Abstract
Liquid chromatography tandem mass spectrometry allows for the measurement of steroid hormone suites in the blubber of marine mammals. By combining this technology with minimally invasive techniques such as remote biopsy, endocrine profiles can be assessed, allowing for studies of hormonal profile variation over time. In this study, we explored associations among different steroidogenic pathways and seasonal differences in blubber hormone profiles of free-ranging common bottlenose dolphins along the coast of South Carolina, USA. Male dolphins experience a peak in testosterone, androstenedione, progesterone, and 17-hydroxyprogesterone in the spring, likely related to an upregulation of the androgen steroidogenic pathway during mating season. We also observed increased cortisol concentrations during summer compared to winter. Among females, there was an increase in androstenedione with elevated progesterone concentrations indicative of pregnancy, highlighting another potential endocrine marker for pregnancy in free-ranging dolphins. This work emphasizes the importance of selecting the appropriate season for studies on endocrine status to effectively uncover physiological variation or disruption in free-ranging cetaceans.
Keywords: Remote biopsy, hormones, cetacean, blubber, reproductive steroids, stress
1. Introduction
Monitoring steroid hormones is an effective method for determining reproductive status and can contribute to the assessment of health status in free-ranging marine mammals (De Mello and De Oliveira, 2016; Kellar et al., 2015; Kellar et al., 2006; Lane et al., 2015; Schwacke et al., 2014; Steinman et al., 2016). Steroid hormones circulate throughout the body in the blood and regulate sexual maturation, reproduction, and stress response. However, collecting blood samples from free-ranging individuals to monitor stress hormones (corticosteroids) in cetaceans is difficult as these species, are highly mobile and require time consuming methods for safe restraint to obtain a blood sample (Schwacke et al., 2009), which in turn provides time for cortisol concentrations to rise in the blood due to the acute stress event. Studies involving temporary capture of some cetacean species have been conducted; however, these are limited to a few small cetacean species and are generally constrained to shallow, nearshore waters (Schwacke et al., 2014; Wells et al., 2004). Capture and handling of the subject animal can also be problematic for the assessment of steroid hormones, particularly corticosteroids, as the time to collection can stress the animals and alter the endocrine profile (Champagne et al., 2017; Kellar et al., 2015). Therefore, identifying an endocrinologically-relevant matrix that can be collected remotely would be highly beneficial for monitoring steroid hormones and improve cross-study comparison.
Remote biopsy sampling of cetaceans is proven to be a safe and efficient methodology to collect blubber from free-ranging individuals (Gorgone et al., 2008; Kiszka et al., 2010; Weller et al., 1997), and blubber is an endocrinologically relevant tissue and has been used to examine male sexual maturity (testosterone), pregnancy (progesterone), and stress in managed individuals or collected post-mortem (cortisol) (Champagne et al., 2017; Champagne et al., 2018; Kellar et al., 2015; Kellar et al., 2009; Kellar et al., 2006; Krutzen et al., 2002). However, because the animal cannot be handled using this method, collection of additional demographic data (size, age, sexual maturity) is not possible in untracked populations and requires a separate analysis for sex determination. Additionally, the technique has been shown to be biased towards sampling of male dolphins (Quérouil et al., 2009). Despite these shortcomings, this sampling technique requires fewer resources than capture-release studies, allowing for increased sample size from less sampling effort, and provides rapid sampling, which limits the changes in stress hormones due to collection processes.
The recent application of liquid chromatography tandem mass spectrometry (LC-MS/MS) methods to measure steroids in blubber provides substantial advantages over the traditional steroid quantification methods, i.e. immunoassays (Boggs et al., 2017; Mary et al., 2017). One major advantage of LC-MS/MS over immunoassay techniques is that multiple hormones are determined simultaneously. Although the main criticism of LC-MS/MS is that immunoassays can attain superior sensitivity, the method developed by Boggs et al. (2017) has demonstrated limits of detection in the pg/g range, making it sufficient for most analyses attempting to detect physiological differences. If greater sensitivity for differences in low concentration hormones is necessary, immunoassays could provide a benefit. None-the-less, by analyzing suites of steroid hormones, collecting broader information on reproductive and stress physiology is possible from a single analysis which can then better inform follow up analyses. While pilot data from Boggs et al. (Boggs et al., 2017) demonstrated that steroid hormones are quantifiable by LC-MS/MS in remote biopsies from common bottlenose dolphins, further analysis is required to determine the biological relevance of these hormones at ambient concentrations in a free-ranging population.
Understanding seasonal hormone variation in free-ranging populations is critical for assessing changes in hormone concentrations induced by reproductive events, endocrine disruption, or chronic stressors. Many species of cetaceans experience seasonal fluctuations in reproductive hormone concentrations (Kellar et al., 2009; Kirby, 1984; Schroeder and Keller, 1989; Yoshioka et al., 1986) that, when not considered during a study design, could confound the interpretation of other exogenous influences on hormones at the population level. Although dolphin reproductive hormones are known to fluctuate with season, little is known about seasonal fluctuations in stress hormones. Annual fluctuations in serum cortisol have been described in managed orcas (Orcinus orca) and common bottlenose dolphins (Orlov et al., 1988; Suzuki et al., 2003) which both displayed elevated circulating cortisol in the winter or spring months. However, these fluctuations have not been described in free-ranging common bottlenose dolphins nor has it been found using blubber biopsies. Elevated seasonal concentrations of cortisol, if they do exist among free-ranging populations, can be a confounding factor when attempting to assess potential disturbances such as acoustic noise, contaminants, or other anthropogenic activities.
Here we describe the analysis of steroid hormone profiles in remote blubber biopsies from common bottlenose dolphin (Tursiops truncatus) in the confluence of the Ashepoo, Combahee, and Edisto Rivers (hereafter referred to as the ACE Basin) and waters in the Charleston area of South Carolina, USA (Charleston Area Waterways System hereafter referred to as CAWS), from October 2011 to August 2012. These populations represent some of the northernmost resident estuarine stocks in the United States, and are hypothesized to display greater seasonal variation in steroid hormones, if such patterns exist. The ACE Basin contains a National Estuarine Research Reserve that is considered to have relatively low anthropogenic activity. Additionally, the ACE Basin and CAWS have no statistical difference in concentrations of persistent organic pollutants (POPs) in the male populations (Neely et al., 2018). Blubber POP concentrations were comparable and intermediate to other southeastern populations of common bottlenose dolphins and are not found in concentrations of high concern (Balmer et al., 2015b; Kucklick et al., 2011). Therefore, these populations were selected to establish seasonal baseline ranges for blubber steroid hormone concentrations. These data constitute the first report on seasonal variation of baseline multi-steroid hormone profiles from blubber of free-ranging common bottlenose dolphins.
