Key Points
Question
Do individuals with tuberous sclerosis–associated neuropsychiatric disorders meet criteria for the neurodegenerative disease frontotemporal dementia (FTD)?
Findings
In this case-control study, quantitative clinical measurements showed that adults with tuberous sclerosis–associated neuropsychiatric disorders exhibit behavioral and cognitive difficulties similar to those seen in FTD. Compared with FTD, significant overlap in cerebrospinal fluid biomarkers was seen in patients with tuberous sclerosis complex, and similarly, a subset of these subjects demonstrated punctate focal retention of the tau PET ligand fluorine 1B–labeled flortaucipir.
Meaning
Additional longitudinal studies should be performed on adults affected with tuberous sclerosis complex to assess risk for developing a neurodegenerative disease such as FTD.
Abstract
Importance
Individuals with tuberous sclerosis complex can develop a progressive neuropsychiatric syndrome known as tuberous sclerosis–associated neuropsychiatric disorders. Tuberous sclerosis–associated neuropsychiatric disorders symptoms overlap with clinical criteria for frontotemporal dementia, yet the association between the 2 has not been explored.
Objective
To investigate the potential association between tuberous sclerosis–associated neuropsychiatric disorders and frontotemporal dementia.
Design, Setting, and Participants
Case-control study that enrolled patients with tuberous sclerosis complex with normal IQs in an observational clinical study at the University of California, San Francisco, from 2017 to 2019 where they underwent a comprehensive clinical evaluation including neuropsychologic testing, cerebral spinal fluid biomarker profiling, and structural neuroimaging. The study included adults who fulfilled the clinical criteria for tuberous sclerosis complex and had normal IQs, had frontotemporal dementia, or were healthy control individuals.
Main Outcomes and Measures
Tuberous sclerosis–associated neuropsychiatric disorders checklist severity score, neuropsychologic test scores, cerebral spinal fluid concentrations of phosphorylated tau181, total tau, amyloid-β 42, and neurofilament light chain. Amyloid and tau positron emission tomography scans were obtained in a subset of patients.
Results
Eighteen patients with tuberous sclerosis complex (mean [SD] age, 48 years [9.54]; 13 women [72%]), 16 with frontotemporal dementia (60 [6.93] years; 7 women [44%]) and 18 healthy control individuals (63 [3.85] years; 9 women [50%]) were included. The tuberous sclerosis–associated neuropsychiatric disorders checklist and neuropsychological test results were not significantly different when the tuberous sclerosis complex and frontotemporal dementia cohorts were compared. The tuberous sclerosis complex cohort exhibited elevated cerebral spinal fluid phosphorylated tau181 and neurofilament light chain with a mean of 32 pg/mL and 2300 pg/mL, respectively, when compared to healthy control individuals. All 3 patients with tuberous sclerosis complex who underwent fluorine 1B–labeled flortaucipir tau positron emission tomographic neuroimaging showed punctate foci of elevated [18F]flortaucipir binding in the frontal and temporal regions.
Conclusions and Relevance
Adults with tuberous sclerosis complex showed phenotypic overlap with frontotemporal dementia. The results support a possible clinical continuum between tuberous sclerosis–associated neuropsychiatric disorders and frontotemporal dementia and highlights a potential pathophysiological link between neurodevelopmental and neurodegenerative processes. Quantitative neuropsychological testing and the tuberous sclerosis–associated neuropsychiatric disorders checklist, potentially supplemented by cerebral spinal fluid and imaging biomarkers, could be used to screen and prognosticate for risk of a neurodegenerative process in adult patients with tuberous sclerosis complex.
This study investigates the potential association between tuberous sclerosis–associated neuropsychiatric disorders and frontotemporal dementia.
Introduction
Tuberous sclerosis complex (TSC) is a childhood-onset disorder with characteristic dermatological findings, tumors, epilepsy, and developmental delay owing to pathogenic genetic variants in the TSC1 (hamartin) or TSC2 (tuberin) genes.1,2,3,4,5,6,7,8,9,10 Many patients with TSC also experience progressive cognitive, behavioral and psychiatric symptoms.2,4 In 2015, de Vries et al11,12 coined the term tuberous sclerosis–associated neuropsychiatric disorders (TAND) to describe this syndrome and developed the TAND checklist to standardize documentation of the constellation of these neuropsychiatric symptoms.11,12 Tuberous sclerosis–associated neuropsychiatric disorders have been reported in the pediatric and adult TSC populations.13,14 In children, TAND has been associated with regression or loss of cognitive skills.13 In adults, features of TAND include abnormalities of comportment, social cognition, language, and executive function, all of which are hallmarks of frontotemporal dementia (FTD).15
Frontotemporal dementia serves as a clinical term for a group of early-onset neurodegenerative disease disorders characterized by behavioral, executive, and sometimes language disorders associated with atrophy of the frontal and temporal lobes.16,17,18 There are 3 primary clinical subtypes: behavioral-variant FTD, semantic variant primary progressive aphasia, and nonfluent variant primary progressive aphasia. Frontotemporal dementia is caused by frontotemporal lobar degeneration (FTLD), which features abnormal aggregation of the microtubule associated protein consisting of tau (FTLD-tau) or the transactivating DNA-binding protein 43.16,18,19,20 In 2017,21 we described a novel genetic variant in the TSC1 gene that represents a potential cause of FTLD-tau, and a deregulation in hamartin, the protein product of TSC1, which is an important upstream modifier of mammalian target of rapamycin (mTOR) activity. Subsequently, TSC1 genetic variant carriers present with mTOR overactivity. Interestingly, an overactivation of other mTOR pathway proteins are also linked to tau pathology.22,23
The TAND checklist and FTD clinical criteria16,17,18 both include progressive changes in behavior (obsessive-compulsive symptoms and altered eating habits), attentional deficits, poor executive function (eg, decreased impulse inhibition and error monitoring), and deficits in language.19,21 Nevertheless, to our knowledge, a systematic comparison of neuropsychiatric symptoms in TSC and FTD has not been previously reported. The long-term clinical outcome, especially of mildly affected adult patients with TSC, remains unclear.13 Here, we analyze the clinical overlap between TAND and FTD in a longitudinal observational cohort, leveraging deep phenotyping of patients with TSC and FTD, including neuropsychological testing, neuroimaging, and biofluid markers.
