Abstract
Supramolecular gels have been an area of interest in many research fields. They provide a means to understand assembly of nanostructures, and through the use of amino acid- and peptide-based gelators they can give insights into the similar assembly pathways of their more complex structural counterparts. Bio-inspired metal coordination, such as histidine–copper coordination, in the supramolecular assembly of these gelators is one method for furthering our understanding and development of these materials. Metal–gelator coordination mimics biologically relevant metal–peptide coordination, thus influencing hydrogel self-assembly and mechanical properties, including biodegradability, biocompatibility, tunability, and recyclablity, while the metal coordination can functionalize the gels to allow for widespread applications in biomedical industries (e.g., drug delivery), waste management, and catalysis. This review aims to discuss recent insights into the supramolecular assembly of gels involving metal ions, as well as a few key areas of application using metal interactions and incorporation.
1. Introduction
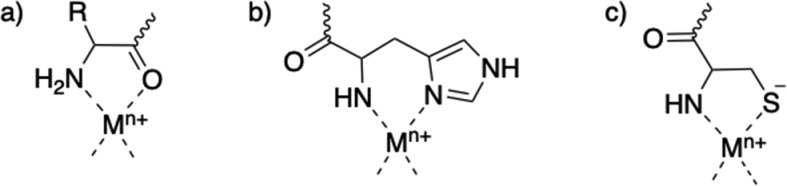
Amino acids and peptides are among the most basic building blocks in the assembly of complex structures in life. They make up proteins that have enzymatic or transport functions, form membrane channels, and have many other capabilities. This is often, in part, a result of their ability to chelate metal ions. This can occur for peptides of amino acids without coordinating side chains, such as alanine or phenylalanine, or with coordinating side chains, such as histidine or cysteine (Scheme 1).1 Those with non-coordinating side chains can chelate metal ions through amino or carboxylate termini (Scheme 1a) or through terminal amino and neighboring carbonyl groups. Peptides containing histidine have site-specific metal ion coordination of the imidazole ring. For example, when histidine is at the N-terminal, its imidazole ring and the terminal amino group can form 6-membered chelates (Scheme 1b); histidine separated from the amino terminal by one amino acid will enhance formation of amide-bonded species and prevent deprotonation of the following amide residues. These will have a different metal–ligand (peptide) stoichiometry and different coordination modes.1,2 Cysteine residues are capable of thiolate formation to bind metals; however, this coordination is more selective. Terminal cysteine residues can form 5-membered chelates with the amino and thiolate group (Scheme 1c) and can also form stable bis(ligand) complexes like C-terminal cysteinyl residues.1 In-depth exploration of the various coordination modes of amino acids can be found in other reviews.1,2
Scheme 1. Examples of Metal Coordination with Peptides through (a) Terminal Amino Group and Carbonyl (R = Amino Acid Side Chain), (b) Terminal Amino Group and Terminal Histidine Imidazole, and (c) Terminal Amino Group and Terminal Cysteine Thiolate.
Metal ion coordination is one aspect of these complex peptides, in terms of functionality; however, they can also be involved in self-assembly and, in turn, the formation of supramolecular gels. These are often composed of solvents encapsulated by nanostructures varying among nanofibers, nanobundles, nanotubes, and others, which can be investigated for further understanding of their more complex, higher order structural counterparts.3,4 Self-assembly of amino acid- and peptide-based gelators is commonly induced in response to external stimuli and triggers such as pH and temperature change.5 Self-assembly occurs via non-covalent interactions such as hydrophobic interactions, hydrogen bonding, ionic bonding, and π–π stacking. These amino acid- and peptide-based supramolecular gels are key areas of development for their biocompatibility, biodegradability, and tunability.3−6 More in-depth exploration of peptide self-assembly and gelation can be found in other reviews.3,4,6
By considering both the metal–ligand coordination and supramolecular assembly potential of amino acids and peptides during gelator design, a different controlling factor in gel formation becomes integral to these biomaterials.7,8 There are times when the addition of metals can cause supramolecular gelation from originally non-gelating compounds. This can occur due to the metal–gelator interactions causing more ordered aggregation and orientation, thus giving rise to enhanced gel stability.7,8 Gelation temperatures can be dependent on metal ion concentrations, while gel strength and robustness can change upon addition of metal ions.7 Metal ions play important roles in structural integrity and biological functionality, and so incorporation of metals into gel design could impart similar properties.9 Metal complexation itself can lead to different functional properties, and in supramolecular gels this can result in catalytic, spectroscopic, as well as redox-active biomaterials, particularly with transition metal complexes.7 This review aims to present the importance of peptide–metal coordination in supramolecular chemistry, with a focus on gelation and some applications of these biomaterials.
