This economic evaluation assesses the incremental cost-effectiveness of combination intravitreal ranibizumab and verteporfin photodynamic therapy compared with ranibizumab monotherapy in patients with polypoidal choroidal vasculopathy.
Key Points
Question
What is the incremental cost-effectiveness of intravitreal ranibizumab with verteporfin photodynamic therapy (combination therapy) relative to ranibizumab monotherapy for patients with polypoidal choroidal vasculopathy?
Findings
In this economic evaluation of a hypothetical cohort of patients with polypoidal choroidal vasculopathy, combination therapy generated slightly greater quality-adjusted life-years (7.87 vs 7.85) at roughly equal lifetime costs during a lifetime horizon. For a 10-year horizon, combination therapy may not be cost-effective.
Meaning
These results suggest that, from a lifetime cost-effectiveness perspective, combination therapy with ranibizumab should be considered as an alternative to standard care for patients with polypoidal choroidal vasculopathy.
Abstract
Importance
The EVEREST II trial showed that for patients with polypoidal choroidal vasculopathy (PCV), intravitreal ranibizumab in combination with verteporfin photodynamic therapy improves visual acuity relative to ranibizumab monotherapy. However, whether combination therapy is incrementally cost-effective relative to monotherapy during a lifetime is unclear.
Objective
To assess the incremental cost-effectiveness of combination therapy compared with ranibizumab monotherapy in patients with PCV.
Design, Setting, and Participants
This model-based, economic evaluation used 2018 unit cost data from a tertiary eye hospital in Singapore, first- and second-year outcomes and resource use data from a multicenter trial across various Asian countries (EVEREST II) to model a hypothetical cohort of patients with symptomatic PCV. Scenario analyses and deterministic and probabilistic sensitivity analyses were performed to examine uncertainty. Data were collected from October 2018 through April 2019 and analyzed from March through October 2019.
Interventions
This model used data from the EVEREST II trial, in which all participants were given 0.5 mg of intravitreal ranibizumab once every 4 weeks for the first 3 months. Subsequent administration occurred as needed. For participants receiving combination therapy, standard fluence (50 J/cm3) photodynamic therapy with 6-mg/m2 verteporfin was administered once during the first 3 months and thereafter as needed.
Main Outcomes and Measures
Incremental cost per quality-adjusted life-year (QALY) gained for combination therapy relative to monotherapy for patients with PCV.
Results
In this model based on a cohort of 1000 patients aged 68 years, a patient with PCV incurred a total cost in Singapore dollars (SGD) of 92 327 (US $67 399) with combination therapy and SGD 92 371 (US $67 431) with monotherapy during a lifetime horizon, generating a modest cost savings of SGD 44 (US $32) per patient undergoing combination therapy. Lifetime QALYs were estimated to be 7.87 for combination therapy and 7.85 for monotherapy, for an incremental gain of 0.02 QALYs. Combination therapy remained cost-saving or cost-effective in all lifetime scenarios modeled, but during shorter time horizons and at lower monotherapy costs, it may not be cost-effective.
Conclusions and Relevance
This study found combination therapy to be a dominant (more effective and less costly) strategy, being similar in costs and slightly more effective than ranibizumab monotherapy during a lifetime horizon. However, decreasing the time horizon to less than 10 years and/or reductions in the cost of monotherapy may result in combination therapy no longer being cost-effective.
Introduction
Polypoidal choroidal vasculopathy (PCV) is a subtype of neovascular age-related macular degeneration, and studies show it is more common in Asian patients with age-related macular degeneration compared with their western counterparts.1,2,3 Left untreated, PCV can lead to irreversible vision loss due to hemorrhage and scarring4,5,6 and decreased vision-related quality of life (QOL).4,7 It is estimated that by 2050, PCV will affect more patients in Asia than in the rest of the world combined.3
The standard of care for PCV is intravitreal administration of anti–vascular endothelial growth factor (anti-VEGF) treatment, such as ranibizumab or aflibercept.8 Patients with PCV may be treated with anti-VEGF monotherapy,9,10 but evidence suggests that verteporfin photodynamic therapy (vPDT) used with intravitreal anti-VEGF agents (combination therapy) results in better visual acuity outcomes and decreased likelihood of PCV recurrence.11,12,13,14,15 EVEREST II, a multicenter randomized clinical trial, demonstrated significant gains in best-corrected visual acuity (BCVA) and regression of polyps with fewer intravitreal ranibizumab injections for patients receiving combination therapy compared with ranibizumab monotherapy.15 However, vPDT may lead to poor vision outcomes16 and is costly and not widely available, and whether combination therapy is a cost-effective treatment compared with monotherapy may be unclear. This study presents an incremental cost-effectiveness analysis from the health system perspective in Singapore using EVEREST II trial data and unit cost data from local sources to compare combination therapy (ranibizumab with vPDT) and ranibizumab monotherapy in patients with symptomatic PCV.
