Abstract
Background:
Data on the prevalence of brain metastases at presentation in patients with non-small cell lung cancer (NSCLC) are limited. We queried the National Cancer Data Base (NCDB) to determine prevalence, clinical risk factors and outcomes of patients with NSCLC presenting with brain metastases.
Methods:
Patients with NSCLC diagnosed between 2010 and 2012 were identified using the NCDB. The risk of brain metastases for individual variables was summarized by odds ratio (OR) and calculated using logistic regression analysis. Kaplan-Meier product limit method was used to calculate the median, 1 year, 2 year and 3 year overall survival (OS).
Results:
Brain metastases were observed in 47,546 (10.4%) of the 457,481 patients with NSCLC overall. The prevalence of brain metastases was much higher (26%) in patients with stage IV disease at presentation. On multivariate analysis younger age, adenocarcinoma or large cell histology, tumor size > 3 cm, tumor grade ≥ II and node positive disease were associated with brain metastases. The prevalence of brain metastases ranged from as low as 0.57% in patients with only 1 risk factor to as high as 22% in patients with all 5 risk factors. The median, 1, 2 and 3-year OS for patients with brain metastases were 6 months 29.9%, 14.3% and 8.4% respectively, with the 3-year OS increasing to 36.2% in those with T1/2 and N0/1 undergoing surgery for the primary site.
Conclusions:
In patients with NSCLC, the risk of brain metastases at presentation may be calculated based on five clinical variables. Selected patients with brain metastases at presentation may achieve prolonged benefit.
Keywords: non-small cell lung cancer, lung cancer, brain metastasis, metastases, NCDB
INTRODUCTION:
Lung cancer is the leading cause of cancer-related death in the United States.1 Non-small cell lung cancer (NSCLC) accounts for 87% of lung cancer cases and approximately 40% of patients are diagnosed with metastatic disease at presentation, with the most common sites of distant metastasis including the brain, liver, adrenal glands and bones.2–4 Brain metastases remain a significant problem in patients with lung cancer for several reasons. First, they are commonly observed, accounting for approximately half of all solid tumor metastases to the brain.5,6 Second, there has been an apparent stage migration resulting from availability of modern staging imaging modalities, such as PET and MRI, which can detect subclinical brain lesions. And finally, with the modest improvement in survival resulting from systemic therapies which lack intracranial penetration, patients tend to live longer, with more time to develop brain metastases.5,7
The frequency of brain metastases at presentation in patients with NSCLC remains unknown. Several estimates have been reported in the literature, which have been limited by small sample size and selection bias due to the setting from which these patient samples are drawn.8–10 Therefore, a large study examining the frequency of brain metastases and validation of previously described clinical risk factors such as age, histology and gender may provide a better understanding of both the prevalence and risk factors for the development of brain metastases.8,11 We sought to systematically address these questions using the National Cancer Data Base (NCDB), which captures data from approximately 70% of cancer cases in the United States and also includes data on brain metastases since 2010.
METHODS:
We searched the NCDB registry data for patients with NSCLC diagnosed between 2010 and 2012 with complete data on brain metastases. Demographic and clinical variables included age at diagnosis, gender, race, histology, tumor size, tumor grade, lymph node status (N) and American Joint Committee on Cancer (AJCC) stage. Age at diagnosis was analyzed as a continuous variable. Tumor size was subdivided into ≤ 3cm, 3.1 to 5cm, 5.1 to 7cm, and > 7cm to match the AJCC 7th edition staging for T1, T2a, T2b, and T3 respectively. The histology was coded according to the International Classification of Diseases for Oncology (ICD-O-3) into adenocarcinoma (8140–8147, 8255, 8260, 8310, 8323, 8480, 8481, 8490, 8550, 8572), squamous cell carcinoma (8050–8052, 8070–8078), large-cell carcinoma (8012–8014), and other histologies including undifferentiated tumors (8020–8022) and carcinomas not otherwise specified (NOS) (8010). We excluded bronchioloalveolar carcinoma (8250–8254) because its natural history and clinical course differs from other subtypes of NSCLC. Among patients with brain metastases Charlson Deyo comorbidity score, use of chemotherapy and surgery at distant site information was collected for additional analyses.
