Short abstract
Background
The nature and extent of inflammation seen in multiple sclerosis (MS) varies throughout the course of the disease. Changes seen in CD4+ T-helper cells in relapsing–remitting (RR) MS and secondary progressive (SP) MS might differ qualitatively and/or quantitatively.
Objective
The objective of this paper is to study the frequencies of all major CD4+ T-helper subtypes – Th17, Th22 and Th1 lineage cells – in relapse, remission and secondary progression alongside CCR6 status, a chemokine receptor involved in migration of these cells into the central nervous system.
Methods
We compared 100 patients (50 RRMS and 50 SPMS) and 50 healthy volunteers and performed flow cytometric analysis of lymphocytes in blood samples.
Results
We demonstrated raised frequencies of various cell types along the Th17 axis; Th17, Th17.1 (IL-17+ interferon gamma+) and dual IL-17+ IL-22+ cells in RRMS. Th22 and CCR6+ Th1 cells (nonclassical Th1) were also increased in RRMS. All these cells were CCR6+. Only Th17 frequencies were elevated in SPMS.
Conclusions
Increased frequencies of Th17 cells are implicated both in RRMS and SPMS. The CCR6 pathway includes Th17, Th22 and Th1 nonclassical cells, of which Th22 and Th1 cells represent the greatest subsets in MS.
Keywords: multiple sclerosis, Th17, Th17.1, Th22, Th1, CCR6
Introduction
Multiple sclerosis (MS) is an autoimmune neurodegenerative disease thought to require myelin-reactive CD4+ T-cells. They are believed to play a dominant role in relapsing–remitting MS (RRMS) in which focal white-matter inflammatory lesions are observed, whereas their role is less clear in secondary progressive MS (SPMS), characterised by pathological changes occurring mainly outside focal inflammatory lesions. Though MS is an organ-specific disease, reduction in inflammatory exacerbations caused by the blockage of the T-cell influx into the central nervous system (CNS) with disease-modifying treatments (DMTs) highlights the role of peripheral immunity.
The myelin-reactive CD4+ cells most commonly implicated in MS are of Th1 and Th17 lineage, defined on the basis of production of interferon gamma (IFNγ) and interleukin 17 (IL-17), respectively. Most studies have not demonstrated any difference in peripheral frequency of Th1 cells in MS. Th17 are a much smaller proportion of CD4+ cells (1%–3%) but their pathogenic potential surpasses their absolute numbers.1 Literature has clearly demonstrated that Th17 cells are intrinsically unstable and functionally heterogeneous, consisting of subpopulations that differentially produce IL-17, alone or in combination with other proinflammatory cytokines, and thereby have various phenotypes. Double-positive IL-17+ IFNγ+ cells, termed Th17.1; and double-positive IL17+ IL-22+ cells are the most frequent ones. IL-22 is not only co-expressed by Th17-lineage cells but also solely produced by Th22, a separate lineage implicated in autoimmune diseases such as psoriasis, but is relatively unexplored in MS.2 Nonclassical Th1, IFNγ-producing cells sharing some features with Th17, have recently been shown to be increased in the cerebrospinal fluid (CSF) of patients with MS as well as other neurological conditions.3
In this study, we set out to look at all the major T-helper cell subsets – Th17, Th17.1, other phenotypes of IL-17 producers – Th22 and Th1 cells in relapse, remission and secondary progression in MS along with their CCR6 status, a chemokine receptor involved in the influx of these cells into the CNS. We demonstrate the presence of higher frequencies of Th17 cells and other Th17-lineage multiple cell phenotypes in RRMS but only of Th17 cells in SPMS. Th22 cells were also increased in MS. All the involved cells are CCR6+ and are of pathogenic significance.
Patients and methods
Study Participants
The study was carried out with ethical approval in accordance with the Declaration of Helsinki (ethics reference: 11/WM/0206). Patients and healthy controls were recruited from Royal Stoke MS Centre of Excellence, University Hospital North Midlands, United Kingdom. Laboratory work was performed at the Centre for Translational Inflammation Research, University of Birmingham and the Institute of Science and Technology in Medicine, Keele University.
A set of i) 100 patients (50 patients with RRMS) and 50 patients with SPMS, and ii) 50 healthy controls were compared (Table 1). MS was diagnosed by using the McDonald 2010 diagnostic criteria.4 The subtypes RRMS and SPMS were defined as per clinical phenotypes given by Lublin et al.5 in 2014; and a minimum of one year of gradual worsening was required to define SPMS.6 Healthy volunteers (HVs) were recruited from patients’ spouses or partners, and family members who had no history or clinical evidence of neurological, systemic inflammatory or autoimmune disease.
Table 1.
