Key Points
Question
What are the clinical implications and prognostic significance of abnormal (positive) exercise electrocardiography but normal stress echocardiography?
Findings
In this cohort study including 15 077 patients undergoing exercise stress echocardiography for suspected coronary artery disease, positive exercise electrocardiography but normal stress echocardiography was associated with higher rates of short- and long-term adverse cardiac events compared with negative exercise electrocardiography and normal stress echocardiography.
Meaning
Abnormal exercise electrocardiography but normal stress echocardiography may identify patients at a slightly increased cardiac risk that was not previously recognized.
Abstract
Importance
Patients with abnormal (positive) exercise electrocardiography, but normal stress echocardiography (+ECG/−Echo) are commonly encountered in clinical practice; however, the prognostic significance of this discordant result is unclear.
Objective
To determine whether patients with +ECG/−Echo have a higher rate of adverse clinical events and a poorer prognosis than patients with negative exercise ECG and normal stress Echo imaging (−ECG/−Echo).
Design, Setting, and Participants
Between January 1, 2000, and February 28, 2014, a total of 47 944 consecutive patients without known coronary artery disease who underwent exercise stress Echo at Duke University Medical Center were evaluated for inclusion in this observational cohort study. Data analysis was conducted from January 1, 2000, to December 31, 2016.
Interventions/Exposures
Patients were categorized as having −ECG/−Echo, +ECG/−Echo, or +Echo (−ECG/+Echo and +ECG/+Echo).
Main Outcomes and Measures
The primary outcome was a composite end point of death, myocardial infarction, hospitalization for unstable angina, and coronary revascularization. Secondary outcomes included individual adverse events and downstream testing.
Results
After excluding submaximal tests and nondiagnostic ECG or stress imaging results, 15 077 patients (mean [SD] age, 52 [13] years; 6228 [41.3%] men) were classified by stress test results. Of these, 12 893 patients (85.5%) had −ECG/−Echo, 1286 patients (8.5%) had +ECG/−Echo, and 898 patients (6.0%) had +Echo. Through a median follow-up of 7.3 (interquartile range, 4.4-10.0) years, the composite end point occurred in 794 patients with −ECG/−Echo (8.5%), 142 patients with +ECG/−Echo (14.6%), and 297 patients with +Echo (37.4%). Death occurred in 425 patients with −ECG/−Echo (4.8%), 50 patients with +ECG/−Echo (5.9%), and 70 patients with +Echo (11.2%). Myocardial infarction occurred in 195 patients with −ECG/−Echo (2.2%), 31 patients with +ECG/−Echo (3.6%), and 59 patients with +Echo (8.7%). The addition of stress ECG findings to clinical and exercise data yielded incremental prognostic value. Patients with −ECG/−Echo imaging results had the least downstream testing (2.3%), followed by +ECG/−Echo (12.8%), and +Echo (33.6%) (P < .001).
Conclusions and Relevance
The presence of +ECG results with normal stress Echo imaging may identify a population of patients who are at slightly increased risk for adverse cardiac events, which was not previously recognized. Further study is needed to determine whether these patients will benefit from intensification of medical management.
This cohort study examines the outcomes of patients who have positive exercise electrocardiographic results but normal stress echocardiographic imaging findings.
Introduction
Exercise stress echocardiography (Echo) is a well-validated and commonly used test for the evaluation of suspected coronary artery disease (CAD).1,2,3 A normal stress echocardiogram, defined as the absence of any left-ventricular wall motion abnormality at rest or with stress, portends an excellent prognosis.4,5,6,7,8 In contrast, exercise stress Echo that reveals regional wall motion abnormalities is specific for CAD (specificity 80%-88%)9 and is associated with an increased risk of adverse cardiac events.10,11,12,13,14
In clinical practice, stress electrocardiography (ECG) is integrated with the results of the stress Echo. However, it is not uncommon to encounter patients who have ischemic ST-segment changes shown on exercise ECG, but normal stress Echo images. The prognostic significance of this discordant finding of positive ECG and negative stress Echo (+ECG/−Echo) is unclear. Prior studies evaluating patients with +ECG/−Echo have shown that, although adverse events (death, myocardial infarction, unstable angina, and/or coronary revascularization) were more frequent among patients with +ECG/−Echo, overall prognosis was similar to that of patients with negative exercise ECG and normal stress echo (−ECG/−Echo).15,16,17 However, these previous studies were small and most had a relatively short follow-up duration, making it difficult to detect a difference in long-term clinical outcomes between patients with +ECG/−Echo and those with −ECG/−Echo.
In light of this uncertainty, we sought to define the +ECG/−ECG population and assess whether these discordant stress results have a prognostic implication. Compared with patients with −ECG/−Echo, we hypothesized that patients with +ECG/−Echo would have a higher rate of adverse clinical events and a worse long-term prognosis. To evaluate this hypothesis, we studied a large cohort of patients who underwent exercise stress Echo for the evaluation of suspected CAD and had long-term clinical follow-up.
Methods
Study Population
Between January 1, 2000, and February 28, 2014, 47 944 patients underwent stress Echo at Duke University Medical Center, Durham, North Carolina. Included patients were those evaluated for suspected CAD and achieved the age-adjusted target heart rate with treadmill stress Echo. Patients were excluded if they had known CAD, heart transplant, congenital heart disease, or an abnormal resting Echo. Pharmacologic stress Echo, submaximal stress tests, and stress tests with nondiagnostic exercise ECG or stress Echo imaging were also excluded. If patients underwent multiple stress Echo, only the results of the first (index) examination were analyzed. All patients gave written or oral informed consent to a stress test. This study was approved by the Duke University Institutional Review Board and was granted a waiver of consent as retrospective research analysis.