Methods
Patient Selection and Study Design
Patients with a clinical or genetic diagnosis of TSC1 who were older than 18 years and were functionally independent were screened for inclusion (eTable 4 in the Supplement). Recruitment of patients with TSC was achieved in collaboration with the Tuberous Sclerosis Alliance from 2017 through June 2019. Patients with a diagnosis of probable behavioral-variant FTD, semantic variant primary progressive,16,17,18 were recruited from the program project on FTD at University of California, San Francisco. Many patients with TSC were found to have a genetic variant (eTable 5 in the Supplement). Healthy control (HC) individuals were recruited through the Hillblom Aging Network at University of California, San Francisco. Institutional review board approval was obtained from the University of California Human Research Protection Program, and written informed consent was obtained from all study participants or their surrogates.
Clinical Evaluation
A comprehensive clinical evaluation included a neurological examination by a behavioral neurologist, neuropsychological testing by a neuropsychologist,24 genetic counseling, and a caregiver interview, as previously described.18 All patients completed the TAND checklist.11 A severity score was obtained by summing the total number of yes responses to questions on the TAND checklist.11 We evaluated 4 cognitive composites (ie, episodic memory, executive functions, language, and visuospatial) on neuropsychologic testing in all 3 cohorts by calculating z scores for individual tests and then averaging across subtests for each cognitive domain, similar to previously published methods.25 An episodic memory composite score was created using a measure of visual memory, Benson Figure Recall,24 and the California Verbal Learning Test, second edition, short-form (CVLT-II26) subscores: immediate recall total, long (20-minute) delay free recall total, and recognition discriminability (d′). The executive functions composite included Stroop interference,27 modified Trail Making Test,24 phonemic fluency (number of D words per minute,24 Digit Span Backward,28 and design fluency).29 Because the 18 HCs included in this study were not administered the same version of the California Verbal Learning Test, the episodic memory composite was not created in this group. The language composite combined an abbreviated, 15-item version of the Boston Naming Test and animal fluency. Finally, a spatial composite included the copy subtask of the Benson Figure task and number location of the Visual Object and Space Perception Battery. Composite scores were created based on the available data for each patient even if some data were missing. An executive composite was created for a patient if they had at least 3 of 5 measures. An episodic memory composite was created if a patient had at least 2 of 4 measures. A language composite was created if patients had at least 1 of 2 measures. A spatial composite was created if subjects had at least 1 of 2 measures. A subset of patients were administered the Wechsler Abbreviated Intelligence Scale28 to quantify general intelligence. All patients with TSC received a brain magnetic resonance imaging (MRI). A lumbar puncture, carbon 11–labeled Pittsburgh compound B (PiB) and fluorine 1B–labeled [18F] flortaucipir (FTP) positron emission tomography (PET) scans were performed on a subset of patients with TSC.15,24
Genetic Testing and Cerebrospinal Fluid Biomarker Investigations
In the TSC cohort, blood-derived DNA was used to sequence the TSC1 and TSC2 genes. Samples were screened using either whole-exome or targeted sequencing as previously described.21,30 Coding and exon-intron boundary regions of TSC1 and TSC2 genes were screened for known or novel pathogenic variants classified as previously described.31 Sanger sequencing was used to confirm pathogenic variants. Confirmed genetic test results were conveyed to the patient with TSC and family members by a licensed genetic counselor and the behavioral neurologist.
The lumbar puncture was performed by the behavioral neurologist. Cerebrospinal fluid (CSF) was stored at –80°C until analysis. The CSF biomarker analysis was performed in collaboration with Anne Fagan’s laboratory at Washington University in St Louis, Missouri. Cerebrospinal fluid was analyzed using the INNO-BIA AlzBio3 (Fujirebio Europe), a multiplexed, fluorimetric bead immunoassay, for the simultaneous quantification of phosphorylated tau181 (P-tau), total tau (T-tau), and amyloid-β 42 (Aβ42). Neurofilament light chain (NfL) was measured with NF-light enzyme-linked immunosorbent assay (UmanDiagnostics AB). Samples underwent a single freeze-thaw cycle prior to assay. Samples were thawed at 4°C for approximately 3 hours prior to analysis, and all were run in duplicate. For all assays, values had to pass quality control criteria, including coefficients of variation (calculated as standard deviation × 100/mean) 25% or less, kit controls within the expected range as defined by the manufacturer (where applicable), and measurement consistency of 2 common pooled CSF samples that were included on each plate.
The CSF biomarker levels reported in the FTD and HC cohorts overlapped with the data reported by Ljubenkov et al32 in 2018. Neurofilament light chain was chosen because in 2014 several groups reported that high CSF NfL levels were associated with more severe cognitive impairment and shorter survival time in patients with FTD.32,33 It is also well established that elevated NfL levels in the CSF implicate a neuronal damage.34 An NfL result greater than 4170 pg/mL was considered abnormal.32 Phosphorylated tau181 and T-tau values greater than 23 pg/mL and 93 pg/mL, respectively, were considered abnormal.35 Sato et al36 report that in the human central nervous system, P-tau exhibits a faster turnover rate, which likely explains the elevated CSF level of this protein in various neurodegenerative conditions. An Aβ42 value less than 350 pg/mL was considered abnormal.35
Neuroimaging Biomarker Investigation
T1-weighted magnetization–prepared rapid gradient echo MRI sequences were acquired at University of California, San Francisco, either on a 3-T Siemens Tim Trio or a 3-T Siemens Prisma Fit scanner. Both scanners had similar acquisition parameters (sagittal slice orientation; slice thickness = 1.0 mm; 160 slices; in-plane resolution = 1 × 1 mm; matrix = 240 × 256; repetition time = 2.300 ms; inversion time = 900 milliseconds; flip angle = 9°), although echo time slightly differed (Trio: 2.98 milliseconds; Prisma: 2.9 milliseconds).
