Abstract
Background
Shoot branching is an important trait of plants that allows them to adapt to environment changes. Strigolactones (SLs) are newly identified plant hormones that inhibit shoot branching in plants. The SL biosynthesis genes CCD7 (carotenoid cleavage dioxygenase 7) and CCD8 have been found to regulate branching in several herbaceous plants by taking advantage of their loss-of-function mutants. However, the role for CCD7 and CCD8 in shoot branching control in grapevine is still unknown due to the lack of corresponding mutants.
Results
Here we employed the CRISPR/Cas9 system to edit the VvCCD7 and VvCCD8 genes in the grape hybrid 41B. The 41B embryogenic cells can easily be transformed and used for regeneration of the corresponding transformed plants. Sequencing analysis revealed that gene editing has been used successfully to target both VvCCD genes in 41B embryogenic cells. After regeneration, six 41B plantlets were identified as transgenic plants carrying the CCD8-sgRNA expression cassette. Among these, four plants showed mutation in the target region and were selected as ccd8 mutants. These ccd8 mutants showed increased shoot branching compared to the corresponding wild-type plants. In addition, no off-target mutation was detected in the tested mutants at predicted off-target sites.
Conclusions
Our results underline the key role of VvCCD8 in the control of grapevine shoot branching.
Keywords: CRISPR/Cas9, CCD8, Mutant, Shoot branching, Strigolactone
Background
The control of shoot branching is an adaptive strategy that allows plants to optimize their growth to adapt to environmental changes. Shoot branching is determined by the number and outgrowth of axillary buds, and bud outgrowth contributes to the flexibility in branching [1]. Auxin and cytokinin are master regulators that control shoot branching in plants. Auxin was considered as inhibitor in bud outgrowth [2, 3], whereas cytokinin was found to promote this process [2]. However, the established hormone signaling pathways cannot fully explain the control of bud outgrowth [4], suggesting the existence of other regulators.
Strigolactones or their derivates (SLs) are newly identified plant hormones that suppress axillary bud outgrowth [5, 6]. SLs are a group of molecules synthesized from carotenoids. Two carotenoid cleavage dioxygenases, CCD7 and CCD8, have been shown to be required for SLs biosynthesis [6, 7]. CCD7 and CCD8 are also known as MORE AXILLARY BRANCHING3 (MAX3) and MAX4 in Arabidopsis [7, 8]. CCD7 and CCD8 orthologs have also been identified in the strigolactone biosynthetic pathway of several plant species, such as DWARF17 (D17) and D10 in rice [9–11], RAMOSUS5 (RMS5) and RMS1 in pea [8, 12] and DECREASED APICAL DOMINANCE3 (DAD3) and DAD1 in petunia [13, 14]. These orthologous proteins were found to be involved in branching control, and a highly branched phenotype has been reported in the corresponding loss-of-function mutants [15, 16]. Additionally, mutations in the α/β-fold hydrolase D14 that functions as a SL receptor in Arabidopsis and rice resulted in an increased shoot branching phenotype [17–19]. SLs inhibited bud outgrowth by increasing the expression of BRANCHED1 (BRC1), which encodes a bud outgrowth repressor [20–22]. Loss-of-function mutations in BRC1 affected bud outgrowth and resulted in increased shoot branching [20, 23]. Likewise, in poplar, knockdown of BRC1 affected shoot architecture [24].
Recently, SLs were proposed to control scion development in response to nitrogen availability in grafted grapevine plants [25]. Additionally, overexpression of grape CCD7 or CCD8 gene in Arabidopsis max3 or max4 mutants background partly reverted their phenotypes [25], suggesting a potential role for CCD7 and CCD8 in grapevine shoot branching. However, up to date, in grapevine no experimental evidence supporting the role of these two genes in the control of shoot branching exists. This role has therefore still to be demonstrated in grapevine. CRISPR/Cas9 (clustered regulatory interspaced short palindromic repeats/CRISPR-associated protein 9) system is a powerful tool for targeted mutagenesis that has been successfully applied in many plant species to achieve genome editing. In grape, this system was efficiently used to edit IdnDH (L-idonate dehydrogenase), PDS (phytoene desaturase), and VvWRKY52 genes [26–28]. This indicates that the CRISPR/Cas9 system can be used for precise genome editing in grapevine.
In this study, we used the CRISPR/Cas9 technology to edit the VvCCD7 and VvCCD8 genes in 41B grapevine rootstock, respectively. As 41B embryogenic cell transformation, selection and regeneration are easy to perform, these cells were chosen to perform gene editing experiments. After regeneration, four VvCCD8 knockout lines were obtained. The recovered ccd8 mutants exhibited increased shoot branching when compared to wild-type plants. Sanger sequencing results showed that VvCCD8 mutant plants carried the targeted mutations, and that no mutation occurred at the putative off-target sites. Altogether, these results underline the efficiency of grape genome editing and provide evidence that VvCCD8 plays a key role in the control of shoot branching in grapevine.
