Abstract
Background
Indications for liver surgery are expanding fast and complexity of procedures increases. Preoperative mortality risk assessment by scoring systems is debatable. A previously published externally validated Mortality Risk Score allowed easy applicable and precise prediction of postoperative mortality. Aim of the study was to compare the performance of the Mortality Risk Score with the standard scores MELD and P-POSSUM.
Methods
Data of 529 patients undergoing liver resection were analysed. Mortality Risk Score, the labMELD Score and the P-POSSUM Scores (PS, OS, P-POSSUM mortality %) were calculated. The ROC curves of the three scoring systems were computed and the areas under the curve (C-index) were calculated using logistic regression models. Comparisons between the ROC curves were performed using the corresponding Wald tests.
Results
Internal validation confirmed that the risk model was predictive for a 90-day mortality rate with a C-index of 0.8421. The labMELD Score had a C-index of 0.7352 and the P-POSSUM system 0.6795 (PS 0.6953, OS 0.5413). The 90-day mortality rate increased with increasing labMELD values (p < 0.0001). Categorized according to the Mortality Risk Score Groups the labMELD Score showed a linear increase while the POSSUM Scores showed variable results.
Conclusions
By accurately predicting the risk of postoperative mortality after liver surgery the Mortality Risk Score should be useful at the selection stage. Prediction can be adjusted by use of the well-established labMELD Score. In contrast, the performance of standard P-POSSUM Scores is limited.
Keywords: Liver resection, Mortality, Prognostic score
Background
Indications for liver surgery are expanding fast and the complexity of procedures has generally increased over the years. The preoperative assessment of the safety of liver surgery and identification of high-risk patients remains challenging. Multiple patient-related factors and procedure-specific variables directly influence the perioperative outcome as well as the 90-day mortality after hepatectomy.
To provide appropriate counseling to the patient regarding the risks and outcome of the planned procedure is the crucial point within a shared decision-making process. Quality of life after surgery has increasingly become an important patient-related endpoint after surgical interventions. Nevertheless, the surgeons’ intuition is not the best when it comes to predicting the outcome after liver resection and “experience-based” indications are no longer acceptable [1, 2].
Various scoring systems such as MELD Score, E-PASS, mE-PASS, Portsmouth-Physiological and Operative Severity Score for the enUmeration of Mortality and morbidity (P-POSSUM), Child-Turcotte-Pugh score or ASA classification have been used in an attempt to accurately predict mortality in liver surgery. However, the results were disappointing. General surgical risk models (E-PASS, mE-PASS and P-POSSUM) tended to overestimate the mortality rate by more than twofold [3]. For prognostication of patients with known liver disease the utility of MELD Score has been well documented. However, heterogenic reports are available regarding its ability to predict morbidity or mortality after elective liver surgery [4, 5]. The P-POSSUM Score is now the most widely validated predictive scoring system used in perioperative care. P-POSSUM aims to calculate the risk of a surgical procedure based on the patient’s physiological condition and operative parameters. In hepato-biliary-pancreatic surgery the predictive value of P-POSSUM is under debate [3, 6, 7]. Previously, we suggested an easy applicable externally validated scoring system for 90-day mortality [8]. The aim of the current study is to compare its performance with already established scoring systems.
Methods
Data of 529 patients undergoing liver resection between 2014 and 2016 at the Department of General and Transplant Surgery, University Hospital Heidelberg were analysed. The institution’s liver resection database contained anonymous information for about 3447 cases performed between 2001 and 2018, which represented more than 99% of all liver resections performed in the department. Patients who underwent liver resection for hepatobiliary trauma as well as after liver transplantation and patients who underwent resections in conjunction with other operations (such as pancreaticoduodenectomy, laparoscopic resections or the deroofing of simple or parasitic cysts, cystectomy and necrosectomy) were excluded from the analysis. Resections performed in patients younger than 18 years of age were also excluded. Written informed consent was obtained by every patient included in the analysis and the study was approved by the ethical committee of the Medical Faculty of Ruprecht-Karls-University Heidelberg S-557/2017.
For all patients the previously suggested Mortality Risk Score, the labMELD Score and the P-POSSUM Score (PS, OS, P-POSSUM mortality %) were calculated.
