Abstract
Renal tubular acidosis (RTA) is a rare disease caused by a defect of urinary acidification. The ammonium chloride loading test is the gold standard method for determining the type of RTA. However, because this test has some side effects (e.g., nausea, vomiting, and stomach discomfort), applying this test for pediatric cases is difficult. Recently, a loading test with the combination of furosemide and fludrocortisone was reported to be an alternative to the ammonium chloride loading test, with 100% sensitivity and specificity in adult’s cases. We report the first pediatric case of distal RTA in a patient who was successfully diagnosed by a drug loading test with the combination of furosemide and fludrocortisone without any side effects. We also performed genetic analysis and detected a known pathogenic variant in the SLC4A1 gene. The combination loading test of furosemide and fludrocortisone is a useful and safe diagnostic tool for pediatric cases of RTA.
Keywords: Furosemide, Fludrocortisone, Loading test, SLC4A1, Autosomal dominant distal renal tubular acidosis, Rickets, Short stature
Introduction
Renal tubular acidosis (RTA) is a group of disorders with urinary acidification disability and it causes metabolic acidosis with a normal plasma anion gap without diarrhea. RTA is classified into four types. Type 1 is distal RTA (dRTA), which is caused by impaired excretion of H+. Type 2 is proximal RTA caused by reabsorption failure of HCO3−. Type 3 is a mixed type of types 1 and 2. Type 4 is hyperkalemic RTA caused by a lack or failure of aldosterone function at the distal tubule. The ammonium chloride loading test is the gold standard method for determining the type of RTA, especially for the differential diagnosis of types 1 and 2 RTA. Using this test, patients in whom urinary pH remains above 5.5, despite strong acidemia with a pH of 7.3 or less, are diagnosed with dRTA. However, ammonium chloride stimulates the stomach. Therefore, this loading test often causes abdominal discomfort, nausea, or vomiting. Therefore, the ammonium chloride loading test is not often applied to cases of suspected RTA, especially for children. Therefore, the furosemide loading test was developed as an alternative to ammonium chloride loading with fewer side effects. When the cut off of this test is set to a urinary pH greater than 5.5 for the diagnosis of dRTA, the sensitivity is 100%, but it has a relatively low specificity of 82–89% [1, 2]. A previous report showed that even healthy volunteers had a urine pH less than 5.5 following a loop diuretic of only 50% with oral administration and 75% after intravenous administration. Guerra-Hernandez NE et al. also reported that the urinary acidification test with furosemide alone was performed with 30 children and showed some false positives [3, 4]. These data suggest that the furosemide loading test still has a problem of specificity.