Statistical analysis:
Univariate and multivariate logistic regression analyses were used to explore the association of age at diagnosis, gender, race, tumor histology, tumor size, tumor grade and N stage with the presence of brain metastases. All patients have data on age at diagnosis, sex, tumor histology (as a function of sample selection) and year of diagnosis. Missing data occurred in tumor grade (42%), tumor size (16.1%) and N stage (10.5%). Imputation of such a large amount of missing data was considered unreliable, and the missing values were included in a separate “unknown” category. Adjusted odds ratios were calculated with 95% confidence intervals. The prevalence of brain metastases according to number of risk factors was also calculated, after dividing age into <70 and ≥70 years. This cutoff was chosen as the median age at diagnosis for lung cancer is 70 years.12 All statistical tests were 2-sided and p < 0.05 was considered significant. Data analyses were performed using SAS 9.4 (SAS Institutes, Cary, NC).
Overall survival (OS) was defined as time from diagnosis to death due to any cause, and patients alive were censored at the last follow up. Only those patients with vital status data available and with at least 1-month of follow-up were included for survival analysis. Kaplan-Meier product limit method was used to calculate the median, 1 year, 2 year and 3 year OS for patients with stage IV with or without brain metastases. Subsequent analyses for patients with brain metastases were performed to evaluate the outcomes according to the Charlson-Deyo Comorbidity score and use of chemotherapy. The differences in Kaplan-Meier curves between these subgroups were compared using log-rank test. Patients with brain metastases, T1/T2 and N0/N1 were further evaluated according to the surgery of the primary site, with or without surgery of a distant site.
RESULTS:
Demographics
A total of 511,050 patients were identified, who met the inclusion criteria, of whom we excluded 53,569 patients due to missing data regarding brain metastases, leaving 457,481 patients in the final cohort (Figure 1). Most patients were men (52%) and of white race (85.2%). The median age was 69 years (range 18–90). The histologic distribution included 235,341 patients (51.4%) with adenocarcinoma, 134,046 (29.3%) with squamous cell carcinoma, 5455 (1.2%) with large-cell carcinoma, and 82,639 (18.1%) with other histologies (Table 1). There were 177,268 tumors ≤ 3cm (38.7%), 104,742 between 3.1 and 5 cm (22.9%) and 101,799 larger than 5 cm (22.2%). Most patients (128,543; 28.1%) had a grade III primary tumor and no node involvement (204,203; 44.6%). The majority of patients had stage IV disease at presentation 178,668 (39%), while stage I, II and III disease was observed in 123,226 (26.9%), 37,190 (8.1%) and 87,739 (19.3%) patients respectively. The stage at presentation was unknown in 6.7% of patients.
Figure 1.

Flow diagram showing patients included in the analysis
Table 1.
Baseline characteristics of patients, and univariate logistic regression analysis of clinical risk factors brain for metastasis
| Variable | N | Brain Mets (%) | Odds Ratio | 95% CL | 95% CU | P |
|---|---|---|---|---|---|---|
| Age (decade) | 457481 | NA | 0.672 | 0.667 | 0.678 | <.001 |
| Sex | ||||||
| M | 237831 | 24861 (10.45) | 1 | |||
| F | 219650 | 22685 (10.33) | 0.987 | 0.968 | 1.006 | 0.165 |
| Race | ||||||
| White | 389631 | 39164 (10.05) | 1 | |||
| Black | 50745 | 6068 (11.96) | 1.215 | 1.181 | 1.251 | <.001 |
| Other/Unknown | 17105 | 2314 (13.53) | 1.401 | 1.339 | 1.465 | <.001 |
| Histology | ||||||
| Squamous | 134046 | 6552 (4.89) | 1 | |||
| Adenocarcinoma | 235341 | 30560 (12.99) | 2.903 | 2.824 | 2.985 | <.001 |