As described previously the Mortality Risk Score was composed of 8 different parameters. One point was assigned for each of the following factors: patient’s age ≥ 60 years, the performance of right trisectionectomy, preoperative INR ≥ 1.1 and preoperative GGT values ≥60 U/l. Absence of these factors resulted in an assignment of 0 points. Two points were assigned for a preoperative platelet count value < 120/nl (preoperative platelet count values ≥120/nl, 0 points). Three points were assigned for a preoperative creatinine value ≥2 mg/dl (preoperative creatinine values < 2 mg/dl, 0 points). The histological diagnosis was stratified according to a 2-point scale; intrahepatic cholangiocarcinoma (1 point), perihilar cholangiocarcinoma (2 points) and all other diagnoses (0 points). ASA classification was stratified according to: ASA IV (5 points), ASA III (1 point) and ASA I or II (0 points). Based on this the patients could be divided into four different Risk Score Groups. Risk Score Group 1 (very low-risk, 0–1 points), Risk Score Group 2 (low-risk, 2–3 points), Risk Score Group 3 (medium-risk, 4–5 points), and Risk Score Group 4 (high-risk, ≥ 6 points) [8]. The labMELD Score was calculated using serum bilirubin, serum creatinine and International Normalized Ratio (INR) according to the following formula: LabMELD = 10x (0.957 x ln (serum creatinine mg/dl) + 0.378 x ln (serum bilirubin mg/dl) + 1.120 x ln (INR) + 0.643). Serum creatinine was capped at 4 mg/dl. For patients receiving dialysis at least 2 times in the previous week the creatinine value was set to 4 mg/dl. The value was rounded to the nearest integer. In order to avoid negative scores, the lower limit for each component of the score was set to 1. LabMELD Score values between 6 and 40 were possible [9–12]. The P-POSSUM equation for in-patient mortality was according to Prytherch et al.: ln (R/(1-R)) = − 9.065 + (0.1692 x physiological score) + (0.1550 x operative severity score). R is the predicted risk of mortality [13]. The score points of the physiological score and the operative severity score were awarded on the basis of the original research study of Copeland et al. [14] For the study population the 12 physiological parameters (age, cardiac history, respiratory history, systolic blood pressure, pulse rate, Glasgow Coma Scale, hemoglobin level, white cell count, urea concentration, sodium level, potassium level and electrocardiography) and the six operative parameters (operative severity, multiple procedures, total blood loss, peritoneal soiling, presence of malignancy and mode of surgery) were analysed and the physiological score (PS), operative severity score (OS) and P-POSSUM mortality % were calculated. For all patients the operative severity was set to major.
Statistical analysis
SAS software (Release 9.4, SAS Institute, Cary, NC, USA) was used for statistical data analysis. Categorical parameters were presented as absolute and relative frequencies. Differences between the 90-day mortality subgroup and the rest of the total cohort were analysed using Fisher’s exact test. Continuous parameters were expressed as the median, the interquartile range (IQR), the mean and the standard deviation (SD). With respect to the continuous parameters, comparisons between the 90-day mortality subgroup and the rest of the total cohort were performed using the Mann-Whitney U test. Logistic regression analyses were performed in order to examine the categorized Risk Score and the categorized MELD Score with respect to the 90-day mortality. Odds ratios (OR) with their corresponding 95% confidence intervals (CI) are presented here. The receiver operating characteristic (ROC) curves of the three scoring systems Mortality Risk Score, MELD Score, and P-POSSUM Score were computed and the areas under the curve (known as the C-index) were calculated using logistic regression models. Comparisons between the ROC curves were performed using the corresponding Wald tests from the underlying logistic regression models. Two-sided p-values of less than 0.05 were considered statistically significant.
Results
Patients’ characteristics and risk profile
Data of 529 patients undergoing liver resection between 2014 and 2016 at the Department of General and Transplant Surgery, University Hospital Heidelberg were analysed for internal validation of the previously reported risk score [8]. The median age was 61.8 years (IQR from 52.8–69.0) and 61.1% (n = 323) of cases were male and 38.9% (n = 206) female. American Society of Anesthesiologists (ASA) classification of ≥ III was found in 45.9% (n = 243) of cases. A BMI ≥ 30 kg/m2 was evident in 18.8% (n = 98). Major co-morbidities were: diabetes 17.6% (n = 93), cardiac disease 19.3% (n = 102), pulmonary disease 8.9% (n = 47) and renal disease 6.2% (n = 33). The further relevant factors for the risk model were the performance of right trisectionectomy (n = 39, 7.4%), intrahepatic cholangiocarcinoma (n = 59, 11.2%), perihilar cholangiocarcinoma (n = 35, 6.6%), preoperative INR ≥ 1.1 (n = 61, 11.5%), preoperative GGT ≥ 60 U/l (n = 285, 53.9%), preoperative platelet count value < 120/nl (n = 32, 6.0%), preoperative creatinine value ≥2 mg/dl (n = 7, 1.3%) and a total bilirubin ≥2 mg/dl (n = 33, 6.2%). Details of the study population and the p-values are shown in Table 1.
Table 1.
