Key Points
Question
What is the effect of low-molecular-weight hydroxyethyl starch (HES 130/0.4) compared with 0.9% saline for intravascular volume expansion on mortality and postoperative complications in high-risk surgical patients?
Findings
In this randomized clinical trial that included 775 patients at increased risk of kidney injury after major abdominal surgery, the primary outcome of mortality or major postoperative complications within 14 days after surgery occurred in 36% in the HES group and 32% in the saline group, a difference that was not statistically significant.
Meaning
The use of HES compared with 0.9% saline resulted in no significant difference in death or postoperative complications among high-risk patients undergoing major abdominal surgery.
Abstract
Importance
It is not known if use of colloid solutions containing hydroxyethyl starch (HES) to correct for intravascular deficits in high-risk surgical patients is either effective or safe.
Objective
To evaluate the effect of HES 130/0.4 compared with 0.9% saline for intravascular volume expansion on mortality and postoperative complications after major abdominal surgery.
Design, Setting, and Participants
Multicenter, double-blind, parallel-group, randomized clinical trial of 775 adult patients at increased risk of postoperative kidney injury undergoing major abdominal surgery at 20 university hospitals in France from February 2016 to July 2018; final follow-up was in October 2018.
Interventions
Patients were randomized to receive fluid containing either 6% HES 130/0.4 diluted in 0.9% saline (n = 389) or 0.9% saline alone (n = 386) in 250-mL boluses using an individualized hemodynamic algorithm during surgery and for up to 24 hours on the first postoperative day, defined as ending at 7:59 am the following day.
Main Outcomes and Measures
The primary outcome was a composite of death or major postoperative complications at 14 days after surgery. Secondary outcomes included predefined postoperative complications within 14 days after surgery, durations of intensive care unit and hospital stays, and all-cause mortality at postoperative days 28 and 90.
Results
Among 826 patients enrolled (mean age, 68 [SD, 7] years; 91 women [12%]), 775 (94%) completed the trial. The primary outcome occurred in 139 of 389 patients (36%) in the HES group and 125 of 386 patients (32%) in the saline group (difference, 3.3% [95% CI, −3.3% to 10.0%]; relative risk, 1.10 [95% CI, 0.91-1.34]; P = .33). Among 12 prespecified secondary outcomes reported, 11 showed no significant difference, but a statistically significant difference was found in median volume of study fluid administered on day 1: 1250 mL (interquartile range, 750-2000 mL) in the HES group and 1500 mL (interquartile range, 750-2150 mL) in the saline group (median difference, 250 mL [95% CI, 83-417 mL]; P = .006). At 28 days after surgery, 4.1% and 2.3% of patients had died in the HES and saline groups, respectively (difference, 1.8% [95% CI, −0.7% to 4.3%]; relative risk, 1.76 [95% CI, 0.79-3.94]; P = .17).
Conclusions and Relevance
Among patients at risk of postoperative kidney injury undergoing major abdominal surgery, use of HES for volume replacement therapy compared with 0.9% saline resulted in no significant difference in a composite outcome of death or major postoperative complications within 14 days after surgery. These findings do not support the use of HES for volume replacement therapy in such patients.
Trial Registration
ClinicalTrials.gov Identifier: NCT02502773
This randomized clinical trial assesses the effect of hydroxyethyl starch 130/0.4 (HES) vs normal saline for intravascular volume expansion in patients at risk of postoperative kidney injury on mortality and postoperative complications up to 14 days after major abdominal surgery.
Introduction
Administration of intravenous fluid therapy is a critical aspect of maintaining fluid balance during surgery and can result in perioperative complications if too much or too little is given.1,2 During surgery, extracellular fluid volume is maintained by giving continuous infusions of intravenous fluids. When hypovolemia occurs, fluid boluses are given to restore intravascular volume.3 It is not known if it is better to use colloid or crystalloid solutions to correct for intravascular volume deficits that occur during surgery.
Hydroxyethyl starches (HES) are semisynthetic colloid solutions that have been used for fluid replacement therapy in patients undergoing major surgery4 because of their hypothetical ability to provide faster hemodynamic stabilization during acute hypovolemia.5,6 In 2013, the US Food and Drug Administration issued warnings about an increased risk of death and acute kidney injury with HES solutions when used in critically ill patients. The European Medicines Agency restricted the use of HES for critically ill patients but retained approval for HES to treat blood loss–related hypovolemia. However, the warnings applied to critically ill patients,7,8 and the effect of HES in surgical patients may be different. In a recent randomized trial of 160 patients undergoing major surgery, intraoperative use of HES resulted in fewer complications than balanced crystalloids.9 A recent open-label randomized trial10 and meta-analyses11,12 found no evidence that adverse effects were more common with low-molecular-weight HES solutions (HES 130/0.4) than with crystalloids. Because of uncertainty regarding outcomes associated with use of HES during surgery, the Fluid Loading in Abdominal Surgery: Saline vs Hydroxyethyl Starch (FLASH) trial was conducted. The hypothesis was that in a population of surgical patients at high risk of postoperative kidney injury, there would be a 10% difference in major morbidity or mortality between groups receiving HES vs saline.
Methods
Study Design
This was a pragmatic, investigator-initiated, multicenter, double-blind, randomized trial conducted in 20 French university hospitals from February 2016 to July 2018. The trial protocol and the statistical analysis plan were published13 and are available in Supplement 1. The trial protocol was approved for all centers by the ethics committee at the Clermont-Ferrand University Hospital. Written informed consent was obtained from all participating patients or next of kin before inclusion in the study. An independent data and safety monitoring board oversaw the study conduct and reviewed blinded safety data.
Patients
Patients were recruited on the eve or on the day of surgery. Consecutive adult patients aged 18 years or older admitted for elective or nonelective abdominal surgery under general anesthesia with an anticipated duration of 2 hours or longer and who had an intermediate to high risk of developing postoperative complications, as indicated by an acute kidney injury risk index14 class 3 or above, were eligible for participation. The acute kidney injury risk index ranges from 1 to 5, with higher classes indicating a greater risk of postoperative acute kidney injury (eAppendix 1 in Supplement 2). Exclusion criteria were preoperative acute heart failure or myocardial ischemia, chronic kidney disease (glomerular filtration rate <30 mL/min/1.73 m2 or requiring renal replacement therapy for end-stage kidney disease), requirement of vasoactive medication before surgery, and contraindications to use of HES, including hypersensitivity to the active substances, critical illness, sepsis, kidney injury, need for renal replacement therapy, severely impaired hepatic function, hyperhydration, and congestive heart failure.
Randomization and Interventions
Eligible patients were randomly assigned in a 1:1 ratio to HES or saline using a dedicated, encrypted web-based randomization system and a minimization algorithm stratified by study site and timing of the surgical procedure (elective or nonelective). Patients were randomly assigned to receive either 6% HES 130/0.4 in 0.9% saline or 0.9% saline alone in indistinguishable 500-mL bags. The study fluid was concealed from patients, clinicians, research staff, the data and safety monitoring board, and the statistician. Patients were given intravenous study fluid according to a stroke volume–guided hemodynamic therapy algorithm.15 Study fluids were manually administered as 250-mL boluses over a 5-minute interval intended to maximize stroke volume. An initial fluid challenge was performed after induction of anesthesia. If there was less than a 10% increase in stroke volume (as measured using devices for this purpose favored by local clinicians) in response to the fluid challenge, study fluid administration was stopped. If stroke volume increased more than 10%, another 250-mL bolus was given. No more than 500 mL of study fluid was administered for the initial fluid challenge. Once the maximal value of the stroke volume was determined, subsequent study fluid boluses during surgery were given if stroke volume decreased by more than 10% (see eAppendix 3 in Supplement 2 for additional details). Study fluid was administered on the day of surgery and for up to 24 hours on the first postoperative day, ending at 7:59 am on the day following the operation, to a maximum daily dose of 30 mL of study fluid per kilogram of body weight, followed by open-label administration of 0.9% saline if volumes of the study fluid were greater than the maximum daily dose. In both groups, lactated Ringer solution was used as maintenance fluid during surgery, given at a maximum infusion rate of 4 mL/kg per hour, and continued postoperatively if clinically indicated (until oral fluid intake was practical).