2. Materials and Methods
2.1. Sample Collection
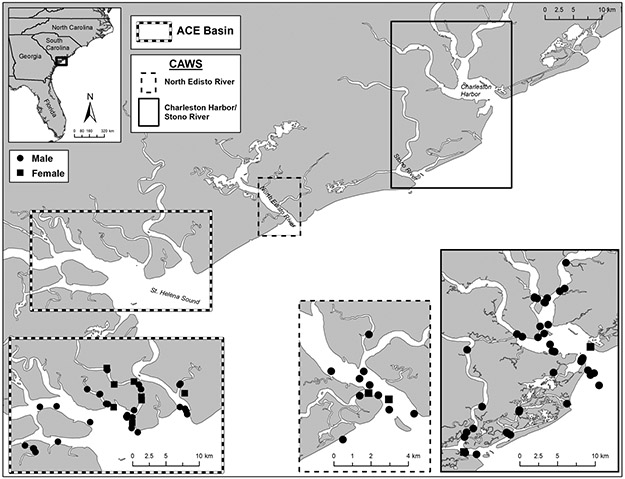
Common bottlenose dolphins from inshore waters of the South Carolina, including the ACE Basin and CAWS, were targeted for this study. The CAWS collectively includes the North Edisto River, the Charleston Harbor, the Cooper, Ashley, and Wando Rivers, and the Stono River estuary (Figure 1). Remote biopsies (n = 93) were collected from October 2011 to August 2012 using the methods described in Balmer et al. (2015a). Seasons were classified by equinoxes and solstices for that collection year (Fall = October 1, 2011 – December 21, 2011, Winter = December 22, 2011 – March 19, 2012, Spring = March 20, 2012 – June 19, 2012, Summer = June 20, 2012 – August 31, 2012). For each season (winter, spring, summer, and fall) the number of males sampled were 18, 14, 33, and 16 respectively and the number of females were 7, 2, 3, and 0 respectively. Upon collection, skin was removed from the biopsy sample and stored in 20 % dimethyl sulfoxide (DMSO) saturated with sodium chloride and were sent to the National Oceanic and Atmospheric Administration National Marine Fisheries Service Southeast Fisheries Science Center, Marine Mammal Molecular Genetics Laboratory for determination of sex and population stock. Using the methods described by Rosel et al. (2003), X and Y chromosomes were amplified using polymerase chain reaction in DNA extracted from the skin to identify sex. Blubber samples were full depth (determined qualitatively by presence of connective tissue, muscle, or gradation of vasculature) and maximally 10 mm X 25 mm deep and 0.8 g. The blubber was halved length-wise to produce two full depth samples approximately 0.4 g to 0.6 g in mass. These sections were placed in separate cryovials then flash frozen in a liquid nitrogen vapor shipper within a mean time of 12 min after collection. One of the frozen blubber subsections was allocated for hormone analysis and was transported to the National Institute of Standards and Technology (NIST) biorepository, in Charleston, SC. There, samples were archived at −80 °C until processing. The remaining subsection was archived for future analyses.
Figure 1:
Map of the Ashepoo, Combahee, Edisto Basin (ACE) and the Charleston Area Waterways System (CAWS) where common bottlenose dolphin remote blubber biopsies were collected. Each dot represents the GPS locations at which an individual was biopsied.
2.2. Sample Preparation and Analysis
Samples were homogenized and steroids extracted according to the methodology described in Boggs et al. (2017). Briefly, an internal standard mixture (concentrations in Supplemental Table 1) consisting of testosterone-13C3, androstenedione-13C3, 17-hydroxyprogesterone-13C3, and cortisol-d4 (Cerilliant; Round Rock, TX, USA; 99.99 % purity) and progesterone-13C3 (Cambridge Isotopes; Tewksbury, MA, USA; 98 % purity) was gravimetrically added to a garnet bead homogenization tube (Mo Bio, San Diego, CA, USA). When exact matched internal standards were not available, the most structurally similar internal standard was substituted (see Boggs et al. 2017 for more details on this method). Remote biopsies were then minced in a glass beaker on dry ice, added to the homogenization tube, and mass of the added blubber recorded. Samples were homogenized at 6500 rpm for 30 s four times using a Precellys bead homogenizer (Bertin Instruments, Montigny-le-Bretonneux, France). Homogenates were extracted and cleaned using the Bond Elut QuEChERS EN Extraction kit and the Bond Elut QuEChERS dispersive-SPE kit for Drug Residues in Meat (Agilent, Santa Clara, CA, USA). The extract was then filtered through a 0.22 μm cellulose acetate spin filter before being reduced to dryness under nitrogen and reconstituted in 200 μL of methanol.
The quality control sample used was NIST Standard Reference Material (SRM) 1945, Organics in Whale Blubber. While there is no matrix matched SRM currently with certified or reference hormone measurements, this material is a large sample of homogenous whale blubber and thus, could be used as quality control across batches. Means and relative standard deviations (RSDs) for SRM 1945 were calculated to track repeatability of the method across the different extraction days. Blanks were also collected on all extraction days to test for contamination.
A six point calibration curve and internal standard blanks were extracted identically to the samples. Calibrants were androstenedione, testosterone, 17-hydroxyprogesterone, progesterone, corticosterone, cortisone, and cortisol (Sigma Aldrich, St. Louis, MO; ≥ 98 % purity), 11-deoxycorticosterone, and 11-deoxycortisol (Steraloids, Newport, RI; ≥ 98 % purity). Calibration standard range of steroid masses (Supplemental Table 2) and internal standard mixture concentrations were calculated and tracked gravimetrically.
2.3. Instrumental method
An Agilent 1200 Series HPLC system coupled to an AB Sciex API4000 QTRAP hybrid triple quadrupole/linear ion trap mass spectrometer was used to monitor two product ions, one for quantitation and one for identity confirmation, using scheduled multiple reaction monitoring for each steroid and internal standard. Chromatography was conducted on a Restek (Bellefonte, PA, USA) Ultra Biphenyl column (250 mm x 4.6 mm, 5.0 μm particle size) with acetonitrile and methanol both containing 0.1 % formic acid (volume fraction) for reproductive hormones. A flow of 500 μL/min was used for a solvent gradient of 20 % acetonitrile increased to 45 % over 30 min., then increased to 80 % over 1 min and held for 4 min, then washed with 100 % acetonitrile for 5 min and re-equilibrated at 20 % for 10 min. Corticosteroids were separated on an Agilent (Santa Clara, CA, USA) Eclipse Plus C18 column (21 mm X 150 mm, 5.0 μm particle size) with methanol and water both containing 0.1 % acetic acid (volume fraction). Column conditions were as follows: flow rate of 250 μL/min, and isocratic method of 46 % methanol for 20 min, a wash of 100 % methanol for 13 min, and re-equilibrated for 10 min. Additional information on compound and instrument parameters can be found in Boggs et al. (Boggs et al., 2017).