2. Metal Ions in the Supramolecular Chemistry of Gelation
The use of metals to induce gelation, as in triggering sol–gel transition, has been demonstrated in the literature.8,9 Beyond sol–gel transitions, metal incorporation into supramolecular gels has also resulted in stimuli-responsiveness, luminescence, and other properties.10,11 The merging of metals and gels has been explored with metal–organic frameworks and polymer gels,12 as well as with metallopolymers.13 However, this review aims to spotlight the influence of metal ion coordination on supramolecular peptide gels. The metal–peptide coordination forms specific orientations, which can allow for non-covalent interactions between the peptide motifs of the gelators. Thus, this section focuses on the effects of metal ions on self-assembled structures, which in turn can affect gelation as well as the mechanical properties of these biomaterials.
2.1. Metal Ions in Self-Assembly
Given the various potential triggers for self-assembly, metal ions have been introduced as a controlling factor in self-assembly and formation of supramolecular gels. They can be used to tune the properties of these biomaterials rather than just acting as fortuitous gel triggers. The functional groups present in peptide-based gelators allow metal coordination to occur. As such, this coordination can influence the supramolecular structural network (Figure 1). With systematic exploration of metal–peptide gelator coordination, these biomaterials can be tuned to obtain desired properties from the resulting library of supramolecular states and assemblies. A brief exploration of the short peptide hydrogelator fluoroenylmethyloxycarbonyl (Fmoc) and carbazole-protected Phe-Phe-Asp has been reported by Martin and co-workers.9 For these gelators, Asp was incorporated to aid metal binding through chelating effects along with the terminal carboxylate. Sodium, calcium, and copper chloride salts induced gelation and could be used to fine-tune fiber morphology. The monovalent cation formed sparse, heterogeneous networks with both gelators (fibers and precipitates), whereas the divalent cations formed denser, more cross-linked fibrous networks with the gelators. In this case, the choice of metal ions was able to affect the density and widths of the self-assembled nanostructures. This demonstrates the fine-tuning effects of metal ions on the self-assembly of peptide hydrogelators, which allows for targeted optimization of the resulting materials.
Figure 1.
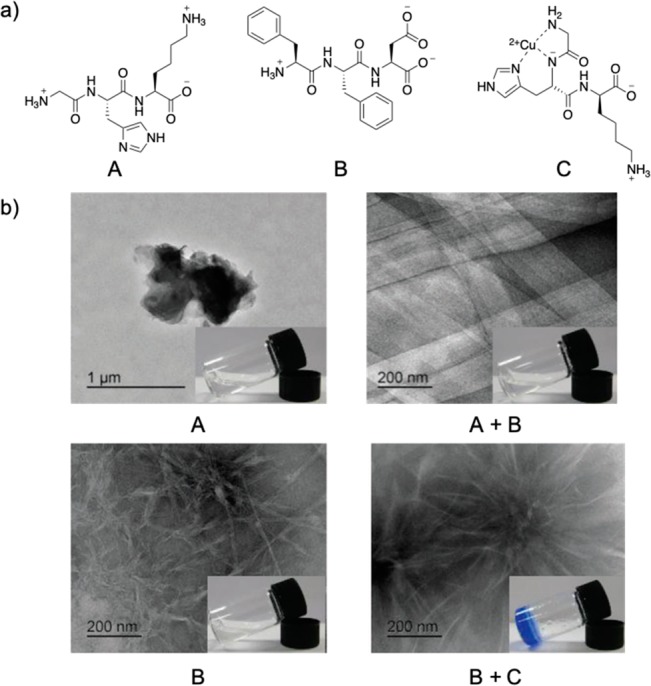
(a) Structures of the tripeptides Phe-Phe-Asp (A), Gly-His-Lys (B), and Gly-His-Lys coordinated with copper ion (C). (b) Changes in the morphology of the tripeptides’ self-assembly (A, B), their co-assembly (A+B), and their co-assembly with copper coordination (A+C), which also resulted in hydrogel formation. This figure was adapted and reproduced from ref (14). Published 2017 by the Royal Society of Chemistry.