Positron emission tomography scans were acquired from a subset of patients with TSC on a Siemens Biograph PET/computed tomography (CT) scanner at the Lawrence Berkeley National Laboratory. 11C-Pittsburgh compound B and FTP were also synthesized and radiolabeled at the Lawrence Berkeley National Laboratory. A low-dose CT scan was performed for attenuation correction prior to PET acquisition, and PET data were reconstructed using an ordered subset expectation maximization algorithm with weighted attenuation and smoothed with a 4-mm gaussian kernel with scatter correction (calculated image resolution 6.5 × 6.5 × 7.25 mm based on Hoffman phantom). Further details of the MRI and PET data acquisition and preprocessing are available in previous publications.35,37,38,39 11C-Pittsburgh compound B–PET standardized uptake value ratio images were read as positive or negative for cortical binding39 and a global neocortical standardized uptake value ratio value was extracted and compared with previously published thresholds.40
Statistical Analyses
Linear regression models covarying for age, sex, and genotype determined group differences in the neuropsychologic composite scores. Overall variations were determined by the Omnibus model or χ2 test. Nonparametric analysis was used to analyze the CSF results. Statistical analyses were performed using Stata, version 15 software (StataCorp). All tests were 2-sided, and P values less than .05 were considered statistically significant.
Results
Follow-up Case Report
We previously published a case report of an individual diagnosed as having FTD who was found to have a novel genetic variant loss of function in the TSC1 gene.21 A 3-year follow up MRI scan of this individual revealed progressive atrophy involving the bilateral temporal lobes and orbitofrontal cortices (eFigure 1A and 1B the Supplement). The fluid-attenuated inversion recovery sequence from 2015 showed a hyperintense signal in the left superior temporal lobe typically referred to as a radial band, which is commonly seen in patients with TSC (eFigure 1C and 1D in the Supplement). No significant progression of the hyperintense signal in the radial band was seen during the 3-year period. This individual met criteria for both FTD and TAND in both 2015 and 2018. To assess whether TAND and FTD are overlapping clinical entities in other patients with TSC, we recruited a group of mildly affected adult individuals with TSC, confirmed their genetic variant in TSC1 or TSC2, and performed a comprehensive clinical phenotyping for both TAND and FTD.
Clinical Phenotyping
Eighteen individuals with TSC, 16 with FTD, and 18 HCs were included. Basic demographic information from all groups include age, sex, mean years of education, and measures of general intelligence are shown in eTable 1 in the Supplement. Most adult patients with TSC held college and postgraduate degrees. Most had mild cognitive issues. The TSC cohort’s age ranged from 27 to 66 years, with a mean age of 48 years. Seventy-two percent of the TSC cohort were women. The mean age of the TSC cohort was approximately 12 years and 15 years younger than the FTD and HC cohorts, respectively. Other basic demographic information for individual patients in all 3 cohorts is shown in Table 1. Ten of 18 patients with TSC had genetic information available, with an equal number of TSC1 and TSC2 genetic variants (eTable 5 in the Supplement). Notably, patients 9 and 10 have a genetic variant in TSC2 of unknown significance or evidence for pathogenicity at this time (eTable 5 in the Supplement). Two TSC study participants displayed skin findings consistent with TSC and had a child with TSC, but whole-exome sequencing of the enrolled participant showed no genetic variants in their TSC1 or TSC2 genes. These individuals may have had a moderate-sized to large insertion or deletion that could not be detected or were mosaic for a mutation. The 6 remaining TSC genetic results were still pending or their DNA was not collected.
Table 1. Demographic Information of All Cohorts.
| Cohort | Sex/Age, y | Years of Education | Diagnosis |
|---|---|---|---|
| TSC | F/27 | 16 | TSC |
| TSC | F/37 | 16 | TSC |
| TSC | F/38 | 16 | TSC |
| TSC | F/40 | 14 | TSC |
| TSC | F/41 | 14 | TSC |
| TSC | F/44 | 16 | TSC |
| TSC | M/44 | 18 | TSC |
| TSC | F/45 | 13 | TSC |
| TSC | F/48 | 13 | TSC |
| TSC | F/49 | 18 | TSC |
| TSC | M/51 | 18 | TSC |
| TSC | M/51 | 16 | TSC |
| TSC | F/53 | 14 | TSC |
| TSC | M/53 | 12 | TSC |
| TSC | M/55 | 12 | TSC |
| TSC | F/59 | 16 | TSC |
| TSC | F/61 | 16 | TSC |
| TSC | F/66 | 18 | TSC |
| FTD | M/68 | 16 | svPPA |
| FTD | F/63 | 12 | bvFTD |
| FTD | M/51 | 14 | bvFTD |
| FTD | F/59 | 17 | bvFTD |
| FTD | M/67 | 14 | bvFTD |
| FTD | M/54 | 12 | bvFTD |
| FTD | F/64 | 19 | bvFTD and svPPA |
| FTD | M/52 | 13 | bvFTD |
| FTD | M/65 | 14 | bvFTD |
| FTD | F/56 | 12 | bvFTD |
| FTD | F/65 | 14 | bvFTD |
| FTD | F/59 | 16 | bvFTD, unspecified PPA |
| FTD | M/58 | 18 | bvFTD |
| FTD | M/65 | 16 | svPPA |
| FTD | F/67 | 16 | Possible bvFTD |
| FTD | M/44 | 16 | CBS |
| HC | M/62 | 18 | None |
| HC | M/67 | 16 | None |
| HC | M/57 | 16 | None |
| HC | M/56 | 16 | None |
| HC | F/64 | 16 | None |
| HC | M/56 | 20 | None |
| HC | F/59 | 19 | None |
| HC | M/58 | 14 | None |
| HC | F/67 | 14 | None |
| HC | F/67 | 18 | None |
| HC | F/66 | 16 | None |
| HC | F/64 | 17 | None |
| HC | F/63 | 19 | None |
| HC | F/64 | 19 | None |
| HC | M/63 | 19 | None |
| HC | F/65 | 19 | None |
| HC | M/66 | 18 | None |
| HC | M/65 | 14 | None |
Abbreviations: bvFTD, behavioral variant frontotemporal dementia; CBS, corticobasal syndrome; FTD, frontotemporal dementia; HC, healthy control individual; MMSE, Mini-Mental State Examination; PPA, primary progressive aphasia; svPPA, semantic variant primary progressive aphasia; TSC, tuberous sclerosis complex; WASI, Weschler Abbreviated Intelligence Scale.
