Abstract
Objectives
To review epidemiology, aetiology and management of childhood pneumonia in low-and-middle-income countries.
Design
Review of published English literature between 2013 and 2019.
Results
Pneumonia remains a major cause of morbidity and mortality. Risk factors include young age, malnutrition, immunosuppression, tobacco smoke or air pollution exposure. Better methods for specimen collection and molecular diagnostics have improved microbiological diagnosis, indicating that pneumonia results from several organisms interacting. Induced sputum increases microbiologic yield for Bordetella pertussis or Mycobacterium tuberculosis, which has been associated with pneumonia in high TB prevalence areas. The proportion of cases due to Streptococcus pneumoniae and Haemophilus influenzae b has declined with new conjugate vaccines; Staphylococcus aureus and H. influenzae non-type b are the commonest bacterial pathogens; viruses are the most common pathogens. Effective interventions comprise antibiotics, oxygen and non-invasive ventilation. New vaccines have reduced severity and incidence of disease, but disparities exist in uptake.
Conclusion
Morbidity and mortality from childhood pneumonia has decreased but a considerable preventable burden remains. Widespread implementation of available, effective interventions and development of novel strategies are needed.
Abbreviations: AAP, ambient air pollution; aP, acellular pertussis; CPAP, continuous positive airway pressure; CRP, C-reactive protein; DTP3, third dose of diphtheria, tetanus and pertussis vaccine; hMPV, human metapneumovirus; IS, induced sputum; LMIC, low-and-middle-income country; MTB, Mycobacteria tuberculosis; NPA, nasopharyngeal aspirate; OI, opportunistic infection; PCV, pneumococcal conjugate vaccine; PCR, polymerase chain reaction; PCP, Pneumocystis pneumonia; PTB, pulmonary tuberculosis; PERCH, Pneumonia Etiology Research for Child Health; RSV, respiratory syncytial virus; TB, tuberculosis; WHO, World Health Organization
Keywords: Lower respiratory tract infection, Epidemiology, Aetiology, Prevention, Management
Introduction
Globally, pneumonia is one of the major causes of death children under the age of five years. In 2015, approximately 700,000 children younger than 5 years died from pneumonia worldwide, despite general improvement in living conditions, improved nutrition and better vaccines [1]. Furthermore, pneumonia continues to be the leading cause of morbidity for young children outside the neonatal period, particularly in low-and-middle-income countries (LMICs) [2]. Understanding the current epidemiology, and diagnostic and management strategies in these settings may improve preventive, diagnostic and treatment approaches.
The aim of this paper was to review the recent literature on (1) the epidemiology and aetiology of childhood pneumonia in LMICs; (2) diagnostic tools; and (3) prevention and management approaches.
Epidemiology and aetiology
Epidemiology
Models of childhood pneumonia from the Global Burden of Disease (GBD) in 2015 show a decreasing trend for pneumonia incidence, severe morbidity, and mortality in LMICs. Approximately 101.8 million pneumonia episodes were estimated in children under5 years in 2015; an incidence of 0.15 episodes per child year. Mortality in children <5 years decreased by 37% between 2005 and 2015, with the highest pneumonia mortality and slowest decreases reported in sub-Saharan Africa. East and South East Asia, Central Europe and tropical Latin America reported the fastest (>50%) reduction in under-5 pneumonia mortality during this period. The lowest and highest under-5 pneumonia mortalities were in Finland, estimated at 0.65 deaths per 100,000, and Somalia, estimated at 546.8 deaths per 100,000 deaths respectively [1]. Population-based surveillance data in the Gambia showed a decline in the incidence of pneumonia in children 2–11 months, and 12–23 months including radiological pneumonia by 23% and 29% respectively; pneumococcal pneumonia by 58% and 75%; and hypoxic pneumonia by 57% and 72% [3]. Marked decreases in the rates of severe pneumonia in vaccinated children and in older unvaccinated populations have also been described in South Africa and Malawi following pneumococcal conjugate vaccine (PCV 7 and PCV-13) introduction [3], [4]. Similarly in Brazil, vaccination with PCV-10 was associated with a substantial reduction in all-cause pneumonia hospitalization [5], [6], [7] in the target age-groups for vaccination and in unvaccinated individuals aged 40–49 years, reflecting the herd protection provided by vaccination [5]. Recent data from a multi-country study in four LMICs showed that measles vaccine was associated with a 15–30% decrease in pneumonia in children in India and Pakistan, and remains an important intervention in the Global Action Plan for Pneumonia and Diarrhoea (GAPPD) [8].
Aetiology
The aetiology of pneumonia has been increasingly ascribed to multiple organisms as detected by molecular testing [9] (Table 1). The increased use of pneumococcal conjugate vaccine (PCV) and Haemophilus influenzae type b (Hib) vaccine has changed pneumonia aetiology, with Staphylococcus aureus and H. influenzae non-type b now the commonest bacterial pathogens and viruses most common as pathogens [9], [10], [11], [12]. However, the identification of aetiological pathogens may be difficult as distinguishing colonizing from pathogenic organisms can be difficult on respiratory specimens and multiple co-pathogens are common [13].
Table 1.
Common aetiology of pneumonia in children in LMICs.
| A. Bacteria | Staphylococcus aureus |
| Haemophilus influenzae | |
| Streptococcus pneumoniae | |
| Mycobacterium tuberculosis | |
| Bordetella pertussis | |
| Klebsiella pneumoniae | |
| B. Viruses | Respiratory syncytial virus |
| rhinovirus Influenza | |
| Human metapneumovirus | |
| Adenovirus | |
| Parainfluenza virus | |
| Rhinovirus | |
| Measles virus | |
| Herpes viruses – CMV, EBV | |
| C. Fungi | Pneumocystis jirovecii |
Viruses
Viruses have been identified in most pneumonia episodes; however ascribing pathogenicity may be difficult unless they are invariably associated with disease. These include respiratory syncytial virus (RSV), rhinovirus influenza, parainfluenza, human metapneumovirus (hMPV), adenovirus and parainfluenza [10], [14]. Global data in 2015 showed that RSV accounted for approximately 36,000 pneumonia deaths in children under 5 years, responsible for approximately 20% of pneumonia cases; most severe cases occurred in young children or infants in LMICs [9], [15]. Influenza was responsible for approximately 10,000 childhood deaths, with an attributable incidence of 10%. Both RSV and influenza were more often associated with non-fatal pneumonia episodes than bacteria [9]. CMV-related pneumonia in HIV-infected or immunosuppressed children is a potentially lethal disease, however data from LMICs are scarce [16], [17], [18]. Although HIV-infected children with CMV-related pneumonitis on ganciclovir in an intensive care unit in South Africa had poor outcomes [18], it was effective in immunocompetent children who were CMV polymerase chain reaction (PCR) positive in bronchoalveolar lavage in a Turkish hospital [16].
