Abstract
Mounting evidence has linked gut microbiome to health benefits of various functional foods. We previously reported that administration of a diet rich in black raspberry (BRB) changed the composition and diverse functional pathways in the mouse gut microbiome. To further characterize the functional profile in the gut microbiome of mice on BRB diet, in this follow-up study, we examined the metabolome differences in the gut microbiome driven by BRB consumption via targeted and untargeted metabolomic approaches. A distinct metabolite profile was observed in the gut microbiome of the mice on BRB diet, likely resulting from a combination of microbiome functional changes and unique precursors in BRBs. A number of functional metabolites, such as tetrahydrobiopterin and butyrate that were significantly increased in the gut microbiome may be linked to the beneficial health effects of BRB consumption. These findings suggest the important role of the gut microbiome in the health effects of BRBs and provide a connection among the health benefits of functional foods and the gut microbiome.
Introduction
The significance of the gut microbiota has been tremendously recognized over the past decade. Accumulating evidence suggested that the metabolic activities in the gut microbiome are profoundly intertwined with human health and disease.1 Maintaining a well-balanced gut microbiome is essential to host fitness; and perturbation in the gut microbial communities can lead to a series of adverse health outcomes.2,3 The metabolic process of dietary precursors by the gut bacteria produces a spectrum of functional metabolites, through which the gut microbiota communicate with the host.1,4 A variety of bacterial metabolites are beneficial. For example, the gut microbiome is an important source of vitamins such as vitamin B family and vitamin K.5 Meanwhile, the fermentation of dietary fiber produces short-chain fatty acids (SCFAs) that can act as signaling molecules in diverse pathways and physiological functions.2,6 Butyrate regulates energy homeostasis and adiposity of the host through stimulating the secretion of glucagon-like peptide-1 in intestinal enteroendocrine L cells and inducing leptin production in adipocytes.7 However, undesirable metabolites can also be produced by the gut microbiota. A typical example is trimethylamine (TMA). The gut bacteria can convert dietary choline and carnitine into TMA,8,9 which is further metabolized to trimethylamine N-oxide in the liver, which is associated with cardiovascular disease and chronic kidney disease.9,10 Thus, the functionality of the gut microbiome and its role in human health and disease is largely established by the metabolite profile.
Consumption of berries including raspberries, cranberries, and currants has diverse health benefits, such as antioxidant, anti-inflammatory, and anticarcinogenic effects.11,12 A standardized black raspberry (BRB)-rich diet has been used to explore the functional role of BRBs in a number of previous studies.13 For example, recent studies clearly demonstrated the anticarcinogenic effects of the BRB diet on esophageal and oral cancer.14,15 The treatment of the BRB diet also inhibited colonic inflammation in an experimental model of colitis.16 It is well accepted that the beneficial effects of berries are attributed to their abundant phytochemicals such as anthocyanins, flavonols, ellagitannins, and phenolic acids.17,18 On the other hand, there is growing evidence that berries can modify the composition of the gut microbiota.19−21 Given the pivotal role of the gut microbiota in human health coupled with the health benefits of berries, investigation of the microbiome changes associated with berry consumption may provide additional evidence regarding the underlying mechanisms of the beneficial health effects from berry consumption. In particular, several seminal studies have reported connections between the gut microbiota and the health benefits of foodstuff. For instance, the health effects of the consumption of walnuts and almonds have been linked to the changes in the gut microbiota.22−24
We previously reported that dietary administration of BRBs changed the composition and diverse functional pathways in the mouse gut microbiome,20 which suggested the involvement of the gut microbial activities in the health effects of BRBs. To further characterize the metabolic profile in the gut microbiome associated with BRB consumption, in the present study we combined untargeted and targeted metabolomics approaches to extensively profile the metabolome signatures of the gut microbiome. The results revealed a significantly distinct metabolite profile in the gut microbiome of mice fed BRB diet compared to mice fed control diet, with increased levels of a number of important metabolites such as tetrahydrobiopterin and butyrate that confer health benefits. We also conducted a comprehensive analysis to integrate functional pathways and metabolic products. Specifically, increased levels of various vitamins and cofactors in concert with enrichment in their biosynthetic pathways were discovered in mice fed BRB diet, indicating that the differences in metabolite profiles could result from the BRB-driven changes in bacterial metabolic pathways. This study further confirmed the role that the gut microbiome played in the beneficial health effects from BRB consumption and provided a functional link among the functional foods, their health benefits, and the gut microbiome.