The TAND checklist serves as a clinical screening tool to assess neuropsychiatric difficulties that include the behavioral, cognitive and psychiatric domains.11 Development of the TAND checklist was necessary because 90% of patients with TSC endorsed neuropsychiatric symptoms, yet only 20% received treatment.11 We collected the TAND checklist from 16 patients with TSC, 15 patients with FTD, and 16 HC individuals (Table 2) and calculated a severity score for each neuropsychiatric domain on all cohorts. The TAND checklist severity score for the TSC and FTD cohorts was not significantly different in the behavioral and cognitive categories (eTable 2 in the Supplement). Both TSC and FTD severity scores in these domains were significantly higher when compared with HC individuals (Table 2). When the total severity score was compared, the TSC group occupied an intermediate space between FTD and HC cohorts, although it was not significantly different from either group (eTable 2 in the Supplement).
Table 2. TAND Checklist Severity Scores From All 3 Cohortsa.
| TAND Checklist Categories and Sections | Severity Score From the TAND Checklist, No. Endorsing/Total No. (%) | ||
|---|---|---|---|
| TSC | FTD | HC | |
| Behavioral total severity score, mean (SD) | 4.6 (3.04) | 5.07 (4.05) | 0.63 (1.2) |
| Outbursts | 5/16 (31) | 7/14 (50) | 0/16 |
| Tantrum | 3/16 (19) | 6/14 (43) | 0/16 |
| Repeating | 5/16 (32) | 4/15 (36) | 0/16 |
| Eye contact | 2/16 (12.5) | 5/14 (36) | 0/16 |
| Difficulty getting along | 3/16 (19) | 1/15 (7) | 0/16 |
| Repetitive behaviors | 5/16 (31) | 6/15 (40) | 0/16 |
| Rigid | 5/16 (31) | 6/15 (40) | 1/16 (6) |
| Overactivity | 6/16 (38) | 5/15 (33) | 1/16 (6) |
| Restlessness | 8/15 (53) | 6/15 (40) | 2/16 (13) |
| Impulsivity | 9/16 (56) | 9/15 (60) | 1/16 (6) |
| Difficulty eating | 6/16 (38) | 8/15 (53) | 0/16 (0) |
| Sleep difficulty | 12/16 (75) | 7/15 (47) | 4/16 (25) |
| Autism spectrum disorder | 0/16 | 0/15 (0) | 0/16 |
| ADHD | 3/16 (19) | 1/14 (7) | 1/16 (6) |
| Cognitive total severity score, mean (SD) | 4.62 (3.66) | 5.33 (3.26) | .5 (.73) |
| Delayed language | 3/15 (20) | 6/15 (40) | 0/16 |
| Difficulty with attention | 10/15 (67) | 9/15 (60) | 1/16 (6) |
| Reading | 4/15 (27) | 3/12 (25) | 2/16 (13) |
| Writing | 3/14 (21) | 1/12 (8) | 0/16 |
| Spelling | 2/14 (14) | 4/12 (33) | 0/16 |
| Mathematic | 10/15 (67) | 3/12 (25) | 2/16 (13) |
| Memory | 10/16 (63) | 9/15 (60) | 3/16 (19) |
| Attention | 10/16 (63) | 12/15 (80) | 0/16 |
| Multitasking | 7/16 (44) | 10/15 (67) | 0/16 |
| Visuospatial | 4/16 (25) | 7/15 (47) | 0/16 |
| Executive | 8/16 (50) | 10/15 (67) | 0/16 |
| Disorientation | 2/15 (13) | 7/15 (47) | 0/16 |
| Psychiatric total severity score, mean (SD) | 2.31 (1.85) | 2.5 (1.22) | .94 (1.06) |
| Anxiety | 13/16 (81) | 12/14 (86) | 4/16 (25) |
| Depressed | 10/16 (63) | 9/14 (64) | 6/16 (38) |
| Shyness | 4/16 (25) | 1/14 (7) | 2/16 (12.5) |
| Mood swing | 7/15 (47) | 10/15 (67) | 3/16 (19) |
| Self-injury | 2/15 (13) | 1/15 (7) | 0/16 |
| Obsessive | 3/16 (19) | 1/15 (7) | 0/16 |
| Psychotic | 0/15 | 1/15 (7) | 0/16 |
Abbreviations: ADHD, attention-deficit/hyperactivity disorder; FTD, frontotemporal dementia; HC, healthy control; TAND, tuberous sclerosis–associated neuropsychiatric disorders; TSC, tuberous sclerosis complex.
The TSC and FTD cohorts endorsed several behavioral and cognitive difficulties along with anxiety and depression. Because not all participants filled out every question, the denominators differ slightly between questions. To retain as much data as possible, we presented the data as percentages to account for differing sample sizes among items.
As part of the diagnostic criteria, patients with FTD completed a standardized set of neuropsychological tests that assessed each cognitive domain. Test scores were compared with norms for age (results were considered abnormal if >1 SD from norm). We also performed standardized cognitive testing on the TSC and HC cohorts. The TSC and FTD cohorts performed similarly in tests of executive function, language, and visuospatial function (Table 3). In contrast, both TSC and FTD performed significantly worse than HC individuals in the executive and language domains (Table 3). The TSC and FTD cohorts were significantly different in memory testing (Table 3). The memory composite in the TSC and FTD cohorts could not be compared with the HC owing to differences in the test battery.
Table 3. Comparison of the Neuropsychologic Profiles in All Cohortsa.
| Domain | Group Differences | TSC vs HC | FTD vs HC | TSC vs FTD | Omnibus Model | ||||
|---|---|---|---|---|---|---|---|---|---|
| β | P Value | β | P Value | β | P Value | Fdf | Model P Value | ||
| Executive | (TSC = FTD) >HC | 1.15 | .002 | 1.44 | <.001 | –0.3 | .39b | 9.854,43 | <.001 |
| Language | (TSC = FTD) >HC | 1.71 | .01 | 2.89 | <.001 | –1.17 | .07b | 6.614,43 | <.001 |
| Spatial | FTD ≠ TSC ≠ HC | 0.07 | .88 | 0.11 | .78 | –0.04 | .93b | 0.684,45 | .61 |
| Memory | TSC >FTD | NA | NA | NA | NA | –1.87 | .04 | 4.433,26 | .01 |
Abbreviations: FTD, frontotemporal dementia; HC, healthy control; TSC, tuberous sclerosis complex.
P values less than .05 were considered significant.
Insignificant changes in all categories when TSC was compared with FTD. Note that the data used to calculate the information for the HC and FTD for neuropsychological testing were different from the participants who completed the TAND checklist.
