Key Points
Question
Is the apolipoprotein E ε4 (APOEε4) genotype associated with tau pathology independently of amyloid-β?
Findings
In this study of 2 cross-sectional cohorts (total n = 489), individuals who were APOEε4 carriers had significantly higher entorhinal and hippocampal tau positron emission tomography signal than APOEε4 noncarriers, controlling for cortical amyloid-β burden, age, sex, and clinical status.
Meaning
Carriership of APOEε4 is associated with tau pathology in medial temporal structures independently of amyloid-β, extending previous reports of greater medial temporal neurodegeneration and memory impairment in APOEε4 carriers.
Abstract
Importance
Apolipoprotein E ε4 (APOEε4) is the single most important genetic risk factor for Alzheimer disease. While APOEε4 is associated with increased amyloid-β burden, its association with cerebral tau pathology has been controversial.
Objective
To determine whether APOEε4 is associated with medial temporal tau pathology independently of amyloid-β, sex, clinical status, and age.
Design, Setting, and Participants
This is a study of 2 cross-sectional cohorts of volunteers who were cognitively normal, had mild cognitive impairment (MCI), or had Alzheimer disease dementia: the Translational Biomarkers in Aging and Dementia (TRIAD) study (data collected between October 2017 and July 2019) and the Alzheimer’s Disease Neuroimaging Initiative (ADNI) (collected between November 2015 and June 2019). The first cohort (TRIAD) comprised cognitively normal elderly participants (n = 124), participants with MCI (n = 50), and participants with Alzheimer disease (n = 50) who underwent tau positron emission tomography (PET) with fluorine 18–labeled MK6240 and amyloid-β PET with [18F]AZD4694. The second sample (ADNI) was composed of cognitively normal elderly participants (n = 157), participants with MCI (n = 83), and participants with Alzheimer disease (n = 25) who underwent tau PET with [18F]flortaucipir and amyloid-β PET with [18F]florbetapir. Exclusion criteria were a history of other neurological disorders, stroke, or head trauma. There were 489 eligible participants, selected based on availability of amyloid-PET, tau-PET, magnetic resonance imaging, and genotyping for APOEε4. Forty-five young adults (<30 years) from the TRIAD cohort were not selected for this study.
Main Outcomes and Measures
A main association between APOEε4 and tau-PET standardized uptake value ratio, correcting for age, sex, clinical status, and neocortical amyloid-PET standardized uptake value ratio.
Results
The mean (SD) age of the 489 participants was 70.5 (7.1) years; 171 were APOEε4 carriers (34.9%), and 230 of 489 were men. In both cohorts, APOEε4 was associated in increased tau-PET uptake in the entorhinal cortex and hippocampus independently of amyloid-β, sex, age, and clinical status after multiple comparisons correction (TRIAD: β = 0.33; 95% CI, 0.19-0.49; ADNI: β = 0.13; 95% CI, 0.08-0.19; P < .001).
Conclusions and Relevance
Our results indicate that the elevated risk of developing dementia conferred by APOEε4 genotype involves mechanisms associated with both amyloid-β and tau aggregation. These results contribute to an evolving framework in which APOEε4 has deleterious consequences in Alzheimer disease beyond its link with amyloid-β and suggest APOEε4 as a potential target for future disease-modifying therapeutic trials targeting tau pathology.
This study investigates whether apolipoprotein E ε4 is associated with medial temporal tau pathology independently of amyloid-β, sex, clinical status, and age.
Introduction
Of genetic risk factors for sporadic Alzheimer disease,1 the apolipoprotein E ε4 (APOEε4) allele is the most well established. The presence of 1 ε4 allele is linked with earlier development of Alzheimer disease,2 and homozygosity for APOEε4 is associated with onset of Alzheimer disease 10 years earlier compared with non-ε4 carriers.3 The APOEε4 allele is associated with increased production of amyloid-β4 as well as with diminished clearance of cerebral amyloid-β compared with ε3 and ε2 alleles.5,6 Consequently, individuals with the APOEε4 genotype demonstrate increased cerebral amyloid-β deposition as measured by amyloid positron emission tomography (PET),7 with amyloid-β positivity beginning earlier in life in APOEε4 carriers than noncarriers.8
However, the APOEε4 allele has been implicated in numerous other processes independent of amyloid-β in preclinical models of Alzheimer disease,9,10 including neuroinflammation and neurodegeneration. In humans, the APOEε4 allele is linked with medial temporal hypometabolism in cognitively normal elderly individuals11 and individuals with Alzheimer disease12 independently of amyloid-β burden, although the mechanisms underlying the process are not known. Because of the spatiotemporal association between tau aggregation and neurodegeneration,13,14,15 aggregation of tau pathology presents a potential pathway for the specific patterns of neurodegeneration observed in APOEε4 carriers.
The goal of this study is therefore to determine whether APOEε4 is associated with cerebral tau pathology, independently of age, sex, clinical status, and amyloid-β deposition. Building on previous reports of specific patterns of neurodegeneration in APOEε4 carriers,11,12,16 we hypothesize that APOEε4 is associated with tau pathology in medial temporal structures.
Methods
Participants
Translational Biomarkers in Aging and Dementia
The Translational Biomarkers in Aging and Dementia (TRIAD) cohort aims at describing biomarker trajectories and interactions as drivers of dementia. The TRIAD study was launched in 2017 as part of the McGill Centre for Studies in Aging. We assessed cognitively normal participants (n = 124), participants with mild cognitive impairment (MCI) (n = 50), and participants with Alzheimer disease dementia (n = 50) who underwent amyloid-β PET with fluorine 18–labeled [18F] AZD4694, tau PET with [18F]MK6240, structural magnetic resonance imaging, and genotyping for APOEε4. All participants had detailed clinical assessments including Mini-Mental State Examination, Clinical Dementia Rating (CDR), and cerebrovascular disease risk with the Hachinski Ischemic scale.17 Cognitively normal control individuals had a CDR of 0, participants with MCI had a CDR of 0.5, and participants with Alzheimer disease had a CDR between 1 and 2, in addition to meeting standard diagnostic criteria.18 Similar to other longitudinal cohort studies of aging and Alzheimer disease,19 the TRIAD cohort is enriched for APOEε4 carriers. Inclusion criteria for all participants are the ability to speak English or French, good general health (no diseases expected to interfere with study participation over time), absence of claustrophobia, and adequate visual and auditory capacities to follow neuropsychologic evaluation. This study’s protocol was approved by McGill University’s institutional review board, and informed written consent was obtained from each participants. There was no attempt to match cases between cohorts.
