Key Points
Question
What are the key similarities and differences in characteristics, comorbidities, therapies, and in-hospital outcomes in patients with chronic and acute heart failure?
Findings
In this cohort study including 18 553 patients from 7 global regions, there were similarities in many regions regarding prevalence of prior heart failure, ejection fraction, and comorbidities. However, there were key differences in outpatient treatment, hospital point of entry, acute heart failure precipitants, and timing and type of inpatient intravenous therapies.
Meaning
These data provide information on the current global burden of acute heart failure, identify region-specific gaps in management, and note differences in practice around the world associated with patient outcomes.
Abstract
Importance
Acute heart failure (AHF) precipitates millions of hospital admissions worldwide, but previous registries have been country or region specific.
Objective
To conduct a prospective contemporaneous comparison of AHF presentations, etiologic factors and precipitants, treatments, and in-hospital outcomes among global regions through the International Registry to Assess Medical Practice with Longitudinal Observation for Treatment of Heart Failure (REPORT-HF).
Design, Setting, and Participants
A total of 18 553 adults were enrolled during a hospitalization for AHF. Patients were recruited from the acute setting in Western Europe (WE), Eastern Europe (EE), Eastern Mediterranean and Africa (EMA), Southeast Asia (SEA), Western Pacific (WP), North America (NA), and Central and South America (CSA). Patients with AHF were approached for consent and excluded only if there was recent participation in a clinical trial. Patients were enrolled from July 23, 2014, to March 24, 2017. Statistical analysis was conducted from April 18 to June 29, 2018; revised analyses occurred between August 6 and 29, 2019.
Main Outcomes and Measures
Heart failure etiologic factors and precipitants, treatments, and in-hospital outcomes among global regions.
Results
A total of 18 553 patients were enrolled at 358 sites in 44 countries. The median age was 67.0 years (interquartile range [IQR], 57-77), 11 372 were men (61.3%), 9656 were white (52.0%), 5738 were Asian (30.9%), and 867 were black (4.7%). A history of HF was present in more than 50% of the patients and 40% were known to have a prior left-ventricular ejection fraction lower than 40%. Ischemia was a common AHF precipitant in SEA (596 of 2329 [25.6%]), WP (572 of 3354 [17.1%]), and EMA (364 of 2241 [16.2%]), whereas nonadherence to diet and medications was most common in NA (306 of 1592 [19.2%]). Median time to the first intravenous therapy was 3.0 (IQR, 1.4-5.6) hours in NA; no other region had a median time above 1.2 hours (P < .001). This treatment delay remained after adjusting for severity of illness (P < .001). Intravenous loop diuretics were the most common medication administered in the first 6 hours of AHF management across all regions (65.4%-89.9%). Despite similar initial blood pressure across all regions, inotropic agents were used approximately 3 times more often in SEA, WP, and EE (11.3%-13.5%) compared with NA and WE (3.1%-4.3%) (P < .001). Older age (odds ratio [OR], 1.0; 95% CI, 1.00-1.02), HF etiology (ischemia: OR, 1.65; 95% CI, 1.11-2.44; valvular: OR, 2.10; 95% CI, 1.36-3.25), creatinine level greater than 2.75 mg/dL (OR, 1.85; 95% CI, 0.71-2.40), and chest radiograph signs of congestion (OR, 2.03; 95% CI, 1.39-2.97) were all associated with increased in-hospital mortality. Similarly, younger age (OR, −0.04; 95% CI, −0.05 to −0.02), HF etiology (ischemia: OR, 0.77; 95% CI, 0.26-1.29; valvular: OR, 2.01; 95% CI, 1.38-2.65), creatinine level greater than 2.75 mg/dL (OR, 1.16; 95% CI, 0.31-2.00), and chest radiograph signs of congestion (OR, 1.02; 95% CI, 0.57-1.47) were all associated with increased in-hospital LOS.
Conclusions and Relevance
Data from REPORT-HF suggest that patients are similar across regions in many respects, but important differences in timing and type of treatment exist, identifying region-specific gaps in medical management that may be associated with patient outcomes.
This cohort study compares the characteristics and management of acute heart failure in global regions comprising 44 countries.
Introduction
Acute heart failure (AHF) precipitates millions of hospital admissions each year.1,2,3 Patients with AHF have a variety of underlying heart diseases, risk factors, comorbidities, and precipitants leading to acute decompensation.4,5,6 Hospitalization for and management of AHF account for the largest proportion of health care resource use in patients living with HF.7,8 Acute and chronic HF management can also affect resource use associated with length of stay (LOS) and early readmission. Furthermore, the route of admission and care pathways (emergency department, ward, or intensive care unit) may affect timing of intravenous therapy for AHF and outcomes.9,10,11
Several registries have compared international differences in patient characteristics, precipitants, initial therapy, and short-term outcomes of AHF, but, to our knowledge, none has simultaneously enrolled patients worldwide.4,5,6,12 Contemporaneous comparisons of AHF presentations, etiologic factors, precipitants, treatments, and outcomes between global regions are lacking. International Registry to Assess Medical Practice With Longitudinal Observation for Treatment of Heart Failure (REPORT-HF) is a global, prospective, and observational cohort study initiated during an index hospitalization for AHF that could be either new-onset (first diagnosis) HF or decompensation of chronic HF. We performed uniform data collection and systematic follow-up, allowing patient characteristics, presentation, management, and outcomes to be compared and contrasted across world regions. This article presents data from the index hospitalization. Our specific goal herein is to describe the initial AHF presentation and evaluation, including baseline characteristics of patients, etiologic factors and precipitants of AHF, timeliness and types of initial intravenous AHF therapies administered in the first 6 hours, and inpatient outcomes, for 7 world regions.
Methods
Study Design and Setting
The REPORT-HF methods have been described in detail previously.13 Briefly, REPORT-HF is a prospective, observational, global cohort study, which enrolled patients at 358 hospitals in 44 countries across Europe, North America, Central and South America, Africa, Asia and the Pacific, and the Middle East. Patients were to be enrolled consecutively (open enrollment) or intermittent consecutively (based on predefined enrollment intervals) depending on the size of the hospital and corresponding number of patients admitted.
This study was conducted in accordance with the Declaration of Helsinki,14 and the protocol received institutional review board and/or ethics committee approval at each participating center. Patients provided written informed consent; provision of financial compensation varied among the study sites.
Participants
All adult patients (age ≥18 years for countries where the adult age is >18 years) who were hospitalized at the sites with a primary diagnosis of AHF (new-onset [first diagnosis] AHF or decompensation of chronic HF) were eligible for inclusion. An AHF diagnosis was made by the treating physician according to local practices and was based on usual care, including history, physical examination, chest radiograph, electrocardiogram, echocardiogram, and natriuretic peptide levels. There were no exclusion criteria except concomitant participation in a clinical trial with any investigational treatment or use of investigational drugs within 5 half-lives of enrollment or until the expected pharmacodynamic effect had returned to baseline, whichever was longer. We used a guideline that the last dose for small molecules should have occurred more than 30 days, and for biologics more than 4 months, before enrollment.
