Abstract
We herein report a case of anti-RANKL monoclonal antibody-associated membranous nephropathy (MN). A 67-year-old woman with a history of rheumatoid arthritis treated with prednisolone and methotrexate for more than 30 years and osteoporosis treated with eldecalcitol and teriparatide for 4 years had achieved a stable disease condition. Her kidney function was normal and her urinalysis was negative for hematuria and proteinuria. An anti-RANKL monoclonal antibody (denosumab) was administered for the treatment of osteoporosis. Four months later, proteinuria appeared (2.3 g/g creatinine) and remained positive for about 6 months, therefore, she was admitted to our hospital. An immunofluorescence study revealed fine granular deposits of immunoglobulin G (IgG) and C3 along the capillary walls. Staining for IgG subclasses showed positive staining for IgG1 (3+), IgG2 (1+), IgG3 (1+), and IgG4 (1+); phospholipase A2 receptor was negative. Electron microscopy showed partial subepithelial and intramembranous deposits and focal thickening of the glomerular basement membrane. No evidence of malignancy or infectious disease was seen. After cessation of denosumab, the proteinuria gradually improved. Based on the renal biopsy results and clinical course (development of marked proteinuria in the presence of denosumab with subsequent amelioration in the absence of the drug), we diagnosed the patient with secondary MN due to denosumab. This is the first reported case of denosumab-associated MN.
Keywords: Membranous nephropathy, Anti-RANKL monoclonal antibody, Rheumatoid arthritis
Introduction
Membranous nephropathy (MN) is the second common etiology of primary nephrotic syndrome in Japan [1]. About 80% of patients with MN are diagnosed with idiopathic MN, whereas about 20–25% are diagnosed with secondary MN due to autoimmune diseases, infections, malignancy, and drugs [2, 3]. Drug-induced MN (DIMN) accounts for 6.6% [4] to 14.0% [5] of all cases of MN. Secondary MN induced by drugs such as antirheumatic drugs, nonsteroidal anti-inflammatory drugs, and antihypertensive drugs has been reported [3].
Denosumab is a fully human monoclonal antibody that selectively binds with the receptor activator of nuclear factor kappa-B ligand (RANKL), a cytokine essential for the formation, function, and survival of osteoclasts [6]. By binding to RANKL on the surface of osteoclasts and their precursors, denosumab inhibits osteoclast-mediated bone resorption [7]. Denosumab is used to treat osteoporosis. Critical adverse effects of denosumab include hypocalcemia [8] and osteonecrosis of the jaw [9]. However, no case reports have described glomerulonephritis associated with denosumab.
We herein report a case of MN that developed during anti-RANKL monoclonal antibody treatment for osteoporosis.
Case report
The patient was a 67-year-old Japanese woman with a history of rheumatoid arthritis treated with prednisolone and methotrexate for more than 30 years and osteoporosis treated with eldecalcitol and teriparatide for 4 years. Her disease condition was stable throughout her treatment history. Her kidney function was normal, and her urinalysis was negative for hematuria and proteinuria. The patient was also being treated with bisphosphonate drugs (risedronate sodium hydrate and minodronic acid hydrate) because of low bone density (young adult mean 55%). However, she could not continue to use these drugs because of the development of fever. Therefore, she was administered denosumab, an anti-RANKL monoclonal antibody, for the treatment of osteoporosis. Three months later, proteinuria appeared (2.3 g/gCr) and remained positive for about 6 months. At this point, she was admitted to our hospital.
On admission, the patient was 155 cm tall and weighed 48 kg. Her blood pressure was 147/88 mmHg. Table 1 shows the examination findings upon admission. The spot urinary protein concentration was 2.8 g/gCr. She showed a decreased serum albumin concentration of 3.6 g/dL and a normal serum creatinine concentration of 0.8 mg/dL. Her serum antinuclear antibody level was × 80, but anti-ds-DNA and anti-ss-DNA antibodies were negative and complement levels were not decreased. Anti-hepatitis B surface antigen and anti-hepatitis C virus antibodies were negative. Computed tomography at the previous hospital revealed no evidence of a tumor.
Table 1.