2.4. Quantitation
Masses of each analyte were calculated using linear regression of calibration standards that bracketed observed sample peak area ratios (area of the analyte divided by the area of the appropriate internal standard; Supplemental Table 2). Concentrations were determined by dividing the calculated mass of each analyte by the extracted sample mass (mass fraction). Therefore, results are presented in ng of steroid per g of wet weight blubber. Steroid concentrations were not normalized by lipid mass because cortisol has been shown to correlate with percent lipid in the blubber (see Galligan et al. (2019) for further explanation). Limit of detection (LOD) was determined as the mean plus three times the standard deviation of the batch blanks (methanol with internal standard extracted identically to the samples) for each analyte. Reporting limit (RL) of the method was defined by the lowest calibration standard in the regression analysis or the LOD if it was higher than the lowest calibration standard. This is a conservative method of defining the RL as discussed by Ragland et al. (2014).
2.5. Statistical Analysis
Values below the RL used the RL as a replacement value and were flagged. Statistical analyses and visualizations were generated using R (Team, 2013) (primarily packages “tidyverse” (Wickham, 2017) and “NADA” (Lee and Lee, 2017)). Concentration data were grouped by analyte, sex, and season of collection. Sample sets under these defined criteria with 100 % detection used standard distribution-based estimates of central tendency and spread as well as standard t-test/ analysis of variance (ANOVA) comparison tests with distribution-appropriate transformation if necessary. Statistical assessment in this traditional manner suffers from the presence of values below the RL. Replacement of values below the RL with an arbitrary value (e.g. zero, RL, RL/2, etc.) modifies the underlying distribution and skews measures of central tendency as well as confounding comparison tests (Helsel, 2012). Helsel’s approaches for central tendency estimates and significance testing as implemented in NADA were used for data sets with < 100 % detection frequency (Helsel, 2012). Briefly, for central tendency estimates, percent of samples above the group maximum RL was used where detection frequency was less than 20 %, sample sets where detection frequencies were 20 % to 50 % used robust regression on order statistics, and sample sets with detection frequencies 50 % to 99.9 % used the Kaplan-Meier method to estimate empirical cumulative distribution functions (ECDFs); measures of central tendency and spread were drawn from these statistics, as appropriate given the parameters of each group, minimizing the impacts of data below the RL. Due to sparsity and detectability, Helsel’s methods (Helsel, 2012) were used for seasonal and geographic comparison between sample sets. This approach 'flips' left-censored data (data below the RL) around an arbitrarily large constant, resulting in right-censored data suitable to survivorship analysis statistics, such as group wise rank order comparison tests across ECDFs. These tests - and others suited to different data set properties - are available and easily implemented using the NADA package in R. Females were excluded from seasonal analysis due to the small number of samples. Hypothesis testing between sample sets with <100 % detection in all sample sets used NADA’s “cendiff()” comparison between ECDFs. Principal components analysis (“prcomp()”) and package “factoextra” (Kassambara and Mundt, 2017) were used for visual data exploration of multivariate analyte pattern relationships. Correlations across hormones, and between hormones and TEO, were assessed using a censored version of Kendall’s tau as implemented in NADA (“NADA::cenken()”) and visualized as tile plots (across hormones) and annotated scatter plots (hormones and TEO, using the Akritas-Theil-Sen slope estimate and the Turnbull intercept estimate). One outlier was identified in the hormone/TEO data set and removed as a case study and the hormone/TEO assessment repeated. Potential seasonal fluctuation of TEO between spring and summer (the only two seasons for which data were available) was assessed by t-test after meeting assumptions of normality and homoscedasticity. Significance levels (α = 0.05) for rejection of H0 were consistent throughout; all tests were two-sided.
3. Results and Discussion
3.1. Quality Control
SRM 1945 had good repeatability for the detectable sex steroids (RSD < 15 %; Table 1). However, this material is from a stranded female pilot whale. Therefore, progesterone concentrations were elevated, while testosterone was not detectable. The RSDs for SRM 1945 were comparable to limits for immunoassays (< 12 %) for all the corticosteroids except for 11-deoxycorticosterone (RSD = 25 %). The high RSD (greater than 15 %) of 11-deoxycorticosterone at concentrations comparable to the dolphin blubber biopsies indicates that the quantification of this hormone is not acceptable for the low concentrations in this study.
Table 1:
Statistics on steroid hormone concentrations for replications (n = 6) of Standard Reference Material 1945, Organics in Whale Blubber.
| Hormone | Mean (ng/g blubber) |
Standard Deviation | RSD |
|---|---|---|---|
| Cortisone | 5.82 | 0.21 | 3.6 % |
| 11-Deoxycortisol | 4.18 | 0.40 | 10 % |
| Corticosterone | 2.46 | 0.28 | 11 % |
| Cortisol | 8.56 | 0.85 | 10 % |
| 11-Deoxycorticosterone* | 2.43 | 0.61 | 25 % |
| 17-Hydroxyprogesterone | 3.37 | 0.18 | 5.4 % |
| Androstenedione | 0.441 | 0.06 | 13 % |
| Testosteroneδ | < RL | ||
| Progesterone | 206 | 31 | 15 % |
Notes: < RL is below the reporting limit of this method; RSD = relative standard deviation
= not measured in dolphin samples
= not detected in 1945 because it is a female whale
3.2. Reproductive Steroids
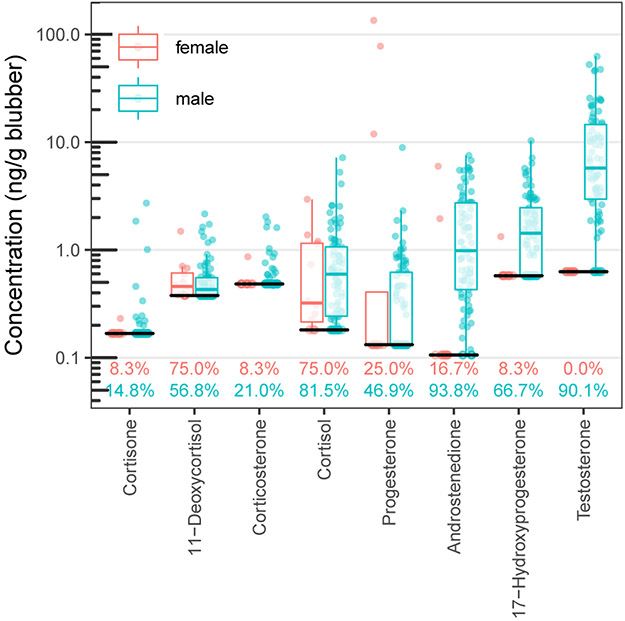
Androstenedione, 17-hydroxyprogesterone, and progesterone were detected in males and females, while testosterone was only quantifiable in males (Figure 2). Of the 12 females sampled, only three (ID: TYP-111215-01, TYP-120314-02, and TYP-120814-06) had quantifiable concentrations of progesterone. Using the blubber progesterone concentration limits for pregnancy (100 ng/g) defined for other dolphin species (Kellar et al., 2013; Kellar et al., 2006; Trego et al., 2013), TYP-120814-06 (summer; 135 ng/g) would be classified as pregnant. TYP-111215-01 (winter; 77.7 ng/g) and TYP-120314-02 (spring; 11.9 ng/g) had elevated progesterone concentrations which would define the individual as a non-pregnant mature female. However, androstenedione was elevated in these two female dolphins exhibiting moderately elevated progesterone signals. Androstenedione production increases in humans, horses, and killer whales (Orcinus orca) during pregnancy (Castracane and Asch, 1995; Kuijper et al., 2013; Legacki et al., 2016; Robeck et al., 2017) potentially as a pathway to increase estrone and estriol production as well as via direct stimulation of luteal progesterone production in early pregnancy (Begumhasan and Murphy, 1992; Carrizo et al., 1994). While demographic information to determine the pregnancy status of female dolphins in this study were not available, it was demonstrated that androstenedione can be quantified in remotely collected blubber of female dolphins should it be found to be an important indicator of pregnancy.