Methods
Overview
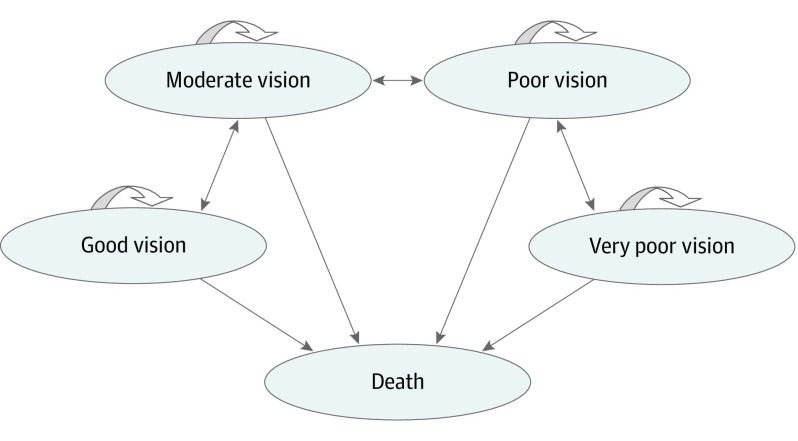
Data were collected from October 2018 through April 2019. This economic evaluation was deemed exempt from review and informed consent by the institutional review board at the National University of Singapore because no human participants were involved. We built a Markov cohort model in TreeAge Pro 2019, version R2.0,17 to estimate the lifetime cost and effectiveness of combination therapy relative to ranibizumab monotherapy from the Singapore health system perspective. In the model, patients cycled among 5 health states (Figure 1).15 Good vision was defined as those with Snellen visual acuity score measured in feet of 20/40 or better; moderate vision, 20/80 to worse than 20/40; poor vision, 20/160 to worse than 20/80; and very poor vision, worse than 20/160 to 20/320.
Figure 1. Model Schematic for Markov Model Showing All Possible Transitions Across the Health States.
During each annual cycle, participants’ BCVA levels could (1) increase by 15 Early Treatment Diabetic Retinopathy Study (ETDRS) letters or more and transition to the next best health state; (2) decrease by 15 ETDRS letters or more and transition to the next worst health state; or (3) gain or lose less than 15 letters and remain in the current health state. We assumed that patients began at the lowest ETDRS letter score of a health state (eg, moderate vision, score of 54). These definitions were necessary to ensure that transitions between health states were reflective of changes of 15 ETDRS letters. Half-cycle correction was implemented to account for patients who may transition at any point during a cycle.
The initial distribution of participants to the 4 vision health states (73 of 168 [43.5%] started with moderate vision during combination therapy and 65 of 153 [42.5%], during monotherapy) and subsequent transitions in the model were based on data from the EVEREST II study (Table 1). To ensure transitions between health states corresponded to changes of 15 ETDRS letters, the initial distributions reported in EVEREST II were modified to reflect our classification of health states; additional information and calculations can be found in eTables 1 and 2 in the Supplement.
Table 1. Baseline, Year 1, and Year 2 Characteristics of EVEREST II Study Population.
| Health State | Initial Distribution, No. (%)a | |
|---|---|---|
| Combination Therapy (n = 168) | Monotherapy (n = 153) | |
| Visionb | ||
| Good | 53 (31.5) | 50 (32.7) |
| Moderate | 73 (43.5) | 65 (42.5) |
| Poor | 34 (20.2) | 27 (17.6) |
| Very poor | 8 (4.8) | 11 (7.2) |
| Death | 0 | 0 |
| Efficacy | ||
| Participants gaining ≥15 letters | ||
| Year 1c,d | 41 (24.5) | 21 (14.0) |
| Year 2d,e | 51 (30.8) | 37 (24.2) |
| Participants losing ≥15 letters, % | ||
| Year 1c,d | 3 (1.9) | 9 (5.9) |
| Year 2d,e | 8 (4.8) | 8 (5.5) |
Initial distributions for poor vision and very poor vision as reported in Koh et al15; initial distributions for good vision and moderate vision are altered to adjust for our different categorization of patients for these 2 health states. Adjustments can be found in eTables 1 and 2 in the Supplement. Combination therapy consists of intravitreal ranibizumab with verteporfin photodynamic therapy; monotherapy, ranibizumab.
Good vision indicates Snellen visual acuity of 20/40 or better; moderate vision, 20/80 to worse than 20/40; poor vision, 20/160 to worse than 20/80; and very poor vision, worse than 20/160 to 20/320.
Data are from Koh et al.15
Numerator estimated from reported proportions and sample size.
Data are from Singh and Chhablani.18
In the base case, patients were treated during a lifetime horizon, but we also used a 10-year time horizon for all scenario and sensitivity analyses to test the effects of this assumption. After year 2, we assumed that treatment helped to maintain but no longer improved a patient’s visual acuity. Therefore, after year 2, no transitions were made between health states, except to death, based on all-cause mortality. We assumed treatment futility for patients in the very poor vision health state after 2 years, because patients in this group were legally blind. Therefore, patients in the very poor vision health state were assumed to no longer receive treatment after 2 years.
Further, we assumed that the efficacy of both treatments did not depend on health state and that equal proportions of patients gained or lost 15 or more letters from each health state. This assumption was necessary because the efficacy of each treatment was only reported in aggregate and not for specific health states. Age-specific all-cause mortality was modeled using 2016 to 2017 Singapore life tables.19 Ocular and nonocular adverse events were not modeled, because the safety profile for both treatments was similar.15
Costs
Patient use of health care resources was obtained from EVEREST II trial data (eTable 3 in the Supplement).15,18 Unit costs were 2018 unsubsidized private charges from the Singapore National Eye Centre and unsubsidized prices for medicines.