Results
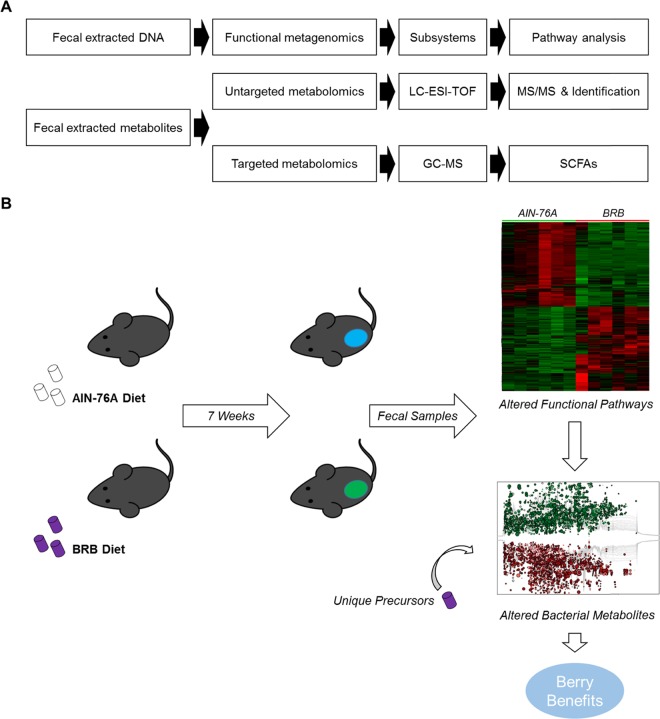
Experimental Workflow
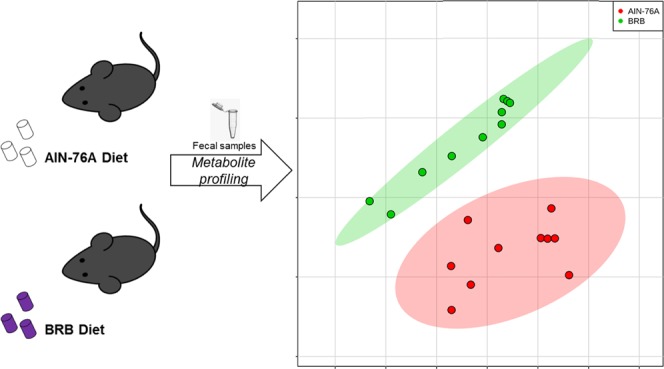
We used a mouse model and a standardized BRB-rich diet to study the metabolic profile in mouse gut microbiome with the consumption of BRBs. The formula of the BRB diet used in the present study has been used in a series of previous studies.13 Fecal samples were collected after 7 weeks of diet treatment to represent the mouse gut microbial contents. Figure 1A summarizes the experimental workflow. We combined untargeted and targeted metabolomics to exhaustively probe gut microbiota-derived metabolites. LC-ESI-TOF was used to conduct untargeted metabolomics, and MS/MS spectra were generated to confirm the metabolite identities. GC/MS was used for the targeted analysis of SCFAs. We also integrated metagenomics and metabolomics to map the changes in metabolic pathways and products.
Figure 1.
Experimental workflow including pathway analysis and metabolite profiling (A). Microbiome changes in functional pathways and metabolites driven by BRB consumption, together with unique precursors in BRB contribute to the health benefits from BRB consumption (B).
Figure 1B summarizes the main point of the present study. Seven weeks of BRB diet treatment substantially changed the metabolic functions of the mouse gut microbiome. The heat map (Figure 1B, upper right) constructed by comparative metagenome analysis shows a consistent clustering pattern within the respective diet groups, indicating a considerably differentiated functional pathway assembly. More importantly, alterations in microbial metabolic pathways together with unique precursors in BRBs lead to significant metabolic changes as shown in the cloud plot (Figure 1, lower right), which may contribute to the underlying mechanisms of the beneficial health effects of BRB consumption.
BRB Consumption Differentiated Gut Microbiome Metabolite Profile Revealed by Untargeted Metabolomics
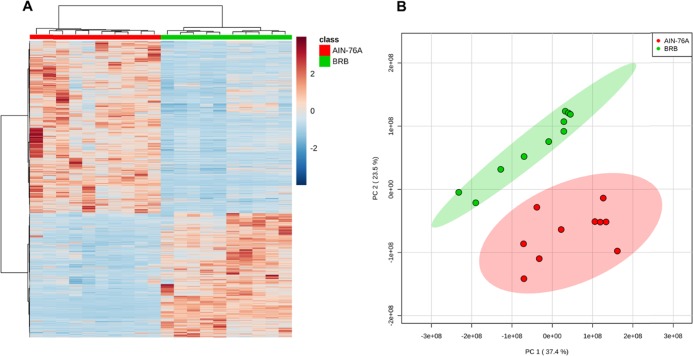
Metabolomics profiling enables us to assess the changes of metabolites in the gut microbiome. As shown in Figure 2A, the hierarchical clustering heat map constructed using significantly changed molecular features shows a consistent clustering pattern within individual groups. Figure 2B also illustrates that the BRB-fed group is well separated from the control diet group by principal component analysis (PCA). We have identified 54 microbiome metabolites that were differentiated by BRB consumption in the gut microbiome (Table S1), for instance, vitamin-related compounds, amino acid derivatives, dipeptides and natural products. Together, the data indicate that the metabolite profiles of these two diet groups are readily differentiated, with diverse bacterial metabolites being directly produced or modified by the gut microbiota.
Figure 2.
Heat map constructed using molecular features shows that BRB consumption significantly changed the metabolite profile in the gut microbiome (A). Fecal metabolite profiles of mice fed BRB were well separated from mice fed control diet by PCA (B).
Elevated Levels of Vitamins and Cofactors in the Gut Microbiome of Mice Fed BRB Diet
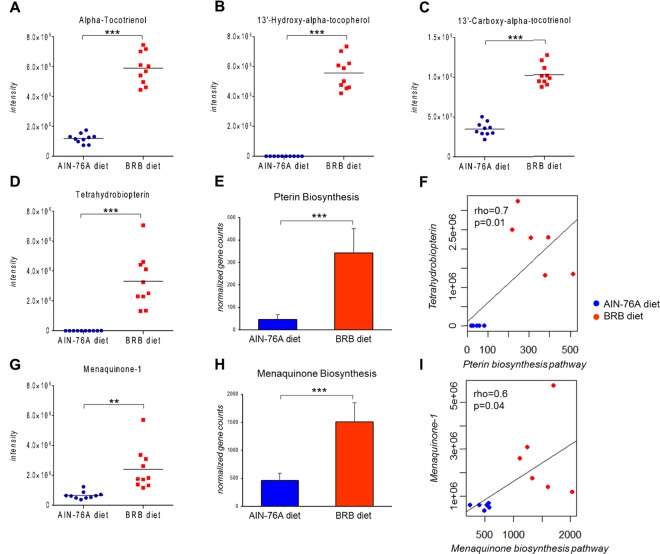
Enrichment in vitamin-related metabolites together with increases in their synthetic pathways were found in mice fed BRB diet compared to those fed control diet (Figure 3). Metabolites that have vitamin E antioxidant activity including tocopherols and tocotrienols were found in higher levels in mice fed BRB diet (Figure 3A–C). Since tocopherols and tocotrienols are usually found in plants and not synthesized by bacteria,25,26 the increased abundances of tocopherols and tocotrienols probably came from BRB per se. Moreover, we observed that tetrahydrobiopterin, a cofactor of multiple important biological reactions, increased 1000-fold in mice fed BRBs (Figure 3D). The pathway of pterin biosynthesis, which is responsible for the biosynthesis of tetrahydrobiopterin, also shows a 7-fold increase (Figure 3E). Likewise, menaquinone-1, which belongs to vitamin K2 family, increased approximately 4-fold in mice fed BRBs (Figure 3G). Gut bacteria are known as important producers of vitamins, and menaquinones can be obtained from the gut bacteria.5 Accordingly, the bacterial pathway synthesizing menaquinones is significantly enriched (Figure 3H). Additionally, to explore the functional correlation between the changes in metabolic pathways and corresponding metabolites, we conducted functional correlation by calculating the Pearson’s correlation coefficient. Clear correlations could be identified between the synthetic pathway abundances and metabolite intensities of tetrahydrobiopterin (Figure 3F) and menaquinone-1 (Figure 3I) (ρ > 0.5, p < 0.05), suggesting that the increased levels of these metabolites resulted from BRB-driven elevation in related synthetic activities. These data suggest that the levels of various vitamins and cofactors are elevated in the gut microbiome of mice fed BRBs, either from BRBs per se or BRB-driven changes in the gut microbiome.