Bacteria
From the 2015 GBD data, approximately 64% of pneumonia deaths in children under5 years were attributed to bacterial aetiology, specifically Streptococcus pneumoniae or H. influenzae [1]. This has changed substantially with widespread use of PCV and Hib conjugate vaccines with S. aureus and H. influenzae non-type b the commonest bacterial pathogens in the context of good coverage with these vaccines [9], [10], [12]. Other bacterial pathogens reported from LMIC settings included Bordetella. pertussis [10], [19], Klebsiella pneumoniae and Escherichia coli [20]. A systematic review showed that 7.5% of paediatric pneumonia was associated with culture confirmed Mycobacterium tuberculosis in three African studies in TB-endemic areas, which is probably a considerable underestimate of the true burden given the difficulties of obtaining microbiologic confirmation in children, Fig. 1 [21].
Fig. 1.
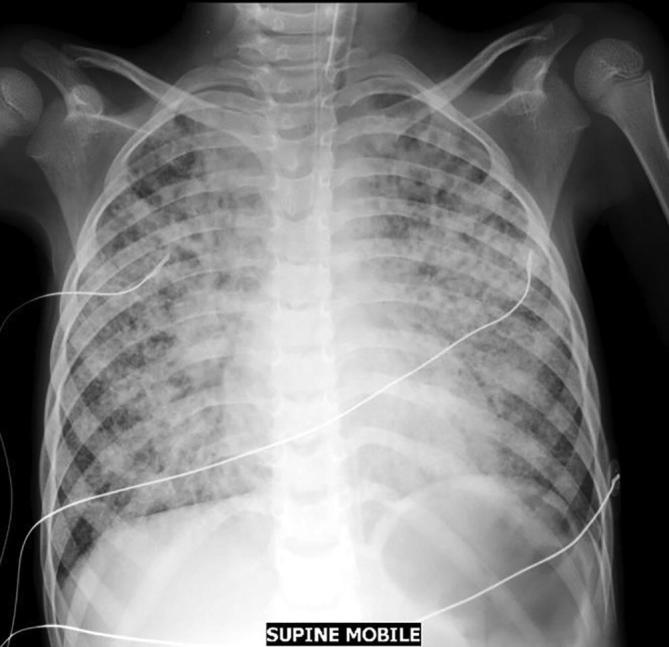
Plain chest radiograph image of a young child with pneumonia who cultured M. tuberculosis on an induced sputum sample.
The burden of pertussis is high in LMICs, especially in Africa. In 2014, it was estimated there were approximately 7.8 million pertussis cases and >92,000 deaths in children <5 years in Africa [22]. Risk factors for pertussis in children include lack of vaccination, being too young to be vaccinated [10], [19], [23], [24], not receiving all three primary doses [23], [24], underweight [19], HIV exposure [19], [23] or HIV infection [23].
In high burden TB settings, pulmonary tuberculosis (PTB) is increasingly reported in children presenting with acute pneumonia, possibly in association with bacterial or viral co-infection [21], [25].
Fungi
Pneumocystis pneumonia (PCP) was the commonest opportunistic infection in HIV-infected children before widespread use of antiretroviral therapy (ART) and effective prevention of mother-to-child transmission programmes [26], [27], [28], but has declined substantially with earlier diagnosis and treatment of HIV [26], [28]. Diagnosis may be hampered by lack of availability of PCR testing for pneumocystis – a South African study detected twice as many children with Pneumocystis jirovecii on PCR than on immunofluorescence or Grocott staining on respiratory samples. HIV-infected children not on ART, HIV exposure in young infants or malnutrition are risk factors for PCP that are still prevalent in sub-Saharan Africa [29].
Risk factors
Risk factors for pneumonia incidence and severity include infancy, lack of immunization, malnutrition, chronic underlying diseases, HIV infection, HIV exposure in young infants, young maternal age, low maternal education, low socio-economic status and smoke exposure/indoor air pollution [30]. From the 2015 GBD analysis, the most important risk factors were malnutrition, household air pollution, ambient particulate matter or sub-optimal breastfeeding [1]. Complex interactions exist between risk factors for pneumonia including HIV exposure, breastfeeding, malnutrition and crowding. For example in a South African study, exclusive breastfeeding was only protective amongst HIV unexposed children, and maternal HIV infection was identified as a risk factor for pneumonia amongst exposed-uninfected infants exclusively breastfed up to 4 months, whereas crowding was a significant risk factor for well nourished children but not underweight children [31]. In LMICs, severe underweight and pallor amongst children with pneumonia were risk factors for death and may be important to consider in deciding the optimal setting for management [32]. HIV-exposed uninfected infants with pneumonia have higher rates of treatment failure and mortality in hospital during the first six months of life compared to unexposed children[33]. In 2015, 246,000 deaths were attributed to ambient air pollution (AAP) pneumonia in children under 5 years. In sub-Saharan Africa, AAP has been estimated to reduce the average life expectancy of children by 4–5 years [34] (Table 2).
Table 2.
Risk factors for pneumonia.
| A. Host characteristics |
| Infants |
| Lack of immunization |
| Lack of exclusive breastfeeding |
| Severe malnutrition |
| Chronic underlying diseases |
| HIV exposure in young infants |
| HIV infection |
| Low birth weight/ prematurity |
| B. Socio-economic and environmental characteristics |
| Young maternal age |
| Low maternal education |
| Low socio-economic status |
| Poor antenatal care |
| Tobacco smoke exposure |
| Indoor air pollution |
| Crowding |
Diagnosis
Classification of pneumonia
In 2013, the World Health Organization (WHO) guidelines for classifying and treating childhood pneumonia were revised. Children presenting with cough or difficulty in breathing were classified into 3 diagnostic categories (pneumonia, severe pneumonia or no pneumonia) according to clinical features. Pneumonia is defined as tachypnea and/or chest indrawing in a child older than 2 months. Severe pneumonia is defined as cough or difficulty breathing with at least one of the following: (i) central cyanosis or oxygen saturation <90% on pulse oximetry, (ii) severe respiratory distress (grunting, very severe chest indrawing), or (iii) any general danger sign (inability to breastfeed or drink, lethargy or unconscious, convulsions). Children without signs of pneumonia or severe pneumonia are classified as no pneumonia: cough or cold [35]. Abnormal oxygen saturation on pulse oximetry predicts oral antibiotic failure [36]. Children with pneumonia seen at outpatient settings without the capacity to perform appropriate investigations such as pulse oximetry, nutritional assessment or HIV testing may need referral [37].
Chest imaging
Chest X-ray remains the primary imaging modality for pneumonia (Fig. 1). The WHO developed standardized criteria for interpretation of chest radiographs in bacterial vaccine trials with three categories of radiological disease: (i) primary end-point pneumonia (end-point consolidation or pleural effusion), (ii) other infiltrate and (iii) no consolidation/infiltrate/effusion [38]. However agreement amongst readers for detection of consolidation was modest (80%, adjusted k = 0.60), and poor for other infiltrates (66.5%, adjusted k = 0.33) [39], [40]. Chest ultrasonography for paediatric pneumonia may be a rapid and safe diagnostic method [41]. Data from a recent meta-analysis show that the diagnostic accuracy of lung ultrasound had a sensitivity of 94% and specificity of 93%, against various reference tests including chest radiographs in nine studies, clinical diagnosis in four studies and computed tomography in one study. However, substantial heterogeneity exists across individual studies, and a reliable reference standard is lacking. Greater methodological rigour is needed in future studies [42].