Figure 2:
Boxplots of blubber hormone concentrations from all common bottlenose dolphins sampled in this study on the Ashepoo, Combahee, Edisto Basin and surrounding Charleston-area waterways in South Carolina. Boxplots represent the median with quartiles. The lower solid black bar represents the reporting limit for this method. Detection frequencies for each hormone are listed by sex above the x-axis.
Androgens were quantifiable in most male samples (testosterone = 90 %, androstenedione = 95 %; Figure 2). Because remote biopsy sampling precludes determination of reproductive maturity using parameters such as age or testis size, we cannot conclude whether individuals were immature, quiescent, or senescent. However, demonstration of the quantification of an additional androgen in remote biopsies provides an additional target to study male maturity.
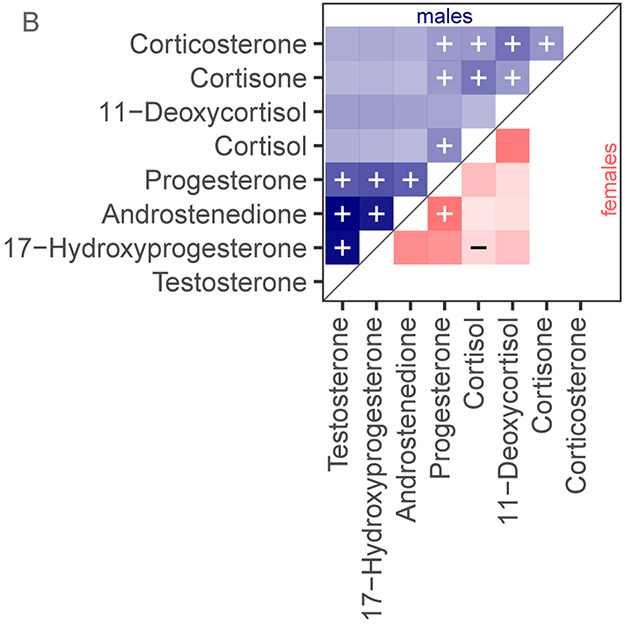
Among males, progestogens and androgens were correlated with each other (Figure 3). A positive relationship between the two androgens is expected (p < 0.001, tau = 0.745) as androstenedione is a precursor hormone to testosterone. There is also a positive correlation between progesterone and 17-hydroxyprogesterone and both androgens (progesterone: testosterone p < 0.001, tau = 0.371, progesterone: androstenedione < 0.001 tau = 0.354, 17-hydroxyprogesterone: testosterone p < 0.001, tau = 0.704, and 17-hydroxyprogesterone: androstenedione p < 0.001 tau = 0.640). Progesterone and 17-hydroxyprogesterone are also precursors to the cortisol pathway, but the correlations between the androgens and progestogens suggest that the testes, rather than the adrenal glands, are the source of circulating progesterone and 17-hydroxyprogesterone in unstressed male common bottlenose dolphins. Thus, caution should be taken in including progestogens in future analyses to assess stress response in remote biopsied males during reproductive events.
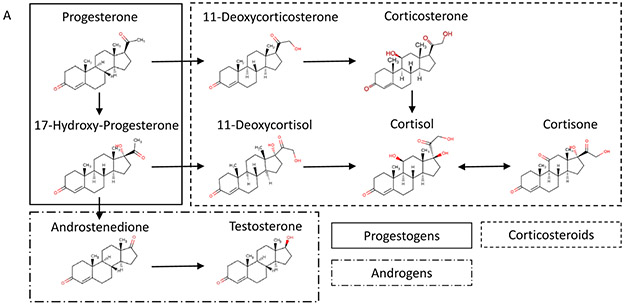
Figure 3:
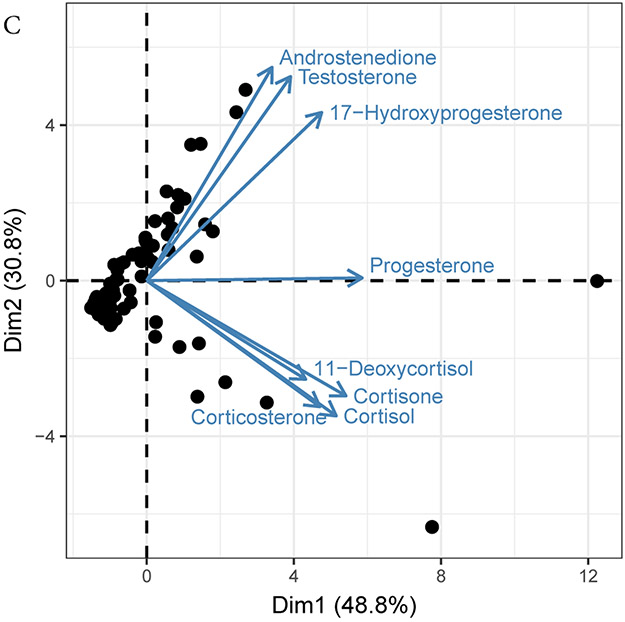
Relationships among hormones in blubber from common bottlenose dolphins. Panel A: The steroid synthesis pathway and classification for hormones measured in this study. Panel B: Correlations heat map using Kendall’s Tau. Intensity approximates tau. Statistical significance (p < 0.05) is represented by (+) for a positive tau and (−) for a negative tau. Spaces are white where no information is available. Sample sizes were 81 for males and 12 for females. Panel C: Principal components analysis of quantified blubber hormones for male common bottlenose dolphins.