Effectiveness
Efficacy data come from the EVEREST II trial involving 322 participants (154 randomized to ranibizumab monotherapy and 168 randomized to combination therapy) with symptomatic PCV. In the EVEREST II trial, all participants were given 0.5 mg of intravitreal ranibizumab once every 4 weeks for the first 3 months. Subsequent administration occurred as needed. For participants receiving combination therapy, standard fluence (50 J/cm3) photodynamic therapy with 6-mg/m2 verteporfin was administered once during the first 3 months and thereafter as needed. Detailed inclusion and exclusion criteria have been published previously.15
Quality-adjusted life-years (QALYs) were calculated by assigning QOL weights to the BCVA ranges used to define the model health states (eTables 3 and 4 in the Supplement) and multiplying these weights by the length of time patients within the model spent in each associated health state. In the base case, QOL weights based on the better-seeing eye were used, because studies have shown that QOL is most affected by vision in this eye.20 The following equation used to derive QOL weights for each health state was estimated by Sharma et al21 through regression analysis:
| QOL (Visual Acuity) = 0.374 × Visual Acuity + 0.514 |
where the dependent variable is the QOL weight associated with a particular visual acuity score. All costs and QALYs were discounted at an annual rate of 3% according to guidelines in Singapore.22
Base Case Analysis
Data were analyzed from March 2019 through October 2019. Incremental cost-effectiveness was calculated by ranking the 2 treatments in order of increasing effectiveness. Treatments that had lower or equal effectiveness and higher costs were considered dominated. Incremental cost-effectiveness ratios (ICERs) were calculated by dividing the difference in cost between combination and monotherapy by the difference in QALYs.
Scenario Analyses
To test the effects of having different types of patients experience improvement or deterioration in their vision as a result of treatment, we first increased the probability of patients with better starting vision moving to a better health state (eg, from moderate vision to good vision) and increased the probability of patients with poor starting vision moving to a worse health state (eg, from poor vision to very poor vision) and then vice versa (eTable 5 in the Supplement). To model different time horizons, we ran 2-, 5-, and 10-year models. To test the effects of our assumptions regarding treatment futility for patients in the very poor vision health state, we modeled all patients continuing on treatment during the entire model time horizon. Then, we assumed treatment futility for patients with poor and very poor vision. We also tested the effect of using QOL weights derived from BCVAs in the treated eye (eTables 3 and 4 in the Supplement)23 because different sources of QOL weights have been shown to affect cost-effectiveness.23,24
Sensitivity Analyses
In 1-way deterministic sensitivity analyses (DSAs), costs were varied using the range from the collected data and probabilities, and QOL weights were varied using the upper and lower boundaries of their 95% CIs. The 95% CIs for QOL weights were calculated using the standard error (SE) from the regression coefficient.21 A 2-way DSA was conducted by simultaneously varying the cost of monotherapy (using a lower and upper bound representative of the cost of bevacizumab [Singapore dollars (SGD) 118 (US $86)] and aflibercept [SGD 1250 (US $913)]) and the probability of improved vision in the first year when receiving combination therapy. In probabilistic sensitivity analysis, 1000 Monte Carlo simulations were conducted. Gamma distributions were fitted to costs, and beta distributions were fitted to QOL weights and probabilities based on each parameter’s mean value and SE (eTable 6 in the Supplement). All sensitivity and scenario analyses were conducted using a lifetime horizon and a 10-year time horizon.
Results
Base Case Analysis
The model used a hypothetical cohort of 1000 patients aged 68 years, among whom those receiving combination therapy incurred lifetime costs of SGD 92 327 (US $67 399) compared with SGD 92 371 (US $67 431) for patients receiving monotherapy. Combination therapy was therefore SGD 44 (US $32) less expensive per patient than ranibizumab monotherapy during a lifetime. Mean estimated QALYs were slightly higher for those receiving combination therapy relative to monotherapy (7.87 vs 7.85 QALYs, for an incremental gain of 0.02 QALYs). Hence, during a lifetime horizon, combination therapy was dominant (ie, more effective and less costly) (Table 2).
Table 2. Cost-effectiveness Results From Base Case and Scenario Analysesa.