Score relevant patients’ characteristics
| Characteristics | Total Cohort (n = 529) | 90-day Mortality (n = 24, 4.54%) |
P-Value | ||
|---|---|---|---|---|---|
| Demographics | n | % | n | % | |
| Age (median; IQR) | 61.8 | (52.8–69.0) | 69.3 | (62.1–74.9) | 0.0049 |
| Male: Female | 323:206 | 61.1%:38.9% | 20:4 | 83.3%:16.7% | 0.0301 |
| Preoperative risk | |||||
| Diabetes mellitus | 93 | 17.6% | 8 | 33.3% | 0.0516 |
| Cardiac disease | 102 | 19.3% | 8 | 33.3% | 0.1064 |
| Renal disease | 33 | 6.2% | 2 | 8.3% | 0.6557 |
| Pulmonary disease | 47 | 8.9% | 1 | 4.2% | 0.7125 |
| ASA III,IV | 243 | 45.9% | 14 | 58.3% | 0.2945 |
| BMI ≥ 30 kg/m2a | 98 | 18.8% | 3 | 13.0% | 0.5939 |
| Tumour entity | |||||
| Intrahepatic cholangiocarcinoma | 59 | 11.2% | 2 | 8.3% | 1.0 |
| Perihilar cholangiocarcinoma | 35 | 6.6% | 8 | 33.3% | < 0.0001 |
| Surgical procedure | |||||
| Right trisectionectomy | 39 | 7.4% | 10 | 41.7% | < 0.0001 |
| Preoperative lab values | |||||
| INR ≥ 1.1 | 61 | 11.5% | 6 | 25.0% | 0.0466 |
| GGT ≥ 60 U/l | 285 | 53.9% | 22 | 91.7% | < 0.0001 |
| Thrombocytes < 120/nl | 32 | 6.0% | 4 | 16.7% | 0.0497 |
| Creatinine≥2 mg/dl | 7 | 1.3% | 1 | 4.2% | 0.2789 |
| Total Bilirubin≥2 mg/dl | 33 | 6.2% | 7 | 29.2% | 0.0003 |
a missing values n = 7
90-day mortality rate and internal validation
The 90-day mortality rate of the study population was 4.5% (n = 24). Internal validation of the risk model confirmed that the risk score points were predictive for a 90-day mortality rate with a C-index of 0.8421. The mortality rate was 0.5% for patients having 0 to 1 point (n = 1, very low-risk group 1, OR 1), 0.8% for those having 2 points (n = 1, low-risk group 2A, OR 0.50, 95% CI 0.03–3.98, p-value 0.5547), 6.1% for those having 3 points (n = 6, low-risk group 2B, OR 4.19, 95% CI 1.08–20.21, p-value 0.0459), 9.1% for those having 4 points (n = 5, medium-risk group 3A, OR 6.50, 95% CI 1.54–32.55, p-value 0.0123), 9.4% for those having 5 points (n = 3, medium-risk group 3B, OR 6.72, 95% CI 1.20–37.86, p-value 0.0234) and 40.0% for those having ≥6 points (n = 8, high-risk group 4, OR 43.33, 95% CI 9.87–234.14, p-value < 0.0001). Table 2.
Table 2.
90-day mortality rate and internal validation of Heidelberg Mortality Risk Score
| Mortality Risk Score Points | Risk Score Groups | N total | N 90-day mortality | % 90-day mortality | OR | 95% CI | p-value |
|---|---|---|---|---|---|---|---|
| 0 + 1 | 1 (very low-risk) | 193 | 1 | 0.5 | 1 | ||
| 2 | 2A (low-risk) | 130 | 1 | 0.8 | 0.50 | 0.03–3.98 | 0.5547 |
| 3 | 2B (low-risk) | 99 | 6 | 6.1 | 4.19 | 1.08–20.21 | 0.0459 |
| 4 | 3A (medium-risk) | 55 | 5 | 9.1 | 6.50 | 1.54–32.55 | 0.0123 |
| 5 | 3B (medium-risk) | 32 | 3 | 9.4 | 6.72 | 1.20–37.86 | 0.0234 |
| ≥6 | 4 (high-risk) | 20 | 8 | 40.0 | 43.33 | 9.87–234.14 | < 0.0001 |
OR Odds ratio CI Confidence interval
Comparison with labMELD score and P-POSSUM scores
The median labMELD Score of the study population of the 529 patients was 7.0 (IQR 6–8) and the mean labMELD Score was 7.5 (SD 2.7). The 90-day mortality rates according to the labMELD Score were as following: labMELD Score 6 1.7% (n = 4 of 236, OR1), labMELD Score 7 3.4% (n = 5 of 148, OR 2.03, 95% CI 0.53–8.31, p-value 0.2979), labMELD Score 8 7.0% (n = 4 of 57, OR 4.38, 95% CI 1.01–19.05, p-value 0.0412), labMELD Score 9 and 10 4.9% (n = 2 of 41, OR 2.97, 95% CI 0.40–15.78, p-value 0.2171), labMELD Score 11–14 14.3% (n = 4 of 28, OR 9.67, 95% CI 2.17–43.31, p-value 0.0021) and labMELD Score ≥ 15 26.3% (n = 5 of 19, OR 20.71, 95% CI 4.98–92.32, p-value < 0.0001). Table 3. The labMELD Score was predictive for a 90-day mortality rate with a C-index of 0.7352.
Table 3.