Investigational Tau-PET Imaging Biomarker
We and others have previously demonstrated a link between TSC and abnormal tau metabolism.21,41 In fact, TSC is considered 1 of a group of juvenile tauopathies.22 Therefore, in addition to comprehensive clinical assessments, we performed tau-PET imaging with the tau tracer FTP.35 Tau-PET neuroimaging was completed in 3 patients with TSC, 2 who were in their mid to late 40s and 1 who was in their late 50s, respectively. All 3 patients demonstrated small foci of FTP uptake predominantly in the frontal and temporal lobes. In 2 of 3 patients, the anatomical location of FTP uptake corresponded with structural changes seen on T1 and fluid attenuation inversion recovery sequences of the brain MRI (eFigure 2-8 in the Supplement). This pattern of distribution has not been reported in the past. The tracer FTP exhibits strong binding to neurofibrillary tangles in Alzheimer disease but also demonstrates low-level uptake to off-target (ie, non–tau-related) lesions such as hemorrhagic foci, calcifications, neuromelanin, and non–tau-related neurodegeneration.42,43,44,45 Therefore, we reviewed susceptibility-weighted imaging sequences in 1 patient and the low-dose CT in the 2 other patients who received tau-PET imaging, depending on availability. These sequences showed no evidence of calcifications in the tubers or other cortical malformations; thus, binding to calcifications appears to be less likely. However, the low-dose CT scans are of low quality, and therefore, our sensitivity to capture calcification in two-thirds of the cases was low. All 3 patients with TSC who had tau-PET scans also underwent PiB-PET scans for amyloid β deposition, which were negative (data not shown).
Cerebrospinal Fluid Biomarkers
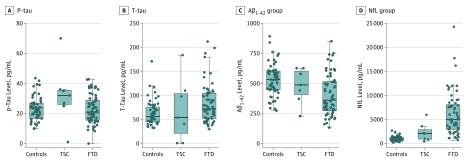
Cerebrospinal fluid was available for biomarker analyses in 7 patients with TSC, 90 with FTD, and 49 HC individuals. The CSF results from the FTD and HC cohorts overlapped with the data used by Ljubenkov et al.32 It was reported that elevated levels of CSF P-tau, with a mean level of 31 pg/mL, is seen in FTD owing to a genetic variant in the MAPT gene,46 which produces the protein product tau. Intriguingly, the mean P-tau level in the TSC cohort was 32 pg/mL. No difference was seen in the CSF P-tau level when the TSC and FTD cohorts were compared (Table 4). In contrast, the P-tau level in the TSC cohort showed a significant difference when compared with HC individuals (Table 4). Ljubenkov et al32 report that the T-tau and Aβ42 levels in patients with FTD are similar to patients with normal cognition. These results were also seen in the TSC cohort (Figure; Table 4), with the exception of TSC patient 2 (eTable 3 in the Supplement). Additionally, Ljubenkov et al32 showed that patients with FTD typically have elevated CSF levels of NfL when compared with control individuals. Comparison of the CSF NfL levels between TSC and HC individuals was underpowered to show a significant difference between the 2 cohorts (Table 4).
Table 4. Statistical Analysis of All 3 Cohorts With Corresponding Biomarkersa.
| Biomarker | Mean (SD) | TSC vs HC | FTD vs HC | TSC vs FTD | χ2 | P Value | |||||
|---|---|---|---|---|---|---|---|---|---|---|---|
| TSC | FTD | HC | z Value | P Value | z Value | P Value | z Value | P Value | |||
| P-tau, pg/mL | 32 (20) | 23 (9.435) | 23 (8.024) | –1.969 | .049 | 0.829 | .41 | 1.668 | .10 | 3.60 | .17 |
| T-tau, pg/mL | 70 (66) | 83 (41) | 63 (27) | 0.058 | .95 | –2.706 | .007 | –0.849 | .40 | 7.91 | .20 |
| Aβ42, pg/mL | 480 (147) | 406 (173) | 530 (137) | 0.587 | .56 | 3.898 | <.001 | 1.312 | .19 | 16.41 | <.001 |
| T-tau to Aβ42 ratio | 0.139 (0.110) | 0.248 (0.197) | 0.124 (0.058) | –1.048 | .30 | –6.017 | <.001 | –1.254 | .21 | 32.26 | <.001 |
| NfL, pg/mL | 2300 (1928) | 6113 (4466) | 999 (499) | –1.877 | .06 | –9.874 | <.001 | –2.777 | .01 | 74.65 | <.001 |
Abbreviations: Aβ42, amyloid-β 42; CSF, cerebral spinal fluid; FTD, frontotemporal dementia; HC, healthy control; NfL, neurofilament light chain; P-tau, phosphorylated tau, T-tau, total tau, TSC, tuberous sclerosis complex.
The elevated P-tau level was found to be statistically significant when TSC was compared with HC in bold. No difference was found when P-tau in TSC was compared with FTD. Comparison of the NfL levels between the TSC and HC cohorts showed no difference.
Figure. Graphic Comparison of 4 Cerebrospinal Fluid (CSF) Biomarkers in All 3 Cohorts.
A, Phosphorylated tau181 (P-tau) was significantly elevated in the tuberous sclerosis complex (TSC) cohort when compared with the control and frontotemporal dementia (FTD) cohorts. B and C, No difference in the total tau (T-tau) or amyloid-β 42 (Aβ42) levels were appreciated when TSC was compared with the other cohorts. D, The mean neurofilament light chain (NfL) level was elevated when TSC was compared with healthy control individuals.
Discussion
It is well established that patients with TSC can experience TAND symptoms, particularly in those more mildly affected. Nevertheless, these symptoms are not well characterized in older adults with TSC. This is important because medical and surgical advancements in TSC during the past decade have allowed affected individuals to live beyond age 50 years.47,48,49 The focus of this study was to characterize the clinical phenotype of adult patients with TSC with quantitative neuropsychologic measurements and biomarkers to evaluate its association with the neurodegenerative disorder FTD. The TSC group illustrated a pattern of impairment predominantly involving the cognitive and behavioral domains that closely mirrored what is seen in patients with FTD.