Demographics of study participants and basic clinical measures.
| HCs | MS | SP | RR | REL | REM | |
|---|---|---|---|---|---|---|
| Patients, n | 50 | 100 | 50 | 50 | 26 | 24 |
| Sex, male, female n (% of women) | 22 M, 28 F (56) | 28 M, 72 F (72) | 13 M, 37 F(74) | 15 M, 35 F (70) | 11 M, 15 F (57.6) | 8 M, 16 F (66.6) |
| Age median (range), y | 49 (19–75) | 49 (19–77) | 56 (37–77) | 45 (19–66) | 45 (19–65) | 45 (19–66) |
| Age at onset median (range), y | NA | 33 (17–52) | 34 (17–52) | 31(18–46) | 31 (18–46) | 31 (18–45) |
| Disease duration median (range), y | NA | 15 (1–52) | 20 (5–52) | 13 (1–32) | 11 (1–31) | 14 (1–32) |
| Smoking status (S, Ex, NS) | 13, 4, 33 | 20, 2, 56 | 9, 17, 24 | 11, 7, 32 | 6, 11, 9 | 7, 7, 10 |
| Relapse in last two years, n | NA | 43 | 5 | 43 | NA | NA |
| DMT status | NA | 15/100a | None | 15/50 | 6/26 | 9/24 |
| Disease severity median (range), EDSS | NA | 6.5 (4.5–7.5) | 3.0 (1.0–4.0) | 6 (5.0–8.0)b | 3.0 (1.0–4.0) | |
| Ambulation | Unrestricted | Unrestricted to WC | 300 m to WC | Unrestricted to 500 m | 100 m with bilateral support to wheel chair bound at time of relapse | Unrestricted to 500 m |
| Fatigue median (range), measured by MFIS-21 | NA | 54 (4–84) | 54 (14–84) | 54 (4–84) | 54 (18–84) | 54 (4–84) |
HC: healthy controls; DMT: disease-modifying therapy; EDSS: Expanded Disability Status Scale; Ex: ex-smoker; F: female; M: male; MFIS: Modified Fatigue Impact Score; MS: multiple sclerosis; NA: not available; NS: nonsmoker; REL: relapsing; REM: remitting; RR: relapsing–remitting multiple sclerosis; S: smoker; SP: secondary progressive multiple sclerosis; WC: wheelchair.
aInterferon beta. bRelapse was confirmed by deterioration of EDSS by at least 0.5.
For the relapse group, patients were recruited at the time of relapse (n = 26) at the first opportunity as they presented to the relapse clinic. Relapse was confirmed by objective evidence of neurological deterioration with worsening of Expanded Disability Status Scale score (EDSS) by at least 0.5. The remission group (n = 39) consisted of RRMS patients who had been stable and had their last clinical relapse at least six months previously. Twenty out of 26 patients recruited at the time of relapse were also recruited to the remission group. Forty-three out of the 50 RR patients had at least one relapse in the two years before the study period. Most patients were treatment naive, and 15 out of 65 RRMS patients were on IFN beta (IFNβ) treatment; these were recruited at the time of relapse. Sixteen out of the 50 SPMS patients recruited were within the first four years of onset of the SP phase. Five out of 50 SPMS patients had superimposed relapses. We measured disease severity by EDSS. Other data collected included smoking status and treatment status: treatment naive vs on DMT. Ambulation was measured by ambulation score, a functional score of EDSS, and fatigue was measured by Modified Fatigue Impact Score-21 (MFIS-21).
Sample preparation and flow cytometry
Blood samples were collected in EDTA tubes. Samples collected were diluted with an equal volume of RPMI-1640 (Sigma-Aldrich, Dorset, UK) layered onto Ficoll-Paque Plus (GE Healthcare Bioscience), and centrifuged at 400 g at 20°C for 30 minutes. The peripheral blood mononuclear cell (PBMC) layer was removed and washed three times in RPMI 1640 before counting. Freshly isolated PBMCs were incubated stimulated with phorbol myristate acetate (50 ng/ml), ionomycin (750 ng/ml) and Brefeldin A (2 mg/ml) for three hours at 37°C (Sigma-Aldrich). Stimulated cells were then stained for surface markers and intracellular cytokine production. Antibodies specific for surface markers were added for 20 minutes at 4°C. PBMCs were then fixed and permeabilised according to the manufacturer’s instructions (FIX & PERM, Life Technologies, UK) before staining with antibodies specific to intracellular markers. The antibodies used were anti-CD3, -CD4, -CCR7, -CCR6 (all four BioLegend); -CD45RA (BD Biosciences); and -IFNγ, -IL-17A, -IL-22 (all three eBioscience). Stained cells were resuspended in phosphate-buffered saline and 2% bovine serum albumin and were analysed using a multicolour Dako-Cyan cytometer. Flow data were analysed using Kaluza Flow Cytometry Analysis Software (Beckman Coulter). Isotype control antibodies or unstimulated controls (for cytokine analysis) were used to determine positivity and define the gating strategy (Supplementary Figure 1).
Statistical analysis
Statistical analysis was performed using GraphPad Prism 6 software. To analyse the differences in cell frequencies, we used the Mann-Whitney test for two-groups comparison and the Kruskal-Wallis test for three-groups comparison. A p value of <0.05 was considered to be statistically significant with descriptions as *p < 0.05, **p < 0.01 and ***p < 0.001.
