Key Points
Question
What is the underlying genetic cause of multiple sudden deaths in young individuals and sudden cardiac arrests observed in 2 large Amish extended families?
Findings
In this molecular autopsy and genetic analysis, a novel homozygous multiexon duplication in RYR2 was identified among young Amish individuals with exertion-related sudden deaths and sudden cardiac arrests without an overt phenotype to suggest RYR2-mediated catecholaminergic polymorphic ventricular tachycardia.
Meaning
Considering that no cardiac tests reliably identify at-risk individuals, and given the high rate of consanguinity in Amish families, identification of unaffected heterozygous carriers may provide potentially lifesaving premarital counseling and reproductive planning.
Abstract
Importance
The exome molecular autopsy may elucidate a pathogenic substrate for sudden unexplained death.
Objective
To investigate the underlying cause of multiple sudden deaths in young individuals and sudden cardiac arrests that occurred in 2 large Amish families.
Design, Setting, and Participants
Two large extended Amish families with multiple sudden deaths in young individuals and sudden cardiac arrests were included in the study. A recessive inheritance pattern was suggested based on an extended family history of sudden deaths in young individuals and sudden cardiac arrests, despite unaffected parents. A family with exercise-associated sudden deaths in young individuals occurring in 4 siblings was referred for postmortem genetic testing using an exome molecular autopsy. Copy number variant (CNV) analysis was performed on exome data using PatternCNV. Chromosomal microarray validated the CNV identified. The nucleotide break points of the CNV were determined by mate-pair sequencing. Samples were collected for this study between November 2004 and June 2019.
Main Outcomes and Measures
The identification of an underlying genetic cause for sudden deaths in young individuals and sudden cardiac arrests consistent with the recessive inheritance pattern observed in the families.
Results
A homozygous duplication, involving approximately 26 000 base pairs of intergenic sequence, RYR2’s 5′UTR/promoter region, and exons 1 through 4 of RYR2, was identified in all 4 siblings of a family. Multiple distantly related relatives experiencing exertion-related sudden cardiac arrest also had the identical RYR2 homozygous duplication. A second, unrelated family with multiple exertion-related sudden deaths and sudden cardiac arrests in young individuals, with the same homozygous duplication, was identified. Several living, homozygous duplication–positive symptomatic patients from both families had nondiagnostic cardiologic testing, with only occasional ventricular ectopy occurring during exercise stress tests.
Conclusions and Relevance
In this analysis, we identified a novel, highly penetrant, homozygous multiexon duplication in RYR2 among Amish youths with exertion-related sudden death and sudden cardiac arrest but without an overt phenotype that is distinct from RYR2-mediated catecholaminergic polymorphic ventricular tachycardia. Considering that no cardiac tests reliably identify at-risk individuals and given the high rate of consanguinity in Amish families, identification of unaffected heterozygous carriers may provide potentially lifesaving premarital counseling and reproductive planning.
This study investigates the underlying cause of multiple sudden deaths and sudden cardiac arrests that occurred in children of 2 large Amish families.
Introduction
Annually, thousands of otherwise healthy individuals between the ages of 1 and 35 years die, at an incidence of 1.3 per 100 000 persons.1 Unfortunately, following a conventional autopsy, 40% of these cases remain inconclusive and are often termed sudden unexplained death in the young (SUDY).1 Although rare, families with multiple SUDYs and no evidentiary clues following autopsy or the postmortem clinical evaluation of surviving relatives are particularly devastated and are seeking answers.
Postmortem genetic testing of SUDY individuals for pathogenic variants in cardiac channelopathy–associated and cardiomyopathy-associated genes may reveal a plausible explanation for the death and is emerging as the new standard of care in decedent evaluation.2 Besides providing closure and clarity regarding the cause and manner of death in a SUDY case, identifying a potential sudden death causing variant may be crucial for surviving family members because it may identify potentially at-risk relatives and enable clinical interventions to prevent a subsequent tragedy. Here, we combined exome sequencing with copy number variant (CNV) analysis to identify the underlying cause for multiple SUDYs and sudden cardiac arrests (SCAs) that occurred in 2 large Amish families with a recessive inheritance pattern.
