Abstract
Momordica charantia (M. charantia) is a medicinal plant, used in traditional practice for treating diseases like hypertension and diabetes mellitus. This study investigated the possible hepato-protective effect of M. charantia following treatment with highly active antiretroviral therapy (HAART) in diabetic rats. 48 adult male Sprague Dawley rats were divided into seven groups (A–G) of 7 animals per group and treated according to protocols. Diabetes was induced with streptozotocin (STZ) by intraperitoneal injection (45 mg/kg body weight). The animals were euthanized on the 10th week with liver removed for examination and blood obtained via cardiac puncture and centrifuged to collect the sera. Blood glucose levels (BGL) were consistently and significantly raised (p < 0.05) in all groups not receiving the adjuvant M. charantia. Treatment with M. charantia reverses the increase in BGL to near normal. Markers of liver injury assayed showed significant increase (p < 0.05) in AST, ALP and ALT levels in groups not receiving M. charantia. Adjuvant HAART and M. charantia caused significant declines in the liver enzymes (p < 0.05). Serum GGT was not markedly altered. Treatment with M. charantia significantly restored liver enzymes elevations to near normal comparable to control. Histopathological observations ranged from severe hepatocellular distortions, necrosis and massive fibrosis following treatment of HAART in diabetic groups not receiving M. charantia. Treatment with M. charantia did not show any sign of hepatotoxicity as judged from the histological and biochemical observations.
Keywords: Highly active antiretroviral therapy, Hepatotoxicity, Liver, Diabetes mellitus, Momordica charantia, Sprague-dawley rats
Introduction
Antiretroviral (ARV) drugs have been shown to suppress HIV replication while reducing morbidity and mortality. However, as a counterweight to this positive impact, ARV drugs carries along deleterious effects, which challenge the management of HIV-infected patients to a great extent [1]. Some of these effects are nephrotoxicity, hepatotoxicity and other metabolic disorders such as hypertension and diabetes mellitus. These effects have been linked to the continuous use of ARV drugs [2].
ARV drugs were shown to inhibit insulin-stimulated glucose disposal by blocking the glucose uptake through glucose transporter isoform 4 (GLUT 4) [3]. Disturbances in glucose-insulin homeostasis through insulin resistance and impaired beta cell function predisposes to hyperglycemia and eventually diabetes mellitus and further complications of diabetes such as diabetic liver disease often called hepatogenous diabetes [4]. It is not clear how diabetic liver disease develop but however scientist have reported Porto systemic shunts and decreased overall liver mass through down- regulation of insulin receptors as a possible mechanism [3].
There are compelling evidences that increased consumption of fruits and vegetables reduces the risk of various pathological events, hence Momordica charantia L. (Cucurbitaceae) known as bitter melon, have been traditionally used worldwide, as an adjuvant in the therapeutic strategy for glycemic control aimed at preventing the development and progression of chronic liver failure associated with uncontrolled diabetes mellitus [5]. Studies have documented the hypoglycemic effects of M. charantia through physiological, pharmacological and biochemical mechanisms. It is believed to act via stimulation of peripheral skeletal muscle glucose utilization, inhibition of intestinal glucose uptake, [6], suppression of key gluconeogenic enzymes, stimulation of key enzyme of hexose mono phosphate (HMP) pathway, and preservation of β cell islet and its functions [5].
However, there is paucity of literature explaining the attenuating influence of M. charantia on the liver injury following treatment with ARV drugs and diabetic condition. Our study therefore sought to investigate the therapeutic potential of M. charantia in treating diabetic liver disease following antiretroviral treatment.
Materials and methods
Preparation of M. charantia fruit ethanolic extract
Fifty kilogram of fresh unripe green fruits of M. charantia was purchased from a local market in Durban South Africa and were authenticated in the Department of Life Science, Westville Campus, University of KwaZulu-Natal, with voucher no: 4617. The fruits of M. charantia was cleaned and sliced into small pieces and the seeds separated from it. The sliced green fruit was weighed and dried in shade and weighed again after drying to obtain the actual weight before pulverizing into fine powered in a grinder and stored at 5 °C until ready for extraction. Fruit powder was extracted with solvent (ethanol) by soxhlet extraction. Solvent was evaporated in a rotary evaporator at 40–50 °C with the percentage yield of 85.25%. The extract was filtered through whatman filter. The concentrated extract was stored at 4 °C until ready for use.