Exercise ECG
All patients underwent symptom-limited, treadmill-exercise ECG. Exercise protocols included Bruce (89.4%), Ekelund (10.1%), and Naughton (0.5%) protocols. Twelve-lead ECG and blood pressure measurements were obtained at baseline, at each stage of the protocol, and during recovery. Whenever possible, β-blockers and calcium channel antagonists were discontinued 24 hours before stress testing. Patients who did not achieve at least 85% of the age-predicted maximum heart rate were considered to have a submaximal exercise stress test and were excluded. The exercise ECG result and the magnitude of ST-segment depression were obtained from the final clinical interpretation. The Duke Treadmill Score was calculated for all patients.18
Stress Echo
Transthoracic Echo was performed at rest and immediately after peak exercise in accordance with guidelines.19 Rest and stress Echo images were acquired in apical and parasternal views using a standard protocol. Echocardiographic contrast was used when necessary to improve endocardial border definition. Patients with poor image quality were not excluded unless it rendered the stress test result nondiagnostic. Stress Echo images were interpreted by experienced cardiologists (level III trained, Echo board certified) according to standardized guidelines.19,20 A new wall motion abnormality on stress Echo imaging or a decrease in ejection fraction of more than 5% at peak stress was considered a positive stress Echo (+Echo), regardless of exercise ECG results. A hypercontractile response in all wall segments was considered normal (−Echo). Patients were categorized into 3 groups based on the clinical interpretation of the exercise ECG and stress Echo results: (1) negative exercise ECG and negative stress Echo (−ECG/−Echo), (2) positive exercise ECG and negative stress Echo (+ECG/−Echo), or (3) positive stress Echo (+Echo).
Clinical Follow-up and Study End Points
The primary outcome was a composite end point of major adverse cardiac events (MACEs), which included all-cause death, myocardial infarction, hospitalization for unstable angina, and coronary revascularization that comprised all percutaneous coronary interventions and coronary artery bypass grafting procedures. Secondary end points included individual adverse event rates and downstream testing. Assessment of downstream testing included any additional noninvasive testing or diagnostic coronary angiography performed in the year following the index stress Echo.
The clinical data sources for the present study population included the Duke Echocardiography Laboratory Database, Duke Enterprise Data Unified Content Explorer, Duke University Health System electronic medical records, and the national Social Security Death Index. Vital statistics for this study population were obtained through the National Death Index through December 31, 2014. After 2014, vital status came from a combination of sources: (1) death records in the Duke Health System, (2) death reported through the Duke Database for Cardiovascular Disease, and (3) subscription to the Social Security Administration Death Master File.
Statistical Analysis
Comparisons of baseline characteristics, exercise data, and MACE rates were made between the −ECG/−Echo, +ECG/−Echo, and +Echo groups. Differences between −ECG/−Echo and +ECG/−Echo were assessed using χ2 test and a 2-tailed, paired t test for pairwise comparisons for categorical and continuous variables, respectively, and analysis of variance or Kruskal-Wallis were used, when appropriate, to compare all 3 groups. Categorical variables are reported as counts with percentages and continuous variables are reported as mean (SD). Multivariable logistic regression was used to identify variables independently associated with +ECG/−Echo; −ECG/−Echo served as the reference group. Variables included age, male sex, hypertension, diabetes, hyperlipidemia, smoking history, peripheral artery disease, cerebrovascular disease, resting and maximum heart rate, percent of predicted heart rate, resting and maximum systolic blood pressure, metabolic equivalents achieved, and exercise time. Cumulative event curves for the MACE composite end point and components were estimated by the Kaplan-Meier method and compared by the log-rank test. Analyses were based on time to first event, and if no event occurred, event time was censored at the end of follow-up or 10 years, whichever occurred first. Multivariable Cox proportional hazards regression was used to examine the association between stress test results and short- and long-term clinical outcomes.
Graphic checks indicated that the assumption of proportional hazards between groups was violated and that the hazard ratio for MACE changed at approximately 30 days. To address this violation, a more flexible Cox model was used that allowed for a separate estimate of group effect on the hazard rate in the immediate posttest period (≤30 days) and during long-term follow-up. Multiple imputation was used for baseline variables included in multivariable analyses that had missing values (<1% missingness in all variables except for Duke Treadmill score, which was missing in 2%). Five imputed data sets were generated with missing values imputed using the fully conditional specification method. Model effect estimates were reported aggregated across imputed data sets. The threshold for statistical significance was 2-sided with a type I error rate of .05. Statistical analyses were performed using SAS software, version 9.4 (SAS Institute). Data analysis was conducted from January 1, 2000, to December 31, 2016.
Results
Study Population
Among consecutive patients referred for stress Echo, 15 077 patients underwent exercise stress Echo for suspected CAD and had diagnostic exercise ECG and stress Echo imaging (Figure 1). Of these, 12 893 patients (85.5%) had −ECG/−Echo, 1286 patients (8.5%) had +ECG/−Echo, and 898 patients (6.0%) had +Echo.
Figure 1. Study Flow Diagram.
ECG indicates electrocardiogram; Echo, echocardiogram.
The baseline and exercise characteristics of the patient population (mean [SD] age, 52 [13] years; 6228 [41.3%] men) are presented in Table 1. Compared with patients with −ECG/−Echo, patients with +ECG/−Echo were older (mean [SD] age, 57 [11] years vs 51 [12] years) and had a greater burden of CAD risk factors. Patients with +ECG/−Echo had lower resting heart rates (mean [SD] bpm, 73 [12] vs 75 [14]), but higher resting systolic blood pressure compared with patients with −ECG/−Echo (mean [SD] mm Hg, 136 [19] vs 130 [18]). Patients with +ECG/−Echo compared with patients with −ECG/−Echo had shorter exercise times (mean [SD] min, 7.5 [2.6] vs 8.0 [2.8]) and lower metabolic equivalents with exercise stress (mean [SD] 9 [3] vs 10 [3]) yet achieved a higher percentage of the age-predicted heart rate (mean [SD], 111% [11%] vs 110% [9%]), potentially indicating greater deconditioning.
