Abstract
This study assessed the number of individuals receiving treatment for opioid use disorder in the United States by age group and sex using a national prescription database to compare between the number of buprenorphine prescriptions filled and the number of US opioid-related overdose deaths.
The increase in opioid-related overdose deaths in the United States has focused attention on extending access to medications for opioid use disorder. Among the 3 medications approved by the US Food and Drug Administration, buprenorphine is widely viewed as offering the greatest opportunity for expanding access. Between 2009-2011 and 2012-2014, US estimates of office-based visits involving buprenorphine prescriptions increased from 1.9 million to 4.3 million.1 In 2015, an estimated 200 per 100 000 privately insured adults filled at least 1 buprenorphine prescription.2 Yet most individuals with opioid use disorder do not receive treatment, and no general population estimates exist for rates of buprenorphine use. We present trends in US buprenorphine use by demographic groups with estimated length and duration of new treatment episodes.
Methods
Buprenorphine prescriptions filled by persons aged 15 to 80 years were identified in the IQVIA Real World Data: Longitudinal Prescription (IQVIA LRx) database from 2009 through 2018, excluding formulations not approved for opioid addiction. This database contains prescriptions from retail and nonretail pharmacies linked to individuals across years, pharmacies, and payment sources. The proportion of the population covered in the data set increased from 76.5% in 2009 to 92.0% in 2018. Calculated buprenorphine rates were based on the US population accounting for changes in IQVIA LRx coverage. Annual rates of filling 1 or more buprenorphine prescriptions per 1000 persons were calculated by age group and sex. New buprenorphine use episodes started on the date of a buprenorphine prescription fill after 180 days or more without a fill and ended after more than 30 days without buprenorphine supply. Because buprenorphine treatment episodes of 180 days or more are a national performance measure,3 percentages of new episodes of this duration were calculated using longitudinal patient data. Because 16 mg/d is the recommended target buprenorphine dosage, percentages of new episodes including 16 mg/d or greater were also calculated. The Yale University institutional review board deemed the analysis exempt from review.
Results
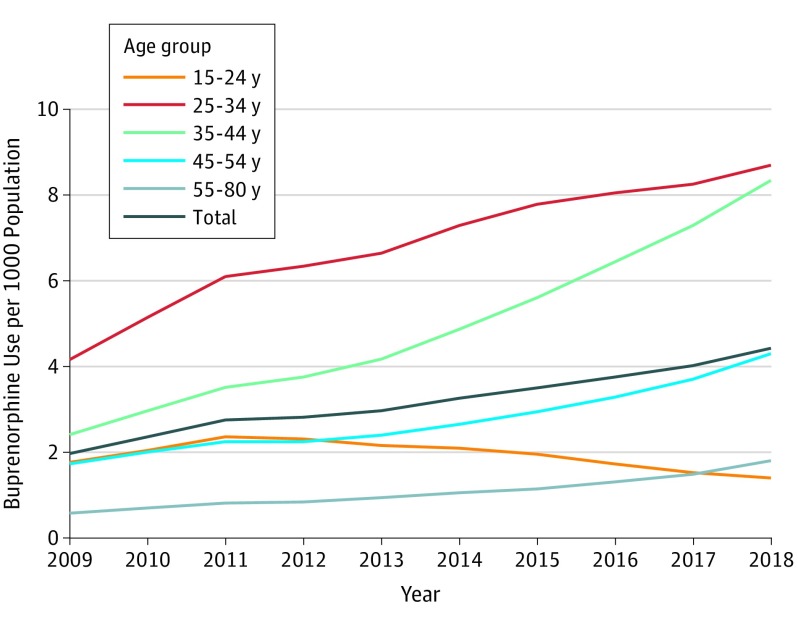
Among persons aged 15 to 80 years, the annual rate per 1000 population of buprenorphine use increased from 1.97 (n = 351 904) in 2009 to 4.43 (n = 1 037 787) in 2018. Between 2009 and 2018, buprenorphine use per 1000 population increased among adults aged 35 to 44 years from 2.41 to 8.34, but it decreased among individuals aged 15 to 24 years from 1.76 to 1.40 (Figure). Between 2009 and 2018, buprenorphine use per 1000 population among males increased from 2.44 to 5.21, and it increased among females from 1.49 to 3.66.
Figure. Trends in Buprenorphine Use in the United States per 1000 Population, Total and by Age Group, 2009-2018.
Data are from IQVIA Real World Data: Longitudinal Prescription, adjusted to the US population. Analysis is limited to persons aged 15 to 80 years.
Approximately 29.3% of buprenorphine use episodes continued for at least 180 days, with similar percentages for males (28.6%) and females (30.2%) (Table). Most new episodes of buprenorphine use (62.9%) included at least 1 prescription for 16 mg/d or greater, with little variation between males (62.8%) and females (62.9%). The percentages of new buprenorphine use episodes that continued for at least 180 days, that included a prescription for at least 16 mg/d, or comprised both characteristics were lower for individuals aged 15 to 24 years than for the other age groups (Table).