Results
Target design and CRISPR/Cas9 vector construction
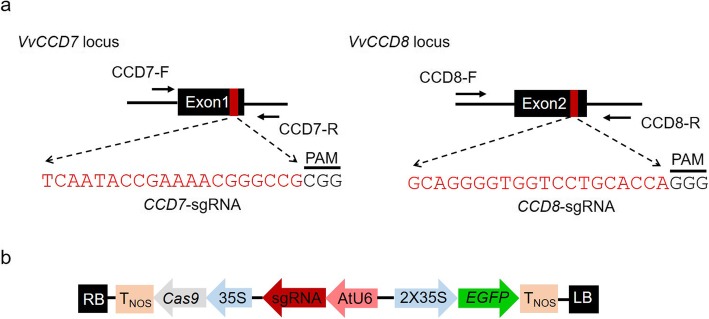
The VvCCD7 (VIT_15s0021g02190) and VvCCD8 (VIT_04s0008g03380) genes contain 6 and 5 exons, respectively. Considering that targeted mutagenesis caused by CRISPR/Cas9 generally resulted in frameshifts or generation of stop codons [26, 27], the upstream exons would be better targets for gene editing to produce non-functional proteins. Thus, the first exon (Exon1) of VvCCD7 and the second exon (Exon2) of VvCCD8 were selected as the targets for CRISPR-Cas9 gene editing, respectively (Fig. 1a). The target regions of these two genes were cloned and verified by Sanger sequencing prior to sgRNA design. The results showed that the amplified sequences of VvCCD7 and VvCCD8 are almost identical to their reference sequences (Additional file 1: Figure S1). The sgRNAs used for targeting VvCCD7 (CCD7-sgRNA) and VvCCD8 (CCD8-sgRNA) were designed accordingly (Fig. 1a). Both sgRNAs were driven by the Arabidopsis U6 promoter (AtU6), while the expression of Streptococcus pyogenes Cas9 was under the control of CaMV35S promoter (35S). The EGFP (enhanced green fluorescent protein) gene was used as a reporter gene to rapidly select efficiently transformed cells (Fig. 1b).
Fig. 1.
Schematic illustration of target design and the binary vector. a Schematic map of the target sites within VvCCD7 and VvCCD8 genes. The sequences of sgRNAs are indicated in red. CCD7-F/R and CCD8-F/R are primers used for PCR amplification. b Schematic diagram of the revised pCACRISPR/Cas9 vector. The EGFP reporter gene was used for rapid selection of transformed cells after transformation. 35S, CaMV35S promoter; AtU6, Arabidopsis small RNA U6 promoter; TNOS, nopaline synthase terminator; RB, right border; LB, left border
Targeted mutagenesis of VvCCD7 and VvCCD8 genes in 41B cells
The constructed CRISPR/Cas9 expression vectors were introduced into 41B grape cells by Agrobacterium-mediated transformation. Successfully transformed cells were selected by EGFP fluorescence, whereas no fluorescence signal could be detected in untransformed cells (Fig. 2a). The 41B cells exhibiting EGFP signal were sampled and subjected to Sanger sequencing in order to reveal the presence of mutations at the target sites. The sequencing chromatograms were manually analyzed for the presence of double tracing peaks at the target regions, considering that the presence of overlapping peaks was a typical indicator of targeted mutations [29]. Our sequencing results (Fig. 2b) revealed the presence of overlapping peaks in the positively transformed 41B cells by contrast to the single peaks of wild-type (WT) cells chromatograms. These results clearly indicate the existence of targeted mutagenesis in VvCCD7 and VvCCD8 genes in transformed 41B cells.
Fig. 2.
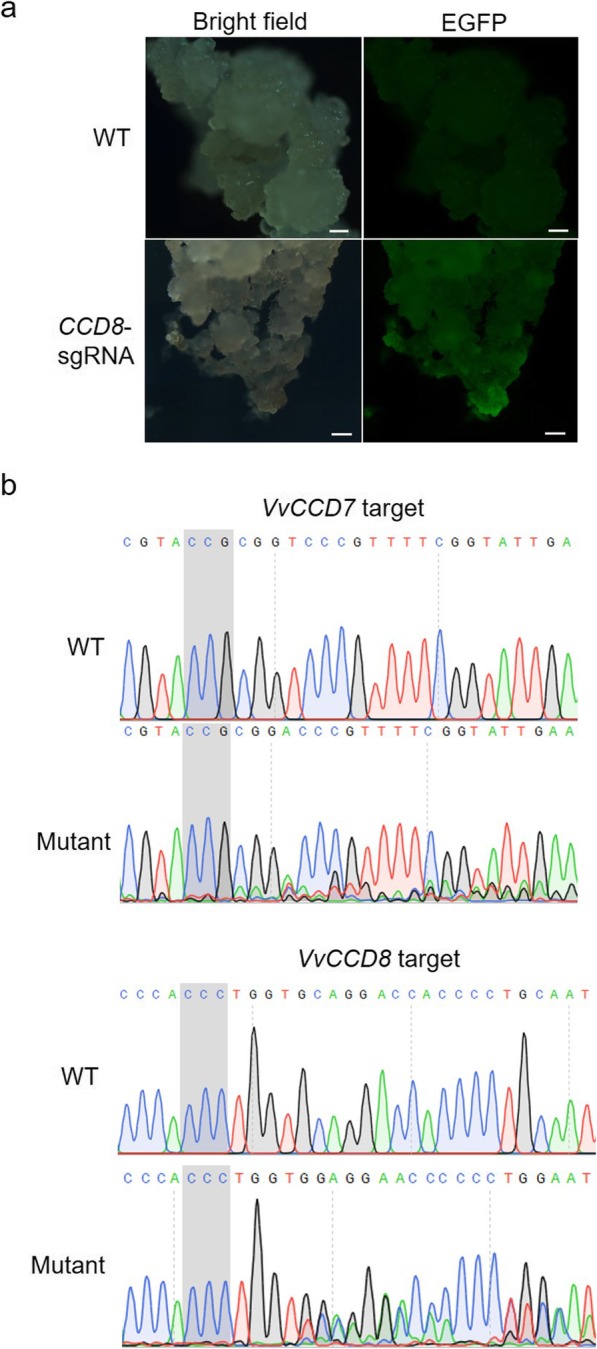
Detection of mutagenesis in transformed 41B cells. a Detection of EGFP signal in 41B cells. The cells transformed with CCD8-sgRNA expression construct was given as an example. Those cells with EGFP signal were considered as transformed cells and were used for subsequent analysis. Scale bars: 100 μm. b Sanger sequencing results of the target sites in VvCCD7 and VvCCD8 genes in transformed 41B cells. The wild-type sequences generated clear sequencing chromatograms, whereas the mutated sequences generated overlapping peaks at the mutation sites. The PAM sequences adjacent to CCD7-sgRNA and CCD8-sgRNA are shadowed
VvCCD8 knockout lines show increased shoot branching phenotype