Blood tests
Although white blood cell count, C-reactive protein (CRP) and erythrocyte sedimentation rate are higher in children with a bacterial aetiology of pneumonia or mixed bacterial–viral aetiology in comparison to viral aetiology, these tests are non-discriminatory [43]. CRP levels of ≥40 mg/L are associated with confirmed bacterial pneumonia especially S. pneumoniae and H. influenzae, and negatively associated with RSV pneumonia. [44] Further research is needed to determine the role of CRP in discriminating confirmed bacterial pneumonia from viral pneumonia and in detecting mixed infections. Blood culture has a low yield between 3 and 7%, with a significantly higher prevalence in children with severe pneumonia, estimated at 6–14% [9], [45]. The microbial yield of blood culture in paediatric pneumonia is estimated at 2% when the blood culture volume is ≤1 ml and increases to ≥6% for volumes ≥3 ml. Conversely, antibiotic exposure reduces blood culture yield by approximately 45% [46]. S. pneumoniae is the most common pathogen isolated on blood culture (77%), followed by H. influenzae (3%) and S. aureus (2%) [45]. The utility of PCR for detection of pneumococcal nucleic acid (lytA gene) in blood amongst microbiologically confirmed pneumococcal pneumonia cases is limited by poor specificity (64%). Furthermore, approximately 1–10% of community controls have a positive test in blood, thus pneumococcal PCR in blood is not specific for pneumonia [47].
Aetiological diagnosis
Nasopharyngeal aspirates (NPA) are frequently used for detection of viruses using molecular techniques and for some bacteria such as B. pertussis. However, distinguishing pathogenic viruses or bacteria may be difficult unless the organism is invariably associated with disease. Amongst viruses, RSV or influenza virus has been shown to be strongly associated with pneumonia in case controlled studies [10], [14]. Recent studies indicate that multiple organisms are involved in pneumonia pathogenesis and that dysbiosis in the nasopharyngeal microbiome may be key in susceptibility to pneumonia or to severe disease [48], [49]. Quantitative analysis of bacterial or viral load in the nasopharynx are not helpful for discriminating colonizing organisms from those that are disease causing although a colonization density on NPA of ≥5.9 log 10 copies/mL was predictive of Hib pneumonia [50]. Induced sputum (IS) is effective for diagnosis of pertussis, pneumocystis pneumonia or tuberculosis using molecular techniques, providing a higher yield than from NPA specimens [51], [52]. B. pertussis PCR on IS specimen provides confirmation earlier than NPA and increases overall diagnostic yield [23]. The yield of P. jirovecii in NPA, IS and non-bronchoscopic bronchoalveolar lavage samples is higher on PCR than using silver staining [53]. In the Gambia, in a study before PCV was widely available, molecular microbiological analyses of lung and pleural aspirates in children with pneumonia, identified pathogens more frequently than culture, and revealed a bacterial predominance, mainly S. pneumoniae or multiple microorganisms including non-typable H. influenzae. [12]. Although M. tuberculosis (MTB) PCR, specifically Xpert or Xpert Ultra is available in LMICs, IS and Xpert MTB/RIF are still infrequently available to diagnose childhood TB, with diagnosis largely based on symptom identification [54]. The sensitivity of Xpert for paediatric TB on IS is around 62% on a single specimen; with an incremental yield of up to 38% on a second specimen [55]. Ultra is an improved version of Xpert with lower limits of detection for MTB; two paediatric studies reported the sensitivity of Ultra on induced sputum of 68–77% compared to culture confirmation [56], [57].
Prevention
Preventive interventions include childhood and maternal immunization, and optimizing nutrition, Table 3.
Table 3.
Current preventive interventions Preventive interventions to avert pneumonia in children in LMICs.
| A. Childhood immunization | Bacillus Calmette–Guérin (BCG) |
| Diptheria and Pertussis (in DTP) | |
| Pneumococcal conjugate vaccine (PCV) | |
| H. influenzae type b vaccine Measles | |
| Influenza | |
| B. Maternal immunization | Influenza |
| Pertussis | |
| C. Nutrition | Breastfeeding |
| Vitamin A supplementation in measles-associated pneumonia | |
| †Vitamin D supplementation | |
| †Zinc supplementation | |
Deficiency is a risk factor but currently no significant evidence of supplementation as a preventive intervention for pneumonia in well-nourished children.
Immunization
Childhood immunization
While global PCV use has rapidly increased with approximately 142 countries introducing it as of 2018 [58], uptake of childhood immunization in LMICs is still sub-optimal. In 2017, PCV-13 coverage globally was estimated at 44%, with estimates for LMICs, specifically Eastern and Southern Africa at 75%, West and Central Africa at 60%, Middle East and North Africa at 40%, Latin America at 77%, South Asia at 23%, and East Asia and Pacific at 15% [59]. Considerable socio-economic inequalities in coverage of the Diptheria, Tetanus and Pertussis vaccine (DTP3), which is used as a proxy for other vaccines administered at 14 weeks including Hib and PCV, have been identified in LMIC settings [60]. In 2017, DTP3 coverage was estimated at 72% in the WHO African region [61], falling short of the WHO recommended goal of >90%. The Global Pertussis Initiative recommends that countries using whole cell pertussis continue to use it to improve primary and toddler booster vaccination coverage [62]. Significant delays exist between scheduled administration age and actual vaccination date, especially for measles vaccine where <40% are administered on schedule [63].
Maternal immunization
Maternal immunization against pertussis, influenza and most recently RSV has been suggested as an effective strategy to protect young infants through the first few months of life when the vulnerability to severe disease is highest [64], [65], [66]. Modelled data simulating LMIC settings showed that maternal pertussis immunization did not substantially impact on infant disease, except where mothers were not immune and eligible for multiple doses; in this group antenatal immunization reduced the incidence of infection in infants less than two months by around 30%, but there was no overall impact in infants less than one year [64]. For maternal influenza, evidence from four trials has shown that maternal immunization prevents illness in pregnant women with an overall vaccine efficacy for lab-confirmed incidence of around 30% in mothers and a vaccine efficacy of 30–63% in infants in the first 6 months of life [65]. Vaccine efficacy is lower in HIV-exposed infants [67]. The duration of protection of maternal influenza vaccination is short, being limited to the first 2–3 months of life [68]. Protection against influenza is lower when the vaccine is given late in pregnancy [69].
Maternal RSV vaccination is a novel strategy that is currently under development [66]. A model using Kenyan data showed that vaccinating pregnant women would boost maternal antibodies in infants by four more months and would potentially reduce RSV infection by >30% [70]. Given that RSV incidence and severity is highest in the first few months of life, an effective vaccine would be an important advance in this context [71]. Recent results of the first Phase 3 trial assessing the efficacy of maternal RSV vaccination, showed a 44% reduction against RSV pneumonia hospitalizations through the first six months of life. Although this trial did not meet the primary objective of significant reduction of RSV pneumonia, other pre-specified endpoints were met including a significant reduction in RSV-associated severe pneumonia, hypoxic pneumonia or hospitalisation. These results are especially important for LMICs where RSV-LRTI is more severe and may be fatal. [72].