Results
Th17 cells increased in RRMS and SPMS, whereas Th17.1– dual IL-17± IFNγ± cells increased only in RRMS
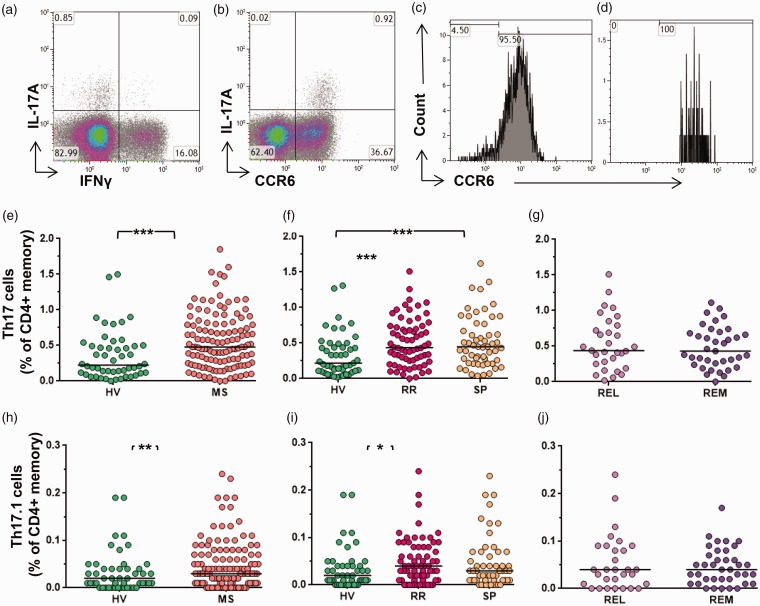
Th17 cells were defined as IL-17A+ cells. Th17.1 cells were defined by dual expression of IL-17 and IFNγ. Both Th17 and Th17.1 were CCR6+ (Figure 1(a–d)).
Figure 1.
Th17 and Th17.1 cells in MS. (a) Representative data demonstrating IL-17A+ and IFNγ+ expression (gated on CD4+ memory cells). Numbers represent the percentage of cells within the quadrant, with negative gates set based on unstimulated controls. (b) Data demonstrating CCR6 expression on IL-17+ cells (gated on CD4+ memory cells). The histograms (Figures (c) and (d)) show CCR6 status of IL-17A+ cells and IL-17A+ IFNγ+ cells, respectively. Th17 as a proportion of CD4+ memory cells (e) in MS vs HV, (f) in RRMS vs SPMS and HV, and (g) in relapse vs remission. Th17.1 as a proportion of CD4+ memory cells (h) in MS vs HV, (i) in RRMS vs SPMS and HV, and (j) in relapse vs remission. Bars indicate the median value for each group. HV: healthy volunteers; IL-17A: interleukin 17A; IFNγ: interferon gamma; MS: multiple sclerosis; REL: relapse; REM: remission; RR: relapsing–remitting multiple sclerosis, SP: secondary progressive multiple sclerosis; Th17: T-helper 17 cell. Mann-Whitney test was performed for comparison of cross data between HV and MS; and between REL and REM. Kruskal-Wallis test was performed for the comparison between HV, RR and SP groups. Bars indicate median values (*p < 0.05, **p < 0.01, ***p < 0.001); all other comparisons were nonsignificant (p > 0.05).
Th17 cells increased in MS as compared to HVs (median 0.55 and 0.35% of memory CD4+ cells. respectively) (p = 0.0002) (Figure 1(e)). Th17 cells increased both in RRMS as compared to HVs, as well as in SPMS as compared to HVs (p = 0.0015) though the difference seen between the RRMS and SPMS groups was not significant (Figure 1(f)). Comparison of RRMS patients in relapse or remission showed no significant difference in Th17 frequencies (Figure 1(g)). There was no difference in Th17-cell frequencies in the female vs male groups either in the MS or in HV categories (data not shown). We also conducted a subgroup analysis of RRMS patients with and without any relapse in the last two years vs SPMS and observed no difference. Similarly, comparison of treatment-naive vs IFNβ-treated patients showed no difference in Th17-cell frequencies (data not shown).
Th17.1, dual–IL-17+ IFNγ+ cells increased in MS as compared to HV (median 0.04 and 0.03, respectively) (p = 0.030) (Figure 1(h)). Increased frequencies were also seen in RRMS as compared to HVs and in SPMS as compared to HVs (p = 0.049) (Figure 1(i)). There was no significant difference noted on comparison of relapse and remission (Figure 1(j)), and in RRMS patients with or without any relapse in the last two years.