Alzheimer’s Disease Neuroimaging Initiative
In this study, we assessed cognitively normal individuals (n = 157), individuals with amnestic mild cognitive impairment (n = 83), and individuals with Alzheimer disease (n = 25) from the Alzheimer’s Disease Neuroimaging Initiative (ADNI) cohort who underwent amyloid-β PET with [18F]florbetapir, tau PET with [18F]flortaucipir, structural MRI, and genotyping for APOEε4. Cognitively normal control individuals had a CDR of 0, participants with MCI had a CDR of 0.5, and participants with Alzheimer disease had a CDR of 1 or greater in addition to meeting standard diagnostic criteria.18 The Alzheimer’s Disease Neuroimaging Initiative (ADNI) study was approved by the institutional review boards of all of the participating institutions. Informed written consent was obtained from all participants at each site. Full information regarding the ADNI inclusion and exclusion criteria can be accessed at http://adni.loni.usc.edu/.
Genetic Analyses
TRIAD
Determination of APOE genotypes for patients recruited at McGill was performed using the polymerase chain reaction amplification technique, followed by restriction enzyme digestion, standard gel resolution and visualization processes. Full details of this procedure can be found elsewhere.20
ADNI
Determination of APOE genotypes for ADNI patients took place at the University of Pennsylvania Alzheimer Disease Biomarker Laboratory. Complete details of genetic methods used in ADNI can be accessed at http://adni.loni.usc.edu/data-samples/clinical-data/.
Positron Emission Tomography Image Acquisition and Processing
TRIAD
All participants had a T1-weighted MRI that was used for coregistration. Full details of MRI acquisition and processing is described in the eMethods 1 in the Supplement. The PET scans were acquired with a Siemens High Resolution Research Tomograph. The [18F]MK6240 images were acquired 90 to 110 minutes postinjection, and scans were reconstructed with the ordered subset expectation maximization algorithm on a 4-dimensional volume with 4 frames (4 × 300 seconds).21 The [18F]AZD4694 images were acquired 40 to 70 minutes following injection, and scans were reconstructed with the ordered subset expectation maximization algorithm on a 4-dimensional volume with 3 frames (3 × 600 seconds).22 Immediately following each PET acquisition, a 6-minute transmission scan was conducted with a rotating cesium 137 point source for attenuation correction. Additionally, the images underwent correction for dead time, decay, and random and scattered coincidences. T1-weighted images were nonuniformity and field-distortion corrected and processed using an in-house pipeline. Then, PET images were automatically registered to the T1-weighted image space, and the T1-weighted images were linearly and nonlinearly registered to the ADNI template space. Subsequently, a PET nonlinear registration was performed using the linear and nonlinear transformations from the T1-weighted image to the ADNI space and the PET to T1-weighted image registration using advanced normalization tools. The PET images were spatially smoothed to achieve a final resolution of 8 mm full width at half maximum. The PET image partial volume correction was carried out using the PETPVC toolbox.23 Briefly, the region-based voxelwise correction technique was used to perform partial volume correction using 10 tissue priors with a gaussian kernel with a full width at half maximum of 2.4 mm. The [18F]MK6240 standardized uptake value ratio (SUVR) maps were generated using the inferior cerebellar gray matter as a reference region, and [18F]AZD4694 SUVR maps were generated using the cerebellar gray matter as a reference region. A global [18F]AZD4694 SUVR value was estimated for each participant by averaging the SUVR from the precuneus, prefrontal, orbitofrontal, parietal, temporal, anterior, and posterior cingulate cortices.24
ADNI
Full information regarding acquisition and preprocessing of PET data in ADNI is provided at http://adni.loni.usc.edu/data-samples/pet/. Preprocessed PET images downloaded from ADNI underwent spatial normalization to the ADNI standardized space using the transformations of PET native to MRI native space and MRI native to the ADNI space. Partial volume correction was carried out using the PETPVC toolbox23 described previously in an effort to diminish off-target binding to the choroid plexus. [18F]flortaucipir (also known as [18F]T807 and/or [18F]AV1451) SUVR maps were generated using the inferior cerebellar gray matter as a reference region,25 and [18F]florbetapir SUVR maps were generated using the cerebellar gray matter as a reference region. A global [18F]florbetapir SUVR value was estimated for each participant by averaging the SUVR from the precuneus, prefrontal, orbitofrontal, parietal, temporal, anterior, and posterior cingulate cortices.24
Statistical Analyses
Two independent samples were investigated: (1) the TRIAD cohort assessed with [18F]MK6240 and [18F]AZD4694 and (2) an ADNI cohort assessed with [18F]flortaucipir and [18F]florbetapir. The primary outcome measure of the study was tau pathology as measured by voxelwise [18F]MK6240 SUVR (TRIAD) and [18F]flortaucipir SUVR (ADNI). In each cohort, we tested whether APOEε4 is associated with tau pathology independently of amyloid-β, sex, or age using voxelwise multivariate linear regression models.
Baseline demographic and clinical data were assessed using t tests and χ2 tests. Neuroimaging analyses were carried out using the VoxelStats toolbox (https://github.com/sulantha2006/VoxelStats), a MATLAB-based analytical framework that allows for the execution of multimodal voxelwise neuroimaging analyses.26 All neuroimaging analyses described in subsequent paragraphs were repeated using partial volume–corrected data. Other statistical analyses were performed using the R Statistical Software Package, version 3.5.3 (the R Foundation). Given the large number of covariates in the statistical models, model diagnostics were carried out using the car package in R to determine the presence of multicollinearity. We computed the variance inflation factor, a measurement of how much variance in regression coefficients are inflated owing to multicollinearity in the statistical models.27
In the TRIAD cohort, the voxel-based model outlined here was built to test whether main effects between APOEε4 carriership are associated with [18F]MK6240 uptake independently of [18F]AZD4694 uptake. To ensure that the results were not driven by an effect of clinical status (ie higher frequency of APOEε4 carriers in the MCI and Alzheimer disease groups), we adjusted the model for clinical diagnosis. The model was also adjusted for age. Because APOEε4 is associated with amyloid-PET uptake, amyloid-β was included as a covariate in every analysis. Sex was included as a covariate owing to sex differences in entorhinal tau aggregation28 and stronger associations between APOEε4 and tau in women.29 Statistical parametric maps were corrected for multiple comparisons using random field theory,30 with a cluster threshold of P < .001. The analysis was repeated using partial volume–corrected data. In every brain voxel, the model was of the form:
| [18F]MK6240 SUVR = βo+ β1([18F]AZD4694 SUVR) + β2(APOEε4) + β3(Clinical Status)+ β4(Age) + β5(Sex)+ ε |
Next, we tested the same hypothesis in the ADNI database, examining whether APOEε4 carriership is associated with [18F]flortaucipir uptake independently of [18F]florbetapir uptake. This model was also adjusted for amyloid-β, sex, age, and clinical status. Statistical parametric maps were corrected for multiple comparisons using random field theory,30 with a cluster threshold of P < .001. The analysis was repeated using partial volume–corrected data. In every brain voxel, the model was of the form:
| [18F]Flortaucipir SUVR = β0 + β1([18F]Florbetapir SUVR) + β2(APOEε4) + β3(Clinical Status)+ β4(Age) + β5(Sex)+ ε |
To better understand the association between APOEε4 and medial temporal tau aggregation, we conducted subgroup analyses, stratifying individuals according to the presence of cognitive impairment (ie, in cognitively unimpaired individuals and cognitively impaired individuals). The cognitively impaired groups consisted of the individuals with MCI and AD pooled together. These models were adjusted for amyloid-β, sex, and age. The analyses were repeated using partial volume–corrected data.