| Large cell | 5455 | 782 (14.34) | 3.256 | 3.006 | 3.526 | <.001 |
| Others | 82639 | 9652 (11.68) | 2.573 | 2.490 | 2.658 | <.001 |
| Tumor size | ||||||
| 0–3cm | 177268 | 11682 (6.59) | 1 | |||
| 3.1–5cm | 104742 | 12013 (11.47) | 1.836 | 1.788 | 1.886 | <.001 |
| >5cm | 101799 | 13961 (13.71) | 2.253 | 2.195 | 2.312 | <.001 |
| Unknown | 73672 | 9890 (13.42) | 2.198 | 2.137 | 2.261 | <.001 |
| Tumor grade | ||||||
| I | 33830 | 762 (2.25) | 1 | |||
| II | 98242 | 4475 (4.56) | 2.071 | 1.916 | 2.238 | <.001 |
| III | 128543 | 13908 (10.82) | 5.264 | 4.889 | 5.668 | <.001 |
| IV | 4737 | 572 (12.08) | 5.959 | 5.322 | 6.672 | <.001 |
| Unknown | 192129 | 27829 (14.48) | 7.349 | 6.832 | 7.905 | <.001 |
| N stage | ||||||
| N0 | 204203 | 10441 (5.11) | 1 | |||
| N1 | 35147 | 4323 (12.3) | 2.602 | 2.507 | 2.702 | <.001 |
| N2 | 124777 | 19210 (15.4) | 3.377 | 3.293 | 3.462 | <.001 |
| N3 | 45366 | 7332 (16.16) | 3.577 | 3.465 | 3.693 | <.001 |
| Unknown | 47988 | 6240 (13) | 2.773 | 2.683 | 2.867 | <.001 |
Risk factors for brain metastases
Brain metastases were detected in 47,546 (10.4%) of the 457,481 patients in the total cohort and the frequency of brain metastases was similar in men (10.4%) and women (10.3%). The prevalence of brain metastases was higher (26%) in patients with stage IV disease at presentation. Based on univariate analysis, the risk factors for brain metastases included younger age, black race, tumor size of over 3 cm, higher grade tumors (grade II or higher) and N1, N2 or N3 lymph node involvement. These variables were included in the multivariate analysis.
On multivariate analysis the following variables were significantly associated with presence of brain metastases (Table 2): age (OR, 0.7 per 10 year increase in age; 95% confidence interval [CI]: 0.7, 0.71); adenocarcinoma histology (OR 2.93; 95% CI:2.84, 3.01), large cell histology (OR 2.46, 95% CI:2.26, 2.68); tumor size > 3 cm (3.1 to 5 cm OR 1.60; 95% CI: 1.56, 1.65 and > 5 cm OR, 1.83; 95% CI: 1.78, 1.88); tumor grade ≥ II (grade II OR 2.16; 95% CI:2.0, 2.34, grade III OR 4.13; 95% CI: 3.83, 4.45 and grade IV OR 3.97; 95% CI: 3.52, 4.47) and node positive disease N1 OR 2.0; 95% CI: 1.93, 2.08, N2 OR 2.24; 95% CI: 2.18, 2.30 and N3 OR 2.1; 95% CI: 2.03, 2.18). The area under the receiver operating curve (ROC) analysis was 0.746.
Table 2.
Multivariate logistic regression analysis exploring the association of age at diagnosis, gender, race, tumor histology, tumor size, tumor grade and N stage with the presence of brain metastasis
| Variable | Odds Ratio | 95%CI Low | 95%CI Up | P value |
|---|---|---|---|---|
| Age (decade) | 0.70 | 0.70 | 0.71 | <.001 |
| Histology | ||||
| Squamous | 1 | |||
| Adenocarcinoma | 2.93 | 2.84 | 3.01 | <.001 |
| Large | 2.46 | 2.26 | 2.68 | <.001 |
| Others | 2.42 | 2.34 | 2.50 | <.001 |
| Tumor size | ||||
| 0–3cm | 1 | |||
| 3.1–5cm | 1.60 | 1.56 | 1.65 | <.001 |
| >5cm | 1.83 | 1.78 | 1.88 | <.001 |
| Unknown | 1.37 | 1.33 | 1.41 | <.001 |
| Tumor grade | ||||
| I | 1 | |||
| II | 2.16 | 2.00 | 2.34 | <.001 |
| III | 4.13 | 3.83 | 4.45 | <.001 |
| IV | 3.97 | 3.52 | 4.47 | <.001 |
| Unknown | 5.42 | 5.04 | 5.84 | <.001 |
| N stage | ||||
| N0 | 1 | |||
| N1 | 2.00 | 1.93 | 2.08 | <.001 |
| N2 | 2.24 | 2.18 | 2.30 | <.001 |
| N3 | 2.10 | 2.03 | 2.18 | <.001 |
| Unknown | 2.13 | 2.06 | 2.21 | <.001 |
Age was re-categorized into < 70 or ≥ 70 years and the prevalence of brain metastases was calculated according to number of risk factors, including age<70, tumor size>3 cm, adenocarcinoma or large cell histology, tumor grade ≥ 2, node positive disease (N1–N3). The prevalence of brain metastases ranged from as low as 0.57% in patients with only 1 risk factor to as high as 22% in patients with all 5 risk factors (Table 3).