Drug and chemical
Highly active antiretroviral (HAART) regimen (Triplavar) (Cipla-Medpro) containing Lamivudine 150 mg, Nevirapine 400 mg and Zidovudine 300 mg, was used for this study. The drug was obtained from Pharmed pharmaceuticals, Pty (Ltd) Durban, South Africa. Streptozotocin (Sigma-Aldrich, St. Louis, MO, USA) which was of analytical grade quality was purchased from Capital Lab Supplies, Durban, South Africa.
Animal management and experimental design
The University of KwaZulu Natal Animal Research Ethics Committee gave full approval of the project and assigned Reference Number: AREC/033/016D. A total of forty-eight (n = 48) adult male Sprague-Dawley rats weighing 178–232 grams (219.31 ± 36.17) were used for the study. Animals were housed in well ventilated plastic cages (4 rats per cage having dimensions of 52 cm long × 36 cm wide and 24 cm high with bedding of soft wood shavings. They were maintained under standardized animal house conditions (temperature: 23–25 °C; light: approximately 12 h natural light per day) and were fed with standard rat pellets from Meadow feeds (Division of Astral Operations Limited, Durban, South Africa) and given tap water ad libitum. The initial body weights of the animals were recorded before treatment. The animals were randomly assigned to the 7 treatment groups, (B–G) with 7 animals per group and 6 animals in the (−ve) control group A.
Group A served as –ve control
Group B served as +ve control (Diabetic)
Group C received Triplavar (Diabetic)
Group D received M. charantia (200 mg/kgbw) (Diabetic)
Group E received M. charantia (400 mg/kgbw) (Diabetic)
Group F received Triplavar + M. charantia (200 mg/kgbw) (Diabetic)
Group G received Triplavar + M. charantia (400 mg/kgbw) (Diabetic)
The therapeutic dose of triplavar was adjusted for animal weight using the human therapeutic dose equivalent for the rat model [7]. These substances were administered daily via oral route with orogastric tube. The study lasted for 10 weeks. Monitoring of the animals was carried out by the research team. Thereafter, the animals were euthanized at the 10th week and the tissues were harvested and prepared for observation.
Induction of diabetes mellitus
All rats were placed on a 12 h fast to obtain baseline fasting blood glucose levels (FBG). The diabetic groups were given intra-peritoneal streptozotocin (STZ) (Sigma-Aldrich Chemical Company, Missouri, St Louis, USA) at 45 mg/kg body weight dissolved in a citrate buffer (pH 4.5) [8]. Successful induction of diabetes was determined by observation of polyuria and polydipsia and confirmed by a 72 h post STZ FBG level ≥ 11 mmol/L.
Measurement of blood glucose
Blood samples were obtained from the tail using sterile needle prick. Glucose levels were measured once a week during the 10 weeks treatment using the one touch ultra-glucometer (Boehringer-Mannheim, Germany).
Body and liver weight
Body weights of animals were recorded on the first day before treatment (initial), thereafter weekly during the 10 weeks treatment till the day of sacrifice (final). Liver weight (LW) was measured by an electronic balance (Mettler Toledo; Microsep (Pty) Ltd, Greifensee, Switzerland).
Assessment of liver function
Blood samples were collected through cardiac puncture and allowed to clot for 30 min and centrifuged for 15 min at 3000 revolutions per minute. The serum was decanted into Eppendorf tubes and prepared for biochemical analyses. Biochemical analyses of the serum enzymes for alanine aminotransferase (ALT), aspartate aminotransferase (AST), alkaline phosphatase (ALP) and gamma-glutamyl transferase (GGT) were spectrophotometrically determined by the method of Reitman and Frankel [9].
Histopathological examination of liver tissues
Liver were weighed and examined for gross pathology. A phosphate buffer solution (PBS) was used to wash out blood before preparation for tissue fixation. They were sectioned at 5 µm thickness using Leica RM 2255 microtome. Tissues were stained with Haematoxylin and Eosin (H and E), for general assessment of liver structure. For histochemical studies, the tissues were stained with Masson’s trichrome (MT) for the assessment of possible liver fibrous architecture [10]. The stained slides were scanned using a Leica SCN 400 (Leica Microsystems GmbH, Wetzlar, Germany) and measured at 200 magnification using image analyser Leica (DMLB) and Leica QWIN software.
Statistical analysis
Continuous variables (liver and body weights and liver function test), were analyzed by one-way analysis of variance (ANOVA) followed by Dunnett’s multiple comparison post-test using Graph pad prism® statistical software 6.02. The results are expressed as mean ± SD (standard deviation). Values were considered significant at p < 0.05.