Table 1. Baseline Clinical and Exercise Characteristics.
| Characteristic | No. (%) | ANOVA P Value | ||
|---|---|---|---|---|
| −ECG/−Echo (n = 12 893) | +ECG/−Echo (n = 1286) | +Echo (n = 898) | ||
| Clinical characteristics | ||||
| Age, y | 51 (12) | 57 (11) | 58 (12) | <.001 |
| Men | 5348 (41.5) | 511 (39.7) | 369 (41.1) | .48 |
| Hypertension | 4661 (36.2) | 591 (46.0) | 396 (44.1) | <.001 |
| Hyperlipidemia | 2691 (20.9) | 378 (29.4) | 255 (28.4) | <.001 |
| Diabetes | 1391 (10.8) | 164 (12.8) | 135 (15.0) | <.001 |
| Smoking | 1154 (9.0) | 98 (7.6) | 95 (10.6) | .06 |
| Positive family history | 289 (2.2) | 29 (2.3) | 13 (1.4) | .29 |
| Cerebrovascular disease | 367 (2.8) | 58 (4.5) | 40 (4.5) | <.001 |
| Peripheral artery disease | 115 (0.9) | 25 (1.9) | 14 (1.6) | <.001 |
| Chronic kidney disease | 139 (1.1) | 17 (1.3) | 10 (1.1) | .73 |
| Indication for stress testing | ||||
| Chest pain | 10 323 (80.1) | 985 (76.6) | 729 (81.2) | .002 |
| Dyspnea | 1574 (12.2) | 183 (14.2) | 110 (12.2) | |
| Abnormal ECG | 513 (4.0) | 73 (5.7) | 41 (4.6) | |
| Syncope | 483 (3.7) | 45 (3.5) | 18 (2.0) | |
| Poor image quality | 906 (7.0) | 98 (7.6) | 121 (13.5) | <.001 |
| Exercise characteristics | ||||
| Heart rate, mean (SD), bpm | ||||
| Resting | 75 (14) | 73 (12) | 75 (14) | <.001 |
| Maximum | 158 (16) | 153 (18) | 148 (19) | <.001 |
| Achieved target HR %, mean (SD) | 110 (9) | 111 (11) | 108 (13) | <.001 |
| Systolic BP, mean (SD), mm Hg | ||||
| Resting | 130 (18) | 136 (19) | 137 (20) | <.001 |
| Maximum | 175 (28) | 180 (28) | 175 (30) | <.001 |
| Double product, mean (SD) | 27 729 (5188) | 27 603 (5467) | 26 031 (5839) | <.001 |
| Exercise time, mean (SD), min | 8.0 (2.8) | 7.5 (2.6) | 6.4 (2.7) | <.001 |
| METS, mean (SD) | 10 (3) | 9 (3) | 8 (3) | <.001 |
| Limiting chest pain | 108 (0.8) | 15 (1.2) | 68 (7.6) | <.001 |
| Duke Treadmill Score, mean (SD) | 7.3 (3.2) | 0.2 (4.2) | 2.0 (6.2) | <.001 |
Abbreviations: ANOVA, analysis of variance; BP, blood pressure; ECG, electrocardiogram; Echo, echocardiogram; HR, heart rate; METS, metabolic equivalents; −, negative result; +, positive result.
Patients with +Echo were older and more likely to be a smoker than patients with +ECG/−Echo. Patients with +Echo had significantly shorter exercise times (mean [SD] 6.4 [2.7] vs 7.5 [2.6] min), lower metabolic equivalents (mean [SD] 8 [3] vs 9 [3]), and were more likely to have limiting chest pain during exercise (7.6% vs 1.2%) compared with patients with +ECG/−Echo. The mean Duke Treadmill Score was 7.3 among patients with −ECG/−Echo (low risk), but indicated moderate risk in patients with +ECG/−Echo (0.2) and +Echo (2.0). After multivariable adjustment, factors independently associated with +ECG/−Echo were age (odds ratio [OR], 1.18; 95% CI, 1.14-1.22; P < .001), hyperlipidemia (OR, 1.10; 95% CI, 1.02-1.18; P = .01), resting systolic blood pressure (OR, 1.12; 95% CI, 1.08-1.11; P < .001), and resting heart rate (OR, 0.83; 95% CI, 0.80-0.87; P < .001) (eTable 1 in the Supplement).
Downstream Testing
In the year following the index stress Echo, patients with −ECG/−Echo had the least downstream testing (2.3%), followed by +ECG/−Echo (12.8%) and +Echo (33.6%) (P < .001). Patients with +ECG/−Echo were 6-fold more likely to have had at least 1 additional noninvasive test (odds ratio [OR], 6.3; 95% CI, 5.16-7.71; P < .001) and over 8 times more likely to have had a diagnostic coronary angiogram (OR, 8.4; 95% CI, 6.19-11.49; P < .001) (eFigure 1 in the Supplement). Coronary angiography was performed in 92 patients with −ECG/−Echo (0.7%), 75 patients with +ECG/−Echo (5.8%), and 250 patients with +Echo (27.8%). Severity of CAD increased with increasingly abnormal stress test results (eTable 2 in the Supplement). Significant CAD, defined as 50% or greater stenosis in 1 or more coronary arteries, was present in 33 patients with +ECG/−Echo. The distribution of significant CAD was 38% in the left anterior descending artery, 29% in the right coronary artery, 25% in the left circumflex artery, and 7% in the left main artery.