3.3. Corticosteroids
Cortisone, 11-deoxycortisol, corticosterone, and cortisol were detected in both males and females (Figure 2). Cortisol was quantified in 81 % of the male samples and 75 % of the female samples. All but 7 samples measured for cortisol were below 2.0 ng/g (Figure 2) with a RL of 0.181 ng/g. The lowest comparable concentrations measured in the blubber from dolphins were 4 ng/g from bycatch dolphins that assumingly died quickly after an acute stress (Kellar et al., 2015) and 1.4 ng/g lipid from volunteered samples from managed dolphins at baseline stress (Champagne et al., 2017). Though this value is in ng/g lipid and not directly comparable, if we generously assume the lipid percentage to be even 75 % of the tissue by weight, this would yield an estimate of 1.1 ng/g, a value comparable to measurements from this study. Three additional corticosteroids were quantified. 11-Deoxycortisol was the next most frequently detected hormone at 57 % and 75 % for male and female samples respectively. Corticosterone and cortisone were quantified in ≤ 20 % of male and female sample sets. Therefore, it is reasonable to conclude that remote biopsy to LC-MS/MS techniques can be used for the assessment of baseline stress hormone concentrations.
Cortisone and cortisol concentrations were correlated (p = 0.001, tau = 0.264) suggesting that either blood cortisone, like cortisol, is transferred to the blubber or that cortisol/cortisone metabolism could occur in blubber from a living dolphin. In vitro enzymatic conversion of cortisol and cortisone occurs in blubber of marine mammals (Galligan et al., 2018). However, cortisone was only detectable in 15 % of the males sampled, and there was a minimum threshold concentration of blubber cortisol of 1.6 ng/g before cortisone was detectable. If the statistical analysis is limited to individuals where both cortisone and cortisol are quantifiable with this method (n = 12), the p-value remains the same and the tau increases to 0.697. Therefore, the relationship between cortisol and cortisone in the blubber potentially is stronger than the detection limits of this method allow us to investigate and a future analysis using immunoassay techniques or dolphins under higher stress conditions could elucidate this relationship.
Progesterone is a precursor to corticosteroid biosynthesis. Thus, as one would expect, analysis of male corticosteroid pathways showed a relationship between progesterone and cortisone, cortisol, and corticosterone (p < 0.05, tau = 0.119, 0.182, 0.114, respectively; Figure 3A). In stressed male cattle, circulating progesterone and corticosteroid concentrations are correlated (Welsh and Johnson, 1981). However the relationship in this study was weaker than the relationship between progesterone and the androgens, as would be expected in a dart biopsied individual that has presumably not experienced a major stressor before sampling.
When the full steroid pathway in males was analyzed using principal components analysis (PCA), reproductive steroids separated from corticosteroids (Figure 3B). As the correlational data and vertebrate steroid hormone pathways suggest, progesterone is a pivotal hormone relating to both the reproductive pathways and the stress pathways in males. Therefore, as previously stated, progesterone should be investigated as a stress hormone, but caution should be given during reproductive events that activate the androgen pathway and could confound results.
3.5. Seasonality
All hormones were assessed for differences among the dolphins sampled in CAWS and ACE. Hormones did not differ significantly in CAWS and ACE dolphins within any season. Therefore, the dolphins sampled in these sites were combined and assessed as one regional group. Insufficient sample sizes were collected for females to conduct seasonal analysis. Thus, all seasonal data discussed are from male dolphins.
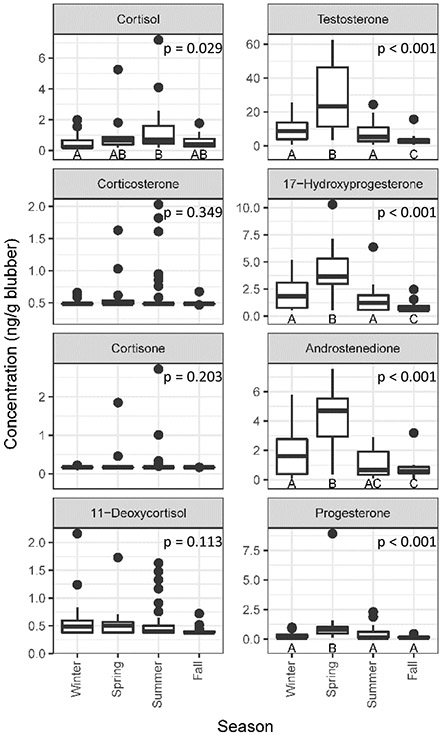
Seasonal differences were found in reproductive steroids among males (Figure 4). A peak in all measured progestogens and androgens (17-hydroxyprogesterone, progesterone, androstenedione, and testosterone; p-values < 0.001 for all hormones) occurred during spring. Spring peaks in reproductive hormones likely coincides with mating seasons in this region (McFee et al., 2014). There was also a nadir in testosterone and 17-hydroxyprogesterone during fall, potentially indicating a seasonal period of reproductive quiescence for this population.
Figure 4:
Comparison of seasonal blubber hormone concentrations in male common bottlenose dolphins. Sample sizes for each season were as follows: Winter n = 18, Spring n = 14, Summer n = 33, Fall n = 16. Significant differences assessed by post-hoc analysis are indicated by different letters.
Progesterone, while normally considered a female hormone for its role in pregnancy, also increased during the spring in males (p < 0.001). However, as discussed previously, progesterone, through its conversion to 17-hydroxyprogesterone, can lead to androgen synthesis. Additionally, progesterone and 17-hydroxyprogesterone were correlated among males in this study (p < 0.001, tau = 0.403; Figure 3A) as well as with the two androgens. This suggests that the significant increase in progesterone in males in spring could be a contributing pathway for the synthesis of androgens for reproductive activities.
We were unable to categorize the remotely sampled dolphins into age-classes, therefore the steroid measurements represent an unknown mix of sexually mature and immature males. This likely contributed to the variance of androgen measures in the spring when sexually mature males would be expected to have elevated androgen concentrations, while immature males would not. Regardless of this disadvantage to remote biopsy techniques, a peak in androgens and progestogens in the spring was detected and the relationship among the androgens and progestogens was defined both through correlations and the PCA. Therefore, this technique can be used to identify reproductive seasons in understudied populations. Additionally, three hormones in addition to testosterone have been identified as possible biomarkers to investigate male maturity through blubber dart biopsy. This study demonstrates the importance of selection of the appropriate hormone targets as well as ideal season for analysis to increase the likelihood of successful determination of male maturity of free-ranging dolphins.