To derive an estimate of the association between APOEε4 and medial temporal tau-PET SUVR across both cohorts, we used the Metafor package in R. We fit a meta-analytic fixed-effects model using β weights and standard errors for the estimates from each population, analyzed using the rma function. The same process was repeated for gene-dose and voxel-based morphometry analyses described subsequently.
The P value level of significance was .001, and all tests were 2-sided. Exploratory gene-dose analyses, APOEε4–voxel-based morphometry analyses, APOEε4 × age interaction analyses, APOEε4 × amyloid-PET interaction analyses, and APOEε4 unadjusted for amyloid-PET analyses are described in eMethods 2 in the Supplement.
Results
Demographic and clinical information for both samples examined in this study is summarized in Table 1. Demographic comparisons between cohorts are reported in eTable 1 in the Supplement. Variance inflation factors (VIFs) for all variables were between 1 and 2, indicating that problematic levels of multicollinearity are not present in our analyses.27
Table 1. Demographic and Key Characteristics of the Samples.
| Cohort | CU | MCI | P Valuea | AD | P Valuea |
|---|---|---|---|---|---|
| TRIAD cohort | |||||
| No. | 124 | 50 | NA | 50 | NA |
| Age, mean (SD), y | 70.41 (6.5) | 70.88 (7.7) | .007 | 66.69 (9.93) | .005 |
| Male, No. (%) | 53 (43) | 25 (50) | .69 | 20 (40) | .61 |
| Education, mean (SD), y | 15.52 (3.86) | 14.26 (3.79) | .06 | 14.2 (3.75) | .04 |
| APOE ε4 carriers, No. (%), % | 38 (31) | 18 (36) | .49 | 26 (52) | .008 |
| MMSE, mean (SD) | 29.05 (1.25) | 27.13 (2.39) | <.001 | 19.1 (7.31) | <.001 |
| CDR SoB, mean (SD) | 0.18 (0.45) | 1.47 (1.23) | <.001 | 6.48 (4.08) | <.001 |
| [18F]AZD4694 SUVR, mean (SD) | 1.48 (0.42) | 1.86 (0.54) | <.001 | 2.42 (0.63) | <.001 |
| ADNI cohort | |||||
| No. | 157 | 83 | NA | 25 | NA |
| Age, mean (SD), y | 70.98 (5.91) | 70.57 (7.09) | .63 | 74.11 (7.65) | .02 |
| Male, No. (%) | 71 (45) | 49 (59) | .04 | 12 (48) | .66 |
| Education, mean (SD), y | 16.65 (2.5) | 15.84 (2.85) | .02 | 16.26 (2.51) | .47 |
| APOE ε4 carriers, No. (%) | 49 (31) | 27 (32.5) | .83 | 13 (52) | .04 |
| MMSE, mean (SD) | 28.97 (1.33) | 28.05 (2.15) | <.001 | 19.67 (5.28) | <.001 |
| CDR SoB, mean (SD) | 0.009 (0.51) | 1.46 (0.93) | <.001 | 7.18 (2.67) | <.001 |
| [18F]Florbetapir SUVR, mean (SD) | 1.2 (0.22) | 1.26 (0.29) | .07 | 1.47 (0.22) | <.001 |
Abbreviations: AD, Alzheimer disease dementia; ADNI, Alzheimer’s Disease Neuroimaging Initiative; CDR SoB, Clinical Dementia Rating sum of boxes; CU, cognitively unimpaired; 18F, fluorine 18 labeled; MCI, mild cognitive impairment; MMSE, Mini-Mental State Examination; SUVR, standardized uptake value ratio; TRIAD, Translational Biomarkers in Aging and Dementia.
P values reported are for comparisons with cognitively unimpaired participants. P values indicate values assessed with independent-samples t tests for each variable except sex and APOE ε4 status, where contingency χ2 tests were performed.
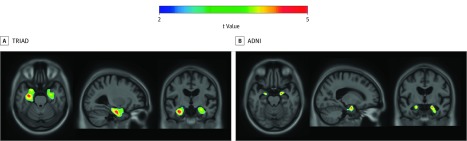
We tested the hypothesis that APOEε4 is associated with greater [18F]MK6240 uptake independently of global [18F]AZD4694 uptake. Voxelwise analyses revealed that APOEε4 carriership was associated with increased [18F]MK6240 SUVR in the bilateral entorhinal cortex and hippocampus (random field theory corrected at P < .001; significant clusters: P <.001; t = 4.42; β = 0.33; 95% CI, 0.19-0.49) (Figure 1A). These results are independent of amyloid-β, clinical diagnosis, age, and sex. Results remained similar when using partial volume–corrected data (eFigure 1A in the Supplement; full model statistics of PVC data presented in Table 2). No statistically significant associations were observed beyond the medial temporal lobes.
Figure 1. Association of Medial Temporal Tau Positron Emission Tomography With Apolipoprotein E ε4 (APOEε4) Independent of Amyloid-β.
T-statistical parametric maps were corrected for multiple comparisons using a random field theory cluster threshold of P < .001, overlaid on the Alzheimer's Disease Neuroimaging Initiative reference template. Age, sex, clinical diagnosis, and amyloid-β standardized uptake value ratio were used as covariates the model. A, Voxelwise analyses revealed that APOEε4 carriership was associated with increased fluorine 18–labeled [18F] MK6240 in the bilateral entorhinal cortex and hippocampus. B, Voxelwise analyses revealed that APOEε4 carriership was associated with increased [18F]flortaucipir in the bilateral entorhinal cortex.