Recent reports showed that a loading test with the combination of furosemide and fludrocortisone could be an alternative to the ammonium chloride loading test with 100% sensitivity and specificity [5, 6]. The authors of these reports regarded a urinary pH greater than 5.3 as the diagnostic criterion for dRTA. However, there have been no reports on this combination loading test applied to children.
We report here a genetically proven case of autosomal dominant dRTA in a patient with a short stature and rickets in childhood. To the best of our knowledge, this is the first pediatric case report of a loading test by furosemide and fludrocortisone.
Case report
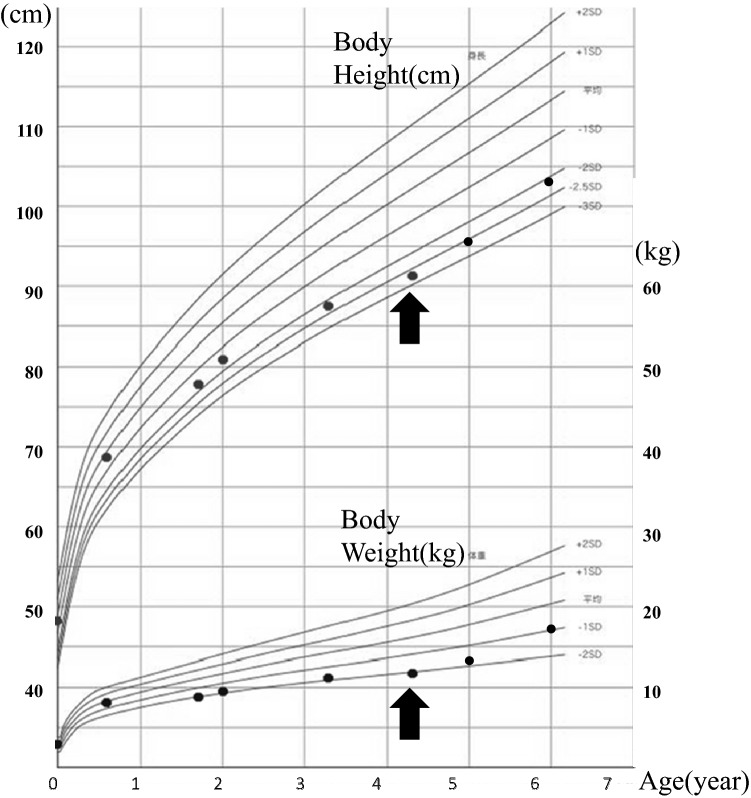
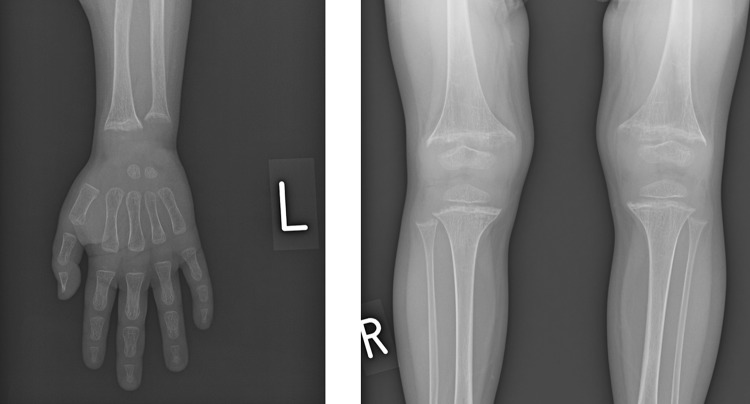
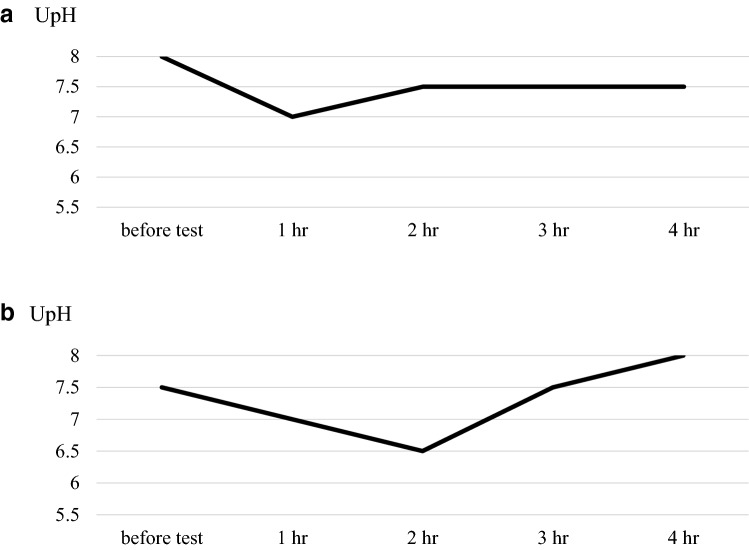
A 4-year-old boy was referred to our hospital because of a short stature and gait abnormality. His parents noticed his gait abnormality and visited a local orthopedic clinic, but a lower limb X-ray examination showed no remarkable findings. He was born at full term, by normal vaginal delivery, with a birth weight of 2780 g, and showed normal development. There was no history of short stature in his family. He often played outside and had no food allergy or unbalanced diet. At the initial visit, his height was 91.9 cm (− 2.7 SD) and his weight was 11.8 kg (− 2.1 SD) (Fig. 1). He walked in a bow-legged manner. A blood examination showed hypokalemia, hyperphosphatasemia, hypophosphatemia, and a low 25(OH) vitamin D level. Blood gas analysis showed hyperchloremic metabolic acidosis with a normal plasma anion gap. Urinalysis showed a relatively high urine pH and hypercalciuria (Table 1). X-ray examinations showed cupping and fraying signs on his wrist and knee (Fig. 2). Abdominal ultrasonography showed nephrocalcinosis (Fig. 3). Diuretic tests with furosemide alone and the combination of furosemide and fludrocortisone were conducted following the protocols previously reported [7], Furosemide was given at a dose of 1 mg/kg by intravenous route and fludrocortisone was given at a dose of 1 mg/1.73 m2 by oral. Urine samples were collected every hour from spontaneous voiding for a period of 4 h. The patient was not fasting before tests and allowed drinking water during tests. We left 24 h between tests. And, both showed urine acidification defects (Fig. 4). Although this case showed typical finding for distal RTA, we conducted this test under the consent of the parents to make a definitive diagnosis and to understand the pathophysiology of this case having severe acidosis. We conducted targeted sequence analysis with a next generation sequencer and detected a known pathogenic missense mutation at exon 14 (c.1766G>A, p.Arg589His) in SLC4A1 (NM_000342.3) [8] (a list of genes is shown in Table 2). This variant was confirmed by the Sanger method. We also performed genetic analysis for the parents and neither of them had the same variant. We diagnosed the patient as a sporadic case of autosomal dominant dRTA. After starting oral alkali therapy, the metabolic acidosis and hypokalemia were soon normalized, and his growth rate improved (Fig. 1).
