropsychological testing, complete blood tests (levels of folic acid – vitamin B9; vitamin B12; thyroid function test; serology for Lyme’s disease and syphilis) and a full neurological examination were performed. Dementia due to AD was diagnosed by using the National Institute on Aging – Alzheimer’s Association (NIA-AA) criteria of McKhann et al. (2011); for MCI diagnosis, the criteria of Petersen et al. (1999) and Albert et al. (2011) were used. FTD was diagnosed according to Neary et al. (1998). The standard MMSE score cut of 24 (23 or below) was used for classification of demented subjects (who were not analyzed in this study), whereas a score cut of 27 (26 or below) was used to classify MCI (O’Bryant et al., Arch Neurol., 2008), as we validated in the elderly Croatian population (Boban et al., 2012). We also used MoCA as it was developed to address the shortcomings of MMSE, particularly those in relation to MCI detection. Its score range is the same as the MMSE, but has additional, more complex tasks including executive function.
Table 1.
Demographic data and levels of errors and times measured in subtests of the HGT.
| MCI | HC | Age-matched controls (older than 52 years) | |||||
|---|---|---|---|---|---|---|---|
| Number of patients | 33 | 91 | 27 | ||||
| Gender | 26 F / 7 M | 66 F / 25 M | 19 F / 8 M | ||||
| Age (years) | 66.1 ± 6.9 | 67 (59 – 71) | 41.6 ± 16.8 | 39 (26 – 54) | 63.3 ± 8.32 | 65 (55 – 69) | |
| Mean ± SD | Median (25th -75th percentile) | Mean ± SD | Median (25th -75th percentile) | Mean ± SD | Median (25th -75th percentile) | ||
| Ego 1st attempt | Error (cm) | 67.96 ± 52.42 | 54.9(30.55 – 81.8) | 29.38 ± 25.29 | 21.1 (12.8 – 42.3) | 31.02 ± 32.01 | 21.6 (12.8 – 47.4) |
| Time (s) | 9.23 ± 5.78 | 10.86 (7.63 – 16.47) | 10.59 ± 5.77 | 8.91 (6.8 – 14.11) | 9.6 ± 4.97 | 7.81 (6.5 – 14.11) | |
| Ego average of last 4 attempts | Error (cm) | 44.76 ± 35.04 | 36.2 (21.6 – 52.7) | 22.14 ± 16.54 | 17.7 (13.2 – 26.2) | 23.83 ± 11.66 | 19 (15.9 – 31.4) |
| Time (s) | 8.13 ± 3.38 | 7.42 (5.23 – 9.88) | 8.92 ± 5.25 | 7.12 (5.74 – 9.58) | 10.07 ± 5.87 | 7.83 (6.63 – 11.74) | |
| Ego average of all 8 attempts | Error (cm) | 48.41 ± 28.67 | 43.4 (25.25 – 63.9) | 23.1 ± 13.69 | 20.6 (15.5 – 25.7) | 25.28 ± 11.47 | 21.5 (18.1 – 29) |
| Time (s) | 9.21 ± 3.3 | 8.18 (6.66 – 12.08) | 9.13 ± 4.19 | 8.03 (6.15 – 11.18) | 9.69 ± 4.36 | 8.03 (6.61 – 11.88) | |
| Allo 1st attempt | Error (cm) | 54.69 ± 52.89 | 29.6 (16.85 – 100) | 26.84 ± 30.56 | 18.4 (10.4 – 31.8) | 42.97 ± 47.46 | 24.7 (14.6 – 38.6) |
| Time (s) | 10.71 ± 6.73 | 8.4 (6.91 – 12.53) | 11.93 ± 10.15 | 8.6 (5.9 – 14.4) | 14.59 ± 12.36 | 8.71 (6.31 – 21.22) | |
| Allo average of last 4 attempts | Error (cm) | 44.65 ± 37.01 | 28.7 (19.45 – 49.55) | 22.87 ± 15.45 | 20.4 (12.3– 27.1) | 26.66 ± 16.78 | 21.5 (14.7 – 34.3) |
| Time (s) | 11.25 ± 5.69 | 9.34 (7.13 – 14.81) | 11.45 ± 5.82 | 10.56 (7.61 – 13.31) | 13.14 ± 7.58 | 11.76 (10.11 – 13.83) | |
| Allo average of all 8 attempts | Error (cm) | 46.39 ± 32.19 | 35.4 (24.5 – 52.95) | 26.14 ± 16.76 | 21.8 (16 – 33) | 30.86 ± 17.03 | 23.8 (18 – 41.8) |
| Time (s) | 12.01 ± 5.27 | 10.59 (7.92– 16.29) | 11.69 ± 5.49 | 10.29 (8.24 – 13.55) | 13.28 ± 6.77 | 11.51 (8.61 – 16.36) | |
| Combined Allo-Ego 1st attempt | Error (cm) | 61.14 ± 39.07 | 47.1 (29.3 – 91.55) | 30.57 ± 23.98 | 24.9 (15.9 – 40.70) | 30.33 ± 18.17 | 30.2 (14.6 – 44.4) |
| Time (s) | 13.22 ± 14.77 | 10.01 (7.74 – 13.88) | 12.52 ± 8.47 | 10.11 (7.6 – 15.41) | 12.02 ± 8.52 | 9.91 (7.4 – 14.3) | |
| Combined Allo-Ego average of last 4 attempts | Error (cm) | 44.35 ± 28.98 | 35.7 (22.95 – 57.3) | 24.12 ± 15.68 | 20.4 (15.5 – 27.8) | 24.4 ± 8.57 | 23.4 (17.7 – 30.1) |
| Time (s) | 10.85 ± 5.59 | 9.21 (7.36 – 12.89) | 10.51 ± 5.94 | 8.56 (6.83 – 12.46) | 10.07 ± 5.87 | 7.83 (6.63 – 11.74) | |
| Combined Allo-Ego average of all 8 attempts | Error (cm) | 48.61 ± 29.16 | 41 (25.8 – 62.45) | 25.91 ± 11.74 | 22.6 (18.3 – 28.9) | 26.19 ± 8.62 | 24.8 (19 – 31.1) |
| Time (s) | 11.69 ± 5.41 | 10.97 (7.81 – 13.25) | 10.98 ± 4.91 | 9.72 (7.5 – 12.99) | 9.69 ± 4.36 | 8.03 (6.61 – 11.88) | |
| Ego average of first 4 attempts | Error (cm) | 51.71 ± 28.85 | 41 (27.3 – 70.1) | 24.08 ± 13.57 | 21.4 (16.1 – 28.7) | 26.71 ± 13.16 | 24.2 (20 – 31.6) |
| Allo average of first 4 attempts | Error (cm) | 48.14 ± 31.16 | 35.6 (27.85 – 60.4) | 29.44 ± 24.64 | 22.9 (14.4 – 35.7) | 35.09 ± 22.66 | 27.9 (21.4 – 45.8) |
| Combined Allo-Ego average of first 4 attempts | Error (cm) | 53.17 ± 33.18 | 45.7 (26.65 – 79.25) | 27.67 ± 13.12 | 24.6 (19.1 – 31.7) | 28 ± 13.31 | 23.6 (18.1 – 32.4) |
| MMSE | 26.5 ± 2.40 | 27 (26 - 28) | 29.99 ± 0.105 | 30 (30 - 30) | 29.96 ± 0.19 | 30 (30 - 30) | |
| MoCA | 24.4 ± 4.36 | 24 (21 - 29) | 29.98 ± 0.147 | 30 (30 - 30) | 29.93 ± 0.27 | 30 (30 - 30) | |
MMSE, mini mental state examination; MoCA, Montreal cognitive assessment.
The inclusion criteria for the HC group were: age over 18 years; MMSE score greater than or equal to 28; corrected for age and number of years of formal education; MoCA sum greater than or equal to 26; corrected for number of formal education years, lack of objectively determined memory impairment or any other cognitive impairment; the absence of any neurological or psychiatric illness known to affect cognitive functioning; the absence of systemic disorders or malignancies; and non-medication for cognitive impairment. The exclusion criteria for subjects with MCI included unspecified dementia, pseudodementia, less than 24 points on MMSE due to AD, suspected mixed dementia as revealed by Hachinski’s ischemic score of 5 or 6, primary psychiatric disease, history or heteroanamnesis of chronic alcoholism, other neurological and endocrinological diseases that can lead to cognitive decline (hypothyroidism, deficiency of vitamins B12 and B9), secondary causes of cognitive impairment, systemic diseases, neurosyphilis, pharmacologic treatment of dementia, and symptoms of anxiety during testing.
2.2. Methods
The test room is located at the Croatian Institute for Brain Research, Zagreb. The testing arena comprises a closed, cylindrical apparatus, 2.9 m in diameter, surrounded by a 2.8 m high dark-blue, opaque curtain (Fig. 1). Eight numerical 7-segment (with an additional dot) light emitting diode (LED) displays were placed on the inside of the arena curtain, at intervals of 45°, at a height of 1.5 m. The subjects could not see the LEDs unless they were turned on. When turned on, the LEDs served as landmarks and consisted of either two horizontal or three vertical lines, or they were showing the starting position as a red dot. Eight laser light sources were pointing into the arena ceiling at 45° intervals, projecting on the floor in the shape of a 12 cm-large red circle representing the target for a period of 5 s. After the single laser light (single red circle) was extinguished, the subject had to determine the location of the target (“hidden object”) as accurately as possible. When the subject felt that they had found the target, they would mark it as closely (accurately) as possible by putting a specially constructed rod at the target location. As there was a LED installed at the bottom of the rod, a video camera placed in the center (“tip”) of the arena recorded the position of the LED, while another camera on the arena wall served the operator to monitor the movements of the subjects.
Figure 1.

Sketch of the testing arena. See text for details.
2.2.1. Hidden-goal test
The HGT was used for testing allocentric and egocentric spatial orientation. The testing procedure consisted of examining the spatial orientation of the subjects on the computer monitor first and then in the testing arena. Both the virtual and the real-life testing parts were comprised of four tests. The first three tests consisted of eight subtests, and the last test consisted of two subtests. The subject’s task was to find a previously displayed object (target; which was the red circle on the floor) that was only visible for a short time (5 s) and then “hidden” (disappeared). For orientation, the subject could only use their initial position (in egocentric testing) or two distal landmarks (in allocentric testing). During each subtest, the starting position, spatial landmarks, and hidden goal, occupied one of the eight possible predetermined positions, and were selected in a semi-random order, while maintaining the same mutual relationships.