In addition to these minute effects, metal ions have also been shown to completely change the morphologies of the self-assembled peptides. Abul-Haija et al. used copper ions to complex two tripeptides (Phe-Phe-Asp and Gly-His-Lys) into cooperative co-assembly of a hydrogel (Figure 1a).14 The tripeptides, both on their own and when combined, were unable to form supramolecular gels, although self-assembly was observed through transmission electron microscopy (TEM) imaging of their nanostructures (Figure 1b). With the addition of copper ions, a change in the co-assembled nanotapes to nanofibers was found, along with hydrogel formation (Figure 1b). This was described to occur due to the peptide complexation with the copper ions, such as in the tripeptide Gly-His-Lys through histidine’s imidazole, the terminal amine, and the amide group (Figure 1a). This demonstrates the possible structural reconfiguration of self- and co-assemblies of peptides due to ion sensitivities and metal coordination. The Gazit group explored the effects of metal ions on the thoroughly studied Fmoc-Phe-Phe motif of Alzheimer’s β-amyloid polypeptide.15 Specifically, they examined the transformation of the amyloid-like β-sheet self-assembly to superhelix or random coil structures (Figure 2) by adding metal ions with different valences, as well as altering the ratios of gelator to ion, through circular dichroism. Through TEM it was observed that trivalent metallogels formed spherical structures, which was attributed to strong peptide–metal ion binding enhancing hydrophobic interactions, thus preventing the transition of the spheres into nanofibrils. Monovalent and divalent metallogels formed entangled nanofibrous networks characteristic of the dipeptide motif, with varying sizes at different peptide-to-metal ion ratios. The differences found in the morphologies suggest the susceptibility of the self-assembly of this motif to peptide–metal coordination and interactions.
Figure 2.
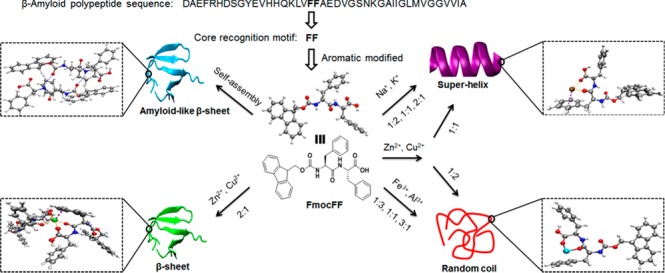
Effect of mono-, di-, and trivalent metal ions on the supramolecular self-assembly of the Fmoc-FF motif. Reproduced with permission from ref (15). Copyright 2019 American Chemical Society.
2.2. Metal Ions Affecting Mechanical Properties
The influence of metal ions on self-assembly can also have resulting effects on the mechanical properties of the formed hydrogels. Chen et al. reported an amphiphilic histidine derivative (LHC18) capable of forming a hydro-metallogel with ferric ions that exhibited shear-triggered self-healing and shrinkage (see Figure 3).16 Resting hydro-metallogels underwent shrinkage, whereas collapsed gels that self-healed and recovered into the gel phase did not. Flexibility in the metal–ligand interactions is a plausible factor that allowed for the shrinkage and thixotropic properties. The morphologies of these supramolecular gels were examined under scanning electron microscopy (SEM), showing that the flexible membrane nanostructure of the ferric gels changed into nanobelt structures after shrinking, whereas shear force destruction and recovery of the ferric gels did not change their original morphology. This was the first example of a metallogel exhibiting both shrinking and self-healing properties. Many self-healing supramolecular gels have compromised toughness and healing rates.17 In an effort to design a supramolecular material that both is tough and has great self-healing properties, Zeng et al. developed a highly stretchable, tough and fast self-healing hydrogel based on cross-linked peptide–-metal ion coordination sites.17 Using a tri- (Gly-Gly-His) and pentapeptide (Gly-His-His-Pro-His) with histidines acting as metal ion binding sites, they demonstrated that the pentapeptide, which has a higher coordination number than the tripeptide, as a hydrogel showed a higher Young’s modulus and was stronger under strain than the tripeptide hydrogel cross-linked by single ligand–metal ion bonds (Figure 4a,b). The dynamic and tensile mechanical properties were investigated, and the pentapeptide displayed greater stiffness and toughness without deducting stretchability and self-healing (Figure 4c). From this, the improvement in mechanical strength of supramolecular hydrogels through higher coordination number was demonstrated.