Methods
An Amish family with exercise-associated SUDY (EA-SUDY) occurring in 4 siblings was referred to the Mayo Clinic Windland Smith Rice Sudden Death Genomics Laboratory. Patient samples were collected for this study between November 2004 and June 2019. Both parents were unaffected clinically. Following written informed consent for this Mayo Clinic institutional review board–approved study, autopsy samples were collected for all 4 siblings who experienced a SUDY as well as whole blood from first-degree living family members, and genomic DNA was isolated. All parents and children of adult age from the families in this study who gave blood for genetic analyses signed the written informed consent form. Exome sequencing and CNV analysis were performed on genomic DNA derived from all 4 SUDY individuals and unaffected parents. See the eMethods in the Supplement for a detailed explanation of the exome molecular autopsy, CNV analysis, chromosomal microarray analysis, mate-pair sequencing, genotyping, and quantitative linkage analysis and estimated logarithm of the odd score (eLOD).
Results
Amish Family With Recessively Inherited EA-SUDY
An extended Amish family with a history of multiple consanguineous unions came to clinical attention following the EA-SUDYs of 4 siblings (Figure 1A, pedigree 1A). The index case was a child who experienced exercise-associated syncope. The electrocardiogram (ECG) displayed a normal sinus rhythm with a ventricular rate of 84 beats per minute, PR interval of 136 milliseconds, QRS duration of 72 milliseconds, and a QTc of 466 milliseconds. The child had a normal exercise stress test result without exercise-induced ectopy and died suddenly several years later during physical exertion. Three siblings also experienced EA sudden cardiac death. Autopsy and toxicology reports were unrevealing.
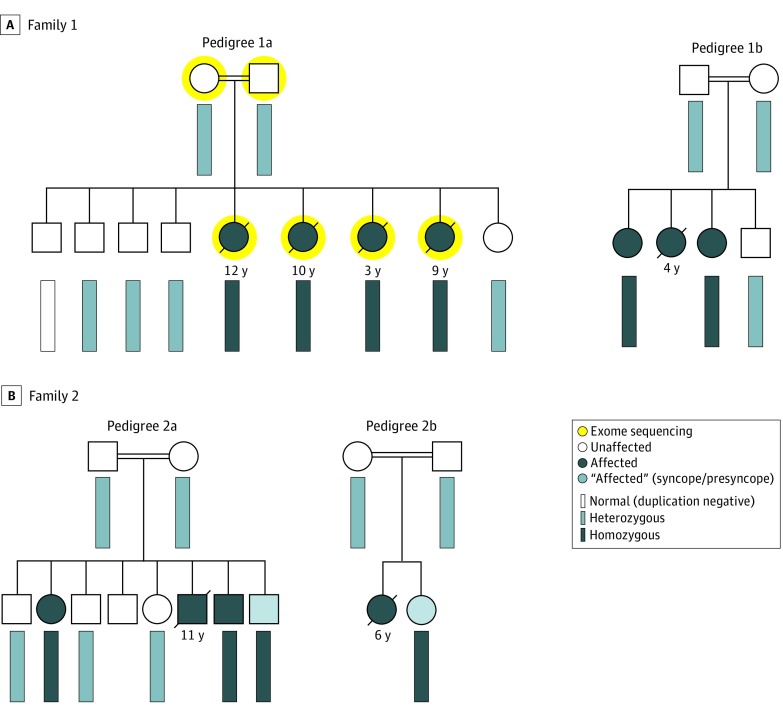
Figure 1. Amish Families With Recessively Inherited Exertion-Associated Sudden Unexplained Death in the Young (SUDY).
Shown are partial pedigrees from 2 unrelated Amish families (pedigree 1a and 1b in panel A and pedigree 2a and 2b in panel B) with autopsy-negative SUDY or sudden cardiac arrests (SCA). Open symbols (circles = female, squares = male) represent unaffected individuals. Dark blue symbols represent affected family members. The white (normal), light blue (heterozygous), and light gray (homozygous) rectangles represent the genotype of the RYR2 duplication in the family members who underwent variant specific genetic testing.