The HGT consisted of four subgroups of the following tests: allocentric-egocentric (Allo-Ego), egocentric (Ego), allocentric (Allo), and Allocentric with Delay (Allo-D). In the first subtest, to find a target, subjects could use the relationship and distance of the target from the starting position, and the two distal landmarks presented (turned on) at all times during the test. In the second subtest, they could only use the starting position for this purpose, while the distal landmarks were not displayed. In the third subtest, only two distal landmarks assisted them in finding the target, while the location of the target was always independent of the starting position. The third and fourth subtests were equal, with the exception of a 30-min delay in the fourth. The virtual and real-life tests were run in alternation, one after the other, in the following manner: the first test was virtual Allo-Ego, then the real Allo-Ego, then the virtual Ego followed by the real Ego, then the virtual and the real Allo, and lastly, virtual and real Allo-D. Subtests of the HGT are listed in the first column of all Tables (1–9). Our original calibrated and tested software, written in in Java (v.8), measured the distance between the actual target and the location of the LED mounted on the rod, and based on this information measured the value of the error made. Subjects were always first shown the correct position of the target at the beginning of the first virtual Allo-Ego subtest, and in each subsequent subtest they were shown once again after the subject had already indicated the location that they thought the target should be located on (and left the freestanding rod on the floor). The computer recorded not only the value of the measured error for each subtest, but also its duration and the entire trajectory of the subject during the test (video stream of the LED on rod movement). However, in this study, we focused mainly on the value of the measured error itself, which we used as the main variable to analyze the accuracy of spatial orientation.
Table 9.
ROC curve analysis between MCI and HC group, adjusted for age and sex.
| MCI vs HC | |||||||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Prev. 5% | Prev. 10% | Prev. 16% | Prev. 20% | Prev. 36.7% | |||||||||||
| Sensitivity | Specificity | Cut-off | AUC, p | PPV | NPV | PPV | NPV | PPV | NPV | PPV | NPV | PPV | NPV | ||
| Ego 1st attempt | Error | 86.7% | 63% | 29.3 cm | 0.805, p=0.001* | 11% | 98.9% | 20.7% | 97.7% | 30.9% | 96.1% | 36.9% | 95% | 57.6% | 89.1% |
| Ego average of last 4 attempts | Error | 100% | 66.7% | 22.65 cm | 0.873, p<0.001* | 13.6% | 100% | 25% | 100% | 36.4% | 100% | 42.9% | 100% | 63.5% | 100% |
| Ego average of all 8 attempts | Error | 93.3% | 77.8% | 29.65 cm | 0.909, p<0.001* | 18.1% | 99.5% | 31.8% | 99.1% | 44.5% | 98.4% | 51.2% | 97.9% | 70.9% | 95.2% |
| Allo 1st attempt | Error | 66.7% | 77.8% | 42 cm | 0.670, p=0.070 | 13.7% | 97.8% | 25% | 95.5% | 36.4% | 92.5% | 42.9% | 90.3% | 63.5% | 80.1% |
| Allo average of last 4 attempts | Error | 66.7% | 81.5% | 37.05 cm | 0.775, p=0.003* | 15.9% | 97.9% | 28.6% | 95.7% | 40.7% | 92.8% | 47.4% | 90.7% | 67.6% | 80.8% |
| Allo average of all 8 attempts | Error | 86.7% | 63% | 33.9 cm | 0.773, p=0.004* | 11% | 98.9% | 20.7% | 97.7% | 30.9% | 96.1% | 36.9% | 95% | 57.6% | 89.1% |
| Combined Allo-Ego 1st attempt | Error | 60% | 96.3% | 68.4 cm | 0.849, p<0.001* | 46% | 97.9% | 64.3% | 95.6% | 75.5% | 92.7% | 80.2% | 90.6% | 90.4% | 80.6% |
| Combined Allo-Ego average of last 4 attempts | Error | 80% | 88.9% | 35.6 cm | 0.914, p<0.001* | 27.5% | 98.8% | 44.5% | 97.6% | 57.9% | 95.9% | 64.3% | 94.7% | 80.7% | 88.5% |
| Combined Allo-Ego average of all 8 attempts | Error | 73.3% | 96.3% | 40.75 cm | 0.902, p<0.001* | 51% | 98.6% | 68.8% | 97% | 79.1% | 95% | 83.2% | 93.5% | 92% | 86.2% |
| Ego average of first 4 attempts | Error | 93.3% | 88.9% | 35.35 cm | 0.904, p<0.001* | 30.7% | 99.6% | 48.3% | 99.2% | 61.6% | 98.6% | 67.8% | 98.2% | 83% | 95.8% |
| Allo average of first 4 attempts | Error | 66.7% | 77.8% | 46.55 cm | 0.753, p=0.007* | 13.7% | 97.8% | 25% | 95.5% | 36.4% | 92.5% | 42.9% | 90.3% | 63.5% | 80.1% |
| Combined Allo-Ego average of first 4 attempts | Error | 66.7% | 96.3% | 54.8 cm | 0.847, p<0.001* | 48.7% | 98.2% | 66.7% | 96.3% | 77.4% | 93.8% | 81.8% | 92% | 91.3% | 83.3% |
Allo, allocentric; AUC, area under the curve; Ego, egocentric; HC, healthy control; MCI, mild cognitive impairment; NPV, negative predictive value; PPV, positive predictive value; Prev, prevalence.
Testing always started first with a detailed verbal explanation of the test procedure itself, which was followed by giving instructions on the monitor. In this way, each subject was introduced to the method and goal of the test. While giving instructions, the arena layout was displayed with the starting position (full red dot), landmarks (two vertical and three horizontal lines) and the target (purple circle), which was useful because it helped the examiners (D.B. and M.B.R.) to give instructions in an unambiguous way (Fig. 2A). A large white circle on a black background represented the floor plan of the arena. The white circle was rotated counterclockwise (with all elements) during the instruction time, in order to provide the subjects with the understanding that there would be changes in the starting position, landmarks activated, and target, during the test (Fig. 2B). Importantly, it was always explained (and confirmed that the subject had understood) that in case of changes in the starting position, landmarks and goal in the arena, their mutual relationships may remain the same, but the goal goal will always be at the same distance from the starting position and landmarks.
Figure 2.
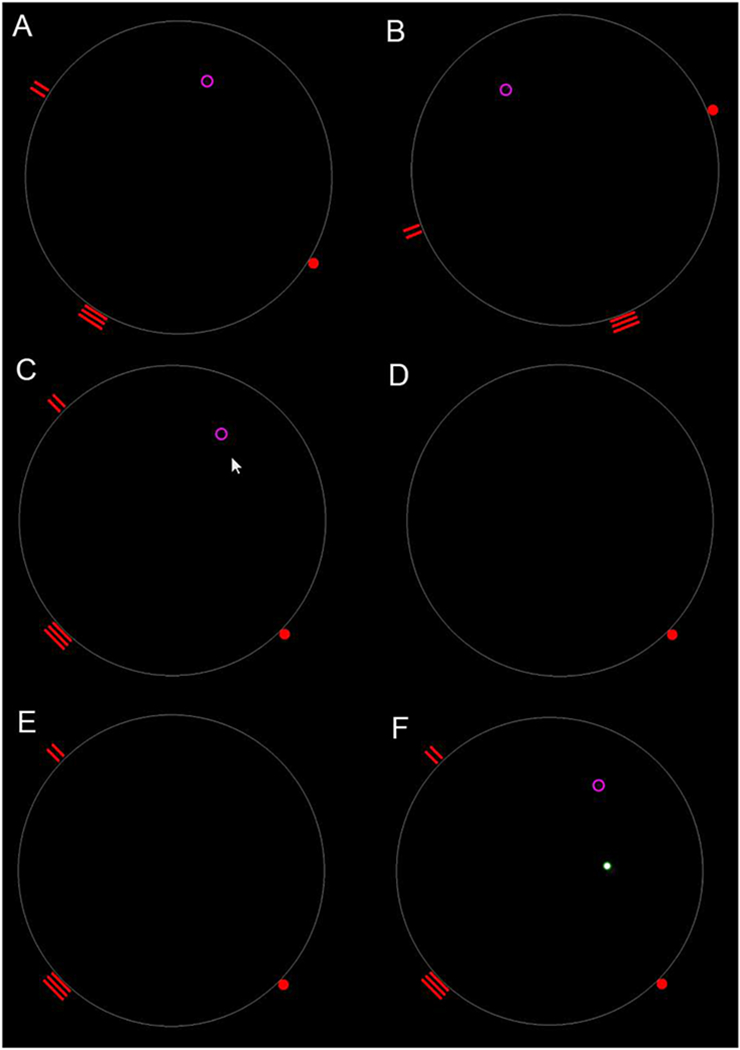
Schematic rendering of the HGT. Each test was first explained and tested on the monitor, and then in the testing arena. See text for details.
After giving instructions, testing began, and the eight subtests were always carried out in the following same sequence. The Allo-Ego orientation test on the computer monitor (Test 1) was conducted in such a way that the subject had to use the starting position and landmarks to move and click the mouse cursor as close as possible to where the target had previously been shown (Fig. 2C). The subject was shown a target at the beginning of the test and it was explained to them that they should remember this position of the target using the starting position and available landmarks. Then both the target and the landmarks were hidden (Fig. 2D). Then the subject had to position the cursor at the starting position, after which the landmarks were shown (Fig. 2E). The subject had thus to move the mouse cursor to mark the position of the previously displayed target by a single left-mouse click (Fig. 2F – full white dot). The examiner then showed the subject the exact position of the target, with a mandatory note to look at how far from the target the subject was, and to look again and remember the exact position of the target (Fig. 2F – purple circle). After the subject had looked at the original position of the target picture shown, the existing display was turned off and a new starting position was lit. The Allo-Ego test on the monitor was repeated in the same manner for seven more times (eight times altogether), followed by the actual (real-life, not virtual) Allo-Ego testing in the arena, again for eight times. The average duration of the test was approximately 25 min. per subject.
For the Allo-Ego orientation testing in the testing arena, a separate code in Java v.8 was made to control the LED displays and 8 laser lights. The examiner did this by monitoring the position of the subject in the arena on the computer screen, i.e. recording the distance between the rod marked and the target given. The upper part of the examiner’s computer monitor included the “Object tracking video stream” that was used to show the actual position of the target and the LED on the rod, by which the subject marked the memorized target. Both positions were shown as green circles with corresponding coordinates inside the arena. The bottom part of the examiner’s computer monitor contained an infrared (IR) video stream, so that the position of subjects in the arena could be monitored using an infrared camera, and also the LED on the rod, which was shown as the white light. The position of the illuminated landmarks and the starting position on the LED screens and the laser lights, by which the target was displayed, was controlled by the examiner from the outside of the testing arena, and according to a predetermined, well-defined scheme, i.e. always according to the same half-random layout that matched that of the virtual part of the test on the monitor. The coordinates of the eight positions of the target were memorized in the program. After the testing, the program calculated the error value, i.e. the distance from the coordinate stored with the one that the subject marked by the deposited rod with LED.