Study Procedures
Data collected during the index admission included patient demographics, clinical symptoms, risk factors and comorbidities, medical history, admission medications, vital signs, physical examination findings, laboratory test values, acute therapies and procedures, and hospital course, including LOS and mortality. All data were captured prospectively in a central electronic database, using the same case report forms across all 44 countries and 358 sites. We did not mandate specific data collection to be done for study purposes. Background HF therapy was left to the discretion of the treating physician, but all sites were encouraged to provide patient care according to standard local practice as informed by current guideline-based recommendations.
Statistical Analysis
The primary analysis focused on describing routine practice and geographic variation in clinical characteristics, HF etiologic factors, AHF precipitants, hospital point of entry, and the timeliness and types of intravenous AHF therapies during the index admission. Geographic groupings of the 44 participating countries were determined using a modification of the World Health Organization classification15 into 7 regions: (1) Western Europe (WE [n = 3594]), including Austria, Germany, Great Britain, Greece, the Netherlands, Norway, Spain, Sweden, and Switzerland; (2) Eastern Europe (EE [n = 2802]), including Bulgaria, Israel, Romania, Russian Federation, and Turkey; (3) Western Pacific (WP [n = 3354]), including Australia, China, Japan, Malaysia, Philippines, Republic of Korea, Taiwan, and Vietnam; (4) Southeast Asia (SEA [n = 2329]), including India, Indonesia, and Thailand; (5) North America (NA [n = 1592]), including Canada and the United States; (6) Central and South America (CSA [n = 2641]), including Argentina, Brazil, Chile, Colombia, Ecuador, Guatemala, Mexico, and Panama; and (7) Eastern Mediterranean Region and Africa (EMA [n = 2241]), including Algeria, Egypt, Jordan, Lebanon, Morocco, Saudi Arabia, South Africa, Tunisia, and United Arab Emirates (eFigure 1 in the Supplement). The final categories were selected to enable meaningful comparisons among geographic regions as well as provide balance between the number of countries and patients in each region.
Medians and interquartile ranges (IQRs) or counts and proportions were used to describe the data. Comparisons between groups used analysis of variance for continuous variables and χ2 testing for categorical variables. Given the large number of patients enrolled and the multiple comparisons, the investigators viewed P values considering the relative effect sizes and clinically important differences. Part of obtaining an understanding of regional differences in initial data was an expectation of nonrandom missingness due to variations in clinical practice and the availability of diagnostic tests and acute therapies. For modeling purposes, we categorized clinical and laboratory tests as positive, negative, or not obtained. This categorization allowed us to include all cases in the analyses without requiring assumptions associated with imputation or other similar approaches. To evaluate differences among regions in the association between point of hospital entry and timing of intravenous therapy, as well as between the timing and type of treatment and both in-hospital LOS and mortality, we used mixed-effects generalized linear models with either a linear or logit link function and with region as a random variable to properly account for clustering within region. Models were adjusted for baseline age, sex, vital signs (systolic blood pressure, pulse), HF etiologic factor, and results of diagnostic testing (blood urea nitrogen level, serum creatinine level, and chest radiograph findings of congestion). Findings were considered significant at P<.05, with 2-tailed testing. Data analysis was performed from April 18 to June 29, 2018; revised analyses occurred between August 6 and 29, 2019. All statistical analyses were conducted using SPSS, version 26.0 (IBM Corp). Graphics were created using R, base 3.5 (R Foundation).
Results
Patient Characteristics
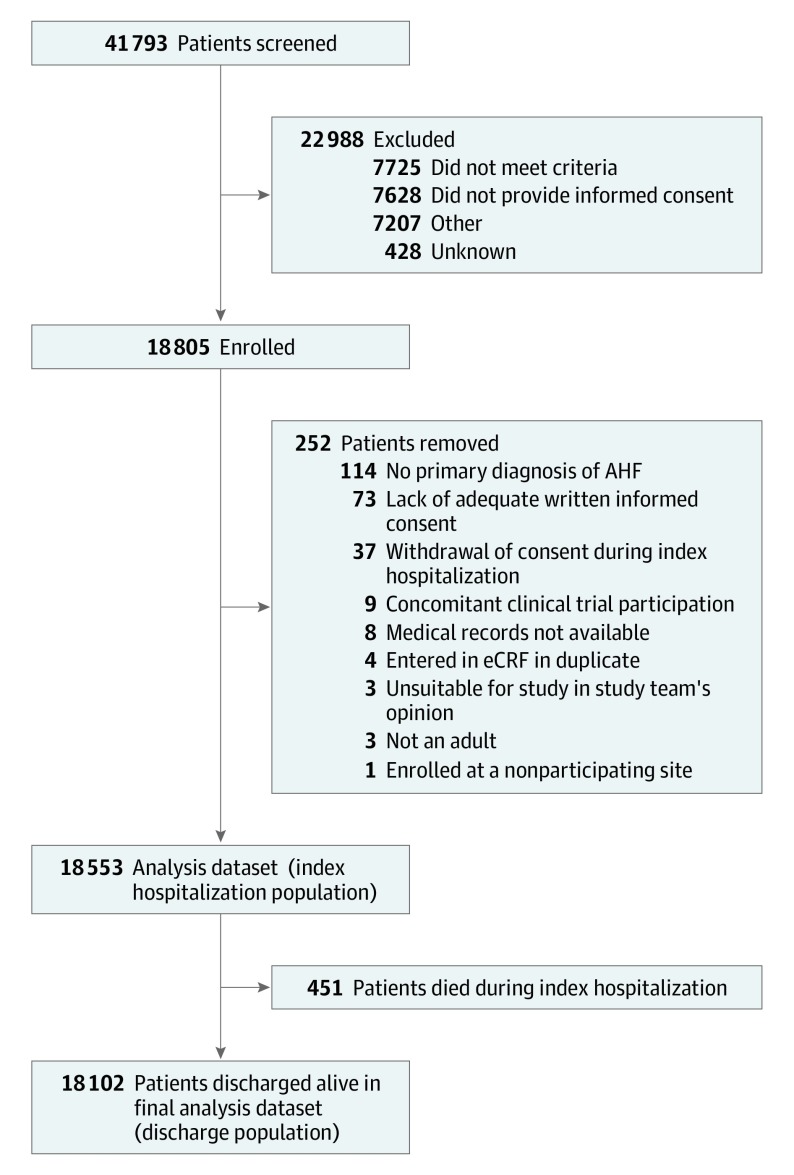
Over 32 months at 358 sites in 44 countries, 41 793 patients were screened and 18 805 patients were enrolled (Figure 1). The first patient was enrolled on July 23, 2014, and the last patient was enrolled on March 24, 2017. The final index hospitalization data set comprised 18 553 patients: 37 patients (0.2%) withdrew consent during the index hospitalization and 215 patients (1.2%) were removed from analysis owing to other factors (Figure 1). Patients who did not consent to participate were similar with respect to age and sex. The median age was 67.0 years (IQR, 57-77), 11 372 were men (61.3%), 9656 were white (52.0%), 5738 were Asian (30.9%), and 867 were black (4.7%). The largest proportion of patients was enrolled in WE (3594 [19.4%]) followed by WP (3354 [18.1%]), EE (2802 [15.1%]), and CSA (2641 [14.2%]) (Table). Comorbidities, such as hypertension (11 807 [63.6%]), type 2 diabetes (6808 [36.7%]), and chronic kidney disease (3766 [20.3%]), were prevalent across all regions. However, hypertension was more prevalent in EE (2241 [80.0%]) and NA (1226 [77.0%]) compared with SEA (1109 [47.6%]), while chronic obstructive pulmonary disease/asthma was more prevalent in NA (432 [27.1%]) compared with WP (340 [10.1%]) and EMA (227 [10.1%]), and a history of atrial arrhythmias was more prevalent in EE (1318 [47.0%]) and WE (1644 [45.7%]) compared with SEA (190 [8.2%]).