Results
Blood glucose levels
The blood glucose concentration for diabetic +ve control (group B) and diabetic treated with HAART-regimen (triplavar) alone was significantly high (p < 0.05) (Fig. 1). This level was sustained throughout and was peaked between 6th and 10th weeks. Treatment with M. charantia alone at both low and high dose as well as co-administration of M. charantia (low and high dose) and HAART showed significant mild hyperglycemia (p < 0.05) compared to normal control. From the 4th to 6th week of treatment, adjuvants treatment with M. charantia in all groups receiving M. charantia extract showed a significant lowering of blood glucose (p < 0.05). Between weeks 8 and 10, groups receiving M. charantia (low and high dose) together with HAART showed significant (p < 0.05) reduction in blood glucose to near normal levels (Fig. 1). This reduction of blood glucose level shows in addition, a dose dependent mitigation of hyperglycemia (Fig. 1).
Fig. 1.
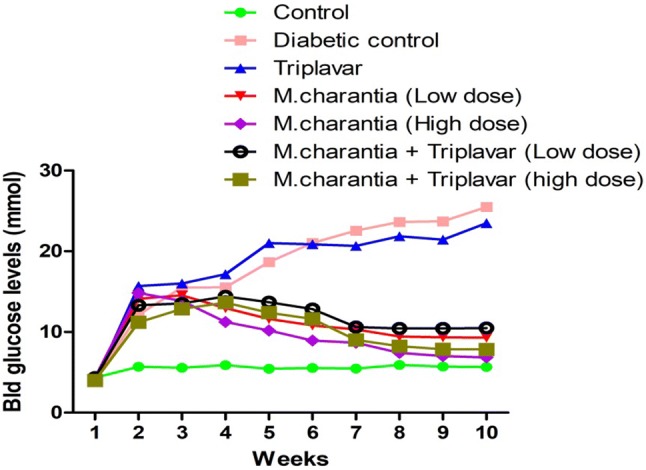
Graphical representation of blood glucose levels
Body weight and organ (liver) weight changes
While there was an overall increase in the final body weight (BW) in all groups, Groups, E and G recorded the highest increase in BW significant at p < 0.05 with the percentage BW gain recording 34.9% and 20.9% respectively, but however, lower when compared to control animals. Although there was a lower rate of body weight gain in all groups when compared to control, this was particularly so in Groups (B, C and F). Diabetes and HAART were related to lower rate of body weight gain as observed in Groups (B and C) with the percentage body weight recording 11.74%, 12.77%. Groups D (administered with low dose (200 mg/kgbw) of M. charantia extract alone), and Group F (administered with low dose of M. charantia extract + HAART) showed a narrower range of weight gain when compared with Groups E (administered with high dose (400 mg/kgbw) and Group G (administered with high dose of M. charantia extract + HAART). Mean liver weight corresponds with the percentage BW gained across groups with the control group having an optimal mean liver weight (Table 1).
Table 1.
Body weight and liver weight of experimental and control groups
| Groups | Initial BW (g) | Final BW (g) | Mean BW | BW difference | BW Diff in (%) | Mean LW | LWBR |
|---|---|---|---|---|---|---|---|
| A | 178.1 | 351.5 | 261.7 ± 127.0 | 173.4 | 66.26 | 12.69 ± 1.41 | 4.85 |
| B | 227.1 | 255.3 | 240.1 ± 18.28 | 28.2 | 11.74 | 10.30 ± 1.79 | 4.29 |
| C | 227.1 | 258.1 | 242.6 ± 21.92 | 31.0 | 12.77 | 10.55 ± 1.22 | 4.35 |
| D | 212.6 | 258.0 | 235.3 ± 32.12 | 45.4 | 19.29* | 10.07 ± 1.56 | 4.28 |
| E | 240.3 | 342.1 | 291.2 ± 72.02 | 101.8 | 34.9* | 12.43 ± 1.89 | 4.27 |
| F | 217.9 | 249.9 | 233.9 ± 22.63 | 32.0 | 13.68 | 10.19 ± 1.58 | 4.36 |
| G | 232.1 | 286.3 | 259.2 ± 38.32 | 54.2 | 20.9* | 11.34 ± 0.75 | 4.37 |
LWBR = (Mean LW/Mean BW) × 100. BW diff in% = (BW diff/Mean BW) × 100
BW body weight of rats, LW liver weight of rats, LWBR liver weight body ratio
*Values are expressed as mean ± SD for each group and considered statistically significant at p < 0.05
Liver function test
As shown in Table 2, the levels of AST, ALT and ALP were significantly decreased (p < 0.05) in animals treated with M. charantia alone as well as M. charantia + HAART (low and high dose) as seen in groups (D, E, F, G) when compared to groups (B and C). Treatment with HAART (group C) as observed showed significant increase in liver function parameters (p < 0.05). Similarly, the liver functional indices were elevated in the diabetic +ve control animals (group B).