The patients with TSC described cognitive and psychiatric symptoms that included executive and memory dysfunction, agitation, and aggressive behavior that are all seen in patients with FTD as well. Significant overlap in both the TAND checklist severity score and neuropsychologic testing19,50,51 was appreciated when the patients with TSC and FTD were compared (Table 2 and 3). P-tau is elevated in FTD owing to a genetic variant in MAPT, which was also elevated in the patients with TSC when compared with the HC cohort (Table 4B). The importance of this is highlighted by the fact that elevated CSF NfL and P-tau levels have a greater predictive value in patients with tau pathology.32 Together, the data establish a striking clinical overlap between TAND and FTD that has major implications for care and treatment of the aging TSC population.
Whether TSC ultimately predisposes to a neurodegenerative disorder, such as FTD, requires further investigation. It can be difficult to perform these types of studies in those with significant intellectual disability from childhood, and therefore our cohort selection was limited to older individuals with normal IQs. An additional limitation of this study is that to achieve an adequate study group for comparison to the patients with TSC, we used different patients with FTD for cognitive testing and CSF biomarker analysis. Nonetheless, our results support future work in which populations of adults with TSC are followed longitudinally with neuropsychologic testing, neuroimaging, and fluid biomarker collection. These types of studies will be key to determining whether TAND symptoms represent a clinical continuum with FTD and for developing targeted interventions and therapeutics.
Limitations
Inclusion criteria for this study required that the patients with TSC be able to complete neuropsychological testing. Subsequently, the results from this study may not be generalizable to all patients with TSC. There is a higher risk of a type 1 error owing to the small sample size. A larger sample size will allow for a more precise clinical phenotyping. Longitudinal clinical and biomarker characterization will also further clarify the clinical trajectory of adult patients with TSC. Additionally, the FTP PET ligand is not specific for tau pathology and can show elevated binding unrelated to tau in normal individuals and in patients with non–tau-related neueodegeneration.42 Therefore, neuropathologic correlation is needed in patients with TSC who receive FTP tau-PET scans to determine whether the FTP is binding to a pathological form of tau. Lastly, the patients with FTD and HC participants who provided CSF results were not the same patients with FTD and HC participants who completed clinical measurements.
Conclusions
Our data suggest a clinical overlap and therefore potential association between TAND and the neurodegenerative disease FTD, in particular, where the latter is associated with the accumulation of pathological tau. This study provides a template by which to screen and prognosticate regarding cognitive difficulties and behavioral changes in the aging TSC population.
eTable 1. General demographic and global cognitive function information
eTable 2. Statistical analysis of the TAND checklist severity score
eTable 3. CSF biomarkers in a subset of TSC subjects
eTable 4. A list of the genetic and clinical criteria for the diagnosis of TSC.
eTable 5. Genetic mutation results of the TSC and FTD cohorts.
eFigure 1. Comparison of the brain MRI T1 sequences from 2015 to 2018 in a patient diagnosed with bvFTD and svPPA due to a TSC1 mutation.
eFigure2. Imaging biomarker results of the left anterior cingulate in the 59 year-old TSC participant.
eFigure 3. Imaging biomarker results of the left temporal neocortex in the same 59 year-old TSC participant in eFigure 2.
eFigure 4. Imaging biomarkers results of the right anterior cingulate in the 48 year-old TSC participant.
eFigure 5. Imaging biomarker results of the right precentral neocortex in the 44 year-old TSC participant.
eFigure 6. Imaging biomarker results of the left orbitofrontal neocortex in the same 44 year-old TSC participant from eFigure 5.
eFigure 7. Imaging biomarker results of the left dorsal occipital neocortex in the same 44 year-old TSC participant from eFigure 5.
eFigure 8. Imaging biomarker results of the left ventral occipital gyrus in the same 44 year-old TSC participant from eFigure 5.
References
- 1.Northrup H, Krueger DA, Roberds S, et al. ; International Tuberous Sclerosis Complex Consensus Group . Tuberous sclerosis complex diagnostic criteria update: recommendations of the 2012 International Tuberous Sclerosis Complex Consensus Conference. Pediatr Neurol. 2013;49(4):243-254. doi: 10.1016/j.pediatrneurol.2013.08.001 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Kwiatkowski DJ, Thiele EA, Whittemore VH. Tuberous Sclerosis Complex Hoboken, NJ: Wiley-Blackwell; 2010. [Google Scholar]
- 3.DiMario FJ Jr, Sahin M, Ebrahimi-Fakhari D. Tuberous sclerosis complex. Pediatr Clin North Am. 2015;62(3):633-648. doi: 10.1016/j.pcl.2015.03.005 [DOI] [PubMed] [Google Scholar]
- 4.Curatolo P, Moavero R, de Vries PJ. Neurological and neuropsychiatric aspects of tuberous sclerosis complex. Lancet Neurol. 2015;14(7):733-745. doi: 10.1016/S1474-4422(15)00069-1 [DOI] [PubMed] [Google Scholar]
- 5.Gipson Tanjala T, Johnston Michael V. New insights into the pathogenesis and prevention of tuberous sclerosis-associated neuropsychiatric disorders (TAND). F1000 Research. 2017;6. doi: 10.12688/f1000research.11110.1 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Ehninger D. From genes to cognition in tuberous sclerosis: implications for mTOR inhibitor-based treatment approaches. Neuropharmacology. 2013;68:97-105. doi: 10.1016/j.neuropharm.2012.05.015 [DOI] [PubMed] [Google Scholar]
- 7.Dabora SL, Jozwiak S, Franz DN, et al. . Mutational analysis in a cohort of 224 tuberous sclerosis patients indicates increased severity of TSC2, compared with TSC1, disease in multiple organs. Am J Hum Genet. 2001;68(1):64-80. doi: 10.1086/316951 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Roach ES, Kwiatkowski DJ. Seizures in tuberous sclerosis complex: hitting the target. Lancet. 2016;388(10056):2062-2064. doi: 10.1016/S0140-6736(16)31576-8 [DOI] [PubMed] [Google Scholar]