Laboratory data at admission
| Peripheral blood | Serological test | ||
| White blood cell | 6570/μL | Immunoglobulin G | 1022 mg/dL |
| Red blood cell count | 428 × 104/μL | Immunoglobulin A | 242 mg/dL |
| Hemoglobin | 12.6 g/dL | Immunoglobulin M | 76 mg/dL |
| Hematocrit | 38.8% | Complement activity | 69 U/mL |
| Platelets | 32.9 × 104/μL | C3 component | 105 mg/dL |
| Blood chemistry | C4 component | 20 mg/dL | |
| Total protein | 6.5 g/dL | Antinuclear antibody | 80 |
| Albumin | 3.6 g/dL | Anti-ds-DNA | 0.8 IU/mL |
| Aspartate aminotransferase | 38 IU/L | Anti-ss-DNA | 1.5AU/mL |
| Alanine aminotransferase | 42 IU/L | Rheumatoid factor | 60 U/mL |
| Lactate dehydrogenase | 283 IU/L | Anti-CCP antibody | 1.1 U/mL |
| Total cholesterol | 235 mg/dL | Anti-RNP antibody | 0.3 U/mL |
| LDL cholesterol | 141 mg/dL | Anti-Scl-70 antibody | 7.0 U/mL |
| Triglycerides | 104 mg/dL | Anti-Sm antibody | 0.3 U/mL |
| Uric acid | 4.5 mg/dL | Anti-SS-A/Ro antibody | 0.2 U/mL |
| Urea nitrogen | 7.8 mg/dL | Anti-SS-B/La antibody | 0.2 U/mL |
| Creatinine | 0.77 mg/dL | Anti-centromere antibody | 1.3 U/mL |
| eGFR | 57 mL/min/1.73 m2 | MPO-ANCA | 0.1 U/mL |
| Sodium | 145 mEq/L | PR3-ANCA | 0.1 U/mL |
| Potassium | 4.2 mEq/L | Hepatitis B virus, surface antigen | (–) |
| Chloride | 106 mEq/L | Hepatitis C virus antibody | (–) |
| C-reactive protein | 0.28 mg/dL | Urinalysis | |
| SAA | 54.1 μg/mL | Gravity | 1.005 |
| pH | 6.5 | ||
| Protein | 2+ | ||
| 2.8 g/gCr | |||
| Occult blood | (–) | ||
| Glucose | (–) | ||
| Ketone | (–) | ||
| NAG | 4.5 U/L | ||
| β2MG | 294 μg/L | ||
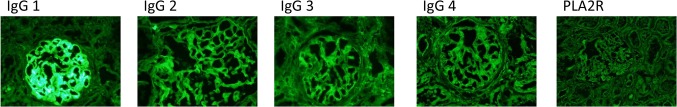
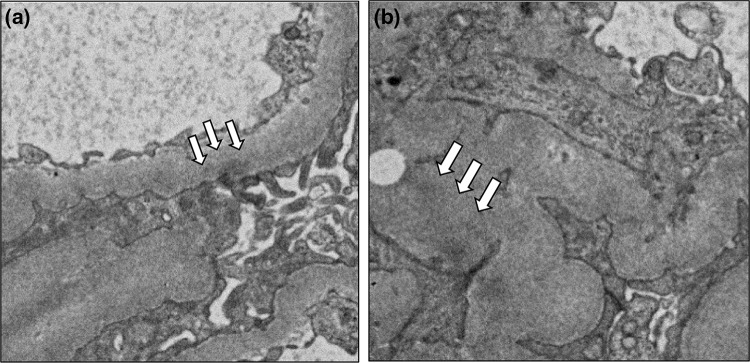
Renal biopsy was performed 6 months after administration of denosumab. Light microscopy showed 12 normal glomeruli and 1 globally sclerotic glomerulus (Fig. 1a). Immunofluorescence staining showed deposition of immunoglobulin G (IgG) and C3 in the glomerular basement membrane. Staining for IgA and IgM was negative (Fig. 1b). IgG subclass analysis revealed predominant deposition of IgG1 and weak deposition of IgG2, IgG3, and IgG4. Staining for the phospholipase A2 receptor (PLA2R) was negative (Fig. 2). Electron microscopy revealed granular electron-dense deposits in the subepithelial and intramembranous regions of the glomerular basement membrane (Fig. 3). The histological features were compatible with the diagnosis of secondary MN.
Fig. 1.