Table 3.
Prevalence of brain metastasis according to number of risk factors (age<70, tumor size>3 cm, adenocarcinoma or large cell histology, tumor grade ≥ 2, node positive disease (N1–N3).
| Number of risk factors | Prevalence of brain metastases |
|---|---|
| 1 | 0.57% |
| 2 | 1.56% |
| 3 | 4.49% |
| 4 | 11.54% |
| 5 | 21.99% |
Survival analysis for patients with stage IV disease
The median follow up time for survivors was 27.5 months (range 1.0–61.3 months). The median OS for all patients with stage IV disease during this time period was 7.1 months (95% CI 7.0 – 7.1), with 1 year, 2 year and 3 year survivals of 33.7%, 16.6% and 9.7% respectively. The median OS for patients with brain metastases was significantly worse than in patients without brain metastases (6 months versus 7.5 months; log rank chi square 324, p<0.001). The 1 year, 2 year and 3 year survival rates were 35.1%, 17.4% and 10.2% respectively for patients without brain metastases, and 29.9%, 14.3% and 8.4% respectively for patients with brain metastases (Figure 2).
Figure 2.
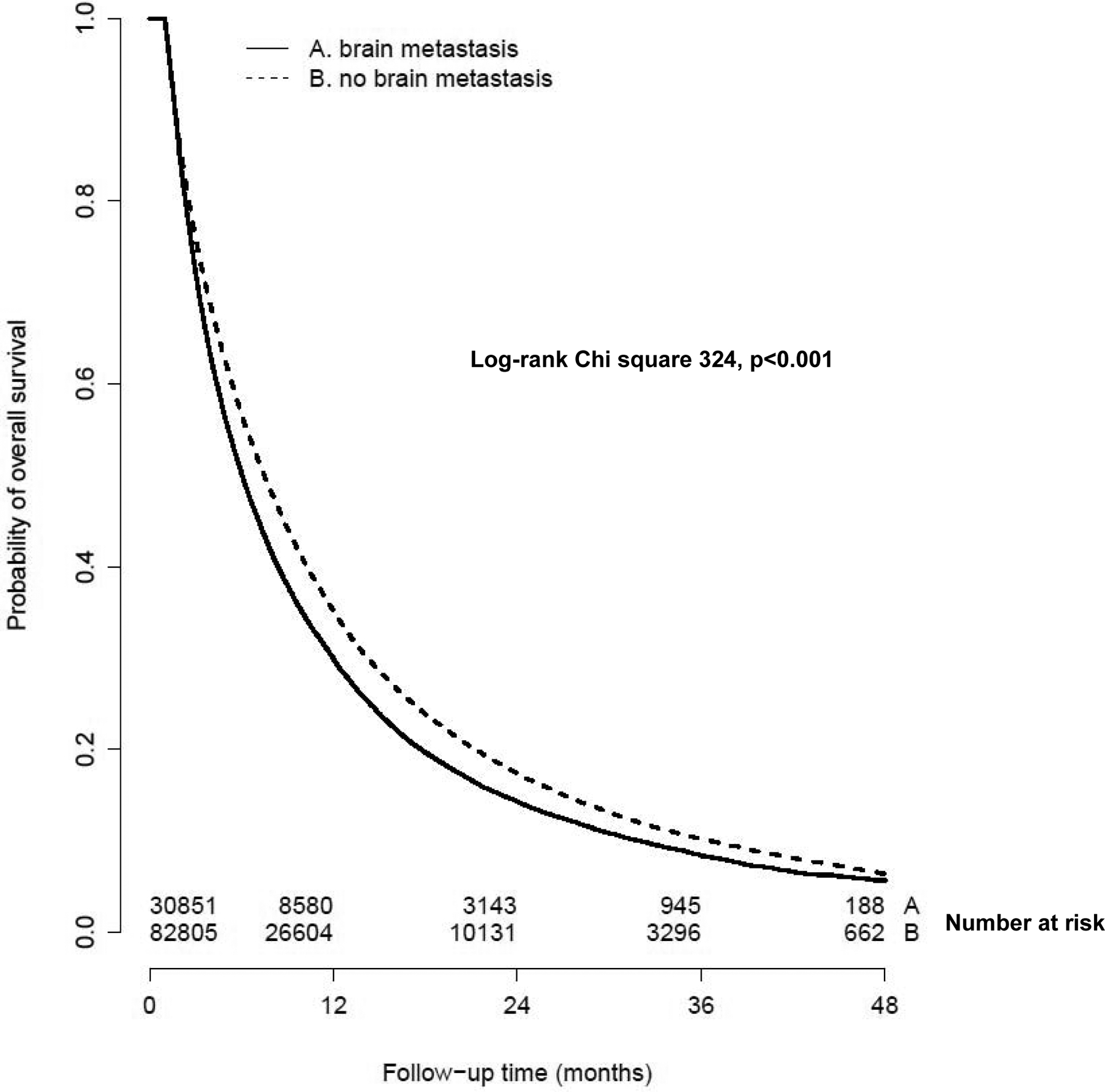
Kaplan Meier curves showing overall survival for patients with stage IV disease according to the presence of brain metastases