Table 2.
Effect on serum AST, ALT ALP and GGT following treatment with HAART and M. charantia
| Groups | AST (IU/L) | ALT (IU/L) | AST/ALT ratio | ALP (IU/L) | GGT (IU/L) |
|---|---|---|---|---|---|
| A | 24.67 ± 3.05 | 27.67 ± 3.22 | 0.89 | 104.7 ± 29.37 | 4.33 ± 4.04 |
| B | 134.7 ± 31.50 | 104.0 ± 22.87* | 1.29 | 558.7 ± 140.6* | 19.00 ± 3.00 |
| C | 113.0 ± 44.54* | 87.00 ± 22.27 | 1.29 | 225.3 ± 40.10 | 11.67 ± 2.52 |
| D | 32.33 ± 6.51* | 33.67 ± 8.51 | 0.96 | 126.7 ± 14.19* | 15.33 ± 6.81 |
| E | 31.67 ± 4.04 | 40.67 ± 6.03* | 0.78 | 119.0 ± 26.63* | 11.67 ± 6.51 |
| F | 29.33 ± 6.81 | 36.00 ± 12.53* | 0.81 | 108.7 ± 27.79 | 9.00 ± 5.00 |
| G | 30.33 ± 5.13* | 37.00 ± 4.58* | 0.82 | 117.7 ± 14.57 | 4.33 ± 2.52 |
AST alanine amino aspartate, ALT alanine amino transferase, ALP alkaline phosphatase, GGT gamma-glutamyl transferase
*Values are expressed as mean ± SD for each group and considered statistically significant at p < 0.05
Histopathological examination (H and E and MT)
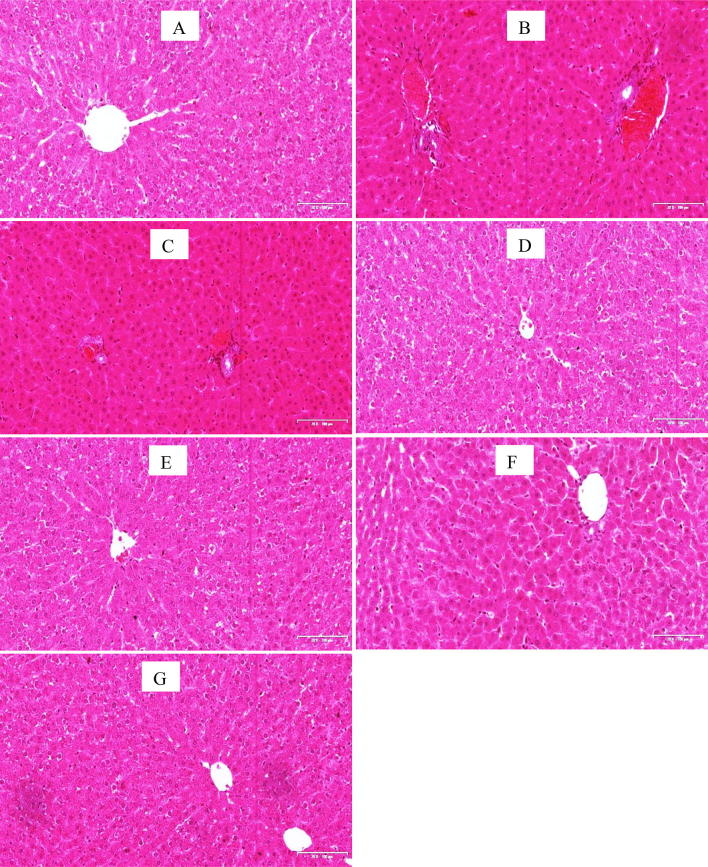
The H&E stained sections of the liver tissues in control animals and animals treated with M. charantia alone and its co-administration with HAART revealed adequate preservation of hepatocellular architecture with the normal hepatocytes and no cytoplasmic vacuolation of liver cells and fine sinusoidal spaces (Fig. 2). The outline of hepatocytes and sinusoidal spaces were seen with no pathologies. Histopathological evaluation of groups treated with HAART alone as well as untreated diabetic group (B and C) showed various degrees of distortions and cytoarchitectural patterns ranging from extensive necrosis of hepatocytes with occlusion of sinusoidal spaces and cytoplasmic vacuolation.
Fig. 2.