Th22 and IL-22-secreting Th17 cells increased in RRMS
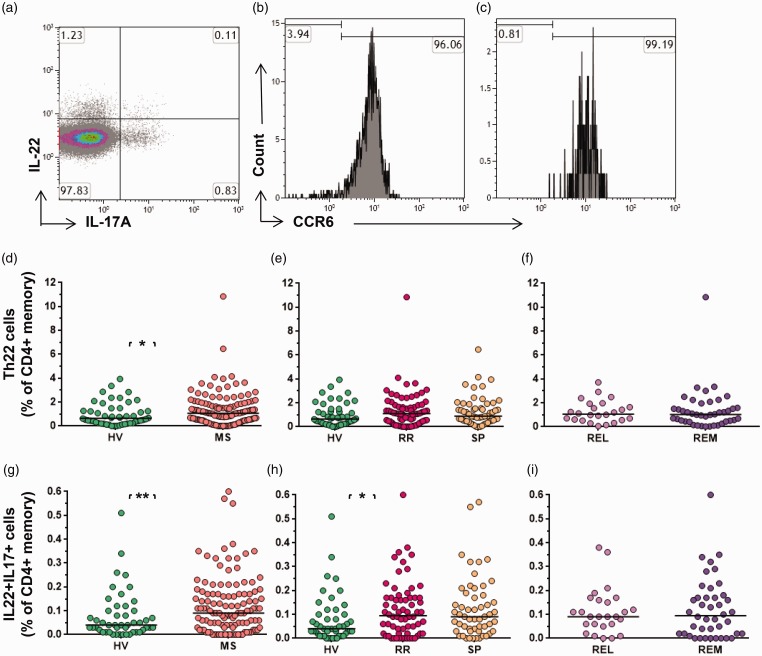
We looked at solo IL-22 producers, and dual IL-22+ IL-17A+ cells (Figure 2(a)). Solo IL-22 producers, called Th22 cells, are of separate T-cell lineage, whereas dual–IL-17A+ IL-22 secretors are considered to be of Th17 lineage. Both these cell populations express CCR6, as shown in Figures 2(b) and (c).
Figure 2.
Th22 and IL-17A+ IL-22+ cells in MS. (a) Representative data demonstrating IL-17A+ and IL-22+ expression (gated on CD4+memory cells). Numbers represent the percentage of cells within the quadrant, with negative gates set based on unstimulated controls. The histograms (b and c) show CCR6 status of IL-22+ cells and IL-17A+ IL-22+ cells, respectively. Th22 as a proportion of CD4+memory cells (d) in MS vs HV, (e) in RRMS vs SPMS and HV, and (f) in relapse vs remission. Dual IL-17A+ IL-22+ cells as a proportion of CD4+memory cells (g) in MS vs HV, (h) in RRMS vs SPMS and HV, and (i) in relapse vs remission. Bars indicate the median value for each group. HV: healthy volunteers; IL: interleukin; MS: multiple sclerosis; REL: relapse; REM: remission; RR: relapsing–remitting multiple sclerosis, SP: secondary progressive multiple sclerosis; Th17: T-helper 17 cell. Mann-Whitney test was performed for comparison of cross data between HV and MS; and between REL and REM. Kruskal-Wallis test was performed for the comparison between HV, RR and SP groups. Bars indicate median values (*p < 0.05, **p < 0.01, ***p < 0.001); all other comparisons were nonsignificant (p > 0.05).
Th22s were significantly elevated in MS as compared to HVs (median 1.35% and 0.95%, respectively) as a percentage of memory CD4+ cells (p = 0.0434) (Figure 2(d)). There was no statistically significant difference observed on comparing the RRMS, SPMS and HV groups (Figure 2(e)). There was no significant difference observed in the relapse vs remission phase (Figure 2(f)).
IL-22+ IL-17A+ dual-expression cells also increased in MS as compared to HV (median 0.07 and 0.12, respectively) (p = 0.0091) (Figure 2(g)), more specifically in the RRMS group as compared to the HVs (p = 0.033) but not as compared to the SPMS group (Figure 2(h)). There was no significant change in the relapse vs remission phase on cross-sectional data (Figure 2(i)).
Although we showed both these cell types frequencies are elevated in MS, we wanted to look for what was the bigger source of IL-22, Th22 or dual IL-17A+ IL-22+ cells. As per our data, a median of 1.35% of memory T cells were Th22 cells but only 0.12% coexpressed the two cytokines in MS, thus Th22 cells were a bigger source of IL-22 than dual expressers (Table 2).
Table 2.
Relative proportion of cells as a percentage of CD4+memory cells in HV and MS. Numbers represent median and range(in brackets). Mann-Whitney tests were performed for comparison of cross-sectional data between HV and MS. Exact P-values are given.