Fig. 1.
Growth curve. The black points represent height and body weight at an examination. The arrow shows the point of starting oral alkali therapy
Table 1.
Laboratory data of the patient on admission
| Blood | Urine | ||
|---|---|---|---|
| Venous blood gas | Urinalysis | ||
| pH | 7.287 | pH | 7.5 |
| pCO2 | 31.1 mmHg | Specific gravity | 1.01 |
| HCO3− | 14.5 mmol/L | Protein | (1+) |
| Base excess | − 10.7 mmol/L | Glucose | (−) |
| Complete blood count | Blood | (−) | |
| White blood cell | 9300/µL | Urine chemistry | |
| Hemoglobin | 16.2 g/dL | Sodium | 61 mmol/L |
| Platelet count | 35 × 104 /µL | Potassium | 25.1 mmol/L |
| Serum chemistry | Chloride | 48 mmol/L | |
| Albumin | 4.9 g/dL | Anion gap | 38.1 mmol/L |
| Asparate amino transferase | 26 U/L | Calcium | 9.3 mg/dL |
| Alanine transaminase | 9 U/L | Creatinine | 28.27 mg/dL |
| Alkaline phosphatase | 2378 U/L | ||
| Blood urea nitrogen | 8.1 mg/dL | ||
| Creatinine | 0.24 mg/dL | ||
| Sodium | 137 mmol/L | ||
| Potassium | 2.5 mmol/L | ||
| Chloride | 113 mmol/L | ||
| Anion gap | 9.5 mmol/L | ||
| Calcium | 8.6 mg/dL | ||
| Phosphorus | 2.4 mg/dL | ||
| Ammonia | 161 µg/dL | ||
Plasma anion gap: sodium − (chloride + HCO3−), uretic anion gap: sodium + potassium − chloride
Fig. 2.
An X-ray at the first examination shows cupping and fraying signs on the patient’s wrist and knee
Fig. 3.
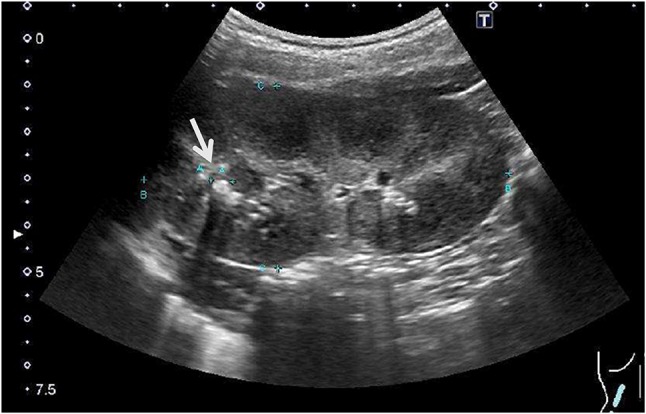
Abdominal ultrasonography at the first examination shows nephrocalcinosis (arrow)
Fig. 4.
Diuretic tests with furosemide alone (a) and the combination of furosemide and fludrocortisone (b) were conducted, and both showed urine acidification defects
Table 2.