Photomicrographs of liver sections (H &E stains). Scale bar 20 × 200 μm. Note the normal architecture of hepatocellular cords sinusoidal spaces and central vein in groups A, D, E, F and G. There are various degrees of distortion (mild to severe) in the radial arrangement of hepatic cords seen in groups B, extensive necrosis are observed in groups C. a –ve Control, b Diabetic +ve Control c Diabetic + HAART d Diabetic + M. charantia 200 mg/kg e Diabetic + M. charantia 400 mg/kg, f Diabetic + M. charantia 200 mg/kg + HAART g Diabetic + M. charantia 400 mg/kg + HAART
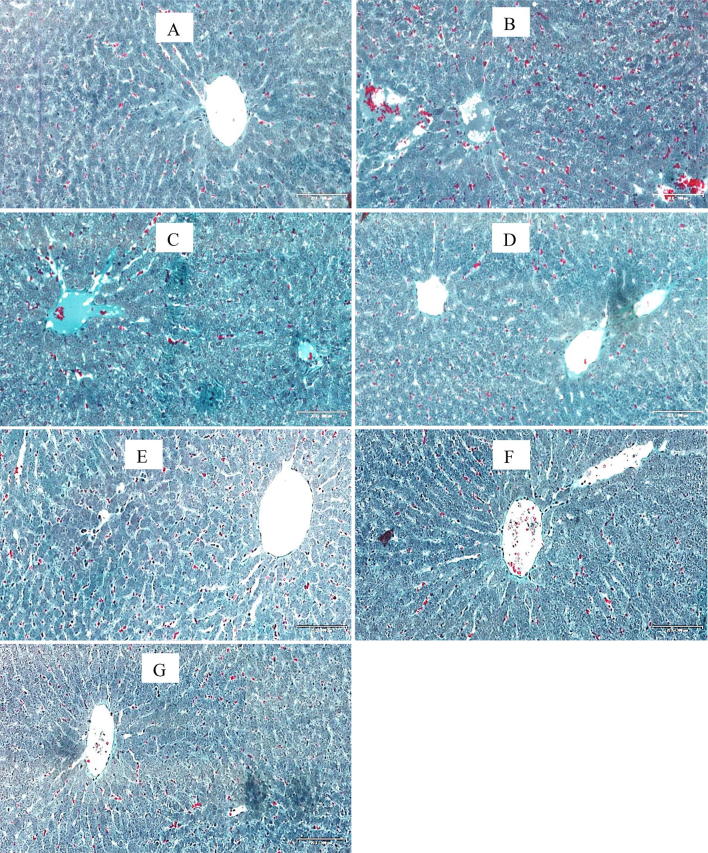
Masson’s trichrome stains fibrous tissue blue while the cytoplasm of hepatocytes are stained red and nuclei could be dark or red as well. Sections of liver in control group and groups treated with M. charantia alone and its co-administration with HAART showed normal red-stained hepatocytes. Photomicrographs from liver tissues of groups treated with HAART alone as well as untreated diabetic group showed extensive network of blue-stained fibrous components in a necrotized, disorganized hepatic tissue cord (Fig. 3).
Fig. 3.
Photomicrographs of liver sections (MT stains). Scale bar 20 × 200 μm. Note the normal architecture of the liver with intact hepatocytes stained red in groups A, D, E, F and G. Nuclei can be seen as dark red to black structures within cells and fibrous elements stained light blue. There is extensive necrosis of central veins, hepatic cords and massive fibrosis in groups B, and C. a –ve Control, b Diabetic +ve Control c Diabetic + HAART d Diabetic + M. charantia 200 mg/kg e Diabetic + M. charantia 400 mg/kg, f Diabetic + M. charantia 200 mg/kg + HAART g Diabetic + M. charantia 400 mg/kg + HAART
Discussion
Despite the substantial benefit in the reduction of morbidity and mortality by HAART, organ toxicities (especially liver) are frequently becoming a major concern for scientist and researchers ranging from mild side effects (elevated liver enzymes) to fatal complications (chronic liver failure, diabetic liver disease etc.) [11]. The liver performs numerous vital metabolic activities, including synthetic and excretory functions of drugs/chemicals. Due to this central role, it is thus exposed to toxic injuries. In other to mitigate some of these toxicities, people living with HIV/AIDS (PLWHAs) in sub-Saharan Africa rely on medicinal plants rich in phytochemical components as a complementary or alternative therapy for the alleviation of some of the toxicities of HAART [12].