Figure 3.
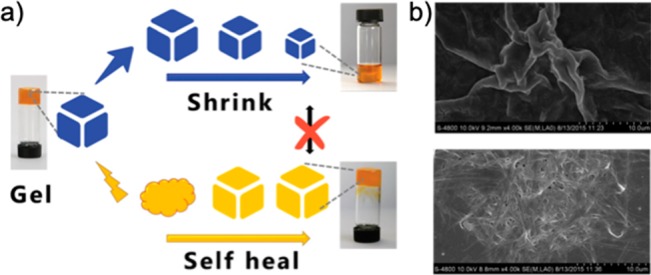
Histidine-based amphiphile hydro-metallogel with ferric ions capable of shear-triggered self-healing and shrinking. Reproduced with permission from ref (16). Copyright 2016 The Royal Society of Chemistry.
Figure 4.
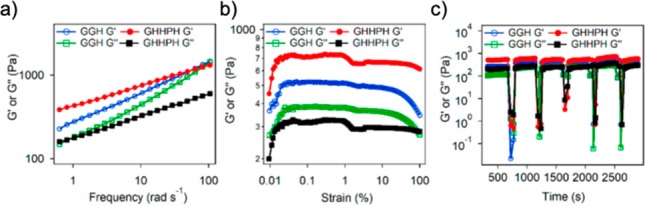
Rheology frequency sweeps of tri- and pentapeptide hydrogels showing (a) gel formation indicated by G′ > G′′ (blue above green and red above black respectively), (b) gel strength seen with maintained gel state despite increasing strain, and (c) self-healing properties of the gels through multiple step–strain cycles. Reproduced from ref (17) (Open Access).
3. Peptide Supramolecular Metallogel Applications
The interactions of metals with these supramolecular gels have allowed observation of assembly pathways for these unique structures and biomaterials. In addition, the ability to incorporate metal ions with these peptide-based materials provides a wide range of applications (Figure 5). This section highlights a small variety of ways supramolecular gels and metals have potential benefits in these fields.
Figure 5.
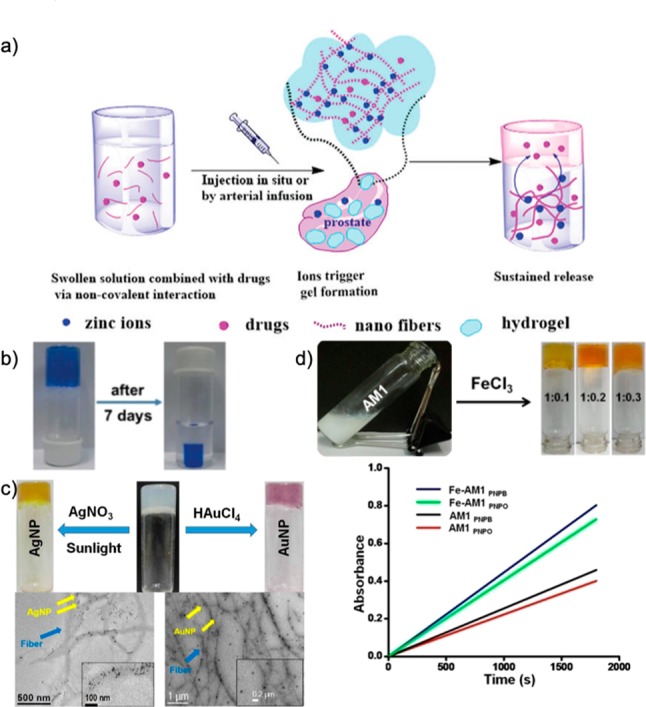
Applications of metallohydrogels. (a) Scheme of injectable drug–gelator solution targeted to zinc-rich prostate, resulting in hydrogel formation in the presence of zinc and sustained drug release. Reproduced with permission from ref (18). Copyright 2018 The Royal Society of Chemistry. (b) Uptake of Pb2+ ions and dyes in a tripeptide hydrogel, with shrinking after 7 days from water expulsion. Reproduced with permission from ref (19). Copyright 2017 The Royal Society of Chemistry. (c) Gold and silver nanoparticle formation in a citric acid-containing dipeptide hydrogel, visualized with TEM imaging. Reproduced with permission from ref (20). Copyright 2018 Wiley-VCH Verlag GmbH & Co. KGaA. (d) Histidine-based amphiphile hydrogel forming with Fe3+, found to have catalytic activity measured with UV–vis. Reproduced with permission from ref (23). Copyright 2018 American Chemistry Society.