Nutrition
Undernutrition is a major risk factor for severe pneumonia [73] and associated mortality [74]. Prevention, and early identification and treatment of malnourished children with pneumonia is paramount including promotion of breastfeeding [75]. In children aged 2–59 months, zinc supplementation is associated with a 13% reduction in pneumonia incidence [76]. Vitamin A supplementation significantly reduces all-cause mortality and the incidence of measles in children aged 6-months to five years [77]. However, a current metanalysis shows that Vitamin A has no significant effect on the incidence of respiratory disease, hospitalization or mortality due to non-measles respiratory disease [77]. Similarly there is no evidence for the effect of vitamin D supplementation on the incidence or severity of pneumonia in children [78]. Further research is needed to determine the vitamin D concentrations associated with increased risk of pneumonia and ideal supplementation regimens [79]. However, vitamin D deficiency is significantly associated with TB infection [80] and disease in children [81], [82].
Management
Antibiotics
First line treatment for pneumonia is high dose oral amoxicillin (40 mg/kg/dose given twice daily) in children aged 2 months to 5 yrs for 5 days in areas with high HIV prevalence, and for 3 days in areas with low HIV prevalence. Intravenous ampicillin/benzylpenicillin and gentamicin for at least 5 days is the first line treatment recommended for children with severe pneumonia, whereas intravenous ampicillin/benzylpenicillin and gentamicin is recommended for 10 days in HIV-infected children. For HIV infected or exposed children aged from 2 months up to 1 year with severe pneumonia, cotrimoxazole is recommended as an additional treatment for suspected PCP. Prophylaxis for PCP using cotrimoxazole is recommended for all HIV-infected children and for all HIV-exposed infants from six weeks of age until HIV is confirmed negative and breast feeding has been discontinued for 6 weeks [35]; PCP prophylaxis is also highly effective for non-HIV immunocompromised children [83].
Corticosteroids
Children hospitalized with PCP should receive oral corticosteroids within 48 h in addition to standard treatment with cotrimoxazole as this significantly reduced mortality in-hospital and 6 months after discharge [84]. Corticosteroids should also be used with tuberculosis medication to reduce nodal compression from tuberculosis if this occurs.
Oxygen and CPAP
Oxygen saturation rates lower than 90–92% have been associated with an increased risk of death in children with pneumonia. Therefore hypoxia is an important prognostic indicator in paediatric pneumonia. However in many LMICs, the regular use of pulse oximetry and the availability of oxygen is limited [85]. An innovative low-pressure oxygen storage system piloted in Uganda, can continuously provide oxygen equivalent to the treatment of one child for 30 days despite power cuts. This system is ready for clinical field trials [86]. Similarly, a large scale implementation effectiveness trial to evaluate the feasibility and sustainability of solar powered oxygen systems in remote health centres in New Papua Guinea is ongoing [87]. In an open randomized controlled trial conducted in Bangladesh, children <5 years with severe pneumonia and hypoxaemia who received oxygen delivered via bubble continuous positive airway pressure (bCPAP) had significantly less treatment failure or death compared to those on standard low-flow oxygen [88]. The safety and efficacy of bCPAP in India has also been established [89]. Low cost bCPAP systems are being utilized in other LMIC settings. In Malawi, physicians have found bCPAP to be useful in the management of children with respiratory distress including pneumonia [90].
Micronutrients
Vitamin A is effective for measles-associated pneumonia [91]. Given as an adjunct to the treatment of severe pneumonia in children, zinc significantly reduces mortality [92]. There is no evidence for the use of other micronutrients in the treatment of acute pneumonia in well-nourished children [93], [94]. However, nutritional support including vitamins and zinc should be given in malnourished children [35].
Conclusion
Pneumonia remains the major cause of morbidity and mortality in children in LMICs, outside the neonatal period. With improved immunization and new conjugate vaccines, viral pathogens have been commonly reported, but multiple co-pathogens are most common. S. penumoniae and H. influenzae type b are declining with better immunisation; S. aureus and non-type b H. influenzae are becoming the commonest bacterial pathogens. TB is increasingly recognized as occurring in acute pneumonia in high burden areas. Malnutrition, HIV infection or exposure, tobacco smoke exposure and AAP are significant modifiable risk factors for pneumonia in these contexts. Available effective preventive and management strategies need to be strengthened in LMICs, while new, more accurate diagnostic and better prevention strategies are needed.
Educational aims
The reader will be able to:
-
•
Review the recent epidemiology and aetiology of childhood pneumonia in low-and-middle-income countries (LMICs).
-
•
Summarize the current evidence for diagnostic tools for paediatric pneumonia in LMICs.
-
•
Review prevention and management approaches for children with pneumonia in these settings.
Directions for future research
Future research directions in childhood pneumonia include:
-
(1)
comprehensive data on disease aetiology and risk factors
-
(2)
improved implementation of effective diagnostics and interventions and
-
(3)
innovative approaches to diagnosis, prevention and treatment including novel vaccines and rapid molecular diagnostics.
Funding
HZ is supported by the SA Medical Research Council; HZ has received funding for studies of childhood pneumonia from the Gates Foundation Bill and Melinda Gates Foundation, USA, NIH National Institute of Health, USA, NRF National Research Foundation SA, SA-MRC SA-Medical Research Council, and NICD National Institute Communicable Diseases, SA.