Figure 1. Enrollment and Patient Flow Through the Study.
AHF indicates acute heart failure; eCRF, electronic case report form.
Table. Demographics and Baseline Clinical Characteristics.
| Characteristic | No. (%) | P Value | |||||||
|---|---|---|---|---|---|---|---|---|---|
| All | Western Europe | Eastern Europe | Western Pacific | South East Asia | North America | Central/South America | Eastern Mediterranean and Africa | ||
| No. of patients (in-hospital population) | 18 553 | 3594 (19.4) | 2802 (15.1) | 3354 (18.1) | 2329 (12.6) | 1592 (8.6) | 2641 (14.2) | 2241 (12.1) | NA |
| Patients who died during index stay, No. (%) | 451 (2.4) | 105 (2.9) | 41 (1.5) | 56 (1.7) | 37 (1.6) | 27 (1.7) | 116 (4.4) | 69 (3.1) | NA |
| Patients discharged alive, No. (%) | 18 102 (97.6) | 3489 (97.1) | 2761 (98.5) | 3298 (98.3) | 2292 (98.4) | 1565 (98.3) | 2525 (95.6) | 2172 (96.9) | NA |
| Countries, No. | 44 | 9 | 5 | 8 | 3 | 2 | 8 | 9 | NA |
| Sites, No. | 358 | 69 | 48 | 73 | 41 | 20 | 62 | 45 | NA |
| Demographic | |||||||||
| Age, mean (SD), y | 66.1 (13.92) | 72.3 (12.47) | 67.7 (11.56) | 65.4 (14.59) | 60.9 (13.02) | 63.2 (14.45) | 66.0 (14.46) | 63.0 (13.81) | <.001 |
| Median (IQR) | 67.0 (57-77) | 75.0 (65-81) | 68.0 (60-77) | 67.0 (56-77) | 61.0 (53-70) | 63.0 (54-74) | 67.0 (57-77) | 64.0 (55-73) | |
| ≥65 | 10 651 (57.4) | 2732 (76.0) | 1764 (63.0) | 1890 (56.4) | 955 (41.0) | 747 (46.9) | 1502 (56.9) | 1061(47.3) | |
| ≥75 | 5817 (31.4) | 1822 (50.7) | 910 (32.5) | 1072 (32.0) | 349 (15.0) | 365 (22.9) | 815 (30.9) | 484 (21.6) | |
| BMI | |||||||||
| No. | 8900 | 1849 | 1247 | 1717 | 959 | 1455 | 894 | 779 | <.001 |
| Mean (SD) | 28.7 (15.78) | 29.6 (7.23) | 29.9 (6.72) | 25.9 (31.63) | 24.6 (4.94) | 32.8 (11.44) | 28.0 (6.20) | 29.3 (7.10) | |
| Median (IQR) | 27.1 (24-32) | 28.1 (25-33) | 28.7 (25-33) | 24.4 (22-28) | 24.1 (21-27) | 30.7 (26-38) | 27.1 (24-31) | 27.7 (25-32) | |
| Male | 11 372 (61.3) | 2317 (64.5) | 1636 (58.4) | 2031 (60.6) | 1483 (63.7) | 937 (58.9) | 1576 (59.7) | 1392 (62.1) | |
| Race | |||||||||
| White | 9656 (52.0) | 3504 (97.5) | 2777 (99.1) | 95 (2.8) | 0 | 795 (49.9) | 1059 (40.1) | 1426 (63.6) | <.001 |
| Black | 867 (4.7) | 14 (0.4) | 0 | 2 (0.1) | 1 (0.0) | 705 (44.3) | 95 (3.6) | 50 (2.2) | |
| Asian | 5738 (30.9) | 24 (0.7) | 9 (0.3) | 3246 (96.8) | 2326 (99.9) | 27 (1.7) | 2 (0.1) | 104 (4.6) | |
| Native American | 375 (2.0) | 2 (0.1) | 0 | 0 | 0 | 8 (0.5) | 365 (13.8) | 0 | |
| Pacific Islander | 7 (0) | 2 (0.1) | 0 | 0 | 0 | 2 (0.1) | 3 (0.1) | 0 | |
| Other | 1910 (10.3) | 48 (1.3) | 16 (0.6) | 11 (0.3) | 2 (0.1) | 55 (3.5) | 1117 (42.3) | 661 (29.5) | |
| Medical history | |||||||||
| Hypertension | 11 807 (63.6) | 2260 (62.9) | 2241 (80.0) | 1846 (55.0) | 1109 (47.6) | 1226 (77.0) | 1785 (67.6) | 1340 (59.8) | <.001 |
| Prior MI/ACS | 3504 (18.9) | 745 (20.7) | 869 (31.0) | 555 (16.5) | 283 (12.2) | 324 (20.4) | 395 (15.0) | 333 (14.9) | |
| Prior CAD | 6381 (34.4) | 1090 (30.3) | 1481 (52.9) | 1180 (35.2) | 601 (25.8) | 628 (39.4) | 495 (18.7) | 906 (404) | |
| Atrial fibrillation/flutter | 5766 (31.1) | 1644 (45.7) | 1318 (47.0) | 824 (24.6) | 190 (8.2) | 608 (38.2) | 703 (26.6) | 479 (21.4) | |
| Valvular surgery or percutaneous valvular procedures | 771 (4.2) | 284 (7.9) | 110 (3.9) | 55 (1.6) | 27 (1.2) | 86 (5.4) | 108 (4.1) | 101 (4.5) | |
| COPD/asthma | 2655 (14.3) | 631 (17.6) | 524 (18.7) | 340 (10.1) | 143 (6.1) | 432 (27.1) | 358 (13.6) | 227 (10.1) | |
| Type 2 diabetes | 6808 (36.7) | 1317 (36.6) | 920 (32.8) | 1076 (32.1) | 972 (41.7) | 661 (41.5) | 815 (30.9) | 1047 (46.7) | |
| CKD | 3766 (20.3) | 965 (26.9) | 642 (22.9) | 519 (15.5) | 243 (10.4) | 539 (33.9) | 466 (17.6) | 392 (17.5) | |
| Anemia | 1614 (8.7) | 430 (12.0) | 380 (13.6) | 158 (4.7) | 61 (2.6) | 266 (16.7) | 149 (5.6) | 170 (7.6) | |
| Smoking status | |||||||||
| Current | 2532 (13.6) | 468 (13.0) | 405 (14.5) | 541 (16.1) | 242 (10.4) | 291 (18.3) | 202 (7.6) | 383 (17.1) | |
| Systolic BP, mm Hg | |||||||||
| No. | 16 995 | 3168 | 2479 | 3110 | 2162 | 1578 | 2363 | 2135 | <.001 |
| Mean (SD) | 133.3 (29.2) | 135.1 (28.1) | 137.3 (28.7) | 132.6 (28.3) | 132.0 (28.7) | 138.1 (31.9) | 128.2 (28.7) | 130.6 (30.4) | |