Decisions regarding all other aspects of patient care during and after surgery were at the discretion of attending physicians according to local expertise and clinical practice. To avoid extremes of practice, general measures for vasopressor administration, blood transfusion, mechanical ventilation, and antibiotic prophylaxis were recommended (eAppendix in Supplement 2).
Primary Outcome
The primary outcome was a composite of death or preselected major postoperative complications, including acute kidney injury of stage 1 or higher according to the Kidney Disease: Improving Global Outcomes (KDIGO) criteria,16 acute respiratory failure requiring invasive or noninvasive mechanical ventilation, acute heart failure, major septic complications, and unplanned reoperation 14 days after surgery. Each of these outcomes was also analyzed separately.
Secondary and Exploratory Outcomes
There were 13 secondary outcomes (definitions of end points are provided in eAppendix 3 in Supplement 2): postoperative kidney dysfunction within 14 days; postoperative pulmonary complications within 14 days; postoperative major adverse cardiovascular events within 14 days; postoperative infectious complications within 14 days; postoperative surgical complications within 14 days; Sequential Organ Failure Assessment score, modified by excluding the Glasgow Coma Scale at postoperative day 217; systemic inflammatory response syndrome score at postoperative day 218; amount of fluids and blood products administered on postoperative days 1 and 2; time to return of bowel function (not reported in this article); duration of intensive care unit and hospital stay; unplanned intensive care unit admission; all-cause mortality at postoperative day 28; and all-cause mortality at postoperative day 90.
Individual components of the postoperative complications composite end points were considered prespecified exploratory outcomes and included KDIGO stage 1, 2, and 3 acute kidney injury, need for renal replacement therapy, cardiac arrhythmia, myocardial infarction, pulmonary embolism, hypoxemia, pneumonia, acute respiratory distress syndrome, surgical site infection, and anastomotic leakage.
Post Hoc Outcomes
Post hoc outcomes included a composite of death or major postoperative complications 28 days after surgery, acute kidney injury 28 days after surgery, sepsis, acute respiratory failure, acute heart failure, unplanned reoperation 28 days after surgery, fluid balance until postoperative day 1, and need for blood transfusion or vasoactive medication.
Statistical Analysis
Two interim analyses were performed after enrollment of 210 and 420 patients, using the Lan-DeMets method.13 There was no stopping rule for efficacy when considering the primary outcome. The data and safety monitoring board did not recommend discontinuation of the trial at the interim analyses. We calculated that 826 patients were needed to have 95% statistical power to show an absolute between-group difference of 10% in the primary outcome at a 2-sided α = .05, assuming 20% morbidity19 and 5% mortality20,21 on postoperative day 14 (thus, 25% for the composite primary outcome). Because data on a clinical difference between HES and crystalloids among patients undergoing major surgery was limited when the study was designed, and because protocol-based hemodynamic management was used in both groups, we assumed that a 10% difference in the primary outcome would be appropriate and clinically relevant, based on the difference in the outcome of major complications found in a previous randomized clinical trial comparing HES vs crystalloids that also used a stroke volume–guided hemodynamic algorithm.22
All prespecified analyses were performed before the randomization code was broken. Patients were analyzed according to their randomization group. The analytic data set included all patients who were randomized except those who withdrew consent to the use of their data and those who never received study fluid during the study because of patient or clinician refusal or inability to implement the hemodynamic algorithm. There were no missing data for the primary and secondary outcome analyses, and complete case analysis was performed. An additional analysis was performed in the per-protocol population of patients who did not have any major protocol violations, as defined in the statistical analysis plan (Supplement 1). The primary outcome was compared between the 2 groups using unadjusted χ2 tests. Other binary outcomes were tested using unadjusted χ2 or Fisher exact tests as appropriate. Results are additionally reported as relative risks with 95% confidence intervals. Multiple logistic mixed regression was used to identify prespecified covariates with a known relationship to the primary outcome (selected if P < .10 in the bivariable analysis) in addition to the stratification variables. Multicollinearity between variables was assessed by computing the variance inflation factor and using the Farrar-Glauber test. The Akaike information criterion and Bayesian information criterion were calculated and used as model diagnostics to determine how well the model fit improved following addition of covariates. Adjusted analyses were performed using robust Poisson generalized linear model regression23 that included a random effect to account for center effects. The Hochberg procedure was used to adjust for multiple testing of components of the composite primary outcome.24 Continuous variables were compared with an unpaired t test or the Mann-Whitney U test. Time-to-event curves were constructed by the Kaplan-Meier method. Follow-up time was censored at 28 days following surgery. The proportional hazard hypothesis was studied using the Schoenfeld test and plotting residuals. Complete case analysis was performed for all outcomes. We did not compensate for dropouts caused by withdrawal of consent or surgery cancellations after randomization. Missing data for baseline and intraoperative clinical variables were not imputed. With the exception of components of the composite primary outcome, no adjustment was made for multiple comparisons. Because of the potential for type I error due to multiple comparisons, findings for analyses of secondary end points should be interpreted as exploratory.
A post hoc subgroup analysis was performed to test for a difference in treatment effect within patients with kidney dysfunction as defined by preoperative serum creatinine level greater than 1.2 mg/dL (yes vs no) at randomization. P values for interaction were derived from the multivariable random-effect logistic regression model including treatment and an interaction term.
All analyses were conducted using Stata software, version 13.0 (StataCorp), using the gllamm module. A 2-sided P < .05 was considered to indicate statistical significance.
Results
Patients
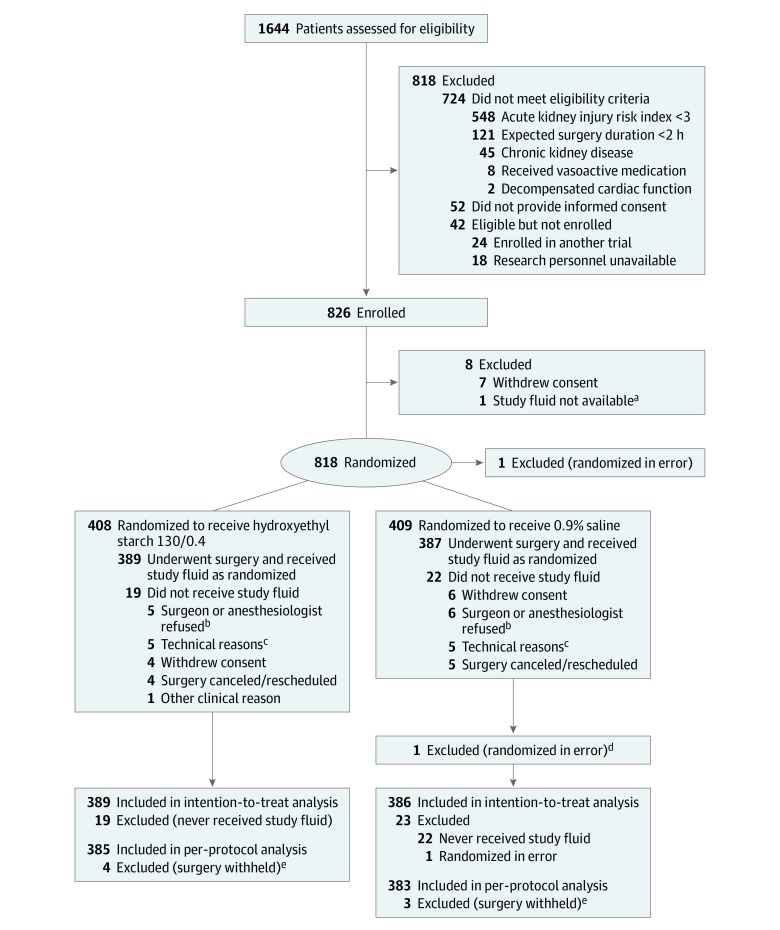
From February 24, 2016, through July 22, 2018, 826 patients provided written informed consent and were enrolled in the trial; 408 were randomly assigned to the HES group and 409 to the saline group. After withdrawals, 775 patients (389 in the HES group and 386 in the saline group) were included in the analysis (Figure 1). Data from 768 patients were included in the per-protocol analysis. The demographic and clinical characteristics of both groups were comparable with the exception of diabetes mellitus, which was more common in the HES group (Table 1 and eTable 1 in Supplement 2). At randomization, 441 of 775 patients (57%) had a class 3 acute kidney risk index, 334 patients (43%) had a class 4 or 5 acute kidney risk index, and 600 patients (77%) had had surgical procedures for cancer. Data for the primary outcome were available for all patients. Missing data for baseline characteristics and urine output are shown in eAppendix 4 in Supplement 2.