Figure 3.
BRB consumption significantly increased vitamin-related metabolites in the mouse gut microbiome, as exemplified by vitamin E metabolites (A–C), cofactor tetrahydrobiopterin (D) and menaquinone-1 (G); meanwhile, gut bacterial pathways involved in the biosynthesis of tetrahydrobiopterin (E) and menaquinone (H) were upregulated in mice fed BRB, which shows clear correlation with the abundance of corresponding metabolites (F, I) (p < 0.05; ρ > 0.5). (***p < 0.001; **p < 0.01; *p < 0.05).
Functional Metabolites That Have Beneficial Health Effects in the Gut Microbiome of Mice Fed BRB Diet
In addition to vitamins and cofactors, we also observed an enrichment of important metabolites that may confer health benefits in the gut microbiome of mice fed BRB diet compared to mice fed control diet (Figure 4). For instance, protocatechuic acid is a phenolic acid and has distinct antioxidant and anti-inflammatory effects. Protocatechuic acid is a typical metabolite of polyphenols, which are abundant in BRBs; therefore, it is expected to observe a 900-fold increase of protocatechuic acid in mice fed BRB diet. Protocatechuic acid has many beneficial health effects that are recently reviewed.27 We also observed that α-linolenic acid (ALA) and its further metabolite stearidonic acid increased 3-fold and 11-fold, respectively. Both of them are important fatty acids that are known to be abundant in berry fruits.28,29 The abundance of oleoylethanolamine (OEA) increased more than 3-fold, which is an important signaling molecule as an endogenous peroxisome proliferator-activated receptor α (PPAR-α) agonist. OEA is involved in the regulation of satiety and bodyweight.30,31 In addition, the abundance of kaempferol 3-sophorotrioside increased approximately 50-fold, which belongs to flavonoid glycosides. Flavonoid and derivatives are typical polyphenolic molecules rich in berry fruits, and kaempferol and its glycosides have many health effects such as antitumor, antioxidant, and anti-inflammatory activities.32,33
Figure 4.
Fecal metabolites (A–E) that have beneficial health effects in the gut microbiome of mice fed BRB diet were enriched compared to mice fed control diet (***p < 0.001; **p < 0.01; *p < 0.05).
BRB Consumption Changed the SCFAs in the Gut Microbiome
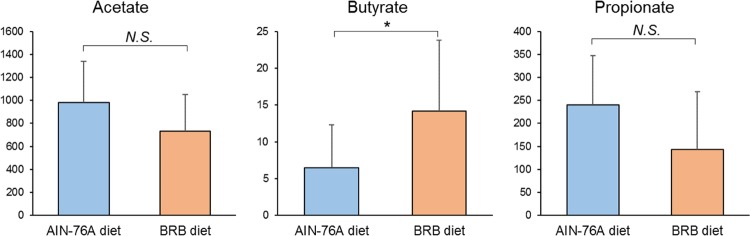
SCFAs are important gut microbial products of microbial fermentation with diverse biological functions. We examined the changes of SCFAs and found that the abundance of butyrate significantly increased in the gut microbiome of mice fed BRB diet compared to mice fed control diet (Figure 5). The levels of acetate and propionate did not show significant differences between two groups. Butyrate exerts anti-inflammatory effects by inhibiting the release of cytokines and chemokines. Meanwhile, the expression of tight junction proteins can be improved by intestinal butyrate.1 Therefore, the increased levels of butyrate in the gut microbiome could contribute to associated health effects of BRBs.
Figure 5.
Comparisons of SCFAs in the gut microbiome of mice fed BRB or control diets (*p < 0.05; N.S., not significant).
Discussion
Our previous study has clearly demonstrated the impact of BRB consumption on mouse gut microbiome at both compositional and functional levels.20 For example, BRB consumption changed multiple microbiome-related functional pathways including vitamin synthesis and carbohydrate metabolism.20 The effects of BRBs on the functional pathways in gut bacteria, together with the interactions of gut bacterial activities to human health, infer that BRB-driven microbiome changes may play a role in the health effects of BRB consumption. Meanwhile, changes in microbial metabolic pathways could lead to different metabolic products. To further investigate the functional profile of the gut microbiome associated with BRB consumption, in this follow-up study we examined the metabolome differences in the gut microbiome of mice fed BRB diet compared to mice fed control diet. A significantly distinct metabolite profile was observed, representing a different functional role of the BRB-shaped gut microbiome. A large number of metabolites were significantly changed in the gut microbiome with BRB consumption. Database search using exact masses coupled with tandem MS/MS analysis identified a number of metabolites that might account for the beneficial effects of BRB consumption. These findings may provide mechanistic insights into the gut microbial activities contributing to the health effects of BRBs.