References
- 1.Estimates of the global regional, and national morbidity, mortality, and aetiologies of lower respiratory tract infections in 195 countries: a systematic analysis for the Global Burden of Disease Study 2015. Lancet Infect Dis. 2017;17(11):1133–1161. doi: 10.1016/S1473-3099(17)30396-1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Chopra M., Mason E., Borrazzo J., Campbell H., Rudan I., Liu L. Ending of preventable deaths from pneumonia and diarrhoea: an achievable goal. Lancet (London, England) 2013;381(9876):1499–1506. doi: 10.1016/S0140-6736(13)60319-0. [DOI] [PubMed] [Google Scholar]
- 3.Mackenzie G.A., Hill P.C., Jeffries D.J., Hossain I., Uchendu U., Ameh D. Effect of the introduction of pneumococcal conjugate vaccination on invasive pneumococcal disease in The Gambia: a population-based surveillance study. Lancet Infect Dis. 2016;16(6):703–711. doi: 10.1016/S1473-3099(16)00054-2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.McCollum E.D., Nambiar B., Deula R., Zadutsa B., Bondo A., King C. Impact of the 13-valent pneumococcal conjugate vaccine on clinical and hypoxemic childhood pneumonia over three years in central malawi: an observational study. PLoS One. 2017;12(1) doi: 10.1371/journal.pone.0168209. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Andrade A.L., Afonso E.T., Minamisava R., Bierrenbach A.L., Cristo E.B., Morais-Neto O.L. Direct and indirect impact of 10-valent pneumococcal conjugate vaccine introduction on pneumonia hospitalizations and economic burden in all age-groups in Brazil: A time-series analysis. PLoS One. 2017;12(9) doi: 10.1371/journal.pone.0184204. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Kurum E., Warren J.L., Schuck-Paim C., Lustig R., Lewnard J.A., Fuentes R. Bayesian model averaging with change points to assess the impact of vaccination and public health interventions. Epidemiology (Cambridge, Mass) 2017;28(6):889–897. doi: 10.1097/EDE.0000000000000719. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Schuck-Paim C., Taylor R.J., Simonsen L., Lustig R., Kurum E., Bruhn C.A. Challenges to estimating vaccine impact using hospitalization data. Vaccine. 2017;35(1):118–124. doi: 10.1016/j.vaccine.2016.11.030. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Bawankule R., Singh A., Kumar K., Shetye S. Does measles vaccination reduce the risk of acute respiratory infection (ARI) and diarrhea in children: a multi-country study? PLoS One. 2017;12(1) doi: 10.1371/journal.pone.0169713. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Causes of severe pneumonia requiring hospital admission in children without HIV infection from Africa and Asia: the PERCH multi-country case-control study. The Pneumonia Etiology Research for Child Health (PERCH) Study Group* Lancet 27 June 2019. 10.1016/S0140-6736(19)30721-4. [DOI] [PMC free article] [PubMed]
- 10.Zar H.J., Barnett W., Stadler A., Gardner-Lubbe S., Myer L., Nicol M.P. Aetiology of childhood pneumonia in a well vaccinated South African birth cohort: a nested case-control study of the Drakenstein Child Health Study. Lancet Resp Med. 2016;4(6):463–472. doi: 10.1016/S2213-2600(16)00096-5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Hammitt L.L., Kazungu S., Morpeth S.C., Gibson D.G., Mvera B., Brent A.J. A preliminary study of pneumonia etiology among hospitalized children in Kenya. Clin Infect Dis. 2012;54(Suppl 2):S190–S199. doi: 10.1093/cid/cir1071. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Howie S.R., Morris G.A., Tokarz R., Ebruke B.E., Machuka E.M., Ideh R.C. Etiology of severe childhood pneumonia in the Gambia, West Africa, determined by conventional and molecular microbiological analyses of lung and pleural aspirate samples. Clin Infect Dis. 2014;59(5):682–685. doi: 10.1093/cid/ciu384. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Feikin D.R., Hammitt L.L., Murdoch D.R., O'Brien K.L., Scott J.A.G. The enduring challenge of determining pneumonia etiology in children: considerations for future research priorities. Clin Infect Dis. 2017;64(suppl_3):S188–S196. doi: 10.1093/cid/cix143. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Shi T., McLean K., Campbell H., Nair H. Aetiological role of common respiratory viruses in acute lower respiratory infections in children under five years: a systematic review and meta-analysis. J Glob Health. 2015;5(1) doi: 10.7189/jogh.05.010408. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Shi T., McAllister D.A., O'Brien K.L., Simoes E.A.F., Madhi S.A., Gessner B.D. Global, regional, and national disease burden estimates of acute lower respiratory infections due to respiratory syncytial virus in young children in 2015: a systematic review and modelling study. Lancet (London, England) 2017;390(10098):946–958. doi: 10.1016/S0140-6736(17)30938-8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Cinel G., Pekcan S., Ozcelik U., Alp A., Yalcin E., Dogru Ersoz D. Cytomegalovirus infection in immunocompetent wheezy infants: the diagnostic value of CMV PCR in bronchoalveolar lavage fluid. J Clin Pharm Ther. 2014;39(4):399–403. doi: 10.1111/jcpt.12169. [DOI] [PubMed] [Google Scholar]
- 17.Restrepo-Gualteros S.M., Jaramillo-Barberi L.E., Gonzalez-Santos M., Rodriguez-Martinez C.E., Perez G.F., Gutierrez M.J. Characterization of cytomegalovirus lung infection in non-HIV infected children. Viruses. 2014;6(5):2038–2051. doi: 10.3390/v6052038. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Gupte A.N., Mave V., Meshram S., Lokhande R., Kadam D., Dharmshale S. The significance of cytomegalovirus in children with pneumonia admitted for mechanical ventilation. Int J Tuberc Lung Dis. 2017;21(12):800–806. doi: 10.5588/ijtld.17.0026. [DOI] [PubMed] [Google Scholar]
- 19.Barger-Kamate B., Deloria Knoll M., Kagucia E.W., Prosperi C., Baggett H.C., Brooks W.A. Pertussis-associated pneumonia in infants and children from low- and middle-income countries participating in the PERCH study. Clin Infect Dis. 2016;63(suppl 4):S187–S196. doi: 10.1093/cid/ciw546. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Harris C., Mills R., Seager E., Blackstock S., Hiwa T., Pumphrey J. Paediatric deaths in a tertiary government hospital setting, Malawi. Paediatr Int Child Health. 2018 doi: 10.1080/20469047.2018.1536873. [DOI] [PubMed] [Google Scholar]
- 21.Oliwa J.N., Karumbi J.M., Marais B.J., Madhi S.A., Graham S.M. Tuberculosis as a cause or comorbidity of childhood pneumonia in tuberculosis-endemic areas: a systematic review. Lancet Resp Med. 2015;3(3):235–243. doi: 10.1016/S2213-2600(15)00028-4. [DOI] [PubMed] [Google Scholar]
- 22.Yeung K.H.T., Duclos P., Nelson E.A.S., Hutubessy R.C.W. An update of the global burden of pertussis in children younger than 5 years: a modelling study. Lancet Infect Dis. 2017;17(9):974–980. doi: 10.1016/S1473-3099(17)30390-0. [DOI] [PubMed] [Google Scholar]
- 23.Muloiwa R., Dube F.S., Nicol M.P., Zar H.J., Hussey G.D. Incidence and diagnosis of pertussis in South African children hospitalized with lower respiratory tract infection. Pediatr Infect Dis J. 2016;35(6):611–616. doi: 10.1097/INF.0000000000001132. [DOI] [PubMed] [Google Scholar]