Figure 1. Participant Flow in the Fluid Loading in Abdominal Surgery: Saline vs Hydroxyethyl Starch (FLASH) Trial.
aStudy fluid not available as a result of dispensing failure.
bSenior clinician refusal due to conflicting ongoing investigations.
cUnavailability or technical problem preventing hemodynamic monitoring.
dViolation of exclusion criteria.
eExtensive disease preventing surgical resection.
Table 1. Baseline Participant Demographic and Perioperative Characteristics.
| Characteristics | Hydroxyethyl Starch 130/0.4 (n = 389) | 0.9% Saline (n = 386) |
|---|---|---|
| Age, mean (SD), y | 68 (7) | 69 (7) |
| Sex, No. (%) | ||
| Men | 340 (87) | 344 (89) |
| Women | 49 (13) | 42 (11) |
| Height, mean (SD), cm | 172 (8) | 172 (7) |
| Weight, mean (SD), kg | 82 (17) | 81 (15) |
| Body mass index, mean (SD)a | 28 (6) | 27 (5) |
| ASA physical status class, No./total (%)b | ||
| I (Healthy) | 3/388 (0.8) | 3/386 (0.8) |
| II | 193/388 (49.7) | 199/386 (51.6) |
| III | 190/388 (48.9) | 175/386 (45.3) |
| IV (Life-threatening severe systemic disease) | 2/388 (0.5) | 9/386 (2.3) |
| Acute kidney injury risk index class, No./total (%)c | ||
| 3 | 213/387 (55) | 228/385 (59) |
| 4 | 146/387 (38) | 133/385 (35) |
| 5 | 28/387 (7) | 24/385 (6) |
| Coexisting medical conditions, No. (%) | ||
| Hypertension | 335 (86) | 338 (88) |
| Diabetes mellitus | 195 (50) | 157 (41) |
| Mild or moderate kidney dysfunctiond | 93 (24) | 89 (23) |
| Coronary artery disease | 57 (15) | 55 (14) |
| Current smoking | 49 (13) | 45 (12) |
| Alcohol use | 48 (12) | 51 (13) |
| Chronic obstructive pulmonary disease | 43 (11) | 44 (11) |
| Chronic heart failure | 18 (5) | 25 (6) |
| Malnutrition | 32 (8) | 33 (9) |
| Cancer diagnosis, No. (%) | 316 (81) | 299 (77) |
| Type of surgery, No. (%)e | ||
| Hepatopancreatobiliary | 167 (43) | 159 (41) |
| Colorectal resection | 119 (31) | 115 (30) |
| Cystectomy | 52 (13) | 61 (16) |
| Gastrectomy | 30 (8) | 29 (8) |
| Vascular | 19 (5) | 15 (4) |
| Otherf | 46 (12) | 57 (15) |
| Laparoscopic surgery, No. (%) | 142 (37) | 151 (39) |
| Cancer surgery, No. (%) | 305 (78) | 295 (76) |
| Emergency surgical procedure, No. (%) | 7 (2) | 6 (2) |
| Duration of surgery, median (IQR), min | 240 (180-345) | 240 (174-330) |
| Baseline serum electrolyte level, mean (SD) | ||
| Sodium, mmol/L | 139 (3) | 139 (3) |
| Chloride, mmol/L | 102 (4) | 102 (4) |
| Serum urea nitrogen, mmol/L | 6.6 (5.2-8.7) | 7.1 (5.6-8.6) |
| Creatinine, mg/dL | 0.96 (0.80-1.15) | 0.95 (0.80-1.17) |
| Estimated glomerular filtration rate, mL/min/1.73 m2g | ||
| Overall, median (IQR) | 80.4 (62.9-99.6) | 81.1 (65.3-100.8) |
| With creatinine >1.2 mg/dL | ||
| No. | 92 | 88 |
| Median (IQR) | 54.2 (46.0-60.5) | 55.3 (47.1-60.4) |
Abbreviation: IQR, interquartile range.
SI conversion: To convert creatinine to μmol/L, multiply by 88.4.
Calculated as weight in kilograms divided by height in meters squared.
The American Society of Anesthesiologists (ASA) physical status class is a grading system for preoperative physical health assessment of surgical patients ranging from I to V, with higher classes indicating more severe systemic disease: class I indicates a completely healthy, fit patient; II, a patient with mild systemic disease that does not limit physical activity; III, a patient with severe systemic disease; IV, a patient with severe systemic disease that is a constant threat to life; and V, a moribund patient who is not expected to live 24 hours with or without surgery.
The acute kidney injury risk index for postoperative kidney injury is a scoring system based on 9 independent preoperative risk factors, with higher classes indicating higher risk of postoperative acute kidney injury.14
Mild or moderate kidney dysfunction was defined as a preoperative serum creatinine level greater than 1.2 mg/dL (105.6 μmol/L).
Patients may have undergone more than 1 type of surgery. Patients were recruited from 20 university hospitals; the number of surgeons per hospital was not recorded.
Common other surgical procedures were cytoreduction surgery, Hartmann procedure reversal, splenectomy, and hysterectomy.
Estimated glomerular filtration rate was calculated with the 4-variable Modification of Diet in Renal Disease equation.
Fluid Therapy
Intraoperatively, the median cumulative volume of maintenance fluid administered (lactated Ringer solution) was 1500 mL (interquartile range [IQR], 1000-2000 mL) in the HES group and 1500 mL (IQR, 1000-2030 mL) in the saline group (median difference, 0 mL [95% CI, −147 to 147 mL]; P = .60) (Table 2 and eTable 2 in Supplement 2). On the day of surgery (day 1; defined as the day when the surgery occurred until 7:59 am the next day), the median volume of study fluid given (including intraoperative fluid administration) was 1250 mL (IQR, 750-2000 mL) in the HES group and 1500 mL (IQR, 750-2150 mL) in the saline group (median difference, 250 mL [95% CI, 83-417 mL]; P = .006), with most fluids administered during surgery. With the aim of maintaining stroke volume, 190 patients (79 in the HES group and 111 in the saline group) required additional open-label study fluid during surgery (difference, −8.5%; 95% CI, −14.5% to −2.4%; P = .006). Because fluids are given in bulk, it is difficult to precisely deliver the exact amount of fluid required by the hemodynamic algorithm; because of this, 100 patients (41 in the HES group and 59 in the saline group) received more study fluid than the protocol-specified maximum dose. There was no statistically significant between-group difference in the total mean dose of study fluid given (33.4 [SD, 3.4] mL/kg in the HES group and 34.6 [SD, 5.8] mL/kg in the saline group; P = .15). The median cumulative total volume of intravenous fluid administered on the day of surgery was statistically significantly lower in the HES group than in the saline group (4000 mL [IQR, 3000-5000 mL] vs 4500 mL [IQR, 3350-6000 mL], respectively; median difference, 500 mL [95% CI, 175-824 mL]; P = .001)
Table 2. Fluid Therapy During the Study Period.