Accumulating evidence suggested the importance of the gut microbiome in host health and disease.34 The composition and functionality of the gut microbiome can be readily affected by various intrinsic and extrinsic factors.35 Among these factors, diet received considerable attention. On one hand, typical Western diet rich in simple sugars and saturated fat is linked to increased risks of adverse health outcomes such as obesity, diabetes, and cardiovascular disease, and the gut microbiome is believed to be involved in this progress.36,37 On the other hand, healthy dietary patterns such as Mediterranean diet beneficially impact the host via effects on the gut microbiota such as promoting bacteria that utilize dietary fiber and produce SCFAs.38 In the present study, we focused on investigating the impact of a BRB-rich diet on the gut microbiota especially its metabolic profile for the exploration of the relationship between BRBs and the gut microbiome. We observed consistent alterations in bacterial pathways and metabolic products driven by BRB consumption, suggesting that bacterial functions can be readily altered by dietary patterns leading to differential metabolic products, thereby differentially influencing the host.
We previously reported that a number of bacterial genes encoding enzymes involved in vitamin and cofactor biosynthesis were enriched in the gut microbiome of mice fed BRBs.20 Accordingly, we observed increased levels of vitamins such as menaquinone-1 (vitamin K2 family) and important cofactor tetrahydrobiopterin in the fecal samples of mice fed BRB. However, aside from the contribution of the gut microbiota, it should be noted that vitamins are naturally present in berry fruits.39 We also observed a 1000-fold increase of tetrahydrobiopterin, which is a crucial cofactor that participates in a series of biological reactions.40 One of these reactions is to convert aromatic amino acids into precursors of neurotransmitters such as serotonin and dopamine.41 Thus, the deficiency of tetrahydrobiopterin is associated with neurological disorders.42 Moreover, insufficient tetrahydrobiopterin also causes reduced levels of nitric oxide, which contributes to insulin resistance.43 In addition, it is reported that the gut microbiome is an exogenous source of tetrahydrobiopterin;44 we discovered increased levels of tetrahydrobiopterin and its biosynthetic pathway in the gut microbiome of mice fed BRBs. It is likely that BRB consumption enhances the gut bacterial activities on the production of tetrahydrobiopterin, providing mechanistic insights regarding the neuroprotective and antidiabetic effects of BRBs.45−47
As important gut microbiome-derived metabolites, the functions of SCFAs have been extensively studied. For example, it is well documented that butyrate has anti-inflammatory activities primarily via the inhibition of nuclear factor κB (NF-κB) activation in human colonic epithelial cells.48,49 NF-κB regulates many cellular genes that are involved in early immune inflammatory responses, and the activity of NF-κB is frequently perturbed in inflammatory bowel disease (IBD)50 and colorectal cancer.51 Butyrate therefore plays a protective role in these diseases.52 Meanwhile, it is demonstrated that BRB diet has effects against the development of colorectal cancer53,54 and IBD.16 Thus, increased levels of butyrate in the gut microbiome may be one of the underlying mechanisms of the anti-inflammatory and anticancer effects of the BRB diet.
Diet is a key factor that influences the gut microbiome; meanwhile, the metabolic activities of the gut microbiota are also indispensable regarding the health benefits of functional foods.55 The role of the gut microbiome in the toxicity of environmental toxic chemicals has been increasingly acknowledged to be of equal importance; future studies on the role of the gut microbiome in the health benefits of various functional foods are warranted. Additionally, the LC–MS analysis in the present study was performed in the positive mode with an electrospray ionization source. More exhaustive examination in the negative mode as well as using different ion sources is warranted.
In summary, we combined untargeted and targeted metabolomics to analyze the fecal samples of mice with the administration of BRBs. Metabolomic profiling revealed that BRB consumption drastically changed the metabolome of the mouse gut microbiome, with many metabolites contributing to the beneficial effects from BRB consumption. Taken together, these results indicate the involvement of the gut microbiome in the health benefits of BRBs, and the connection among the functional foods, their health benefits, and the gut microbiome.
Methods
Animals
Custom purified American Institute of Nutrition (AIN)-76A animal diet (Dyets, Inc., Bethlehem, PA) was used as the control diet. BRB diet was prepared essentially as described.20 Briefly, whole ripe BRBs (Rubus occidentalis) of the Jewel variety were freeze-dried and ground into powder. BRB powder was stored at −20 °C until being incorporated into AIN-76A animal diet pellets by 10% w/w concentration at the expense of cornstarch. The diets were stored at 4 °C until being fed to mice. Mice of the control group were fed AIN-76A diet, mice of the dietary treatment group were fed BRB diet. A total of 20 specific pathogen-free C57BL/6 mice (∼8 week old), purchased from Jackson Laboratories, were housed in the animal facility of the University of Georgia for one week to acclimatize with standard pelleted rodent diet and tap water provided ad libitum (protocol number: A2013 06-033-Y3-A3). In the beginning of the diet treatment, the mice were randomly assigned to either control (AIN-76A diet) or treatment group (BRB diet). The environmental conditions were 22 °C, 40–70% humidity, and a 12:12 h light/dark cycle, and water ad libitum was provided throughout the experiment period. After 7 weeks, their fecal samples from individual mice were collected and kept at −80 °C immediately for further analysis. Regular monitoring of animal health and well-being was done twice weekly. The animal protocol was approved by the University of Georgia Institutional Animal Care and Use Committee.