The EGFP-fluorescent 41B cells were used for plant regeneration. A number of 24 and 73 regenerated plants were obtained for CCD7-sgRNA and CCD8-sgRNA, respectively (Fig. 3a). The recovered plants were selected by PCR using Cas9-specific primers (Additional file 4: Table S1). The PCR results showed that among CCD8-sgRNA regenerated plants 6 plants contained the exogenous Cas9 gene (Fig. 3b), indicating a transformation rate of 8.2% (6/73). By contrast, none of the 24 CCD7-sgRNA selected plants presented the exogenous Cas9 gene (Fig. 3a). Among the 6 CCD8-sgRNA plants, four (Plant #1, Plant #3, Plant #5 and Plant #6) were identified as ccd8 mutants (Fig. 3a). Interestingly, all ccd8 mutants showed increased shoot branching, with Plant #3 and Plant #6 containing 4 shoots, Plant #1 containing 3 shoots, and Plant #5 containing 2 shoots (Fig. 3c and d). In these mutant plants, VvCCD8 target sequences were analyzed by Sanger sequencing. Twenty clones of PCR amplicons were sequenced for each mutant plant. The results showed that Plant #1 and Plant #3 contained two types of mutations at the target site. The first one corresponds to an insertion of one nucleotide and the second one to a deletion of several nucleotides (20 bp for Plant #1 and 11 bp for Plant #3) (Fig. 3e). These results suggest that these two mutant plants might be biallelic. According to the sequencing results, Plant #5 and Plant #6 only contained one type of mutation (Fig. 3e). Plant #6 mutant might be homozygous as almost all its sequenced clones (19/20) contained the same mutation (1-bp insertion). By contrast, Plant #5 mutant might be heterozygote or chimeric as both wild-type and mutated (1-bp deletion) sequences were identified within the sequenced clones (Fig. 3e). These different mutations led to frameshifts changes, resulting in new mutated amino acid sequences (Fig. 3f) or in the production of stop codons (Fig. 3f) that would result in premature termination of translation.
Fig. 3.

Identification of VvCCD8 knockout mutants. a Overview of identification of regenerated plants. b Identification of exogenous T-DNA insertions in regenerated plants by PCR. The specific primers designed for Cas9 gene were used for PCR identification. Only CCD8-sgRNA plants were identified with exogenous T-DNA insertions. Lanes 1–6 represent different individual CCD8-sgRNA plants. The plasmid was used as the positive control (P), while the wild-type genomic DNA was used as the negative control (N). M, DNA marker. The cropped gel image is shown here, and the original, uncropped image is available in Additional file 3: Figure S3. c Phenotypes of VvCCD8 knockout mutants. The shoot branches of VvCCD8 knockout mutants were indicated in black arrows. Scale bars: 0.5 cm. d The branch number of the four VvCCD8 knockout mutants. e Sequencing results of the target sites in the four VvCCD8 knockout mutants. The gene fragments were amplified from each mutant plant and were cloned into pLB vector for Sanger sequencing assay. A number of 20 clonal amplicons for each plant were analyzed. The mutated sequences identified from the mutants were shown. The plant IDs are shown on the left. Mutation types (colored in red) and the corresponding number (indicated in black) of clones were shown on the right. Those undesired sequences were omitted from the analysis. f Mutations of amino acids in mutated sequences shown in e. The altered amino acids are colored in red and the premature stop codons are indicated in red asterisks (*). The number of amino acids (aa) that are not shown in the figure is indicated in parentheses
We also investigated the expression profiles of VvCCD8 in these mutant plants. The results showed that the transcript abundance of VvCCD8 in the four mutants was significantly decreased in comparison with wild-type plants (Fig. 4), suggesting that the targeted mutagenesis observed in VvCCD8 resulted in transcript decay in these ccd8 mutants.
Fig. 4.