Clinical Outcomes
Median follow-up was 7.3 (interquartile range, 4.4-10.0) years. During follow-up, 14 050 patients (93.2%) had at least 1 encounter with the Duke Health System and 1027 patients (6.8%) had no follow-up at the Duke Health System after the index stress Echo was obtained, but 30 patients (0.2%) had an event captured outside of the Duke Health System. Of the remaining 997 patients, 870 individuals (87.3%) had follow-up censored at the last vital status query.
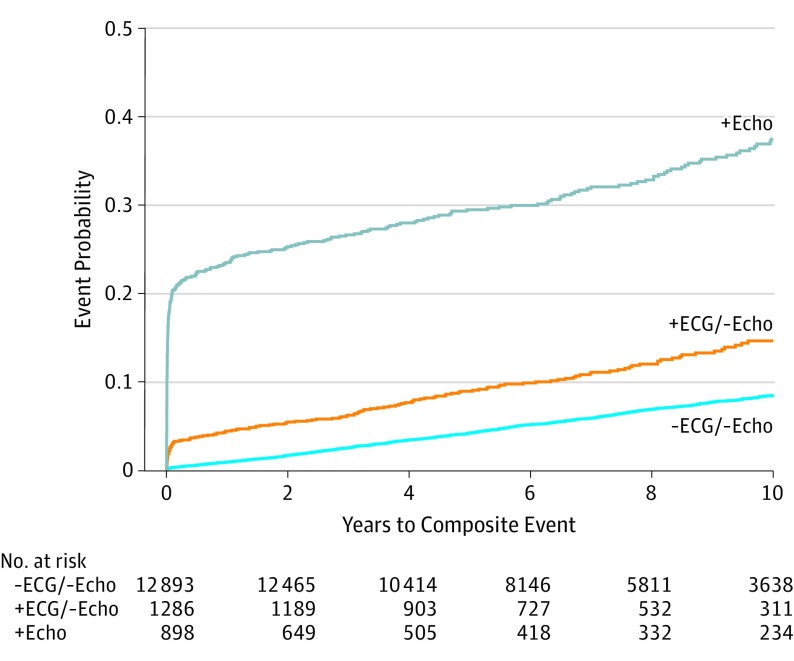
The primary composite MACE end point occurred in 794 patients with −ECG/−Echo (8.5%), 142 patients with +ECG/−Echo (14.6%), and 297 patients with +Echo imaging (37.4%) (Figure 2). The annual incidence of the combined end point was 0.89% with −ECG/−Echo, 1.72% with +ECG/−Echo, and 6.25% with +Echo (P < .001). The individual secondary end points of myocardial infarction, unstable angina, and revascularization were more frequent among patients with +ECG/−Echo than −ECG/−Echo (Figure 3). With percentages estimated using the Kaplan-Meier method, through follow-up, death occurred in 425 patients with −ECG/−Echo (4.8%), 50 patients with +ECG/−Echo (5.9%), and 70 patients with +Echo (11.2%) results. Myocardial infarction occurred in 195 patients with −ECG/−Echo (2.2%), 31 patients with +ECG/−Echo (3.6%), and 59 patients with +Echo (8.7%) results. Unstable angina occurred in 267 patients with −ECG/−Echo (2.7%), 69 patients with +ECG/−Echo (6.7%), and 208 patients with +Echo (25.5%). Revascularization occurred in 129 patients with −ECG/−Echo (1.3%), 44 patients with +ECG/−Echo (4.1%), and 144 patients with +Echo (17.5%) (Table 2).
Figure 2. Cumulative Incidence of the Primary Composite End Point: Death, Myocardial Infarction, Hospitalization for Unstable Angina, and Coronary Revascularization.
Cumulative incidence classified according to exercise electrocardiogram (ECG) and stress echocardiogram (Echo) results. Comparisons were significant at P < .001 using negative ECG and Echo (−ECG/−Echo) as the reference.
Figure 3. Cumulative Incidence of Individual Major Adverse Cardiac Events.
Cumulative incidence of individual end points for patients with negative electrocardiogram/negative echocardiogram (−ECG/−Echo) and positive ECG/negative Echo (+ECG/−Echo). Incidence of myocardial infarction, unstable angina, and coronary revascularization was significantly greater among patients with +ECG/−Echo.
Table 2. Major Adverse Cardiac Events by Stress ECG/Echo Result.