| Variables | Median (IQR), mL | Median Difference (95% CI)a | P Valueb | |
|---|---|---|---|---|
| Hydroxyethyl Starch 130/0.4 (n = 389) | 0.9% Saline (n = 386) | |||
| Day 1c | ||||
| Fluids administered during surgeryd | ||||
| Lactated Ringer solution | 1500 (1000 to 2000) | 1500 (1000 to 2030) | 0 (−147 to 147) | .60 |
| Study fluid | 1000 (750 to 1500) | 1250 (750 to 2000) | 250 (83 to 417) | .005 |
| Open-label study fluid | 500 (500 to 1000) | 750 (500 to 1000) | 233 (0 to 447) | .50 |
| Other fluidse | 500 (250 to 1000) | 500 (500 to 1000) | 0 (−308 to 308) | .11 |
| Blood products | ||||
| Packed red blood cells | 560 (560 to 840) [n = 63] | 560 (560 to 840) [n = 42] | 0 (−54 to 54) | .58 |
| Fresh frozen plasma | 400 (400 to 500) [n = 16] | 400 (400 to 600) [n = 13] | 0 (−175 to 175) | .25 |
| Platelets | 350 (350 to 350) [n = 3] | 350 (350 to 700) [n = 4] | 0 (0 to 700) | .39 |
| Fluids administered following surgery | ||||
| Lactated Ringer solution | 600 (440 to 1000) | 500 (500 to 1000) | −125 (−334 to 84) | .71 |
| Study fluid | 500 (500 to 750) | 500 (500 to 1000) | 0 (−98 to 98) | .31 |
| Open-label study fluid | 660 (500 to 1500) | 500 (500 to 1000) | −158 (−385 to 69) | .09 |
| Other fluidse | 1000 (750 to 1350) | 1000 (750 to 1500) | 0 (−49 to 49) | .12 |
| Blood products | ||||
| Packed red blood cells | 560 (280 to 560) [n = 18] | 560 (280 to 1400) [n = 7] | 0 (−357 to 357) | .97 |
| Fresh frozen plasma | 400 (400 to 400) [n = 6] | 400 (400 to 1000) [n = 4] | 0 (−1200 to 0) | .17 |
| Platelets | 350 (350 to 350) [n = 2] | 700 [n = 1] | ND | |
| Cumulative total intravenous fluids for day 1 | 4000 (3000 to 5000) | 4500 (3350 to 6000) | 500 (175 to 824) | .001 |
| Blood loss | 400 (200 to 800) | 400 (200 to 700) | 0 (−70 to 70) | .52 |
| Urine output | 375 (200 to 550) | 300 (200 to 500) | −80 (−129 to −31) | .03 |
| Fluid balancef | 3200 (2450 to 4200) | 3800 (2650 to 5100) | 575 (304 to 846) | <.001 |
| Day 2g | ||||
| Lactated Ringer solution | 500 (500 to 1040) | 500 (500 to 1000) | 0 (−248 to 248) | .07 |
| Study fluid | 500 (250 to 1000) | 500 (500 to 1000) | 0 (−152 to 152) | .68 |
| Open-label study fluid | 500 (500 to 1000) | 550 (450 to 1000) | 50 (−194 to 294) | .85 |
| Other fluidse | 1400 (1000 to 1500) | 1300 (1000 to 1700) | −36 (−210 to 138) | .87 |
| Blood products | ||||
| Packed red blood cells | 560 (280 to 560) [n = 6] | 560 (560 to 1120) [n = 3] | −280 (−840 to 0) | .12 |
| Fresh frozen plasma | 300 (200 to 600) [n = 4] | [n = 0] | ND | |
| Platelets | [n = 0] | [n = 0] | ND | |
| Cumulative total intravenous fluids for day 2 | 1500 (1000 to 2000) | 1500 (1000 to 2000) | 0 (−86 to 86) | .56 |
| Urine output | 1250 (900 to 1800) | 1400 (1000 to 2000) | 160 (23 to 297) | .02 |
| Fluid balancef | 200 (−600 to 900) | −100 (−900 to 700) | −300 (−543 to −57) | .02 |
Abbreviations: IQR, interquartile range; ND, analysis not done.
Calculated using the quantile regression model.
Calculated using the Mann-Whitney U test.
From the start of surgery to 7:59 am on postoperative day 1.
The amount of fluids and blood products administered were prespecified secondary outcomes.
Other fluids included gelatin, albumin, 5% dextrose, sodium bicarbonate, and 6% hydroxyethyl starch.
Fluid balance was calculated by subtracting the total fluid output from the total fluid intake. Fluid intake was the sum of all intravenous fluids, and fluid output was the sum of the volumes of urine output and blood loss. Insensible fluid losses were not included. Fluid balance was calculated as the median for all patients.
8:00 am on postoperative day 1 to 8:00 am on postoperative day 2.
Primary Outcome
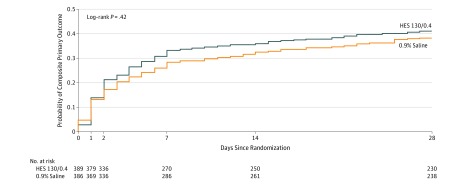
By postoperative day 14, 139 of 389 patients (36%) in the HES group and 125 of 386 patients (32%) in the saline group had died or developed major postoperative complications (difference, 3.3% [95% CI, −3.3% to 10.0%]; relative risk, 1.10 [95% CI, 0.91-1.34]; P = .33) (Table 3 and Figure 2). There were no statistically significant between-group differences in the individual components of the primary outcome.
Table 3. Primary, Secondary, and Exploratory Outcomes.
| Outcomes | Hydroxyethyl Starch 130/0.4 (n = 389) | 0.9% Saline (n = 386) | Absolute Difference (95% CI) | Relative Risk (95% CI)a | P Valueb |
|---|---|---|---|---|---|
| Primary Outcome | |||||
| Primary composite outcome at day 14, No. (%) | 139 (36) | 125 (32) | 3.3 (−3.3 to 10.0) | 1.10 (0.91-1.34) | .33 |
| Components of primary outcome, No. (%)c | |||||
| Death | 12 (3) | 6 (2) | 1.5 (−0.6 to 3.6) | 1.98 (0.75-5.23) | .85 |
| Acute kidney injury stage ≥1 | 85 (22) | 63 (16) | 5.5 (0.1 to 11.1) | 1.34 (1.00-1.80) | .30 |
| Acute respiratory failure | 32 (8) | 31 (8) | 0.2 (−3.7 to 4.0) | 1.02 (0.64-1.64) | .92 |
| Acute heart failure | 7 (2) | 4 (1) | 0.8 (−0.9 to 2.4) | 1.74 (0.51-5.88) | .92 |
| Major sepsis complications | 61 (16) | 65 (17) | −1.2 (−6.4 to 4.0) | 0.93 (0.68-1.28) | .92 |
| Sepsis | 49 (13) | 56 (15) | −1.9 (−6.7 to 2.9) | 0.87 (0.61-1.24) | .70 |
| Severe sepsis or septic shock | 15 (4) | 17 (4) | −0.5 (−3.4 to 2.3) | 0.88 (0.34-2.26) | .70 |
| Unplanned reoperation | 39 (10) | 48 (12) | −2.4 (−6.9 to 2.0) | 0.81 (0.54-1.20) | .92 |
| Secondary Outcomes | |||||
| Day 2 scores, median (IQR) | |||||
| SOFAd | 1 (0-2) | 1 (0-2) | −0.02 (−0.31 to 0.26) | NA | .34 |
| SIRSe | 2 (2-3) | 2 (2-3) | 0.05 (−0.08 to 0.18) | NA | .51 |
| Kidney dysfunction up to day 14, No. (%)f | 85 (22) | 63 (16) | 5.5 (0.1 to 11.1) | 1.34 (1.00-1.80) | .05 |
| Pulmonary complications up to day 14, No. (%)g | 62 (16) | 66 (17) | −1.1 (−6.4 to 4.1) | 0.93 (0.68-1.28) | .66 |
| Infectious complications up to day 14, No. (%)h | 78 (20) | 86 (22) | −2.2 (−8.0 to 3.5) | 0.90 (0.69-1.18) | .45 |
| Surgical complications up to day 14, No. (%)i | 60 (15) | 67 (17) | −1.9 (−7.1 to 3.3) | 0.89 (0.65-1.22) | .47 |