In our study, we evaluated alanine aminotransferase (ALT), aspartate aminotransferase (AST), alkaline phosphatase (ALP) and gamma-glutamyl transferase (GGT) on the serum samples. These liver enzymes are helpful in understanding inflammatory and necrotic changes in the liver [13]. It has been reported that elevated levels of these liver enzymes are associated with complications of diabetes mellitus [14], and commencement of antiretroviral therapy [15]. Although this can be exacerbated by other co-infections like hepatitis B or C virus [16]. Our study revealed an elevation of these liver enzymes (ALT, AST and ALP) above normal levels in the diabetic +ve control animals and the diabetic animals treated with HAART. This elevations perhaps could reflect a major permeability problem or cell disruptions in the liver [17]. Elevations of these enzymes is a sensitive signal for liver injury and may worsen into severe complications of the liver with the continuation of therapy [15].
In the quest for better outcome and well-being of PLWHAs, there is increased utilization of alternative and complementary medicines in association with HAART, although many of these adjuvants have been implicated in drug-induced liver/other organ’s injury [7]. From our study we observed lower levels of ALT, AST and ALP in diabetic rats treated with M. charantia alone and its concomitant treatment with HAART, indicating that consumption of M. charantia had a beneficial effect in suppressing elevation of liver enzymes levels. The decreased levels of these liver enzymes (especially following adjuvant HAART with M. charantia) in our study supports previous reports by [18] on effects of M. charantia on the serum and liver triglyceride levels in rats. While the levels of ALT, AST and ALP were reduced in our study, GGT showed normal levels in all groups. The tendency of M. charantia to suppress the rise in values of the liver enzymes provides substantial evidence that treatment with M. charantia extract could mitigate the progression of liver dysfunction.
Liver dysfunction is known to be associated with complications of diabetes and assessment of blood glucose level plays a critical role in evaluating the functional capacity of the liver following antiretroviral treatment in STZ induced diabetic rats. Elevated levels of blood glucose are associated with complications arising from occlusion of the vascular wall [19]. This pathogenesis is clearly complex and occurs as a result of cytokines secretion increase which induces dysregulation of endothelial and vascular smooth muscle cell growth [20, 21]. In the present study, elevated level of blood glucose level was observed when rats were treated with HAART regimen alone indicating that HAART regimen suppresses glucose uptake by the tissues. While blood glucose levels were elevated in animals treated with HAART alone, it was lowered when M. charantia was used as adjuvant to HAART. It is believed that M. charantia could exert their hypoglycemic properties via stimulation of peripheral skeletal muscle glucose utilization [22, 23] inhibition of adipocyte differentiation [24], stimulation of key enzyme of hexose mono phosphate (HMP) pathway, and preservation of β cell islet and its functions [25].
We correlated our findings with the histopathological assessment of the liver tissues. HAART-treated groups revealed disorganized architecture and extensive network of fibrotic strands and hepatic structural injury. The pathogenic pathway for HAART-mediated hepatic injury has been associated with mitochondrial toxicity especially with NRTIs [26, 27]. HAART inhibit the mitochondrial DNA resulting in inhibition of normal mitochondrial replication which in turn decrease cellular respiratory chain and inhibits fatty acid β-oxidation pathway [26]. Studies have also concretely proved that interruption in electron transport chain results in the release of increased intracellular reactive oxygen species (ROS) leading to oxidative stress [28, 29]. These free radicals can cause lipid peroxidation of fatty acid present in the hepatocytes membrane consequently to release profibrogenic cytokines, and nuclear factor κB to counteract the effect [30]. It is reasonable to believe that a perturbed antioxidant defense system could perhaps be a contributory arm towards the histopathological observations in our study.
Our observations in the H and E stains corresponded with the MT staining intensities of liver sections and there is a supporting link. Photomicrographs from liver tissues of groups B and C showed extensive network of blue-stained fibrous components in a necrotized, disorganized hepatic tissue cord possibly indicating low deposists of collagen fibres. These derangements could be as a result of low glucose metabolism and is supported by [31] involving PIs regimens. It is interesting to note that while all these deleterious effects were seen in HAART treated groups, M. charantia reversed the pathologies and showed good cyto-protective effects and maintained hepatic structural integrity. Although [7] reported that the pharmacokinetic interactions between antiretroviral therapy and plant based adjuvants can be unfavourable due to drug- drug interactions, the exact mechanistic pathway for M. charantia boosting in hepatic tissues in our study still remain a subject for further investigation.
Body and organ weight analysis offers insight on inflammatory changes and thus important in the identification of target organ by toxicants. In this study, HAART with M. charantia induced observational changes in the body weight of experimental animals. While loss of weight may be associated with induction of diabetes, initiation of HAART may as well contribute to the low pace of weight gain due to other metabolic activities relative to lipid and sugar [7]. Our study agrees with [7] that long term liver enzyme elevation in addition to other positive indices tilts the balance towards liver fibrosis. These perturbations were mitigated by M. charantia and its concomitant treatment with HAART.