Metagenomics Sequencing
Shotgun metagenomic sequencing was performed as previously described.56 Briefly, fecal DNA (10 ng/μL) was fragmented using the Bioruptor UCD-300 sonication device. The Kapa Hyper Prep Kit was used for the construction of the sequencing library according to manufacturer’s instructions. The DNA was quantified using Qubit 2.0 Fluorometer. The sequencing was performed using the Illumina NextSeq High Output Flow Cell (300 Cycles; PE150) in the Georgia Genomics Facility of University of Georgia. For bioinformatics analysis, the MG-RAST metagenomics analysis sever (http://metagenomics.anl.gov, version 4.0.3) was applied for the automatic functional pathway analysis of metagenomes using the Subsystems database.57
Untargeted Metabolomics Profiling
The LC–MS analysis was performed as previously described with minor modifications.58 Briefly, 20 mg fecal sample and 50 mg glass beads (Sigma-Aldrich, MO) were added to 400 μL cooled methanol solution (methanol/water, 1:1), followed by homogenization using a TissueLyser (Qiagen, Hilden, Germany) at 50 Hz for 10 min. The supernatant was collected after centrifuging for 10 min at 12 000 rpm, followed by drying up in a speed vacuum (Thermo), and then resuspending for injection. The mass spectrometer was interfaced with an Agilent 1290 Infinity II UPLC system. The LC–MS analysis was performed on a quadrupole time-of-flight (Q-TOF) 6530 mass spectrometer (Agilent Technologies, Santa Clara, CA) with an electrospray ionization source. Metabolic features were analyzed in the positive mode over a m/z range of 50–1000 with a C18 T3 reverse-phased column (Waters Corporation, Milford, MA). The XCMS Online sever (https://xcmsonline.scripps.edu, version 3.5.1) was applied for peak picking, alignment, integration, and extraction of the peak intensities. MS/MS data were generated on the Q-TOF for the identification of differentiated molecular features. The software of MS-DIAL (version 2.90)59 and MS-FINDER (version 2.40)60 were used for the identification of metabolites based on the MS/MS spectrum.
Quantification of Fecal SCFAs
SCFAs (acetate, butyrate, and propionate) in the fecal samples were quantified as previously described.61 Briefly, sample aliquots were added to NaOH solution (0.005 M) containing internal standards (50 μg/mL of acetic acid-d4, 10 μg/mL of propionic acid-d2, and 10 μg/mL of butyric acid-d2), followed by homogenization and centrifugation. The supernatant were transferred, and a mixture of 1-propanol/pyridine (3:2, v/v) and propyl chloroformate was subsequently added for derivatization. After sonication, the propyl formate derivatives were extracted using hexane and then transferred to an autosampler vial for injection. An Agilent 7820A GC-5977B MS system (Santa Clara, CA) coupled with an Agilent DB-5 column (30 m × 0.25 mm × 0.25 μm) was used for the GC/MS analysis.
Statistical Analysis of Data
PCA and hierarchical clustering algorithm were used to visualize the comparison of metabolite profiles. Two-tailed Welch’s t-test was used to analyze metabolites that differed in abundance between groups corrected for the false discovery rate (FDR). The metagenomics sequence count data for functional analysis were processed using DESeq262 for statistical analysis adjusted for multiple testing of FDR. The correlation matrix between bacterial pathways and metabolites was generated using Pearson’s correlation coefficient. Statistical significances of SCFAs between two diet groups were assessed by Student’s t test.
Acknowledgments
We thank the University of Georgia, University of North Carolina at Chapel Hill, and the NIH/NIEHS provided partial financial support (R01ES024950) for this work.
Supporting Information Available
The Supporting Information is available free of charge at https://pubs.acs.org/doi/10.1021/acsomega.9b00237.
List of identified fecal metabolites that are significantly elevated in mice fed BRB diet compared to mice fed control diet (Table S1); MS/MS spectral match of the identified metabolites (Table S2) (PDF)
Author Present Address
∥ Department of Medicine, University of California San Diego, La Jolla, California 92093, United States (B.G.).
The authors declare no competing financial interest.
Supplementary Material
References
- Nicholson J. K.; Holmes E.; Kinross J.; Burcelin R.; Gibson G.; Jia W.; Pettersson S. Host-gut microbiota metabolic interactions. Science 2012, 336, 1262–1267. 10.1126/science.1223813. [DOI] [PubMed] [Google Scholar]
- Tremaroli V.; Bäckhed F. Functional interactions between the gut microbiota and host metabolism. Nature 2012, 489, 242. 10.1038/nature11552. [DOI] [PubMed] [Google Scholar]
- Claus S. P.; Guillou H.; Ellero-Simatos S. The gut microbiota: a major player in the toxicity of environmental pollutants?. npj Biofilms Microbiomes 2016, 2, 16003 10.1038/npjbiofilms.2016.3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sharon G.; Garg N.; Debelius J.; Knight R.; Dorrestein P. C.; Mazmanian S. K. Specialized metabolites from the microbiome in health and disease. Cell Metab. 2014, 20, 719–730. 10.1016/j.cmet.2014.10.016. [DOI] [PMC free article] [PubMed] [Google Scholar]