| Major adverse cardiovascular events up to day 14, No. (%)j | 40 (10) | 44 (11) | −1.1 (−5.5 to 3.3) | 0.90 (0.60-1.35) | .62 |
| Unplanned admission to ICU up to day 28, No. (%) | 39 (10) | 45 (12) | −1.6 (−6.0 to 2.7) | 0.86 (0.57-1.29) | .47 |
| Length of stay, median (IQR), d | |||||
| HDU or ICU | 4 (2-8) | 4 (2-7) | −0.2 (−1.4 to 0.9) | NA | 68 |
| Hospital | 10 (7-17) | 11 (7-17) | −0.4 (−1.5 to 0.7) | NA | .49 |
| Mortality, No. (%) | |||||
| At day 28 | 16 (4) | 9 (2) | 1.8 (−0.7 to 4.3) | 1.76 (0.79-3.94) | .17 |
| At day 90 | 26 (7) | 18 (5) | 2.0 (−1.2 to 5.3) | 1.43 (0.80-2.57) | .23 |
| Prespecified Exploratory Outcomesk | |||||
| Acute kidney injury KDIGO stage, No. (%) | |||||
| Stage 1 | 59 (15) | 39 (10) | 5.5 (0.5 to 10.4) | 1.61 (1.04-2.48) | .03 |
| Stage 2 | 14 (4) | 11 (3) | 0.8 (−1.8 to 3.3) | 1.35 (0.60-3.02) | .46 |
| Stage 3 | 12 (3) | 13 (3) | −0.3 (−2.8 to 2.2) | 0.98 (0.44-2.18) | .96 |
| Need for renal replacement therapy, No. (%) | 6 (2) | 10 (3) | −1.1 (−3.1 to 1.0) | 0.60 (0.22-1.62) | .31 |
| Pulmonary complications, No. (%) | |||||
| Hypoxemia | 55 (14) | 52 (13) | 0.07 (−4.2 to 5.6) | 1.05 (0.74-1.49) | .79 |
| Pneumonia | 15 (4) | 19 (5) | −1.1 (−4.0 to 1.8) | 0.78 (0.40-1.52) | .47 |
| Acute respiratory distress syndrome | 3 (1) | 4 (1) | −0.3 (−1.6 to 1.1) | 0.74 (0.17-3.30) | .70 |
| Surgical site infection up to day 14, No. (%) | 30 (8) | 40 (10) | −2.7 (−6.7 to 1.4) | 0.74 (0.47-1.17) | .20 |
| Anastomotic leak, No./total (%)l | 25/250 (10) | 33/240 (14) | −3.8 (−9.5 to 2.0) | 0.73 (0.45-1.19) | .20 |
| Major adverse cardiovascular events, No. (%) | |||||
| Cardiac arrhythmia | 27 (7) | 31 (8) | −1.1 (−4.8 to 2.6) | 0.90 (0.60-1.35) | .62 |
| Myocardial infarction | 1 (1) | 1 (1) | 0.0 (−0.7 to 0.7) | 0.99 (0.06-15.8) | .99 |
| Pulmonary embolism | 9 (2) | 9 (2) | 0.0 (−2.1 to 2.1) | 0.99 (0.40-2.47) | .99 |
| Post Hoc Outcomes | |||||
| Death or major postoperative complications up to day 28, No. (%)m | 159 (41) | 148 (38) | 2.5 (−4.4 to 9.4) | 1.07 (0.90-1.27) | .47 |
| Acute kidney injury up to day 28, No. (%)n | 88 (23) | 64 (17) | 6.0 (0.5 to 11.6) | 1.36 (1.02-1.82) | .04 |
| Acute respiratory failure up to day 28, No. (%) | 35 (9) | 36 (9) | −0.3 (−4.4 to 3.7) | 0.96 (0.62-1.50) | .87 |
| Sepsis up to day 28, No. (%) | 76 (20) | 83 (22) | −2.0 (−7.7 to 3.7) | 0.91 (0.69-1.20) | .50 |
| Acute heart failure up to day 28, No. (%) | 45 (12) | 45 (12) | 0 (−4.6 to 4.4) | 0.99 (0.67-1.46) | .97 |
| Unplanned reoperation up to day 28, No. (%) | 62 (16) | 63 (16) | −0.4 (−5.6 to 4.8) | 0.98 (0.71-1.35) | .89 |
| Day 1 fluid balance, median (IQR)o | 3200 (2450-4200) | 3800 (2650-5100) | 575 (304 to 846) | NA | <.001 |
| Need for blood transfusion, No. (%) | 75 (19) | 45 (12) | 7.6 (2.6 to 12.7) | 1.65 (1.18-2.33) | .003 |
| Need for vasoactive medication, No. (%) | NA | ||||
| Norepinephrine | 104 (27) | 117 (30) | −3.6 (−9.9 to 2.8) | ||
| Phenylephrine | 64 (16) | 79 (20) | −4.0 (−9.5 to 1.4) | ||
| Ephedrine | 242 (62) | 240 (62) | 0.0 (−6.8 to 6.9) | ||
| Epinephrine | 0 | 3 (1) | −0.8 (−1.7 to 0.0) | ||
Abbreviations: HDU, high dependency unit; ICU, intensive care unit; IQR, interquartile range; KDIGO, Kidney Disease: Improving Global Outcomes; NA, not applicable.
Unadjusted relative risk. For adjusted analysis, see eTable 3 in Supplement 2.
Calculated using the χ2 test or Fisher exact test, as appropriate, for categorical data and the unpaired t test or Mann-Whitney U test for continuous data.
All components of the composite primary outcome were assessed at 14 days after surgery. The Hochberg procedure was used to correct for multiple testing of the components of the composite primary outcome.
Scores on the Sequential Organ Failure Assessment (SOFA) scale range from 0 to 4 for each organ system, with higher scores indicating more severe organ dysfunction.
The systemic inflammatory response syndrome (SIRS) score (range, 0 [best] to 4 [worst]) assigns 1 point for each of the following variables: temperature >38°C or <36°C, white blood cell count >12 000/μL or <4000/μL, heart rate >90/min and respiratory rate >20/min, and Paco2 <32 mm Hg.
Defined as any kidney injury assessed using the 3-category KDIGO classification system.
Defined as hypoxemia (Pao2 <60 mm Hg or peripheral oxygen saturation as measured by pulse oximetry <90% when breathing room air, Pao2 <80 mm Hg when breathing 15 L/min of supplemental oxygen, or Pao2/fraction of inspired oxygen ratio <300 mm Hg within 14 days after surgery), pneumonia, acute respiratory distress syndrome, or acute respiratory failure requiring invasive or noninvasive mechanical ventilation.
Defined as sepsis, severe sepsis, septic shock, or surgical site infection.
Defined as reoperation and anastomotic leak.
Defined as acute heart failure, cardiac arrhythmia, myocardial infarction, or pulmonary embolism.
All prespecified exploratory analyses (individual components of the secondary analyses) were measured up to postoperative day 14.
Anastomotic leak data are expressed as No./total (%) patients who had an operation in which an anastomosis was performed.
Major postoperative complications were KDIGO stage ≥1 acute kidney injury, acute respiratory failure requiring invasive or noninvasive mechanical ventilation, acute heart failure, major sepsis complications, and unplanned reoperation.
Defined as KDIGO stage ≥1 acute kidney injury.
Fluid balance was calculated by subtracting the total fluid output from the total fluid intake. Fluid intake was the sum of all intravenous fluids, and fluid output was the sum of the volumes of urine output and blood loss. Insensible fluid losses were not included. Fluid balance was calculated as the median for all patients.
Figure 2. Kaplan-Meier Estimates of the Probability of the Composite Primary Outcome.