Table 2. Regression Coefficients of APOE4 on Medial Temporal Tau-PET.
| Variable | Medial Temporal Tau-PET | Medial Temporal Tau-PET (PVC) | ||||
|---|---|---|---|---|---|---|
| β (95% CI) | t Value | P Value | β (95% CI) | t Value | P Value | |
| TRIAD cohorta | ||||||
| APOE4 | 0.33 (0.19 to 0.49) | 4.42 | <.001 | 0.26 (0.14 to 0.37) | 4.25 | <.001 |
| Neocortical [18F]AZD4694 SUVR | 0.93 (0.81 to 1.05) | 14.49 | <.001 | 0.76 (0.65 to 0.87) | 9.1 | <.001 |
| Male | −0.17 (−0.31 to −0.04) | −2.52 | .01 | −0.18 (−0.29 to 0.06) | −3.01 | .003 |
| Age | −0.008 (−0.02 to −0.0007) | −2.18 | .02 | −0.008 (−0.01 to 0.0004) | −2.12 | .03 |
| Clinical status | ||||||
| MCI | 0.2 (0.02 to 0.38) | 2.28 | .02 | 0.18 (0.03 to 0.34) | 2.41 | .01 |
| AD | 0.64 (0.44 to 0.85) | 6.2 | <.001 | 0.53 (0.36 to 0.71) | 6.07 | <.001 |
| ADNI cohortb | ||||||
| APOE4 | 0.13 (0.08 to 0.19) | 4.53 | <.001 | 0.12 (0.06 to 0.19) | 3.95 | <.001 |
| Neocortical [18F]florbetapir | 0.23 (0.11 to 0.34) | 4.1 | <.001 | 0.26 (0.13 to 0.38) | 4.05 | <.001 |
| Male | −0.02 (−0.004 to 0.004) | 0.57 | .57 | −0.03 (0.02 to −0.09) | −1.11 | .27 |
| Age | −0.006 (−0.03 to 0.004) | 0.28 | .77 | −0.001 (−0.006 to 0.003) | −0.73 | .46 |
| MCI | 0.13 (0.07 to 0.19) | 4.51 | <.001 | 0.15 (0.09 to 0.22) | 4.65 | <.001 |
| AD | 0.49 (0.4 to 0.59) | 10.27 | <.001 | 0.45 (0.34 to 0.56) | 8.29 | <.001 |
Abbreviations: AD, Alzheimer disease dementia; ADNI, Alzheimer’s Disease Neuroimaging Initiative; APOE4, apolipoprotein E ε4; 18F, fluorine 18 labeled; MCI, mild cognitive impairment; PET, positron emission tomography; PVC, partial volume corrected data; SUVR, standardized uptake value ratio; TRIAD, Translational Biomarkers in Aging and Dementia.
Adjusted R2: 0.61, F = 58.61 (non-PVC); adjusted R2: 0.6, F = 56.39 (PVC).
Adjusted R2: 0.42, F = 0.33 (non-PVC), adjusted R2: 0.35, F = 25.19 (PVC).
We also tested the hypothesis that APOEε4 is associated with greater [18F]flortaucipir uptake independently of global [18F]florbetapir uptake. Voxelwise analyses revealed that APOEε4 carriership was associated with increased [18F]flortaucipir SUVR in the bilateral entorhinal cortex (random field theory corrected at P < .001; significant clusters: t = 4.527; β = 0.13; 95% CI, 0.08-0.19) (Figure 1B). Results remained similar when using partial volume–corrected data (eFigure 1B in the Supplement; full model statistics of PVC data presented in Table 2). These results are independent of amyloid-β, clinical diagnosis, age, and sex. No statistically significant associations were observed beyond the medial temporal lobes.
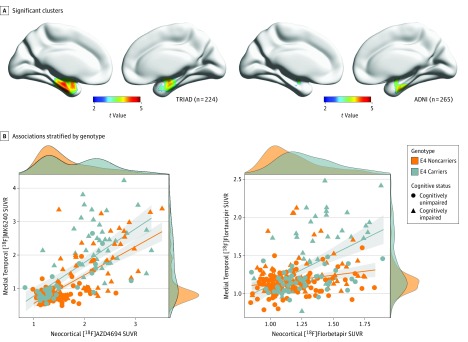
Scatterplots of the association between neocortical amyloid-PET SUVR and medial temporal tau-PET SUVR stratified by APOEε4 status are displayed in Figure 2. Density plots are also provided to visualize distribution of the data.
Figure 2. Associations Between Medial Temporal Tau Positron Emission Tomography (PET) and Neocortical Amyloid PET Stratified by Apolipoprotein E ε4 (APOEε4) Genotype.
A, Clusters that remained significant after multiple comparisons correction with random field theory at P < .001 were used to extract tau-PET standardized uptake value ratio (SUVR) values in the TRIAD cohort (left) and ADNI cohort (right). B, Scatterplots displaying associations between medial temporal tau PET and neocortical amyloid PET stratified by APOEε4 genotype in TRIAD (left) and ADNI (right). Density plots are provided along the x and y axes to visualize the distribution of the data for neocortical amyloid PET and medial temporal tau PET SUVR, respectively. In the TRIAD cohort, APOEε4 carriership was associated with medial temporal fluorine 18–labeled [18F] MK6240 SUVR (t = 4.42; β = 0.33; 95% CI, 0.19-0.49). In the ADNI cohort, APOEε4 carriership was significantly associated with medial temporal [18F]flortaucipir SUVR (t = 4.527; β = 0.13; 95% CI, 0.08-0.19).
Full model statistics are presented in Table 2. While t values for the APOEε4 medial temporal tau–PET SUVR associations were similar across studies, the regression β estimates for the TRIAD cohort were higher (TRIAD: P < .001; t = 4.464; β = 0.33; 95% CI, 0.19-0.49; ADNI: P < .001, t = 4.52; β= 0.13; 95% CI, 0.08-0.19). While the association between APOEε4 and medial temporal tau–PET SUVR was significant in both cohorts, β estimates for APOEε4–medial temporal tau-PET SUVR associations were smaller than those of amyloid-PET in TRIAD (P < .001, t = 14.49; β = 0.93; 95% CI, 0.81-1.05) or a clinical diagnosis of Alzheimer disease in ADNI (P < .001, t = 10.27; β = 0.49; 95% CI, 0.4-0.59).