Seasonal analysis of corticosteroids was conducted on the biopsies of males. Cortisol concentrations were significantly elevated in the summer compared to winter (p = 0.029, Figure 4). This raises the question whether increase in cortisol could be due to the increased temperatures during the summer compared to cold winter temperatures. Champagne et al. (2018) observed that blubber cortisol concentrations are increased in relation to increasing ambient air temperatures during out of water events, potentially due to increased perfusion of the blubber due to dilation of the blood vessels to offload heat. However, if perfusion were the only driving factor in this study, one would expect an increase in all of the hormones due to increased temperatures during summer versus winter seasons as indicated by the water temperature data from the ACE Basin National Estuarine Research Reserve System (the warmest water temperatures during this study occurred July through August with the coldest water temperatures through December and January; http://cdmo.baruch.sc.edu/aqs/). However, the relationship between water temperature, perfusion, and blubber hormone concentrations is more complex. A seasonal study on Indo pacific bottlenose dolphins (Tursiops aduncus) under human care, found that the highest serum cortisol concentrations coincided with highest rectal temperatures, but these occurred in the season with the coldest water temperatures, spring (Funasaka et al., 2011). In other studies on cetaceans under human care, serum cortisol were highest in winter (male orcas and common bottlenose dolphins) or spring (common bottlenose dolphins) (Orlov et al., 1988; Suzuki et al., 2003). However, all of these studies were conducted on animals under human care where water temperatures are often controlled independent of natural environmental factors such as ambient air temperatures and photoperiods. These differences emphasize the need to establish cortisol baselines using consistent collection methods on free-ranging populations rather than applying results from populations under human care to free-ranging populations.
Despite a statistically significant elevation of cortisol concentrations in the summer, these data should be considered in a biological context. The differences in mean seasonal cortisol concentrations are minor (less than 0.7 ng/g between winter and summer). The biological significance of such a small difference may not be relevant. However, the relationship between blood and blubber cortisol concentrations during chronic stress is unknown. Baseline measurements of cortisol from dolphins managed under human care showed a five-fold increase in blood cortisol (ng/mL) compared to blubber cortisol (ng/g) after oral administration of 60 mg of cortisol every 6 h (Champagne et al., 2017). This suggests that blood cortisol concentrations could be much greater than what is reflected in the blubber. However, the five-fold increase was measured from a simulated stress event in managed animals and long term offloading of the initial cortisol response was not studied. Therefore, the results should be applied with caution to this study where the slow partitioning of cortisol during acute or chronic stressors might affect the blubber concentrations of cortisol at the moment of sample collection. This study serves to emphasize the importance of conducting field experiments to understand the baseline seasonal physiology of free-ranging populations of marine mammals before disturbances can be detected.
4. Conclusions
This is the first study to analyze seasonal baseline concentrations of steroid hormones, including reproductive and stress steroids, in the blubber of free-ranging dolphins where momentary stress during sample collection would not have affected the measured hormone concentrations. This allows for the characterization of baseline hormone values, with potential differences likely reflecting seasonal environmental influences and/or exposure to other stressors without the confounding effect of sampling-induced stress.
By coupling remote biopsies with the LC-MS/MS method, suites of reproductive steroid hormones can be measured together from a single sample, providing steroid hormone profiles without the need for stressful and costly capture and release procedures. LC-MS/MS method precision was comparable to immunoassay methods (RSDs < 12 %) for the eight hormones reliably quantified in this study, making it a precise and efficient method for the investigation of hormone pathways compared to running eight separate immunoassays.
Using the approach in this study, demographic reproductive profiles, reproductive health, and stress could be defined using tracked populations and applied in a greater proportion of the free-ranging populations with less stress to this protected species. While more data are needed on female seasonality and reproductive outcomes, this information serves as a starting point to explore progesterone and androstenedione concentrations in blubber as a technique for the detection of pregnancy, thereby improving estimations of miscarriages and successful births. Additionally, this study emphasizes the importance of selecting the appropriate season for the desired investigation. The method clearly demonstrates a significant increase in blubber androgens and other reproductive hormones in male common bottlenose dolphins during seasons of increased reproductive activity, which could serve as useful targets for the investigation of male maturity. Also, understanding seasonal variation in stress hormones is critical to investigating potentially disturbed populations. With this information, scientists in the field can better examine populations of common bottlenose dolphins that may be under chronic stress, and, by doing so, can aid in the monitoring and conservation of this protected marine mammal and potentially other species.
Supplementary Material
Highlights.
Eight hormones were measured in 0.4 g remote blubber biopsies using LC-MS/MS
Reproduction in the spring elevated male androgens and two progestogens
Cortisol was elevated in summer meriting consideration for detecting disturbances
Baselines from captive dolphins cannot be fully applied to free-ranging populations
Selection of season is critical for studying free-ranging dolphin endocrinology
Acknowledgements
We thank the laboratory of Patricia Rosel for the sex determination data. We would also like to thank Leslie Hart, Suzanne Lane, Brian Quigley, Todd Speakman, and John Venturella for assistance with sample collection and data management and analysis, Al Segars and Daniel Barrineau for logistical support and assistance w/ sample collection, Amber Evans, Sarah Carson, Meredith Diskin, Lauren Ryan, Jamie Brusa, Rob Young for sample collection assistance and photo identification processing. Finally, we would like to thank the staff of the National Institute of Standards and Technology (NIST) Biorepository for maintenance and archiving of this sample set until the time of analysis.
Funding
This research was made possible through a grant from the Office of Naval Research Marine Mammals and Biology Program; the National Institute of Standards and Technology; the National Oceanic and Atmospheric Administration; and the National Academies National Research Council Associateship Program. This research was partially funded by the Office of Naval Research (ONR) under grant award numbers N0001412IP20053, N0001411IP20085, and N000141110542.
Abbreviations:
- ACE
Ashepoo, Combahee, Edisto River
- CAWS
Charleston Area Waterways
- ECDFs
Empirical Cumulative Distribution Functions
- DMSO
Dimethyl Sulfoxide
- LC-MS/MS
Liquid Chromatography Tandem Mass Spectrometry
- LOD
Limit of Detection
- PCA
Principal Components Analysis
- POPs
Persistent Organic Pollutants
- RL
Reporting Limit
- RSD
Relative Standard Deviation
- SRM
Standard Reference Material
Footnotes
Declarations of interest: none
Compliance with ethical standards
All research protocols used were approved by a NOAA Institutional Animal Care and Use Committee. Collections were conducted in concordance with ethical standard guidelines provided by the Office of Protected Resources, Marine Mammal Health and Stranding Response Program and Animal Welfare Act and under the NOAA authorization 109(h) of the Marine Mammal Protection Act. The authors declare that they have no competing interests in the publication of this manuscript. Commercial equipment, instruments, or materials are identified to specify adequately the experimental procedure. Such identification does not imply recommendation or endorsement by NIST nor NOAA, nor does it imply that the materials or equipment identified are necessarily the best available for the purpose. All samples were collected under Marine Mammal Protection Act Permit No. 779-1633.
Data accessibility
The datasets supporting this article are publicly available from NIST (MIDAS record ID 1961) at https://doi.org/10.18434/T4/1503309.