Acknowledgements
The study received operational funds from the College of Health Science; University of KwaZulu-Natal awarded to the first author and is also partly supported by the National Research Foundation of South Africa awarded to Prof OO Azu (Unique Grant No. 99053). We thank Drs Sanil Singh and Linda Bester of Biomedical Research Unit, University of KwaZulu-Natal for their technical support.
Compliance with ethical standards
Conflict of interest
The authors declared that there is no conflict of interest.
References
- 1.Havlir DV, Currier JS. CROI 2015: complications of HIV infection and antiretroviral therapy. Top Antivir Med. 2015;23:56–65. [PMC free article] [PubMed] [Google Scholar]
- 2.Bonacci M, Lens S, Mariño Z, Forns X. Challenges in special populations: HIV/HCV coinfection, liver transplantation and patients with end-stage renal disease. Dig Dis. 2016;34:317–326. doi: 10.1159/000444470. [DOI] [PubMed] [Google Scholar]
- 3.Goswami A, Bhargava N, Dadhich S, Kulamarva G. Insulin resistance in euglycemic cirrhosis. Ann Gastroenterol Q Publ Hell Soc Gastroenterol. 2014;27:237. [PMC free article] [PubMed] [Google Scholar]
- 4.Takahashi H, Eguchi Y, Anzai K. Pathogenesis of hepatogenous diabetes. Nihon Rinsho Jpn J Clin Med. 2016;74:587. [PubMed] [Google Scholar]
- 5.Kwatra D, Dandawate P, Padhye S, Anant S. Bitter melon as a therapy for diabetes, inflammation, and cancer: a panacea? Curr Pharmacol Rep. 2016;2:34–44. doi: 10.1007/s40495-016-0045-2. [DOI] [Google Scholar]
- 6.Perumal V, Murugesu S, Lajis N, Khatib A, Saari K, Abdul-Hamid A, et al. Evaluation of antidiabetic properties of Momordica charantia in streptozotocin induced diabetic rats using metabolomics approach. Int Food Res J. 2015;22:1298–1306. [Google Scholar]
- 7.Azu OO, Jegede AI, Ugochukwu O, Onanuga IO, Kharwa S, Naidu EC. Hepatic histomorphological and biochemical changes following highly active antiretroviral therapy in an experimental animal model: does Hypoxis hemerocallidea exacerbate hepatic injury? Toxicol Rep. 2016;3:114–122. doi: 10.1016/j.toxrep.2015.12.013. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Etuk E. Animals models for studying diabetes mellitus. Agric Biol J N Am. 2010;1:130–134. [Google Scholar]
- 9.Reitman S, Frankel S. A colorimetric method for the determination of serum glutamic oxalacetic and glutamic pyruvic transaminases. Am J Clin Pathol. 1957;28:56–63. doi: 10.1093/ajcp/28.1.56. [DOI] [PubMed] [Google Scholar]
- 10.Naruse K, Tang W, Makuuchi M. Artificial and bioartificial liver support: a review of perfusion treatment for hepatic failure patients. World J Gastroenterol. 2007;13:1516. doi: 10.3748/wjg.v13.i10.1516. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Bissell DM, Gores GJ, Laskin DL, Hoofnagle JH. Drug-induced liver injury: mechanisms and test systems. Hepatology. 2001;33:1009–1013. doi: 10.1053/jhep.2001.23505. [DOI] [PubMed] [Google Scholar]
- 12.Offor U, Jegede A, Onanuga I, Naidu E, Azu O. Does Hypoxis hemerocallidea mitigate renal histopathological injuries following highly active antiretroviral therapy? An experimental animal study. Minerva Urol Nefrol. 2016;69:391–399. doi: 10.23736/S0393-2249.16.02651-5. [DOI] [PubMed] [Google Scholar]
- 13.Ramaiah SK. A toxicologist guide to the diagnostic interpretation of hepatic biochemical parameters. Food Chem Toxicol. 2007;45:1551–1557. doi: 10.1016/j.fct.2007.06.007. [DOI] [PubMed] [Google Scholar]
- 14.Ekstedt M, Franzén LE, Mathiesen UL, Thorelius L, Holmqvist M, Bodemar G, et al. Long-term follow-up of patients with NAFLD and elevated liver enzymes. Hepatology. 2006;44:865–873. doi: 10.1002/hep.21327. [DOI] [PubMed] [Google Scholar]
- 15.Carr A, Cooper DA. Adverse effects of antiretroviral therapy. Lancet. 2000;356:1423–1430. doi: 10.1016/S0140-6736(00)02854-3. [DOI] [PubMed] [Google Scholar]