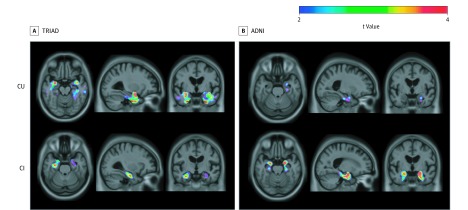
To better understand the association between APOEε4 and medial temporal tau aggregation, we conducted subgroup analyses by stratifying individuals according to cognitive impairment. When stratifying analyses by cognitive status in the TRIAD cohort, we observed that APOEε4 was associated with medial temporal [18F]MK6240 SUVR independently of [18F]AZD4694 SUVR in cognitively normal elderly individuals (n = 124) and in cognitively impaired individuals (n = 100) (Figure 3). When conducting subgroup analyses in the ADNI cohort, we found that APOEε4 was associated with [18F]flortaucipir SUVR in the left entorhinal cortex in cognitively unimpaired elderly individuals (n = 157). The APOEε4 carriership was also associated with [18F]flortaucipir SUVR independently of [18F]florbetapir SUVR in the bilateral entorhinal cortices in cognitively impaired individuals (n = 108). Full model statistics are presented in eTable 2 in the Supplement. Full model statistics for all exploratory analyses are reported in eTables 3-7 in the Supplement. Gene-dose associations are reported in eFigure 2 in the Supplement and associations unadjusted for amyloid-PET are reported in eFigure3 in the Supplement.
Figure 3. Association Between Medial Temporal Tau Positron Emission Tomography (PET) and Apolipoprotein E ε4 (APOEε4) Stratified by Cognitive Status.
A, In cognitively unimpaired participants (n = 124), APOEε4 carriership was associated with fluorine 18–labeled [18F] MK6240 standardized uptake value ratio (SUVR) in the bilateral entorhinal cortex and hippocampus. In cognitively impaired participants (n = 100), APOEε4 carriership was associated with increased [18F]MK6240 in the bilateral hippocampus. B, In cognitively unimpaired patients (n = 157), APOEε4 carriership was associated with [18F]flortaucipir SUVR in the left entorhinal cortex. In cognitively impaired patients (n = 109), APOEε4 carriership was associated with increased [18F]flortaucipir in the bilateral entorhinal cortices and hippocampus. Age, sex, and amyloid-β SUVR were used as covariates in each model. Results remained similar when using partial volume–corrected PET data. CU indicates cognitively unimpaired; CI, cognitively impaired.
Meta-analytic Estimates
When fitting a fixed-effect rma model to the coefficients and standard errors from the models in both cohorts, we found that the main association of APOEε4 on medial temporal tau–PET SUVR was significant (P < .001, meta-analytic β = 0.22; 95% CI, 0.15-0.29).
Discussion
This study provides evidence from 2 independent cohorts that APOEε4 is associated with increased tau pathology in the entorhinal cortex and hippocampus independently of age, clinical status, sex, and amyloid-β. Our study is in agreement with a growing body of research demonstrating greater vulnerability of the medial temporal lobes to hypometabolism11,12 and atrophy31,32,33 in APOEε4 carriers compared with noncarriers, independently of amyloid-β. Because of the topographical concordance between tau pathology and neurodegeneration,13,14,34 our results suggest greater tau pathology may be responsible for the medial temporal neurodegeneration observed in APOEε4 carriers.
Our findings of greater medial temporal tauopathy are consistent with specific neuropsychologic profiles of APOEε4 carriers vs noncarriers. Patients with Alzheimer disease dementia who are APOEε4 carriers perform worse on memory tasks than noncarriers at the same disease stage.35,36 Correspondingly, memory tends to be relatively preserved in ε4-negative patients, while deficits in executive function and processing speed are more severe.37,38 Patients with Alzheimer disease dementia who do not carry an ε4 allele are also more likely to present with nonamnestic phenotypes.39 Taken together, these studies support a framework in which medial temporal structures are specifically vulnerable to the deleterious effects of APOEε4.
An outstanding question is why APOEε4’s association with tau pathology is restricted to the medial temporal lobe. While pyramidal neurons of the entorhinal cortex, subiculum, and CA1 region of the hippocampus are vulnerable to early tau accumulation in Alzheimer disease,40,41,42 limited data exist as to how APOEε4 may preferentially (or selectively) affect tau aggregation in these structures.10 Data from the Allen Brain Atlas suggest that messenger RNA expression of APOE is highest in the medial temporal lobes.43 Apolipoprotein E immunoreactivity is observed in neurons bearing neurofibrillary tangles.44 Furthermore, greater expression of neuronal APOE is associated with increased tau phosphorylation in transgenic animal models45,46,47 and human stem cell models.48 Truncated APOEε4 fragments are also associated with greater tau hyperphosphorylation and neuronal cytoskeletal disruption.49,50
Our study builds on studies of tau-PET distribution across the Alzheimer disease spectrum15 by identifying a unique regional contribution of APOEε4 to tau pathology. Furthermore, neuropathologic14 and tau-PET51 studies that have identified medial temporal tauopathy in the absence of amyloid-β suggest that medial temporal tauopathy may be a consequence of aging. Correspondingly, later age at onset of Alzheimer disease dementia is linked to limbic-predominant or memory-predominant clinical presentations.52 Even in cognitively normal individuals, increased tau pathology in the medial temporal lobe is associated with declines in subjective53 and objective memory function as well as medial temporal gray matter volume.54 Our study extends these findings by identifying APOEε4 as a contributor to medial temporal tauopathy, independent of age and amyloid-β.
While the results of our study implicate APOEε4 in the pathogenesis of both pathological hallmarks of Alzheimer disease,55 APOEε4 is not sufficient for a diagnosis of Alzheimer disease nor to cause dementia. Instead, our study supports a framework in which isocortical/medial temporal (Braak stage 1-2) tau pathology may be a consequence of specific vulnerability factors (such as aging51,56 or genotype9), while amyloid-β facilitates the spread of tau pathology from the medial temporal lobe to neocortical regions,57,58 associated with greater cognitive decline. In fact, significant tau pathology in neocortical regions is seldom observed independently of amyloid-β pathology,59 although exceptions do exist.60 Because accepted Alzheimer disease models suggest that amyloid-β accumulation occurs years before tau accumulation measured with cerebrospinal fluid,61,62 longitudinal imaging studies are needed to clarify APOEε4’s association with medial temporal tau pathology across disease stages.
Strengths and Limitations
Some methodologic limitations should be considered when interpreting this study. The first is that this study is not designed to discover a biological mechanism underlying the association between APOEε4 and tau independently of amyloid-β. It is important to mention that both TRIAD and ADNI cohorts are convenience samples of individuals motivated to participate in a study about Alzheimer disease and thus involve recruitment and sampling biases. Future work is needed to determine whether the effects of APOEε4 on tau result in increased phosphorylation, conformational changes, or increased cortical spreading. Future studies should also investigate possible associations between APOEε4 and amyloid-β in relation to tau pathology. Methodologic strengths of this study include large sample sizes as well as a replication in an independent cohort. In particular, replication of results obtained with first-generation and second-generation tau-PET ligands is an important methodological advance.