| Model and Stress Group | Cumulative Events Through 10 y, %a | Time Period | Mean Event Rate (per 100 Person-Years) | Unadjusted HR (95% CI) | P Value | Adjusted HR (95% CI) | P Value |
|---|---|---|---|---|---|---|---|
| Composite MACEs | |||||||
| −ECG/−Echo | 794 (8.5) | Before 30 d | 3.2 | NA | NA | NA | NA |
| After 30 d | 0.9 | NA | NA | NA | NA | ||
| +ECG/−Echo | 142 (14.6) | Before 30 d | 33.8 | 10.40 (6.49-16.68) | <.0001 | 8.06 (5.02-12.94) | <.0001 |
| After 30 d | 1.3 | 1.54 (1.26-1.89) | <.0001 | 1.25 (1.02-1.53) | .03 | ||
| +Echo | 297 (37.4) | Before 30 d | 283.5 | 81.97 (56.77-118.35) | <.0001 | 41.43 (28.54-60.13) | <.0001 |
| After 30 d | 2.6 | 3.03 (2.50-3.67) | <.0001 | 1.80 (1.48-2.20) | <.0001 | ||
| Death | |||||||
| −ECG/−Echo | 425 (4.8) | Before 30 d | 0.5 | NA | NA | NA | NA |
| After 30 d | 0.5 | NA | NA | NA | NA | ||
| +ECG/−Echo | 50 (5.9) | Before 30 d | 0.9 | 2.00 (0.23-17.2) | .53 | 1.55 (0.18-13.2) | .69 |
| After 30 d | 0.6 | 1.23 (0.91-1.65) | .17 | 0.96 (0.71-1.30) | .81 | ||
| +Echo | 70 (11.2) | Before 30 d | 1.4 | 2.88 (0.34-24.6) | .33 | 1.39 (0.16-11.9) | .76 |
| After 30 d | 1.1 | 2.38 (1.84-3.07) | <.001 | 1.17 (0.89-1.53) | .25 | ||
| Myocardial infarction | |||||||
| −ECG/−Echo | 195 (2.2) | Before 30 d | 0.7 | NA | NA | NA | NA |
| After 30 d | 0.2 | NA | NA | NA | NA | ||
| +ECG/−Echo | 31 (3.6) | Before 30 d | 1.9 | 2.87 (0.60-13.8) | .19 | 2.09 (0.43-10.1) | .36 |
| After 30 d | 0.3 | 1.62 (1.10-2.40) | .02 | 1.19 (0.80-1.77) | .39 | ||
| +Echo | 59 (8.7) | Before 30 d | 24.7 | 37.2 (15.6-89.1) | <.001 | 18.6 (7.69-45.0) | <.001 |
| After 30 d | 0.7 | 3.26 (2.33-4.58) | <.001 | 1.72 (1.20-2.46) | .003 | ||
| Unstable angina | |||||||
| −ECG/−Echo | 267 (2.7) | Before 30 d | 2.1 | NA | NA | NA | NA |
| After 30 d | 0.3 | NA | NA | NA | NA | ||
| +ECG/−Echo | 69 (6.7) | Before 30 d | 26.9 | 12.9 (7.35-22.5) | <.001 | 10.5 (5.97-18.3) | <.001 |
| After 30 d | 0.5 | 1.81 (1.30-2.52) | <.001 | 1.51 (1.08-2.11) | .02 | ||
| +Echo | 208 (25.5) | Before 30 d | 208.5 | 95.4 (60.8-149.7) | <.001 | 52.6 (33.2-83.3) | <.001 |
| After 30 d | 1.5 | 5.37 (4.13-6.99) | <.001 | 3.35 (2.55-4.41) | <.001 | ||
| Coronary revascularization | |||||||
| −ECG/−Echo | 129 (1.3) | Before 30 d | 0.9 | NA | NA | NA | NA |
| After 30 d | 0.1 | NA | NA | NA | NA | ||
| +ECG/−Echo | 44 (4.1) | Before 30 d | 14.3 | 15.1 (6.78-33.6) | <.001 | 11.7 (5.26-26.2) | <.001 |
| After 30 d | 0.3 | 2.60 (1.73-3.90) | <.001 | 2.10 (1.40-3.17) | <.001 | ||
| +Echo | 144 (17.5) | Before 30 d | 153.4 | 156.7 (81.9-300.0) | <.001 | 81.3 (42.0-157.4) | <.001 |
| After 30 d | 0.8 | 5.82 (4.08-8.30) | <.001 | 3.30 (2.28-4.79) | <.001 |
Abbreviations: ECG, electrocardiogram; Echo, echocardiogram; HR, hazard ratio; MACEs, major adverse cardiac events; NA, not applicable; −, negative result; +, positive result.
Percent estimated using Kaplan-Meier method.
The absolute MACE rate was higher among patients with +ECG/−Echo in both short-term and long-term follow-up compared with patients with −ECG/−Echo (eFigure 2 in the Supplement). The time-dependent analysis, which accounts for the hazard rate changing over time, demonstrated that the risk of MACE was greater among the +ECG/−Echo and +Echo groups in both the immediate posttest period (0-30 days) and during long-term follow-up compared with patients with −ECG/−Echo (Table 2). Even after adjustment for clinical risk factors and exercise results, the risk of an adverse cardiac event remained higher among patients with +ECG/−Echo compared with patients with −ECG/−Echo.
Patients with +ECG/−Echo were further stratified according to the Duke Treadmill Score; higher risk was associated with a significantly increased rate of MACE (eFigure 3 in the Supplement). Stratification by degree of ST-segment depression also revealed that adverse events increased with greater ST-segment depression but was not statistically significant (P = .06).
To assess whether exercise ECG findings also had prognostic significance among patients with +Echo, the +Echo population was dichotomized by ECG results and revealed that the exercise ECG also risk stratified patients with +Echo. Among the 521 patients with −ECG/+Echo, the composite end point occurred in 120 patients (23.0%) and in 177 of the 377 patients with +ECG/+Echo (46.9%) (eFigure 4 in the Supplement). Patients with +ECG/+Echo also had an increased risk for the individual end points of death, myocardial infarction, unstable angina, and coronary revascularization (eFigure 5 in the Supplement).
Discussion
This study demonstrated that in a large stress Echo cohort, 8.5% of patients had the discordant findings of +ECG/−Echo, which was associated with increased downstream testing and a higher rate of short- and long-term adverse cardiac events, particularly hospitalization for unstable angina and coronary revascularization in the 30 days following testing. The finding of positive exercise ECG added modest incremental prognostic value to clinical factors and exercise performance and helped to further risk stratify patients with normal stress Echo.