| Cost, Singapore Dollars | QALYs | ICER | ||||
|---|---|---|---|---|---|---|
| Total | Incremental | Effectiveness | Incremental Effectiveness | Singapore Dollars/QALY | US $/QALY | |
| Base Case–Lifetime Horizon | ||||||
| Monotherapy | 92 371 | NA | 7.85 | NA | Dominated | Dominated |
| Combination therapy | 92 327 | −44 | 7.87 | 0.02 | NA | NA |
| Participants With Better Vision Have a Greater Probability of Improving, and Participants With Worse Vision Have a Greater Probability of Deteriorating | ||||||
| Lifetime horizon | ||||||
| Monotherapy | 87 653 | NA | 8.22 | NA | NA | NA |
| Combination therapy | 88 684 | 1031 | 8.34 | 0.13 | 8051 | 5877 |
| 10-y horizon | ||||||
| Monotherapy | 59 476 | NA | 5.36 | NA | NA | NA |
| Combination therapy | 61 136 | 1660 | 5.44 | 0.08 | 19 698 | 14 380 |
| Participants With Better Vision Have a Greater Probability of Deteriorating, and Participants With Worse Vision Have a Greater Probability of Improving | ||||||
| Lifetime horizon | ||||||
| Monotherapy | 99 886 | NA | 8.15 | NA | Dominated | Dominated |
| Combination therapy | 97 803 | −2083 | 8.26 | 0.11 | NA | NA |
| 10-y horizon | ||||||
| Monotherapy | 66 345 | NA | 5.32 | NA | Dominated | Dominated |
| Combination therapy | 66 315 | −30 | 5.39 | 0.07 | NA | NA |
| 2-y Model | ||||||
| Monotherapy | 17 682 | NA | 1.225 | NA | NA | NA |
| Combination therapy | 20 221 | 2540 | 1.229 | 0.004 | 711 565 | 519 442 |
| 5-y Model | ||||||
| Monotherapy | 36 754 | NA | 2.88 | NA | NA | NA |
| Combination therapy | 38 587 | 1833 | 3.89 | 0.01 | 238 475 | 174 087 |
| 10-y Model | ||||||
| Monotherapy | 62 054 | NA | 5.13 | NA | NA | NA |
| Combination therapy | 63 127 | 1073 | 5.14 | 0.01 | 80 655 | 58 878 |
| Participants With Very Poor Vision Continue Treatment | ||||||
| Lifetime horizon | ||||||
| Monotherapy | 97 683 | NA | 7.85 | NA | Dominated | Dominated |
| Combination therapy | 95 860 | −1823 | 7.87 | 0.02 | NA | NA |
| 10-y horizon | ||||||
| Monotherapy | 64 958 | NA | 5.13 | NA | NA | NA |
| Combination therapy | 65 071 | 113 | 5.14 | 0.01 | 8499 | 6204 |
| Assume Treatment Futility for Participants With Poor Vision and Very Poor Vision | ||||||
| Lifetime horizon | ||||||
| Monotherapy | 78 951 | NA | 7.85 | NA | Dominated | Dominated |
| Combination therapy | 78 268 | −683 | 7.87 | 0.02 | NA | NA |
| 10-y horizon | ||||||
| Monotherapy | 54 721 | NA | 5.13 | NA | NA | NA |
| Combination therapy | 55 444 | 723 | 5.14 | 0.01 | 54 380 | 39 697 |
| Using QOL Weights Based on Visual Acuity in the Treated Eye | ||||||
| Lifetime horizon | ||||||
| Monotherapy | 92 371 | NA | 9.69 | NA | Dominated | Dominated |
| Combination therapy | 92 327 | −44 | 9.73 | 0.03 | NA | NA |
| 10-y horizon | ||||||
| Monotherapy | 62 054 | NA | 6.34 | NA | NA | NA |
| Combination therapy | 63 127 | 1073 | 6.36 | 0.02 | 46 830 | 34 186 |
Abbreviations: ICER, incremental cost-effectiveness ratio; NA, not applicable; QALY, quality-adjusted life-year; QOL, quality of life.
Combination therapy indicates ranibizumab with verteporfin photodynamic therapy; monotherapy, ranibizumab. Good vision indicates Snellen visual acuity of 20/40 or better; moderate vision, 20/80 to worse than 20/40; poor vision, 20/160 to worse than 20/80; and very poor vision, worse than 20/160 to 20/320. In Singapore dollars, 1 equals US $0.73 as of September 5, 2018, from XE Currency Converter.
Scenario Analyses
When patients in better starting health states had a greater probability of improving, monotherapy was no longer dominant (ie, less effective and more costly). However, combination therapy remained highly cost-effective (ICER = SGD 8051/QALY [US $5877/QALY] during a lifetime horizon; ICER = SGD 19 698/QALY [US $14 380/QALY] during a 10-year time horizon) based on a willingness to pay of 1 times the gross domestic product per capita of Singapore (SGD 79 357/QALY [US $57 931/QALY]). When patients in better starting health states had a greater probability of deteriorating, monotherapy remained dominated (less effective and more costly) during a lifetime and a 10-year time horizon. Model results were sensitive to changes in the time horizon (eFigure 1 in the Supplement). The ICER for combination therapy was only slightly higher than the cited willingness-to-pay threshold in the 10-year model (SGD 80 655/QALY [US $58 878/QALY]) but was much larger for the 2-year (SGD 711 565/QALY [US $519 442/QALY]) and 5-year (SGD 238 475/QALY [US $174 087/QALY]) models. When all patients continue treatment, when treatment futility is assumed for patients with poor and very poor vision and when using QOL weights based on BCVAs in the treated eye, combination therapy remained dominant (ie, more effective and less costly) during a lifetime horizon. For the same 3 scenarios during a 10-year time horizon, combination therapy was not dominant (more effective and less costly) but remained cost-effective. These analyses are summarized in Table 2.
Sensitivity Analyses
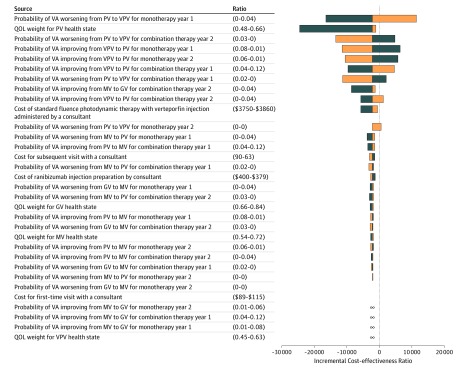
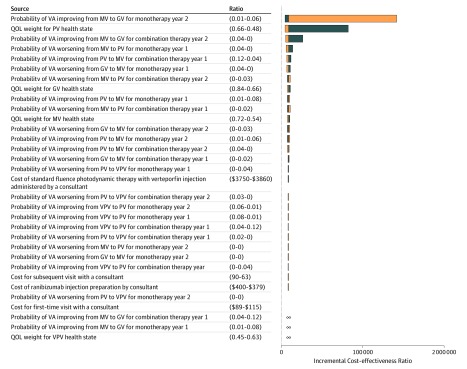
The parameters with the largest effects on the ICER were the transition probabilities of moving between health states and the QOL weight for very poor vision (Figure 2 and Figure 3). All sensitivity analyses when using a lifetime horizon resulted in ICER estimates that were below the commonly accepted threshold for Singapore (SGD 79 357 [US $57 931]) (eTable 7 in the Supplement). When using a 10-year time horizon, ICER estimates were only below the accepted threshold when using the lower bound estimates for each parameter (eTable 8 in the Supplement).