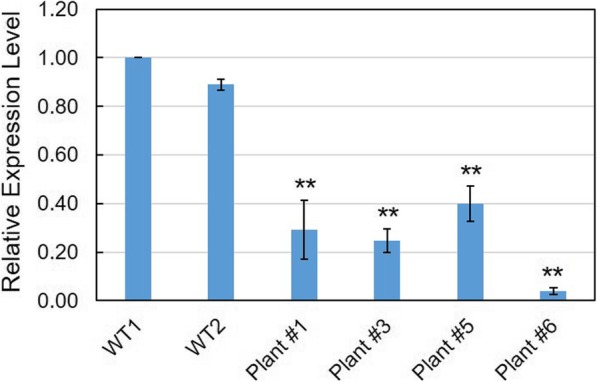
Expression profiles of VvCCD8 in ccd8 mutant plants. The expression of VvCCD8 was determined by quantitative real-time PCR, and the Actin 1 (accession no. AY680701) was used as internal control. The experiment was repeated three times and the data are shown as mean ± SD. The significance of differential expression level was examined by Student’s t-test with P < 0.01 indicating highly significant (**)
Off-target effect was not detected in ccd8 mutants
To make sure that the enhanced shoot branching phenotype observed in ccd8 mutants was not due to off-target effects, we investigated the putative genomic off-target loci of CCD8-sgRNA in Plant #1 and Plant #6. Putative off-target sites were predicted according to their sequence homology with CCD8-sgRNA. Among the 5 highest ranked potential off-target sites, 2 sites were predicted to localize in the exons of VIT_03s0091g00830 and VIT_13s0019g01150 sequences (Additional file 5: Table S2). These two putative off-target sites were therefore selected for further analysis. DNA fragments containing the 2 putative off-target sites were amplified from Plant #1 and Plant #6 by PCR using specific primers (Additional file 4: Table S1). PCR products were cloned into pLB vector and verified by Sanger sequencing. No mutation was detected at the potential off-target sites (Additional file 2: Figure S2), supporting the fact that VvCCD8 editing was efficient in grapevine and that the increased branching phenotype observed in ccd8 mutants was specifically due to the mutation in VvCCD8 and not to off-target effects.
Discussion
The CRISPR/Cas9 system has emerged as a powerful tool for genome editing, and it shows great potential in generating mutants in plants. In Arabidopsis, CRISPR/Cas9 system was successfully used to produce cbfs mutants. The characterization of these mutants revealed the important role played by CBF2 in cold acclimation-dependent freezing [30]. Intriguingly, targeted mutagenesis of SBP-CNR and NAC-NOR transcription factors, which are thought to be master regulators of tomato ripening, resulted in tomato delayed ripening or partial non-ripening. This phenotype was surprisingly distinct from the previously used original tomato mutants [31], suggesting a great potential of CRISPR/Cas9 system in gene functional research. SLs were found to retard bud outgrowth [5, 6], and SL biosynthetic genes CCD7 (MAX3) and CCD8 (MAX4) had been demonstrated to be involved in branching control in multiple herbaceous plants, including Arabidopsis, rice and tomato [8, 32–34]. In woody plant, poplar MAX4 (CCD8) knockdown lines exhibited altered branching patterns [24]. In grape, it has been suggested that SL could be involved in the control of scion architecture in grafted grapevine plants, based on the observation that exudate from grape CCDs-overexpressing transgenic cells could stimulate the germination of Phelipanche ramosa seeds, and that overexpression of grape CCD7 or CCD8 gene in corresponding Arabidopsis mutant can partly revert the mutant phenotype [25]. However, no direct and clear evidence supporting this role exists in grapevine.
In the present study, we employed the CRISPR/Cas9 system to edit VvCCD7 and VvCCD8 genes in grapevine (41B rootstock). After transformation of 41B embryogenic cells, Sanger sequencing assay was performed to detect the targeted mutations. The results showed that the designed sgRNAs could effectively direct the targeted editing in both genes (Fig. 2b). The whole plants were obtained through regeneration, and transgenic plants were identified and selected by PCR. Only 6 CCD8-sgRNA plants were found to contain exogenous Cas9 gene (Fig. 3b). The lack of antibiotics-dependent selection and low regeneration rate of 41B cells in this experiment are probably responsible for low rate of transgenic plants obtained. Among the 6 transgenic plants, 4 were identified as ccd8 mutants (Fig. 3a). As expected, the ccd8 mutants exhibited increased shoot branching, which is in agreement with previous reports [13, 15, 16, 24]. Except for Plant #5, all mutants had at least 3 shoots, whereas WT plants generally had only one shoot (Fig. 3d). According to the sequencing results, Plant #5 might be heterozygous or chimeric (Fig. 3e), suggesting a possible relationship between the number of shoots and SLs concentration in grapevine. Off-target effect is a major concern when applying CRISPR/Cas9 technology. We therefore investigated the putative genomic off-target loci of CCD8-sgRNA, and no off-target mutation was observed (Additional file 2: Figure S2). These results ruled out the possibility that the altered shoot branching observed in ccd8 mutants was caused by the presence of off-target mutations.
Interestingly, the ccd8 mutants obtain with 41B rootstock could serve for grafting experiments in order to further study the role of SLs in the control of grapevine shoot branching. Finally, whether VvCCD7 plays a same role in shoot branching is remains unclear and has yet to be investigated. To go further in this direction, new attempt to regenerate ccd7 mutant plants could be achieved in the future.
Conclusions
Collectively, our results showed that CRISPR/Cas9 system can be successfully used to knock out VvCCD7 and VvCCD8 genes in grape. Additionally, the study of VvCCD8 knockout grapevine plants revealed the key role of this gene in the control of shoot branching, therefore providing a first clue to investigate the mechanisms involved in the regulation of shoot architecture in grapevine.