- 24.du Plessis N.M., Ntshoe G., Reubenson G., Kularatne R., Blumberg L., Thomas J. Risk factors for pertussis among hospitalized children in a high HIV prevalence setting, South Africa. Int J Infect Dis. 2018;68:54–60. doi: 10.1016/j.ijid.2018.01.010. [DOI] [PubMed] [Google Scholar]
- 25.Martinez L., le Roux D.M., Barnett W., At Stadler, Nicol M.P., Zar H.J. Tuberculin Skin test conversion and primary progressive tuberculosis disease in the first 5 years of life: a birth cohort study from Cape Town, South Africa. Lancet Child Adolesc Health. Lancet Child Adolesc Health. 2018;2(1):46–55. doi: 10.1016/S2352-4642(17)30149-9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Zar H.J., Dechaboon A., Hanslo D., Apolles P., Magnus K.G., Hussey G. Pneumocystis carinii pneumonia in South African children infected with human immunodeficiency virus. Pediatr Infect Dis J. 2000;19(7):603–607. doi: 10.1097/00006454-200007000-00004. [DOI] [PubMed] [Google Scholar]
- 27.Ruffini D.D., Madhi S.A. The high burden of Pneumocystis carinii pneumonia in African HIV-1-infected children hospitalized for severe pneumonia. AIDS (London, England) 2002;16(1):105–112. doi: 10.1097/00002030-200201040-00013. [DOI] [PubMed] [Google Scholar]
- 28.Morrow B.M., Hsaio N.Y., Zampoli M., Whitelaw A., Zar H.J. Pneumocystis pneumonia in South African children with and without human immunodeficiency virus infection in the era of highly active antiretroviral therapy. Pediatr Infect Dis J. 2010;29(6):535–539. doi: 10.1097/INF.0b013e3181ce871e. [DOI] [PubMed] [Google Scholar]
- 29.Morrow B.M., Samuel C.M., Zampoli M., Whitelaw A., Zar H.J. Pneumocystis pneumonia in South African children diagnosed by molecular methods. BMC Res Notes. 2014;7:26. doi: 10.1186/1756-0500-7-26. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Sonego M., Pellegrin M.C., Becker G., Lazzerini M. Risk factors for mortality from acute lower respiratory infections (ALRI) in children under five years of age in low and middle-income countries: a systematic review and meta-analysis of observational studies. PLoS One. 2015;10(1) doi: 10.1371/journal.pone.0116380. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Verani J.R., Groome M.J., Zar H.J., Zell E.R., Kapongo C.N., Nzenze S.A. Risk factors for presumed bacterial pneumonia among HIV-uninfected children hospitalized in Soweto, South Africa. Pediatr Infect Dis J. 2016;35(11):1169–1174. doi: 10.1097/INF.0000000000001264. [DOI] [PubMed] [Google Scholar]
- 32.Agweyu A., Lilford R.J., English M. Appropriateness of clinical severity classification of new WHO childhood pneumonia guidance: a multi-hospital, retrospective, cohort study. Lancet Glob Health. 2018;6(1):e74–e83. doi: 10.1016/S2214-109X(17)30448-5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Kelly M.S., Wirth K.E., Steenhoff A.P., Cunningham C.K., Arscott-Mills T., Boiditswe S.C. Treatment failures and excess mortality among HIV-exposed, uninfected children with pneumonia. J Pediatric Infect Dis Soc. 2015;4(4):e117–e126. doi: 10.1093/jpids/piu092. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Lelieveld J., Haines A., Pozzer A. Age-dependent health risk from ambient air pollution: a modelling and data analysis of childhood mortality in middle-income and low-income countries. Lancet Planet Health. 2018;2(7):e292–e300. doi: 10.1016/S2542-5196(18)30147-5. [DOI] [PubMed] [Google Scholar]
- 35.WHO . World Health Organization; Geneva: 2013. Pocket book of hospital care for children: guidelines for the management of common illnesses with limited resources, 2nd ed. [PubMed] [Google Scholar]
- 36.McCollum E.D., King C., Hollowell R., Zhou J., Colbourn T., Nambiar B. Predictors of treatment failure for non-severe childhood pneumonia in developing countries–systematic literature review and expert survey–the first step towards a community focused mHealth risk-assessment tool? BMC Pediatr. 2015;15:74. doi: 10.1186/s12887-015-0392-x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.McCollum E.D., Ginsburg A.S. Outpatient management of children with world health organization chest indrawing pneumonia: implementation risks and proposed solutions. Clin Infect Dis. 2017;65(9):1560–1564. doi: 10.1093/cid/cix543. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Cherian T., Mulholland E.K., Carlin J.B., Ostensen H., Amin R., de Campo M. Standardized interpretation of paediatric chest radiographs for the diagnosis of pneumonia in epidemiological studies. Bull World Health Organ. 2005;83(5):353–359. [PMC free article] [PubMed] [Google Scholar]
- 39.Fancourt N., Deloria Knoll M., Barger-Kamate B., de Campo J., de Campo M., Diallo M. Standardized interpretation of chest radiographs in cases of pediatric pneumonia from the PERCH study. Clin Infect Dis. 2017;64(suppl_3):S253–S261. doi: 10.1093/cid/cix082. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Xavier-Souza G., Vilas-Boas A.L., Fontoura M.S., Araujo-Neto C.A., Andrade S.C., Cardoso M.R. The inter-observer variation of chest radiograph reading in acute lower respiratory tract infection among children. Pediatr Pulmonol. 2013;48(5):464–469. doi: 10.1002/ppul.22644. [DOI] [PubMed] [Google Scholar]
- 41.Najgrodzka P., Buda N., Zamojska A., Marciniewicz E., Lewandowicz-Uszynska A. Lung ultrasonography in the diagnosis of pneumonia in children-a metaanalysis and a review of pediatric lung imaging. Ultrasound Q. 2019 doi: 10.1097/RUQ.0000000000000411. [DOI] [PubMed] [Google Scholar]
- 42.Orso D., Ban A., Guglielmo N. Lung ultrasound in diagnosing pneumonia in childhood: a systematic review and meta-analysis. J Ultrasound. 2018;21(3):183–195. doi: 10.1007/s40477-018-0306-5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Zar H.J., Andronikou S., Nicol M.P. Advances in the diagnosis of pneumonia in children. BMJ. 2017;358 doi: 10.1136/bmj.j2739. [DOI] [PubMed] [Google Scholar]
- 44.Higdon M.M., Le T., O'Brien K.L., Murdoch D.R., Prosperi C., Baggett H.C. Association of C-reactive protein with bacterial and respiratory syncytial virus-associated pneumonia among children aged <5 years in the PERCH study. Clin Infect Dis. 2017;64(suppl_3):S378–S386. doi: 10.1093/cid/cix150. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Iroh Tam P.Y., Bernstein E., Ma X., Ferrieri P. Blood culture in evaluation of pediatric community-acquired pneumonia: a systematic review and meta-analysis. Hosp Pediatr. 2015;5(6):324–336. doi: 10.1542/hpeds.2014-0138. [DOI] [PubMed] [Google Scholar]
- 46.Driscoll A.J., Deloria Knoll M., Hammitt L.L., Baggett H.C., Brooks W.A., Feikin D.R. The effect of antibiotic exposure and specimen volume on the detection of bacterial pathogens in children with pneumonia. Clin Infect Dis. 2017;64(suppl_3):S368–S377. doi: 10.1093/cid/cix101. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Morpeth S.C., Deloria Knoll M., Scott J.A.G., Park D.E., Watson N.L., Baggett H.C. Detection of pneumococcal dna in blood by polymerase chain reaction for diagnosing pneumococcal pneumonia in young children from low- and middle-income countries. Clin Infect Dis. 2017;64(suppl_3):S347–S356. doi: 10.1093/cid/cix145. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Zar H.J. Bacterial and viral pneumonia: new insights from the Drakenstein child health study. Paediatr Respir Rev. 2017;24:8–10. doi: 10.1016/j.prrv.2017.06.009. [DOI] [PubMed] [Google Scholar]