An extended family history of SUDY/SCA was noted in 3 additional children who were related to this family (Figure 1A, pedigree 1B). One child experienced SCA while playing and died 1 month later during a second SCA. One affected child survived a documented ventricular fibrillation SCA while swimming. Clinical evaluation following the SCA, including echocardiography, serial 12-lead ECGs, and exercise stress testing, revealed a normal baseline QTc, normal QTc adaption with exercise, and monomorphic ventricular ectopy that was accentuated mildly with low-level exercise but was attenuated with increasing heart rates/effort (see patient 1 in eFigure 1 in the Supplement). The child experienced a second SCA that required cardiopulmonary resuscitation. A third child experienced swimming-associated syncope having had a normal exercise stress test result a few weeks prior. The deaths occurred during early childhood and adolescence.
Genetic Analysis and Cascade Screening
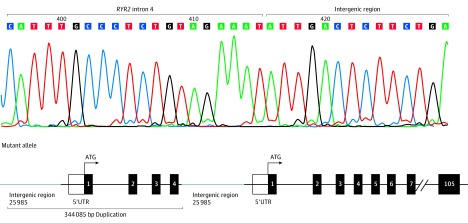
Exome sequencing (Figure 1A; pedigree 1A), followed by recessive inheritance pattern rare variant filtering and analysis, failed to reveal any homozygous or compound heterozygous nonsynonymous candidate variants (eFigure 2 in the Supplement). However, CNV analysis revealed a homozygous tandem duplication of 344 085 base pairs (bp) involving approximately 26 000 bp of intergenic sequence, RYR2’s 5′UTR/promoter region, and exons 1 through 4 of RYR2 (eFigure 3 in the Supplement; Figure 2) in all 4 deceased siblings. Cascade genetic testing revealed that the unaffected living siblings and both parents were heterozygous, and 1 unaffected living sibling was duplication negative. In addition, genotyping of extended family members revealed that both symptomatic women were homozygous (Figure 1A; pedigree 1b). The eLOD generated for the CNV observed in the pedigree using a formula/approach (eResults in the Supplement), endorsed by the Clinical Genome Resource Consortium, was 3.76.3
Figure 2. Mate-Pair Sequencing and Sanger Sequencing to Identify Exact Break Points of the Copy Number Variant (CNV).
Mate-pair sequencing followed by Sanger sequencing determined the exact nucleotide break points of the homozygous duplication. Shown is the Sanger sequencing chromatogram from a family member hosting the homozygous duplication and a graphic representation of the biallelic tandem 344 085 base-pair (bp) duplication involving approximately 26 000 bp of intergenic sequence, RYR2’s 5′UTR/promoter region, and exons 1 to 4 of RYR2.
Validation in a Second Amish Family With EA-SUDY
Subsequently, a second, seemingly unrelated Amish family composed of more than 250 family members, of whom 15 experienced an EA-SUDY (mean [SD] age at death, 11.7 [6.8] years) or survived a SCA, was identified. Figure 1B shows 2 branches of this large family (pedigree 2a and 2b). Because the duplication may represent an Amish founder variant, affected individuals and their first-degree relatives underwent CNV genotyping. Whereas those who experienced an EA-SUDY or SCA were homozygous for the duplication, all unaffected parents, siblings, and offspring tested were heterozygous (Figure 1B).
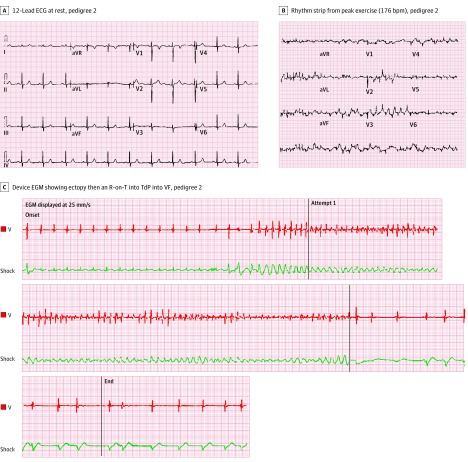
Interestingly, one of the SCA survivors in this family (Figure 1B, pedigree 2a) has experienced 3 appropriate ventricular tachycardia/ventricular fibrillation–terminating implantable cardioverter defibrillator shocks. Review of device tracings revealed premature ventricular contractions with a short coupling interval with R-on-T phenomena triggered torsades de pointes (TdP) that quickly degenerated into ventricular fibrillation (see patient 2 in Figure 3) Importantly, no definitive evidence of a cardiac channelopathy in general, catecholaminergic polymorphic ventricular tachycardia (CPVT) in particular, or other underlying genetic heart disease has been observed in affected individuals. The calculated eLOD score for this second extended family was 2.18. Assuming that the affected children are distantly related, the eLOD score for the CNV would be 6.54. The details of the relationships among these children and families are not being reported to protect privacy (Figure 1).