Methods
Design of sgRNA and construction of genome editing vectors
The target regions of VvCCD7 and VvCCD8 genes were amplified from 41B embryogenic cells by PCR with primers CCD7-F/R and CCD8-F/R, respectively. The amplified fragments were verified by Sanger sequencing. The verified sequences were used as an input for sgRNA design with the online tool CRISPR-P v2.0 [35]. The potential off-target sites were predicted simultaneously with this tool. The designed sgRNAs were then ligated into the pCACRISPR/Cas9 vector via homologous recombination (HR). PCR cloning, sgRNA design and plasmid construction were conducted as previously described [26]. The pCACRISPR/Cas9 vector was digested with SmaI and XhoI to remove the hptII (hygromycin phosphotransferase II) gene, and the EGFP gene (NCBI accession: NC_025025) amplified from pCAMBIA2300-EGFP vector was inserted into the linearized pCACRISPR/Cas9 vector via HR using the ClonExpress II One Step Cloning Kit (Vazyme, China). The primers used in the experiment are available in Additional file 4: Table S1.
Plant material, transformation and regeneration
The embryogenic grape cells derived from 41B rootstock (Vitis vinifera cv. Chasselas × Vitis berlandieri) were graciously provided by Dr. F. Lecourieux (EGFV, Université de Bordeaux), and the cells were cultured as previously described [36]. In brief, the suspension cells were subcultured weekly in 25 mL of liquid glycerol-maltose (GM) medium containing 1 mg L− 1 naphthoxy acetic acid (NOA) in the dark.
The constructed binary vectors were introduced into Agrobacterium tumefaciens strain EHA105 by the freeze-thaw method, and the 41B embryogenic cells were transformed using the A. tumefaciens co-cultivation method [37]. After co-cultivation, the grape cells were first washed twice with liquid GM medium and then subcultured every other day in GM medium supplemented with 200 mg/L timentin for 1 week. Then the cells were collected and divided into small groups (~ 0.5 cm2) for EGFP detection.
For induction of embryogenesis, 41B cells were transferred onto solid hormone-free regeneration medium (GM medium without NOA) under a 16-h photoperiod with white fluorescent lights. Plants regenerated on McCown woody plant medium (Duchefa) supplemented with 3% sucrose, 0.2 mg/L naphthalene acetic acid (NAA), 0.5 mg/L activated charcoal, 7.5 g/L agar under long-day (16 h light/8 h dark) conditions.
Extraction of genomic DNA and PCR identification of exogenous T-DNA insertion
Genomic DNA was prepared using the CTAB plant genomic DNA extraction kit (Aidlab, China) according to the manufacturer’s instructions. The isolated DNA was used as the template for PCR. The PCR reaction was performed with Cas9-specific primers (Additional file 4: Table S1) using Es Taq DNA polymerase (CWBIO, China) according to the manufacturer’s protocol. The PCR products were detected by 1% agarose gel electrophoresis and were further confirmed by Sanger sequencing.
Sanger sequencing assay
The DNA fragments containing the target sites were amplified from 41B cells or regenerated plants by PCR with the primers CCD7-F/R and CCD8-F/R, respectively. The PCR products amplified from grape cells were purified and directly used for Sanger sequencing analysis (Tsingke, Beijing). The amplified fragments from 41B plants were cloned into pLB-Simple vector (TIANGEN, China), and a total of 20 clones for each sample were sequenced.
Quantitative real-time PCR assay
The expression profiles of VvCCD8 gene were investigated using quantitative real-time PCR (qRT-PCR) with VvCCD8 specific primers (Additional file 4: Table S1). The grape Actin 1 (accession no. AY680701) was used as internal control, and the relative expression level was determined using the 2−ΔΔCT method [38]. The qRT-PCR assay was performed as previously reported [26].
Off-target analysis
Off-target analysis was performed in VvCCD8 knockout lines. Two top-ranking putative off-target sites that localize in gene exons were chosen for off-target analysis. The potential off-target regions were amplified using their specific primers (Additional file 4: Table S1), and the fragments were cloned into pLB vector and at least 6 clones were analyzed by Sanger sequencing.
EGFP detection
The EGFP signal was detected using the Eclipse Ni-U fluorescence microscope (Nikon, Japan) with excitation at 487 nm, emission at 505 nm. The wild-type cells were used as the negative control.
Supplementary information
Additional file 1: Figure S1. Sequencing results of VvCCD7 and VvCCD8 fragments amplified from 41B cells. a The target sequence of VvCCD7 gene in 41B. b The target sequence of VvCCD8 gene in 41B. The target sites in VvCCD7 and VvCCD8 genes are highlighted in dark blue.
Additional file 2: Figure S2. Sequencing results of the two putative off-target sites in VvCCD8 knockout lines. The two off-target sites predicted within exons of other genes were selected for off-target analysis. Two VvCCD8 knockout lines, Plant #1 and Plant #6 were used in the experiment. The amplified fragments containing the off-target sites were amplified and cloned into pLB-Simple vector. At least 6 clones for each site were used for Sanger sequencing.
Additional file 3: Figure S3. The original gel image of PCR identification of T-DNA insertions in CCD8-sgRNA plants. The vector plasmid (P1) and the transgenic cells (P2) were used as the positive controls, while wild-type plant was used as the negative control (N). Lanes 1–6 represent individual CCD8-sgRNA plants.
Additional file 4: Table S1. List of primers used in this study.
Additional file 5: Table S2. Putative off-target sites predicted for CCD8-sgRNA.