| Cell type (% of CD4+memory cells) | HV | MS |
|---|---|---|
| Th17 | 0.35 (0 -1.50) | 0.55 (0 -1.85)p value-0.0002 |
| Th17-IFN gamma | 0.03 (0 – 0.19) | 0.04 (0 – 0.24) p value-0.0030 |
| Th22 | 0.95 (0-3.93) | 1.35 (0.48-10.84) p value-0.0434 |
| Th22-17 | 0.07 (0-0.51) | 0.12 (0-0.60) p value-0.0091 |
| Th1 | 10.83 (0.08-29.70) | 9.83 (0.06-31.62) p value-0.5511 |
| Non-classical Th1 | 2.20 (0-8.40) | 2.21(0-9.48)p value-0.0409 |
Nonclassical Th1 cells increased in RRMS
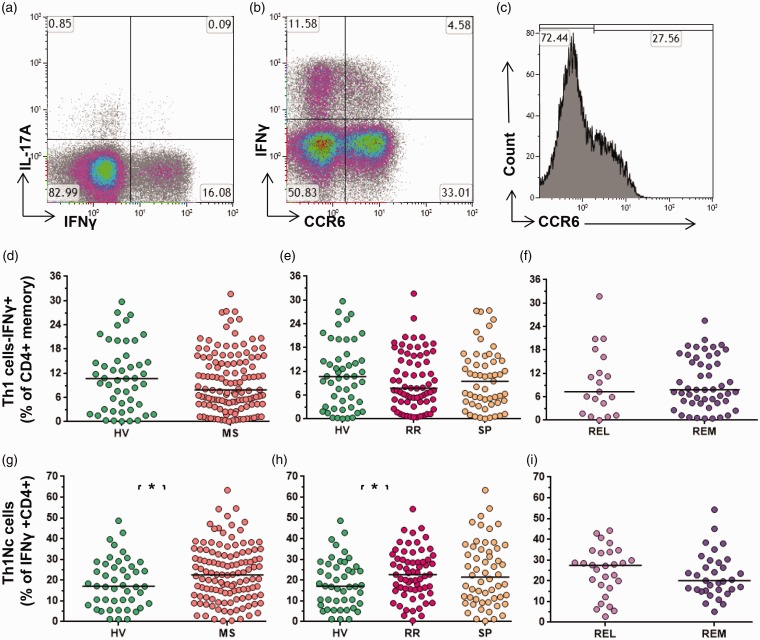
Th1s, defined as IFNγ-secreting memory CD4+ T cells, were analysed alongside their CCR6 status (Figure 3).
Figure 3.
Th1 and nonclassical Th1 cells in MS. (a) Representative data demonstrating IL-17A+ and IFNγ+ expression (gated on CD4+memory cells). Numbers represent the percentage of cells within the quadrant, with negative gates set based on unstimulated controls. (b) Data demonstrating CCR6 expression on IFNγ+ cells (gated on CD4+memory cells). (c) The histogram shows CCR6 status of IFNγ+ cells. Th17, as proportion of CD4+ memory cells, (d) in MS vs HV, (e) in RRMS vs SPMS and HV, and (f) in relapse vs remission. Nonclassical Th1 cells, CCR6+ IFNγ+ CD4+ cells, as a proportion of CD4+ IFNγ+ cells (g) in MS vs HV, in RRMS vs (h) SPMS and HV, and (i) in relapse vs remission. Bars indicate the median value for each group. Mann-Whitney test was performed for comparison of cross data between HV and MS; and between REL and REM. Kruskal-Wallis test was performed for comparison between HV, RR and SP groups. Bars indicate median values (*p < 0.05, **p < 0.01, ***p < 0.001); all other comparisons were nonsignificant (p > 0.05). HV: healthy volunteers; IL-17A: interleukin 17A; IFNγ: interferon gamma; MS: multiple sclerosis; REL: relapse; REM: remission; RR: relapsing–remitting multiple sclerosis, SP: secondary progressive multiple sclerosis; Th17: T-helper 17 cell.
Th1-cell frequencies were unaffected in MS as compared to HV (Figure 3(d)). There was no difference observed in comparing the RRMS, SPMS and HV groups (Figure 3(e)). Th1s were not altered between relapse and remission (Figure 3(f)). This was in keeping with the previous literature.
Almost one-third of Th1s were classical, CCR6– Th1 cells, with the remainder IFNγ+ CCR6+ cells defined as nonclassical Th1 cells (Figure 3(b and c)). Nonclassical Th1 cells increased in MS as compared to the HV group (p = 0.048) (Figure 3(g)). The increased frequencies were also observed in RRMS as compared to SPMS and HVs (p = 0.028) (Figure 3(h)). The difference in relapse and remission phases for nonclassical Th1-cell frequencies did not reach a level of significance (Figure 3(i)). Nonclassical and classical Th1 showed similar median fluorescence intensity for IFNγ, suggesting a similar degree of IFNγ expression (data not shown).
Nonclassical Th1 cells represented a median 2.2% of the memory T-cell population. Thus, the nonclassical Th1 cell population formed a bigger proportion of the memory T-cell population than Th22 and Th17, which represented 0.95% and 0.35% of CD4+ T cells, respectively. Classical Th1 cells constituted a median of 7.5% of memory T cells (Table 2).
Discussion
Elevations in CD4+ Th17-cell frequencies in the blood and CSF, with likely reduction in peripheral blood frequency during relapses, are well reported in RRMS.7–10 Treatment responsiveness to steroids and IFNβ is paralleled by a reduction in Th17 frequency in the blood.8,11 Treatment with natalizumab, on the other hand, showed an elevation in peripheral Th17 frequency because Th17 cells are locked out of the CNS and other tissues.10 Segal alluded to increased frequencies of Th17 cells in SPMS in a review based on his previously unpublished data.12 There is little evidence for changes in Th17 frequency in SPMS. A histopathological study by Tzartos et al. in 2008 reported the presence of Th17 and IL-17 messenger RNA in chronic lesions and normal-appearing white matter, pointing to their role in SPMS also.13 Our study is the first robust clinical evidence of increased blood frequency of Th17 in SPMS.