Conclusions
In summary, we found that APOEε4 is associated with increased tau pathology in medial temporal structures independent of amyloid-β, sex, age, and clinical status. These results, in combination with preclinical data,9,48 suggest that APOEε4 may be an important therapeutic target for future disease-modifying clinical trials.
eMethods 1. Structural MRI acquisition and processing
eReferences
eMethods 2. Exploratory statistical analyses
eTable 1. Between-sample demographic comparisons
eFigure 1. Association of partial volume-corrected medial temporal tau-PET with APOEε4 independent of amyloid-b
eTable 2. Regression coefficients of APOE4 on MTL Tau-PET stratified by cognitive status
eTable 3. Regression coefficients of models investigating gene-dose framework
eFigure 2. Voxelwise gene-dose association of APOE4 on MTL Tau-PET
eTable 4. Regression coefficients of models investigating APOE-VBM relationships
eTable 5. Regression coefficients of models investigating age*apoe4 interactions
eTable 6. Regression coefficients of models investigating APOE4-Tau associations without adjusting for amyloid-PET
eFigure 3. Voxel-vise association of APOE4 on Tau-PET unadjusted for amyloid-PET
eTable 7. Regression coefficients of models including synergistic interaction term
References
- 1.Farrer LA, Cupples LA, Haines JL, et al. ; APOE and Alzheimer Disease Meta Analysis Consortium . Effects of age, sex, and ethnicity on the association between apolipoprotein E genotype and Alzheimer disease: a meta-analysis. JAMA. 1997;278(16):1349-1356. doi: 10.1001/jama.1997.03550160069041 [DOI] [PubMed] [Google Scholar]
- 2.Meyer MR, Tschanz JT, Norton MC, et al. . APOE genotype predicts when, not whether, one is predisposed to develop Alzheimer disease. Nat Genet. 1998;19(4):321-322. doi: 10.1038/1206 [DOI] [PubMed] [Google Scholar]
- 3.Blacker D, Haines JL, Rodes L, et al. . ApoE-4 and age at onset of Alzheimer’s disease: the NIMH genetics initiative. Neurology. 1997;48(1):139-147. doi: 10.1212/WNL.48.1.139 [DOI] [PubMed] [Google Scholar]
- 4.Zerbinatti CV, Wozniak DF, Cirrito J, et al. . Increased soluble amyloid-beta peptide and memory deficits in amyloid model mice overexpressing the low-density lipoprotein receptor-related protein. Proc Natl Acad Sci U S A. 2004;101(4):1075-1080. doi: 10.1073/pnas.0305803101 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Liu CC, Zhao N, Yamaguchi Y, et al. . Neuronal heparan sulfates promote amyloid pathology by modulating brain amyloid-β clearance and aggregation in Alzheimer’s disease. Sci Transl Med. 2016;8(332):332ra44. doi: 10.1126/scitranslmed.aad3650 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Castellano JM, Kim J, Stewart FR, et al. . Human apoE isoforms differentially regulate brain amyloid-β peptide clearance. Sci Transl Med. 2011;3(89):89ra57. doi: 10.1126/scitranslmed.3002156 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Gonneaud J, Arenaza-Urquijo EM, Fouquet M, et al. . Relative effect of APOE ε4 on neuroimaging biomarker changes across the lifespan. Neurology. 2016;87(16):1696-1703. doi: 10.1212/WNL.0000000000003234 [DOI] [PubMed] [Google Scholar]
- 8.Fleisher AS, Chen K, Liu X, et al. . Apolipoprotein E ε4 and age effects on florbetapir positron emission tomography in healthy aging and Alzheimer disease. Neurobiol Aging. 2013;34(1):1-12. doi: 10.1016/j.neurobiolaging.2012.04.017 [DOI] [PubMed] [Google Scholar]
- 9.Shi Y, Yamada K, Liddelow SA, et al. ; Alzheimer’s Disease Neuroimaging Initiative . ApoE4 markedly exacerbates tau-mediated neurodegeneration in a mouse model of tauopathy. Nature. 2017;549(7673):523-527. doi: 10.1038/nature24016 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Zhao N, Liu CC, Qiao W, Bu G. Apolipoprotein E, receptors, and modulation of Alzheimer’s disease. Biol Psychiatry. 2018;83(4):347-357. doi: 10.1016/j.biopsych.2017.03.003 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Jagust WJ, Landau SM; Alzheimer’s Disease Neuroimaging Initiative . Apolipoprotein E, not fibrillar β-amyloid, reduces cerebral glucose metabolism in normal aging. J Neurosci. 2012;32(50):18227-18233. doi: 10.1523/JNEUROSCI.3266-12.2012 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Lehmann M, Ghosh PM, Madison C, et al. . Greater medial temporal hypometabolism and lower cortical amyloid burden in ApoE4-positive AD patients. J Neurol Neurosurg Psychiatry. 2014;85(3):266-273. doi: 10.1371/journal.pone.0178059 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Xia C, Makaretz SJ, Caso C, et al. . Association of in vivo [18 F]AV-1451 tau PET imaging results with cortical atrophy and symptoms in typical and atypical Alzheimer disease. JAMA Neurol. 2017;74(4):427-436. doi: 10.1001/jamaneurol.2016.5755 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Braak H, Braak E. Neuropathological stageing of Alzheimer-related changes. Acta Neuropathol. 1991;82(4):239-259. doi: 10.1007/BF00308809 [DOI] [PubMed] [Google Scholar]
- 15.Whitwell JL, Graff-Radford J, Tosakulwong N, et al. . [18 F]AV-1451 clustering of entorhinal and cortical uptake in Alzheimer’s disease. Ann Neurol. 2018;83(2):248-257. doi: 10.1002/ana.25142 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Filippini N, Rao A, Wetten S, et al. . Anatomically-distinct genetic associations of APOE epsilon4 allele load with regional cortical atrophy in Alzheimer’s disease. Neuroimage. 2009;44(3):724-728. doi: 10.1016/j.neuroimage.2008.10.003 [DOI] [PubMed] [Google Scholar]
- 17.Hachinski VC, Iliff LD, Zilhka E, et al. . Cerebral blood flow in dementia. Arch Neurol. 1975;32(9):632-637. doi: 10.1001/archneur.1975.00490510088009 [DOI] [PubMed] [Google Scholar]
- 18.McKhann G, Drachman D, Folstein M, Katzman R, Price D, Stadlan EM. Clinical diagnosis of Alzheimer’s disease: report of the NINCDS-ADRDA Work Group under the auspices of Department of Health and Human Services Task Force on Alzheimer’s Disease. Neurology. 1984;34(7):939-944. doi: 10.1212/WNL.34.7.939 [DOI] [PubMed] [Google Scholar]