Panel used for gene analysis
| SLC12A1 | CFH | CUL3 | COQ2 | EMP2 |
| KCNJ1 | CFI | WNK4 | COQ6 | FAT1 |
| CLCNKB | MCP (CD46) | COL4A3 | ITGA3 | KANK1 |
| BSND | C3 | COL4A4 | ITGB4 | KANK2 |
| CLCNKA | CFB | COL4A5 | GLEPP1 (PTPRO) | KANK4 |
| SLC12A3 | DGKE | GLA | MYO1E | PDSS2 |
| CASR | THBD | UMOD | ARHGDIA | PTPRO |
| MAGED2 | ADAMTS13 | MUC1 | ADCK4 | WDR73 |
| CFTR | FN1 | SEC61A1 | TTC21B | XPO5 |
| CLCN5 | SLC4A1 | REN | NUP93 | ACTN4 |
| OCRL | ATP6V0A4 | PKD1 | NUP107 | ANLN |
| SLC26A3 | ATP6V1B1 | PKD2 | NUP205 | ARHGAP24 |
| KCNJ10 | SLC4A4 | GANAB | CRB2 | INF2 |
| CLDN16 | CA2 | EYA1 | CUBN | LMX1B |
| CLDN19 | EHHADH | SIX2 | EMP2 | MYH9 |
| FXYD2 | SLC34A1 | CD2AP | FAT1 | PAX2 |
| EGF | CTNS | NPHS1 | KANK1 | TRPC6 |
| TRPM6 | NR3C2 | NPHS2 | KANK2 | WT1 |
| KCNA1 | SCNN1A | PLCE1 (NPHS3) | KANK4 | WDR73 |
| CNNM2 | SCNN1B | SMARCAL1 | PDSS2 | MAGI2 |
| HNF1B | SCNN1G | LAMB2 | PTPRO | |
| SLC41A3 | KLHL3 | SCARB2 | CUBN |
We detected a pathogenic mutation in the SLC4A1 gene
Bold values show the responsible genes for renal tubular acidosis
Discussion
Autosomal dominant dRTA is a rare genetic disease caused by pathogenic variants in the SLC4A1 gene encoding the chloride–bicarbonate anion exchanger 1. Autosomal recessive dRTA is caused by pathogenic variants in either the ATP6V1B1 or ATP6V0A4 gene encoding subunits of the vacuolar H+-ATPase. A defect of urinary acidification in the distal nephron leads to hyperchloremic metabolic acidosis with a normal plasma anion gap, and this is frequently accompanied by hypokalemia, hypercalciuria, and nephrocalcinosis. Affected patients usually show mild phenotypes and present with clinical symptoms in adolescence or adulthood [9–12]. Alonso-Varela et al. reported that the median age at diagnosis of patients with SLC4A1 gene mutations was 10 years old and they showed relatively mild symptoms [13].
Furosemide loading blocks NKCC2, which is a sodium transporter at the thick ascending limb of Henle’s loop, and a lot of sodium flows into the distal renal tubule and collecting ducts. Furosemide loading enhances reabsorption of sodium from these segments and results in a negative charge of the urine. This condition leads to excretion of proton ions and urinary acidification occurs. Addition of fludrocortisone loading enhances sodium reabsorption by expressing sodium channels in the cell membrane in collecting duct epithelial cells, and also has a direct action to enhance the activity of H+-ATPase [5]. Therefore, a combination of furosemide and fludrocortisone loading can enhance urine acidification. However, patients with dRTA lack the ability of excreting proton ions into urine and lack acidification with furosemide loading. In our case, the results of the furosemide loading test and furosemide and fludrocortisone loading test showed a defect of urine acidification. Genetic analysis also detected a known pathogenic variant. We diagnosed our patient as dRTA clinically and genetically.
This study has some limitations, because we conducted this test for only one case and have not conducted any tests on healthy pediatric cases. Recent studies suggested that in incomplete dRTA cases, furosemide and fludrocortisone tests could not replace the ammonium chloride loading test [14–16]. Therefore, careful interpretation of the results is needed with diagnose these cases. Accessing gene diagnostic technology, including next generation sequencing, is becoming easier. However, we believe that the loading test is still a useful tool with high diagnostic value for patients with dRTA, and it can be conducted safely and easily in our facility.
Conclusion
We diagnosed dRTA with a short stature and rickets by diuretic tests using furosemide and furosemide plus fludrocortisone. The combination of furosemide and fludrocortisone as a loading test without side effects in pediatric cases may be useful for diagnosing children with dRTA. A loading test with a combination of furosemide and fludrocortisone is an alternative to the ammonium chloride loading test for pediatric cases. However, more cases need to be collected and analyzed to validate this alternative.
Acknowledgements
We thank Ellen Knapp, PhD, from Edanz Group (https://www.edanzeditingcom/ac) for editing a draft of this manuscript.
Compliance with ethical standards
Conflict of interest
The authors have declared that no conflict of interest exists.
Human and animal rights
This article does not contain any studies with human participants or animals performed by any of the authors.
Informed consent
Informed consent was obtained from participant’s parents included in the study.