Acknowledgements
Not applicable.
Abbreviations
- CCD
Carotenoid cleavage dioxygenase
- CRISPR/Cas9
clustered regulatory interspaced short palindromic repeats/CRISPR-associated protein 9
- EGFP
Enhanced green fluorescent protein
- GM
Glycerol-maltose medium
- hptII
hygromycin phosphotransferase II
- MAX
More axillary branching
- NAA
Naphthalene acetic acid
- NOA
Naphthoxy acetic acid
- qRT-PCR
Quantitative real-time PCR
- sgRNA
Single guide RNA
- SLs
Strigolactones
Authors’ contributions
CR and ZL conceived and designed the experiments. CR, YG and JK conducted the experiments. CR, FL and ZD wrote the manuscript. FL, SL and ZL critically read and revised the manuscript. All authors read and approved the final manuscript.
Funding
This work was funded by the major science and technology program of NingxiaHui Autonomous region (Grant No. 2016BZ06), the National Science Foundation of China (Grant No. 31772266), STS project of Chinese Academy of Sciences (Grant No. KFJ-STS-ZDTP-025) and the Agricultural Breeding Project of Ningxia Hui Autonomous Region (Grant No. NXNYYZ20150203). The funders had no role in the design of the study and collection, analysis, and interpretation of data and in writing the manuscript.
Availability of data and materials
The datasets supporting the conclusions of this article and materials used in this study are available by contacting with the corresponding author (zl249@ibcas.ac.cn).
Ethics approval and consent to participate
Not applicable.
Consent for publication
Not applicable.
Competing interests
The authors declare that they have no competing interests.
Footnotes
Publisher’s Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Contributor Information
Chong Ren, Email: rcarthur@126.com.
Yuchen Guo, Email: GYC1477570428@163.com.
Junhua Kong, Email: kjhua1203@163.com.
Fatma Lecourieux, Email: fatma.lecourieux@bordeaux.inra.fr.
Zhanwu Dai, Email: zhanwu.dai@ibcas.ac.cn.
Shaohua Li, Email: shhli@ibcas.ac.cn.
Zhenchang Liang, Email: zl249@ibcas.ac.cn.
Supplementary information
Supplementary information accompanies this paper at 10.1186/s12870-020-2263-3.
References
- 1.Liang J, Zhao L, Challis R, Leyser O. Strigolactone regulation of shoot branching in chrysanthemum (Dendranthema grandiflorum) J Exp Bot. 2010;61:3069–3078. doi: 10.1093/jxb/erq133. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Sachs T, Thimann V. Role of auxins and cytokinins in release of buds from dominance. Am J Bot. 1967;54:136–144. doi: 10.1002/j.1537-2197.1967.tb06901.x. [DOI] [Google Scholar]
- 3.Nordstrom A, Tarkowski P, Tarkowska D, Norbaek R, Astot C, Dolezal K, Sandberg G. Auxin regulation of cytokinin biosynthesis in Arabidopsis thaliana: a factor of potential importance for auxin-cytokinin-regulated development. Proc Natl Acad Sci U S A. 2004;101:8039–8044. doi: 10.1073/pnas.0402504101. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Mueller D, Waldie T, Miyawaki K, To JPC, Melnyk CW, Kieber JJ, Kakimoto T, Leyser O. Cytokinin is required for escape but not release from auxin mediated apical dominance. Plant J. 2015;82:874–886. doi: 10.1111/tpj.12862. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Gomez-Roldan V, Fermas S, Brewer PB, Puech-Pages V, Dun EA, Pillot JP, Letisse F, Matusova R, Danoun S, Portais JC, et al. Strigolactone inhibition of shoot branching. Nature. 2008;455:189–194. doi: 10.1038/nature07271. [DOI] [PubMed] [Google Scholar]
- 6.Umehara M, Hanada A, Yoshida S, Akiyama K, Arite T, Takeda-Kamiya N, Magome H, Kamiya Y, Shirasu K, Yoneyama K, et al. Inhibition of shoot branching by new terpenoid plant hormones. Nature. 2008;455:195–200. doi: 10.1038/nature07272. [DOI] [PubMed] [Google Scholar]
- 7.Booker J, Auldridge M, Wills S, McCarty D, Klee H, Leyser O. MAX3/CCD7 is a carotenoid cleavage dioxygenase required for the synthesis of a novel plant signaling molecule. Curr Biol. 2004;14:1232–1238. doi: 10.1016/j.cub.2004.06.061. [DOI] [PubMed] [Google Scholar]
- 8.Sorefan K, Booker J, Haurogne K, Goussot M, Bainbridge K, Foo E, Chatfield S, Ward S, Beveridge C, Rameau C, et al. MAX4 and RMS1 are orthologous dioxygenase-like genes that regulate shoot branching in Arabidopsis and pea. Genes Dev. 2003;17:1469–1474. doi: 10.1101/gad.256603. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Ishikawa S, Maekawa M, Arite T, Onishi K, Takamure I, Kyozuka J. Suppression of tiller bud activity in tillering dwarf mutants of rice. Plant Cell Physiol. 2005;46:79–86. doi: 10.1093/pcp/pci022. [DOI] [PubMed] [Google Scholar]
- 10.Arite T, Iwata H, Ohshima K, Maekawa M, Nakajima M, Kojima M, Sakakibara H, Kyozuka J. DWARF10, an RMS1/MAX4/DAD1 ortholog, controls lateral bud outgrowth in rice. Plant J. 2007;51:1019–1029. doi: 10.1111/j.1365-313X.2007.03210.x. [DOI] [PubMed] [Google Scholar]