- 19.Lim YY, Kalinowski P, Pietrzak RH, et al. . Association of β-Amyloid and apolipoprotein e e4 with memory decline in preclinical Alzheimer disease. JAMA Neurol. 2018;75(4):488-494. doi: 10.1001/jamaneurol.2017.4325 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Saykin AJ, Shen L, Yao X, et al. ; Alzheimer’s Disease Neuroimaging Initiative . Genetic studies of quantitative MCI and AD phenotypes in ADNI: progress, opportunities, and plans. Alzheimers Dement. 2015;11(7):792-814. doi: 10.1016/j.jalz.2015.05.009 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Pascoal TA, Shin M, Kang MS, et al. . In vivo quantification of neurofibrillary tangles with [18F]MK-6240. Alzheimers Res Ther. 2018;10(1):74. doi: 10.1186/s13195-018-0402-y [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Cselényi Z, Jönhagen ME, Forsberg A, et al. . Clinical validation of 18F-AZD4694, an amyloid-β-specific PET radioligand. J Nucl Med. 2012;53(3):415-424. doi: 10.2967/jnumed.111.094029 [DOI] [PubMed] [Google Scholar]
- 23.Thomas BA, Cuplov V, Bousse A, et al. . PETPVC: a toolbox for performing partial volume correction techniques in positron emission tomography. Phys Med Biol. 2016;61(22):7975-7993. doi: 10.1088/0031-9155/61/22/7975 [DOI] [PubMed] [Google Scholar]
- 24.Jack CR Jr, Wiste HJ, Weigand SD, et al. . Defining imaging biomarker cut points for brain aging and Alzheimer’s disease. Alzheimers Dement. 2017;13(3):205-216. doi: 10.1016/j.jalz.2016.08.005 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Maass A, Landau S, Baker SL, et al. ; Alzheimer’s Disease Neuroimaging Initiative . Comparison of multiple tau-PET measures as biomarkers in aging and Alzheimer’s disease. Neuroimage. 2017;157(June):448-463. doi: 10.1016/j.neuroimage.2017.05.058 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Mathotaarachchi S, Wang S, Shin M, et al. . VoxelStats: A MATLAB package for multi-modal voxel-wise brain image analysis. Front Neuroinform. 2016;10(6):20. doi: 10.3389/fninf.2016.00020 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.O’Brien RM. A caution regarding rules of thumb for variance inflation factors. Qual Quant. 2007;41(5):673-690. doi: 10.1007/s11135-006-9018-6 [DOI] [Google Scholar]
- 28.Buckley RF, Mormino EC, Rabin JS, et al. . Sex differences in the association of global amyloid and regional tau deposition measured by positron emission tomography in clinically normal older adults. JAMA Neurol. 2019;76(5):542-551. doi: 10.1001/jamaneurol.2018.4693 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Hohman TJ, Dumitrescu L, Barnes LL, et al. ; Alzheimer’s Disease Genetics Consortium and the Alzheimer’s Disease Neuroimaging Initiative . Sex-specific association of apolipoprotein e with cerebrospinal fluid levels of tau. JAMA Neurol. 2018;75(8):989-998. doi: 10.1001/jamaneurol.2018.0821 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Worsley KJ, Taylor JE, Tomaiuolo F, Lerch J. Unified univariate and multivariate random field theory. Neuroimage. 2004;23(suppl 1):S189-S195. doi: 10.1016/j.neuroimage.2004.07.026 [DOI] [PubMed] [Google Scholar]
- 31.Geroldi C, Pihlajamäki M, Laakso MP, et al. . APOE-epsilon4 is associated with less frontal and more medial temporal lobe atrophy in AD. Neurology. 1999;53(8):1825-1832. doi: 10.1212/WNL.53.8.1825 [DOI] [PubMed] [Google Scholar]
- 32.Donix M, Burggren AC, Suthana NA, et al. . Longitudinal changes in medial temporal cortical thickness in normal subjects with the APOE-4 polymorphism. Neuroimage. 2010;53(1):37-43. doi: 10.1016/j.neuroimage.2010.06.009 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Filippini N, MacIntosh BJ, Hough MG, et al. . Distinct patterns of brain activity in young carriers of the APOE-epsilon4 allele. Proc Natl Acad Sci U S A. 2009;106(17):7209-7214. doi: 10.1073/pnas.0811879106 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Ossenkoppele R, Schonhaut DR, Schöll M, et al. . Tau PET patterns mirror clinical and neuroanatomical variability in Alzheimer’s disease. Brain. 2016;139(pt 5):1551-1567. doi: 10.1093/brain/aww027 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Marra C, Bizzarro A, Daniele A, et al. . Apolipoprotein E ε4 allele differently affects the patterns of neuropsychological presentation in early- and late-onset Alzheimer’s disease patients. Dement Geriatr Cogn Disord. 2004;18(2):125-131. doi: 10.1159/000079191 [DOI] [PubMed] [Google Scholar]
- 36.Lehtovirta M, Soininen H, Helisalmi S, et al. . Clinical and neuropsychological characteristics in familial and sporadic Alzheimer’s disease: relation to apolipoprotein E polymorphism. Neurology. 1996;46(2):413-419. doi: 10.1212/WNL.46.2.413 [DOI] [PubMed] [Google Scholar]
- 37.van der Vlies AE, Pijnenburg YAL, Koene T, et al. . Cognitive impairment in Alzheimer’s disease is modified by APOE genotype. Dement Geriatr Cogn Disord. 2007;24(2):98-103. doi: 10.1159/000104467 [DOI] [PubMed] [Google Scholar]
- 38.Wolk DA, Dickerson BC; Alzheimer’s Disease Neuroimaging Initiative . Apolipoprotein E (APOE) genotype has dissociable effects on memory and attentional-executive network function in Alzheimer’s disease. Proc Natl Acad Sci U S A. 2010;107(22):10256-10261. doi: 10.1073/pnas.1001412107 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Schott JM, Ridha BH, Crutch SJ, et al. . Apolipoprotein e genotype modifies the phenotype of Alzheimer disease. [2]. Arch Neurol. 2006;63(1):155-156. doi: 10.1001/archneur.63.1.155 [DOI] [PubMed] [Google Scholar]
- 40.Hyman BT, Van Hoesen GW, Damasio AR, Barnes CL. Alzheimer’s disease: cell-specific pathology isolates the hippocampal formation. Science. 1984;225(4667):1168-1170. doi: 10.1126/science.6474172 [DOI] [PubMed] [Google Scholar]
- 41.Morrison BM, Hof PR, Morrison JH. Determinants of neuronal vulnerability in neurodegenerative diseases. Ann Neurol. 1998;44(3)(suppl 1):S32-S44. doi: 10.1002/ana.410440706 [DOI] [PubMed] [Google Scholar]
- 42.Morrison JH, Hof PR Selective vulnerability of corticocortical and hippocampal circuits in aging and Alzheimer’s disease. In: Progress in Brain Research.; 2002. doi: 10.1016/S0079-6123(02)36039-4 [DOI] [PubMed] [Google Scholar]
- 43.Gryglewski G, Seiger R, James GM, et al. . Spatial analysis and high resolution mapping of the human whole-brain transcriptome for integrative analysis in neuroimaging. Neuroimage. 2018;176(March):259-267. doi: 10.1016/j.neuroimage.2018.04.068 [DOI] [PubMed] [Google Scholar]