The Allo-Ego orientation test in the arena (Test 2), was conducted in such a way that the subject, using the starting position and landmarks, should place the rod on a stand as close as possible to where the object or “target” (which was a red laser light, 10-cm in diameter circle, on the floor) was located. Before the subject entered the arena, the program half-randomly adjusted the starting position and two landmarks (one with three horizontal lines and one with two vertical lines). The subject was then told to enter the arena by carrying a stick with a LED in his hand and to position their back to the LED screen, showing the red dot on one of the displays (the start position sign). The subject was then shown a red circle target on the arena floor for 5 s, after which it was extinguished. The subject was then instructed by the examiner (from outside of the arena) to mark the location of the target as accurately as possible with a free-standing rod (stick). At the moment when the subject started to mark the target, a program for tracking the path and time required for the subject to find the target was started. After the subject put the stick on the floor, i.e. marked the target, a laser light with stimulus was displayed, showing the target in the form of a red circle at the original position on the floor of the arena. The subject was told to look at how wrong they were in marking the goal. The remaining seven subtests of the Allo-Ego test were then conducted in the same manner (altogether, eight times for every subtest).
The Ego orientation test on the computer monitor (Test 3) was conducted in such a way that the subject, from their starting position, had to position the computer cursor as accurately as possible to the location of the hidden goal (target) without any landmarks. The rest of the testing was conducted in the same manner as the Allo-Ego test.
The Ego orientation test in the arena (Test 4) was conducted in such a way that the subject, using only the starting position (without any landmarks), had to place the free-standing rod (stick) at the location of the hidden goal (target).
The Allo orientation test on the computer monitor (Test 5) was conducted in such a way that the subject was required to point the location of the hidden goal (target), with the computer (mouse) cursor with the help of two landmarks, but without using the starting position for orientation. Unlike the previous tests, in this and the following tests, the interrelations between the start, target, and spatial landmarks between the different subtests were not preserved. In other words, the relationship between the goal and the spatial landmarks was preserved, but not the starting position. The rest of the testing was the same as previously stated in the Allo-Ego and Ego tests.
The Allo orientation test in the arena (Test 6) was conducted in such a way that the subject was required to find the location of the target shown, with the help of the landmarks only, as the starting position was not visible (the red spot on one of the displays).
The Delay Orientation Test (Tests 7 and 8) was conducted 30 min after the completion of Test 6. These two tests were conducted in the same manner as Tests 5 and 6, except that they consisted of two repeats, instead of eight, and, after determining the position of the target, the original stimulus was not displayed.
Altogether, the testing time has average duration of only about 20-25 minutes. This includes giving instructions to a subject, testing, and interpreting the results.
2.2.2. Statistical analysis
All results obtained were first analyzed by descriptive statistics. Clinical parameters (MMSE, patient age) are shown as mean ± standard deviation (SD). The main variables analyzed were the measured errors and times elapsed, from the starting position to the target obtained, during the three different HGTs (Allo-Ego, Ego, and Allo). As each of the three tests consisted of eight repeats, the statistical analysis was performed on the errors and times measured on the first attempt, average value of the first four attempts, average value of the last four attempts, and average values obtained in all eight attempts.
The Mann-Whitney test was used for comparison of errors and times between the two different groups of subjects (MCI group and HC group). Values of errors and times measured in each subtest were correlated using Pearson and Spearman correlations. Diagnostic sensitivities, specificities, and cut-off values, were measured by the analysis of Receiver Operating Characteristic (ROC) Area Under Curve (AUC). The best cut-off values were determined when the sum of sensitivity and specificity was maximized. Cut-off values were also determined when sensitivity was set at 90%. PPV (positive predictive value) and NPV (negative predictive value) were determined using information on sensitivities and specificities, with prevalence set at: 5%, 10%, 16%, 20%, and 36.7%. All statistical analyses were performed in SPSS 19.0.1 (SPSS Inc., Chicago, IL, USA), with statistical significance set at α = 0.05.
3. Results
Errors and times measured in Allo, Ego and combined Allo-Ego subtests of HGT for MCI and HC are shown in Table 1. The 8 AD subjects could not handle the testing and only one of them finished all 8 subtests (with extremely low scores). As these and other AD subject were clearly demented, they were not included in further testing using HGT.
3.1. Comparison of errors and times measured in Allo, Ego, and combined Allo-Ego subtests of tent test between MCI and HC subjects
There were significant differences in errors and times measured in Allo, Ego, and combined Allo-Ego subtests of tent test between the MCI patients and all the HC subjects, as well as between MCI patients and age-matched HC subjects (Table 2).
Table 2.
Comparison of errors and times measured in subtests of HGT between MCI and HC subjects.
| MCI vs all HC | MCI vs age-matched HC | ||
|---|---|---|---|
| Ego 1st attempt | Error | U=623; Z=−4.967; p<0.001* | U=188.5; Z=−3.819; p<0.001* |
| Time | U=1217; Z=−1.378; p=0.168 | U=329; Z=−1.567; p=0.117 | |
| Ego average of last 4 attempts | Error | U=671.5; Z=−4.693; p<0.001* | U=230.5; Z=−3.195; p=0.001* |
| Time | U=1426; Z=−0.173; p=0.863 | U=355.5; Z=−1.164; p=0.244 | |
| Ego average of all 8 attempts | Error | U=540.5; Z=−5.434; p<0.001* | U=196; Z=−3.707; p<0.001* |
| Time | U=1345; Z=−0.640; p=0.522 | U=429.5; Z=−0.038; p=0.970 | |
| Allo 1st attempt | Error | U=970; Z=−3.005; p=0.003* | U=384.5; Z=−0.906; p=0.365 |
| Time | U=1412; Z=−0.254; p=0.800 | U=405; Z=−0.411; p=0.681 | |
| Allo average of last 4 attempts | Error | U=869.5; Z=−3.573; p<0.001* | U=307; Z=−2.058; p=0.040* |
| Time | U=1412.5; Z=−0.251; p=0.802 | U=351.5; Z=−1.225; p=0.221 | |
| Allo average of all 8 attempts | Error | U=800; Z=−3.966; p<0.001* | U=308; Z=−2.043; p=0.041* |
| Time | U=1397; Z=−0.340; p=0.734 | U=385; Z=−0.715; p=0.475 | |
| Combined Allo-Ego 1st attempt | Error | U=730; Z=−4.362; p<0.001* | U=234; Z=−3.143; p=0.002* |
| Time | U=1399; Z=−0.329; p=0.742 | U=428; Z=−0.061; p=0.951 | |
| Combined Allo-Ego average of last 4 attempts | Error | U=702; Z=−4.521; p<0.001* | U=232; Z=−3.172; p=0.002* |
| Time | U=1317; Z=−0.801; p=0.423 | U=379; Z=−0.806; p=0.420 | |
| Combined Allo-Ego average of all 8 attempts | Error | U=685; Z=−4.617; p<0.001* | U=211.5; Z=−3.477; p=0.001* |
| Time | U=1276.5; Z=−1.035; p=0.301 | U=416; Z=−0.243; p=0.808 | |
| Ego average of first 4 attempts | Error | U=569.5; Z=−5.270; p<0.001* | U=200.5; Z=−3.641; p<0.001* |
| Allo average of first 4 attempts | Error | U=813.5; Z=−3.890; p<0.001* | U=314; Z=−1.954; p=0.051 |
| Combined Allo-Ego average of first 4 attempts | Error | U=783; Z=−4.063; p<0.001* | U=233; Z=−3.158; p=0.002* |
Allo, allocentric; Ego, egocentric; MCI, mild cognitive impairment.
p<0.05
Significant correlations between errors and times were revealed for Ego (first attempt), Ego (average of last 4 attempts), Allo (first attempt), combined Allo-Ego (last 4 attempts) and combined Allo-Ego (average of all 8 attempts) subtests (Table 3; Figure 3).
Table 3.
Correlation between errors and times measured in subtests of the HGT.
| Error vs time | |||
|---|---|---|---|
| Control | MCI | Control + MCI | |
| Ego 1st attempt | rs=0.174; df=89; p=0.098 | rs=0.231; df=30; p=0.203 | rs=0.229; df=121; p=0.011* |
| r=0.268; df=89; p=0.010* | r=0.158; df=30; p=0.389 | r=0.234; df=121; p=0.009* | |
| Ego average of last 4 attempts | rs=0.099; df=89; p=0.351 | rs=0.185; df=30; p=0.311 | rs=0.122; df=121; p=0.180 |
| r=0.292; df=89; p=0.005* | r=−0.007; df=30; p=0.969 | r=0.149; df=121; p=0.100 | |
| Ego average of all 8 attempts | rs=0.066; df=89; p=0.536 | rs=0.136; df=30; p=0.457 | rs=0.152; df=121; p=0.094 |
| r=0.322; df=89; p=0.002* | r=−0.009; df=30; p=0.959 | r=0.175; df=121; p=0.053 | |
| Allo 1st attempt | rs=0.115; df=89; p=0.276 | rs=0.237; df=30; p=0.191 | rs=0.156; df=121; p=0.085 |
| r=0.229; df=89; p=0.029* | r=0.341; df=30; p=0.056 | r=0.212; df=121; p=0.019* | |
| Allo average of last 4 attempts | rs=0.146; df=89; p=0.167 | rs=0.210; df=30; p=0.250 | rs=0.166; df=121; p=0.067 |
| r=0.130; df=89; p=0.219 | r=−0.023; df=30; p=0.901 | r=0.045; df=121; p=0.618 | |
| Allo average of all 8 attempts | rs=0.098; df=89; p=0.357 | rs=0.083; df=30; p=0.653 | rs=0.128; df=121; p=0.158 |
| r=0.150; df=89; p=0.156 | r=−0.089; df=30; p=0.629 | r=0.060; df=121; p=0.059 | |
| Combined Allo-Ego 1st attempt | rs=−0.098; df=89; p=0.355 | rs=0.129; df=30; p=0.483 | rs=−0.050; df=121; p=0.584 |
| r=−0.068; df=89; p=0.523 | r=0.265; df=30; p=0.143 | r=0.097; df=121; p=0.284 | |
| Combined Allo-Ego average of last 4 attempts | rs=0.318; df=89; p=0.002* | rs=0.145; df=30; p=0.428 | rs=0.263; df=121; p=0.003* |
| r=0.193; df=89; p=0.067 | r=0.545; df=30; p=0.001* | r=0.291; df=121; p=0.001* | |
| Combined Allo-Ego average of all 8 attempts | rs=0.316; df=89; p=0.002* | rs=0.324; df=30; p=0.070 | rs=0.328; df=121; p<0.001* |
| r=0.304; df=89; p=0.004* | r=0.606; df=30; p<0.001* | r=0.394; df=121; p<0.001* | |
Allo, allocentric; Ego, egocentric; MCI, mild cognitive impairment; p<0.05.