Little is known about the clinical relevance and frequency of various other cell phenotypes of Th17 lineage in MS. We showed an elevation of Th17.1 in MS, especially in RRMS. Th17.1 cells are Th1-like cells of Th17 origin.1 A number of definitions of Th17.1 cells are in use in the literature, based on the cytokine coexpression IL-17 with IFNγ, like ours, and/or the presence of chemokines CCR6 and CXCR3 with or without CCR4+, and/or based on coexpression of transcription factor T-bet, RORC.14,15 Th17.1 cells are considered pathogenic in experimental autoimmune encephalomyelitis (EAE).16 Th17.1 cells may be more pathogenic than Th17 cells in MS but there are not enough data out to make definite conclusions on their clinical importance and frequencies. Van Langelaar and colleagues have shown that increased frequencies of Th17.1 cells are associated with MS onset, disease activity, the tendency to relapse and early conversion from clinically isolated syndrome to clinically definite MS.14
Dual-positive IL-17A+ IL-22+ cells have a separate pathogenic relevance to Th22 cells, another IL-22 producer. This is because the function of IL-22 depends on its coproduction with IL-17A, which also regulates its expression.17 The presence or absence of IL-17A governs the balance between proinflammatory vs tissue-protective effects of IL-22.17 We showed that Th22 cells were a bigger source of IL-22 than dual expressers in MS. This is in parallel to observations by Muls et al. in 2017.18 Th22 cells have been implicated in a number of inflammatory and autoimmune conditions.19 Rolla and colleagues reported that Th22 cells are elevated in RRMS patients and express high levels of CCR6, which confers a CNS-homing property but lower levels of IFNAR1 as compared to Th17 cells, and were therefore not sensitive to IFNβ therapy, unlike Th17, conferring treatment resistance.20
All these CD4+ Th subsets express CCR6, which is relevant for their influx into the CNS, with high expression of CCL20, the ligand for CCR6, in the choroid plexus.10,21
Our results show increases in the Th17 axis, both in RRMS and SPMS, though the extent of involvement is much less in SPMS. RRMS shows elevation in all components of the Th17 axis, i.e. Th17, Th17.1, IL-17+ IL-22+ dual-expression cells, but the inflammation in SPMS is characterised by a rise in Th17 cells only. This differential involvement of immune cell subsets and mediators during RRMS and SPMS holds important implications for the pathogenesis and therapeutics of these disease subtypes. The cumulative evidence from various pathology and imaging studies shows that the nature of immune dysregulation evolves during the course of MS with the presence of widespread microglial activation in macroscopically normal-appearing white matter and lymphoid follicles in the leptomeninges of SPMS.22,23 This, along with our data, suggests that there is ongoing neuroinflammation in the pathogenic process, but perhaps via pathways that are distinct from those dominant in the RR phase.
We also demonstrate that various cell frequencies that were altered in MS belong to the CCR6 pathway. Though the Th17-axis cells forms an important part of the CCR6 pathway through Th17, Th17.1 and IL-17+ IL-22 dual-expression cells, and is most addressed, Th22 and nonclassical Th1 form an even bigger proportion of this pathway. Nonclassical Th1 cells also form the dominant CCR6+ T-helper subset in CSF of MS patients as well as other neurological conditions.3
Nonclassical Th1s are interesting cell subsets. Recent literature has suggested that these cells are of Th17 origin, transitioning into Th1, thus ex-Th17.1,3 They have an overlapping transcriptional profile with Th17 cells because they express RORC, alongside classical Th1 markers like IFNG and TBX21, along with the CXCR3 surface marker expression.1,3 More studies are needed to understand their pathogenic potential.
The EAE model of MS demonstrates that Th17 and Th1 both are encephalitogenic. Even though Th17 cells may be more potent inducers of myelin damage than Th1, the jury is still out as to whether Th17 or a high Th17 to Th1 ratio–driven EAE produces a different or severer phenotype of EAE than that of Th1. Th17 and Th1 cells both are capable of causing spinal cord inflammation and brainstem, cerebellar, supraspinal inflammatory as well as optic nerve inflammatory lesions with similar degrees of inflammation, demyelination and axonal damage, and regional predisposition with variable Th17 to Th1 ratio has not been proven.24–28 Studies in MS are relatively fewer and have shown that RRMS with predominant spinal cord lesions was mainly driven by Th17 cells, whereas brain lesion–dominant MS is associated with Th1 cells, possibly alongside their counterpart innate lymphoid cells.29,30 It is also worth highlighting that Th17 cells are not just restricted to MS but are also involved in other T-cell–mediated neuroinflammatory conditions including in neuroinflammation seen in traditional neurodegenerative diseases such as Parkinson disease and Alzheimer disease and their animal models.28 Th17 cells there too are believed to contribute to chronic neuroinflammation, perpetuating neurodegenerative processes.
We acknowledge that we have studied multiple cell types and made multiple comparisons. We made three comparisons (HV vs MS; HV vs RRMS and SPMS; and relapse vs remission) for each of the cell subtypes we studied (Th17, Th17.1, Th22, dual IL22+ IL17+ cells; total Th1 and nonclassical Th1 cells), and therefore, there is a chance of type 1 statistical error; however, all of the comparisons made were a priori and hypothesis driven, and along the Th17/Th1 cell axis. Also, for some measures, e.g. Th17, frequencies were determined in more than one analysis, which provides some reassurance about the validity of these results.