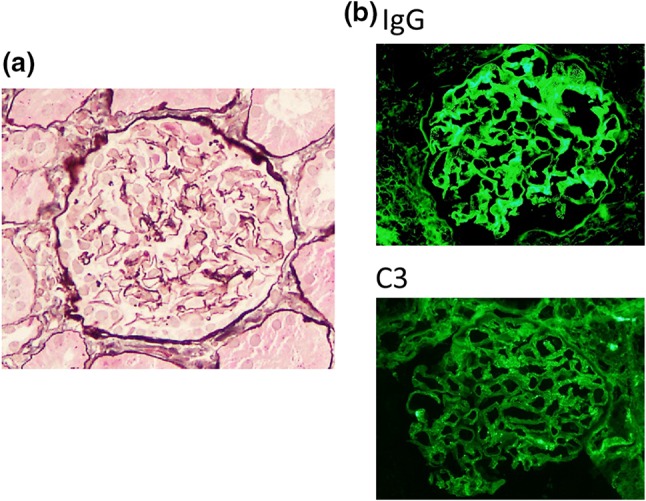
Light microscopy and immunofluorescence findings. a Light microscopy findings. Renal biopsy samples revealed 12 normal glomeruli and 1 globally sclerotic glomerulus. There were no remarkable changes (original magnification × 200). b Immunofluorescence findings. Granular deposition of IgG was noted as a peripheral pattern. IgA and IgM were not seen. Granular deposition of C3 was also noted as a peripheral pattern
Fig. 2.
IgG subclass findings. Immunofluorescence IgG subclass analysis showed a predominance of IgG1 deposition followed by IgG2, IgG3, and IgG4 in a peripheral granular pattern. Anti-PLA2R deposits were negative
Fig. 3.
Electron microscopic findings. Focal thickening of the glomerular basement membrane was seen. In some glomerular capillary walls, a subepithelial (arrows) and b intramembranous small dense deposits (arrows) were seen
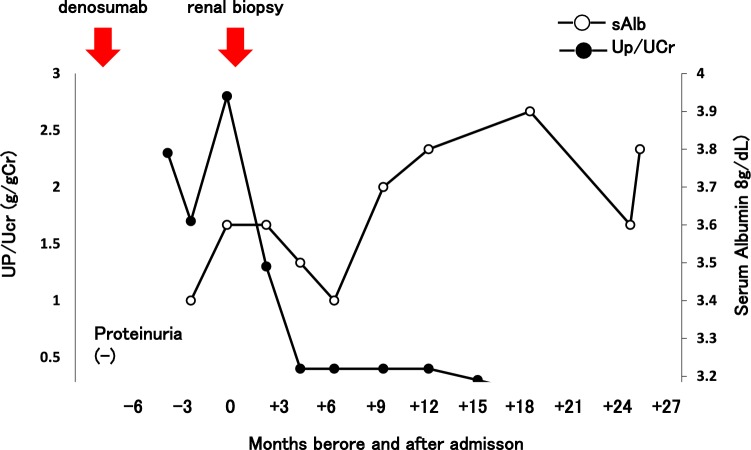
At 4–6 months after beginning the administration of denosumab, the urinary protein concentration was about 2 g/gCr, but it gradually decreased. In contrast, the serum albumin concentration was elevated (Fig. 4). We diagnosed MN associated with denosumab and did not administer denosumab thereafter. In patients with severe symptoms of nephrotic syndrome and/or lack of improvement after withdrawal, immunosuppressive therapy may be warranted [10]. In the present case, the patient had no symptoms and the urinary protein concentration was not severe. Therefore, she underwent follow-up with no treatment for MN. About 2.5 years after beginning the administration of denosumab, her urinary protein concentration was almost normal (0.1 g/gCr).
Fig. 4.
Clinical course. After administration of denosumab, urinary protein was positive. After cessation of denosumab, urinary protein gradually decreased and the serum albumin concentration gradually recovered. About 2.5 years after administration of denosumab, urinary protein was almost normal (0.1 g/gCr). sAlb serum albumin, UP/UCr urine protein/urine creatinine ratio, eGFR estimated glomerular filtration rate
Discussion
We here reported a case of denosumab-associated MN in a patient with rheumatoid arthritis. The Japan renal biopsy registry showed that glomerular disorders are the most common cases of drug-induced kidney diseases, in particular, MN accounts for 60% of glomerular disorders [11]. Several culprit drugs of MN have been reported, including gold salts, penicillamine, bucillamine, mercury, captopril, and nonsteroidal anti-inflammatory drugs [10]. The pathogenesis of drug-induced MN involves an immune response to a therapeutic agent or its byproduct; specifically, cationic drug-derived antigens that traverse the glomerular basement membrane are planted at the subepithelial aspect of the glomerular basement membrane, and become bound in situ to circulating antibodies directed against these antigens [10]. Pathologic findings do not distinguish between primary MN and drug-induced MN, highlighting the importance of obtaining a detailed clinical history regarding drug use [10].