Figure 3.
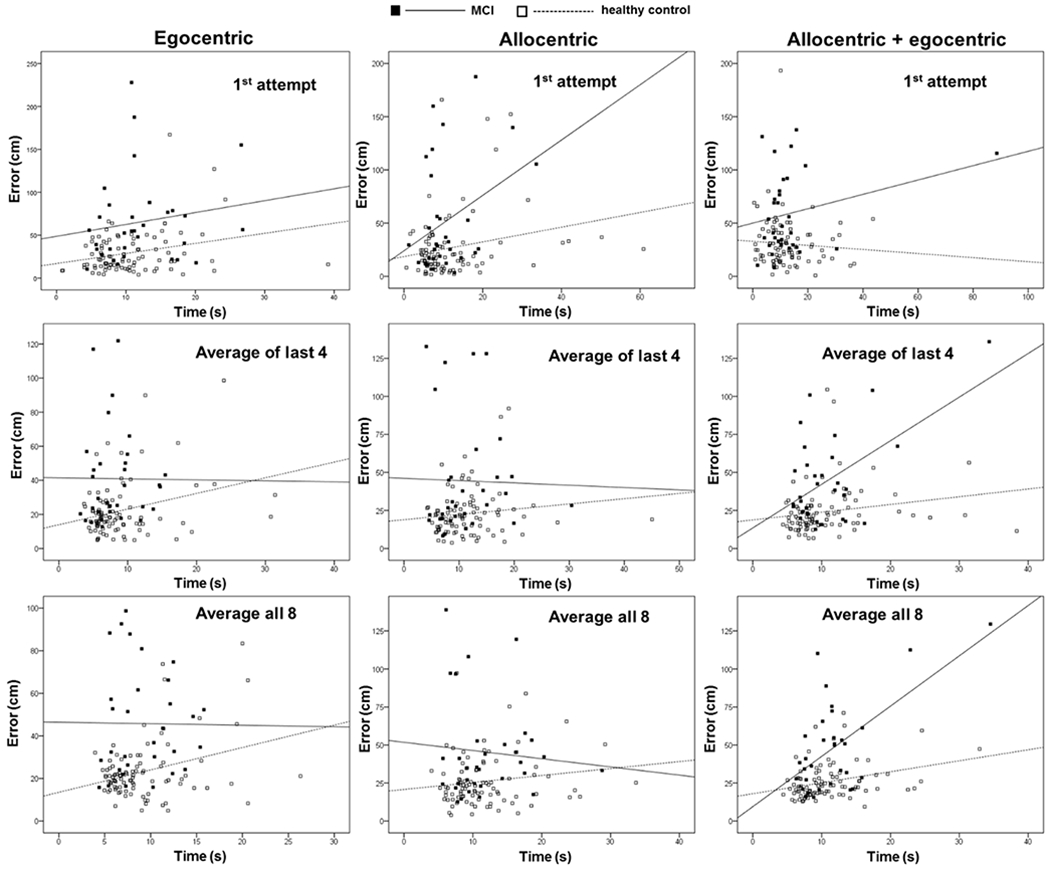
Correlation between errors and times measured in subtests of the HGT.
3.2. Diagnostic performance of errors and times measured in Allo, Ego, and combined Allo-Ego subtests in the HGT
Diagnostic performance was determined for errors and times measured in Allo, Ego, and combined Allo-Ego subtests in the HGT. Sensitivity, specificity, cut-off, positive predictive values (PPV) and negative predictive values (NPV) were determined for errors and times measured in each subtest. These values were determined to detect MCI subjects among all the HC subjects (Table 4; Table 5), and in the group of age-matched HC (Table 6; Table 7). All analyses were performed in the groups unadjusted for age and sex (Table 4; Table 6), and in the groups adjusted for age and sex (Table 5; Table 7).
Table 4.
ROC curve analysis determined between MCI and HC, unadjusted for age and sex.
| MCI vs HC |
|||||||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Prev. 5 % |
Prev. 10 % |
Prev. 16 % |
Prev. 20 % |
Prev. 36.7 % |
|||||||||||
| Sensitivity | Specificity | Cut-off | AUC, p | PPV | NPV | PPV | NPV | PPV | NPV | PPV | NPV | PPV | NPV | ||
| Ego 1st attempt | Error | 57.6 % | 89 % | 51.65 cm | 0.793, p < 0.001* | 21.6 % | 97.6 % | 36.8 % | 95 % | 49.9 % | 91.7 % | 56.7 % | 89.4 % | 75.2 % | 78.4 % |
| Time | 65.6 % | 54.9 % | 9.7085 s | 0.582, p = 0.168 | 7.1 % | 96.8 % | 13.9 % | 93.5 % | 21.7 % | 89.3 % | 26.7 % | 86.5 % | 45.7 % | 73.4 % | |
| Ego average of last 4 attempts | Error | 78.7 % | 67 % | 21.5 cm | 0.776, p < 0.001* | 11.2 % | 98.4 % | 20.9 % | 96.6 % | 31.2 % | 94.3 % | 37.4 % | 92.6 % | 58 % | 84.4 % |
| Time | 59.4 % | 49.5 % | 7.0425 s | 0.490, p = 0.863 | 5.8 % | 95.9 % | 11.6 % | 91.6 % | 18.3 % | 86.5 % | 22.7 % | 83 % | 40.5 % | 67.8 % | |
| Ego average of all 8 attempts | Error | 81.8 % | 72.5 % | 24.05 cm | 0.820, p < 0.001* | 13.5 % | 98.7 % | 24.8 % | 97.3 % | 36.2 % | 95.4 % | 42.6 % | 94.1 % | 63.3 % | 87.3 % |
| Time | 43.8 % | 73.6 % | 10.2205s | 0.538, p = 0.522 | 8 % | 96.1 % | 15.6 % | 92.2 % | 24 % | 87.3 % | 29.3 % | 84 % | 49 % | 69.3 % | |
| Allo 1st attempt | Error | 63.6 % | 68.1 % | 25.40 cm | 0.677, p = 0.003* | 9.5 % | 97.3 % | 18.1 % | 94.4 % | 27.5 % | 90.8 % | 33.3 % | 88.2 % | 53.6 % | 76.3 % |
| Time | 78.1 % | 35.2 % | 6.857 s | 0.515, p = 0.800 | 6 % | 96.8 % | 11.8 % | 93.5 % | 18.7 % | 89.4 % | 23.2 % | 86.5 % | 41.1 % | 73.5 % | |
| Allo average of last 4 attempts | Error | 60.6 % | 74.7 % | 26.6 cm | 0.710, p < 0.001* | 11.2 % | 97.3 % | 21 % | 94.5 % | 31.3 % | 90.9 % | 37.5 % | 88.4 % | 58.1 % | 76.6 % |
| Time | 28.1 % | 80.2 % | 14.0975 s | 0.485, p = 0.802 | 7 % | 95.5 % | 13.6 % | 90.9 % | 21.3 % | 85.4 % | 26.2 % | 81.7 % | 42.6 % | 65.2 % | |
| Allo average of all 8 attempts | Error | 69.7 % | 68.1 % | 26.95 cm | 0.734, p < 0.001* | 10.3 % | 97.7 % | 19.5 % | 95.3 % | 29.4 % | 92.2 % | 35.3 % | 90 % | 55.9 % | 79.5 % |
| Time | 68.8 % | 41.8 % | 9.131 s | 0.520, p = 0.734 | 5.9 % | 96.2 % | 11.6 % | 92.3 % | 18.4 % | 87.6 % | 22.8 % | 84.3 % | 46.7 % | 85.7 % | |
| Combined Allo-Ego 1st attempt | Error | 48.5 % | 91.2 % | 53.35 cm | 0.757, p < 0.001* | 22.5 % | 97.1 % | 38 % | 94.1 % | 51.2 % | 90.3 % | 57.9 % | 87.6 % | 76.2 % | 75.3 % |
| Time | 96.9 % | 9.9 % | 3.1535s | 0.480, p = 0.742 | 5.4 % | 98.4 % | 10.7 % | 96.9 % | 17 % | 94.4 % | 21.2 % | 92.7 % | 38.4 % | 84.6 % | |
| Combined Allo-Ego average of last 4 attempts | Error | 63.6 % | 79.1 % | 28.75 cm | 0.766, p < 0.001* | 13.8 % | 97.6 % | 25.3 % | 95.1 % | 36.7 % | 91.9 % | 43.2 % | 89.7 % | 63.8 % | 78.9 % |
| Time | 90.6 % | 25.3 % | 6.8440 s | 0.548, p = 0.423 | 6 % | 98.1 % | 11.9 % | 96 % | 18.8 % | 93.4 % | 23.3 % | 91.5 % | 41.3 % | 82.3 % | |
| Combined Allo-Ego average of all 8 attempts | Error | 66.7 % | 81.3 % | 31.8 cm | 0.772, p < 0.001* | 15.8 % | 97.9 % | 28.4 % | 95.6 % | 40.5 % | 92.8 % | 47.1 % | 90.7 % | 67.4 % | 80.8 % |
| Time | 59.4 % | 56 % | 10.1015s | 0.562, p = 0.301 | 6.6 % | 96.3 % | 13 % | 92.5 % | 20.5 % | 87.9 % | 25.2 % | 84.7 % | 43.9 % | 70.4 % | |
| Ego average of first 4 attempts | Error | 66.7 % | 87.9 % | 34.7 cm | 0.810, p < 0.001* | 22.5 % | 98 % | 38 % | 96 % | 51.2 % | 93.3 % | 57.9 % | 91.3 % | 76.2 % | 82 % |
| Allo average of first 4 attempts | Error | 75.8 % | 64.8 % | 28.3 cm | 0.729, p < 0.001* | 10.2 % | 98.1 % | 19.3 % | 96 % | 29.1 % | 93.4 % | 35 % | 91.5 % | 55.5 % | 82.2 % |
| Combined Allo-Ego average of first 4 attempts | Error | 75.8 % | 71.4 % | 29.7 cm | 0.739, p < 0.001* | 12.2 % | 98.2 % | 22.7 % | 96.4 % | 33.5 % | 93.9 % | 39.9 % | 92.2 % | 60.6 % | 83.6 % |
| MMSE | 90 % | 98.9 % | 29.5 | 0.949, p < 0.001* | 81.2 % | 99.5 % | 90.1 % | 98.9 % | 94 % | 98.1 % | 95.3 % | 97.5 % | 97.9 % | 94.5 % | |
| MoCA | 86.7 % | 97.8 % | 29.5 | 0.930, p < 0.001* | 68.3 % | 99.5 % | 81.4 % | 98.5 % | 88.2 % | 97.5 % | 90.8 % | 96.7 % | 95.8 % | 92.7 % | |
Allo, allocentric; AUC, area under the curve; Ego, egocentric; HC, healthy control; MCI, mild cognitive impairment; MMSE, mini-mental state examination; MoCA, Montreal cognitive assessment; NPV, negative predictive value; PPV, positive predictive value; Prev, prevalence.