90-day mortality rate according to labMELD Score
| labMELD Score points | N total | N (%) 90-day mortality | OR | 95% CI | p-value |
|---|---|---|---|---|---|
| 6 | 236 | 4 (1.7) | 1 | ||
| 7 | 148 | 5 (3.4) | 2.03 | 0.53–8.31 | 0.2979 |
| 8 | 57 | 4 (7.0) | 4.38 | 1.01–19.05 | 0.0412 |
| 9 + 10 | 41 | 2 (4.9) | 2.97 | 0.40–15.78 | 0.2171 |
| 11–14 | 28 | 4 (14.3) | 9.67 | 2.17–43.31 | 0.0021 |
| ≥15 | 19 | 5 (26.3) | 20.71 | 4.98–92.32 | < 0.0001 |
OR Odds ratio CI Confidence interval
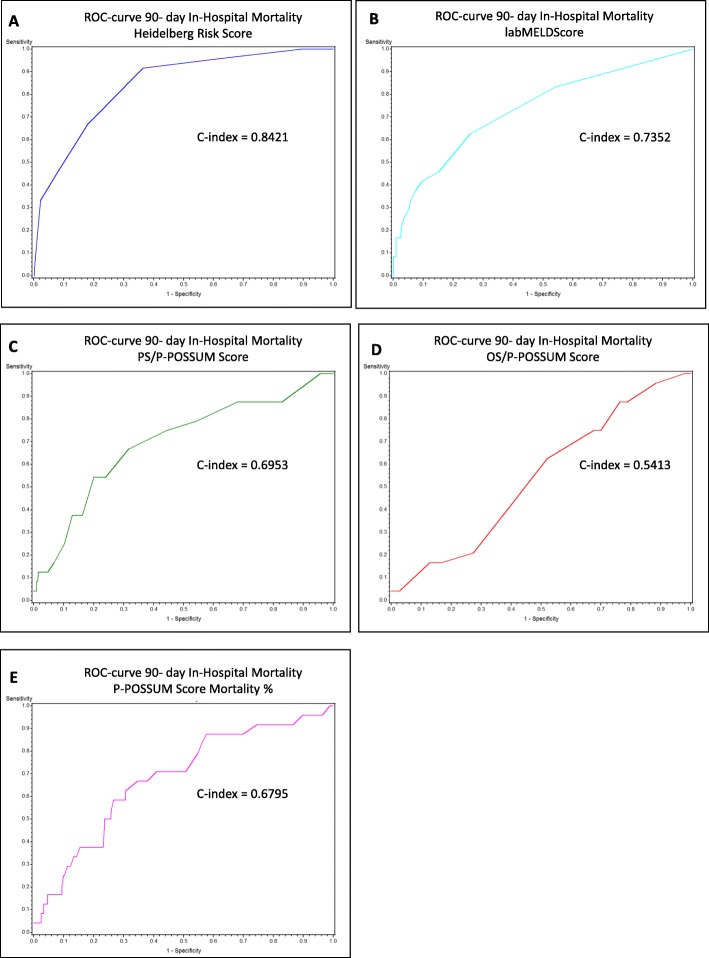
The P-POSSUM scoring system consists of two parts, the physiological score (PS) and the operative severity score (OS). The mortality rate is defined as in-patient mortality. The median PS was 16.0 (IQR 14–18.8) and the mean PS was 17.1 (SD 4.5). The median OS was 17.0 (IQR 13–19) and the mean OS was 16.2 (SD 4.2). The median mortality rate of the P-POSSUM scoring system amounted to 2.3% (IQR 1.4–4.4) and the mean mortality rate amounted to 4.3% (SD 7.3). The C-value for AUC for the prediction of in-patient mortality for the PS part of P-POSSUM scoring system was 0.6953, for the OS part 0.5413 and for the mortality rate of the P-POSSUM system 0.6795 Table 4 Fig. 1.
Table 4.
AUC value of the Mortality Risk Score compared to the AUC values of the labMELD Score and the P-POSSUM Scores
| Risk score | AUC value | 95% CI | p-value |
|---|---|---|---|
| Mortality Risk Score | 0.8421 | 0.7675–0.9167 | |
| labMELD Score | 0.7352 | 0.6252–0.8452 | 0.0163 |
| PS/P-POSSUM | 0.6953 | 0.5794–0.8112 | 0.0278 |
| OS/P-POSSUM | 0.5413 | 0.4348–0.6478 | < 0.0001 |
| P-POSSUM mortality % | 0.6795 | 0.5682–0.7908 | 0.0166 |
AUC Area under the curve CI Confidence interval
Fig. 1.
Receiver operating characteristic (ROC) curve obtained for (a) Mortality Risk Score; (b) labMELD Score; (c) PS/P-POSSUM Score; (d) OS/P-POSSUM Score and (e) P-POSSUM mortality % to predict the 90-day mortality rate in 529 patients after liver resection
Performance of labMELD score and P-POSSUM scores according to mortality risk score groups
The median labMELD Scores and mean labMELD Scores categorized according to Mortality Risk Score Groups were Risk Score Group 1 (very low-risk) n = 193 median labMELD 6.0 (IQR 6–7) and mean labMELD 6.5 (SD 0.8); Group 2A (low-risk) n = 130 median labMELD 7.0 (IQR 6–8) and mean labMELD 7.0 (SD 1.4); Group 2B (low-risk) n = 99 median labMELD 7.0 (IQR 6–8) and mean labMELD 7.7 (SD 2.4); Group 3A (medium-risk) n = 55 median labMELD 8.0 (IQR 7–9) and mean labMELD 8.4 (SD 2.8); Group 3B (medium-risk) n = 32 median labMELD 9.0 (IQR 7–13) and mean labMELD 10.6 (SD 4.0) and Group 4 (high-risk) n = 20 median labMELD 13.0 (IQR 8–17) and mean labMELD 13.4 (SD 6.1), p-value < 0.0001.