- LeBlanc J. G.; Milani C.; de Giori G. S.; Sesma F.; van Sinderen D.; Ventura M. Bacteria as vitamin suppliers to their host: a gut microbiota perspective. Curr. Opin. Biotechnol. 2013, 24, 160–168. 10.1016/j.copbio.2012.08.005. [DOI] [PubMed] [Google Scholar]
- Smith P. M.; Howitt M. R.; Panikov N.; Michaud M.; Gallini C. A.; Bohlooly-y M.; Glickman J. N.; Garrett W. S. The microbial metabolites, short-chain fatty acids, regulate colonic Treg cell homeostasis. Science 2013, 341, 569–573. 10.1126/science.1241165. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Samuel B. S.; Shaito A.; Motoike T.; Rey F. E.; Backhed F.; Manchester J. K.; Hammer R. E.; Williams S. C.; Crowley J.; Yanagisawa M.; Gordon J. I. Effects of the gut microbiota on host adiposity are modulated by the short-chain fatty-acid binding G protein-coupled receptor, Gpr41. Proc. Natl. Acad. Sci. U.S.A. 2008, 105, 16767–16772. 10.1073/pnas.0808567105. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Koeth R. A.; Wang Z.; Levison B. S.; Buffa J. A.; Org E.; Sheehy B. T.; Britt E. B.; Fu X.; Wu Y.; Li L.; et al. Intestinal microbiota metabolism of L-carnitine, a nutrient in red meat, promotes atherosclerosis. Nat. Med. 2013, 19, 576. 10.1038/nm.3145. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wang Z.; Klipfell E.; Bennett B. J.; Koeth R.; Levison B. S.; DuGar B.; Feldstein A. E.; Britt E. B.; Fu X.; Chung Y.-M.; et al. Gut flora metabolism of phosphatidylcholine promotes cardiovascular disease. Nature 2011, 472, 57. 10.1038/nature09922. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tang W. W.; Wang Z.; Kennedy D. J.; Wu Y.; Buffa J. A.; Agatisa-Boyle B.; Li X. S.; Levison B. S.; Hazen S. L. Gut Microbiota-Dependent Trimethylamine N-Oxide (TMAO) Pathway Contributes to Both Development of Renal Insufficiency and Mortality Risk in Chronic Kidney Disease Novelty and Significance. Circ. Res. 2015, 116, 448–455. 10.1161/CIRCRESAHA.116.305360. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Beattie J.; Crozier A.; Duthie G. G. Potential health benefits of berries. Curr. Nutr. Food Sci. 2005, 1, 71–86. 10.2174/1573401052953294. [DOI] [Google Scholar]
- Paredes-López O.; Cervantes-Ceja M. L.; Vigna-Pérez M.; Hernández-Pérez T. Berries: improving human health and healthy aging, and promoting quality life—a review. Plant Foods Hum. Nutr. 2010, 65, 299–308. 10.1007/s11130-010-0177-1. [DOI] [PubMed] [Google Scholar]
- Stoner G. D. Foodstuffs for preventing cancer: the preclinical and clinical development of berries. Cancer Prev. Res. 2009, 187–194. 10.1158/1940-6207.CAPR-08-0226. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chen T.; Rose M. E.; Hwang H.; Nines R. G.; Stoner G. D. Black raspberries inhibit N-nitrosomethylbenzylamine (NMBA)-induced angiogenesis in rat esophagus parallel to the suppression of COX-2 and iNOS. Carcinogenesis 2006, 27, 2301–2307. 10.1093/carcin/bgl109. [DOI] [PubMed] [Google Scholar]
- Oghumu S.; Casto B. C.; Ahn-Jarvis J.; Weghorst L. C.; Maloney J.; Geuy P.; Horvath K. Z.; Bollinger C. E.; Warner B. M.; Summersgill K. F. inhibition of Pro-inflammatory and anti-apoptotic Biomarkers during experimental Oral cancer chemoprevention by Dietary Black raspberries. Front. Immunol. 2017, 8, 1325 10.3389/fimmu.2017.01325. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Montrose D. C.; Horelik N. A.; Madigan J. P.; Stoner G. D.; Wang L.-S.; Bruno R. S.; Park H. J.; Giardina C.; Rosenberg D. W. Anti-inflammatory effects of freeze-dried black raspberry powder in ulcerative colitis. Carcinogenesis 2011, 32, 343–350. 10.1093/carcin/bgq248. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Seeram N. P.Berry Fruits: Compositional Elements, Biochemical Activities, and the Impact of Their Intake on Human Health, Performance, and Disease; ACS Publications, 2008. [DOI] [PubMed] [Google Scholar]
- Nile S. H.; Park S. W. Edible berries: Bioactive components and their effect on human health. Nutrition 2014, 30, 134–144. 10.1016/j.nut.2013.04.007. [DOI] [PubMed] [Google Scholar]
- Anhê F. F.; Roy D.; Pilon G.; Dudonné S.; Matamoros S.; Varin T. V.; Garofalo C.; Moine Q.; Desjardins Y.; Levy E.; Marette A. A polyphenol-rich cranberry extract protects from diet-induced obesity, insulin resistance and intestinal inflammation in association with increased Akkermansia spp. population in the gut microbiota of mice. Gut 2015, 64, 872–883. 10.1136/gutjnl-2014-307142. [DOI] [PubMed] [Google Scholar]
- Tu P.; Bian X.; Chi L.; Gao B.; Ru H.; Knobloch T. J.; Weghorst C. M.; Lu K. Characterization of the Functional Changes in Mouse Gut Microbiome Associated with Increased Akkermansia muciniphila Population Modulated by Dietary Black Raspberries. ACS Omega 2018, 3, 10927–10937. 10.1021/acsomega.8b00064. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bekiares N.; Krueger C. G.; Meudt J. J.; Shanmuganayagam D.; Reed J. D. Effect of Sweetened Dried Cranberry Consumption on Urinary Proteome and Fecal Microbiome in Healthy Human Subjects. OMICS: J. Integr. Biol. 2018, 22, 145–153. 10.1089/omi.2016.0167. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Holscher H. D.; Taylor A. M.; Swanson K. S.; Novotny J. A.; Baer D. J. Almond consumption and processing affects the composition of the gastrointestinal microbiota of healthy adult men and women: a randomized controlled trial. Nutrients 2018, 10, 126 10.3390/nu10020126. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Holscher H. D.; Guetterman H. M.; Swanson K. S.; An R.; Matthan N. R.; Lichtenstein A. H.; Novotny J. A.; Baer D. J. Walnut consumption alters the gastrointestinal microbiota, microbially derived secondary bile acids, and health markers in healthy adults: a randomized controlled trial. J. Nutr. 2018, 148, 861–867. 10.1093/jn/nxy004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Byerley L. O.; Samuelson D.; Blanchard E. IV; Luo M.; Lorenzen B. N.; Banks S.; Ponder M. A.; Welsh D. A.; Taylor C. M. Changes in the gut microbial communities following addition of walnuts to the diet. J. Nutr. Biochem. 2017, 48, 94–102. 10.1016/j.jnutbio.2017.07.001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sen C. K.; Khanna S.; Roy S. Tocotrienols: Vitamin E beyond tocopherols. Life Sci. 2006, 78, 2088–2098. 10.1016/j.lfs.2005.12.001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fritsche S.; Wang X.; Jung C. Recent advances in our understanding of tocopherol biosynthesis in plants: An overview of key genes, functions, and breeding of vitamin E improved crops. Antioxidants 2017, 6, 99 10.3390/antiox6040099. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kakkar S.; Bais S. A review on protocatechuic acid and its pharmacological potential. ISRN Pharmacol. 2014, 2014, 1–9. 10.1155/2014/952943. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Barceló-Coblijn G.; Murphy E. J. Alpha-linolenic acid and its conversion to longer chain n– 3 fatty acids: Benefits for human health and a role in maintaining tissue n– 3 fatty acid levels. Prog. Lipid Res. 2009, 48, 355–374. 10.1016/j.plipres.2009.07.002. [DOI] [PubMed] [Google Scholar]
- Whelan J. Dietary stearidonic acid is a long chain (n-3) polyunsaturated fatty acid with potential health benefits. J. Nutr. 2009, 139, 5–10. 10.3945/jn.108.094268. [DOI] [PubMed] [Google Scholar]
- Verme J. L.; Gaetani S.; Fu J.; Oveisi F.; Burton K.; Piomelli D. Regulation of food intake by oleoylethanolamide. Cell. Mol. Life Sci. 2005, 62, 708. 10.1007/s00018-004-4494-0. [DOI] [PubMed] [Google Scholar]
- Gaetani S.; Oveisi F.; Piomelli D. Modulation of meal pattern in the rat by the anorexic lipid mediator oleoylethanolamide. Neuropsychopharmacology 2003, 28, 1311. 10.1038/sj.npp.1300166. [DOI] [PubMed] [Google Scholar]
- Kim T. H.; Ku S.-K.; Lee I.-C.; Bae J.-S. Anti-inflammatory effects of kaempferol-3-O-sophoroside in human endothelial cells. Inflammation Res. 2012, 61, 217–224. 10.1007/s00011-011-0403-9. [DOI] [PubMed] [Google Scholar]
- Wang J.; Fang X.; Ge L.; Cao F.; Zhao L.; Wang Z.; Xiao W. Antitumor, antioxidant and anti-inflammatory activities of kaempferol and its corresponding glycosides and the enzymatic preparation of kaempferol. PLoS One 2018, 13, e0197563 10.1371/journal.pone.0197563. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cho I.; Blaser M. J. The human microbiome: at the interface of health and disease. Nat. Rev. Genet. 2012, 13, 260. 10.1038/nrg3182. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sommer F.; Bäckhed F. The gut microbiota—masters of host development and physiology. Nat. Rev. Microbiol. 2013, 11, 227. 10.1038/nrmicro2974. [DOI] [PubMed] [Google Scholar]
- Turnbaugh P. J.; Ridaura V. K.; Faith J. J.; Rey F. E.; Knight R.; Gordon J. I. The effect of diet on the human gut microbiome: a metagenomic analysis in humanized gnotobiotic mice. Sci. Transl. Med. 2009, 1, 6ra14 10.1126/scitranslmed.3000322. [DOI] [PMC free article] [PubMed] [Google Scholar]
- David L. A.; Maurice C. F.; Carmody R. N.; Gootenberg D. B.; Button J. E.; Wolfe B. E.; Ling A. V.; Devlin A. S.; Varma Y.; Fischbach M. A.; et al. Diet rapidly and reproducibly alters the human gut microbiome. Nature 2014, 505, 559. 10.1038/nature12820. [DOI] [PMC free article] [PubMed] [Google Scholar]
- De Filippis F.; Pellegrini N.; Vannini L.; Jeffery I. B.; La Storia A.; Laghi L.; Serrazanetti D. I.; Di Cagno R.; Ferrocino I.; Lazzi C. High-level adherence to a Mediterranean diet beneficially impacts the gut microbiota and associated metabolome. Gut 2016, 65, 1812–1821. 10.1136/gutjnl-2015-309957. [DOI] [PubMed] [Google Scholar]
- Howard L. R.; Hager T. J.. Berry Fruit Phytochemicals. Food Science and Technology; Marcel Dekker: New York, 2007; Vol. 168, p 73. [Google Scholar]
- Kappock T. J.; Caradonna J. P. Pterin-dependent amino acid hydroxylases. Chem. Rev. 1996, 96, 2659–2756. 10.1021/cr9402034. [DOI] [PubMed] [Google Scholar]
- Werner-Felmayer G.; Golderer G.; Werner E. R. Tetrahydrobiopterin biosynthesis, utilization and pharmacological effects. Curr. Drug Metab. 2002, 3, 159–173. 10.2174/1389200024605073. [DOI] [PubMed] [Google Scholar]
- Foxton R. H.; Land J. M.; Heales S. J. Tetrahydrobiopterin availability in Parkinson’s and Alzheimer’s disease; potential pathogenic mechanisms. Neurochem. Res. 2007, 32, 751–756. 10.1007/s11064-006-9201-0. [DOI] [PubMed] [Google Scholar]
- Wu G.; Meininger C. J. Nitric oxide and vascular insulin resistance. Biofactors 2009, 35, 21–27. 10.1002/biof.3. [DOI] [PubMed] [Google Scholar]