Raw data for the Kaplan-Meier probability of death or major postoperative complications were censored at 28 days after surgery. Major postoperative complications were acute kidney injury stage 1 or higher according to the Kidney Disease: Improving Global Outcomes (KDIGO) criteria, acute respiratory failure requiring invasive or noninvasive mechanical ventilation, acute heart failure, major septic complications, and unplanned reoperation. The median observation time was 28 days (interquartile range, 4-28 days) for the HES group and 28 days (interquartile range, 6-28 days) for the saline group.
Secondary and Exploratory Outcomes
Kidney dysfunction within 14 days after surgery occurred in 22% of patients in the HES group and 16% of patients in the saline group (difference, 5.5% [95% CI, 0.1%-11.1%]; relative risk, 1.34 [95% CI, 1.00-1.80]; P = .05). The result was unaffected by adjustment for stratification variables and covariates (adjusted relative risk, 1.27; 95% CI, 0.96-1.70; P = .10) (eTable 3 in Supplement 2).
More patients in the HES group had KDIGO stage 1 acute kidney injury 14 days after surgery (15% vs 10%; difference, 5.1% [95% CI, 0.1%-10.1%]; relative risk, 1.61 [95% CI, 1.04-2.48]; P = .03). Covariate adjustment had little effect on these results (adjusted relative risk, 1.45; 95% CI, 1.15-1.83; P = .002). There were no statistically significant between-group differences in KDIGO stage 2 or stage 3 acute kidney injury or in use of renal replacement therapy (eTable 4 in Supplement 2). There were no other statistically significant between-group differences in the rates of the other secondary trial outcomes (Table 3 and eFigures 1 and 2 in Supplement 2). By 28 days after surgery, 16 patients (4.1%) in the HES group and 9 (2.3%) in the saline group had died (relative risk, 1.76; 95% CI, 0.79-3.94; P = .17).
Additional Analyses
The results for the primary outcome were essentially unchanged in the adjusted analysis (adjusted relative risk, 1.09 [95% CI, 0.91-1.31]; P = .35) (eTables 3 and 4 in Supplement 2) and in the per-protocol analysis (36% vs 33%; difference, 3.2% [95% CI, −3.5% to 9.9%]; unadjusted relative risk, 1.10 [95% CI, 0.90-1.34]; adjusted relative risk, 1.09 [95% CI, 0.90-1.31]; P = .38) (eAppendix 5 in Supplement 2).
Post Hoc Analyses
During the intraoperative period, post hoc analyses showed that patients in the HES group had less positive fluid balance (median, 3200 mL [IQR, 2450-4200 mL] in the HES group and 3800 mL [IQR, 2650-5100 mL] in the saline group; difference, 575 mL [95% CI, 304-846 mL]; P < .001). Patients in the HES group also had a significantly higher stroke volume at the end of surgery (mean, 47 [SD, 13] mL/m2 in the HES group and 43 [SD, 13] mL/m2 in the saline group; difference, 4 mL/m2 [95% CI, 2-6 mL/m2]; P < .001), had a significantly higher urine output (median, 375 mL [IQR, 200-550 mL] in the HES group and 300 mL [IQR, 200-500 mL] in the saline group; difference, 80 mL [95% CI, 31-129 mL]; P = .03), and received significantly lower doses of norepinephrine (median, 0.04 μg/kg per minute [IQR, 0.02-0.08 μg/kg per minute] in the HES group and 0.06 μg/kg per minute [IQR, 0.03-0.12 μg/kg per minute] in the saline group; difference, −0.02 μg/kg per minute [95% CI, −0.03 to −0.01 μg/kg per minute]; P = .01). Patients in the HES group were also more likely to receive red blood cell transfusion (19% vs 12%; difference, 7.6%; 95% CI, 2.6%-12.7%; P = .003) (eTable 1 in Supplement 2).
There was no significant interaction between kidney dysfunction at enrollment and treatment group with respect to postoperative acute kidney injury (39% with HES vs 22% with saline; difference, 17.4% [95% CI, 4.3%-30.4%] in patients with kidney dysfunction at enrollment; 17% with HES and 15% with saline; difference, 1.7% [95% CI, −4.1% to 7.6%] in patients without kidney dysfunction at enrollment; P = .36 for interaction). At 28 days after surgery, acute kidney injury had occurred in 88 patients (23%) in the HES group and 64 patients (17%) in the saline group (relative risk, 1.36; 95% CI, 1.02-1.82; P = .04) (eFigure 1 in Supplement 2).
Discussion
In this multicenter, double-blind randomized trial involving patients at risk of postoperative kidney injury undergoing major abdominal surgery, rates of death and major postoperative complications within 14 days after surgery did not differ significantly between those receiving bolus infusions of HES 130/0.4 diluted in 0.9% saline or 0.9% saline alone for volume replacement therapy.
Inappropriate administration of intravenous fluid during surgery can be harmful, resulting in an increased risk of acute kidney injury and death.2 Additionally, concerns about the use of HES have been raised about kidney injury and other serious adverse effects, including an increased risk of bleeding and need for blood products resulting from HES-induced coagulopathy,25 with no evidence of benefit in terms of patient outcome measures. However, results from a meta-analysis of 32 trials including 16 647 patients showed that administration of colloids, including low-molecular-weight HES, did not increase mortality or risk of acute kidney injury in surgical patients.26
This trial was conducted to clarify the clinical effectiveness and adverse events of colloid HES in patients at high risk of complications that have been attributed to HES. Previous studies in surgical patients suggested that HES solutions may be more effective than crystalloids in expanding the intravascular space with a volume-sparing effect.9,22 Arguments against the use of HES in surgical patients include that no previous large, randomized perioperative study has demonstrated harm.11,26,27 A strength of the current trial was the use of a protocolized hemodynamic algorithm to titrate fluid administration.28 Previous studies have suggested beneficial effects of cardiac output–guided hemodynamic therapy to improve outcomes in high-risk patients.19,29 For this reason, clinical practice guidelines recommend the use of protocol-based hemodynamic management to prevent development of kidney injury in the perioperative setting.30 However, the role of the type of fluid in improving outcomes remains unclear. The findings of the current trial show that HES was better than crystalloids at expanding intravascular volume in patients with hypovolemia,5,6 as shown by requiring significantly less HES than crystalloids to achieve similar hemodynamic outcomes. The observation that postoperative stroke volume was higher in the HES group is consistent with previous findings that HES was associated with more potent and prolonged plasma volume expansion than crystalloids.31 Previous studies reported that colloids may be more effective at maintaining cardiac output and osmotic pressure than are rapidly extravasating crystalloid solutions.32 Despite these differences, there was no significant difference in major postoperative complication rates between the 2 groups in this study. These findings corroborate those of a recent subgroup analysis of the Colloids Compared to Crystalloids in Fluid Resuscitation of Critically Ill Patients (CRISTAL) randomized clinical trial,33 which included critically ill surgical patients with hypovolemic shock. Although that trial was not designed to evaluate the effect of any particular type of fluid, no difference was found between colloids and crystalloids in risk of death or organ failure.
A small proprotion of patients overall (10.5% in the HES group and 15.3% in the saline group) were given study fluid at doses higher than the protocol-specified maximum daily dose (ie, 30 mL/kg), with similar rates of fluid infusion in the 2 groups. Higher-than-targeted doses of the study drugs resulted from the need to administer more fluid than specified in the protocol to optimize stroke volume. The dose of study fluid in the 2 groups was defined a priori to comply with the maximum daily dose of 6% HES recommended by the manufacturers and to limit the potential harm to patients from high doses of HES. Although the maximum daily dose of HES in the study was lower than that used in other large randomized clinical trials,7,34 the possibility that high doses of study fluid affected the results cannot be totally excluded.