Table 5.
ROC curve analysis determined between MCI and HC, adjusted for age and sex.
| MCI vs HC |
|||||||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Prev. 5 % |
Prev. 10 % |
Prev. 16 % |
Prev. 20 % |
Prev. 36.7 % |
|||||||||||
| Sensitivity | Specificity | Cut-off | AUC, p | PPV | NPV | PPV | NPV | PPV | NPV | PPV | NPV | PPV | NPV | ||
| Ego 1st attempt | Error | 93.9 % | 80.7 % | 0.2519 | 0.917, p < 0.001* | 20.3 % | 99.6 % | 35 % | 99.1 % | 48 % | 98.5 % | 54.8 % | 98.1 % | 73.8 % | 95.6 % |
| Time | 96.9 % | 78.4 % | 0.2466 | 0.900, p < 0.001* | 19.1 % | 99.8 % | 33.3 % | 99.6 % | 46.1 % | 99.3 % | 52.9 % | 99 % | 72.2 % | 87.9 % | |
| Ego average of last 4 attempts | Error | 87.9 % | 83 % | 0.3153 | 0.901, p < 0.001* | 21.4 % | 99.2 % | 36.5 % | 98.4 % | 49.6 % | 97.3 % | 56.4 % | 96.5 % | 75 % | 92.2 % |
| Time | 100 % | 71.6 % | 0.1511 | 0.899, p < 0.001* | 15.6 % | 100 % | 28.1 % | 100 % | 40.1 % | 100 % | 46.8 % | 100 % | 67.1 % | 100 % | |
| Ego average of all 8 attempts | Error | 93.9 % | 76.1 % | 0.1956 | 0.915, p < 0.001* | 17.1 % | 99.6 % | 30.4 % | 99.1 % | 42.8 % | 98.5 % | 49.6 % | 98 % | 69.5 % | 95.6 % |
| Time | 100 % | 73.9 % | 0.2053 | 0.888, p < 0.001* | 16.8 % | 100 % | 29.9 % | 100 % | 42.2 % | 100 % | 48.9 % | 100 % | 69 % | 100 % | |
| Allo 1st attempt | Error | 100 % | 72.7 % | 0.1951 | 0.887, p < 0.001* | 16.2 % | 100 % | 28.9 % | 100 % | 41.1 % | 100 % | 47.8 % | 100 % | 68 % | 100 % |
| Time | 100 % | 75 % | 0.2109 | 0.895, p < 0.001* | 17.4 % | 100 % | 30.8 % | 100 % | 43.2 % | 100 % | 50 % | 100 % | 69.9 % | 100 % | |
| Allo average of last 4 attempts | Error | 100 % | 70.5 % | 0.1707 | 0.879, p < 0.001* | 15.1 % | 100 % | 27.4 % | 100 % | 39.2 % | 100 % | 45.9 % | 100 % | 63.3 % | 100 % |
| Time | 100 % | 70.5 % | 0.2059 | 0.892, p < 0.001* | 15.1 % | 100 % | 27.4 % | 100 % | 39.2 % | 100 % | 45.9 % | 100 % | 66.3 % | 100 % | |
| Allo average of all 8 attempts | Error | 100 % | 71.6 % | 0.1642 | 0.896, p < 0.001* | 15.6 % | 100 % | 28.1 % | 100 % | 40.1 % | 100 % | 46.8 % | 100 % | 67.1 % | 100 % |
| Time | 100 % | 73.9 % | 0.1831 | 0.890, p < 0.001* | 16.8 % | 100 % | 29.9 % | 100 % | 42.2 % | 100 % | 48.9 % | 100 % | 69 % | 100 % | |
| Combined Allo-Ego 1st attempt | Error | 90.9 % | 80.7 % | 0.2294 | 0.920, p < 0.001* | 15.5 % | 99.4 % | 34.4 % | 98.8 % | 47.3 % | 97.9 % | 54.1 % | 97.3 % | 73.2 % | 93.9 % |
| Time | 96.9 % | 77.3 % | 0.2367 | 0.886, p < 0.001* | 18.3 % | 99.8 % | 32.2 % | 99.6 % | 44.8 % | 99.2 % | 51.6 % | 99 % | 71.2 % | 97.7 % | |
| Combined Allo-Ego average of last 4 attempts | Error | 97 % | 73.9 % | 0.1787 | 0.906, p < 0.001* | 16.4 % | 99.8 % | 29.2 % | 99.6 % | 41.4 % | 99.2 % | 48.2 % | 99 % | 68.3 % | 97.7 % |
| Time | 100 % | 73.9 % | 0.1886 | 0.889, p < 0.001* | 16.8 % | 100 % | 29.9 % | 100 % | 42.2 % | 100 % | 48.9 % | 100 % | 69 % | 100 % | |
| Combined Allo-Ego average of all 8 attempts | Error | 93.9 % | 80.7 % | 0.2453 | 0.917, p < 0.001* | 20.4 % | 99.6 % | 35.1 % | 99.2 % | 48.1 % | 98.6 % | 54.9 % | 98.1 % | 73.8 % | 95.8 % |
| Time | 96.9 % | 77.3 % | 0.2624 | 0.888, p < 0.001* | 18.3 % | 99.8 % | 32.2 % | 99.6 % | 44.8 % | 99.2 % | 51.6 % | 99 % | 71.2 % | 97.7 % | |
| Ego average of first 4 attempts | Error | 93.9 % | 77.3 % | 0.1874 | 0.920, p < 0.001* | 17.9 % | 99.6 % | 31.5 % | 99.1 % | 44.1 % | 98.5 % | 50.8 % | 98.1 % | 70.6 % | 95.6 % |
| Allo average of first 4 attempts | Error | 100 % | 70.5 % | 0.1658 | 0.893, p < 0.001* | 15.1 % | 100 % | 27.4 % | 100 % | 39.2 % | 100 % | 45.9 % | 100 % | 66.3 % | 100 % |
| Combined Allo-Ego average of first 4 attempts | Error | 90.9 % | 83 % | 0.2774 | 0.917, p < 0.001* | 22 % | 99.4 % | 37.3 % | 98.8 % | 50.5 % | 98 % | 57.2 % | 97.3 % | 75.6 % | 94 % |
Allo, allocentric; AUC, area under the curve; Ego, egocentric; HC, healthy control; MCI, mild cognitive impairment; NPV, negative predictive value; PPV, positive predictive value; Prev, prevalence.
Table 6.
ROC curve analysis determined between MCI patients and age-matched healthy controls, unadjusted for age and sex.
| MCI vs HC |
|||||||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Prev. 5 % |
Prev. 10 % |
Prev. 16 % |
Prev. 20 % |
Prev. 36.7 % |
|||||||||||
| Sensitivity | Specificity | Cut-off | AUC, p | PPV | NPV | PPV | NPV | PPV | NPV | PPV | NPV | PPV | NPV | ||
| Ego 1st attempt | Error | 57.6 % | 88.9 % | 54.85 cm | 0.788, p < 0.001* | 21.5 % | 97.6 % | 36.6 % | 95 % | 49.7 % | 91.7 % | 56.5 % | 89.3 % | 75.1 % | 78.3 % |
| Time | 65.6 % | 59.3 % | 9.607 s | 0.619, p = 0.117 | 7.8 % | 97 % | 15.2 % | 93.9 % | 23.5 % | 90 % | 28.7 % | 87.3 % | 48.3 % | 74.8 % | |
| Ego average of last 4 attempts | Error | 78.8 % | 63 % | 21.5 cm | 0.741, p = 0.001* | 10.1 % | 98.3 % | 19.1 % | 96.4 % | 28.9 % | 94 % | 34.7 % | 92.2 % | 55.3 % | 83.7 % |
| Time | 43.8 % | 59.3 % | 8.445 s | 0.411, p = 0.244 | 5.4 % | 95.2 % | 10.7 % | 90.5 % | 17 % | 84.7 % | 21.2 % | 80.8 % | 38.4 % | 64.5 % | |
| Ego average of all 8 attempts | Error | 81.8 % | 70.4 % | 23.80 cm | 0.780, p < 0.001* | 12.7 % | 98.7 % | 23.5 % | 97.2 % | 34.5 % | 95.3 % | 40.9 % | 93.9 % | 61.6 % | 87 % |
| Time | 43.8 % | 66.7 % | 9.9705 s | 0.497, p = 0.970 | 6.5 % | 95.8 % | 12.8 % | 91.4 % | 20 % | 86.2 % | 24.7 % | 82.6 % | 43.3 % | 67.2 % | |
| Allo 1st attempt | Error | 63.6 % | 59.3 % | 25.4 cm | 0.568, p = 0.365 | 7.6 % | 96.9 % | 14.8 % | 93.6 % | 22.9 % | 89.5 % | 28.1 % | 86.7 % | 47.5 % | 73.8 % |
| Time | 81.3 % | 29.6 % | 6.4035 s | 0.469, p = 0.681 | 5.7 % | 96.8 % | 11.4 % | 93.4 % | 18 % | 89.3 % | 22.4 % | 86.4 % | 40.1 % | 73.2 % | |
| Allo average of last 4 attempts | Error | 60.6 % | 66.7 % | 26.6 cm | 0.655, p = 0.040* | 8.7 % | 97 % | 16.8 % | 93.8 % | 25.7 % | 89.9 % | 31.3 % | 87.1 % | 51.3 % | 74.5 % |
| Time | 84.4 % | 14.8 % | 6.7665 s | 0.407, p = 0.221 | 5 % | 94.7 % | 9.9 % | 89.5 % | 15.9 % | 83.3 % | 19.8 % | 79.1 % | 36.5 % | 62.1 % | |
| Allo average of all 8 attempts | Error | 78.8 % | 51.9 % | 24.05 cm | 0.654, p = 0.041* | 7.9 % | 97.9 % | 15.4 % | 95.7 % | 23.8 % | 92.8 % | 29.1 % | 90.7 % | 48.7 % | 80.9 % |
| Time | 34.4 % | 70.4 % | 13.628 s | 0.446, p = 0.475 | 5.8 % | 95.3 % | 11.4 % | 90.6 % | 18.1 % | 84.9 % | 22.5 % | 81.1 % | 40.3 % | 64.9 % | |
| Combined Allo-Ego 1st attempt | Error | 42.4 % | 96.3 % | 68.4 cm | 0.737, p = 0.002* | 37.6 % | 96.9 % | 56 % | 93.8 % | 68.6 % | 89.8 % | 74.1 % | 87 % | 89 % | 74.3 % |
| Time | 78.1 % | 33.3 % | 7.709 s | 0.505, p = 0.951 | 5.8 % | 96.7 % | 11.5 % | 93.2 % | 18.2 % | 88.9 % | 22.6 % | 85.9 % | 40.4 % | 72.4 % | |
| Combined Allo-Ego average of last 4 attempts | Error | 48.5 % | 96.3 % | 37.7 cm | 0.740, p = 0.002* | 40.8 % | 97.3 % | 59.3 % | 94.4 % | 71.4 % | 90.8 % | 76.6 % | 88.2 % | 88.4 % | 76.3 % |
| Time | 34.4 % | 70.4 % | 11.8715 s | 0.439, p = 0.420 | 5.8 % | 95.3 % | 11.4 % | 90.6 % | 18.1 % | 84.9 % | 22.5 % | 81.1 % | 40.3 % | 64.9 % | |