| Median (IQR) | 130 (110-150) | 131 (115-150) | 133 (120-155) | 130 (110-149) | 130 (110-150) | 134 (115-156) | 125 (109-143) | 126 (110-148) | |
| Heart rate, bpm | |||||||||
| No. | 16 932 | 3135 | 2477 | 3096 | 2159 | 1578 | 2362 | 2125 | <.001 |
| Mean (SD) | 89.4 (22.9) | 87.4 (23.4) | 88.5 (24.0) | 89.0 (22.4) | 94.0 (21.9) | 89.6 (21.2) | 87.3 (23.2) | 91.7 (22.3) | |
| Median (IQR) | 86 (73-102) | 84 (70-100) | 82 (70-100) | 86 (73-101) | 92 (80-107) | 87 (74-103) | 84 (71-100) | 90 (76-104) | |
| eGFR (MDRD), mL/min/1.73 m2 | |||||||||
| No. (%) | 11 282 (60.8) | 2055 (57.2) | 820 (29.3) | 1150 (34.3) | 2129 (91.4) | 1441 (90.5) | 2280 (86.3) | 1407 (62.8) | <.001 |
| Median (IQR) | 64.4 (44.7-87.1) | 63.8 (45.0-85.5) | 71.5 (50.4-96.3) | 63.8 (41.7-87.5) | 64.4 (45.3-87.2) | 56.8 (37.9-78.3) | 65.7 (46.2-86.6) | 67.1 (46.6-91.5) | |
Abbreviations: ACS, acute coronary syndrome; BMI, body mass index (calculated as weight in kilograms divided by height in meters squared); BP, blood pressure; CAD, coronary artery disease; CKD, chronic kidney disease; COPD, chronic obstructive pulmonary disease; eGFR, estimated glomerular filtration rate; IQR, interquartile range; MDRD, Modification of Diet in Renal Disease; MI, myocardial infarction; NA, not applicable.
Regional Differences
Prior New York Heart Association functional class was well distributed across the regions, although patients in SEA (423 [18.2%]) and EMA (401 [17.9%]) were more likely to have New York Heart Association class IV HF compared with other regions (all ≤12.6%) (eTable 1 in the Supplement). Patients in WE (622 [17.3%]) and NA (479 [29.5%]) were more likely to have an implantable cardioverter-defibrillator or pacemaker compared with the other regions (all ≤11.5%). Decompensated chronic HF was associated with more than 50% of the AHF presentations in all regions except SEA, which reported only 495 (21.3%) of patients with a history of chronic HF. Baseline medications for chronic HF differed across regions: β-blockers were most prevalent in NA (1127 [70.8%]) and least common in SEA (580 [24.9%]), while angiotensin-converting enzyme inhibitor or angiotensin receptor blocker use was most prevalent in CSA (377 [61.2%]) and least prevalent in SEA (617 [26.5%]). Overall, there were 4194 patients (22.6%) with an ejection fraction of 50% or greater, ranging from 363 patients (16.2%) in EMA to 950 patients (33.9%) in EE.
An ischemic etiologic factor of HF (6195 [33.4%]) was most common in the overall cohort and in all regions except NA. In decompensated chronic HF, ischemia was more common in EE (843 of 1867 [45.2%]) and EMA (570 of 1387 [41.1%]), while hypertension was most prevalent in SEA (x [18.6%]) and primary cardiomyopathy in NA (x [25.3%]). In patients with de novo HF, an ischemic etiologic factor was more common in WP (604 of 1572 [38.4%]) and EMA (320 of 854 [37.5%]), and hypertension was most common in EE (271 of 935 [29.0%]) and primary cardiomyopathy in SEA (348 of 1834 [19.0%]). Ischemia was a common precipitant in SEA (596 of 2329 [25.6%]), WP (572 of 3354 [17.1%]), and EMA (364 of 2241 [16.2%]), whereas nonadherence to diet or medications was most common in NA (306 of 1592 [19.2%]).
Vital signs at presentation were similar across all regions (eTable 2 in the Supplement). Signs and symptoms of congestion were highly prevalent across all regions, but dyspnea at rest was lower in NA (605 [38.0%]) compared with other regions (all ≥70.1%). Differences in local practice were reflected in the variability of ordering of laboratory tests: availability of natriuretic peptide and troponin level measurements ranged from 87.9% and 79.0% in NA to 19.7% and 29.8% in EE, respectively. Serum creatinine level was measured in more than 90% of patients in NA and SEA, but only 29.3% of patients in EE. Among available measurements, median natriuretic peptide levels were elevated and similar across regions, while renal function was mildly reduced (estimated glomerular filtration rate <90 mL/min/1.73 m2) in most regions and moderately reduced in NA (estimated glomerular filtration rate 56.8 mL/min/1.73 m2) (IQR, 37.9-78.3) (eTable 2 in the Supplement).