These findings have several important clinical implications. First, this study has identified a possible at-risk population that is commonly encountered in clinical practice but has been underrecognized and not well characterized. Second, previous studies have been either too small or too short to definitively differentiate patients with +ECG/−Echo from those with −ECG/−Echo, and thus these populations have been considered to have an equivalent prognosis with a similar benign clinical trajectory.15,16,17 This study suggests that patients with +ECG/−Echo are at slightly higher risk for adverse outcomes. Furthermore, a positive ECG was additive to and independent of the risk conferred by baseline clinical characteristics and exercise performance. However, the increased risk from −ECG to +ECG with −Echo was smaller than the increased risk associated with a +Echo.
Prognostic Value of +ECG/−Echo
Patients with −ECG/−Echo had a low incidence of adverse events, which has been previously demonstrated, and this study supports the good prognosis associated with negative exercise ECG and normal stress Echo.4,6,14,21 In comparison, the annualized event rate among patients with +ECG/−Echo was 1.72%, which is still low in absolute terms, but higher than the 0.89% annualized event rate of patients with −ECG/−Echo. Prior studies and a meta-analysis of patients with normal stress Echo found the annualized event rates to range between 0.5% and 2%; however, these studies did not stratify by ECG results.4,5,6,7,8 Prior studies, such as that of Marwick et al,16 found that irrespective of ECG results, patients with positive stress Echo (n = 200) had poorer outcomes than those with negative stress Echo (n = 300), and among patients with normal Echo imaging and ST-segment changes (+ECG/−Echo) had a worse prognosis than those without ST-segment changes (−ECG/−Echo); however, annualized event rates were not reported. Another study of patients with normal stress Echo found that 79 of 677 patients (11.7%) had +ECG/−Echo imaging results, but event rates did not differ between ECG groups at 1, 2, or 5 years.15 However, by the end of the follow-up period (9.6 years), there was a significant difference in the rate of events: 8% among patients with −ECG/−Echo compared with 15% among patients with +ECG/−Echo, which was similar to the 10-year event rate observed in this study.
Potential Pathophysiologic Characteristics of +ECG/−Echo
The underlying pathophysiologic characteristics of this discordant stress result remain unclear. The presence of ischemic ECG changes in the absence of wall motion abnormalities defies the order of the ischemic cascade, which postulates that exercise-induced wall motion abnormalities appear before ST-segment changes or angina.22,23 Although patients with submaximal levels of exercise were excluded and stress Echo imaging was obtained immediately post peak, it is possible that wall motion abnormalities present at peak stress could have resolved by the time stress Echo images were acquired at lower heart rates. However, rapid heart rate recovery has been associated with improved clinical outcomes, whereas patients with +ECG/−Echo imaging results experienced an increase in adverse events.24 Suboptimal acoustic imaging could affect the results by potentially obscuring wall motion abnormalities. Yet, few patients with +ECG/−Echo (7.6%) had poor image quality. In addition, 13.5% of patients with +Echo imaging results had poor image quality but regional wall motion abnormalities were still detected. Another explanation could be that patients with +ECG/−Echo had a greater proportion of obstructive CAD in the left circumflex artery for which stress Echo is the least sensitive.19,25 Yet, among the subgroup of patients with +ECG/−Echo who underwent cardiac catheterization, CAD in the left circumflex artery did not predominate. However, cardiac catheterization was performed in only a small fraction of the patients with +ECG/−Echo and, in most patients with +ECG/−Echo, the CAD burden was not known. It is also possible that patients with +ECG/−Echo have a greater incidence of nonobstructive disease and/or a higher burden of vulnerable coronary plaque, both of which are associated with increased adverse outcomes.26,27 A future study incorporating cardiac computed tomographic angiography could delineate the underlying anatomy and plaque morphologic characteristics in patients with +ECG/−Echo, provide mechanistic insight for the elevated risk, and act as a gatekeeper to the cardiac catheterization lab in this population.
Clinical Implications
This study has important clinical implications, particularly for primary care and cardiology. First, we may have identified a population with increased cardiac risk that until now, was not well defined and unknowingly grouped with a lower risk cohort, which obscured the prognostic significance of +ECG/−Echo. Second, these findings have possible ramifications for the dissemination of clinical results. At Duke University Medical Center, to alert referring clinicians of the increased risk associated with +ECG/−Echo, we now report these stress test findings as, “abnormal stress echocardiogram; positive ECG without wall motion abnormalities at rest and stress.” In addition, by recognizing that patients with +ECG/−Echo are at increased cardiac risk, there is an opportunity to intervene and potentially decrease adverse outcomes in this population.
Limitations
This study has limitations. As in any retrospective, observational study, unmeasured confounding may account for part of the observed differences. This was a single-center study and, aside from death, which was confirmed with the national death registry, events occurring outside of Duke University Medical Center may not have been captured. However, to our knowledge, this is the largest cohort of patients with +ECG/−Echo studied to date and as such provides adequate power to suggest an increase in cardiac risk among patients with +ECG/−Echo compared with patients with −ECG/−Echo. The increased MACE rate was largely driven by a higher rate of hospitalization for unstable angina and coronary revascularization in the first 30 days, which may be a response to the +ECG results despite normal stress Echo imaging. Stress Echos were conducted on a treadmill with stress Echo imaging performed immediately after peak stress. Although poststress imaging may be less sensitive for wall-motion abnormalities than imaging during peak with bicycle exercise Echo imaging, the workloads are usually higher with treadmill exercise, partially offsetting the advantage of peak exercise imaging.19
Conclusions
This study suggests that in a large cohort of patients undergoing stress Echo imaging, those with the discordant findings of +ECG/−Echo had a slightly higher rate of short- and long-term adverse cardiac events than patients with −ECG/−Echo. Further study is needed to assess the impact of intensification of medical management, promotion of lifestyle modification, and increased clinical follow-up in the population with +ECG/−Echo.