The median and mean PS categorized according to Mortality Risk Score Groups were Risk Score Group 1 (very low-risk) median PS 15.0 (IQR 13–17) and mean PS 15.4 (SD 2.8); Group 2A (low-risk) median PS 16.0 (IQR 14–18) and mean PS 16.8 (SD 3.7); Group 2B (low-risk) median PS 17.0 (IQR 15–21) and mean PS 19.1 (SD 6.2); Group 3A (medium-risk) median PS 17.0 (IQR 15–21) and mean PS 18.6 (SD 4.8); Group 3B (medium-risk) median PS 18.0 (IQR 16–23) and mean PS 19.2 (SD 5.2) and Group 4 (high-risk) median PS 19.0 (IQR 19–24) and mean PS 22.0 (SD 10.5), p-value < 0.0001. The median and mean OS categorized according to Mortality Risk Score Groups were Risk Score Group 1 (very low-risk) median OS 16.0 (IQR 12–19) and mean OS 16.0 (SD 4.1); Group 2A (low-risk) median OS 17.0 (IQR 15–19) and mean OS 16.7 (SD 3.9); Group 2B (low-risk) median OS 17.0 (IQR 13–19) and mean OS 16.8 (SD 4.5); Group 3A (medium-risk) median OS 16.0 (IQR 11–17) and mean OS 15.7 (SD 4.5); Group 3B (medium-risk) median OS 13.0 (IQR 11–17) and mean OS 14.2 (SD 4.2) and Group 4 (high-risk) median OS 17.0 (IQR 13–19) and mean OS 17.3 (SD 5.2), p-value 0.0132. The median and mean mortality rates of the P-POSSUM system were for Risk Score Group 1 (very low-risk) median mortality rate 1.7% (IQR 1.2–2.8) and mean mortality rate 2.9% (SD 4.5); for Risk Score Group 2A (low-risk) median mortality rate 2.4% (IQR 1.4–4.9) and mean mortality rate 4.0% (SD 5.3); for Risk Score Group 2B (low-risk) median mortality rate 3.3% (IQR 1.9–6.8) and mean mortality rate 6.6% (SD 11.3); for Risk Score Group 3A (medium-risk) median mortality rate 2.8% (IQR 1.1–5.6) and mean mortality rate 5.6% (SD 9.5); for Risk Score Group 3B (medium-risk) median mortality rate 1.7% (IQR 1.1–5.2) and mean mortality rate 5.0% (SD 7.6) and for Risk Score Group 4 (high-risk) median mortality rate 3.4% (IQR 2.4–5.3) and mean mortality rate 13.2% (SD 29.0), p-value < 0.0001. Table 5.
Table 5.
Comparison of different scoring systems according to the 90-day Mortality Risk Score groups
| Mortality Risk Score | labMELD Score | P-POSSUM % mortality | PS/P-POSSUM | OS/P-POSSUM | |||||
|---|---|---|---|---|---|---|---|---|---|
| Score Points | Score Group | Median Score (IQR) | Mean Score (SD) | Median Score (IQR) | Mean Score (SD) | Median Score (IQR) | Mean Score (SD) | Median Score (IQR) | Mean Score (SD) |
| 0 + 1 | 1 (very low-risk) | 6 (6–7) | 6.5 (0.8) | 1.7 (1.2–2.8) | 2.9 (4.5) | 15 (13–17) | 15.4 (2.8) | 16 (12–19) | 16.0 (4.1) |
| 2 | 2A (low-risk) | 7 (6–8) | 7.0 (1.4) | 2.4 (1.4–4.9) | 4.0 (5.3) | 16 (14–18) | 16.8 (3.7) | 17 (15–19) | 16.7 (3.9) |
| 3 | 2B (low-risk) | 7 (6–8) | 7.7 (2.4) | 3.3 (1.9–6.8) | 6.6 (11.3) | 17 (15–21) | 19.1 (6.2) | 17 (13–19) | 16.8 (4.5) |
| 4 | 3A (medium-risk) | 8 (7–9) | 8.4 (2.8) | 2.8 (1.1–5.6) | 5.6 (9.5) | 17 (15–21) | 18.6 (4.8) | 16 (11–17) | 15.7 (4.5) |
| 5 | 3B (medium-risk) | 9 (7–13) | 10.6 (4.0) | 1.7 (1.1–5.2) | 5.0 (7.6) | 18 (16–23) | 19.2 (5.2) | 13 (11–17) | 14.2 (4.2) |
| ≥6 | 4 (high-risk) | 13 (8–17) | 13.4 (6.1) | 3.4 (2.4–5.3) | 13.2 (29.0) | 19 (19–24) | 22.0 (10.5) | 17 (13–19) | 17.3 (5.2) |
| p-value | < 0.0001 | < 0.0001 | < 0.0001 | 0.0132 | |||||
Discussions
Selection criteria for hepatectomy and prediction of the individual mortality risk are debatable. This study aimed at evaluating prognostic models of postoperative mortality in patients undergoing hepatectomy. By accurately predicting the risk of 90-day mortality the Mortality Risk Score should be useful at the selection stage. Using only preoperatively available parameters it is easy applicable and does not need subjective judgement of patients’ condition.