- 44.Huang Y, Liu XQ, Wyss-Coray T, Brecht WJ, Sanan DA, Mahley RW. Apolipoprotein E fragments present in Alzheimer’s disease brains induce neurofibrillary tangle-like intracellular inclusions in neurons. Proc Natl Acad Sci U S A. 2001;98(15):8838-8843. doi: 10.1073/pnas.151254698 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Brecht WJ, Harris FM, Chang S, et al. . Neuron-specific apolipoprotein e4 proteolysis is associated with increased tau phosphorylation in brains of transgenic mice. J Neurosci. 2004;24(10):2527-2534. doi: 10.1523/JNEUROSCI.4315-03.2004 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Tesseur I, Van Dorpe J, Spittaels K, Van den Haute C, Moechars D, Van Leuven F. Expression of human apolipoprotein E4 in neurons causes hyperphosphorylation of protein tau in the brains of transgenic mice. Am J Pathol. 2000;156(3):951-964. doi: 10.1016/S0002-9440(10)64963-2 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Tesseur I, Van Dorpe J, Bruynseels K, et al. . Prominent axonopathy and disruption of axonal transport in transgenic mice expressing human apolipoprotein E4 in neurons of brain and spinal cord. Am J Pathol. 2000;157(5):1495-1510. doi: 10.1016/S0002-9440(10)64788-8 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Wadhwani AR, Affaneh A, Van Gulden S, Kessler JA. Neuronal apolipoprotein E4 increases cell death and phosphorylated tau release in alzheimer disease. Ann Neurol. 2019;85(5):726-739. doi: 10.1002/ana.25455 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Huang Y. Abeta-independent roles of apolipoprotein E4 in the pathogenesis of Alzheimer’s disease. Trends Mol Med. 2010;16(6):287-294. doi: 10.1016/j.molmed.2010.04.004 [DOI] [PubMed] [Google Scholar]
- 50.Mahley RW, Weisgraber KH, Huang Y. Apolipoprotein E4: a causative factor and therapeutic target in neuropathology, including Alzheimer’s disease. Proc Natl Acad Sci U S A. 2006;103(15):5644-5651. doi: 10.1073/pnas.0600549103 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51.Johnson KA, Schultz A, Betensky RA, et al. . Tau positron emission tomographic imaging in aging and early Alzheimer disease. Ann Neurol. 2016;79(1):110-119. doi: 10.1002/ana.24546 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52.Murray ME, Graff-Radford NR, Ross OA, Petersen RC, Duara R, Dickson DW. Neuropathologically defined subtypes of Alzheimer’s disease with distinct clinical characteristics: a retrospective study. Lancet Neurol. 2011;10(9):785-796. doi: 10.1016/S1474-4422(11)70156-9 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53.Buckley RF, Hanseeuw B, Schultz AP, et al. . Region-specific association of subjective cognitive decline with tauopathy independent of global β-amyloid burden. JAMA Neurol. 2017;74(12):1455-1463. doi: 10.1001/jamaneurol.2017.2216 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54.Maass A, Lockhart SN, Harrison TM, et al. . Entorhinal tau pathology, episodic memory decline, and neurodegeneration in aging. J Neurosci. 2018;38(3):530-543. doi: 10.1523/JNEUROSCI.2028-17.2017 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 55.Jack CR Jr, Bennett DA, Blennow K, et al. ; Contributors . NIA-AA research framework: toward a biological definition of Alzheimer’s disease. Alzheimers Dement. 2018;14(4):535-562. doi: 10.1016/j.jalz.2018.02.018 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 56.Power MC, Mormino E, Soldan A, et al. . Combined neuropathological pathways account for age-related risk of dementia. Ann Neurol. 2018;84(1):10-22. doi: 10.1002/ana.25246 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 57.Sepulcre J, Grothe MJ, d’Oleire Uquillas F, et al. . Neurogenetic contributions to amyloid beta and tau spreading in the human cortex. Nat Med. 2018;24(12):1910-1918. doi: 10.1038/s41591-018-0206-4 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 58.Gibbons GS, Lee VMY, Trojanowski JQ. Mechanisms of cell-to-cell transmission of pathological tau: a review. JAMA Neurol. 2019;76(1):101-108. doi: 10.1001/jamaneurol.2018.2505 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 59.Jagust W. Imaging the evolution and pathophysiology of Alzheimer disease. Nat Rev Neurosci. 2018;19(11):687-700. doi: 10.1038/s41583-018-0067-3 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 60.Crary JF, Trojanowski JQ, Schneider JA, et al. . Primary age-related tauopathy (PART): a common pathology associated with human aging. Acta Neuropathol. 2014;128(6):755-766. doi: 10.1007/s00401-014-1349-0 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 61.Jack CR Jr, Knopman DS, Jagust WJ, et al. . Tracking pathophysiological processes in Alzheimer’s disease: an updated hypothetical model of dynamic biomarkers. Lancet Neurol. 2013;12(2):207-216. doi: 10.1016/S1474-4422(12)70291-0 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 62.Bateman RJ, Xiong C, Benzinger TLS, et al. ; Dominantly Inherited Alzheimer Network . Clinical and biomarker changes in dominantly inherited Alzheimer’s disease. N Engl J Med. 2012;367(9):795-804. doi: 10.1056/NEJMoa1202753 [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
eMethods 1. Structural MRI acquisition and processing
eReferences
eMethods 2. Exploratory statistical analyses
eTable 1. Between-sample demographic comparisons
eFigure 1. Association of partial volume-corrected medial temporal tau-PET with APOEε4 independent of amyloid-b
eTable 2. Regression coefficients of APOE4 on MTL Tau-PET stratified by cognitive status
eTable 3. Regression coefficients of models investigating gene-dose framework
eFigure 2. Voxelwise gene-dose association of APOE4 on MTL Tau-PET
eTable 4. Regression coefficients of models investigating APOE-VBM relationships
eTable 5. Regression coefficients of models investigating age*apoe4 interactions
eTable 6. Regression coefficients of models investigating APOE4-Tau associations without adjusting for amyloid-PET
eFigure 3. Voxel-vise association of APOE4 on Tau-PET unadjusted for amyloid-PET
eTable 7. Regression coefficients of models including synergistic interaction term