Footnotes
Publisher's Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
References
- 1.Weger W, Kotanko P, Weger M, Deutschmann H, Skrabal F. Prevalence and characterization of renal tubular acidosis in patients with osteopenia and osteoporosis and in non-porotic controls. Nephrol Dial Transpl. 2000;15(7):975–980. doi: 10.1093/ndt/15.7.975. [DOI] [PubMed] [Google Scholar]
- 2.Reynolds TM, Burgess N, Matanhelia S, Brain A, Penney MD. The frusemide test: simple screening test for renal acidification defect in urolithiasis. Br J Urol. 1993;72(2):153–156. doi: 10.1111/j.1464-410X.1993.tb00677.x. [DOI] [PubMed] [Google Scholar]
- 3.Han SW, Kim HJ, Oh MS. Comparison of the urine acidification tests of torsemide vs furosemide in healthy volunteers. Nephrol Dial Transpl. 2005;20(11):2582–2583. doi: 10.1093/ndt/gfi085. [DOI] [PubMed] [Google Scholar]
- 4.Guerra-Hernandez NE, Ordaz-Lopez KV, Escobar-Perez L, et al. Distal renal tubular acidosis screening by urinary acidification testing in Mexican children. Rev Invest Clin. 2015;67(3):191–198. [PubMed] [Google Scholar]
- 5.Schwartz GJ. Diagnosis of distal renal tubular acidosis: use of furosemide plus fludrocortisone versus ammonium chloride. Nat Clin Pract Nephrol. 2007;3(11):590–591. doi: 10.1038/ncpneph0596. [DOI] [PubMed] [Google Scholar]
- 6.Walsh SB, Shirley DG, Wrong OM, Unwin RJ. Urinary acidification assessed by simultaneous furosemide and fludrocortisone treatment: an alternative to ammonium chloride. Kidney Int. 2007;71(12):1310–1316. doi: 10.1038/sj.ki.5002220. [DOI] [PubMed] [Google Scholar]
- 7.Fernando S, Ordóñez FA, Claramunt-Taberner D, et al. Clinical and laboratory approaches in the diagnosis of renal tubular acidosis. Pediatr Nephrol. 2015;30:2099–2107. doi: 10.1007/s00467-015-3083-9. [DOI] [PubMed] [Google Scholar]
- 8.Bruce LJ, Cope D, Jones GK, et al. Familial distal renal tubular acidosis is associated with mutations in the red cell anion exchanger (Band 3, AE1) J Clin Invest. 1997;100(7):1693–1707. doi: 10.1172/JCI119694. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Batlle D, Haque SK. Genetic causes and mechanisms of distal renal tubular acidosis. Nephrol Dial Transpl. 2012;27(10):3691–3704. doi: 10.1093/ndt/gfs442. [DOI] [PubMed] [Google Scholar]
- 10.Besouw MTP, Bienias M, Walsh P, Kleta R, Van't Hoff WG, Ashton E, et al. Clinical and molecular aspects of distal renal tubular acidosis in children. Pediatr Nephrol. 2017;32(6):987–996. doi: 10.1007/s00467-016-3573-4. [DOI] [PubMed] [Google Scholar]
- 11.Fry AC, Karet FE. Inherited renal acidoses. Physiology (Bethesda). 2007;22:202–211. doi: 10.1152/physiol.00044.2006. [DOI] [PubMed] [Google Scholar]
- 12.Ito N, Ihara K, Kamoda T, Akamine S, Kamezaki K, Tsuru N, et al. Autosomal dominant distal renal tubular acidosis caused by a mutation in the anion exchanger 1 gene in a Japanese family. CEN Case Rep. 2015;4(2):218–222. doi: 10.1007/s13730-015-0172-3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Alonso-Varela M, Gil-Pena H, Coto E, Gomez J, Rodriguez J, Rodriguez-Rubio E, et al. Distal renal tubular acidosis. Clinical manifestations in patients with different underlying gene mutations. Pediatr Nephrol. 2018;33(9):1523–1529. doi: 10.1007/s00467-018-3965-8. [DOI] [PubMed] [Google Scholar]
- 14.Both T, Hoorn EJ, Zietse R, et al. Prevalence of distal renal tubular acidosis in primary Sjögren's syndrome. Rheumatology (Oxford) 2015;54(5):933–939. doi: 10.1093/rheumatology/keu401. [DOI] [PubMed] [Google Scholar]
- 15.Shavit L, Chen L, Ahmed F, et al. Selective screening for distal renal tubular acidosis in recurrent kidney stone formers: initial experience and comparison of the simultaneous furosemide and fludrocortisone test with the short ammonium chloride test. Nephrol Dial Transpl. 2016;31(11):1870–1876. doi: 10.1093/ndt/gfv423. [DOI] [PubMed] [Google Scholar]
- 16.Dhayat NA, Gradwell MW, Pathare G, et al. Furosemide/fludrocortisone test and clinical parameters to diagnose incomplete distal renal tubular acidosis in kidney stone formers. Clin J Am Soc Nephrol. 2017;12(9):1507–1517. doi: 10.2215/CJN.01320217. [DOI] [PMC free article] [PubMed] [Google Scholar]