eFigure 1. Downstream Testing in the Year Following the Index Stress Echo
eFigure 2. Short- and Long-term Adverse Event Rates
eFigure 3. Adverse Cardiac Events in +ECG/-Echo Patients Stratified by Duke Treadmill Score and Degree of ST Depression
eFigure 4. Unadjusted Event-Free Survival From the Composite Endpoint Dichotomized by Exercise ECG Result
eFigure 5. Event-Free Survival From Individual Major Adverse Cardiac Events
eTable 1. Factors Independently Associated With +ECG/-Echo
eTable 2. Coronary Artery Disease Severity by Stress ECG/Echo Result
References
- 1.Ryan T, Feigenbaum H. Exercise echocardiography. Am J Cardiol. 1992;69(20):82H-89H. doi: 10.1016/0002-9149(92)90650-N [DOI] [PubMed] [Google Scholar]
- 2.Fihn SD, Gardin JM, Abrams J, et al. ; American College of Cardiology Foundation; American Heart Association Task Force on Practice Guidelines; American College of Physicians; American Association for Thoracic Surgery; Preventive Cardiovascular Nurses Association; Society for Cardiovascular Angiography and Interventions; Society of Thoracic Surgeons . 2012 ACCF/AHA/ACP/AATS/PCNA/SCAI/STS Guideline for the diagnosis and management of patients with stable ischemic heart disease: a report of the American College of Cardiology Foundation/American Heart Association Task Force on Practice Guidelines, and the American College of Physicians, American Association for Thoracic Surgery, Preventive Cardiovascular Nurses Association, Society for Cardiovascular Angiography and Interventions, and Society of Thoracic Surgeons. J Am Coll Cardiol. 2012;60(24):e44-e164. doi: 10.1016/j.jacc.2012.07.013 [DOI] [PubMed] [Google Scholar]
- 3.Armstrong WF, Ryan T. Stress echocardiography from 1979 to present. J Am Soc Echocardiogr. 2008;21(1):22-28. doi: 10.1016/j.echo.2007.11.005 [DOI] [PubMed] [Google Scholar]
- 4.Metz LD, Beattie M, Hom R, Redberg RF, Grady D, Fleischmann KE. The prognostic value of normal exercise myocardial perfusion imaging and exercise echocardiography: a meta-analysis. J Am Coll Cardiol. 2007;49(2):227-237. doi: 10.1016/j.jacc.2006.08.048 [DOI] [PubMed] [Google Scholar]
- 5.Leischik R, Dworrak B, Littwitz H, Gülker H. Prognostic significance of exercise stress echocardiography in 3329 outpatients (5-year longitudinal study). Int J Cardiol. 2007;119(3):297-305. doi: 10.1016/j.ijcard.2006.07.190 [DOI] [PubMed] [Google Scholar]
- 6.Sawada SG, Ryan T, Conley MJ, Corya BC, Feigenbaum H, Armstrong WF. Prognostic value of a normal exercise echocardiogram. Am Heart J. 1990;120(1):49-55. doi: 10.1016/0002-8703(90)90159-U [DOI] [PubMed] [Google Scholar]
- 7.Ismail G, Lo E, Sada M, Conant RD, Shapiro SM, Ginzton LE. Long-term prognosis of patients with a normal exercise echocardiogram and clinical suspicion of myocardial ischemia. Am J Cardiol. 1995;75(14):934-936. doi: 10.1016/S0002-9149(99)80690-0 [DOI] [PubMed] [Google Scholar]
- 8.McCully RB, Roger VL, Mahoney DW, et al. Outcome after normal exercise echocardiography and predictors of subsequent cardiac events: follow-up of 1,325 patients. J Am Coll Cardiol. 1998;31(1):144-149. doi: 10.1016/S0735-1097(97)00427-0 [DOI] [PubMed] [Google Scholar]
- 9.Fordyce CB, Douglas PS. Optimal non-invasive imaging test selection for the diagnosis of ischaemic heart disease. Heart. 2016;102(7):555-564. doi: 10.1136/heartjnl-2015-307764 [DOI] [PubMed] [Google Scholar]
- 10.McCully RB, Roger VL, Mahoney DW, et al. Outcome after abnormal exercise echocardiography for patients with good exercise capacity: prognostic importance of the extent and severity of exercise-related left ventricular dysfunction. J Am Coll Cardiol. 2002;39(8):1345-1352. doi: 10.1016/S0735-1097(02)01778-3 [DOI] [PubMed] [Google Scholar]
- 11.Elhendy A, Mahoney DW, Khandheria BK, Paterick TE, Burger KN, Pellikka PA. Prognostic significance of the location of wall motion abnormalities during exercise echocardiography. J Am Coll Cardiol. 2002;40(9):1623-1629. doi: 10.1016/S0735-1097(02)02338-0 [DOI] [PubMed] [Google Scholar]
- 12.Marwick TH, Case C, Vasey C, Allen S, Short L, Thomas JD. Prediction of mortality by exercise echocardiography: a strategy for combination with the Duke treadmill score. Circulation. 2001;103(21):2566-2571. doi: 10.1161/01.CIR.103.21.2566 [DOI] [PubMed] [Google Scholar]
- 13.Shaw LJ, Vasey C, Sawada S, Rimmerman C, Marwick TH. Impact of gender on risk stratification by exercise and dobutamine stress echocardiography: long-term mortality in 4234 women and 6898 men. Eur Heart J. 2005;26(5):447-456. doi: 10.1093/eurheartj/ehi102 [DOI] [PubMed] [Google Scholar]