- 49.de Steenhuijsen Piters W.A., Heinonen S., Hasrat R., Bunsow E., Smith B., Suarez-Arrabal M.C. Nasopharyngeal microbiota, host transcriptome, and disease severity in children with respiratory syncytial virus infection. Am J Respir Crit Care Med. 2016;194(9):1104–1115. doi: 10.1164/rccm.201602-0220OC. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.Park D.E., Baggett H.C., Howie S.R.C., Shi Q., Watson N.L., Brooks W.A. Colonization density of the upper respiratory tract as a predictor of Pneumonia-Haemophilus influenzae, Moraxella catarrhalis, Staphylococcus aureus, and Pneumocystis jirovecii. Clin Infect Dis. 2017;64(suppl_3):S328–S336. doi: 10.1093/cid/cix104. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51.DeLuca A.N., Hammitt L.L., Kim J., Higdon M.M., Baggett H.C., Brooks W.A. Safety of induced sputum collection in children hospitalized with severe or very severe pneumonia. Clin Infect Dis. 2017;64(suppl_3):S301–S308. doi: 10.1093/cid/cix078. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52.Planting N.S., Visser G.L., Nicol M.P., Workman L., Isaacs W., Zar H.J. Safety and efficacy of induced sputum in young children hospitalised with suspected pulmonary tuberculosis. Int J Tuberc Lung Dis. 2014;18(1):8–12. doi: 10.5588/ijtld.13.0132. [DOI] [PubMed] [Google Scholar]
- 53.Samuel C.M., Whitelaw A., Corcoran C., Morrow B., Hsiao N.Y., Zampoli M. Improved detection of Pneumocystis jirovecii in upper and lower respiratory tract specimens from children with suspected pneumocystis pneumonia using real-time PCR: a prospective study. BMC Infect Dis. 2011;11:329. doi: 10.1186/1471-2334-11-329. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54.Ballif M., Renner L., Claude Dusingize J., Leroy V., Ayaya S., Wools-Kaloustian K. Tuberculosis in pediatric antiretroviral therapy programs in low- and middle-income countries: diagnosis and screening practices. J Pediatr Infect Dis Soc. 2015;4(1):30–38. doi: 10.1093/jpids/piu020. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 55.Detjen A.K., DiNardo A.R., Leyden J., Steingart K.R., Menzies D., Schiller I. Xpert MTB/RIF assay for the diagnosis of pulmonary tuberculosis in children: a systematic review and meta-analysis. Lancet Respir Med. 2015;3(6):451–461. doi: 10.1016/S2213-2600(15)00095-8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 56.Sabi I., Rachow A., Mapamba D., Clowes P., Ntinginya N.E., Sasamalo M. Xpert MTB/RIF Ultra assay for the diagnosis of pulmonary tuberculosis in children: a multicentre comparative accuracy study. J Infect. 2018;77(4):321–327. doi: 10.1016/j.jinf.2018.07.002. [DOI] [PubMed] [Google Scholar]
- 57.Nicol M.P., Workman L., Prins M., Bateman L., Ghebrekristos Y., Mbhele S. Accuracy of Xpert Mtb/Rif ultra for the diagnosis of pulmonary tuberculosis in children. Pediatr Infect Dis J. 2018;37(10):e261–e263. doi: 10.1097/INF.0000000000001960. [DOI] [PubMed] [Google Scholar]
- 58.VIEW-hub report: Global Vaccine Introduction and Implementation. June 2018. A report on current global access to new childhood vaccines.
- 59.UNICEF Immunization Data. Immunization coverage by antigen (country, regional, and global trends).
- 60.Hosseinpoor A.R., Bergen N., Schlotheuber A., Gacic-Dobo M., Hansen P.M., Senouci K. State of inequality in diphtheria-tetanus-pertussis immunisation coverage in low-income and middle-income countries: a multicountry study of household health surveys. Lancet Glob Health. 2016 doi: 10.1016/S2214-109X(16)30141-3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 61.VanderEnde K., Gacic-Dobo M., Diallo M.S., Conklin L.M., Wallace A.S. Global routine vaccination coverage – 2017. MMWR Morb Mortal Wkly Rep. 2018;67(45):1261–1264. doi: 10.15585/mmwr.mm6745a2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 62.Muloiwa R., Wolter N., Mupere E., Tan T., Chitkara A.J., Forsyth K.D. Pertussis in Africa: findings and recommendations of the Global Pertussis Initiative (GPI) Vaccine. 2018;36(18):2385–2393. doi: 10.1016/j.vaccine.2018.03.025. [DOI] [PubMed] [Google Scholar]
- 63.Hoest C., Seidman J.C., Lee G., Platts-Mills J.A., Ali A., Olortegui M.P. Vaccine coverage and adherence to EPI schedules in eight resource poor settings in the MAL-ED cohort study. Vaccine. 2017;35(3):443–451. doi: 10.1016/j.vaccine.2016.11.075. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 64.Campbell P.T., McVernon J., McIntyre P., Geard N. Influence of population demography and immunization history on the impact of an antenatal pertussis program. Clin Infect Dis. 2016;63(suppl 4):S213–S220. doi: 10.1093/cid/ciw520. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 65.Fell D.B., Azziz-Baumgartner E., Baker M.G., Batra M., Beaute J., Beutels P. Influenza epidemiology and immunization during pregnancy: final report of a World Health Organization working group. Vaccine. 2017;35(43):5738–5750. doi: 10.1016/j.vaccine.2017.08.037. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 66.Simoes E.A.F., Bont L., Manzoni P., Fauroux B., Paes B., Figueras-Aloy J. Past, present and future approaches to the prevention and treatment of respiratory syncytial virus infection in children. Infect Dis Ther. 2018;7(1):87–120. doi: 10.1007/s40121-018-0188-z. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 67.Madhi S.A., Cutland C.L., Kuwanda L., Weinberg A., Hugo A., Jones S. Influenza vaccination of pregnant women and protection of their infants. N Engl J Med. 2014;371(10):918–931. doi: 10.1056/NEJMoa1401480. [DOI] [PubMed] [Google Scholar]
- 68.Nunes M.C., Madhi S.A. Influenza vaccination during pregnancy for prevention of influenza confirmed illness in the infants: a systematic review and meta-analysis. Human Vaccines Immunother. 2018;14(3):758–766. doi: 10.1080/21645515.2017.1345385. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 69.Schlaudecker E.P., Ambroggio L., McNeal M.M., Finkelman F.D., Way S.S. Declining responsiveness to influenza vaccination with progression of human pregnancy. Vaccine. 2018;36(31):4734–4741. doi: 10.1016/j.vaccine.2018.05.117. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 70.Poletti P., Merler S., Ajelli M., Manfredi P., Munywoki P.K., Nokes D. Evaluating vaccination strategies for reducing infant respiratory syncytial virus infection in low-income settings. BMC Med. 2015;13:49. doi: 10.1186/s12916-015-0283-x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 71.Buchwald A.G., Tamboura B., Tennant S.M., Haidara F.C., Coulibaly F., Doumbia M. Epidemiology, risk factors, and outcomes of respiratory syncytial virus infections in newborns in Bamako, Mali. Clin Infect Dis. 2019 doi: 10.1093/cid/ciz157. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 72.Novavax Announces Topline Results from Phase 3 PrepareTM Trial of ResVax™ for Prevention of RSV Disease in Infants via Maternal Immunization [Available from: http://ir.novavax.com/news-releases/news-release-details/novavax-announces-topline-results-phase-3-preparetm-trial].