- 11.Alder A, Jamil M, Marzorati M, Bruno M, Vermathen M, Bigler P, Ghisla S, Bouwmeester H, Beyer P, Al-Babili S. The path from beta-carotene to carlactone, a strigolactone-like plant hormone. Science. 2012;335:1348–1351. doi: 10.1126/science.1218094. [DOI] [PubMed] [Google Scholar]
- 12.Johnson X, Brcich T, Dun EA, Goussot M, Haurogne K, Beveridge CA, Rameau C. 2006. Branching genes are conserved across species. Genes controlling a novel signal in pea are coregulated by other long-distance signals. Plant Physiol. 2006;142:1014–1026. doi: 10.1104/pp.106.087676. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Snowden KC, Simkin AJ, Janssen BJ, Templeton KR, Loucas HM, Simons JL, Karunairetnam S, Gleave AP, Clark DG, Klee HJ. The decreased apical dominance 1/Petunia hybrida CAROTENOID CLEAVAGE DIOXYGENASE8 gene affects branch production and plays a role in leaf senescence, root growth, and flower development. Plant Cell. 2005;17:746–759. doi: 10.1105/tpc.104.027714. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Drummond RSM, Martinez-Sanchez NM, Janssen BJ, Templeton KR, Simons JL, Quinn BD, Karunairetnam S, Snowden KC. Petunia hybrid CAROTENOID CLEAVAGE DIOXYGENASE7 is involved in the production of negative and positive branching signals in Petunia. Plant Physiol. 2009;151:1867–1877. doi: 10.1104/pp.109.146720. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Dun EA, Ferguson BJ, Beveridge CA. Apical dominance and shoot branching. Divergent opinions or divergent mechanisms? Plant Physiol. 2006;142:812–819. doi: 10.1104/pp.106.086868. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Leyser O. The control of shoot branching: an example of plant information processing. Plant Cell Environ. 2009;32:694–703. doi: 10.1111/j.1365-3040.2009.01930.x. [DOI] [PubMed] [Google Scholar]
- 17.Arite T, Umehara M, Ishikawa S, Hanada A, Maekawa M, Yamaguchi S, Kyozuka J. d14, a strigolactone-insensitive mutant of rice, shows an accelerated outgrowth of tillers. Plant Cell Physiol. 2009;50:1416–1424. doi: 10.1093/pcp/pcp091. [DOI] [PubMed] [Google Scholar]
- 18.Hamiaux C, Drummond RSM, Janssen BJ, Ledger SE, Cooney JM, Newcomb RD, Snowden KC. DAD2 is an alpha/beta hydrolase likely to be involved in the perception ofthe plant branching hormone, strigolactone. Curr Biol. 2012;22:2032–2036. doi: 10.1016/j.cub.2012.08.007. [DOI] [PubMed] [Google Scholar]
- 19.Waters MT, Nelson DC, Scaffidi A, Flematti GR, Sun YK, Dixon KW, Smith SM. Specialisation within the DWARF14 protein family confers distinct responses to karrikins and strigolactones in Arabidopsis. Development. 2012;139:1285–1295. doi: 10.1242/dev.074567. [DOI] [PubMed] [Google Scholar]
- 20.Aguilar-Martinez JA, Poza-Carrion C, Cubas P. Arabidopsis BRANCHED1 acts as an integrator ofbranching signals within axillary buds. Plant Cell. 2007;19:458–472. doi: 10.1105/tpc.106.048934. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Braun N, de Saint GA, Pillot J-P, Boutet-Mercey S, Dalmais M, Antoniadi I, Li X, Maia-Grondard A, Le Signor C, Bouteiller N, et al. The pea TCP transcription factor PsBRC1 acts downstream of strigolactones to control shoot branching. Plant Physiol. 2012;158:225–238. doi: 10.1104/pp.111.182725. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Dun EA, de Saint GA, Rameau C, Beveridge CA. Antagonistic action of strigolactone and cytokinin in bud outgrowth control. Plant Physiol. 2012;158:487–498. doi: 10.1104/pp.111.186783. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Finlayson SA. Arabidopsis TEOSINTE BRANCHED1-LIKE 1 regulates axillary bud outgrowth and is homologous to monocot TEOSINTE BRANCHED1. Plant Cell Physiol. 2007;48:667–677. doi: 10.1093/pcp/pcm044. [DOI] [PubMed] [Google Scholar]
- 24.Muhr M, Prufer N, Paulat M, Teichmann T. Knockdown of strigolactone biosynthesis genes in Populus affects BRANCHED1 expression and shoot architecture. New Phytol. 2016;212:613–626. doi: 10.1111/nph.14076. [DOI] [PubMed] [Google Scholar]