Patients in the current trial had lower risks of adverse kidney outcomes compared with critically ill intensive care unit patients.7,8 The observed overall rate of 19% of acute kidney injury in this trial was consistent with rates previously reported among surgical patients.35 The results mirror those of a large observational study assessing the adverse events of perioperative colloids that suggested an increased risk of acute kidney injury in association with use of HES.36 The interaction in the current study between preoperative kidney dysfunction and acute kidney injury was not significant. Although the study may not be powered enough to detect a significant difference among subgroups, this result suggests a consistency of effect. These findings are important because even mild and transient changes in kidney function after major surgery may affect short- and long-term patient outcomes.37,38
Another randomized clinical trial involving patients undergoing major abdominal surgery showed no adverse effect of HES on kidney outcomes.10 One possible explanation for why the findings of the current trial differ is the inclusion of patients with a higher risk of developing postoperative kidney dysfunction. In contrast to the study by Kabon et al,10 patients in this study were older (a mean of 68 years vs 52 years), had more comorbidities, and had an overall rate of kidney injury (19.6% in this study vs 3.5% in the study by Kabon et al) that was comparable with rates reported in previous studies after major abdominal surgery (6.7%-39.3%).35 Other potential explanations include the lack of blinding of attending physicians to treatment allocation in the study by Kabon et al and an enrollment period of 10 years, during which care of patients may have changed, which might have affected results.
Limitations
This study has several limitations. First, the trial protocol restricted the use of study fluid to the day of surgery and the next 24 hours; administration of fluid later in the hospital course was not controlled. However, this limitation is unlikely to have affected the results because most intravenous fluids are usually administered during the early postoperative period in adults. Second, the trial was pragmatic and was aimed to replicate routine practice; however, all co-interventions undertaken during the study period were not assessed. Third, the study population did not include patients with lower risk of morbidity. Fourth, as discussed previously, 100 patients received study fluid at higher doses than the protocol-specified maximum daily dose. Such protocol violations are difficult to prevent in multicenter trials, even though adherence to the trial protocol was regularly assessed among study centers. Fifth, the use of 0.9% saline rather than a balanced crystalloid solution may have affected the results. Although 0.9% saline can cause hyperchloremic metabolic acidosis and impair renal perfusion,39 0.9% saline was used to allow comparable chemical composition of the study fluid.
Conclusions
Among patients at risk of postoperative kidney injury undergoing major abdominal surgery, use of HES for volume replacement therapy, compared with 0.9% saline, resulted in no significant difference in a composite outcome of death or major postoperative complications within 14 days after surgery. These findings do not support the use of HES for volume replacement therapy in such patients.
Trial Protocol and Statistical Analysis Plan
eAppendix 1. Trial Inclusion and Exclusion Criteria
eAppendix 2. Supplementary Methods for Intravenous Fluid Administration
eAppendix 3. Outcome Definitions
eAppendix 4. Handling of Missing Data
eAppendix 5. Per-Protocol Analysis
eFigure 1. Time-to-Event Curve for Postoperative Acute Kidney Injury Within 28 Days After Surgery
eFigure 2. Time-to-Event Curve for Postoperative Death Within 28 Days After Surgery
eTable 1. Additional Details on Perioperative Characteristics
eTable 2. Additional Details on Fluid Therapy and Blood Products
eTable 3. Results of the Adjusted Analysis for Outcomes
eTable 4. Univariable and Multivariable Analysis of Factors Associated With the Primary Outcome, and Model Diagnostics
eReferences
Data Sharing Statement
References
- 1.Myles PS, Bellomo R, Corcoran T, et al. ; Australian and New Zealand College of Anaesthetists Clinical Trials Network and the Australian and New Zealand Intensive Care Society Clinical Trials Group . Restrictive versus liberal fluid therapy for major abdominal surgery. N Engl J Med. 2018;378(24):2263-2274. doi: 10.1056/NEJMoa1801601 [DOI] [PubMed] [Google Scholar]
- 2.Shin CH, Long DR, McLean D, et al. . Effects of intraoperative fluid management on postoperative outcomes: a hospital registry study. Ann Surg. 2018;267(6):1084-1092. doi: 10.1097/SLA.0000000000002220 [DOI] [PubMed] [Google Scholar]
- 3.Moritz ML, Ayus JC. Maintenance intravenous fluids in acutely ill patients. N Engl J Med. 2015;373(14):1350-1360. doi: 10.1056/NEJMra1412877 [DOI] [PubMed] [Google Scholar]
- 4.Myburgh JA, Mythen MG. Resuscitation fluids. N Engl J Med. 2013;369(13):1243-1251. doi: 10.1056/NEJMra1208627 [DOI] [PubMed] [Google Scholar]
- 5.Annane D, Siami S, Jaber S, et al. ; CRISTAL Investigators . Effects of fluid resuscitation with colloids vs crystalloids on mortality in critically ill patients presenting with hypovolemic shock: the CRISTAL randomized trial. JAMA. 2013;310(17):1809-1817. doi: 10.1001/jama.2013.280502 [DOI] [PubMed] [Google Scholar]
- 6.Trof RJ, Sukul SP, Twisk JW, Girbes AR, Groeneveld AB. Greater cardiac response of colloid than saline fluid loading in septic and non-septic critically ill patients with clinical hypovolaemia. Intensive Care Med. 2010;36(4):697-701. doi: 10.1007/s00134-010-1776-x [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Perner A, Haase N, Guttormsen AB, et al. ; 6S Trial Group; Scandinavian Critical Care Trials Group . Hydroxyethyl starch 130/0.42 versus Ringer’s acetate in severe sepsis. N Engl J Med. 2012;367(2):124-134. doi: 10.1056/NEJMoa1204242 [DOI] [PubMed] [Google Scholar]
- 8.Myburgh JA, Finfer S, Bellomo R, et al. ; CHEST Investigators; Australian and New Zealand Intensive Care Society Clinical Trials Group . Hydroxyethyl starch or saline for fluid resuscitation in intensive care. N Engl J Med. 2012;367(20):1901-1911. doi: 10.1056/NEJMoa1209759 [DOI] [PubMed] [Google Scholar]
- 9.Joosten A, Delaporte A, Ickx B, et al. . Crystalloid versus colloid for intraoperative goal-directed fluid therapy using a closed-loop system: a randomized, double-blinded, controlled trial in major abdominal surgery. Anesthesiology. 2018;128(1):55-66. doi: 10.1097/ALN.0000000000001936 [DOI] [PubMed] [Google Scholar]
- 10.Kabon B, Sessler DI, Kurz A; Crystalloid-Colloid Study Team . Effect of intraoperative goal-directed balanced crystalloid versus colloid administration on major postoperative morbidity: a randomized trial. Anesthesiology. 2019;130(5):728-744. doi: 10.1097/ALN.0000000000002601 [DOI] [PubMed] [Google Scholar]
- 11.Gillies MA, Habicher M, Jhanji S, et al. . Incidence of postoperative death and acute kidney injury associated with IV 6% hydroxyethyl starch use: systematic review and meta-analysis. Br J Anaesth. 2014;112(1):25-34. doi: 10.1093/bja/aet303 [DOI] [PubMed] [Google Scholar]
- 12.Raiman M, Mitchell CG, Biccard BM, Rodseth RN. Comparison of hydroxyethyl starch colloids with crystalloids for surgical patients: a systematic review and meta-analysis. Eur J Anaesthesiol. 2016;33(1):42-48. doi: 10.1097/EJA.0000000000000328 [DOI] [PubMed] [Google Scholar]