Figure 2. Tornado Diagram for 1-Way Sensitivity Analyses of Combination vs Monotherapy Therapy During a Lifetime Horizon.
Negative incremental cost-effectiveness ratios (ICERs) in the tornado diagram represent 1-way sensitivity analyses in which combination therapy (intravitreal ranibizumab and verteporfin photodynamic therapy) dominates ranibizumab monotherapy (ie, combination therapy has higher quality-adjusted life-years [QALYs] and lower costs compared with monotherapy). For 4 of the parameters, the 1-way sensitivity analysis resulted in combination therapy being less effective and less costly than monotherapy, but an ICER below the accepted threshold or monotherapy being dominated by combination therapy. These parameters are labeled with ∞. Additional details of the 1-way sensitivity analyses for these 4 parameters are also provided in eTable 6 in the Supplement. See the Overview subsection of the Methods section for definitions of the vision categories. Blue bars indicate results when using lower parameter value; orange bars, results when using upper parameter value. GV indicates good vision; MV, moderate vision; QOL, quality of life; PV, poor vision; VA, visual acuity; and VPV, very poor vision.
Figure 3. Tornado Diagram for 1-Way Sensitivity Analyses of Combination vs Monotherapy Therapy During a 10-Year Horizon.
Negative incremental cost-effectiveness ratios (ICERs) in the tornado diagram represent 1-way sensitivity analyses where combination therapy (intravitreal ranibizumab and verteporfin photodynamic therapy) dominates ranibizumab monotherapy (ie, combination therapy has higher quality-adjusted life-years [QALYs] and lower costs compared with monotherapy). For 3 parameters, the 1-way sensitivity analysis resulted in combination therapy being both more effective and more costly then monotherapy, but an ICER below the accepted threshold or combination therapy being dominated by monotherapy. These parameters are labeled with ∞. Additional details of the 1-way sensitivity analyses for these 3 parameters are also provided in eTable 6 in the Supplement. See the Overview subsection of the Methods section for definitions of the vision categories. Blue bars indicate results when using lower parameter value; orange bars, results when using upper parameter value. GV indicates good vision; MV, moderate vision; QOL, quality of life; PV, poor vision; VA, visual acuity; and VPV, very poor vision.
The results of the 2-way DSA highlight that during a lifetime horizon and at the lowest cost of anti-VEGF monotherapy (SGD 118 [US $86]), combination therapy would never be cost-effective over the range of plausible values of improving vision while receiving combination therapy. Only at costs of anti-VEGF monotherapy slightly less than SGD 850 (US $621) did combination therapy become cost-effective at probability values in the middle and high range (eFigure 2A in the Supplement). A similar trend was also observed during a 10-year time horizon, with the exception that monotherapy costs slightly greater than SGD 850 were required for combination therapy to be cost-effective at the middle and low probability values (eFigure 2B in the Supplement).
Using a willingness-to-pay threshold of 1 times the gross domestic product per capita of Singapore (SGD 79 357 [US $57 931]), combination therapy was cost-effective in 65% and 49% of the iterations of the probabilistic sensitivity analysis for the lifetime and 10-year time horizons, respectively (eFigure 3A-B in the Supplement). However, for the lifetime and 10-year time horizons, combination therapy was less effective and more costly than monotherapy in approximately 14% and 33% of the iterations, respectively (eFigure 4A-B in the Supplement).
Discussion
Our study reveals that for patients with PCV, ranibizumab with vPDT (combination therapy) is a dominant (ie, more effective and less costly) treatment strategy, because it generates higher QALYs (7.87 vs 7.85) at a comparable cost (cost saving by SGD 44 [US $32]) relative to ranibizumab monotherapy during a lifetime horizon. Combination therapy remained dominant (more effective and less costly) or cost-effective in all lifetime scenario analyses. However, results from 1-way DSAs using a 10-year time horizon and 2-way DSAs indicate that ICER estimates for combination therapy were only less than the accepted threshold when using the lower-bound estimates for each parameter and that relatively small decreases in the cost of monotherapy required large increases in the probability of vision improvement for combination therapy to remain cost-effective. Furthermore, results from 2- and 5-year scenarios found the ICER for combination therapy to be well above commonly cited willingness-to-pay thresholds. These findings suggest that an up-front cost and time investment is required before the benefits of combination therapy relative to monotherapy materialize. Therefore, if other inexpensive anti-VEGF therapies with effectiveness similar to ranibizumab monotherapy become available in the near future, combination therapy likely would not be cost-effective.