- 25.Cochetel N, Météier E, Merlin I, Hévin C, Pouvreau JB, Coutos-Thévenot P, Hernould M, Vivin P, Cookson SJ, Ollat N, et al. Potential contribution of strigolactones in regulating scion growth and branching in grafted grapevine in response to nitrogen availability. J Exp Bot. 2018;69:4099–4112. doi: 10.1093/jxb/ery206. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Ren C, Liu X, Zhang Z, Wang Y, Duan W, Li S, Liang Z. CRISPR/Cas9-mediated efficient targeted mutagenesis in Chardonnay (Vitis vinifera L.) Sci Rep. 2016;6:32289. doi: 10.1038/srep32289. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Nakajima I, Ban Y, Azuma A, Onoue N, Moriguchi T, Yamamoto T, Toki S, Endo M. CRISPR/Cas9-mediated targeted mutagenesis in grape. PLoS One. 2017;12:e0177966. doi: 10.1371/journal.pone.0177966. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Wang X, Tu M, Wang D, Liu J, Li Y, Li Z, Wang Y, Wang X. CRISPR/Cas9-mediated efficient targeted mutagenesis in grape in the first generation. Plant Biotechnol J. 2018;16:844–855. doi: 10.1111/pbi.12832. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Ma X, Chen L, Zu Q, Chen Y, Liu YG. Rapid decoding of sequence-specific nuclease-induced heterozygous and biallelic mutations by direct sequencing of PCR products. Mol Plant. 2015;8:1285–1287. doi: 10.1016/j.molp.2015.02.012. [DOI] [PubMed] [Google Scholar]
- 30.Zhao C, Zhang Z, Xie S, Si T, Li Y, Zhu JK. Mutational evidence for the critical role of CBF transcription factors in cold acclimation in Arabidopsis[J] Plant Physiol. 2016;171:2744–2759. doi: 10.1104/pp.16.00533. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Gao Y, Zhu N, Zhu X, Wu M, Jiang CZ, Grierson D, Luo Y, Shen W, Zhong S, Fu DQ, et al. Diversity and redundancy of the ripening regulatory networks revealed by the fruitENCODE and the new CRISPR/Cas9 CNR and NOR mutants. Hortic Res. 2019;6:39. doi: 10.1038/s41438-019-0122-x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Umehara M, Hanada A, Magome H, Takeda-Kamiya N, Yamaguchi S. (2010) contribution of strigolactones to the inhibition of tiller bud outgrowth under phosphate deficiency in rice. Plant Cell Physiol. 2010;51:1118–1126. doi: 10.1093/pcp/pcq084. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Yang X, Chen L, He J, Yu W. Knocking out of carotenoid catabolic genes in rice fails to boost carotenoid accumulation, but reveals a mutation in strigolactone biosynthesis. Plant Cell Rep. 2017;36:1533–1545. doi: 10.1007/s00299-017-2172-6. [DOI] [PubMed] [Google Scholar]
- 34.Vogel JT, Walter MH, Giavalisco P, Lytovchenko A, Kohlen W, Charnikhova T, Simkin AJ, Goulet C, Strack D, Bouwmeester HJ, et al. SlCCD7 controls strigolactone biosynthesis, shoot branching and mycorrhiza-induced apocarotenoid formation in tomato. Plant J. 2010;61:300–311. doi: 10.1111/j.1365-313X.2009.04056.x. [DOI] [PubMed] [Google Scholar]
- 35.Lei Y, Lu L, Liu HY, Li S, Xing F, Chen LL. CRISPR-P: a web tool for synthetic single-guide RNA design of CRISPR-system in plants. Mol Plant. 2014;7:1494–1496. doi: 10.1093/mp/ssu044. [DOI] [PubMed] [Google Scholar]
- 36.Lecourieux F, Lecourieux D, Vignault C, Delrot S. A sugar-inducible protein kinase, VvSK1, regulates hexose transport and sugar accumulation in grapevine cells. Plant Physiol. 2010;125:1096–1106. doi: 10.1104/pp.109.149138. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Mauro MC, Toutain S, Walter B, Pinck L, Otten L, Coutos-Thevenot P, Deloire A, Barbier P. High efficiency regeneration of grapevine plants transformed with the GFLV coat protein gene. Plant Sci. 1995;112:97–106. doi: 10.1016/0168-9452(95)04246-Q. [DOI] [Google Scholar]
- 38.Livak KJ, Schmittgen TD. Analysis of relative gene expression data using real-time quantitative PCR and the 2−ΔΔCT method. Methods. 2001;25:402–408. doi: 10.1006/meth.2001.1262. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Additional file 1: Figure S1. Sequencing results of VvCCD7 and VvCCD8 fragments amplified from 41B cells. a The target sequence of VvCCD7 gene in 41B. b The target sequence of VvCCD8 gene in 41B. The target sites in VvCCD7 and VvCCD8 genes are highlighted in dark blue.
Additional file 2: Figure S2. Sequencing results of the two putative off-target sites in VvCCD8 knockout lines. The two off-target sites predicted within exons of other genes were selected for off-target analysis. Two VvCCD8 knockout lines, Plant #1 and Plant #6 were used in the experiment. The amplified fragments containing the off-target sites were amplified and cloned into pLB-Simple vector. At least 6 clones for each site were used for Sanger sequencing.
Additional file 3: Figure S3. The original gel image of PCR identification of T-DNA insertions in CCD8-sgRNA plants. The vector plasmid (P1) and the transgenic cells (P2) were used as the positive controls, while wild-type plant was used as the negative control (N). Lanes 1–6 represent individual CCD8-sgRNA plants.
Additional file 4: Table S1. List of primers used in this study.
Additional file 5: Table S2. Putative off-target sites predicted for CCD8-sgRNA.
Data Availability Statement
The datasets supporting the conclusions of this article and materials used in this study are available by contacting with the corresponding author (zl249@ibcas.ac.cn).