References
- Balmer BC, Ylitalo GM, McGeorge LE, Baugh KA, Boyd D, Mullin KD, Rosel PE, Sinclair C, Wells RS, Zolman ES, Schwacke LH, 2015a. Persistent organic pollutants (POPs) in blubber of common bottlenose dolphins (Tursiops truncatus) along the northern Gulf of Mexico coast, USA. Sci. Total Environ 527-528, 306–312. [DOI] [PubMed] [Google Scholar]
- Balmer BC, Ylitalo GM, McGeorge LE, Baugh KA, Boyd D, Mullin KD, Rosel PE, Sinclair C, Wells RS, Zolman ES, Schwacke LH, 2015b. Persistent organic pollutants (POPs) in blubber of common bottlenose dolphins (Tursiops truncatus) along the northern Gulf of Mexico coast, USA. Sci. Total Environ 527-528, 306–312. [DOI] [PubMed] [Google Scholar]
- Begumhasan J, Murphy BEP, 1992. Invitro stimulation of placental progesterone production by 19-nortestostereone and C-19 steroids in early human-pregnancy. J. Clin. Endocrinol. Metab 75, 838–845. [DOI] [PubMed] [Google Scholar]
- Boggs ASP, Schock TB, Schwacke LH, Galligan TM, Morey JS, McFee WE, Kucklick JR, 2017. Rapid and reliable steroid hormone profiling in Tursiops truncatus blubber using liquid chromatography tandem mass spectrometry (LC-MS/MS). Anal. Bioanal. Chem 409, 5019–5029. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Carrizo DG, Rastrilla AM, Tellería CM, Aguado LI, 1994. Androstenedione stimulates progesterone production in corpora lutea of pregnant rats: an effect not mediated by oestrogen. The Journal of Steroid Biochemistry and Molecular Biology 51, 191–197. [DOI] [PubMed] [Google Scholar]
- Castracane VD, Asch RH, 1995. Testosterone and androstenedione in premature ovarian failure pregnancies: Evidence for an ovarian source of androgens in early-pregnancy. Hum. Reprod 10, 677–680. [DOI] [PubMed] [Google Scholar]
- Champagne CD, Kellar NM, Crocker DE, Wasser SK, Booth RK, Trego ML, Houser DS, 2017. Blubber cortisol qualitatively reflects circulating cortisol concentrations in bottlenose dolphins. Mar. Mamm. Sci 33, 134–153. [Google Scholar]
- Champagne CD, Kellar NM, Trego ML, Delehanty B, Boonstra R, Wasser SK, Booth RK, Crocker DE, Houser DS, 2018. Comprehensive endocrine response to acute stress in the bottlenose dolphin from serum, blubber, and feces. Gen. Comp. Endocrinol 266, 178–193. [DOI] [PubMed] [Google Scholar]
- De Mello DMD, De Oliveira CA, 2016. Biological matrices for sampling free-ranging cetaceans and the implications of their use for reproductive endocrine monitoring. Mamm. Rev 46, 77–91. [Google Scholar]
- Funasaka N, Yoshioka M, Suzuki M, Ueda K, Miyahara H, Uchida S, 2011. Seasonal Difference of Diurnal Variations in Serum Melatonin, Cortisol, Testosterone, and Rectal Temperature in Indo-Pacific Bottlenose Dolphins (Tursiops aduncus). Aquat. Mamm 37, 433–442. [Google Scholar]
- Galligan TM, Balmer BC, Schwacke LH, Bolton JL, Quigley BM, Rosel PE, Ylitalo GM, Boggs ASP, 2019. Examining the relationships between blubber steroid hormones and persistent organic pollutants in common bottlenose dolphins. Environ. Pollut [DOI] [PubMed] [Google Scholar]
- Galligan TM, Schwacke LH, McFee WE, Boggs ASP, 2018. Evidence for cortisol–cortisone metabolism by marine mammal blubber. Mar. Biol 165, 114. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gorgone AM, Haase PA, Griffith ES, Hohn AA, 2008. Modeling Response of Target and Nontarget Dolphins to Biopsy Darting. J. Wildl. Manage 72, 926–932. [Google Scholar]
- Helsel D, 2012. Statistics for Censored Environmental Data Using Minitab and R. [Google Scholar]
- Kassambara A, Mundt F, 2017. factoextra: Extract and Visualize the Results of Multivariate Data Analyses. R package version 1.0.5 https://CRAN.R-project.org/package=factoextra
- Kellar NM, Catelani KN, Robbins MN, Trego ML, Allen CD, Danil K, Chivers SJ, 2015. Blubber Cortisol: A Potential Tool for Assessing Stress Response in Free-Ranging Dolphins without Effects due to Sampling. PLoS One 10, 16. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kellar NM, Trego ML, Chivers SJ, Archer FI, 2013. Pregnancy patterns of pantropical spotted dolphins (Stenella attenuata) in the eastern tropical Pacific determined from hormonal analysis of blubber biopsies and correlations with the purse-seine tuna fishery. Mar. Biol 160, 3113–3124. [Google Scholar]
- Kellar NM, Trego ML, Marks CI, Chivers SJ, Danil K, Archer FI, 2009. Blubber testosterone: A potential marker of male reproductive status in short-beaked common dolphins. Mar. Mamm. Sci 25, 507–522. [Google Scholar]
- Kellar NM, Trego ML, Marks CI, Dizon AE, 2006. Determining pregnancy from blubber in three species of delphinids. Mar. Mamm. Sci 22, 1–16. [Google Scholar]
- Kirby VL, 1984. Hormonal evidence of spontaneous ovulation in captive dolphins (Tursiops truncatus and Delphinus delphis). Rep int Whal Commn 6, 459–464. [Google Scholar]
- Kiszka JJ, Simon-Bouhet B, Charlier F, Pusineri C, Ridoux V, 2010. Individual and group behavioural reactions of small delphinids to remote biopsy sampling. Anim. Welfare 19, 411–417. [Google Scholar]
- Krutzen M, Barre LM, Moller LM, Heithaus MR, Simms C, Sherwin WB, 2002. A biopsy system for small cetaceans: Darting success and wound healing in Tursiops SPP. Mar. Mamm. Sci 18, 863–878. [Google Scholar]
- Kucklick J, Schwacke L, Wells R, Hohn A, Guichard A, Yordy J, Hansen L, Zolman E, Wilson R, Litz J, Nowacek D, Rowles T, Pugh R, Balmer B, Sinclair C, Rosel P, 2011. Bottlenose Dolphins as Indicators of Persistent Organic Pollutants in the Western North Atlantic Ocean and Northern Gulf of Mexico. Environ. Sci. Technol 45, 4270–4277. [DOI] [PubMed] [Google Scholar]
- Kuijper EAM, Ket JCF, Caanen MR, Lambalk CB, 2013. Reproductive hormone concentrations in pregnancy and neonates: a systematic review. Reprod. Biomed. Online 27, 33–63. [DOI] [PubMed] [Google Scholar]