3.1. Peptide Supramolecular Gels in Drug Applications
Peptide supramolecular hydrogels present a vehicle of increasing interest as drug delivery systems due to their biocompatibility and likeness to biological structures and properties, such as tissue elasticity.18 Although hydrogel formation can be triggered in vitro, in vivo formation can also be stimulated by using physiological conditions such as metal ion localization. This aims to increase target specificity and efficacy of biomedical applications such as drug delivery. For example, to deliver anti-cancer drugs to the prostate, which has a high concentration of zinc ions, Tao et al. developed a novel class of oligo-peptides.18 These short peptides have a unique forky structure due to the presence of three adjacent glutamic residues, along with varying numbers phenylalanine residues to aid gelation. Among these, the hexapeptide (three glutamic residues and three phenylalanine residues) was found to form hydrogels in the presence of zinc ions, while not forming in other bivalent cations found in the blood or tissue fluids. This allows for injectable prostate-targeted drug delivery, in which there is a high concentration of zinc ions (Figure 5a). The capabilities of this peptide were demonstrated with the anti-cancer drug docetaxel and showed excellent anti-cancer efficacy with prostate cancer cells along with no cytotoxicity from the peptide material to normal liver cells. The development of this highly specific, in vivo-triggered peptide hydrogel formation is based on targeted metal ion interactions, presenting a new route to developing targeted drug delivery systems based on localized metal ions.
3.2. Peptide Supramolecular Gels in Waste Management
Water pollution is an increasing area of concern due to the negative impacts and consequences for human and ecosystem health due to toxic organic dyes and heavy metals from industrial waste. In response to this issue, supramolecular gels present an intriguing countermeasure due to their waste sensitivity, reusability, and proper biodegradability. Their porous fibrous structures can trap pollutants, and the peptide basis can aid metal absorption. The Banerjee group has reported a tripeptide-based self-shrinking hydrogel capable of removing toxic dyes and heavy metal ions from wastewater.19 The tripeptide-based gelator demonstrated self-shrinking properties, allowing for easy removal of water pollutants through instant syneresis. It was observed that, after hydrogel formation of the triphenylalanine amphiphile with Pb2+ ions, the supramolecular gel immediately began shrinking through expulsion of water while retaining the majority of heavy metal ions (leaving negligible amounts in the water) (Figure 5b).19 The coordination of metal ions in these cases entrapped them in the hydrogels, allowing for waste management, recycling, and reuse of these simple, efficient biomaterials.
3.3. Peptide Supramolecular Gels as a Scaffold for Nanoparticles Formation
Supramolecular gels form a three-dimensional matrix that can serve as a scaffold for nanoparticle formation. They can be functionalized to fabricate metal nanoparticles, resulting in gel–nanoparticle hybrid materials. Paul et al. investigated a diphenylalanine peptide amphiphile that formed a hydrogel in acidic and basic pH, but not neutral pH.20 By including citric acid, they induced hydrogel formation while also providing gel functionality to fabricate gold and silver nanoparticles (Figure 5c). Gold nanoparticle formation was successful through the addition of an HAuCl4 solution on top of a preformed hydrogel, with nanoparticle formation within the gel matrix visible by a violet color change. Silver nanoparticle formation was successful through the addition of a AgNO3 solution to the peptide–citric acid solution pre-hydrogel formation. Upon cooling and sunlight irradiation, a yellow color change indicated the presence of nanoparticle formation within the gel matrix. Through this, a simple system for nanoparticle formation is demonstrated that does not require the presence of external reducing or stabilizing agents. The resulting gel–nanoparticle hybrid material could then be used as a recyclable catalyst.