The median OS for patients with brain metastases and Charlson-Deyo Comorbidity score of 0, 1 and 2 being 7.1 months, 5.4 months and 4.4 months respectively (Log-rank chisquare 321.6, 2 df, p<0.001). The median OS for patients with brain metastases was significantly higher in those who received chemotherapy, compared to those who did not receive chemotherapy (9.4 months versus 3.3 months, log-rank chi square 2279.1, 1 df, p<0.0001).
A subset of 561 patients with brain metastases who had T1/T2 and N0/N1 stage and underwent surgery of the primary site had a median OS of 21.4 months, with OS rates at 1, 2 and 3 years being 69.2%, 45.5% and 36.2% respectively (Figure 3). Within this patient subset, 237 patients (42%) also had surgical resection of a distant site. In this patient population, the median, 1, 2 and 3-year OS were 26.5 months, 79.1%, 53.2% and 48.6% respectively.
Figure 3.
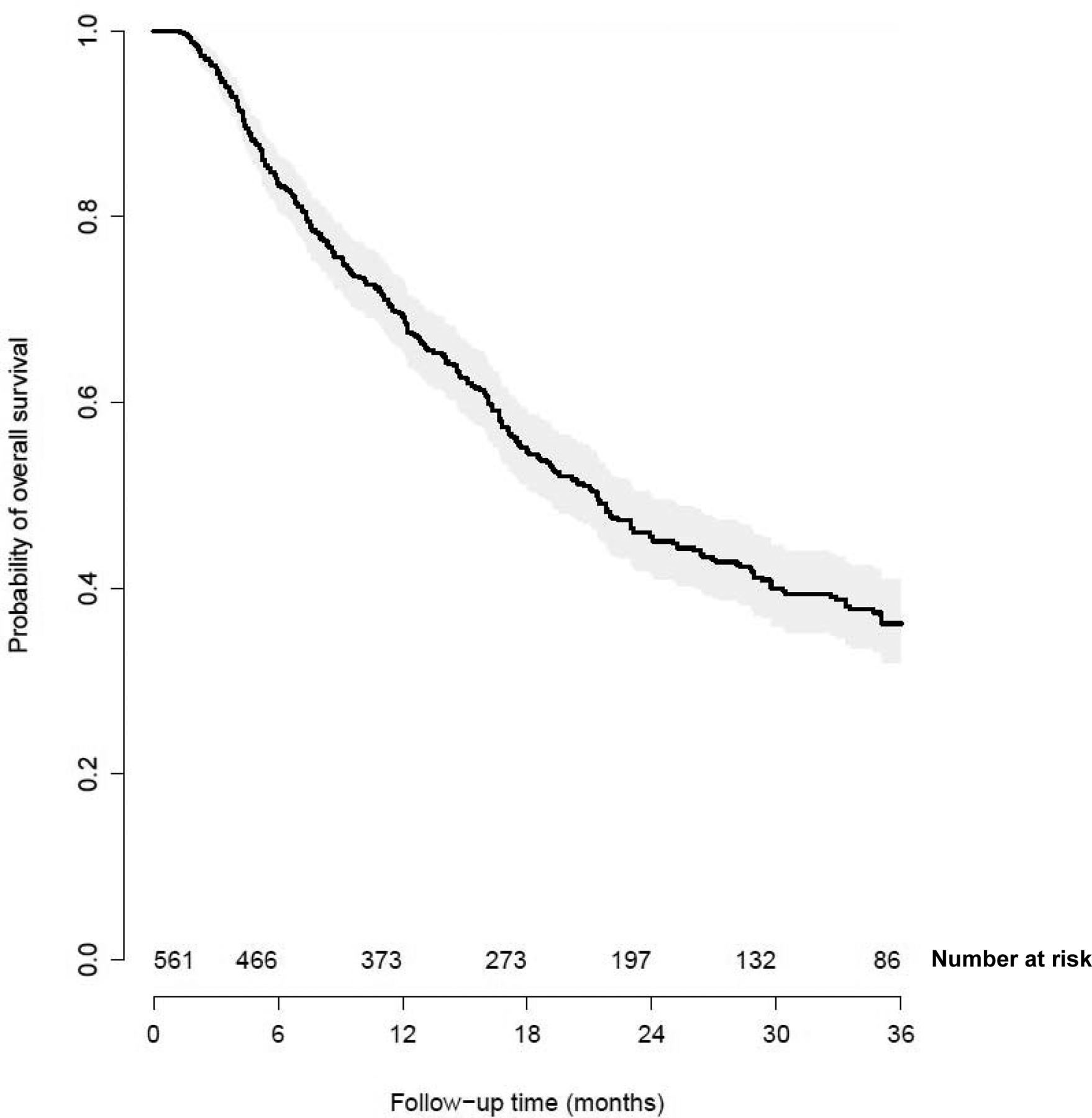
Kaplan Meier curve for patients with brain metastases, T1–T2, N0–N1 disease and undergoing surgery to the primary site
DISCUSSION:
In our study, brain metastases were found in 10.4% of patients with newly diagnosed NSCLC and 26% of patients with stage IV disease. This underscores the importance of routine brain MRI for staging in patients with newly diagnosed NSCLC. This rate of brain metastases is numerically higher than the 8.3% that we had previously described in patients diagnosed between 1988–1997, likely due to the widespread availability of brain MRI during the time period of the present study.13 Our study substantiates findings from previous studies that link non-squamous histology, younger age, tumor size and nodal station involvement to the development of brain metastases.13–16
Patients with brain metastases and T1/T2 and N0/N1 disease who underwent surgery are a unique patient subset with better prognosis. The increased percentage of long-term survivors in this patient population may reflect the current practice of aggressive surgical and modern stereotactic radiotherapy interventions for the treatment of brain metastases in this selected subgroup.17–21
Our study has several limitations including its retrospective nature, lack of information regarding how patients were staged, knowledge of molecular subtype, and specific chemotherapy drugs or targeted therapies given. Nevertheless, this is the largest study of patients with brain metastases at presentation to date, allowing for a more detailed evaluation of its risk factors.