- Lane SM, Smith CR, Mitchell J, Balmer BC, Barry KP, McDonald T, Mori CS, Rosel PE, Rowles TK, Speakman TR, Townsend FI, Tumlin MC, Wells RS, Zolman ES, Schwacke LH, 2015. Reproductive outcome and survival of common bottlenose dolphins sampled in Barataria Bay, Louisiana, USA, following the Deepwater Horizon oil spill. Proc. R. Soc. B-Biol. Sci 282, 9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lee L, Lee ML, 2017. NADA: Nondetects and Data Analysis for Environmental Data. R package version 1.6–1 . https://CRAN.R-project.org/package=NADA. [Google Scholar]
- Legacki EL, Scholtz EL, Ball BA, Stanley SD, Berger T, Conley AJ, 2016. The dynamic steroid landscape of equine pregnancy mapped by mass spectrometry. Reproduction 151, 421–430. [DOI] [PubMed] [Google Scholar]
- Mary H, Ruchika B, John E, Clinton H, O.H. TM, David K, Susan SF, Masoud ZM, G.C. CAJ, 2017. Nanospray liquid chromatography/tandem mass spectrometry analysis of steroids from gray whale blubber. Rapid Commun. Mass Spectrom 31, 1088–1094. [DOI] [PubMed] [Google Scholar]
- McFee WE, Speakman TR, Balthis L, Adams JD, Zolman ES, 2014. Reproductive seasonality of a recently designated bottlenose dolphin stock near Charleston, South Carolina, U.S.A. Mar. Mamm. Sci 30, 528–543. [Google Scholar]
- Neely MG, Morey JS, Anderson P, Balmer BC, Ylitalo GM, Zolman ES, Speakman TR, Sinclair C, Bachman MJ, Huncik K, Kucklick J, Rosel PE, Mullin KD, Rowles TK, Schwacke LH, Van Dolah FM, 2018. Skin Transcriptomes of common bottlenose dolphins (& IT;Tursiops truncatus & IT;) from the northern Gulf of Mexico and southeastern US Atlantic coasts. Mar. Genom 38, 45–58. [DOI] [PubMed] [Google Scholar]
- Orlov MM, Mukhlia AM, Kulikov NA, 1988. Hormonal indices of the normal dolphin Turpsiops truncatus and in the dynamics of experimental stress. Zh Evol Biokhim Fiziol 24, 557–563. [PubMed] [Google Scholar]
- Quérouil S, Freitas L, Dinis A, Alves F, Cascão I, Prieto R, Silva MA, Magalhães S, Matos JA, Santos RS, 2009. Sex bias in biopsy samples collected from free-ranging dolphins. European Journal of Wildlife Research 56, 151–158. [Google Scholar]
- Ragland JM, Liebert D, Wirth E, 2014. Using procedural blanks to generate analyte-specific limits of detection for persistent organic pollutants based on GC-MS analysis. Anal. Chem 86, 7696–7704. [DOI] [PubMed] [Google Scholar]
- Robeck TR, Steinman KJ, O'Brien JK, 2017. Characterization and longitudinal monitoring of serum androgens and glucocorticoids during normal pregnancy in the killer whale (Orcinus orca). Gen. Comp. Endocrinol 247, 116–129. [DOI] [PubMed] [Google Scholar]
- Rosel P, 2003. PCR-based determination in Odontocete cetaceans. [Google Scholar]
- Schroeder JP, Keller KV, 1989. Seasonality of serum testosterone levels and sperm density in Tursiops truncatus. The Journal of experimental zoology 249, 316–321. [DOI] [PubMed] [Google Scholar]
- Schwacke LH, Hall AJ, Townsend FI, Wells RS, Hansen LJ, Hohn AA, Bossart GD, Fair PA, Rowles TK, 2009. Hematologic and serum biochemical reference intervals for free-ranging common bottlenose dolphins (Tursiops truncatus) and variation in the distributions of clinicopathologic values related to geographic sampling site. Am. J. Vet. Res 70, 973–985. [DOI] [PubMed] [Google Scholar]
- Schwacke LH, Smith CR, Townsend FI, Wells RS, Hart LB, Balmer BC, Collier TK, De Guise S, Fry MM, Guillette LJ, Lamb SV, Lane SM, McFee WE, Place NJ, Tumlin MC, Ylitalo GM, Zolman ES, Rowles TK, 2014. Health of Common Bottlenose Dolphins (Tursiops truncatus) in Barataria Bay, Louisiana Following the Deepwater Horizon Oil Spill (vol 48, pg 93, 2014). Environ. Sci. Technol 48, 10528–10528. [DOI] [PubMed] [Google Scholar]
- Steinman KJ, Robeck TR, O’Brien JK, 2016. Characterization of estrogens, testosterone, and cortisol in normal bottlenose dolphin (Tursiops truncatus) pregnancy. Gen. Comp. Endocrinol 226, 102–112. [DOI] [PubMed] [Google Scholar]
- Suzuki M, Uchida S, Ueda K, Tobayama T, Katsumata E, Yoshioka M, Aida K, 2003. Diurnal and annual changes in serum cortisol concentrations in Indo-Pacific bottlenose dolphins Tursiops aduncus and killer whales Orcinus orca. Gen. Comp. Endocrinol 132, 427–433. [DOI] [PubMed] [Google Scholar]
- Team, R.C., 2013. R: A language and environment for statistical computing. R Foundation for Statistical Computing, Vienna, Austria. [Google Scholar]
- Trego ML, Kellar NM, Danil K, 2013. Validation of Blubber Progesterone Concentrations for Pregnancy Determination in Three Dolphin Species and a Porpoise. PLoS One 8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Weller DW, Cockcroft VG, Wursig B, Lynn SK, Fertl D, 1997. Behavioral responses of bottlenose dolphins to remote biopsy sampling and observations of surgical biopsy wound healing. Aquat. Mamm 231, 49–58. [Google Scholar]
- Wells RS, Rhinehart HL, Hansen LJ, Sweeney JC, Townsend FI, Stone R, Casper D, Scott MD, Hohn AA, Rowles TK, 2004. Bottlenose dolphins as marine ecosystem sentinels: Developing a health monitoring system. EcoHealth 1, 246–254. [Google Scholar]
- Welsh TH, Johnson BH, 1981. Stress-Induced Alterations in Secretion of Corticosteroids, Progesterone, Luteinizing Hormone, and Testosterone in Bulls. Endocrinology 109, 185–190. [DOI] [PubMed] [Google Scholar]
- Wickham H, 2017. tidyverse: Easily install and load “Tidyverse” packages (Version 1.1. 1). R Core Team: Vienna, Austria: https://CRAN.R-project.org/package=tidyverse. [Google Scholar]
- Yoshioka M, Mohri E, Tobayama T, Aida K, Hanyu I, 1986. Annual Changes in Serum Reproductive Hormone Levels in the Captive Female Bottle-nosed Dolphins. Nippon Suisan Gakkaishi 52, 1939–1946. [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.