- 73.Wiens M.O., Pawluk S., Kissoon N., Kumbakumba E., Ansermino J.M., Singer J. Pediatric post-discharge mortality in resource poor countries: a systematic review. PLoS One. 2013;8(6) doi: 10.1371/journal.pone.0066698. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 74.United Nations Children’s Fund. Committing to child survival: a promise renewed. Progress report 2015. http://www.unicef.org/publications/index_83078.html.
- 75.Ginsburg A.S., Izadnegahdar R., Berkley J.A., Walson J.L., Rollins N., Klugman K.P. Undernutrition and pneumonia mortality. Lancet Glob Health. 2015;3(12):e735–e736. doi: 10.1016/S2214-109X(15)00222-3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 76.Lassi Z.S., Moin A., Bhutta Z.A. Zinc supplementation for the prevention of pneumonia in children aged 2 months to 59 months. Cochr Database Syst Rev. 2016;12 doi: 10.1002/14651858.CD005978.pub3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 77.Imdad A., Mayo-Wilson E., Herzer K., Bhutta Z.A. Vitamin A supplementation for preventing morbidity and mortality in children from six months to five years of age. Cochr Database Syst Rev. 2017;3 doi: 10.1002/14651858.CD008524.pub3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 78.Yakoob M.Y., Salam R.A., Khan F.R., Bhutta Z.A. Vitamin D supplementation for preventing infections in children under five years of age. Cochr Database Syst Rev. 2016;11 doi: 10.1002/14651858.CD008824.pub2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 79.Esposito S., Lelii M. Vitamin D and respiratory tract infections in childhood. BMC Infect Dis. 2015;15:487. doi: 10.1186/s12879-015-1196-1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 80.Venturini E., Facchini L., Martinez-Alier N., Novelli V., Galli L., de Martino M. Vitamin D and tuberculosis: a multicenter study in children. BMC Infect Dis. 2014;14:652. doi: 10.1186/s12879-014-0652-7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 81.Buonsenso D., Sali M., Pata D., Masiello E., Salerno G., Ceccarelli M. Vitamin D levels in active TB, latent TB, non-TB pneumonia and healthy children: a prospective observational study. Fetal Pediatr Pathol. 2018;37(5):337–347. doi: 10.1080/15513815.2018.1509407. [DOI] [PubMed] [Google Scholar]
- 82.Gou X., Pan L., Tang F., Gao H., Xiao D. The association between vitamin D status and tuberculosis in children: a meta-analysis. Medicine (Baltimore) 2018;97(35) doi: 10.1097/MD.0000000000012179. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 83.Stern A., Green H., Paul M., Vidal L., Leibovici L. Prophylaxis for Pneumocystis pneumonia (PCP) in non-HIV immunocompromised patients. Cochr Database Syst Rev. 2014;10 doi: 10.1002/14651858.CD005590.pub3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 84.Newberry L., O'Hare B., Kennedy N., Selman A., Omar S., Dawson P. Early use of corticosteroids in infants with a clinical diagnosis of Pneumocystis jirovecii pneumonia in Malawi: a double-blind, randomised clinical trial. Paediatr Int Child Health. 2017;37(2):121–128. doi: 10.1080/20469047.2016.1260891. [DOI] [PubMed] [Google Scholar]
- 85.Lazzerini M., Sonego M., Pellegrin M.C. Hypoxaemia as a mortality risk factor in acute lower respiratory infections in children in low and middle-income countries: systematic review and meta-analysis. PLoS One. 2015;10(9) doi: 10.1371/journal.pone.0136166. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 86.Rassool R.P., Sobott B.A., Peake D.J., Mutetire B.S., Moschovis P.P., Black J.F. A low-pressure oxygen storage system for oxygen supply in low-resource settings. Respir Care. 2017;62(12):1582–1587. doi: 10.4187/respcare.05532. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 87.Duke T., Hwaihwanje I., Kaupa M., Karubi J., Panauwe D., Sa'avu M. Solar powered oxygen systems in remote health centers in Papua New Guinea: a large scale implementation effectiveness trial. J Glob Health. 2017;7(1) doi: 10.7189/jogh.07.010411. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 88.Chisti M.J., Salam M.A., Smith J.H., Ahmed T., Pietroni M.A., Shahunja K.M. Bubble continuous positive airway pressure for children with severe pneumonia and hypoxaemia in Bangladesh: an open, randomised controlled trial. Lancet (London, England) 2015;386(9998):1057–1065. doi: 10.1016/S0140-6736(15)60249-5. [DOI] [PubMed] [Google Scholar]
- 89.Jayashree M., KiranBabu H.B., Singhi S., Nallasamy K. Use of Nasal bubble CPAP in children with hypoxemic clinical pneumonia-report from a resource limited set-up. J Trop Pediatr. 2016;62(1):69–74. doi: 10.1093/tropej/fmv063. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 90.Machen H.E., Mwanza Z.V., Brown J.K., Kawaza K.M., Newberry L., Richards-Kortum R.R. Outcomes of patients with respiratory distress treated with bubble CPAP on a pediatric ward in Malawi. J Trop Pediatr. 2015;61(6):421–427. doi: 10.1093/tropej/fmv052. [DOI] [PubMed] [Google Scholar]
- 91.Ellison J.B. Intensive vitamin therapy in measles. Br Med J. 1932;2(3745):708–711. doi: 10.1136/bmj.2.3745.708. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 92.Wang L., Song Y. Efficacy of zinc given as an adjunct to the treatment of severe pneumonia: a meta-analysis of randomized, double-blind and placebo-controlled trials. Clin Respir J. 2018;12(3):857–864. doi: 10.1111/crj.12646. [DOI] [PubMed] [Google Scholar]
- 93.Taneja S., Strand T.A., Kumar T., Mahesh M., Mohan S., Manger M.S. Folic acid and vitamin B-12 supplementation and common infections in 6–30-mo-old children in India: a randomized placebo-controlled trial. Am J Clin Nutr. 2013;98(3):731–737. doi: 10.3945/ajcn.113.059592. [DOI] [PubMed] [Google Scholar]
- 94.Das R.R., Singh M., Naik S.S. Vitamin D as an adjunct to antibiotics for the treatment of acute childhood pneumonia. Cochr Database Syst Rev. 2018;7 doi: 10.1002/14651858.CD011597.pub2. [DOI] [PMC free article] [PubMed] [Google Scholar]