3.4. Peptide Supramolecular Gels in Catalysis Applications
The incorporation of inorganic components into hydrogels constitutes a way to introduce unique properties such as redox, catalysis, and photochemical properties to the resulting materials. It is of great interest to construct metallogel-based soft, functional materials with appropriate functionality and a suitable metal ion that can show catalytic activity. Embedding catalytic sites in terms of a metal center in gelator molecules has been reported to be the most logical way of designing supramolecular gel-based catalytic systems.21 Xing et al. was the first to report a catalytic metallogel containing Pd(II) which catalyzed the oxidative reaction of benzyl alcohol to benzaldehyde.22 The catalytic turnovers of the polymer cross-linked metallogels were twice as as those of free Pd(OAc)2, and it was reported that the higher catalytic turnovers of the metallogels were related to the superior stability of the catalysts under the gel reaction conditions. Not only do cross-linked metallo-polymeric gels have the ability to act as catalytic materials, but recent literature shows that supramolecular gels composed of peptides and amino acids also have this capability. Gayen et al. constructed an amino acid-based amphiphilic gel composed of N-histidyl-N′-myristyl ethyl amine and synthesized it in such a way that it contains the histidine imadalzole ring to bind transition metal ions, an amide group for intermolecular hydrogel bonding interactions, and a fatty acyl chain to promote hydrophobic interactions.23 The amino acid-based amphiphile binds with Fe3+ and Hg2+ ions separately and forms a hydrogel at pH 6.6. This hydrogel shows the ability to act as a catalyst for ester hydrolysis and shows an esterase-like activity toward a series of p-nitrophenyl esters (Figure 5d).23 Other examples of amino acid and peptide metallogels as catalytic materials include the works by Ulijn and Marchesan.24,25
4. Conclusion and Future Outlook
There are many different ways to trigger supramolecular gel formation.3,5,6,9 The use of coordinating metal ions is one of the lesser explored methods yet is no less important in understanding the supramolecular chemistry and development of these biomaterials. Peptide-based gelators can be triggered into forming supramolecular gels through complexation of the functional groups with the addition of metal ions.3,7−10 The peptide–metal ion coordination gives rise to tunable self-assembly, from controlled gelation to changing nanostructure morphology.9,14,15 Supramolecular metallogels have also been found to have interesting mechanical properties, which could be attributed to the role of metal ions in their structural integrity.16,17 However, there is still a gap in understanding the peptide–metal ion coordination modes when in their self-assembled supramolecular state, and so further research efforts are required in the fields of crystallography and other spectroscopic techniques. The interaction between metal ions and these peptide supramolecular gels has allowed for applications in targeted drug delivery,18 waste management,19 nanoparticle scaffold design,20 and catalysis.23 Metal ions and interactions represent an avenue for these biomaterials that, with more in-depth exploration, holds great potential for understanding more complex, biological structure assembly and for developing efficient, tunable, functionalized materials.
Acknowledgments
We thank the Natural Science and Engineering Research Council (NSERC), and the University of Toronto (U of T) for financial support. N.F. thanks NSERC for a postgraduate scholarship (CGS-D).
Glossary
Abbreviations
- Gly
glycine
- His
histidine
- Pro
proline
- Lys
lysine
- Asp
aspartic acid
Biographies

Tsuimy Shao received her H. BSc. in Biochemistry and Molecular Biology at the University of Toronto, Canada (2019). She recently joined the Kraatz research group as an M.Sc. student in Chemistry. Her research interests focus on developing peptide amphiphiles to investigate hierarchical self-assembly of nanostructures.

Natashya Falcone received her H. BSc. in Biological Chemistry and Molecular Biology at the University of Toronto, Canada (2016). Since 2016, she has been a Ph.D. student in Applied Chemistry in the Kraatz research group. Her research interests center around synthesizing novel biomaterials including peptide hydrogels for various biomedical engineering applications.