- 9.Kwiatkowski DJ, Short MP. Tuberous sclerosis. Arch Dermatol. 1994;130(3):348-354. doi: 10.1001/archderm.1994.01690030080013 [DOI] [PubMed] [Google Scholar]
- 10.Han JM, Sahin M. TSC1/TSC2 signaling in the CNS. FEBS Lett. 2011;585(7):973-980. doi: 10.1016/j.febslet.2011.02.001 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.de Vries PJ, Whittemore VH, Leclezio L, et al. . Tuberous sclerosis associated neuropsychiatric disorders (TAND) and the TAND checklist. Pediatr Neurol. 2015;52(1):25-35. doi: 10.1016/j.pediatrneurol.2014.10.004 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.de Vries PJ, Belousova E, Benedik MP, et al. ; TOSCA Consortium and TOSCA Investigators . TSC-associated neuropsychiatric disorders (TAND): findings from the TOSCA natural history study. Orphanet J Rare Dis. 2018;13(1):157. doi: 10.1186/s13023-018-0901-8 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Kingswood JC, d’Augères GB, Belousova E, et al. ; TOSCA consortium and TOSCA investigators . Tuberous Sclerosis registry to increase disease Awareness (TOSCA): baseline data on 2093 patients. Orphanet J Rare Dis. 2017;12(1):2. doi: 10.1186/s13023-016-0553-5 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Peron A, Vignoli A, Briola F, et al. ; TSC Study Group of the San Paolo Hospital of Milan . Deep phenotyping of patients with Tuberous Sclerosis Complex and no mutation identified in TSC1 and TSC2. Eur J Med Genet. 2018;61(7):403-410. doi: 10.1016/j.ejmg.2018.02.005 [DOI] [PubMed] [Google Scholar]
- 15.Staffaroni AM, Ljubenkov PA, Kornak J, et al. . Longitudinal multimodal imaging and clinical endpoints for frontotemporal dementia clinical trials. Brain. 2019;142(2):443-459. doi: 10.1093/brain/awy319 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Olney NT, Spina S, Miller BL. Frontotemporal dementia. Neurol Clin. 2017;35(2):339-374. doi: 10.1016/j.ncl.2017.01.008 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Ratnavalli E, Brayne C, Dawson K, Hodges JR. The prevalence of frontotemporal dementia. Neurology. 2002;58(11):1615-1621. doi: 10.1212/WNL.58.11.1615 [DOI] [PubMed] [Google Scholar]
- 18.Cairns NJ, Bigio EH, Mackenzie IR, et al. ; Consortium for Frontotemporal Lobar Degeneration . Neuropathologic diagnostic and nosologic criteria for frontotemporal lobar degeneration: consensus of the Consortium for Frontotemporal Lobar Degeneration. Acta Neuropathol. 2007;114(1):5-22. doi: 10.1007/s00401-007-0237-2 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Rascovsky K, Hodges JR, Knopman D, et al. . Sensitivity of revised diagnostic criteria for the behavioural variant of frontotemporal dementia. Brain. 2011;134(pt 9):2456-2477. doi: 10.1093/brain/awr179 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.van Swieten J, Spillantini MG. Hereditary frontotemporal dementia caused by tau gene mutations. Brain Pathol. 2007;17(1):63-73. doi: 10.1111/j.1750-3639.2007.00052.x [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Olney NT, Alquezar C, Ramos EM, et al. . Linking tuberous sclerosis complex, excessive mTOR signaling, and age-related neurodegeneration: a new association between TSC1 mutation and frontotemporal dementia. Acta Neuropathol. 2017;134(5):813-816. doi: 10.1007/s00401-017-1764-0 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Sarnat HB, Flores-Sarnat L. Infantile tauopathies: hemimegalencephaly; tuberous sclerosis complex; focal cortical dysplasia 2; ganglioglioma. Brain Dev. 2015;37(6):553-562. doi: 10.1016/j.braindev.2014.08.010 [DOI] [PubMed] [Google Scholar]
- 23.Tang Z, Ioja E, Bereczki E, et al. . mTor mediates tau localization and secretion: implication for Alzheimer’s disease. Biochim Biophys Acta. 2015;1853(7):1646-1657. doi: 10.1016/j.bbamcr.2015.03.003 [DOI] [PubMed] [Google Scholar]
- 24.Kramer JH, Jurik J, Sha SJ, et al. . Distinctive neuropsychological patterns in frontotemporal dementia, semantic dementia, and Alzheimer disease. Cogn Behav Neurol. 2003;16(4):211-218. doi: 10.1097/00146965-200312000-00002 [DOI] [PubMed] [Google Scholar]
- 25.Pankov A, Binney RJ, Staffaroni AM, et al. . Data-driven regions of interest for longitudinal change in frontotemporal lobar degeneration. Neuroimage Clin. 2015;12(C):332-340. doi: 10.1016/j.nicl.2015.08.002 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Delis DC, Kramer JH, Kaplan E, Ober BA. California Verbal Learning Test, Second Edition, Adult Version: Manual San Antonio, TX: Psychological Corporation; 2000. [Google Scholar]
- 27.Stroop JR. Studies of interference in serial verbal reaction. J Exp Psychol. 1935;18(6):643-662. doi: 10.1037/h0054651 [DOI] [Google Scholar]
- 28.Wechsler D. Wechsler Adult Intelligence Scale. New York, NY: The Psychological Corporation; 1997:3. [Google Scholar]
- 29.Delis DC, Kaplan E, Kramer J. Delis-Kaplan Executive Function System: Examiner’s Manual. San Antonio, TX: The Psychological Corporation; 2001. [Google Scholar]
- 30.Ramos EM, Dokuru DR, Van Berlo V, et al. . Genetic screen in a large series of patients with primary progressive aphasia. Alzheimers Dement. 2019;15(4):553-560. doi: 10.1016/j.jalz.2018.10.009 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Richards S, Aziz N, Bale S, et al. ; ACMG Laboratory Quality Assurance Committee . Standards and guidelines for the interpretation of sequence variants: a joint consensus recommendation of the American College of Medical Genetics and Genomics and the Association for Molecular Pathology. Genet Med. 2015;17(5):405-424. doi: 10.1038/gim.2015.30 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Ljubenkov PA, Staffaroni AM, Rojas JC, et al. . Cerebrospinal fluid biomarkers predict frontotemporal dementia trajectory. Ann Clin Transl Neurol. 2018;5(10):1250-1263. doi: 10.1002/acn3.643 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Skillbäck T, Farahmand B, Bartlett JW, et al. . CSF neurofilament light differs in neurodegenerative diseases and predicts severity and survival. Neurology. 2014;83(21):1945-1953. doi: 10.1212/WNL.0000000000001015 [DOI] [PubMed] [Google Scholar]