NSCLC is a disease characterized by both intertumor and intratumor molecular heterogeneity. The molecular analysis of patient-matched paired primary lung and brain metastases has implicated several including PI3K/AKT/mTOR, HER2/EGFR and MAPK CDK pathways in driving brain metastases, which may be therapeutically targeted.22,23 Several agents including small molecule EGFR- tyrosine kinase inhibitors and next generation ALK inhibitors are already approved by the FDA for patients with EGFR-mutant and ALK rearrangement positive NSCLC, with promising intracranial activity. With the advent of immunotherapy and approval of pembrolizumab and nivolumab for the treatment of metastatic NSCLC, the intracranial activity of these agents is also being investigated.24–26 Our study provides the most comprehensive clinical predictor data for the brain metastases at presentation and may serve as a baseline for the contribution of individual clinical variables as predictors for brain metastases to be used in combination with the new molecular predictors.
CONCLUSIONS:
Brain metastases are seen in 10.4% of patients with NSCLC at presentation. The incidence of brain metastases at presentation may be estimated based on age, histology, tumor size, tumor grade and lymph node involvement. Selected patients with brain metastases at presentation may achieve prolonged benefit.
ACKNOWLEDGEMENTS:
This publication was supported by the National Cancer Institute of the National Institutes of Health (NIH), Grant Number 1K12CA167540 and the Clinical Translational Science Award (CTSA) program of the National Center for Advancing Translational Sciences at the National Institutes of Health, Grant Number UL1RR024992.
Footnotes
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
REFERENCES:
- 1.Siegel RL, Miller KD, Jemal A: Cancer statistics, 2016. CA Cancer J Clin 66:7–30, 2016 [DOI] [PubMed] [Google Scholar]
- 2.Govindan R, Page N, Morgensztern D, et al. : Changing epidemiology of small-cell lung cancer in the United States over the last 30 years: analysis of the surveillance, epidemiologic, and end results database. J Clin Oncol 24:4539–44, 2006 [DOI] [PubMed] [Google Scholar]
- 3.Morgensztern D, Waqar S, Subramanian J, et al. : Improving survival for stage IV non-small cell lung cancer: a surveillance, epidemiology, and end results survey from 1990 to 2005. J Thorac Oncol 4:1524–9, 2009 [DOI] [PubMed] [Google Scholar]
- 4.Lee DS, Kim YS, Kay CS, et al. : Distinctive Patterns of Initially Presenting Metastases and Clinical Outcomes According to the Histological Subtypes in Stage IV Non-Small Cell Lung Cancer. Medicine (Baltimore) 95:e2795, 2016 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Patel RR, Mehta MP: Targeted therapy for brain metastases: improving the therapeutic ratio. Clinical cancer research : an official journal of the American Association for Cancer Research 13:1675–83, 2007 [DOI] [PubMed] [Google Scholar]
- 6.Delattre JY, Krol G, Thaler HT, et al. : Distribution of brain metastases. Archives of neurology 45:741–4, 1988 [DOI] [PubMed] [Google Scholar]
- 7.Smedby KE, Brandt L, Backlund ML, et al. : Brain metastases admissions in Sweden between 1987 and 2006. Br J Cancer 101:1919–24, 2009 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Ryan GF, Ball DL, Smith JG: Treatment of brain metastases from primary lung cancer. International journal of radiation oncology, biology, physics 31:273–8, 1995 [DOI] [PubMed] [Google Scholar]
- 9.Davis FG, Dolecek TA, McCarthy BJ, et al. : Toward determining the lifetime occurrence of metastatic brain tumors estimated from 2007 United States cancer incidence data. Neuro Oncol 14:1171–7, 2012 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Schouten LJ, Rutten J, Huveneers HA, et al. : Incidence of brain metastases in a cohort of patients with carcinoma of the breast, colon, kidney, and lung and melanoma. Cancer 94:2698–705, 2002 [DOI] [PubMed] [Google Scholar]