Table. US Percentages of New Buprenorphine Use Episodes of 180 Days or Greater and 16 mg/d or Greater by Sex and Age Groups, 2017a.
| Buprenorphine Use | Age, y | |||||
|---|---|---|---|---|---|---|
| 15-80 | 15-24 | 25-34 | 35-44 | 45-54 | 55-80 | |
| Total No.b | 457 166 | 41 961 | 181 067 | 123 759 | 63 889 | 46 490 |
| Malesb | 266 232 | 23 063 | 104 957 | 74 069 | 37 125 | 27 018 |
| Femalesb | 190 934 | 18 898 | 76 110 | 49 690 | 26 764 | 19 472 |
| ≥180 Days of use, No. (%) | 133 915 (29.3) | 8109 (19.3) | 50 368 (27.8) | 39 123 (31.6) | 20 618 (32.3) | 15 697 (33.8) |
| Males | 76 162 (28.6) | 4210 (18.3) | 28 114 (26.8) | 23 174 (31.3) | 11 757 (31.7) | 8907 (33.0) |
| Females | 57 753 (30.2) | 3899 (20.6) | 22 254 (29.2) | 15 949 (32.1) | 8861 (33.1) | 6790 (34.9) |
| Use of ≥16 mg/d, No. (%) | 287 408 (62.9) | 21 593 (51.5) | 113 846 (62.9) | 82 421 (66.6) | 41 691 (65.3) | 27 857 (59.9) |
| Males | 167 300 (62.8) | 11 579 (50.2) | 65 390 (62.3) | 49 409 (66.7) | 24 436 (65.8) | 16 486 (61.0) |
| Females | 120 108 (62.9) | 10 014 (53.0) | 48 456 (63.7) | 33 012 (66.4) | 17 255 (64.5) | 11 371 (58.4) |
| ≥180 Days of use and ≥16 mg/d, No. (%) | 105 744 (23.1) | 6270 (14.9) | 40 101 (22.1) | 31 323 (25.3) | 16 351 (25.6) | 11 699 (25.2) |
| Males | 60 070 (22.6) | 3209 (13.9) | 22 260 (21.2) | 18 556 (25.1) | 9354 (25.2) | 6691 (24.8) |
| Females | 45 674 (23.9) | 3061 (16.2) | 17 841 (23.4) | 12 767 (25.7) | 6997 (26.1) | 5008 (25.7) |
Data source: 2016-2018 IQVIA Real World Data: Longitudinal Prescription, results for 2017.
Numbers denote new buprenorphine use episodes.
Discussion
Annual buprenorphine treatment per 1000 population increased between 2009 and 2018 from 1.97 to 4.43. Yet these rates are below national estimates of the combined rates of prescription opioid use disorder and heroin use.4,5 Findings suggest that the treatment gap may be widening for individuals aged 15 to 24 years, who experienced a decline in buprenorphine use and who received relatively low buprenorphine doses and short treatment episodes during a period when young people had increasing rates of opioid-related overdose deaths.6
Study limitations include uncertain accuracy of population coverage of IQVIA LRx data, particularly in earlier years, error in measurement of treatment length, and inability to exclude off-label use for pain. Prescription data measured purchased rather than consumed buprenorphine.
Although buprenorphine use has increased for most age groups, individuals aged 15 to 24 years experienced a decrease in use and low treatment retention, as reflected in a low proportion receiving long prescription episodes. Findings suggest a widening treatment gap for young people and underscore the importance of improving buprenorphine treatment services for this age group.
Section Editor: Jody W. Zylke, MD, Deputy Editor.
References
- 1.Wen H, Borders TF, Cummings JR. Trends in buprenorphine prescribing by physician specialty. Health Aff (Millwood). 2019;38(1):24-28. doi: 10.1377/hlthaff.2018.05145 [DOI] [PubMed] [Google Scholar]
- 2.Roberts AW, Saloner B, Dusetzina SB. Buprenorphine use and spending for opioid use disorder treatment: trends from 2003 to 2015. Psychiatr Serv. 2018;69(7):832-835. doi: 10.1176/appi.ps.201700315 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.National Quality Forum Behavioral Health 2016-2017: Final Report Washington, DC: National Quality Forum; August 2017. https://www.qualityforum.org/Publications/2017/08/Behavioral_Health_2016-2017_Final_Report.aspx. Accessed October 11, 2019.
- 4.Substance Abuse and Mental Health Services Administration Reports and detailed tables from the 2017 National Survey of Drug Use and Health; Table 7.2B. https://www.samhsa.gov/data/nsduh/reports-detailed-tables-2017-NSDUH. Accessed October 11, 2019.
- 5.Saha TD, Kerridge BT, Goldstein RB, et al. Nonmedical prescription opioid use and DSM-5 nonmedical prescription opioid use disorder in the United States. J Clin Psychiatry. 2016;77(6):772-780. doi: 10.4088/JCP.15m10386 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Gaither JR, Shabanova V, Leventhal JM. US national trends in pediatric deaths from prescription and illicit opioids, 1999-2016. JAMA Netw Open. 2018;1(8):e186558. doi: 10.1001/jamanetworkopen.2018.6558 [DOI] [PMC free article] [PubMed] [Google Scholar]