- 34.Khalil M, Teunissen CE, Otto M, et al. . Neurofilaments as biomarkers in neurological disorders. Nat Rev Neurol. 2018;14(10):577-589. doi: 10.1038/s41582-018-0058-z [DOI] [PubMed] [Google Scholar]
- 35.La Joie R, Bejanin A, Fagan AM, et al. . Associations between [18F]AV1451 tau PET and CSF measures of tau pathology in a clinical sample. Neurology. 2018;90(4):e282-e290. doi: 10.1212/WNL.0000000000004860 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Sato C, Barthélemy NR, Mawuenyega KG, et al. . Tau kinetics in neurons and the human central nervous system. Neuron. 2018;97(6):1284-1298.e7. doi: 10.1016/j.neuron.2018.02.015 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Maass A, Landau S, Baker SL, et al. ; Alzheimer’s Disease Neuroimaging Initiative . Comparison of multiple tau-PET measures as biomarkers in aging and Alzheimer’s disease. Neuroimage. 2017;157:448-463. doi: 10.1016/j.neuroimage.2017.05.058 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Baker SL, Maass A, Jagust WJ. Considerations and code for partial volume correcting [18F]-AV-1451 tau PET data. Data Brief. 2017;15:648-657. doi: 10.1016/j.dib.2017.10.024 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Rabinovici GD, Rosen HJ, Alkalay A, et al. . Amyloid vs FDG-PET in the differential diagnosis of AD and FTLD. Neurology. 2011;77(23):2034-2042. doi: 10.1212/WNL.0b013e31823b9c5e [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Villeneuve S, Rabinovici GD, Cohn-Sheehy BI, et al. . Existing Pittsburgh compound-B positron emission tomography thresholds are too high: statistical and pathological evaluation. Brain. 2015;138(pt 7):2020-2033. doi: 10.1093/brain/awv112 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Qin W, Chan JA, Vinters HV, et al. . Analysis of TSC cortical tubers by deep sequencing of TSC1, TSC2 and KRAS demonstrates that small second-hit mutations in these genes are rare events. Brain Pathol. 2010;20(6):1096-1105. doi: 10.1111/j.1750-3639.2010.00416.x [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Baker SL, Harrison TM, Maass A, La Joie R, Jagust WJ. Effect of off-target binding on 18F-flortaucipir variability in healthy controls across the lifespan. J Nucl Med. 2019;60(10):1444-1451. doi: 10.2967/jnumed.118.224113 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Makaretz SJ, Quimby M, Collins J, et al. . Flortaucipir tau PET imaging in semantic variant primary progressive aphasia. J Neurol Neurosurg Psychiatry. 2018;89(10):1024-1031. doi: 10.1136/jnnp-2017-316409 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Smith R, Santillo AF, Waldö ML, et al. . 18F-Flortaucipir in TDP-43 associated frontotemporal dementia. Sci Rep. 2019;9(1):6082. doi: 10.1038/s41598-019-42625-9 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Tsai RM, Bejanin A, Lesman-Segev O, et al. . 18F-flortaucipir (AV-1451) tau PET in frontotemporal dementia syndromes. Alzheimers Res Ther. 2019;11(1):13. doi: 10.1186/s13195-019-0470-7 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Jack CR Jr, Lowe VJ, Senjem ML, et al. . 11C PiB and structural MRI provide complementary information in imaging of Alzheimer’s disease and amnestic mild cognitive impairment. Brain. 2008;131(pt 3):665-680. doi: 10.1093/brain/awm336 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Rosso SM, van Herpen E, Pijnenburg YAL, et al. . Total tau and phosphorylated tau 181 levels in the cerebrospinal fluid of patients with frontotemporal dementia due to P301L and G272V tau mutations. Arch Neurol. 2003;60(9):1209-1213. doi: 10.1001/archneur.60.9.1209 [DOI] [PubMed] [Google Scholar]
- 48.French JA, Lawson JA, Yapici Z, et al. . Adjunctive everolimus therapy for treatment-resistant focal-onset seizures associated with tuberous sclerosis (EXIST-3): a phase 3, randomised, double-blind, placebo-controlled study. Lancet. 2016;388(10056):2153-2163. doi: 10.1016/S0140-6736(16)31419-2 [DOI] [PubMed] [Google Scholar]
- 49.Wu JY, Salamon N, Kirsch HE, et al. . Noninvasive testing, early surgery, and seizure freedom in tuberous sclerosis complex. Neurology. 2010;74(5):392-398. doi: 10.1212/WNL.0b013e3181ce5d9e [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.Possin KL, Chester SK, Laluz V, et al. . The frontal-anatomic specificity of design fluency repetitions and their diagnostic relevance for behavioral variant frontotemporal dementia. J Int Neuropsychol Soc. 2012;18(5):834-844. doi: 10.1017/S1355617712000604 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51.Ramanan S, Bertoux M, Flanagan E, et al. . Longitudinal executive function and episodic memory profiles in behavioral-variant frontotemporal dementia and Alzheimer’s Disease. J Int Neuropsychol Soc. 2017;23(1):34-43. doi: 10.1017/S1355617716000837 [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
eTable 1. General demographic and global cognitive function information
eTable 2. Statistical analysis of the TAND checklist severity score
eTable 3. CSF biomarkers in a subset of TSC subjects
eTable 4. A list of the genetic and clinical criteria for the diagnosis of TSC.
eTable 5. Genetic mutation results of the TSC and FTD cohorts.
eFigure 1. Comparison of the brain MRI T1 sequences from 2015 to 2018 in a patient diagnosed with bvFTD and svPPA due to a TSC1 mutation.
eFigure2. Imaging biomarker results of the left anterior cingulate in the 59 year-old TSC participant.
eFigure 3. Imaging biomarker results of the left temporal neocortex in the same 59 year-old TSC participant in eFigure 2.
eFigure 4. Imaging biomarkers results of the right anterior cingulate in the 48 year-old TSC participant.
eFigure 5. Imaging biomarker results of the right precentral neocortex in the 44 year-old TSC participant.
eFigure 6. Imaging biomarker results of the left orbitofrontal neocortex in the same 44 year-old TSC participant from eFigure 5.
eFigure 7. Imaging biomarker results of the left dorsal occipital neocortex in the same 44 year-old TSC participant from eFigure 5.
eFigure 8. Imaging biomarker results of the left ventral occipital gyrus in the same 44 year-old TSC participant from eFigure 5.