Heinz-Bernhard Kraatz received his Ph.D. in 1993 from the University of Calgary and is a Professor of Chemistry at the University of Toronto Scarborough. His research interests are in the area of peptide bioconjugates with a focus on materials and bioelectroanalytical applications.
The authors declare no competing financial interest.
References
- Sóvágó I.; Kállay C.; Várnagy K. Peptides as complexing agents: Factors influencing the structure and thermodynamic stability of peptide complexes. Coord. Chem. Rev. 2012, 256, 2225–2233. 10.1016/j.ccr.2012.02.026. [DOI] [Google Scholar]
- Sóvágó I.; Várnagy K.; Lihi N.; Grenács Á. Coordinating properties of peptides containing histidyl residues. Coord. Chem. Rev. 2016, 327–328, 43–54. 10.1016/j.ccr.2016.04.015. [DOI] [Google Scholar]
- Dasgupta A.; Das D. Designer Peptide Amphiphiles: Self-Assembly to Applications. Langmuir 2019, 35, 10704–10724. 10.1021/acs.langmuir.9b01837. [DOI] [PubMed] [Google Scholar]
- De Santis E.; Ryadnov M. G. Peptide self-assembly for nanomaterials: the old new kid on the block. Chem. Soc. Rev. 2015, 44, 8288–8300. 10.1039/C5CS00470E. [DOI] [PubMed] [Google Scholar]
- Falcone N.; Shao T.; Sun X.; Kraatz H.-B. Systematic exploration of the pH dependence of a peptide hydrogel. Can. J. Chem. 2019, 97, 430–434. 10.1139/cjc-2018-0419. [DOI] [Google Scholar]
- Du X.; Zhou J.; Shi J.; Xu B. Supramolecular Hydrogelators and Hydrogels: From Soft Matter to Molecular Biomaterials. Chem. Rev. 2015, 115, 13165–13307. 10.1021/acs.chemrev.5b00299. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Li Y.; Qin M.; Cao Y.; Wang W. Designing the mechanical properties of peptide-based supramolecular hydrogels for biomedical applications. Sci. China: Phys., Mech. Astron. 2014, 57, 849–858. 10.1007/s11433-014-5427-z. [DOI] [Google Scholar]
- Tam A. Y. Y.; Yam V. W. W. Recent advances in metallogels. Chem. Soc. Rev. 2013, 42, 1540–1567. 10.1039/c2cs35354g. [DOI] [PubMed] [Google Scholar]
- McEwen H.; Du E. Y.; Mata J. P.; Thordarson P.; Martin A. D. Tuning hydrogels through metal-based gelation triggers. J. Mater. Chem. B 2017, 5, 9412–9417. 10.1039/C7TB02140B. [DOI] [PubMed] [Google Scholar]
- Ma X.; Yu D.; Tang N.; Wu J. Tb3+-containing supramolecular hydrogels: luminescence properties and reversible sol-gel transitions induced by external stimuli. Dalton Trans. 2014, 43, 9856–9859. 10.1039/c4dt00110a. [DOI] [PubMed] [Google Scholar]
- Falcone N.; Kraatz H.-B. Supramolecular Assembly of Peptide and Metallopeptide Gelators and Their Stimuli-Responsive Properties in Biomedical Applications. Chem. - Eur. J. 2018, 24, 14316–14328. 10.1002/chem.201801247. [DOI] [PubMed] [Google Scholar]
- Ishiwata T.; Furukawa Y.; Sugikawa K.; Kokado K.; Sada K. Transformation of Metal-Organic Framework to Polymer Gel by Cross-Linking the Organic Ligands Preorganized in Metal-Organic Framework. J. Am. Chem. Soc. 2013, 135, 5427–5432. 10.1021/ja3125614. [DOI] [PubMed] [Google Scholar]