| Combined Allo-Ego average of all 8 attempts | Error | 48.5 % | 100 % | 48.65 cm | 0.763, p = 0.001* | 100 % | 97.4 % | 100 % | 94.6 % | 100 % | 91.1 % | 100 % | 88.6 % | 100 % | 77 % |
| Time | 93.8 % | 11.1 % | 7.112 s | 0.519, p = 0.808 | 5.3 % | 97.1 % | 10.5 % | 94.2 % | 16.7 % | 90.4 % | 20.9 % | 87.7 % | 38 % | 75.5 % | |
| Ego average of first 4 attempts | Error | 63.6 % | 88.9 % | 35.35 cm | 0.775, p < 0.001* | 23.3 % | 97.9 % | 38.9 % | 95.6 % | 52.2 % | 92.8 % | 58.9 % | 90.7 % | 76.9 % | 80.8 % |
| Allo average of first 4 attempts | Error | 75.8 % | 55.6 % | 28.3 cm | 0.648, p = 0.051 | 8.2 % | 97.8 % | 15.9 % | 95.4 % | 24.5 % | 92.3 % | 29.9 % | 90.2 % | 49.7 % | 79.8 % |
| Combined Allo-Ego average of first 4 attempts | Error | 75.8 % | 70.4 % | 29.2 cm | 0.738, p = 0.002* | 11.9 % | 98.2 % | 22.2 % | 96.3 % | 32.8 % | 93.9 % | 39 % | 92.1 % | 59.8 % | 83.4 % |
| MMSE | 90 % | 96.3 % | 29.5 | 0.947, p < 0.001* | 56.1 % | 99.5 % | 73 % | 98.9 % | 82.2 % | 98.1 % | 85.9 % | 97.5 % | 93.4 % | 94.3 % | |
| MoCA | 86.7 % | 92.6 % | 29.5 | 0.922, p < 0.001* | 38.1 % | 99.2 % | 56.6 % | 98.4 % | 69.1 % | 97.3 % | 74.5 % | 96.5 % | 87.2 % | 92.3 % | |
Allo, allocentric; AUC, area under the curve; Ego, egocentric; HC, healthy control; MCI, mild cognitive impairment; MMSE, mini-mental state examination; MoCA, Montreal cognitive assessment; NPV, negative predictive value; PPV, positive predictive value; Prev, prevalence.
Table 7.
ROC curve analysis determined between MCI and age-matched HC, adjusted for age and sex.
| attempts | Time | 68.8% | 66.7% | 0.5438 | 0.679, p=0.018* | 9.8% | 97.6% | 18.7% | 95.1% | 28.2% | 91.8% | 34.1% | 89.5% | 54.5% | 78.7% |
| Ego average of all 8 attempts | Error | 75.8% | 77.8% | 0.4393 | 0.783, p<0.001* | 15.2% | 98.4% | 27.5% | 96.7% | 39.4% | 94.4% | 46.1% | 92.8% | 66.4% | 84.7% |
| Time | 68.8% | 70.4% | 0.5390 | 0.642, p=0.061 | 10.9% | 97.7% | 20.5% | 95.3% | 30.7% | 92.2% | 36.8% | 90% | 57.4% | 79.6% | |
| Allo 1st attempt | Error | 75.8% | 51.9% | 0.4920 | 0.622, p=0.105 | 7.7% | 97.6% | 14.9% | 95.1% | 23.1% | 91.8% | 28.3% | 89.6% | 47.7% | 78.7% |
| Time | 71.9% | 55.6% | 0.5228 | 0.641, p=0.063 | 7.9% | 7.4% | 15.2% | 94.7% | 23.6% | 91.2% | 28.8% | 88.8% | 48.4% | 77.3% | |
| Allo average of last 4 attempts | Error | 78.8% | 55.6% | 0.4522 | 0.694, p=0.010* | 8.5% | 98% | 16.5% | 95.9% | 25.3% | 93.2% | 30.7% | 91.3% | 50.7% | 81.9% |
| Time | 59.4% | 74.1% | 0.5654 | 0.639, p=0.068 | 10.8% | 97.2% | 20.3% | 94.3% | 30.4% | 90.5% | 36.4% | 88% | 57.1% | 75.9% | |
| Allo average of all 8 attempts | Error | 66.7% | 59.3% | 0.4916 | 0.673, p=0.022* | 7.9% | 97.1% | 15.4% | 94.1% | 23.8% | 90.3% | 29.1% | 87.7% | 48.7% | 75.4% |
| Time | 68.8% | 70.4% | 0.5524 | 0.635, p=0.075 | 10.9% | 97.7% | 20.5% | 95.3% | 30.7% | 92.2% | 36.8% | 90% | 57.4% | 79.6% | |
| Combined Allo-Ego 1st attempt | Error | 84.8% | 59.3% | 0.4454 | 0.767, p<0.001* | 9.9% | 98.7% | 18.8% | 97.2% | 28.4% | 95.3% | 34.2% | 94% | 54.7% | 87.1% |
| Time | 75% | 44.4% | 0.4908 | 0.620, p=0.114 | 6.6% | 97.1% | 13% | 94.1% | 20.4% | 90.3% | 25.2% | 87.7% | 43.9% | 75.4% | |
| Combined Allo-Ego average of last 4 attempts | Error | 60.6% | 81.5% | 0.5968 | 0.745, p=0.001* | 14.7% | 97.5% | 26.7% | 94.9% | 38.4% | 91.6% | 45% | 89.2% | 65.5% | 78.1% |
| Time | 59.4% | 70.4% | 0.5644 | 0.620, p=0.114 | 9.6% | 97.1% | 18.2% | 94% | 27.7% | 90.1% | 33.4% | 87.4% | 53.8% | 74.9% | |
| Combined Allo-Ego average of all 8 attempts | Error | 45.5% | 100% | 0.8049 | 0.774, p<0.001* | 100% | 97.2% | 100% | 94.3% | 100% | 90.6% | 100% | 88% | 100% | 76% |
| Time | 46.9% | 81.5% | 0.6105 | 0.617, p=0.124 | 11.8% | 96.7% | 22% | 93.2% | 32.6% | 89% | 38.8% | 86% | 59.5% | 72.6% | |
| Ego average of first 4 attempts | Error | 69.7% | 85.2% | 0.5262 | 0.776, p<0.001* | 19.9% | 98.2% | 34.4% | 96.2% | 47.3% | 93.7% | 54.1% | 91.8% | 73.2% | 82.9% |
| Allo average of first 4 attempts | Error | 81.8% | 44.4% | 0.4397 | 0.648, p=0.051 | 7.2% | 97.9% | 14.1% | 95.6% | 21.9% | 92.8% | 26.9% | 90.7% | 46% | 80.8% |
| Combined Allo-Ego average of first 4 attempts | Error | 75.8% | 70.4% | 0.4603 | 0.772, p<0.001* | 11.9% | 98.2% | 22.2% | 96.3% | 32.8% | 93.9% | 39% | 92.1% | 59.8% | 83.4% |
Allo, allocentric; AUC, area under the curve; Ego, egocentric; HC, healthy control; MCI, mild cognitive impairment; NPV, negative predictive value; PPV, positive predictive value; Prev, prevalence.
Information on the prevalence of MCI was necessary for the determination of PPV and NPV. As data on the prevalence of MCI varies across different studies, we determined several PPVs and NPVs. According to Sachdev et al. (2015), the prevalence of MCI is 5-36.7%, while Roberts et al. (2013), stated that the prevalence of MCI is 16-20%. The average of 16-20% estimated prevalence of MCI reported by Roberts and Knopman (2013) resulted from a meta-analysis of 15 large studies, of which 10 were population-based. However, these authors noted that estimates of MCI my be higher in studies that had issues with non-participation and in studies conducted in urban sites, multiethnic cohorts, and in clinic-based studies. The 5-36.7% MCI prevalence estimate reported by Sachdev et al. (2013) was based on uniform criteria applied to harmonized data from 11 longitudinal, population-based studies from USA, Europe, Asia, and Australia (Sachdev et al., 2013). As we could not estimate precisely the prevalence for the Croatian population, we determined the PPVs and NPVs when the prevalence is set at 5%, 10%, 16%, 20% and 36.7%.
After analyzing ROC curves between the MCI and HC groups, high sensitivities and specificities were observed for the combined Allo-Ego error average of all 8 attempts (85.7% sensitivity / 84.6% specificity), and the Ego error average of the first 4 attempts (92.9% / 89%, results marked in bold in Tables 8 and 9). The very high NPVs (over 90% in almost all subtests and at all prevalences) suggest high discriminative capacity and diagnostic potential and indicate that the HGT could be an excellent test for screening (i.e. to find a subject in a healthy population who will progress to MCI). Importantly, although the test had significantly lower PPVs than NPVs, it still further improved the differentiation of the MCI subjects from HC. In some cases, PPVs were above 80%, and even above 90% (bold italics in Tables 8 and 9). If the PPV was 92% (as in the case of combined Allo-Ego average error of all 8 attempts at the prevalence set at 36.7%, see Table 9), there is an 8% probability of no MCI with a positive test. Thus, if analyzing a “risk” MCI group showing worsening performance on neuropsychological tests or altered levels of other different biomarkers, the HGT may also serve as a relatively good confirmatory test.