Despite some indication that vPDT is a useful and versatile tool for ophthalmologists in treating PCV,25 the evidence of the potential benefit of combining vPDT with anti-VEGFs for PCV remains limited. Two clinical trials (EVEREST II and PLANET [Aflibercept in Polypoidal Choroidal Vasculopathy]) have assessed the efficacy of vPDT in combination with anti-VEGFs relative to anti-VEGF monotherapy.10,15 Both trials considered changes in 2 main outcomes: visual acuity gain and polyp regression; however, only EVEREST II examined vPDT in combination with ranibizumab at baseline as a true combination first-line treatment. Our analysis is based on the EVEREST II trial results and therefore addresses the question of whether combination therapy of ranibizumab with vPDT as first-line treatment is a cost-effective therapy. As such, our results cannot be extrapolated to other treatment regimens, such as the rescue treatment in PLANET. In addition, our analysis solely focused on using data from a randomized comparison of 2 treatments approved by regulatory agencies for use in patients with PCV in Singapore. Of note, bevacizumab is available, although not approved by regulatory agencies, for PCV. It has been shown to have noninferior visual acuity outcomes to ranibizumab or aflibercept in randomized clinical trials when given monthly for neovascular age-related macular degeneration,26 at the primary outcome time in macular edema from central retinal vein occlusion,27,28 and for diabetic macular edema when visual acuity ranged from 20/32 to 20/40.29 We are not aware of any randomized clinical trials comparing bevacizumab with ranibizumab or aflibercept for PCV, but only small nonrandomized studies.30 If the effectiveness of bevacizumab was noninferior to ranibizumab for PCV, and if we were to consider bevacizumab as a comparator in our analysis (eg, low-cost anti-VEGF therapy), the incremental cost-effectiveness of combination therapy would be greatly reduced, as shown by our 2-way DSAs.
Although we show a slight improvement in QALYs for the average patient during a lifetime, given the heterogeneity in outcomes, many patients will experience visual acuity and QALY gains far from the average and therefore may or may not benefit from combination therapy. Future research should therefore aim to identify which subset of patients is most likely to benefit.
There is reason to believe that our estimates may be conservative. EVEREST II indicated a relatively small gain in ETDRS letters for patients receiving combination therapy relative to monotherapy (mean gain of 9.7 compared with 5.6 ETDRS letters).18 However, greater benefits in total polyp regression were observed for patients in combination therapy than in monotherapy in the EVEREST II study after 2 years (55.6% compared with 26.7%).18 Owing to the lack of QOL weights for different levels of polyp regression, we were unable to model the effect of this outcome in our analysis, which would have led to an even more favorable result in favor of combination therapy.
An additional consideration consists of potential differences in compliance with treatment.6 Because combination therapy requires fewer injections than monotherapy (mean, 8.1 vs 12.5 injections in 2 years), there are likely to be differences in compliance18 that may influence treatment effectiveness. Incorporating differences in compliance should also be an area of future research, because these differences will affect costs and QALYs.
Limitations
This study is subject to several limitations. A primary limitation is that we used mean values as reported in the EVEREST II trial and hence assumed that the key model parameters (eg, transition probabilities) were the same regardless of the patients’ starting visual acuity. This assumption was necessary because we did not have access to patient-level data.
Further, our model did not necessarily capture the increased likelihood of transitioning to better health states through the gradual accumulation of visual acuity improvements for individuals who entered the model in the middle or higher range for a given health state. This limitation was due to our assumption that patients began at the lowest ETDRS letter score of a health state. We suspect that if our base case was modeled with transition probabilities that accounted for smaller ETDRS letter changes, our results would further favor combination therapy.
We were also limited by the trial duration, which concluded after 2 years. Model inputs were determined based on the 2-year data and propagated through a lifetime horizon in our model. If dosages and/or treatment duration vary beyond the 2-year period, then our results may be biased in unknown directions.
A final limitation was the use of QOL weights converted from BCVA that were developed based on anchoring at perfect vision rather than perfect health. Although this approach is commonly used,20 this limitation should be noted. Anchoring at perfect vision has been shown to result in lower QOL weights compared with anchoring at perfect health, which can result in greater benefit being observed with successful treatment.31 To avoid these issues, it would have been preferable to have trial participants complete a validated preference-based instrument, such as the EuroQoL 5-dimension questionnaire, from which QOL weights could have been indirectly estimated, but unfortunately those data were not collected.
Conclusions
Our base case analysis and scenario and sensitivity analyses indicate that combination therapy consisting of ranibizumab with vPDT is slightly more cost-effective and potentially cost saving for patients with PCV compared with ranibizumab monotherapy from a lifetime perspective. However, decreasing the time horizon to less than 10 years and/or small reductions in the cost of monotherapy may result in combination therapy no longer being cost-effective.
eTable 1. Visual Acuity Ranges and Participant Distribution to Visual Acuity Ranges as Reported in EVEREST II
eTable 2. Visual Acuity Ranges and Participant Distributions Used in Model, Stratified by Health State
eTable 3. Parameters and Base-Case Values
eTable 4. Calculation of Utilities for Model Health States Using Best-Seeing Eye and Treated Eye
eTable 5. Transition Probabilities for Base Case and Scenario Analyses
eTable 6. Model Values for Deterministic and Probabilistic Sensitivity Analyses
eTable 7. Incremental Cost-effectiveness Ratio Estimates From 1-Way Deterministic Sensitivity Analysis–Lifetime Horizon
eTable 8. Incremental Cost-effectiveness Ratio Estimates From 1-Way Deterministic Sensitivity Analysis–10-Year Time Horizon
eFigure 1. Incremental Cost-effectiveness Ratio Across 20-Year Decision-Making Time-Horizon
eFigure 2. Two-Way Sensitivity Analysis
eFigure 3. Cost-effectiveness Acceptability Curve Showing Proportion of Cost-effective Iterations Across Willingness-to-Pay Thresholds, Stratified by Treatment
eFigure 4. Incremental Cost-effectiveness Scatter Plot
eReferences.