Several risk assessment scores have been proposed for the prediction of outcomes after liver resection previously [15–24]. The labMELD and P-POSSUM scoring systems are well-established, frequently used and have been developed or validated in large patient cohorts [7, 9–14, 25–28]. The MELD Score showed heterogenic predictive power in varying cohorts of patients undergoing hepatectomy [29, 30]. The P-POSSUM scoring systems include patient’s physiological condition and operative parameters and were found suitable for predicting postoperative mortality after hepato-biliary-pancreatic surgery [6].
The current study identifies a precise prediction of the postoperative mortality risk by the previously suggested Mortality Risk Score compared to the labMELD and P-POSSUM scoring systems. Depending on the individual risk profile an increasing risk for 90-day mortality could be identified with high predictive power. In contrast, the labMELD Score showed quite heterogenic results with medium predictive power. One has to recognize that despite increasing score points the mortality risk was evaluated to be not linear increasing as one might have suspected. The predictive power of the P-POSSUM Score was low with the OS clearly underperforming.
When the labMELD Scores, the PS and OS as well as the whole P-POSSUM system were categorized according to the suggested Mortality Risk Score groups it could be demonstrated that the median labMELD as well as the median PS/P-POSSUM Score values increase gradually within the different risk groups and were able to dynamically reflect low risk to high risk patients. However, further studies are needed; especially on eastern populations.
In summary, the trend of expanding the pool of patients undergoing liver resection towards high risk surgical patients continues. The 90-day mortality rate improved over the past decade but still varies across cohorts. Overall, risk scores will never replace comprehensive preoperative assessment and proper patient selection criteria. However, they might help to estimate the individual risk profile and support planning of a risk adapted operation strategy including pre-treatment to enhance the functional liver reserve, staged resections or combined surgical and interventional radiological concepts to reduce the postoperative morbidity and mortality rates. This will not only have significant implications on the patients’ quality of life but also on healthcare-economics.
Conclusions
The Mortality Risk score shows a precise prediction of the risk of postoperative mortality after liver surgery and should be used at the selection stage. The well-established labMELD Score estimates the postoperative mortality risk with a medium predictive power, the standard P-POSSUM Scores fail in predicting the 90-day mortality after liver resection.
Acknowledgements
Not applicable.
Abbreviations
- ASA
American Society of Anesthesiologists
- AUC
Area under the curve
- BMI
Body Mass Index
- CI
Confidence interval
- E-PASS
Estimation of Physiologic Ability and Surgical Stress
- GGT
Gamma-Glutamyltransferase
- INR
International Normalized Ratio
- IQR
Interquartile range
- labMELD
Calculated Model for End-Stage Liver Disease
- MELD
Model for End-Stage Liver Disease
- ME-PASS
Modified Estimation of Physiologic Ability and Surgical Stress
- OR
Odds ratio
- OS
Operative severity score
- P-POSSUM
Portsmouth-Physiological and Operative Severity Score for the enUmeration of Mortality and morbidity
- PS
Physiological score
- ROC
Receiver operating characteristic
- SD
Standard deviation
Authors’ contributions
TK analysed and interpreted the patient data regarding the three scoring systems. UH designed and computed the statistical analysis. CS and MRS provided and analysed the data. KH designed the trial, analysed the data, wrote and edited the manuscript. AM and MWB edited and revised the manuscript. All authors have read and approved the manuscript.
Funding
Sources of financial support: none.
Availability of data and materials
The datasets used and/or analysed during the current study are available from the corresponding author on reasonable request.
Ethics approval and consent to participate
Written informed consent was obtained by every patient included in the analysis and the study was approved by the ethical committee of the Medical Faculty of Ruprecht-Karls-University Heidelberg S-557/2017.
Consent for publication
Not applicable.
Competing interests
The authors declare that they have no competing interests.