Hospital Entry and Treatment Timing
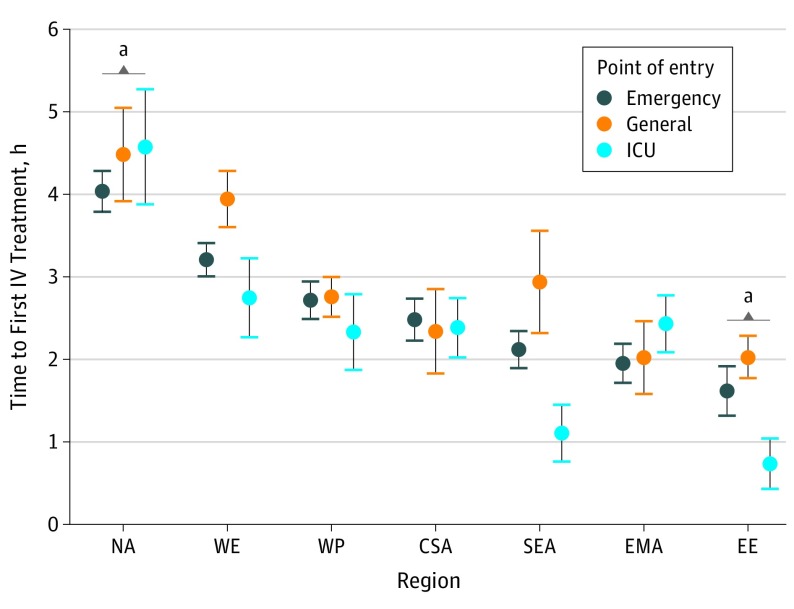
There were prominent differences in the hospital point of entry across regions. Patients in CSA (2085 of 2641 [78.9%]), NA (1237 of 1592 [77.7%]), and WE (2760 of 3594 [76.8%]) were more likely to enter the hospital via the emergency department compared with patients in EE (1048 of 2802 [37%]) and WP (1526 of 3354 [46%]) who were more likely to be admitted directly to the cardiac ward. There were also differences in timeliness of intravenous therapy across regions (Figure 2). Median time to the first intravenous therapy was 3.0 (IQR, 1.4-5.6) hours in NA regardless of the point of entry, while no other region had a median time above 1.2 hours (P < .001). Compared with the WP region, participants untreated at arrival in NA had the longest time to treatment after adjusting for potential confounders (1.7, 95% CI 1.1-2.3). Although there were general tendencies for decreased time to therapy when patients entered via the cardiac or intensive care unit, these differences were most pronounced in EE and SEA where patients who entered via the intensive or cardiac care unit were more likely to be treated earlier (median [IQR] time, 0.0 [0.0-0.5] hours in EE and 0.4 [0.0-0.4] hours in SEA) compared with patients who entered via other portals (median [IQR] time, EE, emergency department: 0.5 [0.2-1.5] and general: 0.8 [0.1-2.0], and for SEA, emergency department: 0.8 [0.2-2.2] and general: 1.1 [0.5-3.3]). When considering the interaction of point of entry and region, after adjusting for HF severity, time to therapy remained significantly shorter for patients entering via the cardiac or intensive care unit in EE (−1.3 hours; 95%, CI, −2.1 to −0.6 hours; P < .001) or SEA (−1.4 hours; 95%, CI, −2.4 to −0.4 hours; P < .001) and the emergency department in SEA (−0.9 hours; 95% CI, −1.7 to −0.1 hours; P = .04).
Figure 2. Time to First Intravenous (IV) Treatment Based on Point of Entry.
CSA indicates Central and South America; EE, Eastern Europe; EMA, Eastern Mediterranean Region and Africa; ICU, intensive care unit; NA, North America; SEA, Southeast Asia; WE, Western Europe; and WP, Western Pacific.
aTime to IV therapy relative to the WP region, P < .001.
Timing and Type of Therapy and In-Hospital Outcomes
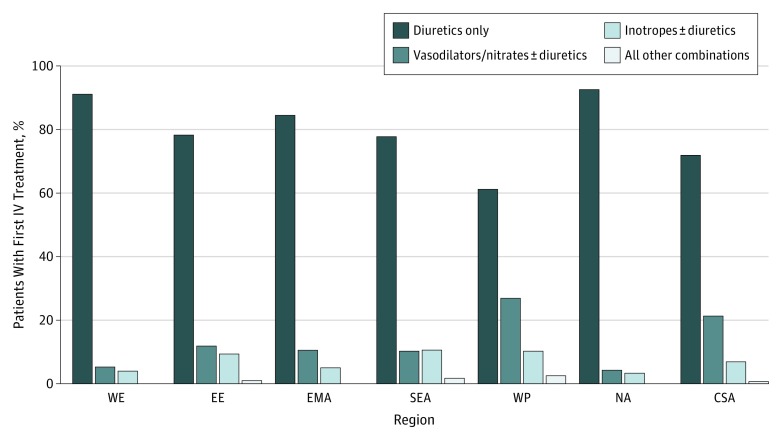
Although the frequency among regions varied, intravenous loop diuretics were the most common medication administered within the first 6 hours of hospital presentation across all regions (65.4% for WP and 89.9% for EE), followed by intravenous vasodilators or nitrates (5.4%for NA and 30.6% for WP) and intravenous inotropic agents (3.1% for NA and 13.5% for SEA) (eTable 3 in the Supplement; Figure 3). While the proportion of patients with systolic blood pressure less than 90 mm Hg was similar across all regions (≤4.4%), inotropic agents were used approximately 3 times more often in EE, WP, and SEA (11.3%, 13.4%, and 13.5%, respectively) compared with NA and WE (3.1% and 4.3%). These findings remained significant after adjustment for known indicators of AHF severity. A greater proportion of patients in WP (1026 [30.6%]) and CSA (593 [22.5%]) received intravenous vasodilators compared with other regions (5.6% fore WE and 16.4% for EE). Median time to initiation of intravenous vasodilators was similar across all regions (0.9 [IQR, 0.3-2.2] hours). However, time-to-treatment was significantly different between regions after adjusting for known indicators of AHF severity and were most pronounced for intravenous loop diuretics in NA (3.5 [IQR, 2.0-5.7] hours) compared with EE (0.4 [IQR, 0.0-1.5] hours) and SEA (0.7 [IQR, 0.1-2.1] hours) (Figure 2).
Figure 3. Type of Initial Therapy Stratified by Region.
CSA indicates Central and South America; EE, Eastern Europe; EMA, Eastern Mediterranean Region and Africa; IV, intravenous; NA, North America; SEA, Southeast Asia; WE, Western Europe; and WP, Western Pacific.
LOS and In-Hospital Mortality
Median in-hospital LOS was the same among NA, EMA, and SEA (6.0 days), and lower than WE, EE, and WP (9.0 days) (eTable 3 in the Supplement). Overall in-hospital mortality among participants was 2.4% but was highest in CSA (4.4%) and lowest in EE (1.5%). In multivariable models, LOS was significantly associated with age, blood pressure, HF etiologic factors, radiographic signs of congestion, and renal function (eTable 4 and eFigure 2 in the Supplement). Younger age (OR, −0.04; 95% CI, −0.05 to −0.02), HF etiology (ischemia: OR, 0.77; 95% CI, 0.26-1.29; valvular: OR, 2.01; 95% CI, 1.38-2.65), creatinine level greater than 2.75 mg/dL (OR, 1.16; 95% CI, 0.31-2.00), and chest radiograph signs of congestion (OR, 1.02; 95% CI, 0.57-1.47) were all associated with increased in-hospital LOS. The effect of time to treatment was marginal (P = .08), and the only treatment type associated with LOS was use of vasodilators. Effects of region were mitigated (P = .09). Similarly, for mortality, HF etiologic factors, radiographic signs of congestion, renal function, and age were all associated with increased odds of death. Older age (odds ratio [OR], 1.0; 95% CI, 1.00-1.02), HF etiology (ischemia: OR, 1.65; 95% CI, 1.11-2.44; valvular: OR, 2.10; 95% CI, 1.36-3.25), creatinine level greater than 2.75 mg/dL (OR, 1.85; 95% CI, 0.71-2.40), and chest radiograph signs of congestion (OR, 2.03; 95% CI, 1.39-2.97) were all associated with increased in-hospital mortality, but there was no effect of time to first intravenous therapy, type of first intravenous therapy, or blood pressure. Differences in mortality between regions was also somewhat attenuated (P = .12) (eTable 4 and eFigure 3 in the Supplement).