References
- 1.Maruko I, Iida T, Saito M, Nagayama D, Saito K. Clinical characteristics of exudative age-related macular degeneration in Japanese patients. Am J Ophthalmol. 2007;144(1):15-22. doi: 10.1016/j.ajo.2007.03.047 [DOI] [PubMed] [Google Scholar]
- 2.Kawasaki R, Yasuda M, Song SJ, et al. The prevalence of age-related macular degeneration in Asians: a systematic review and meta-analysis. Ophthalmology. 2010;117(5):921-927. doi: 10.1016/j.ophtha.2009.10.007 [DOI] [PubMed] [Google Scholar]
- 3.Wong CW, Yanagi Y, Lee WK, et al. Age-related macular degeneration and polypoidal choroidal vasculopathy in Asians. Prog Retin Eye Res. 2016;53:107-139. doi: 10.1016/j.preteyeres.2016.04.002 [DOI] [PubMed] [Google Scholar]
- 4.Berdeaux GH, Nordmann JP, Colin E, Arnould B. Vision-related quality of life in patients suffering from age-related macular degeneration. Am J Ophthalmol. 2005;139(2):271-279. doi: 10.1016/j.ajo.2004.09.028 [DOI] [PubMed] [Google Scholar]
- 5.Cheung CM, Yang E, Lee WK, et al. The natural history of polypoidal choroidal vasculopathy: a multi-center series of untreated Asian patients. Graefes Arch Clin Exp Ophthalmol. 2015;253(12):2075-2085. doi: 10.1007/s00417-015-2933-2 [DOI] [PubMed] [Google Scholar]
- 6.Cheung CMG, Lai TYY, Ruamviboonsuk P, et al. Polypoidal choroidal vasculopathy: definition, pathogenesis, diagnosis, and management. Ophthalmology. 2018;125(5):708-724. doi: 10.1016/j.ophtha.2017.11.019 [DOI] [PubMed] [Google Scholar]
- 7.Fenwick EK, Cheung CMG, Ong PG, et al. The impact of typical neovascular age-related macular degeneration and polypoidal choroidal vasculopathy on vision-related quality of life in Asian patients. Br J Ophthalmol. 2017;101(5):591-596. doi: 10.1136/bjophthalmol-2016-308541 [DOI] [PubMed] [Google Scholar]
- 8.Mitchell P, Liew G, Gopinath B, Wong TY. Age-related macular degeneration. Lancet. 2018;392(10153):1147-1159. doi: 10.1016/S0140-6736(18)31550-2 [DOI] [PubMed] [Google Scholar]
- 9.Lee WK, Iida T, Ogura Y, et al. ; PLANET Investigators . Efficacy and safety of intravitreal aflibercept for polypoidal choroidal vasculopathy in the PLANET study: a randomized clinical trial. JAMA Ophthalmol. 2018;136(7):786-793. doi: 10.1001/jamaophthalmol.2018.1804 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Wong TY, Ogura Y, Lee WK, et al. Efficacy and safety of intravitreal aflibercept for polypoidal choroidal vasculopathy: 2-year results of the PLANET study. Am J Ophthalmol. 2019;204:80-89. doi: 10.1016/j.ajo.2019.02.027 [DOI] [PubMed] [Google Scholar]
- 11.Ng WY, Cheung CM, Mathur R, et al. Trends in age-related macular degeneration management in Singapore. Optom Vis Sci. 2014;91(8):872-877. doi: 10.1097/OPX.0000000000000283 [DOI] [PubMed] [Google Scholar]
- 12.Ruamviboonsuk P, Tadarati M, Vanichvaranont S, Hanutsaha P, Pokawattana N. Photodynamic therapy combined with ranibizumab for polypoidal choroidal vasculopathy: results of a 1-year preliminary study. Br J Ophthalmol. 2010;94(8):1045-1051. doi: 10.1136/bjo.2009.173120 [DOI] [PubMed] [Google Scholar]
- 13.Lee YH, Lee EK, Shin KS, Lee KM, Kim JY. Intravitreal ranibizumab combined with verteporfin photodynamic therapy for treating polypoidal choroidal vasculopathy. Retina. 2011;31(7):1287-1293. doi: 10.1097/IAE.0b013e3182003ccd [DOI] [PubMed] [Google Scholar]
- 14.Kim M, Kim K, Kim DG, Yu SY, Kwak HW. Two-year results of photodynamic therapy combined with intravitreal anti–vascular endothelial growth factor for polypoidal choroidal vasculopathy. Ophthalmologica. 2011;226(4):205-213. doi: 10.1159/000330793 [DOI] [PubMed] [Google Scholar]
- 15.Koh A, Lai TYY, Takahashi K, et al. ; EVEREST II study group . Efficacy and safety of ranibizumab with or without verteporfin photodynamic therapy for polypoidal choroidal vasculopathy: a randomized clinical trial. JAMA Ophthalmol. 2017;135(11):1206-1213. doi: 10.1001/jamaophthalmol.2017.4030 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Wong CW, Cheung CMG, Mathur R, et al. Three-year results of polypoidal choroidal vasculopathy treated with photodynamic therapy: retrospective study and systematic review. Retina. 2015;35(8):1577-1593. doi: 10.1097/IAE.0000000000000499 [DOI] [PubMed] [Google Scholar]
- 17.TreeAge Pro, R2 [computer program]. Williamstown, MA: TreeAge Software; 2018.