Figure 3. Cardiologic Testing of Patient 2 Hosting the Homozygous RYR2 Duplication.
A, 12-Lead electrocardiogram at rest; pedigree 2. B, Rhythm strip from peak exercise (176 bpm) during exercise stress testing; pedigree 2. C, Device electrogram (EGM) during an appropriate implantable cardioverter defibrillator (ICD) therapy occurring in the setting of exertion that shows ectopy, then an R-on-T into torsade de pointes (TdP)–triggered ventricular fibrillation (VF) for an affected family member.
Discussion
In this analysis, we show that the same large homozygous tandem duplication of 344 085 bp involving approximately 26 000 bp of intergenic sequence, RYR2’s 5′UTR/promoter region, and exons 1 through 4 of RYR2 is a novel genetic basis for recessively inherited EA-SUDY/SCD in 2 seemingly unrelated, large multigenerational Amish families. Despite pedigree expansion efforts across several generations, a common ancestor has not yet been identified. Nevertheless, we suspect that this RYR2 duplication represents a founder mutation in the Amish community. Of approximately 70 000 clinical chromosomal microarray tests performed by the Mayo Clinic Genomics Laboratory, this duplication was seen only 4 times in a heterozygous state, adding additional support for the speculation that this duplication is likely an Amish founder mutation.
RYR2 mutations are known to predispose to ventricular arrhythmias/SCD through 2 distinct mechanisms: (1) CPVT1-causative gain-of-function variants that result in excessive calcium leak during sympathetic activation/delayed after depolarizations, and (2) so-called short-coupled TdP –causative loss-of-function variants that result in gradual sarcoplasmic reticulum calcium overload/triggered early after depolarizations. Whereas most CPVT1-causative and sc-TdP–causative RYR2 variants have been missense, CNVs involving the in-frame deletion of RYR2 exon 3 (35 amino acids, Asn57_Gly91) that leads to RyR2 gain of function have been reported to cause a wide spectrum of clinical phenotypes. including autosomal dominant CPVT, sinoatrial node dysfunction, bradycardia, atrial fibrillation, atrioventricular block, dilated cardiomyopathy, left ventricular noncompaction, and SCA.4,5,6,7,8
Unlike classic RYR2-mediated autosomal dominantly inherited CPVT (CPVT1), herein, we describe 2 large multigenerational Amish families with a highly penetrant and lethal autosomal recessively inherited RYR2 pathogenic substrate. Among the 2 families hosting the identical large homozygous tandem duplication involving exons 1 through 4 of RYR2, a total of 23 affected individuals have been identified so far, with 22 of 23 (96%) being symptomatic and 18 of 23 (78%) dying suddenly. Unlike typical CPVT, individuals homozygous for the RYR2 duplication have displayed typically only intermittently prolonged QT intervals or prominent U-waves, mild ventricular ectopy, or had a completely normal exercise stress test result/epinephrine challenge or 24-hour Holter monitor. Thus, cardiologic tests, such as ECG, stress testing, and echocardiogram, are currently unable to reliably identify or further risk stratify family members likely to be homozygous for the RYR2 duplication.
Because the clinical phenotype of the affected individuals is similar to patients with RyR2 loss of function dominantly inherited missense variants, we speculate that this homozygous duplication may result in at least partial LOF of RyR2, possibly through a significant attenuation in RYR2’s transcription and translation that leads to at least haploinsufficiency of the protein. If confirmed, this mechanism and pathogenic process could be referred to as calcium release channel deficiency syndrome. It is inconceivable that complete loss of RyR2 is occurring in the patients who are homozygous for this duplication because RyR2 null status would most likely be embryonically lethal.9 Provided that family members who are heterozygous for the duplication are clinically unaffected, this specific gene imbalance of a single RYR2 allele is likely not enough to produce a pathogenic substrate.