Discussion
REPORT-HF is a contemporary, prospective global AHF study spanning 7 worldwide regions with uniform collection of detailed data from the point of hospital entry and including systematic postdischarge follow-up. We describe the index hospitalization for these regions, presenting, to our knowledge, the first contemporaneous global contrast. This initial analysis presents a unique opportunity to identify regional differences and similarities in AHF presentation and contrast practice differences in both evaluation and management. We have 5 main findings. First, our results suggest a complex burden of disease in patients with HF around the world and identify diabetes, hypertension, and chronic kidney disease as the possible major targets for global prevention strategies in all regions. Prior studies suggest previously lower prevalence of these diseases in some regions of the world.16,17
Second, we found significant variability in AHF etiologic factors and precipitants, yet similarities in the prevalence of comorbidities, preexisting HF, and presenting signs and symptoms from one region to the next. Decompensated chronic HF was responsible for more than 50% of the AHF presentations in all regions except SEA, which reported only 21.3% of patients with a history of chronic HF. This difference may be due to the younger age of patients from this region compared with other regions in our registry. Similar results have been characterized in the International Congestive Heart Failure Study registry covering 16 countries from this region.18 Along with the prevalence of HF with reduced ejection fraction, these de novo presentations may have been associated with the variability in the use of angiotensin-converting enzyme inhibitors, angiotensin receptor blockers, and β-blockers in our cohort as well. However, given the similar recommendations for chronic HF therapy among US and European guidelines, it is not surprising that prescribing practices were similar across regions with a comparable prevalence of HF with reduced ejection fraction and prior HF, such as WE, EE, CSA, EMA, and NA.1,19 The regional differences that we observed in background medical therapy were also prevalent in the ASTRONAUT and ASCEND-HF global clinical trials20,21 and may reflect the care delivery systems of the various regions and be associated with LOS and intensive care unit use.
Compared with US-based registries, the prevalence of hypertension, diabetes, and chronic kidney disease in the patients from NA in our study are higher and the age is lower.4,22 This difference may reflect the high proportion of nonwhite patients enrolled and may account for many of the differences observed between patients enrolled in NA compared with WE. Our participants from EE and WE had findings similar to those seen in prior European registries and the ASTRONAUT global clinical trial with regard to median age, sex, and prevalence of hypertension.21,23 In SEA and WP, we found a lower prevalence of hypertension, chronic kidney disease, and left-ventricular ejection fraction less than 40%, but a higher proportion of de novo HF compared with data from the ADHERE International Asia–Pacific registry.24 However, the proportions of these comorbidities and de novo HF are similar to those seen in the Korean Acute Heart Failure Registry.25 Compared with a previous sub-Saharan registry, the proportion of women in our registry was lower and the age of patients was higher, but there was a similar median ejection fraction as well as prevalence of hypertension and atrial fibrillation/flutter.26
Our third finding is regional differences in the point of hospital entry and time to treatment that persisted after adjusting for known indicators of AHF severity. Despite a similar proportion of patients entering via the emergency department in NA, WE, and CSA, the time to the first intravenous AHF therapy was significantly longer in NA compared with other regions. This difference could be the result of fewer patients in NA having dyspnea at rest or congestion identified on their chest radiograph. The short time to treatment in some regions in REPORT-HF reflects prehospital treatment.
Fourth, there were significant regional differences in the type of initial therapy. Although some variations have been previously described, to our knowledge, the report has never been done simultaneously across multiple regions.4,5,12,27,28 While diuretics were the intravenous medication used with the greatest frequency in all regions, administration of intravenous vasodilators and inotropes varied considerably across the 7 regions. ALARM-HF saw similar disparities in inotropic and vasodilator use across a smaller number of countries in EE and WE.27 Despite a similar presenting blood pressure level in REPORT-HF and a similar proportion of patients in each region with systolic blood pressure less than 90 mm Hg, patients in EE, WP, and SEA were more likely to receive inotropic agents compared with the rest of the regions, even after adjustment for known markers of AHF severity. This difference may reflect variations in treatment algorithms, factors other than systolic blood pressure affecting this decision making, granular differences in presenting signs and symptoms, lack of guideline implementation, local availability of medications, variability in regional practice patterns, or the difficulty in generalizing clinical trial data to different regions of the world.
Fifth, we have reported on the differences in routine practice by collecting data available as standard care, rather than mandating study-specific data collection. We found significant variability in routine measurement of natriuretic peptide levels, renal function, and cardiac troponin levels. This difference may be a result of (1) the cost and availability of these tests; (2) the subtlety of AHF presentation, as natriuretic peptide levels may be measured only when the diagnosis is not clear after the initial evaluation; and (3) what health care professionals in different regions consider to be part of the standard diagnostic evaluation.
In addition, to our knowledge, a comparison of the type of initial intravenous AHF treatment, in-hospital mortality, and LOS across all 7 regions of the world has not previously been reported. Age, HF etiologic factors, radiographic signs of congestion, systolic blood pressure, and renal function were all associated with in-hospital mortality and LOS after adjusting for region of the world. Raw regional differences were also somewhat attenuated, suggesting that patient characteristics are important regardless of the location of care delivery. While we did not see an association between AHF treatment and mortality, others have reported improved in-hospital outcomes with use of intravenous vasodilators.27,29 Future clinical trial design of novel therapeutic agents will be better informed by understanding the timing and type of standard care medication exposure before randomization in the countries involved.30,31 Subsequent analyses from REPORT-HF will evaluate the relationship between regional differences in initial management, patient characteristics, and their association with intermediate- and long-term outcomes.
Limitations
This study has limitations to consider when interpreting the results. REPORT-HF reflects practice outside of clinical trials and exemplifies the challenge when resources are limited. As a result, the lack of universal use of natriuretic peptide level measurements could have affected the diagnosis of AHF. The in-hospital mortality of the patients was 2.4%, which is lower than that seen in many prior registries. This difference may reflect selection or participation bias, given that the design of the registry included a long-term follow-up after the index hospitalization, despite our attempts to include consecutive unselected patients in each region. Accordingly, inpatient mortality might be expected to be similar to that reported in clinical trials of AHF, which is typically lower than 2%,32,33,34 rather than that observed in registries or medical record reviews not requiring patient consent where inpatient where inpatient mortality exceeding 10% may be observed.35 This difference may limit the generalizability of our findings to cohorts with similarly low mortality. In addition, race was self-reported at the time of enrollment and errors in assigning racial categories could have resulted in misattribution.