- 18.Singh SR, Chhablani J. Analysis of 2-year data from the EVEREST II and PLANET trials. Retin Physician. 2018;15:36-38. https://www.retinalphysician.com/issues/2018/july-august-2018/analysis-of-2-year-data-from-the-everest-ii-and-pl. Accessed November 4, 2019. [Google Scholar]
- 19.Department of Statistics Complete Life Tables for Singapore Resident Population, 2016-2017 Republic of Singapore: Ministry of Trade and Industry; 2018. [Google Scholar]
- 20.Herring W, Carrico J, Mladsi D, Pierson R, Lofland J. A review of vision-related utility values and their suitability for use in cost-effectiveness models in age-related macular degeneration. Value Health. 2017;20(5):A159. [Google Scholar]
- 21.Sharma S, Brown GC, Brown MM, et al. Converting visual acuity to utilities. Can J Ophthalmol. 2000;35(5):267-272. doi: 10.1016/S0008-4182(00)80077-0 [DOI] [PubMed] [Google Scholar]
- 22.Agency for Care Effectiveness Drug Evaluation Methods and Process Guide—Version 1.0. Singapore: Ministry of Health; 2018. [Google Scholar]
- 23.Mitchell P, Annemans L, Gallagher M, et al. Cost-effectiveness of ranibizumab in treatment of diabetic macular oedema (DME) causing visual impairment: evidence from the RESTORE trial. Br J Ophthalmol. 2012;96(5):688-693. doi: 10.1136/bjophthalmol-2011-300726 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Hutton DW, Stein JD, Bressler NM, Jampol LM, Browning D, Glassman AR; Diabetic Retinopathy Clinical Research Network . Cost-effectiveness of intravitreous ranibizumab compared with panretinal photocoagulation for proliferative diabetic retinopathy: secondary analysis from a Diabetic Retinopathy Clinical Research Network randomized clinical trial. JAMA Ophthalmol. 2017;135(6):576-584. doi: 10.1001/jamaophthalmol.2017.0837 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Kumar A, Kumawat D, Sundar M D, et al. Polypoidal choroidal vasculopathy: a comprehensive clinical update. Ther Adv Ophthalmol. 2019;11:2515841419831152. doi: 10.1177/2515841419831152 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Martin DF, Maguire MG, Ying GS, Grunwald JE, Fine SL, Jaffe GJ; CATT Research Group . Ranibizumab and bevacizumab for neovascular age-related macular degeneration. N Engl J Med. 2011;364(20):1897-1908. doi: 10.1056/NEJMoa1102673 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Scott IU, VanVeldhuisen PC, Ip MS, et al. ; SCORE2 Investigator Group . Effect of bevacizumab vs aflibercept on visual acuity among patients with macular edema due to central retinal vein occlusion: the SCORE2 randomized clinical trial. JAMA. 2017;317(20):2072-2087. doi: 10.1001/jama.2017.4568 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Hykin P, Prevost AT, Vasconcelos JC, et al. ; LEAVO Study Group . Clinical effectiveness of intravitreal therapy with ranibizumab vs aflibercept vs bevacizumab for macular edema secondary to central retinal vein occlusion: a randomized clinical trial [published online August 29, 2019]. JAMA Ophthalmol. doi: 10.1001/jamaophthalmol.2019.3305 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Cai S, Bressler NM. Aflibercept, bevacizumab or ranibizumab for diabetic macular oedema: recent clinically relevant findings from DRCR.net Protocol T. Curr Opin Ophthalmol. 2017;28(6):636-643. doi: 10.1097/ICU.0000000000000424 [DOI] [PubMed] [Google Scholar]
- 30.Cho HJ, Kim JW, Lee DW, Cho SW, Kim CG. Intravitreal bevacizumab and ranibizumab injections for patients with polypoidal choroidal vasculopathy. Eye (Lond). 2012;26(3):426-433. doi: 10.1038/eye.2011.324 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Lee BS, Kymes SM, Nease RF Jr, Sumner W, Siegfried CJ, Gordon MO. The impact of anchor point on utilities for 5 common ophthalmic diseases. Ophthalmology. 2008;115(5):898-903.e4. doi: 10.1016/j.ophtha.2007.06.008 [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
eTable 1. Visual Acuity Ranges and Participant Distribution to Visual Acuity Ranges as Reported in EVEREST II
eTable 2. Visual Acuity Ranges and Participant Distributions Used in Model, Stratified by Health State
eTable 3. Parameters and Base-Case Values
eTable 4. Calculation of Utilities for Model Health States Using Best-Seeing Eye and Treated Eye
eTable 5. Transition Probabilities for Base Case and Scenario Analyses
eTable 6. Model Values for Deterministic and Probabilistic Sensitivity Analyses
eTable 7. Incremental Cost-effectiveness Ratio Estimates From 1-Way Deterministic Sensitivity Analysis–Lifetime Horizon
eTable 8. Incremental Cost-effectiveness Ratio Estimates From 1-Way Deterministic Sensitivity Analysis–10-Year Time Horizon
eFigure 1. Incremental Cost-effectiveness Ratio Across 20-Year Decision-Making Time-Horizon
eFigure 2. Two-Way Sensitivity Analysis
eFigure 3. Cost-effectiveness Acceptability Curve Showing Proportion of Cost-effective Iterations Across Willingness-to-Pay Thresholds, Stratified by Treatment
eFigure 4. Incremental Cost-effectiveness Scatter Plot
eReferences.