Footnotes
Publisher’s Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
References
- 1.Farges O, Vibert E, Cosse C, Pruvot FR, Le Treut YP, Scatton O, et al. “Surgeons' intuition” versus “prognostic models”: predicting the risk of liver resections. Ann Surg. 2014;260(5):923–928. doi: 10.1097/SLA.0000000000000961. [DOI] [PubMed] [Google Scholar]
- 2.Woodfield JC, Pettigrew RA, Plank LD, Landmann M, van Rij AM. Accuracy of the surgeons' clinical prediction of perioperative complications using a visual analog scale. World J Surg. 2007;31(10):1912–1920. doi: 10.1007/s00268-007-9178-0. [DOI] [PubMed] [Google Scholar]
- 3.Haga Y, Ikejiri K, Takeuchi H, Ikenaga M, Wada Y. Value of general surgical risk models for predicting postoperative liver failure and mortality following liver surgery. J Surg Oncol. 2012;106(7):898–904. doi: 10.1002/jso.23160. [DOI] [PubMed] [Google Scholar]
- 4.Ross SW, Seshadri R, Walters AL, Augenstein VA, Heniford BT, Iannitti DA, Martinie JB, Vrochides D, Swan RZ. Mortality in hepatectomy: model for end-stage liver disease as a predictor of death using the National Surgical Quality Improvement Program database. Surgery. 2016;159(3):777–792. doi: 10.1016/j.surg.2015.08.021. [DOI] [PubMed] [Google Scholar]
- 5.Schroeder RA, Marroquin CE, Bute BP, Khuri S, Henderson WG, Kuo PC. Predictive indices of morbidity and mortality after liver resection. Ann Surg. 2006;243(3):373–379. doi: 10.1097/01.sla.0000201483.95911.08. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Chen T, Wang H, Wang H, Song Y, Li X, Wang J. POSSUM and P-POSSUM as predictors of postoperative morbidity and mortality in patients undergoing hepato-biliary-pancreatic surgery: a meta-analysis. Ann Surg Oncol. 2013;20(8):2501–2510. doi: 10.1245/s10434-013-2893-x. [DOI] [PubMed] [Google Scholar]
- 7.Lam CM, Fan ST, Yuen AW, Law WL, Poon K. Validation of POSSUM scoring systems for audit of major hepatectomy. Br J Surg. 2004;91(4):450–454. doi: 10.1002/bjs.4515. [DOI] [PubMed] [Google Scholar]
- 8.Hoffmann K, Hinz U, Stravodimos C, Knoblich T, Schön MR, Büchler MW, Mehrabi A. Risk assessment for liver resection. Surgery. 2018;164(5):998–1005. doi: 10.1016/j.surg.2018.06.024. [DOI] [PubMed] [Google Scholar]
- 9.Kamath PS, Wiesner RH, Malinchoc M, Kremers W, Therneau TM, Kosberg CL, D'Amico G, Dickson ER, Kim WR. A model to predict survival in patients with end-stage liver disease. Hepatology. 2001;33(2):464–470. doi: 10.1053/jhep.2001.22172. [DOI] [PubMed] [Google Scholar]
- 10.Singal AK, Kamath PS. Model for end-stage liver disease. J Clin Exp Hepatol. 2013;3(1):50–60. doi: 10.1016/j.jceh.2012.11.002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Wiesner R, Edwards E, Freeman R, Harper A, Kim R, Kamath P, Kremers W, Lake J, Howard T, Merion RM, Wolfe RA, Krom R. Model for end-stage liver disease (MELD) and allocation of donor livers. Gastroenterology. 2003;124(1):91–96. doi: 10.1053/gast.2003.50016. [DOI] [PubMed] [Google Scholar]
- 12.Wiesner RH, McDiarmid SV, Kamath PS, Edwards EB, Malinchoc M, Kremers WK, Krom RA, Kim WR. MELD and PELD: application of survival models to liver allocation. Liver Transpl. 2001;7(7):567–580. doi: 10.1053/jlts.2001.25879. [DOI] [PubMed] [Google Scholar]
- 13.Prytherch DR, Whiteley MS, Higgins B, Weaver PC, Prout WG, Powell SJ. POSSUM and Portsmouth POSSUM for predicting mortality. Physiological and operative severity score for the enUmeration of mortality and morbidity. Br J Surg. 1998;85:1217–1220. doi: 10.1046/j.1365-2168.1998.00840.x. [DOI] [PubMed] [Google Scholar]
- 14.Copeland GP, Jones D, Walters M. POSSUM: a scoring system for surgical audit. Br J Surg. 1991;78(3):355–360. doi: 10.1002/bjs.1800780327. [DOI] [PubMed] [Google Scholar]
- 15.Mullen JT, Ribero D, Reddy SK, Donadon M, Zorzi D, Gautam S, et al. Hepatic insufficiency and mortality in 1,059 noncirrhotic patients undergoing major hepatectomy. J Am Coll Surg. 2007;204(5):854–862. doi: 10.1016/j.jamcollsurg.2006.12.032. [DOI] [PubMed] [Google Scholar]
- 16.Rahbari NN, Reissfelder C, Koch M, Elbers H, Striebel F, Büchler MW, et al. The predictive value of postoperative clinical risk scores for outcome after hepatic resection: a validation analysis in 807 patients. Ann Surg Oncol. 2011;18(13):3640–3649. doi: 10.1245/s10434-011-1829-6. [DOI] [PubMed] [Google Scholar]