- Yan X.; Cook T. R.; Pollock J. B.; Wei P.; Zhang Y.; Yu Y.; Huang F.; Stang P. J. Responsive Supramolecular Polymer Metallogel Constructed by Orthogonal Coordination-Driven Self-Assembly and Host/Guest Interactions. J. Am. Chem. Soc. 2014, 136, 4460–4463. 10.1021/ja412249k. [DOI] [PubMed] [Google Scholar]
- Abul-Haija Y. M.; Scott G. G.; Kishore Sahoo J.; Tuttle T.; Ulijn R. V. Cooperative, ion-sensitive co-assembly of tripeptide hydrogels. Chem. Commun. 2017, 53, 9562–9565. 10.1039/C7CC04796G. [DOI] [PubMed] [Google Scholar]
- Ji W.; Yuan C.; Zilberzwige-Tal S.; Xing R.; Chakraborty P.; Tao K.; Gilead S.; Yan X.; Gazit E. Metal-Ion Modulated Structural Transformation of Amyloid-Like Dipeptide Supramolecular Self-Assembly. ACS Nano 2019, 13, 7300–7309. 10.1021/acsnano.9b03444. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chen J.; Wang T.; Liu M. A hydro-metallogel of an amphiphilic L-histidine with ferric ions: shear-triggered self-healing and shrinkage. Inorg. Chem. Front. 2016, 3, 1559–1565. 10.1039/C6QI00238B. [DOI] [Google Scholar]
- Zeng L.; Song M.; Gu J.; Xu Z.; Xue B.; Li Y.; Cao Y. A Highly Stretchable, Tough, Fast Self-Healing Hydrogel Based on Peptide-Metal Ion Coordination. Biomimetics 2019, 4, 36. 10.3390/biomimetics4020036. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tao M.; Xu K.; He S.; Li H.; Zhang L.; Luo X.; Zhong W. Zinc-ion-mediated self-assembly of forky peptides for prostate cancer-specific drug delivery. Chem. Commun. 2018, 54, 4673–4676. 10.1039/C8CC00604K. [DOI] [PubMed] [Google Scholar]
- Basak S.; Nandi N.; Paul S.; Hamley I. W.; Banerjee A. A tripeptide-based self-shrinking hydrogel for waste-water treatment: removal of toxic organic dyes and lead (Pb2+) ions. Chem. Commun. 2017, 53, 5910–5913. 10.1039/C7CC01774J. [DOI] [PubMed] [Google Scholar]
- Paul S.; Basu K.; Sundar Das K.; Banerjee A. Peptide-Based Hydrogels as a Scaffold for In Situ Synthesis of Metal Nanoparticles: Catalytic Activity of the Nanohybrid System. ChemNanoMat 2018, 4, 882–887. 10.1002/cnma.201800227. [DOI] [Google Scholar]
- Dawn A. Supramolecular Gel as the Template for Catalysis, Inorganic Superstructure, and Pharmaceutical Crystallization. Int. J. Mol. Sci. 2019, 20, 781–803. 10.3390/ijms20030781. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Xing B.; Choi M.-F.; Xu B. Design of Coordination Polymer Gels as Stable Catalytic Systems. Chem. - Eur. J. 2002, 8, 5028–5032. . [DOI] [PubMed] [Google Scholar]
- Gayen K.; Basu K.; Bairagi D.; Castelletto V.; Hamley I. W.; Banerjee A. Amino-Acid-Based Metallo-Hydrogel That Acts Like an Esterase. ACS Appl. Bio. Mater. 2018, 1, 1717–1724. 10.1021/acsabm.8b00513. [DOI] [PubMed] [Google Scholar]
- Zhang C.; Shafi R.; Lampel A.; MacPherson D.; Pappas C. G.; Narang V.; Wang T.; Maldarelli C.; Ulijn R. V. Switchable Hydrolase Based on Reversible Formation of Supramolecular Catalytic Site Using a Self-Assembling Peptide. Angew. Chem., Int. Ed. 2017, 56, 14511–14515. 10.1002/anie.201708036. [DOI] [PubMed] [Google Scholar]
- Garcia A. M.; Kurbasic M.; Kralj S.; Melchionna M.; Marchesan S. A biocatalytic and thermoreversible hydrogel from a histidine-containing tripeptide. Chem. Commun. 2017, 53, 8110–8113. 10.1039/C7CC03371K. [DOI] [PubMed] [Google Scholar]