Limitations
Although the identified homozygous duplication variant cosegregates properly with the observed recessively inherited phenotype of sudden unexplained death/cardiac arrest in these families, the functional consequence of this RYR2 duplication is currently unknown. Patient-specific inducible pluripotent stem cell–derived cardiomyocyte phenotyping of individuals either homozygous or heterozygous for this RYR2 duplication may provide mechanistic and hopefully therapeutic insights in the future.
Conclusions
Given the high penetrance and severe expressivity noted thus far, cascade RYR2 duplication testing may enable a way to identify at-risk family members and provide a preemptive/prophylactic ICD. In addition, this RYR2 duplication test may help the Amish community with screening to identify the unaffected heterozygous duplication carriers, thereby providing an opportunity for genotype-guided, premarriage counseling in the Amish community akin to cystic fibrosis carrier testing that is offered for white European ancestry populations.
eMethods.
eResults.
eFigure 1. Cardiologic testing of Patient 1 hosting the homozygous RYR2 duplication
eFigure 2. Exome sequencing variant filtering strategy
eFigure 3. PatternCNV and CytoscanHD copy number variant (CNV) analysis
References
- 1.Bagnall RD, Weintraub RG, Ingles J, et al. A prospective study of sudden cardiac death among children and young adults. N Engl J Med. 2016;374(25):2441-2452. doi: 10.1056/NEJMoa1510687 [DOI] [PubMed] [Google Scholar]
- 2.Ackerman MJ, Priori SG, Willems S, et al. HRS/EHRA expert consensus statement on the state of genetic testing for the channelopathies and cardiomyopathies this document was developed as a partnership between the Heart Rhythm Society (HRS) and the European Heart Rhythm Association (EHRA). Heart Rhythm. 2011;8(8):1308-1339. doi: 10.1016/j.hrthm.2011.05.020 [DOI] [PubMed] [Google Scholar]
- 3.Strande NT, Riggs ER, Buchanan AH, et al. Evaluating the clinical validity of gene-disease associations: an evidence-based framework developed by the Clinical Genome Resource. Am J Hum Genet. 2017;100(6):895-906. doi: 10.1016/j.ajhg.2017.04.015 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Bhuiyan ZA, van den Berg MP, van Tintelen JP, et al. Expanding spectrum of human RYR2-related disease: new electrocardiographic, structural, and genetic features. Circulation. 2007;116(14):1569-1576. doi: 10.1161/CIRCULATIONAHA.107.711606 [DOI] [PubMed] [Google Scholar]
- 5.Medeiros-Domingo A, Bhuiyan ZA, Tester DJ, et al. The RYR2-encoded ryanodine receptor/calcium release channel in patients diagnosed previously with either catecholaminergic polymorphic ventricular tachycardia or genotype negative, exercise-induced long QT syndrome: a comprehensive open reading frame mutational analysis. J Am Coll Cardiol. 2009;54(22):2065-2074. doi: 10.1016/j.jacc.2009.08.022 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Marjamaa A, Laitinen-Forsblom P, Lahtinen AM, et al. Search for cardiac calcium cycling gene mutations in familial ventricular arrhythmias resembling catecholaminergic polymorphic ventricular tachycardia. BMC Med Genet. 2009;10(1):12. doi: 10.1186/1471-2350-10-12 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Liu Y, Wang R, Sun B, et al. Generation and characterization of a mouse model harboring the exon-3 deletion in the cardiac ryanodine receptor. PLoS One. 2014;9(4):e95615. doi: 10.1371/journal.pone.0095615 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Ohno S, Omura M, Kawamura M, et al. Exon 3 deletion of RYR2 encoding cardiac ryanodine receptor is associated with left ventricular non-compaction. Europace. 2014;16(11):1646-1654. doi: 10.1093/europace/eut382 [DOI] [PubMed] [Google Scholar]
- 9.Takeshima H, Komazaki S, Hirose K, Nishi M, Noda T, Iino M. Embryonic lethality and abnormal cardiac myocytes in mice lacking ryanodine receptor type 2. EMBO J. 1998;17(12):3309-3316. doi: 10.1093/emboj/17.12.3309 [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
eMethods.
eResults.
eFigure 1. Cardiologic testing of Patient 1 hosting the homozygous RYR2 duplication
eFigure 2. Exome sequencing variant filtering strategy
eFigure 3. PatternCNV and CytoscanHD copy number variant (CNV) analysis