- 13.Futier E, Biais M, Godet T, et al. ; FLASH Trial Management Committee . Fluid Loading in Abdominal Surgery—Saline vs Hydroxyethyl Starch (FLASH trial): study protocol for a randomized controlled trial. Trials. 2015;16:582. doi: 10.1186/s13063-015-1085-3 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Kheterpal S, Tremper KK, Heung M, et al. . Development and validation of an acute kidney injury risk index for patients undergoing general surgery: results from a national data set. Anesthesiology. 2009;110(3):505-515. doi: 10.1097/ALN.0b013e3181979440 [DOI] [PubMed] [Google Scholar]
- 15.Vallet B, Blanloeil Y, Cholley B, Orliaguet G, Pierre S, Tavernier B; Société Française d’Anesthésie et de Réanimation . Guidelines for perioperative haemodynamic optimization. Ann Fr Anesth Reanim. 2013;32(10):e151-e158. doi: 10.1016/j.annfar.2013.09.010 [DOI] [PubMed] [Google Scholar]
- 16.Kidney Disease Improving Global Outcomes (KDIGO) Acute Kidney Injury Work Group KDIGO clinical practice guideline for acute kidney injury. Kidney Int. 2012;2(1)(suppl):1-138. [Google Scholar]
- 17.Vincent JL, Moreno R, Takala J, et al. ; Working Group on Sepsis-Related Problems of the European Society of Intensive Care Medicine . The SOFA (Sepsis-Related Organ Failure Assessment) score to describe organ dysfunction/failure. Intensive Care Med. 1996;22(7):707-710. doi: 10.1007/BF01709751 [DOI] [PubMed] [Google Scholar]
- 18.Talmor M, Hydo L, Barie PS. Relationship of systemic inflammatory response syndrome to organ dysfunction, length of stay, and mortality in critical surgical illness: effect of intensive care unit resuscitation. Arch Surg. 1999;134(1):81-87. doi: 10.1001/archsurg.134.1.81 [DOI] [PubMed] [Google Scholar]
- 19.Pearse RM, Harrison DA, MacDonald N, et al. ; OPTIMISE Study Group . Effect of a perioperative, cardiac output-guided hemodynamic therapy algorithm on outcomes following major gastrointestinal surgery: a randomized clinical trial and systematic review. JAMA. 2014;311(21):2181-2190. doi: 10.1001/jama.2014.5305 [DOI] [PubMed] [Google Scholar]
- 20.Pearse RM, Moreno RP, Bauer P, et al. ; European Surgical Outcomes Study Group for the Trials Groups of the European Society of Intensive Care Medicine and the European Society of Anaesthesiology . Mortality after surgery in Europe: a 7 day cohort study. Lancet. 2012;380(9847):1059-1065. doi: 10.1016/S0140-6736(12)61148-9 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Ghaferi AA, Birkmeyer JD, Dimick JB. Variation in hospital mortality associated with inpatient surgery. N Engl J Med. 2009;361(14):1368-1375. doi: 10.1056/NEJMsa0903048 [DOI] [PubMed] [Google Scholar]
- 22.Yates DR, Davies SJ, Milner HE, Wilson RJ. Crystalloid or colloid for goal-directed fluid therapy in colorectal surgery. Br J Anaesth. 2014;112(2):281-289. doi: 10.1093/bja/aet307 [DOI] [PubMed] [Google Scholar]
- 23.Zou G. A modified Poisson regression approach to prospective studies with binary data. Am J Epidemiol. 2004;159(7):702-706. doi: 10.1093/aje/kwh090 [DOI] [PubMed] [Google Scholar]
- 24.Hochberg Y. A sharper Bonferroni procedure for multiple tests of significance. Biometrika. 1988;75:800-802. doi: 10.1093/biomet/75.4.800 [DOI] [Google Scholar]
- 25.Rasmussen KC, Johansson PI, Højskov M, et al. . Hydroxyethyl starch reduces coagulation competence and increases blood loss during major surgery: results from a randomized controlled trial. Ann Surg. 2014;259(2):249-254. doi: 10.1097/SLA.0000000000000267 [DOI] [PubMed] [Google Scholar]
- 26.Qureshi SH, Rizvi SI, Patel NN, Murphy GJ. Meta-analysis of colloids versus crystalloids in critically ill, trauma and surgical patients. Br J Surg. 2016;103(1):14-26. doi: 10.1002/bjs.9943 [DOI] [PubMed] [Google Scholar]
- 27.Van Der Linden P, James M, Mythen M, Weiskopf RB. Safety of modern starches used during surgery. Anesth Analg. 2013;116(1):35-48. doi: 10.1213/ANE.0b013e31827175da [DOI] [PubMed] [Google Scholar]
- 28.Vincent JL, Pelosi P, Pearse R, et al. . Perioperative cardiovascular monitoring of high-risk patients: a consensus of 12. Crit Care. 2015;19:224. doi: 10.1186/s13054-015-0932-7 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Cecconi M, Corredor C, Arulkumaran N, et al. . Goal-directed therapy—what is the evidence in surgical patients? the effect on different risk groups. Crit Care. 2013;17(2):209. doi: 10.1186/cc11823 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Kellum JA, Lameire N; KDIGO AKI Guideline Work Group . Diagnosis, evaluation, and management of acute kidney injury: a KDIGO summary (part 1). Crit Care. 2013;17(1):204. doi: 10.1186/cc11454 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Hahn RG. Volume kinetics for infusion fluids. Anesthesiology. 2010;113(2):470-481. doi: 10.1097/ALN.0b013e3181dcd88f [DOI] [PubMed] [Google Scholar]
- 32.Verheij J, van Lingen A, Beishuizen A, et al. . Cardiac response is greater for colloid than saline fluid loading after cardiac or vascular surgery. Intensive Care Med. 2006;32(7):1030-1038. doi: 10.1007/s00134-006-0195-5 [DOI] [PubMed] [Google Scholar]
- 33.Heming N, Lamothe L, Jaber S, et al. . Morbidity and mortality of crystalloids compared to colloids in critically ill surgical patients: a subgroup analysis of a randomized trial. Anesthesiology. 2018;129(6):1149-1158. doi: 10.1097/ALN.0000000000002413 [DOI] [PubMed] [Google Scholar]
- 34.Brunkhorst FM, Engel C, Bloos F, et al. ; German Competence Network Sepsis (SepNet) . Intensive insulin therapy and pentastarch resuscitation in severe sepsis. N Engl J Med. 2008;358(2):125-139. doi: 10.1056/NEJMoa070716 [DOI] [PubMed] [Google Scholar]
- 35.O’Connor ME, Kirwan CJ, Pearse RM, Prowle JR. Incidence and associations of acute kidney injury after major abdominal surgery. Intensive Care Med. 2016;42(4):521-530. doi: 10.1007/s00134-015-4157-7 [DOI] [PubMed] [Google Scholar]
- 36.Opperer M, Poeran J, Rasul R, Mazumdar M, Memtsoudis SG. Use of perioperative hydroxyethyl starch 6% and albumin 5% in elective joint arthroplasty and association with adverse outcomes: a retrospective population based analysis. BMJ. 2015;350:h1567. doi: 10.1136/bmj.h1567 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Bihorac A, Yavas S, Subbiah S, et al. . Long-term risk of mortality and acute kidney injury during hospitalization after major surgery. Ann Surg. 2009;249(5):851-858. doi: 10.1097/SLA.0b013e3181a40a0b [DOI] [PubMed] [Google Scholar]
- 38.O’Connor ME, Hewson RW, Kirwan CJ, Ackland GL, Pearse RM, Prowle JR. Acute kidney injury and mortality 1 year after major non-cardiac surgery. Br J Surg. 2017;104(7):868-876. doi: 10.1002/bjs.10498 [DOI] [PubMed] [Google Scholar]
- 39.Yunos NM, Kim IB, Bellomo R, et al. . The biochemical effects of restricting chloride-rich fluids in intensive care. Crit Care Med. 2011;39(11):2419-2424. doi: 10.1097/CCM.0b013e31822571e5 [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Trial Protocol and Statistical Analysis Plan
eAppendix 1. Trial Inclusion and Exclusion Criteria
eAppendix 2. Supplementary Methods for Intravenous Fluid Administration
eAppendix 3. Outcome Definitions
eAppendix 4. Handling of Missing Data
eAppendix 5. Per-Protocol Analysis
eFigure 1. Time-to-Event Curve for Postoperative Acute Kidney Injury Within 28 Days After Surgery
eFigure 2. Time-to-Event Curve for Postoperative Death Within 28 Days After Surgery
eTable 1. Additional Details on Perioperative Characteristics
eTable 2. Additional Details on Fluid Therapy and Blood Products
eTable 3. Results of the Adjusted Analysis for Outcomes
eTable 4. Univariable and Multivariable Analysis of Factors Associated With the Primary Outcome, and Model Diagnostics
eReferences
Data Sharing Statement