- 11.Alsan Cetin I, Akgun Z, Atasoy BM, et al. : Who may benefit from prophylactic cranial irradiation amongst stage III non-small cell lung cancer patients? Journal of B.U.ON. : official journal of the Balkan Union of Oncology 18:453–8, 2013 [PubMed] [Google Scholar]
- 12.Miller KD, Siegel RL, Lin CC, et al. : Cancer treatment and survivorship statistics, 2016. CA Cancer J Clin, 2016 [DOI] [PubMed] [Google Scholar]
- 13.Waqar SN, Waqar SH, Trinkaus K, et al. : Brain Metastases at Presentation in Patients With Non-Small Cell Lung Cancer. Am J Clin Oncol, 2015 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Yawn BP, Wollan PC, Schroeder C, et al. : Temporal and gender-related trends in brain metastases from lung and breast cancer. Minnesota medicine 86:32–7, 2003 [PubMed] [Google Scholar]
- 15.Mujoomdar A, Austin JH, Malhotra R, et al. : Clinical predictors of metastatic disease to the brain from non-small cell lung carcinoma: primary tumor size, cell type, and lymph node metastases. Radiology 242:882–8, 2007 [DOI] [PubMed] [Google Scholar]
- 16.Gaspar LE, Chansky K, Albain KS, et al. : Time from treatment to subsequent diagnosis of brain metastases in stage III non-small-cell lung cancer: a retrospective review by the Southwest Oncology Group. J Clin Oncol 23:2955–61, 2005 [DOI] [PubMed] [Google Scholar]
- 17.Qin H, Wang C, Jiang Y, et al. : Patients with single brain metastasis from non-small cell lung cancer equally benefit from stereotactic radiosurgery and surgery: a systematic review. Med Sci Monit 21:144–52, 2015 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Scorsetti M, Alongi F, Navarria P, et al. : Overall and disease-free survival greater than 12 years in metastatic non-small cell lung cancer after linear accelerator-based stereotactic radiosurgery for solitary brain metastasis. Tumori 98:31e–34e, 2012 [DOI] [PubMed] [Google Scholar]
- 19.Mariya Y, Sekizawa G, Matsuoka Y, et al. : Outcome of stereotactic radiosurgery for patients with non-small cell lung cancer metastatic to the brain. J Radiat Res 51:333–42, 2010 [DOI] [PubMed] [Google Scholar]
- 20.Flannery TW, Suntharalingam M, Kwok Y, et al. : Gamma knife stereotactic radiosurgery for synchronous versus metachronous solitary brain metastases from non-small cell lung cancer. Lung Cancer 42:327–33, 2003 [DOI] [PubMed] [Google Scholar]
- 21.Bougie E, Masson-Cote L, Mathieu D: Comparison Between Surgical Resection and Stereotactic Radiosurgery in Patients with a Single Brain Metastasis from Non-Small Cell Lung Cancer. World Neurosurg 83:900–6, 2015 [DOI] [PubMed] [Google Scholar]
- 22.Brastianos PK, Carter SL, Santagata S, et al. : Genomic Characterization of Brain Metastases Reveals Branched Evolution and Potential Therapeutic Targets. Cancer Discov 5:1164–77, 2015 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Paik PK, Shen R, Won H, et al. : Next-Generation Sequencing of Stage IV Squamous Cell Lung Cancers Reveals an Association of PI3K Aberrations and Evidence of Clonal Heterogeneity in Patients with Brain Metastases. Cancer Discov 5:610–21, 2015 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Kazandjian D, Suzman DL, Blumenthal G, et al. : FDA Approval Summary: Nivolumab for the Treatment of Metastatic Non-Small Cell Lung Cancer With Progression On or After Platinum-Based Chemotherapy. Oncologist 21:634–42, 2016 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Goldberg SBGS, Mahajan A, Chiang AC, Herbst RS, Sznol M, Tsiouris AJ, Cohen J, Vortmeyer A, Jilaveanu L, Yu J, Hegde U, Speaker S, Madura M, Ralabate A, Rivera A, Rowen E, Gerrish H, Yao X, Chiang V, Kluger HM: Pembrolizumab for patients with melanoma or non-small-cell lung cancer and untreated brain metastases: early analysis of a non-randomised, open-label, phase 2 trial. Lancet Oncology Published Online June 3, 2016 10.1016/S1470-2045(16)30053-5, 2016 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Sul J, Blumenthal GM, Jiang X, et al. : FDA Approval Summary: Pembrolizumab for the Treatment of Patients With Metastatic Non-Small Cell Lung Cancer Whose Tumors Express Programmed Death-Ligand 1. Oncologist 21:643–50, 2016 [DOI] [PMC free article] [PubMed] [Google Scholar]