Conclusions
To our knowledge, this is the first global AHF registry collecting contemporary data systematically from the point of hospital admission. REPORT-HF suggests that patients are similar across regions in many respects, but differences in timing and type of treatment exist, identifying region-specific gaps in medical management that may be associated with patient outcomes. These data might assist discussion and debate in the international research community about the nature of the patients they treat and the strengths and weaknesses of how patient care is managed in different regions. Learning from best practices around the world and implementing the findings, subject to available resources, may also help to improve outcomes for patients with HF.
eFigure 1. Geographic Groupings
eTable 1. HF Characteristics/History, AHF Precipitants and Prior Outpatient Cardiovascular Medication: Overall and Divided by Region of the World
eTable 2. Presenting Vital Signs, Signs and Symptoms and Laboratory Values: Overall and Divided by Region of the World
eTable 3. Hospital Point of Entry, Time to Initial AHF Treatment and Types of Initial AHF Therapy Administered, and In-Hospital Outcomes
eTable 4. Multivariable Model Evaluating the Association of Timeliness and Type of IV Therapy With In-Hospital Mortality and LOS Adjusting for Severity
eFigure 2. Predicted Length of Stay With 95% Confidence Intervals
eFigure 3. Predicted Probability of Death With 95% Confidence Intervals
References
- 1.Ponikowski P, Voors AA, Anker SD, et al. ; ESC Scientific Document Group . 2016 ESC Guidelines for the diagnosis and treatment of acute and chronic heart failure: the Task Force for the diagnosis and treatment of acute and chronic heart failure of the European Society of Cardiology (ESC)Developed with the special contribution of the Heart Failure Association (HFA) of the ESC. Eur Heart J. 2016;37(27):2129-2200. doi: 10.1093/eurheartj/ehw128 [DOI] [PubMed] [Google Scholar]
- 2.Cook C, Cole G, Asaria P, Jabbour R, Francis DP. The annual global economic burden of heart failure. Int J Cardiol. 2014;171(3):368-376. doi: 10.1016/j.ijcard.2013.12.028 [DOI] [PubMed] [Google Scholar]
- 3.Maggioni AP, Dahlström U, Filippatos G, et al. ; Heart Failure Association of the European Society of Cardiology (HFA) . EURObservational Research Programme: regional differences and 1-year follow-up results of the Heart Failure Pilot Survey (ESC-HF Pilot). Eur J Heart Fail. 2013;15(7):808-817. doi: 10.1093/eurjhf/hft050 [DOI] [PubMed] [Google Scholar]
- 4.Adams KF Jr, Fonarow GC, Emerman CL, et al. ; ADHERE Scientific Advisory Committee and Investigators . Characteristics and outcomes of patients hospitalized for heart failure in the United States: rationale, design, and preliminary observations from the first 100,000 cases in the Acute Decompensated Heart Failure National Registry (ADHERE). Am Heart J. 2005;149(2):209-216. doi: 10.1016/j.ahj.2004.08.005 [DOI] [PubMed] [Google Scholar]
- 5.Cleland JG, Swedberg K, Follath F, et al. ; Study Group on Diagnosis of the Working Group on Heart Failure of the European Society of Cardiology . The EuroHeart Failure Survey Programme—a survey on the quality of care among patients with heart failure in Europe; part 1: patient characteristics and diagnosis. Eur Heart J. 2003;24(5):442-463. doi: 10.1016/S0195-668X(02)00823-0 [DOI] [PubMed] [Google Scholar]
- 6.Fonarow GC, Stough WG, Abraham WT, et al. ; OPTIMIZE-HF Investigators and Hospitals . Characteristics, treatments, and outcomes of patients with preserved systolic function hospitalized for heart failure: a report from the OPTIMIZE-HF Registry. J Am Coll Cardiol. 2007;50(8):768-777. doi: 10.1016/j.jacc.2007.04.064 [DOI] [PubMed] [Google Scholar]
- 7.Kim E, Kwon HY, Baek SH, et al. Medical costs in patients with heart failure after acute heart failure events: one-year follow-up study. J Med Econ. 2018;21(3):288-293. doi: 10.1080/13696998.2017.1403922 [DOI] [PubMed] [Google Scholar]
- 8.Ziaeian B, Heidenreich PA, Xu H, et al. Medicare expenditures by race/ethnicity after hospitalization for heart failure with preserved ejection fraction. JACC Heart Fail. 2018;6(5):388-397. doi: 10.1016/j.jchf.2017.12.007 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Fonarow GC, Corday E; ADHERE Scientific Advisory Committee . Overview of acutely decompensated congestive heart failure (ADHF): a report from the ADHERE registry. Heart Fail Rev. 2004;9(3):179-185. doi: 10.1007/s10741-005-6127-6 [DOI] [PubMed] [Google Scholar]
- 10.Wong YW, Mentz RJ, Felker GM, et al. Nesiritide in patients hospitalized for acute heart failure: does timing matter? implication for future acute heart failure trials. Eur J Heart Fail. 2016;18(6):684-692. doi: 10.1002/ejhf.487 [DOI] [PubMed] [Google Scholar]
- 11.Wong YW, Fonarow GC, Mi X, et al. Early intravenous heart failure therapy and outcomes among older patients hospitalized for acute decompensated heart failure: findings from the Acute Decompensated Heart Failure Registry Emergency Module (ADHERE-EM). Am Heart J. 2013;166(2):349-356. doi: 10.1016/j.ahj.2013.05.014 [DOI] [PubMed] [Google Scholar]
- 12.Lam CS, Teng TK, Tay WT, et al. Regional and ethnic differences among patients with heart failure in Asia: the Asian Sudden Cardiac Death in Heart Failure Registry. Eur Heart J. 2016;37(41):3141-3153. doi: 10.1093/eurheartj/ehw331 [DOI] [PubMed] [Google Scholar]
- 13.Filippatos G, Khan SS, Ambrosy AP, et al. International Registry to Assess Medical Practice With Longitudinal Observation for Treatment of Heart Failure (REPORT-HF): rationale for and design of a global registry. Eur J Heart Fail. 2015;17(5):527-533. doi: 10.1002/ejhf.262 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.World Medical Association World Medical Association Declaration of Helsinki: ethical principles for medical research involving human subjects. JAMA. 2013;310(20):2191-2194. doi: 10.1001/jama.2013.281053 [DOI] [PubMed] [Google Scholar]
- 15.World Health Organization Health statistics and information systems. https://www.who.int/healthinfo/global_burden_disease/definition_regions/en/. Accessed November 27, 2019.