Denosumab is an anti-RANKL monoclonal neutralizing antibody used to treat osteoporosis and cancer-induced bone diseases. RANKL and its receptor RANK are important regulators of T cells [12]; however, RANKL and RANK mechanism of kidney disease are not fully understood. Liu et al. reported that RANKL and RANK were increased in the two human podocyte diseases: minimal change nephrotic syndrome and MN, and RANK was localized in podocytes in a podocyte injury rat model [13]. Based on these previous reports, we speculate that denosumab can bind to RANKL/RANK on podocytes, which alters the RANK structure and induces the formation of antibodies against RANK. This results in the formation of subepithelial immune complexes and the subsequent development of MN. Another possibility is that administration of denosumab directly affects T-cell functions, thereby resulting in the formation of renal subepithelial RANK–RANKL immune complexes.
Recently, the PLA2R has been identified as a target antigen in primary MN. The serum anti-PLA2R antibody titer is positive in 70% of patients with primary MN [14]. Conversely, the prevalence of anti-PLA2R antibody in Japanese patients with primary MN is 50% [15]. Considering histological findings, the prevalence of anti-PLA2R antibody increased to 70% [16]. Furthermore, the main IgG subclass of IgG deposition in idiopathic MN is IgG4. In contrast, IgG1 and IgG2 are dominant in secondary MN due to systemic lupus erythematosus [17]. In cases of MN associated with bucillamine, which is used for rheumatoid arthritis therapy, IgG1 or IgG2 is equivalent or dominant to IgG4 [18]. Moreover, the IgG subclasses in patients with malignancy-associated MN are similar to those in patients with bucillamine-associated MN [19].
In the present case, immunostaining of glomerular anti-PLA2R antibody was negative, and IgG1 immunostaining was dominantly positive. Although the renal histology findings did not confirm primary or secondary MN, other causes of secondary MN, such as infection, malignancy, autoimmune disease other than rheumatoid arthritis, and potential drugs were ruled out. We are aware that this patient had a history of rheumatoid arthritis, which is also a main cause of secondary MN, for more than 30 years; however, at the time of this writing, her renal function was still very stable and she was negative for proteinuria. Furthermore, the patient had been transiently treated with a gold salt drug more than 30 years previously and had never been treated with bucillamine.
The most important point in this patient’s clinical course is that marked proteinuria appeared after a single administration of denosumab, and it was ameliorated after discontinuation of the drug. With respect to the very slow improvement of this patient’s proteinuria, we speculate that because denosumab is a very long-acting drug that should be administered once every 6 months, improvement of the patient’s proteinuria took a long time.
The culprit drugs of drug-induced MN are mainly oral drugs administered every day. However, we must pay attention to the fact that long-acting drugs such as denosumab (administered every 6 months) can also cause drug-induced MN.
The patient’s renal biopsy results and clinical course (appearance of marked proteinuria in the presence of denosumab with gradual amelioration after discontinuation of the drug) led to the diagnosis of secondary MN associated with denosumab. Denosumab is now used as an antiresorptive drug for the treatment of osteoporosis in multiple countries, thus, routine urinalysis should be considered whenever denosumab is administered.
Acknowledgements
We thank Angela Morben, DVM, ELS, from Edanz Group (http://www.edanzediting.com/ac), for editing a draft of this manuscript.
Compliance with ethical standards
Conflict of interest
All the authors have declared that they have no competing interest.
Human and animal rights
This article does not contain any studies with human participants or animals performed by any of the authors.
Informed consent
Informed consent was obtained from all individual participants included in the study.