Our study reports that the Th17-axis cell phenotypes are increased in MS. We confirm and expand on previous immunological findings seen along the Th17 axis in RRMS but also make some novel observations in SPMS. We show the relative contributions various CCR6+ Th cells make toward the inflammation in MS in this relatively large, robust clinical study. Although our study alludes to the involvement of Th17 cells in the secondary progression of MS, further studies are needed to study their role in the progression and pathogenesis of MS.
Supplemental Material
Supplemental material, MSO899695 Supplemental material for Th17 cells increase in RRMS as well as in SPMS, whereas various other phenotypes of Th17 increase in RRMS only by S Kalra, C Lowndes, L Durant, RC Strange, A Al-Araji, Clive P Hawkins and S John Curnow in Multiple Sclerosis Journal—Experimental, Translational and Clinical
Supplemental material, MSO899695 Supplemental Figure for Th17 cells increase in RRMS as well as in SPMS, whereas various other phenotypes of Th17 increase in RRMS only by S Kalra, C Lowndes, L Durant, RC Strange, A Al-Araji, Clive P Hawkins and S John Curnow in Multiple Sclerosis Journal—Experimental, Translational and Clinical
Acknowledgements
We are grateful to the patients, their relatives and carers for participating in this study, and MS clinical and research team members for their general support for this work.
Contributor Information
S Kalra, Royal Stoke MS Centre of Excellence, Neurology Department, University Hospital North Midlands NHS Trust, UK; Institute for Science and Technology in Medicine, Keele University Medical School, UK.
C Lowndes, Royal Stoke MS Centre of Excellence, Neurology Department, University Hospital North Midlands NHS Trust, UK.
L Durant, Centre for Translational Inflammation Research, Institute of Immunology and Immunotherapy, College of Medical and Dental Sciences, University of Birmingham, UK.
RC Strange, Institute for Science and Technology in Medicine, Keele University Medical School, UK.
A Al-Araji, Royal Stoke MS Centre of Excellence, Neurology Department, University Hospital North Midlands NHS Trust, UK.
Clive P Hawkins, Royal Stoke MS Centre of Excellence, Neurology Department, University Hospital North Midlands NHS Trust, UK; Institute for Science and Technology in Medicine, Keele University Medical School, UK.
S John Curnow, Centre for Translational Inflammation Research, Institute of Immunology and Immunotherapy, College of Medical and Dental Sciences, University of Birmingham, UK.
Conflict of Interests
The author(s) declared no potential conflicts of interestwith respect to the research, authorship, and/or publicationof this article.
Funding
The author(s) disclosed receipt of the following financial support for the research, authorship, and/or publication of this article: This study was supported by the Keele MS Research Fund and a North Staffordshire Medical Institute charity grant (Grant number B269).
ORCID iD
Supplemental Material
Supplemental material for this article is available online.
References
- 1.Annunziato F, Cosmi L, Liotta Fet al. Main features of human T helper 17 cells. Ann N Y Acad Sci 2013; 1284: 66–70. [DOI] [PubMed] [Google Scholar]
- 2.Trifari S, Kaplan CD, Tran EHet al. Identification of a human helper T cell population that has abundant production of interleukin 22 and is distinct from T(H)-17, T(H)1 and T(H)2 cells. Nat Immunol 2009; 10: 864–871. [DOI] [PubMed] [Google Scholar]
- 3.Restorick SM, Durant L, Kalra Set al. CCR6+ Th cells in the cerebrospinal fluid of persons with multiple sclerosis are dominated by pathogenic non-classic Th1 cells and GM-CSF-only-secreting Th cells. Brain Behav Immun 2017; 64: 71–79. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Polman CH, Reingold SC, Banwell Bet al. Diagnostic criteria for multiple sclerosis: 2010 revisions to the McDonald criteria. Ann Neurol 2011; 69: 292–302. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Lublin FD, Reingold SC, Cohen JA. Defining the clinical course of multiple sclerosis. The 2013 revisions. Neurology 2014; 83: 278–286. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Lorscheider J, Buzzard K, Jokubaitis Vet al. Defining secondary progressive multiple sclerosis. Brain 2016; 139: 2395–2405. [DOI] [PubMed] [Google Scholar]
- 7.Brucklacher-Waldert V, Stuerner K, Kolster Met al. Phenotypical and functional characterization of T helper 17 cells in multiple sclerosis. Brain 2009; 132: 3329–3341. [DOI] [PubMed] [Google Scholar]
- 8.Durelli L, Conti L, Clerico Met al. T-helper 17 cells expand in multiple sclerosis and are inhibited by interferon-β. Ann Neurol 2009; 65: 499–509. [DOI] [PubMed] [Google Scholar]
- 9.Edwards LJ, Robins RA, Constantinescu CS. Th17/Th1 phenotype in demyelinating disease. Cytokine 2010; 50: 19–23. [DOI] [PubMed] [Google Scholar]
- 10.Haas J, Schneider K, Schwarz Aet al. Th17 cells: A prognostic marker for MS rebound after natalizumab cessation? Mult Scler 2017; 23: 114–118. [DOI] [PubMed] [Google Scholar]