Table 8.
ROC curve analysis between MCI and HC group, unadjusted for age and sex.
| MCI vs HC | |||||||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Prev. 5% | Prev. 10% | Prev. 16% | Prev. 20% | Prev. 36.7% | |||||||||||
| Sensitivity | Specificity | Cut-off | AUC, p | PPV | NPV | PPV | NPV | PPV | NPV | PPV | NPV | PPV | NPV | ||
| Ego 1st attempt | Error | 85.7% | 68.1% | 33.6 cm | 0.807, p<0.001* | 12.4% | 98.9% | 23% | 97.7% | 33.9% | 96.2% | 40.2% | 95% | 60.9% | 89.1% |
| Ego average of last 4 attempts | Error | 100% | 71.4% | 23.2 cm | 0.875, p<0.001* | 15.5% | 100% | 28% | 100% | 40% | 100% | 46.6% | 100% | 67% | 100% |
| Ego average of all 8 attempts | Error | 92.9% | 82.4% | 24.05 cm | 0.918, p<0.001* | 21.7% | 99.5% | 37% | 99.1% | 50.1% | 98.4% | 56.9% | 97.9% | 75.4% | 95.2% |
| Allo 1st attempt | Error | 64.3% | 87.3% | 44 cm | 0.747, p=0.003* | 21% | 97.9% | 36% | 95.7% | 49.1% | 92.8% | 55.9% | 90.7% | 74.6% | 80.8% |
| Allo average of last 4 attempts | Error | 71.4% | 82.4% | 30.5 cm | 0.794, p<0.001* | 17.6% | 98.2% | 31.1% | 96.3% | 43.6% | 93.8% | 50.4% | 92% | 70.2% | 83.2% |
| Allo average of all 8 attempts | Error | 85.7% | 78% | 33.9 cm | 0.825, p<0.001* | 17% | 99% | 30.2% | 98% | 42.6% | 96.6% | 49.3% | 95.6% | 69.3% | 90.4% |
| Combined Allo-Ego 1st attempt | Error | 64.3% | 91.2% | 53.35 cm | 0.849, p<0.001* | 27.8% | 98% | 44.8% | 95.8% | 58.2% | 93.1% | 64.6% | 91.1% | 80.9% | 81.5% |
| Combined Allo-Ego average of last 4 attempts | Error | 78.6% | 90.1% | 38.35 cm | 0.902, p<0.001* | 29.5% | 98.8% | 46.9% | 97.4% | 60.2% | 95.7% | 66.5% | 94.4% | 82.2% | 87.9% |
| Combined Allo-Ego average of all 8 attempts | Error | 85.7% | 84.6% | 35.55 cm | 0.895, p<0.001* | 22.7% | 99.1% | 38.2% | 98.2% | 51.5% | 96.9% | 58.2% | 95.9% | 76.3% | 91.1% |
| Ego average of first 4 attempts | Error | 92.9% | 89% | 35.35 cm | 0.917, p<0.001* | 30.8% | 99.6% | 48.4% | 99.1% | 61.7% | 98.5% | 67.9% | 98% | 83% | 95.6% |
| Allo average of first 4 attempts | Error | 64.3% | 87.9% | 46.55 cm | 0.797, p<0.001* | 21.9% | 97.9% | 37.1% | 95.7% | 50.3% | 92.8% | 57.1% | 90.8% | 75.5% | 80.9% |
| Combined Allo-Ego average of first 4 attempts | Error | 71.4% | 89% | 43.85 cm | 0.832, p<0.001* | 25.5% | 98.3% | 41.9% | 96.6% | 55.3% | 94.2% | 61.9% | 92.6% | 79% | 84.3% |
Allo, allocentric; AUC, area under the curve; ego, egocentric; HC, healthy control; MCI, mild cognitive impairment; NPV, negative predictive value; PPV, positive predictive value; Prev, prevalence.
4. Discussion
Errors measured in Allo, Ego, and combined Allo-Ego subtests of the HGT showed significantly higher sensitivities, specificities and AUCs than times needed for completion of these subtests. Times needed for completion of these subtests (either in the first attempt, average of the last 4 attempts, or the average of all 8 attempts) did not reach sufficient AUCs, sensitivities, and specificities in any of the subtests. However, when comparing MCI subjects with all HC, several measurements reached high sensitivities and specificities: Ego average error of the last 4 attempts (78.7% sensitivity / 67% specificity); Ego average error of all 8 attempts (81.8% / 72.5%, respectively); combined Allo-Ego average error of all 8 attempts (66.7% / 81.3%); Ego average error of the first 4 attempts (66.7% / 87.9%); combined Allo-Ego average error of the first 4 attempts (75.8% / 71.4%). In most subtests, adjustment for age and sex further improved their discriminative properties: Ego average error of the last 4 attempts (78.8% sensitivity / 63% specificity); Ego average error of all 8 attempts (81.3%/70.4%); Ego average error of the first 4 attempts (63.6% / 88.9%); combined Allo-Ego average error of the first 4 attempts (75.8% / 71.4 %). Overall, the error measured in the Ego subtest (average of all 8 attempts) besides having the best diagnostic performance (i.e. highest sensitivity and specificity combined) also showed the best discriminative properties (highest AUC: 0.82, p<0.001).
Because the exact value for prevalence of MCI is still not determined in any population and data on prevalence of MCI vary among different studies (Sachdev et al., 2013; Roberts and Knopman, 2013), PPVs and NPVs (diagnostic indicators that depend on prevalence of disease and values of sensitivity and specificity) were determined when prevalence of MCI was set at 5%, 10%, 16%, 20% and 36.7%. None of the examined data reached sufficient PPVs and NPVs (above 95%). However, the error measured in the egocentric subtest (average of all 8 attempts) showed the best discriminative properties and diagnostic performance, reached high NPVs (above 90% except for 36.7% prevalence - above 85%), but insufficient PPVs (below 65%) (Table 4; Table 6). These results indicate that the HGT could be a good screening test (due to high NPVs), but also that it does not have high potential as a confirmatory test (due to low PPVs). For example, error measured in the egocentric subtest (average of all 8 attempts; cut-off = 23.8 cm) at prevalence set at 20% has 40.9% PPV and 93.9% NPV. As such, there is 59.1% probability of no MCI with a positive test, and 6.1% probability of MCI with a negative test, which makes the error measured in the egocentric subtest (average of all 8 attempts) a good screening test, but not a single confirmatory test.
What distinguishes ALZENTIA from other screening tests is that it provides a fast (testing takes only about 20-25 minutes) and non-invasive diagnostic procedure, and that it is an integrated solution for all test-system steps that allows setting, calibration, and sequence test management, as well as tracking and saving results in one place. Therefore, this detection system for spatial navigation deficit is intended for the scientific community, pharmaceutical industry, healthcare institutions and organizations caring for elderly populations where early detection of cognitive impairment and dementia is important.
The limitation of any test, including the HGT, is that it may neglect certain domains of cognitive function that are affected in the early stages of MCI, with the risk that some persons may achieve high score despite impairment of some domains not involved in spatial orientation and navigational ability. Another issue may be the usage of MMSE scores validated in our Croatian population (Boban et al., 2012), which may need adjustments when ALZENTIA is used in other populations. While we had not randomized the order of testing in this research work, we will consider this approach in the future investigations.
The ALZENTIA system is relatively simple and compact: it consists of original software and hardware as well as a few non-specific elements (3 m diameter tent with 8 laser pointers, displays for starting point and landmarks’ marking) that are needed for carrying out the testing. Additionally, there should be a single trained operator running the test by guiding the subject and giving her/him the appropriate instructions.
Altogether, this study confirms that the HGT is a good screening tool for detecting MCI, which is, in most cases, followed by progression to AD. It could also improve the reliability of predicting MCI progression to AD, especially when used with other biological markers of AD. This is important considering that MMSE and MoCA have low sensitivity in detecting MCI and dementia, as well as poor specificity, NPV and PPV values (Arevalo-Rodriguez et al., 2015). The use of the HGT in combination with other neuropsychological tests and cerebrospinal fluid and neuroimaging biomarkers in both clinical assessments and for research purposes will be helpful to attain a higher probability of correct diagnosis and identify MCI patients in elderly populations at risk of progressing to AD dementia. Although we have not studied the potential of the HGT for the differential diagnosis of dementia, we would encourage other groups and investigators to do so. In the future, we intend to use the ALZENTIA system in other aged cohorts to assess replicability of the results obtained in the present study and applicability to other aged populations, such as centenarians.
Highlights.
a new system to detect early mild cognitive impairment (MCI) is introduced
the system is based on a hidden goal task (HGT) test
HGT testing consists of egocentric, allocentric, and combined variants
significant differences were observed between MCI and healthy controls
high negative predictive values suggested strong screening potential for HGT
Acknowledgments
Funding and author contribution
This work was funded by The Croatian Science Foundation grants IP-2014-09-9730 and IP-2019-04-3584 to GŠ, by the Scientific Centre of Excellence for Basic, Clinical and Translational Neuroscience CoRE-NEURO (“Experimental and clinical research of hypoxic-ischemic damage in perinatal and adult brain”; GA KK01.1.1.01.0007 funded by the European Union through the European Regional Development Fund), by HAMAG-BICRO (University of Zagreb), and in part by the NIH grant P50 AG005138 to PRH. GŠ conceived and directed the project, coordinated testing, and wrote the first draft of the paper; DB and MBR performed testing of subjects; IF designed the software and hardware of the ALZENTIA system with the help of GŠ, DB and MBL; MBL, GŠ, and DB performed the statistical analysis; PRH substantially contributed to the interpretation of data; all authors contributed to revising and editing the manuscript critically for important intellectual content. All authors approved the final version of the manuscript.
Footnotes
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
Ethics approvals
All procedures were in accordance with the Helsinki Declaration (World Health Organization, 2013) and were approved by the Ethical Committee of the Clinical Hospital Center Zagreb (case no. 02/21 AG, class 8.1-18/82-2 from April 24, 2018) and by the Central Ethical Committee of the University of Zagreb School of Medicine (case no. 380-59-10106-18-111/126, class 641-01/18-02/01 from June 20, 2018).
Conflict of interest
The authors declare no conflict of interest.