Footnotes
Publisher's Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
References
- 1.Yokoyama H, Taguchi T, Sugiyama H, et al. Membranous nephropathy in Japan: analysis of the Japan Renal Biopsy Registry (J-RBR) Clin Exp Nephrol. 2012;16:557–563. doi: 10.1007/s10157-012-0593-7. [DOI] [PubMed] [Google Scholar]
- 2.Ronco P, Debiec H. Molecular pathomechanisms of membranous nephropathy: from Heymann nephritis to alloimmunization. J Am Soc Nephrol. 2005;16:1205–1213. doi: 10.1681/ASN.2004121080. [DOI] [PubMed] [Google Scholar]
- 3.Nawaz FA, Larsen CP, Troxell ML. Membranous nephropathy and nonsteroidal anti-inflammatory agents. Am J Kidney Dis. 2013;62:1012–1017. doi: 10.1053/j.ajkd.2013.03.045. [DOI] [PubMed] [Google Scholar]
- 4.Glassock RJ. Secondary membranous glomerulonephritis. Nephrol Dial Transplant. 1992;7(Suppl 1):64–71. [PubMed] [Google Scholar]
- 5.Rihova Z, Honsova E, Merta M, et al. Secondary membranous nephropathy—one center experience. Ren Fail. 2005;27:397–402. [PubMed] [Google Scholar]
- 6.Boyle WJ, Simonet WS, Lacey DL. Osteoclast differentiation and activation. Nature. 2003;423:337–342. doi: 10.1038/nature01658. [DOI] [PubMed] [Google Scholar]
- 7.Delmas PD. Clinical potential of RANKL inhibition for the management of postmenopausal osteoporosis and other metabolic bone diseases. J Clin Densitom. 2008;11:325–338. doi: 10.1016/j.jocd.2008.02.002. [DOI] [PubMed] [Google Scholar]
- 8.Chen Q, Hu C, Liu Y, et al. Pharmacokinetics, pharmacodynamics, safety, and tolerability of single-dose denosumab in healthy Chinese volunteers: a randomized, single-blind, placebo-controlled study. PLoS One. 2018;13:e0197984. doi: 10.1371/journal.pone.0197984. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Aljohani S, Gaudin R, Weiser J, et al. Osteonecrosis of the jaw in patients treated with denosumab: a multicenter case series. J Craniomaxillofac Surg. 2018;46:1515–1525. doi: 10.1016/j.jcms.2018.05.046. [DOI] [PubMed] [Google Scholar]
- 10.Hogan JJ, Markowitz GS, Radhakrishnan J. Drug-induced glomerular disease: immune-mediated injury. Clin J Am Soc Nephrol. 2015;10:1300–1310. doi: 10.2215/CJN.01910215. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Yokoyama H, Narita I, Sugiyama H, et al. Drug-induced kidney disease: a study of the Japan Renal Biopsy Registry from 2007 to 2015. Clin Exp Nephrol. 2016;20:720–730. doi: 10.1007/s10157-015-1201-4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Anderson DM, Maraskovsky E, Billingsley WL, et al. A homologue of the TNF receptor and its ligand enhance T-cell growth and dendritic-cell function. Nature. 1997;390:175–179. doi: 10.1038/36593. [DOI] [PubMed] [Google Scholar]
- 13.Liu S, Shi W, Xiao H, et al. Receptor activator of NF-kappa B and podocytes: towards a function of a novel receptor-ligand pair in the survival response of podocyte injury. PLoS One. 2012;7:e41331. doi: 10.1371/journal.pone.0041331. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Beck LH, Bonegio RG, Lambeau G, et al. M-type phospholipase A2 receptor as target antigen in idiopathic membranous nephropathy. N Engl J Med. 2009;361:11–21. doi: 10.1056/NEJMoa0810457. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Akiyama S, Akiyama M, Imai E, et al. Prevalence of anti-phospholipase A2 receptor antibodies in Japanese patients with membranous nephropathy. Clin Exp Nephrol. 2015;19:653–660. doi: 10.1007/s10157-014-1054-2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Hayashi N, Akiyama S, Okuyama H, et al. Clinicopathological characteristics of M-type phospholipase A2 receptor (PLA2R)-related membranous nephropathy in Japanese. Clin Exp Nephrol. 2015;19:797–803. doi: 10.1007/s10157-014-1064-0. [DOI] [PubMed] [Google Scholar]
- 17.Imai H, Hamai K, Komatsuda A, et al. IgG subclasses in patients with membranoproliferative glomerulonephritis, membranous nephropathy, and lupus nephritis. Kidney Int. 1997;51:270–276. doi: 10.1038/ki.1997.32. [DOI] [PubMed] [Google Scholar]
- 18.Nagahama K, Matsushita H, Hara M, et al. Bucillamine induces membranous glomerulonephritis. Am J Kidney Dis. 2002;39:706–712. doi: 10.1053/ajkd.2002.31987. [DOI] [PubMed] [Google Scholar]
- 19.Ohtani H, Wakui H, Komatsuda A, et al. Distribution of glomerular IgG subclass deposits in malignancy-associated membranous nephropathy. Nephrol Dial Transplant. 2004;19:574–579. doi: 10.1093/ndt/gfg616. [DOI] [PubMed] [Google Scholar]