- 11.Ramgolam VS, Sha Y, Jin Jet al. IFN-beta inhibits human Th17 cell differentiation. J Immunol 2009; 183: 5418–5427. [DOI] [PubMed] [Google Scholar]
- 12.Segal BM. Stage-specific immune dysregulation in multiple sclerosis. J Interf Cytokine Res 2014; 34: 633–640. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Tzartos JS, Friese MA, Craner MJet al. Interleukin-17 production in central nervous system-infiltrating T cells and glial cells is associated with active disease in multiple sclerosis. Am J Pathol 2008; 172: 146–155. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Acosta-Rodriguez EV, Rivino L, Geginat Jet al. Surface phenotype and antigenic specificity of human interleukin 17-producing T helper memory cells. Nat Immunol 2007; 8: 639–646. [DOI] [PubMed] [Google Scholar]
- 15.van Langelaar J, van der Vuurst de Vries RM, Janssen Met al. T helper 17.1 cells associate with multiple sclerosis disease activity: Perspectives for early intervention. Brain 2018; 141: 1334–1349. [DOI] [PubMed] [Google Scholar]
- 16.Domingues HS, Mues M, Lassmann Het al. Functional and pathogenic differences of Th1 and Th17 cells in experimental autoimmune encephalomyelitis. PLoS One 2010; 5: e15531. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Sonnenberg GF, Nair MG, Kirn TJet al. Pathological versus protective functions of IL-22 in airway inflammation are regulated by IL-17A. J Exp Med 2010; 207: 1293–1305. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Muls N, Nasr Z, Dang HAet al. IL-22, GM-CSF and IL-17 in peripheral CD4+T cell subpopulations during multiple sclerosis relapses and remission. Impact of corticosteroid therapy. PLoS One 2017; 12: 1–16. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Xu W, Li R, Dai Yet al. IL-22 secreting CD4+ T cells in the patients with neuromyelitis optica and multiple sclerosis. J Neuroimmunol 2013; 261: 87–91. [DOI] [PubMed] [Google Scholar]
- 20.Rolla S, Bardina V, De Mercanti Set al. Th22 cells are expanded in multiple sclerosis and are resistant to IFN-β. J Leukoc Biol 2014; 96: 1155–1164. [DOI] [PubMed] [Google Scholar]
- 21.Reboldi A, Coisne C, Baumjohann Det al. C-C chemokine receptor 6-regulated entry of TH-17 cells into the CNS through the choroid plexus is required for the initiation of EAE. Nat Immunol 2009; 10: 514–523. [DOI] [PubMed] [Google Scholar]
- 22.Matthews PM, Roncaroli F, Waldman Aet al. A practical review of the neuropathology and neuroimaging of multiple sclerosis. Pract Neurol 2016; 16: 279–287. [DOI] [PubMed] [Google Scholar]
- 23.Magliozzi R, Howell OW, Nicholas Ret al. Inflammatory intrathecal profiles and cortical damage in multiple sclerosis. Ann Neurol 2018; 83: 739–755. [DOI] [PubMed] [Google Scholar]
- 24.Axtell RC, Steinman L. Gaining entry to an uninflamed brain. Nat Immunol 2009; 10: 453–455. [DOI] [PubMed] [Google Scholar]
- 25.Pierson E, Simmons SB, Castelli Let al. Mechanisms regulating regional localization of inflammation during CNS autoimmunity. Immunol Rev 2012; 248: 205–215. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Robinson AP, Harp CT, Noronha Aet al. The experimental autoimmune encephalomyelitis (EAE) model of MS: Utility for understanding disease pathophysiology and treatment. Handb Clin Neurol 2014; 122: 173–189. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Carbajal KS, Mironova Y, Ulrich-Lewis JTet al. Th cell diversity in experimental autoimmune encephalomyelitis and multiple sclerosis. J Immunol 2015; 195: 2552–2559. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.González H, Pacheco R. T-cell-mediated regulation of neuroinflammation involved in neurodegenerative diseases. J Neuroinflammation 2014; 11: 201. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Johnson MC, Pierson ER, Spieker AJet al. Distinct T cell signatures define subsets of patients with multiple sclerosis. Neurol Neuroimmunol Neuroinflamm 2016; 3: e278. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Gross CC, Schulte-Mecklenbeck A, Hanning Uet al. Distinct pattern of lesion distribution in multiple sclerosis is associated with different circulating T-helper and helper-like innate lymphoid cell subsets. Mult Scler 2017; 23: 1025–1030. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Supplemental material, MSO899695 Supplemental material for Th17 cells increase in RRMS as well as in SPMS, whereas various other phenotypes of Th17 increase in RRMS only by S Kalra, C Lowndes, L Durant, RC Strange, A Al-Araji, Clive P Hawkins and S John Curnow in Multiple Sclerosis Journal—Experimental, Translational and Clinical
Supplemental material, MSO899695 Supplemental Figure for Th17 cells increase in RRMS as well as in SPMS, whereas various other phenotypes of Th17 increase in RRMS only by S Kalra, C Lowndes, L Durant, RC Strange, A Al-Araji, Clive P Hawkins and S John Curnow in Multiple Sclerosis Journal—Experimental, Translational and Clinical