- 14.Olmos LI, Dakik H, Gordon R, et al. Long-term prognostic value of exercise echocardiography compared with exercise 201Tl, ECG, and clinical variables in patients evaluated for coronary artery disease. Circulation. 1998;98(24):2679-2686. doi: 10.1161/01.CIR.98.24.2679 [DOI] [PubMed] [Google Scholar]
- 15.Al-Mallah M, Alqaisi F, Arafeh A, Lakhdar R, Al-Tamsheh R, Ananthasubramaniam K. Long term favorable prognostic value of negative treadmill echocardiogram in the setting of abnormal treadmill electrocardiogram: a 95 month median duration follow-up study. J Am Soc Echocardiogr. 2008;21(9):1018-1022. doi: 10.1016/j.echo.2008.06.005 [DOI] [PubMed] [Google Scholar]
- 16.Marwick TH, Mehta R, Arheart K, Lauer MS. Use of exercise echocardiography for prognostic evaluation of patients with known or suspected coronary artery disease. J Am Coll Cardiol. 1997;30(1):83-90. doi: 10.1016/S0735-1097(97)00148-4 [DOI] [PubMed] [Google Scholar]
- 17.Mahenthiran J, Bangalore S, Yao SS, Chaudhry FA. Comparison of prognostic value of stress echocardiography versus stress electrocardiography in patients with suspected coronary artery disease. Am J Cardiol. 2005;96(5):628-634. doi: 10.1016/j.amjcard.2005.04.032 [DOI] [PubMed] [Google Scholar]
- 18.Mark DB, Shaw L, Harrell FE Jr, et al. Prognostic value of a treadmill exercise score in outpatients with suspected coronary artery disease. N Engl J Med. 1991;325(12):849-853. doi: 10.1056/NEJM199109193251204 [DOI] [PubMed] [Google Scholar]
- 19.Pellikka PA, Nagueh SF, Elhendy AA, Kuehl CA, Sawada SG; American Society of Echocardiography . American Society of Echocardiography recommendations for performance, interpretation, and application of stress echocardiography. J Am Soc Echocardiogr. 2007;20(9):1021-1041. doi: 10.1016/j.echo.2007.07.003 [DOI] [PubMed] [Google Scholar]
- 20.Hoffmann R, Lethen H, Marwick T, et al. Standardized guidelines for the interpretation of dobutamine echocardiography reduce interinstitutional variance in interpretation. Am J Cardiol. 1998;82(12):1520-1524. doi: 10.1016/S0002-9149(98)00697-3 [DOI] [PubMed] [Google Scholar]
- 21.Bouzas-Mosquera A, Peteiro J, Alvarez-García N, et al. Prediction of mortality and major cardiac events by exercise echocardiography in patients with normal exercise electrocardiographic testing. J Am Coll Cardiol. 2009;53(21):1981-1990. doi: 10.1016/j.jacc.2009.01.067 [DOI] [PubMed] [Google Scholar]
- 22.Nesto RW, Kowalchuk GJ. The ischemic cascade: temporal sequence of hemodynamic, electrocardiographic and symptomatic expressions of ischemia. Am J Cardiol. 1987;59(7):23C-30C. doi: 10.1016/0002-9149(87)90192-5 [DOI] [PubMed] [Google Scholar]
- 23.Stillman AE, Oudkerk M, Bluemke DA, et al. Imaging the myocardial ischemic cascade. Int J Cardiovasc Imaging. 2018;34(8):1249-1263. doi: 10.1007/s10554-018-1330-4 [DOI] [PubMed] [Google Scholar]
- 24.Cole CR, Blackstone EH, Pashkow FJ, Snader CE, Lauer MS. Heart-rate recovery immediately after exercise as a predictor of mortality. N Engl J Med. 1999;341(18):1351-1357. doi: 10.1056/NEJM199910283411804 [DOI] [PubMed] [Google Scholar]
- 25.Marwick TH, Nemec JJ, Pashkow FJ, Stewart WJ, Salcedo EE. Accuracy and limitations of exercise echocardiography in a routine clinical setting. J Am Coll Cardiol. 1992;19(1):74-81. doi: 10.1016/0735-1097(92)90054-Q [DOI] [PubMed] [Google Scholar]
- 26.Lin FY, Shaw LJ, Dunning AM, et al. Mortality risk in symptomatic patients with nonobstructive coronary artery disease: a prospective 2-center study of 2,583 patients undergoing 64-detector row coronary computed tomographic angiography. J Am Coll Cardiol. 2011;58(5):510-519. doi: 10.1016/j.jacc.2010.11.078 [DOI] [PubMed] [Google Scholar]
- 27.Motoyama S, Sarai M, Harigaya H, et al. Computed tomographic angiography characteristics of atherosclerotic plaques subsequently resulting in acute coronary syndrome. J Am Coll Cardiol. 2009;54(1):49-57. doi: 10.1016/j.jacc.2009.02.068 [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
eFigure 1. Downstream Testing in the Year Following the Index Stress Echo
eFigure 2. Short- and Long-term Adverse Event Rates
eFigure 3. Adverse Cardiac Events in +ECG/-Echo Patients Stratified by Duke Treadmill Score and Degree of ST Depression
eFigure 4. Unadjusted Event-Free Survival From the Composite Endpoint Dichotomized by Exercise ECG Result
eFigure 5. Event-Free Survival From Individual Major Adverse Cardiac Events
eTable 1. Factors Independently Associated With +ECG/-Echo
eTable 2. Coronary Artery Disease Severity by Stress ECG/Echo Result