- 17.Balzan S, Belghiti J, Farges O, Ogata S, Sauvanet A, Delefosse D, et al. The “50-50 criteria” on postoperative day 5: an accurate predictor of liver failure and death after hepatectomy. Ann Surg. 2005;242(6):824–828. doi: 10.1097/01.sla.0000189131.90876.9e. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Akamatsu N, Sugawara Y, Kanako J, Arita J, Sakamoto Y, Hasegawa K, et al. Low platelet counts and prolonged Prothrombin time early after operation predict the 90 days morbidity and mortality in living-donor liver transplantation. Ann Surg. 2017;265(1):166–172. doi: 10.1097/SLA.0000000000001634. [DOI] [PubMed] [Google Scholar]
- 19.Golriz M, Ghamarnejad O, Khajeh E, Sabagh M, Mieth M, Hoffmann K, et al. Preoperative thrombocytopenia may predict poor surgical outcome after extended hepatectomy. Can J Gastroenterol Hepatol. 2018;2018:1275720. doi: 10.1155/2018/1275720. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Mehrabi A, Golriz M, Khajeh E, Ghamarnejad O, Probst P, Fonouni H, et al. Meta-analysis of the prognostic role of perioperative platelet count in posthepatectomy liver failure and mortality. Br J Surg. 2018;105(10):1254–1261. doi: 10.1002/bjs.10906. [DOI] [PubMed] [Google Scholar]
- 21.Wang YY, Zhong JH, Su ZY, Huang JF, Lu SD, Xiang BD, et al. Albumin–bilirubin versus child–Pugh score as a predictor of outcome after liver resection for hepatocellular carcinoma. Br J Surg. 2016;103(6):725–734. doi: 10.1002/bjs.10095. [DOI] [PubMed] [Google Scholar]
- 22.Toyoda H, Kumada T, Tada T, Kaneoka Y, Maeda A. A laboratory marker, FIB-4 index, as a predictor for long-term outcomes of hepatocellular carcinoma patients after curative hepatic resection. Surgery. 2015;157(4):699–707. doi: 10.1016/j.surg.2014.10.022. [DOI] [PubMed] [Google Scholar]
- 23.Maithel SK, Kneuertz PJ, Kooby DA, Scoggins CR, Weber SM, Martin RC, II, et al. Importance of low preoperative platelet count in selecting patients for resection of hepatocellular carcinoma: a multi-institutional analysis. J Am Coll Surg. 2011;212(4):638–648. doi: 10.1016/j.jamcollsurg.2011.01.004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Dasari BV, Hodson J, Sutcliffe RP, Marudanayagam R, Roberts KJ, Abradelo M, et al. Developing and validating a preoperative risk score to predict 90-day mortality after liver resection. J Surg Oncol. 2019;119(4):472–478. doi: 10.1002/jso.25350. [DOI] [PubMed] [Google Scholar]
- 25.Botta F, Giannini E, Romagnoli P, Fasoli A, Malfatti F, Chiarbonello B, Testa E, Risso D, Colla G, Testa R. MELD scoring system is useful for predicting prognosis in patients with liver cirrhosis and is correlated with residual liver function: a European study. Gut. 2003;52:134–139. doi: 10.1136/gut.52.1.134. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Malinchoc M, Kamath PS, Gordon FD, Peine CJ, Rank J, ter Borg PC. A model to predict poor survival in patients undergoing transjugular intrahepatic portosystemic shunts. Hepatology. 2000;31(4):864–871. doi: 10.1053/he.2000.5852. [DOI] [PubMed] [Google Scholar]
- 27.Teh SH, Christein J, Donohue J, Que F, Kendrick M, Farnell M, Cha S, Kamath P, Kim R, Nagorney DM. Hepatic resection of hepatocellular carcinoma in patients with cirrhosis: model of end-stage liver disease (MELD) score predicts perioperative mortality. J Gastrointest Surg. 2005;9(9):1207–1215. doi: 10.1016/j.gassur.2005.09.008. [DOI] [PubMed] [Google Scholar]
- 28.Whiteley MS, Prytherch DR, Higgins B, Weaver PC, Prout WG. An evaluation of the POSSUM surgical scoring system. Br J Surg. 1996;83:812–815. doi: 10.1002/bjs.1800830628. [DOI] [PubMed] [Google Scholar]
- 29.Fromer M, Aloia T, Gaughan J, Atabek U, Spitz F. The utility of the MELD score in predicting mortality following liver resection for metastasis. Eur J Surg Oncol (EJSO) 2016;42(10):1568–1575. doi: 10.1016/j.ejso.2016.05.035. [DOI] [PubMed] [Google Scholar]
- 30.Delis SG, Bakoyiannis A, Biliatis I, Athanassiou K, Tassopoulos N, Dervenis C. Model for end-stage liver disease (MELD) score, as a prognostic factor for postoperative morbidity and mortality in cirrhotic patients, undergoing hepatectomy for hepatocellular carcinoma. HPB. 2009;11(4):351–357. doi: 10.1111/j.1477-2574.2009.00067.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Data Availability Statement
The datasets used and/or analysed during the current study are available from the corresponding author on reasonable request.