- 16.Htet AS, Bjertness MB, Oo WM, et al. Changes in prevalence, awareness, treatment and control of hypertension from 2004 to 2014 among 25-74-year-old citizens in the Yangon Region, Myanmar. BMC Public Health. 2017;17(1):847. doi: 10.1186/s12889-017-4870-y [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Midha T, Idris MZ, Saran RK, Srivastav AK, Singh SK. Prevalence and determinants of hypertension in the urban and rural population of a north Indian district. East Afr J Public Health. 2009;6(3):268-273. [PubMed] [Google Scholar]
- 18.Dokainish H, Teo K, Zhu J, et al. ; INTER-CHF Investigators . Heart failure in Africa, Asia, the Middle East and South America: the INTER-CHF study. Int J Cardiol. 2016;204:133-141. doi: 10.1016/j.ijcard.2015.11.183 [DOI] [PubMed] [Google Scholar]
- 19.Yancy CW, Jessup M, Bozkurt B, et al. 2017 ACC/AHA/HFSA focused update of the 2013 ACCF/AHA guideline for the management of heart failure: a report of the American College of Cardiology/American Heart Association Task Force on Clinical Practice Guidelines and the Heart Failure Society of America. Circulation. 2017;136(6):e137-e161. doi: 10.1161/CIR.0000000000000509 [DOI] [PubMed] [Google Scholar]
- 20.Metra M, Mentz RJ, Hernandez AF, et al. Geographic differences in patients in a global acute heart failure clinical trial (from the ASCEND-HF Trial). Am J Cardiol. 2016;117(11):1771-1778. doi: 10.1016/j.amjcard.2016.03.002 [DOI] [PubMed] [Google Scholar]
- 21.Greene SJ, Fonarow GC, Solomon SD, et al. ; ASTRONAUT Investigators and Coordinators . Global variation in clinical profile, management, and post-discharge outcomes among patients hospitalized for worsening chronic heart failure: findings from the ASTRONAUT trial. Eur J Heart Fail. 2015;17(6):591-600. doi: 10.1002/ejhf.280 [DOI] [PubMed] [Google Scholar]
- 22.Fonarow GC, Abraham WT, Albert NM, et al. ; OPTIMIZE-HF Investigators and Coordinators . Influence of beta-blocker continuation or withdrawal on outcomes in patients hospitalized with heart failure: findings from the OPTIMIZE-HF program. J Am Coll Cardiol. 2008;52(3):190-199. doi: 10.1016/j.jacc.2008.03.048 [DOI] [PubMed] [Google Scholar]
- 23.Lenzen MJ, Boersma E, Reimer WJ, et al. Under-utilization of evidence-based drug treatment in patients with heart failure is only partially explained by dissimilarity to patients enrolled in landmark trials: a report from the Euro Heart Survey on Heart Failure. Eur Heart J. 2005;26(24):2706-2713. doi: 10.1093/eurheartj/ehi499 [DOI] [PubMed] [Google Scholar]
- 24.Atherton JJ, Hayward CS, Wan Ahmad WA, et al. ; ADHERE International–Asia Pacific Scientific Advisory Committee . Patient characteristics from a regional multicenter database of acute decompensated heart failure in Asia Pacific (ADHERE International–Asia Pacific). J Card Fail. 2012;18(1):82-88. doi: 10.1016/j.cardfail.2011.09.003 [DOI] [PubMed] [Google Scholar]
- 25.Lee SE, Lee HY, Cho HJ, et al. clinical characteristics and outcome of acute heart failure in Korea: results from the Korean Acute Heart Failure Registry (KorAHF). Korean Circ J. 2017;47(3):341-353. doi: 10.4070/kcj.2016.0419 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Damasceno A, Mayosi BM, Sani M, et al. The causes, treatment, and outcome of acute heart failure in 1006 Africans from 9 countries. Arch Intern Med. 2012;172(18):1386-1394. doi: 10.1001/archinternmed.2012.3310 [DOI] [PubMed] [Google Scholar]
- 27.Mebazaa A, Parissis J, Porcher R, et al. Short-term survival by treatment among patients hospitalized with acute heart failure: the global ALARM-HF registry using propensity scoring methods. Intensive Care Med. 2011;37(2):290-301. doi: 10.1007/s00134-010-2073-4 [DOI] [PubMed] [Google Scholar]
- 28.Fonarow GC, Abraham WT, Albert NM, et al. Organized Program to Initiate Lifesaving Treatment in Hospitalized Patients with Heart Failure (OPTIMIZE-HF): rationale and design. Am Heart J. 2004;148(1):43-51. doi: 10.1016/j.ahj.2004.03.004 [DOI] [PubMed] [Google Scholar]
- 29.Abraham WT, Adams KF, Fonarow GC, et al. ; ADHERE Scientific Advisory Committee and Investigators; ADHERE Study Group . In-hospital mortality in patients with acute decompensated heart failure requiring intravenous vasoactive medications: an analysis from the Acute Decompensated Heart Failure National Registry (ADHERE). J Am Coll Cardiol. 2005;46(1):57-64. doi: 10.1016/j.jacc.2005.03.051 [DOI] [PubMed] [Google Scholar]
- 30.Collins SP, Levy PD, Fermann GJ, et al. what’s next for acute heart failure research? Acad Emerg Med. 2018;25(1):85-93. doi: 10.1111/acem.13331 [DOI] [PubMed] [Google Scholar]
- 31.Collins S, Storrow AB, Albert NM, et al. ; SAEM/HFSA Acute Heart Failure Working Group . Early management of patients with acute heart failure: state of the art and future directions: a consensus document from the Society for Academic Emergency Medicine/Heart Failure Society of America Acute Heart Failure Working Group. J Card Fail. 2015;21(1):27-43. doi: 10.1016/j.cardfail.2014.07.003 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Voors AA, Dittrich HC, Massie BM, et al. Effects of the adenosine A1 receptor antagonist rolofylline on renal function in patients with acute heart failure and renal dysfunction: results from PROTECT (Placebo-Controlled Randomized Study of the Selective Adenosine A1 Receptor Antagonist Rolofylline for Patients Hospitalized with Acute Decompensated Heart Failure and Volume Overload to Assess Treatment Effect on Congestion and Renal Function). J Am Coll Cardiol. 2011;57(19):1899-1907. doi: 10.1016/j.jacc.2010.11.057 [DOI] [PubMed] [Google Scholar]
- 33.Packer M, O’Connor C, McMurray JJV, et al. ; TRUE-AHF Investigators . Effect of ularitide on cardiovascular mortality in acute heart failure. N Engl J Med. 2017;376(20):1956-1964. doi: 10.1056/NEJMoa1601895 [DOI] [PubMed] [Google Scholar]
- 34.Teerlink JR, Cotter G, Davison BA, et al. ; RELAXin in Acute Heart Failure (RELAX-AHF) Investigators . Serelaxin, recombinant human relaxin-2, for treatment of acute heart failure (RELAX-AHF): a randomised, placebo-controlled trial. Lancet. 2013;381(9860):29-39. doi: 10.1016/S0140-6736(12)61855-8 [DOI] [PubMed] [Google Scholar]
- 35.Cleland JG, McDonagh T, Rigby AS, Yassin A, Whittaker T, Dargie HJ; National Heart Failure Audit Team for England and Wales . The national heart failure audit for England and Wales 2008-2009. Heart. 2011;97(11):876-886. doi: 10.1136/hrt.2010.209171 [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
eFigure 1. Geographic Groupings
eTable 1. HF Characteristics/History, AHF Precipitants and Prior Outpatient Cardiovascular Medication: Overall and Divided by Region of the World
eTable 2. Presenting Vital Signs, Signs and Symptoms and Laboratory Values: Overall and Divided by Region of the World
eTable 3. Hospital Point of Entry, Time to Initial AHF Treatment and Types of Initial AHF Therapy Administered, and In-Hospital Outcomes
eTable 4. Multivariable Model Evaluating the Association of Timeliness and Type of IV Therapy With In-Hospital Mortality and LOS Adjusting for Severity
eFigure 2. Predicted Length of Stay With 95% Confidence Intervals
eFigure 3. Predicted Probability of Death With 95% Confidence Intervals