References
- Aguirre GK, D’Esposito M, 1999. Topographical disorientation: a synthesis and taxonomy. Brain 122, 1613–1628. [DOI] [PubMed] [Google Scholar]
- Albert MS, DeKosky ST, Dickson D, Dubois B, Feldman HH, Fox NC, Gamst A, Holtzman DM, Jagust WJ, Petersen RC, Snyder PJ, Carrillo MC, Thies B, Phelps CH, 2011. The diagnosis of mild cognitive impairment due to Alzheimer’s disease: recommendations from the National Institute on Aging-Alzheimer’s Association workgroups on diagnostic guidelines for Alzheimer’s disease. Alzheimers Dement. 7, 270–279. [DOI] [PMC free article] [PubMed] [Google Scholar]
- American Psychiatric Association, 2013. Diagnostic and Statistical Manual of Mental Disorders (Fifth ed.). Arlington, VA, USA: American Psychiatric Publishing. [Google Scholar]
- Arevalo-Rodriguez I, Smailagic N, Roqué I Figuls M, Ciapponi A, Sanchez-Perez E, Giannakou A, Pedraza OL, Bonfill Cosp X, Cullum S, 2015. Mini-Mental State Examination (MMSE) for the detection of Alzheimer’s disease and other dementias in people with mild cognitive impairment (MCI). Cochrane Database Syst. Rev 3, CD010783. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Astur RS, Taylor LB, Mamelak AN, Philpott L, Sutherland RJ, 2002. Humans with hippocampus damage display severe spatial memory impairments in a virtual Morris water task. Behav. Brain Res 132, 77–84. [DOI] [PubMed] [Google Scholar]
- Babić Leko M, Borovečki F, Dejanović N, Hof PR, Šimić G, 2016. Predictive value of cerebrospinal fluid visinin-like protein-1 levels for Alzheimer’s disease early detection and differential diagnosis in patients with mild cognitive impairment. J. Alzheimers Dis 50, 765–778. [DOI] [PubMed] [Google Scholar]
- Boban M, Malojčić B, Mimica N, Vuković S, Zrilić I, Hof PR, Šimić G, 2012. The reliability and validity of the Mini-Mental State Examination in the elderly Croatian population. Dement. Geriatr. Cogn. Disord 33, 385–392. [DOI] [PubMed] [Google Scholar]
- Bohbot VD, Jech R, Růžička E, Nadel L, Kalina M, Stepánková K, Bureš J, 2002. Rat spatial memory tasks adapted for humans: characterization in subjects with intact brain and subjects with selective medial temporal lobe thermal lesions. Physiol. Res 51 (Suppl. 1), S49–56. [PubMed] [Google Scholar]
- Bohbot VD, Kalina M, Stêpánková K, Spackova N, Petrides M, Nadel LY, 1998 Spatial memory deficits in patients with lesions to the right hippocampus and to the right parahippocampal cortex. Neuropsychologia 1998; 36: 1217–1238. [DOI] [PubMed] [Google Scholar]
- Burgess N, Maguire EA, O’Keefe J, 2002. The human hippocampus and spatial and episodic memory. Neuron 35, 625–641. [DOI] [PubMed] [Google Scholar]
- Coughlan G, Laczó J, Hort J, Minihane AM, Hornberger M, 2018. Spatial navigation deficits - overlooked cognitive marker for preclinical Alzheimer disease? Nat. Rev. Neurol 14, 496–506. [DOI] [PubMed] [Google Scholar]
- Feigenbaum JD, Morris RG, 2004. Allocentric versus egocentric spatial memory after unilateral temporal lobectomy in humans. Neuropsychology 18, 462–472. [DOI] [PubMed] [Google Scholar]
- Filimon F, 2015. Are all spatial reference frames egocentric? Reinterpreting evidence for allocentric, object-centered, or world-centered reference frames. Front. Hum. Neurosci 9: 648. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hampel H, Prvulovic D, Teipel S, Jessen F, Luckhaus C, Frölich L, Riepe MW, Dodel R, Leyhe T, Bertram L, Hoffmann W, Faltraco F, German Task Force on Alzheimer’s Disease (GTF-AD), 2011. The future of Alzheimer’s disease: the next 10 years. Prog. Neurobiol 95, 718–728. [DOI] [PubMed] [Google Scholar]
- Hort J, Laczó J, Vyhnálek M, Bojar M, Bures J, Vlček K, 2007. Spatian navigation deficit in amnestic mild cognitive impairment. Proc. Natl. Acad. Sci. U. S. A 104, 4042–4047. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Howett D, Castegnaro A, Krzywicka K, Hagman J, Marchment D, Henson R, Rio M, King JA, Burgess N, Chan D, 2019. Differentiation of mild cognitive impairment using an entorhinal cortex-based test of virtual reality navigation. Brain 142, 1751–1766. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Iaria G, Petrides M, Dagher A, Pike B, Bohbot VD, 2003. Cognitive strategies dependent on the hippocampus and caudate nucleus in human navigation: variability and change with practice. J. Neurosci 23, 5945–5952. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jheng SS, Pai MC, 2009. Cognitive map in patients with mild Alzheimer’s disease: A computer-generated arena study. Behav. Brain Res 200, 42–47. [DOI] [PubMed] [Google Scholar]
- Kiđemet-Piskač S, Babić Leko M, Blažeković A, Langer Horvat L, Klepac N, Sonicki Z, Kolenc D, Hof PR, Boban M, Mimica N, Borovečki F, Šimić G, 2018. Evaluation of cerebrospinal fluid phosphorylated tau231 as a biomarker in the differential diagnosis of Alzheimer’s disease and vascular dementia. CNS Neurosci. Ther 24, 734–740. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lehnung M, Leplow B, Friege L, Herzog A, Ferstl R, Mehdorn M. Development of spatial memory and spatial orientation in preschoolers and primary school children. Brit. J. Psychol 1998; 89: 463–480. [DOI] [PubMed] [Google Scholar]
- Maguire EA, Burgess N, Donnett JG, Frackowiak RS, Frith CD, O’Keefe J, 1998. Knowing where and getting there: a human navigation network. Science 280, 921–924. [DOI] [PubMed] [Google Scholar]
- McKhann GM, Knopman DS, Chertkow H, Hyman BT, Jack CR Jr., Kawas CH, Klunk WE, Koroshetz WJ, Manly JJ, Mayeux R, Mohs RC, Morris JC, Rossor MN, Scheltens P, Carrillo MC, Thies B, Weintraub S, Phelps CH, 2011. The diagnosis of dementia due to Alzheimer’s disease: recommendations from the National Institute on Aging-Alzheimer’s Association workgroups on diagnostic guidelines for Alzheimer’s disease. Alzheimers Dement. 7, 263–269. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Modrego PJ. Predictors of conversion to dementia of probable Alzheimer type in patients with mild cognitive impairment. Curr. Alzheimer Res 2006; 3: 161–170. [DOI] [PubMed] [Google Scholar]
- Monacelli AM, Cushman LA, Kavcic V, Duffy CJ, 2003. Spatial disorientation in Alzheimer’s disease: the remembrance of things passed. Neurology 61, 1491–1497. [DOI] [PubMed] [Google Scholar]
- Morris RG, Garrud P, Rawlins JA, O’Keefe J, 1982. Place navigation impaired in rats with hippocampal lesions. Nature 297, 681–683. [DOI] [PubMed] [Google Scholar]
- Neary D, Snowden JS, Gustafson L, Passant U, Stuss D, Black S, Freedman M, Kertesz A, Robert PH, Albert M, Boone K, Miller BL, Cummings J, Benson DF, 1998. Frontotemporal lobar degeneration: a consensus on clinical diagnostic criteria. Neurology 51, 1546–1554. [DOI] [PubMed] [Google Scholar]
- O’Bryant SE, Humphreys JD, Smith GE, Ivnik RJ, Graff-Radford NR, Petersen RC, Lucas JA, 2008. Detecting dementia with the Mini-Mental State Examination in highly educated individuals. Arch. Neurol 65, 963–967. [DOI] [PMC free article] [PubMed] [Google Scholar]
- O’Keefe J, Nadel L, 1978. The hippocampus as a cognitive map. Oxford University Press: London, UK. [Google Scholar]
- Overman WH, Pate BJ, Moore K, Peuster A, 1996. Ontogeny of place learning in children as measured in the radial arm maze, Morris search task, and open field task. Behav. Neurosci 110, 1205–1228. [DOI] [PubMed] [Google Scholar]
- Parslow DM, Morris RG, Fleminger S, Rahman Q, Abrahams S, Recce M, 2005. Allocentric spatial memory in humans with hippocampal lesions. Acta Psychol. 118: 123–147. [DOI] [PubMed] [Google Scholar]
- Petersen RC, Smith GE, Waring SC, Ivnik RJ, Tangalos EG, Kokmen E, 1999. Mild cognitive impairment: clinical characterization and outcome. Arch. Neurol 56, 303–308. [DOI] [PubMed] [Google Scholar]
- Roberts R, Knopman DS, 2013. Classification and epidemiology of MCI. Clin. Geriatr. Med 29, 753–772. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sachdev PS, Lipnicki DM, Kochan NA, Crawford JD, Thalamuthu A, Andrews G, Brayne C, Matthews FE, Stephan BC, Lipton RB, Katz MJ, Ritchie K, Carrière I, Ancelin ML, Lam LC, Wong CH, Fung AW, Guaita A, Vaccaro R, Davin A, Ganguli M, Dodge H, Hughes T, Anstey KJ, Cherbuin N, Butterworth P, Ng TP, Gao Q, Reppermund S, Brodaty H, Schupf N, Manly J, Stern Y, Lobo A, Lopez-Anton R, Santabárbara J, Cohort Studies of Memory in an International Consortium (COSMIC), 2013. The prevalence of mild cognitive impairment in diverse geographical and ethnocultural regions: the COSMIC collaboration. PLoS One 10, e0142388. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Stěpánková K, Pastalkova E, Kalova E, Kalina M, Bureš J, 2003. A battery of tests for quantitative examination of idiothetic and allothetic place navigation modes in humans. Behav. Brain Res 147: 95–105. [DOI] [PubMed] [Google Scholar]
- Vlček K, Laczó J, 2014. Neural correlates of spatial navigation changes in mild cognitive impairment and Alzheimer’s disease. Front. Behav. Neurosci 8, 89. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Weniger G, Ruhleder M, Lange C, Wolf S, Irle E, 2011. Egocentric and allocentric memory as assessed by virtual reality in individuals with amnestic mild cognitive impairment. Neuropsychologia 49: 518–527. [DOI] [PubMed] [Google Scholar]
- Weniger G, Siemerkus J, Schmidt-Samoa C, Mehlitz M, Baudewig J, Dechent P, Irle E, 2010. The human parahippocampal cortex subserves egocentric spatial learning during navigation in a virtual maze. Neurobiol. Learn. Mem 93, 46–55. [DOI] [PubMed] [Google Scholar]
- World Medical Association, 2013. World Medical Association Declaration of Helsinki: ethical principles for medical research involving human subjects. JAMA 310, 2191–2194. [DOI] [PubMed] [Google Scholar]