- 16.Weber R, Sabin CA, Friis-Moller N, Reiss P, El-Sadr WM, Kirk O, et al. Liver-related deaths in persons infected with the human immunodeficiency virus: the D:A:D study. Arch Intern Med. 2006;166:1632–1641. doi: 10.1001/archinte.166.15.1632. [DOI] [PubMed] [Google Scholar]
- 17.Kubota N, Tobe K, Terauchi Y, Eto K, Yamauchi T, Suzuki R, et al. Disruption of insulin receptor substrate 2 causes type 2 diabetes because of liver insulin resistance and lack of compensatory beta-cell hyperplasia. Diabetes. 2000;49:1880–1889. doi: 10.2337/diabetes.49.11.1880. [DOI] [PubMed] [Google Scholar]
- 18.Senanayake GV, Maruyama M, Shibuya K, Sakono M, Fukuda N, Morishita T, et al. The effects of bitter melon (Momordica charantia) on serum and liver triglyceride levels in rats. J Ethnopharmacol. 2004;91:257–262. doi: 10.1016/j.jep.2003.12.026. [DOI] [PubMed] [Google Scholar]
- 19.Kohner EM, Patel V, Rassam SM. Role of blood flow and impaired autoregulation in the pathogenesis of diabetic retinopathy. Diabetes. 1995;44:603–607. doi: 10.2337/diab.44.6.603. [DOI] [PubMed] [Google Scholar]
- 20.Vlassara H, Palace M. Diabetes and advanced glycation endproducts. J Intern Med. 2002;251:87–101. doi: 10.1046/j.1365-2796.2002.00932.x. [DOI] [PubMed] [Google Scholar]
- 21.Ahmed N. Advanced glycation endproducts—role in pathology of diabetic complications. Diabetes Res Clin Pract. 2005;67:3–21. doi: 10.1016/j.diabres.2004.09.004. [DOI] [PubMed] [Google Scholar]
- 22.Abas R, Othman F, Thent ZC. Effect of Momordica charantia fruit extract on vascular complication in type 1 diabetic rats. EXCLI J. 2015;14:179. doi: 10.17179/excli2014-539. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Kang J, Zeng B, Tang S, Wang M, Han X, Zhou C, et al. Effects of Momordica charantia Saponins on in vitro ruminal fermentation and microbial population. Asian-Australas J Anim Sci. 2016;29:500–508. doi: 10.5713/ajas.15.0402. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Perumal V, Khoo W, Abdul Hamid A, Ismail A, Shaari K, Murugesu S, et al. Evaluation of antidiabetic properties of Momordica charantia in streptozotocin induced diabetic rats using metabolomics approach. Int Food Res J. 2015;22:1298–1306. [Google Scholar]
- 25.Aljohi A, Matou-Nasri S, Al-Khafaji N, Slevin M, Ahmed N. Momordica charantia (bitter melon) extracts promote angiogenesis in vitro via the receptor for advanced glycation endproducts (RAGE) J Int Soc Antioxid Nutr Health. 2016;3:514–515. [Google Scholar]
- 26.Barve S, Kapoor R, Moghe A, Ramirez JA, Eaton JW, Gobejishvili L, et al. Focus on the liver: alcohol use, highly active antiretroviral therapy, and liver disease in HIV-infected patients. Alcohol Res Health. 2010;33:229. [PMC free article] [PubMed] [Google Scholar]
- 27.McInnes J. Mitochondrial-associated metabolic disorders: foundations, pathologies and recent progress. Nutr Metab. 2013;10:63. doi: 10.1186/1743-7075-10-63. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Zhang DX, Gutterman DD. Mitochondrial reactive oxygen species-mediated signaling in endothelial cells. Am J Physiol Heart Circ Physiol. 2007;292:H2023–H2031. doi: 10.1152/ajpheart.01283.2006. [DOI] [PubMed] [Google Scholar]
- 29.Forbes JM, Coughlan MT, Cooper ME. Oxidative stress as a major culprit in kidney disease in diabetes. Diabetes. 2008;57:1446–1454. doi: 10.2337/db08-0057. [DOI] [PubMed] [Google Scholar]
- 30.Browning JD, Horton JD. Molecular mediators of hepatic steatosis and liver injury. J Clin Investig. 2004;114:147–152. doi: 10.1172/JCI200422422. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Herman J, Easterbrook P. The metabolic toxicities of antiretroviral therapy. Int J STD AIDS. 2001;12:555–564. doi: 10.1258/0956462011923714. [DOI] [PubMed] [Google Scholar]