- Belik J.; Shifrin Y.; Arning E.; Bottiglieri T.; Pan J.; Daigneault M. C.; Allen-Vercoe E. Intestinal microbiota as a tetrahydrobiopterin exogenous source in hph-1 mice. Sci. Rep. 2017, 7, 39854 10.1038/srep39854. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Subash S.; Essa M. M.; Al-Adawi S.; Memon M. A.; Manivasagam T.; Akbar M. Neuroprotective effects of berry fruits on neurodegenerative diseases. Neural Regener. Res. 2014, 9, 1557. 10.4103/1673-5374.139483. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yang H.; Tian T.; Wu D.; Guo D.; Lu J. Prevention and treatment effects of edible berries for three deadly diseases: Cardiovascular disease, cancer and diabetes. Crit. Rev. Food Sci. Nutr. 2018, 1–10. 10.1080/10408398.2018.1432562. [DOI] [PubMed] [Google Scholar]
- Kirakosyan A.; Seymour E. M.; Kondoleon N.; Gutierrez E.; Wolforth J.; Bolling S. The intake of red raspberry fruit is inversely related to cardiac risk factors associated with metabolic syndrome. J. Funct. Foods 2018, 41, 83–89. 10.1016/j.jff.2017.12.033. [DOI] [Google Scholar]
- Inan M. S.; Rasoulpour R. J.; Yin L.; Hubbard A. K.; Rosenberg D. W.; Giardina C. The luminal short-chain fatty acid butyrate modulates NF-κB activity in a human colonic epithelial cell line. Gastroenterology 2000, 118, 724–734. 10.1016/S0016-5085(00)70142-9. [DOI] [PubMed] [Google Scholar]
- Segain J.; De La Blétiere D. R.; Bourreille A.; Leray V.; Gervois N.; Rosales C.; Ferrier L.; Bonnet C.; Blottiere H.; Galmiche J. Butyrate inhibits inflammatory responses through NFκB inhibition: implications for Crohn’s disease. Gut 2000, 47, 397–403. 10.1136/gut.47.3.397. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Schreiber S.; Nikolaus S.; Hampe J. Activation of nuclear factor κB in inflammatory bowel disease. Gut 1998, 42, 477–484. 10.1136/gut.42.4.477. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lind D. S.; Hochwald S. N.; Malaty J.; Rekkas S.; Hebig P.; Mishra G.; Moldawer L. L.; Copeland E. M. III; MacKay S. Nuclear factor-κB is upregulated in colorectal cancer. Surgery 2001, 130, 363–369. 10.1067/msy.2001.116672. [DOI] [PubMed] [Google Scholar]
- Canani R. B.; Di Costanzo M.; Leone L.; Pedata M.; Meli R.; Calignano A. Potential beneficial effects of butyrate in intestinal and extraintestinal diseases. World J. Gastroenterol. 2011, 17, 1519 10.3748/wjg.v17.i12.1519. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wang L.-S.; Arnold M.; Huang Y.-W.; Sardo C.; Seguin C.; Martin E.; Huang T. H.-M.; Riedl K.; Schwartz S.; Frankel W.; et al. Modulation of genetic and epigenetic biomarkers of colorectal cancer in humans by black raspberries: a phase I pilot study. Clin. Cancer Res. 2011, 17, 598–610. 10.1158/1078-0432.CCR-10-1260. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bi X.; Fang W.; Wang L.-S.; Stoner G. D.; Yang W. Black raspberries inhibit intestinal tumorigenesis in apc1638+/– and Muc2–/– mouse models of colorectal cancer. Cancer Prev. Res. 2010, 1443–1450. 10.1158/1940-6207.CAPR-10-0124. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zmora N.; Suez J.; Elinav E. You are what you eat: diet, health and the gut microbiota. Nat. Rev. Gastroenterol. Hepatol. 2018, 35–56. 10.1038/s41575-018-0061-2. [DOI] [PubMed] [Google Scholar]
- Chi L.; Bian X.; Gao B.; Tu P.; Ru H.; Lu K. The effects of an environmentally relevant level of arsenic on the gut microbiome and its functional metagenome. Toxicol. Sci. 2017, 160, 193–204. 10.1093/toxsci/kfx174. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Keegan K. P.; Glass E. M.; Meyer F.. MG-RAST, a Metagenomics Service for Analysis of Microbial Community Structure and Function. In Microbial Environmental Genomics (MEG); Springer, 2016; pp 207–233. [DOI] [PubMed] [Google Scholar]
- Lu K.; Abo R. P.; Schlieper K. A.; Graffam M. E.; Levine S.; Wishnok J. S.; Swenberg J. A.; Tannenbaum S. R.; Fox J. G. Arsenic exposure perturbs the gut microbiome and its metabolic profile in mice: an integrated metagenomics and metabolomics analysis. Environ. Health Perspect. 2014, 122, 284–291. 10.1289/ehp.1307429. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tsugawa H.; Cajka T.; Kind T.; Ma Y.; Higgins B.; Ikeda K.; Kanazawa M.; VanderGheynst J.; Fiehn O.; Arita M. MS-DIAL: data-independent MS/MS deconvolution for comprehensive metabolome analysis. Nat. Methods 2015, 12, 523. 10.1038/nmeth.3393. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tsugawa H.; Kind T.; Nakabayashi R.; Yukihira D.; Tanaka W.; Cajka T.; Saito K.; Fiehn O.; Arita M. Hydrogen rearrangement rules: computational MS/MS fragmentation and structure elucidation using MS-FINDER software. Anal. Chem. 2016, 88, 7946–7958. 10.1021/acs.analchem.6b00770. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zheng X.; Qiu Y.; Zhong W.; Baxter S.; Su M.; Li Q.; Xie G.; Ore B. M.; Qiao S.; Spencer M. D.; et al. A targeted metabolomic protocol for short-chain fatty acids and branched-chain amino acids. Metabolomics 2013, 9, 818–827. 10.1007/s11306-013-0500-6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Love M. I.; Huber W.; Anders S. Moderated estimation of fold change and dispersion for RNA-seq data with DESeq2. Genome Biol. 2014, 15, 550 10.1186/s13059-014-0550-8. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.